Abstract
The Chlorobaculum tepidum genome contains two paralogous genes, CT2256 and CT1232, whose products are members of the FixC dehydrogenase superfamily and have sequence similarity to geranylgeranyl reductases. Each gene was insertionally inactivated, and the resulting mutants were characterized. CT2256 encodes geranylgeranyl reductase (BchP); CT1232 is not involved in bacteriochlorophyll or chlorophyll biosynthesis.
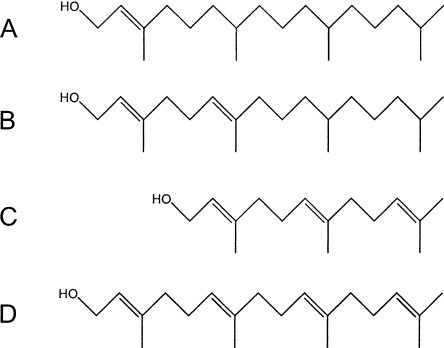
Chlorophyll (Chl) and bacteriochlorophyll (BChl) molecules are composed of two parts: a hydrophilic tetrapyrrole moiety, the “head,” and a hydrophobic alcohol moiety, the “tail.” A Chl or BChl synthase esterifies the C-172 propionic acid carboxylate of the corresponding chlorophyllide (Chlide) or bacteriochlorophyllide (BChlide) head with the appropriate alcohol tail group (1, 13, 14, 20). BChls can be esterified with either isoprenoid or alkyl alcohol moieties (14). Green sulfur bacteria (GSB) synthesize three types of (B)Chl: BChl a, Chl a, and an antenna BChl, which can be BChl c, d, or e (10). Each of these pigments is esterified with a different isoprenoid tail (7, 18) (Fig. 1). In Chlorobaculum tepidum, BChl aP, which is associated with the type 1 reaction centers, the Fenna-Matthews-Olson protein, and CsmA, is esterified with phytol (Fig. 1A). The C-172 position of Chl aPD, which serves as the primary electron acceptor in the reaction centers, is esterified with Δ2,6-phytadienol (Fig. 1B) (18). Finally, the antenna BChl cF in the chlorosomes is esterified with farnesol (7, 23) (Fig. 1C). A small amount (typically <1%) of BChl c has esterifying alcohols other than farnesol (6). The esterification reaction occurs only after the (B)Chlide head group has been completely synthesized and has been shown to be the last or possibly the penultimate step in the biosynthesis of various (B)Chls (5, 14, 16, 20).
FIG. 1.
Esterifying alcohols of GSB (B)Chls. (A) phytol; (B) Δ2,6-phytadienol; (C) farnesol; and (D) geranylgeraniol.
The only reaction in the biosynthesis of (B)Chls that may occur after the attachment of the alcohol moiety is the hydrogenation of geranylgeraniol (GG) to yield phytol or Δ2,6-phytadienol (2, 20). In Arabidopsis thaliana and other plants, the geranylgeranyl reductase (ChlP) appears to be a multifunctional enzyme that can reduce the double bonds of geranylgeranyl-Chl a as well as geranylgeranyl diphosphate (16, 21). Studies with the purple bacterium Rhodobacter sphaeroides, which, like GSB, synthesizes BChl aP, have shown that BchP catalyzes the three double-bond reductions that produce phytol from GG (3). Addlesee and Hunter (3) obtained a bchP mutant of R. sphaeroides that accumulated BChl a esterified with GG. Another mutant that encoded a partially active BchP protein accumulated BChl a esterified with GG, dihydro-GG, tetrahydro-GG (Δ2,6-phytadienol), and phytol. These results strongly imply that BchP is responsible for all three hydrogenation reactions in R. sphaeroides (3). The genomes of purple bacteria sequenced to date contain only one copy of the bchP gene. However, the C. tepidum genome contains two paralogous genes, CT1232 and CT2256, whose products have sequence similarity to the bchP products of other organisms and which belong to the FixC dehydrogenase/oxidoreductase superfamily (8).
