Abstract
The metalloprotease (Mpl) of Listeria monocytogenes is a thermolysin-like protease that mediates the maturation of a broad-range phospholipase C, whose function contributes to the ability of this food-borne bacterial pathogen to survive intracellularly. Mpl is made as a proprotein that undergoes maturation by proteolytic cleavage of a large N-terminal prodomain. In this study, we identified the N terminus of mature Mpl and generated Mpl catalytic mutants to investigate the mechanism of Mpl maturation. We observed that Mpl activity was a prerequisite for maturation, suggesting a mechanism of autocatalysis. Furthermore, using a strain of L. monocytogenes expressing both the wild-type form and a catalytic mutant form of Mpl simultaneously, we determined that in vivo maturation of Mpl occurs exclusively by an intramolecular autocatalysis mechanism.
Listeria monocytogenes is a facultative intracellular bacterial pathogen that multiplies in the cytosol of host cells and uses an actin-based mechanism of motility to spread from cell to cell without exiting the intracellular milieu (26). Upon cell-to-cell spread, bacteria are temporarily located in double-membrane vacuoles from which they must exit to perpetuate the intracellular life cycle. Among the factors contributing to vacuolar lysis is a bacterial phospholipase C (PC-PLC) (24, 28). PC-PLC is made as a proenzyme whose activation requires cleavage of a 24-amino-acid propeptide. The metalloprotease of Listeria (Mpl) contributes to PC-PLC activation (21). Moreover, Mpl-mediated maturation of PC-PLC is regulated in a temporal and spatial manner during the intracellular life cycle of L. monocytogenes (15).
Mpl possesses the HEXXH motif that is characteristic of the Zincins superfamily of metalloproteases. Within this family, Mpl is most closely related to thermolysin, which is the prototype member of the M4 family of metalloproteases (20). Thermolysin is made by Bacillus thermoproteolyticus, and all members of the thermolysin family originate from bacteria. The active site zinc ion of these enzymes is coordinated by a water molecule and three amino acid residues, including the two histidines present within the HEXXH motif and a glutamic acid located 20 residues downstream of this motif (2, 8). In addition, the glutamic acid residue located within the HEXXH motif and a histidine residue located 83 residues downstream of this motif interact with a water molecule at the active site and are required for catalysis (2, 3).
Thermolysin and related metalloproteases are synthesized as preproenzymes. The prodomain, which accounts for ∼40% of the proenzyme, serves as a chaperone and as an inhibitor of catalysis (17, 23, 29). Processing of the prodomain generates the mature active form of the protease. Autocatalysis has been suggested to be the mechanism of maturation of thermolysin-like proteases, as catalytic site mutants fail to generate mature proteases (10, 11, 13, 16). Furthermore, a mechanism of intramolecular autocatalysis was suggested for thermolysin itself, as purified active thermolysin failed to process a catalytic mutant of thermolysin (13).
Similar to other thermolysin-like metalloproteases, Mpl is synthesized as a preproprotein, and the 55-kDa proenzyme is predicted to give rise to a 35-kDa mature enzyme (19). In this study, we used an in vivo approach to investigate the mechanism of Mpl maturation. Our results indicate that Mpl maturation occurs exclusively by an intramolecular autocatalysis mechanism.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
L. monocytogenes strains and relevant genotypes are listed in Table 1. For J774 infections, bacteria were grown overnight at 30°C in brain heart infusion broth. For all other experiments. L. monocytogenes was grown at 37°C in Luria-Bertani broth containing 50 mM morpholinepropanesulfonic acid (MOPS), adjusted to pH 7.3, and supplemented with 0.2% activated charcoal and 10 to 20 mM glucose (LB-MOPS-Glc).
TABLE 1.
L. monocytogenes strains
| Strain | Genotype and relevant features | Reference or source |
|---|---|---|
| 10403S | Wild-type strain; serotype 1/2a | 4 |
| DP-L1935 | ΔplcB in strain 10403S | 24 |
| NF-L943 | PrfA G155S in strain 10403S; the PrfA-dependent genes, including mpl and plcB, are overexpressed | 22 |
| HEL-469 | Δmpl in NF-L943 | This study |
| HEL-583 | mpl(E350Q) in 10403S | This study |
| HEL-587 | mpl(E350Q) in NF-L943 | This study |
| HEL-772 | mpl(His6)in NF-L943 | This study |
| HEL-793 | HEL-772 ΔplcB | This study |
| HEL-798 | mpl(Flag) in NF-L943 | This study |
| HEL-800 | mpl(E350Q-Flag) in NF-L943 | This study |
| HEL-813 | mpl(E350Q-Flag) integrated at the tRNAArg site in HEL-772 | This study |
| HEL-817 | mpl(H437A-His6) integrated at the tRNAArg site in HEL-798 | This study |
Construction of Mpl with a C-terminal His6 tag and a Flag tag.
