Abstract
Several lines of evidence suggest that targeting pre-erythrocytic-stage parasites for malaria vaccine development can provide sterile immunity. The objectives of this study were (i) to evaluate preclinically the safety and immunogenicity of a new recombinant pre-erythrocytic-stage antigen, liver-stage antigen 1 (LSA1), in nonhuman primates; and (ii) to investigate the potential for immune interference between LSA1 and the leading malaria vaccine candidate, RTS,S, by comparing the immune responses after single-antigen vaccination to responses after simultaneous administration of both antigens at separate sites. Using a rhesus monkey model, we found that LSA1 formulated with the GlaxoSmithKline proprietary adjuvant system AS01B (LSA1/AS01B) was safe and immunogenic, inducing high titers of antigen-specific antibody and CD4+ T-cell responses, as monitored by the production of interleukin-2 and gamma interferon, using intracellular cytokine staining. RTS,S/AS01B vaccination was well tolerated and demonstrated robust antibody and moderate CD4+ T-cell responses to circumsporozoite protein (CSP) and HBsAg. Positive CD8+ T-cell responses to HBsAg were detected, whereas the responses to CSP and LSA1 were negligible. For both LSA1/AS01B and RTS,S/AS01B, no statistically significant differences were observed between individual and concurrent administration in the magnitude or duration of antibody and T-cell responses. Our results revealed that both pre-erythrocytic-stage antigens were safe and immunogenic, administered either separately or simultaneously to rhesus monkeys, and that no significant immune cross interference occurred with concurrent separate-site administration. The comparison of the profiles of immune responses induced by separate-site and single-site vaccinations with LSA1 and RTS,S warrants further investigation.
Several observations suggest that it is possible to develop effective vaccines against malaria by targeting pre-erythrocytic-stage parasites. In the first demonstration, immunization of mice with radiation-attenuated mouse malaria sporozoites induced protection against challenge with live sporozoites (24). A similar approach was then studied with humans and found to consistently elicit protection after challenge with Plasmodium falciparum sporozoites (7, 15, 26). During the last 10 years, there has been a significant advance in the clinical study of pre-erythrocytic-stage malaria vaccines. Two distinct P. falciparum pre-erythrocytic-stage vaccines, a protein/adjuvant vaccine and a heterologous prime-boost vaccine (12), recently entered field trials (phase 2b). Unlike the DNA/MVA ME-TRAP prime-boost vaccine (23), the RTS,S/AS02A protein/adjuvant vaccine consistently exhibited considerable efficacy, as measured by sterile protection in the clinic (17, 18, 31) and by demonstration of reduced infection and clinical and severe disease in the field (1, 2, 5). The recombinant RTS,S protein consists of two polypeptides, RTS and S, that are expressed in Saccharomyces cerevisiae. RTS is a single polypeptide that corresponds to amino acids 207 to 395 of P. falciparum circumsporozoite protein (CSP) derived from strain NF54 fused to the hepatitis B virus surface antigen (HBsAg). S is a polypeptide that corresponds to HBsAg. The two peptides self-assemble in yeast cells to form the RTS,S particulate antigen. AS02A is a multicomponent adjuvant system, developed by GlaxoSmithKline Biologicals (Rixensart, Belgium), consisting of an oil-in-water emulsion, 3-O-desacyl-4′-monophosphoryl lipid A, and a saponin derivative, QS21 (11).
A recent field trial with children (1 to 4 years of age) in Mozambique provided 30% efficacy during the first clinical attack of malaria and 58% efficacy against severe P. falciparum malaria for a 6-month period (2). A follow-up study to evaluate the longevity of the protective effect of the vaccine showed that RTS,S/AS02A protected children in the area of endemicity against clinical disease and severe disease for 18 months (1).
Despite this significant protective efficacy, further improvement of malaria vaccine efficacy might be achievable. Several strategies to further increase vaccine efficacy are being explored, including the use of novel antigen delivery systems (more potent adjuvant systems, DNA vaccines, and various heterologous prime-boost vaccine strategies) (28) and the combination of RTS,S with other pre-erythrocytic-stage antigens.
In addition to CSP, other potentially protective pre-erythrocytic-stage antigens, such as thrombospondin-related adhesive protein, the sporozoite threonine- and asparagine-rich protein, liver-stage antigen 1 (LSA1), and LSA3, have been described. The LSA1 protein is synthesized in significant quantities by growing hepatic schizonts in infected hepatocytes. Accumulating evidence supports the use of P. falciparum LSA1 as a candidate pre-erythrocytic-stage vaccine (20). Although the functional role of LSA1 remains poorly understood, several epidemiological studies have linked specific immune responses to LSA1 with resistance to malaria infection (16, 21, 22). LSA1-specific immune responses were detected in protected individuals immunized with attenuated sporozoites, suggesting that LSA1 may also contribute to protective immunity in this vaccine regimen (19).
A recombinant LSA1 protein consisting of the N- and C-terminal regions of the protein and two of the centrally placed 17-amino-acid repeats was recently produced from a recombinant Escherichia coli strain (14). Studies with mice demonstrated that immunization with recombinant LSA1 elicited strong antibody and cell-mediated immune responses in BALB/c and A/J mice but not in C57BL/6J mice (6).
The vaccine development pipeline has witnessed the increasing use of rhesus monkeys because they are much more closely related phylogenetically to humans than are mice. A recent study from our group demonstrated that whereas murine dendritic cell subsets differ substantially from their human counterparts, rhesus dendritic cells share the functionality of Toll-like receptors with human dendritic cells (C. Ketloy, unpublished data). These findings support the use of the rhesus model for testing and optimizing human vaccines.
In the present study, we preclinically evaluated the safety and immunogenicity of clinical-grade recombinant LSA1 formulated with the AS01B adjuvant system in rhesus monkeys. The malaria blood-stage antigens MSP1 and RTS,S formulated with AS01B have previously been shown to induce stronger cell-mediated immune responses in rhesus monkeys than those formulated with AS02A (25, 29, 30). Furthermore, RTS,S/AS01B demonstrated improved efficacy and superior antibody and cellular immunity compared to RTS,S/AS02A in a human challenge study (K. Kester, personal communication). Therefore, the AS01B adjuvant was selected for use in our study. In order to initially assess the potential for immune cross interference between LSA1 and the RTS,S antigen in a multiantigen vaccine, both RTS,S/AS01B and LSA1/AS01B were administered concurrently at separate sites for one group and individually for two separate groups.
MATERIALS AND METHODS
Clinical-grade vaccines and immunization.
All animal procedures were reviewed and approved by the institutional animal care and use committee and were performed in a facility accredited by the AAALAC, in compliance with the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals (23a) and in accordance with all applicable USDA, Office of Laboratory Animal Welfare, and Department of Defense guidelines. Clinical-grade recombinant LSA1 protein from the P. falciparum 3D7 strain was produced in E. coli (19). Fermentation, purification, and aliquoting were performed with good manufacturing practices at the BioProduction Facility, Walter Reed Army Institute of Research, Silver Spring, MD. The final protein was >98% pure, contained an undetectable amount of endotoxin (<0.005 endotoxin units per 50 μg of protein), and passed the pyrogen test in rabbits. Clinical-grade recombinant RTS,S protein and the AS01B adjuvant system (liposomes plus 3-O-desacyl-4′-monophosphoryl lipid A plus QS21) were obtained from GlaxoSmithKline Biologicals (Rixensart, Belgium). Both LSA1 and RTS,S vaccines were formulated by mixing the AS01B adjuvant system with lyophilized proteins for 1 min on the day of vaccination.