To determine which of the two C. tepidum bchP paralogs encodes the geranylgeranyl reductase for Chl/BChl biosynthesis, both genes were insertionally inactivated with aadA from pSRA2 (11). Mutants were constructed by the megaprimer PCR method (see references 11 and 13), and segregation was confirmed by analytical PCR (11) (data not shown). The pigments from a 1-ml aliquot of a dense culture were extracted with acetone-methanol (7:2, vol/vol) and analyzed by high-performance liquid chromatography (HPLC) as described previously (12). BChls were extracted from cultures of R. sphaeroides mutants T6G5 and T6G4 (3), which synthesize only BChl aGG and BChl a esterified with phytol, GG, dihydro-GG, and tetrahydro-GG, respectively, and used as standards (12).
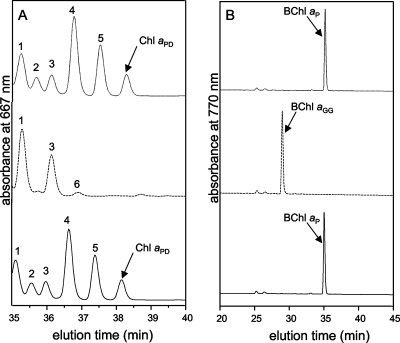
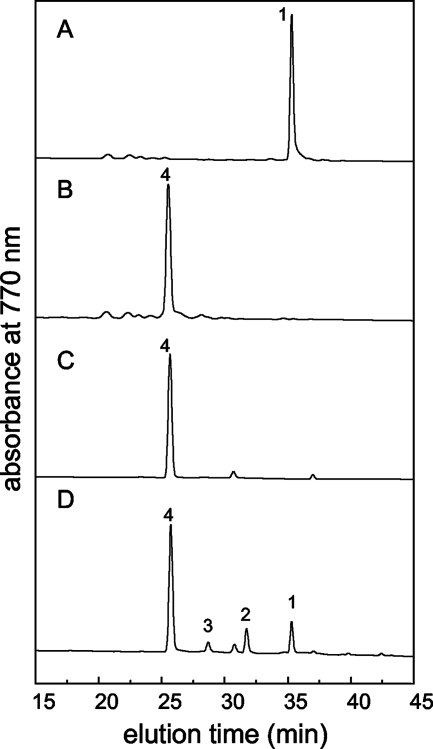
The HPLC elution times of BChl aP and Chl aPD from the CT1232 mutant were identical to those for these compounds from the wild type (Fig. 2). However, the HPLC elution times for BChl a and Chl a from the CT2256 mutant were different (Fig. 2A and B). BChl a from the CT2256 mutant eluted ∼8 min earlier than BChl aP (Fig. 2B). To confirm the identity of the esterifying alcohol of BChl a produced by the CT2256 mutant, a pigment extract of R. sphaeroides T6G5, which exclusively synthesizes BChl aGG (3), was analyzed (Fig. 3C). BChl a from the CT2256 mutant and BChl a from R. sphaeroides T6G5 had identical elution times (Fig. 3B and C). Thus, the esterifying alcohol of BChl a in the CT2256 mutant was determined to be GG.
FIG. 2.
HPLC elution profile of pigment extracts from the C. tepidum wild type and the CT2256 and CT1232 mutant strains. (A) HPLC elution profile recorded at 667 nm for pigment extracts from the wild type (solid line), the CT2256 mutant (dashed line), and the CT1232 mutant (dotted line). Peaks 1 through 6 have absorption spectra identical to those of BChl c but are esterified with alcohols other than farnesol, including phytol. (B) HPLC elution profile of pigment extracts recorded at 770 nm, the absorbance maximum of BChl a in HPLC solvents, for the wild type (solid line), the CT2256 mutant (dashed line), and the CT1232 mutant (dotted line).
FIG. 3.
