Abstract
Metabolic flux analysis indicated that the heterofermentative Lactobacillus reuteri strain ATCC 55730 uses both the Embden-Meyerhof pathway (EMP) and phosphoketolase pathway (PKP) when glucose or sucrose is converted into the three-carbon intermediate stage of glycolysis. In all cases studied, the main flux is through the PKP, while the EMP is used as a shunt. In the exponential growth phase, 70%, 73%, and 84% of the flux goes through the PKP in cells metabolizing (i) glucose plus fructose, (ii) glucose alone, and (iii) sucrose alone, respectively. Analysis of the genome of L. reuteri ATCC 55730 confirmed the presence of the genes for both pathways. Further evidence for the simultaneous operation of two central carbon metabolic pathways was found through the detection of fructose-1,6-bisphosphate aldolase, phosphofructokinase, and phosphoglucoisomerase activities and the presence of phosphorylated EMP and PKP intermediates using in vitro 31P NMR. The maximum specific growth rate and biomass yield obtained on glucose were twice as low as on sucrose. This was the result of low ATP levels being present in glucose-metabolizing cells, although the ATP production flux was as high as in sucrose-metabolizing cells due to a twofold increase of enzyme activities in both glycolytic pathways. Growth performance on glucose could be improved by adding fructose as an external electron acceptor, suggesting that the observed behavior is due to a redox imbalance causing energy starvation.
Lactic acid bacteria (LAB) employ a few glycolytic pathways to funnel carbohydrates into the common three-carbon intermediate stage of glycolysis. In general, homofermentative LAB convert carbohydrates into lactate using the Embden-Meyerhof pathway (EMP), whereas heterofermentative LAB produce lactate, ethanol, and carbon dioxide using the phosphoketolase pathway (PKP) (8). The PKP is usually used by LAB to ferment pentoses (9), and it has a poor energy yield compared to that of the EMP (16). This disadvantage can be compensated for by the addition of external electron acceptors, which creates alternative pathways for NAD(P)H reoxidation and may stimulate growth (33). A part of the acetyl phosphate can then be converted into acetate instead of ethanol, thereby gaining one additional ATP, making the PKP as efficient as the EMP.
Lactobacillus reuteri ATCC 55730 has been described as a heterofermentative bacterium using the PKP (9) for converting glucose to lactate, ethanol, and CO2. Sucrose is converted into lactate, acetate, ethanol, CO2, and mannitol with this strain, whereby the latter is formed from the fructose half that functions solely as an electron acceptor (2).
Heterofermentative lactobacilli take up sugars by nonphosphorylating permease systems, but several possess the phosphotransferase system for the uptake of fructose, which is inevitably connected to a fully operating EMP instead of the PKP because of the requirement of the production of two phosphoenolpyruvates (PEP) per sugar unit (18, 25).
In the current study, we describe and show evidence for L. reuteri ATCC 55730's simultaneous use of the PKP and EMP under batch growth conditions. The use of two different glycolytic pathways has so far only been genetically indicated in other lactobacilli, including L. plantarum, under stress conditions (20), and L. salivarius (5), and has been proven to exist in a few other bacteria and archaea, such as Oenococcus oeni (23, 24), Thermotoga maritima, and Thermoproteus tenax (26). Except for O. oeni, which uses a mixture of the proper and an alternative PKP, all these organisms use the EMP as their dominant glycolytic pathway. Surprisingly, when L. reuteri ATCC 55730 was grown under normal conditions with sucrose as the carbon source, the main flux went through the PKP while the EMP was used as a mere shunt. Cells metabolizing glucose without fructose had higher activities of the enzymes of both pathways and displayed (i) a relatively low growth rate, (ii) low biomass yield, and (iii) low ATP levels. That these are resultants of a redox imbalance will be discussed in light of the fact that, in the presence of an external electron acceptor, these phenomena were absent.
MATERIALS AND METHODS
Culture conditions and analysis.
L. reuteri strain ATCC 55730, obtained from BioGaia AB, Stockholm, Sweden, was grown under anaerobic conditions on sucrose (0.15 mol liter−1), glucose (0.11 and 0.28 mol liter−1) and glucose plus fructose (0.14 mol liter−1 each) at 37°C and pH 5.5 using a semidefined medium (11) without lactose, but with 4 g liter−1 acetate as a growth factor (7). The pH was adjusted by automatic titration with 5 M NaOH, and the stirring speed was set to 100 rpm. Anaerobic conditions were created and maintained by the production of carbon dioxide in the culture. The levels of sucrose, glucose, and fermentation products were determined by high-pressure liquid chromatography. Lactate, acetate, and ethanol were separated on an Aminex HPX-87H column (Bio-Rad, Hercules, CA) (20). Sucrose, glucose, fructose, and mannitol were separated on an Aminex HPX-87C column (Bio-Rad, Hercules, CA) at 85°C with Milli-Q water as the mobile phase (19).
