Abstract
Agrobacterium tumefaciens can grow anaerobically via denitrification. To learn more about how cells regulate production of nitrite and nitric oxide, experiments were carried out to identify proteins involved in regulating expression and activity of nitrite and nitric oxide reductase. Transcription of NnrR, required for expression of these two reductases, was found to be under control of FnrN. Insertional inactivation of the response regulator actR significantly reduced nirK expression and Nir activity but not nnrR expression. Purified ActR bound to the nirK promoter but not the nor or nnrR promoter. A putative ActR binding site was identified in the nirK promoter region using mutational analysis and an in vitro binding assay. A nirK promoter containing mutations preventing the binding of ActR showed delayed expression but eventually reached about 65% of the activity of an equivalent wild-type promoter lacZ fusion. Truncation of the nirK promoter revealed that truncation up to and within the ActR binding site reduced expression, but fragments lacking the ActR binding site and retaining the NnrR binding site showed expression as high as or higher than the full-length fragment. Additional experiments revealed that expression of paz, encoding the copper protein pseudoazurin, was highly reduced in the actR or fnrN mutants and that ActR binds to the paz promoter. Inactivation of paz reduced Nir activity by 55%. These results help explain why Nir activity is very low in the actR mutant even though a nirK promoter with mutations in the ActR binding site showed significant expression.
Denitrification is the dissimilatory reduction of nitrate to gaseous end products and is utilized as an alternative form of respiration under oxygen-limited conditions (42). Nitrate is considered to be the initial substrate in the denitrification pathway because it is the nitrogen oxide in the pathway with the highest oxidation state. Nitrate is reduced to nitrite, which is then reduced to nitric oxide, nitrous oxide, and finally nitrogen. During denitrification, expression of nitrite reductase (Nir) and nitric oxide reductase (Nor), which catalyze nitrite and nitric oxide reduction, respectively, is coupled at the transcriptional level to mitigate nitric oxide toxicity (43). In many members of the alphaproteobacteria including Agrobacterium tumefaciens, a member of the FNR family that is frequently designated DNR or NNR controls expression of the genes encoding Nir and Nor. This protein has been designated NnrR in A. tumefaciens (3). Evidence suggests that these proteins are activated by nitric oxide (NO), but the mechanism is unclear (43).
Besides available substrates for each nitrogen oxide reductase, optimal expression of the denitrification genes requires low oxygen. This aspect of regulation utilizes regulatory systems that are controlled by oxygen levels or the cellular redox state. For example, Fnr and Prr/Reg have been implicated in the regulation of nirK or nor gene expression (2, 18). Fnr is a global regulator of gene expression utilizing an O2-labile [Fe-S] cluster (38). The Prr/Reg two-component system is a highly conserved global regulatory system in alphaproteobacteria that provides redox control for a variety of cellular processes such as photosynthesis, carbon dioxide fixation, nitrogen fixation, hydrogen oxidation, denitrification, and aerotaxis (8, 34). In this regulatory system PrrB/RegB is a membrane-bound histidine sensor kinase that is autophosphorylated, and PrrA/RegA is a transcription regulator activated by phosphorylation (8).
Work done with Rhodobacter species suggests that the activity of PrrB (RegB) is regulated by components of the electron transport chain. In Rhodobacter sphaeroides evidence suggests that the turnover of the cbb3-oxidase inhibits PrrB activity (13, 24). In Rhodobacter capsulatus the redox state of the ubiquinone pool has been shown to regulate RegB activity (35). Ubiquinone, which is the oxidized form of this redox mediator, was found to bind to RegB and thereby inhibit its autophosphorylation (35). This implies that when electron transport is active, such as when O2 levels are high, there will be low levels of phosphorylated RegA.
This work was undertaken to assess the role of Fnr and related proteins as well as Prr/Reg orthologs in controlling expression of nirK and nor in A. tumefaciens. Nir and Nor have been shown to be expressed when A. tumefaciens is infiltrated into plant leaves and occasionally when cells become associated with roots (3). Denitrification has been found to benefit bacteria that form close associations with plants, so it is important to understand what environmental and physiological signals are required for expression of genes required for nitrogen oxide respiration in a model organism like A. tumefaciens (9). There are four homologs of Fnr, designated FnrN, FixK, SinR, and NnrR, in the genome of A. tumefaciens C58 (28). Only FnrN is predicted to have an [Fe-S] cluster. SinR has been shown to control biofilm development (28). The A. tumefaciens C58 genome also contains an ortholog of the PrrAB two-component system designated ActRS (41). Inactivation of either actR or fnrN decreased expression of both nirK and nor. Further experimentation revealed that ActR is directly involved in regulating nirK while the involvement of FnrN is indirect. ActR was also found to regulate genes whose products are required for optimal Nir activity, further demonstrating the importance of the ActRS system in controlling nitrogen oxide respiration in A. tumefaciens.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
A. tumefaciens C58 (ATCC 33970) was used in this study. Escherichia coli strains DH5α and S17-1 were used for cloning and biparental matings, respectively. Growth conditions, antibiotic concentrations, and nitrate amendments were previously described (3).
Construction of strains and plasmids.
actR, fixK, paz, and fixN were insertionally inactivated by Campbell insertion of a suicide vector containing an internal fragment of each gene. Locus tags for each gene are given using the format given in Wood et al. (41). Internal DNA fragments of the actR (Atu0050) and paz (Atu2283) open reading frames (ORFs) were amplified by PCR and cloned into pJP5603 (26) to create pACTR-I and pPAZ-I, respectively. Internal DNA fragments of the fixK (Atu1037) and fixN (Atu1537) ORFs were cloned into pSUP201 (32) to create pFIXK-I and pFIXN-I, respectively (Table 1). pACTR-I and pPAZ-I were transformed into E. coli S17-1 (λ pir), and pFIXK-I and pFIXN-I were transformed into E. coli S17-1. Each vector was conjugated into A. tumefaciens C58. Exconjugants with kanamycin resistance were selected for pACTR-I and pPAZ-I, and exconjugants with tetracycline resistance were selected for pFIXK-I and pFIXN-I. Inactivation of each gene was confirmed by PCR after genomic DNA was extracted from the exconjugants. The fnrN and sinR mutants were a generous gift from Clay Fuqua, University of Indiana.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strains or plasmid | Genotype or description | Reference or source |
|---|---|---|
| Strains | ||
| C58 | Wild-type pathogenic strain of A. tumefaciens | ATCC 33970 |
| A002 | nir strain of A. tumefaciens with Campbell insertion of pSUP202 into nirK ORF; Tcr | 3 |
| A011 | nnrR strain of A. tumefaciens with nnrR being replaced by ΩSprSmr cassette; Smr Spr | 3 |
| BER3 | fnrN strain of A. tumefaciens: fnrN::pBER213; Cbr, Sucs | 28 |
| RG8 | sinR strain of A. tumefaciens: sinR::TnMod-OKm′; Kmr | 28 |
| A059 | actR strain of A. tumefaciens with Campbell insertion of pJP5603 into actR ORF; Kmr | This study |
| A071 | fixN strain of A. tumefaciens with Campbell insertion of pSUP202 into fixN ORF; Tcr | This study |
