Abstract
To characterize the roles of SigB and SigF in sigma factor regulation in Mycobacterium tuberculosis, we used chemically inducible recombinant strains to conditionally overexpress sigB and sigF. Using whole genomic microarray analysis and quantitative reverse transcription-PCR, we investigated the resulting global transcriptional changes after sigB induction, and we specifically tested the relative expression of other sigma factor genes after knock-in expression of sigB and sigF. Overexpression of sigB resulted in significant upregulation of genes encoding several early culture filtrate antigens (ESAT-6-like proteins), ribosomal proteins, PE-PGRS proteins, the keto-acyl synthase, KasA, and the regulatory proteins WhiB2 and IdeR. Of note, the induction of sigB did not alter the expression of other sigma factor genes, indicating that SigB is likely to serve as an end regulator for at least one branch of the M. tuberculosis sigma factor regulatory cascade. Analysis of the 5′-untranslated region (UTR) of SigB-dependent transcripts revealed a putative consensus sequence of NGTGG-N14-18-NNGNNG. This sequence appeared upstream of both sigB (Rv2710) and the gene following it, ideR (Rv2711), and in vitro transcription analysis with recombinant SigB-reconstituted RNA polymerase confirmed SigB-dependent transcription from each of these promoters. Knock-in expression of sigF revealed that only the sigC gene was significantly upregulated 6 and 12 h after sigF induction. The previously identified SigF promoter consensus sequence AGTTTG-N15-GGGTTT was identified in the 5′ UTR of the sigC gene, and SigF-dependent in vitro transcription of the promoter upstream of sigC was confirmed by using recombinant SigF-reconstituted RNA polymerase. These two knock-in recombinant strains were tested in a macrophage model of infection which showed that overexpression of sigB and sigF resulted in reduced rates of M. tuberculosis intracellular growth. These results define the SigB promoter consensus recognition sequence and members of the SigB regulon. Moreover, the data suggest that, in addition to serving as an end regulator in a sigma factor cascade, SigB may auto-amplify its own expression under certain conditions.
Mycobacterium tuberculosis remains one of the most important human pathogens since its discovery over a century ago. A unique feature of M. tuberculosis is its ability to persist in a latent state without causing disease. Because M. tuberculosis is exposed to stresses such as nutrient limitation and antimicrobial products of the immune system during host infection, its adaptation to different environments through regulation of genetic programs is an essential feature of virulence. Tubercle bacilli can survive in granulomas, which represent a stringent and nutrient-deficient environment (36).
Gene expression is initiated by the binding of RNA polymerase to the promoter sequence of target genes. Bacterial RNA polymerase is composed of a core enzyme (α2 ββ′) and one of several different sigma factors. The sigma factor determines promoter specificity by recognizing and binding to the sequence of the promoter. Thus, investigating the role of sigma factors in M. tuberculosis is important for understanding the genetic adaptation of this pathogen during infection (19). M. tuberculosis has 13 sigma factor genes (10). SigA, the principal sigma factor, is constitutively expressed and governs the transcription of numerous housekeeping genes in M. tuberculosis (27, 41). M. tuberculosis sigB, which encodes a principal-like sigma factor 62% homologous to SigA (Fig. 1A and see Fig. S1 in the supplemental material), is induced under various stress conditions, including exposure to sodium dodecyl sulfate (SDS), heat shock, cold shock, low aeration, and stationary phase (27). SigF, which shares 32% homology to SigB, is classified as a stress response sigma factor based on homology to sporulation and stress response sigma factors in Streptomyces coelicolor (4) and Bacillus subtilis (4, 13, 38) and to the stationary sigma factor RpoS in Vibrio spp. (Fig. 1A and see Fig. S1 in the supplemental material). sigF is induced by heat shock and mild cold shock and in nutrient starvation conditions (27, 30). The other sigma factor genes (sigC, sigD, sigE, sigG, sigH, sigI, sigJ, sigK, sigL, and sigM) are classified as extracytoplasmic function sigma factors, which control cell envelope synthesis, secretory functions, and periplasmic protein repair and degradation (10, 25, 34).
FIG. 1.
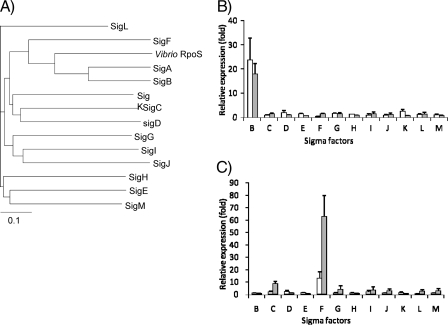
Analysis of sigma factor protein similarity and real-time RT-PCR analysis of sigma factor gene expression in the M. tuberculosis sigB and sigF KI recombinant strains. (A) A phylogenetic tree of the 13 M. tuberculosis sigma factors was generated by comparison of the amino acid sequences of these sigma factors and the sequence of Vibrio RpoS (obtained from NCBI GenBank accession no. AAK09363). (B) Changes in gene expression of the 13 sigma factors in the M. tuberculosis sigB KI recombinant strain were measured 6 and 12 h after acetamide induction by using real-time RT-PCR. The CT value was normalized to that of sigA, and the relative expression was calculated by the ΔΔCT method compared to the control strain. Bars: □, 6 h; ░⃞,12 h. (C) Same analysis as in panel B but with the M. tuberculosis sigF KI recombinant strain.
Previous studies have investigated the role of M. tuberculosis sigma factors under various in vitro stress conditions and in animal models of infection. A sigC-deficient mutant was shown to cause reduced mortality in mice (39), and the infection of guinea pigs with M. tuberculosis lacking a functional SigC resulted in the absence of necrotic granulomas (21), suggesting that sigC is essential for immunopathogenesis. A sigD-deficient mutant was also found to be attenuated in mice (7), and a sigE-deficient mutant showed attenuation within macrophages, as well as delayed mortality of mice (3, 28). A sigH-deficient mutant demonstrated increased susceptibility to oxidative stress (22, 33), and a sigF-deficient mutant was found to have reduced survival compared to the isogenic wild-type strain in mice (14). Recently, a sigL deletion mutant also showed less pathogenicity in mice than the wild-type strain (12, 16). However, other sigma factor-deficient mutants, such as those lacking sigJ and sigM, did not show a distinct phenotype in mouse or macrophage infection models (1, 20). Phenotypic differences between various sigma factor-deficient mutants suggest underlying differences in the regulatory networks controlled by each sigma factor. M. tuberculosis exposed to nutrient starvation conditions in vitro revealed significantly increased expression of RelMtb (ppGpp synthase I). ppGpp (hyperphosphorylated guanine) acts as a molecular signal in many bacteria to regulate the expression of sigma factors (18, 24). Lack of RelMtb in M. tuberculosis resulted in decreased mycobacterial survival in mice (11) and reduced the expression of sigD (11). Nutrient starvation conditions increased the expression of sigB, sigD, sigE, and sigF, while the expression of rpoA (α subunit) and rpoC (β′) was decreased (5).