Preparation of cell extracts and NMR conditions.
Rapid filtration of bacterial media followed by immediate freezing in liquid nitrogen of the unwashed filters has been shown to result in accurate imaging of the levels of phosphorylated intracellular metabolites from perchloric acid-extracted LAB (29) and was used in this study. L. reuteri ATCC 55730 cultures were harvested by rapid filtration, perchloric acid extracts were made, and the final volumes of the extracts were brought up to 2.5 ml. 31P nuclear magnetic resonance (NMR) spectra were obtained at 25°C on a Bruker Avance 400 NMR spectrometer operating at the 31P frequency of 162 MHz using a 10-mm broadband probe. The experimental parameters employed were as follows: a 60-degree pulse length (6.6 μs), a sweep width of 12 kHz (16,000 complex data points), 30,000 scans, a 2.0-s recycle time with waltz16 proton decoupling applied in a bilevel power scheme to minimize the effects of heating. The data were zero filled to 32,000 and subjected to a 1.0- Hz line broadening prior to Fourier transformation. The assignment of the peaks was made by adding known standards to the extracts and by comparison with the results of previous studies of LAB (12, 29) and pentose-metabolizing yeast (14). The 31P spectra of the ATP region were normalized to reflect the differences in the dry weights of the samples, and, hence, the signal-to-noise levels vary between the spectra. Duplicate samples from different batch cultures were analyzed in this study, and the spectra showed the same results.
Enzyme assays.
Cell extracts were prepared from cultures harvested in the late exponential phase (t = 6 h), washed in 50 mM triethanolamine buffer (pH 7.0), disrupted by 0.1-mm silica beads in a bead beater (Mini-BeadBeater; Biospec Products, Inc., Bartlesville, OK), and freed from debris through centrifugation. All enzyme assays were evaluated spectrophotometrically by measuring the appearance of NADPH or NADH at 340 nm (ɛ = 6.22 mM−1 cm−1). The measurements of glucose-6-phosphate dehydrogenase (G6PDH), 6-phosphogluconate dehydrogenase (6PGDH), fructose-1,6-bisphosphate aldolase (ALD), phosphofructokinase (PFK), alcohol dehydrogenase (ADH), and lactate dehydrogenase activities were performed according to the methods of Bergmeyer (4). Triethanolamine buffer (50 mM, pH 7.0) with MgCl2 (10 mM) was used in all assays. The method used for the inhibition study of phosphoglucose isomerase (PGI) by 6-phosphogluconate was similar to the method of Richter et al. (22) except that the perchloric acid treatment step was done according to the method of Palmfeldt et al. (19).
Metabolic flux analysis.
The metabolic flux analysis for sucrose-grown cells consisted of eight different fluxes: four intracellular reactions and four extracellular fluxes, i.e., mannitol, lactate, acetate, and ethanol, were measured. Fructose is used solely as an electron acceptor and not as a carbon and energy source in strain ATCC 55730 (2). For glucose-grown cells, the metabolic flux analysis consisted of six fluxes: three intracellular reactions and three extracellular fluxes, i.e., lactate, acetate, and ethanol, were measured. The fluxes (QI [mol metabolite I·mol biomass−1·h−1], where Q is flux and I is metabolite), were calculated from the relationship between the maximum specific growth rate [μMAX (h−1)] and the specific production of metabolite I (YI) [mol I·mol biomass−1] according to the equation QI = μMAX·YI. The metabolic matrix was constructed based on the fundamental law of mass conservation. The annotated draft genome sequencing of strain ATCC 55730 (3) was used in the analysis for genes known to be involved in sucrose and glucose metabolism. The carbon balances for sucrose-, glucose-, and glucose-plus-fructose-grown cells, estimated during the exponential growth phase of the batch cultures, were closing on the C-mol basis at 99%, 103%, and 110%, respectively (Table 1). The ATP fluxes can be calculated from the fluxes to acetate and lactate, assuming that two and one ATPs are produced via lactate and acetate, respectively.
TABLE 1.
Carbon balances of L. reuteri ATCC 55730 cultures on glucose, sucrose, and glucose plus fructosea
| Carbon source | S | G | F | M | L | A | E | CO2 | % C balance (mean ± SD) |
|---|---|---|---|---|---|---|---|---|---|
| G | 8.6 | 7.7 | 0 | 2.1 | 2.1 | 103 ± 3 | |||
| S | 8.8 | 0.24 | 1.5 | 2.6 | 2.4 | 0.74 | 0.25 | 0.78 | 99 ± 1 |
| G and F | 5.9 | 11 | 11 | 5.3 | 3.0 | 0 | 2.2 | 110 ± 1 |
Cultures were grown on 50 g liter−1 glucose, 50 g liter−1 sucrose, and 25 g liter−1 each glucose plus fructose. S, consumed sucrose; G, produced/consumed glucose; F, produced/consumed fructose; M, mannitol; L, lactate; A, acetate; E, ethanol. All values except those for C balance are given in g liter−1 and are the means of the results of duplicate experiments.
