Abstract
Saframycin A (SFM-A), produced by Streptomyces lavendulae NRRL 11002, belongs to the tetrahydroisoquinoline family of antibiotics, and its core is structurally similar to the core of ecteinascidin 743, which is a highly potent antitumor drug isolated from a marine tunicate. In this study, the biosynthetic gene cluster for SFM-A was cloned and localized to a 62-kb contiguous DNA region. Sequence analysis revealed 30 genes that constitute the SFM-A gene cluster, encoding an unusual nonribosomal peptide synthetase (NRPS) system and tailoring enzymes and regulatory and resistance proteins. The results of substrate prediction and in vitro characterization of the adenylation specificities of this NRPS system support the hypothesis that the last module acts in an iterative manner to form a tetrapeptidyl intermediate and that the colinearity rule does not apply. Although this mechanism is different from those proposed for the SFM-A analogs SFM-Mx1 and safracin B (SAC-B), based on the high similarity of these systems, it is likely they share a common mechanism of biosynthesis as we describe here. Construction of the biosynthetic pathway of SFM-Y3, an aminated SFM-A, was achieved in the SAC-B producer (Pseudomonas fluorescens). These findings not only shed new insight on tetrahydroisoquinoline biosynthesis but also demonstrate the feasibility of engineering microorganisms to generate structurally more complex and biologically more active analogs by combinatorial biosynthesis.
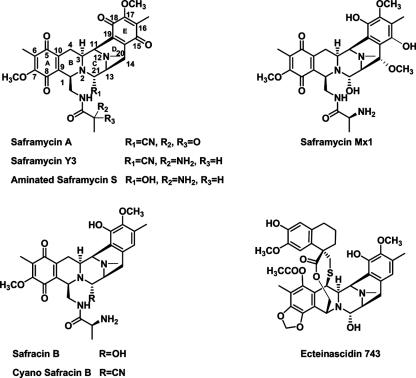
Saframycins, belonging to the tetrahydroisoquinoline family of antibiotics, are a group of microbial natural products isolated from Streptomyces lavendulae strain 314 (deposited in NRRL with the accession number 11002) (40). Saframycin A (SFM-A), which has antiproliferative activity against a variety of tumor cell lines at low doses, is one of the most potent members of this class of compounds (3, 29). As shown in Fig. 1, SFM-A contains a characteristic bisquinone core with a α-amino nitrile moiety (at the C-21 position), and the departure of the nitrile moiety from C-21 in the presence of reduced cofactors allows the formation of an electrophilic iminium ion that alkylates the guanine residues of double-stranded DNA (17, 18, 23, 34, 35). Although it has frequently been speculated that covalent modification of DNA is essential for the antitumor activity of SFM-A, recently the identification of glyceraldehyde-3-phosphate dehydrogenase (a putative key transcriptional coactivator necessary for entry into the S phase of cell proliferation) as a protein target of SFM-DNA adducts suggested that the action of SFM-A involves a protein-drug-DNA interaction; thus, a distinct pathway may be involved for SFM-A antiproliferative activity (31, 48).
FIG. 1.
Structures of saframycins, safracins, and ecteinascidin 743.
Ecteinascidin 743 (ET743) (Fig. 1), isolated from marine invertebrates, has a core that is structurally similar to that of SFM-A and is currently in phase II/III clinical trails as an anticancer drug (36, 38, 43). The antiproliferative activity of ET743 is higher than those of many clinically used drugs, such as taxol, by 1 to 3 orders of magnitude. However, the natural scarcity (1 mg of ET743 per 1 kg of tunicate) and the structural complexity may ultimately limit the practical value of preparing the drug either by extraction from the natural source or by total synthesis. Recently, advances in biotechnology have provided a promising alternative to make complex natural products by genetic engineering of the biosynthetic pathways in microorganisms (5, 11, 13). Therefore, an alternative to economically producing ET743 or analogs is by reconstructing the biosynthetic pathway in a recombinant microorganism (8), and the success of this approach critically depends on characterization of the biosynthetic mechanism of ET743-like natural products. The structural similarities between ET743 and SFM-A suggest that they have a common biosynthetic strategy to form a similar intermediate, despite their difference in origin (in fact, tunicates often harbor many symbiotic bacteria that are assumed to be the “real” source of numerous biologically active compounds [12, 20]). Thus, SFM-A, a terrestrial microbial metabolite, serves as a model for this family of compounds to identify a biosynthetic paradigm, and the results of studies with SFM-A should provide a genetic and biochemical basis for rationally engineering these complex metabolites and serve as a starting point to access other tetrahydroisoquinoline natural products, such as ET743.
Previous feeding experiments using isotope-labeled substrates showed that the backbone of SFM-A is derived from one alanine (Ala), one glycine (Gly), and two tyrosine (Tyr) residues, suggesting that it is of tetrapeptide origin (28), which is also probably shared among other structurally related analogs, such as saframycin Mx1 (SFM-Mx1) and safracin B (SAC-B) as shown in Fig. 1. The partial biosynthetic gene cluster of SFM-Mx1 (with a hydroquinone form of the E ring, a hydroxy group at the C-21 position, and a reserved α-amino group of Ala in comparison to SFM-A) and the entire biosynthetic gene cluster of SAC-B (one of the structurally simplest members in the SFM family) were cloned from Myxococcus xanthus in 1995 (32, 33) and Pseudomonas fluorescens in 2005 (46), respectively, indeed revealing a nonribosomal peptide synthetase (NRPS) system for the formation of an identical tetrapeptide intermediate. In both cases, sequential incorporation of Ala, Gly, and Tyr derivatives into the backbone was speculated to be catalyzed by NRPSs in a colinear way according to the substrate specificity of the NRPS modules. This biosynthetic logic was formulated using bioinformatics analysis, but to date, no biochemical studies on SFM-Mx1 and SAC-B have been reported.
We hypothesized that SFM-A is biosynthesized in a manner similar to that of SFM-Mx1 and SAC-B according to their conserved structure. Here we report the cloning and sequencing of the SFM-A biosynthetic gene cluster from S. lavendulae NRRL 11002 and propose biochemical functions for the deduced gene products. Sequence analysis and genetic comparison revealed a common strategy of NRPS-directed tetrapeptide assembly during the biosynthesis of SFM-A, SFM-Mx1, and SAC-B. However, in contrast to speculations from prior reports regarding SFM-Mx1 and SAC-B, we predicted that the same tetrapeptide backbone is catalyzed by NRPSs using the last module in an iterative manner rather than following the typical colinear rule. To confirm this prediction, we heterologously produced and purified proteins containing each adenylation domain of the NRPS modules and determined their substrate specificity using an ATP-PPi exchange assay. Thus, these findings shed new insight into tetrahydroisoquinoline biosynthesis and afford the opportunity to study iterative events during nonribosomal peptide biosynthesis. Finally, production of SFM-Y3, an aminated analog of SFM-A, was achieved by heterologous expression of the hydroxylase SfmO4 in the SAC-B producer P. fluorescens FERM BP-14, demonstrating the feasibility of producing tetrahydroisoquinoline analogs by rationally engineering of an established biosynthetic pathway in microorganisms. The availability of the gene clusters and biosynthetic pathways of SFM-A, SFM-Mx1, and SAC-B has paved the way for future studies regarding the unusual biochemistry found in this pathway and subsequent attempts of applying this knowledge for combinatorial biosynthesis.
MATERIALS AND METHODS
Bacterial strains, plasmids, and reagents.
