Abstract
Pili are a major surface feature of the human pathogen Streptococcus pyogenes (group A streptococcus [GAS]). The T3 pilus is composed of a covalently linked polymer of protein T3 (formerly Orf100 or Fct3) with an ancillary protein, Cpa, attached. A putative signal peptidase, SipA (also called LepA), has been identified in several pilus gene clusters of GAS. We demonstrate that the SipA2 allele of a GAS serotype M3 strain is required for synthesis of T3 pili. Heterologous expression in Escherichia coli showed that SipA2, along with the pilus backbone protein T3 and the sortase SrtC2, is required for polymerization of the T3 protein. In addition, we found that SipA2 is also required for linkage of the ancillary pilin protein Cpa to polymerized T3. Despite partial conservation of motifs of the type I signal peptidase family proteins, SipA lacks the highly conserved and catalytically important serine and lysine residues of these enzymes. Substitution of alanine for either of the two serine residues closest to the expected location of an active site serine demonstrated that these serine residues are both dispensable for T3 polymerization. Therefore, it seems unlikely that SipA functions as a signal peptidase. However, a T3 protein mutated at the P-1 position of the signal peptide cleavage site (alanine to arginine) was unstable in the presence of SipA2, suggesting that there is an interaction between SipA and T3. A possible chaperone-like function of SipA2 in T3 pilus formation is discussed.
Streptococcus pyogenes (group A streptococcus [GAS]) is an important gram-positive human pathogen that is capable of causing a broad range of diseases (11). Most of these diseases are mild, self-limiting infections of the throat (pharyngitis) or the skin (impetigo or pyoderma). However, occasionally, GAS causes severe, invasive diseases, including streptococcal toxic shock syndrome and necrotizing fasciitis, that are associated with a high mortality rate. Additionally, GAS infections can result in serious sequelae, including rheumatic fever, rheumatic heart disease, and glomerulonephritis. Recently, it has been demonstrated that GAS produces pili on its surface (23). The presence of these pili facilitates attachment of GAS to host cells and internalization (1, 22), as well as formation of biofilms, which may be important for disease development (22).
Pili are composed of multiple subunits of a single major backbone protein (pilin). As originally demonstrated by Ton-That and coworkers for Corynebacterium diphtheriae (42, 43), on gram-positive bacteria the pilin subunits are covalently attached to each other, unlike the pili on gram-negative bacteria (for reviews, see references 35, 39, and 43). In addition, the polymerized pilus is covalently attached to the gram-positive cell wall. In many cases, pili on gram-positive bacteria also contain ancillary pilin proteins, which are not required for assembly of the pilin structure. The location of the ancillary proteins on the pilus structure and the method of attachment of these proteins to the pilus are not well understood and may be different in different organisms. Like other proteins that are transported through the cell membrane, pilin proteins have an N-terminal signal sequence which is removed during secretion. In addition, like other proteins that are covalently linked to the gram-positive cell wall, pilins contain a C-terminal hydrophobic domain, followed by an LPXTG or similar motif, like QVPTG or V(V/P)PTG, and a charged tail. This arrangement, known as a “cell wall sorting signal”(CWSS), serves as the substrate for a transpeptidase or “housekeeping” sortase, which cleaves the protein between the T and G residues of the CWSS and attaches it to the growing peptidoglycan chain.
The first step in biosynthesis of gram-positive pili involves Sec-dependent secretion of the pilus proteins, which are retained on the cell surface by the C-terminal hydrophobic membrane-anchoring domain. Following this, a sortase family transpeptidase (pilin polymerase) cleaves the CWSS between the threonine and glycine residues in the LPXTG motif, producing an acyl enzyme intermediate in which the threonine of the CWSS is linked to a cysteine residue of the transpeptidase. However, instead of attaching the pilin to the peptidoglycan, the pilin polymerase attaches it to another pilin subunit in a reaction that has not been thoroughly investigated. Finally, after this process is reiterated many times, the growing pilus is attached to the peptidoglycan in a reaction that may involve the housekeeping sortase, as well as the pilin polymerase (for reviews, see references 35, 39, and 43).
GAS pili are encoded in gene clusters located in a highly variable genomic region known as the FCT region (fibronectin-binding, collagen-binding T antigen) (7). The T (trypsin-resistant) antigen, a surface protein, has long been used for serological typing of GAS (31). Surprisingly, Mora et al. recently found that the T antigen is actually the major subunit or backbone protein of a pilus (23). In some GAS strains, this pilus also has Cpa covalently attached to it. Cpa was previously identified as a collagen-binding protein that mediates adhesion to host cells (20).
To date, the GAS FCT regions whose complete sequences are available fall into six classes (FCT-1 to FCT-6) which differ in gene content and gene order (19). FCT-2, FCT-3 (encoding T3), and FCT-4 are present in strains of the serotypes most commonly associated with disease in the Western world, serotypes M1, M3, M5, and M18 (19). A curiosity of pilin proteins encoded in the FCT-2, FCT-3, and FCT-4 regions is the presence of a CWSS containing QVPTG or V(V/P)PTG instead of the canonical LPXTG motif (3).
In addition to the pilin polymerase and the pilin proteins, some FCT gene clusters also encode a homolog of type I signal peptidases, SipA (3, 19, 23, 26). When sipA (also known as lepA) is present, its location within the gene cluster is conserved: it lies between cpa and the gene for the pilus backbone. The SipA gene is present in FCT-2, FCT-3, and FCT-4 (23), but its sequence in the FCT-2 region (SipA1) differs from that in the FCT-3 and FCT-4 regions (SipA2) (3, 19). sipA homologs have also been identified in some, but not all, GBS clusters and in a putative pilus gene cluster in Streptococcus suis (13, 29). The S. suis homolog was found to be highly upregulated when S. suis interacted with porcine brain microvascular endothelial cells, suggesting that it may have a role in the infection process of this pathogen (13).
Type I signal peptidases are required for cleavage of the N-terminal signal sequence of secreted proteins during translocation and secretion by the Sec pathway (33, 47). They belong to a unique class of serine proteases whose catalytic activity depends on a serine-lysine dyad (9, 24, 38). The active site serine serves as the nucleophile in the hydrolytic signal peptide cleavage mechanism, and a lysine residue provides the general base to activate the nucleophilic serine. Gram-negative bacteria typically have only a single type I signal peptidase, and it is essential for growth of these organisms (17). In contrast, many gram-positive bacteria have several signal peptidases, which may either have redundant functions or differ in substrate specificity (46).
In this work, we investigated the possible role of sipA2 in T3 pilus polymerization. We found that sipA2 is essential for polymerization of the T3 protein and for addition of Cpa to this polymer. We also found that in SipA2, which lacks the conserved catalytic site serine and lysine residues of type I signal peptidases, the serine residues nearest the predicted location of the active site are not required for polymerization of T3. In addition, our results imply that there is an interaction between SipA2 and the T3 pilin protein. We suggest that SipA2 may have a chaperone-like function in formation of the gram-positive pili.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
GAS strain JRS4 is a spontaneous streptomycin-resistant derivative of serotype M6 clinical isolate D471 (34). GAS strain AM3 was used as a source of DNA for PCR amplification of FCT-3-specific sequences (37). JRS4/pJRS1317 is a derivative of JRS4 containing a plasmid encoding the sipA-tee3HA-srtC2 region from serotype M3 strain AM3 (3), in which T3 has been tagged with the hemagglutinin (HA) epitope. Escherichia coli strain TOP10 (Invitrogen) was used for plasmid propagation and protein analysis.
