Abstract
LmrCD is an ABC-type multidrug transporter in Lactococcus lactis. LmrR encodes a putative transcriptional regulator. In a ΔlmrR strain, lmrCD is up-regulated. LmrR binds the promoter region of lmrCD and interacts with drugs that cause lmrCD up-regulation. This suggests that LmrR is a drug-dependent transcriptional regulator of lmrCD expression.
In recent years, the exposure of human-pathogenic bacteria to antibiotics and toxic drugs has led to a major boost in the emergence of (multi)drug resistant pathogens (24), which are now a serious problem in public health, causing millions of death worldwide (6, 22). The overexpression of a multidrug efflux pump(s) is one of the causes of the resistance phenotype observed in bacteria (17, 19, 26). Bacteria possess various genes that encode putative multidrug resistance (MDR) transporters, but for most of these systems the exact physiological function is unclear (25). Resistance readily develops when cells are exposed to drugs or antibiotics, and the immediate response usually involves the up-regulation of low-expression MDR transporters through local or global transcriptional regulators (1, 11, 12). The gram-positive bacterium Lactococcus lactis is widely used in fermented food production. The genomes of L. lactis IL-1403 (5) and MG1363 (30) contain about 40 genes that encode putative MDR transporters. LmrA and LmrP of L. lactis have been implicated in the MDR phenotype, but gene inactivation analysis of a number of putative MDR transporter genes suggests that the intrinsic MDR of L. lactis is due to the expression of the heterodimeric ATP-binding cassette (ABC) transporter LmrCD (20). Exposure of L. lactis cells to the compounds daunomycin, ethidium bromide, and rhodamine 6G readily resulted in the development of an MDR phenotype (4). DNA microarray analysis revealed that in these strains the expression of lmrCD is strongly increased (four- to eightfold), whereas several other genes are up- or down-regulated in a strain-specific manner (18). This suggests that LmrCD is also a major determinant of acquired drug resistance. The DNA region upstream of the lmrCD genes specifies a putative regulatory protein, LmrR (formerly YdaF), that by homology belongs to the PadR family of transcriptional regulators (Pfam PF03551). PadR proteins are involved in the regulation of expression of the phenolic acid decarboxylase (pad) genes, which are required for the detoxification (13) and metabolism (10, 23, 28) of phenolic acid compounds. In lactic acid bacteria, phenolic acids are converted to 4-vinyl derivatives, which are further reduced to 4-ethyl derivatives (3). The PadR family is related to the bacterial and archaeal MarR family of transcriptional regulators of multiple antibiotic resistance. These proteins share a common domain organization which comprises an N-terminal winged helix-turn-helix DNA binding motif that via a conserved hinge region is connected to a highly divergent C-terminal domain (2). The latter region has been postulated to be involved in substrates binding. Interestingly, in L. lactis MDR strains, the lmrR gene contains either a frameshift mutation or a point mutation (T82I in the hinge region) (18). This suggests that the up-regulation of lmrCD observed in these strains is related to a defective LmrR protein.