| A105 | fixK strain of A. tumefaciens with Campbell insertion of pSUP202 into fixK ORF; Tcr | This study |
| A111 | paz strain of A. tumefaciens with Campbell insertion of pJP5603 into paz ORF; Kmr | This study |
| Plasmids | ||
| pWL-CYCA | Derivative of pYSW35 with PrrnB′-cycA fusion | 18 |
| pBBRAnirK | Derivative of pBBR1MCS-5 (18) with 426 bp upstream of nirK ORF (Gmr) | This study |
| pETactR | Derivative of pET-16b with ORF of actR | This study |
| pACTR-I | Derivative of pJP5603 containing an internal fragment of actR (35 bp-347 bp); Kmr | This study |
| pPAZ-I | Derivative of pJP5603 containing an internal fragment of paz (81 bp-313 bp); Kmr | This study |
| pFIXK-I | Derivative of pSUP201 containing an internal fragment of fixK (56 bp-594 bp); Tcr | This study |
| pFIXN-I | Derivative of pSUP201 containing an internal fragment of fixN (669 bp-1101 bp); Kmr | This study |
| pAnorCZ | Derivative of pRK415 with norB-lacZ transcriptional fusion (Tcr Kmr) | 3 |
| pAnirKZ | Derivative of pRK415 with nirK-lacZ transcriptional fusion (Tcr Kmr) | 3 |
| pAnnrRZ | Derivative of pRK415 with nnrR-lacZ transcriptional fusion (Tcr Kmr) | This study |
| pApazZ | Derivative of pRK415 with paz-lacZ transcriptional fusion (Tcr Kmr) | This study |
| pAnirKZ | Derivative of pRK415 with nirK-lacZ transcriptional fusion containing 208 bp upstream of the nirK start (Tcr Kmr) | This study |
| pAnirK2Z | Derivative of pRK415 with nirK-lacZ transcriptional fusion containing 149 bp upstream of the nirK start (Tcr Kmr) | This study |
| pAnirK3Z | Derivative of pRK415 with nirK-lacZ transcriptional fusion containing 78 bp upstream of the nirK start (Tcr Kmr) | This study |
| pAnirK4Z | Derivative of pRK415 with nirK-lacZ transcriptional fusion containing 127 bp upstream of the nirK start (Tcr Kmr) | This study |
| pAnirK5Z | Derivative of pRK415 with nirK-lacZ transcriptional fusion containing 110 bp upstream of the nirK start (Tcr Kmr) | This study |
| pAnirK6Z | Derivative of pRK415 with nirK-lacZ transcriptional fusion containing 97 bp upstream of the nirK start (Tcr Kmr) | This study |
| pAnirK7Z | Derivative of pRK415 with nirK-lacZ transcriptional fusion containing 92 bp upstream of the nirK start (Tcr Kmr) | This study |
| pnirM4 | Derivative of pRK415 with a mutation in the ActR binding site of the nirK promoter (G→C, C→A, and C→T; Tcr) | This study |
| pnirM5 | Derivative of pRK415 with a mutation in the ActR binding site of the nirK promoter (C→A and C→T; Tcr) | This study |
| pnirM6 | Derivative of pRK415 with a mutation in the ActR binding site of the nirK promoter (G→C; Tcr) | This study |
| pnirM4Z | Derivative of pNIRM4 with nirKM4-lacZ transcriptional fusion (Tcr Kmr) | This study |
| ppazM1 | Derivative of pRK415 with a mutation in the ActR binding site of the paz promoter (G→T, G→T, and C→T; Tcr) | This study |
The nnrR-lacZ fusion was generated by amplifying a DNA fragment with the upstream primer 5′-GACGAATTCAGGATACGCGGTGGTATG-3′ and the downstream primer 5′-GGCGGATCCTCTGTTCGACGAATTGCG-3′. The underlined bases indicate restriction sites added for cloning purposes. The 565-bp fragment contained 211 bp upstream of the putative translation start. The amplicon was digested with EcoRI and BamHI and ligated along with the lacZ cassette from pKOK6 (14), digested with BamHI, into the broad-host-range vector pRK415 (12). The construct was designated pAnnrRZ. The paz-lacZ fusion was constructed using a similar strategy with the upstream primer 5′-CGCGAATTCCGATGAAAATACCGACCTG-3′ and the downstream primer 5′-GGCGGATCCGGGTGCATTTGACGACATA-3′ (restriction sites are underlined). The 823-bp fragment contained 511 bp upstream of the putative start of translation.
Four truncated nirK-lacZ fusions, designated pAnirK4Z, pAnirK5Z, pAnirK6Z, and pAnirK7Z, were constructed with the following oligonucleotides: 5′-GCCGAATTCCGCCTGCCAAAATGTCG-3′, 5′-GGGGAATTCCAGGGCGCCGAAAGC-3′, 5′-GCCGAATTCGCGAAGTTTGTTCCATCT-3′ and 5′-CGCGAATTCAGTTTGTTCCATCTCAAATTG-3′ (restriction sites are underlined), respectively. The downstream oligonucleotide used for these constructs was the same as described for the full-length nirK-lacZ fusion (3). These fusions contained 127, 110, 97, and 92 bp upstream of the putative nirK translation start, respectively.
Mutation of the nirK and paz promoters.
Mutation of a putative ActR binding site in the nirK promoter was introduced by the overlap extension method using complementary primers carrying substitutions of one to three bases (1). A mutated ActR binding site carrying a three-base substitution was made with two complementary primers, the upstream primer 5′-CCGCCTCCCAAAATGTAGTAGG-3′ and the downstream primer 5′-CCTACTACATTTTGGGAGGCGG-3′ (substitutions are underlined). The first two amplifications were done with the upstream primer of the complementary primers and the downstream primer for the nirK-lacZ fusion and with the downstream primer of the complementary primers and the upstream primer for the nirK-lacZ fusion. The second PCR was carried out with the upstream and downstream primers for the nirK-lacZ fusion using the two first PCR products as templates. Dimethyl sulfoxide was added to the second PCR mixture to a final concentration of 10%. The 1,020-bp final product was cloned into pRK415 to create pnirM4 followed by the lacZ cassette. A similar approach was used to generate mutated ActR binding sites carrying one- and two-base substitutions, using pnirM4 as a template, to create pnirM6 or pnirM5, respectively. Introduction of three substitutions into a putative ActR binding site in the paz promoter was done as described above using two complementary primers (5′-GCTGCTTCCGGTTGCTTCATT-3′ and 5′-AAATGAAGCAACCGGAAGCAGC-3′; substitutions are underlined) and two primers used for the paz-lacZ fusion. All of the mutated DNA sequences were confirmed by sequencing the final cloned fragments.
Purification of ActR.
ActR was modified to contain an N-terminal hexahistidine tag for rapid purification. To do this the ORF of actR was amplified using the oligonucleotides 5′-GGAATTCCATATGAAGATTGAAGACCAGACC-3′ and 5′-GCGGGATCCTCACTTCGGAGCGCGTTT-3′ (restrictions sites are underlined), and then the product was cloned into pET-16b to create pETactR. The modified ActR protein, with an N-terminal extension of MGHHHHHHHHHHSSGHIEGRH, was expressed in E. coli BL21(DE3)pLysS, which was grown at 30°C in 1 liter of LB medium (pH 7.5) containing 0.5% glucose, 100 μg/ml ampicillin, and 25 μg/ml chloramphenicol. When the culture reached an optical density at 600 nm (OD600) of 0.5, 0.5 mM isopropyl-beta-d-thiogalactopyranoside was added, and then the culture was grown for an additional 3 h. The cells were harvested and washed and resuspended with a buffer containing 50 mM Tris-HCl (pH 8.0), 300 mM NaCl, and 10% glycerol and then broken by passage through a French pressure cell at 19,000 lb/in2. Following DNase treatment in the presence of MgCl2, the crude extracts were centrifuged at 15,000 × g for 15 min, and the resulting supernatant was passed through a column containing Ni-nitrilotriacetic acid resin (Qiagen Co.). Imidazole (10 mM) was added to the crude extract before it was passed through the column. The column was washed with buffer containing 50 mM imidazole, and bound protein was eluted with 250 mM imidazole. Protein from the final elution was pooled and dialyzed overnight against a buffer containing 20 mM Tris-HCl (pH 8.0), 50 mM KCl, and 5% glycerol. Phosphorylation was carried out in the binding buffer with 20 mM acetyl phosphate and 10 mM MgCl2 at 30°C for 30 min. Purified protein was confirmed as ActR by matrix-assisted laser desorption ionization-time of flight mass spectrometry using an Applied Biosystems 4700 mass spectrometer after trypsin digestion.