Bacterial strains and plasmids used in this study are summarized in Table 1. Biochemicals, chemicals, media, restriction enzymes, and other molecular biological reagents were from standard commercial sources.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Characteristic(s) | Source or reference |
|---|---|---|
| E. coli strains | ||
| DH5α | Host for general cloning | Invitrogen |
| CC118 (λpir) | Host for general cloning | 9 |
| XL1-Blue MRF′ | Host for constructing the genomic library | Stratagene |
| S17-1 | Donor strain for conjugation between E. coli and Streptomyces | 26 |
| S17-1 (λpir) | Donor strain for conjugation between E. coli and Pseudomonas | 9 |
| BL21(DE3) | Donor strain for protein overexpression | Novagen |
| S. lavendulae strains | ||
| NRRL 11002 | Wild-type strain, SFM-A producing | NRRL |
| TL2001 | NRPS allele mutant of P1, SFM-A producing | This study |
| TL2002 | NRPS allele mutant of P2, SFM-A nonproducing | This study |
| TL2003 | ΔsfmB gene replacement mutant, SFM-A nonproducing | This study |
| TL2004 | TL2003 derivative containing pTL2007, expression of sfmB under the control of PermE* promoter, SFM-A producing | This study |
| TL2005 | orf(−1) gene disruption mutant, SFM-A producing | This study |
| TL2006 | orf(+1) gene disruption mutant, SFM-A producing | This study |
| P. fluorescens strains | ||
| FERM BP-14 | Wild-type strain, SAC-B producing | FERMa |
| TL2101 | FERM BP-14 containing pTL2011, expression of sfmO to sfmK under the control of the Ptac promoter | This study |
| TL2102 | FERM BP-14 containing pTL2014, expression of sfmO4 under the control of the Ptac promoter, aminated SFM-S producing | This study |
| Plasmids | ||
| pGEM-T Easy | E. coli subcloning vector | Promega |
| pGEM-7zf | E. coli subcloning vector | Promega |
| pSP72 | E. coli subcloning vector | Promega |
| pANT841 | E. coli subcloning vector | GenBank (accession no. AF438749) |
| pVLT33 | Heterologous expression vector in P. fluorescens A2-2 | 9 |
| pTL2001 | 1.2-kb PCR product of the NRPS gene (P1) in pGEM-T Easy | This study |
| pTL2002 | 1.2-kb PCR product of the NRPS gene (P2) in pGEM-T Easy | This study |
| pTL2003 | 0.8-kb PCR product of the NRPS RE gene (P3) in pGEM-T Easy | This study |
| pTL2004 | 1.2-kb EcoRI/SpeI internal fragment of pTL2001 in pOJ260 | This study |
| pTL2005 | 1.2-kb EcoRI/HindIII internal fragment of pTL2002 in pKC1139 | This study |
| pTL2006 | sfmB replacement construct in which sfmB was inactivated by insertion of ermE | This study |
| pTL2007 | 5.2-kb fragment containing sfmB under the control of PermE* in pTGV-4 | This study |
| pTL2008 | 1.4-kb PCR fragment containing sfmO3 to -K in pANT841 | This study |
| pTL2009 | 1.4-kb NdeI/HindIII fragment containing sfmO3 to -K in pET28a | This study |
| pTL2010 | 1.4-kb XbaI/HindIII fragment containing sfmO3 to -K in pVLT33 | This study |
| pTL2011 | 1.4-kb PCR fragment containing sfmO4 in pANT841 | This study |
| pTL2012 | 1.4-kb NdeI/HindIII fragment containing sfmO4 in pET28a | This study |
| pTL2013 | 1.4-kb XbaI/HindIII fragment containing sfmO4 in pVLT33 | This study |
| pTL2014 | 2.0-kb EcoRI/HindIII fragment that encodes the N terminus of SfmA in pSP72 | This study |
| pTL2015 | 5.5-kb fragment that encodes SfmA in pET28a | This study |
| pTL2016 | 3.4-kb fragment that encodes the truncated SfmA (C1-A1-PCP1) in pET28a | This study |
| pTL2017 | 0.6-kb EcoRI/PstI fragment that encodes the N terminus of SfmB in pSP72 | This study |
| pTL2018 | 0.5-kb EcoRI/HindIII fragment that encodes the C terminus of SfmB in pSP72 | This study |
| pTL2019 | 3.3-kb fragment that encodes SfmB in pET28a | This study |
| pTL2020 | 1.7-kb EcoRI/SphI fragment that encodes the N terminus of SfmC in pSP72 | This study |
| pTL2021 | 0.2-kb XbaI/HindIII fragment that encodes the C terminus of SfmC in pGEM-7zf | This study |
| pTL2022 | 4.5-kb fragment that encodes SfmC in pET28a | This study |
| pTL2023 | 0.3-kb PCR product encoding the internal fragment of orf(−1) in pSP72 | This study |
| pTL2024 | 0.4-kb PCR product encoding the internal fragment orf(+1) in pSP72 | This study |
| pTL2025 | 0.3-kb EcoRI/XbaI internal fragment of pTL2023 in pKC1139 | This study |
| pTL2026 | 0.4-kb EcoRI/XbaI internal fragment of pTL2024 in pKC1139 | This study |
| pTL2101 | S. lavendulae NRRL 11002 genomic library cosmid | This study |
| pTL2102 | S. lavendulae NRRL 11002 genomic library cosmid | This study |
| pTL2103 | S. lavendulae NRRL 11002 genomic library cosmid | This study |
FERM, Fermentation Research Institute, Agency of Industrial Science and Technology, Japan.
DNA isolation, manipulation, and sequencing.
DNA isolation and manipulation in Escherichia coli and Streptomyces were carried out according to standard methods (19, 37). PCR amplifications were carried out on an Eppendorf authorized thermal cycler (Eppendorf AG, Hamburg, Germany) using either Taq DNA polymerase or PfuUltra high-fidelity DNA polymerase. Primer synthesis and DNA sequencing were performed at the Shanghai GeneCore Biotechnology, Inc., and Chinese National Human Genome Center.
Genomic library construction and screening.
A genomic library of S. lavendulae NRRL 11002 was constructed in Super-Cos1 according to a previously published protocol (22). E. coli VCS257 and Gigapack III XL packaging extract (Stratagene, La Jolla, CA) were used for library construction according to the manufacturer's instructions. The NRPS gene probes for library screening were obtained by PCR amplification and confirmed by sequencing. For PCR products P1 and P2, a 1.2-kb fragment was obtained by using the primers 5′-TACACGTCCGGCACSACSGGCAARCCNAARGG-3′ and 5′-AWCGAGKSGCCSGGGSMGAAGAA-3′. For PCR product P3, a 0.8-kb fragment was amplified by using the primers 5′-GACAACTTCTTCGAGCTGGGSGGSSAYTC-3′ and 5′-GCGGACCAACTTCTCCGCSRCCCAYTTRCT-3′. The genomic library (6.0 × 103 colonies) was screened by colony hybridization with P2 as a probe, and resultant positive clones were further confirmed by Southern hybridization with P2 and P3 as probes.
Sequence analysis.
The open reading frames (ORFs) were deduced from the sequence by performing FramePlot 3.0beta program (http://watson.nih.go.jp/∼jun/cgi-bin/frameplot-3.0b.pl). The corresponding deduced proteins were compared with other known proteins in the databases by available BLAST methods (http://www.ncbi.nlm.nih.gov/BLAST/). Amino acid sequence alignments were performed by the CLUSTALW method, and the DRAWTREE and DRAWGRAM methods, respectively, from BiologyWorkBench 3.2 software (http://workbench.sdsc.edu). Prediction of amino acid specificity of individual NRPS A domains was performed by using the BLAST server provided at the website http://bix.umbi.umd.edu/Projects/nrps/ (see Table S2 in the supplemental material).
Production, isolation, and analysis of SFM-A in S. lavendulae.
S. lavendulae wild-type and recombinant strains were grown on ISP-2 (0.4% glucose, 0.4% yeast extract, and 1% malt extract [pH 7.2]) agar plates (with the appropriate antibiotic for recombinant strains) at 30°C for sporulation. For fermentation, 200 μl of spore suspension (1.0 × 106 to 1.0 × 107/ml) of each S. lavendulae strain was inoculated onto a YSA (0.1% yeast, 0.5% soluble starch, 1.5% agar, pH 7.5) plate and incubated at 27°C for 7 days. A piece of YSA with spores was then transferred into a 500-ml flask containing 50 ml of fermentation medium (0.1% glucose, 1% soluble starch, 0.5% NaCl, 0.1% K2HPO4, 0.5% casein acid hydrolysate, 0.5% meat extract, pH 7.0) and incubated at 27°C and 250 rpm for 30 to 36 h.
For SFM-A isolation, each 50 ml of the culture broth was filtered and adjusted to a pH of 6.8. After treatment with 1 mM KCN at 35°C for 30 min, the filtered broth was extracted thrice with 30 ml of ethyl acetate, and the combined extract was finally concentrated to 100 μl in a vacuum.
High-performance liquid chromatography (HPLC) analysis was carried out on a Microsorb-MV 100-5 C18 column (4.6 by 250 mm) (catalog no. SN 281505; Varian). The column was equilibrated with 50% solvent A (H2O, 0.05% trifluoroacetic acid) and solvent B (CH3CN, 0.05% trifluoroacetic acid) and developed with the following program: 0 to 5 min, 90% solvent A and 10% solvent B; 5 to 25 min, a linear gradient from 90% solvent A and 10% solvent B to 15% solvent A and 85% solvent B; 25 to 27 min, constant 15% solvent A and 85% solvent B; 27 to 29 min, a linear gradient from 15% solvent A and 85% solvent B to 90% solvent A and 10% solvent B; and 29 to 30 min, constant 90% solvent A and 10% solvent B. This was carried out at a flow rate of 1 ml/min and UV detection at 270 nm using a Agilent 1100 HPLC system (Agilent Technologies, Palo Alto, CA). The identity of a compound was confirmed by coinjection with standard SFM-A and liquid chromatography-mass spectrometry (LC-MS) analysis performed on an LCMS-2010 A liquid chromatograph mass spectrometer (Shimadzu, Japan) under the same conditions. SFM-A showed [M+H]+ ion at m/z 563.0, consistent with the molecular formula C29H30N4O8.
Production, isolation, and analysis of SACs and SFMs in P. fluorescens.
For fermentation, 50 μl of frozen vegetative stock of each P. fluorescens strain was transferred into a 250-ml flask containing 50 ml of YMP3 medium (1% glucose, 0.25% beef extract, 0.5% Bacto peptone, and 0.8% CaCO3, pH 6.5). After incubation at 27°C for 30 h, 2% of this seed medium was then transferred into a 500-ml flask containing 100 ml of M-16B medium [15.2% d-mannitol, 3.5% dried brewer's yeast, 1.4% (NH4)2SO4, 0.001% FeCl3, and 2.6% CaCO3, pH 6.5]. Strains were incubated at 27°C for 40 h, and the expression of sfmO3 to sfmK or expression of sfmO4 was induced by the addition of isopropyl-β-d-thiogalactopyranoside (IPTG) to a final concentration of 0.2 mM, and cultures were incubated at 24°C for an additional 72 to 96 h. The same fermentation procedure was applied to the wild-type strain as a compatible control.
For the production of cyano-substituted analogs, the supernatants of fermentation cultures were treated with 1 mM KCN at 35°C for 30 min.
