Abstract
Tannerella forsythia is a gram-negative anaerobe strongly associated with chronic human periodontitis. This bacterium expresses a cell surface-associated and secreted protein, designated BspA, which has been recognized as an important virulence factor. The BspA protein belongs to the leucine-rich repeat (LRR) and bacterial immunoglobulin-like protein families. BspA is, moreover, a multifunctional protein which interacts with a variety of host cells, including monocytes which appear to respond to BspA through Toll-like receptor (TLR) signaling. Since gingival epithelium forms a barrier against periodontal pathogens, this study was undertaken to determine if gingival epithelial cells respond to BspA challenge and if TLRs play any role in BspA recognition. This study was also directed towards identifying the BspA domains responsible for cellular activation. We provide direct evidence for BspA binding to TLR2 and demonstrate that the release of the chemokine interleukin-8 from human gingival epithelial cells by BspA is TLR2 dependent. Furthermore, the LRR domain of BspA is involved in activation of TLR2, while TLR1 serves as a signaling partner. Thus, our findings suggest that BspA is an important modulator of host innate immune responses through activation of TLR2 in cooperation with TLR1.
Tannerella forsythia is a gram-negative rod-shaped anaerobic periodontal pathogen implicated in periodontal diseases characterized by inflammatory destruction of the tooth-supporting tissue and subsequent tooth loss if they are untreated (28, 31). Although periodontal diseases are multifactorial, the primary etiology involves the presence of subgingival bacterial plaque or biofilm in the oral cavity which triggers host inflammatory responses. Among the bacterial species implicated in the progression of periodontal disease, such as Porphyromonas gingivalis and Treponema denticola, accumulated evidence indicates that T. forsythia is a major etiologic agent of chronic periodontitis (28, 30, 32). In addition, several recent reports have implicated T. forsythia in atherosclerosis (4, 21, 23). Despite accumulating evidence for the correlation between T. forsythia and diseases, few studies have addressed the pathogenic mechanisms of T. forsythia, probably because of the fastidious growth requirements of this organism and difficulties associated with its genetic manipulation.
We previously identified a cell surface-associated and secreted protein with pathogenic attributes, designated BspA, from T. forsythia (26). The BspA protein is characterized by the presence of two leucine-rich repeat (LRR) domains separated by a short segment in the N-terminal region of the molecule. The LRR domains are thought to function in binding to other proteins via protein-protein interactions (16, 17). In addition, the BspA protein contains two tandem immunoglobulin (Ig)-like domains in the C-terminal region. Recent studies in our laboratory have suggested multiple virulence functions for BspA which are likely to play significant roles in periodontitis. In this regard, in vitro studies have shown that BspA triggers release of proinflammatory cytokines from human monocytic THP-1 cells (6), as well as release of chemokines from murine osteoblasts (24), and BspA is required for uptake of T. forsythia by epithelial cells in vitro (12). The bacterial uptake is dependent upon host protein phosphorylation in response to interactions of BspA with a cellular receptor(s). Additionally, we have shown that periodontitis patients harboring T. forsythia elicit BspA-specific serum antibodies (26) and that BspA expression is required for alveolar bone loss in mice, indicating an in vivo role for BspA as well (25). Further, analysis of the recently completed draft sequence of the T. forsythia genome deposited in the Los Alamos oral pathogen database (www.oralgen.lanl.gov) has indicated the presence of multiple BspA-like homolog proteins in T. forsythia.
Toll-like receptors (TLRs) recognize a variety of microbe-associated molecular patterns and trigger innate immunity, and they also play an instructive role in the induction of the adaptive immunity (29). In general, different TLRs recognize distinct microbial structures. For example, TLR2 responds to lipid moieties and peptides, TLR4 responds to lipopolysaccharide (LPS), and TLR5 responds to flagellin. In this regard, T. forsythia and other gram-negative periodontal bacteria have been shown to activate TLR2 (15). Antibody inhibition data obtained by our group have suggested that TLR2 may be involved in BspA-induced release of proinflammatory cytokines by the human monocytic cell line THP-1 (6). This may represent a mechanism by which T. forsythia induces periodontal inflammation. Although macrophages are considered to be key players in innate immunity, since they are phagocytic cells as well as targets for activation by T cells, epithelial cells also play essential roles in providing innate defense against microbial challenge through the production of antimicrobial molecules, as well as cytokines and chemokines necessary for leukocyte recruitment (14). The overall objectives of this study were to determine if BspA is involved in epithelial cell activation leading to chemokine secretion and to elucidate the mechanism involved in this activation. Importantly, we were interested in determining the role of TLRs in BspA-mediated activation of epithelial cells. Our findings show that the BspA LRR domains are involved in the activation of epithelial cells via TLR2 binding. In addition, we demonstrated that TLR1 cooperates with TLR2 in the activation of epithelial cells in response to BspA.
MATERIALS AND METHODS
Reagents.
Zymosan (yeast cell wall particle), Pam3Cys, and highly purified LPS from Escherichia coli K-12 were purchased from InvivoGen (San Diego, CA). HEK293 (hTLR2/hTLR1) cells stably expressing human TLR2 and human TLR1 were purchased from InvivoGen. Anti-TLR2 monoclonal antibody (MAb) and isotype control MAb (IgG2a) were obtained from BioLegend (San Diego, CA). Polymyxin B sulfate was purchased from Sigma (St. Louis, MO).
Bacteria and rBspA.
