Abstract
Trypanosoma cruzi infection of host cells is a complex process in which many proteins participate but only a few of these proteins have been identified experimentally. One parasite factor likely to be involved is the protein product of LYT1, a single-copy gene cloned, sequenced, and characterized by Manning-Cela et al. (Infect. Immun. 69:3916-3923, 2001). This gene was potentially associated with infectivity, since the deletion of both LYT1 alleles in the CL Brenner strain (the wild type [WT]) resulted in a null mutant T. cruzi clone (L16) that shows an attenuated phenotype in cell culture models. The aim of this work was to characterize the infective behavior of L16 in the insect vector and murine models. The infection of adult Swiss mice with 103 trypomastigotes of both clones revealed a significant reduction in infective behavior of L16, as shown by direct parasitemia, spleen index, and quantitation of tissue parasite burden, suggesting the loss of virulence in the null mutant clone. Although L16 blood counts were almost undetectable, blood-based PCRs indicated the presence of latent and persistent infection during all of the study period and epimastigotes were reisolated from hemocultures until 12 months postinfection. Nevertheless, virulence was not restored in L16 by serial passages in mice, and reisolated parasites lacking the LYT1 gene and bearing the antibiotic resistance genes revealed the stability of the genetic manipulation. Histopathological studies showed a strong diminution in the muscle inflammatory response triggered by L16 compared to that triggered by the WT group, consistent with a lower tissue parasite load. A strong protection against a virulent challenge in both L16- and WT-infected mice was observed; however, the immunizing infection by the genetically modified parasite was highly attenuated.
Trypanosoma cruzi is the causative agent of Chagas' disease and infects an estimated 18 million people in the American continents (27). This protozoan parasite is transmitted by three main routes: via the feces of infected triatomine insects, via blood transfusions, and from mother to fetus during pregnancy. Parasites persist through the entire life of the patient, and the disease normally progresses from an acute stage characterized only by systemic parasitemia to a virtually asymptomatic stage that can last for several decades. Eventually, one-third of chronically infected patients manifest symptoms of Chagas' disease, developing severe cardiac and/or gastrointestinal disease, which leads to death in most cases. Nowadays there is neither an effective cure nor a vaccine against T. cruzi infection, and the only methods for combating the disease are the control of the vector in areas of endemicity and the prevention of transmission via blood supply.
The invasion of host cells by T. cruzi involves several steps: attachment of the parasite to the cell surface, internalization mediated by the recruitment and fusion of host cell lysosomes, and escape of the parasite from the parasitophorous vacuole to multiply freely in the cytosol as amastigotes (2, 3, 26). Though many parasite proteins are very important for T. cruzi infection, surprisingly, only a few have been identified experimentally. One parasite factor likely involved in the infection is the protein product of the LYT1 gene. Manning-Cela et al. (22) isolated a LYT1 cDNA clone from a Y strain T. cruzi amastigote library by virtue of its cross-reactivity to antibodies against the human complement component C9. On this basis, the researchers proposed that the 552-amino-acid protein encoded by the LYT1 open reading frame could be involved in a lytic pathway, mediating the escape of T. cruzi from the acidic parasitophorous vacuole into the cytosol, as was previously shown for the T. cruzi protein Tc-TOX with pore-forming activity at a low pH (4). However, a bioinformatics analysis of all available DNA and protein databases failed to identify paralogs. To gain insight into the possible role of the LYT1 gene product, the researchers generated a biallelic, LYT1 deletion in the CL strain and the biological characteristics of a mutant clone (clone L16) were assessed. The deletion of both alleles did not impair the capacity of epimastigote parasites to proliferate in axenic cultures. Nevertheless, the LYT1 null parasites exhibited reduced infectivity in cell culture experiments and also displayed accelerated in vitro-stage transition and diminished hemolytic activity at an acidic pH (23). These distinct phenotypes were attributed to the fact that different forms of the protein are expressed as a result of alternative trans splicing. Through this genetic regulation process of the primary transcript, two LYT1 protein derivates were obtained, differing only in the presence or absence of the first 28 amino acids. The trans-splicing processing was developmentally regulated, since the complete form was expressed mostly in the mammalian stages and the shorter one in epimastigotes (23). Recently, Benabdellah et al. (9) revealed how these two proteins can be involved in processes so different as developmental regulation and cell invasion. The researchers found that the shorter form, named kLYT1, localizes in the kinetoflagellar zone and is responsible for the accelerated-stage development phenotype, while the full-size LYT1, named mLYT1, was associated with impaired infectivity. The latter is a type II membrane-anchored protein, whose mature active form is released by cleavage of the signal sequence resident in the amino-terminal end. Hemolytic activity has been correlated with infectivity, and this behavior was shown to be displayed only by the parasites expressing LYT1 on their surfaces (9).
Although several targeted gene deletion studies allowed the identification of parasite factors involved in phenotypic expression of T. cruzi virulence, only a few progressed to in vivo experiments testing the ability of mutant parasites to produce disease in experimental hosts (7, 11, 17).
On these bases, the main aim of this work was to characterize the infective behavior of the L16 clone in the insect vector and murine models. The deletion of LYT1 produced a sharp and stable loss of virulence in the parasite for mice, without significant differences in the percentage of infection to the triatomine vector and in the number of parasites released in the feces of the bugs. The mutant clone displayed an attenuated pathogenicity and was able to protect against virulent T. cruzi challenge.
MATERIALS AND METHODS
Parasites.
The CL Brenner strain of T. cruzi, referred to here as the wild type (WT), and its mutant LYT1 null derivative L16 clone (22) were used. Transfection, selection, and cloning of L16 were fulfilled by homologous recombination at the Instituto de Parasitología y Biomedicina (CSIC, Granada, Spain). The lines were kept frozen in liquid nitrogen until used and serially cultured in liver infusion-tryptose medium (LIT) supplemented with 10% fetal bovine serum, 1% hemin, and antibiotics (LIT-HSP). Mid-log-phase cultures were used in all experiments. Cultures were submitted to antibiotic pressure, G418 (250 μg/ml) or hygromycin B (250 μg/ml), to check the stability of the mutation. Metacyclogenesis was carried out by adding 1% triatomine gut homogenate (19) to epimastigote cultures and harvesting the parasites after 7 to 10 days. Complement-resistant forms (CRF) purified using normal nondecomplemented serum were quantified in a Neubauer chamber and further used to inoculate experimental animals. To test for immune protection against virulent challenge, the highly virulent Tulahuen mouse passage strain was used (8).
