Abstract
Enteropathogenic Escherichia coli (EPEC) and Shigella flexneri are human host-specific pathogens that infect intestinal epithelial cells. However, each bacterial species employs a different infection strategy within this environmental niche. EPEC attaches to the apical surface of small intestine enterocytes, causing microvillus effacement and rearrangement of the host cell cytoskeleton beneath adherent bacteria. In contrast, S. flexneri invades the large intestine epithelium at the basolateral membrane, replicates, and spreads cell to cell. Both EPEC and S. flexneri rely on type three secretion systems (T3SS) to secrete effectors into host cells, and both pathogens recruit polymorphonuclear leukocytes (PMNs) from the submucosa to the lumen of the intestine. In this report, we compared the virulence functions of the EPEC T3SS effector NleE and the homologous Shigella protein Orf212. We discovered that Orf212 was secreted by the S. flexneri T3SS and renamed this protein OspZ. Infection of polarized T84 intestinal epithelial cells with an ospZ deletion mutant of S. flexneri resulted in reduced PMN transepithelial migration compared to infection by the wild type. An nleE deletion mutant of EPEC showed a similar reduction of PMN migration. The ability to induce PMN migration was restored in both mutants when either ospZ or nleE was expressed from a plasmid. An infection of T84 cells with the ΔospZ mutant resulted in reduced extracellular signal-related kinase phosphorylation and NF-κB activation compared to infection with the wild type. Therefore, we conclude that OspZ and NleE have similar roles in the upstream induction of host signaling pathways required for PMN transepithelial migration in Shigella and EPEC infections.
Shigella species and enteropathogenic Escherichia coli (EPEC) are gram-negative bacterial pathogens that mediate infections in the human intestine, often causing life-threatening diarrhea in immunocompromised individuals (1, 3, 12, 20, 22, 25). Those most at risk are children in underdeveloped nations, where contamination of food and water is common (1, 25). Remarkable progress has been made to identify virulence determinants required to mediate the pathogenesis of both species of bacteria. However, there are still aspects of virulence that are not fully understood, efficacious vaccines have not been produced, and antibiotic resistance across all diarrheagenic E. coli and Shigella species is increasing (41).
While infections caused by these enteric pathogens cause similar symptoms, they exhibit different pathogenesis strategies. Shigella flexneri invades only colonic epithelial cells at the basolateral membrane, which the bacteria may reach by transcytosis through M cells and tight junction modification (44, 45). Alternatively, S. flexneri may reach the basolateral membrane through the activation of polymorphonuclear leukocyte (PMN) migration. Secreted proteins and lipopolysaccharide activate host cell signaling pathways, including the extracellular signal-related kinase 1/2 (ERK1/2) pathway, leading to the production of molecules that attract PMNs from the bloodstream to the lumen of the large intestine (24, 31, 39, 40, 55). Shigella then passes through the gaps created between epithelial cells by the migrating PMNs to gain access to the submucosa (39, 45).
In contrast to S. flexneri, EPEC attaches intimately to the apical surface of the intestinal epithelium, where the bacteria induce characteristic attaching and effacing (A/E) lesions (20, 22). EPEC remains extracellular and recruits focal adhesion proteins and other proteins required for the actin polymerization, which forms a pedestal on the surface of the cell (20, 22). Despite these differences, both S. flexneri and EPEC recruit PMNs to the lumen from the submucosa, a process which requires attachment and interleukin-8 (IL-8) secretion in EPEC infections (49). PMN transepithelial migration associated with S. flexneri infections in vitro requires the activation of the ERK1/2 pathway and IL-8 secretion (24). EPEC infections also activate the ERK1/2 pathway and IL-8 secretion (49), but this activity has not yet been linked to PMN transepithelial migration. Regardless, the transmigration of PMNs across the intestinal epithelium is a pathogenesis trait shared by S. flexneri and EPEC infections (31, 39, 49).
Both S. flexneri and EPEC also use type three secretion systems (T3SS) to secrete effector proteins into the host cell to manipulate the environment to one that fosters replication and spread of the bacteria (5, 11, 20, 32, 46). NleE is a T3SS effector discovered in Citrobacter rodentium that is encoded in a two-gene operon downstream of nleB (9, 21) and has homologues in Shigella and A/E E. coli species (53). Recently, it was shown that infection of mice with an nleE mutant of C. rodentium results in a reduction of both bacterial load and colonic hyperplasia, suggesting that nleE plays a role in colonization and disease (53). In this study, we characterized the NleE homologue Orf212 from S. flexneri. Orf212 is encoded on the virulence plasmid of S. flexneri and other Shigella species and is found between two sets of insertion elements. We discovered that Orf212 was secreted by S. flexneri in a T3SS-dependent manner. A deletion mutation of orf212 indicated that the gene was dispensable for invasion and cell-to-cell spread but was required for effective transepithelial migration of PMNs in an in vitro assay. Interestingly, we discovered that nleE was required for EPEC-induced PMN migration and that either nleE or orf212 could complement the EPEC and S. flexneri deletion mutations across species. Therefore, we renamed orf212 ospZ, because it is the last open reading frame (ORF) (number 212 of 212 ORFs) that was discovered on the virulence plasmid (5) and because it has a function similar to that of other Osp proteins (55).
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All E. coli and S. flexneri strains used in this study are listed in Table 1. E. coli and S. flexneri were cultured in Luria broth (LB) or in tryptic soy broth, both at 37°C with aeration. Antibiotics were used in growth media and on plates, when required for selection, at the following concentrations: kanamycin, 50 μg/ml; chloramphenicol, 25 μg/ml; spectinomycin, 100 μg/ml; streptomycin, 25 μg/ml; and ampicillin, 200 μg/ml.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype/description | Referencea |
|---|---|---|
| Shigella strains | ||
| 2457T | Wild-type S. flexneri 2a | 13 |
| BS103 | Virulence plasmid-cured derivative of 2457T | 30 |
| BS508 | S. flexneri 1a | CDC |
| BS510 | S. flexneri 3a | CDC |
| BS652 | 2457T/Δspa47 (spa47::aadA); Specr | 55 |
| BS685 | S. boydii 12 | CDC |
| BS766 | 2457T transformed with pKM208; Ampr | 55 |
| BS819 | 2457T/ΔospZ (ospZ::cat); Cmr | This study |
| BS821 | 2457T/ΔospZ, unmarked | This study |
| BS822 | BS821 transformed with pDZ9; Cmr | This study |
| BS824 | BS652 transformed with pDZ9; Cmr | This study |
| BS833 | BS821 transformed with pBAD24::ospZ; Cmr | This study |
| BS834 | BS821 transformed with pBAD24::nleE; Cmr | This study |
| BS840 | 2457T transformed with pTrc-NleE-2HA; Ampr | This study |
| BS841 | BS821 transformed with pTrc-OspZ-2HA; Ampr | This study |
| E. coli strains | ||
| DH5α | endA1 hsdR17 supE44 thi-1 recA1 gyrA96 relA1 (ΔlacIZYA-argF) U169 deoR (Φ80dlacΔ [lacZ] M15) | 16 |
| EIEC | EIEC serotype O124:NM | 17 |
| EPEC | EPEC E2348/69 wild-type O127:H6 | 28 |
| ΔnleE | EPEC E2348/69/ΔnleE; Cmr | This study |
| EPEC/pTrc-NleE-2HA | EPEC transformed with pTrc-NleE-2HA; Ampr | This study |
| ATM897 | ΔnleE transformed with pBAD24::ospZ; Cmr | This study |
| ATM900 | ΔnleE transformed with pTrc-NleE-2HA; Ampr | This study |
| ATM928 | ΔnleE transformed with pTrc-OspZ-2HA; Ampr | This study |
| Plasmids | ||
| pKD3 | bla cat oriR6K; Ampr Cmr | 7 |
| pKM208 | Temperature-sensitive red-, gam-, and lacI-expressing plasmid driven by PTac promoter; Ampr | 35 |
| pCP20 | Plasmid expressing FLP recombination target recombinase; Ampr | 7 |
| pDZ2 | Cloning intermediate; Cmr | 55 |
| pDZ9 | OspZ-2HA fusion. Expression driven by PospZ; Cmr | This study |
| pTrc-NleE-2HA | NleE-2HA fusion. Expression driven by Ptrc; Ampr | This study |
| pTrc-OspZ-2HA | OspZ-2HA fusion. Expression driven by Ptrc; Ampr | This study |
| pBAD24 | Arabinose-inducible vector; pBR322ori; Ampr | 14 |
| pBAD24::nleE | nleE cloned into NcoI/HindIII sites of pBAD24; Ampr | This study |
| pBAD24::ospZ | ospZ cloned into NcoI/HindIII sites of pBAD24; Ampr | This study |
| pDsRed2-C1 | RFP vector; Kanr | Clontech |
| pEGFP-C1 | GFP vector; Kanr | Clontech |
| EGFP::NleE | nleE cloned into pEGFP-C1; Kanr | This study |
| EGFP::OspZ | ospZ cloned into pEGFP-C1; Kanr | This study |
CDC, Nancy Strockbine, Centers for Disease Control and Prevention.
