Abstract
The role of flagellin glycosylation on motility was investigated in Pseudomonas syringae pv. tabaci. The swimming activity of glycosylation-defective mutants was prominently decreased in a highly viscous medium. The mutants showed differences in polymorphic transitions and in the bundle formation of flagella, indicating that glycosylation stabilizes the filament structure and lubricates the rotation of the bundle.
Pseudomonas syringae pv. tabaci 6605 is a phytopathogenic bacterium that causes wildfire disease in tobacco plants (10, 26). The cell possesses several flagella at the cell pole when grown in liquid cultures. Our previous study revealed that the flagella of this bacterial strain are indispensable for intrinsic virulence on the host tobacco plant and that flagellin, the major component protein of the flagellum, is a major elicitor of hypersensitive cell death in nonhost plants (10, 24, 26-28). Furthermore, the flagellin of P. syringae was found to be glycosylated at six serine residues by the products of the orf1 and orf2 genes that are located in a glycosylation island (26, 29, 30). Recently, the orf1 and orf2 genes in the P. syringae glycosylation island have been renamed fgt1 (flagellar glycosyltransferase 1) and fgt2, respectively. To examine the roles of glycosylation in bacterial virulence and interactions with plants, we constructed a glycosylation-defective mutant (Δfgt1), a partially defective mutant (Δfgt2), mutants replacing a single Ser with Ala (S143A, S164A, S176A, S183A, S193A, and S201A) and a mutant replacing six serines with alanines (6 S/A) (26). Using these mutants, we demonstrated that the glycosylation of flagellin is required for virulence toward host tobacco plants and swarming and adhesion abilities; thus, glycosylation may play an important role in determining host specificity (26).
In this study, swimming ability, polymorphic flagellar transitions at various pH and salt concentrations, and bundle formation were analyzed to compare the structural and functional differences between flagella of the wild type (WT) bacteria and glycosylation-defective mutants.
Effect of viscosity on swimming of the WT and glycosylation-defective mutants.
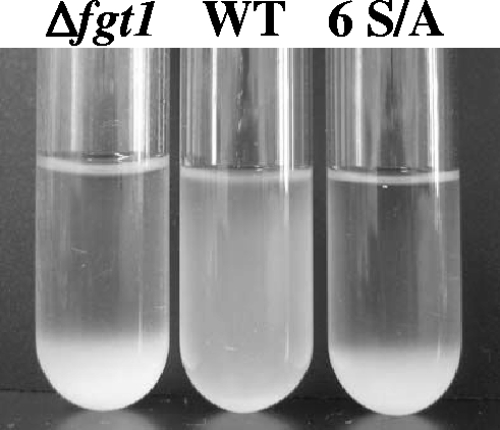
WT and mutant strains were grown in LB medium supplemented with 10 mM MgCl2 with vigorous agitation at 25°C for 24 h. The overnight cultures were left standing without agitation for another 6 h. While WT cells remained in suspension, the Δfgt1 and 6 S/A mutant cells were precipitated (Fig. 1). To investigate the cause of this phenomenon, the proportions of swimming cells in the supernatant and precipitate from each sample were compared. The swimming bacteria were observed by using a phase contrast microscope equipped with a video recording system. Approximately 200 cells were counted to calculate the percentage of swimming cells. More than 60% of WT cells in the suspension culture swam, whereas 18% of Δfgt1 and 19% of 6 S/A mutant cells in the supernatant of each culture did. Furthermore, only 6% of Δfgt1 and 7% of 6 S/A mutant cells in the culture precipitates swam. These results might indicate that a defect of swimming ability in these mutants causes the aggregation of cells.
FIG. 1.
Two-night culture of the WT, the fgt1-deleted mutant (Δfgt1), and six-serine-replacement mutants (6 S/A). Bacterial strains were incubated in LB supplemented with 10 mM MgCl2 for 24 h at 25°C with agitation and then for 20 h without agitation.