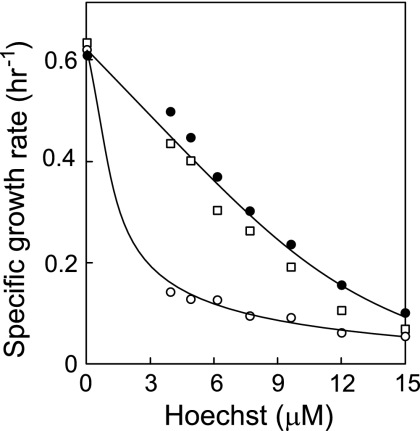
Since the previously characterized MDR strains of L. lactis were obtained by experiments involving long-term drug challenge, repeated transfer, and growth (4), there is a risk that other factors besides LmrR contribute to the MDR phenotype. To evaluate the exact role of LmrR in lmrCD expression, the lmrR gene was deleted by chromosomal replacement (15, 16). A PCR fragment containing the complete lmrR gene and the flanking regions was amplified from genomic DNA of L. lactis NZ9000 (8) using the primer pair lmrR FW2/lmrR RV2 (Table 1). The PCR product and the plasmid pORI280 were digested with BglII/BamHI and ligated, resulting in pORIYdaF. Subsequently, the complete lmrR gene was removed from this plasmid by a PCR method using phosphorylated primers facing back to back, i.e., lmrR FW3/lmrR RV3 (Table 1). The obtained linear PCR product was self-ligated, resulting in pORIYdaFDel; this plasmid was introduced into L. lactis NZ9000 cells containing the temperature-sensitive pVE6007 plasmid, which bears the repA gene necessary for the replication of pORIYdaFDel. Single transformants were grown overnight at an elevated growth temperature (37°C) to induce the loss of pVE6007. Integrants were selected by growth in M17 medium (Difco) containing 0.5% (wt/vol) glucose (GM17) and erythromycin (5 μg/ml) and grown further for 30 to 40 generations in medium without the antibiotic to allow excision of the integrated structure. The deletion was confirmed by PCR and nucleotide sequencing of the corresponding region of the chromosome. L. lactis NZ9000 parental and ΔlmrR cells were grown at 30°C in GM17 and harvested at an optical density at 660 of 1 (late log phase), and their transcriptomes were compared by DNA microarray analysis (7, 29). Expression of a gene was considered to be significantly altered when the Cyber T Baysian P value was ≤1E−05. lmrC and lmrD are highly up-regulated (>4.5-fold) upon deletion of lmrR, confirming our previous assumption that LmrR is a transcriptional repressor of lmrCD. A limited number of other genes are significantly and strongly (∼2-fold) transcribed differentially. These are mostly related to the intracellular redox state, such as the trxA, thioredoxin, and superoxide dismutase genes. Genes that were down-regulated more than twofold are glnR (glutamine synthetase repressor), cysK (cysteine synthase), and rplD (50S ribosomal protein L4). The L. lactis NZ9000 ΔlmrR strain showed similar growth and resistance against Hoechst 33342 (Fig. 1) and daunomycin (data not shown) as did L. lactis NZ9000 cells but was significantly more resistant than the ΔlmrCD strain, as expected for the derepression of lmrCD. Interestingly, we have previously shown that overexpression of lmrCD restores the drug-sensitive phenotype of the ΔlmrCD strain to parental levels only, despite the increased drug extrusion activity relative to the parental strain (18).
TABLE 1.
Primers used
| Primer | Sequence (5′ to 3′)a | Endonuclease |
|---|---|---|
| lmrR FW1 | GGCCCATGGGGGCAGAAATACCAAAAGAAATG | NcoI |
| lmrR RV1 | GCGTCTAGATTTAATCGCTTTCACTCTTCTTAT | XbaI |
| lmrR FW2 | TATAGATCTGCAATTCGAAGTCCAATTAAG | BglII |
| lmrR RV2 | TATGGATCCGTAAGTTGCTTCACGAACGTC | BamHI |
| lmrR FW3 | TAAATTCACGATTCATTCCTTACTT | |
| lmrR RV3 | TCTTTTTCCTTTCTATCATTTTAAACA | |
| lmrCDpmtr FW1 | ATTGTAATCTTTAACAGCATTAAC | |
| lmrCDpmtr FW2 | ACAAATAACGTCGTAAATCG | |
| lmrCDpmtr RV1 | GGCAACCCATTTATGCTTCA | |
| lmrRpmtr FW1 | TGTCGCAAACGCAATTTGTC | |
| lmrRpmtr FW2 | TCAAGGAAAGTTGTCTTCCACCGCTAA | |
| lmrRpmtr RV1 | CTGCCATTCTTTTTCCTTTC | |
| lmrRpmtr RV2 | GGGCTCGTAACATTTCTTTTGGTATTTCTG | |
| lmrC RT-PCR FW | GTTGAAGAACGTGGGAATAATTTCTCAGGTGG | |
| lmrC RT-PCR RV | CCTCCTGTGCTTTCTGTGTATCGTAGATTTC | |
| lmrD RT-PCR FW | CGTTTCTGATGATGAATCAGTCTTCTCAGTTGG | |
| lmrD RT-PCR RV | CAAAAACGAATTGATTATGATAAAGTTCAGAG | |
| lmrR RT-PCR FW | ATGGCAGAAATACCAAAAGAAATG | |
| lmrR RT-PCR RV | TTATTTAATCGCTTCACTCTTCTTAT | |
| secY RT-PCR FW | TACAACTGCTCCAGCTACGA | |
| secY RT-PCR RV | GTTCCTCCAAGAGCGACAAT |
Endonuclease sites are in bold.