Tissue culture.
HeLa cells and L2 mouse fibroblast cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum. The human epithelial colon cancer-derived cell line T84 (passages 46 to 66) was maintained in DMEM/F-12 medium supplemented with 15 mM HEPES (pH 7.5) and 10% fetal bovine serum. To obtain polarized monolayers, T84 cells were grown on 0.33-cm2 (PMN migration) or 4.7-cm2 (harvesting, immunoblotting) collagen-coated permeable polycarbonate filters (Costar) as previously described (24, 31) and were utilized after they reached a confluent and differentiated state. All tissue culture media were purchased from Invitrogen, and all cell lines were maintained in the presence of 5% CO2 at 37°C.
Plasmid construction.
All plasmids used in this study are described in Table 1. ospZ from S. flexneri 2457T was amplified by PCR using Taq (Qiagen) polymerase with upstream (5′-GGTACCCACATATAAAACGTCTTTATTG-3′) and downstream (5′-GGATCCATAGACTTTAATCTCTGGCGA-3′) primers, cloned into pGEM-T (Promega), and sequenced. Subsequently, ospZ was subcloned into pDZ2 and EGFP-C1 using KpnI and BamHI restriction sites. In order to generate arabinose-inducible constructs for cross-species complementation, both nleE and ospZ were cloned into pBAD24. ospZ was amplified with upstream (5′-CCATGGATGATTAGTCCCATCAAG-3′) and downstream (5′-AAGCTTATAGACTTTAATCTC-3′) primers. nleE was amplified from EPEC with upstream (5′-CCATGGATGATTAATCCTGTTACTAAT-3′) and downstream (5′-AAGCTTCGAATTCTCCTCAATTTTAGAAAGTTT-3′) primers. Both genes were cut with NcoI/HindIII restriction enzymes and ligated into pBAD24 cut with the same enzymes. The pTrcNleE-2HA plasmid was made by amplifying nleE using upstream (5′-CATGCCATGGTTAATCCTGTTAC-3′) and downstream (5′-CGGAATTCCTACGCATAATCCGGCACATCATACGGATACGCATAATCCGGCACATCATACGGATACTCAATTTTAGAAAG-3′) primers cloned into the pTrc99A vector. The pTrcOspZ-2HA plasmid was generated by cutting an intermediate OspZ-2HA from pBAD24 using NcoI and HindIII and ligating into pTrc99A cut with the same restriction enzymes. Lastly, nleE was amplified using upstream (5′-AAGAATTCATGATTAATCCTGTTACTA-3′) and downstream (5′-TCCCCGCGGCTCAATTTTAGAAAG-3′) primers and was cloned into pEGFP-C1 to generate an N-terminal GFP fusion.
Mutant construction.
The ΔospZ deletion mutant of S. flexneri was generated by allelic exchange using a modification of the method of Datsenko and Wanner (7, 35) as previously described (55). Primers were used to amplify the chloramphenicol resistance cassette (cat) with sequences at the 5′ and 3′ ends identical to sequences 20 bp internal to and 30 bp upstream (5′-TGTTTATATTTGAGTATAGAGATTAAAAATGATTAGTCCCATCAAGAATTGTGTAGGCTGGAGCTGCTTC-3′) and downstream (5′-CATTTCACGAGCAATAATAATCTCAGATTTAATAGACTTTAATCTCTGGCATATGAATACCTCCTTAGTTCC-3′) of ospZ. After transformation of BS766 and recombination, bacteria were plated on tryptic soy broth plates containing Congo red and chloramphenicol at a concentration of 5 μg/ml. Cmr colonies on these plates were purified and screened by PCR using three different primer sets to identify the ΔospZ deletion mutant. To remove cat from BS819, the strain was transformed with pCP20 and incubated at 42°C (7) to generate BS821. The EPEC ΔnleE mutant was also generated by the method of Datsenko and Wanner (7, 35) using pKD3 as a template and the primer pairs 5′-GCGTGTCCCCTATAAATACTAAATATGCTGAACATGTGGTGAAAAATATTTACCTGTGTAGGCTGGAGCTGCTTC-3′ and 5′-CAATTTTAGAAAGTTTATTATTTATGTATTTCATATAACTGTCTATTTCCCCAGGCCATATGAATATCCTCCTTA-3′. Lastly, we verified that all mutations created in S. flexneri or EPEC did not have an effect on growth in LB.
Secretion assays.
The secretion of T3SS effector proteins from S. flexneri was analyzed as previously described (55). Briefly, bacterial cultures were subcultured in LB and grown at 37°C. Once late log phase was reached, bacterial samples were normalized to the same optical density at 600 nm, and Congo red (7 μg/ml) was added. After 1 h, whole-cell lysates and supernatant fractions were prepared. For whole-cell preparations, 1 ml of culture was centrifuged and resuspended in 100 μl of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer. Supernatant fractions were prepared by trichloroacetic acid precipitation of 25 ml of culture passed through a 0.2-micron filter to ensure the removal of bacteria. Secretion of T3SS effectors from EPEC was analyzed as previously described (54). Briefly, overnight cultures were washed, subcultured in DMEM, and grown at 37°C for 5 h, and whole-cell and supernatant fractions were prepared as described above.
PAGE and immunoblot analysis of proteins.
For protein analysis, samples were resolved on 10% or 13% Tris-glycine SDS-PAGE gels (26). For immunoblotting, proteins were transferred to pure nitrocellulose membranes (Bio-Rad), and two-hemagglutinin (2HA)-tagged proteins were detected by the addition of mouse anti-HA monoclonal antibody HA.11 (Covance). To analyze ERK and NF-κB signaling, T84 cells were seeded on six-well plates and infected, and cells were scraped at different time points. Infected cells were pelleted, resuspended in sample buffer, and frozen until they were boiled and run on SDS-PAGE. Samples were immunoblotted using mouse monoclonal antibody against phospho-ERK1/2 (Thr202/Tyr204) and rabbit monoclonal antibody against phospho-Iκκ α/β (Ser176/180) (Cell Signaling, Inc.). Total protein amounts were evaluated using rabbit polyclonal antibody against ERK1/2 (Cell Signaling, Inc.). Bands were visualized using sheep anti-mouse and sheep anti-rabbit secondary antibodies conjugated to horseradish peroxidase (Amersham). Primary and secondary antibodies were used at a 1:1,000 dilution. Blots were developed using Visualizer (Upstate), and images were captured with a charge-coupled device camera from an LAS-3000 CH imaging system (Fuji). Densitometry of visualized bands was determined using Image Gauge V4.22.
Shigella virulence assays.
S. flexneri invasion assays were carried out as previously described (15), and CFU were counted and compared to the amount of input bacteria to calculate the invasion efficiency. Plaque assays were performed as previously described (37). The Serény test (50) was used to assess invasion and the in vivo inflammatory response in guinea pigs. Three guinea pigs were used to evaluate each strain used for each experiment, and symptoms were monitored for 4 days.