A His6 tag was added at the C terminus of Mpl. The tag was preceded by a TEV cleavage site (ENLYFQS) flanked by 2- and 8-amino-acid linkers. The sequence of the entire fragment is GAENLYFQSSSGVDLGTHHHHHH. To generate the construct, primer pairs Marq306/Marq307 (Table 2) and Marq308/Marq309 were used to amplify DNA from the 3′ end and downstream region of mpl, respectively, using strain 10403S genomic DNA as the template. The PCR fragments were digested with BglII and ligated. The ligation product was amplified with primer pair Marq306/Marq309, and the resulting 1,152-bp fragment was digested with BamHI and EcoRI and cloned into the temperature-sensitive shuttle vector pKSV7 (5), creating plasmid pAB771. The construct was verified by sequencing and introduced into the genome of strain NF-L943 by allelic exchange as described previously (5), creating strain HEL-772.
TABLE 2.
Oligonucleotide primers and characteristics
| Primer | Sequence (5′→3′)a | Characteristics |
|---|---|---|
| Marq237 | CTGCTGCAGGATTGTTCAAGTGGACG | PstI |
| Marq239 | CATGCGTTAGTTGATGACCAACA | |
| Marq241 | TGTTGGTCATCAACTAACGCATG | |
| Marq242 | GGAGCTCGGCCTTCATTTTCACTAAT | SstI |
| Marq306 | AAGGATCCAGTATTCGGCGGGATTG | BamHI |
| Marq307 | AAAGATCTACGCCAGAAGAGGATTGGAAGTACAGGTTCTCCGCACCGTTAACCCCAACTGCTTCCCAAGC | BglII and Tev site |
| Marq308 | GTAGATCTGGGAACCCACCACCATCATCATCACTGATTAACAAATGTTAGAGAAAAATTAATTCTCCAAGTG | BglII and His6 tag |
| Marq309 | GGGAATTCTGGATGACGACGCTCCACTT | EcoRI |
| Marq319 | GTAGATCTGGATTACAAGGATGACGATGACAAGTGATTAACAAATGTTAGAGAAAAATTAATTCTCCAAGTG | BglII and Flag tag |
| Marq334 | TAGAGCTCAGAAGAATTAACAAATGTAAAAG | SstI |
| Marq335 | ATACTGCAGATCACTTGTCATCGTCATCC | PstI |
| Marq336 | ACGGATCCAGAATTTAGTTCC | BamHI |
| Marq337 | ACCACTATTATAAGCGACTCCGCCCC | |
| Marq338 | GGGGCGGAGTCGCTTATAATAGTGGT | |
| Marq339 | TTCCTGCAGATCAGTGATGATGATGGTGG | PstI |
Restriction sites, the TEV cleavage site, the His6 tag, and the FLAG tag are underlined.
Strain HEL-793 is a derivative of HEL-772 with an internal in-frame deletion of the gene coding for PC-PLC (plcB). The plcB deletion was generated by allelic exchange using plasmid pDP-1888 (24).
A Flag tag (DYKDDDDK) was added at the C terminus of Mpl. The tag was preceded by a TEV cleavage site flanked by 2- and 6-amino-acid linkers. The sequence of the entire fragment is GAENLYFQSSSGVDLDYKDDDDK. To generate the construct, primer pairs Marq306/Marq307 and Marq319/Marq309 were used to amplify DNA from the 3′ end and downstream region of mpl, respectively, using strain 10403S genomic DNA as the template. The PCR fragments were digested with BglII and ligated. The ligation product was amplified with primer pair Marq306/Marq309, and the resulting 1,152-bp fragment was digested with BamHI and EcoRI and cloned into pKSV7, creating plasmid pAB796. The construct was verified by sequencing and introduced into the genome of strain NF-L943 by allelic exchange, creating strain HEL-798.
Construction of mpl mutant strains.