The analysis on each filtered culture was carried out by using the same set of HPLC and LC-MS conditions as those for SFM-A detection described above. The column was equilibrated with 50% solvent A (10 mM NH4Ac, 1% diethanolamine, pH 4.0) and solvent B (CH3CN), and was developed with the following program: 0 to 5 min, 93% solvent A and 7% solvent B; 5 to 25 min, a linear gradient from 93% solvent A and 7% solvent B to 15% solvent A and 85% solvent B; 25 to 27 min, constant 15% solvent A and 85% solvent B; 27 to 29 min, a linear gradient from 15% solvent A and 85% solvent B to 93% solvent A and 7% solvent B; and 29 to 30 min, constant 93% solvent A and 7% solvent B. This was carried out at a flow rate of 1 ml/min and UV detection at 268 nm. The identity of compound was confirmed by LC-MS analysis under the same conditions. SAC-B showed [M+H]+ ion at m/z 541.2, consistent with the molecular formula C28H36N4O7. Cyano-substituted SAC-B showed [M+H]+ ion at m/z 550.2, consistent with the molecular formula C29H35N5O6. Aminated SFM-S showed [M+H]+ ion at m/z 555.2, consistent with the molecular formula C28H34N4O8. SFM-Y3 showed [M+H]+ ion at m/z 564.0, consistent with the molecular formula C29H33N5O7.
Determination of substrate specificities of SfmA, SfmB, and SfmC.
For amino acid-dependent ATP-PPi exchange assay, a typical reaction (100 μl) was carried out in 75 mM Tris-HCl (pH 8.0) buffer, containing 50 to 100 nM NRPS protein, 5 mM ATP, 1.0 mM PPi with 0.5 μCi of 32PPi (40.02 Ci/mmol; NEN Life Science Products, Boston, MA), 10 mM MgCl2, 5.0 mM dithiothreitol, and a 1.0 mM concentration of each of various amino acids. After incubation at 30°C for 30 min, each assay was stopped by the addition of 0.5 ml of 1% (wt/vol) activated charcoal in 4.5% (wt/vol) tetrasodium pyrophosphate and 3.5% (vol/vol) perchloric acid. The precipitate was collected on a glass-fiber filter (2.4 cm) (G-4; Fisher, Pittsburgh, PA), washed successively with 10 ml of 40 mM sodium pyrophosphate plus 1.4% perchloric acid, 10 ml of water, and 5 ml of 95% ethanol and briefly dried in air. The filter was mixed with 5 ml of scintillation fluid (ScintiSafe gel; Fisher) and counted on a Beckman LS-6800 scintillation counter to determine the radioactivity.
Supplemental material.
See the supplemental material for supporting data including deduced function of open reading frames beyond the SFM-A biosynthetic gene cluster boundary, prediction of amino acid recognitions of NRPS A domains, structures of SFMs, inactivation and complementation in S. lavendulae, biotransformation in P. fluorescens, overexpression and purification of SFM NRPSs, and chemical synthesis of Tyr derivatives in this study.
Nucleotide sequence accession number.
The sequence reported in this paper has been deposited into GenBank under the accession number DQ838002.
RESULTS
Cloning and sequencing of the SFM-A gene cluster from S. lavendulae NRRL 11002.
NRPSs catalyze the assembly of nonribosomal peptides from proteinogenic and nonproteinogenic amino acids and usually possess a multimodular structure (42). Each module consists minimally of an adenylation domain (A domain) responsible for amino acid activation; a peptidyl carrier protein (PCP) domain, which usually resides adjacent to the A domain, for thioesterification of the activated amino acid; and a condensation domain (C domain) for transpeptidation between the upstream and downstream peptidyl and amino acyl thioesters to elongate the growing peptide chain. Based on the high sequence similarity among various A and PCP domains in the database, two conserved motifs (YTSGTTGKPKG in A domains and FFXLGGXSX in PCP domains) were used to design a pair of degenerate primers to clone the putative SFM-A NRPS genes by PCR. With the genomic DNA of S. lavendulae as the template, a distinct product with the expected size of 1.2 kb was readily amplified. Sequencing and analyzing of the selected clones revealed two gene sequences (P1 and P2), both of which are highly similar to those of known NRPS genes. To determine their roles in SFM-A biosynthesis, we set out to inactive the target alleles in S. lavendulae. While the P1 allele mutant strain TL2001 retained the ability to produce SFM-A and even produced SFM-A to a level higher than that of the wild-type strain, the P2 allele mutant strain TL2002 completely lost the ability to produce SFM-A, confirming that the P2-contained NRPS gene is involved in SFM-A biosynthesis (data not shown). As a result, after screening approximately 6 × 103 clones of the S. lavendulae genomic library with the 1.2-kb P2 fragment as a probe, we isolated 20 overlapping cosmids that span a 60- to 65-kb DNA region, as exemplified by pTL2101, pTL2102, and pTL2103 (see Fig. S2 in the supplemental material).
Previous studies on SFM-Mx1 and SAC-B biosynthesis revealed a relatively rare reductase (RE) domain that contains a NAD(P)H binding site at the C-terminal ends of SafA and SacC (33, 46), both of which are NRPSs involved in the tetrapeptidyl backbone formation. These RE domains may act on the PCP-tethered polypeptidyl intermediate and reductively release it from the PCP as a linear aldehyde (21), instead of the thioesterase (TE) functionality at the C-terminal ends of typical NRPSs. Since the RE domain is conserved and always resides next to the last PCP, we adopted an alternative strategy to specifically clone the putative RE gene fragment by PCR according to two motifs [DNFFEL(G/D)GHS in the PCP domain and RVLKEAVWKS in the RE domain]. A single product with the expected size of 0.8 kb was readily amplified and cloned from the genomic DNA of S. lavendulae. Sequence analysis of the randomly selected clones confirmed that 80% of them contain an identical product, P3, and the sequence of P3 exhibits significant similarity to the sequences of the RE domains of the safA and sacC genes. To identify the locus on the chromosome, Southern analysis of the previously identified cosmids obtained by P2 screening was performed using the 0.8-kb P3 fragment as a probe. Intriguingly, most cosmids showed a positive signal, and a single 7.4-kb BamHI fragment that harbors both P2 and P3 was detected. All together, these results provided strong support that we have cloned the SFM-A gene cluster in S. lavendulae.
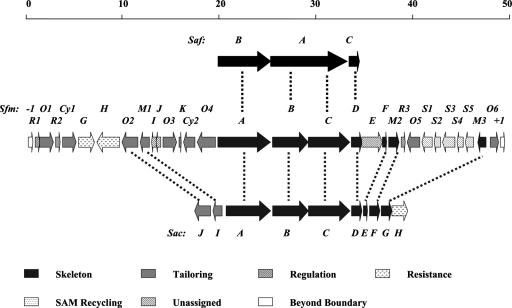
The DNA region represented by cosmid pTL2101 (partial), pTL2102 (entire), and pTL2103 (partial) was selected for sequencing, yielding a 62,804-bp contiguous sequence with 71.86% of the overall GC content that is characteristic for Streptomyces DNA. Bioinformatic analysis of the sequenced region revealed 47 ORFs, and 30 of the ORFs from sfmR1 to sfmO6 were proposed to constitute the SFM-A gene cluster according to functional assignment of their deduced products and genetic comparison with the gene cluster for SAC-B biosynthesis (Fig. 2 and Table 2; see also Table S1 in the supplemental material). Mutant strains with inactivated orf(−1) and orf(+1) retained the ability to produce SFM-A as deduced from HPLC analysis (see Fig. S3 in the supplemental material), supporting the idea that they are outside the sfm gene cluster. Consistent with the structure of SFM-A, the ORFs within the sfm cluster presumably include eight genes encoding enzymes involved in the biosynthesis of the SFM-A tetrapeptidyl backbone, nine genes encoding the tailoring enzymes, as well as three regulatory genes, two resistance genes, five genes involved in S-adenosylmethionine (SAM) recycling, and three additional genes whose functions could not be predicted or assigned for SFM-A production.
FIG. 2.
Genetic organization and comparison of the saf (SFM-Mx1), sfm (SFM-A), and sac (SAC-B) biosynthetic gene clusters. The proposed functions of individual ORFs are shown and summarized in Table 1.
TABLE 2.