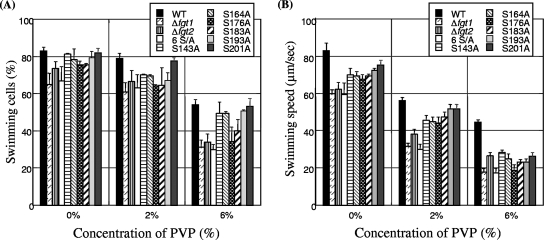
For a more-precise investigation of the ability of the flagella to propel the cell in liquid culture, the effect of viscosity was examined by conventional phase contrast microscopy according to a previously reported method (3). Cells were cultured overnight in LB medium supplemented with 10 mM MgCl2 and inoculated into MMMF minimal medium (50 mM potassium phosphate buffer, 7.6 mM (NH4)2SO4, 1.7 mM MgCl2, and 1.7 mM NaCl, pH 5.7) supplemented with 10 mM each of mannitol and fructose and cultured at 23°C for 24 h. The viscosity was increased by the addition of polyvinylpyrrolidone K 90 (PVP; Wako Pure Chemical Industries) to the MMMF culture medium. As shown in Fig. 2A, approximately 83% of WT cells swam in the absence of PVP, while about 65 to 75% of cells from the Δfgt1, Δfgt2, six-serine-replaced (6 S/A), S176A, and S183A mutant strains swam. In the presence of 2% PVP, the percentages of swimming cells of all bacterial strains except the WT were decreased. In the presence of 6% PVP, the rates of swimming cells of the WT and four single-serine-replaced mutant strains (S143A, S164A, S193A, and S201A) were 50 to 55%, and those of other mutant strains (Δfgt1, Δfgt2, 6 S/A, S176A, and S183A) were decreased to about 30 to 38%. We previously reported that Δfgt1 (Δorf1), Δfgt2 (Δorf2), 6 S/A, S176A, and S183A mutants were impaired in pathogenicity on host tobacco plants and had reduced adhesion and swarming abilities on an SWM plate (0.5% agar, 0.5% peptone, 0.3% yeast extract) (26). The lower swimming ability of the glycosylation-defective mutants in viscous medium may be one of the causes of these phenotypes.
FIG. 2.
(A) Effect of viscosity on swimming motility of the WT and glycosylation-defective mutants (Δfgt1, fgt2-deleted mutant [Δfgt2], 6 S/A, and six mutants replacing a single Ser with Ala [S143A, S164A, S173A, S183A, S193A, and S201A]) from P. syringae pv. tabaci 6605. The percentage of swimming cells is indicated for each. Viscosity was increased by the addition of PVP. (B) Effect of viscosity on swimming speed of the WT and glycosylation-defective mutants from P. syringae pv. tabaci 6605.
The swimming speed was calculated by tracing the tracks of individual bacteria recorded on videotape and measuring the distance traveled in a period of time (3). The profiles for swimming speed against viscosity of the WT and mutant strains are shown in Fig. 2B. In the absence of PVP, WT cells swam in MMMF medium at 83 μm/s, cells of four single-serine-replaced mutant strains (S143A, S164A, S193A, and S201A) swam at 70 to 76 μm/s, and cells of the other mutant strains (Δfgt1, Δfgt2, 6 S/A, S176A, and S183A) swam at 59 to 69 μm/s. In 2% PVP, the swimming speed of all bacterial strains was slightly decreased. The viscosity effect was more prominent in 6% PVP; the cell swimming speeds of the WT, four single-serine-replaced mutants (S143A, S164A, S193A, and S201A), and the Δfgt1, Δfgt2, 6 S/A, S176A, and S183A mutants were 45 μm/s, 24 to 28 μm/s, and 17 to 23 μm/s, respectively.
Because the reductions of the percentage of swimming cells and the swimming speed might be due to a regulatory effect on gene expression, we performed an immunoblot analysis to measure flagellin protein accumulation. Each overnight culture (LB with 10 mM MgCl2) was centrifuged, and the concentration of cells was adjusted to 2 × 108 CFU ml−1. Proteins were separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and an antibody that was raised against purified flagellin from P. syringae pv. tabaci was used (27). However, the amounts of flagellin protein from each mutant were almost identical (data not shown), indicating that there are no significant differences in flagella numbers in WT and glycosylation-defective mutant strains.