GAS strains were grown in Todd-Hewitt medium supplemented with 0.2% yeast extract (Difco), and E. coli strains were grown in LB medium (32) at 37°C. When plasmids were selected for, media were supplemented with 50 μg/ml kanamycin, 34 μg/ml chloramphenicol, or 100 μg/ml spectinomycin for E. coli and with 5 μg/ml chloramphenicol or 100 μg/ml spectinomycin for GAS.
SipA2 deletion in a SipA-T3HA-SrtC2-expressing GAS strain.
Plasmid pJRS1317 (3) was amplified with inverse primers del-SipA2-F and del-SipA2-R (Table 1), which deleted the coding region of sipA2. The resulting 8.1-kb PCR product was digested with BamHI and religated with T4 DNA ligase to produce pJRS1332.
TABLE 1.
Primers used in this study
| Primer | Sequence (5′-3′)a |
|---|---|
| del-SipA2-F | gcatggatccACGTATTTCATATTGAATGA |
| del-SipA2-R | gcatggatccCAATAGTGGATTCTCATTTA |
| del_SipA_F | gcgcgtcgacTCAACTCTATTAAGAGTGAGAGG |
| del_SipA_R | gcgcgtcgacATTTAAGCGATTTAGGTAATTAG |
| sipA_F_BamHI | ggatccGGTTTGGCTATTTGGTCGTAAAG |
| sipA_R_XhoI | ctcgagGTTGCTAAGATTGCAGTAGCAAG |
| Orf100_F_BamHI | ggatccCTCAACTCTATTAAGAGTGAGAGG |
| Orf100_R_XhoI | ctccgaATAAGAATGAGAGTATCAATGGC |
| SrtC2_F_BamHI | ggatccGGTGGAGTTATCTATATTACAAAACG |
| SrtC2_R_XhoI | ctcgagCAATATTTTCTCAAAAGTCTCCTC |
| Orf100A28R_F | CTTTAAATCAAAACGTAAAAcgTGAGACGGCAGGAGTGTCC |
| Orf100A28R_R | GGACACTCCTGCCGTCTCAcgTTTTACGTTTTGATTTAAAG |
| SipA2-S50A-F | CATGATTGTTAACACAAATCAGATGgcTCCTGCTGTAAGTGCTGGT |
| SipA2-S50A-R | ACCAGCACTTACAGCAGGAgcCATCTGATTTGTGTTAACAATCATG |
| SipA2-S55A-F | CAAATCAGATGAGTCCTGCTGTAgcTGCTGGTGATGGAGTCTTATA |
| SipA2-S55A-R | TATAAGACTCCATCACCAGCAgcTACAGCAGGACTCATCTGATTTG |
| Cpa-F3 | gcatctgcagGCTACTTTAGTTTAGAAGGAGGAG |
| Cpa-HA-F | tatccatatgatgttccagattatgctACTGAAAAAACATCAGTCATTATCAG |
| Cpa-HA-R | agcataatctggaacatcatatggataTTTAGCCGGAGGCTCTTCTCC |
| SrtC2-SmaI-R2 | gcatcccgggATTTTCCAATATTTTCTCAAAAGTCTCCTCC |
Introduced restriction sites are indicated by bold type. Uppercase letters indicate bases complementary to the GAS sequence. Lowercase letters indicate bases added or changed to facilitate cloning or mutagenesis. Underlined regions are bases incorporated to produce an HA tag (Cpa-HA-F and Cpa-HA-R).
Construction of a plasmid for complementation of sipA2 deletion mutants.
Chromosomal DNA of S. pyogenes strain AM3 was used as a template to amplify sipA2 and 43 bp of the region upstream of this gene by PCR using Herculase (Stratagene) and primers sipA_F_BamHI and sipA_R_XhoI (Table 1). The PCR product was cloned into pCR2.1 using a TOPO-TA cloning kit (Invitrogen). The insert was digested with BamHI and XhoI and ligated into pJRS9508 (2) to produce pJRS9535.
Constructs encoding combinations of sipA2, tee3, and srtC2.
sipA2, tee3, and srtC2 were amplified in different combinations (Fig. 1) using primers located at the 5′ ends of sipA2 (sipA_F_BamHI), tee3 (Orf100_F_BamHI), and srtC2 (SrtC2_F_BamHI) and the 3′ ends of sipA2 (sipA_R_BamHI), tee3 (Orf100_R_BamHI), and srtC2 (SrtC2_R_BamHI) and were cloned into the pCR2.1 vector using the TOPO-TA cloning kit (Invitrogen). The resulting plasmids were introduced into E. coli TOP10, and the strains were used for protein analysis. In all constructs, the inserts were in the sense orientation with respect to the T7 promoter of the vector pCR2.1. Neither E. coli TOP10 nor plasmid pCR2.1 encodes T7 polymerase; hence, transcription was due to leakage of either the T7 promoter or other promoters present on pCR2.1.
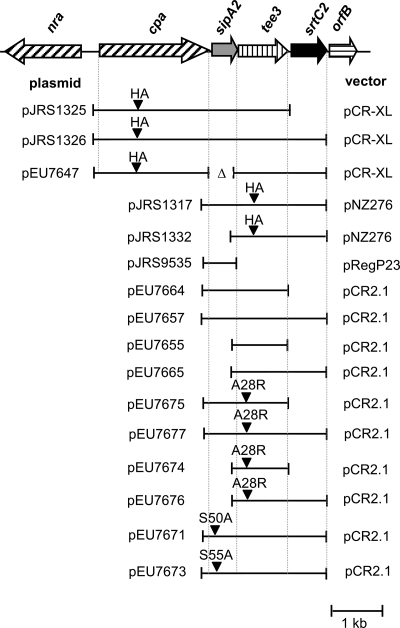
FIG. 1.
FCT region of serotype M3 GAS strain AM3 and derived constructs. The positions of HA tags and mutations introduced by site-specific mutagenesis are indicated by arrowheads. Vectors pCR2.1 and pCR-XL are E. coli cloning vectors (Invitrogen), and pNZ276 (25) and pReg696 (14) are gram-positive-E. coli shuttle vectors.
Site-specific mutagenesis of SipA2 and T3.
Site-specific mutagenesis was performed using a QuikChange 2XL kit (Stratagene) according to the protocol provided by the manufacturer, except that strain TOP10 was used as the recipient strain for the mutagenized plasmids. The mutagenic primers used for a serine-to-alanine change at position 50 (S50A) in SipA2 were SipA2-S50A-F and SipA2-S50A-R, and the mutagenic primers used for a serine-to-alanine change at position 55 (S55A) were SipA2-S55A-F and SipA2-S55A-R (Table 1). The mutagenic primers used for an alanine-to-arginine change at position 28 of tee3 were Orf100A28R_F and Orf100A28R_R (Table 1).
Construction of bacterial strains expressing CpaHA and the downstream segment of the FCT region.
A sequence encoding an HA tag (Sigma) was introduced into the cpa gene from GAS strain MGAS315 by two-step overlapping PCR (16). Primers Cpa-F3 and CpaHA-R (Table 1) were used to amplify the 5′ 810 bp of cpa and 33 bp of the upstream sequence. Similarly, primers CpaHA-F and SrtC2-SmaI-R2 (Table 1) were used to amplify the 3′ 1,425 bp of cpa and 2,320 bp of downstream sequence containing sipA2, tee3, and srtC2 (Fig. 1). The two PCR products were gel purified, combined, and used as the template for a second-round reaction.
A DNA fragment containing cpaHA-sipA2-tee3 was amplified from this template using primers CpaF3 and Orf100-R3 (Table 1) and cloned into pCR-XL using a TOPO-XL PCR cloning kit (Invitrogen), resulting in pJRS1325. Similarly, a DNA fragment containing cpaHA-sipA2-tee3-srtC2 was amplified using primers CpaF3 and SrtC2-SmaI-R2 (Table 1) and subcloned in pCR-XL to produce pJRS1326.
