Abstract
To stimulate both local and systemic immune responses against Trypanosoma cruzi, Salmonella enterica serovar Typhimurium aroA was exploited as a DNA delivery system for cruzipain (SCz). In a murine model we compared SCz alone (GI) or coadministered with Salmonella carrying a plasmid encoding granulocyte-macrophage colony-stimulating factor (GII), as well as protocols in which SCz priming was followed by boosting with recombinant cruzipain (rCz) admixed with either CpG-ODN (GIII) or MALP-2, a synthetic derivative of a macrophage-activating lipopeptide of 2 kDa from Mycoplasma fermentans (GIV). The results showed that protocols that included four oral doses of SCz (GI) elicited mainly a mucosal response characterized by immunoglobulin A (IgA) secretion and proliferation of gut-associated lymphoid tissue cells, with weak systemic responses. In contrast, the protocol that included a boost with rCz plus CpG (GIII) triggered stronger systemic responses in terms of Cz-specific serum IgG titers, splenocyte proliferation, gamma interferon (IFN-γ) secretion, and delayed-type hypersensitivity response. Trypomastigote challenge of vaccinated mice resulted in significantly lower levels of parasitemia compared to controls. Protection was abolished by depletion of either CD4+ or CD8+ T cells. Parasite control was also evident from the reduction of tissue damage, as revealed by histopathologic studies and serum levels of enzymes that are markers of muscle injury in chronic Chagas' disease (i.e., creatine kinase, aspartate aminotransferase, and lactate dehydrogenase). Enhanced release of IFN-γ and interleukin-2 was observed in GI and GII upon restimulation of splenocytes in the nonparasitic phase of infection. Our results indicate that Salmonella-mediated delivery of Cz-DNA by itself promotes the elicitation of an immune response that controls T. cruzi infection, thereby reducing parasite loads and subsequent damage to muscle tissues.
The etiologic agent of Chagas' disease (American trypanosomiasis) is an obligate intracellular protozoan parasite, Trypanosoma cruzi, which is transmitted by feces of reduviid insects. Chagas' disease is prevalent in almost all Latin American countries, where ∼18 million people are infected and ∼50,000 children and adults die every year as a result of clinical complications of T. cruzi-induced heart disease and lack of effective treatments (51). Because of continuous population migration from areas of endemicity towards developed countries, Chagas' disease also threatens populations outside the traditional geographic boundaries (e.g., Europe, Canada, and the United States) (23). Currently available drugs, though effective against acute infection, are highly toxic and ineffective in arresting or attenuating symptoms in chronic patients. Thus, there is an urgent need for a vaccine against T. cruzi.
T. cruzi contains a major cysteine proteinase called cruzipain (Cz), which is displayed on the surface of the parasite and also accumulates in the lysosomes located near the flagellar pocket, from where it is secreted (46). Cz exhibits a number of attractive properties as a candidate antigen for vaccine development: (i) it is highly immunogenic in natural infection (34); (ii) it is present in the three main developmental stages of the parasite (i.e., epimastigote, amastigote, and trypomastigote) in all tested strains (40); (iii) it is a secreted antigen and its ability to cleave immunoglobulins has been proposed as an immunoescape mechanism (5); (iv) it plays an important role in the process of parasite internalization within mammalian cells (46); and (v) it is able to induce a protective immune response when coadministered with synthetic oligodeoxynucleotides carrying immunostimulatory CpG motifs (CpG-ODN) (17).
Considering the portals of T. cruzi entry, skin and mucosae, the capacity of a candidate vaccine to efficiently stimulate both systemic and local immune responses would represent a real asset. This could be achieved by vaccination through the mucosal route. In this context, different strategies have been exploited to promote mucosal responses, such as antigen expression by live attenuated bacterial or viral carriers, or their coadministration with mucosal adjuvants (7, 41). Attenuated pathogens are very attractive, since protection against the pathogen itself and immune responses specific for the heterologous antigen can be simultaneously achieved (12, 13, 15, 22, 38, 39, 54). Bacteria can be attenuated by generating deletions in genes that are essential for either the virulence process or bacterial metabolism. The introduction of several independent attenuating deletions makes the risk of reversion as a result of recombination events almost negligible. Additional work also demonstrated that bacterial vectors can be used as a delivery system for the so-called “DNA vaccines” (19). Traditional naked DNA vaccination is extremely inefficient, because multiple administrations and high dosages are needed. The use of bacterial carriers as a delivery system eliminates the requirement for DNA purification and allows specific targeting of antigen-presenting cells (APC). The carrier also acts as a natural adjuvant by the presence of pathogen-associated molecular patterns, such as cell wall degradation products or unmethylated microbial DNA, which promote the recruitment of innate immunity masters and APC activation through the stimulation of pattern recognition receptors (4, 14). Thus, a local environment conducive to successful antigen processing and presentation is specifically created. Additional advantages are cost-effective large-scale production and easy administration logistics, which are essential for implementing an effective vaccination program in underdeveloped and developing countries, as those are most affected by Chagas' disease (28).
The main goal of this work was to determine the ability of Cz-DNA immunization through a Salmonella-based delivery system to induce protection in a murine model of T. cruzi infection. Previous studies have documented the usefulness of Cz-based vaccines in eliciting protective immunity to T. cruzi (17, 44, 45). On the other hand, Salmonella is an ideal delivery system because is easy to handle and to manipulate genetically. In addition, Salmonella can specifically target foreign antigens to APC and act as a natural adjuvant, thereby maximizing adaptive immune responses. In our novel approach, orally administered Salmonella was used as a Cz-DNA delivery system to ensure strong activation of innate immunity and efficient stimulation of both local and systemic anti-T. cruzi responses. Attempts were also carried out to further improve the efficacy of this vaccine. To this end, we evaluated mucosal immunization regimens in which animals received Salmonella carrying a plasmid coding for Cz (SCz) together with bacteria containing a granulocyte-macrophage colony-stimulating factor encoding plasmid (SGM-CSF). As an additional strategy, prime-boost protocols were established in which mice primed with SCz were subsequently boosted with recombinant Cz (rCz) coadministered with different adjuvants. Two potent adjuvants were exploited, namely, (i) CpG-ODN motifs that enhance Th1 immune responses (11, 27, 42) by stimulating Toll-like receptor 9 (TLR-9) (24), previously reported as an efficacious adjuvant for native Cz to confer protection against T. cruzi after parenteral vaccination (17), and (ii) MALP-2, a synthetic derivative of a macrophage-activating lipopeptide of 2 kDa from Mycoplasma fermentans which acts as TLR-2/6 agonist after mucosal administration (6, 7, 41). Our results demonstrated that Salmonella-mediated delivery of Cz-DNA, alone or in combination with a boost with rCz and CpG-ODN or MALP-2, promotes the elicitation of an immune response able to control T. cruzi infection and the collateral damage to muscle tissues.
MATERIALS AND METHODS
Native and recombinant cruzipain.