FIG. 1.
Sensitivity of L. lactis NZ9000, ΔlmrR, and ΔlmrCD cells to Hoechst 33342. L. lactis NZ9000 (•), ΔlmrCD (○), and ΔlmrR (□) cells were grown in GM17 medium in the presence of increasing concentrations of Hoechst 33342. The specific growth rates are plotted as a function of the drug concentration.
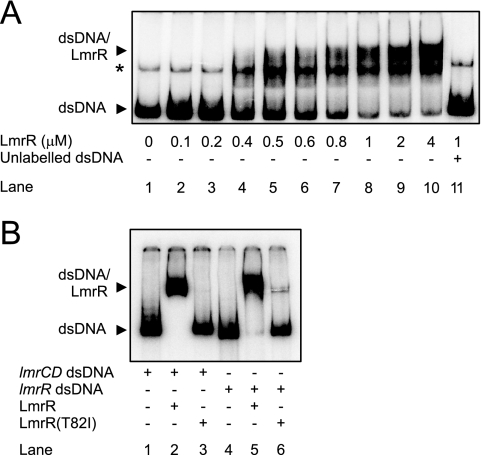
To determine the function of LmrR, the lmrR gene (351 bp) was PCR amplified from L. lactis MG1363 (9) genomic DNA using the primers lmrR FW1 and lmrR RV1 (Table 1). The lmrR gene was inserted between the NcoI/XbaI sites of the pNSC8048 expression vector (encoding a C-terminal streptactin tag), yielding pNSC8048-lmrR. By a similar method, the lmrR gene was amplified from the rhodamine-resistant L. lactis MG1363 strain (4), which contains the point mutation T82I, yielding pNSC8048-lmrRRho. Cells of L. lactis NZ9000, an MG1363 derivative containing pepN::nisR/K (8), were transformed with these plasmids and grown at 30°C in GM17 with 5 μg/ml chloramphenicol to the mid-log phase (optical density at 660 nm of 0.7 to 0.8), and then expression was induced by the addition of nisin to 5 ng/ml (8). Growth was continued for 1 h, and cells were harvested by centrifugation, resuspended in Tris-Cl (pH 7.0), and lysed by incubation with 10 mg/ml freshly prepared lysozyme for 45 min at 30°C, followed by the addition of 10 mM MgSO4, 100 μg/ml DNase I, and complete protease inhibitor (Roche), and subsequent French press treatment at 15,000 lb/in2. Cellular debris and membranes were removed by low-speed centrifugation and ultracentrifugation, and LmrR was purified to homogeneity via Streptactin Sepharose column chromatography (IBA GmbH) according to the manufacturer's protocol. To remove associated DNA, LmrR was further purified by HiTrap heparin HP column chromatography (Amersham) in a buffer containing 20 mM Tris-Cl (pH 8.0), 0.2 mM EDTA, and 0.5 mM dithiothreitol. The protein was eluted using a linear gradient of 0.15 to 1.5 M NaCl in the same buffer. LmrR-containing fractions were pooled and concentrated using a Microcon centrifugal 10-kDa-cutoff filter (Millipore Corporation, Bedford, MA). Purified LmrR migrates as a 13.5-kDa protein on sodium dodecyl sulfate-polyacrylamide gel electrophoresis and mostly as a dimer in gel filtration (data not shown). The ability of LmrR to bind the promoter region of lmrCD was assessed by an electrophoretic mobility shift assay (EMSA) (14). DNA fragments of 205 and 387 bp containing the predicted promoter regions of lmrCD and lmrR, respectively, were amplified with Pwo DNA polymerase (Roche) using the PCR primer pairs lmrCDpmtr FW1/lmrCDpmtr RV1 and lmrRpmtr FW1/lmrCDpmtr RV1 (Table 1). After [γ-32P]ATP end labeling, the probes were