Abstract
We constructed monocopy lac operon control regions in which the operators O1-lac and O3-lac were replaced by NarL and NarP binding sites from the nirB or napF operon control regions. The results support the hypothesis that DNA-bound dimers of phospho-NarL can participate in higher-order cooperative interactions.
Anaerobic respiratory gene expression in Escherichia coli K-12 is controlled in part by nitrate and nitrite, acting through the Nar regulatory system. The paralogous sensors NarX and NarQ respond to nitrate and nitrite to control phosphorylation of the paralogous response regulators NarL and NarP. Phosphorylation increases the affinity of the NarL and NarP proteins for their specific DNA binding sites, from whence they activate and repress target operon expression (for a review, see reference 39).
Most phospho-NarL and -NarP binding sites consist of inverted heptamer sequences (consensus sequence, TACYYMT, where Y = C or T and M = A or C) separated by 2 nucleotides (nt) (11, 24). Binding sites with this geometry are termed 7-2-7 heptamer pairs. Specificity determinants that discriminate between binding by one regulator or the other have not yet been defined, although most sites characterized to date appear to have greater affinity for the phospho-NarL protein (11, 37).
The control regions for many nitrate-regulated operons contain heptamer sequences in addition to an essential 7-2-7 heptamer pair (for a review, see reference 39). These extra heptamers are critical for NarL-dependent regulation of nrfA and fdnG operon expression (10, 11, 19, 20, 42). Evidence suggests that a dimer of the phospho-NarL protein bound to the 7-2-7 heptamer pair has cooperative interactions with additional phospho-NarL molecules, thereby promoting their binding to the extra heptamer sequences. By contrast, phospho-NarP dimers apparently interact weakly if at all and thus are restricted to binding single 7-2-7 heptamer pairs (10, 11).
Transcription of the lacZYA operon for lactose catabolism is repressed by the LacI protein. The primary operator (O1-lac) for LacI repressor binding consists of inverted half-sites centered at position 11 with respect to the transcription initiation site (Fig. 1). At least one of the two auxiliary operators is required for maximal repression (32). LacI repressor binds cooperatively to the primary operator and to one of the auxiliary operators, thereby increasing its local concentration (for a review, see reference 29).
FIG. 1.
Control region sequences. The modified lac operon control region sequence from O3 through O1 is shown at the top. The complete sequence for the lac operon control region used has been described previously (37). Sequences of O1 and O3 substitutions are indicated below the corresponding lac operators. The deletion and insertions were employed to make the spacing variants used for the experiments whose results are shown in Fig. 2 and Tables 2 to 4. The deletion is indicated by a vertical line, whereas inserted sequences are enclosed in boxes. The transcription initiation site is labeled +1. The arrowheads indicate centers of protein-binding half-sites.
Previously, we described construction of synthetic lac operon control regions in which the primary operator O1 is replaced by 7-2-7 heptamer pairs from different Nar-regulated operons (37). Transcription from these constructs is repressed by the phospho-NarL and -NarP proteins in response to added nitrate. Here, we describe additional constructs in which the auxiliary operator O3 was also replaced by different 7-2-7 heptamer pairs. In narL+ strains, these double-operator-substituted constructs exhibited increased repression, supporting the hypothesis that two dimers of the phospho-NarL protein bind cooperatively to specific DNA sites.
Strains.
Strains are listed in Table 1. Construction of the synthetic lac operon control region has been described previously (37). New versions reported here, with substitutions at operator O3, were constructed by the same methods. All constructs were based on lacZ gene fusion plasmid pVJS3253 (37) and were crossed into bacteriophage λInCh (5).
TABLE 1.