The Mpl E350Q mutation was created by site-directed mutagenesis with overlap extension (SOEing) PCR (7). Briefly, primer pairs Marq237/Marq239 and Marq241/Marq242 were used to amplify DNA from regions upstream and downstream of the mutation site, respectively, using strain 10403S genomic DNA as the template. The two PCR products and primer pair Marq237/Marq242 were used for SOEing PCR. The final DNA fragment was digested with PstI and SstI and cloned into pKSV7, creating plasmid pAB791. The mutation was introduced into strains 10403S and NFL-943 by allelic exchange. Mutant clones were identified by amplifying the mpl gene from genomic DNA by PCR and digesting the DNA fragment with BspHI, as a BspHI restriction site was lost by creating the E350Q mutation. The strains containing the Mpl E350Q mutation were designated HEL-583 (10403S background) and HEL-587 (NF-L943 background). In addition, strain HEL-800 expressing Mpl E350Q-Flag was created by allelic exchange after electroporation of plasmid pAB796 into strain HEL-587.
A strain expressing Mpl-His6 and Mpl E350Q-Flag was created as follows. The entire mpl structural gene with approximately 180 bp of upstream sequence was amplified by PCR with primer pair Marq334/Marq335, using strain HEL-800 genomic DNA as the template. The 1,825-bp DNA fragment was digested with SstI and PstI and cloned into the integration vector pPL2 (12), creating plasmid pAB812. The construct was verified by sequencing and integrated into the genome of strain HEL-772, creating strain HEL-813. Proper integration of pAB812 at the tRNAArg site of HEL-813 was verified by amplifying the DNA at the integration site using primers NC16 and PL95 (12).
A strain expressing Mpl-Flag and Mpl H437A-His6 was created as follows. The Mpl H437A-His6 construct was created by SOEing PCR as described above using HEL-772 genomic DNA as the template and primer pairs Marq336/Marq337 and Marq338/Marq339. The two products were used in a second PCR with primer pair Marq336/Marq339, generating a 815-bp product. A 1,273-bp PCR fragment encompassing the mpl promoter and 5′ end of the structural gene was generated using pAB812 plasmid DNA as the template and primer pair Marq334/Marq239. The 815- and 1,273-bp fragments were digested with BamHI and ligated. The final PCR product was generated using the ligated DNA fragments as the template and primer pair Marq334/Marq339. The 1,825-bp fragment was digested with SstI and PstI and cloned into pPL2, creating plasmid pAB816. The clone was verified by sequencing and integrated into HEL-798, creating strain HEL-817. Integration of pAB816 at the tRNAArg site of HEL-798 was verified as described above.
An mpl deletion (Δmpl) in strain NF-L943 was generated using plasmid DP-2264. DP-2264 is a derivative of pKSV7 containing the mpl gene with an internal in-frame deletion of 684 bp, encompassing approximately one-half of the structural mpl gene. DP-2264 was originally constructed to create strain DP-L2296, a 10403S Δmpl strain (14). The mpl deletion was integrated into the genome of strain NF-L943 by allelic exchange, creating strain HEL-469.
Protein purification.
Mpl-His6 was purified from strain HEL-793. Bacteria were grown in 300 ml of LB-MOPS-Glc to an optical density at 600 nm (OD600) of ∼1.0. The culture was cooled on ice, and everything was kept cold from this point on. The supernatant was filtered, and proteins were precipitated overnight with ammonium sulfate (70% saturation). The precipitate was dissolved in 6 ml of sterile water, and the solution was desalted using a 10DG buffer exchange column (Bio-Rad) preequilibrated in binding buffer (50 mM sodium phosphate, 300 mM sodium chloride; pH 7.7). The protein sample was reacted overnight with 0.5 ml of BD Talon metal affinity resin. The resin was washed with 50 ml of binding buffer, and Mpl was eluted with 50 mM sodium phosphate-300 mM sodium chloride-150 mM imidazole (pH 7.0). The eluate was precipitated with 5% trichloroacetic acid, and the precipitate was washed with acetone before it was dissolved in sample buffer. Purified Mpl was resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to an Immobilon PSQ membrane (Millipore). The membrane was stained with amido black to visualize the blotted proteins, and Edman sequence analysis of the N terminus of the 42-kDa Mpl species was performed by the proteomics facility of the Wistar Institute (Philadelphia, PA).