Deduced functions of ORFs in the SFM-A biosynthetic gene cluster
| Gene | Sizea | Protein homologb |
|---|---|---|
| orf(−1 to −10) | Beyond the sfm cluster boundary | |
| sfmR1 | 195 | StropDRAFT_1652 (ZP_01431206; 57/71), TetR type regulatory protein from Salinispora tropica CNB-440 |
| sfmO1 | 304 | SAMR0789 (CAJ88498; 62/75), putative oxidoreductase from Streptomyces ambofaciens ATCC 23877 |
| sfmR2 | 169 | MitQ (AAD28455; 63/78), putative regulatory protein in mitomycin biosynthesis |
| sfmCy1 | 512 | MitR (AAD28454; 67/78), similar to mitomycin C oxidase McrA |
| sfmG | 479 | DQ915964.1 (ABL09967; 37/56), AraJ-like transmembrane efflux protein from Streptomyces echinatus |
| sfmH | 819 | CmrX (CAE17542; 57/72), UV repair protein in chromomycin biosynthesis |
| sfmO2 | 521 | SacJ (AAL33754; 43/58), putative monooxygenase/hydroxylase in SAC-B biosynthesis |
| sfmM1 | 199 | SacI (AAL33755; 43/56), SAM-dependent methyltransferase in SAC-B biosynthesis |
| sfmI | 160 | MflvDRAFT_0798 (ZP_01194885; 35/46), unknown protein from Mycobacterium flavescens PYR-GCK |
| sfmJ | 165 | MflvDRAFT_0799 (ZP_01194886; 41/58), unknown protein from M. flavescens PYR-GCK |
| sfmO3 | 395 | Orf3 (AAD28449; 50/67), cytochrome P450 hydroxylase from S. lavendulae |
| sfmK | 61 | Fas2 (P46374, 44/57), ferredoxin-like protein from Rhodococcus fascians |
| sfmCy2 | 504 | MitR (AAD28454; 45/60), similar to mitomycin C oxidase McrA |
| sfmO4 | 475 | HctH (AAY42400; 25/45), cytochrome P450 monooxygenase from Lyngbya majuscula |
| sfmA | 1,836 | SafB (AAC44128; 38/51), NRPS in SFM-Mx1 biosynthesis |
| sfmB | 1,082 | SacB (AAL33757; 42/58), NRPS in SAC-B biosynthesis |
| sfmC | 1,485 | SacC (AAL33758; 47/60), NRPS in SAC-B biosynthesis |
| sfmD | 365 | SacD (AAL33759; 38/51), hydroxylase in SAC-B biosynthesis |
| sfmE | 789 | Orf (−15) (AAN85499; 32/43), putative peptidase from Streptomyces atroolivaceus |
| sfmF | 73 | SacE (AAL33760; 60/68), protein containing MbtH-like domain in SAC-B biosynthesis |
| sfmM2 | 366 | SacF (AAL33761, 63/76), SAM-dependent methyltransferase in SAC-B biosynthesis |
| sfmR3 | 178 | MitQ (AAD28455; 51/69), regulatory protein in mitomycin biosynthesis |
| sfmO5 | 389 | ComPD (AAK81837; 42/52), prephenate dehydrogenase in complestatin biosynthesis |
| sfmS1 | 484 | Fnq16 (CAL34094; 91/95), putative adenosylhomocysteinase from Streptomyces cinnamonensis |
| sfmS2 | 313 | Fnq15 (CAL34093, 76/85), putative 5,10-methylene-tetrahydrofolate reductase from S. cinnamonensis |
| sfmS3 | 1,160 | Fnq14 (CAL34092; 86/92), putative methionine synthase from S. cinnamonensis |
| sfmS4 | 327 | Fnq13 (CAL34091; 67/76), putative adenosine kinase from S. cinnamonensis |
| sfmS5 | 401 | Fnq12 (CAL34090, 85/91), putative S-adenosylmethionine synthase from S. cinnamonensis |
| sfmM3 | 334 | SacG (AAL33762; 43/62), SAM-dependent methyltransferase in SAC-B biosynthesis |
| sfmO6 | 376 | MtmOII (CAK50777; 37/46), FAD-dependent oxygenase in mithramycin biosynthesis |
| orf(+1 to +10) | Beyond the sfm cluster boundary |
The size of each protein is shown as the number of amino acids.
NCBI accession numbers and percent identity/percent similarity are given in parentheses.
Genes encoding NRPSs and associated enzymes for the biosynthesis of the core.
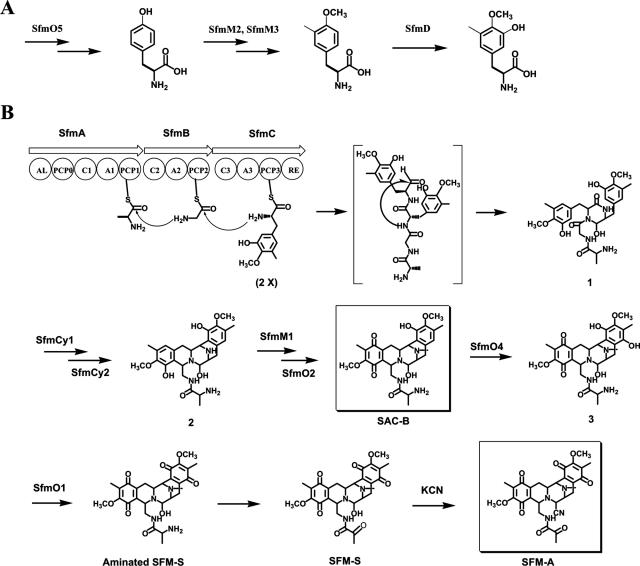
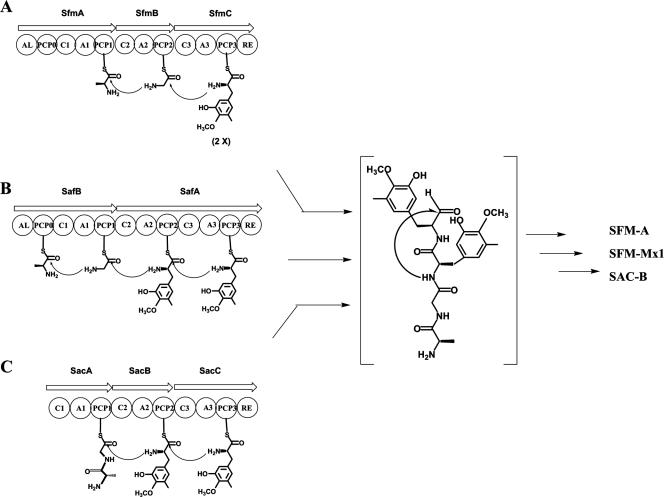
Three NRPS genes, sfmA, sfmB and sfmC, were identified within the sfm cluster (Fig. 2). As shown in Fig. 3B (also see Fig. 6A), their deduced products constitute an NRPS system that contains a set of characteristic domains arranged as follows: acyl coenzyme A ligase (AL)-PCP0-C1-A1-PCP1-C2-A2-PCP2-C3-A3-PCP3-RE. The products are similar from head to tail in both domain organization and amino acid sequence to those for SFM-Mx1 and SAC-B biosynthesis (see Fig. 6B and C [the SAC-B NRPS system lacks the first module AL-PCP0]). The sfmB mutant strain TL2003 completely lost its ability to produce SFM-A (Fig. 4A, panel III), which was restored by expressing sfmB in trans (Fig. 4A, panel IV), confirming the essential role of this NRPS system for SFM-A biosynthesis. The first module of SfmA resembles a family of NRPS N-terminal modules, which consist of a domain with high similarity to ALs and an PCP-like domain, such as BlmVI NRPS-5 (40% identity) that is presumably involved in the biosynthesis of the β-aminoalaniamide moiety of bleomycin as a starter module (10). Although its function on initiation of the peptidyl intermediate biosynthesis remains unclear, sequence alignment revealed that the SfmA AL-like domain lacks most of the conserved motifs of A domains, supporting that the first module of SfmA does not function as a typical NRPS module to incorporate amino acid residues into the tetrapeptidyl skeleton. To predict the substrate of the SFM-A NRPS system, the eight specificity-conferring codes for each A domain were identified as follows by sequence alignment with the A domain of PheA (gramicidin synthase A) (6, 44) (see Table S2 in the supplemental material): DLFNNALT for SfmA-A1 (100% identity to those for SafB-A1 and SacA-A1), DILQLGLI for SfmB-A2 (87.5% identity to those for SafA-A2 and 100% identity to those for SacB-A2), and DPWGLGLI for SfmC-A3 (100% identity to those for SafA-A3 and SacC-A3). Among the SFM-A, SFM-Mx1, and SAC-B NRPS systems, the identity of these eight codes for each group of A domains (with the exception that the Val at residue position 330 in SafA-A2 is replaced by Ile in SfmB-A2 or SacB-A2) indicates that they recognize and activate a same amino acid substrate. Together with the same order of modules for substrate incorporation (see Fig. 6), a common strategy of assembling the tetrapeptidyl intermediate is proposed to occur in SFM-A, SFM-Mx1, and SAC-B biosynthesis. Subsequently, SfmA-A1, SfmB-A2, and SfmC-A3 were predicted to activate Ala or Gly, Gly or 3-hydroxy-5-methy-O-methyltyrosine (3h5mOmTyr), and 3h5mOmTyr, respectively, by an analysis program provided at the website http://bix.umbi.umd.edu/Projects/nrps/. Further biochemical determination of substrate specificities of these NRPS A domains in vitro and mechanistic analysis of this NRPS system for the tetrapeptidyl intermediate assembly are described below. The RE domain of SfmC exhibits high similarity to a few NRPS C-terminal reductase domains that release peptidyl intermediates from NRPSs as reductive products including aldehydes, alcohols, and macrocyclic imines. Very recently, the terminal reductase domain of NcpB, an NRPS for nostocyclopeptide biosynthesis, was biochemically characterized to catalyze the reductive release of the matured peptide chain as an aldehyde and then trigger the spontaneous formation of the imino head-to-tail linkage (21), instead of the more commonly found TE domains for hydrolysis, lactamization, or lactonization. In a mechanistic analogy, SfmC-RE may catalyze the reductive release of the resulting tetrapeptidyl intermediate tethered to SfmC-PCP3 as an aldehyde and then trigger the spontaneous intramolecular cyclization to close the C ring (Fig. 3B).
FIG. 3.
Proposed biosynthetic pathways for 3-hydroxy-5-methy-O-methyltyrosine (A) and saframycin A (B).
FIG. 6.