Mice.
Male Swiss mice (1.5 to 2.5 months old) bred in our colony were inoculated by the intraperitoneal (i.p.) route with either 1 × 103 CRF or 1 × 104 bloodstream forms as specified. Animal care guidelines adopted by the School of Health Sciences, National University of Salta (18), were strictly followed.
Parasitological determinations.
Blood (10 μl) was drawn from the tail tips of mice under slight ether anesthesia, and the number of parasites per 100 fields (parasitemia) was recorded from fresh blood mounts (FBM) under the microscope (magnification, ×400). Hemocultures were performed by seeding 200 μl of heparinized blood into 2 ml LIT-HSP under sterile conditions; the cultures were incubated at 28°C and analyzed at 15, 30, 45, and 60 days.
Immunization tests.
To test the immunological protection induced by the L16 clone, the inoculated mice were challenged, after different periods of time, with 104 blood trypomastigotes of the Tulahuen strain.
PCR.
For parasite determination, 700 μl of blood was processed following strict decontamination procedures. Sample storage, DNA extraction, and amplification using primers #121 (5′-AAATAATGTACGGGTGAGATGCATGA-3′) and #122 (5′-GGTTCGATTGGGGTTGGTGTAATATA-3′) were performed as previously described (10).
To amplify genomic fragments at the LYT1 locus, DNA was extracted by standard protocols. Three pairs of primers were used. Pair 1 consisted of LYT1F (5′-ATGCGGAAGAAAGCCGCAG-3′), annealing to the initiation codon, and LYT2R (5′-TCAATCAGCTGCCAGCATGT-3′), which anneals to the stop codon amplifying the whole coding sequence (CDS) of the LYT1 gene (1,659 bp). Pair 2 consisted of LYT3F (5′-TGCATTGAACAGACAGCTAGA-3′), which anneals to 520 bp downstream from the LYT1 initiation codon, and LYT2R (the expected size of the product was 1,140 bp). Pair 3 consisted of LYT1F and LYT4R (5′-TGCTCCTGAGACAAAGCATG-3′), which amplifies an internal 640-bp fragment of the coding sequence of the LYT1 gene. To verify the presence of the antibiotic resistance genes, two pairs of primers were used; pair A consisted of N1F (5′-CGGCCATTGAACAAGATGGA-3′) and N2R (5′-TCGCCTTCTT GACGAGTTCT-3′), which amplifies the CDS of the neomycin gene (850 bp), and pair B consisted of H1F (5′-ACAGCGTCTCCGACCTGAT-3′) and H2R (5′-GAAGTACTCGCCGATAGTG-3′), amplifying the CDS of the hygromycin gene (960 bp).
Histopathology.
Tissue samples (heart, quadriceps muscle, and urinary bladder) were fixed in 10% formaldehyde, and histological hematoxylin-eosin-stained sections were studied. The lesions found were scored blindly as severe, moderate, slight, or absent by two observers. We searched T. cruzi amastigote nests in areas averaging 53 mm2 for heart, 38 mm2 for quadriceps muscle, and 14 mm2 for urinary bladder, scanning at least three sections per organ. For all autopsied animals, body and spleen weights were determined to calculate the spleen index, an indirect measurement of infection severity (spleen index = spleen weight × 100/body weight).
Real-time PCR for quantitation of parasite load in infected tissues.
In brief, tissue specimens of about 25 mg each were extracted separately with the QIAamp DNA Mini kit (Qiagen, Valencia, CA) by following the manufacturer's instructions for tissue DNA extraction. A 146-bp sequence of the satellite nuclear repeat (accession no. K01771) was amplified by using novel primers SatFw (5′-GCAGTCGGCKGATCGTTTTCG-3′) and SatRv (5′-TTCAGRGTTGTTTGGTGTCCAGTG-3′) targeted to sequence tracts conserved between T. cruzi lineage I and II satellite repeats (T. Duffy et al., unpublished results). The 20-μl reaction mixture tube contained 0.5 μM of primers SatFw and SatRv, 3 mM MgCl2, 250 μM of each deoxynucleoside triphosphate, 0.5 U of Platinum Taq polymerase, SYBR green (both from Invitrogen Life Technologies) at a final concentration of 0.5×, and 2 μl of sample DNA. Thermal cycling comprised an initial denaturation step of 5 min at 95°C, followed by 40 cycles of 94°C for 10 s, 65°C for 10 s, and 72°C for 10 s on an MJR-Opticon II device (Promega). The number of parasites refers to the number for 106 or 103 host cells, as described previously by Mary et al. (24). This number was assessed by quantifying the single-copy, murine-specific, tumor necrosis factor alpha gene by using primers TNF-5241 (5-TCCCTCTCATCAGTTCTATGGCCCA-3) and TNF-5411 (5-CAGCAAGCATCTATGCACTTAGACCCC-3) (13) at a final concentration of 1 μM. All other PCR reagents and cycling conditions were the same as those used for the T. cruzi satellite DNA amplification. The standard curve was established from DNA extracted from the NSF-60 murine cell line.
Serological determinations.
Titers of total immunoglobulin G antibodies against T. cruzi were measured by the enzyme-linked immunosorbent assay using T. cruzi epimastigote homogenates as an antigen. The antibody concentration was expressed as the optical density at a 492-nm wavelength.
Studies of insect vectors (xenodiagnosis).
Noninfected, laboratory-bred Triatoma infestans was used. Mice showing the same parasitemia level for both parasite lines (WT and L16) were selected, anesthetized with pentobarbital, and used to feed groups of 50 second- or third-instar bugs fasted for 1 month for 30 min in the dark. Nonengorged insects were discarded, and the remaining ones (engorged) were kept in a chamber at 29°C with 75% humidity and examined after 20 and 40 days. We recorded the parasite concentration in a Neubauer chamber.