SipA deletion in a CpaHA-SipA-T3-SrtC2-expressing E. coli strain.
Plasmid pJRS1326 was amplified with inverse primers del_SipA_F and del_SipA_R (Table 1), excluding the coding region of sipA2. The resulting 7.3-kb PCR product was treated with polynucleotide kinase and religated with T4 DNA ligase to produce pEU7647.
Preparation of cell lysates and cell wall extracts and immunoblot studies.
To obtain E. coli cell lysates, overnight cultures were grown in LB medium with antibiotic selection, and the cells were collected by centrifugation and resuspended to an optical density at 600 nm of 10 in saline or, when indicated below, in RIPA buffer (150 mM NaCl, 1.0% Igepal CA-630, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 50 mM Tris; pH 8.0). Cells were disrupted by sonication, and 10 μl was used as a sample for analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (PAGE).
For GAS cell wall extraction, overnight cultures were grown in Todd-Hewitt medium supplemented with 0.2% yeast extract, collected by centrifugation, and washed in saline. The cell density was adjusted to 10 cell units per ml in lysin buffer A (50 mM ammonium acetate, 10 mM CaCl2, 1 mM dithiothreitol; pH 6.2); 1 cell unit was defined as 1 ml of culture at an optical density at 600 nm of 2.0. Ten cell units of culture was centrifuged, and the cell pellet was resuspended in 500 μl of lysin buffer A supplemented with 30% raffinose and 200 U/ml recombinant phage lysin from bacteriophage B30 of Streptococcus agalactiae (28). Cells were digested for 3 h at 30°C with constant rotation and pelleted by centrifugation. The supernatant containing the cell wall fraction was used for further analysis. Twenty microliters of cell wall extract was used as a sample for SDS-PAGE analysis. Recombinant phage lysin from bacteriophage B30 was purified from E. coli BL21(DE3)/pSD101 (12, 28), and its activity was determined with GAS strain JRS4 as described by Pritchard et al. (28).
Protein extracts were separated by SDS-PAGE on 10% or 4 to 12% gradient gels (NuPAGE; Invitrogen) using morpholineethanesulfonic acid (MES) electrophoresis buffer (50 mM MES [pH 7.2], 50 mM Tris base, 0.1% SDS, 1 mM EDTA [pH 7.3]) and transferred to a nitrocellulose membrane (Bio-Rad) for immunoblot analysis. HA-7 monoclonal antibody (Sigma) was used at a 1:2.000 dilution, and polyclonal T3 antiserum was used at a dilution of 1:250.
RESULTS
SipA2 is required for T3 pilus formation on the GAS surface.
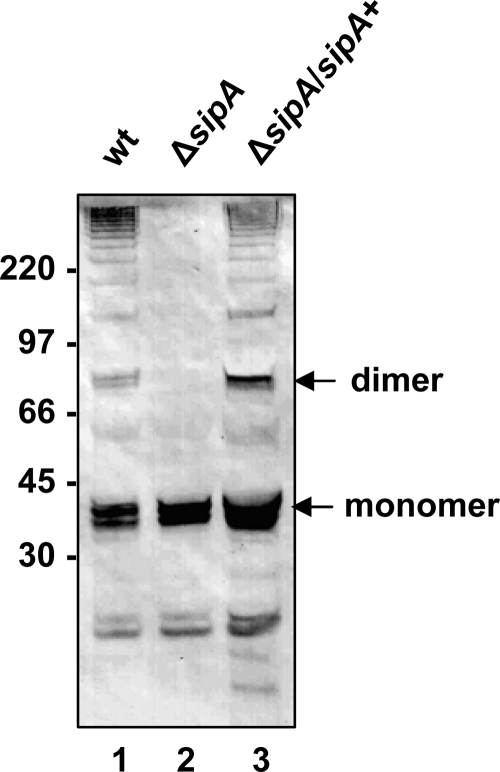
A protein is usually considered to be part of a gram-positive pilus if it is present as a high-molecular-weight ladder in a cell wall extract (35, 44). In our previous studies of the FCT region, we tagged the T3 protein from the serotype M3 strain AM3 with an HA epitope and introduced it on plasmid pJRS1317 (Fig. 1) into a heterologous serotype M6 GAS strain, JRS4 (3). Strain JRS4 has an FCT-1 region which encodes no signal peptidase homolog (3). Because the T6 pili produced by JRS4 do not cross-react with anti-HA antibody (data not shown), we were able to analyze formation of T3 pili in this strain. When we introduced the sipA2, tee3HA, and srtC2 genes of the GAS serotype M3 pilus gene cluster from strain AM3 on plasmid pJRS1317 (3) into strain JRS4, the high-molecular-weight ladder pattern characteristic of pili in gram-positive bacteria was identified in cell wall extracts by immunoblot analysis with anti-HA antibody. To evaluate whether SipA2 was required for the observed T3 polymers, sipA2 was deleted from plasmid pJRS1317 to produce pJRS1332 (Fig. 1), and cell wall extracts were analyzed by immunoblotting. Figure 2 shows that deletion of sipA2 resulted in loss of high-molecular-weight T3 species in cell wall extracts (Fig. 2, compare lanes 1 and 2). The presence of monomeric T3HA (molecular mass, around 32 kDa) indicates that this protein was still expressed in this strain. Complementation with sipA2 on a second plasmid in the strain with the deletion (JRS4/pJRS1332/pJRS9535) resulted in the presence of the high-molecular-weight forms at least at the levels present in parental strain JRS4/pJRS1317 (Fig. 2, lane 3). We concluded that SipA2 is essential for T3 pilus formation in GAS.
FIG. 2.
Effect of sipA2 deletion on T3HA pilus formation in S. pyogenes: Western immunoblot analysis of cell wall extracts of JRS4/pJRS1317 (lane 1), JRS4/pJRS1332 (lane 2), and JRS4/pJRS1332/pJRS9535 (lane 3) reacted with anti-HA monoclonal antibody. The positions of the T3HA monomer and dimer are indicated on the right. The sizes of molecular mass standards (in kilodaltons) are indicated on the left. wt, wild type.
SipA2 is required for T3 polymerization in E. coli.
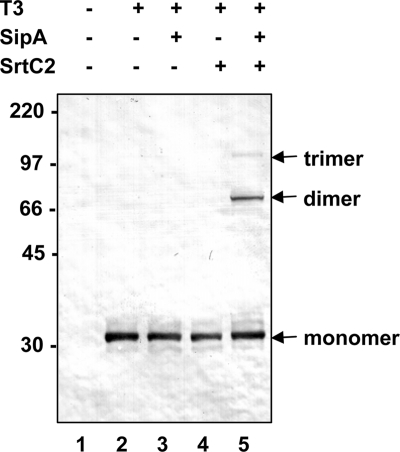
To facilitate further characterization of this system, we investigated expression of tee3 alone and in combination with sipA2 and srtC2 subcloned into vector pCR2.1 in E. coli in plasmids pEU7655, pEU7664, pEU7665, and pEU7657 (Fig. 1 and 3). As expected, expression of T3 alone (pEU7655) produced monomeric T3 (Fig. 3, lane 2). In the presence of SipA2 and SrtC2 (pEU7657), two high-molecular-weight T3 bands were detected with anti-HA antibody (Fig. 3, lane 5). The molecular masses of these bands corresponded to those expected for T3 dimers (64 kDa) and trimers (97 kDa). This demonstrated polymerization of the T3 pilin in the heterologous E. coli host. We also found that in addition to tee3, sipA2 and srtC2 are both required for this polymerization, since deletion of either resulted in the presence of only monomeric T3 (Fig. 3, lanes 3 and 4). These results demonstrate that T3 polymerization does occur in E. coli and that it requires both SipA2 and SrtC2.