Construction of the D74A ActR mutant.
Mutagenesis of actR was carried out using a strategy similar to that described for making mutants of the nirK and paz promoters. Two complementary primers, 5′-GCGGTCGTCGCTTTGAGGCT-3′ and 5′-AGCCTCAAAGCGACGACCGC-3′, along with the two primers for construction of pETactR were used to amplify the mutated fragment. Sequencing was used to confirm the presence of the mutation, and the fragment was cloned into pET-16b for heterologous expression as described above. To express the D74A mutant in A. tumefaciens, the upstream primer 5′-CGCGGTACCCAATTCCCCTCTAGAAATAAT-3′ (restriction site is underlined) and the downstream primer used in generating the actR fragment cloned into pETactR were used to amplify the actR fragment from pET-16b containing the D74A mutant. This fragment was cloned into the multiple cloning site of pBBR-MCS-5 in an orientation that would allow expression to be driven from the plasmid's lac promoter (16). A similar fragment was amplified from pETactR and cloned into pBBR-MCS-5 as a wild-type control. Both constructs were moved into A. tumefaciens strain A059 by conjugation.
Enzymatic and protein assays.
In vivo cytochrome c oxidase activity was measured amperometrically in a magnetically stirred chamber of an O2 electrode (Rank Brothers, Cambridge, England). Fifty milliliters of cell culture (OD600 of 0.55 to 0.60) grown under microoxic conditions was harvested by centrifugation at 10,000 × g for 10 min, and the cells were resuspended in 5 ml of 50 mM phosphate buffer (pH 7.0). Oxygen uptake was measured using 100 μl of the resuspended cells added to 1 ml of the same air-saturated buffer. The oxygen consumption rates were monitored after the addition of 10 mM sodium ascorbate and 0.2 mM N,N,N′,N′-tetramethyl-p-phenylenediamine (TMPD). As a control the rate of oxygen consumption in the absence of the resuspended cells was monitored.
β-Galactosidase assays were performed as described previously (37). β-Galactosidase activities were determined in duplicate on at least three independently grown cultures. Nir activity of whole cells was measured as previously described (18). Protein was quantified using the Bio-Rad protein assay using bovine serum albumin (BSA) as a standard protein. The phosphorylation of purified ActR was measured as described previously (39). Horse heart cytochrome c and BSA were used as controls.
EMSA.
The ability of purified ActR to bind to the promoters of nirK, nor, nnrR, and paz was tested using electrophoretic mobility shift assays (EMSAs). A fragment extending from 173 bp to 15 bp upstream of the putative nirK translation start was amplified using the following oligonucleotides: 5′-CGCGAATTCCGAACAGGCAAAGAGGAG-3′ and 5′-TCTTAGGGCAGAGCATGTT-3′. A fragment spanning the region from 165 bp to 44 bp upstream of the putative paz translation start was amplified using 5′-CGCGAATTCAATACGTTTCCTTCTGCCG-3′ and 5-GTCGTCTTAGCGCAGGAG-3′. A nor fragment spanning the region from 194 bp to 40 bp upstream of the putative norC translation start was amplified using the following oligonucleotides: 5′-CGCGAATTCTTCCTGCAGGGCATTTTG-3′ and 5′-CTGGCTTGATTGAACCCTC-3′. The nnrR promoter fragment spanned the region from 105 bp upstream to 71 bp downstream of the putative nnrR translation start and was amplified using the following oligonucleotides: 5′-CGCGAATTCTCTTCAGCCAGTGATAGTGC-3′ and 5′-TCCGTCCAGCTCATCGTC-3′.
DNA was labeled at the 5′ ends with [γ-32P]ATP by polynucleotide kinase. The buffer used in the gel shift assay was composed of 20 mM Tris-HCl (pH 8.0), 50 mM KCl, 5% glycerol, 1 mM MgCl2, 1 mM dithiothreitol, 50 μg/ml BSA, and 5 μg/ml salmon sperm DNA. All targeted promoter DNAs were added to a final level of 0.280 fmol. All samples were incubated at 30°C for 30 min and then separated on a 6% polyacrylamide gel with a 45 mM Tris-borate buffer. After gel drying, the gel was visualized by using a STORM PhosphorImager, and the DNA was quantified by using ImageQuant software (Molecular Dynamics).
RESULTS
Regulation of nirK and nnrR by Fnr homologs.
It has been previously shown that nirK and nor expression in A. tumefaciens is under the control of NnrR, a member of the FNR family of transcriptional regulators (3). Upstream of nnrR there is a nearly perfect FNR consensus binding site, TTGAT(N4)GTCAA (where N is any nucleotide). This suggests that one of the FNR homologs in the genome regulates transcription of nnrR. Regulation by an FNR homolog was supported by the observation that nnrR expression in wild-type C58 was enhanced about sixfold when the cell culture was converted from oxic to microoxic conditions (Table 2). Expression of nnrR in a strain in which fnrN (Atu1537) had been inactivated was about 85% lower than wild type under microoxic conditions. As expected, nirK and nor expression also decreased significantly in the fnrN mutant, likely due to the reduced level of NnrR (Table 2). Inactivation of sinR reduced expression of nnrR about 50% under microoxic conditions. This caused only about a 20% reduction of nirK expression under microoxic conditions in nitrate-supplemented medium. Expression of both nnrR and nirK was not significantly affected by inactivation of fixK (Table 2).
TABLE 2.
Expression of nirK-lacZ, norC-lacZ, and nnrR- lacZ in A. tumefaciens strains
| Strain (genotype) | β-Galactosidase activity (Miller units) under the indicated conditionsa
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
|
nirK-lacZ
|
norC-lacZ
|
nnrR-lacZ
|
|||||||
| Aerobic | O2 limited | O2 limited + NO3− | Aerobic | O2 limited | O2 limited + NO3− | Aerobic | O2 limited | O2 limited + NO3− | |
| C58 | 5 ± 2 | 805 ± 25 | 1307 ± 51 | 3 ± 2 | 591 ± 21 | 902 ± 27 | 130 ± 11 | 637 ± 33 | 753 ± 28 |
| BER3 (fnrN) | 3 ± 2 | 48 ± 8 | 71 ± 9 | 8 ± 3 | 80 ± 5 | 67 ± 9 | 77 ± 4 | 88 ± 6 | 115 ± 7 |
| RG8 (sinR) | 6 ± 4 | 433 ± 29 | 1063 ± 56 | ND | ND | ND | 115 ± 7 | 281 ± 13 | 386 ± 19 |
| A011 (nnrR) | 4 ± 2 | 137 ± 10 | 110 ± 9 | 1 ± 1 | 33 ± 5 | 28 ± 4 | 136 ± 9 | 450 ± 12 | 411 ± 27 |
| A059 (actR) | 4 ± 3 | 17 ± 8 | 129 ± 13 | 6 ± 2 | 52 ± 5 | 278 ± 12 | 124 ± 14 | 411 ± 24 | 697 ± 25 |
| A071 (fixN) | 5 ± 3 | 736 ± 31 | 1288 ± 43 | ND | ND | ND | 131 ± 8 | 514 ± 18 | 426 ± 16 |
| A105 (fixK) | 6 ± 2 | 607 ± 38 | 1206 ± 30 | ND | ND | ND | 129 ± 6 | 506 ± 34 | 603 ± 30 |
Activities ± standard deviations were determined in duplicate assays of at least three independently grown cultures. ND, not determined.
