Abstract
Phosphorylation can affect the function of microtubule-associated protein tau. Here, the human brain tau with 441 amino acids was phosphorylated by cyclic-AMP-dependent protein kinase (PKA) or glycogen synthase kinase-3β. PKA-phosphorylated tau (2.7 mol phosphates/mol) does not promote tubulin assembly as judged by spectrophotometric and atomic force microscopy measurements, unless trimethylamine N-oxide (TMAO), a natural occurring osmolyte, is included in these assays. TMAO is also found to promote tubulin assembly of glycogen synthase kinase-3β-phosphorylated tau (1.6 mol phosphates/mol). TMAO does not act by causing a chemical dephosphorylation of phosphorylated tau, but it acts to overcome the functional deficit caused by phosphorylation. PKA-phosphorylated tau binds to tubulin in the presence of TMAO and lowers the critical concentration of tubulin needed for assembly. From these data, we conclude that PKA-phosphorylated tau retains the ability to bind tubulin and promote tubulin assembly. TMAO is required, however, to sensitize the reaction. Possible uses of TMAO in relation to studies of tubulin assembly in vitro, in intact cells, and in relation to Alzheimer’s disease are presented in this report.
Hyperphosphorylation or abnormal phosphorylation of tau has been proposed as one potential cause of Alzheimer’s disease (1–4). It is believed that this hyperphosphorylation reduces the affinity of tau for tubulin and can contribute the self association of tau and formation of neurofibrillary tangles or paired helical filaments (PHF) (1–4), major hallmarks of Alzheimer’s disease. A recent study shows, however, that some phosphorylation reactions of tau may protect it against formation of PHFs (5). At least 25 sites have been identified in the tau of paired helical filaments (6, 7). These sites can be classified as either proline-directed or nonproline-directed phosphorylation sites (6, 7). Both types of phosphorylation can affect tau’s function. For instance, tau phosphorylated by either cyclic-AMP-dependent protein kinase (PKA) or glycogen synthase kinase-3β (GSK-3β) reduces its ability to stimulate the formation of microtubules (MTs) (8, 9). PKA, a nonproline-directed kinase, phosphorylates tau on Ser-214, Ser-262, Ser-316, Ser-320, Ser-356, Ser-409, Ser-416, and one of residues Ser-235, Ser-237, or Ser-238 (5–8, 10, 12). GSK-3β, a proline-directed kinase, phosphorylates tau on Ser-199, Thr-231, Ser-235, Ser-262, Ser-396, Ser-400, and Ser-413, most of which are in Ser/Thr-Pro motifs (6, 7, 9, 11). Activation of PKA in SH-SY5Y neuroblastoma cells can increase phosphorylation of Ser-262/Ser-356 in tau and cause the disassociation of tau from MTs (13). GSK-3β phosphorylation has been proposed to play a role in Alzheimer’s disease (14, 15). A previous study showed that endogenous tau can be phosphorylated by GSK-3β in cultured human NT2N neurons (16).
Tau exists as six isoforms in the human brain, and these forms are derived from a single gene by different mRNA splicing reactions (17–19). The largest brain tau containing 441 amino acids possesses two N-terminal inserts and four MT-binding repeats, but the smallest form possesses no N-terminal insert and has three MT-binding repeats instead (17, 18). The tau structure for all isoforms is enriched with proline, glycine, and hydrophilic amino acids (19). Early biophysical studies of tau by using analytical centrifugation, electron microscopy, gel filtration, CD, and 1H-NMR spectroscopy suggest that tau is a highly flexible extended molecule with little secondary structure (20–25). Recent studies suggest that hyperphosphorylation with mitogen-activated protein kinase induced structure into the C-terminal region of tau (23), and that the secondary structure of Alzheimer’s disease-hyperphosphorylated tau can be changed by dephosphorylation (26). Studies by Hagestedt et al. by using paracrystal imaging showed that Ca/calmodulin protein kinase II can reduce tau’s flexibility by phosphorylation (27). In addition, binding of tau to MTs can also generate some ordered structures, indicating that MTs can induce some conformational changes in tau (25).
Small molecular weight organic osmolytes found in elasmobranchs, e.g., sharks and rays, act to stabilize proteins by counteracting the denaturing effect of urea in vivo (28–31). In a recent paper, it was reported that trimethylamine N-oxide (TMAO) can force thermodynamically unfolded proteins to fold (32). RNase T1 with its disulfide bonds reduced and chemically derivatized and staphylococcal nuclease mutant protein (T62P) exist as unfolded biologically inactive structures (32). However, in the presence of TMAO these proteins become folded and biologically active. The authors state, “The ability of TMAO to force thermodynamically unstable proteins to fold presents an opportunity for structure determination and functional studies of an important emerging class of proteins that have little or no structure without the presence of TMAO” (32). A previous report of ours showed that 200 mM TMAO, a physiological concentration (28, 29), could increase the formation of tau-inducted MTs and counteract inhibition of assembly caused by urea (33). On the basis of this and of the background information on tau suggesting it exists as an unfolded protein, we initiated work on the effect of TMAO on tau phosphorylated by either PKA or GSK-3β and their interactions with tubulin. The results, shown herein, provide evidence that low concentrations of TMAO can overcome the functional deficit of phosphorylation of tau by PKA or GSK-3β.