E. coli K-12 strains
| Strain | Genotype | Reference |
|---|---|---|
| VJS632 | F− λ− prototroph | 38 |
| VJS676 | As VJS632 but Δ(argF-lacIZYA)U169 | 38 |
| VJS8364 | As VJS632 but ΔlacZ | 21 |
| Derivatives of strain VJS676 | ||
| VJS7449 | λ− Δ(attλ-lom)::bla [O3-lac O1-napF lacZ+] | 37 |
| VJS7475 | λ− Δ(attλ-lom)::bla [O3-lac O1-napF lacZ+]/narL215::Tn10 | 37 |
| VJS7476 | λ− Δ(attλ-lom)::bla [O3-lac O1-napF lacZ+]/narP253::Tn10d(Cm) | 37 |
| VJS7446 | λ− Δ(attλ-lom)::bla [O3-nirB O1-napF lacZ+] | This study |
| VJS7473 | λ− Δ(attλ-lom)::bla [O3-nirB O1-napF lacZ+]/narL215::Tn10 | This study |
| VJS7474 | λ− Δ(attλ-lom)::bla [O3-nirB O1-napF lacZ+]/narP253::Tn10d(Cm) | This study |
| VJS7902 | λ− Δ(attλ-lom)::bla [O3-nirB O1-napF (95 nt) lacZ+] | This study |
| VJS7922 | λ− Δ(attλ-lom)::bla [O3-nirB O1-napF (102 nt) lacZ+] | This study |
| VJS7923 | λ− Δ(attλ-lom)::bla [O3-nirB O1-napF (106 nt) lacZ+] | This study |
| VJS7929 | λ− Δ(attλ-lom)::bla [O3-nirB O1-napF (110 nt) lacZ+] | This study |
| VJS8206 | λ− Δ(attλ-lom)::bla [O3-nirB O1-napF (95 nt) lacZ+]/narL215::Tn10 | This study |
| VJS8207 | λ− Δ(attλ-lom)::bla [O3-nirB O1-napF (95 nt) lacZ+]/narP253::Tn10d(Cm) | This study |
| Derivatives of strain VJS8364 | ||
| VJS8880 | λ− Δ(attλ-lom)::bla [O3-lac O1-napF lacZ+] | This study |
| VJS8881 | λ− Δ(attλ-lom)::bla [O3-lac O1-napF lacZ+]/ΔnarL261 | This study |
| VJS8882 | λ− Δ(attλ-lom)::bla [O3-lac O1-napF lacZ+]/ΔnarP262 | This study |
| VJS8883 | λ− Δ(attλ-lom)::bla [O3-napF O1-napF lacZ+] | This study |
| VJS8884 | λ− Δ(attλ-lom)::bla [O3-napF O1-napF lacZ+]/ΔnarL261 | This study |
| VJS8885 | λ− Δ(attλ-lom)::bla [O3-napF O1-napF lacZ+]/ΔnarP262 | This study |
| VJS8886 | λ− Δ(attλ-lom)::bla [O3-lac O1-lac lacZ+] | This study |
| VJS8899 | λ− Δ(attλ-lom)::bla [O3-napF O1-napF (87 nt) lacZ+] | This study |
| VJS9000 | λ− Δ(attλ-lom)::bla [O3-napF O1-napF (87 nt) lacZ+]/ΔnarP262 | This study |
| VJS9001 | λ− Δ(attλ-lom)::bla [O3-napF O1-napF (87 nt) lacZ+]/ΔnarL261 | This study |
| VJS9002 | λ− Δ(attλ-lom)::bla [O3-napF O1-lac (87 nt) lacZ+] | This study |
| VJS9003 | λ− Δ(attλ-lom)::bla [O3-napF O1-lac lacZ+] | This study |
| VJS9004 | λ− Δ(attλ-lom)::bla [O3-lac O1-napF lacZ+]/ΔnarL261 ΔnarP262 | This study |
| VJS9005 | λ− Δ(attλ-lom)::bla [O3-napF O1-napF lacZ+]/ΔnarL261 ΔnarP262 | This study |
| VJS9006 | λ− Δ(attλ-lom)::bla [O3-napF O1-napF (87 nt) lacZ+]/ΔnarL261 ΔnarP262 | This study |
Genetic crosses were performed by bacteriophage P1kc-mediated generalized transduction (26). Null alleles of nar regulatory genes (Table 1) have been described previously (21, 33). Standard methods were used for restriction endonuclease digestion, ligation, transformation, and PCR amplification of DNA (22).
Culture media and conditions.
Defined, complex, and indicator media for genetic manipulations were used as described previously (22). The defined medium used to grow cultures for enzyme assays was buffered with 3-(N-morpholino)propanesulfonic acid (MOPS) as described previously (38). The medium used for overnight cultures arrested in the mid-exponential phase (13) contained glucose (6 mM) or glucose plus NaNO3 (4 and 10 mM, respectively) (37). The concentrations were determined empirically to support growth to the mid-exponential phase (about 35 to 40 Klett units). Isopropyl-β-d-thiogalactoside (IPTG) was added as indicated to inactivate the LacI repressor in cultures of O1-lac derivatives.
Cultures were grown at 37°C. Culture densities were monitored with a Klett-Summerson photoelectric colorimeter (Klett Manufacturing Co., New York, NY) equipped with a number 66 (red) filter. Anaerobic cultures used for enzyme assays were grown in screw-cap tubes as described previously (38).
Enzyme assay.