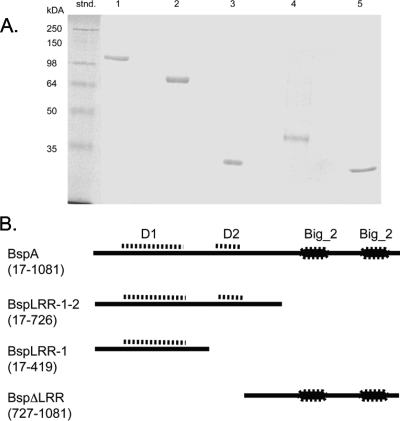
T. forsythia parent strain ATCC 47037 and a BspA-defective mutant of this strain were grown using previously described culture conditions (11), Briefly, T. forsythia ATCC 43037 was grown anaerobically (5% CO2, 10% H2, 85% N2) in brain heart infusion (Difco Laboratories, Detroit, MI) broth containing 5 μg/ml hemin, 0.5 μg/ml menadione, 0.001% N-acetylmuramic acid, and 5% fetal bovine serum (Life Technologies, Grand Island, NY). The BspA-deficient mutant BFM571 (11) was grown anaerobically in the same medium containing 5 μg/ml tetracycline. The full-length and truncated polypeptides of BspA were expressed as His-tagged fusion proteins in E. coli utilizing the pQE80 expression plasmid system (Qiagen, Valencia, CA). The recombinant polypeptides included (Fig. 1A) recombinant BspA (rBspA) (residues 17 to 1081 of the mature protein), rBspLRR-1-2 (residues 17 to 726, including both LRR domains, domains D1 and D2), rBspLRR-1 (residues 17 to 419, including only the D1 domain), and rBspΔLRR (residues 727 to 1081, containing only the C-terminal Big_2 region). The DNA fragments encoding these recombinant polypeptides were amplified from T. forsythia genomic DNA using forward primer 5′-GCGCGGATCCTTGACGACCCTGGGCGCTACGGC-3′ and reverse primer 5′-GCCCAAGCTTTCACTTTATAAGAATTTTGGTTACCCG-3′ (rBspA) or the same forward primer and either reverse primer 5′-GCCCAAGCTTTCAGGCGTCAACGAAGGAGAGC-3′ (rBspLRR-1-2) or reverse primer 5′-GCCCAAGCTTTCATACGGTCACGTCCTT-3′ (rBspLRR-1). The DNA fragment encoding the rBspΔLRR polypeptide was amplified with forward primer 5′-GCGCGGATCCGGCGGAACGAAGCCGATCAC-3′ and reverse primer 5′-GCCCAAGCTTTCACTTTATAAGAATTTTGGTTACCCG-3′. The underlined residues indicate a BamHI restriction site and a HindIII restriction site in the forward and reverse primers, respectively. The amplified DNA fragments were cloned into the BamH-HindIII site of the pQE80 plasmid vector, and sequences of the inserts were confirmed by DNA sequencing at the DNA Core Facility at Roswell Park Cancer Institute (Buffalo, NY) to confirm correct in-frame cloning. All plasmids were introduced into E. coli BL21 (Novagen, Madison, WI). Expression of recombinant polypeptides was induced with isopropyl-β-d-thiogalactoside, and the recombinant BspA polypeptides were purified from the soluble extracts of E. coli by affinity chromatography using a His·Bind resin column (Novagen). The purity of the recombinant proteins was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. All protein preparations were also analyzed by quantitative Limulus amebocyte lysate assays (using kits obtained from BioWhittaker, Walkersville, MD, or from Charles River Endosafe, Charleston, SC) to measure incidental endotoxin contamination. All polypeptide preparations were essentially free of LPS (≤0.0064 ng/μg of protein). A His-glutathione S-transferase fusion protein (His-GST) was likewise expressed and purified, and it was used as a negative control in epithelial cell activation assays. The purity of recombinant proteins was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Fig. 1B) and quantitative Limulus amebocyte lysate assays (using kits obtained from BioWhittaker, Walkersville, Md., or Charles River Endosafe, Charleston, SC) to determine endotoxic activity. All BspA preparations were essentially free of incidental LPS contamination (≤0.0064 ng/μg of protein). All recombinant BspA polypeptides were treated further with polymyxin B (10 mg/ml) to block any undetectable LPS in the preparation. Preliminary experiments showed that there was no significant cytokine induction with polymyxin B at a concentration of 10 μg/ml compared to a no-stimulation control in human gingival epithelial cells (HGECs) or even in primary monocytic cells (data not shown).
FIG. 1.
(A) Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of rBspA and its derivatives. Lane stnd, protein marker; lane 1, rBspA; lane 2, rBspLRR-1-2; lane 3, rBspLRR-1; lane 4, rBspΔLRR; lane 5, His-GST. The numbers on the left indicate the molecular masses of protein standards. (B) Schematic diagrams of BspA and its truncated derivatives expressed as recombinant proteins. The amino acid residues for each derivative are indicated in parentheses. Abbreviations: D1, LRR domain D1 containing 14 tandem LRRs (residues 91 to 412); D2, LRR domain D2 containing six tandem LRRs; Big_2, bacterial Ig-like domains.
TLR expression plasmids.
Expression plasmids containing human TLR-1, -2, -4, and -6 and empty plasmids (pFLAG-CMV) were kindly provided by R. I. Tapping (University of Illinois). Reporter plasmids containing cDNA (Rluc) encoding Renilla luciferase (pRL-TK) and firefly luciferase (pGL4) were obtained from Promega Corporation (Madison, WI).
Transient transfection and luciferase assay.
HEK293 cells were seeded at a concentration of 6 × 104 cells per well in a 48-well plate. After incubation until they reached about 60% confluence, HEK293 cells were transiently transfected with the indicated TLR plasmids and simultaneously cotransfected with a firefly luciferase gene conjugated with the NF-κB promoter as a reporter gene and pRL-TK containing a Renilla luciferase gene as an internal control (Promega, Madison, WI). Transfection was performed using the FuGENE 6 transfection reagent at a reagent-to-DNA ratio of 3:1 (vol/wt) (Roche Diagnostics, Indianapolis, IN) according to the manufacturer's instructions. The total amount of transfected plasmid DNA was equalized by supplementation with an empty vector, pFLAG-CMV. Polymyxin B sulfate (10 μg/ml) was added to each recombinant protein prior to stimulation to block any contaminating LPS in the preparation. At 24 h posttransfection, the cells were stimulated with the indicated reagents for 6 h, and cells were collected for further analysis. Firefly luciferase and Renilla luciferase activities were determined using dual-luciferase reporter assay systems (Promega) according to the manufacturer's instructions. The firefly luciferase activity was normalized to that of Renilla luciferase to correct the transfection efficiency and cell viability.