Bioinformatic analyses.
To identify genes encoding enzymes involved in the glycolytic pathways, the predicted open reading frames in the draft genome of L. reuteri (3) were screened by BlastP (1) searches at a local server using sequences of enzymes from related bacteria. The identified proteins were analyzed at the National Centre for Biotechnology Information (NCBI) website (http://www.ncbi.nlm.nih.gov/BLAST/) to identify similarities with other proteins and with conserved domains from the Clusters of Orthologous Groups (COG) database (http://www.ncbi.nlm.nih.gov/COG/) (31). The organization of different genomes from LAB was analyzed at the website of the Joint Genome Institute (http://www.jgi.doe.gov/).
RESULTS
Growth behavior on glucose and sucrose.
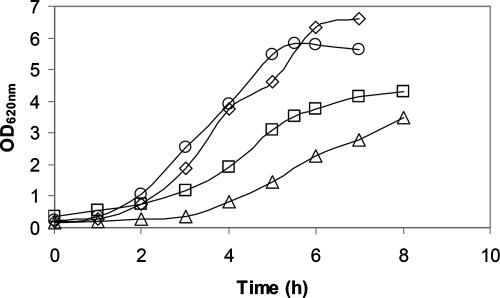
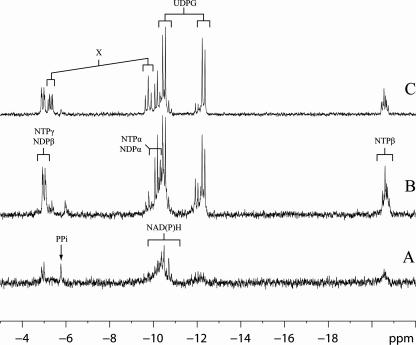
The choice of carbon source clearly affected the growth performance of L. reuteri ATCC 55730. Growth on sucrose resulted in a high growth rate and an appropriate biomass yield, whereas growth with glucose was characterized by a maximum specific growth rate and a YATP that were each about a factor of two lower (Table 2). Although growth on 0.11 and 0.28 M glucose was initially exponential, after 2 h and 4 h, respectively, it changed to linear growth (Fig. 1). Because of the longer exponential phase, we only considered the culture on 0.28 M glucose in the remaining study. Surprisingly, in comparison to the levels in sucrose-metabolizing cells, the glucose-grown cells showed low levels of nucleoside tri-/diphosphates, NAD(P)H, and UDP-glucose (UDPG) (Fig. 2A and B). The intracellular metabolite PEP, normally only observed when cells are starved, was evident here (data not shown) despite the fact that the cultures were harvested when high extracellular glucose levels still remained in the media. A duplicate sample from a different culture gave identical results. Growth could be greatly improved in the presence of fructose (Table 2), and the cells contained higher levels of ATP (Fig. 2C). L. reuteri ATCC 55730 is not able to use fructose as a carbon and energy source (2) but uses it as an external electron acceptor, indicating that the reduced growth on glucose alone is due to a redox imbalance. In addition, peaks of an unknown compound (Fig. 2C) with an as-yet-unknown function, which have also been observed previously in Streptococcus thermophilus (29), were found.
TABLE 2.
Maximum specific growth rate, molar growth yield (YX), and YATP of L. reuteri ATCC 55730 growing on glucose, sucrose, and glucose plus fructosea
| Substrate | μMAX (h−1) | YX (g biomass·mol substrate−1) | YATP (g biomass·mol ATP−1) |
|---|---|---|---|
| Glucose (0.28 M) | 0.45 ± 0.01 | 15.3 ± 2.3 | 5.2 ± 0.1 |
| Sucrose | 0.82 ± 0.16 | 22.0 ± 2.0 | 10.5 ± 1.6 |
| Glucose and fructose | 0.66 ± 0.01 | 33.2 ± 1.7 | 7.7 ± 0.2 |
Values are the means ± standard deviations of the results of duplicate experiments.
FIG. 1.
Growth of L. reuteri ATCC 55730 on 50 g·liter−1 glucose (□), 50 g·liter−1 sucrose (⋄), 25 g·liter−1 glucose plus 25 g·liter−1 fructose (○), and 20 g·liter−1 glucose (▵). OD620nm, optical density at 620 nm.
FIG. 2.
31P NMR spectra of L. reuteri ATCC 55730 metabolizing glucose (A), sucrose (B), and glucose plus fructose (C). Labels: PPi, pyrophosphate; NTP- or NDPα, -β, or -γ, phosphates of nucleoside tri- and diphosphates; NAD(P)H, NAD(phosphate) reduced and oxidized; X, unknown compound.
Two simultaneously operating glycolytic pathways.