Phospholipase activity assay.
PC-PLC activity was detected as previously described (30), using LB-egg yolk agar.
Protein preparation for Western blotting.
Bacteria were grown in LB-MOPS-Glc to an OD600 of ∼1.0. Cultures were cooled on ice and centrifuged, and supernatants were decanted. Cell wall fractions were prepared by treating bacteria with purified Listeria-specific phage endolysin A118 as previously described (25). Secreted proteins were precipitated on ice for 1 h with 5% trichloroacetic acid, and the precipitates were washed with acetone before they were dissolved in sample buffer. The equivalent of 1 ml of a culture having an OD600 of ∼1.0 was loaded in each lane for detection of Mpl, Mpl-His6, Mpl-Flag, and Mpl E350Q-Flag. For pPL2 integrants (strains HEL-813 and HEL-817), the equivalent of 8 ml of a culture having an OD600 of ∼1.0 was used for detection of Mpl E350Q-Flag and Mpl H437A-His6, as less protein was generated from these constructs.
Western blotting.
Western immunoblot assays were performed as described previously (25). Rabbit polyclonal antibody to Mpl was used at a dilution of 1/500. Anti-His6 (Clontech) and anti-Flag M2 (Sigma) mouse monoclonal antibodies were used at dilutions of 1/10,000 and 1/5,000, respectively.
Immunoprecipitation.
The mouse macrophage-like cell line J774 was used for metabolic labeling of intracellular bacteria, and the experiment was performed as described previously (30). Briefly, J774 cells infected with L. monocytogenes were labeled with [35S]methionine and chased in nigericin-containing buffer at pH 7.3 or 6.5. Infected cells were lysed, and bacteria were pelleted. Bacteria were treated with a cell wall hydrolase. PC-PLC was immunoprecipitated from the host cell lysate (secreted fraction) and from the bacterial lysate (bacterium-associated fraction). Immunoprecipitates were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and detected by autoradiography.
RESULTS AND DISCUSSION
Determination of the N terminus of mature Mpl.
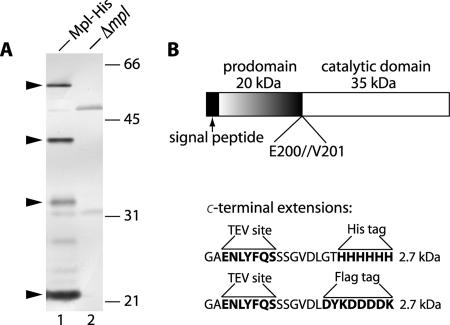
Mpl is synthesized as a 510-amino-acid preproprotein with a predicted signal sequence consisting of 24 residues. On the basis of amino acid sequence comparison, it was estimated that maturation of the protease would result in cleavage between Glu-200 and Val-201, generating 20- and 35-kDa species (6). Detection of secreted Mpl-His6 by Western immunoblotting using a rabbit anti-Mpl antibody revealed the presence of four major reacting bands at approximately 58, 42, 33, and 22 kDa (Fig. 1A). However, we detected only the 58-, 42-, and 33-kDa species with a mouse anti-His6 monoclonal antibody, suggesting that the 22-kDa species is the prodomain, as the His6 tag is connected to the C terminus of Mpl (data not shown). Although the sizes of the 58- and 22-kDa species closely matched the predicted sizes of the proprotein and prodomain, respectively, it was not clear which one of the other two species corresponded to the mature protease. To elucidate this, we purified Mpl-His6 and sequenced the N terminus of the 42-kDa band. The sequence obtained was VERADTH, corresponding to amino acids 201 to 208 of Mpl, indicating that maturation of Mpl occurs between Glu-200 and Val-201 (Fig. 1B). This result is consistent with the predicted maturation site identified by comparing the amino acid sequences of Mpl and other proteases belonging to the themolysin-like family (6). This result is also consistent with the general concept that thermolysin-like proteases prefer hydrophobic or bulky P1′ residues.
FIG. 1.