Native cruzipain (nCz) was obtained from epimastigotes as previously described (10). The expression and purification of rCz will be described elsewhere (S. I. Cazorla, et al., unpublished results). Briefly, the amplified 1,037-bp fragment (encompassing bp 367 to 1404) was digested with BamHI and XhoI and ligated into pET-23a. The resulting vector was transformed into Escherichia coli BL21(DE3) cells for expression. rCz was purified under denaturing conditions from the supernatant using a Ni2+-nitrilotriacetic acid-Sepharose matrix. Properly folded rCz was obtained by extensive dialysis against phosphate-buffered saline (PBS), purified by ion exchange, and stored at −70°C until use. Endotoxin was removed by a column of polymyxin B-agarose (Sigma, St. Louis, MO). Endotoxin levels in the final protein preparations were <100 units/mg, as determined using a Limulus amoebocyte lysate analysis kit (Whittaker Bioproducts, Walkersville, MD).
Construction of eukaryotic expression plasmids encoding Cz and GM-CSF.
The purified DNA from T. cruzi was used as a template for a PCR amplification using a forward primer encompassing an initial TGA, a BamHI site, and a Kozak sequence and a reverse primer with a XhoI site and a stop codon. The amplified 1,404-bp fragment (positions 1 to 1404), which included the signal peptide and the prodomains of the protein, was digested and ligated into pCDNA 3.1(+) (Invitrogen) treated with the same restriction enzymes. The resulting vector (pCDNA-Cz) was transformed into E. coli DH5 host cells. The expression of the protein in mammalian cells was analyzed by transfecting BHK-A cells with pCDNA-Cz. Briefly, BHK-A cells were transfected with 5 μg of the plasmid carrying Cz, or an empty plasmid as control (pCDNA), using Lipofectamine (Gibco-BRL). After 48 h of incubation, the supernatants were collected, centrifuged to eliminate cellular debris, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and blotted onto nitrocellulose. After blocking with 1% skin milk in Tris-HCl buffer solution, nitrocellulose was incubated with a murine polyclonal antibody raised by several inoculations with nCz and complete Freund's adjuvant. The reaction was revealed using peroxidase-conjugated goat anti-mouse immunoglobulins (Sigma) and H2O2-4-Cl-1-naphtol.
The vector LmGMSN/2 (kindly provided by P. Paglia) was used as a template for the PCR amplification of a 459-bp fragment encompassing the gene coding for the murine GM-CSF using the primers 5′-ATAAGAATGCGGCCGCCATGTGGCTGCAGAATTTACTTTTC-3′ and 5′-ATAAGAATGCGGCCGCTCATTTTTGGCTTGGTTTTTTGCAT-3′. The resulting fragment was then digested with NotI and cloned into the NotI sites of pCMVβ (Clontech), thereby generating pCGA2, in which the lacZ gene is replaced by the murine GM-CSF-encoding gene. The corresponding expression vectors were transferred into the attenuated S. enterica serovar Typhimurium aroA 7207 strain (an S. enterica serovar Typhimurium 2337-65 derivative, hisG46 DEL407 [aroA::Tn105Tc-s6]) (25).
Adjuvants.
CpG-ODN 1826 (5′-TCCATGACGTTCCTGACGTT-3′) was synthesized under Good Manufacturing Practices conditions with a nuclease-resistant phosphorothioate backbone by Oligos Etc. (Wilsonville, OR) and used at a dose of 100 μg per mouse where indicated. A derivative of the macrophage-activating lipopeptide from M. fermentans (MALP-2) was synthesized according to established protocols (37), and 0.5 μg of the active moiety was employed per mouse per dose where indicated. Endotoxin was undetectable in both adjuvant preparations (<1.0 endotoxin units/mg), as determined by the Limulus test.
Immunizations and challenge studies.
All studies were carried out in inbred female 6- to 8-week-old C3H/HeN mice. Each group consisted of 12 mice maintained in three cages under standard conditions. Oral administration was achieved by feeding 20 μl of PBS containing 109 bacteria to mice that were deprived of drinking water for 2 h. Mice were immunized on days 0, 10, 20, and 30 with one of the following regimens: GI, four doses of Salmonella carrying Cz DNA (SCz); GII, four doses of SCz together with 109 Salmonella organisms carrying the GM-CSF-encoding vector pCGA2 (SGM-CSF); GIII, two doses of SCz on days 0 and 10 with two boosts of rCz (10 μg/dose) plus CpG-ODN (100 μg/dose) by the intradermal route on days 20 and 30; GIV, two doses of SCz on days 0 and 10 with two boosts of rCz (10 μg/dose) plus MALP (0.5 μg/dose) by the intranasal route on days 20 and 30; GV, plasmidless Salmonella (control group). Two weeks after the last immunization, half of the mice were killed by cervical dislocation and their spleens were aseptically removed, whereas the remaining animals were challenged with 50 bloodstream trypomastigotes (RA strain). We used this chronic model of T. cruzi infection in order to simulate the human chronic infection. Parasitemia was monitored by counting peripheral blood parasites every 2 days with a Neubauer chamber. Mice were killed by cervical dislocation, and their organs were removed at 100 days postinfection.
Antibody determination.
Cz-specific serum immunoglobulin G (IgG) antibody titers were determined by enzyme-linked immunosorbent assay (ELISA) as described previously (17). Total IgA was detected by ELISA using plates coated with anti-IgA antibodies (Sigma) and purified IgA (Sigma) for the generation of the standard curve. The amount of Cz-specific IgA present in intestinal lavage samples was determined as previously described (41). To compensate for variations in the efficiency of recovery of secretory antibodies among animals, the Cz-specific IgA in each sample was normalized with the total IgA present in the lavage.
DTH reactions.
The delayed-type hypersensitivity (DTH) test was performed 13 days after the last immunization by intradermal injection of rCz (5 μg in the left footpad) or nCz (5 μg in the right footpad). The thickness of both hind footpads was measured before and 48 h after the injection of antigen. Results are expressed as the difference in thickness of the corresponding footpad after and before the inoculation.
Proliferation assay and cytokine quantification.
Mesenteric lymph nodes, Peyer's patches, and spleen cells from mice were aseptically removed, and cell suspensions were prepared. Cell proliferation was assayed as previously reported (17). The production of cytokines in spleen cell supernatants was determined by capture ELISA (R&D Systems, Minneapolis, MN). To estimate the number of gamma interferon (IFN-γ)-secreting CD8+ T cells, the murine IFN-γ enzyme-linked immunospot (ELISPOT) kit from BD Biosciences (San Jose, CA) was used according to the manufacturer's instructions. Colored spots were counted with a CTL ELISPOT reader and analyzed using the ImmunoSpot image analyzer software v3.2.
In vivo depletion of CD4+ and CD8+ T cells.
The hybridomas secreting monoclonal antibody (MAb) anti-CD4 (GK1.5; rat IgG2a) or anti-CD8 (53.6.7; rat IgG2a) were kindly provided by Gerardo Mirkin (University of Buenos Aires). Antibodies were purified by chromatography on a MonoQ column using ÁKTA (GE Healthcare, Buckinghamshire, England). At days −3 and −4 before challenge, SCz-immunized (GI) mice were treated with daily intraperitoneal injections of 1 mg of anti-CD4 MAb, anti-CD8 MAb, or isotype-matched rat IgG. Seven days after challenge, each mouse received one additional dose of the corresponding MAb or control IgG. The efficacy of the depletion was estimated by flow cytometry analysis of spleen cells and found to be >90% and >99% for CD4+ and CD8+ cells, respectively.
Measurement of muscle damage.