FIG. 3.
T3 polymerization in E. coli: Western immunoblot analysis of cell lysates of E. coli TOP10 strains containing the vector pCR2.1 (lane 1), plasmid pEU7655 (lane 2), pEU7664 (lane 3), pEU7665 (lane 4), and pEU7657 (lane 5) reacted with polyclonal anti-T3 antiserum. The positions of the T3 monomer, dimer, and trimer are indicated on the right. The sizes of molecular mass standards (in kilodaltons) are indicated on the left.
SipA2 is not required for T3 signal peptide cleavage in E. coli.
To determine whether SipA2 acts as a signal peptidase on the T3 precursor, we asked whether the T3 monomers shown in Fig. 3 were full-length T3 preproteins or if they had undergone processing of the N-terminal signal peptide. Cleavage of the signal peptide of T3 is predicted to occur after alanine 28, which should decrease the molecular mass of the resulting protein by about 3 kDa. Because all monomeric T3 proteins in Fig. 3 appear to be the same size independent of the presence of SipA2, it appears that if processing occurs, it can be performed by E. coli housekeeping signal peptidase I (LepB). This is expected since the signal peptide predicted for T3 should be recognized by gram-negative as well as gram-positive signal peptidases, according to the signal prediction algorithm of SignalP3.0 (6).
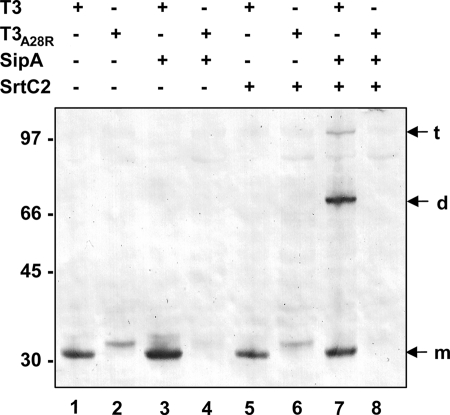
To determine whether processing of the T3 signal peptide occurs in E. coli, the alanine at position 28 was changed to arginine. This mutation at the P-1 position of the signal peptide cleavage site has been shown to abolish processing by the E. coli signal peptidase LepB (36). In the absence of any other proteins encoded by the pilus gene cluster, monomeric T3-A28R appeared as a band at slightly higher molecular weight than wild-type T3 (Fig. 4, lanes 1 and 2), consistent with the expected size difference between T3 with the signal peptide and T3 without the signal peptide. Therefore, it appears that the signal sequence of T3 was removed in this heterologous host, presumably by LepB. Therefore, we could not determine whether the introduced SipA2 protein has a similar function.
FIG. 4.
Effect of signal sequence mutations on size and polymerization of T3: Western immunoblot analysis of cell lysates of E. coli TOP10 containing plasmids pEU7655 (lane 1), pEU7674 (lane 2), pEU7664 (lane 3), pEU7675 (lane 4), pEU7665 (lane 5), pEU7676 (lane 6), pEU7657 (lane 7), and pEU7677 (lane 8) reacted with polyclonal anti-T3 antiserum. The positions of the T3 monomer (m), dimer (d), and trimer (t) are indicated on the right. The sizes of molecular mass standards (in kilodaltons) are indicated on the left.
As expected, the presence of SrtC2 in the absence of SipA2 had no effect on the cleavage of monomeric T3 (Fig. 4, lanes 5 and 6). However, surprisingly, in strains expressing SipA2 either with or without SrtC2, no T3-A28R was detected (Fig. 4, lanes 4 and 8). This suggests that SipA2 acts on, or interacts with, the mutant T3 protein.
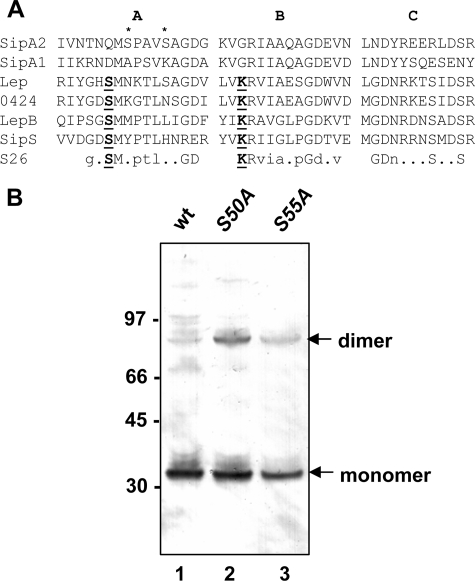
SipA2 lacks the conserved “active site serine” of signal peptidases, and serine residues near its expected location are not required for polymerization of T3.
Although SipA2 shares some homology to signal peptidases (3, 19, 23), the conserved serine residue in the active site of proteins in this family and the conserved lysine residue required for signal peptide cleavage are not present in the SipA proteins of GAS (Fig. 5A). Two serine residues are located near the expected location of the conserved serine and might substitute for it in the serine-lysine dyad mechanism described for signal peptidase I enzymes. To test whether these serine residues are essential for SipA2 activity, we replaced each residue with an alanine in plasmid pEU7657 and analyzed E. coli strains containing the resultant mutant plasmids (pEU7671 and pEU7673 [Fig. 1]) for the ability to polymerize T3 protein. We found that cell lysates of both mutants contained T3 polymers (Fig. 5B). This result indicates that neither of these serine residues is essential for SipA2 function in pilin polymerization.
FIG. 5.
Effect of putative active site serines in SipA2 on T3 polymerization. (A) Alignment of sections of GAS SipA1 and SipA2 and putative signal peptidases encoded by pilus gene clusters of GBS (Lep) and S. suis Ssu0424 (0424) with conserved regions in the primary structure of signal peptidases of E. coli (LepB) and Bacillus subtilis (SipS) and the consensus sequence of type I signal peptidase family S26 (PDOC00418) (28a). The GenBank accession numbers for are as follows: SipA2 (serotype M3 strain MGAS315), NP_66390; SipA1 (serotype M1 strain SF370), NP_268516; Lep (GBS strain A909), YP_330051; E. coli, NP_417063; and B. subtilis, CAA77871. The sequence of Ssu0424 (S. suis strain P1/7) was obtained from http://www.sanger.ac.uk/Projects/S_suis. Catalytically active residues in the signal peptidase motif are indicated by bold type and underlined. The serine residues in SipA2 changed by site-specific mutagenesis to alanine are indicated by asterisks. (B) Western immunoblot with cell lysates of E. coli TOP10 strains containing pEU7657 (lane 1), pEU7672 (lane 2), and pEU7673 (lane 3) reacted with polyclonal anti T3-antiserum. The positions of the T3 monomer and dimer are indicated on the right. The sizes of molecular mass standards (in kilodaltons) are indicated on the left. wt, wild type.
SrtC2 and SipA2 are required for linkage of Cpa-HA to the T3 pilin protein.
