Abstract
LuxS catalyzes the synthesis of the quorum-sensing signaling molecule autoinducer 2. We show that in Salmonella enterica serovar Typhimurium, deletion of the luxS gene polarizes flagellar phase variation toward the more immunogenic phase 1 flagellin. This phenotype is complementable by luxS in trans but is independent of quorum-sensing signals.
Quorum sensing in bacteria involves the production and detection of signaling molecules, called autoinducers (AI), which allow bacteria to coordinate gene expression (1, 2, 15, 20, 30). Following detection of a critical density of AI, bacteria coordinate their gene expression and behave in a “multicellular” fashion. Quorum sensing is implicated in the regulation of a range of processes from bioluminescence to virulence (11, 27, 32). LuxS is involved in the production of the AI-2 signal molecule (4, 21, 25) and also plays an important role in central metabolism, being part of the activated methyl cycle (29, 31). Taga et al. demonstrated that luxS in Salmonella enterica serovar Typhimurium affects the expression of, surprisingly, only a single operon encoding the Lsr AI-2 sensor system (28). S. enterica serovar Typhimurium is an important food-borne pathogen (18). Salmonellae swim using two different flagellar subunit types, either FliC (phase 1) or FljB (phase 2) (3, 33). Flagella have previously been implicated in invasion of host cells by Salmonella (14, 17). They also stimulate the host immune response by binding to Toll-like receptor 5 (23). Recently, FliC has been shown to play a unique role in macrophage-induced bacterial killing by its unorthodox secretion through the type 3 secretion system (8, 19). In this study, we highlight the contribution of luxS to flagellar phase variation in a quorum-sensing-independent manner.
A complete deletion of luxS from the start codon to the stop codon was constructed in S. enterica serovar Typhimurium SL1344 (Table 1) using λ-red recombination (6), to generate SL1344LS. The deletion was confirmed by PCR, Western blotting with LuxS antibodies, and AI-2 detection using the well-established Vibrio bioluminescence reporter bioassay (24).
TABLE 1.
Strains, plasmids, and primers used in the study
| Strain, plasmid, or primer | Genotype, description, or sequencea | Reference | Restriction site/comment |
|---|---|---|---|
| Strains | |||
| SL1344 | Parent strain | 13 | |
| SL1344LS | SL1344 luxS | This work | |
| TH1077 | LT2 fliC5050::MudJ | 9 | |
| SL1344F | SL1344 fliC5050::MudJ | This work | |
| SL1344LSF | SL1344 luxS fliC5050::MudJ | This work | |
| SL1344LSF(pBR322) | SL1344 luxS fliC5050::MudJ with pBR322 | This work | |
| SL1344LSF(pBRluxS) | SL1344 luxS fliC5050::MudJ with pBRluxS | This work | |
| Plasmids | |||
| pBR322 | Cloning vector | 26 | |
| pBRluxS | pBR322 with HindIII-BamHI fragment containing luxS region | This work | |
| Primers | |||
| OMW41 | GCGAAGCTTACCGAGCCGTTTGCCGCGTGG | HindIII | |
| OMW42 | GCGGGATCCATTTAACAGGCCAGGCATTAC | BamHI | |
| STM2817FOR | ATGCCATTATTAGATAGCTT | luxS/Northern | |
| STM2817REV | CTAATACGACTCACTATAGGGAGATGGTCGCGCATAAAGCCAGC | luxS/Northern | |
| STM2772FOR | CCTGGTGGCGTTAATATCAG | hin/Northern | |
| STM2772REV | CTAATACGACTCACTATAGGGAGAGCCCTCCCAGTCGTCCTTGC | hin/Northern | |
| STM1959FOR | AGTACTTTTAAAGCCTCGGC | fliC/Northern | |
| STM1959REV | CTAATACGACTCACTATAGGGAGAAGCGGGGAAGTCGCACCGCC | fliC/Northern | |
| STM2771FOR | TCGGGTCTTGATGATGCAGC | fljB/Northern | |
| STM2771REV | CTAATACGACTCACTATAGGGAGAGCCGCAAGGGTTACTGTACC | fljB/Northern |
Restriction sites are underlined.