BspA binding to TLR2.
TLR2 binding was measured as previously described (18). Briefly, 96-well microtiter wells were coated overnight at 4°C with 20 μg/ml of purified recombinant mouse TLR2 (R&D Systems, Minneapolis, MN). After washing and blocking of nonspecific binding sites, biotinylated BspA (2.5 to 40 μg/ml in phosphate-buffered saline [PBS] containing 1% bovine serum albumin) was incubated for 2 h at 37°C. After washing, bound protein was detected with peroxidase-conjugated streptavidin. Alternatively, nonbiotinylated BspA was used, and bound protein was detected with specific IgG anti-BspA antibody followed by peroxidase-conjugated goat anti-rabbit IgG (adsorbed against human or mouse IgG). The peroxidase reaction was performed using the 3,3′,5,5′-tetramethylbenzidene chromogenic substrate, and the optical density at 450 nm was determined with a microplate reader (Bio-Tek Instruments, Winooski, VT). The dissociation constant (Kd) was calculated using GraphPad Prism-4 software based on the law of mass action, according to which the Kd (in moles per liter) equals the concentration of ligand which occupies one-half of the receptors at equilibrium.
Epithelial cells.
Following approval by the University of Louisville Institutional Review Board, primary HGECs were obtained from healthy patients after third-molar extraction as described previously (3). Briefly, the HGECs were grown in serum-free keratinocyte medium (Invitrogen, Carlsbad, CA) containing 10 μg/ml of insulin, 5 μg/ml of transferrin, 10 μM 2-mercaptoethanol, 10 μM 2-aminoethanol, 10 nM sodium selenite, 50 μg/ml of bovine pituitary extract, 100 U/ml of penicillin/streptomycin, and 50 ng/ml of amphotericin B (complete medium). The cells were seeded in 60-mm-diameter plastic tissue culture dishes coated with type I collagen and incubated in 5% CO2-95% air at 37°C. When the cells reached 80% confluence, they were harvested and subcultured in complete medium. HEK293 cells were cultured in Dulbecco's modified Eagle's medium (Mediatech Inc.) supplemented with 10% fetal bovine serum (Gibco, Carlsbad, CA) and 1% Antibiotic-Antimycotic (Gibco). HEK293 (hTLR2/hTLR1) cells stably expressing human TLR2 and human TLR1 were purchased from InvivoGen (San Diego, CA). These cells were cultured in normal growth medium supplemented with 0.1% blasticidin (InvivoGen).
Cytokine ELISA.
HGECs were plated on 96-well plates at a concentration of 2 × 105 cells per well containing 100 μl of normal growth medium. The cells were grown for 4 days to obtain confluence prior to challenge. At 24 h prior to challenge, the cells were washed with PBS and 100 μl of fresh normal growth medium was added to the wells. Cells were preincubated with anti-TLR2 MAb (TL2.1) or an Ig isotype-matched control (IgG2a) for 30 min and then incubated with Pam3Cys or BspA for 16 h at 37°C in humidified air containing 5% (vol/vol) CO2. HEK293 (hTLR2/hTLR1) or HEK293 cells were plated on 24-well plates at a concentration of 1 × 105 cells per well in 500 μl of normal growth medium. The cells were grown to confluence and then washed with PBS, and 500 μl of fresh Dulbecco's modified Eagle's medium with 2.5% fetal bovine serum was added to the wells. Cells were treated with Pam3Cys or rBspA for 16 h. The culture supernatants were collected, clarified by centrifugation, diluted 1:50 or 1:100, and stored at −70°C until they were assayed. The culture supernatants were assayed for interleukin-1β (IL-1β) and IL-6 (eBioscience, San Diego, CA) and for IL-8 (Pelikine, Sanquin, Amsterdam, The Netherlands) by performing enzyme-linked immunosorbent assays (ELISAs) as directed by the manufacturer.
RESULTS
BspA activates HGECs.
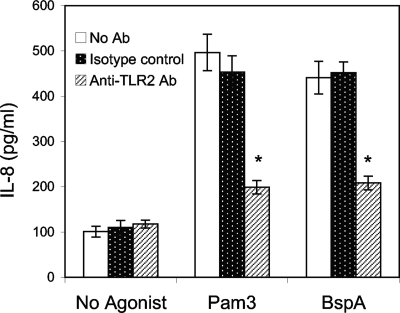
Our previous studies have shown that the BspA protein induces secretion of IL-1β and tumor necrosis factor alpha from the human monocytic THP-1 cell line (6). In this study we found that BspA also induces secretion of the proinflammatory cytokines IL-1β and IL-6 from HGECs (Table 1). The HGECs can also play roles in defense against periodontal bacteria by secreting chemokines such as IL-8, leading to neutrophil migration and bacterial killing. The HGECs express TLR1, -2, -4, and -6 but not CD14, which can function as a TLR coreceptor (3). Therefore, we sought to determine whether HGECs respond to the BspA protein through TLR activation, leading to chemokine secretion. For this purpose, HGECs were challenged with serum-free keratinocyte medium containing rBspA protein, and the IL-8 response was determined. The results showed that the rBspA protein significantly upregulated IL-8 secretion in HGECs compared to medium alone (Fig. 2). As expected, cells challenged with the control TLR2 agonist Pam3Cys (a TLR2/TLR1 agonist) also responded by secreting IL-8. Further, anti-TLR2 MAb, but not isotype control Ig, significantly inhibited IL-8 release by either the BspA protein or Pam3Cys (Fig. 2).
TABLE 1.
BspA stimulates proinflammatory cytokine expression in HGECs
| Agonist | Concn (μg/ml) | IL-1β concn (pg/ml)a | IL-6 concn (pg/ml)a |
|---|---|---|---|
| None | 23.56 ± 4.5 | 50.23 ± 5.21 | |
| Pam3Cys | 0.5 | 87.23 ± 7.98b | 175.54 ± 15.23b |
| Zymosan | 10 | 110.23 ± 10.56b | 220.23 ± 10.23b |
| BspA | 10 | 80.23 ± 6.56b | 168.56 ± 12.56b |
The data are the means ± standard deviations for triplicate determinations in one of two independent sets of experiments that yielded similar results.