Previous work using sigma factor knockout mutants or knock-in (KI; conditional overexpression) recombinants has suggested the interdependence of sigma factor gene expression. For example, sigH is required for maximal expression of sigE and sigB (28, 33). Likewise, deletion of sigF resulted in decreased expression of sigC (14), suggesting that SigF may exert its regulatory activity at a level close to those of SigC and SigB. In vitro transcription studies have demonstrated that SigE-, SigF-, SigH-, and SigL-containing RNA polymerases can transcribe sigB (12, 33). These results suggest that multiple sigma factor signaling pathways might converge at sigB. Therefore, SigB may be a key end-effector regulator, since deletion of sigC, sigE, sigF, sigH, and sigL (resulting in decreased sigB expression) demonstrated reduced immunopathology in the mouse (3, 12, 14, 16, 22, 39). Despite the potential significance of these sigma factors in M. tuberculosis, the roles of SigB and SigF within the complex sigma factor regulatory network remain unclear. We investigated here the roles of SigB and SigF in sigma factor regulation in M. tuberculosis.
MATERIALS AND METHODS
Bacterial strains and media.
Escherichia coli DH5α was used in DNA cloning procedures, and M. tuberculosis CDC1551 (Oshkosh) was cultivated in Middlebrook 7H9 liquid broth (supplemented with 0.05% Tween 80, 10% oleic acid-albumin-dextrose-catalase, 5% glycerol) or 7H10 agar for transformation and screening with appropriate antibiotics. For the selection of the sigB knock-in strain, kanamycin (10 μg/ml) was added to cultures.
Conditionally overexpressing recombinants.
Previously, we constructed a sigF overexpression vector pSCW35 containing an acetamide promoter-sigF fusion gene (40), as well as a control vector pSCW38 containing only the promoter (1, 40). To overexpress sigB in M. tuberculosis, the entire sigB gene was substituted for the sigF gene in vector pSCW35 using restriction enzymes NdeI and PacI, resulting in vector pSCW40. The entire sigB gene was amplified by PCR using primers the pACEB1 (5′-GGCCCATATGATGGCCGATGCACCCACAAG-3′; the NdeI site was incorporated) and pACEB2 (5′-GGCCTTAATTAACTACCTGGCTCAGGATGTCC-3′; the PacI site was incorporated). Vector pSCW40 was transformed into M. tuberculosis by electroporation (2.0 kV), and transformants were screened by using kanamycin (10 μg/ml)-containing 7H10 plates.
Real-time RT-PCR of sigB and sigF KI strains.
sigB and sigF expression was confirmed by real-time reverse transcription-PCR (RT-PCR). To induce sigB and sigF, 0.2% of acetamide solution was added to a culture at an optical density at 600 nm of ∼0.5, and at 6 and 12 h after induction the cells were pelleted, washed with phosphate-buffered saline, and resuspended in TRIzol reagent (Invitrogen). Mycobacterial membranes were disrupted by using 0.1-mm diameter silica beads and a bead beater at 5,000 rpm. After centrifugation at 10,000 × g for 5 min at 4°C, the supernatants were collected and treated with chloroform and centrifuged again to collect the aqueous phase. Finally, RNA was precipitated with isopropyl alcohol and washed with 75% ethanol and dried. The RNA was treated with DNase I and subjected to 30 cycles of PCR to confirm the removal of all DNA during the RNA extraction procedure. About 1 μg of DNase I-treated RNA was reverse transcribed by using Superscript II reverse transcriptase (Invitrogen) and random primers (Invitrogen). Real-time RT-PCR was performed using an IQ SYBR green I PCR kit (Bio-Rad). The primers used in real-time RT-PCR are listed in Table S1 in the supplemental material.
The cycle threshold value (CT) obtained for each gene of interest was normalized with that of sigA, a housekeeping gene, in order to obtain relative fold-change in gene expression [ΔCT = CT(gene of interest) − CT(housekeeping gene)]. The regulation of individual genes was calculated by using the formula 2−(ΔCT(s) − ΔCT(c)), Where, s represents the sigB- or sigF-inducible strain and c represents the control strain (pSCW38).
Microarray of sigB overexpression.
sigB overexpression was also analyzed by using microarrays. Microarray analysis was performed with poly-l-lysine coated glass slides containing oligonucleotides corresponding to all annotated M. tuberculosis genes. Total RNA was extracted from the control strain and the sigB KI mutant 12 h after acetamide exposure. RNA was reverse transcribed with random hexamers (Invitrogen). The resulting cDNA was labeled with either Cy3-dCTP or Cy5-dCTP and competitively hybridized to whole-genome arrays (one 70mer oligonucleotide probe per M. tuberculosis open reading frame spotted onto glass slides). Hybridization to microarray slides was performed as described previously (39). Arrays were scanned by using an Axon 4000B scanner. The image data were quantified by using GenePix pro 4.0 software and then normalized with the total intensity of spots. The ratios of Cy5 and Cy3 were compared and calculated.
In vitro transcription assay.
SigB and SigF were purified as C-terminal His-tagged recombinant fusion proteins. The entire open reading frame of the M. tuberculosis sigB gene was amplified with the primers pETsigB1 (5′-GGGCCCATATGGCCGATGCACCCACAAGGGC-3′) and pETsigB2 (5′-GGGCCCTCGAGGCTGGCGTACGA CCGCAGCC-3′), and the sigF gene was amplified with the primers pETsigF1 (5′-GGGCCCATATGACGGCGCGCGCTGCCGG-3′) and pETsigF2 (5′-GGGCCAAG CTTCTCCAACTGATCCCGTAGCCG-3′). Each amplicon was digested with NdeI and XhoI, ligated to NdeI- and XhoI-digested pET22b(+) (Novagen), and transformed into E. coli BL21(DE3) strain. Sigma factor overexpression was induced with 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside), and after 3 h of induction the cells were harvested and disrupted by sonication in binding buffer (50 mM Tris-Cl, 0.5 M NaCl, 10 mM imidazole). The supernatant was recovered by centrifugation at 10,000 × g for 10 min at 4°C, and passed through a nickel-nitrilotriacetic acid agarose column. The column was washed with 10 volumes of washing buffer (50 mM Tris-Cl, 0.5 M NaCl, 50 mM imidazole) and finally eluted with buffer (50 mM Tris-Cl, 0.5 M NaCl, 500 mM imidazole). The purity of the protein was checked by SDS-polyacrylamide gel electrophoresis, and the protein concentration was determined by the Bradford method. The SigF protein was purified by using the same techniques.
Short (template 1) and long (template 2) DNA templates which, including the putative promoter regions, were prepared by PCR with sets of three primers. For the sigB promoter region, Binv10 (5′-TGCACGTCACAGGGCGTCAGAT-3′) and Binv12 (5′-TGCGCTTGGCCAGTTCGACT-3′) were used to create sigB template 1, and Binv10 with Binv14 (5′-CAGGTCGCGTTTTCGGTTCT-3′) were used to create sigB template 2. For the ideR promoter region, primers iderinv10 (5′-TGGACATCCTGAGCCAGGTAGC-3′) and iderinv12 (5′-AAGTAGCCCATCGCGCTCCATC-3′) were used to produce ideR template 1, and iderinv10 with iderinv14 (5′-CTTGCGCATCACGGCGATGG-3′) were used to produce ideR template 2. Finally, for the sigC promoter region, Cinv10 (5′-GGGGAGATCGACCGAATGTC-3′) and Cinv12 (5′-ACAACCTTGCCGGCCGGAGC-3′) were used to generate sigC template 1, and Cinv10 with Cinv14 (5′-CTGGTAGCGATGGCAATGCTG-3′) were used to generate sigC template 2. In vitro transcription assays were performed as previously described (39). Briefly, 2 pmol of E. coli RNA core polymerase (Epicenter) was incubated with 20 pmol of purified sigma factor protein for 30 min in transcription assay buffer (10 mM Tris, 50 mM KCl, 10 mM MgCl2, 0.1 mM EDTA, 0.25 μg of bovine serum albumin/μl) and then 0.09 μg of template DNA was added and incubated with 0.25 mM ATP, GTP, UTP, and [32P]CTP. The transcription mixture was subjected to 6% denaturing polyacrylamide gel electrophoresis and developed on film (Kodak BioMax MR).