With sucrose, part of the NAD(P)+ could be regenerated via the reduction of the fructose half to mannitol, allowing part of the acetyl phosphate flux to be directed to acetate formation (Table 1). With glucose, all redox equivalents could only be directed to lactate and ethanol production and, consequently, no acetate could be formed. When fructose was present, the redox balance could be maintained by the production of mannitol, which enabled the formation of acetate instead of ethanol.
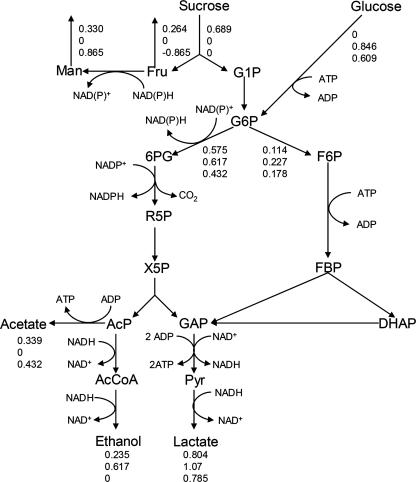
Heterofermentatives are often quoted to have a stoichiometric ratio of 1:1 between lactate and ethanol plus acetate (see, e.g., reference 8). However, on both sugars, L. reuteri ATCC 55730 clearly produced relatively more lactate. To elucidate the biased stoichiometry, a metabolic flux analysis was performed of the fluxes during the exponential growth phase, but no satisfactory solution could be obtained with various modifications of the PKP unless the upper part of the EMP was introduced in the flux model. The metabolic flux analysis revealed that, with sucrose, 16% of the carbon flux went via the EMP and 84% through the PKP (Fig. 3). With glucose (0.28 M)- and glucose-plus-fructose-grown cells, the fluxes through the EMP were raised to 27% and 30%, respectively. Several approaches were undertaken to find evidence for the simultaneous operation of the two pathways.
FIG. 3.
Pathways of sucrose and glucose metabolism in L. reuteri ATCC 55730 as proposed by the metabolic flux analysis, annotated genome sequence, and in vitro enzyme assays. The fluxes (mol·mol biomass−1·h−1) represent those of cultures on 50 g·liter−1 sucrose, 50 g·liter−1 glucose, and 25 g·liter−1 glucose plus 25 g·liter−1 fructose. Fru, fructose; Man, mannitol; G1P, glucose-1-phosphate; G6P, glucose-6-phosphate; 6PG, 6-phosphogluconate; R5P, ribulose-5-phosphate; X5P, xylulose-5-phosphate; AcP, acetyl phosphate; AcCoA, acetyl coenzyme A; F6P, fructose-6-phosphate; FBP, fructose-1,6-bisphosphate; DHAP, dihydroxyacetone phosphate; GAP, glyceraldehyde-3-phosphate; Pyr, pyruvate.
Bioinformatics and enzyme activities.
Genome annotation of L. reuteri ATCC 55730 confirmed the presence of the genes encoding the following enzymes of the upper EMP: phosphoglucose isomerase (lr0459; GenBank accession no. DQ466584), fructose bisphosphate aldolase (lr1682; GenBank accession no. DQ470843), and triose-phosphate isomerase (TPI) (lr1683; GenBank accession no. DQ857798), but not PFK (COG0205; EC 2.7.1.11). By searching in GenBank, the latter gene could not be identified in any of the obligate heterofermentative Lactobacillales. In comparing the genome organization of 13 sequenced Lactobacillales, L. reuteri (ATCC 55730 and DSM 20016) is one of the four exceptions that do not have the PFK gene (pfkA) between the genes encoding the DNA polymerase III β subunit and pyruvate kinase. This indicates that L. reuteri does not have the usual PFK gene, but PFK activity is detected in ATCC 55730 (Table 3). Interestingly, strain ATCC 55730 has two genes (lr0160 and lr0378, with GenBank accession numbers EF547651 and EF547653, respectively) belonging to the same cluster of orthologous groups as pfkB (i.e., COG0524), which encodes a minor PFK in Escherichia coli (6). Thus, there is a possibility that one of these genes is an ortholog to pfkB. The L. reuteri genes involved in the EMP are only partly organized together. The genes encoding ALD and TPI are located in the same operon (together with three genes involved in metabolic processes), but the PGI gene and the putative PFK gene are encoded by monocistronic genes not located close to each other.
TABLE 3.
Specific activities of several enzymes in glucose- and sucrose-grown cultures of L. reuteri ATCC 55730a
| Enzyme | Activity (μmol·min−1 mg protein−1) on:
|
|
|---|---|---|
| Glucose | Sucrose | |
| ALD | 0.21 ± 0.01 | 0.11 ± 0.004 |
| PFK | 0.27 ± 0.16 | 0.18 ± 0.16 |
| PGI | 0.59 ± 0.19 | 0.23 ± 0.07 |
Values are the means ± standard deviations of the results of triplicate experiments.