β-Galactosidase activities were determined at room temperature (approximately 21°C) by monitoring the hydrolysis of o-nitrophenyl-β-d-galactoside in CHCl3-sodium dodecyl sulfate-permeabilized cells. Specific activities are expressed in arbitrary units (26). All cultures were assayed in duplicate, and the reported values are averages from at least two independent experiments.
Nar-dependent repression of lacZ gene expression from O1 substitution control regions.
Previously, we used site-specific mutagenesis to replace the primary operator O1-lac with 7-2-7 heptamer pairs from different Nar-responsive control regions (37). One of the constructs, the O1-nirB construct, exhibits about 100-fold repression of lacZ gene expression in response to nitrate, whereas the O1-napF construct exhibits only about 10-fold repression. However, the O1-nirB construct displays only about fivefold repression in a narP+ narL null strain, indicating that the phospho-NarP protein binds relatively poorly at O1-nirB. By contrast, the O1-napF construct displays similar repression in both narL+ narP null and narP+ narL null strains, indicating that the phospho-NarL and -NarP proteins bind equally well at O1-napF (37).
For the experiments described below, we used constructs with the O1-napF substitution so that (i) enhanced repression could be more readily detected and (ii) relative levels of repression by the phospho-NarL and -NarP proteins could be compared directly.
Enhanced repression by the O3-nirB substitution.
The LacI repressor consists of two DNA-binding dimers assembled as a homotetramer (for reviews, see references 7, 18, 29, and 47). Full repression of lac operon expression requires not only the high-affinity primary operator O1-lac but also at least one of two lower-affinity auxiliary operators, O2-lac (within the lacZ gene) or O3-lac (32). Occupancy of the operator O1-lac by one dimer is increased through cooperative binding of the other dimer to an auxiliary operator (6, 32, 34), resulting in a more stable repressor complex (14, 17, 46). This cooperative binding accounts for the nonlinear response of lacZYA operon transcription to an inducer (30).
Mutant LacI repressors that form essentially normal dimers but fail to assemble into tetramers have been described (1, 2, 6, 8, 32). Mutant dimeric LacI proteins and wild-type LacI protein both repress transcription from O1-lac to about the same extent. However, repression by a dimeric repressor is not enhanced by the presence of an auxiliary operator (27, 32), because the repressor does not bind cooperatively to two operators (6, 8, 23, 32). Thus, specific interaction between two dimers, each bound at a separate operator, enhances the overall stability of the repression complex. Analogous observations have been made with other repressors (9, 35), including the GalR repressor (15, 23, 36).
Therefore, we reasoned that the lac operon control region might provide a means to study specific interactions between phospho-NarL dimers (23). Accordingly, we used site-specific mutagenesis to replace the auxiliary operator O3-lac (positions −92 through −72 [Fig. 1]) with 7-2-7 heptamer pairs from the nirB or napF control regions. These operators were placed in the O1-napF, O1-nirB, O1-fdnG, and O1-lac constructs (Fig. 1), forming various combinations of O3- and O1-substituted lac control regions.
In a narL+ narP+ background, the O3-lac O1-napF construct exhibited about 10-fold repression of lacZ gene expression in response to nitrate, whereas the O3-nirB construct exhibited about 30-fold repression (Table 2). Similar results were observed with the congruent O1-nirB and O1-fdnG constructs (data not shown). Thus, placing a Nar 7-2-7 heptamer pair in operator O3 resulted in a modest enhancement of nitrate repression.
TABLE 2.
Interoperator spacing influences O3-nirB enhancement of NarL-mediated repression at O1-napF
| Strain | Construct | Spacinga | Genotype
|
LacZ sp act (arbitrary units)b
|
Repression (fold) | ||
|---|---|---|---|---|---|---|---|
| narL | narP | No NO3− | NO3− | ||||
| VJS7449 | O3-lac O1-napF | 91 | + | + | 850 | 66 | 13 |
| VJS7476 | O3-lac O1-napF | 91 | + | − | 850 | 71 | 12 |
| VJS7475 | O3-lac O1-napF | 91 | − | + | 920 | 100 | 9.2 |
| VJS7446 | O3-nirB O1-napF | 91 | + | + | 790 | 24 | 33 |
| VJS7474 | O3-nirB O1-napF | 91 | + | − | 970 | 20 | 49 |
| VJS7473 | O3-nirB O1-napF | 91 | − | + | 950 | 100 | 10 |
| VJS7902 | O3-nirB O1-napF | 95 | + | + | 800 | 4 | 200 |
| VJS8207 | O3-nirB O1-napF | 95 | + | − | 830 | 4 | 208 |
| VJS8206 | O3-nirB O1-napF | 95 | − | + | 820 | 70 | 12 |
Interoperator spacing (see Fig. 1).