Macrophage infections.
Alveolar macrophage J774A.1 cells were cultivated in cRPMI (2 mM glutamine, 10% fetal bovine serum) and activated by gamma interferon (500 U/ml) for 12 h, followed by lipopolysaccharide (200 ng/ml) for 3 h before infection. After the macrophages were washed three times with cRPMI, 105 bacteria were used to infect macrophage cultures at an MOI of 1:1. After 2 h of infection, macrophages were washed five times with RPMI media and cultivated with cRPMI with 5% CO2 at 37°C. Macrophages were harvested, washed three times with RPMI, and then lysed by using 0.1% Triton X-100 in PBS at each time point (2 h, 3 days, and 6 days after infection). Th macrophage lysate was plated on 7H10 plates for CFU counting.
RESULTS
Conditionally inducible sigB- and sigF-expressing KI strains.
The entire sigB and sigF genes were introduced downstream of the acetamidase promoter and regulatory cassette to yield sigB-inducible and sigF-inducible recombinant M. tuberculosis strains, respectively. The identity of these constructs was confirmed by PCR and sequencing. Quantitative RT-PCR was used to confirm upregulation of sigB and sigF at 6 and 12 h after acetamide exposure. The data obtained from real-time PCR experiments was normalized to sigA expression levels. In these experiments, sigA expression remained constant in all strains and under all conditions. Using real-time RT-PCR, we found sigB expression was increased (23.7 ± 9.1)-fold and (17.9 ± 4.5)-fold at 6 and 12 h after induction, respectively, and sigF was increased (12.7 ± 5.5)-fold and (62.7 ± 17)-fold at 6 and 12 h after induction, respectively.
Using these KI strains, we examined the possibility that sigB or sigF may control in vitro growth of M. tuberculosis. Sigma factor expression was induced with 0.2% acetamide, and the growth of each sigma factor-inducible strain was compared to that of the corresponding control strain containing only the acetamidase promoter and regulatory cassette region. Overexpression of sigB or sigF did not alter M. tuberculosis in vitro growth rates (see Fig. S2 in the supplemental material), nor did colony morphology differ in the recombinant strains when these were plated on acetamide-containing 7H10 plates (data not shown).
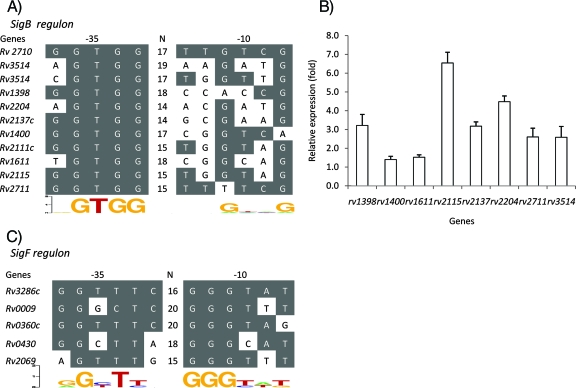
Next, we examined the relative expression of M. tuberculosis sigma factor genes after induction of sigB and sigF. Although the majority of genes encoding sigma factors were not differentially regulated after overexpression of these two genes, the expression of sigC was consistently found to be increased after sigF induction (Fig. 1C). Specifically, sigC was upregulated by (1.8 ± 0.9)-fold and (8.3 ± 2.2)-fold at 6 and 12 h, respectively, after overexpression of sigF. Consistent with these gene expression data, the previously identified sigF promoter recognition sequence AGTTTG-N15-GGGTTT was identified in the 5′ untranslated region (UTR) of the sigC gene (Rv2069) (Fig. 2C). Overexpression of sigB did not significantly alter expression of other genes encoding sigma factors (Fig. 1B).
FIG. 2.
Putative promoter consensus recognition sequences of SigB and SigF and confirmation of putative SigB-dependent genes by real-time RT-PCR analysis after sigB induction in the M. tuberculosis sigB KI strain. (A) The SigB putative promoter consensus recognition sequence was identified by analyzing the microarray data from Table 1. Genes found to be upregulated after sigB induction were collected and aligned to determine the sigB promoter consensus sequence. (B) Genes found by microarray to have a putative SigB-dependent promoter were evaluated by real-time RT-PCR for their level of expression before and after sigB KI expression. RNA was obtained 12 h after sigB induction in the M. tuberculosis sigB KI strain. The relative expression values shown are the ratios of postinduction RT-PCR signal normalized to the preinduction levels. The primers used in this experiment are described in Table S1 in the supplemental material. (C) The SigF putative promoter consensus recognition sequence identified in the present study by sigF KI overexpression, as well as those of genes shown to be regulated by SigF in previous studies, were also aligned. The SigF promoter recognition sequence was identified in the 5′ UTR region of the sigC gene (Rv2069). SigB and SigF promoter consensus sequences were derived using weblogo software (http://weblogo.berkeley.edu/logo.cgi).
M. tuberculosis global gene expression profile after sigB overexpression.
To further characterize the sigB regulon, global gene expression analysis was performed using the sigB-inducible strain. Mycobacterial RNA was isolated at 12 h after acetamide induction. The microarray data were normalized to total intensity of spots and sigA expression. Table 1 shows genes found to be significantly increased (≥4-fold, Q value of <0.05) after sigB overexpression. Genes found to be highly upregulated include those encoding proteins involved in cell wall processes, PE-PPE family genes, as well as essential genes, such as 50S ribosomal proteins (Rv0709, Rv0701, Rv0641, and Rv2904c), and the chaperonin proteins groEL2 (Rv0440) and groES (Rv3418c). In addition, sigB induction led to increased expression of esxB (Rv3874, 10-kDa culture filtrate antigen), which encodes a major secreted antigen, as well as ideR, which is located downstream of the sigB gene.
TABLE 1.