Muscle injury was evaluated through the determination of a panel of myopathy-linked enzyme markers. Serum levels of creatine kinase (CK), aspartate aminotransferase (AST), and lactate dehydrogenase (LDH) were measured at 100 days postinfection. The assays were done with UV spectrophotometry following the specifications of the kit's manufacturer (Wiener Lab, Buenos Aires, Argentina). In addition, skeletal muscles (quadriceps) were removed from control, immunized, and infected mice on day 100 after trypomastigote challenge. The organs were rinsed with PBS and fixed for 24 h in 10% buffered formalin. Fixed tissues were embedded in paraffin, sectioned, stained with hematoxylin-eosin, and examined by light microscopy. A blind histological test of quadriceps was done, analyzing 10 microscope fields in 10 slices of each organ.
Statistical analysis.
The statistical analysis was carried out with the Prisma 3.0 software (GraphPad, San Diego, CA) using a one-way analysis of variance for proliferation, cytokine, antibody, and enzyme assay results and the parasitemia data. All the comparisons were referred to the empty vector control group (GV). P values of <0.05 were considered significant.
RESULTS
Eukaryotic expression of Cz.
A eukaryotic expression plasmid was engineered by insertion of the Cz gene, including the signal peptide and the prodomain, into the plasmid pCDNA3.1 between the BamHI and the XhoI sites. We analyzed mammal expression of Cz by transient transfection of BHK-A cells with the pCDNA3.1 vector encoding Cz. The presence of Cz in the supernatant of BHK-A cells cultured for 48 h was confirmed by Western blot analysis with a murine polyclonal anti-nCz antibody. These results showed not only that Cz is expressed by mammalian cells transfected with our vector, but also that the recombinant protein is released into the culture medium. This is expected in turn to facilitate the elicitation of an immune response following vaccination.
Vaccination with Salmonella carrying Cz-DNA results in the elicitation of a humoral immune response.
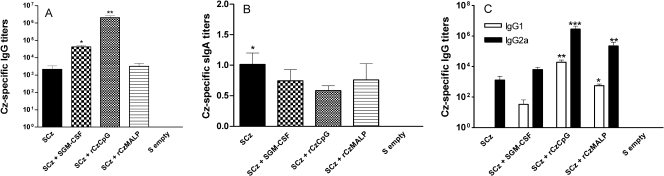
To analyze whether Salmonella carrying Cz-DNA is able to elicit an immune response, mice were orally immunized with four doses of SCz (GI). To evaluate if the performance of this DNA vaccine could be fine-tuned, a group of mice received SCz coadministered with Salmonella carrying GM-CSF (SGM-CSF) (GII). We also analyzed the effect of establishing prime-boost protocols in which mice orally primed with SCz were boosted with rCz coadministered with either CpG-ODN (GIII) or MALP-2 (GIV) by an intradermal or intranasal route, respectively. Blood samples were taken 2 weeks after the last immunization, and the specific IgG antibodies against Cz were measured by ELISA (Fig. 1A). Cz-specific serum antibodies were stimulated in all mice receiving SCz and were not detected in animals receiving Salmonella carrying an empty vector. These antibodies were capable of cross-recognizing both native and recombinant Cz (data not shown). The coadministration of SGM-CSF (GII) greatly increased Cz-specific IgG responses. The mice primed with SCz and boosted by intradermal route with rCz plus CpG-ODN showed the highest titer, (1.79 ± 0.52) ×106 (mean ± standard error of the mean [SEM]), whereas boosting with rCz plus MALP by the intranasal route did not improve significantly the level of Cz-specific serum IgG over that observed in mice that received SCz alone (Fig. 1A). We then evaluated in intestinal lavage fluids whether the vaccination regimens were able to stimulate Cz-specific secretory IgA (sIgA). The obtained results showed that Cz-specific sIgA was detected in all vaccinated animals; nevertheless, only in mice from GI were the levels significantly higher compared to the control (Fig. 1B). By and large, these results show that Salmonella, as a delivery system for a Cz-based DNA vaccine, can induce an antibody-specific response at both the mucosal and systemic levels.
FIG. 1.
Humoral immune responses in mice immunized using Salmonella as a delivery system for a Cz-based DNA vaccine. Two weeks after the last immunization, sera and intestinal lavage fluids were collected and assayed by ELISA for the presence of rCz-specific antibodies. (A) Antigen-specific serum IgG titers. (B) Antigen-specific sIgA titers in intestinal lavage fluids. Results are expressed as Cz-specific sIgA titers per μg of total IgA. (C) Antigen-specific serum IgG isotype titers. Results are expressed as endpoint titers. The SEMs are indicated by vertical lines. The results are representative of three independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Salmonella-mediated DNA vaccination with Cz induces a Th1-biased immune response.
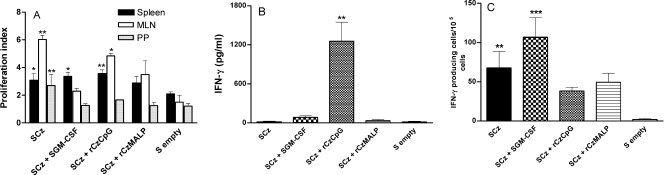
To obtain an overview of the dominant Th pattern stimulated following immunization, the titers of the major Cz-specific IgG isotypes were determined. The results showed that in all vaccination groups IgG2a was the main isotype, suggesting that a Th1 dominant response was promoted by all protocols (Fig. 1C). Cz-specific IgG1 was also detected in animals from GII, GIII, and GIV; however, the values were statistically different compared to the control group only in mice boosted with rCz and adjuvants (GIII and GIV). Then, the ability of the vaccination protocols to promote antigen-specific cellular responses was assessed in spleen and gut-associated lymphoid tissue (GALT). A proliferative response of splenocytes was detected in all immunized animals after in vitro restimulation with either rCz (Fig. 2A) or nCz (data not shown). In contrast, when we analyzed the cellular responses stimulated at the level of GALT, only mice immunized with SCz showed a significant proliferative response in both mesenteric lymph nodes and Peyer′s patches (Fig. 2A). These results suggest that SCz immunization is sufficient per se to trigger a Cz-specific cellular immune response at both systemic and mucosal levels.
FIG. 2.
Cellular responses stimulated in vaccinated mice. (A) Proliferative responses of mesenteric lymph node (MLN), Peyer's patch (PP), and spleen cells from immunized mice. Fifteen days after the last immunization, cells were collected and stimulated with 10 μg/ml of rCz. Four days later, 1 μCi of [3H]thymidine per well was added and the counts per minute were measured 18 h later. Results are expressed as the ratio of values from stimulated and nonstimulated samples (proliferation index). Each bar represents the group mean ± SEM. *, P < 0.05; **, P < 0.01. (B) Detection of IFN-γ produced by spleen cells of vaccinated mice. Cells were harvested 2 weeks after the last immunization and cultured by duplicate in the presence of rCz (10 μg/ml). Supernatant fluids were collected 48 h later and assayed in duplicate by capture ELISA for the presence of IFN-γ. **, P < 0.01. (C) Detection of IFN-γ-secreting CD8+ T cells. Spleen CD8+ T-cell-enriched preparations recovered from immunized mice were incubated for 16 h in the presence of rCz, and secretion of IFN-γ was determined. Then, the number of IFN-γ-producing cells was determined by ELISPOT. Results are presented as spot-forming units per 105 cells (mean ± SEM of triplicate wells), which were subtracted from the values obtained from nonstimulated cells. **, P < 0.01; ***, P < 0.001. The results are representative of three independent experiments.