HPLC elution profile of pigment extracts recorded at 770 nm for the C. tepidum wild type (A), the CT2256 mutant of C. tepidum (B), and the R. sphaeroides strains T6G5 (C) and T6G4 (D). The identified peaks are BChl aP (1), BChl a esterified with tetrahydro-GG (Δ2,6-phytadienol) (2), BChl a esterified with dihydro-GG (3), and BChl aGG (4).
Chl aPD represents less than 1% of the total (B)Chl produced by C. tepidum (6), and it elutes ∼2 min after the last of several minor BChl cP homologs (Fig. 2A). No peak corresponding to Chl aPD was observed at about 38 min in the HPLC elution profile for the CT2256 mutant (Fig. 2A). A mutant lacking BchP is expected to produce Chl aGG, and this compound should elute at about 32 to 33 min together with much more abundant BChl cGG homologs. Because BChl c and Chl a have very similar absorption properties (17), we were unable to detect Chl aGG (Fig. 2A).
The results described above show that CT2256 encodes the geranylgeranyl reductase BchP, while CT1232 does not appear to have a role in (B)Chl biosynthesis. Database analyses show that orthologs of bchP (CT2256) are present in all 10 recently sequenced GSB genomes, while orthologs of CT1232 are found only in C. tepidum, Chlorobium clathratiforme DSMZ 5477, and Prosthecochloris aestuarii DSMZ 271. This distribution supports the conclusion that CT1232 is not involved in (B)Chl biosynthesis and that it must have a specialized function of limited occurrence in GSB. The genome of the archaeon Archaeoglobus fulgidus encodes four proteins with similarity to geranylgeranyl reductases (15). One of these proteins was recently shown to reduce the isoprenoid tail of menaquinone (15). However, the elution time of menaquinone in the CT1232 mutant was the same as that for the wild type (data not shown). A second reductase, AF0464, which appears to be orthologous to CT1232, was very recently shown to reduce the geranylgeranyl moieties of isoprenoid lipids in A. fulgidus (19). These observations suggest that some GSB may produce lipids with reduced isoprenoid tails.
The results reported here clearly demonstrate that C. tepidum BchP is responsible for the saturation of the GG moieties of both BChl a and Chl a, which produce phytol and Δ2,6-phytadienol, respectively. These results invalidate a previous hypothesis that CT2256 encodes a BChl a-specific saturase while CT1232 encodes a Chl a-specific saturase (9) and additionally exclude the possibility that both gene products are required for GG reduction. In Rhodospirillum rubrum, BchP acts after BChl a synthase to produce bacteriopheophytin aP from bacteriopheophytin aGG, and neither the isoprenyl diphosphates nor BChl aGG was a substrate (4). Thus, BchP can have very specific substrate requirements and might produce products different from BChl a and Chl a. However, in R. capsulatus and plants, both the free isoprenyl diphosphates and the tails of (B)Chls are substrates of GG reductase (3, 16, 20, 21). The presence of exclusively Chl aPD and BChl aP in wild-type C. tepidum suggests two possibilities. Either BchP acts after the esterification of GG to Chlide a or BChlide a by the respective ChlG or BchG synthase, or these (B)Chl synthases must have nearly absolute specificity for both the appropriate isoprenyl diphosphate and the head group. BChl c synthase (BchK) does not have absolute specificity for the esterifying alcohol (6, 13). Similarly, indicating that BchG does not have absolute specificity for phytol, wild-type levels of BChl a (as BChl aGG) occur in the C. tepidum bchP mutant (Fig. 2 and 3). Thus, as observed for the Chl/BChl synthases from plant and purple bacterial synthases (20, 22), it seems likely that BchP preferentially acts after esterification of Chlide a and BChlide a with GG by ChlG and BchG, respectively. Biochemical experiments in vitro will be required to resolve these issues conclusively.
Acknowledgments
We thank C. Neil Hunter (University of Sheffield) for the R. sphaeroides TGC5 and TGC4 strains.
This work was supported by grant DE-FG02-94ER20137 from the U.S. Department of Energy to D.A.B.