MATERIALS AND METHODS
Materials.
The plasmid of the human tau cDNA encoding 441 amino acids was provided by K. Iqbal from New York State Institutes for Basic Research in Developmental Disabilities (Staten Island, NY). PKA and GSK-3β were purchased from Sigma; Escherichia coli BL21(DE3) cells from Novagen; and [γ-32P]ATP from ICN. TMAO was purchased from Sigma, recrystallized from deionized water, dried, and stored in a desiccator at room temperature. All other reagents were reagent grade.
Phosphorylation of Tau.
Human recombinant tau was purified as described previously (33). Pure recombinant tau (20 μM, based on a molecular mass of 45.7 kDa) was phosphorylated by kinases in the phosphorylation solution (1 mM ATP/10 mM MgCl/1 mM EDTA/0.5 μg/ml leupeptin/0.7 μg/ml pepstatin/1 μg/ml aprotinin/85 μg/ml PMSF/1 μM microcystin-LR/25 mM Hepes, pH 7.5). The concentrations of kinases in the phosphorylation reactions are 0.2 units/μl PKA for PKA phosphorylation and 0.4 units/μl GSK-3β for GSK-3β phosphorylation. The phosphorylation reactions were carried out at 30°C and terminated by heating the reaction solutions at 95°C for 10 min. The cooled phosphorylated tau was centrifuged at 10,000 × g for 10 min to remove protein aggregates, and the supernatants were ready for use in the MT assembly. Nonphosphorylated tau was treated as the same condition as phosphorylated tau, except that no ATP was added. To determine the phosphorylation stoichiometry, 1 μCi of [γ-32P]ATP was included in the phosphorylation mixture. After incubation, a portion of reaction mixture was spotted on a ET-31 paper disc, washed with trichloroacetic acid, and analyzed by liquid scintillation counting (34). This filter paper assay was also used to evaluate dephosphorylation of phosphorylated tau by TMAO.
Tubulin Preparation and Tubulin Assembly.
The preparation of porcine brain tubulin followed a method described by Williams and Lee (36), except that a SP-cation exchange column (high-performance SP from Amersham Pharmacia) was used rather than a phosphocellulose column (33, 36). The elution rate in the chromatography was 0.5 ml/min. The elution and collection of tubulin were the same as described previously (36). The assembly condition and the turbidity measurement were described in a previous paper (33). In brief, thawed tubulin was centrifuged at 15,000 × g for 10 min at 4°C to remove tubulin aggregates. This supernatant was collected and used for assembly reactions. Assembly started with the addition of tubulin (8 μM, based on a molecular mass of 55 kDa) to assembly mixture that contained without/with 200 mM TMAO/with/without 0.8 μM tau/1 mM GTP/1 mM DTT/1 mM MgCl2/1 mM EGTA/0.1 mM EDTA/100 mM Mes, pH 6.4. The assembly process was monitored by following changes in absorbance at 350 nm by using a Beckman (Beckman Dickinson) DU640 spectrophotometer. The presence of a small amount of ATP from the phosphorylation reaction did not affect tubulin assembly. The determination of tubulin-critical concentrations followed the principle described by Timasheff’s laboratory (37, 38). The concentrations of tau and PKA-phosphorylated tau (2.8 mol phosphates/mol) were 0.8 μM. TMAO was 200 mM. The assembly buffer and the turbidity measurement were the same as above, except that the incubation time was 20 min. At least four different concentrations of tubulin were applied in each assembly group. A linear curve fitting method was used to obtain a line, which fits the data in the same assembly group. The x-intercepts of the fitted lines were designated to be the tubulin-critical concentrations.
Analysis of Phosphorylated Tau–Tubulin Complex.
Tau was phosphorylated with PKA in the presence of [γ-32P]ATP. Phosphorylated tau was incubated with or without tubulin in the assembly buffer containing 200 mM TMAO. After 15 min assembly incubation, the pellets were collected by a centrifugation of 50,000 × g for 30 min at 25°C. The pellets were washed three times with prewarmed (30°C) assembly buffer containing 200 mM TMAO. The washed pellets were resuspended in 50 μl cold assembly buffer (0–4°C) and analyzed for radioactivity.
Atomic Force Microscopy (AFM).
The assembly mixtures were incubated under the assembly condition described above, followed by a fixation with 0.8% glutaraldehyde for 30 s. Ten microliters of the fixed samples was deposited onto a disc with a freshly cleaved mica surface and incubated for 5 min, followed by gentle rinsing with 1 ml HPLC grade water. The mica discs were then dried under a lamp for 5 min, during which time the temperature of the mica surface was gradually raised to 45°C. After this, sample discs were transferred into a 55°C oven with vacuum and incubated for 24 hr. The samples were imaged in air with a NanoScope III AFM (Digital Instruments, Santa Barbara, CA) by using a Tapping Mode. The imaging conditions usually were: height and amplitude modes, Ultra levers (Park Scientific, Sunnyvale, CA), scanning rate 1.97 Hz, and <50% relative humidity. Captured AFM images were processed and analyzed by using nanoscope software. Micropipette tips used in this AFM experiment section have been cut off to avoid shearing.