Polymorphic transition and bundle formation of flagellar filaments from WT and glycosylation-defective mutants.
The bacterial flagellum is a filament consisting of flagellin protein, and the helical shape, which is defined by the helical parameters of pitch and diameter, is essential for movement. Despite the different primary structures of flagellins, flagellar helices are similar among members of the same family (23). In peritrichously flagellated species, such as Escherichia coli and Salmonella enterica serovar Typhimurium, the left-handed helical filament named “Normal” is the common form in smoothly swimming cells, and the right-handed form called “Curly” appears only transiently during cell tumbling (16). These two shapes are reversibly converted under various environmental conditions, such as changes in pH, salt concentration, and temperature. Other polymorphs include “Coiled” and “SemiCoiled,” which are not very effective for movement. In an extreme case, the Straight form was found in nonmotile mutants with amino acid substitutions (18). However, in polar-flagellated species, such as the marine bacterium Idiomarina loihiensis and Pseudomonas aeruginosa, the helical parameters are smaller than those of peritrichously flagellated species. For example, the Normal form of the polar flagellum is similar to the Curly form of the peritrichous flagellum in pitch and diameter but is left-handed. (23). We categorized the left-handed Curly-like filaments as small-Normal (S-Normal) and assumed that P. syringae pv. tabaci flagella might belong to this flagellar group.
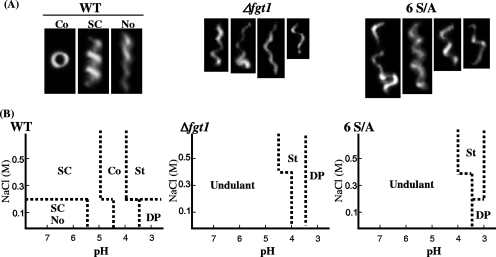
To compare the nature of flagellar filaments of the WT and glycosylation-defective mutants of P. syringae pv. tabaci 6605, the helical parameters of each polymorph were measured, as shown in Table 1. The polymorphic transitions due to changes in pH and salt concentration were examined as described by Kamiya and Asakura (13). Flagellar filaments were purified as described previously (23). The polymorphic shapes of filaments observed by dark-field light microscopy and diagrams of the polymorphs observed are shown in Fig. 3A and B. At low pH (pH 5.0 to 7.0), the shapes of flagellar filaments were predominantly Semi-Coiled, and S-Normal (left-handed Curly-like) filaments were also found at a low NaCl concentration (0.1 M). When the pH was further shifted to the acidic, the filaments were changed to the Coiled form and then the Straight form. At pH 3.0 and 0.1 M NaCl concentration, flagellar filaments were depolymerized.
TABLE 1.
Helical parameters of flagellar filaments of P. syringae
| Polymorphic form | Handedness | Pitch (μm) | Diam (μm) |
|---|---|---|---|
| S-Normal | Left | 1.59 | 0.18 |
| Semi-Coiled | Left | 1.49 | 0.39 |
| Coiled | Left | 1.04 | 0.65 |
FIG. 3.
Polymorphic transitions of flagellar filaments from the WT and glycosylation-defective mutants of P. syringae pv. tabaci 6605. (A) Dark-field light micrographs of flagella. Typical images of Coiled, Semi-Coiled, and a mixture of Semi-Coiled and Normal filaments prepared from the WT and undulant filaments prepared from Δfgt1 and 6 S/A mutant strains. (B) Phase diagrams of polymorphs by pH and NaCl concentration. SC, Semi-Coiled; No, Normal; Co, Coiled; St, Straight; DP, depolymerized.