A total of 29 healthy rhesus monkeys (Macaca mulatta) were selected and randomized into four groups, as follows: (i) control saline (n = 5); (ii) LSA1/AS01B (n = 8); (iii) RTS,S/AS01B (n = 8); and (iv) LSA1/AS01B and RTS,S/AS01B injected concurrently into different legs (n = 8). Each vaccine contained 50 μg of protein in a total volume of 0.5 ml of AS01B adjuvant system. Thus, the monkeys administered the LSA1 and RTS,S vaccines received 100 μg protein altogether, with twice the amount of AS01B adjuvant, at each immunization session. The monkeys were immunized on a three-dose schedule, at weeks 0, 4, and 8. All vaccines were administered by intramuscular injection into rectus femoris muscles.
Safety assessments.
Injection sites were examined for reactions, including skin warmth, erythema, swelling, muscle induration, ulceration, abscess, or other abnormalities, at baseline and on days 1, 2, 3, and 14 after each vaccine injection. The grading scale was as follows: 0, absent; 1, mild; 2, moderate; and 3, severe (30). Hematologic (hematocrit, hemoglobin level, and white blood cell count) and biochemical (blood urea nitrogen, serum creatinine, total protein, albumin, aspartate aminotransferase, and gamma glutamyl transpeptidase levels) analyses were performed on days 0, 1, 2, and 3 after each immunization.
Antibody and T-cell responses.
Serum samples and peripheral blood mononuclear cells (PBMC) were collected and frozen in liquid nitrogen until use. Antibody responses against LSA1 were assessed by enzyme-linked immunosorbent assay (ELISA). Briefly, 96-well Immulux plates (Dynex, Chantilly, VA) were coated with 0.125 μg/ml of the LSA1 vaccine antigen in phosphate-buffered saline (PBS) and incubated in a humidity chamber at 4°C overnight (at least 15 h). The plates were then washed on an automated plate washer (Molecular Devices, Sunnyvale, CA), using a wash buffer of PBS with 0.05% Tween 20 (Sigma, St. Louis, MO). All wells were filled with blocking buffer composed of 0.5% boiled casein in PBS with 1% Tween 20. All incubations from this point on were conducted in a humidity chamber at 22°C. The diluent for all sera and subsequent steps, except for the substrate, was 0.5% boiled casein with 0.04% Tween 20. Samples, including controls, were incubated on coated, blocked plates for 2 h in triplicate serial twofold dilutions from 1:50 to 1:6,400. Anti-monkey immunoglobulin Gγ peroxidase conjugate (Sigma) was prepared at a 1:9,000 dilution in diluent. The plates were washed on an automated plate washer, loaded with the second antibody, and incubated for 1 h. Before the end of the hour, the substrate for development was made using equal parts of solutions A [2,2′-azino-di-(3-ethylbenzthiazoline-6-sulfonate)] and B (H2O2) (KPL, Gaithersburg, MD). The plates were washed on an automated plate washer, loaded with substrate, and incubated for 1 h. At the end of this incubation, the reaction was stopped using a 20% sodium dodecyl sulfate stop solution. The plates were read at 414 nm on a SPECTRAmax plate reader (Molecular Devices Inc.), and serial dilution curves were analyzed using SoftMax Pro 4.8 software. Titer is defined as the dilution factor needed to give an optical density of 1.0 in our assay. Data received a “passing” designation if plates met preset limits for the positive and negative controls and blanks and if individual samples met preset limits for range and curve fit. Samples with titers in excess of 6,400 were repeated at dilutions of 1:500 to 1:64,000; a few were subsequently tested at a starting dilution of 1:5,000. Operators were blinded regarding the test groups of samples.
Antibody responses to the CSP tandem repeat epitope were measured by ELISA, with recombinant R32LR as the capture antigen for antibodies, as described previously (30). Antibodies against HBsAg were measured using a commercially available kit (Abbott Laboratories, Abbott Park, IL).
Intracellular cytokine staining (ICS) was used to assess T-cell responses. Cryopreserved PBMC (106 cells) in 200 μl of complete medium (RPMI 1640, 2 mM glutamine, 0.1 mM sodium pyruvate, 1 mM nonessential amino acids, 0.055 mM 2-mercaptoethanol, and 5% heat-inactivated, lowly mitogenic fetal calf serum) were stimulated with LSA1 peptide pool 1 (38 15-mer peptides overlapping by 11 amino acids, covering the N terminus through the second repeat), LSA1 peptide pool 2 (73 15-mer peptides overlapping by 11 amino acids, covering the second repeat through the C terminus), a CSP peptide pool (30 15-mer peptides overlapping by 11 amino acids), or an HBsAg peptide pool (54 15-mer peptides overlapping by 11 amino acids) at a final concentration of each peptide of 1 μg/ml. All peptide-stimulated PBMC cultures contained 1 μg/ml of anti-CD28 (BD Pharmingen, San Diego, CA) and 1 μg/ml of anti-CD49 (BD Pharmingen). Staphylococcal enterotoxin B (SEB) (4 μg/ml; Sigma) and no peptide were used as positive and negative controls, respectively. After the initial 2 h of stimulation, Golgiplug (diluted 1/1,000 from the stock; BD Pharmingen) was added to inhibit cytokine secretion, and the cell cultures were further incubated overnight. After 18 to 20 h of incubation, cells were washed and stained for cell surface markers with fluorescence-conjugated monoclonal antibodies (MAbs) to CD4 (clone L200; BD Pharmingen) and CD8 (clone SK1; BD Pharmingen). The stained cells were washed and treated with fixation/permeabilization solution (BD Pharmingen) and then stained with fluorescence-conjugated MAbs against IFN-γ (clone B27; BD Pharmingen) and IL-2 (clone MQ1-17H12; BD Pharmingen). Isotype-matched control MAbs were used to confirm the staining specificity. Finally, stained cells were analyzed by four-color flow cytometry (FACSCalibur; Becton Dickinson, Mountain View, CA). Approximately 200,000 to 400,000 events in the lymphocyte gate were acquired. The samples considered positive were those in which the percentage of cytokine-staining cells was at least twice that for the background or in which there was a distinct population of brightly cytokine-positive cells. The CD4+ T-cell population was analyzed by excluding CD8+ T cells, and vice versa for analysis of the CD8+ T-cell population.
Statistical analysis.
The data were analyzed using SPSS 12.0 for Windows (SPSS Inc., Chicago, IL) and StatXact-7 (Cytel Software Corporation, MA). Antibody titers were log transformed before testing of the differences. Differences in reactivity were analyzed using the McNemar-Bowker test. Differences among groups in systemic toxicity and antibody and T-cell responses were analyzed by using the Kruskal-Wallis test and the Mann-Whitney rank sum test (for non-normally distributed data), with the Bonferroni correction for multiple comparisons. P values of <0.05 were considered statistically significant.