In vitro metacyclogenesis assay.
One percent triatomine gut homogenate (19) was added to mid-log cultures of either WT or L16 clones (1 ml of parasite culture), and the number of trypo- and epimastigotes was recorded periodically during 14 days. Results were expressed as a percentage of trypomastigote cells.
Statistical analysis.
Comparisons based on the proportion of infected vectors were made with Fisher's exact test. Continuous variables, such as antibody titers and parasite concentrations in feces or blood samples, were analyzed with the two-tailed Wilcoxon signed-rank test for time course plots and with the Mann-Whitney or Kruskal-Wallis test for single-day measurements. Values are expressed as means ± standard errors of the means from at least three separate experiments.
RESULTS
Stability of the mutation after long-term culture.
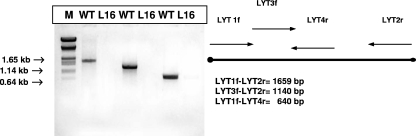
Gene replacement in the LYT1 gene was completed at the Instituto de Parasitología y Biomedicina (CSIC, Granada, Spain) before 2001, and the L16 clone was transferred from there to our laboratory in Argentina in 2004. Before we began infectivity studies of mice, PCR amplifications of the LYT1 locus were performed to exclude the possibilities of cross-contamination, LYT1 locus instability, or genomic alterations at the deletion site in the null mutant. Three amplification reactions were performed using three pairs of primers: (i) pair LYT1F-LYT2R, which yields an amplification product of 1,659 bp, corresponding to all the gene CDSs; (ii) pair LYT3F-LYT2R, which amplifies an 1,140-bp internal fragment of the CDS from nucleotide 520 to the stop codon; and (iii) pair LYT1F-LYT4R, which amplifies another CDS internal fragment (640 bp) running from the start codon to nucleotide 640. The WT strain showed PCR amplification products for all of these primer arrangements, while there was no amplification for any primer set when DNA from clone L16 was used, as expected (Fig. 1). Furthermore, to confirm the LYT1 gene replacement, comparative Southern blot analysis was carried out for genomic DNA from the WT and L16 clones digested with PstI restriction enzyme. Hybridization with a probe from nucleotides 1 to 640 of the LYT1 coding region revealed a restriction pattern consistent with the deletion of the LYT1 loci in the L16 clone. While the WT showed the three restriction fragments described previously (16) (a 2.5-kb band was common to both alleles; 3.7 kb was specific for allele a, and 1.2 kb corresponded to allele b), the null mutant (the L16 clone) lacked the hybridization signal according to the deletion of both LYT1 sequences (data not shown). The differential antibiotic resistance of the L16 clone was also assayed. Only the L16 clone showed sustained growth in the presence of either 250 μg/ml G418 antibiotic or 250 μg/ml hygromycin, conferred by the presence of the hygromycin B and neomycin phosphotransferase genes at the deleted LYT1 locus (data not shown). These findings indicated that the L16 strain maintained the deletion introduced by homologous recombination and did not suffer contaminations, genetic reversion, or genomic rearrangement in the time elapsed, since the mutant was selected until it was tested in vivo.
FIG. 1.
Stability of the mutation after long-term culture. PCR to discard contamination or instability of the gene-targeted mutation directed towards the LYT1 gene. The bar represents the gene, with its initiation and termination codons (black dots), and the approximate location and orientation of primers. The reaction was performed using genomic DNA isolated from either the L16 clone (LYT1 null mutant) or the WT CL Brenner strain as the template. Three sets of primers were used. The absence of amplification products on the L16 clone demonstrated that both alleles of LYT1 were completely replaced. M, DNA size marker.
Infectivity for mice.
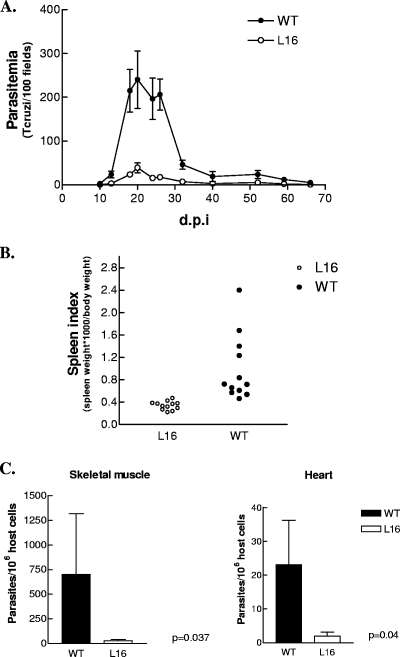
To determine whether LYT1 mutant lines display a reduced infectivity in vivo, adult Swiss mice were used. T. cruzi lines (WT and L16 clone) were transformed into infective stages (CRF), and 103 trypomastigotes were inoculated i.p. and the number of bloodstream forms were recorded for FBM until extinction (day 65). Figure 2A shows the parasitemia levels for both groups, where L16 exhibited a significantly reduced infectivity (P < 0.001). The infection of mice with T. cruzi produces a major expansion of B- and T-lymphoid cell populations (25) manifested macroscopically as a marked splenomegaly. The spleen index thus becomes, in the experimental model, an indirect but easily measurable effect of infection severity. This parameter was also significantly decreased (50-fold) in L16 compared to that in the WT (Fig. 2B). To test whether this observation correlated with different tissue parasitisms between both experimental groups, the tissue parasite load during the acute phase of infection was quantified by real-time PCR. Although the parasites were distributed throughout the heart and the skeletal muscle, this sensitive method showed that the L16-infected mice displayed a significantly reduced parasite load (P ≤ 0.04) (Fig. 2C). Finally, to test whether the lower parasitemia was a consequence of the LYT1 gene deletion or an effect of long axenic cultivation, the virulence of mouse-passaged L16 was checked. Parasitemia levels of BALB/c mice (1 month old) inoculated with parasites recovered by hemoculture from previous mouse passages were measured in FBM. The L16 clone displayed, in spite of serial mouse passages, much lower parasitemia values than the WT did, mirroring the results of L16 complement-resistant parasites produced in culture (data not shown).