(A) Detection of Mpl by Western immunoblotting. L. monocytogenes strain HEL-772 expressing Mpl-His6 and HEL-469, an isogenic strain with a deletion in the mpl gene, were grown in LB-MOPS-Glc. Mpl secreted into the bacterial supernatant was detected using a rabbit anti-Mpl antibody. The positions of Mpl-specific bands at approximately 58, 42, 33, and 22 kDa are indicated on the left by arrowheads. The positions of molecular mass standards (in kDa) are indicated on the right. (B) Schematic diagram of Mpl preproprotein with the calculated molecular masses of the prodomain and catalytic domain and amino acid residues flanking the cleavage site, as determined by Edman N-terminal sequencing of the mature form. Also shown are the amino acid sequences and molecular masses of the C-terminal His6 tag extension present in strains HEL-772, HEL-793, and HEL-817 and the C-terminal Flag tag extension present in strains HEL-798, HEL-800, and HEL-813.
The 33-kDa species detected by Western immunoblotting (Fig. 1A) reacted with the His tag antibody (date not shown), indicating that it is a C-terminal fragment of Mpl. It is very unlikely that this truncated form of Mpl has activity, as it would be missing nearly 20% of the mature enzyme. However, we have not experimentally eliminated this possibility.
Mpl maturation occurs by intramolecular autocatalysis.
We considered four potential mechanisms of Mpl maturation. (i) There could be an Mpl-activating protease; (ii and iii) Mpl could be activated by intramolecular and/or intermolecular autocatalysis; and (iv) the proform of Mpl could have sufficient activity to activate an Mpl-dependent Mpl-activating protease.
To assess the possibility that Mpl maturation is dependent on an Mpl-activating protease, we created a catalytically inactive form of Mpl by mutating residue E350 in the HEXXH motif. The mutation was inserted into the genome of L. monocytogenes by allelic exchange. The phenotype of L. monocytogenes Mpl E350Q was identical to that of an isogenic mpl deletion mutant (30). L. monocytogenes Mpl E350Q was negative for PC-PLC activity on egg yolk agar (Fig. 2A). Mpl-dependent maturation of PC-PLC was not observed in cells infected with the L. monocytogenes strain expressing Mpl E350Q (Fig. 2B). By using Western immunoblotting, the Mpl E350Q proprotein and a 33-kDa species identified as a C-terminal fragment of Mpl were detected, but the mature protein and the prodomain were undetectable (Fig. 2C). These results indicated that Mpl E350Q is inactive and that Mpl catalytic activity is required to generate the mature form of Mpl. These results also indicated that the smaller C-terminal fragment of Mpl is not a degradation product of mature Mpl and that this product is generated by another protease.
FIG. 2.
Mpl proteolytic activity is required for PC-PLC and Mpl maturation. (A) Detection of PC-PLC activity on LB-egg yolk agar. The agar was inoculated with strain NF-L943 expressing Mpl, strain HEL-587 expressing Mpl E350Q, and strain HEL-469, a Δmpl mutant. PC-PLC hydrolysis of egg yolk phospholipids generated a zone of opacity around the bacterial colony, which was seen only with the strain expressing wild-type Mpl. (B) Detection of bacterium-associated and secreted PC-PLC from infected J774 cells. J774 cells were infected with L. monocytogenes strain 10403S (wild type), HEL-583 (Mpl E350Q), or DP-L1935 (ΔplcB [plcB is the gene coding for PC-PLC]). Infected cells were pulse-labeled with [35S]methionine and chased in buffer adjusted to pH 7.3 or 6.5 supplemented with nigericin. Infected cells were lysed, and bacteria were pelleted. Bacteria were treated with a cell wall hydrolase and lysed. Bacterium-associated PC-PLC (lanes B) was immunoprecipitated from the bacterial lysates, whereas secreted PC-PLC (lanes S) was immunoprecipitated from the host cell lysates. PC-PLC was detected by autoradiography. The mature form of PC-PLC migrated faster than the proprotein because it was missing the 24-amino-acid propeptide. Maturation and secretion of PC-PLC are dependent on a decrease in pH and on Mpl activity. (C) Detection of Mpl by Western immunoblotting. L. monocytogenes strain HEL-798 expressing Mpl-Flag, strain HEL-800 expressing Mpl E350Q-Flag, and strain HEL-469 harboring a deletion in the mpl gene were grown in LB-MOPS-Glc. Mpl secreted in the bacterial supernatant was detected using a rabbit anti-Mpl antibody. The positions of the proform, mature form, and prodomain of Mpl are indicated on the left. The positions of molecular mass standards (in kDa) are indicated on the right. The mature form and the prodomain are missing in Mpl E350Q.