Domain organizations and proposed enzymatic mechanisms of NRPS systems for SFM-A (A), SFM-Mx1 (B) (33), and SAC-B (C) (46) biosynthesis.
FIG. 4.
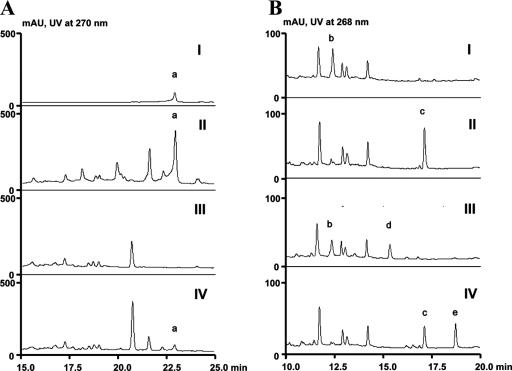
HPLC analysis of saframycin and safracins. (A) Saframycin A (a) isolated from an authentic standard (I), S. lavendulae wild-type strain (II), strain TL2003 (ΔsfmB) (III), and strain TL2004 (PermE*::sfmB) (IV). (B) Safracin B (b) isolated from a P. fluorescens wild-type strain (I), cyano-substituted safracin B (c) isolated from a P. fluorescens wild-type strain treated with 1 mM KCN (II), aminated saframycin S (d) isolated from strain TL2102 (Ptac::sfmO4) (III), and saframycin Y3 (e) isolated from strain TL2102 (Ptac::sfmO4) treated with 1 mM KCN (IV). Absorbance of UV at 270 nm (A) or 268 nm (B) in milliabsorbance units (mAU) is shown on the y axes, and time (in minutes) is shown on the x axes.
Four genes, sfmD, sfmF, sfmM2, and sfmM3, are proposed to encode the enzymes involved in biosynthesis of the nonproteinogenic amino acid 3h5mOmTyr (Fig. 3A), the pathway of which was established in SAC-B biosynthesis by heterologous expression of homologous genes and cocultivation among mutant strains (46). SfmO5, closely related to the prephenate dehydrogenases (e.g., ComPD in complestatin biosynthesis [7]; 42% identity) that catalyze the p-hydroxyphenylpyruvate formation, might enhance the biosynthesis of Tyr that serves as the precursor of 3h5mOmTyr. SfmF, with high similarity to SacE (60% identity), belongs to a family of MbtH-like proteins that contain three fully conserved tryptophan (Trp) residues. Most members of this family are found in known antibiotic gene clusters, such as VioN (60% identity) in viomycin biosynthesis (45); however, their roles remain to be established. SfmM2 exhibits high sequence similarity to SacF (63% identity) and likely functions as a C-methyltransferase to introduce a methyl group at the C-3 position of Tyr. SfmM3 exhibits high sequence homology to SacG (43% identity) and various O-methyltransferases (e.g., CalO6 in calicheamicin biosynthesis [1]; 36% identity), supporting its role for O methylation at the C-4 position. SfmD, with no other homologous proteins found in the database, exhibits relatively high sequence similarity to SacD (38% identity), the function of which was deduced to be responsible for the hydroxyl group substitution at C-5 to convert 3-methyl-O-methyltyrosine (3mOmTyr) into 3h5mOmTyr.
Genes encoding tailoring enzymes.
Postmodifications on the tetrapeptidyl intermediate compound 1, including cyclization, methylation, oxidoreduction, and nitrile moiety substitution, are postulated to proceed with a set of tailoring enzymes in the SFM-A biosynthetic pathway as outlined in Fig. 3B. The gene products of sfmCy1 and sfmCy2 exhibit high sequence similarity to MitR (67% and 45% identity, respectively), which might be responsible for the C8a-C9 bond formation in mitomycin biosynthesis (24). In a mechanistic analogy, SfmCy1 and SfmCy2 may act on compound 1 as cyclases to close the B and D rings at C9-C1 and C19-C11, although their regiospecificities need to be determined. Noticeably, such homologous genes have not been identified within the SAC-B biosynthetic gene cluster in P. fluorescens. It would be interesting to further determine whether these homologs can be identified in the P. fluorescens chromosome or whether they have any function regarding the formation of the B and E rings.
sfmM1 and sfmO2 encode proteins that have high sequence similarity to SacI (43% identity) and SacJ (43% identity), respectively, both of which have been functionally assigned to catalyze the last two steps for SAC-B biosynthesis on the basis of identifying shunt metabolites resulted from sacI or sacJ inactivation (46). Although the catalytic order remains to be established, it is likely that SfmM1 acts as a N-methyltransferase to introduce a methyl group at the N-12 position, and SfmO2 serves as a monooxygenase responsible for hydroxylation at the C-5 position on the A ring, which then undergoes a dehydrogenation to form the quinone ring of SAC-B. Based on this hypothesis, interestingly, SAC-B might be a key intermediate in the SFM-A biosynthetic pathway (Fig. 3B).
SFM-A structurally differs from SAC-B with a heavily oxidized E ring. Heterologous expression of SfmO4 in the SAC-B producer resulted in aminated SFM-S production (described below), supporting the hypothesis that SfmO4 acts on SAC-B at the C-15 position for a hydroxyl substitution. sfmO1, encoding a putative NAD(P)+-dependent oxidoreductase, might catalyze oxidation of the resultant hydroxyl on the E ring in vivo, producing the characteristic bisquinone core scaffold (Fig. 3B). The further desamination of Ala and substitution of a nitrile moiety at the C-21 position are proposed to yield SFM-A. Alternatively, it could not be excluded that the oxidative desamination step, which is predicted to be catalyzed by a putative FAD-dependent monooxygenase SfmO6, occurs at an earlier stage during the tailoring process. Since previous studies showed that treatment of SFM-S (an SFM-A precursor with a hydroxyl group instead of a nitrile moiety) with sodium cyanide led to the formation of SFM-A (2), the substitution of a nitrile moiety may be spontaneous, consisting with no obvious gene candidate identified within the sfmA cluster.
The S. lavendulae wild-type strain also produces a series of SFM derivatives with additional oxidation or O methylation at the C-14 position (as shown as a hydroxyl, methoxy, or keto group in Fig. 1) (40), suggesting that the branched biosynthetic pathways may start with the intermediate compound 3 and aminated SFM-S (or their desamino derivatives). The putative cytochrome P450 enzyme SfmO3 (coupled with the ferredoxin-like protein SfmK) presumably initiates the oxidative bioconversion at this position.
Genes encoding regulation, resistance, and other functions.
Three genes (Fig. 2B), sfmR1, sfmR2, and sfmR3, are presumed to encode pathway-specific regulatory proteins. While SfmR1 resembles the TetR family of transcriptional regulators widely found in many microorganisms, SfmR2 and SfmR3, with high similarity to MitQ (63% and 51% identity, respectively) in the mitomycin biosynthetic pathway (24), belong to the OmpR family of DNA binding regulators in the two-component system.
Two resistance genes (Fig. 2B), sfmG and sfmH, were found in the sfm cluster. In contrast to SfmG that belongs to a family of transmembrane efflux permeases that usually exhibits multiple drug resistance, such as AraJ (37% identity) in the aranciamycin biosynthetic pathway (41), SfmH shows high sequence similarity to a family of UV repair proteins, such as CmrX (57% identity) in the chromomycin biosynthetic pathway (27), representing a more specific resistance protein in agreement with the mechanism of action of SFM-A as a DNA alkylation agent.
Sequence analysis within the sfm cluster revealed five genes (Fig. 2B), sfmS1 to sfmS5 as a complete set for the recycling of SAM from S-adenosylhomocysteine (SAH, a by-product in the SAM-dependent methylation reaction). SfmS1, a putative S-adenosyl-l-homocysteine hydrolase, may cleave SAH to adenosine and homocysteine. The latter could be methylated and converted into methionine by SfmS2, a putative methionine synthase, with N5-methyl tetrahydrofolate (N5-methyl THF) as the cosubstrate. SfmS5, closely related to a family of SAM synthetases, might be responsible for the generation of SAM from methionine and ATP. N5-methyl THF as a methyl donor originates from N5,N10-methylene THF, requiring SfmS2 as a putative N5,N10-methylene THF reductase. SfmS4 shows high sequence similarity to a family of adenosine kinases, presumably in charge of ATP regeneration by converting adenosine to AMP. The pathway for recycling SAH to SAM has been well established in primary metabolism and recently was identified to be involved in a few biosynthetic pathways for secondary metabolites, such as the polyketide-isoprenoid compound furanonaphthoquinone I (16). Since multiple SAM-dependent methylations at C, O, and N positions occur in the SFM-A biosynthetic process, the advantage of involvement of this complete pathway might facilitate enhancement of the supply of the SAM precursor.
Finally, three genes within the sfm cluster could not be functionally assigned on the basis of sequence analysis alone (Fig. 2B). sfmE encodes a protein that resembles proteins in the peptidase M28 family. The deduced products of two coupled genes, sfmI and sfmJ, exhibit high sequence similarity to MflvDRAFT_0798 (35% identity) and MflvDRAFT_0799 (41% identity), respectively, and the genes encoding MflvDRAFT_0798 and MflvDRAFT_0799 are clustered within the genome of Mycobacterium flavescens PYR-GCK (under the NCBI accession number NZ_AAPA01000017). Although SfmJ contains a putative pyridoxamine 5′-phosphate oxidase domain, their roles in SFM-A biosynthesis could not be speculated.
Determination of substrate specificities of SfmA, SfmB, and SfmC by utilizing amino acid-dependent ATP-PPi exchange assay.