Footnotes
Published ahead of print on 9 November 2007.
REFERENCES
- 1.Addlesee, H. A., L. Fiedor, and C. N. Hunter. 2000. Physical mapping of bchG, orf427, and orf177 in the photosynthesis gene cluster of Rhodobacter sphaeroides: functional assignment of the bacteriochlorophyll synthetase gene. J. Bacteriol. 1823175-3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Addlesee, H. A., L. C. D. Gibson, P. E. Jensen, and C. N. Hunter. 1996. Cloning, sequencing and functional assignment of the chlorophyll biosynthesis gene, chlP, of Synechocystis sp. PCC 6803. FEBS Lett. 389126-130. [DOI] [PubMed] [Google Scholar]
- 3.Addlesee, H. A., and C. N. Hunter. 1999. Physical mapping and functional assignment of the geranylgeranyl-bacteriochlorophyll reductase gene, bchP, of Rhodobacter sphaeroides. J. Bacteriol. 1817248-7255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Addlesee, H. A., and C. N. Hunter. 2002. Rhodospirillum rubrum possesses a variant of the bchP gene, encoding geranylgeranyl-bacteriopheophytin reductase. J. Bacteriol. 1841578-1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beale, S. I. 1999. Enzymes of chlorophyll biosynthesis. Photosynth. Res. 6043-73. [Google Scholar]
- 6.Borrego, C. M., P. D. Gerola, M. Miller, and R. P. Cox. 1999. Light intensity effects on pigment composition and organisation in the green sulfur bacterium Chlorobium tepidum. Photosynth. Res. 59159-166. [Google Scholar]
- 7.Caple, M. B., H. Chow, and C. E. Strouse. 1978. Photosynthetic pigments of green sulfur bacteria: the esterifying alcohols of bacteriochlorophylls c from Chlorobium limicola. J. Biol. Chem. 2536730-6737. [PubMed] [Google Scholar]
- 8.Eisen, J. A., K. E. Nelson, I. T. Paulsen, J. F. Heidelberg, M. Wu, R. J. Dodson, R. Deboy, M. L. Gwinn, W. C. Nelson, D. H. Haft, E. K. Hickey, J. D. Peterson, A. S. Durkin, J. L. Kolonay, F. Yang, I. Holt, L. A. Umayam, T. Mason, M. Brenner, T. P. Shea, D. Parksey, W. C. Nierman, T. V. Feldblyum, C. L. Hansen, M. B. Craven, D. Radune, J. Vamathevan, H. Khouri, O. White, T. M. Gruber, K. A. Ketchum, J. C. Venter, H. Tettelin, D. A. Bryant, and C. M. Fraser. 2002. The complete genome sequence of Chlorobium tepidum TLS, a photosynthetic, anaerobic, green-sulfur bacterium. Proc. Natl. Acad. Sci. USA 999509-9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frigaard, N.-U., A. Gomez Maqueo Chew, H. Li, J. A. Maresca, and D. A. Bryant. 2003. Chlorobium tepidum: insights into the structure, physiology, and metabolism of a green sulfur bacterium derived from the complete genome sequence. Photosynth. Res. 7893-117. [DOI] [PubMed] [Google Scholar]
- 10.Frigaard, N.-U., A. Gomez Maqueo Chew, J. A. Maresca, and D. A. Bryant. 2006. Bacteriochlorophyll biosynthesis in green bacteria, p. 201-221. In B. Grimm, R. J. Porra, W. Rüdiger, and H. Scheer (ed.), Advances in photosynthesis and respiration, vol. 25. Chlorophylls and bacteriochlorophylls: biochemistry, biophysics, functions and applications. Springer, Dordrecht, The Netherlands. [Google Scholar]
- 11.Frigaard, N.-U., Y. Sakuragi, and D. A. Bryant. 2004. Gene inactivation in the cyanobacterium Synechococcus sp. PCC 7002 and the green sulfur bacterium Chlorobium tepidum using in vitro-made DNA constructs and natural transformation. Methods Mol. Biol. 274325-340. [DOI] [PubMed] [Google Scholar]