RESULTS
Safety assessment of tested vaccines.
Most animals showed transient skin erythema, skin swelling, and muscle induration from vaccination. These reactions were most notable after the third dose. A summary of local skin reactions at the injection sites after the third dose of vaccine is presented in Table 1. Skin erythema, swelling, and muscle induration at the injection sites were the most frequent local effects observed, but they subsided by day 3 after each injection. No local ulceration or abscess was observed. There were no statistically significant differences in local skin reactions when LSA1/AS01B and RTS,S/AS01B were administered singly or concurrently. Differences in muscle induration among the three vaccine groups compared to the control group were observed (P values ranged from 0.0000 to 0.0006). Biochemistry and hematology values for all four animal groups were determined as scheduled, at baseline and on days 1, 2, 3, and 14 after each immunization. Some of these values showed statistically significant differences compared to those before immunization, such as total protein (for LSA1/AS01B and RTS,S/AS01B groups, P = 0.03 and 0.02, respectively), serum creatinine levels (for LSA1/AS01B and RTS,S/AS01B groups, P = 0.03 and 0.02, respectively), and aspartate aminotransferase levels (for LSA1/AS01B and LSA1/AS01B-plus-RTS,S/AS01B groups, P = 0.008 and 0.008, respectively). However, all values remained within the normal reference range. Hematology data showed that the mean white blood cell counts for all vaccine groups were transiently increased about twofold on day 1 after each vaccination and then subsided on days 2 to 3 (data not shown). No differences in hematology and biochemistry values after doses 1, 2, and 3 were noted. The data presented in Table 2 list the laboratory parameters assessed for evidence of systemic toxicity on day 3 after the third dose of vaccine. Overall, the tested vaccines were safe and well tolerated.
TABLE 1.
Overall incidence of reactogenicity at the injection site after third vaccine dosea
| Group (n) | Incidence of symptomb
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Skin redness
|
Skin swelling
|
Muscle induration
|
||||||||||
| Preinjection | Dose 1 | Dose 2 | Dose 3 | Preinjection | Dose 1 | Dose 2 | Dose 3 | Preinjection | Dose 1 | Dose 2 | Dose 3 | |
| Control (5) | 0.60 ± 0.25 | 0.80 ± 0.37 | 0.80 ± 0.20 | 0.80 ± 0.20 | 0.20 ± 0.20 | 0.20 ± 0.20 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.20 ± 0.20 | 0.12 ± 0.20 | 1.00 ± 0.32 | 0.20 ± 0.20 |
| LSA1/AS01B (8) | 0.25 ± 0.25 | 1.25 ± 0.31 | 0.63 ± 0.26 | 0.38 ± 0.26 | 0.13 ± 0.13 | 1.38 ± 0.26 | 0.63 ± 0.26 | 0.13 ± 0.13 | 0.00 ± 0.00 | 2.38 ± 0.18 | 1.88 ± 0.30 | 1.00 ± 0.27 |
| RTS,S/AS01B (8) | 0.38 ± 0.26 | 1.25 ± 0.31 | 0.88 ± 0.30 | 0.50 ± 0.19 | 0.25 ± 0.25 | 1.00 ± 0.38 | 0.75 ± 0.49 | 0.38 ± 0.38 | 0.00 ± 0.00 | 2.38 ± 0.18 | 2.00 ± 0.27 | 1.25 ± 0.31 |
| LSA1/AS01B plus RTS,S/AS01B | ||||||||||||
| LSA1 leg (8) | 0.38 ± 0.18 | 1.13 ± 0.23 | 0.88 ± 0.30 | 0.63 ± 0.26 | 0.00 ± 0.00 | 1.38 ± 0.53 | 1.13 ± 0.44 | 0.13 ± 0.13 | 0.25 ± 0.16 | 2.63 ± 0.18 | 2.75 ± 0.16 | 2.00 ± 0.19 |
| RTS,S leg (8) | 0.38 ± 0.18 | 1.00 ± 0.27 | 0.88 ± 0.30 | 0.63 ± 0.26 | 0.00 ± 0.00 | 1.00 ± 0.38 | 1.13 ± 0.44 | 0.38 ± 0.26 | 0.00 ± 0.00 | 2.25 ± 0.31 | 2.38 ± 0.26 | 1.38 ± 0.18 |
Grade 3 muscle induration was frequently observed on days 1 and 2 after the third dose of vaccine. This subsided on day 3 and disappeared on day 14.
Data are mean intensities ± SE for scores ranging from 0 to 3 (0, absent; 1, mild; 2, moderate; and 3, severe).
TABLE 2.
Systemic toxicity on day 3 after the third dosea
| Parameter | Value for group
|
|||
|---|---|---|---|---|
| Control (n = 5) | LSA1/AS01B (n = 8) | RTS,S/AS01B (n = 8) | LSA1/AS01B plus RTS,S/AS01B (n = 8) | |
| WBC (μl) | 4,600 ± 372 | 6,687 ± 497 | 5,875 ± 450 | 6,125 ± 505 |
| RBC (million/μl) | 4.86 ± 0.20 | 5.20 ± 0.14 | 5.24 ± 0.10 | 4.98 ± 0.08 |
| HGB (g/dl) | 11.80 ± 0.34 | 13.08 ± 0.60 | 12.70 ± 0.13 | 11.81 ± 0.22 |
| HCT (%) | 36.20 ± 1.32 | 39.00 ± 0.85 | 39.63 ± 0.46 | 36.63 ± 0.68 |
| MCV (fl) | 74.48 ± 0.75 | 75.20 ± 1.05 | 75.53 ± 0.90 | 73.73 ± 1.09 |
| MCH (pg) | 24.34 ± 0.42 | 25.13 ± 0.79 | 24.25 ± 0.31 | 23.71 ± 0.36 |
| MCHC (g/dl) | 32.66 ± 0.28 | 33.43 ± 1.12 | 32.11 ± 0.17 | 32.19 ± 0.16 |
| PLT (/μl) | 269,800 ± 18,464 | 230,125 ± 20,187 | 293,000 ± 14,067 | 275,750 ± 37,736 |
| BUN (mg/dl) | 21.36 ± 2.05 | 18.09 ± 1.64 | 21.76 ± 1.66 | 20.39 ± 1.03 |
| CRE (mg/dl) | 0.86 ± 0.05 | 0.94 ± 0.04 | 0.90 ± 0.04 | 0.93 ± 0.06 |
| TP (g/dl) | 6.50 ± 0.11 | 6.84 ± 0.09 | 6.99 ± 0.08 | 6.98 ± 0.13 |
| ALB (g/dl) | 4.38 ± 0.14 | 4.33 ± 0.09 | 4.38 ± 0.08 | 4.25 ± 0.08 |
| AST (U/l) | 61.20 ± 5.34 | 54.38 ± 3.50 | 66.25 ± 8.32 | 58.00 ± 6.79 |
| GGT (U/l) | 42.60 ± 2.32 | 41.10 ± 5.94 | 59.25 ± 7.66 | 43.25 ± 2.61 |
Data are mean values ± SE. WBC, white blood cells; RBC, red blood cells; HGB, hemoglobin; HCT, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; PLT, platelets; BUN, blood urea nitrogen; CRE, serum creatinine; TP, total protein; ALB, albumin; AST, aspartate aminotransferase; GGT, gamma glutamyl transpeptidase.