Protein Concentration Determination.
Porcine brain tubulin was dissolved in 6 M guanidine⋅HCl, and its absorbance was determined by spectrophotometric measurement at 275 nm. The extinction coefficient for tubulin in guanidine⋅HCl at 275 nm is 1.03 ml/mg/cm (37). Tau concentration was measured by the Bradford method (39), by using tau solutions with known concentrations as standards.
RESULTS
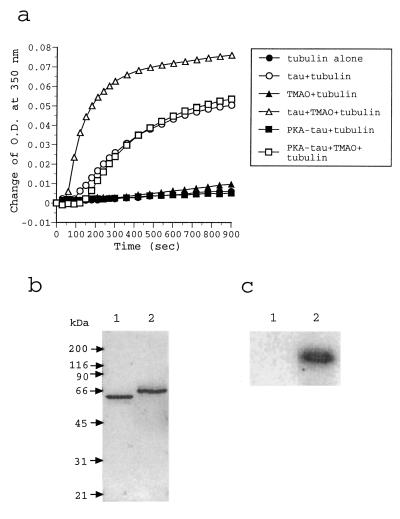
Human brain recombinant tau was phosphorylated with PKA to an extent of 2.7 ± 0.1 mol phosphates/mol. Using conditions that promote tubulin assembly, we found, like others (8), that PKA-phosphorylated tau does not promote tubulin polymerization as judged by spectrophotometric measurements (Fig. 1a). However, if 200 mM TMAO is included in the assembly mixture, turbidity increases, and the rate and extent of the reaction are equivalent to that seen with nonphosphorylated tau. The upper curve in Fig. 1a shows that stimulatory effect of TMAO by using nonphosphorylated tau, and it is similar to what was reported earlier by us (33). If the temperature is lowered in an assembly mixture, the formed MTs can disassemble (40). We found that the change in absorbance caused by the presence of mixtures of tau–tubulin or tau—tubulin–TMAO observed at 30°C could be reversed by altering the temperature to 4°C (results not shown).
Figure 1.
(a) Spectrophotometric measurement of tubulin assembly. Tubulin was incubated (30°C, 15 min) alone (solid circles) or with TMAO (solid triangles), and the assemblies were monitored by an increase in the absorbance at 350 nm. By using the same conditions, tubulin was assembled in the presence of tau (open circles) or tau and TMAO (open triangles). Finally, tubulin was assembled with PKA-phosphorylated tau in the presence (open squares) or absence (solid squares) of TMAO. The phosphorylation stoichometry of PKA-phosphorylated tau was 2.7 ± 0.1 phosphates/mol. (b) SDS/PAGE analysis of tau proteins. Nonphosphorylated tau (lane 1) and PKA-phosphorylated tau with 2.8 phosphates/mol (lane 2) were analyzed in a 10% SDS/PAGE gel. Proteins in the gel were visualized by a Coomassie brilliant blue R staining. (c) Radioautogram of 32P-labeled tau in a SDS/PAGE analysis. Tau was phosphorylated with PKA in the presence of [γ-32P]ATP. Any aggregates of phosphorylated tau–TMAO (lane 1) and phosphorylated tau—tubulin–TMAO (lane 2) were collected by centrifugation and analyzed by SDS/PAGE.
SDS/gel electrophoresis profiles of tau and tau phosphorylated by PKA are shown in Fig. 1b. Nonphosphorylated tau (lane 1) migrates as a single band, and phosphorylated tau (lane 2) migrates slower, as expected (8). One major band is found for phosphorylated tau. A slower moving minor band is found, indicating some heterogeneity in the phosphorylated tau preparation. However, no nonphosphorylated tau is detected by SDS/PAGE analysis. Similar results were seen with capillary isoelectric focusing electrospray ionization mass spectrometry of PKA-phosphorylated tau containing 2.6 mol phosphates/mol (12). These results strongly suggest that PKA-phosphorylated tau induces tubulin polymerization judged by the spectrophotometric measurements of Fig. 1a. Results shown in Fig. 1a cannot be explained by the presence of nonphosphorylated tau in the PKA-phosphorylated preparation.
The possibility existed that TMAO might act to cause a dephosphorylation of tau, because it has been reported that TMAO can act as a nucleophile to remove phosphate from phosphorylated γ-picoline monoanion (41). To test this possibility, we generated 32P-labeled tau by phosphorylation with PKA and incubated phosphorylated tau with 200 mM TMAO at 30°C. After incubation of 60 min, we found no loss of radioactivity from phosphorylated tau (data not shown). Thus, the effect of TMAO on phosphorylated tau cannot be explained by a chemical reaction leading to the formation of nonphosphorylated tau.