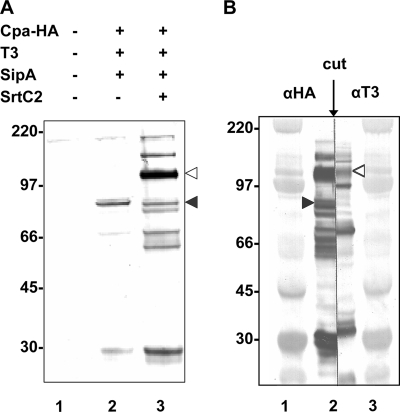
Since SipA2 is required for polymerization of the T3 pilus backbone protein, we also wished to determine whether it is needed for linkage of the ancillary protein Cpa to T3. We designed an HA-tagged Cpa derivative and expressed it together with the downstream sipA2-tee3 region and with the downstream sipA2-tee3-srtC2 region in pJRS1325 and pJRS1326, respectively, in E. coli TOP10 (Fig. 1). Immunoblot analysis with anti-HA antibody of cell lysates of strain TOP10/pJRS1325 containing SipA2 and T3 but not SrtC2 showed that there was a weak band at about 75 kDa, corresponding to the size of the Cpa monomer (Fig. 6A). In the presence of SrtC2 in strain TOP10/pJRS1326, an additional band at about 105 kDa was detected, which is approximately the molecular mass expected for a complex of CpaHA and T3. To confirm that this band was indeed due to such a complex, proteins from a cell lysate of TOP10/pJRS1326 were separated by SDS-PAGE and transferred to nitrocellulose. The membrane was then cut in the center of the sample lane, and one side was reacted with anti-HA antibody while the other side was reacted with anti-T3 antiserum (Fig. 6B). Both antibodies reacted with the band at ∼105 kDa, demonstrating that both proteins were present in this band. The presence of this complex following heat denaturation and reaction with SDS suggests that CpaHA and T3 are covalently linked to each other in a reaction that requires SrtC2. Three additional strong bands visible in the blot reacted with anti-T3 antiserum (Fig. 6B) had molecular sizes consistent with their being the monomer, dimer, and trimer of T3, as expected from the previous results obtained with T3, SipA2, and SrtC2 in the absence of Cpa.
FIG. 6.
CpaHA-T3 linkage in E. coli. (A) Western immunoblot with cell lysates of E. coli TOP10 strains containing vector pCR2.1 (lane 1), plasmid pJRS1325 (lane 2), and pJRS1326 (lane 3) reacted with HA-7 monoclonal antibody. The positions of monomeric CpaHA (filled arrowhead) and the putative CpaHA-T3 complex (open arrowhead) are indicated on the right. (B) Western immunoblot with cell lysates of E. coli TOP10 containing plasmid pJRS1326 (lane 2) cut after transfer to a membrane and developed with HA-7 monoclonal antibody (left side) and polyclonal anti-T3 antiserum (right side). Lanes 1 and 3 contained the High-Range Rainbow molecular weight marker (GE Healthcare).
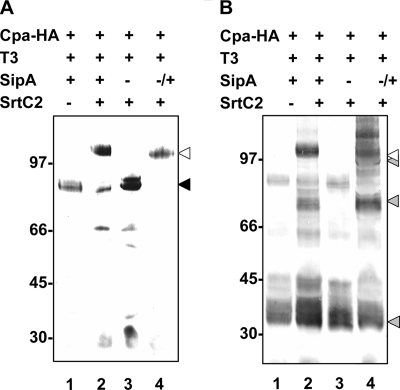
To analyze whether sipA2 is required for the formation of the Cpa-T3 complex, the sipA2 gene was deleted from plasmid pJRS1326 by inverse PCR, and the resulting plasmid (pEU7647) was introduced into E. coli TOP10. Immunoblots of cell lysates of this mutant showed that the level of resolution of CpaHA bands by SDS-PAGE was reduced compared to the results obtained for wild-type strain TOP10/pJRS1326 (data not shown). Because the C-terminal CWSS of CpaHA should cause the protein to be associated with the membrane prior to its further processing by SrtC2, it seemed possible that the poor resolution of the CpaHA protein by SDS-PAGE resulted from attachment to the membrane. Therefore, we resuspended cells in detergent-containing RIPA buffer to improve the solubilization of the protein. Immunoblots of the cell lysates developed with anti-HA antibody indeed showed improved resolution of CpaHA (Fig. 7, lane 3). The location of the CpaHA monomer was determined using the extract of strain TOP10/pJRS1325 (without sortase) (Fig. 7A, lane 1), and the location of the CpaHA-T3 complex was determined using the extract of strain TOP10/pJRS1326 (with sortase) (Fig. 7A, lane 2). In the sipA2 deletion strain, no CpaHA-T3 complex was seen (Fig. 7A, lane 3). Instead, only the CpaHA monomer and the characteristic degradation products of CpaHA were present. To verify that the absence of the CpaHA-T3 complex was due to deletion of the sipA2 gene, sipA2 was introduced into the deletion strain on plasmid pJRS9535 (Fig. 1). Analysis of cell lysates of the complemented mutant showed that the complex consisting of CpaHA and T3 was restored Fig. 7A, lane 4). This indicates that SipA2 is required for linkage of CpaHA to T3.
FIG. 7.
Effect of sipA2 deletion on CpaHA-T3 polymerization: Western immunoblots with cell lysates of E. coli TOP10 strains resuspended in RIPA buffer and disrupted by sonication. The lanes contained cell lysates from cells with vector pCR2.1 (lane 1), pJRS1326 (lane 2), pEU7647 (lane 3), and pEU7647/pJRS9535 (lane 4) and were reacted with HA-7 monoclonal antibody (A) and polyclonal anti-T3 antiserum (B). The position of the CpaHA monomer is indicated by a black arrowhead, the positions of the T3 monomer and polymers are indicated by gray arrowheads, and the position of the CpaHA-T3 complex is indicated by an open arrowhead. The sizes of molecular mass standards (in kilodaltons) are indicated on the left.
In an additional experiment, the cell lysates that were used to obtain Fig. 7A were used for immunoblotting but were developed with T3 antiserum (Fig. 7B). The location of T3 monomers is shown in Fig. 7B, lane 1 (strain without sortase), and the locations of the polymerized T3 and the Cpa-T3 complex are shown in Fig. 7B, lane 2. Confirming our previous results, in the sipA2 deletion strain no T3 polymers were visible (Fig. 7B, lane 3), and their presence was restored by complementation with sipA2 (Fig. 7B, lane 4). Therefore, SipA2 is essential for attachment of CpaHA to the T3 pilus backbone protein, as well as for polymerization of T3.
DISCUSSION
SipA2 is essential for T3 pilus formation in GAS.
Bacterial type 1 signal peptidases (encoded by a gene designated sip or lep) are required for cleavage of the N-terminal secretion signal peptide from proteins secreted through the cell membrane by the Sec system. The genome sequences of gram-positive bacteria often reveal the presence of more than one signal. In the cases studied, most of these peptidases have at least partially redundant functions, although for at least one of them substrate preferences are evident (40, 41, 46).
Because pili are located outside the cell membrane, the proteins that they are composed of must be secreted. The gene cluster required for synthesis of most pili on gram-positive bacteria is composed only of genes encoding a sortase family pilin polymerase and genes encoding the major and minor pilins that constitute the pilus structure. However, in some gram-positive bacteria, including some strains of GAS, the pilin gene cluster also contains a sipA homolog. In this work we studied the role of SipA2 in one such GAS strain that produces T3 pili.
We found that the signal peptidase homolog SipA2, encoded in the T3 gene cluster of a serotype M3 strain of GAS, is essential for pilus biogenesis. Deletion of the sipA2 gene from the strain resulted in production of only monomeric T3 protein, and complementation with the sipA2 gene restored production of the high-molecular-weight forms of T3 that indicate the presence of pili. This is consistent with the possible role of SipA2 as a pilin-specific signal peptidase required for transport of these proteins through the cell membrane. However, the current model for pilus formation in gram-positive bacteria proposes that these structural proteins are secreted via the Sec pathway involving cleavage of the N-terminal secretion signal by a housekeeping signal peptidase. In agreement with this model, we observed that monomeric T3 protein, the major subunit composing the backbone protein of T3 pili on the GAS surface, is secreted through the cell membrane in the absence of SipA2, since it is found in cell wall preparations. Therefore, it appears that the housekeeping signal peptidase is sufficient for this process, and SipA2 appears to play a different essential role in pilus biogenesis.