purified and mixed with LmrR (0 to 50 μg/ml). After 10 min of incubation at 30°C, the samples were subjected to 6% polyacrylamide gel electrophoresis to separate the bound LmrR from the free DNA probe. LmrR causes a mobility shift of the DNA fragment containing the lmrCD promoter, with an apparent dissociation constant of 0.45 μM (Fig. 2A). The observed shift was efficiently prevented by the addition of excess unlabeled DNA probe for the lmrCD promoter. Interestingly, the LmrR(T82I) mutant failed to induce a DNA mobility shift (Fig. 2B), demonstrating that this mutant is deficient in promoter binding. Since earlier microarray studies suggested that lmrR expression is under the control of an autoregulatory mechanism (18), the ability of LmrR to bind to its own promoter region was also analyzed by EMSA. LmrR effectively binds to the DNA fragment containing the lmrR promoter region, while the LmrR(T82I) mutant fails to bind (Fig. 2B). We conclude that LmrR binds specifically to both the lmrR and lmrCD promoter regions, consistent with its proposed role as a transcriptional regulator.
FIG. 2.
Interaction of LmrR with the lmrCD and lmrR promoter regions. EMSA was performed with increasing amounts of purified LmrR incubated with 0.7 μM of 32P-labeled 369-bp and 387-bp double-stranded DNA (dsDNA) probes comprising the promoter regions of lmrCD (A) and lmrR (B), respectively. Where indicated, wild-type LmrR was substituted for the LmrR(T82I) mutant. *, single-stranded probe DNA. Unless indicated otherwise, LmrR was used at a concentration of 3.7 μM.
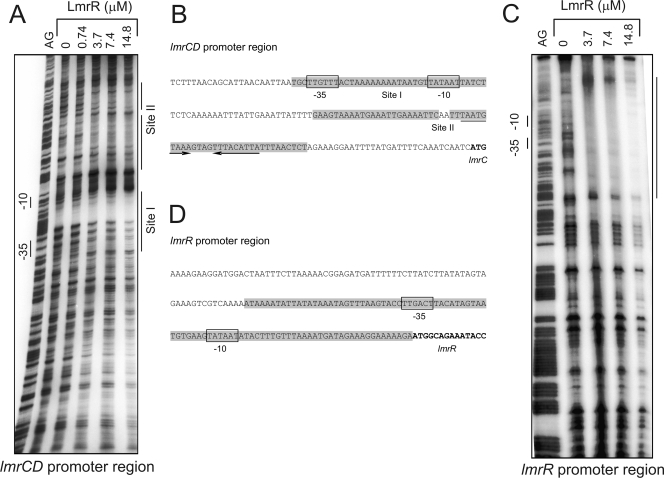
To map the binding regions on the lmrCD and lmrR promoters, DNase I protection assays were performed (14). DNA fragments containing the promoter regions of lmrCD and lmrR were amplified by PCR using the primer sets lmrCDpmtr FW2/lmrCDpmtr RV1 and lmrRpmtr FW2/lmrCDpmtr RV2, respectively. Various amounts of purified LmrR (0 to 200 μg/ml) were added, and then LmrR-protected DNA sequences were determined by the Maxam-Gilbert DNA sequencing method (27). LmrR protects two sites on the lmrCD promoter that are separated by 29 base pairs: site I corresponds to the putative −10 and −35 regions, and site II contains two direct inverted repeats, i.e., ATGT-10N-ACAT (Fig. 3A and B). Interestingly, a similar motif, ATGT-8N-ACAT, is conserved among PadR-like regulators (13). A screen of the L. lactis genome for potential binding sites using the site II motif yielded only the promoter region of lmrCD, consistent with our transcriptome analysis results, which show that LmrR is a local transcriptional regulator. LmrR protected a much longer stretch of DNA, with no apparent structural features, on its own promoter region (Fig. 3C and D).