Immunofluorescence and transfection analysis.
Immunofluorescence was carried out as described before with S. flexneri using mouse anti-HA monoclonal antibody HA.11 (Covance) and goat anti-mouse antibody conjugated to AlexaFluor 488 (Invitrogen) (54), except that ImageiT-FX blocker (Invitrogen) was used to further eliminate background staining. EPEC-infected cells were fixed with 3% paraformaldehyde and stained with anti-HA antibody as described above. Transfections were carried out as described before (55) except that Lipofectamine 2000 or Lipofectamine LTX (Invitrogen) was used as the transfection reagent according to the manufacturer's instructions. Cells were sometimes stained with 4′,6′-diamidino-2-phenylindole (DAPI) (0.5 μg/ml) for 20 min. Images of Shigella-infected and EPEC-infected cells were acquired with an Olympus BX51 microscope using an Olympus DP-70 digital camera and merged using DP controller software, version 1.1.1.71. Images of transfected cells were acquired with an Olympus 1X81 microscope using a SensiCam charge-coupled device camera (Cooke) and merged with IPLabs software, version 3.1, or an Olympus BX51 microscope and the software mentioned above.
PMN transepithelial migration assay.
The PMN migration assay for S. flexneri and EPEC was performed as previously described (31, 49). Briefly, S. flexneri and EPEC were subcultured in LB, washed in Hank's buffered salt solution (HBSS), and added to the basolateral and apical compartments, respectively, of Transwells at a multiplicity of infection (MOI) of ∼100 for 90 min. After 90 min, bacteria were thoroughly washed away. Human PMNs were purified from whole blood by a gelatin sedimentation technique (31) and added to the basolateral compartment. Transmigration to the apical compartment was quantified by assaying for the PMN azurophilic granule marker myeloperoxidase. For inducible complementation of deletion mutations, arabinose was added at a final concentration of 0.6% to the HBSS during the PMN migration assay. Unpaired Student's t tests were used to analyze statistical significances in comparisons between wild-type EPEC and S. flexneri and experimental strains.
RESULTS
Orf212 is a homologue of the EPEC T3SS effector NleE.
It was shown previously that NleE has homologues in Citrobacter, EHEC, and Shigella species (53). In A/E effacing pathogens, the two T3SS effectors nleB and nleE are encoded together in the same operon (21). Interestingly, Shigella species evolved to incorporate only an nleE homologue, orf212, on the virulence plasmid. In contrast, Salmonella enterica serovar Typhimurium species evolved to incorporate only an nleB homologue, STM4157, on its chromosome (BLAST analysis). It should also be noted that Salmonella enterica serovars Typhi and Paratyphi do not have a copy of nleB or nleE (BLAST analysis).
Further study of orf212, the S. flexneri nleE homologue, found that this gene harbored a frameshift mutation that creates a stop codon which truncates the protein product at amino acid 188 in the sequenced strains of S. flexneri 2a (301 and 2457T) and S. flexneri 5 M90T. We resequenced orf212 from S. flexneri 2a 2457T, the wild-type strain used in this study, to confirm the mutation. We also sequenced orf212 from clinical isolates of the same related cluster of Shigella (27), which included S. flexneri types 1a and 3a, and Shigella boydii type 12 to see if the frameshift mutation was conserved in these isolates. Lastly, we sequenced orf212 from enteroinvasive E. coli (EIEC), a close relative of Shigella species.
We found that S. flexneri 1a does indeed have the same frameshift mutation which leads to a stop codon at amino acid 188 (Fig. 1), whereas S. flexneri 3a has an insertion element (IS3) that is inserted after just 34 base pairs of DNA sequence, leading to a gene deletion (data not shown). The EIEC orf212 also had a frameshift mutation that leads to a stop codon, but the truncation was at amino acid 152 (Fig. 1). S. boydii 12 had the same mutation that results in a protein product truncated at amino acid 152 (Fig. 1). Therefore, it appears that there is some plasticity within this region of the virulence plasmid, but the majority of related S. flexneri serotypes (1, 2, and 5) have a frameshift mutation leading to a truncated protein product at amino acid 188.
FIG. 1.
NleE and Orf212 (OspZ) are homologues found in Shigella species and A/E strains of E. coli. Sequences from S. flexneri 1a, S. flexneri 2a 2457T, S. flexneri 5a M90T (GenBank accession no. AF348706), S. dysenteriae (YP406222), S. sonnei (YP313493), S. boydii (YP406371), S. boydii 12, EPEC E2348/69 (epath100c11_p1cb_ORF_3157; coliBase), and EIEC were aligned using ClustalW (CLSTALW; http://www.ebi.ac.uk/clustalw/) and generated by BioEdit (http://www.mbio.ncsu.edu/BioEdit/bioedit.html). Shaded regions are homologous residues, where asterisks (*) represent identical amino acids, colons (:) are conservative substitutions, and periods (.) are semiconservative substitutions, all based on the GONNET250 matrix.
Orf212 is secreted by the S. flexneri T3SS.
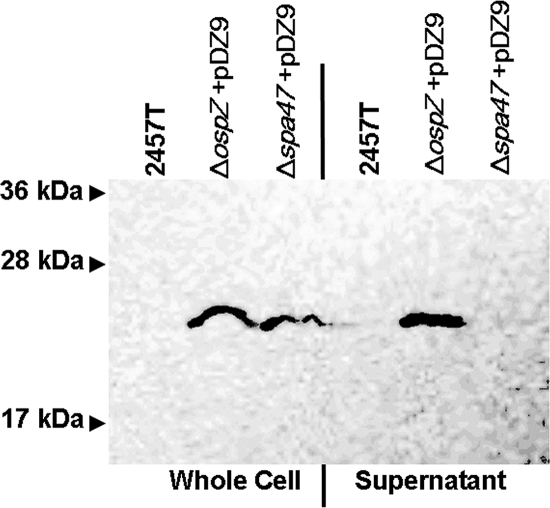
NleE is a T3SS effector protein that is secreted and translocated into host eukaryotic cells by EPEC (9, 21). Since orf212 on the S. flexneri virulence plasmid has such a high degree of homology to nleE (53), we wanted to determine if Orf212 was secreted by the S. flexneri T3SS. Using a strategy implemented previously (55), we generated a 2HA-tagged version of Orf212. The pDZ9 (Orf212-2HA) plasmid was transformed into an Δorf212 deletion mutant (BS821) that was generated by allelic exchange using a lambda red recombination system. The resulting strain was designated BS822. This plasmid was also transformed into a Δspa47 mutant (BS652) to generate BS824. Spa47 is the ATPase required for a functional T3SS of S. flexneri (19), and we previously used this background strain to test the T3SS-dependent secretion of putative effectors (55). After 1 h of exposure to Congo red to induce the S. flexneri T3SS, Orf212-2HA (∼23 kDa) was found in the whole-cell and supernatant fractions of BS822 (Fig. 2). In contrast, no signal was detected in either the untransformed 2457T or in the supernatant of the T3SS mutant BS824 (Fig. 2). Therefore, Orf212 is secreted by the S. flexneri T3SS, and we renamed this protein OspZ because it is the last annotated ORF on the virulence plasmid (5), and the potential exists for more T3SS effectors to be discovered (orf9 to orf186).
FIG. 2.
T3SS-dependent secretion of OspZ. 2457T, ΔospZ/pDZ9 (BS822), and Δspa47/pDZ9 (BS824) whole-cell fractions and supernatants were prepared after 1 h of induction with Congo red. Samples were run on a 13% SDS-PAGE gel and immunoblotted with anti-HA antibody to visualize OspZ-2HA (∼22-kDa band).
Intracellular localization of OspZ and NleE.