FIG. 2.
Double LYT1 allele replacement decreases in vivo infectivity of CL Brenner T. cruzi. Adult Swiss mice (2 months old) were infected with 103 CRF of either the WT CL Brenner or the L16 clone. Parasite number and infections were monitored as described in Materials and Methods. (A) Parasitemia levels show a significant decrease in blood counts of L16 parasites (P < 0.001) during the entire time course studied. (B) Spleen index, an indirect measurement of infection severity, displayed a significant reduction (P < 0.0001). A representative experiment of five different independent repeats is shown. (C) Parasitic loads in skeletal and cardiac muscles at 28 days postinfection (d.p.i.). Data are expressed as number of parasites for 106 host cells. Values are given as means; error bars indicate standard errors of the means.
Persistent infection.
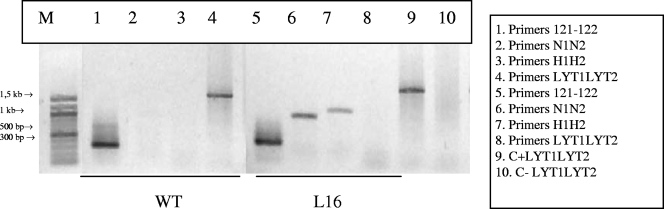
To test whether infection with L16 was extinguished during the time course of the study, hemocultures and PCR were assessed for the L16-infected mice. Blood samples for hemoculture were taken on days 90, 150, 210, and 360 after infection. PCRs were performed at 150 and 240 days after the inoculation. Both determinations yielded positive results for both the L16 group and the WT group at all the evaluated time points. These results showed that even though the L16 parasitemia levels were almost undetectable and the spleen index was lower, the LYT1 null mutant produced a latent and persistent infection. To reject the notion that the in vivo persistence of the infection was a consequence of the wild-type phenotype recovery by redundancy or selection of revertants, the stability of the genetic manipulation was evaluated. For this purpose, a PCR to amplify the complete CDS of the LYT1 gene and the antibiotic resistance genes using DNA isolated from parasites recovered by hemoculture after 1 year of infection was performed. The L16 clone showed the presence of both antibiotic genes and the complete absence of the LYT1-deleted gene by homologous recombination (Fig. 3).
FIG. 3.
Stability of the mutation after long-term infection. PCR to discard that the in vivo persistence of the infection was due to the recovery of the wild-type phenotype by redundancy or selection of revertants. The reaction was performed using genomic DNA isolated from parasites recovered by hemoculture from chronically infected mice, with the L16 clone or the WT strain as the template. Four sets of primers were used: (i) 121F-122R, to check that the isolated DNA belonged to T. cruzi, (ii) N1N2 to amplify the neomycin gene (850 bp), (iii) H1H2 to amplify the hygromycin gene (960 bp), and (iv) LYT1LYT2 to amplify the complete CDS of LYT1 (1,659 bp). The presence of amplification products for the antibiotic resistance genes as well as the absence of amplification for the LYT1 gene on the L16 clone demonstrated that the genetic replacement kept stable. M, DNA size marker.
L16 development in T. infestans vectors and in vitro transformation experiments.
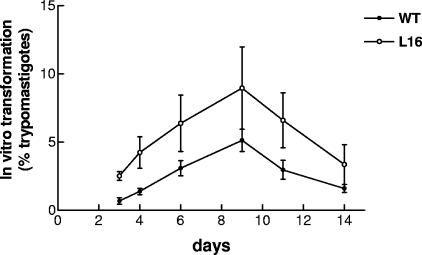
To assess whether the LYT1 gene deletion affects the stage transition in the guts of Reduviidae insects, WT development and L16 development were compared in T. infestans vectors. Groups of 50 second- or third-instar bugs were fed on infected mice inoculated with 103 CRF of each experimental strain (WT or L16 clone) and selected for having equivalent levels of parasitemia on the day of feeding. Trypomastigote concentrations were recorded in the feces of the bugs on days 20 and 40 after feeding. Three experiments were performed, and no significant differences were found. Parasites were detected in 30 of 50 (61%) or 29 of 50 (58%) of the T. infestans vectors fed with the L16 clone or the WT strain, respectively (data not shown). These results show that LYT1 mutation does not affect the ability of the parasite to multiply and complete the transition to infective stages in the insect vector. Moreover, this transition was even enhanced in the in vitro metacyclogenesis assays (Fig. 4), consistent with the finding of Manning-Cela et al. (22). The L16 line rapidly converted to metacyclic trypomastigotes, reaching an 8% peak at day 9. In contrast, less than 4% of the wild-type parasites converted to this form in parallel tests (P = 0.031). This observation demonstrates that the reduced infectivity of the LYT1-deficient parasites was not a consequence of an inability to complete the life cycle.
FIG. 4.
In vitro transformation experiments. By their abilities to convert to metacyclic trypomastigotes, we determined the transition efficiencies of WT and L16 clone epimastigotes by adding 1% triatomine gut homogenate to the cultures. The L16 clone showed a significant increase in the percentage of trypomastigotes compared to that of the WT (P < 0.05). The results plotted are the means ± standard errors of the means (error bars) of two different experiments.
Antibody production in persistent infection.
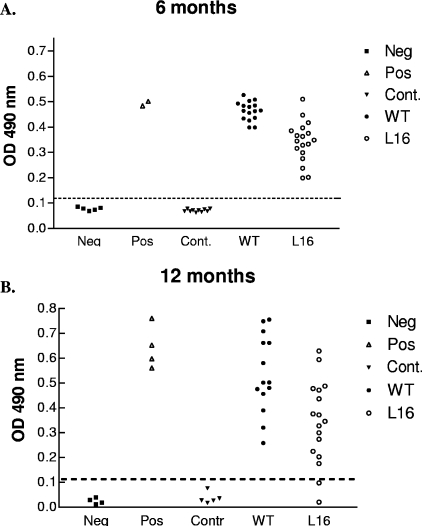
The antibody levels in the blood of mice infected with the WT or the L16 clone were compared. CRF, 103 per mouse, were inoculated intraperitoneally into groups of five mice each. Serum samples were taken at 6 and 12 months postinoculation. Specific antibodies against T. cruzi were detected throughout the study in both experimental groups. Nevertheless, the levels showed by the WT were at all times about twofold higher (P < 0.005) than those corresponding to the L16 clone (Fig. 5), displaying similar findings for parasite burden and severity of disease.