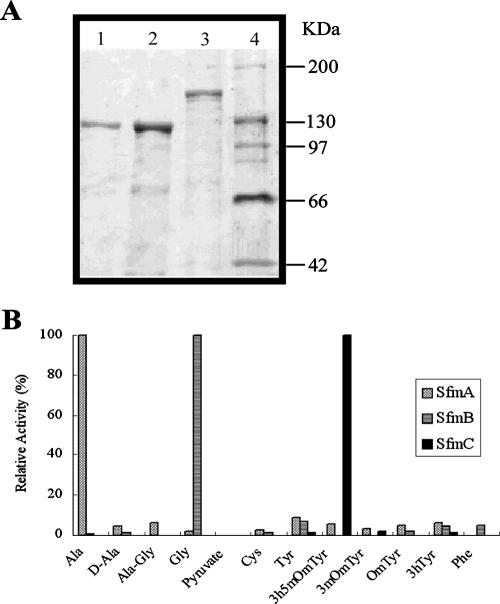
Initial attempts to directly determine substrate specificities of individual A domains (i.e., SfmA-A1, SfmB-A2, and SfmC-A3) or intact SfmA from the SFM-A NRPS system were hampered by either poor solubility of the resultant proteins in E. coli or low enzymatic activities (data not shown). Thus, truncated SfmA (C1-A1-PCP1) and the intact SfmB (C2-A2-PCP2) and SfmC (C3-A3-PCP3-RE) were heterologously expressed in E. coli by using pET28a yielding soluble N-terminal His-tagged proteins with a yield around 5 to 10 mg/liter. Using nickel affinity chromatography, gel filtration, or anion-exchange chromatography in tandem, all proteins were purified to near homogeneity as shown in Fig. 5A, and sodium dodecyl sulfate-polyacrylamide gel electrophoresis revealed a dominant band consistent with their deduced molecular masses (124 kDa, 119 kDa, and 163 kDa, respectively). Among the substrates used in the ATP-PPi exchange assay (l-Ala, d-Ala, l-Ala-l-Gly, l-Gly, pyruvate, l-Cys, l-Tyr, l-3h5mOmTyr, l-3mOmTyr, l-OmTyr, l-3hTyr, and l-Phe), as shown in Fig. 5B, truncated SfmA (C1-A1-PCP1), SfmB (C2-A2-PCP2), and SfmC (C3-A3-PCP3-RE) specifically recognized and activated l-Ala, l-Gly, and l-3h5mOmTyr, respectively.
FIG. 5.
(A) Purified SfmA-C1-A1-PCP1 (lane 1), SfmB-C2-A2-PCP2 (lane 2), and SfmC-C3-A3-PCP3-RE (lane 3) as analyzed by electrophoresis on a 7.5% sodium dodecyl sulfate-polyacrylamide gel. The positions of molecular mass markers (lane 4) (in kilodaltons) are shown to the right of the gel. (B) Substrate specificities as determined by the ATP-PPi exchange reaction with the amino acids predicted to be incorporated into SFM-A (100% relative activity corresponds to 51,540 cpm for SfmA-C1-A1-PCP1, 44,300 cpm for SfmB-C2-A2-PCP2, and 51,500 cpm for SfmC-C3-A3-PCP3-RE).
Construction of the SFM-Y3 biosynthetic pathway in the SAC-B producer.
According to the proposed SFM-A biosynthetic pathway (Fig. 3B), SAC-B, originally produced by P. fluorescens, may serve as a key intermediate. Two putative cytochrome P450 hydroxylase genes, sfmO3 and sfmO4, were identified within the sfm cluster, and one of these genes encodes a protein that may be involved in further oxidation of the E ring. To verify this hypothesis, the constructs that carry sfmO3 to sfmK (SfmK is a ferredoxin-like protein as a putative electron donor) and sfmO4 were individually introduced into the SAC-B-producing strain, yielding the recombinant strains TL2101 and TL2102, respectively. Using the P. fluorescens wild-type strain as a control, strains TL2101 and TL2102 were cultured, and the resulting compounds were detected by HPLC analysis. While the culture of strain TL2101 exhibited an HPLC profile similar to that of the wild-type strain (Fig. 4B, panel I), the production of SAC-B in TL2102 decreased. Accordingly, it was partially transformed into a new compound (Fig. 4B, panel III). LC-MS analysis revealed this compound with an [M+H]+ ion at m/z 555.2, consistent with the molecular formula C28H34N4O8. By comparison with SAC-B and SFM-A, it was deduced to be an aminated SFM-S that contains a heavily oxidized quinone E ring. Thus, SfmO4 may act at C-15 position and yield the hydroquinone derivative compound 3, which may not be stable and is rapidly converted into aminated SFM-S during the purification process. To further confirm this oxidation step, the cultures of wild-type and mutant strains were treated with potassium cyanide at an earlier stage of purification. Upon HPLC (Fig. 4B, panels II and IV) and LC-MS analyses, as we expected, SAC-B from the wild-type strain and aminated SFM-S from strain TL2013 were correspondingly transformed into cyano-SAC-B and SFM-Y3 (the structure of the latter was further supported by tandem MS spectrometry analysis. See the supplemental material for detailed information), respectively. SFM-Y3 that is distinct from SFM-A only by a preserved amino group on Ala was previously found in the S. lavendulae strain culture supplemented with additional Ala and Gly or Ala-Gly dipeptide as substrates (Fig. 1) (4). These results not only clearly confirmed the function of SfmO4 but also showed that the SAC-B biosynthetic machinery in P. fluorescens is amenable to be rationally engineered for the production of structurally more complex analogs by introducing additional tailoring genes from the SFM-A biosynthetic pathway.
DISCUSSION
SFM-A is a bisquinone alkaloid that has significant antiproliferative activity. Previous feeding experiments indicated that the skeleton of SFM-A originates from a highly modified tetrapeptide (28), and studies with the structurally related compounds, SFM-Mx1 (33) and SAC-B (46), revealed that the biosynthesis of these compounds is mediated by an NRPS system (Fig. 1). Based on the assumption that SFM-A is biosynthesized in a similar manner, we attempted to clone the sfm cluster by amplifying putative NRPS gene fragments by PCR from S. lavendulae NRRL 11002. Using the PCR-amplified fragment P2 (obtained with the general NRPS primer set designed according to the conserved motifs of A and PCP domains) and P3 (obtained with the specific NRPS primer set designed according to the conserved motifs of PCP and RE domains) as probes, we screened the genomic library of S. lavendulae and identified the NRPS gene locus. Genetic analysis of a sequenced 62,804-bp DNA region revealed 47 ORFs, 30 of which are proposed to constitute the sfm gene cluster that contains a set of unusual NRPS genes with numerous novel genes for the subsequent tailoring steps to produce SFM-A and its analogs (Fig. 2 and 3B). The inactivation of NRPS gene sfmB completely abolished the ability to produce SFM-A, and subsequent complementation of this mutation by expressing sfmB in trans restored the production of SFM-A, unambiguously confirming that the cloned gene cluster is essential for SFM-A biosynthesis (Fig. 4A).
SfmA, SfmB, and SfmC constitute an NRPS system that exhibits similarities in domain organization and amino sequence from head to tail to those for SFM-Mx1 and SAC-B biosynthesis (Fig. 6). With the exception of the first module that is not found in the SAC-B NRPS system, the eight specificity-conferring amino acids for each A domain in the remaining three modules are almost identical, suggesting a common logic for the assembly of the tetrapeptide intermediate during SFM-A, SFM-Mx1, and SAC-B biosynthesis. Based on the colinearity rule (25), wherein the NRPS module organization parallels the order of the amino acid residues in the resultant polypeptide, sequential incorporation of Ala, Gly, and two Tyr derivatives into the tetrapeptide in SAM-Mx1 biosynthesis was previously speculated to be directed by four successive modules (i.e., SafB-AL-PCP0, SafB-C1-A1-PCP1, SafA-C2-A2-PCP2, and SafA-C3-A3-PCP3-RE) (Fig. 6B) (33). Since SacA in SAC-B biosynthesis lacks the first module AL-PCP0, a bifunctional adenylation activation by SacA or direct incorporation of an Ala-Gly dipeptide into the tetrapeptide by SacA was hypothesized (Fig. 6C) (46). In both systems, the last two modules (SafA-C2-A2-PCP2 and SafA-C3-A3-PCP3-RE or SacB-C2-A2-PCP2 and SacC-C3-A3-PCP3-RE) were suggested to be responsible for activation and incorporation of each Tyr derivative, 3h5mOmTyr.