To further characterize the cellular responses stimulated by different vaccination protocols, we quantified by capture ELISA the concentration of the Th1-associated cytokine IFN-γ in the supernatant fluids of spleen cells, mainly CD4+ derived, restimulated in vitro with rCz (Fig. 2B) or nCz (data not shown). Splenocytes from mice primed with SCz and boosted with rCz plus CpG-ODN produced significantly higher levels of IFN-γ (1,255 ± 292 pg/ml) than those detected in the other four experimental groups. These results confirm our previous report (17) in which CpG-ODN administered intramuscularly with nCz was able to induce a strong IFN-γ response.
Despite the importance of CD4+ Th1 in the provision of help for cell-mediated responses in Chagas' disease, CD8+ T cells seem to play a critical role in the control of T. cruzi infection (2). Thus, we determined by ELISPOT assay the number of IFN-γ-secreting cells present after in vitro restimulation of CD4+-depleted splenocytes with rCz (Fig. 2C). The obtained results showed an incremental increase in the number of antigen-specific IFN-γ-secreting cells in all immunization groups with 33-, 53-, 19-, and 24-fold-higher spot counts (GI to GIV, respectively) than those observed in control mice. The highest values were observed in animals receiving SCz alone or together with SGM-CSF (P < 0.01 and 0.001, respectively). These results suggest that in the immunization groups receiving four doses of SCz (GI and GII) a higher number of cytotoxic CD8+ T cells is induced compared to the groups receiving only two doses (GIII and GIV).
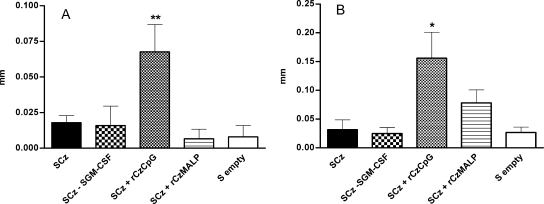
Skin tests were also performed in immunized mice to investigate the efficacy of the different vaccination regimens in terms of stimulating delayed-type responses in vivo. Thirteen days after the fourth immunization, the thickness of the hind footpads was measured before and 48 h after intradermal injection of 5 μg of rCz (left footpad) or nCz (right footpad). Both nCz and rCz evoked a significant delayed-type hypersensitivity response, but only in mice primed with SCz and boosted intradermally with rCz plus CpG-ODN (Fig. 3). As expected, the hypersensitivity reaction was 2.3 times stronger after injection of the recombinant protein (0.16 mm) than after using native Cz purified from parasites (0.07 mm).
FIG. 3.
Delayed-type hypersensitivity test in vaccinated mice. Mice were immunized as indicated previously and intradermally challenged on day 13 after the last immunization with 5 μg of either nCz in the right footpad (A) or rCz in the left footpad (B). The results are expressed as the difference between the thickness of the footpad 48 h after and before the inoculation. Each bar represents the group mean (n = 6) ± SEM. The results are representative of three independent experiments. *, P < 0.05; **, P < 0.01.
Taken together, these results show that a vaccination protocol based on four oral doses of SCz (GI) mainly triggered a strong mucosal response characterized by the presence of Cz-specific sIgA (Fig. 1B) and T cells in the GALT (Fig. 2A), with a comparatively weaker systemic response. In contrast, the protocols in which mice were boosted with rCz plus CpG (GIII) elicited a stronger systemic response in terms of serum IgG (Fig. 1A and C), splenocyte proliferation (Fig. 2A), IFN-γ secretion (Fig. 2B), and delayed-type hypersensitivity (Fig. 3).
SCz-based immunization protocols are able to control T. cruzi infection.
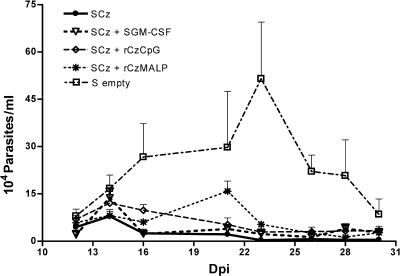
To determine if the different vaccination strategies were able to promote protection against T. cruzi, 15 days after the last immunization mice were challenged by the intraperitoneal route with bloodstream trypomastigotes, and the individual parasitemia levels were assessed (Fig. 4). An important decrease in the number of circulating parasites was observed in all immunization groups compared to control mice (i.e., plasmidless Salmonella) during the acute phase of infection. In vaccinated mice, the parasitemia was significantly lower (P < 0.01) between days 16 and 28 postchallenge, when the control group reached the highest level. The differences in parasitemia among the immunization groups GI to GIV were not statistically significant. These results are somehow surprising, since mice in GI and GII, which had a weaker systemic immune response, were also able to control infection. This suggests that when mice are challenged with parasites, effector lymphocytes located at the mucosal level rapidly respond and migrate to effector sites in order to destroy the parasites.
FIG. 4.
Parasitemia levels during the acute phase of T. cruzi infection. Mice were challenged 15 days after the last immunization with 50 trypomastigotes of the RA strain by the intraperitoneal route. Parasitemia was determined by counting in a Neubauer chamber the number of trypomastigotes in 5 μl of fresh blood collected from the tail vein every 2 to 3 days. The results are representative of three independent experiments. Each bar represents the group mean ± SEM.
Although the analysis of the immune responses showed differences at the level of systemic or mucosal stimulation among mice receiving the different immunization protocols, the immunoprotection against T. cruzi challenge was similarly efficient in all groups. These observations led us to investigate the immune response in a nonparasitic stage of the infection. To this end, on day 100 after the challenge, spleen cells were in vitro stimulated with a complex soluble antigen (F105) from T. cruzi. Surprisingly, cytokine production differed from that observed prior to trypomastigote challenge. Before challenge, GIII (SCz plus rCz-CpG) exhibited the highest splenic level of IFN-γ, whereas 100 days after challenge the level of secreted IFN-γ was almost negligible. In contrast, animals in GI (SCz), which had no detectable IFN-γ before challenge (Fig. 3B), showed the highest levels of both IFN-γ (1,364.0 ± 350.5 pg/ml) and IL-2 (174.0 ± 29.9 pg/ml) in spleen cell supernatants at a late stage after infection (Fig. 5). This result suggests that the GI immunization protocol is able to induce a long-standing Th1-driven immune response that would be helpful in case of reactivation or reinfection.
FIG. 5.
Cytokine secretion by spleen cells from vaccinated mice after challenge with T. cruzi. One hundred days after challenge with trypomastigotes (chronic stage of the infection), spleen cells from mice were aseptically removed. Cultures were performed in duplicate in the presence of whole soluble antigens of T. cruzi (F105; 10 μg/ml). Supernatants were collected 48 h later and assayed by capture ELISA for IFN-γ (A) and IL-2 (B). Each bar represents the group mean ± SEM. The results presented are representative of three independent experiments. *, P < 0.05; **, P < 0.01. Spleen cells from all groups exhibited demonstrable concanavalin A responsiveness (not shown).
T cells are key elements for the control of T. cruzi infection in vaccinated mice.