FIG. 3.
DNase I protection of the lmrCD and lmrR promoter regions by LmrR, showing site specificity of binding of LmrR to the lmrCD (A and B) and lmrR (B and D) promoter regions. The DNase I-digested promoter fragments (A and C) are flanked by the Maxam-Gilbert ladder on the left (AG). Poly(dI-dC) was present to suppress unspecific binding. Nucleotide sequences of the lmrC (B) and lmrR (D) promoter regions show the LmrR-protected regions (shaded), the putative −35 and −10 regions (boxed), the inverted repeats (arrows), and the structural genes (bold).
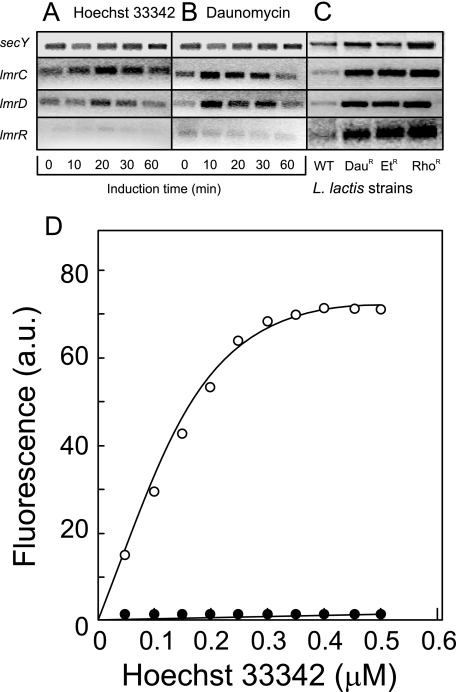
The expression of the lmrCD and lmrR genes was further investigated by reverse transcription-PCR (RT-PCR) using the primer sets listed in Table 1. Transcript levels were followed upon challenge of L. lactis MG1363 cells with the chemically unrelated drugs daunomycin (5 μM) (Fig. 4A) and Hoechst 33342 (50 μM) (Fig. 4B), both of which are substrates of LmrCD (20). The expression of lmrC and lmrD transiently increased up to twofold within 10 min. Unlike in the MDR strains (Fig. 4C), no detectable change in lmrR expression was detected in the drug-challenged cells (Fig. 4A and B). This shows that the lmrCD genes are up-regulated in response to challenge with toxic drugs and suggests that the autoregulatory mechanism of lmrR differs, at least in timing, from that of the structural genes lmrCD.
FIG. 4.
Expression of lmrC, lmrD, and lmrR in L. lactis MG1363 and MDR strains and binding of Hoechst 33342 to LmrR. (A to C) The expression of lmrC, lmrD, lmrR, and the control gene secY was measured by RT-PCR using specific primer pairs and total RNA isolated from the parental L. lactis MG1363 incubated in the presence of Hoechst 33342 (5 μM) (A) or daunomycin (50 μM) (B) and from the drug-resistant Daur, Ethr, and Rhor derivatives in the absence of drugs (C). WT, wild type. (D) Binding of Hoechst 33342 to LmrR (○) and the LmrR(T82I) mutant (•). Binding was measured as an increase in Hoechst 33342 fluorescence (a.u., arbitrary units).