Our sequence analysis revealed that the N-terminal domain of SfmA in SFM-A biosynthesis, as well as that of SafB in SFM-Mx1 biosynthesis, lacks the expected conserved motifs of A domains and closely resembles the AL family, suggesting that SfmA-AL-PCP0 or SafB-AL-PCP0 might not be involved in amino acid incorporation like typical NRPS modules. Furthermore, the lack of this complete module in SAC-B NRPS system suggests that it may not be essential for tetrapeptide biosynthesis. Comparisons of the eight specificity-conferring amino acids of the A2 domain (DILQLGLI) and the A3 domain (DPWGLGLI) show they are significantly different, and thus, it is unlikely that the third module (SfmB-C2-A2-PCP2, SafA-C2-A2-PCP2, or SacB-C2-A2-PCP2) activates and incorporates the same 3h5mOmTyr residue as the fourth module does (SfmC-C3-A3-PCP3-RE, SafA-C3-A3-PCP3-RE, or SacC-C3-A3-PCP3-RE). In fact, question on the original assignments of substrate specificities for the SFM-Mx1 NRPS system was raised in bleomycin biosynthesis (10), in which reexamination of the SafAB sequence suggested that SafB-A1 serves as a candidate for Ala activation, while SafA-A2 recognizes and activates Gly. Consequently, these NRPS systems most likely catalyze the formation of a tetrapeptide intermediate using the last module in an iterative manner rather than following a typical colinearity rule, as shown in Fig. 6A. To confirm this hypothesis, we performed an amino acid-dependent ATP-PPi exchange assay to determine the substrate specificities of SfmA, SfmB, and SfmC. As we anticipated, truncated SfmA (C1-A1-PCP1), SfmB (C2-A2-PCP2), and SfmC (C3-A3-PCP3-RE) showed exclusive activities with l-Ala, l-Gly, and L-3h5mOmTyr, respectively, strongly supporting the hypothesis that SfmC acts twice to incorporate two L-3h5mOmTyr residues into the tetrapeptide (Fig. 6A). This finding, as well as the recent emergence of many unusual NRPS systems (30, 39, 47), indicates a rich variety of biochemistry and architecture of NRPSs beyond those previously appreciated.
NRPS systems that are distinct from the current paradigm fall into one of two categories: they either contain at least one module with an atypical arrangement of the core domains, exemplified by the syringomycin biosynthesis (15), or use all their modules iteratively for product assembly with the TE domain channeling the multimeric intermediates, exemplified by enterobactin biosynthesis (14). While the SFM-A NRPS system shares the classical A-PCP-(C-A-PCP)n domain organization found in linear NRPSs, the last module (SfmC), in contrast to the SfmA and SfmB modules, acts in an iterative fashion to produce a tetrapeptide intermediate. Furthermore, the TE domain that is typically found as the C-terminal domain of NRPS is substituted with a terminal RE domain. Since the C domain catalyzes transpeptidation between amino acyl thioesters without covalently binding the intermediate and since NRPSs are usually recognized to function as monomers (39), SfmC might channel the tripeptidyl or tetrapeptidyl intermediate in an unusual iterative manner that is distinct from any known NRPS system and mechanistically different from iterative events found in type I polyketide synthases (47). Knowledge of the structure of SfmC will be necessary to understand the selectivity and interaction of the domains involved and to eventually engineer novel NRPS enzymes, like SfmC, for combinatorial biosynthesis.
As shown in Fig. 3B, a RE domain that resides at the C terminus of SfmC may reductively release the tetrapeptide intermediate from the PCP3 as a linear aldehyde. A series of intramolecular cyclizations lead to the formation of the B, C, and D rings, characteristic of the tetrahydroisoquinoline family. Subsequently, regiospecific methylation, oxidation, desamination, and substitution of a cyano group successively occur to produce SFM-A. Genetic comparison revealed that the sfm gene cluster contains all the structural genes for SAC-B biosynthesis (Fig. 2), supporting our hypothesis that SAC-B, which was originally isolated from a Pseudomonas strain, might serve as a key intermediate for SFM-A biosynthesis. It is not surprising that the sfm cluster harbors additional genes, since their functions are required for further modifications of the shared intermediate, such as multiple oxidations of the E ring. Heterologous expression of SfmO4 (a hydroxylase responsible for the introduction of a hydroxyl group at C-15 position) in the SAC-B producer resulted in a bisquinone derivative, aminated SFM-S, which was then converted into an aminated SFM-A analog, SFM-Y3, by treatment of the fermentation culture with potassium cyanide (Fig. 4B). Thus, our results are consistent with parallel biosynthetic pathways for SFMA-A and SAC-B production.
In conclusion, the availability of the sfm biosynthetic gene cluster described here provides an excellent opportunity to access the unusual enzymatic mechanism of SFM-A biosynthesis. Sequence analysis and genetic comparison revealed a common strategy for tetrapeptidyl assembly among SFM-A and its analogs (SFM-Mx1 and SAC-B), and biochemical determination of substrate specificities for the A domains supported that the backbone formation is catalyzed by a multimodular NRPS system in a semi-iterative manner rather than following a previously proposed colinear rule. On the basis of the SAC-B biosynthetic machinery, we reconstructed an aminated SFM-A pathway by heterologously expressing a regiospecific hydroxylase SfmO4 in the SAC-B-producing Pseudomonas strain, thereby demonstrating a unified mechanism of biosynthesis among the tetrahydroisoquinoline compounds and the feasibility of engineering bacterial strains to generate new or otherwise scarce bisquinone alkaloid analogs.
Supplementary Material
Acknowledgments
We thank Steven G. Van Lanen, School of Pharmacy, University of Wisconsin—Madison, for reading the manuscript and providing helpful comments; Andrew G. Myers, Department of Chemistry and Chemical Biology, Harvard University, for providing the authentic SFM-A standard; Victor De Lorenzo, Centro de Astrobiología (Instituto Nacional de Técnica Aeroespacial-CSIC), Spain, for providing the expression system in a Pseudomonas strain; and Linquan Bai, Shanghai Jiaotong University, China, for helpful discussions.
This work was supported in part by grants from the National Natural Science Foundation of China (20621062, 20402021, 30425003, and 30525001), the Ministry of Science and Technology of China (2006AA02Z185), the Chinese Academy of Science (KJCX2-YW-H08), and the Science and Technology Commission of Shanghai Municipality (04DZ14901 and 05QMX1466).
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
Published ahead of print on 2 November 2007.
REFERENCES
- 1.Ahlert, J., E. Shepard, N. Lomovskaya, E. Zazopoulos, A. Staffa, B. O. Bachmann, K. Huang, L. Fonstein, A. Czisny, R. E. Whitwam, C. M. Farnet, and J. S. Thorson. 2002. The calicheamicin gene cluster and its iterative type I enediyne PKS. Science 2971173-1176. [DOI] [PubMed] [Google Scholar]
- 2.Arai, T., K. Takahashi, K. Ishiguro, and K. Yazawa. 1980. Increased production of saframycin A and isolation of saframycin S. J. Antibiot. 33951-960. [DOI] [PubMed] [Google Scholar]
- 3.Arai, T., K. Takahashi, K. Ishiguro, and Y. Mikami. 1980. Some chemotherapeutic properties of two new antitumor antibiotics, saframycins A and C. Gann 71790-796. [PubMed] [Google Scholar]
- 4.Arai, T., K. Yazawa, K. Takahashi, A. Maeda, and Y. Mikami. 1985. Directed biosynthesis of new saframycin derivatives with resting cells of Streptomyces lavendulae. Antimicrob. Agents Chemother. 285-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baltz, R. H. 2006. Molecular engineering approaches to peptide, polyketide and other antibiotics. Nat. Biotechnol. 241533-1540. [DOI] [PubMed] [Google Scholar]
- 6.Challis, G. L., J. Ravel, and C. A. Townsend. 2000. Predictive, structure-based model of amino acid recognization by nonribosomal peptide synthetase adenylation domains. Chem. Biol. 7211-224. [DOI] [PubMed] [Google Scholar]
- 7.Chiu, H. T., B. K. Hubbard, A. N. Shah, J. Eide, R. A. Fredenburg, C. T. Walsh, and C. Khosla. 2001. Molecular cloning and sequence analysis of the complestatin biosynthetic gene cluster. Proc. Natl. Acad. Sci. USA 988548-8553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuevas, C., M. Perez, M. J. Martin, J. L. Chicharro, C. Fernandez-Rivas, M. Flores, A. Francesch, P. Gallego, M. Zarzuelo, F. De La Calle, J. Garcia, C. Polanco, I. Rodriguez, and I. Manzanares. 2000. Synthesis of ecteinascidin ET-743 and phthalascidin Pt-650 from cyanosafracin B. Org. Lett. 22545-2548. [DOI] [PubMed] [Google Scholar]
- 9.De Lorenzo, V., L. Eltis, B. Kessler, and K. N. Timmis. 1993. Analysis of Pseudomonas gene products using lacIq/Ptrp-lac plasmids and transposons that confer conditional phenotypes. Gene 12317-24. [DOI] [PubMed] [Google Scholar]
- 10.Du, L., C. Sanchez, M. Chen, D. J. Edwards, and B. Shen. 2000. The biosynthetic gene cluster for the antitumor drug bleomycin from Streptomyces verticillus ATCC 15003 supporting functional interactions between nonribosomal peptide synthetases and a polyketide synthase. Chem. Biol. 7623-642. [DOI] [PubMed] [Google Scholar]
- 11.Fischbach, M. A., and C. T. Walsh. 2006. Directing biosynthesis. Science 314603-605. [DOI] [PubMed] [Google Scholar]
- 12.Fortman, J. L., and D. H. Sherman. 2005. Utilizing the power of microbial genetics to bridge the gap between the promise and the application of marine natural products. Chembiochem 6960-978. [DOI] [PubMed] [Google Scholar]
- 13.Galm, U., and B. Shen. 2006. Expression of biosynthetic gene clusters in heterologous hosts for natural product production and combinatorial biosynthesis. Expert Opin. Drug Discov. 1409-437. [DOI] [PubMed] [Google Scholar]
- 14.Gehring, A. M., I. Mori, and C. T. Walsh. 1998. Reconstitution and characterization of the Escherichia coli enterobactin synthetase from EntB, EntE, and EntF. Biochemistry 372648-2659. [DOI] [PubMed] [Google Scholar]
- 15.Guenzi, E., G. Galli, I. Grgurina, D. C. Gross, and G. Grandi. 1998. Characterization of the syringomycin synthetase gene cluster. A link between prokaryotic and eukaryotic peptide synthetases. J. Biol. Chem. 27332857-32863. [DOI] [PubMed] [Google Scholar]
- 16.Haagen, Y., K. Gluck, K. Fay, B. Kammerer, B. Gust, and L. Heide. 2006. A gene cluster for prenylated naphthoquinone and prenylated phenazine biosynthesis in Streptomyces cinnamonensis DSM 1042. Chembiochem 72016-2027. [DOI] [PubMed] [Google Scholar]
- 17.Ishiguro, K., K. Takahashi, K. Yazawa, S. Sakiyama, and T. Arai. 1981. Binding of saframycin A, a heterocyclic quinone anti-tumor antibiotic to DNA as revealed by the use of the antibiotic labeled with [14C]tyrosine or [14C]cyanide. J. Biol. Chem. 2562162-2167. [PubMed] [Google Scholar]
- 18.Ishiguro, K., S. Sakiyama, K. Takahashi, and T. Arai. 1978. Mode of action of saframycin A, a novel heterocyclic quinone antibiotic. Inhibition of RNA synthesis in vivo and in vitro. Biochemistry 172545-2550. [DOI] [PubMed] [Google Scholar]
- 19.Kieser, T., M. Bibb, M. Butter, K. F. Chater, and D. A. Hopwood. 2000. Practical Streptomyces genetics. The John Innes Foundation, Norwich, United Kingdom.