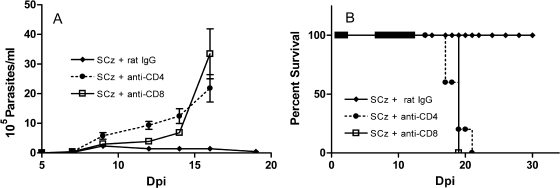
The results of our experimental T. cruzi vaccine suggest that elicitation of protective immunity is associated with the generation of specific CD4+ and CD8+ T cells. To confirm this, we treated SCz -vaccinated mice (GI) with rat anti-CD4 or anti-CD8 MAb prior to the challenge with T. cruzi. Parasitemia levels of T-cell-depleted mice were significantly higher than those recorded in vaccinated control animals inoculated with rat IgG (Fig. 6A). All vaccinated and rat IgG-treated mice survived infection, whereas 100% of the CD4+ and CD8+ T-cell-depleted animals died between days 17 and 20 after trypomastigote challenge (Fig. 6B). These results show that treatment with each of the MAbs dramatically increased mouse susceptibility to infection in comparison to control animals receiving normal rat IgG. These findings indicate that in mice vaccinated with Salmonella as Cz-DNA delivery systems, both CD8+ and CD4+ T cells are central to efficiently reducing the number of circulating parasites and improving survival from T. cruzi infection.
FIG. 6.
T. cruzi challenge in vaccinated mice depleted of CD4+ or CD8+ T cells. Intraperitoneal challenge with 50 trypomastigotes of the RA strain was performed 15 days after the last immunizing dose. SCz-vaccinated mice were treated with rat IgG, anti-CD4, or anti-CD8 MAb. Parasitemia was determined by counting in a Neubauer chamber every 2 to 3 days (A). Mortality was recorded daily (B). Results are representative of three independent experiments.
SCz immunization reduces the tissue damage triggered by T. cruzi infection.
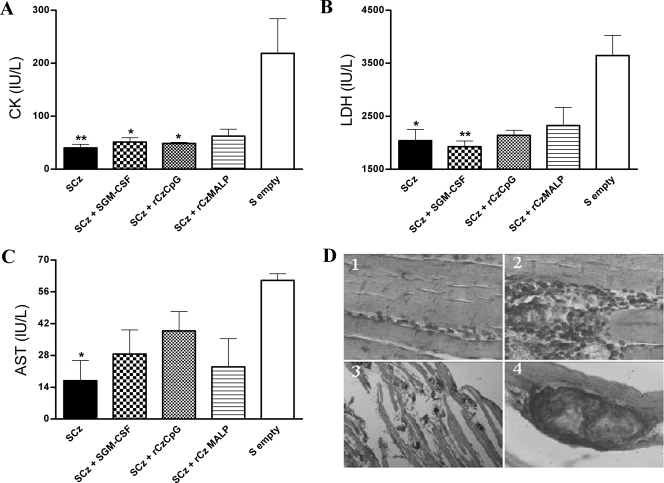
We further investigated if the immunization protocols that were able to reduce parasitemia were also effective in terms of limiting tissue injury. To this end, we measured the serum levels of the cardiomyopathy-associated enzymes CK, LDH, and AST in infected mice at 100 days postinfection. Vaccinated and challenged mice exhibited a marked decrease in the level of circulating enzymes compared to animals receiving empty Salmonella (Fig. 7). The absolute values of these myopathy-linked markers determined in immunized mice were similar to baseline levels detected in normal mouse serum (28.67 ± 10.41, 1,087 ± 240, and 8.6 ± 4.6 IU/liter for CK, LDH, and AST, respectively). We also analyzed the effect of immunization and challenge on the tissues most affected by Chagas' disease on day 100 after infection. We were not able to identify any abnormality in hearts from vaccinated or control animals by microscopic examination. However, skeletal tissue sections stained with hematoxylin and eosin showed severe inflammatory foci and fiber muscle infarction in the control group, whereas SCz -immunized animals showed only small foci containing mononuclear cells (Fig. 7D; Table 1). The results of the skeletal muscle histology in groups GII to GIV were similar to GI (Table 1).
FIG. 7.
(A to C) Serum levels of myopathy-linked enzyme markers and histopathological studies on skeletal muscle from immunized mice after challenge. Blood was collected on day 100 postinfection with trypomastigotes, and assays were performed to determine the levels of CK (A), LDH (B), and AST (C) by spectrophotometry. The bars represent the average of six determinations; the SEMs are indicated by vertical lines. The results are representative of three independent experiments. *, P < 0.05; **, P < 0.01. (D) Tissue sections were collected at the same time and stained with hematoxylin and eosin. Histological sections of skeletal muscle from mice immunized with Salmonella as a Cz-DNA delivery system, showing limited focal cellular infiltrates (panel 1), and reactions in control groups (panels 2, 3, and 4), showing severe inflammatory foci and fiber muscle infarction. Magnification: ×40 (panels 1, 2, and 4) or ×5 (panel 3).
TABLE 1.
Score of skeletal muscle damage from vaccinated mice after T. cruzi challenge
| Group | Muscle damage ina:
|
|
|---|---|---|
| Cellular infiltrate | Fiber muscle infarction | |
| SCz | + | − |
| SCz + SGM-CSF | + | − |
| SCz + rCz + CpG-ODN | ++ | − |
| SCz + rCz + MALP | ++ | − |
| S empty vector | ++++ | ++ |
Tissue observations were arbitrarily scored on a gradual scale from − to ++++, representing normal tissue and maximal injury, respectively.
DISCUSSION
The exploration of vaccines against T. cruzi has been widely avoided, due to the fear that such intervention would exacerbate rather than prevent an illness that many still consider to have an autoimmune etiology. However, a growing body of evidence indicates that it is the persistence of T. cruzi in diseased tissues rather than a parasite-induced immune response to self molecules which correlates best with the induction and maintenance of the inflammatory process (3, 8, 30, 31, 48, 50). These results strongly support the fact that immune therapeutic or prophylactic interventions directed to control T. cruzi infection and parasitemia would prevent or arrest the development of Chagas' disease.
An efficient protective response against T. cruzi requires the induction of Th1 cytokines, lytic antibodies, and the concerted activities of macrophages, T helper cells, and cytotoxic T cells (1, 18, 21, 26, 33, 49, 52) and, more importantly, a careful selection of target antigens capable of eliciting the desired immune responses at a level that confers protection from infection. We have previously demonstrated that Cz isolated from T. cruzi epimastigotes coadministered with CpG-ODN is able to induce a protective immune response in acutely infected mice (17). Nevertheless, native antigens require complex manufacturing and are considered unstable and expensive for vaccine development. In this work, we generated a recombinant Cz protein, as well as a Cz-encoding DNA plasmid, and evaluated the vaccine potential of both preparations in a murine model of T. cruzi infection.
In the attempt to construct an efficacious immunogen, we transferred the Cz expression vector into attenuated S. enterica serovar Typhimurium, which has been characterized as a successful delivery system for orally administered vaccines (12, 13, 14, 20, 22, 36, 38, 39, 54), but it has never been tested as a delivery system for oral DNA vaccines against Chagas' disease. However, T. cruzi invades the host via mucosal surfaces. Consequently, the relevance of eliciting an efficient local immune response at the site where the first line of defense is laid is apparent. Despite this, most experimental approaches have used needle vaccines, which promote systemic rather than mucosal immunity and are poorly accepted by the public, as well as being more reactogenic than needle-free oral vaccines.