SipA2 is essential for T3 pilin polymerization in E. coli.
To investigate the role of SipA2 in pilus formation, we expressed the T3 pilus gene cluster in E. coli. In samples prepared for gel analysis by boiling in SDS, we found monomers, dimers, trimers, and tetramers of the T3 protein in extracts of this heterologous organism. This indicates that the T3 pilin protein is polymerized and that the pilin polymers are linked by covalent bonds in E. coli, just as they are in GAS. However, not surprisingly, long pili, characterized by the presence of high-molecular-weight bands in Western blots, are not found in E. coli. We also observed that attempts to increase the expression level of the T3 pilus genes in E. coli led to lethality (data not shown). This is consistent with the possibility that production of high-molecular-weight T3 polymers in E. coli might kill the cells, possibly because the polymers might accumulate in the membrane. The ability to study pilin polymerization in E. coli, which is more malleable genetically than GAS, and the reduced complexity of the pilus polymers in this heterologous organism facilitate analysis of the formation of defined pilus multimers and make the study of specific individual protein interactions in pilus biogenesis more accessible.
By expressing different combinations of T3 pilus cluster genes, we found that SipA2 is required for polymerization of the T3 protein in E. coli, as it is in GAS. Furthermore, covalent association of the minor pilin Cpa with T3 in E. coli also requires SipA2. This may indicate either that SipA2 interacts specifically with Cpa, as well as with T3, or that T3 must first interact with SipA2 before Cpa can be attached to it. The requirement for SipA2 for T3 polymerization in E. coli demonstrates that no E. coli protein can substitute for SipA2 in this process. In addition, it demonstrates that no other GAS-specific proteins are required for pilus polymerization. Thus, our studies show the value of using E. coli as a surrogate host in which to study the process of gram-positive pilus assembly.
The essential role of SipA2 for polymerization of T3 in E. coli is not as a signal peptidase.
It appears that processing of the signal peptide of T3 occurs in E. coli. This was determined by comparing the size of the monomer of the wild-type T3 protein with the size of the T3-A28R mutant protein, in which a residue essential for signal peptidase cleavage by the housekeeping signal peptidase LepB was mutated (Fig. 4). We found that signal peptidase processing occurs even in the absence of sipA2, suggesting that the E. coli enzyme is sufficient for cleavage of the signal peptide from T3. Therefore, because SipA2 is required for polymerization of T3, its essential role in this process in E. coli is not as a signal peptidase.
SipA lacks the conserved serine and lysine residues of type I signal peptidases.
Type I signal peptidases belong to a class of serine proteases (9, 24, 38) whose catalytic activity depends on a serine-lysine dyad mechanism, as described in the Introduction (8, 45). Based on conservation of the three domains that characterize this family, bioinformatic analysis indicated that SipA2 belongs to this family of proteins. However, the active site serine (corresponding to Ser90 in LepB of E. coli), which acts as the nucleophile in the hydrolytic mechanism of signal peptide cleavage, is absent from SipA2. Additionally, the lysine residue (Lys145) which provides the general base required to activate Ser90 is also absent (Fig. 5A). Thus, unless other nearby residues can substitute for these essential amino acids, SipA2 should not be able to function as a type I signal peptidase. In SipA2, there are two serine residues, Ser50 and Ser55, near the expected location of the active site serine. We found that substitution of alanines for both of these serine residues in SipA2 in E. coli did not abolish T3 polymerization, indicating that these residues are dispensable for pilus polymerization. This evidence, as well as that described above, demonstrates that the role of SipA2 in T3 pilin polymerization is not as a serine protease with the catalytic mechanism described for type I signal peptidases. However, the presence of the conserved secondary structure of the type I signal peptidase family in SipA2 suggests that SipA2 may be able to interact with other proteins.
Like SipA2 in GAS, there are signal peptidase-like proteins (designated SpsA and SipA, respectively) in Staphylococcus aureus and Staphylococcus carnosus which lack the “active site serine” and have a serine residue at the position corresponding to S50 in GAS SipA2 (10) (Fig. 5A). Due to the lack of critical residues, these SpsA signal peptidase homologs have been considered inactive (10). In a recent extensive screen for serine protease activities in S. aureus, it was confirmed experimentally that SpsA lacks signal peptidase activity (18). Further work is required to determine the function of these proteins.
SipA2 interacts with the T3 protein.
In the course of the analysis of N-terminal signal processing of the T3 protein in E. coli, we constructed a T3 mutant with a A28R mutation. Although the amount of this protein was significantly less than the amount of the wild-type T3 protein in the absence of SipA2, in the presence of SipA2 the mutant protein was undetectable (Fig. 4). Furthermore, no defined lower-molecular-weight proteolytic products were detected in any strain containing T3-A28R, indicating that proteolysis was complete. These results suggest that SipA2 interacts with T3. Our results also indicate that the interaction of SipA2 with the T3 protein is independent of the presence of the pilin polymerase SrtC2 (Fig. 4). We cannot distinguish between the following two alternative explanations for the instability of T3-A28R in the presence of SipA2: (i) SipA2 may be an enzyme that alters or modifies T3-A28R so that the mutant protein is degraded, or (ii) SipA2 may be a chaperone that binds to T3 to protect it from proteolytic cleavage by an E. coli protease and SipA2 may be unable to bind to T3-A28R. Both alternatives involve an interaction of SipA2 with T3. Considering the similarity of SipA2 to type I signal peptidases, is seems possible that SipA2 may have retained the ability of these enzymes to interact with protein substrates while it lost enzymatic activity.
Similar putative pilus gene cluster in a strain of Streptococcus pneumoniae.
A SipA homolog (CGSSp11BS70_06113) in the partial genome sequence of S. pneumoniae strain SP11-BS70 (Center for Genomic Sciences accession number EDK62593) shows 38% identity (58% similarity) to SipA2, and this predicted protein is more similar to SipA2 encoded in the different GAS genomes than to the other members of the signal peptidase family. As in GAS SipA2, the conserved active site serine and lysine residues are absent. In this strain, the sipA2 homolog is located immediately upstream of a putative pilus backbone protein gene in a gene cluster resembling the FCT-3-FCT-4 region in GAS. As observed for GAS FCT-3-FCT-4 major pilin proteins, the S. pneumoniae putative major pilus protein has a noncanonical CWSS. Instead of LPXTG, VTPTG precedes the hydrophobic region and charged tail of this pilin. Further, neither the S. pneumoniae putative pilin nor the related GAS pilins contain the additional motifs described for pili of gram-positive bacteria (i.e., the pilin motif and the E box) (42, 44). Therefore, the putative pilus gene cluster in this S. pneumoniae strain seems to be more closely related to the pilus gene clusters in some GAS strains than to the pilus gene cluster in the rlr pathogenicity islet (15) of other strains of S. pneumoniae (5, 21). This may suggest that there has been horizontal gene transfer between strains of these two species of streptococci.
Signal peptidase-like proteins in other pilus gene clusters have the conserved residues.
In group B streptococci (GBS), a SipA homolog has been identified in the PI-2b pilus gene cluster (29). However, in contrast to the GAS SipAs, the conserved serine and lysine residues of the type I signal peptidases are fully conserved in the GBS homolog (Fig. 5A). Similarly, in the SipA homolog present in one of the several pilus gene clusters in S. suis (18), the conserved active site residues are also present (Fig. 5A). In neither of these cases has either the enzymatic activity or the role of the encoded protein in pilus production been determined. However, the SipA proteins and the organization of the genes in the pilus clusters of S. suis are more similar to those of GBS than to those of GAS.