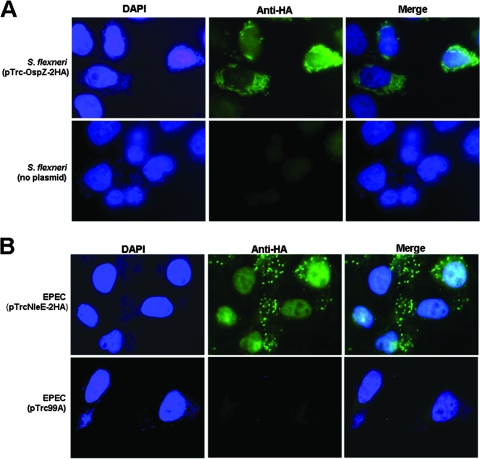
Since OspZ and NleE are homologues, we hypothesized that these proteins may share a similar localization inside the host cell. Therefore, a semiconfluent monolayer of HeLa cells was infected with 2457T or ΔospZ/pTrc-OspZ-2HA (BS841) for 4 h, and the OspZ-2HA fusion protein was detected using indirect immunofluorescence with an anti-HA antibody and an Alexa Fluor 488 secondary antibody. We counterstained with DAPI, which stains the DNA of the bacteria and nucleus of the host cell. We found that the anti-HA signal was localized primarily with the bacteria or in the vicinity of the bacteria in the cytoplasm (Fig. 3A). To a lesser degree, some signal was also found inside the nucleus (Fig. 3A). As a negative control, cells infected with wild-type 2457T (no pTrc-OspZ-2HA) displayed no anti-HA signal above background levels (Fig. 3A).
FIG. 3.
Localization of OspZ-2HA and NleE-2HA in infected host cells. (A) ΔospZ/pTrc-OspZ-2HA (BS841) was used to infect a semiconfluent monolayer of HeLa cells. After 4 h, cells were fixed and evaluated by immunofluorescence. Images are representative of DAPI staining, anti-HA, and the merged fields. The negative control is HeLa cells infected with wild-type 2457T (minus pTrc-OspZ-2HA). (B) EPEC/pTrc-NleE-2HA and EPEC (pTrc99A, empty plasmid) were used to infect a semiconfluent monolayer of HeLa cells. After 5 h, cells were fixed and evaluated by immunofluorescence. Images in the middle column are representative of the immunofluorescence anti-HA signal observed with each strain, the left column shows nuclei and bacteria stained with DAPI, and the right column shows the merged fields.
To determine if NleE exhibited a similar localization in EPEC-infected cells, we cloned nleE in frame with a 2HA tag (pTrc-NleE-2HA) and transformed this plasmid into EPEC E2348/69. HeLa cell monolayers were infected with wild-type EPEC (no pTrc-NleE-2HA) or EPEC harboring the pTrc-NleE-2HA plasmid for 5 h, and the secreted NleE-2HA fusion protein was detected using immunofluorescence with anti-HA antibody. We observed that the immunofluorescence signal localized with the bacteria but also more definitively in the nucleus of the host cells compared to OspZ (Fig. 3B). No HA signal was found in the nucleus infected with the negative control (Fig. 3B).
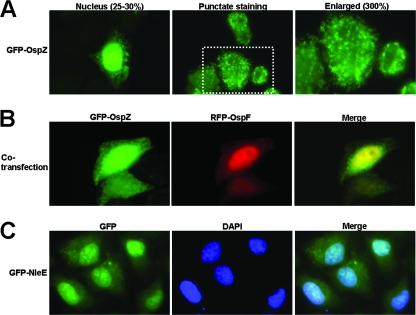
To confirm the localization of NleE and OspZ, we ectopically expressed these proteins fused to green fluorescent protein (GFP) at their N terminus. OspZ and NleE were cloned in frame with enhanced GFP (EGFP) to generate the N-terminal fusion proteins. A semiconfluent monolayer of HeLa cells was transiently transfected with plasmids expressing GFP-OspZ or GFP-NleE, and cells were fixed after 12 h. Upon analysis, we observed two distinct localization phenotypes in the host cell. GFP-OspZ was found in punctate staining in the cytoplasm of transfected cells (Fig. 4A) and, occasionally, GFP-OspZ localized in the nucleus (∼25 to 30% of transfected cells) (Fig. 4A). To confirm the nuclear localization of GFP-OspZ, the GFP-OspZ plasmid was cotransfected with red fluorescent protein (RFP)-OspF, which has been shown previously to localize in the nucleus of transfected cells (55). In cotransfected cells, GFP-OspZ and RFP-OspF were found in the nucleus together (Fig. 4B). We also confirmed this observation with DAPI staining (data not shown). In contrast, GFP-NleE was found primarily in the nucleus of the transfected HeLa cells (Fig. 4C), and no punctate staining was observed. The nuclear staining with the ectopically expressed proteins was consistent with the immunofluorescence results observed with infected cells.
FIG. 4.
Ectopic expression of GFP-OspZ and GFP-NleE in HeLa cells. Semiconfluent monolayers of HeLa cells were transiently transfected for 12 h with mammalian expression vectors encoding GFP-OspZ (A), GFP-OspZ and RFP-OspF (B), or GFP-NleE (C). Panel A has two different representative fields of GFP-OspZ (left and middle column), nuclear and cytoplasmic staining. The far right column is the middle column (dotted white box) enlarged to highlight the punctate staining in the cytoplasm. In panel C, nuclei were stained with DAPI.
Virulence phenotypes of a ΔospZ mutant.
Since OspZ from S. flexneri was secreted in a T3SS-dependent manner, we wanted to determine whether the ΔospZ deletion mutant (BS821) was attenuated in various tests of S. flexneri virulence. First, we compared ΔospZ to wild-type 2457T in an invasion assay using HeLa cells and polarized T84 cells and found no discernible difference in invasion efficiency (data not shown).
Next, we compared ΔospZ and 2457T in a plaque assay to determine the ability of each strain to invade, replicate intracellularly, and spread cell to cell. Again, no significant differences were observed between ΔospZ and 2457T, with the efficiencies of plaque formation (2457T, 1.1% ± 0.13%; ΔospZ, 1.3% ± 0.15%) and sizes of plaques being equivalent (∼1.5 mm). Lastly, we compared ΔospZ and 2457T infections using the Serény test, one of a limited number of animal infection models for S. flexneri (50). Three guinea pigs were infected with ΔospZ or 2457T at a dose of 2.5 × 108 CFU administered into one eye of each animal. Over the course of 4 days, guinea pigs infected with either ΔospZ or 2457T developed conjunctivitis and a strong inflammatory response with no discernible differences between results with the wild type and with ΔospZ.
PMN transepithelial migration assays.
PMN transepithelial migration is a hallmark of S. flexneri infection and requires the T3SS (31). We identified two T3SS effectors, OspF and OspC1, that contribute to Shigella-induced PMN migration (55). The PMN migration assay allowed us to detect subtle differences between strains which other S. flexneri virulence assays do not detect (24, 31, 55). We postulated that PMN migration requires multiple, upstream factors and wanted to see if OspZ contributed to this aspect of S. flexneri pathogenesis.
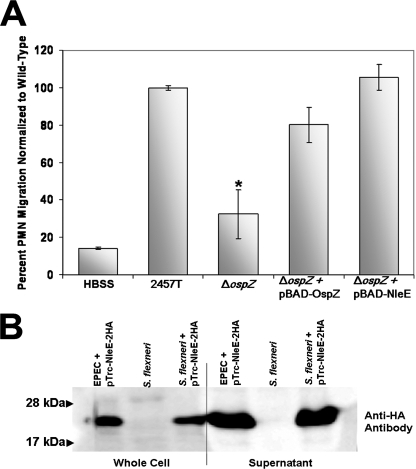
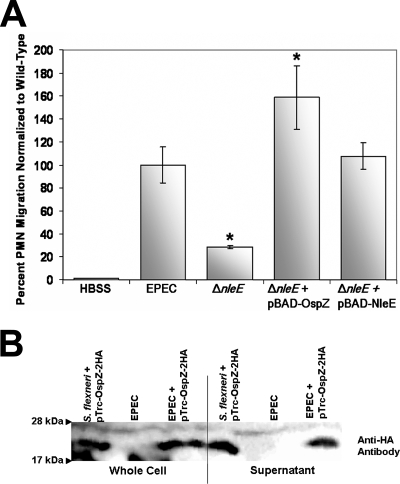
Mutant and wild-type strains of S. flexneri were used to infect polarized T84 monolayers. After 90 min of infection, isolated human PMNs were added to the basolateral side of the Transwell and transepithelial migration was evaluated. When ΔospZ (BS821) was compared to 2457T, we observed a statistically significant decrease in the amount of neutrophils that migrated from the basolateral to the apical compartment, the physiologic direction of PMN recruitment/infiltration (Fig. 5A).