Strains were cultured overnight in MOPS defined medium with limiting glucose.
We next introduced narL or narP null alleles into the strains. The O3-nirB-dependent enhanced repression of lacZ gene expression was eliminated in the narP+ narL null strain but increased to nearly 50-fold in the narL+ narP null strain (Table 2). Thus, the O3-nirB-dependent enhanced repression of lacZ gene expression required the narL+ gene and appeared to be slightly inhibited by the narP+ gene.
Interoperator spacing affects enhanced repression by the O3-nirB substitution.
Spacing between the O3-lac and O1-lac operators is critical for effective repression, because the repressor-operator interaction depends on the helical phase of the two operators in order to allow formation of the intervening DNA loop (14, 17, 28, 29, 31). Loop formation (and therefore cooperative repressor binding) is also influenced by factors such as DNA superhelicity (12, 16, 34, 40, 44, 45) and host proteins (3, 27).
The wild-type interoperator spacing between operators O1-lac and O3-lac is nearly optimal for cooperative LacI repression (28). (Our constructs have a slightly shorter interoperator space, 91 versus 92.5 nt [Fig. 1].) We wished to determine the optimal spacing for cooperative phospho-NarL repression. We therefore varied the spacing between the O3-nirB and O1-napF operators over the range from 95 to 110 nt (Fig. 1). The variant with 95-nt spacing was constructed by filling in the 4-nt BglII overhang with Klenow polymerase, and the remaining variants were constructed by consecutive rounds of site-specific mutagenesis.
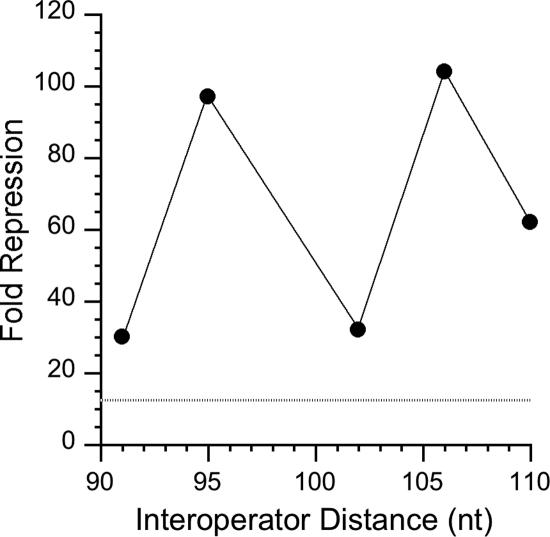
The interoperator spacing strongly affected O3-nirB-dependent enhanced repression of lacZ gene expression (Fig. 2). For the O3-nirB O1-napF constructs with 91-nt (native) and 102-nt spacing, nitrate repression of lacZ gene expression was about 30-fold (Table 2), whereas for the constructs with 95- and 106-nt spacing, nitrate repression of lacZ gene expression was about 100-fold (Fig. 2). The level of nitrate repression of lacZ gene expression for the construct with 110-nt spacing was intermediate, about 60-fold. These results, based on the limited number of spacing variants examined (28), are consistent with the notion that the helical phase influences the magnitude of the O3-nirB-dependent enhanced nitrate repression of lacZ gene expression (for a review, see reference 25).
FIG. 2.
Interoperator spacing influences O3-nirB enhancement of repression at O1-napF. The fold repression of lacZ gene expression by nitrate is plotted against the distance between the centers of operator elements O1-napF and O3-nirB. The dashed line indicates the ≤15-fold repression observed for the O3-lacZ O1-napF construct (see Table 2). Strains carry both narL+ and narP+ alleles.
The experiments described above indicated that the native O3-O1 spacing is the least optimal spacing for monitoring O3-dependent enhanced repression of lacZ gene expression. We therefore examined the influence of narL and narP null alleles on enhanced nitrate repression of lacZ gene expression in the O3-nirB O1-napF construct with 95-nt spacing. In this experiment, the nitrate repression of lacZ expression was more than 200-fold in the narL+ narP null strain, whereas the repression was only 12-fold in the narP+ narL null strain (Table 2).
Enhanced repression by the O3-napF substitution.