Microarray analysis of M. tuberculosis genes found to be upregulated after sigB KI expression
| Code and gene (or locus tag) | Rv no. | Avg | SD | Q value | Description |
|---|---|---|---|---|---|
| Virulence, detoxification, and adaptation | |||||
| groEL2 | Rv0440 | 4.4 | 0.7 | 0.02 | 60-kDa chaperonin 2 |
| groES | Rv3418c | 4.2 | 3.5 | 0.03 | 10-kDa chaperonin |
| Lipid metabolism | |||||
| pgsA | Rv2612c | 21.8 | 0.6 | 0.00 | Phosphatidylinositol synthase |
| fadD31 | Rv1925 | 18.3 | 5.6 | 0.00 | Acyl-coenzyme A ligase |
| kasA | Rv2245 | 6.4 | 1.5 | 0.01 | 3-Oxoacyl-[acyl-carrier protein] synthase 1 |
| pgsA2 | Rv1822 | 4.1 | 0.9 | 0.01 | Phosphatidyltransferase |
| ppsD | Rv2934 | 4.1 | 0.3 | 0.01 | Phenolphthiocerol synthesis type-I polyketide synthase |
| Information pathways | |||||
| sigB | Rv2710 | 56.2 | 7.2 | 0.00 | RNA polymerase sigma factor |
| dinP | Rv3056 | 6.3 | 0.9 | 0.01 | DNA-damage-inducible protein P |
| dinF | Rv2836c | 5.6 | 0.9 | 0.01 | DNA-damage-inducible protein |
| rpmC | Rv0709 | 5.5 | 1.4 | 0.01 | 50S ribosomal protein l29 |
| tuf | Rv0685 | 5.5 | 0.7 | 0.01 | Iron-regulated elongation factor |
| nrdH | Rv3053c | 5.4 | 0.1 | 0.00 | Glutaredoxin electron transport component of nrdef |
| rplC | Rv0701 | 4.8 | 0.7 | 0.01 | 50S ribosomal protein l3 |
| rplA | Rv0641 | 4.8 | 1.6 | 0.00 | 50S ribosomal protein |
| rplS | Rv2904c | 4.8 | 1.5 | 0.01 | 50S ribosomal protein l19 |
| greA | Rv1080c | 4.2 | 0.9 | 0.02 | Transcription elongation factor |
| Cell wall and cell processes | |||||
| esxB | Rv1398c | 27.4 | 2.2 | 0.01 | 10-kDa culture filtrate antigen |
| Rv2625c | Rv2625c | 7.2 | 2.5 | 0.00 | Conserved transmembrane alanine and leucine-rich protein |
| Rv0463 | Rv0463 | 7.2 | 0.7 | 0.00 | Conserved membrane protein |
| Rv1792 | Rv1792 | 7.1 | 3.7 | 0.01 | ESAT-6 like protein |
| wag31 | Rv2145c | 4.7 | 0.7 | 0.01 | Conserved hypothetical protein |
| Rv2115 | Rv2115 | 4.7 | 0.2 | 0.01 | ATPase |
| secE | Rv0638 | 4.7 | 0.5 | 0.01 | Preprotein translocase |
| mmpS3 | Rv2198c | 4.6 | 0.8 | 0.01 | Conserved membrane protein |
| Rv1410c | Rv1410c | 4.3 | 1.4 | 0.02 | Aminoglycosides |
| yjcE | Rv2287 | 4.3 | 0.2 | 0.00 | Conserved integral membrane transport protein |
| Rv0559c | Rv0559c | 4.2 | 0.4 | 0.01 | Conserved secreted protein |
| Rv1845c | Rv1845c | 4.1 | 0.4 | 0.01 | Conserved hypothetical transmembrane protein |
| Rv2446c | Rv2446c | 4.1 | 0.4 | 0.00 | Conserved integral membrane protein |
| Rv2206 | Rv2206 | 4.1 | 0.8 | 0.00 | Conserved transmembrane protein |
| Rv2136c | Rv2136c | 4.0 | 0.8 | 0.00 | Conserved transmembrane protein |
| Rv0383c | Rv0383c | 4.0 | 0.7 | 0.01 | Conserved secreted protein |
| secF | Rv2586c | 4.0 | 1.1 | 0.00 | Protein-export membrane protein |
| Insertion sequences and phages | |||||
| Rv2978c | Rv2978c | 4.2 | 0.3 | 0.00 | Transposase |
| PE/PPE | |||||
| PE_PGRS | Rv3514 | 49.4 | 4.6 | 0.00 | PE PGRS family protein |
| PE_PGRS | Rv3508 | 11.9 | 1.5 | 0.01 | PE PGRS family protein |
| PE_PGRS | Rv2591 | 9.6 | 0.8 | 0.00 | PE PGRS family protein |
| PPE | Rv0442c | 5.5 | 0.1 | 0.01 | PPE family protein |
| PE_PGRS | Rv0872c | 4.9 | 2.2 | 0.02 | PE PGRS family protein |
| PPE | Rv3136 | 4.8 | 1.7 | 0.01 | PPE family protein |
| PE_PGRS | Rv2490c | 4.7 | 0.3 | 0.00 | PE PGRS family protein |
| PE_PGRS | Rv1818c | 4.2 | 1.1 | 0.01 | PE PGRS family protein |
| PPE | Rv3022c | 4.1 | 0.2 | 0.00 | PPE family protein |
| PE_PGRS | Rv2162c | 4.1 | 0.8 | 0.00 | PE PGRS family protein |
| Intermediary metabolism and respiration | |||||
| lipI | Rv1400c | 10.1 | 1.5 | 0.01 | Lipase |
| cbhK | Rv2202c | 6.8 | 2.9 | 0.00 | Carbohydrate kinase |
| nadB | Rv1595 | 6.1 | 0.4 | 0.00 | l-Aspartate oxidase |
| trpC | Rv1611 | 5.8 | 0.4 | 0.00 | Indole-3-glycerol phosphate synthase |
| ctaC | Rv2200c | 5.3 | 1.3 | 0.01 | Transmembrane cytochrome c oxidase |
| nadC | Rv1596 | 5.0 | 1.2 | 0.01 | Nicotinate-nucleotide pyrophosphatase |
| cysS2 | Rv2130c | 4.8 | 1.0 | 0.01 | Glucopyranoside ligase |
| lldD2 | Rv1872c | 4.6 | 1.1 | 0.02 | l-Lactate dehydrogenase |
| lipN | Rv2970c | 4.5 | 0.9 | 0.01 | Lipase/esterase |
| Rv2850c | Rv2850c | 4.2 | 0.1 | 0.01 | Magnesium chelatase |
| aroG | Rv2178c | 4.1 | 1.0 | 0.00 | 3-Deoxy-d-arabino-heptulosonate 7-phosphate synthase |
| ribC | Rv1412 | 4.1 | 0.8 | 0.01 | Riboflavin synthase alpha chain |
| prcB | Rv2110c | 4.0 | 1.7 | 0.02 | Proteasome (β-subunit) |
| Unknown | |||||
| Rv1046c | Rv1046c | 4.1 | 2.7 | 0.01 | Hypothetical protein |
| Regulatory proteins | |||||
| whiB2 | Rv3260c | 4.0 | 2.1 | 0.01 | Transcriptional regulatory protein |
| Conserved hypotheticals | |||||
| Rv2204c | Rv2204c | 18.2 | 5.0 | 0.00 | Conserved hypothetical protein |
| Rv2137c | Rv2137c | 15.6 | 7.2 | 0.01 | Conserved hypothetical protein |
| Rv2111c | Rv2111c | 10.2 | 2.8 | 0.01 | Conserved hypothetical protein |
| Rv1827 | Rv1827 | 7.0 | 1.1 | 0.00 | Conserved hypothetical protein |
| Rv2182c | Rv2182c | 6.9 | 1.2 | 0.00 | 1-Acylglycerol-3-phosphate O-acyltransferase |
| Rv2974c | Rv2974c | 4.3 | 0.3 | 0.01 | Conserved hypothetical alanine rich protein |
| Rv2132 | Rv2132 | 4.2 | 0.5 | 0.01 | Conserved hypothetical protein |
| Rv1211 | Rv1211 | 4.2 | 1.4 | 0.01 | Conserved hypothetical protein |
| Rv0863 | Rv0863 | 4.1 | 1.8 | 0.02 | Conserved hypothetical protein |
| Rv1429 | Rv1429 | 4.0 | 0.2 | 0.01 | Conserved hypothetical protein |
| Rv0637 | Rv0637 | 4.0 | 0.7 | 0.02 | Conserved hypothetical protein |
| Rv0750 | Rv0750 | 4.0 | 0.8 | 0.01 | Conserved hypothetical protein |
aThe sigB gene was overexpressed using acetamide-inducing promoter at an optical density at 600 nm of ∼0.5, and cells were harvested after 12 h. Code (i.e., the functional categories) and gene annotation data were obtained from the TubercuList database (http://genolist.pasteur.fr/TubercuList/). The Q value was calculated by using SAM software (http://www-stat.stanford.edu/∼tibs/SAM/) with 1% FDR. We performed the real-time RT-PCR on the subset of genes to confirm microarray results (Fig. 2B).