FIG. 5.
Levels of T. cruzi antibodies elicited in mice inoculated with either L16 clone or CL Brenner WT T. cruzi. Dispersion diagrams of antibody levels in control (untreated) animals and those inoculated with 103 CRF of the L16 clone or the WT strain. Serum samples were taken at 6 (A) and 12 (B) months postinoculation. The results are expressed as the ratio of the absorbance of each serum sample at a 490-nm optical density (OD) to the cutoff value. Dotted lines indicate the cutoff adopted for positivity, calculated as the mean of the values determined for the controls (Cont.) plus three times the standard deviation. Each symbol represents a mouse. Neg, negative serum standard; Pos, positive serum standard. L16-inoculated mice showed significantly reduced antibody titers compared to those of mice inoculated with WT strain. (P values of L16 versus WT mice were 0.0031 [A] and 0.004 [B]).
Histopathology and tissue parasite quantitation by real-time PCR at the chronic phase of infection.
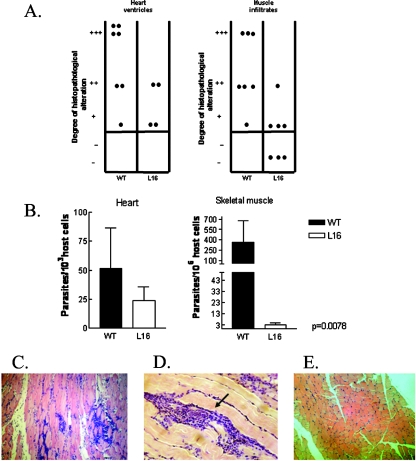
Although the deletion of the LYT1 gene from T. cruzi resulted in an attenuated phenotype, displaying a very low parasitemia, low spleen indexes, and a high survival rate compared to those of WT-infected control mice, hemocultures and parasite DNA detection by PCR indicated parasite persistence during the entire lifetime of the hosts. To test the effects of latent infection on tissue damage, animals inoculated with 103 CRF were sacrificed 7 months after infection and skeletal muscle, heart and urinary bladder samples were processed histologically. By processing skeletal muscle, we found that most samples of the WT group had extensive and severe lesions, consisting of interstitial inflammatory mononuclear infiltrates, incipient degeneration of muscle fibers, and scarce amastigote nests, while of the L16 group, only few mice showed a slight inflammatory infiltrate. The difference in the levels of inflammatory infiltrate between both groups was statistically significant (P < 0.05) (Fig. 6A). Lesions in the heart were not as severe or extensive as those in skeletal muscle. This result is consistent with previous observations of other groups (28), showing that as the infection progresses towards the chronic phase, distribution of the lineage II T. cruzi parasite appears to be more restricted to the skeletal muscle (as was the case for CL WT). The predominant lesions depicted widely scattered mononuclear infiltrates in either atrial or ventricular tissues. Although differences in heart tissue damage between mutant and WT infections were not significant, L16-infected mice displayed less inflammation than the WT mice (Fig. 6A). No histological alterations were found in the urinary bladder. It is noteworthy that when tissue parasitism at the chronic phase of infection was quantified by real-time PCR, the parasite load was significantly greater in the skeletal muscles of WT-infected mice than in those of the L16 group (P < 0.007), whereas no significant difference was detected in the cardiac muscles between both groups (Fig. 6B). We suggest that the absence of statistical significance of the real-time PCR results obtained for heart tissue could be due to the fact that the number of parasites found was in the lower limit of the linear range of quantitation (approximately 1 parasite/1,000 cells) (T. Duffy et al., unpublished results).
FIG. 6.
Inflammatory infiltrates and tissue parasitism in the heart and skeletal muscles of mice chronically infected with either the L16 clone or the WT CL Brenner strain. (A) Histopathological analysis was plotted as dispersion diagrams indicating the intensities of inflammatory infiltrates graded as absent (−), slight (+), moderate (++), and severe (+++). Each dot represents a mouse. Mice inoculated with 103 CRF were autopsied at 7 months postinfection. The L16 group showed a significantly low inflammatory response in muscle tissue (P < 0.05) and a low degree of inflammation in heart tissue compared to the levels of the WT group. (B) Parasite burden in skeletal and cardiac muscles at the chronic phase of infection. Data are expressed as the number of parasites for 106 or 103 host cells. (C and D) Microphotograph of muscle from mice infected with WT strain; note the amastigote nest inside the inflammatory infiltrates (arrow) (magnification, ×25). Values are given as means; error bars indicate standard errors of the means. (E) Muscle of L16-infected mouse, showing the absence of dense inflammatory infiltrates relative to the WT group.
This finding supports a less pathogenic behavior for the mutant clone, consistent with a low-virulence phenotype.
Protective immunization with mutant epimastigotes against virulent T. cruzi challenge.