On the other hand, various abnormal shapes of flagellar filaments were observed in the Δfgt1 and 6 S/A mutants between pH 4.0 to 7.0 in a wide range of salt concentrations. These results suggest that the filaments from nonglycosylated mutants show no distinct polymorphic forms and do not take on proper polymorphs in response to the change of environmental conditions. Because single filaments of the nonglycosylated mutant showed different shapes at the same time, we called them “undulant” filaments. When the pH was further shifted to the acidic, filaments from both mutants changed to the Straight form and then were depolymerized. In the cases of mutants with single Ser-to-Ala replacements, the polymorphic transition of flagellar filaments was similar to that of the WT. Moreover, there was no correlation between the polymorphic transition and viscosity in flagellar filaments from both the WT and mutants (data not shown).
We suspected that undulant filaments from nonglycosylated mutants might be structurally unstable and, thus, measured the amounts of unpolymerized flagellin present in the spent medium. The protein from the supernatant of overnight cultures of each strain was precipitated by the addition of trichloroacetic acid at a final concentration of 10% (wt/vol) and dissolved in 1/100 of the original volume of PBST buffer (137 mM NaCl, 8.1 mM Na2HPO4, 2.68 mM KCl, 1.47 mM KH2PO4, and Tween 20, pH 7.4). The immunoblot analysis revealed no significant difference in the amounts of intact flagellin from each mutant and the WT strain. Furthermore, we did not detect broken filaments in the spent media by electron microscopy (data not shown). These results suggest that the filament formation of glycosylation-defective mutants was normal.
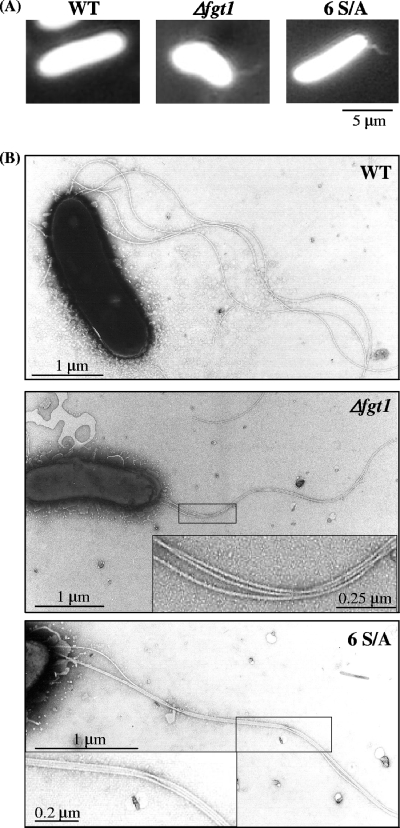
Peritrichous flagella form a bundle when the cell is swimming smoothly. The bundle formation of flagella on WT and nonglycosylated mutant cells was compared by using dark-field microscopy. The shapes of flagella have been reported as bright particles with twisted bundles when seen by dark-field microscopy under strong illumination (19). With this method, bundled flagella were observed only on slowly moving or resting cells. The flagellar bundles on WT cells were too loose to observe, while irregular flagellar bundles were constantly observed in Δfgt1 and 6 S/A mutants (Fig. 4A). The binding between filaments appeared tight with electron microscopy, although this seldom occurs in WT cells (Fig. 4B). We also examined the bundle formation using flagella ejected from WT and nonglycosylated mutants, followed by polyethylene glycol precipitation (23). Many thick flagellar bundles were observed only in preparations of the mutants. Furthermore, bundled flagella were also found frequently with dark-field microscopy and electron microscopy (data not shown) in the precipitated samples from nonglycosylated mutants shown in Fig. 1. These results suggested that the surface charge or hydrophobic properties of the filament of the nonglycosylation mutants might be changed so that more than two filaments interact tightly along their length. This irregular entanglement of filaments would result in a reduction of swimming ability in these mutants.
FIG. 4.
Bundle formation of flagella from glycosylation-defective mutants. (A) Bundle formation of flagella in swimming bacteria (the WT and Δfgt1 and 6 S/A mutants) under a dark-field microscope. (B) Electron micrographs of each strain. Insets are magnifications of the entangled flagella.