The spectrophotometric results in Fig. 1a suggest that phosphorylated tau bound to tubulin in the presence of TMAO. To test this idea more directly, we incubated tubulin with 32P-labeled tau (2.7 ± 0.1 mol phosphates/mol) and collected the polymerized tubulin by centrifugation (Materials and Methods). This fraction contained radioactivity (30.0 pCi) but little or no 32P-labeled tau sedimented in the control (0.27 pCi). An autoradiogram of the SDS/PAGE gel of phosphorylated tau and phosphorylated tau—TMAO–tubulin is shown in Fig. 1c. Only lane 2 shows detectable radioactivity. These results suggest that PKA-phosphorylated tau associates with tubulin in the presence of TMAO, and that TMAO does not promote self aggregation of PKA-phosphorylated tau in our assembly conditions.
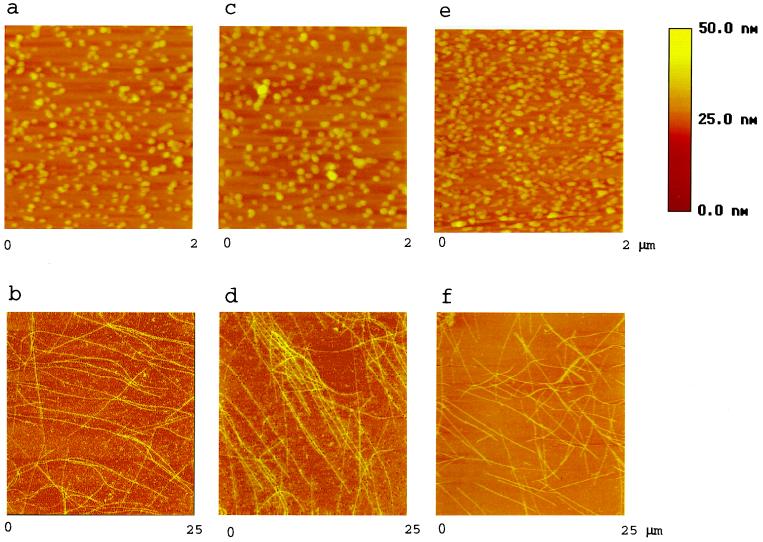
The change in absorbance at 350 nm shown in Fig. 1a is characteristic of the formation of MTs (37), but it is not clear what exact structures are being formed, particularly in the new reactions containing TMAO. To examine the morphology of the structures formed in the reaction mixtures, we used AFM. First, as expected from the data of Fig. 1, tubulin alone (Fig. 2a) and tubulin plus 200 mM TMAO (Fig. 2c) do not aggregate. But when tau is added, MTs are formed (Fig. 2 b and d). Note there are no apparent differences in the structures of MTs formed in the absence (Fig. 2b) and presence (Fig. 2d) of TMAO. Thus, at 200 mM, TMAO has no adverse effect on the morphology of MTs. Under these conditions, the MTs are generally straight and are several micrometers long (Fig. 2 b, d, and f). In contrast, those formed by using phosphorylated tau (Fig. 2f) tend to be shorter. As expected from the spectrophotometric studies, PKA-phosphorylated tau (2.7 ± 0.1 mol phosphate/mol) plus tubulin does not make any MTs (Fig. 2e) unless 200 mM TMAO is present (Fig. 2f). The heights of all MTs in Fig. 2 b, d, and f are about 20 ± 5 nm. The widths of all MTs shown above are about the same. Because of the association of 32P-labeled tau with tubulin in the presence of TMAO, we conclude that the polymerized MTs shown in Fig. 2f are caused by the combination of phosphorylated tau with tubulin.
Figure 2.
AFM images of tubulin assembly. Tubulin (a) was assembled by using standard reaction conditions (Fig. 1) and fixed. The mixture was then spotted onto a mica disc, dried, and scanned by AFM. Other reaction mixtures that included tau (b), TMAO (c), tau + TMAO (d), PKA-phosphorylated tau with 2.7 phosphates/mol (e), or PKA-phosphorylated tau + TMAO (f) were also scanned. The sample height is color coded, as indicated by the height scale bar. Note field sizes for a, c, and e (2 μm × 2 μm) are smaller than those for b, d, and f (25 μm × 25 μm) to highlight features. The heights of MTs shown in b, d, and f are all about 20 nm, in general.