Several of these enzymes were proven to be active in cultures growing on sucrose or glucose (Table 3). Interestingly, all enzymes tested showed higher activities in glucose-grown cells than in sucrose-grown cells (Table 3).
The PKP enzymes G6PDH and 6PGDH are both NADPH dependent in sucrose-grown cells, but in glucose-grown cells, G6PDH can use both NADPH and NADH, albeit with a lower activity with the latter. ADH uses both NADH and NADPH and thus can be used to regenerate NADP+ if needed. Previous work has shown that mannitol dehydrogenase prefers NADPH over NADH in this strain (2).
An interesting question is whether the flux through the EMP is controlled by the PKP. In O. oeni, 6-phosphogluconate is responsible for controlling the channeling of fructose into PKP by the inhibition of PGI (22). However, no notable inhibition of PGI by 6-phosphogluconate in the tested range of 0 to 500 μM could be observed in both sucrose- and glucose-grown cells (data not shown). In addition, the internal concentration of 6-phosphogluconate found in strain ATCC 55730, 59 ± 29 (mean ± standard deviation) μM, is much lower than that found in O. oeni and it might, therefore, not be a good allosteric regulator.
In vitro 31P NMR analysis of glycolytic metabolites.
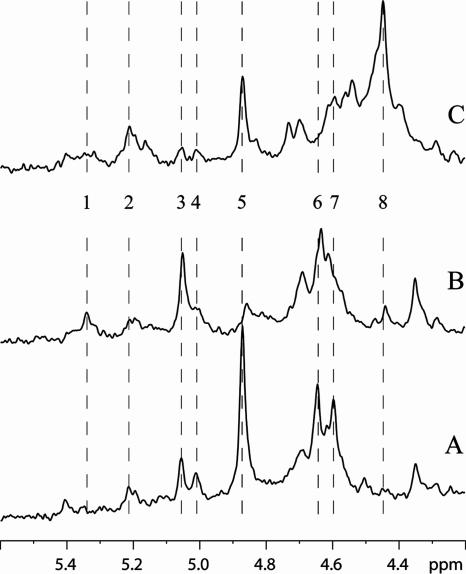
The presence of intermediates of the PKP and upper and lower EMPs in exponentially growing cells was checked with 31P NMR. The expanded phosphomonoester region showed the simultaneous presence of fructose-6-P with many of the PKP metabolites, such as xylose-5-P, ribulose-5-P, and 6-phosphogluconate (Fig. 4), except in cells grown on sucrose. Surprisingly, fructose-1,6 bisphosphate, normally the principle component of LAB utilizing the glycolytic pathway, was not detectible in these extracts. Notably, the level of 3-phosphoglycerate, a starvation intermediate in LAB (12), is relatively high in glucose-metabolizing cells but is also visible in the other fermentations.
FIG. 4.
31P NMR spectra of the expanded phosphomonoester region of L. reuteri ATCC 55730 metabolizing glucose (A), sucrose (B), and glucose plus fructose (C). Peaks: 1, 6-phosphogluconate; 2, glucose-6-P; 3, xylulose-5-P; 4, ribulose-5-P; 5, 3-phosphoglycerate; 6, ribose-5-P; 7, fructose-6-P; 8, unidentified.
DISCUSSION
This study presents evidence for the operation of two glycolytic pathways in L. reuteri ATCC 55730. (i) Metabolic flux analysis exposed the flux distribution, (ii) bioinformatics provided information about the presence of the genes encoding both pathways on the genome, (iii) enzyme assays proved the activities of the key enzymes, and (iv) 31P NMR showed the simultaneous presence of metabolites of both pathways. So far, the operation of two glycolytic pathways has been described in L. plantarum and in L. salivarius, but only from a genetic level (2, 20).
Metabolic flux analysis revealed that the flux through the PKP is superior to that through the EMP in L. reuteri ATCC 55730, and hence, it utilizes the latter as a mere shunt. To our knowledge, this is the first time this change in the order of glycolytic pathways has been observed. Pieterse et al. (20) observed that the genes encoding the PKP were upregulated, thereby creating a bypass in the homofermentative L. plantarum, in response to lactic acid stress. This observed hierarchy is more obvious here, since the EMP is considered to be Nature's optimized design for maximizing the flux to ATP production, whereas the PKP gains poor energetic yields (16). The latter would suggest that the presence of an active EMP in L. reuteri ATCC 55730, even if it services only a small flux, should reflect a gain in ATP production. Indeed, estimations from the metabolic flux analysis (i.e., the difference between the observed results and hypothetical results in fermentations if the whole sugar flux is through the PKP) indicate that cells on glucose and on sucrose will acquire maximally about 28% and 7%, respectively, extra ATP via the EMP. In the latter case, the extra ATP gained in the production of acetate might compensate for the lower flux through the EMP, because with fructose present, the PKP becomes as energetically efficient as the EMP. The culture on glucose with fructose is estimated to gain maximally 42% extra ATP from the EMP shunt.