There was statistically significant (P < 0.05) induction of the cytokine compared to the no-agonist control.
FIG. 2.
IL-8 induction in epithelial cells challenged with the BspA protein. HGECs were treated with rBspA (5 μg/ml) or the TLR2 agonist Pam3Cys (Pam3) (0.1 μg/ml) for 6 h. Induction of IL-8 in culture supernatants was determined by an ELISA. The data are the means ± standard deviations of triplicate determinations in one of three independent sets of experiments that yielded similar findings. Statistically significant (P < 0.05) blocking of cytokine release by TLR2 antibody (Ab) is indicated by an asterisk.
BspA activates epithelial cells through TLR2 in cooperation with TLR1 but not through TLR2 in cooperation with TLR6.
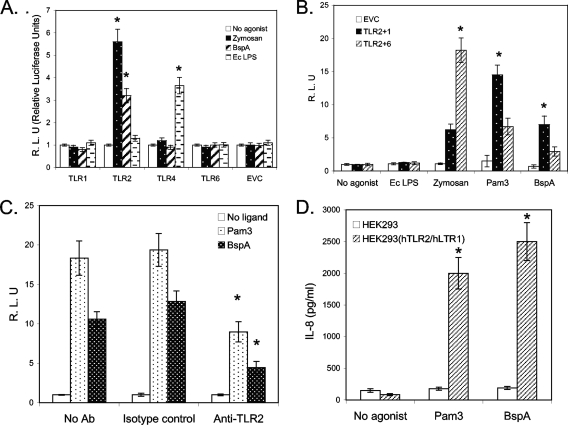
In order to conclusively establish the role of TLRs in BspA-induced activation of epithelial cells, we determined the ability of the BspA protein to activate NF-κB in human embryonic kidney (HEK293) cells transiently expressing different TLRs alone or in combination. Briefly, HEK293 cells were transiently transfected with a TLR1, -2, -4, or -6-expressing plasmid along with an NF-κB promoter-conjugated luciferase reporter plasmid. Transfected HEK293 cells were then used to determine the abilities of zymosan (a TLR2 agonist), highly purified E. coli LPS (a TLR4 agonist), or BspA to induce NF-κB activation. HEK293 cells transfected with pCMV4 alone were used as a control. Both zymosan and BspA activated NF-κB in HEK293 cells expressing TLR2 but not in HEK293 cells expressing other TLRs (Fig. 3A). As expected, E. coli LPS activated NF-κB in cells expressing TLR4 and not other TLRs (Fig. 3A), and treatment of E. coli LPS (1 μg/ml) with polymyxin B (10 μg/ml) blocked NF-κB induction in TLR4-expressing cells (data not shown). Since rBspA preparations used in this study were treated with polymyxin B, the results confirm that the responses obtained with rBspA preparations were not due to residual LPS in the preparations that might have escaped detection.
FIG. 3.
(A) TLR2 and TLR1 recognize BspA. HEK293 cells were transiently transfected with plasmids expressing the indicated human TLRs or an empty vector (EVC). Cells were incubated for 6 h with zymosan (10 μg/ml), BspA (5 μg/ml), highly purified E. coli LPS (Ec LPS) (1 μg/ml), or no agonist. NF-κB activity in supernatants was quantified by the luciferase reporter assay. (B) NF-κB is activated by BspA through TLR2 and TLR1 but not through TLR2 and TLR6. Cells were transiently transfected with the TLR2 and TLR1 plasmids or with the TLR2 and TLR6 plasmids and incubated for 6 h with the indicated reagents. The data are the averages ± standard deviations for triplicate wells, normalized to the unstimulated cells for each TLR combination. Statistically significant cellular activation (P < 0.05) compared with the corresponding unstimulated control (A) or a statistically significant difference between TLR2/TLR1 and TLR2/TLR6 (B) as calculated by Student's t test is indicated by an asterisk. Pam3, Pam3Cys. (C) Anti-human TLR2 antibody (Ab) inhibited BspA-mediated activation of NF-κB activity in HEK293 cells stably expressing human TLR2 and human TLR1. HEK293 (hTLR2/hTLR1) stable cells were transiently transfected with reporter plasmids, the firefly luciferase gene conjugated with NF-κB promoters (NF-κB-luc), and Renilla luciferase genes (R-luc). Cells were preincubated with anti-TLR2 MAb (TL2.1; 5 mg/ml) or an Ig isotype-matched control (IgG2a; 5 mg/ml) for 30 min before stimulation with Pam3Cys (1 μg/ml) or BspA (5 μg/ml). After 6 h of stimulation, supernatants were collected, and the NF-κB activity in supernatants was quantified by a luciferase reporter assay. The data are the averages ± standard deviations for triplicate determinations. Statistically significant inhibition (P < 0.05) as calculated by Student's t test compared to the isotype control treatment is indicated by an asterisk. (D) HEK293 or HEK293 (hTLR2/hTLR1) cells were stimulated with BspA protein (5 μg/ml) for 16 h, and the amount of IL-8 released in the medium was determined by an ELISA. The data are the means ± standard deviations for triplicate determinations. Statistical significance (P < 0.05) as calculated by Student's t test is indicated by an asterisk.
A number of studies have demonstrated that TLR2 functionally cooperates with other TLRs (namely, TLR1 and TLR6) to recognize and discriminate the structural differences in ligands (1, 9, 22, 23). This specific feature of TLR2 prompted us to further examine which TLRs may cooperate with TLR2 in the epithelial cell response to BspA. Therefore, HEK293 cells were transiently transfected with TLR2 and TLR1 or with TLR2 and TLR6, and the ability of BspA to activate NF-κB was determined. The combination of TLR2 with TLR1, but not the combination of TLR2 with TLR6, was found to significantly enhance the response of HEK293 cells to BspA (Fig. 3B). NF-κB was not activated in response to rBspA by transfection with either TLR1 or TLR6 alone (Fig. 3A). Zymosan and Pam3Cys were used as positive control agonists for the TLR2/TLR6 and TLR/TLR1 receptor combinations, respectively. The significant response to BspA in cells transfected with TLR2 alone was likely due to heterodimerization of recombinantly expressed TLR2 with endogenous TLR1, which is present at low levels in HEK293 cells.