Glycosylation of the surface structure has been reported for pili (6, 7), for S-layers (21), and for flagella (1, 5, 8, 9, 12, 27, 29, 31). In gram-negative bacteria, glycosylation has been shown to play important roles in adhesion (4, 14), solubility (17), immune response (2, 20, 25, 32), motility, and flagellar filament assembly (22). Further, it was also pointed out that glycosylation of flagellin in Archae may increase the structural stability of the filament and its resistance to proteolysis (15). We previously demonstrated that all the glycosylation sites of the flagellin molecule in P. syringae pv. tabaci 6605 are located on the putative surface-exposed region and that glycosylation might be involved in pathovar-specific recognition (11, 26, 30). This exposed surface region is also considered to be a major antigen for the adaptive immune system in mammals (33). Very recently, we identified the glycan structure at serine 201 (S201) of flagellin from P. syringae pv. tabaci and pv. glycinea by sugar composition analysis, mass spectrometry, and 1H and 13C nuclear magnetic resonance spectroscopy. The S201 glycan was composed of a unique trisaccharide consisting of two rhamnosyl residues and one modified 4-amino-4,6-dideoxyglucosyl residue (29). Further analysis to elucidate the glycan structure is in progress.
The results obtained in this study revealed that flagellin glycosylation facilitates proper flagellar suprastructures that contribute to the proper swimming ability of the bacterium. The regular transitions of flagella morphology indicate that glycosylation of the filament surface increases the slippage between filaments and contributes to smooth swimming. Previously we found that the glycosylation of flagellin is required for bacterial virulence (26, 30). The reduction of motility eventually impairs the virulence of glycosylation-defective mutants. In nature, flagellin glycosylation may enhance the swimming ability on the viscous and sticky surface of tobacco leaves. Together with our previous results, it is likely that the glycosylation of flagellin in P. syringae pv. tabaci 6605 is indispensable for virulence on the host tobacco plant.
Acknowledgments
We thank the Leaf Tobacco Research Laboratory of Japan Tobacco, Inc., for providing P. syringae pv. tabaci 6605.
This work was supported in part by a Grant-in-Aid for Scientific Research (B) (grant 18380035) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and by the program for Promotion of Basic Research Activities for Innovative Bioscience (PROBRAIN), the Okayama University COE Program for the Establishment of Plant Health Science (grant to Y.I.), and the Soft-Nano Machine Project, Japan Science and Technology Agency (grant to S.-I.A).
Footnotes
Published ahead of print on 16 November 2007.
REFERENCES
- 1.Arora, S. K., M. Bangera, S. Lory, and R. Ramphal. 2001. A genomic island in Pseudomonas aeruginosa carries the determinants of flagellin glycosylation. Proc. Natl. Acad. Sci. USA 989342-9347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arora, S. K., A. N. Neely, B. Blair, S. Lory, and R. Ramphal. 2005. Role of motility and flagellin glycosylation in the pathogenesis of Pseudomonas aeruginosa burn wound infections. Infect. Immun. 734395-4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atsumi, T., Y. Maekawa, T. Yamada, I. Kawagishi, Y. Imae, and M. Homma. 1996. Effect of viscosity on swimming by the lateral and polar flagella of Vibrio alginolyticus. J. Bacteriol. 1785024-5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benz, L., and M. A. Schmidt. 2001. Glycosylation with heptose residues mediated by the aah gene A product is essential for adherence of the AIDA-I adhesion. Mol. Microbiol. 401403-1413. [DOI] [PubMed] [Google Scholar]