- 20.Konig, G. M., S. Kehraus, S. F. Seibert, A. Abdel-Lateff, and D. Muller. 2006. Natural products from marine organisms and their associated microbes. Chembiochem 7229-238. [DOI] [PubMed] [Google Scholar]
- 21.Kopp, F., C. Mahlert, J. Grunewald, and M. A. Marahiel. 2006. Peptide macrocyclization: the reductase of the nostocyclopeptide synthetase triggers the self-assembly of a macrocyclic imine. J. Am. Chem. Soc. 12816478-16479. [DOI] [PubMed] [Google Scholar]
- 22.Liu, W., and B. Shen. 2000. Genes for production of the enediyne antitumor antibiotic C-1027 in Streptomyces globisporus are clustered with the cagA gene that encodes the C-1027 apoprotein. Antimicrob. Agents Chemother. 44382-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lown, J. W., A. V. Joshua, and J. S. Lee. 1982. Molecular mechanisms of binding and single-strand scission of deoxyribonucleic acid by the antitumor antibiotics saframycins A and C. Biochemistry 21419-428. [DOI] [PubMed] [Google Scholar]
- 24.Mao, Y., M. Varoglu, and D. H. Sherman. 1999. Molecular characterization and analysis of the biosynthetic gene cluster for the antitumor antibiotic mitomycin C from Streptomyces lavendulae NRRL 2564. Chem. Biol. 6251-263. [DOI] [PubMed] [Google Scholar]
- 25.Marahiel, M. A., T. Stachelhaus, and H. D. Mootz. 1997. Modular peptide synthetases involved in nonribosomal peptide synthesis. Chem. Rev. 972651-2674. [DOI] [PubMed] [Google Scholar]
- 26.Mazodier, H., R. Petter, and C. Thompson. 1989. Intergeneric conjugation between Escherichia and Streptomyces species. J. Bacteriol. 1713583-3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Menendez, N., M. Nur-e-Alam, A. F. Brana, J. Rohr, J. A. Salas, and C. Mendez. 2004. Biosynthesis of the antitumor chromomycin A3 in Streptomyces griseus: analysis of the gene cluster and rational design of novel chromomycin analogs. Chem. Biol. 1121-32. [DOI] [PubMed] [Google Scholar]
- 28.Mikami, Y., K. Takahashi, K. Yazawa, T. Arai, M. Namikoshi, S. Iwasaki, and S. Okuda. 1985. Biosynthetic studies on saframycin A, a quinone antitumor antibiotic produced by Streptomyces lavendulae. J. Biol. Chem. 260344-348. [PubMed] [Google Scholar]
- 29.Mikami, Y., K. Yokoyama, H. Tabeta, K. Nakagaki, and T. Arai. 1981. Saframycin S, a new saframycin group antibiotic. J. Pharmacobiol. Dyn. 4282-286. [DOI] [PubMed] [Google Scholar]
- 30.Mootz, H. D., D. Schwarzer, and M. A. Marahiel. 2002. Ways of assembling complex natural products on modular nonribosomal peptide synthetases. Chembiochem 3490-504. [DOI] [PubMed] [Google Scholar]
- 31.Plowright, A. T., S. E. Schaus, and A. G. Myers. 2002. Transcriptional response pathways in a yeast strain sensitive to saframycin A and a more potent analog: evidence for a common basis of activity. Chem. Biol. 9607-618. [DOI] [PubMed] [Google Scholar]
- 32.Pospiech, A., B. Cluzel, J. Bietenhader, and T. Schupp. 1995. A new Myxococcus xanthus gene cluster for the biosynthesis of the antibiotic saframycin Mx1 encoding a peptide synthetase. Microbiology 1411793-1803. [DOI] [PubMed] [Google Scholar]
- 33.Pospiech, A., J. Bietenhader, and T. Schupp. 1996. Two multifunctional peptide synthetases and an O-methyltransferase are involved in the biosynthesis of the DNA-binding antibiotic and antitumor agent saframycin Mx1 from Myxococcus xanthus. Microbiology 142741-746. [DOI] [PubMed] [Google Scholar]
- 34.Rao, K. E., and J. W. Lown. 1990. Mode of action of saframycin antitumor antibiotics: sequence selectivities in the covalent binding of saframycins A and S to deoxyribonucleic acid. Chem. Res. Toxicol. 3262-267. [DOI] [PubMed] [Google Scholar]
- 35.Rao, K. E., and J. W. Lown. 1992. DNA sequence selectivities in the covalent bonding of antibiotic saframycins Mx1, Mx3, A, and S deduced from MPE. Fe(II) footprinting and exonuclease III stop assays. Biochemistry 3112076-12082. [DOI] [PubMed] [Google Scholar]
- 36.Rinehart, K. L. 2000. Antitumor compounds from tunicates. Med. Res. Rev. 201-27. [DOI] [PubMed] [Google Scholar]
- 37.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 38.Schwartsmann, G., A. Brondani da Rocha, R. G. Berlinck, and J. Jimeno. 2001. Marine organisms as a source of new anticancer agents. Lancet Oncol. 2221-225. [DOI] [PubMed] [Google Scholar]
- 39.Schwarzer, D., R. Finking, and M. A. Marahiel. 2003. Nonribosomal peptides: from genes to products. Nat. Prod. Rep. 20275-287. [DOI] [PubMed] [Google Scholar]
- 40.Scott, J. D., and R. M. Williams. 2002. Chemistry and biology of the tetrahydroisoquinoline antitumor antibiotics. Chem. Rev. 1021669-1730. [DOI] [PubMed] [Google Scholar]
- 41.Sianidis, G., S. E. Wohlert, C. Pozidis, S. Karamanou, A. Luzhetskyy, A. Vente, and A. Economou. 2006. Cloning, purification and characterization of a functional anthracycline glycosyltransferase. J. Biotechnol. 125425-433. [DOI] [PubMed] [Google Scholar]
- 42.Sieber, S. A., and M. A. Marahiel. 2005. Molecular mechanisms underlying nonribosomal peptide synthesis: approaches to new antibiotics. Chem. Rev. 105715-738. [DOI] [PubMed] [Google Scholar]
- 43.Simmons, T. L., E. Andrianansolo, K. McPhail, P. Flatt, and W. H. Gerwick. 2005. Marine natural products as anticancer drugs. Mol. Cancer Ther. 4333-342. [PubMed] [Google Scholar]
- 44.Stachelhaus, T., H. D. Mootz, and M. A. Marahiel. 1999. The specificity-conferring code of adenylation domains in nonribosomal peptide synthetases. Chem. Biol. 6493-505. [DOI] [PubMed] [Google Scholar]
- 45.Thomas, M. G., Y. A. Chan, and S. G. Ozanick. 2003. Deciphering tuberactinomycin biosynthesis: isolation, sequencing, and annotation of the viomycin biosynthetic gene cluster. Antimicrob. Agents Chemother. 472823-2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Velasco, A., P. Acebo, A. Gomez, C. Schleissner, P. Rodriguez, T. Aparicio, S. Conde, R. Munoz, F. De La Calle, J. L. Garcia, and J. M. Sanchez-Puelles. 2005. Molecular characterization of the safracin biosynthetic pathway from Pseudomonas fluorescens A2-2: designing new cytotoxic compounds. Mol. Microbiol. 56144-154. [DOI] [PubMed] [Google Scholar]
- 47.Wenzel, S. C., and R. Muller. 2005. Formation of novel secondary metabolites by bacterial multimodular assembly lines: deviations from textbook biosynthetic logic. Curr. Opin. Chem. Biol. 9447-458. [DOI] [PubMed] [Google Scholar]
- 48.Xing, C., J. R. Lacob, J. K. Barbay, and A. G. Myers. 2004. Identification of GAPDH as a protein target of the saframycin antiproliferative agents. Proc. Natl. Acad. Sci. USA 1015862-5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.