The involvement of TLR2/TLR1 coreceptors in cellular activation by BspA was further confirmed using HEK293 cells stably expressing TLR2/TLR1 coreceptors [HEK293 (hTLR2/hTLR1) cells]. The results showed that anti-TLR2 MAb significantly blocked BspA-induced NF-κB activation in HEK293 (hTLR2/hTLR1) cells compared to activation with BspA alone or with rBspA in the presence of an isotype control antibody (Fig. 3C). Furthermore, the BspA protein induced secretion of IL-8 from HEK293 (hTLR2/hTLR1) cells but not from HEK293 cells (Fig. 3D). Additionally, to determine the function of the BspA protein in the context of the whole bacterial cell, we compared the TLR2/TLR1 activation abilities of the wild-type T. forsythia strain and its BspA mutant. The wild-type T. forsythia strain induced IL-8 secretion in HEK293 (hTLR2/hTLR1) cells. A BspA-defective T. forsythia mutant (BFM571) was found to be significantly less potent (P < 0.05) in terms of the ability to induce IL-8 secretion in these cells at a multiplicity of infection (MOI) of 20:1. However, at a higher MOI, there was no significant difference between the ability of the wild type to induce IL-8 and the ability of the mutant to induce IL-8 (Table 2). This is not surprising since other surface molecules, including homologs of BspA encoded in the T. forsythia genome, are likely to also be involved in TLR2 induction and thus may have compensatory functions. Moreover, surface lipoproteins in T. forsythia have previously been shown to activate TLR2 (10). Our results suggest that at a low MOI (i.e., at early stages of infection), BspA could play a significant role in the induction of TLR2-mediated proinflammatory responses. Interestingly, a recent study showed that the expression of BspA in T. forsythia is induced severalfold in vivo (35). Thus, estimation of the TLR2 induction by in vitro-grown T. forsythia cells and comparison with a BspA mutant as described above are likely to underestimate the true potential of BspA in host inflammation in vivo.
TABLE 2.
Activation of TLR2 by T. forsythia parent strain ATCC 43037 and BspA mutant strain BFM571
| MOI | IL-8 secretion (pg/ml) in response toa:
|
|
|---|---|---|
| Parent strain | BspA mutant | |
| 20 | 1,680 ± 178 | 1,038 ± 110 |
| 200 | 2,897 ± 326 | 2,734 ± 301 |
Monolayers of stable HEK293 (hTLR2/hTLR1) cells were stimulated with T. forsythia parent strain ATCC 43037 or BspA mutant strain BFM571 at different MOIs. Culture supernatants were collected after 12 h and assayed for IL-8 by an ELISA. There was a significant difference (P < 0.05) in the IL-8 response between the parent and mutant strains at an MOI of 20, but there was not a significant difference at an MOI of 200.
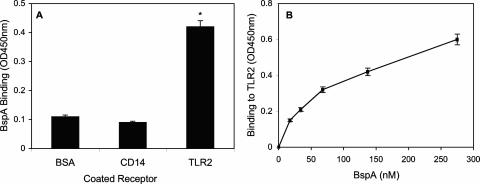
Direct binding of BspA to TLR2.
Consistent with its ability to activate TLR2-dependent cell activation, BspA was shown to bind to TLR2 (Fig. 4A). BspA could bind immobilized TLR2 even when the ligand was used in a nonbiotinylated form probed with specific antibody (not shown). However, BspA failed to bind CD14; indeed, the level of binding to CD14 was quite low and similar to the background binding determined using bovine serum albumin-coated wells (Fig. 4A). This finding is consistent with the ability of BspA to induce cell activation in a CD14-independent way, as observed in HGECs. Because recombinant TLR2 was expressed as a fusion protein with the Fc region of human IgG (R&D Systems), the lack of BspA binding to recombinant CD14 (which is similarly fused to the Fc region of human IgG) ensured that BspA did not bind IgG Fc. BspA binds TLR2 with a Kd of 1.6 × 10−7 M, calculated using data from a dose-response binding curve (Fig. 4B), as described in Materials and Methods.
FIG. 4.
BspA binds TLR2 but not CD14. (A) Binding of biotinylated BspA (10 μg/ml) to the indicated receptors was determined colorimetrically using receptor-coated microtiter wells probed with peroxidase-conjugated streptavidin. (B) Dose-response binding curve at the indicated concentrations of BspA. The data are means ± standard deviations of duplicate determinations in typical experiments, which were repeated three times with similar findings. BSA, bovine serum albumin.
LRR domain D1 of BspA activates TLR2.
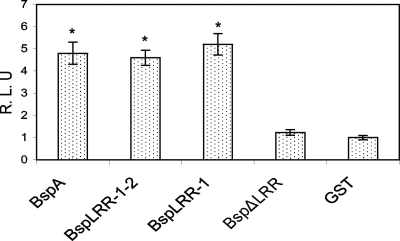
The predicted domain map of the BspA protein is shown in Fig. 1A. The BspA protein contains two LLR domains, domains D1 and D2, in the N-terminal region, as well as two adjacent bacterial Ig-like domains in the C-terminal portion. The D1 and D2 domains are composed of 14 and 6 tandem repeats of a 23-amino-acid LRR motif, respectively. Both LRR and Big_2 domains have been proposed to be involved in protein-protein interactions. Therefore, it was of great interest to identify which of the domain(s) is likely to be responsible for TLR2 activation. For this purpose, truncated polypeptide derivatives of the BspA protein were generated as His6-tagged fusion proteins, purified, and tested in in vitro assays for NF-κB activation in HEK293 (hTLR2/hTLR1) stable cells. We used a His-tagged glutathione S-transferase fusion protein (His-GST) as a negative control. The results showed that rBspA, rBspLRR-1-2, and rBspLRR-1 significantly upregulated NF-κB activation compared to His-induced activation. However, the LRR-deficient recombinant derivative rBspΔLRR failed to significantly induce NF-kB activation compared to the His-GST control (Fig. 5). These studies demonstrated that LRR domain D1 of BspA is the primary region involved in TLR2 activation.