In the presence of nonlimiting concentrations of glucose, the growth of L. reuteri ATCC 55730 was severely restricted, as was concluded from the relatively low growth rate and low biomass yield (Table 2). The UDPG and ATP levels in L. reuteri on glucose were also very low (Fig. 2A), and enhanced concentrations of 3-phosphoglycerate (Fig. 4A) and PEP (data not shown) were found, indicating that the cells were energy limited. Slow growth accompanied by low levels of ATP and UDPG has also been described for immobilized Candida tropicalis on xylose (13). The ATP production fluxes in cells on sucrose and glucose were estimated to be 1.94 and 2.14 mol ATP·mol biomass−1·h−1, respectively. Thus, the ATP production flux in cells growing on glucose was quite similar to that of cells growing on sucrose. Therefore, the ATP limitation seen on glucose must have been caused by an increased consumption flux (including, most importantly, the ATP investment in phosphorylation of glucose of 0.73 mol ATP·mol biomass−1·h−1) which was about five times higher than for sucrose. Although both glycolytic pathways had higher fluxes on glucose, especially the one through the EMP, which was doubled (Fig. 3), glucose was not able to supply the required ATP demand. These higher fluxes are partly due to higher activities of the enzymes of the PKP and EMP (Tables 3 and 4).
TABLE 4.
Cofactor preferences of different dehydrogenases in PKP and in final-product formation of glucose- or sucrose- grown L. reuteri ATCC 55730a
| Enzyme | Activity (μmol·min−1 mg protein−1) on:
|
|||
|---|---|---|---|---|
| Glucose
|
Sucrose
|
|||
| NADPH | NADH | NADPH | NADH | |
| G6PDH | 2.3 ± 0.09 | 0.73 ± 0.04 | 0.87 ± 0.02 | 0 |
| 6PGDH | 0.64 ± 0.0 | 0 | 0.33 ± 0.01 | 0 |
| LDH | 0 | 16 ± 0.3 | 0 | 9.3 ± 0.2 |
| ADH | 2.4 ± 0.1 | 11 ± 2 | 1.0 ± 0.1 | 3.9 ± 0.6 |
Specific activities of G6PDH, 6PGDH, LDH, and alcohol dehydrogenase (ADH). Values are the means ± standard deviations of the results of triplicate experiments.
This growth limitation on glucose could be alleviated by the presence of the electron acceptor fructose (Table 2), indicating that the limitation was imposed by a redox imbalance. A similar phenomenon has been described so far only for the anaerobic growth of the Saccharomyces cerevisiae strain C1 on xylose (27). The mutant strain C1 had evolved as a subpopulation in a culture that was under carbon-limited selective pressure for anaerobic growth on xylose. The authors concluded that in strain C1, the ATP production flux had increased above the ATP maintenance requirements, the flux allowing growth at a very low rate (27). Apparently, redox imbalance imposes higher ATP demands, at least in L. reuteri ATCC 55730, probably due to stress mechanisms, and thus merits further investigation.
In addition to L. reuteri ATCC 55730, genome analyses of other lactobacilli, including the homofermentatives L. plantarum (10) and L. salivarius (5), have revealed the presence of the genes for both EMP and PKP. This strongly indicates that the possession of both pathways might be widespread in this genus. It can be expected that, in the classic homofermentatives, the EMP is dominant, whereas in (facultative) heterofermentatives, it is the PKP. The presence of two central carbon pathways provides these organisms with greater metabolic flexibility. However, the question remains why L. reuteri ATCC 55730 while growing in an optimal medium prefers the PKP over the EMP when all the enzymes of both pathways are active. The lack of optimal organization of the genes involved in the EMP might be a clue to the answer. The performance of this strain indicates that it is adapted to an environment in which (external) electron acceptors are as abundantly present as carbon and energy sources. Such a situation guarantees the production of two ATPs per sugar unit via the PKP, with acetate instead of ethanol production, and might therefore exclude the superior presence of the EMP in this organism. Similar conclusions have been drawn for other lactobacilli and for O. oeni (15, 21, 23, 28), and the subject has recently been reviewed (33). L. reuteri strains can use other organic compounds as electron acceptors, including malate, fumarate, and glycerol (28, 30). Hence, one might wonder whether this adaptation of L. reuteri extends to the gastrointestinal tract of humans and animals, where this organism has frequently been isolated. Indeed, although many of the LAB in feces are described as obligate homofermentatives, it has been reported that a majority of them are of the obligate or facultative heterofermentative type (see, e.g., reference 17). The milieu of the gastrointestinal tract might provide electron acceptors, including potentially toxic compounds, such as butadiene, glyoxal, and Strecker aldehydes, which are products of protein degradation (32), which might be reduced by heterofermentatives into harmless products. This would mean that the physiologic behavior of L. reuteri ATCC 55730 described here could be an auxiliary requisite to its suggested probiotic properties. This hypothesis merits further pursuit through dedicated in-depth studies.