These results indicate that FnrN plays an important role in expression of nirK and nor by regulating nnrR expression. SinR also seems to be involved in nnrR regulation as well. sinR expression has been shown to be regulated by FnrN (28), so the decrease in nnrR expression in the fnrN mutant may be partly due to a decrease in sinR expression. However, a decrease in sinR expression cannot account for the large decrease in nnrR expression observed in the fnrN mutant.
Interestingly, expression of nnrR in wild-type C58 is about 20% higher in nitrate-supplemented medium than in medium without supplementation (Table 2). This may indicate that NnrR can enhance its own expression. This conclusion was supported by the observation that nnrR expression decreased in the NnrR-deficient strain when it was grown under microoxic conditions (Table 2). In addition, 1 mM sodium nitroprusside, a NO+ donor, increased nnrR expression by 2.5-fold in the fnrN mutant (data not shown). Sodium nitroprusside can activate expression of genes regulated by NnrR (43).
nirK expression in actR and fixN mutants.
nirK showed very limited expression in an actR mutant (Table 2). This decrease was not due to a change in nnrR expression since its expression under denitrifying conditions decreased only about 10% in the actR mutant. Unlike the nnrR mutation, which decreased expression of both nirK and nor by >90%, inactivation of actR did not impact nor expression as much as nirK. nirK expression was nearly identical in the nnrR and actR mutants under denitrifying conditions, but nor expression was 10-fold higher in the actR mutant than in the nnrR mutant under the same conditions (Table 2). This suggests that ActR may play a direct role in regulating nirK expression, but its role in regulating nor expression may only be a consequence of its effect on Nir activity. Nir activity in whole cells of the actR mutant was only about 1% of the activity of wild type (Table 3).
TABLE 3.
Whole-cell Nir activity in A. tumefaciens strains
| Strain (genotype) | Added plasmid(s) | Nir activitya |
|---|---|---|
| C58 | None | 1.53 ± 0.11 |
| BER3 (fnrN) | None | 0 |
| A002 (nirK) | None | 0 |
| A011 (nnrR) | None | 0.16 ± 0.03 |
| A059 (actR) | None | 0.01 ± 0.01 |
| A059 (actR) | pBBRAnirK | 0.28 ± 0.04 |
| A059 (actR) | pWL-CYCA | 0.09 ± 0.03 |
| A059 (actR) | pBBRAnirK, pWL-CYCA | 1.48 ± 0.15 |
| A071 (fixN) | None | 1.52 ± 0.10 |
| A111 (paz) | None | 0.73 ± 0.06 |
In vivo activity of nitrite reductase from cultures measured as previously described (18). Data represent activity from at least two independently grown cultures. Units are defined in Materials and Methods (see “Assays for enzymatic activities”).
In the related denitrifier R. sphaeroides 2.4.3, loss of the cbb3 oxidase leads to an unexpected loss of Nir activity (18). The reason for this is unclear but may be due to an impact on PrrBA activity, which is influenced by the cbb3 oxidase. To determine if this was the case in A. tumefaciens fixN (Atu1537), a cbb3 oxidase structural gene, was insertionally inactivated. Loss of the cbb3 oxidase caused no detectable change in the oxic growth of A. tumefaciens, as previously observed (30). The only detectable change was an ∼75% decrease in TMPD oxidase activity of cells grown under microoxic conditions (data not shown). The absence of this oxidase had no impact on nirK expression or Nir activity (Tables 2 and 3). However, there was a noticeable decrease in nnrR expression when the mutant was grown under denitrifying conditions (Table 2).
Binding of ActR to nirK and nor.
To provide evidence that ActR is directly involved in the regulation of nitric oxide metabolism, an N-terminal histidine-tagged variant of A. tumefaciens ActR was purified from E. coli. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of the protein that eluted at high imidazole concentrations revealed a major band (>95%) at about 30 kDa, which is slightly higher than the predicted 24 kDa (data not shown). Matrix-assisted laser desorption ionization-time of flight mass spectrometry analysis of the trypsin-digested protein indicated that the purified protein was ActR.
Since expression of nirK was significantly decreased in an actR mutant, the ability of the purified ActR to bind to DNA was initially tested using nirK. The as-isolated ActR was found to bind with high affinity to nirK but only when Mg2+ was present in the reaction mixture (Fig. 1). Incubation of the protein with acetyl phosphate as a phosphate donor did not change the binding characteristics. The dissociation constant of the as-isolated protein was estimated at about 50 nM while that of the acetyl phosphate protein was about 30 nM. The nearly identical dissociation constants for the as-purified and acetyl phosphate-treated forms of ActR suggested that they might not be chemically distinct. This was confirmed by a phosphate analysis that revealed that the as-purified ActR was 75% phosphorylated. While the phosphorylation of the purified ActR may account for its high-affinity binding, it is also possible that phosphorylation is not a critical factor since nonphosphorylated orthologs of ActR can bind DNA (10). To determine if this was the case with ActR, aspartic acid 74, predicted to be the site of phosphorylation based on comparison with other characterized ActR orthologs, was replaced with an alanine. The purified mutant protein contained approximately three- to fourfold less phosphate than the native protein. In spite of this decrease, the D74A mutant was found to bind nirK with the same affinity as wild type (data not shown). To determine if mutation of this residue affected ActR function in vivo, its ability to restore Nir activity in A059 was tested. Cells containing the D74A form of ActR had levels of Nir activity that were as low as those measured in the ActR-deficient strain (data not shown). A wild-type actR construct identical to the D74A mutant restored Nir activity to wild-type levels.
FIG. 1.
The ability of ActR to bind to the promoters of nirK, nor, nnrR, and paz (PnirK, Pnor, PnnrR, and Ppaz, respectively) was tested using EMSAs as described in Materials and Methods. Concentrations of purified ActR are indicated. To phosphorylate ActR, 20 mM acetyl phosphate (acetyl P) together with MgCl2 was added to purified protein.
To further investigate the interactions of ActR with other genes involved in NO metabolism during denitrification, its interaction with nnrR and nor was assessed by EMSA. Neither as-purified ActR or acetyl phosphate-treated protein would bind to nnrR or nor (Fig. 1). The lack of interaction with nnrR is consistent with the minimal impact seen on nnrR expression in the actR mutant (Table 1). The inability to bind to nor confirms that the decrease in nor expression in the actR mutant is an indirect result of the decrease in Nir activity of this strain (Table 3).
Characterization of a putative ActR binding site.