FIG. 5.
BspA LRR domain D1 activates TLR2/TLR1. HEK293 cells cotransfected with human TLR2 and human TLR1 plasmids along with the reporter plasmid were incubated with rBspA, rBspLRR-1-2, rBspLRR-1, rBspΔLRR, or His-GST (negative control protein), each at a final concentration of 5 nM, for 6 h. The cells were harvested and lysed for further determination of NF-κB activity. The data are expressed in relative luciferase units and are averages ± standard deviations for triplicate determinations, normalized to His-GST-treated cells (control). A statistically significant difference (P < 0.05) compared to the rBspΔLRR or His-GST treatment as calculated by Student's t test is indicated by an asterisk.
DISCUSSION
Although T. forsythia is considered an important pathogen associated with the development of chronic periodontitis, its pathogenic abilities and its interactions with host response mechanisms have yet to be fully elucidated. We have identified a cell surface-associated and secreted virulence factor, the BspA protein, in T. forsythia (26) which may contribute to the pathogenesis of chronic periodontitis. The BspA protein belongs to the LRR and bacterial Ig-like (Big_2) protein families. Moreover, a recently assembled draft sequence of the genome of T. forsythia indicated that there are multiple BspA-like protein homologs in the bacterium. Therefore, the studies of the role of the BspA protein in inflammation mediated via the TLRs described here may shed light on the biological functions of its homologs as well. The BspA protein has been suggested to play roles in inflammation by triggering inflammatory responses in monocytes (6). Our previous studies utilizing TLR blocking antibodies suggested that TLR2 may be responsible for the inflammatory response induced by BspA in monocytes (6). In the present study we explored the role of BspA in epithelial cell activation through TLRs. Our results provide evidence that HGECs respond to the BspA protein by releasing IL-8, an important leukocyte-attracting chemokine. Moreover, we show that BspA-induced activation is mediated by TLR2 in cooperation with TLR1. This finding is consistent with our previous findings, based on antibody inhibition data, which suggested that TLR2 may be responsible for the inflammatory response induced by BspA in monocytes (6). We also demonstrated the direct binding and estimated the binding affinity of the BspA protein to TLR2 using a ligand binding assay. Since murine TLR2 was used in the ligand binding assays, it is important to note that the binding affinity of BspA for human TLR2, as well as the mechanism of binding, might be different. This is due to the fact that there are subtle differences in the ligand specificities of mouse and human TLR2 (5). However, a recent study involving crystal analyses of human and mouse TLR2 molecules showed that the overall backbone structures of the two molecules are identical and that the bacterial lipopeptides bind to human and mouse TLR2 in essentially identical ways (13). The ability of BspA to activate epithelial cells is CD14 independent, which is consistent with the lack of CD14 binding to BspA protein demonstrated in our ligand binding assay. Our observations with the BspA protein, as well as the observations of other workers made with other protein ligands (namely, the Salmonella CsgA protein [33], the Yersinia LcrV protein [27], the neisserial outer membrane protein PorB [19], and the P. gingivalis FimA protein [7, 8]), indicate that TLR2 may recognize specific protein epitopes in addition to lipid moieties in cooperation with other coreceptors. The fimA-encoded fimbriae of P. gingivalis have been shown to induce proinflammatory cytokine expression in monocytes through activation of the TLR2 pathway. Interestingly, gingival epithelial cells do not respond to P. gingivalis fimbriae (3). This is because P. gingivalis fimbriae require membrane-expressed CD14 as a coreceptor for TLR2 (8) and because epithelial cells, unlike monocytes, do not express membrane CD14 (3). In contrast, BspA does not require CD14 for activating gingival epithelial cells (Fig. 3) and, in fact, does not bind CD14 (Fig. 4). P. gingivalis fimbriae, therefore, may function as a colonization adhesin in the context of the gingival epithelium as it may allow bacterial colonization without causing inflammatory reactions. On the other hand, the T. forsythia BspA protein functions as a pathogenic factor inducing substantial inflammatory responses in gingival epithelial cells. Thus, virulence factors of periodontal pathogens either have evolved to evade epithelial cell recognition, as is the case for P. gingivalis fimbriae, or have a propensity to induce the excessive inflammation which is a feature of periodontitis, as is the case for BspA. Although P. gingivalis and T. forsythia are both successful periodontal pathogens, they appear to use different tactics to modulate disease, and consequently, their bacterial components are likely to interact differently with the gingival epithelium. In this regard, we believe that the T. forsythia BspA protein plays a more direct role in stimulating the epithelial cell-mediated inflammatory response. Since BspA is a secreted protein, it can activate epithelial cells away from the site of bacterial colonization as well. In the context of the whole bacterial cell, our results indicated that in addition to BspA, other homologs, as well as bacterial associated lipoproteins, contribute in TLR2 activation. A recent study also showed that the expression of BspA is induced severalfold in vivo (35), and therefore this could lead to enhanced TLR2-mediated host inflammatory responses.
We are also interested in identifying the BspA epitopes responsible for binding to TLR2 in the hope of deciphering common molecular peptide patterns recognized by TLRs. Since genes encoding BspA-like homologs have also been identified in several recently completed genomes of other oral pathogens (2), we predict that TLRs may be involved in recognition of these pathogens as well via BspA-like homologs. Interestingly, TLRs contain LRR domains in their extracellular ligand binding domains. The LRR domains have been implicated in protein-protein interactions (16, 17). Thus, we hypothesized that the LRR of BspA may be involved in the interaction with TLR2. This hypothesis was tested by determining the activity of truncated BspA derivatives in TLR2 activation. The results clearly demonstrated that LRR domain D1 of BspA is responsible for the NF-κB activation in HEK293 cells through TLR2. Although the mechanisms of their interactions remain to be elucidated, this finding implies that both LRR regions in the extracellular ligand binding domain of TLR2 and in BspA are likely to be involved in TLR2-mediated cellular activation in human epithelial cells.