For the experiments described above, we used the nirB 7-2-7 heptamer pair substituted at operator O3. Our intention was to use a site that binds phospho-NarL strongly, in an attempt to maximize repression resulting from cooperative interactions between operators O1 and O3 (28, 31). Indeed, with nearly optimal interoperator spacing, the O3-nirB sequence increased NarL-dependent repression by roughly 10-fold (Fig. 2 and Table 2). As noted above, however, the O1-nirB operator substitution yields feeble NarP-dependent repression, suggesting that it bound phospho-NarP weakly. Thus, the O3-nirB substitution constructs did not permit conclusions regarding cooperative binding by the phospho-NarP protein.
To examine this point, we made constructs with O3-napF operator substitutions (Table 3). In narL+ narP null strains, the O3-napF substitution resulted in repression that was threefold greater than that observed in O3-lac strains. The enhancement was even more pronounced (more than fivefold) with a construct in which the interoperator spacing was decreased to 87 nt (constructed by deleting the GATC overhang resulting from BglII digestion). By contrast, repression in the narP+ narL null strains was increased less than twofold in the presence of the O3-napF operator substitution (Table 3). This indicates that the phospho-NarP protein has only weak cooperative interactions that result in enhanced O3-dependent repression.
TABLE 3.
Interoperator spacing influences O3-napF enhancement of NarL-mediated repression at O1-napF
| Strain | Construct | Spacinga | Genotype
|
LacZ sp act (arbitrary units)b
|
Repression (fold) | ||
|---|---|---|---|---|---|---|---|
| narL | narP | No NO3− | NO3− | ||||
| VJS8880 | O3-lac O1-napF | 91 | + | + | 1,190 | 100 | 12 |
| VJS8882 | O3-lac O1-napF | 91 | + | − | 1,170 | 110 | 11 |
| VJS8881 | O3-lac O1-napF | 91 | − | + | 1,020 | 140 | 7.3 |
| VJS9004 | O3-lac O1-napF | 91 | − | − | 1,010 | 650 | 1.6 |
| VJS8883 | O3-napF O1-napF | 91 | + | + | 1,150 | 55 | 21 |
| VJS8885 | O3-napF O1-napF | 91 | + | − | 1,200 | 35 | 34 |
| VJS8884 | O3-napF O1-napF | 91 | − | + | 1,100 | 130 | 8.5 |
| VJS9005 | O3-napF O1-napF | 91 | − | − | 1,100 | 720 | 1.5 |
| VJS8899 | O3-napF O1-napF | 87 | + | + | 870 | 23 | 38 |
| VJS9001 | O3-napF O1-napF | 87 | + | − | 980 | 17 | 58 |
| VJS9000 | O3-napF O1-napF | 87 | − | + | 1,100 | 100 | 11 |
| VJS9006 | O3-napF O1-napF | 87 | − | − | 970 | 620 | 1.6 |
Interoperator spacing (see Fig. 1).
Strains were cultured overnight in MOPS defined medium with limiting glucose.
The level of repression with the O3-napF operator substitution was lower in narL+ narP+ strains than in narL+ narP null strains but was similar in the O3-lac strain (Table 3). This indicates that the phospho-NarP protein, which competes with the phospho-NarL protein for binding to the napF site, inhibits the enhanced repression resulting from phospho-NarL cooperative interactions. This further suggests that the phospho-NarP protein has weak, if any, cooperative interactions.
Control experiments.
In the lacZYA operon control region, the auxiliary operators cannot mediate repression in the absence of the primary operator, O1-lac (27, 32). To document this for the synthetic lac control regions described here, we made O3-napF O1-lac constructs with 91-nt (native) and 87-nt interoperator spacing. Neither construct exhibited Nar-dependent repression (Table 4). This established that the repression observed with O3 substitution constructs (Tables 2 and 3; Fig. 2) was wholly dependent upon substitution also at operator O1.
TABLE 4.
Neither O3-napF nor interoperator spacing influences expression from O1-lac
| Strain | Construct | Spacinga | Genotype
|
LacZ sp act (arbitrary units)b
|
Repression (fold) | ||
|---|---|---|---|---|---|---|---|
| narL | narP | No NO3− | NO3− | ||||
| VJS8886 | O3-lac O1-lac | 91 | + | + | 3,290 | 2,050 | 1.6 |
| VJS9003 | O3-napF O1-lac | 91 | + | + | 2,680 | 2,210 | 1.2 |
| VJS9002 | O3-napF O1-lac | 87 | + | + | 2,770 | 2,200 | 1.3 |
Interoperator spacing (see Fig. 1).
Strains were cultured overnight in MOPS defined medium with limiting glucose and 0.1 M IPTG.
Analysis of NarL-NarP chimeras.