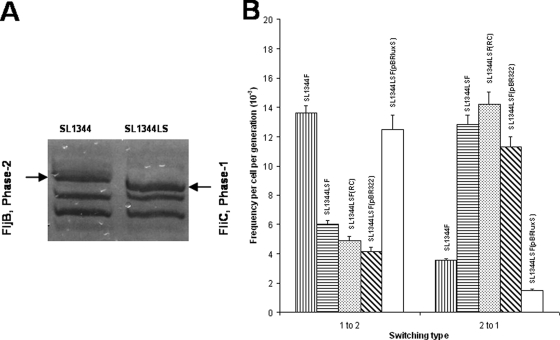
We assessed the secretion profiles of SL1344LS and its isogenic parent by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Coomassie blue staining of proteins. This revealed a major difference in the levels of FliC and FljB flagellins, with FliC being predominant in SL1344LS as identified via matrix-assisted laser desorption ionization-time-of-flight mass spectrometry (Fig. 1A). No differences in motility were observed between SL1344 and SL1344LS (data not shown) (22). We assessed phase variation using triphenyltetrazolium chloride (TTC) indicator plates (10). The fliC::mudJ reporter was transduced from TH1077 (10) into either SL1344 or SL1344LS, giving rise to SL1344F and SL1344LSF, respectively. A single Lac+ (white on TTC) or Lac− (red on TTC) colony of either SL1344F or SL1344LSF was grown to mid-log phase in LB and plated for single colonies on TTC plates. Experiments were repeated in triplicate. Although in SL1344F inversion to phase 2 predominates, in SL1344LSF we discovered a bias toward expression of phase 1 flagellin (Fig. 1B). This switching phenotype was complementable in trans using luxS expressed from its natural promoter (Table 1, pBRluxS), which restored switching frequencies back to parental levels, highlighting the importance of luxS in modulating flagellar phase variation (Fig. 1B).
FIG. 1.
Flagella phase variation is LuxS dependent in S. enterica serovar Typhimurium. (A) Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of secreted protein from SL1344 and the isogenic luxS mutant, SL1344LS, showing the flagellar protein bands identified by matrix-assisted laser desorption ionization-time-of-flight mass spectrometry; (B) rates of flagellar phase switching. Expression of LuxS from pBRluxS in SL1344LSF(pBRluxS) restores switching frequencies back to parental levels as measured by the TTC plate assay. However, supplementation with reconstituted cell supernatants [SL1344LSF(RC)] containing AI-2 (or other diffusible signaling molecules) fails to restore phase variation back to parental levels in SL1344LSF. Standard errors are shown.
To determine whether AI-2 or other diffusible signals in SL1344 supernatant supplemented with LB (reconstituted supernatant) could complement flagellar phase variation, SL1344LSF was cultured for 16 h in reconstituted supernatant to stimulate physiological adaptation mediated by AI-2 or another diffusible metabolite. Phase variation frequencies were then determined using the TTC indicator plate method and reconstituted medium to grow the relevant strains. Growing SL1344LSF in reconstituted supernatants did not reinstate parental flagellar phase variation frequencies (Fig. 1B), suggesting that neither AI-2 nor other diffusible signals mediate flagellar phase variation under these conditions.
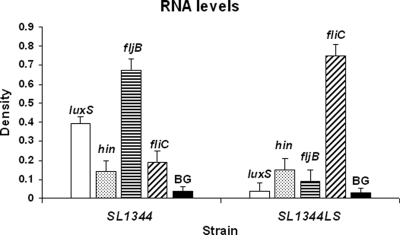
Since flagellar phase variation in S. enterica serovar Typhimurium is Hin recombinase dependent, we determined hin transcript levels in SL1344LS by Northern dot blots (16) and found no significant differences (Fig. 2). Phase 1 and phase 2 transcript levels correlated well with the observations made independently by the methods described above (Fig. 1 and 2). This may imply the existence of either an indirect posttranscriptional effect on Hin recombinase or a Hin-independent regulatory mechanism. The importance of phase variation in Salmonella pathogenicity remains to be fully elucidated. FliC is known to directly elicit an Ipaf-dependent macrophage response, leading to macrophage death and inflammation (8, 19). Furthermore, at early stages of infection, FliC expression is restricted to the small intestine and is dramatically repressed by intracellular bacteria (5, 7). We hypothesize that the microenvironment within the macrophage may trigger a luxS-dependent “stealth” response to enhance expression of the less immunogenic FljB. Phase variation control in Salmonella may be delicately balanced by pathways involving luxS, creating an advantage to the population under specific environmental conditions.
FIG. 2.
Transcript levels of the hin recombinase are unaffected in SL1344LS. Control transcript (luxS) levels are similar to background (BG) levels in SL1344LS, and those of phase 1 (fliC) and phase 2 (fljB) flagellins appear altered, with phase 1 flagellin predominating in SL1344LS. Standard errors are shown.