FIG. 5.
NleE can complement the S. flexneri ΔospZ in PMN transepithelial migration assays. (A) T84 polarized monolayers were infected basolaterally with 2457T, ΔospZ (BS821), ΔospZ/pBAD24::ospZ (BS833), and ΔospZ/pBAD24::nleE (BS834) and were evaluated after 90 min for PMN migration. HBSS represents uninfected monolayers (buffer only). All strains were normalized to wild-type 2457T. Experiments were performed three times in triplicate. The data are means ± standard deviations (error bars) of triplicate samples and represent one of the three experiments performed in which similar results were obtained. Test samples were individually compared to 2457T using an unpaired Student t test, and statistically significant differences (P values of ≤0.01) are indicated by an asterisk. (B) 2457T transformed with pTrc-NleE-2HA (BS840) was grown in secretion conditions and compared to the negative control, untransformed wild-type 2457T. Whole-cell fractions and supernatant were prepared. Samples were boiled, run on a 13% SDS-PAGE gel, and immunoblotted with anti-HA antibody to visualize Nle-2HA (∼25-kDa band).
EPEC is also capable of inducing PMN transepithelial migration (49). Therefore, we wanted to see if infection with a ΔnleE deletion mutant resulted in a deficiency similar to that observed with the deletion of ospZ of S. flexneri. PMN transmigration induced by the EPEC ΔnleE mutant was significantly reduced in comparison to wild-type EPEC (Fig. 6A), and this reduction mirrored the reduction observed with the ΔospZ deletion mutant of S. flexneri.
FIG. 6.
OspZ can complement EPEC ΔnleE in PMN transepithelial migration assays. (A) T84 polarized monolayers were infected apically with EPEC E2348/69, ΔnleE, ΔnleE/pBAD24::ospZ (ATM897), and ΔnleE/pBAD24::nleE (ATM898) and after 90 min were evaluated for PMN migration. HBSS represents uninfected monolayers (buffer only). All strains were normalized to wild-type EPEC E2348/69. Experiments were performed three times in triplicate. The data are means ± standard deviations (error bars) of triplicate samples and represent one of the three experiments performed in which similar results were obtained. Test samples were individually compared to 2457T using an unpaired Student t test, and statistically significant differences (P values of ≤0.01) are indicated by an asterisk. (B) ΔnleE/pTrc-OspZ-2HA was grown in EPEC secretion conditions and compared to the negative control EPEC E2348/69 with no plasmid and the positive control, ΔospZ/pTrc-OspZ-2HA (BS841). Whole-cell fractions and supernatant were prepared. Samples were boiled, run on a 13% SDS-PAGE gel, and immunoblotted with anti-HA antibody to visualize OspZ-2HA (∼22-kDa band).
Since OspZ and NleE have nearly identical primary amino acid sequences (Fig. 1) and the deletion mutants showed a comparable phenotype in the PMN transmigration assay, we hypothesized that nleE should complement the Shigella ΔospZ deletion mutant and, conversely, ospZ should complement the EPEC ΔnleE mutant in the PMN migration assay. To address each hypothesis, we generated two arabinose-inducible, untagged constructs by cloning ospZ and nleE into pBAD24, and each plasmid was transformed in a cross-species manner into the corresponding deletion mutant.
ΔospZ/pBAD24::nleE (BS834) was used to infect polarized T84 cells in Transwells in the presence of 0.6% arabinose and compared to 2457T. ΔospZ/pBAD24::ospZ (BS833) served as a positive control. When normalized to 2457T, T84 cells infected with BS834 induced nearly equivalent levels of PMN migration to the apical compartment of the Transwell compared to 2457T (Fig. 5A). Similarly, the reduction could be restored by an OspZ-expressing plasmid, pBAD24::ospZ (BS833) (Fig. 5A). These results implied that nleE was secreted by the S. flexneri T3SS and complemented the function of ospZ in the ΔospZ deletion mutant. We verified that NleE was secreted by 2457T by analyzing supernatants of 2457T transformed with pTrc-NleE-2HA (BS840) (Fig. 5B).
Since NleE expression could complement an ospZ deletion mutant of S. flexneri, we hypothesized that OspZ expression should complement the PMN transepithelial migration defect for the ΔnleE mutant of EPEC. ΔnleE/pBAD24::ospZ (ATM897) was used to infect polarized T84 cells, and the level of PMN migration was compared to that seen with wild-type EPEC infection. ΔnleE/pBAD24::nleE (ATM898) was used as a positive control. When normalized to wild-type EPEC infection levels, ΔnleE/pBAD24::ospZ complemented the ΔnleE mutant, and actually induced significantly more PMN migration than wild-type EPEC and ΔnleE/pBAD24::nleE (Fig. 6A). Therefore, ospZ can substitute for nleE in the PMN transepithelial assay.
Lastly, to verify that OspZ was secreted by the heterologous EPEC T3SS, we transformed pTrc-OspZ-2HA into wild-type EPEC (ATM928). ATM928 and wild-type EPEC were grown overnight and subcultured into DMEM, and secretion was evaluated after 5 h. As expected from the PMN migration results, OspZ was secreted by the EPEC T3SS (Fig. 6B).
OspZ plays a role in the induction of host signaling pathways.
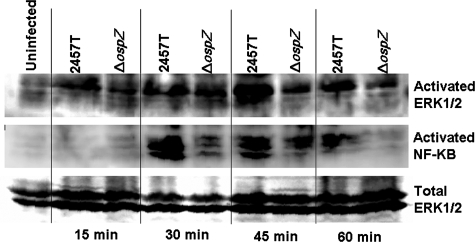
Since we have previously shown that S. flexneri infections with other osp deletion mutants have a deficiency in PMN transepithelial migration and display a deficiency in ERK1/2 pathway activation (55), we evaluated ERK1/2 phosphorylation in BS821, a ΔospZ deletion mutant, during infection. We infected T84 cells with 2457T or BS821 and evaluated ERK1/2 phosphorylation by Western blotting over the first hour of infection using a phosphospecific antibody that recognizes the activation of ERK1/2. As a loading control, we blotted for total ERK2 protein. We saw a marked reduction in ERK1/2 phosphorylation after 30 min of infection in T84 cells infected with BS821 compared to 2457T (Fig. 7). We also evaluated NF-κB activation, since it has been shown to be activated the first hour of S. flexneri infection (40). As shown in Fig. 7, we used an antibody against phosphospecific residues indicating NF-κB activation to show that cells infected with BS821 also displayed reduced NF-κB activation with respect to wild-type infection (Fig. 7). We used densitometry on blots from three different experiments at the 45-min time point and found that cells infected with the ΔospZ had 63.53% ± 3.92% of the amount of ERK1/2 phosphorylation and 52.82% ± 11.95% of NF-κB activation compared to that seen with wild-type infection (normalized to 100%). Therefore, we conclude that OspZ plays a role in activating host inflammatory pathways required for PMN transepithelial migration.
FIG. 7.
ERK1/2 and NF-κB have reduced activation in cells infected with the S. flexneri ΔospZ mutant. T84 cells were infected with wild-type 2457T or ΔospZ (BS821). Cells were scraped at each time point, boiled, run on a 10% SDS-PAGE gel, and immunoblotted with anti-phospho-ERK1/2 and anti-phospho NF-κB antibody to visualize signaling pathway activation. Samples were blotted with anti-ERK1/2 antibody to monitor equal loading. The blot is representative of an experiment repeated three times.