The NarL protein dimerizes through its carboxyl-terminal DNA-binding domain (24). Since the phospho-NarL and -NarP proteins differ in the ability to have cooperative interactions, we hypothesized that this is a property of the corresponding amino-terminal receiver domains. As one attempt to examine this, we constructed chimeric NarL-NarP proteins by fusing the receiver domain from one protein to the DNA-binding effector domain of the other protein. We made four chimeras with all possible combinations of receiver-linker-effector arrangements, based on a study of OmpR-PhoB chimeras (43). As observed in other studies of chimeric response regulators (4, 41, 43), transcription control by these NarL-NarP chimeras responded poorly or not at all to inducing signal, compromising analysis of enhanced repression at O3 substitution constructs. Although the results (data not shown) were consistent with the notion that the NarL receiver domain mediates cooperative interaction, the overall magnitude of the response was weak.
Concluding remarks.
This study exploited well-documented observations that cooperative interactions between LacI dimers, mediated by formation of a DNA loop, can enhance repression of lacZYA operon transcription by about 50-fold (32). We were curious to determine whether the lac control region could provide an artificial means to examine cooperative interactions between DNA-binding proteins unrelated to the LacI repressor. Previous studies revealed cooperative binding by phospho-NarL dimers to immediately adjacent sites in the fdnG and nrfA operon control regions (10, 11, 19, 20, 42). Accordingly, binding to the O1 and O3 operator substitutions, spaced approximately 90 nt apart, represents a nonnative context for the Nar regulators. On the other hand, the Nar system allows internal comparison of otherwise similar proteins that differ in the ability to form cooperative interactions.
The operator substitution O3-napF enhanced NarL-dependent repression from an O1-napF synthetic lac control region up to fivefold, whereas NarP-dependent repression was virtually unaltered (Table 3). This result provides independent support for the hypothesis that phospho-NarL dimers have cooperative interactions in binding to specific DNA sites, whereas the phospho-NarP protein has poor or no cooperative interactions. Additional analysis with the operator substitution O3-nirB revealed NarL-dependent repression that was enhanced up to 15-fold (Table 2) and was dependent on the spacing between the two operators (Fig. 2). Thus, the results mimic those obtained for the native lac system and suggest that the lac control region may provide a general assay for analyzing cooperative protein-DNA interactions.
Acknowledgments
This study was supported by Public Health Service grant GM36877 from the National Institute of General Medical Sciences.
Footnotes
Published ahead of print on 26 October 2007.
REFERENCES
- 1.Alberti, S., S. Oehler, B. von Wilcken-Bergmann, H. Krämer, and B. Müller-Hill. 1991. Dimer-to-tetramer assembly of Lac repressor involves a leucine heptad repeat. New Biol. 357-62. [PubMed] [Google Scholar]
- 2.Alberti, S., S. Oehler, B. von Wilcken-Bergmann, and B. Müller-Hill. 1993. Genetic analysis of the leucine heptad repeats of Lac repressor: evidence for a 4-helical bundle. EMBO J. 123227-3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker, N. A., J. D. Kahn, and L. J. Maher III. 2005. Bacterial repression loops require enhanced DNA flexibility. J. Mol. Biol. 349716-730. [DOI] [PubMed] [Google Scholar]
- 4.Bock, A., M. Bantscheff, A. L. Perraud, K. Rippe, V. Weiss, M. O. Glocker, and R. Gross. 2001. Rational design and molecular characterization of a chimaeric response regulator protein. J. Mol. Biol. 310283-290. [DOI] [PubMed] [Google Scholar]
- 5.Boyd, D., D. S. Weiss, J. C. Chen, and J. Beckwith. 2000. Towards single-copy gene expression systems making gene cloning physiologically relevant: lambda InCh, a simple Escherichia coli plasmid-chromosome shuttle system. J. Bacteriol. 182842-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenowitz, M., N. Mandal, A. Pickar, E. Jamison, and S. Adhya. 1991. DNA-binding properties of a lac repressor mutant incapable of forming tetramers. J. Biol. Chem. 2661281-1288. [PubMed] [Google Scholar]
- 7.Chakerian, A. E., and K. S. Matthews. 1992. Effect of lac repressor oligomerization on regulatory outcome. Mol. Microbiol. 6963-968. [DOI] [PubMed] [Google Scholar]