Next, we tested the ability of Mpl to process Mpl E350Q directly (by intermolecular autocatalysis) or indirectly (by activating an Mpl-dependent Mpl-activating protease). There is evidence that Mpl-mediated activation of PC-PLC occurs at the membrane cell wall interface before translocation of PC-PLC across the bacterial cell wall (30), indicating that Mpl maturation occurs before translocation across the cell wall. Although the mature form of Mpl is found predominantly in the bacterial supernatant, there is no evidence that it is active in this environment. In fact, when an Mpl− strain of L. monocytogenes was cultured with a PC-PLC− strain, PC-PLC activity was not detected even though the proform of PC-PLC and the mature form of Mpl were both secreted (data not shown). Therefore, it appears that the optimal conditions for Mpl activity are encountered before protein translocation across the bacterial cell wall. To favor an encounter of Mpl and Mpl E350Q in the appropriate compartment and under the optimal conditions for Mpl activity, we expressed Mpl-His6 and Mpl E350Q-Flag in the same strain of L. monocytogenes. Both forms of Mpl were detected by Western immunoblotting using antibodies specific for the corresponding tags. Under these conditions, we observed the proform and mature form of Mpl, but we did not observe maturation of Mpl E350Q (Fig. 3). However, under the same conditions we observed that Mpl mediates PC-PLC maturation (data not shown). This result confirmed that Mpl E350Q is not a substrate for Mpl or for a putative Mpl-dependent Mpl-activating protease.
FIG. 3.
Mpl maturation occurs by intramolecular autocatalysis. L. monocytogenes strains expressing Mpl E350Q-Flag (HEL-800), Mpl-His6 (HEL-772), Mpl-His6 and Mpl E350Q-Flag (HEL-813), Mpl E350Q (HEL-587), or Mpl-Flag and Mpl H437A-His6 (HEL-817) were grown in LB-MOPS-Glc. Sets of samples were loaded in duplicate on protein gels. Mpl secreted in the bacterial supernatant was detected by Western immunoblotting using an anti-His6 monoclonal antibody for one half of the blot and the anti-Flag M2 monoclonal antibody for the other half of the blot. The positions of the proform and mature form of Mpl are indicated on the left. The positions of the molecular mass standards (in kDa) are indicated on the right. Mpl did not mediate maturation of Mpl E350Q or Mpl H437A.
A different Mpl catalytic mutant was generated to verify that the results obtained with Mpl E350Q-Flag were not a consequence of an intrinsic feature of this particular construct. The new catalytic mutant was generated by mutating H437, an active site histidine involved in stabilizing the transition state during catalysis (3). We also inverted the tags to eliminate the possibility that either tag interfered with substrate binding or catalysis. In addition, we confirmed that neither tag affected the ability of Mpl to mediate PC-PLC activation or the compartmentalization of Mpl (see Fig. S1 in the supplemental material). Similar to the previous experiment, Mpl and Mpl H437A were expressed in the same strain of L. monocytogenes. Both forms of Mpl were detected by Western immunoblotting using antibodies specific for the corresponding tags. Under these conditions, we observed the proform and mature form of Mpl but only the proform of Mpl H437A, indicating that Mpl did not mediate maturation of Mpl H437A (Fig. 3). Together, these results indicated that Mpl maturation occurs exclusively by intramolecular autocatalysis.
Various types of proteases have the ability to undergo proteolytic maturation by intramolecular and intermolecular autocatalysis (1, 9, 18), although exclusive intramolecular autocatalysis has rarely been reported. One example is the bacterial metalloprotease thermolysin. Purified active thermolysin does not mediate maturation of a catalytic mutant, suggesting that thermolysin maturation occurs exclusively by an intramolecular autocatalysis mechanism (13). Nevertheless, this result does not eliminate the possibility that intermolecular autocatalysis occurs in vivo. In our study, we used an in vivo approach to ask whether the maturation of Mpl, a thermolysin-like protease made by L. monocytogenes, occurs by intramolecular and/or intermolecular autocatalysis. Mpl and a catalytic mutant of Mpl were expressed in the same bacterial cell to sustain the in vivo conditions favorable for Mpl maturation. Under these conditions, maturation of wild-type Mpl was observed, but maturation of the catalytic mutant was not observed, indicating that Mpl maturation occurs exclusively by intramolecular autocatalysis. The nature of this event remains to be elucidated. There is evidence that proenzymes exist in two states, inactive and active. Presumably, in the inactive state the prodomain is associated with the catalytic domain, whereas in the active state the prodomain dissociates from the catalytic domain, which would be a prerequisite for intramolecular autocatalysis (18, 27). The activation state of a proenzyme may not matter when it comes to intermolecular autocatalysis, as long as the cleavage site is accessible to the mature enzyme. Presumably, the proform of Mpl does not allow external access to its cleavage site, independent of its activation state.