- 5.Brimer, C. D., and T. C. Montie. 1998. Cloning and comparison of fliC genes and identification of glycosylation in the flagellin in the flagella of Pseudomonas aeruginosa A-type strains. J. Bacteriol. 1783209-3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castric, P. 1995. pilO, a gene required for glycosylation of Pseudomonas aeruginosa 1244 pilin. Microbiology 1411247-1254. [DOI] [PubMed] [Google Scholar]
- 7.Castric, P., F. J. Cassels, and R. W. Carlson. 2001. Structural characterization of the Pseudomonas aeruginosa 1244 pilin. J. Biol. Chem. 27626479-26485. [DOI] [PubMed] [Google Scholar]
- 8.Che, F.-S., Y. Nakajima, N. Tanaka, M. Iwano, T. Yoshida, S. Takayama, I. Kadota, and A. Isogai. 2000. Flagellin from an incompatible strain of Pseudomonas avenae induces a resistance response in cultured rice cells. J. Biol. Chem. 27532347-32356. [DOI] [PubMed] [Google Scholar]
- 9.Doig, P., N. Kinsella, P. Guerry, and T. J. Trust. 1996. Characterization of a post-translational modification of Campylobacter flagellin: identification of a sero-specific glycosyl moiety. Mol. Microbiol. 19379-387. [DOI] [PubMed] [Google Scholar]
- 10.Ichinose, Y., R. Shimizu, Y. Ikeda, F. Taguchi, M. Marutani, T. Mukaihara, Y. Inagaki, K. Toyoda, and T. Shiraishi. 2003. Need for flagella for complete virulence of Pseudomonas syringae pv. tabaci: genetic analysis with flagella-defective mutants ΔfliC and ΔfliD in host tobacco plants. J. Gen. Plant Pathol. 69244-249. [Google Scholar]
- 11.Ishiga, Y., K. Takeuchi, F. Taguchi, Y. Inagaki, K. Toyoda, T. Shiraishi, and Y. Ichinose. 2005. Defense responses of Arabidopsis thaliana inoculated with Pseudomonas syringae pv. tabaci wild type and defective mutants for flagellin (ΔfliC) and flagellin-glycosylation (Δorf1). J. Gen. Plant Pathol. 71302-307. [Google Scholar]
- 12.Josenhans, C., R. L. Ferrero, A. Labigne, and S. Suerhaum. 1999. Cloning and allelic exchange mutagenesis of two flagellin genes of Helicobacter felis. Mol. Microbiol. 33350-362. [DOI] [PubMed] [Google Scholar]
- 13.Kamiya, R., and S. Asakura. 1977. Flagellar transformations at alkaline pH. J. Mol. Biol. 108513-518. [DOI] [PubMed] [Google Scholar]
- 14.Lindenthal, C., and E. A. Elsinghorst. 1999. Identification of a glycoprotein production by enterotoxigenic Escherichia coli. Infect. Immun. 674087-4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Logan, S. M. 2006. Flagellar glycosylation: a new component of the motility repertoire? Microbiology 1521249-1262. [DOI] [PubMed] [Google Scholar]
- 16.Macnab, R. M., and M. K. Ornston. 1977. Normal-to-curly flagellar transitions and their role in bacterial tumbling. Stabilization of an alternative quaternary structure by mechanical force. J. Mol. Biol. 1121-30. [DOI] [PubMed] [Google Scholar]
- 17.Marceau, M., and X. Nassif. 1999. Role of glycosylation at Ser63 in production of soluble pilin in pathogenic Neisseria. J. Bacteriol. 181656-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuura, S., R. Kamiya, and S. Asakura. 1978. Transformation of straight flagella and recovery of motility in a mutant Escherichia coli. J. Mol. Biol. 118431-440. [DOI] [PubMed] [Google Scholar]