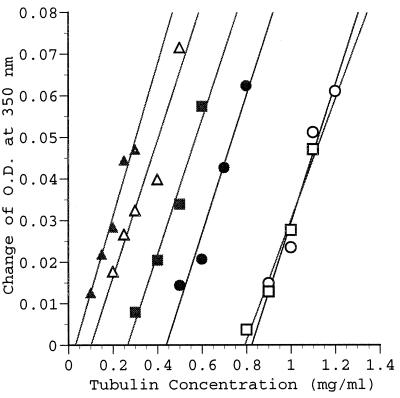
To learn how TMAO affects MT assembly, we determined the critical concentration of tubulin needed for assembly (Fig. 3). The critical concentration of tubulin required for tubulin self assembly is about 0.8 mg/ml (Fig. 3) similar to what was reported previously (37, 38, 42). TMAO (200 mM) lowers the critical concentration of tubulin to about 0.4 mg/ml (Fig. 3), whereas that in the presence of tau is 0.1 mg/ml, or TMAO plus tau is about 0.03 mg/ml (Fig. 3). Note that when PKA-phosphorylated tau is used there is no change in the critical concentration of tubulin (0.79 mg/ml), but when TMAO is added the critical concentration drops to a value of about 0.25 mg/ml (Fig. 3). This value is significantly lower than that found with TMAO and tubulin. Thus, TMAO not only lowers the concentration of tubulin needed for assembly but also sensitizes the assembly process to tau and phosphorylated tau, which was completely ineffective under these experimental conditions.
Figure 3.
The determination of tubulin-critical concentrations. Six assembly groups were analyzed: (i) tubulin alone (open circles); (ii) tubulin plus TMAO (close circles); (iii) tubulin plus tau (open triangles); (iv) tubulin, tau, plus TMAO (closed triangles); (v) tubulin plus phosphorylated tau (open squares); (vi) tubulin, phosphorylated tau, plus TMAO (closed squares). The detailed assembly conditions are described in Materials and Methods. The lines are the results after a linear curve fitting processing for each group.
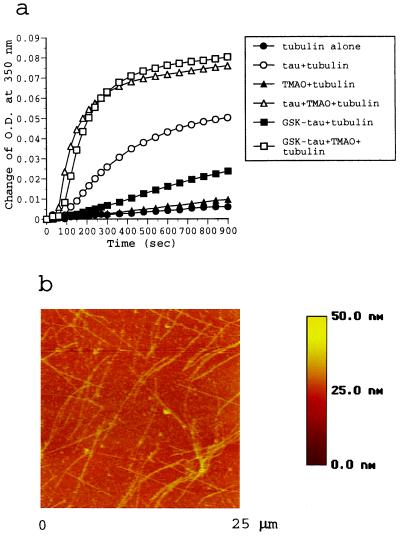
Phosphorylation of GSK-3β is known to affect tau function (9, 11, 16). In contrast to PKA, GSK-3β has a substrate preference with the sequence for Ser/Thr-Pro, which characterizes a “proline-directed” kinase (6). To examine whether TMAO can affect tubulin polymerization with proline-directed phosphorylated tau, we generated GSK-3β–phosphorylated tau and tested it with TMAO. As shown in Fig. 4a, the assembly of MTs caused by 0.8 μM GSK-3β-phosphorylated tau (1.6 mol phosphates/mol) was significantly decreased compared with nonphosphorylated tau. However, tubulin assembly with this phosphorylated tau could be promoted by the addition of 200 mM TMAO to an extent seen with nonphosphorylated tau (Fig. 4a). SDS gel electrophoresis of GSK-3β-phosphorylated tau (1.6 mol phosphates/mol) shows two major bands that migrate slower than the control nonphosphorylated tau. A small amount of nonphosphorylated tau (approximately 10%) may be present in the GSK-3β-phosphorylated sample (results not shown). On this basis and from our earlier results of the amount of nonphosphorylated tau needed for assembly (33), we conclude that the results of Fig. 4a can be explained only by the participation of GSK-3β-phosphorylated tau in the tubulin assembly. To correlate these data with a physical structure, we used AFM to observe the final products in assembly mixture. Again, it was found, as with PKA-phosphorylated tau, that MTs are assembled in the copresence of GSK-3β-phosphorylated tau and TMAO (Fig. 4b). These results show that TMAO can also restore GSK-3β-phosphorylated tau–MT assembly.
Figure 4.
Analysis of tubulin assembly with GSK-3β-phosphorylated tau. (a) Spectrophotometric measurement of tubulin assembly. Reaction mixtures contained tubulin alone (solid circles) or tubulin + TMAO (solid triangles). Other reactions contained tubulin in the presence of tau (open circles), tau + TMAO (open triangles), GSK-3β-phosphorylated tau (solid squares), or GSK-3β-phosphorylated tau + TMAO (open squares). The phosphorylation stoichometry of GSK-3β-phosphorylated tau is 1.6 phosphates/mol. (b) AFM image of the reaction mixture that contained tubulin, GSK-3β-phosphorylated tau, and TMAO.
DISCUSSION
It is thought that in Alzheimer’s disease abnormal phosphorylation of tau causes tau to lose its ability to promote tubulin assembly. This functional deficit can be overcome by enzymatic dephosphorylation in vitro (1–4, 43). Yet, how might the consequence of abnormal phosphorylation be overcome in the brain in Alzheimer’s disease? Much research is being done to study which specific protein phosphorylations-dephosphorylations occur and how they are regulated in the diseased state. In this paper, we report new findings suggesting that a small organic osmolyte TMAO can overcome the functional deficit of tau caused by specific enzymatic phosphorylation reactions. Because no dephosphorylation occurs, the effect of TMAO is unlike that caused by phosphatases acting on tau.