Assessment of malaria antigen-specific antibody responses.
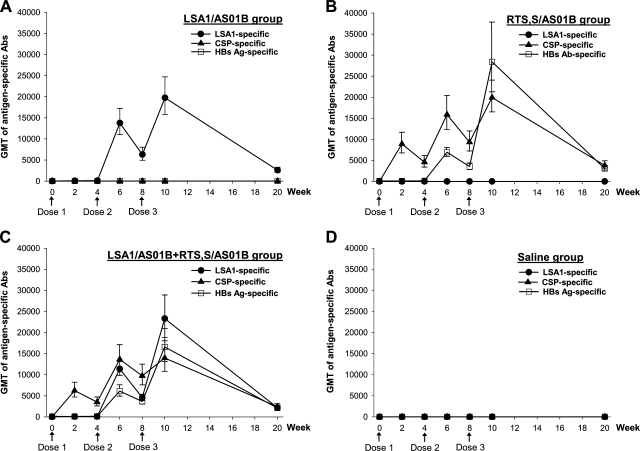
The time course of the antibody responses to LSA1 for the group receiving only LSA1/AS01B is depicted in Fig. 1A. LSA1/AS01B elicited high titers of antibodies to LSA1 antigen, which peaked after the third dose, with a group geometric mean titer (GMT) of 19,750 (95% confidence interval [95% CI] = 12,737 to 30,623). We were interested in studying the potential immune cross interference between LSA1 and the RTS,S antigen. Thus, we also investigated the immune responses elicited in rhesus monkeys receiving RTS,S/AS01B alone (Fig. 1B) and in those receiving LSA1/AS01B and RTS,S/AS01B concurrently at separate sites (Fig. 1C).
FIG. 1.
Kinetics of antigen-specific antibody responses to LSA1, CSP, and HBsAg. Rhesus monkeys were immunized with LSA1/AS01B (A), RTS,S/AS01B (B), LSA1/AS01B and RTS,S/AS01B simultaneously at separate sites (C), or a saline control (D) on a three-dose schedule (indicated by arrows). ELISA was used to assess antibody titers. Each data point represents the GMT ± standard error (SE) for anti-LSA1 (•), anti-CSP (▴), or anti-HBsAg (□).
Robust anti-CSP and anti-HBsAg responses were also detected in animals receiving RTS,S/AS01B (peak GMT of anti-CSP = 19,945 [95% CI = 13,790 to 28,848]; peak GMT of anti-HBsAg = 28,398 [95% CI = 14,399 to 56,006]). Two weeks after the first dose, all animals receiving either LSA1/AS01B or RTS,S/AS01B seroconverted to immunized antigens (>2-fold compared to preimmunization values). The follow-up study indicated that the titers of anti-LSA1, anti-CSP, and anti-HBsAg antibodies declined >80% by 3 months after the last dose of vaccine (Fig. 1A and B).
We next evaluated the antigen-specific antibody responses in monkeys receiving LSA1/AS01B and RTS,S/AS01B concurrently. As shown in Fig. 1C, the magnitudes and durations of antibody responses specific to LSA1, CSP, and HBsAg for this group were not significantly different from those observed for monkeys that received single-antigen vaccines. Lower antibody responses to CSP (peak GMT = 13,979; 95% CI = 8,467 to 23,079) and HBsAg (peak GMT = 16,606; 95% CI = 9,566 to 28,829) than those for animals receiving RTS,S/AS01B alone were observed, but the differences did not reach statistical significance (P = 0.28 and 0.16, respectively). No antibody response specific to LSA1, CSP, or HBsAg was detected in the control saline group (Fig. 1D).
Assessment of malaria antigen-specific T-cell responses by ICS.
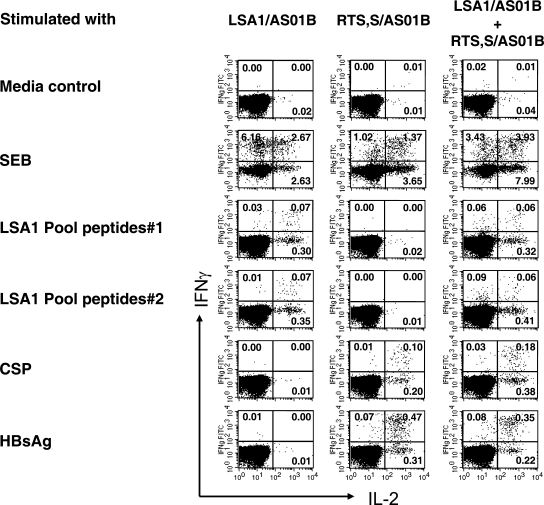
Although IFN-γ production is commonly used to assess T-cell responses to malaria vaccines in humans and nonhuman primates, this single-cytokine analysis is not sufficient to identify the extent of the antigen-specific T-cell responses. Therefore, we simultaneously assessed antigen-specific CD4+ and CD8+ T cells for IL-2 and IFN-γ production at the single-cell level by ICS. The assay identifies three functionally distinct populations, producing IL-2 alone, IFN-γ alone, and IL-2 plus IFN-γ. Figure 2 depicts the flow cytometry scattergrams of antigen-specific CD4+ T cells producing IL-2, IFN-γ, and IL-2 plus IFN-γ in response to LSA1 (peptide pools 1 and 2), CSP, and HBsAg from single representative vaccinated monkeys receiving either LSA/AS01B alone, RTS,S/AS01B alone, or LSA1/AS01B and RTS,S/AS01B simultaneously. A medium control, stimulated only with anti-CD28 MAb and anti-CD49 MAb, produced negligible amounts of IL-2 and IFN-γ. SEB-stimulated PBMC, a positive control, elicited large amounts of IL-2 and IFN-γ. The magnitude of antigen-specific CD4+ or CD8+ T-cell responses was defined as the combined frequency of IL-2-, IFN-γ-, and IL-2-plus-IFN-γ-producing cells within the CD4+ or CD8+ T-cell population.
FIG. 2.
Use of ICS to assess the frequencies of antigen-specific IL-2-, IFN-γ-, and IL-2-plus-IFN-γ-secreting CD4+ T cells. PBMC harvested from rhesus monkeys receiving LSA1/AS01B, RTS,S/AS01B, or LSA1/AS01B and RTS,S/AS01B simultaneously were stimulated with pooled peptides of LSA1 (pools 1 and 2), CSP, or HBsAg in the presence of anti-CD28 MAb and anti-CD49 MAb. SEB and no-peptide medium controls were used as positive and negative controls, respectively. After 18 to 20 h, the stimulated cells were stained with anti-CD4, anti-CD8, anti-IL-2, and anti-IFN-γ MAbs. CD4+ CD8− T cells were gated and then analyzed for cytokine production (IL-2, IFN-γ, and IL-2 plus IFN-γ) by four-color flow cytometry. The data are representative of one monkey in each vaccine group.