DNA binding sequences among orthologs of ActR are not highly conserved (20). There have been consensus binding sites reported, but they show variability. This may be due to the fact that DNA structure as well as DNA sequence is an important factor in determining binding of PrrA and RegA (17). As a consequence of the poor sequence conservation, it is difficult to predict the likely binding site for ActR binding to nirK through sequence comparisons. Therefore, as an initial step in an attempt to identify an ActR binding site in nirK, the curvature in the upstream region was estimated using the program bend.it. This program has proven effective in identifying regions that have a high likelihood of being binding sites for Reg/Prr orthologs (11, 17, 29, 40). A sequence with high curvature, 5′-CTGCCAAAATGTCGC-3′, was identified in the nirK fragment used in the EMSAs. This site has significant similarity with a previously predicted consensus binding site for the two ActR orthologs PrrA and RegA, G(T/C)G(C/G)(C/G)(A/G)NN(A/T)(T/A)NNC(G/A)C (17, 20). The predicted site is located 19 bp upstream of the putative NnrR binding site in the nirK promoter.
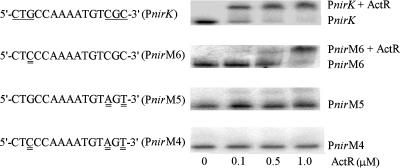
To determine if the predicted site is the actual ActR binding site, nucleotides in the triplet of bases at both the 5′ and 3′ ends of the predicted binding site were mutated. These bases were targeted since they have been shown to be the most highly conserved residues in the predicted binding sites for PrrA and RegA (17, 20). A mutation of G3 →C3 caused a significant decrease in the ActR binding affinity since a 0.5 μM concentration of protein caused only a partial shift of the mutated fragment whereas the native fragment was completely shifted at this level of protein (Fig. 2). The fragment containing the double mutation of C13→A13 and C15→T15 showed even weaker binding than the single G3 mutation, with only a small amount of shifted DNA found at 1.0 μM protein. There was no detectable shifting of the triple mutant with the substitutions G3→C3, C13→A13, and C15→T15 even at the highest protein concentration (Fig. 2). These results demonstrate that this region of DNA is critical for binding of ActR to the nirK regulatory region.
FIG. 2.
Binding of ActR to mutated nirK promoters (Pnir sequences). Concentrations of purified ActR are indicated. In the sequences at left, the wild-type sequence is shown at the top with the region to be targeted underlined. In other sequences, mutated bases are underlined twice. Conditions were the same as for the experiments shown in Fig. 1.
Expression of truncated and mutated nirK promoters.
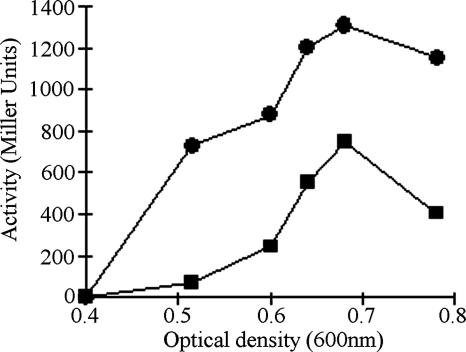
Since nirK expression in an actR mutant decreased significantly, it would be expected that mutation of the ActR binding site in nirK would also lead to a large decrease in nirK expression. This was tested by measuring expression of a nirK fusion containing the triple mutations G3→C3, C13→A13, and C15→T15 of the ActR binding site. While the expression of the mutated nirK promoter was somewhat delayed, its maximal expression was about 65% of the activity of the wild-type promoter during denitrifying conditions (Fig. 3). This higher than predicted expression could indicate the presence of additional regulatory elements in the nirK promoter that are critical for expression and that were not affected by the mutations. It is also possible that the large decrease in nirK expression in the actR mutant is a result of a direct effect on nirK regulation, coupled with a change in the regulation of other genes whose products are required for optimal nirK expression.
FIG. 3.
Expression of nirK-lacZ (•) and nirM4-lacZ (▪). Each mark is the average value of β-galactosidase activities measured in duplicate from at least three independent cultures grown in nitrate-amended medium under microoxic conditions. The standard deviation of all data was lower than 10% of each value of β-galactosidase activity. nirK expression was negligible before an OD600 of ∼0.4, so the initial reported measurement is at this OD.
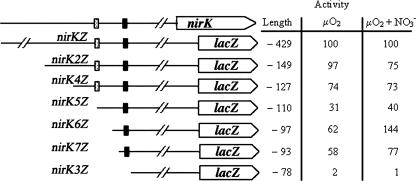
To determine if there are additional regulatory elements in the nirK promoter, expression from a series of truncated fragments was measured. pAnirK4Z, which contained 3 bp upstream of the ActR binding site, was still nitrate inducible and retained 75% of the expression level of the full-length fusion (Fig. 4). Removal of an additional 17 bp, leaving only a single base of the ActR binding site, decreased expression to 40% of pAnirKZ. Interestingly, two fusions with truncations in the region between the ActR binding site and the NnrR binding site, pAnirK6Z and pAnirK7Z, showed levels of expression as high or higher than the full-length fusion (Fig. 4). pAnirK6Z has only 7 bp upstream of the NnrR binding site but showed expression that was about 150% of the full-length fusion. nirK expression in these fusions was still dependent on NnrR since their expression was similar to that of the full-length fragment in the nnrR mutant strain (Fig. 4). Further truncations that removed the NnrR binding site completely eliminated nirK expression, as reported previously (3).
FIG. 4.
Expression of truncated nirK-lacZ fusions. In the table at right, activity is the percentage of the full-length fusion, pAnirKZ (nirKZ), shown at top, and length is relative to the predicted start of translation. Cells for these experiments were grown under O2-restricted conditions with or without nitrate. Activities were determined in duplicate assays from at least two independently grown cultures. Standard deviations were always less than 5% of the average activity with the exception of the pAnirK3Z (nirK3Z) fusion, where the standard deviation was about 10%. The stippled rectangle indicates the location of the ActR binding site, and the filled rectangle indicates the location of the NnrR binding site.
paz expression activated by ActR and FnrN.
The truncation of the nirK regulatory region did not provide evidence there are additional elements upstream of the ActR binding site required for expression. This suggests that the large decrease in nirK expression measured in the actR mutant may be due to both direct and indirect effects. Since Nir activity is essential for optimal nirK expression, it is possible that loss of ActR decreases expression of proteins required for Nir activity. To test this, efforts were made to identify a gene encoding a protein that could be part of the electron transport chain to Nir. In related alphaproteobacteria Nir can receive electrons from either a c-type cytochrome or a copper protein (25). A. tumefaciens has genes encoding both types of proteins. To narrow the possibilities, only those genes encoding copper proteins were examined. A. tumefaciens has two genes encoding copper proteins that could transfer electrons to Nir. One (Atu2283) is highly conserved throughout its predicted sequence, with orthologs in other denitrifiers such as Paracoccus denitrificans and Achromobacter cycloclastes (19, 23). The other (Atu3393) is similar to Atu2283 with the exception of an N-terminal region that is not found in other proteins of this type. It is also part of an operon that includes three other genes whose products form an ABC-type transporter, making its physiological function uncertain. These comparisons suggested that Atu2283, which will be referred to as paz, is the more likely of the two to play a role in denitrification. Insertional inactivation of paz resulted in a 55% decrease in whole-cell Nir activity (Table 3). In other denitrifiers inactivation of both the copper protein and cytochrome are required before any decrease in Nir activity is detected (25). The observation that inactivation of paz causes a measurable decrease in Nir activity indicates that pseudoazurin is an important electron donor to Nir in A. tumefaciens.