To determine whether LmrR interacts directly with drugs, binding studies with Hoechst 33342 were performed. This drug is essentially nonfluorescent in aqueous medium but becomes highly fluorescent when bound to DNA or protein (21). Addition of increasing amounts of Hoechst 33342 to purified LmrR (5.7 μg/ml) in 50 mM Tris-Cl (pH 7.0) results in a saturable increase in fluorescence (excitation and emission wavelengths of 355 and 457 nm, respectively) (Fig. 4D). Binding saturates at ∼1 mol of Hoechst 33342 per 1.7 mol of LmrR. In contrast, no fluorescence increase was observed upon addition of Hoechst 33342 to the LmrR(T82I) mutant. Therefore, these data suggest that LmrR interacts directly with drugs and that it acts as a drug-regulated local transcriptional regulator of lmrCD. Interestingly, many of the PadR regulators are involved in the regulation of the expression of enzymes involved in phenolic acid degradation and detoxification, whereas LmrR regulates the expression of an MDR transporter that expels toxic molecules from the cell. However, L. lactis NZ9000, ΔlmrCD, and ΔlmrR cells showed similar sensitivities to phenolic acid derivatives (data not shown), which excludes a role of LmrR in the regulation of phenolic acid metabolism.
Based on the current findings, we propose that the regulation of the MDR phenotype in L. lactis occurs according to the following mechanism. When cells are exposed to toxic compounds in the medium, these compounds may permeate the cell membrane and bind LmrR. This binding event likely alters the LmrR conformation, whereupon its interaction with the lmrCD promoter region is weakened, allowing the RNA polymerase to initiate transcription. This results in a derepression of the lmrCD genes and hence initiates the expression of an MDR transporter that expels the drugs from the cell. In due course, drug-free LmrR will rebind to the promoter region of lmrCD and prevent further expression. The phenotype of the MDR strains of L. lactis can now be partially explained as a constitutive deregulation of lmrCD expression due to a defective LmrR that is unable to bind the lmrCD promoter region. However, since the MDR strains show an increased resistance to drugs compared to the parental strain, other, possibly strain-specific, mechanisms seem to contribute to the phenotype as well. The previous transcriptome analysis of these MDR strains (18) showed a significant increase in transcript levels of the lmrR gene, suggesting that LmrR is under control of autoregulation. Consistent with this hypothesis, LmrR was found to protect a long stretch of DNA on its own promoter, but this region is less defined than that on the lmrCD promoter region. Since no significant increase in the levels of the lmrR mRNA was observed upon a drug challenge, we hypothesize that the binding is either more extensive or tighter, allowing only a low level of lmrR expression. Autoregulation may be necessary for subtle tuning of the LmrR levels in the cell, as an excess of LmrR will interfere with a rapid response of cells toward toxic compounds entering the cells. Also the derepression of lmrR might be only weakly influenced by, or even be independent of, drug binding to LmrR. This will be a subject for future studies.
Transcriptome data accession number.
All transcriptome data discussed here have been deposited in the NCBI Gene Expression Omnibus (GEO) (http://www.ncbi.nlm.nih.gov/geo/) and are accessible through GEO series accession number GSE9168.
Acknowledgments
We thank Andy-Mark Thunnissen, Jan Kok, Anne de Jong, Pramod Madoori, and Eveline Peeters for discussion and valuable suggestions and S. Olga Bayraktaroglu for assistance.
Footnotes
Published ahead of print on 9 November 2007.