Salmonella carrying Cz DNA was able to confer protection against T. cruzi, mainly by inducing a Th1-biased cell-mediated immune response, similar to that originally described for native Cz plus CpG-ODN (17). Most probably, the activated cells were mainly CD8+ T lymphocytes and NK cells, a critical source for IFN-γ in this parasite infection (43). Nevertheless, both CD4+ and CD8+ T cells are likely involved in the control of parasitemia achieved through SCz vaccination, as demonstrated by depletion studies.
Independently of the immunostimulatory capacities of the tested vaccines, every strategy applied in the present study evoked a marked decrease of acute parasitemia levels in T. cruzi-challenged mice. It can be hypothesized that Salmonella-based DNA vaccines lead to better early control of T. cruzi infection, perhaps due in part to the activation of innate factors. The adjuvant ability of Salmonella was effective in eliciting a Th1 protective response that could not be further improved by addition of well-known enhancers of innate immunity (e.g., GM-CSF, CpG-ODN, or MALP-2). An important finding of this study is that in mice receiving SCz the boost with rCz plus CpG-ODN seemed unnecessary to enhance vaccine performance. Although boosting increases humoral and cellular immunity to Cz, a weaker response was able to confer the same protection after challenge. This is important because an exacerbated immune response could raise negative effects on certain individuals, and there are reports suggesting that there is a higher risk for hepatotoxicity associated with the use of CpG-ODN (32).
It had been previously reported that GM-CSF contributes to confer protection when coadministered with IL-12 plus different T. cruzi antigens, such as amastigote and trypomastigote surface proteins, pore-forming protein LYT1, and flagellar Ca2+-binding protein (16, 18, 53). In the case of Cz, we were not able to greatly enhance immunoprotection against T. cruzi by coadministering Salmonella as the vehicle of a GM-CSF-encoding plasmid. The reason for this discrepancy remains elusive, but codelivery of IL-12 could be a key element for optimizing genetic immunization. Other adjuvant-induced cytokines, such as IL-10 released by regulatory T cells, may have affected the vaccine efficacy, as demonstrated in murine Leishmania major infection, where high pre- and postchallenge IL-10 determined the failure of prime-boost immunization to improve host protection (47).
When the immune profile of mice at the chronic stage of infection was explored, we found that Th1-associated cytokines (IFN-γ and IL-2) were mainly produced upon antigen stimulation by splenocytes from mice in GI and GII. In our model, the generation and expansion of Cz-specific IFN-γ-secreting memory and effector T cells could be attributed to a persistent antigenic stimulus only achieved in those mice receiving more doses of Cz DNA-carrying bacteria, since GI and GII received four doses of SCz.
Cardiomyopathy is considered the most distinctive clinical entity associated with Chagas' disease in symptomatic chronic patients. Nevertheless, around 60% of subjects chronically infected by T. cruzi present skeletal muscle inflammatory pathology (29). In this context, the finding that SCz-based vaccines limit skeletal muscle injury caused by a sublethal T. cruzi infection should be considered as a remarkable aspect of the immunoprotection achieved through our strategy. More specifically, murine chronic infection with the RA strain of T. cruzi is characterized by the development of neuromyopathic inflammatory lesions (35). In our study, the serum levels of myopathy-associated enzyme markers present at 100 days postinfection were considerably lower in vaccinated animals than in nonimmunized and infected mice, exhibiting values within the normal range. This observation could be linked to the reduced number of trypomastigotes circulating in the bloodstream of vaccinated mice at early stages of infection. Severity of muscle injury in chronically infected hosts has been correlated with elevated parasitemia, tissue parasitism, and inflammatory infiltration during the acute phase of Chagas' disease. Accordingly, our histopathologic studies revealed more intense inflammation and muscle fiber damage in quadriceps from chronically infected controls than in vaccinated animals.
Taken together, our results indicate that oral immunization with Salmonella as a Cz-DNA delivery system induces a complex and efficient immune response, including humoral and cellular components, which is able to provide protection at the early stages of T. cruzi infection by drastically reducing parasitemia. As Cz is not only one of the major antigens but is also expressed in all parasite strains studied so far (9), our findings suggest that Cz-based immunization could be effective in protecting from T. cruzi infection in different regions of endemicity. More interestingly, the immunoprotection proved to be long-lasting and also effective at the chronic stage, during which the occurrence of myopathy was prevented. These observations further support the potential use of Cz, preferentially in combination with other protective antigens, in the design of future vaccines against this human pathogen. The complexity of the parasite life cycle, the expression of developmentally regulated proteins in different morphological stages, and the multifaceted manifestations of Chagas' disease caused by different T. cruzi strains render critical the identification of multiple antigenic targets. Rational development of multivalent vaccines, consisting of a synergistic mixture of diverse antigens, constitutes an attractive strategy to create broad-ranging protection against parasitic infections. This kind of vaccine has a greater amount of protective epitopes and could be effective in a larger proportion of the population. Unfortunately, given the parasite's ability to evade immune detection and survive long term in an immunocompetent host, it is unlikely that anti-T. cruzi vaccines would be effective in preventing infection (i.e., sterilizing immunity). However, vaccines capable of eliciting immune responses that are sufficient to keep the parasite burden below a threshold level would be able to control parasite-mediated immune dysregulation and tissue injury, thereby arresting the onset and progression of Chagas' disease.
Acknowledgments
Financial support was received from Consejo Nacional de Investigaciones Científicas y Técnicas and Agencia Nacional de Investigaciones Científicas y Técnicas (PICT 12217), Argentina. S.I.C. was in part supported by a fellowship from the German Academic Exchange Service (DAAD).
We are grateful to Gerardo Mirkin for critical discussion of the experimental setting for the CD4+ and CD8+ T-cell immunodepletion studies.
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 29 October 2007.