Upstream of the predicted paz translation start is a DNA sequence with significant identity with the FNR consensus site, 5′-TTGAT(N4)CGCAA-3′. Immediately upstream of this is the sequence 5′-GCGTCCGGTTGCCGC-3′, which shares identity with predicted consensus RegA and PrrA binding sites (underlined). This site is also predicted to have high curvature (data not shown). These sites suggest that paz expression is controlled by FnrN or one of the other FNR homologs as well as ActR. To confirm this, expression of a paz-lacZ fusion was measured in wild type and fnrN and actR mutants. Maximum expression of the fusion was measured under microoxic conditions (Table 4). The addition of nitrate to the medium did not increase paz expression. There was only very limited expression of paz-lacZ in the fnrN mutant, indicating that this FNR ortholog is controlling paz expression. Expression of paz-lacZ in the actR mutant was also significantly reduced although not as much as in the fnrN mutant (Table 4).
TABLE 4.
Expression of paz-lacZ in A. tumefaciens strains
| Strain (genotype) | β-Galactosidase activity (Miller units) under the indicated condtionsa
|
||
|---|---|---|---|
| Aerobic | O2 limited | O2 limited + nitrate | |
| C58 | 15 ± 2 | 809 ± 21 | 833 ± 29 |
| BER3 (fnrN) | 12 ± 3 | 24 ± 4 | 43 ± 5 |
| A011 (nnrR) | 13 ± 2 | 675 ± 17 | 703 ± 18 |
| A059 (actR) | 16 ± 1 | 102 ± 12 | 187 ± 14 |
Activities ± standard deviations were determined in duplicate assays of at least three independently grown cultures.
To confirm that paz is directly regulated by ActR, its binding to the paz regulatory region was assessed by EMSA. The as-purified ActR caused a shift in the electrophoretic mobility of the paz fragment, demonstrating that paz is a member of the ActR regulon (Fig. 1). A mutated paz promoter with a change of G3→T3, G14→ T14, and C13→ T13 in the predicted ActR binding site did not show any change in electrophoretic mobility in the presence of ActR, indicating that this segment of DNA is required for ActR binding (data not shown).
DISCUSSION
nirK expression in A. tumefaciens depends on at least three transcriptional activators, NnrR, FnrN, and ActR. As O2 concentrations decrease, FnrN will activate nnrR expression and phosphorylated ActR levels will increase due to changes in the redox status of the electron transport chain (8). If nitrate or nitrite is present, this will lead to the low-level production of NO activating NnrR, and it, together with phosphorylated ActR, will increase the transcription of nirK. NnrR will also activate nor expression, preventing NO accumulation. nor expression is not directly dependent on ActR. The hierarchical organization and control exerted by these various regulators ensure that A. tumefaciens will continue aerobic respiration, its preferred mode of respiration, until O2 levels can no longer support growth. This level is likely to be very low given the presence of high-affinity oxidases (30). However, once aerobic respiration is no longer efficient, the cells can rapidly activate nirK and nor if nitrogen oxides are present in the environment, facilitating a switch to anaerobic respiration.
Loss of any one of these regulatory proteins essentially eliminates nirK expression and Nir activity (Tables 2 and 3). Since removal of the NnrR binding site prevents nirK transcription, the requirement for NnrR is easily understood (3). However, the observation that nirK expression and Nir activity is no longer enhanced by low O2 levels and nitrate in an actR mutant is seemingly inconsistent with the observation that a nirK fusion with its ActR binding site removed by either truncation or site-directed mutagenesis retains significant expression and is nitrate inducible (Fig. 3 and 4). The expression pattern observed in the ActR mutant suggests that both direct and indirect effects are equally important in determining the level of nirK transcription in this strain. Since nirK expression is dependent on Nir activity, it is not unexpected that there would be indirect regulatory effects caused by the loss of a global regulator (3, 36). Any change that impacts Nir activity, such as the loss of an electron donor, will consequently impact nirK expression. The principal electron donors to Nir in alphaproteobacteria are small copper proteins such as azurin or pseudoazurin and c-type cytochromes (42). In denitrifiers such as Paracoccus denitrificans, cytochrome c and azurin supply electrons to Nir but are functionally redundant since the loss of both is required for a measurable decrease in Nir activity (25). Nir activity of a strain of C58 lacking pseudoazurin dropped to about half that of wild type, demonstrating that other respiratory proteins cannot fully compensate for the loss of Paz (Table 3). Since paz expression requires ActR and FnrN, the absence of these regulatory proteins will indirectly lead to a decrease in Nir activity due to the absence of a critical component in the denitrification electron transport chain. This change in electron supply limits Nir turnover, so the loss of ActR has a larger impact on nirK expression than does the loss of the ActR binding site in nirK. There may be additional components of the respiratory chain required for optimal Nir activity that, like paz, are poorly expressed in the ActR and FnrN mutants, which helps to depress nirK expression.
The binding site of ActR homologs is frequently adjacent to or overlapped with other transcriptional factors, thereby helping or inhibiting binding of the other transcriptional factor (5, 6, 33). The ActR binding site in nirK is 19 bp from the predicted NnrR binding site. The ActR binding site in paz is only 2 bp from the putative FnrN binding site. Truncation into the ActR site of nirK decreased its expression (Fig. 4), suggesting that ActR binding is critical for efficient binding of NnrR. Unexpectedly, however, truncations into the region between the ActR and NnrR binding sites restored nirK expression to levels that were higher than the level of the full-length fragment. This result demonstrates that NnrR binding to the nirK promoter can occur independently of ActR if the ActR site is absent (Fig. 4). The restoration of nirK expression upon loss of the ActR binding site could suggest that, instead of recruiting NnrR, binding of phosphorylated ActR to the full-length promoter might change the structure of DNA, facilitating NnrR binding, or it could interfere with the binding of a repressor. Since the EMSAs demonstrate that binding of ActR is not dependent on phosphorylation, as seen in ActR orthologs (10), phosphorylation must be critical for postbinding functions allowing nirK expression. Restricting nirK expression until it is activated by ActR could be useful in preventing nirK expression under undesirable conditions, such as when O2 is present at concentrations high enough to react with NO and generate even more toxic nitrogen oxides (27).
Expression of a copper-type nitrite reductase has also been studied in R. sphaeroides 2.4.3 (18, 36). While many of the details of nirK regulation, including the involvement of ActRS and NnrR orthologs, are similar in R. sphaeroides and A. tumefaciens, there are some noteworthy differences. In particular, while FnrN and its homologs are required for nnrR expression in A. tumefaciens, expression of nnrR does not increase under conditions of low O2 in R. sphaeroides (Table 2) (36). The only known regulator of nnrR in R. sphaeroides is NnrR which negatively autoregulates its own expression. The reason for this difference may reflect the fact that R. sphaeroides has an alternative mode of anoxic growth, photosynthesis. Denitrification in this bacterium may be important as a way of disposing of excess reducing equivalents and not as important for supporting growth under anoxic conditions (21). Disposal of excess reducing equivalents may not be important in A. tumefaciens, so increases in NnrR levels whenever oxygen is limited may be used to optimize the cells' response to the presence of nitrogen oxides. Bradyrhizobium japonicum also has been shown to use the transcriptional regulators FixLJ-FixK to up-regulate nnrR expression when O2 is low (22). This bacterium is also nonphotosynthetic, suggesting that the regulatory pattern seen in R. sphaeroides may not occur in nonphotosynthetic denitrifiers.