DISCUSSION
The large virulence plasmid of S. flexneri encodes a T3SS and at least 20 effector proteins that are secreted by this apparatus (5, 23, 32, 45, 55). Many of these proteins were identified in a previous study that used N-terminal sequencing to identify the secreted proteins from a constitutive secretion mutant (ΔipaB) of S. flexneri serotype 5a (5). OspZ was not among the proteins identified. However, the amount of OspZ secreted by this strain may not have been sufficient for N-terminal sequencing. In this study, OspZ piqued our interest because of its similarity to the EPEC T3SS effector NleE, and we confirmed that OspZ is indeed a secreted S. flexneri T3SS effector. OspZ can therefore be added to a growing list of Shigella T3SS effectors that have homologues in other gram-negative pathogens. For example, OspF of Shigella has homologues in Salmonella species, Pseudomonas syringae, and Chromobacterium violaceum (4). It has also been shown that a family of effectors, IpgB1/B2 (Shigella), SifA/B (Salmonella), and Map (EPEC/EHEC) mimic the function of RhoGTPases in the host cell (2). In addition, Shigella and EPEC share other homologous effectors, such as VirA (Shigella) and EspG/G2 (EPEC), which disrupt host microtubules (10, 52).
While some of these shared T3SS effectors have highly homologous amino acid sequences, it is nevertheless important to confirm their related functions, since even small changes can affect protein function. For example, the Shigella OspF localizes in the nucleus of host cells, while the homologous SpvC (>70% homology) from Salmonella remains in the cytoplasm (55). At the same time, OspF and SpvC share a function as a lyase to remove phosphate groups from mitogen-activated protein kinases (MAPK) (29). In the current study, we encountered a similar localization difference between OspZ and NleE. While OspZ-2HA and GFP-OspZ were found in the nucleus in some instances, the tagged NleE was found in the nucleus 100% of the time. A classic or nonclassical bipartite or monopartite nuclear localization signal (NLS) was not detected in either NleE or OspZ by using NLS scanning software. However, it is possible that each protein interacts directly with a host protein that has an NLS. Another possibility exists where OspZ/NleE could enter the nucleus via an interaction with another secreted bacterial protein which also localizes in the host nucleus. However, the ectopic expression of GFP-OspZ and GFP-NleE showed that both proteins can enter the nucleus in the absence of bacterial infection. The localization difference observed between NleE and OspZ (including the punctuate staining of GFP-OspZ) could be attributed to the additional 36 amino acids found at the C terminus of NleE (Fig. 1). While the additional C-terminal domain does not contain an NLS, it is possible that this domain serves to more effectively target NleE to the nucleus. We are currently investigating this possibility and have preliminary data which suggest that the C terminus is required for NleE nuclear localization (L. Badea and E. L. Hartland, unpublished data). In contrast, the OspZ nuclear localization could be a product of diffusion, since it was not found consistently in the nucleus, and both tagged proteins are smaller than the nuclear pore (∼60 kDa). The importance of nuclear localization is unclear, since both ospZ and nleE can complement the PMN migration deficiency during infection. Therefore, the nuclear localization is unlinked to the PMN migration phenotype.
Since OspZ and NleE both played a role in the induction of PMN migration across a polarized T84 monolayer during S. flexneri and EPEC infections, we speculate that the function these proteins share is linked to influencing host signaling pathways. Interestingly, the expression of OspZ in the ΔnleE mutant led to increased PMN migration compared to that in wild-type EPEC. Therefore, the S. flexneri version of this T3SS effector may be more active than its EPEC counterpart. This hypermigration is not unexpected given that S. flexneri infections cause more acute inflammation than EPEC infections (20), and it could be attributed to the missing C-terminal 36 amino acids.
It is clear from previous work that the activation of the ERK pathway is required for Shigella-induced PMN migration (24, 55). Our data suggest that OspZ is required for the full activation of the ERK1/2 and NF-κB pathways by an unknown, upstream mechanism (Fig. 7). EPEC stimulates ERK1/2 phosphorylation to induce an inflammatory response which includes NF-κB activation and IL-8 production (47, 48). We are currently investigating if NleE plays a role in ERK1/2 and NF-κB activation and if it can be linked to the PMN migration phenotype, but it would not be a surprising connection given the homology to and function shared with OspZ and the reduced hyperplasia observed for infections with the ΔnleE mutant of Citrobacter (53). It should be noted that IL-8 (a product of NF-κB activation) is secreted basolaterally in vivo, and a separate factor, hepoxilin A3 (HXA3) has been identified to be essential in recruiting PMN across the epithelial layer to the apical side in Pseudomonas and Salmonella infections (18, 33, 34). Therefore, it is likely that the same host signaling pathways required for PMN migration are required for HXA3 production and apical secretion, and we are investigating this possibility. EPEC and Shigella may target ERK1/2 and NF-κB directly, but it is more likely that receptors or kinases upstream of ERK1/2 and NF-κB are being activated, and OspZ and NleE play a role in this activation. Therefore, ERK1/2 and NF-κB may be only a fraction of the host signaling pathways whose activation is required to mediate the PMN migration phenotype in EPEC and Shigella-infected epithelial cells (8, 34). One upstream pathway we are investigating is the phosphatidylinositol 3 (PI3) kinase pathway. S. flexneri and EPEC activate PI3 kinase (38, 42), but EPEC can also inhibit PI3 kinase at a different time point during infection and in macrophages (6), so it would be interesting if these responses were altered during infection with ΔospZ/ΔnleE mutants.
The initial activation of the inflammatory response is just one aspect of Shigella and EPEC interactions with the innate immune system. At later time points, both Shigella and EPEC downregulate inflammation in a T3SS-dependent fashion. In the case of Shigella, OspG binds to UbcH5 to promote the ubiquitination of phosphorylated inhibitor of NF-κB type alpha (phospho-IκBα) (23). OspF removes the phosphate groups of members of the MAPK family in the nucleus and plays a role in chromatin remodeling to downregulate AP-1-regulated genes (4, 29). EPEC also downregulates MAPK family members in a T3SS-dependent manner, but the identity of the effector(s) has not been elucidated (43). In addition, MOI plays a role in whether EPEC induces a pro- or anti-inflammatory response in the host (51). When a lower MOI of EPEC is used to infect HT-29 intestinal cells, IL-8 production is increased with contributions from an unidentified T3SS effector and flagellin (51). A higher MOI of EPEC leads to the downregulation of IL-8 in a T3SS-dependent manner (51). Lastly, EPEC rearranges host proteins from the basolateral to the apical membrane, which again may impact the kinetics of inflammatory signaling (36, 42). Interestingly, Shigella lacks flagella, which could be why the bacteria evolved to use multiple T3SS effectors (Osp proteins and IpgD) to activate the signaling pathways required to induce inflammation and PMN transepithelial migration (8, 38, 55). Therefore, in both Shigella and EPEC infections, the regulation of inflammatory signaling is a complex and dynamic balance. While the activation of PMN migration and other inflammatory processes contributes to pathogenesis, too much inflammation may activate the host immune system to clear the infection (8, 39, 40, 45). The challenge in understanding this paradox will be to identify the T3SS effectors involved (in this case, OspZ and NleE) and their function at different times during infection. Shigella and EPEC appear to utilize T3SS effectors with different kinetics and in different host compartments (e.g., cytoplasm versus nucleus) with most likely different host targets to balance the innate immune response.
In conclusion, this study identifies for the first time the facts that OspZ is a secreted Shigella T3SS effector and is required for virulence. OspZ has a role, along with OspF and OspC1 (55) and most likely other secreted proteins, in mediating the PMN migration phenotype. NleE is the first T3SS effector of EPEC shown to play a role in PMN transepithelial migration, but given the shared virulence strategies of related bacterial pathogens, we anticipate that other EPEC T3SS effectors also contribute to this phenotype.