How might TMAO work to overcome the functional deficit of tau caused by phosphorylation? We found that in the presence of 200 mM TMAO, PKA-phosphorylated tau binds to tubulin and promotes formation of MTs (Fig. 1). TMAO can lower the critical concentration of tubulin, and the addition of PKA-phosphorylated tau lowers the concentration further (Fig. 3). No effect on critical concentration of tubulin is seen with PKA-phosphorylated tau in the absence of TMAO, suggesting that PKA-phosphorylated tau has little or no effect by itself on tubulin assembly. Studies done by Lee and Timasheff (38) showed that observed critical concentration of tubulin required for assembly is a thermodynamic indicator of assembly; the lower the critical concentration, the more negative the apparent free energy for assembly (38, 42). On this basis, a lower apparent free energy for the assembly could be expected in the presence of TMAO, PKA-phosphorylated tau, and tubulin, compared with that observed for TMAO and tubulin.
Extensive studies from Timasheff’s laboratory showed that osmolytes like TMAO stabilize proteins by causing a preferential hydration of the proteins (44–47). TMAO has been found to raise the free energy of the unfolded state of proteins and to promote folding by a “solvophobic” effect on proteins (32, 47). In the presence of a cosolvent with solvophobic properties, water is preferred to surround the protein surface, and the cosolvent is excluded (46). Release of bound water molecules occurring during folding or in specific association reactions (46, 47) contributes to the favorable energetics. The release of bound water was found in tubulin assembly (39) and may explain how TMAO can lower the critical concentration of tubulin for tubulin self assembly, as well as for tau–tubulin assembly.
How can TMAO change the properties of phosphorylated tau? Studies from Urry’s laboratory suggest that covalently bound phosphate in elastin polypeptides can influence a conformational transition and change local interactions by influencing hydration (48, 49). Phosphate groups tend to prevent the formation of more ordered water structure around hydrophobic moieties (48). Possibly TMAO, by influencing hydration, could alter key contacts needed for the association of phosphorylated tau with tubulin.
We found the concentration of TMAO needed for assembly is far less than that required to promote folding of unfolded proteins. Chemically derivatized RNase and a staphylococcal nuclease mutant, both of which are unfolded, require 2–3 M concentrations of TMAO to restore structure and function of these proteins. An earlier study showed that molar concentration of TMAO affected tubulin assembly (35). In this work, we found that TMAO can act at lower concentrations (200 mM) on tau. However, TMAO at this concentration has no effect on the CD spectrum of tau (33). One possibility is that the requirement for the amount of TMAO needed to induce structure may be lower for proteins that associate with each other if one protein serves as a folding template for the other. Because tau and its phosphorylated forms are thought to be highly extended molecules with little secondary structures, tubulin may alter tau’s flexibility. 1H-NMR studies suggest that the flexibility of tau can be changed, as shown by the fact that half of the methyl-containing residues of tau become immobilized on tau binding to tubulin (25). Therefore, specific binding of tau with tubulin may explain why 200 mM TMAO is so effective in promoting tau–tubulin assembly.
The fact that concentrations of TMAO found in living tissues can affect tubulin assembly in vitro suggests there may be some application for studies of effects of TMAO on tubulin assembly in cells. Experiments with the XR1 glial-like cell line, isolated from Xenopus retinal neuroepithelium, show that the amounts of cellular MTs are increased by adding TMAO into medium, but that they return to the basal level by removing TMAO (H.-C.T., P. S. Sakaguchi, D. B. Fulton, and D.J.G., unpublished work).
Last, both PKA-phosphorylated tau (Figs. 1a and 2) and GSK-3β-phosphorylated tau (Fig. 4) can promote MT formation in the presence of TMAO, and the appearances of the MTs formed in both cases are similar. Because GSK-3β is known to phosphorylate different sites than PKA in tau (6, 7), our results suggest that the effects of TMAO are not related just to one specific type of phosphorylation. Studies are in progress to define the different sites of phosphorylation with PKA and GSK-3β. Preliminary mass spectrometry studies show that Ser-214 and Ser-356 are major sites of phosphorylation in PKA-phosphorylated tau containing 2.6 mol of phosphate/mol (12). Overall, our studies suggest that TMAO may be used as a tool to study MT-associated protein-induced MT assembly and MT disassembly in relation to neurodegenerative disease, such as Alzheimer’s disease, which may be caused in part by tau phosphorylation.
Acknowledgments
We thank K. Iqbal and N. Haque of New York State Institutes for Basic Research in Developmental Disabilities for their generous gift of tau cDNA and M. Pete for comments about the manuscript. This research was supported by a grant (#96–180) from the Roy J. Carver Charitable Trust (D.J.G.). This is Journal Paper No. J-18463 of the Iowa Agriculture and Home Economics Experiment Station (Ames, IA), Project 3392.