One other noteworthy difference between A. tumefaciens and R. sphaeroides 2.4.3 is that inactivation of the cbb3 oxidase drastically reduced nirK and nor expression in the latter while in the former it had no effect (Table 1). It has been suggested that the loss of cbb3 oxidase in R. sphaeroides may cause a PrrBA-dependent repression of nirK expression (18). The data reported here do not support the hypothesis that nirK is repressed by the Prr/Act regulators although more work is necessary to understand how the loss of cbb3 affects nirK expression in 2.4.3.
One of the features common to the regulation of nirK and nor expression in R. sphaeroides and A. tumefaciens is that both utilize ActRS orthologs. ActRS and their orthologs almost always activate gene expression in conjunction with a second transcriptional regulator (6, 7, 33). nirK, coregulated by ActR and NnrR, will be up-regulated only when O2 is low and NO is present. Genes, such as paz, that are regulated by ActR and FnrN will be expressed when O2 is low (Table 4). This arrangement ensures that when conditions are favorable for nirK expression, paz will also be expressed. paz is likely not in the NnrR regulon because it is part of the microxic respiratory pathway. It is interesting that nnrR is not in the ActR regulon. Since its activity requires Nir activity and low O2, this may obviate any requirement for an additional layer of regulation. This regulatory arrangement also allows nor to be expressed without ActRS involvement, indicating that nirK and nor expression could potentially be decoupled. This form of decoupling would lead to nor but not nirK expression, which could be useful if cells were exposed to an exogenous source of NO.
Previous studies examining how the expression of the various nitrogen oxide reductases varies as a function of O2 concentration have typically observed that Nir and Nor require lower O2 concentrations for expression than nitrate and nitrous oxide reductase (4, 15, 31). This study has revealed some of the principal regulatory mechanisms that could give rise to this expression hierarchy. In particular, it seems likely that most proteobacterial denitrifiers that utilize a copper nitrite reductase will utilize ActRS orthologs to integrate denitrification into the general energy metabolism of the cell via this critical redox control system. It will be of interest to determine if denitrifiers that utilize the heme nitrite reductase or the NorR regulator also use ActRS orthologs.
Acknowledgments
We thank Clay Fuqua for generous gifts of strains, J. W. Lee for providing assistance with certain experiments, and Shiratsuchi Iichiro for reviewing the draft of the manuscript.
This work was supported by Department of Energy grant 95ER20206.
Footnotes
Published ahead of print on 2 November 2007.
REFERENCES
- 1.Aiyar, A., Y. Xiang, and J. Leis. 1996. Site-directed mutagenesis using overlap extension PCR. Methods Mol. Biol. 57177-191. [DOI] [PubMed] [Google Scholar]
- 2.Arai, H., T. Kodama, and Y. Igarashi. 1997. Cascade regulation of the two CRP/FNR-related transcriptional regulators (ANR and DNR) and the denitrification enzymes in Pseudomonas aeruginosa. Mol. Microbiol. 251141-1148. [DOI] [PubMed] [Google Scholar]
- 3.Baek, S.-H., and J. P. Shapleigh. 2005. Expression of nitrite and nitric oxide reductases in free-living and plant-associated Agrobacterium tumefaciens C58 cells. Appl. Environ. Microbiol. 714427-4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cuypers, H., J. Berghofer, and W. G. Zumft. 1995. Multiple nosZ promoters and anaerobic expression of nos genes necessary for Pseudomonas stutzeri nitrous oxide reductase and assembly of its copper centers. Biochim. Biophys. Acta 1264183-190. [DOI] [PubMed] [Google Scholar]
- 5.Du, S., T. H. Bird, and C. E. Bauer. 1998. DNA binding characteristics of RegA*. A constitutively active anaerobic activator of photosynthesis gene expression in Rhodobacter capsulatus. J. Biol. Chem. 27318509-18513. [DOI] [PubMed] [Google Scholar]
- 6.Dubbs, J. M., T. H. Bird, C. E. Bauer, and F. R. Tabita. 2000. Interaction of CbbR and RegA* transcription regulators with the Rhodobacter sphaeroides cbbI promoter-operator region. J. Biol. Chem. 27519224-19230. [DOI] [PubMed] [Google Scholar]
- 7.Dubbs, J. M., and F. R. Tabita. 2003. Interactions of the cbbII promoter-operator region with CbbR and RegA (PrrA) regulators indicate distinct mechanisms to control expression of the two cbb operons of Rhodobacter sphaeroides. J. Biol. Chem. 27816443-16450. [DOI] [PubMed] [Google Scholar]
- 8.Elsen, S., L. R. Swem, D. L. Swem, and C. E. Bauer. 2004. RegB/RegA, a highly conserved redox-responding global two-component regulatory system. Microbiol. Mol. Biol. Rev. 68263-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghiglione, J. F., F. Gourbiere, P. Potier, L. Philippot, and R. Lensi. 2000. Role of respiratory nitrate reductase in ability of Pseudomonas fluorescens YT101 to colonize the rhizosphere of maize. Appl. Environ. Microbiol. 664012-4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hemschemeier, S. K., U. Ebel, A. Jäger, A. Balzer, M. Kirndörfer, and G. Klug. 2000. In vivo and in vitro analysis of RegA response regulator mutants of Rhodobacter capsulatus. J. Mol. Microbiol. Biotechnol. 2291-300. [PubMed] [Google Scholar]
- 11.Karls, R. K., J. R. Wolf, and T. J. Donohue. 1999. Activation of the cycA P2 promoter for the Rhodobacter sphaeroides cytochrome c2 gene by the photosynthesis response regulator. Mol. Microbiol. 34822-835. [DOI] [PubMed] [Google Scholar]
- 12.Keen, N. T., S. Tamaki, D. Kobayashi, and D. Trollinger. 1988. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene 70191-197. [DOI] [PubMed] [Google Scholar]
- 13.Kim, Y. J., I. J. Ko, J. M. Lee, H. Y. Kang, Y. M. Kim, S. Kaplan, and J. I. Oh. 2007. Dominant role of the cbb3 oxidase in regulation of photosynthesis gene expression through the PrrBA system in Rhodobacter sphaeroides 2.4.1. J. Bacteriol. 1895617-5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kokotek, W., and W. Lotz. 1989. Construction of a lacZ-kanamycin-resistance cassette, useful for site-directed mutagenesis and as a promoter probe. Gene 84467-471. [DOI] [PubMed] [Google Scholar]
- 15.Korner, H., and W. G. Zumft. 1989. Expression of denitrification enzymes in response to the dissolved oxygen and respiratory substrate in continuous culture of Pseudomonas stutzeri. Appl. Environ. Microbiol. 551670-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kovach, M. E., P. H. Elzer, D. S. Hill, G. T. Robertson, M. A. Farris, R. M. Roop, I. I., and K. M. Peterson. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166175-176. [DOI] [PubMed] [Google Scholar]