Since oral epithelial cells represent the first line of host defense against oral bacterial challenge by triggering host innate and inflammatory responses important in recruiting neutrophils, BspA may have an important role in the pathogenesis of T. forsythia-induced periodontal diseases. While neutrophils function to clear oral pathogens at infected sites, they may also contribute to the destruction of the periodontium (20). In fact, neutrophils have recently been implicated as key players in host-mediated inflammatory tissue injury in periodontitis (34). It is possible that uncontrolled polymorphonuclear leukocyte recruitment may lead to the tissue destruction observed in periodontitis associated with T. forsythia. Moreover, since BspA is also secreted, it is likely to cause inflammation at sites distant from the site of bacterial colonization. Thus, our findings suggest that BspA is an important modulator of host innate immune responses through activation of TLR2 in cooperation with TLR1. It would also be of interest to determine if other BspA homologs identified in T. forsythia have overlapping roles by triggering TLRs to cause inflammatory responses.
Acknowledgments
We thank Satoru Inagaki and Ramanujam Prativadi for help with recombinant protein production.
This study was supported by grants from the NIDCR (grant DE014749 to A.S., grant DE015254 to G.H., and grant DE017384 to D.K.).
Editor: S. R. Blanke
Footnotes
Published ahead of print on 29 October 2007.
REFERENCES
- 1.Bulut, Y., E. Faure, L. Thomas, O. Equils, and M. Arditi. 2001. Cooperation of Toll-like receptor 2 and 6 for cellular activation by soluble tuberculosis factor and Borrelia burgdorferi outer surface protein A lipoprotein: role of Toll-interacting protein and IL-1 receptor signaling molecules in Toll-like receptor 2 signaling. J. Immunol. 167987-994. [DOI] [PubMed] [Google Scholar]
- 2.Duncan, M. J. 2003. Genomics of oral bacteria. Crit. Rev. Oral Biol. Med. 14175-187. [DOI] [PubMed] [Google Scholar]
- 3.Eskan, M. A., G. Hajishengallis, and D. F. Kinane. 2007. Differential activation of human gingival epithelial cells and monocytes by Porphyromonas gingivalis fimbriae. Infect. Immun. 75892-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ford, P. J., E. Gemmell, A. Chan, C. L. Carter, P. J. Walker, P. S. Bird, M. J. West, M. P. Cullinan, and G. J. Seymour. 2006. Inflammation, heat shock proteins and periodontal pathogens in atherosclerosis: an immunohistologic study. Oral Microbiol. Immunol. 21206-211. [DOI] [PubMed] [Google Scholar]
- 5.Grabiec, A., G. Meng, S. Fichte, W. Bessler, H. Wagner, and C. J. Kirschning. 2004. Human but not murine Toll-like receptor 2 discriminates between tri-palmitoylated and tri-lauroylated peptides. J. Biol. Chem. 27948004-48012. [DOI] [PubMed] [Google Scholar]
- 6.Hajishengallis, G., M. Martin, H. T. Sojar, A. Sharma, R. E. Schifferle, E. DeNardin, M. W. Russell, and R. J. Genco. 2002. Dependence of bacterial protein adhesins on Toll-like receptors for proinflammatory cytokine induction. Clin. Diagn. Lab Immunol. 9403-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hajishengallis, G., P. Ratti, and E. Harokopakis. 2005. Peptide mapping of bacterial fimbrial epitopes interacting with pattern recognition receptors. J. Biol. Chem. 28038902-38913. [DOI] [PubMed] [Google Scholar]
- 8.Hajishengallis, G., R. I. Tapping, E. Harokopakis, S. Nishiyama, P. Ratti, R. E. Schifferle, E. A. Lyle, M. Triantafilou, K. Triantafilou, and F. Yoshimura. 2006. Differential interactions of fimbriae and lipopolysaccharide from Porphyromonas gingivalis with the Toll-like receptor 2-centred pattern recognition apparatus. Cell. Microbiol. 81557-1570. [DOI] [PubMed] [Google Scholar]
- 9.Hajjar, A. M., D. S. O'Mahony, A. Ozinsky, D. M. Underhill, A. Aderem, S. J. Klebanoff, and C. B. Wilson. 2001. Cutting edge: functional interactions between Toll-like receptor (TLR) 2 and TLR1 or TLR6 in response to phenol-soluble modulin. J. Immunol. 16615-19. [DOI] [PubMed] [Google Scholar]
- 10.Hasebe, A., A. Yoshimura, T. Into, H. Kataoka, S. Tanaka, S. Arakawa, H. Ishikura, D. T. Golenbock, T. Sugaya, N. Tsuchida, M. Kawanami, Y. Hara, and K. Shibata. 2004. Biological activities of Bacteroides forsythus lipoproteins and their possible pathological roles in periodontal disease. Infect. Immun. 721318-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Honma, K., H. K. Kuramitsu, R. J. Genco, and A. Sharma. 2001. Development of a gene inactivation system for Bacteroides forsythus: construction and characterization of a BspA mutant. Infect. Immun. 694686-4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inagaki, S., S. Onishi, H. K. Kuramitsu, and A. Sharma. 2006. Porphyromonas gingivalis vesicles enhance attachment, and the leucine-rich repeat BspA protein is required for invasion of epithelial cells by “Tannerella forsythia”. Infect. Immun. 745023-5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin, M. S., S. E. Kim, J. Y. Heo, M. E. Lee, H. M. Kim, S.-G. Paik, H. Lee, and J.-O. Lee. 2007. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell 1301071-1082. [DOI] [PubMed] [Google Scholar]