Quorum sensing allows interbacterial communication to coordinate survival and virulence (12). As well as quorum sensing, luxS also has a key metabolic function in the activated methyl cycle (29). Here we demonstrate that in S. enterica serovar Typhimurium luxS plays a role in flagellar phase variation, highlighting its importance in triggering expression of the less immunogenic FljB and repressing the highly immunogenic FliC. Although luxS is an important component of the AI-2-mediated signaling system, in flagellar phase switching we have identified a phenotype independent of diffusible signaling molecules and quorum sensing. Further characterization of the luxS signaling pathway in Salmonella will allow us to better understand the multiple roles of luxS in mediating gene regulation and bacterial fitness.
Acknowledgments
We thank Colin Harwood, Philip Aldridge, Emma McGhie, Vassilis Koronakis, Colin Hughes, Charlotte Perrett, and Mark Jepson, for help, advice, and useful discussions. We also gratefully acknowledge Kelly Hughes for providing the flagellar reporter strain.
This work was supported by grants from the Biotechnology and Biological Sciences Research Council (BBSRC), United Kingdom.
Footnotes
Published ahead of print on 16 November 2007.
REFERENCES
- 1.Ahmer, B. M. M. 2004. Cell-to-cell signalling in Escherichia coli and Salmonella enterica. Mol. Microbiol. 52933-945. [DOI] [PubMed] [Google Scholar]
- 2.Bassler, B. L., M. Wright, R. E. Showalter, and M. R. Silverman. 1993. Intercellular signaling in Vibrio harveyi—sequence and function of genes regulating expression of luminescence. Mol. Microbiol. 9773-786. [DOI] [PubMed] [Google Scholar]
- 3.Bonifield, H. R., and K. T. Hughes. 2003. Flagellar phase variation in Salmonella enterica is mediated by a posttranscriptional control mechanism. J. Bacteriol. 1853567-3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, X., S. Schauder, N. Potier, A. Van Dorsselaer, I. Pelczer, B. L. Bassler, and F. M. Hughson. 2002. Structural identification of a bacterial quorum-sensing signal containing boron. Nature 415545-549. [DOI] [PubMed] [Google Scholar]
- 5.Cummings, L. A., W. D. Wilkerson, T. Bergsbaken, and B. T. Cookson. 2006. In vivo, fliC expression by Salmonella enterica serovar Typhimurium is heterogeneous, regulated by ClpX, and anatomically restricted. Mol. Microbiol. 61795-809. [DOI] [PubMed] [Google Scholar]
- 6.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 976640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eriksson, S., S. Lucchini, A. Thompson, M. Rhen, and J. C. D. Hinton. 2003. Unravelling the biology of macrophage infection by gene expression profiling of intracellular Salmonella enterica. Mol. Microbiol. 47103-118. [DOI] [PubMed] [Google Scholar]
- 8.Franchi, L., A. Amer, M. Body-Malapel, T. D. Kanneganti, N. Ozoren, R. Jagirdar, N. Inohara, P. Vandenabeele, J. Bertin, A. Coyle, E. P. Grant, and G. Nunez. 2006. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1β in Salmonella-infected macrophages. Nat. Immunol. 7576-582. [DOI] [PubMed] [Google Scholar]
- 9.Gillen, K. L., and K. T. Hughes. 1991. Molecular characterization of flgM, a gene encoding a negative regulator of flagellin synthesis in Salmonella typhimurium. J. Bacteriol. 1736453-6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gillen, K. L., and K. T. Hughes. 1991. Negative regulatory loci coupling flagellin synthesis to flagellar assembly in Salmonella typhimurium. J. Bacteriol. 1732301-2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenberg, E. P. 2003. Bacterial communication and group behavior. J. Clin. Investig. 1121288-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henke, J. M., and B. L. Bassler. 2004. Bacterial social engagements. Trends Cell Biol. 14648-656. [DOI] [PubMed] [Google Scholar]
- 13.Hoiseth, S. K., and B. A. D. Stocker. 1981. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature 291238-239. [DOI] [PubMed] [Google Scholar]