ABBREVIATIONS
- MT
microtubule
- PKA
cyclic-AMP-dependent protein kinase
- GSK-3β
glycogen synthase kinase-3β
- AFM
atomic force microscopy
- TMAO
trimethylamine N-oxide
References
- 1.Iqbal K, Grundke-Iqbal I, Zaidi T, Merz P A, Wen G Y, Shaikh S S, Wisniewski H M, Alzfuzoff I, Winblad B. Lancet. 1986;2:421–426. doi: 10.1016/s0140-6736(86)92134-3. [DOI] [PubMed] [Google Scholar]
- 2.Grundke-Iqbal I, Iqbal K, Tung Y-C, Quinlan M, Wisniewski H M, Binder L I. Proc Natl Acad Sci USA. 1986;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mandelkow E-M, Mandelkow E. Trends Biochem Sci. 1993;18:480–483. doi: 10.1016/0968-0004(93)90011-b. [DOI] [PubMed] [Google Scholar]
- 4.Goedert M. Trends Neurosci. 1993;16:460–465. doi: 10.1016/0166-2236(93)90078-z. [DOI] [PubMed] [Google Scholar]
- 5.Schneider A, Biernat J, von Bergen M, Mandelkow E, Mandelkow E-M. Biochemistry. 1999;38:3549–3558. doi: 10.1021/bi981874p. [DOI] [PubMed] [Google Scholar]
- 6.Morishima-Kawashima M, Hasegawa M, Takio K, Suzuki M, Yoshida H, Titani K, Ihara Y. J Biol Chem. 1995;270:823–829. doi: 10.1074/jbc.270.2.823. [DOI] [PubMed] [Google Scholar]
- 7.Hanger D P, Betts J C, Loviny T L, Blackstock W P, Anderton B H. J Neurochem. 1998;71:2465–2476. doi: 10.1046/j.1471-4159.1998.71062465.x. [DOI] [PubMed] [Google Scholar]
- 8.Scott C W, Spreen R C, Herman J L, Chow F P, Davison M D, Young J, Caputo C B. J Biol Chem. 1993;268:1166–1173. [PubMed] [Google Scholar]
- 9.Song J S, Yang S D. J Protein Chem. 1995;14:95–105. doi: 10.1007/BF01888367. [DOI] [PubMed] [Google Scholar]
- 10.Drewes G, Trinczek B, Illenberger S, Biernat J, Schmitt-Ulms G, Meyer H E, Mandelkow E-M, Mandelkow E. J Biol Chem. 1995;270:7679–7688. doi: 10.1074/jbc.270.13.7679. [DOI] [PubMed] [Google Scholar]
- 11.Ishiguro K, Omori A, Takamatsu M, Sato K, Arioka M, Uchida T, Imahori K. Neurosci Lett. 1992;148:202–206. doi: 10.1016/0304-3940(92)90839-y. [DOI] [PubMed] [Google Scholar]
- 12.Wei J. Ph.D. dissertation. Ames, IA: Iowa State University; 1998. [Google Scholar]
- 13.Xie H, Litersky J M, Hartigan J A, Jope R S, Johnson G V. Brain Res. 1998;798:173–183. doi: 10.1016/s0006-8993(98)00407-7. [DOI] [PubMed] [Google Scholar]
- 14.Lovestone S, Reynolds C H, Latimer D, Davis D R, Anderton B H, Gallo J M, Hanger D, Mulot S, Marquardt B, Stabel S, et al. Curr Biol. 1994;4:1077–1086. doi: 10.1016/s0960-9822(00)00246-3. [DOI] [PubMed] [Google Scholar]
- 15.Lovestone S, Hartley C L, Pearce J, Anderton B H. Neuroscience. 1996;73:1145–1157. doi: 10.1016/0306-4522(96)00126-1. [DOI] [PubMed] [Google Scholar]
- 16.Hong M, Chen D C, Klein P S, Lee V M. J Biol Chem. 1997;272:25326–25332. doi: 10.1074/jbc.272.40.25326. [DOI] [PubMed] [Google Scholar]
- 17.Goedert M, Spillantini M G, Potier M C, Ulrich J, Crowther R A. EMBO J. 1989;8:393–399. doi: 10.1002/j.1460-2075.1989.tb03390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andreadis A, Brown W M, Kosik K S. Biochemistry. 1992;31:10626–10633. doi: 10.1021/bi00158a027. [DOI] [PubMed] [Google Scholar]
- 19.Lee G, Cowan M, Krischner M W. Science. 1988;239:285–288. doi: 10.1126/science.3122323. [DOI] [PubMed] [Google Scholar]
- 20.Cleveland D W, Hwo S Y, Kirschner M W. J Mol Biol. 1977;116:227–247. doi: 10.1016/0022-2836(77)90214-5. [DOI] [PubMed] [Google Scholar]
- 21.Ruben G C, Iqbal K, Grundke-Iqbal I, Wisniewski H M, Ciardelli T L, Johnson J E., Jr J Biol Chem. 1991;266:22019–22027. [PubMed] [Google Scholar]