Possible chaperone role for SipA2.
Since the discovery of pili on gram-positive bacteria (other than C. diphtheriae) is quite recent, the mechanisms of biogenesis of these structures are not yet well understood. In contrast, a fairly detailed molecular understanding of the morphogenesis of some types of pili in gram-negative bacteria has been developed. In particular the “chaperone-usher” pathway used by the type 1 and pap pili of uropathogenic E. coli (4) and the “alternative chaperone-usher” pathway of the CS1-related pili of enterotoxigenic E. coli (27, 30) have been studied. In these pathways, the pilin proteins remain associated with a chaperone which prevents them from associating prematurely with each other to form unstructured aggregates. In the absence of the chaperone, the pilins are degraded. Association, in the correct order, of the pilin proteins with the outer membrane pore formed by the usher protein releases each pilin in turn from its noncovalent attachment to the chaperone. So far, no chaperone protein has been implicated in the biogenesis of pili in gram-positive organisms. In this work, we identified SipA2, which is encoded in the T3 pilin gene cluster, as a protein essential for T3 polymerization. We also obtained evidence suggesting that these two proteins interact with each other. Although SipA2 has homology to the class of proteases that includes type 1 signal peptidases, we showed that its essential function is not as a signal peptidase in T3 pilus polymerization. Furthermore, in other gram-positive bacterial pilus gene clusters in which the canonical LPXTG cell wall sorting motif sequence is replaced with another sequence, genes encoding similar SipA homologs that lack the conserved active site Ser and Lys residues are also present. We suggest, therefore, that some pili of gram-positive bacteria may require a SipA protein as a chaperone to play a role similar to that played by pilin-specific chaperones in gram-negative bacteria. Further work is required to test this hypothesis.
Acknowledgments
This work was supported by grant AI055605 from the National Institutes of Health to J.R.S.
We thank Aman R. Patel and Timothy C. Barnett for constructing JRS4/pJRS1332, TOP10/pJRS1325, and TOP10/pJRS1326. We thank David Pritchard for providing an E. coli strain with cloned phage lysin and Bernard Beall for supplying anti-T3 antiserum.
Footnotes
Published ahead of print on 9 November 2007.
REFERENCES
- 1.Abbot, E. L., W. D. Smith, G. P. Siou, C. Chiriboga, R. J. Smith, J. A. Wilson, B. H. Hirst, and M. A. Kehoe. 2007. Pili mediate specific adhesion of Streptococcus pyogenes to human tonsil and skin. Cell. Microbiol. 91822-1833. [DOI] [PubMed] [Google Scholar]
- 2.Barnett, T. C., J. V. Bugrysheva, and J. R. Scott. 2007. Role of mRNA stability in growth phase regulation of gene expression in the group A streptococcus. J. Bacteriol. 1891866-1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnett, T. C., A. R. Patel, and J. R. Scott. 2004. A novel sortase, SrtC2, from Streptococcus pyogenes anchors a surface protein containing a QVPTGV motif to the cell wall. J. Bacteriol. 1865865-5875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnhart, M. M., J. S. Pinkner, G. E. Soto, F. G. Sauer, S. Langermann, G. Waksman, C. Frieden, and S. J. Hultgren. 2000. PapD-like chaperones provide the missing information for folding of pilin proteins. Proc. Natl. Acad. Sci. USA 977709-7714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barocchi, M. A., J. Ries, X. Zogaj, C. Hemsley, B. Albiger, A. Kanth, S. Dahlberg, J. Fernebro, M. Moschioni, V. Masignani, K. Hultenby, A. R. Taddei, K. Beiter, F. Wartha, A. von Euler, A. Covacci, D. W. Holden, S. Normark, R. Rappuoli, and B. Henriques-Normark. 2006. A pneumococcal pilus influences virulence and host inflammatory responses. Proc. Natl. Acad. Sci. USA 1032857-2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bendtsen, J. D., H. Nielsen, G. von Heijne, and S. Brunak. 2004. Improved prediction of signal peptides: SignalP 3.0. J. Mol. Biol. 340783-795. [DOI] [PubMed] [Google Scholar]
- 7.Bessen, D. E., and A. Kalia. 2002. Genomic localization of a T serotype locus to a recombinatorial zone encoding extracellular matrix-binding proteins in Streptococcus pyogenes. Infect. Immun. 701159-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Black, M. T. 1993. Evidence that the catalytic activity of prokaryote leader peptidase depends upon the operation of a serine-lysine catalytic dyad. J. Bacteriol. 1754957-4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Black, M. T., J. G. Munn, and A. E. Allsop. 1992. On the catalytic mechanism of prokaryotic leader peptidase 1. Biochem. J. 282539-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cregg, K. M., I. Wilding, and M. T. Black. 1996. Molecular cloning and expression of the spsB gene encoding an essential type I signal peptidase from Staphylococcus aureus. J. Bacteriol. 1785712-5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cunningham, M. W. 2000. Pathogenesis of group A streptococcal infections. Clin. Microbiol. Rev. 13470-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donovan, D. M., J. Foster-Frey, S. Dong, G. M. Rousseau, S. Moineau, and D. G. Pritchard. 2006. The cell lysis activity of the Streptococcus agalactiae bacteriophage B30 endolysin relies on the cysteine, histidine-dependent amidohydrolase/peptidase domain. Appl. Environ Microbiol. 725108-5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fittipaldi, N., M. Gottschalk, G. Vanier, F. Daigle, and J. Harel. 2007. Use of selective capture of transcribed sequences to identify genes preferentially expressed by Streptococcus suis upon interaction with porcine brain microvascular endothelial cells. Appl. Environ Microbiol. 734359-4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grady, R., and F. Hayes. 2003. Axe-Txe, a broad-spectrum proteic toxin-antitoxin system specified by a multidrug-resistant, clinical isolate of Enterococcus faecium. Mol. Microbiol. 471419-1432. [DOI] [PubMed] [Google Scholar]
- 15.Hava, D. L., C. J. Hemsley, and A. Camilli. 2003. Transcriptional regulation in the Streptococcus pneumoniae rlrA pathogenicity islet by RlrA. J. Bacteriol. 185413-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho, S. N., H. D. Hunt, R. M. Horton, J. K. Pullen, and L. R. Pease. 1989. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 7751-59. [DOI] [PubMed] [Google Scholar]
- 17.Inada, T., D. L. Court, K. Ito, and Y. Nakamura. 1989. Conditionally lethal amber mutations in the leader peptidase gene of Escherichia coli. J. Bacteriol. 171585-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kavanaugh, J. S., M. Thoendel, and A. R. Horswill. 2007. A role for type I signal peptidase in Staphylococcus aureus quorum sensing. Mol. Microbiol. 65780-798. [DOI] [PubMed] [Google Scholar]