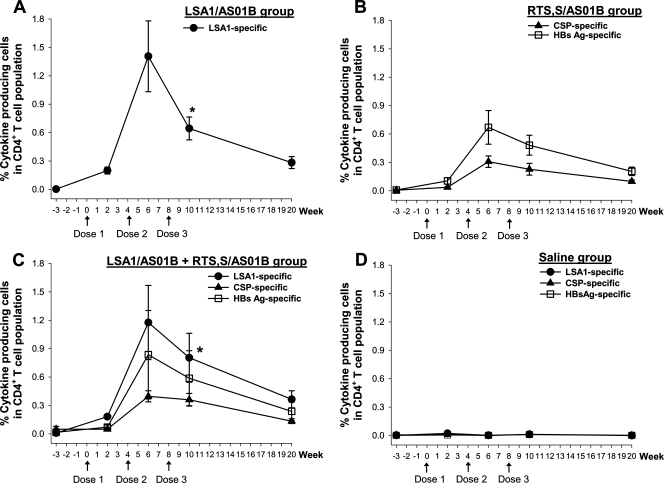
Magnitude and duration of malaria antigen-specific CD4+ T-cell responses.
PBMC from monkeys receiving LSA1/AS01B were stimulated with either peptide pool 1 (peptides 1 to 38, covering the N terminus through the second repeat) or peptide pool 2 (peptides 35 to 108, covering the second repeat through the C terminus). T-cell epitopes are located at the N and C termini of the LSA1 protein but not in the repeat region (14). The LSA1-specific cytokine response was therefore defined as the sum of cytokine responses against peptide pool 1 and peptide pool 2. Using this definition, LSA1/AS01B elicited a high level of T-cell responses whether given by itself or simultaneously with RTS,S/AS01B (Fig. 3).
FIG. 3.
Kinetics of CD4+ T-cell responses specific to LSA1, CSP, and HBsAg. Rhesus monkeys were immunized with LSA1/AS01B (A), RTS,S/AS01B (B), LSA1/AS01B and RTS,S/AS01B simultaneously at separate sites (C), or a saline control (D). PBMC from each monkey were stimulated with pooled peptides in vitro in a short-term restimulation assay and then analyzed for the production of IL-2 and IFN-γ by CD4+ CD8− T cells, using ICS, at various time points. Data shown are means ± SE for specific responses to LSA1 (•), CSP (▴), and HBsAg (□). *, P < 0.05 compared with the LSA1-specific responses at week 6.
After the first dose of vaccine, all animals receiving LSA1/AS01B demonstrated antigen-specific cytokine production by CD4+ T cells. As depicted in Fig. 3A, the magnitude of LSA1-specific T-cell responses within the CD4+ T-cell population peaked after the second dose (mean percentage of cytokine-producing cells within the CD4+ T-cell population = 1.4%). Surprisingly, the third dose of vaccine was followed by a significant reduction in the magnitude of CD4+ T-cell responses (48% reduction; P < 0.05 compared to the response after the second dose).
Moderate CD4+ T-cell responses to peptide pools for CSP and HBsAg were detected in animals receiving RTS,S/AS01B vaccine alone (Fig. 3B). Similar to the LSA1 group, most animals (seven of eight) receiving RTS,S/AS01B demonstrated antigen-specific T-cell responses after the first dose. The responses to CSP (mean percentage of cytokine-producing cells = 0.31%) and HBsAg (mean percentage of cytokine-producing cells = 0.67%) peaked after the second dose (Fig. 3B). The third dose of RTS,S/AS01B did not enhance antigen-specific CD4+ T-cell responses. The magnitudes of CD4+ T-cell responses to HBsAg were higher than those of the responses to CSP, but the difference did not reach statistical significance.
An evaluation of the duration of antigen-specific CD4+ T-cell responses indicated that they persisted for at least 3 months after the last dose of vaccine. The magnitudes of CD4+ T-cell responses decreased approximately 60% from peak responses in animals receiving LSA1/AS01B alone and RTS,S/AS01B alone.
In comparisons of specific T-cell responses to LSA1 and the RTS,S antigen when the two vaccines were administered simultaneously, responses to LSA1, CSP, and HBsAg were evaluated (Fig. 3C) and then compared with the specific responses in animals receiving the corresponding single-antigen vaccines. This comparison revealed no significant differences in the magnitudes or durations of antigen-specific CD4+ T-cell responses (P values ranged from 0.18 to 0.93). In the control saline group, we detected no specific T-cell responses to LSA1, CSP, or HBsAg at any time point (Fig. 3D).
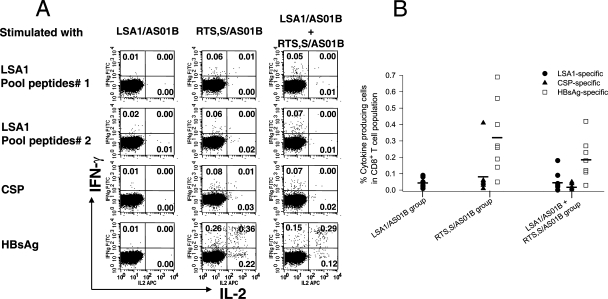
Malaria antigen-specific CD8+ T-cell responses.
It is recognized that in nonhuman primates, protein/adjuvant vaccines elicit poorer CD8+ T-cell responses than do viral vector vaccines (27, 33; S. Pichyangkul, unpublished data). We evaluated whether pre-erythrocytic-stage malaria vaccines formulated with the AS01B adjuvant system were able to elicit CD8+ T-cell responses in rhesus monkeys. As shown in Fig. 4A and B, there were only negligible LSA1- and CSP-specific CD8+ T-cell responses after vaccination; however, distinct CD8+ T-cell responses to HBsAg were detected after dose 2 and dose 3 of the vaccines. The magnitudes of CD8+ T-cell responses to HBsAg in animals that received RTS,S/AS01B alone were always higher than HBsAg-specific CD8+ T-cell responses in animals receiving LSA1/AS01B and RTS,S/AS01B simultaneously (Fig. 4B). However, the differences did not reach statistical significance (P values ranged from 0.07 to 0.54).
FIG. 4.
Induction of CD8+ T-cell responses to LSA1, CSP, and HBsAg. PBMC from rhesus monkeys immunized with either LSA1/AS01B, RTS,S/AS01B, or LSA1/AS01B and RTS,S/AS01B concurrently were collected 2 weeks after dose 2. They were then stimulated with different pooled peptides in a short-term restimulation in vitro assay, and then the production of IL-2 and IFN-γ by CD8+ CD4− T cells was analyzed. (A) Data are representative of one monkey in each vaccine group. (B) Frequencies of antigen-specific cytokine production from CD8+ T cells. Each symbol represents the value for an individual animal, and horizontal lines are means.
Cytokine production patterns at the single-cell level.