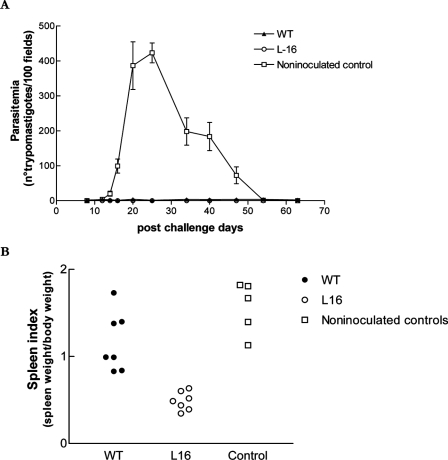
In preliminary experiments (5), we observed that mice recovering or recovered from primary WT infections became resistant to challenge with a secondary, virulent T. cruzi Tulahuen strain. Moreover, the same effect was observed in L16-preinoculated mice, even though these animals had experienced only a highly attenuated primary infection. To confirm these observations and to test how long L16 inocula would protect against virulent challenge, Swiss, 40-day-old, male mice received an i.p. inoculum of 103 L16 epimastigotes. Fourteen months (471 days) later, these animals, together with sham- and WT-preinoculated controls, were challenged i.p. with 104 WT Tulahuen strain blood trypomastigotes. Parasitemia was measured in FBM twice a week for all animals. Figure 7A depicts the evolution of parasitemia in both groups during 60 days. Mice preinoculated with L16 epimastigotes 14 months before were still strongly protected against Tulahuen challenge. A similar effect was observed with the WT-preinoculated group (P < 0.001), but when blood parasite counts were compared between WT- and L16-primed groups, the LYT1 null mutant clone definitely displayed a stronger protective effect (P < 0.01). Furthermore, the spleen indexes on day 62 postchallenge were significantly lower in the L16-primed mice than in the WT group (P = 0.05) (Fig. 7B). The virulent challenge killed 26% (4 of 15) of the naïve mice within 2 months, without affecting the survival rate of the T. cruzi-primed groups. Overall, these observations indicate that both L16 and WT strain inocula bestowed a strong and long-lasting protection against secondary infections. This protection was obtained in WT mice at the expense of a virulent primary infection not suffered by L16-primed mice.
FIG. 7.
Long-term protective immunization with WT or L16 clone epimastigotes against challenge with virulent T. cruzi Tulahuen. Adult Swiss mice (n = 15) were primed with 103 CRF of either the L16 clone or the WT strain. Fourteen months later, the mice were challenged, together with naive controls (n = 5), with 104 bloodstream forms of the highly virulent T. cruzi Tulahuen strain. (A) Parasitemia levels periodically measured during 60 days. Note the significant protection (P < 0.001) in L16-preinoculated mice. Values are given as means; error bars indicate standard errors of the means. (B) Spleen indexes on day 65 postchallenge. Note the significant protective effect of the genetically altered parasites (P < 0.002). A representative experiment of three independent assays is shown.
Protection against tissue lesions, bestowed by previous L16 inoculation.
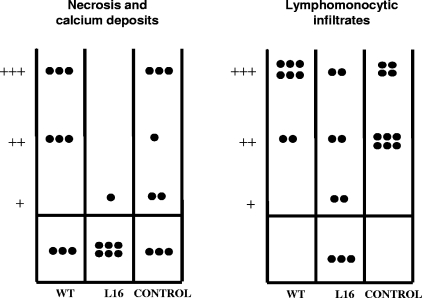
Autopsies were performed on mice 14 months after priming with L16 or the WT and 2 months after virulent T. cruzi challenge. The tissue lesions found in skeletal muscle revealed a protective effect conferred only by previous infection with the L16 mutant (Fig. 8). Both the lymphomonocytic interstitial infiltrates and the presence of necrotic foci with calcium deposits were significantly prevented (P values were 0.0085 and 0.018, respectively). In heart, intestine, and urinary bladder, less intense but characteristic lesions of T. cruzi infection were found, without evidence of protection by the mutant strain.
FIG. 8.
Histopathological observations in muscles of mice infected for 2 months with the virulent T. cruzi Tulahuen strain. The animals had been primed 14 months before the Tulahuen infection, with either WT, L16, or saline (see the text). Each dot represents a mouse. Note the significant degree of protection against necrosis and calcium deposits (P = 0.018) (left panel) or lymphomonocytic infiltrates (P = 0.0085) (right panel) bestowed by previous L16 infection, but not by WT infection. −, absent; +, slight; ++, moderate; +++, severe.
DISCUSSION
Several research groups have recently been able to gain insight into the functions of a growing number of T. cruzi genes. Two powerful research tools allowing these advances are the DNA sequences of the parasite generated by the Tritryp genome initiative and the protocols for targeted gene deletion. Virulence or infectivity is a complex function involving the highly evolved ability of T. cruzi to transform into infective stages, penetrate epithelia and cells, and evade the complex immunologic reactions of the mammalian host (2). These mechanisms are related to the expression of certain genes, loosely referred to as “virulence genes or factors.” In fact, very diverse genetic manipulations converge into less infective parasites (1, 7, 11, 15, 17).
In a previous work reviewed by Kierszenbaum (20), noninfectious epimastigotes of spontaneously attenuated T. cruzi strains were shown to behave as potent immunogens, bestowing protection against virulent T. cruzi challenge. This protection was strong and long lasting compared to that obtained with killed parasites (6). However, the risk of reversion from attenuated toward more virulent phenotypes could not be excluded, since the genetic bases for attenuation in these strains were unknown. The complete deletion of both allelic copies of a T. cruzi gene has been shown to be compatible with indefinite survival of the parasite in culture, as shown at least with the gp72 (13) and LYT1 (22) genes. This feature, coupled to an attenuated phenotype and to a lack of endogenous availability of the missing gene, prompted us to test the possible attenuation and immunogenicity of double-knockout mutants both for the gp72 gene (7) and, in this work, for the LYT1 gene. An irreversibly attenuated T. cruzi mutant would constitute a valuable research tool for studies on pathogenic interactions with virulent strains and for experimental immunization.