- 8.Chakerian, A. E., V. M. Tesmer, S. P. Manly, J. K. Brackett, M. J. Lynch, J. T. Hoh, and K. S. Matthews. 1991. Evidence for leucine zipper motif in lactose repressor protein. J. Biol. Chem. 2661371-1374. [PubMed] [Google Scholar]
- 9.Court, D. L., A. B. Oppenheim, and S. L. Adhya. 2007. A new look at bacteriophage lambda genetic networks. J. Bacteriol. 189298-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darwin, A. J., J. Li, and V. Stewart. 1996. Analysis of nitrate regulatory protein NarL-binding sites in the fdnG and narG operon control regions of Escherichia coli K-12. Mol. Microbiol. 20621-632. [DOI] [PubMed] [Google Scholar]
- 11.Darwin, A. J., K. L. Tyson, S. J. Busby, and V. Stewart. 1997. Differential regulation by the homologous response regulators NarL and NarP of Escherichia coli K-12 depends on DNA binding site arrangement. Mol. Microbiol. 25583-595. [DOI] [PubMed] [Google Scholar]
- 12.Eismann, E. R., and B. Müller-Hill. 1990. lac repressor forms stable loops in vitro with supercoiled wild-type lac DNA containing all three natural lac operators. J. Mol. Biol. 213763-775. [DOI] [PubMed] [Google Scholar]
- 13.Haldimann, A., L. L. Daniels, and B. L. Wanner. 1998. Use of new methods for construction of tightly regulated arabinose and rhamnose promoter fusions in studies of the Escherichia coli phosphate regulon. J. Bacteriol. 1801277-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsieh, W. T., P. A. Whitson, K. S. Matthews, and R. D. Wells. 1987. Influence of sequence and distance between two operators on interaction with the lac repressor. J. Biol. Chem. 26214583-14591. [PubMed] [Google Scholar]
- 15.Irani, M. H., L. Orosz, and S. Adhya. 1983. A control element within a structural gene: the gal operon of Escherichia coli. Cell 32783-788. [DOI] [PubMed] [Google Scholar]
- 16.Krämer, H., M. Amouyal, A. Nordheim, and B. Müller-Hill. 1988. DNA supercoiling changes the spacing requirement of two lac operators for DNA loop formation with lac repressor. EMBO J. 7547-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krämer, H., M. Niemoller, M. Amouyal, B. Revet, B. von Wilcken-Bergmann, and B. Müller-Hill. 1987. lac repressor forms loops with linear DNA carrying two suitably spaced lac operators. EMBO J. 61481-1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis, M. 2005. The lac repressor. C. R. Biol. 328521-548. [DOI] [PubMed] [Google Scholar]
- 19.Li, J., S. Kustu, and V. Stewart. 1994. In vitro interaction of nitrate-responsive regulatory protein NarL with DNA target sequences in the fdnG, narG, narK and frdA operon control regions of Escherichia coli K-12. J. Mol. Biol. 241150-165. [DOI] [PubMed] [Google Scholar]
- 20.Li, J., and V. Stewart. 1992. Localization of upstream sequence elements required for nitrate and anaerobic induction of fdn (formate dehydrogenase-N) operon expression in Escherichia coli K-12. J. Bacteriol. 1744935-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin, H.-Y., P. J. Bledsoe, and V. Stewart. 2007. Activation of yeaR-yoaG operon transcription by nitrate-responsive regulator NarL is independent of oxygen-responsive regulator Fnr in Escherichia coli K-12. J. Bacteriol. 1897539-7548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maloy, S. R., V. J. Stewart, and R. K. Taylor. 1996. Genetic analysis of pathogeneic bacteria. A laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 23.Mandal, N., W. Su, R. Haber, S. Adhya, and H. Echols. 1990. DNA looping in cellular repression of transcription of the galactose operon. Genes Dev. 4410-418. [DOI] [PubMed] [Google Scholar]
- 24.Maris, A. E., M. R. Sawaya, M. Kaczor-Grzeskowiak, M. R. Jarvis, S. M. Bearson, M. L. Kopka, I. Schröder, R. P. Gunsalus, and R. E. Dickerson. 2002. Dimerization allows DNA target site recognition by the NarL response regulator. Nat. Struct. Biol. 9771-778. [DOI] [PubMed] [Google Scholar]
- 25.Matthews, K. S. 1992. DNA looping. Microbiol. Rev. 56123-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 27.Müller, J., A. Barker, S. Oehler, and B. Müller-Hill. 1998. Dimeric lac repressors exhibit phase-dependent co-operativity. J. Mol. Biol. 284851-857. [DOI] [PubMed] [Google Scholar]
- 28.Müller, J., S. Oehler, and B. Müller-Hill. 1996. Repression of lac promoter as a function of distance, phase and quality of an auxiliary lac operator. J. Mol. Biol. 25721-29. [DOI] [PubMed] [Google Scholar]