To further analyze the functional capacity of antigen-specific CD4+ T-cell responses after immunization, the frequency of cells that produced IL-2 alone, IFN-γ alone, or IL-2 plus IFN-γ was evaluated at the individual cell level. A large proportion of antigen-specific CD4+ T cells produced IL-2 alone or IL-2 plus IFN-γ in animals receiving either LSA1/AS01B or RTS,S/AS01B (Fig. 5A and B). Very few antigen-specific CD4+ T cells produced only IFN-γ. A similar pattern of antigen-specific cytokine production at the individual cell level was also observed for animals receiving both LSA1/AS01B and RTS,S/AS01B simultaneously (P values ranged from 0.19 to 0.86) (Fig. 5C). There were approximately equal frequencies of HBsAg-specific CD8+ T cells producing IL-2 alone, IFN-γ alone, and IL-2 plus IFN-γ in animals receiving either RTS,S/AS01B alone or LSA1/AS01B and RTS,S/AS01B simultaneously (data not shown).
FIG. 5.
Analysis of frequencies of CD4+ T cells producing IL-2, IFN-γ, and IL-2 plus IFN-γ by ICS. PBMC from rhesus monkeys receiving LSA1/AS01B (A), RTS,S/AS01B (B), or LSA1/AS01B and RTS,S/AS01B simultaneously (C) were collected 2 weeks after the last immunization and then were stimulated with different pooled peptides in a short-term restimulation in vitro assay. Cytokine-producing CD4+ CD8− Τ cells were separated into three distinct populations based on the production of IL-2 (black bars), IFN-γ (hatched bars), and IL-2 plus IFN-γ (white bars). Data shown are means ± SE.
DISCUSSION
Several studies have reported associations between LSA1-specific antibody and cytokine responses and reduced malaria incidence (16, 21, 22), suggesting that LSA1 may have potential use as a vaccine candidate for P. falciparum malaria. In the present study, we evaluated the safety and immunogenicity of a new recombinant LSA1 protein formulated with AS01B adjuvant in a rhesus monkey model. Our results revealed that LSA1/AS01B was safe, with acceptable reactogenicity and no detectable systemic toxicity. Importantly, the vaccine induced robust antigen-specific antibody and cellular immune responses. Antibody responses peaked after the third dose of vaccine, whereas the CD4+ T-cell responses peaked after the second dose. Surprisingly, the LSA1-specific CD4+ T-cell response decreased significantly after the third immunization. It is not understood why the last dose of vaccination led to a significant reduction in the number of LSA1-specific CD4+ T cells in circulating blood. A redistribution of antigen-specific CD4+ T cells could not be ruled out.
The role of antigen-specific T cells and the pattern of secreted cytokines are currently under intense investigation as potential immune correlates of vaccine efficacy. In this study, we applied ICS to assess IL-2 and IFN-γ production from rhesus T cells at the individual cell level. Our data demonstrated that LSA1/AS01B induced a large proportion of antigen-specific CD4+ T cells that secreted IL-2 alone or both IL-2 and IFN-γ. The ability of antigen-specific CD4+ T cells to produce IL-2 is critical, since IL-2 is essential for clonal expansion. Recent studies have demonstrated that antigen-specific CD4+ T cells that produce IL-2 or IL-2 plus IFN-γ are important in protective immunity against human immunodeficiency virus, simian/human immunodeficiency virus, and Leishmania major infection (4, 32, 34). Recent evidence also suggests that T cells that produce IL-2 plus IFN-γ are highly associated with memory responses to malaria infection (3).
Only a negligible LSA1-specific CD8+ T-cell response was detected in the present study. Data on LSA1-specific CD8+ T-cell responses in protection against P. falciparum infection remain limited. It was first reported 15 years ago that a conserved cytotoxic T-lymphocyte epitope from LSA1 bound to HLA-B53 was associated with resistance to severe malaria in Gambian volunteers (13). Other findings suggested that IFN-γ produced from CD8+ T cells plays a role in immunity to liver-stage P. falciparum infection (8). However, the precise role, if any, of LSA1-specific CD8+ T cells in protective immunity remains to be demonstrated.
In the present study, both antibody and CD4+ T-cell responses to LSA1, although declining over time, persisted and were still detectable for at least 3 months after the last vaccine dose.
RTS,S/AS01B was well tolerated and elicited strong antibody and significant CD4+ T-cell responses to CSP and HBsAg. We did not detect CSP-specific CD8+ T-cell responses, but substantial HBsAg-specific CD8+ T-cell responses were observed. The induction of HBsAg-specific CD8+ T-cell responses may reflect the intrinsic nature of HBsAg, which is able to promote major histocompatibility complex class I priming via cross presentation. This notion may be supported by the finding from a recent human study showing that HBsAg formulated with alum, an adjuvant known to elicit only negligible cellular responses, also induced CD8+ T-cell responses (10).
Vaccination with more than one antigen in the same formulation or simultaneously in the same arm can lead to reduced immune responses. Separate arm injections of vaccines sharing a common epitope can also result in diminished immune responses, a phenomenon termed carrier-induced epitope suppression (9). Very little is known regarding the effects of concurrent vaccination in separate arms with completely different malaria antigens formulated with potent adjuvants. In the single clinical study examining this issue, volunteers received two protein adjuvant vaccines, RTS,S/AS02A and MSP1/AS02A, either alone, concurrently in separate arms, or as a combination. There was no evidence that concurrent, separate-site injection affected antigen-specific antibody or IFN-γ enzyme-linked immunospot responses (J. Cummings, personal communication).
In anticipation of potential clinical trials, we undertook the present study to rule out the possibility of interference. In our detailed analyses of safety and immunogenicity, concurrent administration of LSA1/AS01B and RTS,S/AS01B at separate sites elicited similar profiles of safety and antigen-specific immune responses compared with those induced by single-antigen vaccination. A trend for reduced CSP and HBsAg antibody responses was observed in the two-antigen group, although this did not achieve statistical significance, perhaps due to the small sample sizes. It should be noted that the antibody response to CSP assessed in this study was against immunodominant B-cell epitopes in the central repeat region. We could not rule out possible interference regarding antibody responses to the C-terminal region of CSP.
No statistically significant differences were observed in the magnitudes and durations of T-cell responses. In addition, the cytokine profiles of antigen-specific T cells were the same regardless of whether animals received antigens singly or concurrently. The apparent reduced antibody response is not congruent with the equivalent magnitudes of CD4+ T-cell responses and similar cytokine profiles seen in all animals receiving either concurrent or individual vaccines. Overall, we find no statistical evidence that the doubling of the total adjuvant dose in the concurrently immunized animals impaired or augmented their humoral or cellular responses to the antigens.
In conclusion, our study shows that immunization with LSA1/AS01B or RTS,S/AS01B was safe and elicited strong antibody and CD4+ T-cell responses in the rhesus monkey model. Simultaneous administration of both vaccines at separate sites did not alter the safety profiles or impair the antibody and T-cell responses specific to LSA1, CSP, and HBsAg. To the best of our knowledge, this is the first published study to systematically evaluate immune responses, focusing on immune interference between two pre-erythrocytic-stage antigens, LSA1 and RTS,S. The findings provide important justification for future development of multiantigen malaria vaccines. At the same time, further studies are needed to compare the immune responses elicited by separate-site and single-site vaccinations with these two malaria antigens.
Acknowledgments
This work was funded by the Military Infectious Diseases Research Program, Fort Detrick, MD.