Manning-Cela et al. (22) have already characterized the LYT1 null mutant (the L16 clone) by in vitro experiments that demonstrated a reduction in the efficiency to infect monolayers of NIH-3T3 cells. In this work, this defect is shown to correlate with the impairment of several pathogenic effects in the living animal, such as parasitemia, spleen index, tissue parasite loads, antibody production and tissue damage. A significant loss of the ability to develop blood parasitism (P < 0.001) was shown by inoculations in mice. This loss was not reverted by serial mouse passages, indicating the stability of this mutation. We have not repeated reconstitution experiments for the infective phenotype by retransfecting the LYT1 gene, since these were previously performed for the same clone, with positive results, by Manning-Cela et al. (22). Compared to the levels of the wild type, during the acute phase, L16 produces attenuated infections displaying blood parasite counts 87-fold lower and a decreased spleen weight index, indicative of a less severe infection and lower tissue parasite load. Antibody titers were significantly lower, as also shown for another spontaneously attenuated strain, TCC (8). Although the hemocultures and PCR determinations showed a persistence of the L16 clone infection in inoculated mice for at least 1 year, the histopathological observations displayed a significant reduction in the muscle mononuclear infiltrates, complemented by the presence of a significantly lower number of parasites in this tissue. In the heart, the L16 clone displayed a slight, nonsignificant trend toward less aggressiveness relative to the WT CL Brenner strain without significant differences in the real-time PCR quantitation. The 30-fold higher density of parasites observed between skeletal muscle and cardiac tissues also points to the tissue tropism of the T. cruzi strain or lineage (12, 14, 16). Indeed, this feature could explain the lack of significance found when WT and L16 parasitic loads were compared in cardiac tissue samples.
Manning-Cela et al. (22) also demonstrated that the reduced infectivity of the LYT1-deficient parasites was not a consequence of an inability to complete the life cycle, since the mutant epimastigotes converted to metacyclic trypomastigotes more efficiently than the WT strain did. We observed the same phenotype by inducing metacyclogenesis with triatome gut homogenate added to the culture. However, when growth in the insect vector T. infestans was assessed, we did not find significant differences either in the numbers of parasitized bugs or in the parasite concentrations in feces. Possibly, the environment of the living insect was supplying one or more factors absent in the in vitro experiments, thus concealing the differential behavior observed in culture.
T. cruzi displays a remarkable ability to maintain latent low-burden infections for the lifetime of the host. This ability was apparently not impaired in L16, since both PCR and parasite recovery by hemoculture showed the capacity of the mutant to maintain lifelong infection. It is noteworthy to emphasize that L16-persistent parasites reisolated from long-term infection hemocultures were depicted to be genetically stable without experiencing genomic rearrangement and reversion to the wild-type phenotype (Fig. 3).
To assess the immunoprotective capacity of the L16 clone, mice primed with epimastigotes of the mutant or the WT or sham inoculated with saline were challenged with the highly virulent T. cruzi Tulahuen strain. Fourteen months after inoculation with L16, mice were still highly resistant to reinfection with virulent T. cruzi, as shown by measurements of parasitemia, mortality, and spleen weight.
Chronic parasitism by T. cruzi is protective against reinfections, regardless of whether the primary infection is acute or inapparent. The skeletal muscles of mice are targets for the pathogenic effects of T. cruzi, allowing the measurement of acute or chronic inflammation, parasitism, and degenerative and necrotizing lesions (21). Preinoculations with either WT or L16 were relatively protective against some of these pathogenic effects. However, this protection was obtained at the expense of some initial damage, which was severe in the case of the WT and slight in the case of the L16 mutant. These effects were manifested mainly in skeletal muscle. Similar studies of the heart and other organs did not show significant results, probably because of the moderate intensity of lesions and the limited number of samples available for analysis.
In summary, the results presented here show that the complete replacement of the LYT1 gene markedly attenuates the in vivo infectivity of an otherwise highly virulent T. cruzi strain (CL Brenner), an alteration which is fully compatible with the in vitro propagation of the parasite. This supports the participation of this gene in the phenotypic expression of virulence and suggests that similar manipulations may result in noninfectious parasite strains.
Acknowledgments
This investigation was financially supported by grant HHMI 55003663 from the Howard Hughes Medical Institute and by grant PICT 2005 32739 to M. Basombrío and partially by PIP 5469 (CONICET) and PICT 33955 (FONCYT) to A. G. Schijman. M. Basombrío was an HHMI International Research Scholar. M. P. Zago was a postdoctoral fellow from CONICET.
We are grateful to Antonio Gonzalez from Instituto de Biomedicina CSIC, Granada, Spain, who kindly provided the L16 clone and the CL (WT) strain to our lab, and Mirella Ciaccio, Cecilia Perez Brandán, and Marcelo Padilla for their scientific advice. Skillful technical assistance was provided by Alejandro Uncos, Rosa M. Corrales, M. Asunción Segura, Fernanda García Bustos, Ricardo Rossi, Federico Ramos, and Clara Romero.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 15 October 2007.
REFERENCES
- 1.Allaoui, A., C. Francois, K. Zemzoumi, E. Guilvard, and A. Ouaissi. 1999. Intracellular growth and metacyclogenesis defects in Trypanosoma cruzi carrying a targeted deletion of a Tc52 protein-encoding allele. Mol. Microbiol. 321273-1286. [DOI] [PubMed] [Google Scholar]