- 29.Müller-Hill, B. 1998. The function of auxiliary operators. Mol. Microbiol. 2913-18. [DOI] [PubMed] [Google Scholar]
- 30.Oehler, S., S. Alberti, and B. Müller-Hill. 2006. Induction of the lac promoter in the absence of DNA loops and the stoichiometry of induction. Nucleic Acids Res. 34606-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oehler, S., M. Amouyal, P. Kolkhof, B. von Wilcken-Bergmann, and B. Müller-Hill. 1994. Quality and position of the three lac operators of E. coli define efficiency of repression. EMBO J. 133348-3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oehler, S., E. R. Eismann, H. Krämer, and B. Müller-Hill. 1990. The three operators of the lac operon cooperate in repression. EMBO J. 9973-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rabin, R. S., and V. Stewart. 1993. Dual response regulators (NarL and NarP) interact with dual sensors (NarX and NarQ) to control nitrate- and nitrite-regulated gene expression in Escherichia coli K-12. J. Bacteriol. 1753259-3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sasse-Dwight, S., and J. D. Gralla. 1988. Probing co-operative DNA-binding in vivo. The lac O1:O3 interaction. J. Mol. Biol. 202107-119. [DOI] [PubMed] [Google Scholar]
- 35.Schleif, R. 2000. Regulation of the l-arabinose operon of Escherichia coli. Trends Genet. 16559-565. [DOI] [PubMed] [Google Scholar]
- 36.Semsey, S., M. Geanacopoulos, D. E. Lewis, and S. Adhya. 2002. Operator-bound GalR dimers close DNA loops by direct interaction: tetramerization and inducer binding. EMBO J. 214349-4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stewart, V., and P. J. Bledsoe. 2003. Synthetic lac operator substitutions for studying the nitrate- and nitrite-responsive NarX-NarL and NarQ-NarP two-component regulatory systems of Escherichia coli K-12. J. Bacteriol. 1852104-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stewart, V., and J. Parales, Jr. 1988. Identification and expression of genes narL and narX of the nar (nitrate reductase) locus in Escherichia coli K-12. J. Bacteriol. 1701589-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stewart, V., and R. S. Rabin. 1995. Dual sensors and dual response regulators interact to control nitrate- and nitrite-responsive gene expression in Escherichia coli, p. 233-252. In J. A. Hoch and T. J. Silhavy (ed.), Two-component signal transduction. ASM Press, Washington, DC.
- 40.Swigon, D., B. D. Coleman, and W. K. Olson. 2006. Modeling the Lac repressor-operator assembly: the influence of DNA looping on Lac repressor conformation. Proc. Natl. Acad. Sci. USA 1039879-9884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tapparel, C., A. Monod, and W. L. Kelley. 2006. The DNA-binding domain of the Escherichia coli CpxR two-component response regulator is constitutively active and cannot be fully attenuated by fused adjacent heterologous regulatory domains. Microbiology 152431-441. [DOI] [PubMed] [Google Scholar]
- 42.Tyson, K. L., J. A. Cole, and S. J. W. Busby. 1994. Nitrite and nitrate regulation at the promoters of two Escherichia coli operons encoding nitrite reductase: identification of common target heptamers for both NarP- and NarL-dependent regulation. Mol. Microbiol. 131045-1055. [DOI] [PubMed] [Google Scholar]
- 43.Walthers, D., V. K. Tran, and L. J. Kenney. 2003. Interdomain linkers of homologous response regulators determine their mechanism of action. J. Bacteriol. 185317-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Whitson, P. A., W. T. Hsieh, R. D. Wells, and K. S. Matthews. 1987. Supercoiling facilitates lac operator-repressor-pseudooperator interactions. J. Biol. Chem. 2624943-4946. [PubMed] [Google Scholar]
- 45.Whitson, P. A., W. T. Hsieh, R. D. Wells, and K. S. Matthews. 1987. Influence of supercoiling and sequence context on operator DNA binding with lac repressor. J. Biol. Chem. 26214592-14599. [PubMed] [Google Scholar]
- 46.Whitson, P. A., and K. S. Matthews. 1986. Dissociation of the lactose repressor-operator DNA complex: effects of size and sequence context of operator-containing DNA. Biochemistry 253845-3852. [DOI] [PubMed] [Google Scholar]
- 47.Wilson, C. J., H. Zhan, L. Swint-Kruse, and K. S. Matthews. 2007. The lactose repressor system: paradigms for regulation, allosteric behavior and protein folding. Cell. Mol. Life Sci. 643-16. [DOI] [PMC free article] [PubMed] [Google Scholar]