REFERENCES
- 1.Aliberti, J. C., J. T. Souto, A. P. Marino, J. Lannes-Vieira, M. M. Teixeira, J. Farber, R. T. Gazzinelli, and J. S. Silva. 2001. Modulation of chemokine production and inflammatory responses in interferon-gamma- and tumor necrosis factor-R1-deficient mice during Trypanosoma cruzi infection. Am. J. Pathol. 581433-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araujo, A. F., B. C. de Alentar, J. R. Vasconcelos, M. I. Hiyane, C. R. Marinho, M. L. Penido, S. B Boscardin, D. F. Hoft, R. T. Gazzinelli, and M. M. Rodrigues. 2005. CD8+-T-cell-dependent control of Trypanosoma cruzi infection in a highly susceptible mouse strain after immunization with recombinant proteins based on amastigote surface protein 2. Infect. Immun. 736017-6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bellotti, G., E. A. Bocchi, A. V. de Moraes, M. L. Higuchi, M. Barbero-Marcial, E. Sosa, A. Esteves-Filho, R. Kalil, R. Weiss, A. Jatene, and F. Pileggi. 1996. In vivo detection of Trypanosoma cruzi antigens in hearts of patients with chronic Chagas' heart disease. Am. Heart J. 131301-307. [DOI] [PubMed] [Google Scholar]
- 4.Beyer, T., M. Hermann, C. Reiser, W. Bertling, and J. Hess. 2001. Bacterial carriers and virus-like-particles as antigen delivery device: role of dendritic cells in antigen presentation. Curr. Drug Targets Infect. Disord. 1287-302. [DOI] [PubMed] [Google Scholar]
- 5.Bontempi, E., and J. J. Cazzulo. 1990. Digestion of human immunoglobulin G by the major cysteine proteinase (cruzipain) from Trypanosoma cruzi. FEMS Microbiol. Lett. 58337-341. [DOI] [PubMed] [Google Scholar]
- 6.Borsutzky, S., K. Kretschmer, P. D. Becker, P. F. Muhlradt, C. J. Kirschning, S. Weiss, and C. A. Guzman. 2005. The mucosal adjuvant macrophage-activating lipopeptide-2 directly stimulates B lymphocytes via the TLR2 without the need of accessory cells. J. Immunol. 1746308-6313. [DOI] [PubMed] [Google Scholar]
- 7.Borsutzky, S., V. Fiorelli, T. Ebensen, A. Tripiciano, F. Rharbaoui, A. Scoglio, C. Link, F. Nappi, M. Morr, S. Butto, A. Cafaro, P. F. Muhlradt, B. Ensoli, and C. A. Guzman. 2003. Efficient mucosal delivery of the HIV-1 Tat protein using the synthetic lipopeptide MALP-2 as adjuvant. Eur. J. Immunol. 331548-1556. [DOI] [PubMed] [Google Scholar]
- 8.Brandariz, S., A. Schijman, C. Vigliano, P. Arteman, R. Viotti, C. Beldjord, and M. J. Levin. 1995. Detection of parasite DNA in Chagas' heart disease. Lancet 3461370-1371. [DOI] [PubMed] [Google Scholar]
- 9.Campetella, O., J. Henriksson, L. Aslund, A. C. Frasch, U. Pettersson, and J. J. Cazzulo. 1992. The major cysteine proteinase (cruzipain) from Trypanosoma cruzi is encoded by multiple polymorphic tandemly organized genes located on different chromosomes. Mol. Biochem. Parasitol. 50225-234. [DOI] [PubMed] [Google Scholar]
- 10.Carbonetto, C. H., E. L. Malchiodi, M. Chiaramonte, E. L. Durante de Isola, C. A Fossati, and R. A. Margni. 1990. Isolation of a Trypanosoma cruzi antigen by affinity chromatography with a monoclonal antibody. Preliminary evaluation of its possible applications in serological tests. Clin. Exp. Immunol. 8293-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chu, R. S., O. S. Targoni, A. M. Krieg, P. V. Lehmann, and C. V. Harding. 1997. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J. Exp. Med. 1861623-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Darji, A., C. A. Guzman, B. Gerstel, P. Wachholz, K. N. Timmis, J. Wehland, T. Chakraborty, and S. Weiss. 1997. Oral somatic transgene vaccination using attenuated Salmonella typhimurium. Cell 91765-775. [DOI] [PubMed] [Google Scholar]
- 13.Darji, A., S. zur Lage, A. I. Garbe, T. Chakraborty, and S. Weiss. 2000. Oral delivery of DNA vaccines using attenuated Salmonella typhimurium as carrier. FEMS Immunol. Med. Microbiol. 27341-349. [DOI] [PubMed] [Google Scholar]
- 14.Dietrich, G., S. Spreng, D. Favre, J. F. Viret, and C. A. Guzman. 2003. Live attenuated bacteria as vectors to deliver plasmid DNA vaccines. Curr. Opin. Mol. Ther. 510-19. [PubMed] [Google Scholar]
- 15.Ebensen, T., S. Paukner, C. Link, P. Kudela, C. de Domenico, W. Lubitz, and C. A. Guzman. 2004. Bacterial ghosts are an efficient delivery system for DNA vaccines. J. Immunol. 1726858-6865. [DOI] [PubMed] [Google Scholar]
- 16.Fralish, B. H., and R. L. Tarleton. 2003. Genetic immunization with LYT1 or a pool of trans-sialidase genes protects mice from lethal Trypanosoma cruzi infection. Vaccine 213070-3080. [DOI] [PubMed] [Google Scholar]
- 17.Frank, F. M., P. B. Petray, S. I. Cazorla, M. C. Muñoz, R. S. Corral, and E. L. Malchiodi. 2003. Use of a purified Trypanosoma cruzi antigen and CpG oligodeoxynucleotides for immunoprotection against a lethal challenge with trypomastigotes. Vaccine 2277-86. [DOI] [PubMed] [Google Scholar]
- 18.Garg, N., and R. L. Tarleton. 2002. Genetic immunization elicits antigen-specific protective immune responses and decreases disease severity in Trypanosoma cruzi infection. Infect. Immun. 705547-5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garmony, H. S., R. W. Titball, K. A. Brown, and A. M. Benett. 2002. Construction and evaluation of eukariotic expression plasmid for stable delivery using attenuated Salmonella. Microb. Pathogen. 34115-119. [DOI] [PubMed] [Google Scholar]
- 20.Garmory, H. S., K. F. Griffin, K. A. Brown, and R. W. Titball. 2003. Oral immunisation with live aroA attenuated Salmonella enterica serovar Typhimurium expressing the Yersinia pestis V antigen protects mice against plague. Vaccine 213051-3057. [DOI] [PubMed] [Google Scholar]
- 21.Gazzinelli, R. T., I. P. Oswald, S. Hieny, S. L. James, and A. Sher. 1992. The microbicidal activity of IFN-γ-treated macrophages against Trypanosoma cruzi involves an L-arginine-dependent, NO-mediated mechanism inhibitable by IL-10 and TGF-β. Eur. J. Immunol. 222501-2506. [DOI] [PubMed] [Google Scholar]
- 22.Gentschev, I., J. Fensterle, A. Schmidt, T. Potapenko, J. Troppmair, W. Goebel, and U. R. Rapp. 2005. Use of a recombinant Salmonella enterica serovar Typhimurium strain expressing C-Raf for protection against C-Raf induced lung adenoma in mice. BMC Cancer 515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guzman-Bracho, C. 2001. Epidemiology of Chagas disease in Mexico: an update. Trends Parasitol. 17372-376. [DOI] [PubMed] [Google Scholar]
- 24.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408740-745. [DOI] [PubMed] [Google Scholar]
- 25.Hoiseth, S. K., and B. A. Stocker. 1981. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature 291238-239. [DOI] [PubMed] [Google Scholar]
- 26.Krautz, G. M., J. C. Kissinger, and A. U. Krettli. 2000. The targets of the lytic antibody response against Trypanosoma cruzi. Parasitol. Today 1631-34. [DOI] [PubMed] [Google Scholar]
- 27.Krieg, A. M., A. K. Yi., S. Matson, T. J. Waldschmidt, G. A. Bishop, R. Teasdale, G. A. Koretzky, and D. M. Klinman. 1995. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 374546-549. [DOI] [PubMed] [Google Scholar]