- 22.Hirokawa N, Shiomura Y, Okabe S. J Cell Biol. 1988;107:1449–1459. doi: 10.1083/jcb.107.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uversky V N, Winter S, Galzitskaya O V, Kittler L, Lober G. FEBS Lett. 1998;439:21–25. doi: 10.1016/s0014-5793(98)01303-9. [DOI] [PubMed] [Google Scholar]
- 24.Lichtenberg B, Mandelkow E-M, Hagestedt T, Mandelkow E. Nature (London) 1988;334:359–362. doi: 10.1038/334359a0. [DOI] [PubMed] [Google Scholar]
- 25.Woody R W, Clark D C, Roberts G C, Martin S R, Bayley P M. Biochemistry. 1983;22:2186–2192. doi: 10.1021/bi00278a020. [DOI] [PubMed] [Google Scholar]
- 26.Ruben G C, Ciardelli T L, Grundke-Iqbal I, Iqbal K. Synapse. 1997;27:208–229. doi: 10.1002/(SICI)1098-2396(199711)27:3<208::AID-SYN7>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 27.Hagestedt T, Lichtenberg B, Wille H, Mandelkow E-M, Mandelkow E. J Cell Biol. 1989;109:1643–1651. doi: 10.1083/jcb.109.4.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yancey P H, Somero G N. J Exp Zool. 1980;212:205–213. [Google Scholar]
- 29.Yancey P H, Clark M E, Hand S C, Bowlus R D, Somero G N. Science. 1982;217:1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]
- 30.Lin T Y, Timasheff S N. Biochemistry. 1992;33:12695–12701. doi: 10.1021/bi00208a021. [DOI] [PubMed] [Google Scholar]
- 31.Welch W J, Brown C R. Cell Stress Chaperones. 1996;1:109–115. doi: 10.1379/1466-1268(1996)001<0109:iomacc>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baskakov I, Bolen D W. J Biol Chem. 1998;273:4831–4834. doi: 10.1074/jbc.273.9.4831. [DOI] [PubMed] [Google Scholar]
- 33.Tseng H-C, Graves D J. Biochem Biophys Res Commun. 1998;250:726–730. doi: 10.1006/bbrc.1998.9382. [DOI] [PubMed] [Google Scholar]
- 34.Reimann E M, Walsh D, Kreb E G. J Biol Chem. 1971;246:1986–1995. [PubMed] [Google Scholar]
- 35.Sackett D L. Am J Physiol. 1997;273:R669–R676. doi: 10.1152/ajpregu.1997.273.2.R669. [DOI] [PubMed] [Google Scholar]
- 36.Williams R C, Jr, Lee J C. Methods Enzymol. 1982;85:376–385. doi: 10.1016/0076-6879(82)85038-6. [DOI] [PubMed] [Google Scholar]
- 37.Na G C, Timasheff S N. Methods Enzymol. 1982;85:393–408. doi: 10.1016/0076-6879(82)85040-4. [DOI] [PubMed] [Google Scholar]
- 38.Lee J C, Timasheff S N. Biochemistry. 1977;16:1754–1764. doi: 10.1021/bi00627a037. [DOI] [PubMed] [Google Scholar]
- 39.Bradford M. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 40.Cleveland D W, Hwo S Y, Kirschner M W. J Mol Biol. 1977;116:207–225. doi: 10.1016/0022-2836(77)90213-3. [DOI] [PubMed] [Google Scholar]
- 41.Herschlag D, Jencks W P. J Am Chem Soc. 1990;112:1942–1950. [Google Scholar]
- 42.Lee J C, Timasheff S N. Biochemistry. 1975;14:5183–5187. doi: 10.1021/bi00694a025. [DOI] [PubMed] [Google Scholar]
- 43.Alonso A C, Zaidi T, Grundke-Iqbal I, Iqbal K. Proc Natl Acad Sci USA. 1994;91:5562–5566. doi: 10.1073/pnas.91.12.5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Timasheff S N. Biochemistry. 1992;31:9857–9864. doi: 10.1021/bi00156a001. [DOI] [PubMed] [Google Scholar]
- 45.Arakawa T, Timasheff S N. Biophys J. 1985;47:411–414. doi: 10.1016/S0006-3495(85)83932-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Timasheff S N. Annu Rev Biophys Biomol Struct. 1993;22:67–97. doi: 10.1146/annurev.bb.22.060193.000435. [DOI] [PubMed] [Google Scholar]
- 47.Lee J C, Gekko K, Timasheff S N. Methods Enzymol. 1979;61:26–49. doi: 10.1016/0076-6879(79)61005-4. [DOI] [PubMed] [Google Scholar]
- 48.Urry D W. Angew Chem Int Ed Engl. 1993;32:819–841. [Google Scholar]
- 49.Pattanaik A, Gowda D C, Urry D W. Biochem Biophys Res Commun. 1991;178:539–545. doi: 10.1016/0006-291x(91)90141-s. [DOI] [PubMed] [Google Scholar]