Acknowledgments
We thank Nancy Adams and Reinaldo Fernandez for technical assistance and Stephanie Perry for bioinformatics help.
This work was supported by National Institutes of Allergy and Infectious Diseases grant AI24656 (to A.T.M.). B.A.M. is supported by National Institutes of Health grants DK56754 and DK33506. K.L.M. is supported by a T32 training grant sponsored by Harvard Medical School and the Department of Surgery at Massachusetts General Hospital. J.A.P. was supported by an Australian Postgraduate Scholarship, and E.L.H. is supported by grants from the Australian National Health and Medical Research Council and the Australian Research Council.
The opinions or assertions contained herein are the private ones of the authors and are not to be construed as official or reflecting the views of the Department of Defense of the United States or the Uniformed Services University of the Health Sciences.
Editor: J. B. Bliska
Footnotes
Published ahead of print on 5 November 2007.
REFERENCES
- 1.Ahmetagic, S., E. Jusufovic, J. Petrovic, V. Stojic, and Z. Delibegovic. 2003. Acute infectious diarrhea in children. Med. Arh. 5787-92. [PubMed] [Google Scholar]
- 2.Alto, N. M., F. Shao, C. S. Lazar, R. L. Brost, G. Chua, S. Mattoo, S. A. McMahon, P. Ghosh, T. R. Hughes, C. Boone, and J. E. Dixon. 2006. Identification of a bacterial type III effector family with G protein mimicry functions. Cell 124133-145. [DOI] [PubMed] [Google Scholar]
- 3.Aragon, T. J., D. J. Vugia, S. Shallow, M. C. Samuel, A. Reingold, F. J. Angulo, and W. Z. Bradford. 2007. Case-control study of shigellosis in San Francisco: the role of sexual transmission and HIV infection. Clin. Infect. Dis. 44327-334. [DOI] [PubMed] [Google Scholar]
- 4.Arbibe, L., D. W. Kim, E. Batsche, T. Pedron, B. Mateescu, C. Muchardt, C. Parsot, and P. J. Sansonetti. 2007. An injected bacterial effector targets chromatin access for transcription factor NF-kappaB to alter transcription of host genes involved in immune responses. Nat. Immunol. 847-56. [DOI] [PubMed] [Google Scholar]
- 5.Buchrieser, C., P. Glaser, C. Rusniok, H. Nedjari, H. D'Hauteville, F. Kunst, P. Sansonetti, and C. Parsot. 2000. The virulence plasmid pWR100 and the repertoire of proteins secreted by the type III secretion apparatus of Shigella flexneri. Mol. Microbiol. 38760-771. [DOI] [PubMed] [Google Scholar]
- 6.Celli, J., M. Olivier, and B. B. Finlay. 2001. Enteropathogenic Escherichia coli mediates antiphagocytosis through the inhibition of PI 3-kinase-dependent pathways. EMBO J. 201245-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 976640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delbridge, L. M., and M. X. O'Riordan. 2007. Innate recognition of intracellular bacteria. Curr. Opin. Immunol. 1910-16. [DOI] [PubMed] [Google Scholar]
- 9.Deng, W., J. L. Puente, S. Gruenheid, Y. Li, B. A. Vallance, A. Vazquez, J. Barba, J. A. Ibarra, P. O'Donnell, P. Metalnikov, K. Ashman, S. Lee, D. Goode, T. Pawson, and B. B. Finlay. 2004. Dissecting virulence: systematic and functional analyses of a pathogenicity island. Proc. Natl. Acad. Sci. USA 1013597-3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elliott, S. J., E. O. Krejany, J. L. Mellies, R. M. Robins-Browne, C. Sasakawa, and J. B. Kaper. 2001. EspG, a novel type III system-secreted protein from enteropathogenic Escherichia coli with similarities to VirA of Shigella flexneri. Infect. Immun. 694027-4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elliott, S. J., L. A. Wainwright, T. K. McDaniel, K. G. Jarvis, Y. K. Deng, L. C. Lai, B. P. McNamara, M. S. Donnenberg, and J. B. Kaper. 1998. The complete sequence of the locus of enterocyte effacement (LEE) from enteropathogenic Escherichia coli E2348/69. Mol. Microbiol. 281-4. [DOI] [PubMed] [Google Scholar]
- 12.Faruque, A. S., M. A. Malek, A. I. Khan, S. Huq, M. A. Salam, and D. A. Sack. 2004. Diarrhoea in elderly people: aetiology, and clinical characteristics. Scand. J. Infect. Dis. 36204-208. [DOI] [PubMed] [Google Scholar]
- 13.Formal, S. B., G. J. Dammin, E. H. Labrec, and H. Schneider. 1958. Experimental Shigella infections: characteristics of a fatal infection produced in guinea pigs. J. Bacteriol. 75604-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guzman, L. M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 1774121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hale, T. L., and S. B. Formal. 1981. Protein synthesis in HeLa or Henle 407 cells infected with Shigella dysenteriae 1, Shigella flexneri 2a, or Salmonella typhimurium W118. Infect. Immun. 32137-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166557-580. [DOI] [PubMed] [Google Scholar]
- 17.Harris, J. R., I. K. Wachsmuth, B. R. Davis, and M. L. Cohen. 1982. High-molecular-weight plasmid correlates with Escherichia coli enteroinvasiveness. Infect. Immun. 371295-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurley, B. P., D. Siccardi, R. J. Mrsny, and B. A. McCormick. 2004. Polymorphonuclear cell transmigration induced by Pseudomonas aeruginosa requires the eicosanoid hepoxilin A3. J. Immunol. 1735712-5720. [DOI] [PubMed] [Google Scholar]
- 19.Jouihri, N., M. P. Sory, A. L. Page, P. Gounon, C. Parsot, and A. Allaoui. 2003. MxiK and MxiN interact with the Spa47 ATPase and are required for transit of the needle components MxiH and MxiI, but not of Ipa proteins, through the type III secretion apparatus of Shigella flexneri. Mol. Microbiol. 49755-767. [DOI] [PubMed] [Google Scholar]
- 20.Kaper, J. B., J. P. Nataro, and H. L. Mobley. 2004. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2123-140. [DOI] [PubMed] [Google Scholar]
- 21.Kelly, M., E. Hart, R. Mundy, O. Marches, S. Wiles, L. Badea, S. Luck, M. Tauschek, G. Frankel, R. M. Robins-Browne, and E. L. Hartland. 2006. Essential role of the type III secretion system effector NleB in colonization of mice by Citrobacter rodentium. Infect. Immun. 742328-2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kenny, B. 2002. Mechanism of action of EPEC type III effector molecules. Int. J. Med. Microbiol. 291469-477. [DOI] [PubMed] [Google Scholar]
- 23.Kim, D. W., G. Lenzen, A. L. Page, P. Legrain, P. J. Sansonetti, and C. Parsot. 2005. The Shigella flexneri effector OspG interferes with innate immune responses by targeting ubiquitin-conjugating enzymes. Proc. Natl. Acad. Sci. USA 10214046-14051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Köhler, H., S. P. Rodrigues, and B. A. McCormick. 2002. Shigella flexneri interactions with the basolateral membrane domain of polarized model intestinal epithelium: role of lipopolysaccharide in cell invasion and in activation of the mitogen-activated protein kinase ERK. Infect. Immun. 701150-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kotloff, K. L., J. P. Winickoff, B. Ivanoff, J. D. Clemens, D. L. Swerdlow, P. J. Sansonetti, G. K. Adak, and M. M. Levine. 1999. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull. W. H. O. 77651-666. [PMC free article] [PubMed] [Google Scholar]
- 26.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227680-685. [DOI] [PubMed] [Google Scholar]