In order to identify putative sigB-dependent promoter sequences, genes found to be upregulated by sigB were aligned, and their 5′ UTRs were compared. First, we examined the 5′ UTR sequences of upregulated genes encoding hypothetical proteins that were not in apparent operons, such as Rv3514, Rv1398c, Rv2204c, Rv2137c, Rv2115, Rv2111c, lipI (Rv1400c), Rv1611, and ideR (Rv2711). A putative SigB promoter recognition sequence was identified as NGTGG-N14-18-NNGNNG (Fig. 2A). Interestingly, this promoter recognition motif was also identified upstream of the sigB gene itself, suggesting that SigB may be autoregulatory. Real-time RT-PCR was used to confirm upregulation of genes containing the SigB promoter consensus sequence after sigB overexpression (Fig. 2B).
In vitro transcription assays.
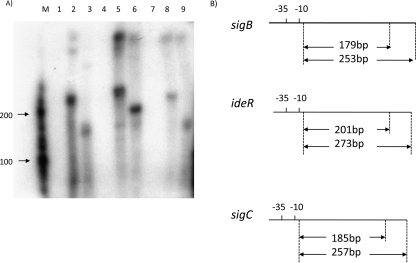
Microarray and quantitative RT-PCR data suggested that the sigC gene might be transcribed by a SigF-containing RNA polymerase (RNAP) and that ideR might be transcribed by a SigB-containing RNAP. To verify that SigB and SigF could specifically recognize and initiate transcription at the promoter regions of ideR and sigC, respectively, we performed in vitro transcription assays. The SigB and SigF proteins were overexpressed in E. coli and purified by nickel affinity chromatography as shown in Fig. S3 in the supplemental material. DNA templates (200 to 300 bp) containing the putative promoter regions of sigB, ideR, and sigC were prepared by PCR amplification. DNA templates were incubated with RNAP holoenzyme (with corresponding sigma factor) or RNAP core enzyme alone (with no sigma factor). As shown in Fig. 3A (lanes 4, 5, and 6), the presence of SigB was required for transcription of ideR, resulting in approximately 200- and 273-nucleotide transcripts using ideR template 1 and ideR template 2, respectively.
FIG. 3.
In vitro transcription assays using purified SigB and SigF sigma factors. (A) In vitro transcription assay using sigma factor protein and DNA template involving the 5′ UTRs of the sigB, ideR, and sigC genes. Lane M, RNA size markers (Ambion). All lanes correspond to complete in vitro transcription reaction mixes with the presence or absence of sigma factor and the presence of either long (template 2) or short (template 1) promoter-containing target templates. Lanes: 1, no added SigB/+sigB long template; 2, SigB added/+sigB long template; 3, SigB added/+sigB short template; 4, no added SigB/+ideR long template; 5, SigB added/+ideR long template; 6, SigB added/ideR short template; 7, no SigF added/+sigC long template; 8, SigF added/+sigC long template; 9, SigF added/+sigC short template. (B) Diagram of expected lengths of transcript products from the short and long templates. The transcription start site of sigB was previously identified (19).
We also investigated the possibility that sigB is autoregulated, since we identified the SigB promoter consensus motif (Table 1 and Fig. 2A) in the sigB upstream promoter region. As shown in Fig. 3A (lanes 1, 2, and 3), the addition of SigB resulted in a sigB transcript measuring about 179 and 253 nucleotides, using sigB template 1 and sigB template 2, respectively, as expected. These results, together with the gene expression data, indicate that sigB is autoregulatory and also transcribes the ideR gene, which encodes a major iron regulator.
The sigF promoter recognition sequence has been reported to be NGNTtg-N14-18-GGGTAt (40). This sequence is located upstream of the rsbW-sigF operon. Consistent with our real-time RT-PCR results, we identified a putative SigF consensus sequence in the 5′ UTR region of the sigC gene. In vitro transcription assays demonstrated that sigC has a SigF-dependent promoter (Fig. 3A, lanes 7, 8, and 9). The putative sigC promoter was identified as AGTTTG-N15-GGGTTT (the consensus sequence is underlined) 46 bp upstream of the ATG start codon of the sigC gene.
Macrophage infection.
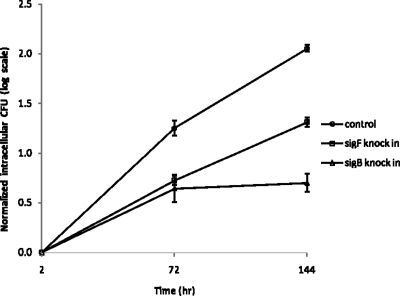
We found that most genes regulated by SigB and SigF encoded secreted antigens or proteins involved in the cell envelope (Table 1) (40). However, the specific regulons of these sigma factors differ significantly. To directly compare the physiological effects of transcriptional activation of each of these regulons, we tested the growth of the sigB and sigF KI strains during macrophage infection. The bacteria were incubated with J774A.1 macrophages at a multiplicity of infection of 1:1, and the bacteria were counted 2 h, 3 days, and 6 days after infection. In these experiments, KI expression was not specifically induced by addition of exogenous acetamide. However, an RT-PCR study showed that baseline sigB and sigF expressions in the KI strains were (10.1 ± 3.4)- and (3.5 ± 1.2)-fold higher, respectively, than in the corresponding control strains due to the increased gene dosage and leakiness of the acetamide promoter system. Thus, even under acetamide-free conditions, these M. tuberculosis recombinants overexpress sigB and sigF. Interestingly, the sigB KI strain showed a growth defect in macrophages, since it failed to replicate at the same rate as the control strain (Fig. 4). Specifically, at 3 and 6 days after infection, the normalized CFU counts in the sigB KI strain were 0.6 and 1.4 log units lower than those of the control strain, both of which were significant (P = 0.029 and 0.04, respectively). Likewise, as shown in Fig. 4, the normalized CFU counts displayed a reduced growth rate (slope) for the sigB-overexpressing strain compared to the control strain at both the early and the late time intervals of the intracellular infection. The CFU counts for the sigF-overexpressing strain also indicated a slowed intracellular growth rate; however, the magnitude of the rate reduction was less than that of the sigB-overexpressing strain and did not achieve statistical significance (Fig. 4).