- 17.Laguri, C., M. K. Phillips-Jones, and M. P. Williamson. 2003. Solution structure and DNA binding of the effector domain from the global regulator PrrA (RegA) from Rhodobacter sphaeroides: insights into DNA binding specificity. Nucleic Acids Res. 316778-6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laratta, W. P., P. S. Choi, I. E. Tosques, and J. P. Shapleigh. 2002. Involvement of the PrrB/PrrA two-component system in nitrite respiration in Rhodobacter sphaeroides 2.4.3: evidence for transcriptional regulation. J. Bacteriol. 1843521-3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu, M.-Y., M.-C. Liu, W. J. Payne, and J. Legall. 1986. Properties and electron transfer specificity of copper proteins from the denitrifier “Achromobacter cycloclastes”. J. Bacteriol. 166604-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mao, L., C. Mackenzie, J. H. Roh, J. M. Eraso, S. Kaplan, and H. Resat. 2005. Combining microarray and genomic data to predict DNA binding motifs. Microbiology 1513197-3213. [DOI] [PubMed] [Google Scholar]
- 21.McEwan, A. G., J. B. Jackson, and S. J. Ferguson. 1984. Rationalisation of properties of nitrate reductases in Rhodopseudomonas capsulata. Arch. Microbiol. 137344-349. [Google Scholar]
- 22.Mesa, S., E. J. Bedmar, A. Chanfon, H. Hennecke, and H. M. Fischer. 2003. Bradyrhizobium japonicum NnrR, a denitrification regulator, expands the FixLJ-FixK2 regulatory cascade. J. Bacteriol. 1853978-3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moir, J. W. B., and S. J. Ferguson. 1994. Properties of a Paracoccus denitrificans mutant deleted in cytochrome c550 indicate that a copper protein can substitute for this cytochrome in electron transport to nitrite, nitric oxide and nitrous oxide. Microbiology 140389-397. [Google Scholar]
- 24.Oh, J.-I., and S. Kaplan. 2000. Redox signaling: globalization of gene expression. EMBO J. 194237-4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pearson, I. V., M. D. Page, R. J. van Spanning, and S. J. Ferguson. 2003. A mutant of Paracoccus denitrificans with disrupted genes coding for cytochrome c550 and pseudoazurin establishes these two proteins as the in vivo electron donors to cytochrome cd1 nitrite reductase. J. Bacteriol. 1856308-6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Penfold, R. J., and J. M. Pemberton. 1992. An improved suicide vector for construction of chromosomal insertion mutations in bacteria. Gene 118145-146. [DOI] [PubMed] [Google Scholar]
- 27.Radi, R., G. Peluffo, M. N. Alvarez, M. Naviliat, and A. Cayota. 2001. Unraveling peroxynitrite formation in biological systems. Free Radic. Biol. Med. 30463-488. [DOI] [PubMed] [Google Scholar]
- 28.Ramey, B. E., A. G. Matthysse, and C. Fuqua. 2004. The FNR-type transcriptional regulator SinR controls maturation of Agrobacterium tumefaciens biofilms. Mol. Microbiol. 521495-1511. [DOI] [PubMed] [Google Scholar]
- 29.Ranson-Olson, B., D. F. Jones, T. J. Donohue, and J. H. Zeilstra-Ryalls. 2006. In vitro and in vivo analysis of the role of PrrA in Rhodobacter sphaeroides 2.4.1 hemA gene expression. J. Bacteriol. 1883208-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schluter, A., S. Ruberg, M. Kramer, S. Weidner, and U. B. Priefer. 1995. A homolog of the Rhizobium meliloti nitrogen fixation gene fixN is involved in the production of a microaerobically induced oxidase activity in the phytopathogenic bacterium Agrobacterium tumefaciens. Mol. Gen. Genet. 247206-215. [DOI] [PubMed] [Google Scholar]
- 31.Sears, H. J., G. Sawers, B. C. Berks, S. J. Ferguson, and D. J. Richardson. 2000. Control of periplasmic nitrate reductase gene expression (napEDABC) from Paracoccus pantotrophus in response to oxygen and carbon substrates. Microbiology 1462977-2985. [DOI] [PubMed] [Google Scholar]
- 32.Simon, R., U. Priefer, and A. Pühler. 1983. A broad host range mobilization system for in nivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Bio/Technology 1784-791. [Google Scholar]
- 33.Smart, J. L., J. W. Willett, and C. E. Bauer. 2004. Regulation of hem gene expression in Rhodobacter capsulatus by redox and photosystem regulators RegA, CrtJ, FnrL, and AerR. J. Mol. Biol. 3421171-1186. [DOI] [PubMed] [Google Scholar]
- 34.Swem, L. R., S. Elsen, T. H. Bird, D. L. Swem, H. G. Koch, H. Myllykallio, F. Daldal, and C. E. Bauer. 2001. The RegB/RegA two-component regulatory system controls synthesis of photosynthesis and respiratory electron transfer components in Rhodobacter capsulatus. J. Mol. Biol. 309121-138. [DOI] [PubMed] [Google Scholar]
- 35.Swem, L. R., X. Gong, C. A. Yu, and C. E. Bauer. 2006. Identification of a ubiquinone-binding site that affects autophosphorylation of the sensor kinase RegB. J. Biol. Chem. 2816768-6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tosques, I. E., A. V. Kwiatkowski, J. Shi, and J. P. Shapleigh. 1997. Characterization and regulation of the gene encoding nitrite reductase in Rhodobacter sphaeroides 2.4.3. J. Bacteriol. 1791090-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tosques, I. E., J. Shi, and J. P. Shapleigh. 1996. Cloning and characterization of nnrR, whose product is required for the expression of proteins involved in nitric oxide metabolism in Rhodobacter sphaeroides 2.4.3. J. Bacteriol. 1784958-4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Unden, G., S. Achebach, G. Holighaus, H. G. Tran, B. Wackwitz, and Y. Zeuner. 2002. Control of FNR function of Escherichia coli by O2 and reducing conditions. J. Mol. Microbiol. Biotechnol. 4263-268. [PubMed] [Google Scholar]
- 39.Van Veldhoven, P. P., and G. P. Mannaerts. 1987. Inorganic and organic phosphate measurements in the nanomolar range. Anal. Biochem. 16145-48. [DOI] [PubMed] [Google Scholar]
- 40.Vlahovicek, K., L. Kajan, and S. Pongor. 2003. DNA analysis servers: plot. it, bend.it, model.it and IS. Nucleic. Acids Res. 313686-3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wood, D. W., J. C. Setubal, R. Kaul, D. E. Monks, J. P. Kitajima, V. K. Okura, Y. Zhou, L. Chen, G. E. Wood, N. F. J. Almeida, L. Woo, Y. Chen, I. T. Paulsen, J. A. Eisen, P. D. Karp, D. S. Bovee, P. Chapman, J. Clendenning, G. Deatherage, W. Gillet, C. Grant, T. Kutyavin, R. Levy, M. J. Li, E. McClelland, A. Palmieri, C. Raymond, G. Rouse, C. Saenphimmachak, Z. Wu, P. Romero, D. Gordon, S. Zhang, H. Yoo, Y. Tao, P. Biddle, M. Jung, W. Krespan, M. Perry, B. Gordon-Kamm, L. Liao, S. Kim, C. Hendrick, Z. Y. Zhao, M. Dolan, F. Chumley, S. V. Tingey, J. F. Tomb, M. P. Gordon, M. V. Olson, and E. W. Nester. 2001. The genome of the natural genetic engineer Agrobacterium tumefaciens C58. Science 2942317-2323. [DOI] [PubMed] [Google Scholar]
- 42.Zumft, W. G. 1997. Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. Rev. 61533-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zumft, W. G. 2002. Nitric oxide signaling and NO dependent transcriptional control in bacterial denitrification by members of the FNR-CRP regulator family. J. Mol. Microbiol. Biotechnol. 4277-286. [PubMed] [Google Scholar]