The views of the authors do not purport to reflect official policy of the U.S. Department of the Army or the Department of Defense.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 22 October 2007.
REFERENCES
- 1.Alonso, P. L., J. Sacarlal, J. J. Aponte, A. Leach, E. Macete, P. Aide, B. Sigauque, J. Milman, I. Mandomando, Q. Bassat, C. Guinovart, M. Espasa, S. Corachan, M. Lievens, M. M. Navia, M. C. Dubois, C. Menendez, F. Dubovsky, J. Cohen, R. Thompson, and W. R. Ballou. 2005. Duration of protection with RTS,S/AS02A malaria vaccine in prevention of Plasmodium falciparum disease in Mozambican children: single-blind extended follow-up of a randomised controlled trial. Lancet 3662012-2018. [DOI] [PubMed] [Google Scholar]
- 2.Alonso, P. L., J. Sacarlal, J. J. Aponte, A. Leach, E. Macete, J. Milman, I. Mandomando, B. Spiessens, C. Guinovart, M. Espasa, Q. Bassat, P. Aide, O. Ofori-Anyinam, M. M. Navia, S. Corachan, M. Ceuppens, M. C. Dubois, M. A. Demoitie, F. Dubovsky, C. Menendez, N. Tornieporth, W. R. Ballou, R. Thompson, and J. Cohen. 2004. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet 3641411-1420. [DOI] [PubMed] [Google Scholar]
- 3.Bejon, P., S. Keating, J. Mwacharo, O. K. Kai, S. Dunachie, M. Walther, T. Berthoud, T. Lang, J. Epstein, D. Carucci, P. Moris, J. Cohen, S. C. Gilbert, N. Peshu, K. Marsh, and A. V. Hill. 2006. Early gamma interferon and interleukin-2 responses to vaccination predict the late resting memory in malaria-naive and malaria-exposed individuals. Infect. Immun. 746331-6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boaz, M. J., A. Waters, S. Murad, P. J. Easterbrook, and A. Vyakarnam. 2002. Presence of HIV-1 Gag-specific IFN-gamma+IL-2+ and CD28+IL-2+ CD4 T cell responses is associated with nonprogression in HIV-1 infection. J. Immunol. 1696376-6385. [DOI] [PubMed] [Google Scholar]
- 5.Bojang, K. A., P. J. Milligan, M. Pinder, L. Vigneron, A. Alloueche, K. E. Kester, W. R. Ballou, D. J. Conway, W. H. Reece, P. Gothard, L. Yamuah, M. Delchambre, G. Voss, B. M. Greenwood, A. Hill, K. P. McAdam, N. Tornieporth, J. D. Cohen, and T. Doherty. 2001. Efficacy of RTS,S/AS02 malaria vaccine against Plasmodium falciparum infection in semi-immune adult men in The Gambia: a randomised trial. Lancet 3581927-1934. [DOI] [PubMed] [Google Scholar]
- 6.Brando, C., L. A. Ware, H. Freyberger, A. Kathcart, A. Barbosa, S. Cayphas, M. A. Demoitie, P. Mettens, D. G. Heppner, and D. E. Lanar. 2007. Murine immune responses to liver-stage antigen 1 protein FMP011, a malaria vaccine candidate, delivered with adjuvant AS01B or AS02A. Infect. Immun. 75838-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clyde, D. F., H. Most, V. C. McCarthy, and J. P. Vanderberg. 1973. Immunization of man against sporozite-induced falciparum malaria. Am. J. Med. Sci. 266169-177. [DOI] [PubMed] [Google Scholar]
- 8.Connelly, M., C. L. King, K. Bucci, S. Walters, B. Genton, M. P. Alpers, M. Hollingdale, and J. W. Kazura. 1997. T-cell immunity to peptide epitopes of liver-stage antigen 1 in an area of Papua New Guinea in which malaria is holoendemic. Infect. Immun. 655082-5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dagan, R., D. Goldblatt, J. R. Maleckar, M. Yaich, and J. Eskola. 2004. Reduction of antibody response to an 11-valent pneumococcal vaccine coadministered with a vaccine containing acellular pertussis components. Infect. Immun. 725383-5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Rosa, S. C., F. X. Lu, J. Yu, S. P. Perfetto, J. Falloon, S. Moser, T. G. Evans, R. Koup, C. J. Miller, and M. Roederer. 2004. Vaccination in humans generates broad T cell cytokine responses. J. Immunol. 1735372-5380. [DOI] [PubMed] [Google Scholar]
- 11.Garcon, N., D. G. Heppner, and J. Cohen. 2003. Development of RTS,S/AS02: a purified subunit-based malaria vaccine candidate formulated with a novel adjuvant. Expert Rev. Vaccines 2231-238. [DOI] [PubMed] [Google Scholar]
- 12.Hill, A. V. 2006. Pre-erythrocytic malaria vaccines: towards greater efficacy. Nat. Rev. Immunol. 621-32. [DOI] [PubMed] [Google Scholar]
- 13.Hill, A. V., J. Elvin, A. C. Willis, M. Aidoo, C. E. Allsopp, F. M. Gotch, X. M. Gao, M. Takiguchi, B. M. Greenwood, A. R. Townsend, A. J. McMichael, and H. C. Whittle. 1992. Molecular analysis of the association of HLA-B53 and resistance to severe malaria. Nature 360434-439. [DOI] [PubMed] [Google Scholar]
- 14.Hillier, C. J., L. A. Ware, A. Barbosa, E. Angov, J. A. Lyon, D. G. Heppner, and D. E. Lanar. 2005. Process development and analysis of liver-stage antigen 1, a preerythrocyte-stage protein-based vaccine for Plasmodium falciparum. Infect. Immun. 732109-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffman, S. L., L. M. Goh, T. C. Luke, I. Schneider, T. P. Le, D. L. Doolan, J. Sacci, P. de la Vega, M. Dowler, C. Paul, D. M. Gordon, J. A. Stoute, L. W. Church, M. Sedegah, D. G. Heppner, W. R. Ballou, and T. L. Richie. 2002. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J. Infect. Dis. 1851155-1164. [DOI] [PubMed] [Google Scholar]
- 16.John, C. C., A. M. Moormann, D. C. Pregibon, P. O. Sumba, M. M. McHugh, D. L. Narum, D. E. Lanar, M. D. Schluchter, and J. W. Kazura. 2005. Correlation of high levels of antibodies to multiple pre-erythrocytic Plasmodium falciparum antigens and protection from infection. Am. J. Trop. Med. Hyg. 73222-228. [PubMed] [Google Scholar]
- 17.Kester, K. E., D. A. McKinney, N. Tornieporth, C. F. Ockenhouse, D. G. Heppner, Jr., T. Hall, B. T. Wellde, K. White, P. Sun, R. Schwenk, U. Krzych, M. Delchambre, G. Voss, M. C. Dubois, R. A. Gasser, Jr., M. G. Dowler, M. O'Brien, J. Wittes, R. Wirtz, J. Cohen, and W. R. Ballou. 2007. A phase I/IIa safety, immunogenicity, and efficacy bridging randomized study of a two-dose regimen of liquid and lyophilized formulations of the candidate malaria vaccine RTS,S/AS02A in malaria-naive adults. Vaccine 255359-5366. [DOI] [PubMed] [Google Scholar]