FIG. 4.
Intracellular growth of M. tuberculosis sigB and sigF KI strains in activated J774A.1 macrophages in vitro. Murine macrophage J774A.1 cells were activated with gamma interferon and lipopolysaccharide (as in Materials and Methods) and then infected with M. tuberculosis strains harboring the empty vector, the sigB KI vector, or the sigF KI vector at a multiplicity of infection of 1:1. After incubation for 2 h, the macrophage monolayer was extensively washed, and samples were taken to determine the initial intracellular CFU titer. The bacterial growth rate was determined by CFU counting of bacilli at 3 and 6 days after infection. The log CFU counts are normalized to the initial intracellular CFU counts.
DISCUSSION
Bacterial sigma factors are classified as principal and alternative sigma factors. Principal sigma factors are essential for bacterial survival, while alternative sigma factors are nonessential and mediate the adaptive response of bacteria to the extracellular environment. The extracytoplasmic function sigma factors comprise a large cluster of alternative sigma factors that regulate cell wall, periplasmic, and secretion-associated functions. These proteins are of interest as potential regulators of virulence factors in bacterial pathogens. For example, a stationary-phase alternative sigma factor, RpoS, plays a role in quorum sensing in the gram-negative bacterium Vibrio sp., and sigma factors such as SigH play a role in cell-to-cell communication in Bacillus subtilis and Streptococcus pneumoniae (23).
Transcriptional regulation of the sigma factor network in M. tuberculosis is complex. Previous studies have suggested the presence of two different sigma factor-dependent promoters upstream of sigB, one recognized by a SigF-containing RNA polymerase and the other recognized by RNA polymerase containing SigE, SigH, or SigL (12). Recently, the two-component response regulator MprAB was found to regulate expression of SigB and SigE but not of SigH (17). Interestingly, Triton X-100 treatment induced the expression of sigB, but not of sigE, in an mprA deletion mutant, suggesting independent regulation of the SigB, SigE, and SigH regulons. Evidence that sigC may be regulated by SigF was provided by the observation that sigC was downregulated in an M. tuberculosis sigF-deficient mutant during stationary phase (14); however, conclusive data that the sigC promoter is SigF dependent are lacking.
In the present study, we explored the roles of SigB and SigF in the M. tuberculosis sigma factor network. We performed an experimental and computational approach to identify SigB- and SigF-regulated sigma factors by overexpressing sigB and sigF in M. tuberculosis, respectively. Previous studies showed that the chemically regulated promoter Pace is useful to study gene expression in M. tuberculosis (1, 26, 40). In the present study, we used this inducible promoter system to characterize genes regulated by SigB and SigF in M. tuberculosis.
Importantly, our microarray and real-time RT-PCR data revealed that the relative expression of other sigma factors was unchanged after sigB induction, suggesting that, at least during exponential growth, SigB is an end regulator of at least one branch of the sigma factor network in M. tuberculosis (12). In addition to previous work (12), we found that SigB-containing RNA polymerase is capable of transcribing sigB, suggesting that SigB may auto-amplify its expression under certain conditions.
Overexpression of sigB resulted in induction of many genes encoding secreted antigens and cell wall-associated proteins, including Rv1925, which encodes an acyl-coenzyme A-synthase, and Rv2612e, which encodes phosphatidylinositol synthase, both of which contribute components for membrane synthesis. In addition, sigB induction led to increased expression of many genes encoding PE/PPE family proteins, several members of which have been localized to the cell membrane and shown to influence mycobacterium-host cell interactions (2, 6). In particular, the gene product of Rv1818c (PE-PGRS 33) was shown to induce strong CD8+ cytotoxic lymphocyte and Th1-type responses, with high levels of gamma interferon and low levels of interleukin-4 in mice (8). Differential regulation of PE and PPE proteins has been described previously for the M. tuberculosis alternative RNA polymerase sigma factors SigF (16, 40) and SigL (16), suggesting that these sigma factors may indirectly regulate PE/PPE genes through the regulation of sigB.
We also found that expression of the ideR gene (Rv2711), which is located immediately downstream of sigB (Rv2710), was increased after sigB induction; however, relative induction of ideR (2.6-fold as determined by real-time RT-PCR) was lower than that of sigB (17-fold as determined by real-time RT-PCR). Previous data suggested that sigB and ideR are not cotranscribed in Mycobacterium sp. (37), a finding consistent with our data showing distinct SigB-dependent promoters upstream of each of these genes. The sigB promoter consensus recognition sequence NGTGG-N14-19-NNGNNG is found 14 bp upstream of the previously identified transcription start site of the sigB gene (19) as GGTGG-N17-TTGTCG and in the 5′ UTR of the ideR gene as GGTGG-N15-TTGTCG. This sequence also resembles the previously described promoter consensus recognition sequences for SigE (TGGGAAC-N17-CGTTA) (28) and SigH (TGGGAA-N18-CGTTA) (33). Of note, the trinucleotide TGG in the −35 region appears to be highly conserved in the upstream promoter region of all SigB-regulated genes in the present study and is also seen in the SigE- and SigH-dependent promoters.
Interestingly, principal-like sigma factors, such as SigB, are not present in E. coli, Bacillus sp., or Vibrio sp. (32). On the other hand, Streptomyces coelicolor has three principal-like sigma factors, HrdA, HrdC, and HrdD, whose functions remain unclear but which are not essential for growth (15). Although SigB contains significant amino acid sequence similarity to the C-terminal portion of SigA, it appears to be dispensable for growth in M. smegmatis and M. tuberculosis (31, 35). In M. tuberculosis, sigB is induced after exposure to heat shock and oxidative stress, which appears to be mediated by SigH (29), as well as in response to SDS-induced surface stress, which appears to be mediated by SigE (28). Consistent with these findings, unpublished data suggest that a M. tuberculosis mutant deficient in SigB is more sensitive to SDS-induced surface stress, heat shock, and oxidative stress (35; I. Smith, personal communication), suggesting that SigB may play a role in the general stress response of M. tuberculosis. Overexpression of M. tuberculosis sigB in M. smegmatis results in a prolonged generation time and markedly altered colony morphology, which has been attributed to the constitutive production of surface hyperglycosylated polar glycopeptidolipids (31). These molecules, which are unique to M. smegmatis and M. avium species, are usually produced in response to carbon starvation, suggesting a role for SigB in mycobacterial adaptation to nutrient-limited conditions. However, overexpression of sigB in M. tuberculosis in our study did not lead to altered growth characteristics or colony morphology, suggesting divergent roles for SigB in the two different mycobacterial species. Although unpublished data reported that SigB is not required for normal M. tuberculosis growth in human macrophages (35), our study revealed a growth defect of M. tuberculosis overexpressing sigB in J774A.1 macrophages. This effect may be due to increased expression of sigB itself or because of increased expression of another gene directly or indirectly regulated by SigB.