Acknowledgments
This study was supported by VINNOVA, the Swedish Agency for Innovation Systems, and the Nordic Innovation Centre.
The NMR samples were run at the Bio-NMR center of the University of Calgary, and Deane McIntyre is thanked especially for his assistance in normalizing spectra to dry weight of cells.
Footnotes
Published ahead of print on 26 October 2007.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215403-410. [DOI] [PubMed] [Google Scholar]
- 2.Årsköld, E., M. Svensson, H. Grage, S. Roos, P. Rådström, and E. W. J. van Niel. 2007. Environmental influences on exopolysaccharide formation in Lactobacillus reuteri ATCC 55730. Int. J. Food Microbiol. 116159-167. [DOI] [PubMed] [Google Scholar]
- 3.Båth, K., S. Roos, T. Wall, and H. Jonsson. 2005. The cell surface of Lactobacillus reuteri ATCC 55730 highlighted by identification of 126 extracellular proteins from the genome sequence. FEMS Microbiol. Lett. 25375-82. [DOI] [PubMed] [Google Scholar]
- 4.Bergmeyer, H. U. 1974. Methods of enzymatic analysis, 2nd ed. Academic Press, Inc., New York, NY.
- 5.Claesson, M. J., Y. Li, S. Leahy, C. Canchaya, J. P. van Pijkeren, A. M. Cerdeño-Tárraga, J. Parkhill, S. Flynn, G. C. O'Sullivan, J. K. Collins, D. Higgins, F. Shanahan, G. F. Fitzgerald, D. van Sinderen, and P. W. O'Toole. 2006. Multireplicon genome architecture of Lactobacillus salivarius. Proc. Natl. Acad. Sci. USA 1036718-6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daldal, F., and D. G. Fraenkel. 1981. Tn10 insertions in the pfkB region of Escherichia coli. J. Bacteriol. 147935-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Man, J. C., M. Rogosa, and M. E. Sharpe. 1960. A medium for the cultivation of lactobacilli. J. Appl. Bacteriol. 23130-135. [Google Scholar]
- 8.DeMoss, R. D., R. C. Bard, and I. C. Gunsalus. 1951. The mechanism of the heterolactic fermentation: a new route of ethanol formation. J. Bacteriol. 62499-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kandler, O. 1983. Carbohydrate metabolism in lactic acid bacteria. Antonie van Leeuwenhoek 49209-224. [DOI] [PubMed] [Google Scholar]
- 10.Kleerebezem, M., J. Boekhorst, R. van Kranenburg, D. Molenaar, O. P. Kuipers, R. Leer, R. Tarchini, S. A. Peters, H. M. Sandbrink, M. W. Fiers, W. Stiekema, R. M. Lankhorst, P. A. Bron, S. M. Hoffer, M. N. Groot, R. Kerkhoven, M. de Vries, B. Ursing, W. M. de Vos, and R. J. Siezen. 2003. Complete genome sequence of Lactobacillus plantarum WCFS1. Proc. Natl. Acad. Sci. USA 1001990-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levander, F., M. Svensson, and P. Rådström. 2001. Small-scale analysis of exopolysaccharides from Streptococcus thermophilus grown in a semi-defined medium. BMC Microbiol. 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lohmeier-Vogel, E. M., B. Hahn-Hägerdal, and H. J. Vogel. 1986. Phosphorus-31 NMR studies of maltose and glucose metabolism in Strepotococcus lactis. Appl. Microbiol. Biotechnol. 2543-51. [Google Scholar]
- 13.Lohmeier-Vogel, E. M., B. Hahn-Hägerdal, and H. J. Vogel. 1995. Phosphorus-31 and carbon-13 nuclear magnetic resonance study of glucose and xylose metabolism in agarose-immobilized Candida tropicalis. Appl. Environ. Microbiol. 611420-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lohmeier-Vogel, E. M., K. Skoog, H. Vogel, and B. Hahn-Hägerdal. 1989. 31P nuclear magnetic resonance study of the effect of azide on xylose fermentation by Candida tropicalis. Appl. Environ. Microbiol. 551974-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maicas, S., S. Ferrer, and I. Pardo. 2002. NAD(P)H regeneration is the key for heterolactic fermentation of hexoses in Oenococcus oeni. Microbiology 148325-332. [DOI] [PubMed] [Google Scholar]
- 16.Meléndez-Hevia, E., T. G. Waddell, R. Heinrich, and F. Montero. 1997. Theoretical approaches to the evolutionary optimization of glycolysis, chemical analysis. Eur. J. Biochem. 244527-543. [DOI] [PubMed] [Google Scholar]
- 17.Mikelsaar, M., H. Annuk, J. Schepetova, R. Mändar, E. Sepp, and B. Björksten. 2002. Intestinal lactobacilli of Estonian and Swedish children. Microbiol. Ecol. Health Dis. 1475-80. [Google Scholar]