Supplementary Material
Acknowledgments
We greatly appreciate the help of Kaye Speicher and Nicole DiFlorio of the proteomics facility of the Wistar Institute for Edman sequence analysis. We thank Nancy Freitag for providing strain NF-L943 and Kyung-Bok Song for helping with the construction of the Mpl E350Q mutant. We also thank Heather O'Neil, Marci Scidmore, and Emily Slepkov for critical reading of the manuscript.
This work was supported by Public Health Service grant AI52154 from NIAID to H.M.
Footnotes
Published ahead of print on 26 October 2007.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Ahmed, S. A., P. McPhie, and L. A. Smith. 2003. Autocatalytically fragmented light chain of botulinum a neurotoxin is enzymatically active. Biochemistry 4212539-12549. [DOI] [PubMed] [Google Scholar]
- 2.Banbula, A., J. Potempa, J. Travis, C. Fernandez-Catalan, K. Mann, R. Huber, W. Bode, and F. Medrano. 1998. Amino-acid sequence and three-dimensional structure of the Staphylococcus aureus metalloproteinase at 1.72 A resolution. Structure 61185-1193. [DOI] [PubMed] [Google Scholar]
- 3.Beaumont, A., M. J. O'Donohue, N. Paredes, N. Rousselet, M. Assicot, C. Bohuon, M. C. Fournie-Zaluski, and B. P. Roques. 1995. The role of histidine 231 in thermolysin-like enzymes. A site-directed mutagenesis study. J. Biol. Chem. 27016803-16808. [DOI] [PubMed] [Google Scholar]
- 4.Bishop, D. K., and D. J. Hinrichs. 1987. Adoptive transfer of immunity to Listeria monocytogenes: the influence of in vitro stimulation on lymphocyte subset requirements. J. Immunol. 1392005-2009. [PubMed] [Google Scholar]
- 5.Camilli, A., L. G. Tilney, and D. A. Portnoy. 1993. Dual roles of plcA in Listeria monocytogenes pathogenesis. Mol. Microbiol. 8143-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Domann, E., M. Leimeister-Wächter, W. Goebel, and T. Chakraborty. 1991. Molecular cloning, sequencing, and identification of a metalloprotease gene from Listeria monocytogenes that is species specific and physically linked to the listeriolysin gene. Infect. Immun. 5965-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ho, S. N., H. D. Hunt, R. M. Horton, J. K. Pullen, and L. R. Pease. 1989. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 7751-59. [DOI] [PubMed] [Google Scholar]
- 8.Holmes, M. A., and B. W. Matthews. 1982. Structure of thermolysin refined at 1.6 A resolution. J. Mol. Biol. 160623-639. [DOI] [PubMed] [Google Scholar]
- 9.Hu, Z., K. Haghjoo, and F. Jordan. 1996. Further evidence for the structure of the subtilisin propeptide and for its interactions with mature subtilisin. J. Biol. Chem. 2713375-3384. [DOI] [PubMed] [Google Scholar]
- 10.Kawamoto, S., Y. Shibano, J. Fukushima, N. Ishii, K. Morihara, and K. Okuda. 1993. Site-directed mutagenesis of Glu-141 and His-223 in Pseudomonas aeruginosa elastase: catalytic activity, processing, and protective activity of the elastase against Pseudomonas infection. Infect. Immun. 611400-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kooi, C., C. R. Corbett, and P. A. Sokol. 2005. Functional analysis of the Burkholderia cenocepacia ZmpA metalloprotease. J. Bacteriol. 1874421-4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lauer, P., M. Y. N. Chow, M. J. Loessner, D. A. Portnoy, and R. Calendar. 2002. Construction, characterization, and use of two Listeria monocytogenes site-specific phage integration vectors. J. Bacteriol. 1844177-4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marie-Claire, C., B. P. Roques, and A. Beaumont. 1998. Intramolecular processing of prothermolysin. J. Biol. Chem. 2735697-5701. [DOI] [PubMed] [Google Scholar]