- 12.Frigaard, N.-U., S. Takaichi, M. Hirota, K. Shimada, and K. Matsuura. 1997. Quinones in chlorosomes of green sulfur bacteria and their role in the redox-dependent fluorescence studied in chlorosome-like bacteriochlorophyll c aggregates. Arch. Microbiol. 167343-349. [Google Scholar]
- 13.Frigaard, N.-U., G. D. Voigt, and D. A. Bryant. 2002. Chlorobium tepidum mutant lacking bacteriochlorophyll c made by inactivation of the bchK gene, encoding bacteriochlorophyll c synthase. J. Bacteriol. 1843368-3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomez Maqueo Chew, A., and D. A. Bryant. 2007. Chlorophyll biosynthesis in bacteria: the origins of structural and functional diversity. Annu. Rev. Microbiol. 61113-129. [DOI] [PubMed] [Google Scholar]
- 15.Hemmi, H., Y. Takahashi, K. Shibuya, T. Nakayama, and T. Nishino. 2005. Menaquinone-specific prenyl reductase from the hyperthermophilic archaeon Archaeoglobus fulgidus. J. Bacteriol. 1871937-1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keller, Y., F. Bouvier, A. D'Harlingue, and B. Camara. 1998. Metabolic compartmentation of plastid prenyllipid biosynthesis—evidence for the involvement of a multifunctional geranylgeranyl reductase. Eur. J. Biochem. 251413-417. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi, M., M. Akiyama, H. Kano, and H. Kise. 2006. Spectroscopy and structure determination, p. 79-94. In B. Grimm, R. J. Porra, W. Rüdiger, and H. Scheer (ed.), Advances in photosynthesis and respiration, vol. 25. Chlorophylls and bacteriochlorophylls: biochemistry, biophysics, functions and applications. Springer, Dordrecht, The Netherlands. [Google Scholar]
- 18.Kobayashi, M., H. Oh-Oka, S. Akutsu, M. Akiyama, K. Tominaga, H. Kise, F. Nishida, T. Watanabe, J. Amesz, M. Koizumi, N. Ishida, and H. Kano. 2000. The primary electron acceptor of green sulfur bacteria, bacteriochlorophyll 663, is chlorophyll a esterified with Δ2,6-phytadienol. Photosynth. Res. 63269-280. [DOI] [PubMed] [Google Scholar]
- 19.Murakami, M., K. Shibuya, T. Nakayama, T. Nishino, T. Yosimura, and H. Hemmi. 2007. Geranylgeranyl reductase involved in the biosynthesis of archaeal membrane lipids in the hyperthermophilic archaeon Archaeoglobus fulgidus. FEBS J. 274805-814. [DOI] [PubMed] [Google Scholar]
- 20.Rüdiger, W. 2006. Biosynthesis of chlorophylls a and b: the last steps, p. 189-200. In B. Grimm, R. J. Porra, W. Rüdiger, and H. Scheer (ed.), Advances in photosynthesis and respiration, vol. 25. Chlorophylls and bacteriochlorophylls: biochemistry, biophysics, functions and applications. Springer, Dordrecht, The Netherlands. [Google Scholar]
- 21.Soll, J., G. Schultz, W. Rüdiger, and J. Benz. 1983. Hydrogenation of geranylgeraniol: two pathways exist in spinach chloroplasts. Plant Physiol. 71849-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzuki, J. Y., D. W. Bollivar, and C. E. Bauer. 1997. Genetic analysis of chlorophyll biosynthesis. Annu. Rev. Genet. 3161-89. [DOI] [PubMed] [Google Scholar]
- 23.van Noort, P. I., C. Francke, N. Schoumans, S. C. M. Otte, T. J. Aartsma, and J. Amesz. 1994. Chlorosomes of green sulfur bacteria: pigment composition and energy transfer. Photosynth. Res. 41193-203. [DOI] [PubMed] [Google Scholar]