- 18.Nagasaki, H., K. Ito, S. Matsuzaki, and S. Tanaka. 1992. Existence of phosphoenolpyruvate:carbohydrate phosphotransferase systems in Lactobacillus fermentum, an obligate heterofermenter. Microbiol. Immunol. 36533-538. [DOI] [PubMed] [Google Scholar]
- 19.Palmfeldt, J., M. Paese, B. Hahn-Hägerdal, and E. W. J. van Niel. 2004. The pool of ADP and ATP regulates anaerobic product formation in resting cells of Lactococcus lactis. Appl. Environ. Microbiol. 705477-5484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pieterse, B., R. J. Leer, F. H. J. Schuren, and M. J. van der Werf. 2005. Unravelling the multiple effects of lactic acid stress on Lactobacillus plantarum by transcription profiling. Microbiology 1513881-3894. [DOI] [PubMed] [Google Scholar]
- 21.Ragout, A., F. Siñeriz, H. Diekmann, and G. F. de Valdez. 1996. Shift in the fermentation balance of Lactobacillus reuteri in the presence of glycerol. Biotechnol. Lett. 181105-1108. [Google Scholar]
- 22.Richter, H., A. A. de Graaf, I. Hamann, and G. Unden. 2003. Significance of phosphoglucose isomerase for the shift between heterolactic and mannitol fermentation of fructose by Oenococcus oeni. Arch. Microbiol. 180465-470. [DOI] [PubMed] [Google Scholar]
- 23.Richter, H., I. Hamann, and G. Unden. 2003. Use of the mannitol pathway in fructose fermentation of Oenococcus oeni due to limiting redox regeneration capacity of the ethanol pathway. Arch. Microbiol. 179227-233. [DOI] [PubMed] [Google Scholar]
- 24.Richter, H., D. Vlad, and G. Unden. 2001. Significance of pantothenate for glucose fermentation by Oenococcus oeni and for suppression of the erythritol and acetate production. Arch. Microbiol. 17526-31. [DOI] [PubMed] [Google Scholar]
- 25.Saier, M. H., Jr., J.-J. Ye, S. Klinke, and E. Nino. 1996. Identification of an anaerobically induced phosphoenolpyruvate-dependent fructose-specific phosphotransferase system and evidence for the Embden-Meyerhof glycolytic pathway in the heterofermentative bacterium Lactobacillus brevis. J. Bacteriol. 178314-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Selig, M., K. B. Xavier, H. Santos, and P. Schönheit. 1997. Comparative analysis of Embden-Meyerhof and Entner-Doudoroff glycolytic pathways in hyperthermophilic archaea and the bacterium Thermotoga. Arch. Microbiol. 167217-232. [DOI] [PubMed] [Google Scholar]
- 27.Sonderegger, M., M. Jeppsson, B. Hahn-Hägerdal, and U. Sauer. 2004. Molecular basis for anaerobic growth of Saccharomyces cerevisiae on xylose, investigated by global gene expression and metabolic flux analysis. Appl. Environ. Microbiol. 702307-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stolz, P., R. F. Vogel, and W. P. Hammes. 1995. Utilization of electron acceptors by lactobacilli isolated from sourdough. Z. Lebensm. Unters. Forsch. 201402-410. [Google Scholar]
- 29.Svensson, M., E. Lohmeier-Vogel, E. Waak, U. Svensson, and P. Rådström. 2007. Altered nucleotide sugar metabolism in Streptococcus thermophilus interferes with nitrogen metabolism. Int. J. Food Microbiol. 113195-200. [DOI] [PubMed] [Google Scholar]
- 30.Talarico, T. L., I. A. Casas, T. C. Chung, and W. J. Dobrogosz. 1988. Production and isolation of reuterin, a growth inhibitor produced by Lactobacillus reuteri. Antimicrob. Agents Chemother. 321854-1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tatusov, R. L., N. D. Fedorova, J. D. Jackson, et al. 2003. The COG database: an updated version includes eukaryotes. BMC Bioinform. 441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tuohy, K. M., D. J. S. Hinton, S. J. Davies, M. J. C. Crabbe, G. R. Gibson, and J. M. Ames. 2006. Metabolism of Maillard reaction products by the human gut microbiota—implications for health. Mol. Nutr. Food Res. 50847-857. [DOI] [PubMed] [Google Scholar]
- 33.Zaunmüller, T., M. Eichert, H. Richter, and G. Unden. 2006. Variations in the energy metabolism of biotechnologically relevant heterofermentative lactic acid bacteria during growth on sugars and organic acids. Appl. Microbiol. Biotechnol. 72421-429. [DOI] [PubMed] [Google Scholar]