REFERENCES
- 1.Ahmed, M., C. M. Borsch, S. S. Taylor, N. Vazquez-Laslop, and A. A. Neyfakh. 1994. A protein that activates expression of a multidrug efflux transporter upon binding the transporter substrates. J. Biol. Chem. 26928506-28513. [PubMed] [Google Scholar]
- 2.Alekshun, M. N., S. B. Levy, T. R. Mealy, B. A. Seaton, and J. F. Head. 2001. The crystal structure of MarR, a regulator of multiple antibiotic resistance, at 2.3 Å resolution. Nat. Struct. Biol. 8710-714. [DOI] [PubMed] [Google Scholar]
- 3.Barthelmebs, L., C. Divies, and J. F. Cavin. 2000. Knockout of the p-coumarate decarboxylase gene from Lactobacillus plantarum reveals the existence of two other inducible enzymatic activities involved in phenolic acid metabolism. Appl. Environ. Microbiol. 663368-3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolhuis, H., D. Molenaar, G. Poelarends, H. W. van Veen, B. Poolman, A. J. M. Driessen, and W. N. Konings. 1994. Proton motive force-driven and ATP-dependent drug extrusion systems in multidrug-resistant Lactococcus lactis. J. Bacteriol. 1766957-6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolotin, A., P. Wincker, S. Mauger, O. Jaillon, K. Malarme, J. Weissenbach, S. D. Ehrlich, and A. Sorokin. 2001. The complete genome sequence of the lactic acid bacterium Lactococcus lactis ssp. lactis IL1403. Genome Res. 11731-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen, M. L. 2000. Changing patterns of infectious disease. Nature 406762-767. [DOI] [PubMed] [Google Scholar]
- 7.den Hengst, C. D., S. A. van Hijum, J. M. Geurts, A. Nauta, J. Kok, and O. P. Kuipers. 2005. The Lactococcus lactis CodY regulon: identification of a conserved cis-regulatory element. J. Biol. Chem. 28034332-34342. [DOI] [PubMed] [Google Scholar]
- 8.de Ruyter, P. G., O. P. Kuipers, and W. M. de Vos. 1996. Controlled gene expression systems for Lactococcus lactis with the food-grade inducer nisin. Appl. Environ. Microbiol. 623662-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gasson, M. J. 1983. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J. Bacteriol. 1541-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gasson, M. J., Y. Kitamura, W. R. McLauchlan, A. Narbad, A. J. Parr, E. L. Parsons, J. Payne, M. J. Rhodes, and N. J. Walton. 1998. Metabolism of ferulic acid to vanillin. A bacterial gene of the enoyl-SCoA hydratase/isomerase superfamily encodes an enzyme for the hydration and cleavage of a hydroxycinnamic acid SCoA thioester. J. Biol. Chem. 2734163-4170. [DOI] [PubMed] [Google Scholar]
- 11.Grkovic, S., M. H. Brown, N. J. Roberts, I. T. Paulsen, and R. A. Skurray. 1998. QacR is a repressor protein that regulates expression of the Staphylococcus aureus multidrug efflux pump QacA. J. Biol. Chem. 27318665-18673. [DOI] [PubMed] [Google Scholar]
- 12.Grkovic, S., M. H. Brown, and R. A. Skurray. 2002. Regulation of bacterial drug export systems. Microbiol. Mol. Biol. Rev. 66671-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gury, J., L. Barthelmebs, N. P. Tran, C. Divies, and J. F. Cavin. 2004. Cloning, deletion, and characterization of PadR, the transcriptional repressor of the phenolic acid decarboxylase-encoding padA gene of Lactobacillus plantarum. Appl. Environ. Microbiol. 702146-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kloosterman, T. G., W. T. Hendriksen, J. J. Bijlsma, H. J. Bootsma, S. A. van Hijum, J. Kok, P. W. Hermans, and O. P. Kuipers. 2006. Regulation of glutamine and glutamate metabolism by GlnR and GlnA in Streptococcus pneumoniae. J. Biol. Chem. 28125097-25109. [DOI] [PubMed] [Google Scholar]