- 14.Marquis, H., V. Doshi, and D. A. Portnoy. 1995. The broad-range phospholipase C and a metalloprotease mediate listeriolysin O-independent escape of Listeria monocytogenes from a primary vacuole in human epithelial cells. Infect. Immun. 634531-4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marquis, H., H. Goldfine, and D. A. Portnoy. 1997. Proteolytic pathways of activation and degradation of a bacterial phospholipase C during intracellular infection by Listeria monocytogenes. J. Cell Biol. 1371381-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McIver, K., E. Kessler, and D. E. Ohman. 1991. Substitution of active-site His-223 in Pseudomonas aeruginosa elastase and expression of the mutated lasB alleles in Escherichia coli show evidence for autoproteolytic processing of proelastase. J. Bacteriol. 1737781-7789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McIver, K. S., E. Kessler, J. C. Olson, and D. E. Ohman. 1995. The elastase propeptide functions as an intramolecular chaperone required for elastase activity and secretion in Pseudomonas aeruginosa. Mol. Microbiol. 18877-889. [DOI] [PubMed] [Google Scholar]
- 18.Menard, R., E. Carmona, S. Takebe, E. Dufour, C. Plouffe, P. Mason, and J. S. Mort. 1998. Autocatalytic processing of recombinant human procathepsin L. Contribution of both intermolecular and unimolecular events in the processing of procathepsin L in vitro. J. Biol. Chem. 2734478-4484. [DOI] [PubMed] [Google Scholar]
- 19.Mengaud, J., C. Geoffroy, and P. Cossart. 1991. Identification of a new operon involved in Listeria monocytogenes virulence: its first gene encodes a protein homologous to bacterial metalloproteases. Infect. Immun. 591043-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyoshi, S., and S. Shinoda. 2000. Microbial metalloproteases and pathogenesis. Microbes Infect. 291-98. [DOI] [PubMed] [Google Scholar]
- 21.Poyart, C., E. Abachin, I. Razafimanantsoa, and P. Berche. 1993. The zinc metalloprotease of Listeria monocytogenes is required for maturation of phosphatidylcholine phospholipase C: direct evidence obtained by gene complementation. Infect. Immun. 611576-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shetron-Rama, L. M., K. Mueller, J. M. Bravo, H. G. Bouwer, S. S. Way, and N. E. Freitag. 2003. Isolation of Listeria monocytogenes mutants with high-level in vitro expression of host cytosol-induced gene products. Mol. Microbiol. 481537-1551. [DOI] [PubMed] [Google Scholar]
- 23.Shinde, U., and M. Inouye. 2000. Intramolecular chaperones: polypeptide extensions that modulate protein folding. Semin. Cell Dev. Biol. 1135-44. [DOI] [PubMed] [Google Scholar]
- 24.Smith, G. A., H. Marquis, S. Jones, N. C. Johnston, D. A. Portnoy, and H. Goldfine. 1995. The two distinct phospholipases C of Listeria monocytogenes have overlapping roles in escape from a vacuole and cell-to-cell spread. Infect. Immun. 634231-4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Snyder, A., and H. Marquis. 2003. Restricted translocation across the cell wall regulates secretion of the broad-range phospholipase C of Listeria monocytogenes. J. Bacteriol. 1855953-5958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tilney, L. G., and D. A. Portnoy. 1989. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J. Cell Biol. 1091597-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Wart, H. E., and H. Birkedal-Hansen. 1990. The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc. Natl. Acad. Sci. USA 875578-5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vazquez-Boland, J.-A., C. Kocks, S. Dramsi, H. Ohayon, C. Geoffroy, J. Mengaud, and P. Cossart. 1992. Nucleotide sequence of the lecithinase operon of Listeria monocytogenes and possible role of lecithinase in cell-to-cell spread. Infect. Immun. 60219-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wetmore, D. R., S. L. Wong, and R. S. Roche. 1992. The role of the prosequence in the processing and secretion of the thermolysin-like neutral protease from Bacillus cereus. Mol. Microbiol. 61593-1604. [DOI] [PubMed] [Google Scholar]
- 30.Yeung, P. S., N. Zagorski, and H. Marquis. 2005. The metalloprotease of Listeria monocytogenes controls cell wall translocation of the broad-range phospholipase C. J. Bacteriol. 1872601-2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.