- 18.Kester, K. E., D. A. McKinney, N. Tornieporth, C. F. Ockenhouse, D. G. Heppner, T. Hall, U. Krzych, M. Delchambre, G. Voss, M. G. Dowler, J. Palensky, J. Wittes, J. Cohen, and W. R. Ballou. Efficacy of recombinant circumsporozoite protein vaccine regimens against experimental Plasmodium falciparum malaria. J. Infect. Dis. 183640-647. [DOI] [PubMed]
- 19.Krzych, U., J. A. Lyon, T. Jareed, I. Schneider, M. R. Hollingdale, D. M. Gordon, and W. R. Ballou. 1995. T lymphocytes from volunteers immunized with irradiated Plasmodium falciparum sporozoites recognize liver and blood stage malaria antigens. J. Immunol. 1554072-4077. [PubMed] [Google Scholar]
- 20.Kurtis, J. D., M. R. Hollingdale, A. J. Luty, D. E. Lanar, U. Krzych, and P. E. Duffy. 2001. Pre-erythrocytic immunity to Plasmodium falciparum: the case for an LSA-1 vaccine. Trends Parasitol. 17219-223. [DOI] [PubMed] [Google Scholar]
- 21.Luty, A. J., B. Lell, R. Schmidt-Ott, L. G. Lehman, D. Luckner, B. Greve, P. Matousek, K. Herbich, D. Schmid, F. Migot-Nabias, P. Deloron, R. S. Nussenzweig, and P. G. Kremsner. 1999. Interferon-gamma responses are associated with resistance to reinfection with Plasmodium falciparum in young African children. J. Infect. Dis. 179980-988. [DOI] [PubMed] [Google Scholar]
- 22.Luty, A. J., B. Lell, R. Schmidt-Ott, L. G. Lehman, D. Luckner, B. Greve, P. Matousek, K. Herbich, D. Schmid, S. Ulbert, F. Migot-Nabias, B. Dubois, P. Deloron, and P. G. Kremsner. 1998. Parasite antigen-specific interleukin-10 and antibody responses predict accelerated parasite clearance in Plasmodium falciparum malaria. Eur. Cytokine Netw. 9639-646. [PubMed] [Google Scholar]
- 23.Moorthy, V. S., E. B. Imoukhuede, P. Milligan, K. Bojang, S. Keating, P. Kaye, M. Pinder, S. C. Gilbert, G. Walraven, B. M. Greenwood, and A. S. Hill. 2004. A randomised, double-blind, controlled vaccine efficacy trial of DNA/MVA ME-TRAP against malaria infection in Gambian adults. PLoS Med. 1e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23a.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC.
- 24.Nussenzweig, R. S., J. Vanderberg, H. Most, and C. Orton. 1967. Protective immunity produced by the injection of X-irradiated sporozoites of Plasmodium berghei. Nature 216160-162. [DOI] [PubMed] [Google Scholar]
- 25.Pichyangkul, S., M. Gettayacamin, R. S. Miller, J. A. Lyon, E. Angov, P. Tongtawe, D. L. Ruble, D. G. Heppner, Jr., K. E. Kester, W. R. Ballou, C. L. Diggs, G. Voss, J. D. Cohen, and D. S. Walsh. 2004. Pre-clinical evaluation of the malaria vaccine candidate P. falciparum MSP1(42) formulated with novel adjuvants or with alum. Vaccine 223831-3840. [DOI] [PubMed] [Google Scholar]
- 26.Rieckmann, K. H., R. L. Beaudoin, J. S. Cassells, and K. W. Sell. 1979. Use of attenuated sporozoites in the immunization of human volunteers against falciparum malaria. Bull. W. H. O. 57(Suppl. 1)261-265. [PMC free article] [PubMed] [Google Scholar]
- 27.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caufield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415331-335. [DOI] [PubMed] [Google Scholar]
- 28.Stewart, V. A., S. M. McGrath, P. M. Dubois, M. G. Pau, P. Mettens, J. Shott, M. Cobb, J. R. Burge, D. Larson, L. A. Ware, M. A. Demoitie, G. J. Weverling, B. Bayat, J. H. Custers, M. C. Dubois, J. Cohen, J. Goudsmit, and D. G. Heppner, Jr. 2007. Priming with an adenovirus 35-circumsporozoite protein (CS) vaccine followed by RTS,S/AS01B boosting significantly improves immunogenicity to Plasmodium falciparum CS compared to that with either malaria vaccine alone. Infect. Immun. 752283-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stewart, V. A., D. S. Walsh, S. M. McGrath, K. E. Kester, J. F. Cummings, G. Voss, M. Delchambre, N. Garcon, J. D. Cohen, and D. G. Heppner, Jr. 2006. Cutaneous delayed-type hypersensitivity (DTH) in a multi-formulation comparator trial of the anti-falciparum malaria vaccine candidate RTS,S in rhesus macaques. Vaccine 246493-6502. [DOI] [PubMed] [Google Scholar]
- 30.Stewart, V. A., S. M. McGrath, D. S. Walsh, S. Davis, A. S. Hess, L. A. Ware, K. E. Kester, J. F. Cummings, J. R. Burge, G. Voss, M. Delchambre, N. Garcon, D. B. Tang, J. D. Cohen, and D. G. Heppner, Jr. 2006. Pre-clinical evaluation of new adjuvant formulations to improve the immunogenicity of the malaria vaccine RTS,S/AS02A. Vaccine 246483-6492. [DOI] [PubMed] [Google Scholar]
- 31.Stoute, J. A., M. Slaoui, D. G. Heppner, P. Momin, K. E. Kester, P. Desmon, B. T. Wellde, N. Garcon, U. Krzych, and M. Marchand. 1997. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. N. Engl. J. Med. 33686-91. [DOI] [PubMed] [Google Scholar]
- 32.Sun, Y., J. E. Schmitz, P. M. Acierno, S. Santra, R. A. Subbramanian, D. H. Barouch, D. A. Gorgone, M. A. Lifton, K. R. Beaudry, K. Manson, V. Philippon, L. Xu, H. T. Maecker, J. R. Mascola, D. Panicali, G. J. Nabel, and N. L. Letvin. 2005. Dysfunction of simian immunodeficiency virus/simian human immunodeficiency virus-induced IL-2 expression by central memory CD4+ T lymphocytes. J. Immunol. 1744753-4760. [DOI] [PubMed] [Google Scholar]
- 33.Wille-Reece, U., B. J. Flynn, K. L. Lore, R. A. Koup, R. M. Kedl, J. J. Mattapallil, W. R. Weiss, M. Roederer, and R. A. Seder. 2005. HIV Gag protein conjugated to a Toll-like receptor 7/8 agonist improves the magnitude and quality of Th1 and CD8+ T cell responses in nonhuman primates. Proc. Natl. Acad. Sci. USA 10215190-15194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zaph, C., J. Uzonna, S. M. Beverley, and P. Scott. 2004. Central memory T cells mediate long-term immunity to Leishmania major in the absence of persistent parasites. Nat. Med. 101104-1110. [DOI] [PubMed] [Google Scholar]