- 15.Law, J., G. Buist, A. Haandrikman, J. Kok, G. Venema, and K. Leenhouts. 1995. A system to generate chromosomal mutations in Lactococcus lactis which allows fast analysis of targeted genes. J. Bacteriol. 1777011-7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leenhouts, K., G. Buist, A. Bolhuis, B. A. ten, J. Kiel, I. Mierau, M. Dabrowska, G. Venema, and J. Kok. 1996. A general system for generating unlabelled gene replacements in bacterial chromosomes. Mol. Gen. Genet. 253217-224. [DOI] [PubMed] [Google Scholar]
- 17.Lewis, K. 1994. Multidrug resistance pumps in bacteria: variations on a theme. Trends Biochem. Sci. 19119-123. [DOI] [PubMed] [Google Scholar]
- 18.Lubelski, J., A. de Jong, R. van Merkerk, H. Agustiandari, O. P. Kuipers, J. Kok, and A. J. M. Driessen. 2006. LmrCD is a major multidrug resistance transporter in Lactococcus lactis. Mol. Microbiol. 61771-781. [DOI] [PubMed] [Google Scholar]
- 19.Lubelski, J., W. N. Konings, and A. J. Driessen. 2007. Distribution and physiology of ABC-type transporters contributing to multidrug resistance in bacteria. Microbiol. Mol. Biol. Rev. 71463-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lubelski, J., P. Mazurkiewicz, R. van Merkerk, W. N. Konings, and A. J. M. Driessen. 2004. ydaG and ydbA of Lactococcus lactis encode a heterodimeric ATP-binding cassette-type multidrug transporter. J. Biol. Chem. 27934449-34455. [DOI] [PubMed] [Google Scholar]
- 21.Mazurkiewicz, P., A. J. M. Driessen, and W. N. Konings. 2004. Energetics of wild-type and mutant multidrug resistance secondary transporter LmrP of Lactococcus lactis. Biochim. Biophys. Acta 1658252-261. [DOI] [PubMed] [Google Scholar]
- 22.Neu, H. C. 1992. The crisis in antibiotic resistance. Science 2571064-1073. [DOI] [PubMed] [Google Scholar]
- 23.Overhage, J., H. Priefert, and A. Steinbuchel. 1999. Biochemical and genetic analyses of ferulic acid catabolism in Pseudomonas sp. strain HR199. Appl. Environ. Microbiol. 654837-4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palumbi, S. R. 2001. Humans as the world's greatest evolutionary force. Science 2931786-1790. [DOI] [PubMed] [Google Scholar]
- 25.Paulsen, I. T. 2003. Multidrug efflux pumps and resistance: regulation and evolution. Curr. Opin. Microbiol. 6446-451. [DOI] [PubMed] [Google Scholar]
- 26.Saier, M. H., Jr., I. T. Paulsen, M. K. Sliwinski, S. S. Pao, R. A. Skurray, and H. Nikaido. 1998. Evolutionary origins of multidrug and drug-specific efflux pumps in bacteria. FASEB J. 12265-274. [DOI] [PubMed] [Google Scholar]
- 27.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 28.Segura, A., P. V. Bunz, D. A. D'Argenio, and L. N. Ornston. 1999. Genetic analysis of a chromosomal region containing vanA and vanB, genes required for conversion of either ferulate or vanillate to protocatechuate in Acinetobacter. J. Bacteriol. 1813494-3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Hijum, S. A., J. A. de, R. J. Baerends, H. A. Karsens, N. E. Kramer, R. Larsen, C. D. den Hengst, C. J. Albers, J. Kok, and O. P. Kuipers. 2005. A generally applicable validation scheme for the assessment of factors involved in reproducibility and quality of DNA-microarray data. BMC Genomics 677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wegmann, U., M. O'Connell-Motherway, A. Zomer, G. Buist, C. Shearman, C. Canchaya, M. Ventura, A. Goesmann, M. J. Gasson, O. P. Kuipers, D. van Sinderen, and J. Kok. 2007. Complete genome sequence of the prototype lactic acid bacterium Lactococcus lactis subsp. cremoris MG1363. J. Bacteriol. 1893256-3270. [DOI] [PMC free article] [PubMed] [Google Scholar]