- 27.Lan, R., M. C. Alles, K. Donohoe, M. B. Martinez, and P. R. Reeves. 2004. Molecular evolutionary relationships of enteroinvasive Escherichia coli and Shigella spp. Infect. Immun. 725080-5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levine, M. M., E. J. Bergquist, D. R. Nalin, D. H. Waterman, R. B. Hornick, C. R. Young, and S. Sotman. 1978. Escherichia coli strains that cause diarrhoea but do not produce heat-labile or heat-stable enterotoxins and are non-invasive. Lancet i1119-1122. [DOI] [PubMed] [Google Scholar]
- 29.Li, H., H. Xu, Y. Zhou, J. Zhang, C. Long, S. Li, S. Chen, J. M. Zhou, and F. Shao. 2007. The phosphothreonine lyase activity of a bacterial type III effector family. Science 3151000-1003. [DOI] [PubMed] [Google Scholar]
- 30.Maurelli, A. T., B. Blackmon, and R. Curtiss III. 1984. Loss of pigmentation in Shigella flexneri 2a is correlated with loss of virulence and virulence-associated plasmid. Infect. Immun. 43397-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCormick, B. A., A. M. Siber, and A. T. Maurelli. 1998. Requirement of the Shigella flexneri virulence plasmid in the ability to induce trafficking of neutrophils across polarized monolayers of the intestinal epithelium. Infect. Immun. 664237-4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Menard, R., P. Sansonetti, and C. Parsot. 1994. The secretion of the Shigella flexneri Ipa invasins is activated by epithelial cells and controlled by IpaB and IpaD. EMBO J. 135293-5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mrsny, R. J., A. T. Gewirtz, D. Siccardi, T. Savidge, B. P. Hurley, J. L. Madara, and B. A. McCormick. 2004. Identification of hepoxilin A3 in inflammatory events: a required role in neutrophil migration across intestinal epithelia. Proc. Natl. Acad. Sci. USA 1017421-7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mumy, K. L., and B. A. McCormick. 2005. Events at the host-microbial interface of the gastrointestinal tract. II. Role of the intestinal epithelium in pathogen-induced inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 288G854-G859. [DOI] [PubMed] [Google Scholar]
- 35.Murphy, K. C., and K. G. Campellone. 2003. Lambda Red-mediated recombinogenic engineering of enterohemorrhagic and enteropathogenic E. coli. BMC Mol. Biol. 411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muza-Moons, M. M., A. Koutsouris, and G. Hecht. 2003. Disruption of cell polarity by enteropathogenic Escherichia coli enables basolateral membrane proteins to migrate apically and to potentiate physiological consequences. Infect. Immun. 717069-7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oaks, E. V., T. L. Hale, and S. B. Formal. 1985. Plaque formation by virulent Shigella flexneri. Infect. Immun. 48124-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pendaries, C., H. Tronchere, L. Arbibe, J. Mounier, O. Gozani, L. Cantley, M. J. Fry, F. Gaits-Iacovoni, P. J. Sansonetti, and B. Payrastre. 2006. PtdIns(5)P activates the host cell PI3-kinase/Akt pathway during Shigella flexneri infection. EMBO J. 251024-1034. 16482216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perdomo, J. J., P. Gounon, and P. J. Sansonetti. 1994. Polymorphonuclear leukocyte transmigration promotes invasion of colonic epithelial monolayers by Shigella flexneri. J. Clin. Investig. 93633-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Philpott, D. J., S. Yamaoka, A. Israel, and P. J. Sansonetti. 2000. Invasive Shigella flexneri activates NF-kappa B through a lipopolysaccharide-dependent innate intracellular response and leads to IL-8 expression in epithelial cells. J. Immunol. 165903-914. [DOI] [PubMed] [Google Scholar]
- 41.Pickering, L. K. 2004. Antimicrobial resistance among enteric pathogens. Semin. Pediatr. Infect. Dis. 1571-77. [DOI] [PubMed] [Google Scholar]
- 42.Roxas, J. L., A. Koutsouris, and V. K. Viswanathan. 2007. Enteropathogenic E. coli-induced epidermal growth factor receptor activation contributes to physiological alterations in intestinal epithelial cells. Infect. Immun. 752316-2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruchaud-Sparagano, M. H., M. Maresca, and B. Kenny. 2007. Enteropathogenic Escherichia coli (EPEC) inactivate innate immune responses prior to compromising epithelial barrier function. Cell. Microbiol. 91909-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sakaguchi, T., H. Kohler, X. Gu, B. A. McCormick, and H. C. Reinecker. 2002. Shigella flexneri regulates tight junction-associated proteins in human intestinal epithelial cells. Cell. Microbiol. 4367-381. [DOI] [PubMed] [Google Scholar]
- 45.Sansonetti, P. J. 2001. Microbes and microbial toxins: paradigms for microbial-mucosal interactions III. Shigellosis: from symptoms to molecular pathogenesis. Am. J. Physiol. Gastrointest. Liver Physiol. 280G319-G323. [DOI] [PubMed] [Google Scholar]
- 46.Sasakawa, C., K. Komatsu, T. Tobe, T. Suzuki, and M. Yoshikawa. 1993. Eight genes in region 5 that form an operon are essential for invasion of epithelial cells by Shigella flexneri 2a. J. Bacteriol. 1752334-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Savkovic, S. D., A. Ramaswamy, A. Koutsouris, and G. Hecht. 2001. EPEC-activated ERK1/2 participate in inflammatory response but not tight junction barrier disruption. Am. J. Physiol. Gastrointest. Liver Physiol. 281G890-G898. [DOI] [PubMed] [Google Scholar]
- 48.Savkovic, S. D., A. Koutsouris, and G. Hecht. 1997. Activation of NF-kappaB in intestinal epithelial cells by enteropathogenic Escherichia coli. Am. J. Physiol. 273C1160-C1167. [DOI] [PubMed] [Google Scholar]
- 49.Savkovic, S. D., A. Koutsouris, and G. Hecht. 1996. Attachment of a noninvasive enteric pathogen, enteropathogenic Escherichia coli, to cultured human intestinal epithelial monolayers induces transmigration of neutrophils. Infect. Immun. 644480-4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Serény, B. 1957. Experimental keratoconjunctivitis shigellosa. Acta Microbiol. Acad. Sci. Hung. 4367-376. [PubMed] [Google Scholar]
- 51.Sharma, R., S. Tesfay, F. L. Tomson, R. P. Kanteti, V. K. Viswanathan, and G. Hecht. 2006. Balance of bacterial pro- and anti-inflammatory mediators dictates net effect of enteropathogenic Escherichia coli on intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 290G685-G694. [DOI] [PubMed] [Google Scholar]
- 52.Tomson, F. L., V. K. Viswanathan, K. J. Kanack, R. P. Kanteti, K. V. Straub, M. Menet, J. B. Kaper, and G. Hecht. 2005. Enteropathogenic Escherichia coli EspG disrupts microtubules and in conjunction with Orf3 enhances perturbation of the tight junction barrier. Mol. Microbiol. 56447-464. [DOI] [PubMed] [Google Scholar]
- 53.Wickham, M. E., C. Lupp, A. Vazquez, M. Mascarenhas, B. Coburn, B. K. Coombes, M. A. Karmali, J. L. Puente, W. Deng, and B. B. Finlay. 2007. Citrobacter rodentium virulence in mice associates with bacterial load and the type III effector NleE. Microbes Infect. 9400-407. [DOI] [PubMed] [Google Scholar]
- 54.Wilson, R. K., R. K. Shaw, S. Daniell, S. Knutton, and G. Frankel. 2001. Role of EscF, a putative needle complex protein, in the type III protein translocation system of enteropathogenic Escherichia coli. Cell. Microbiol. 3753-762. [DOI] [PubMed] [Google Scholar]
- 55.Zurawski, D. V., C. Mitsuhata, K. L. Mumy, B. A. McCormick, and A. T. Maurelli. 2006. OspF and OspC1 are Shigella flexneri type III secretion system effectors that are required for postinvasion aspects of virulence. Infect. Immun. 745964-5976. [DOI] [PMC free article] [PubMed] [Google Scholar]