- 28.Kwon, Y. M., M. M. Cox, and L. N. Calhoun. 2007. Salmonella-based vaccines for infectious diseases. Expert. Rev. Vaccines 6147-152. [DOI] [PubMed] [Google Scholar]
- 29.Laguens, R. P., P. M. Cossio, C. Diez, A. Segal, C. Vasquez, E. Kreutzer, E. Khoury, and R. M. Arana. 1975. Immunopathologic and morphologic studies of skeletal muscle in Chagas' disease. Am. J. Pathol. 80153-162. [PMC free article] [PubMed] [Google Scholar]
- 30.Lane, J. E., D. Olivares-Villagomez, C. L. Vnencak-Jones, T. L. McCurley, and C. E. Carter. 1997. Detection of Trypanosoma cruzi with the polymerase chain reaction and in situ hybridization in infected murine cardiac tissue. Am. J. Trop. Med. Hyg. 5588-595. [DOI] [PubMed] [Google Scholar]
- 31.Laucella, S. A., M. Postan, D. Martin, B. Hubby Fralish, M. C. Albareda, M. G. Alvarez, B. Lococo, G. Barbieri, R. J. Viotti, and R. L. Tarleton. 2004. Frequency of IFN-γ-producing T cells specific for Trypanosoma cruzi inversely correlates with disease severity in chronic human Chagas disease. J. Infect. Dis. 189909-918. [DOI] [PubMed] [Google Scholar]
- 32.Loise, S., C. Le Gall, L. Doucet, C. Ferec, and V. Floch. 2001. Contribution of plasmid DNA to hepatotoxicity after systemic administration of lipoplexes. Hum. Gene Ther. 12685-696. [DOI] [PubMed] [Google Scholar]
- 33.Low, H. P., M. A. Santos, B. Wizel, and R. L. Tarleton. 1998. Amastigote surface proteins of Trypanosoma cruzi are targets for CD8+ CTL. J. Immunol. 1601817-1823. [PubMed] [Google Scholar]
- 34.Malchiodi, E. L., M. G. Chiaramonte, N. J. Taranto, N. W. Zwirner, and R. A. Margni. 1994. Cross reactivity studies and differential serodiagnosis of Trypanosoma cruzi and Leishmania spp. human infection. Use of immunoblotting and ELISA with a purified antigen (Ag 163B6). Clin. Exp. Immunol. 97417-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mirkin, G. A., A. M. Celentano, E. L. Malchiodi, M. Jones, and S. M. Gonzalez Cappa. 1997. Different Trypanosoma cruzi strains promote neuromyopathic damage mediated by distinct T lymphocyte subsets. Clin. Exp. Immunol. 107328-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Motameni, A. R., I. J. Juncadella, S. K. Ananthanarayanan, M. N. Hedrick, Y. Huet-Hudson, and J. Anguita. 2004. Delivery of the immunosuppressive antigen Salp15 to antigen-presenting cells by Salmonella enterica serovar Typhimurium aroA mutants. Infect. Immun. 723638-3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mühlradt, P. F., M. Kiess, H. Meyer, R. Süssmuth, and G. Jung. 1997. Isolation, structure elucidation, and synthesis of a macrophage stimulatory lipopeptide from Mycoplasma fermentans acting at picomolar concentration. J. Exp. Med. 1851951-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paglia, P., E. Medina, I. Arioli, C. A. Guzman, and M. P. Colombo. 1998. Gene transfer in dendritic cells, induced by oral DNA vaccination with Salmonella typhimurium, results in protective immunity against a murine fibrosarcoma. Blood 923172-3176. [PubMed] [Google Scholar]
- 39.Paglia, P., N. Terrazzini, K. Schulze, C. A. Guzman, and M. P. Colombo. 2000. In vivo correction of genetic defects of monocyte/macrophages using attenuated Salmonella as oral vectors for targeted gene delivery. Gene Ther. 71725-1730. [DOI] [PubMed] [Google Scholar]
- 40.Parussini, F., V. G. Duschak., and J. J. Cazzulo. 1998. Membrane-bound cysteine proteinase isoforms in different developmental stages of Trypanosoma cruzi. Cell. Mol. Biol. (Noisy-le-grand) 44513-519. [PubMed] [Google Scholar]
- 41.Rharbaoui, F., B. Drabner, S. Borsutzky, U. Winckler, M. Morr, B. Ensoli, P. F. Muhlradt, and C. A. Guzman. 2002. The Mycoplasma-derived lipopeptide MALP-2 is a potent mucosal adjuvant. Eur. J. Immunol. 322857-2865. [DOI] [PubMed] [Google Scholar]
- 42.Roman, M., E. Martin-Orozco, J. S. Goodman, M. D. Nguyen, Y. Sato, A. Ronaghy, R. S. Kornbluth, D. D. Richman, D. A. Carson, and E. Raz. 1997. Immunostimulatory DNA sequences function as T helper-1-promoting adjuvants. Nat. Med. 3829-831. [DOI] [PubMed] [Google Scholar]
- 43.Sardinha, L. R., R. M. Elias, T. Mosca, K. R. Bastos, C. R. Marinho, M. R. D'Imperio Lima, and J. M. Alvarez. 2006. Contribution of NK, NK T, γδ T, and αβ T cells to the gamma interferon response required for liver protection against Trypanosoma cruzi. Infect. Immun. 742031-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schnapp, A. R., C. S. Eickhoff, J. Scharfstein, and D. F. Hoft. 2002. Induction of B- and T-cell responses to cruzipain in the murine model of Trypanosoma cruzi infection. Microbes Infect. 4805-813. [DOI] [PubMed] [Google Scholar]
- 45.Schnapp, A. R., C. S. Eickhoff, D. Sizemore, R. Curtiss III, and D. F. Hoft. 2002. Cruzipain induces both mucosal and systemic protection against Trypanosoma cruzi in mice. Infect. Immun. 705065-5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Souto-Padron, T., O. Campetella, J. J. Cazzulo, and W. De Souza. 1990. Cysteine proteinases in T. cruzi immunocytochemical localization and involvement in parasite-host cell interaction. J. Cell Sci. 96485-490. [DOI] [PubMed] [Google Scholar]
- 47.Stober, C. B., U. G. Lange, M. T. Roberts, A. Alcami, and J. M. Blackwell. 2005. IL-10 from regulatory T cells determines vaccine efficacy in murine Leishmania major infection. J. Immunol. 1752517-2524. [DOI] [PubMed] [Google Scholar]
- 48.Tarleton, R. L., and L. Zhang. 1999. Chagas disease etiology: autoimmunity or parasite persistence? Parasitol. Today 1594-99. [DOI] [PubMed] [Google Scholar]
- 49.Tarleton, R. L., B. H. Koller, A. Latour, and M. Postan. 1992. Susceptibility of beta 2-microglobulin-deficient mice to Trypanosoma cruzi infection. Nature 356338-340. [DOI] [PubMed] [Google Scholar]
- 50.Tarleton, R. L., L. Zhang, and M. O. Downs. 1997. “Autoimmune rejection” of neonatal heart transplants in experimental Chagas' disease is a parasite-specific response to infected host tissue. Proc. Natl. Acad. Sci. USA 943932-3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.WHO. 1999. Twelfth programme report of the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Disease (TDR). World Health Organization, Geneva, Switzerland.
- 52.Wizel, B., M. Nunes, and R. L. Tarleton. 1997. Identification of Trypanosoma cruzi trans-sialidase family members as targets of protective CD8+ TC1 responses. J. Immunol. 1596120-6130. [PubMed] [Google Scholar]
- 53.Wizel, B., N. Garg, and R. L. Tarleton. 1998. Vaccination with trypomastigote surface antigen 1-encoding plasmid DNA confers protection against lethal Trypanosoma cruzi infection. Infect. Immun. 665073-5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang, X. L., W. C. Liu, W. W. Yang, D. Zhong, Y. H. Liu, J. D. Zhang, J. H. Jiang, and S. S. Li. 2005. Oral immunization of mice with vaccine of attenuated Salmonella typhimurium expressing Helicobacter pylori urease B subunit. Biomed. Environ. Sci. 18411-418. [PubMed] [Google Scholar]