- 2.Andrade, L. O., and N. W. Andrews. 2005. The Trypanosoma cruzi-host-cell interplay: location, invasion, retention. Nat. Rev. Microbiol. 3819-823. [DOI] [PubMed] [Google Scholar]
- 3.Andrews, N. W. 1993. Living dangerously: how Trypanosoma cruzi uses lysosomes to get inside host cells and then escapes into the cytoplasm. Biol. Res. 2665-67. [PubMed] [Google Scholar]
- 4.Andrews, N. W., C. K. Abrams, S. L. Slatin, and G. Griffiths. 1990. A T. cruzi-secreted protein immunologically related to the complement component C9: evidence for membrane pore-forming activity at low pH. Cell 611277-1287. [DOI] [PubMed] [Google Scholar]
- 5.Basombrio, M. A. 1981. Resistance to re-infection by Trypanosoma cruzi in Chagasic mice. Medicina (B Aires) 41(Suppl.)230-232. (In Spanish.) [PubMed] [Google Scholar]
- 6.Basombrio, M. A. 1990. Trypanosoma cruzi: partial prevention of the natural infection of guinea pigs with a killed parasite vaccine. Exp. Parasitol. 711-8. [DOI] [PubMed] [Google Scholar]
- 7.Basombrío, M. A., L. Gomez, A. M. Padilla, M. Ciaccio, T. Nozaki, and G. A. Cross. 2002. Targeted deletion of the gp72 gene decreases the infectivity of Trypanosoma cruzi for mice and insect vectors. J. Parasitol. 88489-493. [DOI] [PubMed] [Google Scholar]
- 8.Basombrío, M. A., M. A. Segura, and J. R. Nasser. 2002. Relationship between long-term resistance to Trypanosoma cruzi and latent infection, examined by antibody production and polymerase chain reaction in mice. J. Parasitol. 881107-1112. [DOI] [PubMed] [Google Scholar]
- 9.Benabdellah, K., E. Gonzalez-Rey, and A. Gonzalez. 2007. Alternative trans-splicing of the Trypanosoma cruzi LYT1 gene transcript results in compartmental and functional switch for the encoded protein. Mol. Microbiol. 651559-1567. [DOI] [PubMed] [Google Scholar]
- 10.Britto, C., M. A. Cardoso, P. Wincker, and C. M. Morel. 1993. A simple protocol for the physical cleavage of Trypanosoma cruzi kinetoplast DNA present in blood samples and its use in polymerase chain reaction (PCR)-based diagnosis of chronic Chagas disease. Mem. Inst. Oswaldo Cruz 88171-172. [DOI] [PubMed] [Google Scholar]
- 11.Caler, E. V., S. Vaena de Avalos, P. A. Haynes, N. W. Andrews, and B. A. Burleigh. 1998. Oligopeptidase B-dependent signaling mediates host cell invasion by Trypanosoma cruzi. EMBO J. 174975-4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camargos, E. R., D. J. Franco, C. M. Garcia, A. P. Dutra, A. L. Teixeira, Jr., E. Chiari, and C. R. Machado. 2000. Infection with different Trypanosoma cruzi populations in rats: myocarditis, cardiac sympathetic denervation, and involvement of digestive organs. Am. J. Trop. Med. Hyg. 62604-612. [DOI] [PubMed] [Google Scholar]
- 13.Cooper, R., A. R. de Jesus, and G. A. Cross. 1993. Deletion of an immunodominant Trypanosoma cruzi surface glycoprotein disrupts flagellum-cell adhesion. J. Cell Biol. 122149-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cummings, K. L., and R. L. Tarleton. 2003. Rapid quantitation of Trypanosoma cruzi in host tissue by real-time PCR. Mol. Biochem. Parasitol. 12953-59. [DOI] [PubMed] [Google Scholar]
- 15.de Jesus, A. R., R. Cooper, M. Espinosa, J. E. Gomes, E. S. Garcia, S. Paul, and G. A. Cross. 1993. Gene deletion suggests a role for Trypanosoma cruzi surface glycoprotein GP72 in the insect and mammalian stages of the life cycle. J. Cell Sci. 1061023-1033. [DOI] [PubMed] [Google Scholar]
- 16.Franco, D. J., A. R. Vago, E. Chiari, F. C. Meira, L. M. Galvao, and C. R. Machado. 2003. Trypanosoma cruzi: mixture of two populations can modify virulence and tissue tropism in rat. Exp. Parasitol. 10454-61. [DOI] [PubMed] [Google Scholar]
- 17.Garzón, E., M. C. Borges, A. Cordeiro-da-Silva, V. Nacife, N. Meirelles Mde, E. Guilvard, M. F. Bosseno, A. G. Guevara, S. F. Breniere, and A. Ouaissi. 2003. Trypanosoma cruzi carrying a targeted deletion of a Tc52 protein-encoding allele elicits attenuated Chagas disease in mice. Immunol. Lett. 8967-80. [DOI] [PubMed] [Google Scholar]
- 18.Grossblatt, N. 1996. Guide for the care and use of laboratory animals. National Academic Press, Washington, DC.
- 19.Isola, E. L., E. M. Lammel, and S. M. Gonzalez Cappa. 1986. Trypanosoma cruzi: differentiation after interaction of epimastigotes and Triatoma infestans intestinal homogenate. Exp. Parasitol. 62329-335. [DOI] [PubMed] [Google Scholar]
- 20.Kierszenbaum, F. 1989. Chagas disease, p. 174-195. In F. Y. Liew (ed.), Vaccination strategies for tropical diseases. CRC Press, Boca Raton, FL.
- 21.Laguens, R. P., P. Cabeza Meckert, M. A. Basombrio, G. J. Chambo, P. M. Cossio, R. M. Arana, and R. Gelpi. 1980. Chronic infection of mice with Trypanosome cruzi. Experimental model of Chagas disease. Medicina (B Aires) 40(Suppl. 1)33-39. (In Spanish.) [PubMed] [Google Scholar]
- 22.Manning-Cela, R., A. Cortes, E. Gonzalez-Rey, W. C. Van Voorhis, J. Swindle, and A. Gonzalez. 2001. LYT1 protein is required for efficient in vitro infection by Trypanosoma cruzi. Infect. Immun. 693916-3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manning-Cela, R., A. Gonzalez, and J. Swindle. 2002. Alternative splicing of LYT1 transcripts in Trypanosoma cruzi. Infect. Immun. 704726-4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mary, C., F. Faraut, L. Lascombe, and H. Dumon. 2004. Quantification of Leishmania infantum DNA by a real-time PCR assay with high sensitivity. J. Clin. Microbiol. 425249-5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minoprio, P., S. Itohara, C. Heusser, S. Tonegawa, and A. Coutinho. 1989. Immunobiology of murine T. cruzi infection: the predominance of parasite-nonspecific responses and the activation of TCRI T cells. Immunol. Rev. 112183-207. [DOI] [PubMed] [Google Scholar]
- 26.Tan, H., and N. W. Andrews. 2002. Don't bother to knock—the cell invasion strategy of Trypanosoma cruzi. Trends Parasitol. 18427-428. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization. 2002. Control of Chagas disease: second report of the WHO expert committee. WHO Technical Report Series 905. UNDP/World Bank/WHO, Geneva, Switzerland.
- 28.Zhang, L., and R. L. Tarleton. 1999. Parasite persistence correlates with disease severity and localization in chronic Chagas' disease. J. Infect. Dis. 180480-486. [DOI] [PubMed] [Google Scholar]