- 14.Jones, B. D., C. A. Lee, and S. Falkow. 1992. Invasion by Salmonella typhimurium is affected by the direction of flagellar rotation. Infect. Immun. 602475-2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaplan, H. B., and E. P. Greenberg. 1985. Diffusion of autoinducer is involved in regulation of the Vibrio fischeri luminescence system. J. Bacteriol. 1631210-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karavolos, M. H., M. Wilson, J. Henderson, J. J. Lee, and C. M. A. Khan. 2005. Type III secretion of the Salmonella effector protein SopE is mediated via an N-terminal amino acid signal and not an mRNA sequence. J. Bacteriol. 1871559-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.La Ragione, R. M., W. A. Cooley, P. Velge, M. A. Jepson, and M. J. Woodward. 2003. Membrane ruffling and invasion of human and avian cell lines is reduced for aflagellate mutants of Salmonella enterica serotype Enteritidis. Int. J. Med. Microbiol. 293261-272. [DOI] [PubMed] [Google Scholar]
- 18.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miao, E. A., C. M. Alpuche-Aranda, M. Dors, A. E. Clark, M. W. Bader, S. I. Miller, and A. Aderem. 2006. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1β via Ipaf. Nat. Immunol. 7569-575. [DOI] [PubMed] [Google Scholar]
- 20.Miller, M. B., and B. L. Bassler. 2001. Quorum sensing in bacteria. Annu. Rev. Microbiol. 55165-199. [DOI] [PubMed] [Google Scholar]
- 21.Schauder, S., K. Shokat, M. G. Surette, and B. L. Bassler. 2001. The LuxS family of bacterial autoinducers: biosynthesis of a novel quorum-sensing signal molecule. Mol. Microbiol. 41463-476. [DOI] [PubMed] [Google Scholar]
- 22.Sperandio, V., A. G. Torres, J. A. Giron, and J. B. Kaper. 2001. Quorum sensing is a global regulatory mechanism in enterohemorrhagic Escherichia coli O157:H7. J. Bacteriol. 1835187-5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steiner, T. S. 2007. How flagellin and Toll-like receptor 5 contribute to enteric infection. Infect. Immun. 75545-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Surette, M. G., and B. L. Bassler. 1998. Quorum sensing in Escherichia coli and Salmonella typhimurium. Proc. Natl. Acad. Sci. USA 957046-7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Surette, M. G., M. B. Miller, and B. L. Bassler. 1999. Quorum sensing in Escherichia coli, Salmonella typhimurium, and Vibrio harveyi: a new family of genes responsible for autoinducer production. Proc. Natl. Acad. Sci. USA 961639-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sutcliffe, J. G. 1979. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harbour Symp. Quant. Biol. 4377-90. [DOI] [PubMed] [Google Scholar]
- 27.Taga, M. E., and B. L. Bassler. 2003. Chemical communication among bacteria. Proc. Natl. Acad. Sci. USA 10014549-14554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taga, M. E., J. L. Semmelhack, and B. L. Bassler. 2001. The LuxS-dependent autoinducer Al-2 controls the expression of an ABC transporter that functions in Al-2 uptake in Salmonella typhimurium. Mol. Microbiol. 42777-793. [DOI] [PubMed] [Google Scholar]
- 29.Vendeville, A., K. Winzer, K. Heurlier, C. M. Tang, and K. R. Hardie. 2005. Making ‘sense’ of metabolism: autoinducer-2, LuxS and pathogenic bacteria. Nat. Rev. Microbiol. 3383-396. [DOI] [PubMed] [Google Scholar]
- 30.Williams, P., N. J. Bainton, S. Swift, S. R. Chhabra, M. K. Winson, G. S. A. B. Stewart, G. P. C. Salmond, and B. W. Bycroft. 1992. Small molecule-mediated density-dependent control of gene expression in prokaryotes—bioluminescence and the biosynthesis of carbapenem antibiotics. FEMS Microbiol. Lett. 100161-167. [DOI] [PubMed] [Google Scholar]
- 31.Winzer, K., K. R. Hardie, and P. Williams. 2002. Bacterial cell-to-cell communication: sorry, can't talk now—gone to lunch! Curr. Opin. Microbiol. 5216-222. [DOI] [PubMed] [Google Scholar]
- 32.Winzer, K., and P. Williams. 2001. Quorum sensing and the regulation of virulence gene expression in pathogenic bacteria. Int. J. Med. Microbiol. 291131-143. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto, S., and K. Kutsukake. 2006. FljA-mediated posttranscriptional control of phase 1 flagellin expression in flagellar phase variation of Salmonella enterica serovar Typhimurium. J. Bacteriol. 188958-967. [DOI] [PMC free article] [PubMed] [Google Scholar]