We also studied the role of M. tuberculosis SigF in the sigma factor network using a conditionally inducible sigF recombinant strain. In contrast to previously published data that M. tuberculosis sigB may have a SigF-dependent promoter (12), we did not observe significant upregulation of sigB after overexpression of sigF. In addition, among the 73 genes that were upregulated after sigB induction (Table 1), only 5 were also upregulated after sigF induction (40), suggesting distinct regulons controlled by each of these sigma factors. We found that sigC was the only sigma factor gene whose expression was increased after sigF induction. Indeed, in vitro transcription assays showed that M. tuberculosis SigF is, in fact, capable of transcribing sigC, and we identified the putative SigF-dependent promoter sequence as AGTTT-N15-GGGTTT (the consensus sequence is underlined) 46 bp upstream of the ATG start codon of the sigC gene. These data are consistent with microarray data indicating downregulation of sigC in a sigF knockout strain during stationary-phase growth (14) and strongly suggest that sigC expression is SigF dependent. Earlier studies of the M. tuberculosis sigF knockout mutant indicated that its gene product is important for bacterial survival and immunopathology in mice (14), although SigF does not appear to be required for M. tuberculosis proliferation within in human monocytes cultured in vitro (9). Consistent with the concept that coordinate expression of SigF-dependent genes is an important virulence mechanism, we observed a reduced intracellular growth rate in activated murine J774 macrophages by the recombinant M. tuberculosis KI strain that overexpresses sigF constitutively.
It will be important to define the degrees of transcriptional redundancy among the M. tuberculosis sigma factors and also determine how stress conditions affect the relative expression patterns of the SigB and SigF regulons—a group of genes that are likely to play important roles in pathogenesis.
Supplementary Material
Acknowledgments
The advice and assistance of Deborah Geiman and Ernest Williams is gratefully acknowledged.
The support for this research from NIH grants AI36973, AI37856, and AI43846 is gratefully acknowledged.
Footnotes
Published ahead of print on 9 November 2007.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Agarwal, N., S. C. Woolwine, S. Tyagi, and W. R. Bishai. 2007. Characterization of the Mycobacterium tuberculosis sigma factor SigM by assessment of virulence and identification of SigM-dependent genes. Infect. Immun. 75:452-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen, P., A. B. Andersen, A. L. Sorensen, and S. Nagai. 1995. Recall of long-lived immunity to Mycobacterium tuberculosis infection in mice. J. Immunol. 154:3359-3372. [PubMed] [Google Scholar]
- 3.Ando, M., T. Yoshimatsu, C. Ko, P. J. Converse, and W. R. Bishai. 2003. Deletion of Mycobacterium tuberculosis sigma factor E results in delayed time to death with bacterial persistence in the lungs of aerosol-infected mice. Infect. Immun. 71:7170-7172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bentley, D. D., K. F. Chater, A. M. Cerdeno-Tarraga, G. L. Challis, N. R. Thomson, K. D. James, D. E. Harris, M. A. Quail, H. Kieser, D. Harper, A. Bateman, S. Brown, G. Chandra, C. W. Chen, M. Collins, A. Cronin, A. Fraser, A. Goble, J. Hidalgo, T. Hornsby, S. Howarth, C. H. Huang, T. Kieser, L. Larke, L. Murphy, K. Oliver, S. O'Neil, E. Rabbinowitsch, M. A. Rajandream, K. Rutherford, S. Rutter, K. Seeger, D. Saunders, S. Sharp, R. Squares, S. Squares, K. Taylor, T. Warren, A. Wietzorrek, J. Woodward, B. G. Barrell, J. Parkhill, and D. A. Hopwood. 2002. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 9:141-147. [DOI] [PubMed] [Google Scholar]
- 5.Betts, J., P. L. Lukey, L. C. Robb, R. A. McAdam, and K. Duncan. 2002. Evaluation of a nutrition model of Mycobacterium tuberculosis persisience by gene and protein expression profiling. Mol. Microbiol. 43:717-731. [DOI] [PubMed] [Google Scholar]
- 6.Brennan, M. J., G. Delogu, Y. Chen, S. Bardarov, J. Kriakov, M. Alavi, and W. R. Jacobs. 2001. Evidence that mycobacterial PE_PGRS proteins are cell surface constituents that influence interactions with other cells. Infect. Immun. 69:7326-7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calamita, H., C. Ko, S. Tyagi, T. Yoshimatsu, N. E. Morrison, and W. R. Bishai. 2005. The Mycobacterium tuberculosis SigD sigma factor controls the expression of ribosome-associated gene products in stationary phase and is required for full virulence. Cell Microbiol. 7:233-244. [DOI] [PubMed] [Google Scholar]
- 8.Chaitra, M. G., M. S. Shaila, and R. Nayak. 2007. Evaluation of T-cell responses to peptides with MHC class I-binding motifs derived from PE_ PGRS 33 protein of Mycobacterium tuberculosis. J. Med. Microbiol. 56:466-474. [DOI] [PubMed] [Google Scholar]
- 9.Chen, P., R. E. Ruiz, Q. Li, R. F. Silver, and W. R. Bishai. 2000. Construction and characterization of Mycobacterium tuberculosis mutant lacking the alternate sigma factor gene, sigF. Infect. Immun. 68:5575-5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, A. Krogh, J. McLean, S. Moule, L. Murphy, K. Oliver, J. Osborne, M. A. Quail, M. A. Rajandream, J. Rogers, S. Rutter, K. Seeger, J. Skelton, R. Squares, S. Squares, J. E. Sulston, K. Taylor, S. Whitehead, and B. G. Barrell. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 11.Dahl, J. L., C. N. Kraus, H. I. Boshoff, B. Doan, K. Foley, D. Avarbock, G. Kaplan, V. Mizrahi, H. Rubin, and C. E. Barry. 2003. The role of RelMtb-mediated adaptation to stationary phase in long-term persistence of Mycobacterium tuberculosis in mice. Proc. Natl. Acad. Sci. USA 100:10026-10031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dainese, E., S. Rodrigue, G. Delogu, R. Provvedi, L. Laflamme, R. Brzezinski, G. Fadda, I. Smith, L. Gaudreau, G. Palu, and R. Manganelli. 2006. Posttranslational regulation of Mycobacterium tuberculosis extracytoplasmic function sigma factor L and roles in virulence and in global regulation of gene expression. Infect. Immun. 74:2457-2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeMaio, J., Y. Zhang, C. Ko, D. B. Young, and W. R. Bishai. 1996. A stationary-phase stress-response sigma factor from Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2:2790-2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geiman, D. E., D. Kaushal, C. Ko, S. Tyagi, Y. C. Manabe, J. M. Chen, B. G. Schroedor, R. D. Fleischmann, N. E. Morrison, P. J. Converse, P. Chen, and W. R. Bishai. 2004. Attenuation of late-stage disease in mice infected by the Mycobacterium tuberculosis mutant lacking the SigF alternate sigma factor and identification of SigF-dependent genes by microarray analysis. Infect. Immun. 72:1733-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gruber, T. M., and C. A. Gross. 2003. Multiple sigma factor subunits and the partitioning of bacterial transcription space. Annu. Rev. Microbiol. 57:441-466. [DOI] [PubMed] [Google Scholar]