- 19.Mitani, M., and T. Iino. 1965. Electron microscopy of bundled flagella of the curly mutant of Salmonella abortivoequina. J. Bacteriol. 901096-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romain, F., C. Horn, P. Pescher, A. Namane, M. Riviere, G. Puzo, O. Barzu, and G. Marchal. 1999. Deglycosylation of the 45/47-kilodalton antigen complex of Mycobacteria tuberculosis decreases its capacity to elicit in vivo or in vitro cellular immune responses. Infect. Immun. 675567-5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schaffer, C., and P. Messner. 2001. Glycobiology of surface layer proteins. Biochimie 83591-599. [DOI] [PubMed] [Google Scholar]
- 22.Schirm, M., S. K. Arora, A. Verma, E. Vinogradov, P. Thibault, R. Ramphal, and S. M. Logan. 2004. Structural and genetic characterization of glycosylation of type-A flagellin in Pseudomonas aeruginosa. J. Bacteriol. 1862523-2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shibata, S., M. Alam, and S. Aizawa. 2005. Flagellar filaments of the deep-sea bacteria Idiomarina loihiensis belong to a family different from those of Salmonella typhimurium. J. Mol. Biol. 352510-516. [DOI] [PubMed] [Google Scholar]
- 24.Shimizu, R., F. Taguchi, M. Marutani, T. Mukaihara, Y. Inagaki, K. Toyoda, T. Shiraishi, and Y. Ichinose. 2003. The ΔfliD mutant of Pseudomonas syringae pv. tabaci, which secretes flagellin monomers, induces a strong hypersensitive reaction (HR) in non-host tomato cells. Mol. Genet. Genomics 26921-30. [DOI] [PubMed] [Google Scholar]
- 25.Szymanski, C. M., D. H. Burr, and P. Guerry. 2002. Campylobactor protein glycosylation affects host cell interactions. Infect. Immun. 702242-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taguchi, F., K. Takeuchi, E. Katoh, K. Murata, T. Suzuki, M. Marutani, T. Kawasaki, M. Eguchi, S. Katoh, H. Kaku, C. Yasuda, Y. Inagaki, K. Toyoda, T. Shiraishi, and Y. Ichinose. 2006. Identification of glycosylation genes and glycosylated amino acids of flagellin in Pseudomonas syringae pv. tabaci. Cell. Microbiol. 8923-938. [DOI] [PubMed] [Google Scholar]
- 27.Taguchi, F., R. Shimizu, Y. Inagaki, K. Toyoda, T. Shiraishi, and Y. Ichinose. 2003. Post-translational modification of flagellin determines the specificity of HR induction. Plant Cell Physiol. 44342-349. [DOI] [PubMed] [Google Scholar]
- 28.Taguchi, F., R. Shimizu, R. Nakajima, K. Toyoda, T. Shiraishi, and Y. Ichinose. 2003. Differential effects of flagellins from Pseudomonas syringae pv. tabaci, tomato and glycinea on plant defense response. Plant Physiol. Biochem. 41165-174. [Google Scholar]
- 29.Takeuchi, K., H. Ono, M. Yoshida, T. Ishii, E. Katoh, F. Taguchi, R. Miki, K. Murata, H. Kaku, and Y. Ichinose. 2007. Flagellin glycans from two pathovars of Pseudomonas syringae contain rhamnose at different ratios of d- and l-configurations and modified 4-amino-4,6-dideoxyglucose. J. Bacteriol. 1896945-6956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takeuchi, K., F. Taguchi, Y. Inagaki, K. Toyoda, T. Shiraishi, and Y. Ichinose. 2003. Flagellin glycosylation island in Pseudomonas syringae pv. glycinea and its role in host specificity. J. Bacteriol. 1856658-6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thibault, P., S. M. Logan, J. F. Kelly, J. R. Brisson, C. P. Ewing, T. J. Trust, and P. Guerry. 2001. Identification of the carbohydrate moieties and glycosylation motifs in Campylobacter jejuni flagellin. J. Biol. Chem. 27634862-34870. [DOI] [PubMed] [Google Scholar]
- 32.Verma, A., S. K. Arora, S. K. Kuravi, and R. Ramphal. 2005. Roles of specific amino acids in the N terminus of Pseudomonas aeruginosa flagellin and of flagellin glycosylation in the innate immune response. Infect. Immun. 738237-8246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zipfel, C., and G. Felix. 2005. Plants and animals: a different taste for microbes? Curr. Opin. Plant Biol. 8353-360. [DOI] [PubMed] [Google Scholar]