- 19.Kratovac, Z., A. Manoharan, F. Luo, S. Lizano, and D. E. Bessen. 2007. Population genetics and linkage analysis of loci within the FCT region of Streptococcus pyogenes. J. Bacteriol. 1891299-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kreikemeyer, B., M. Nakata, S. Oehmcke, C. Gschwendtner, J. Normann, and A. Podbielski. 2005. Streptococcus pyogenes collagen type I-binding Cpa surface protein. Expression profile, binding characteristics, biological functions, and potential clinical impact. J. Biol. Chem. 28033228-33239. [DOI] [PubMed] [Google Scholar]
- 21.LeMieux, J., D. L. Hava, A. Basset, and A. Camilli. 2006. RrgA and RrgB are components of a multisubunit pilus encoded by the Streptococcus pneumoniae rlrA pathogenicity islet. Infect. Immun. 742453-2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manetti, A. G., C. Zingaretti, F. Falugi, S. Capo, M. Bombaci, F. Bagnoli, G. Gambellini, G. Bensi, M. Mora, A. M. Edwards, J. M. Musser, E. A. Graviss, J. L. Telford, G. Grandi, and I. Margarit. 2007. Streptococcus pyogenes pili promote pharyngeal cell adhesion and biofilm formation. Mol. Microbiol. 64968-983. [DOI] [PubMed] [Google Scholar]
- 23.Mora, M., G. Bensi, S. Capo, F. Falugi, C. Zingaretti, A. G. Manetti, T. Maggi, A. R. Taddei, G. Grandi, and J. L. Telford. 2005. Group A Streptococcus produce pilus-like structures containing protective antigens and Lancefield T antigens. Proc. Natl. Acad. Sci. USA 10215641-15646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paetzel, M., and R. E. Dalbey. 1997. Catalytic hydroxyl/amine dyads within serine proteases. Trends Biochem. Sci. 2228-31. [DOI] [PubMed] [Google Scholar]
- 25.Platteeuw, C., G. Simons, and W. M. de Vos. 1994. Use of the Escherichia coli β-glucuronidase (gusA) gene as a reporter gene for analyzing promoters in lactic acid bacteria. Appl. Environ Microbiol. 60587-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Podbielski, A., M. Woischnik, B. A. Leonard, and K. H. Schmidt. 1999. Characterization of nra, a global negative regulator gene in group A streptococci. Mol. Microbiol. 311051-1064. [DOI] [PubMed] [Google Scholar]
- 27.Poole, S. T., A. L. McVeigh, R. P. Anantha, L. H. Lee, Y. M. Akay, E. A. Pontzer, D. A. Scott, E. Bullitt, and S. J. Savarino. 2007. Donor strand complementation governs intersubunit interaction of fimbriae of the alternate chaperone pathway. Mol. Microbiol. 631372-1384. [DOI] [PubMed] [Google Scholar]
- 28.Pritchard, D. G., S. Dong, J. R. Baker, and J. A. Engler. 2004. The bifunctional peptidoglycan lysin of Streptococcus agalactiae bacteriophage B30. Microbiology 1502079-2087. [DOI] [PubMed] [Google Scholar]
- 28a.Rawling, N. D., and N. J. Barrett. 1994. Families of serine peptidases. Methods Enzymol. 24419-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosini, R., C. D. Rinaudo, M. Soriani, P. Lauer, M. Mora, D. Maione, A. Taddei, I. Santi, C. Ghezzo, C. Brettoni, S. Buccato, I. Margarit, G. Grandi, and J. L. Telford. 2006. Identification of novel genomic islands coding for antigenic pilus-like structures in Streptococcus agalactiae. Mol. Microbiol. 61126-141. [DOI] [PubMed] [Google Scholar]
- 30.Sakellaris, H., and J. R. Scott. 1998. New tools in an old trade: CS1 pilus morphogenesis. Mol. Microbiol. 30681-687. [DOI] [PubMed] [Google Scholar]
- 31.Schneewind, O., K. F. Jones, and V. A. Fischetti. 1990. Sequence and structural characteristics of the trypsin-resistant T6 surface protein of group A streptococci. J. Bacteriol. 1723310-3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott, J. R. 1974. A turbid plaque-forming mutant of phage P1 that cannot lysogenize Escherichia coli. Virology 62344-349. [DOI] [PubMed] [Google Scholar]
- 33.Scott, J. R., and T. C. Barnett. 2006. Surface proteins of gram-positive bacteria and how they get there. Annu. Rev. Microbiol. 60397-423. [DOI] [PubMed] [Google Scholar]
- 34.Scott, J. R., P. C. Guenthner, L. M. Malone, and V. A. Fischetti. 1986. Conversion of an M− group A streptococcus to M+ by transfer of a plasmid containing an M6 gene. J. Exp. Med. 1641641-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scott, J. R., and D. Zähner. 2006. Pili with strong attachments: Gram-positive bacteria do it differently. Mol. Microbiol. 62320-330. [DOI] [PubMed] [Google Scholar]
- 36.Shen, L. M., J. I. Lee, S. Y. Cheng, H. Jutte, A. Kuhn, and R. E. Dalbey. 1991. Use of site-directed mutagenesis to define the limits of sequence variation tolerated for processing of the M13 procoat protein by the Escherichia coli leader peptidase. Biochemistry 3011775-11781. [DOI] [PubMed] [Google Scholar]
- 37.Stamp, T. C., and E. B. Hendry. 1937. The immunising activity of certain chemical fractions isolated from haemolytic streptococci. Lancet i257-259. [Google Scholar]
- 38.Sung, M., and R. E. Dalbey. 1992. Identification of potential active-site residues in the Escherichia coli leader peptidase. J. Biol. Chem. 26713154-13159. [PubMed] [Google Scholar]
- 39.Telford, J. L., M. A. Barocchi, I. Margarit, R. Rappuoli, and G. Grandi. 2006. Pili in gram-positive pathogens. Nat. Rev. Microbiol. 4509-519. [DOI] [PubMed] [Google Scholar]
- 40.Tjalsma, H., M. A. Noback, S. Bron, G. Venema, K. Yamane, and J. M. van Dijl. 1997. Bacillus subtilis contains four closely related type I signal peptidases with overlapping substrate specificities. Constitutive and temporally controlled expression of different sip genes. J. Biol. Chem. 27225983-25992. [DOI] [PubMed] [Google Scholar]
- 41.Tjalsma, H., A. G. Stöver, A. Driks, G. Venema, S. Bron, and J. M. van Dijl. 2000. Conserved serine and histidine residues are critical for activity of the ER-type signal peptidase SipW of Bacillus subtilis. J. Biol. Chem. 27525102-25108. [DOI] [PubMed] [Google Scholar]
- 42.Ton-That, H., L. A. Marraffini, and O. Schneewind. 2004. Sortases and pilin elements involved in pilus assembly of Corynebacterium diphtheriae. Mol. Microbiol. 53251-261. [DOI] [PubMed] [Google Scholar]
- 43.Ton-That, H., and O. Schneewind. 2004. Assembly of pili in Gram-positive bacteria. Trends Microbiol. 12228-234. [DOI] [PubMed] [Google Scholar]
- 44.Ton-That, H., and O. Schneewind. 2003. Assembly of pili on the surface of Corynebacterium diphtheriae. Mol. Microbiol. 501429-1438. [DOI] [PubMed] [Google Scholar]
- 45.van Dijl, J. M., A. de Jong, G. Venema, and S. Bron. 1995. Identification of the potential active site of the signal peptidase SipS of Bacillus subtilis. Structural and functional similarities with LexA-like proteases. J. Biol. Chem. 2703611-3618. [DOI] [PubMed] [Google Scholar]
- 46.van Roosmalen, M. L., N. Geukens, J. D. Jongbloed, H. Tjalsma, J. Y. Dubois, S. Bron, J. M. van Dijl, and J. Anne. 2004. Type I signal peptidases of Gram-positive bacteria. Biochim. Biophys. Acta 1694279-297. [DOI] [PubMed] [Google Scholar]
- 47.van Wely, K. H., J. Swaving, R. Freudl, and A. J. Driessen. 2001. Translocation of proteins across the cell envelope of Gram-positive bacteria. FEMS Microbiol. Rev. 25437-454. [DOI] [PubMed] [Google Scholar]