- 14.Kagnoff, M. F., and L. Eckmann. 1997. Epithelial cells as sensors for microbial infection. J. Clin. Investig. 1006-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kikkert, R., M. L. Laine, L. A. Aarden, and A. J. van Winkelhoff. 2007. Activation of Toll-like receptors 2 and 4 by gram-negative periodontal bacteria. Oral Microbiol. Immunol. 22145-151. [DOI] [PubMed] [Google Scholar]
- 16.Kobe, B., and J. Deisenhofer. 1994. The leucine-rich repeat: a versatile binding motif. Trends Biochem. Sci. 19415-421. [DOI] [PubMed] [Google Scholar]
- 17.Kobe, B., and A. V. Kajava. 2001. The leucine-rich repeat as a protein recognition motif. Curr. Opin. Struct. Biol. 11725-732. [DOI] [PubMed] [Google Scholar]
- 18.Liang, S., M. Wang, K. Triantafilou, M. Triantafilou, H. F. Nawar, M. W. Russell, T. D. Connell, and G. Hajishengallis. 2007. The A subunit of type IIb enterotoxin (LT-IIb) suppresses the proinflammatory potential of the B subunit and its ability to recruit and interact with TLR2. J. Immunol. 1784811-4819. [DOI] [PubMed] [Google Scholar]
- 19.Massari, P., A. Visintin, J. Gunawardana, K. A. Halmen, C. A. King, D. T. Golenbock, and L. M. Wetzler. 2006. Meningococcal porin PorB binds to TLR2 and requires TLR1 for signaling. J. Immunol. 1762373-2380. [DOI] [PubMed] [Google Scholar]
- 20.Nathan, C. 2006. Neutrophils and immunity: challenges and opportunities. Nat. Rev. Immunol. 6173-182. [DOI] [PubMed] [Google Scholar]
- 21.Noack, B., R. J. Genco, M. Trevisan, S. Grossi, J. J. Zambon, and E. De Nardin. 2001. Periodontal infections contribute to elevated systemic C-reactive protein level. J. Periodontol. 721221-1227. [DOI] [PubMed] [Google Scholar]
- 22.Omueti, K. O., J. M. Beyer, C. M. Johnson, E. A. Lyle, and R. I. Tapping. 2005. Domain exchange between human Toll-like receptors 1 and 6 reveals a region required for lipopeptide discrimination. J. Biol. Chem. 28036616-36825. [DOI] [PubMed] [Google Scholar]
- 23.Ozinsky, A., D. M. Underhill, J. D. Fontenot, A. M. Hajjar, K. D. Smith, C. B. Wilson, L. Schroeder, and A. Aderem. 2000. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between Toll-like receptors. Proc. Natl. Acad. Sci. USA 9713766-13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruddy, M. J., F. Shen, J. B. Smith, A. Sharma, and S. L. Gaffen. 2004. Interleukin-17 regulates expression of the CXC chemokine LIX/CXCL5 in osteoblasts: implications for inflammation and neutrophil recruitment. J. Leukoc. Biol. 76135-144. [DOI] [PubMed] [Google Scholar]
- 25.Sharma, A., S. Inagaki, K. Honma, C. Sfintescu, P. J. Baker, and R. T. Evans. 2005. Tannerella forsythia-induced alveolar bone loss in mice involves leucine-rich-repeat BspA protein. J. Dent. Res. 84462-467. [DOI] [PubMed] [Google Scholar]
- 26.Sharma, A., H. T. Sojar, I. Glurich, K. Honma, H. K. Kuramitsu, and R. J. Genco. 1998. Cloning, expression, and sequencing of a cell surface antigen containing a leucine-rich repeat motif from Bacteroides forsythus ATCC 43037. Infect. Immun. 665703-5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sing, A., D. Reithmeier-Rost, K. Granfors, J. Hill, A. Roggenkamp, and J. Heesemann. 2005. A hypervariable N-terminal region of Yersinia LcrV determines Toll-like receptor 2-mediated IL-10 induction and mouse virulence. Proc. Natl. Acad. Sci. USA 10216049-16054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Socransky, S. S., A. D. Haffajee, M. A. Cugini, C. Smith, and R. L. Kent, Jr. 1998. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 25134-144. [DOI] [PubMed] [Google Scholar]
- 29.Takeda, K., T. Kaisho, and S. Akira. 2003. Toll-like receptors. Annu. Rev. Immunol. 21335-376. [DOI] [PubMed] [Google Scholar]
- 30.Tanner, A., M. F. Maiden, P. J. Macuch, L. L. Murray, and R. L. Kent, Jr. 1998. Microbiota of health, gingivitis, and initial periodontitis. J. Clin. Periodontol. 2585-98. [DOI] [PubMed] [Google Scholar]
- 31.Tanner, A. C., B. J. Paster, S. C. Lu, E. Kanasi, R. Kent, Jr., T. Van Dyke, and S. T. Sonis. 2006. Subgingival and tongue microbiota during early periodontitis. J. Dent. Res. 85318-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tran, S. D., J. D. Rudney, B. S. Sparks, and J. S. Hodges. 2001. Persistent presence of Bacteroides forsythus as a risk factor for attachment loss in a population with low prevalence and severity of adult periodontitis. J. Periodontol. 721-10. [DOI] [PubMed] [Google Scholar]
- 33.Tukel, C., M. Raffatellu, A. D. Humphries, R. P. Wilson, H. L. Andrews-Polymenis, T. Gull, J. F. Figueiredo, M. H. Wong, K. S. Michelsen, M. Akcelik, L. G. Adams, and A. J. Baumler. 2005. CsgA is a pathogen-associated molecular pattern of Salmonella enterica serotype Typhimurium that is recognized by Toll-like receptor 2. Mol. Microbiol. 58289-304. [DOI] [PubMed] [Google Scholar]
- 34.Van Dyke, T. E., and C. N. Serhan. 2003. Resolution of inflammation: a new paradigm for the pathogenesis of periodontal diseases. J. Dent. Res. 8282-90. [DOI] [PubMed] [Google Scholar]
- 35.Yoo, J. Y., H. C. Kim, W. Zhu, S. M. Kim, M. Sabet, M. Handfield, J. Hillman, A. Progulske-Fox, and S. W. Lee. 2007. Identification of Tannerella forsythia antigens specifically expressed in patients with periodontal disease. FEMS Microbiol. Lett. 275344-352. [DOI] [PubMed] [Google Scholar]