- 16.Hahn, M., S. Raman, M. Anaya, and R. N. Husson. 2005. The Mycobacterium tuberculosis extracytoplasmic-function sigma factor SigL regulates polyketide synthases and secreted or membrane proteins and its required for virulence. J. Bacteriol. 187:7062-7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He, H., R. Hovey, J. Kane, V. Singh, and T. C. Zahrt. 2006. MprAB is a stress-responsive two-component system that directly regulates expression of sigma factors SigB and SigE in Mycobacterium tuberculosis. J. Bacteriol. 188:2134-2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirsch, M., and T. Elliott. 2002. Role of ppGpp in RpoS stationary-phase regulation in Escherichia coli. J. Bacteriol. 184:5077-5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu, Y., and A. R. Coates. 1999. Transcription of two sigma 70 homologue genes, sigA and sigB, in stationary-phase Mycobacterium tuberculosis. J. Bacteriol. 181:469-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu, Y., S. Kendall, N. G. Stoker, and A. R. Coates. 2004. The Mycobacterium tuberculosis sigJ gene controls sensitivity of the bacterium to hydrogen peroxide. FEMS Microbiol. Lett. 273:415-423. [DOI] [PubMed] [Google Scholar]
- 21.Karls, R. K., J. Guarner, D. N. McMurray, K. A. Birkness, and F. D. Quinn. 2006. Examination of Mycobacterium tuberculosis sigma factor mutants using low-dose aerosol infection of guinea pigs suggests a role for SigC in pathogenesis. Microbiology 152:1591-1600. [DOI] [PubMed] [Google Scholar]
- 22.Kaushal, D., B. G. Schroeder, S. Tyagi, T. Yoshimatsu, C. Scott, C. Ko, L. Carpenter, J. Mehrotra, Y. C. Manabe, R. D. Fleischmann, and W. R. Bishai. 2002. Reduced immunopathology and mortality despite tissue persistence in Mycobacterium tuberculosis mutant lacking alternative sigma factor, sigH. Proc. Natl. Acad. Sci. USA 99:8300-8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee, M. S., and D. A. Morrison. 1999. Identification of a new regulator in Streptococcus pneumoniae linking quorum sensing to competence for genetic transformation. J. Bacteriol. 181:5004-5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loewen, P. C., B. Hu, J. Strutinsky, and R. Sparling. 1998. Regulation in the rpoS regulon of Escherichia coli. Can. J. Microbiol. 44:707-717. [DOI] [PubMed] [Google Scholar]
- 25.Lonetto, M., K. Brown, K. Rudd, and M. Buttner. 1994. Analysis of the Streptomyces coelicor sigE gene reveals the existence of subfamily of eubacterial RNA polymerase (sigma) factors involved in the regulation of extracytoplasmic functions. Proc. Natl. Acad. Sci. USA 91:7573-7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manabe, Y. C., J. M. Chen, C. G. Ko, P. Chen, and W. R. Bishai. 1999. Conditional sigma factor expression, using the inducible acetamidase promoter reveals that the Mycobacterium tuberculosis sigF gene modulates expression of the 16-kilodalton alpha-crystallin homologue. J. Bacteriol. 181:7629-7633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manganelli, R., E. Dubnau, S. Tyagi, F. R. Kramer, and I. Smith. 1999. Differential expression of 10 sigma factor genes in Mycobacterium tuberculosis. Mol. Microbiol. 31:715-724. [DOI] [PubMed] [Google Scholar]
- 28.Manganelli, R., M. Voskuil, G. Schoolnik, and I. Smith. 2001. The Mycobacterium tuberculosis ECF sigma factor E: role in global gene expression and survival in macrophages. Mol. Microbiol. 41:423-437. [DOI] [PubMed] [Google Scholar]
- 29.Manganelli, R., M. I. Voskuil, G. K. Schoolnik, E. Dubnau, M. Gomez, and I. Smith. 2002. Role of the extracytoplasmic-function sigma factor sigma(H) in Mycobacterium tuberculosis global gene express. Mol. Microbiol. 45:365-374. [DOI] [PubMed] [Google Scholar]
- 30.Manganelli, R., R. Proveddi, S. Rodrigue, J. Beaucher, L. Gaudreau, and I. Smith. 2004. σ factors and global gene regulation in Mycobacterium tuberculosis. J. Bacteriol. 186:895-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mukherjee, R., M. Gomez, N. Jayaraman, I. Smith, and D. Chatterji. 2005. Hyperglycosylation of glycopeptide of Mycobacterium smegmatis under nutrient starvation: structural studies. Microbiology 151:2385-2392. [DOI] [PubMed] [Google Scholar]
- 32.Paget, M. S., and J. D. Helmann. 2003. The sigma 70 family of sigma factors. Genome Biol. 4:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raman, S., T. Song, X. Puyang, S. Bardarov, W. R. Jacob, Jr., and R. N. Husson. 2001. The alternative sigma factor sigH regulates major components of oxidative and heat stress responses in Mycobacterium tuberculosis. J. Bacteriol. 183:6119-6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raman, S., X. Puyang, T. Y. Cheng, D. C. Young, D. B. Moody, and R. N. Husson. 2006. Mycobacterium tuberculosis SigM positively regulates Esx secreted protein and nonribosomal peptide synthetase genes and downregulates virulence-associated surface lipid synthesis. J. Bacteriol. 188:8460-8468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodrigue, S., R. Provvedi, P. E. Jacques, L. Gaudreau, and R. Manganelli. 2006. The sigma factors of Mycobacterium tuberculosis. FEMS Microbiol. Rev. 30:926-941. [DOI] [PubMed] [Google Scholar]
- 36.Saunders, B. M., A. A. Frank, and I. M. Orme. 1999. Granuloma formation is required to contain bacillus growth and delay mortality in mice chronically infected with Mycobacterium tuberculosis. Immunology 98:324-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith, I., O. Dussurget, G. M. Rodriguez, J. Timm, M. Gomez, J. Dubnau, B. Gold, and R. Manganelli. 1998. Extra and intracellular expression of Mycobacterium tuberculosis genes. Tuber. Lung Dis. 79:91-97. [DOI] [PubMed] [Google Scholar]
- 38.Smith, I., W. R. Bishai., and V. Nagaraja. 2005. Control of mycobacterial transcription, p. 219-231. In S. T. Cole, K. D. Eisenach, D. N. McMurray, and W. R. Jacobs, Jr (ed.), Tuberculosis and the tubercle bacillus. ASM Press, Washington, DC.
- 39.Sun, R., P. J. Converse, C. Ko, S. Tyagi, N. E. Morrison, and W. R. Bishai. 2004. Mycobacterium tuberculosis sigC is an ECF sigma factor required for lethality in mice and for the conditional expression of defined gene set. Mol. Microbiol. 52:25-38. [DOI] [PubMed] [Google Scholar]
- 40.Williams, E. P., J. H. Lee, W. R. Bishai, C. Colantuoni, and P. C. Karakousis. 2007. Mycobacterium tuberculosis SigF regulates genes encoding cell wall-associated proteins and directly regulates the transcriptional regulatory gene phoY1. J. Bacteriol. 189:4234-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu, S., S. T. Howard, D. L. Lakey, A. Kipnis, B. Samten, H. Safi, V. Gruppo, B. Wizel, H. Shams, R. J. Basaraba, I. M. Orme, and P. F. Barnes. 2004. The principal sigma factor sigA mediates enhanced growth of Mycobacterium tuberculosis in vivo. Mol. Microbiol. 51:1551-1562. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.