Abstract
It has been suggested that sensitivity of ovarian carcinomas to cisplatin is in part related to an endogenous folate deficiency. In this work, we investigated whether overexpression of the folate-binding protein (FBP), a receptor involved in folate transport, might be associated with cisplatin sensitivity. The results obtained on a panel of ten ovarian carcinoma cell lines that overexpress different levels of the FBP showed a statistically significant relationship between FBP overexpression and cisplatin responsiveness, with the most sensitive cell lines expressing higher FBP levels on their membrane than the less sensitive ones. The relationship was observed both in cells growing in standard medium-containing high-folate concentrations (2.3 microM) and in cells adapted to growth in low-folate (20 nM) medium. Analysis of two cisplatin-resistant cell lines derived from the cisplatin-sensitive IGROV1 ovarian carcinoma cell line indicated that resistance was associated with a significant decrease in FBP expression. However, the receptor does not appear to be directly responsible for drug sensitivity per se as different cell lines transfected with FBP cDNA did not become more sensitive to the drug. Together, the data suggest the possible predictive value of FBP in ovarian carcinoma, as higher levels of expression can be indirectly but significantly associated with increased drug sensitivity.
Full text
PDF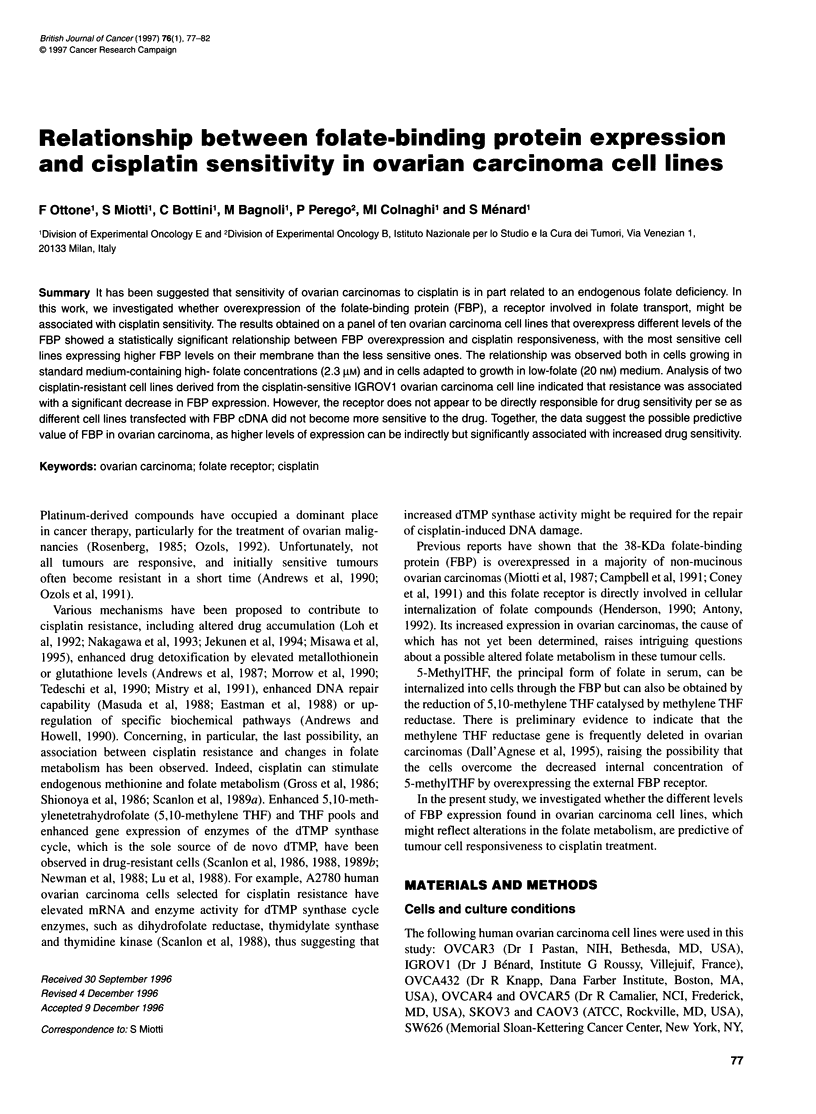
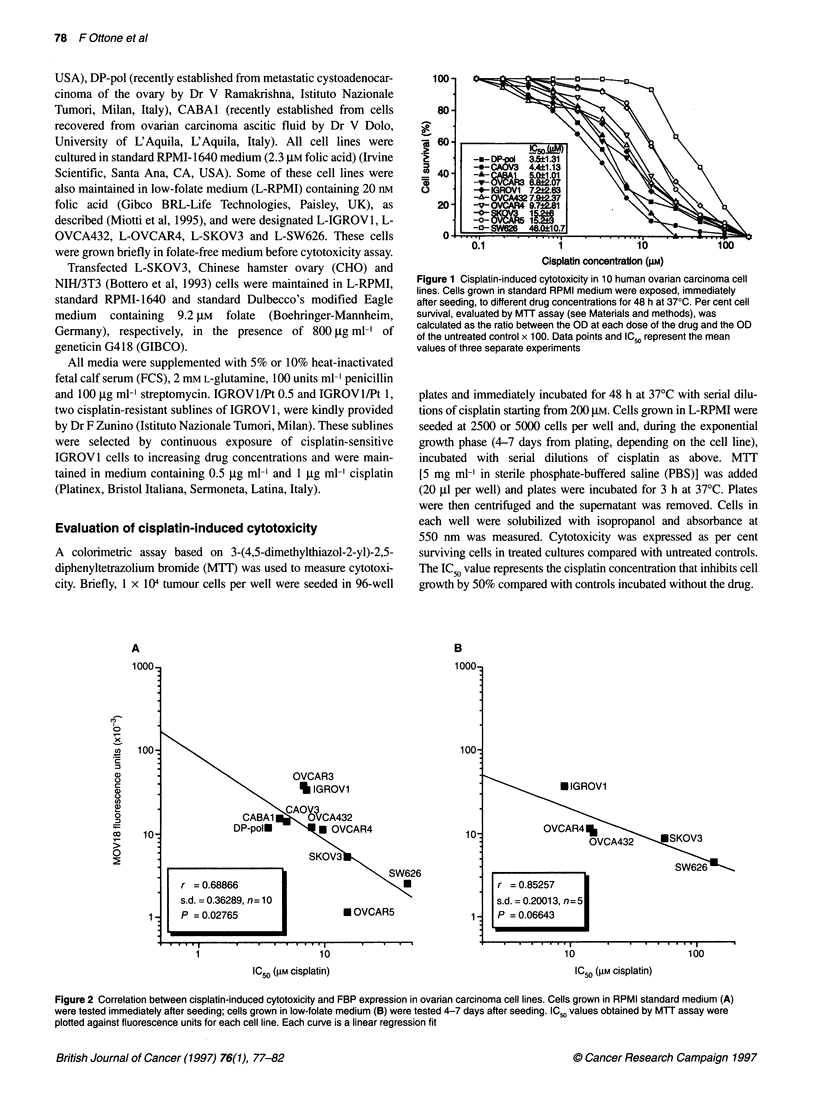
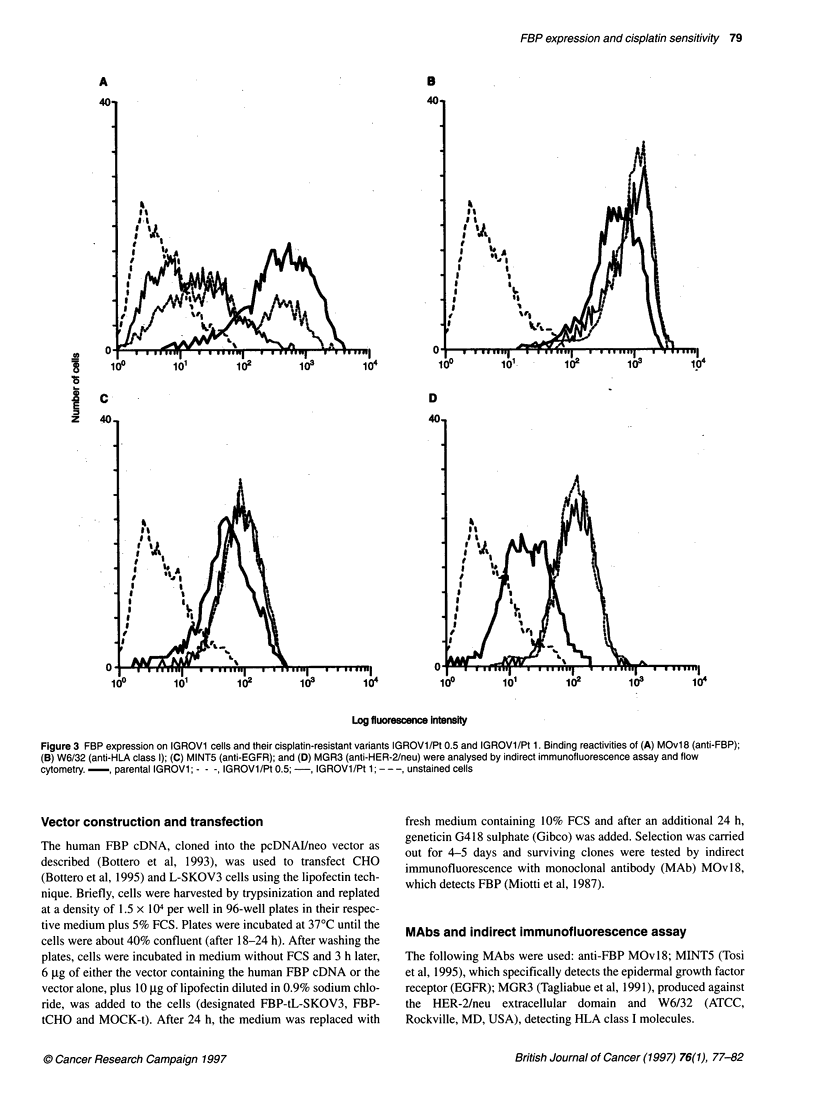
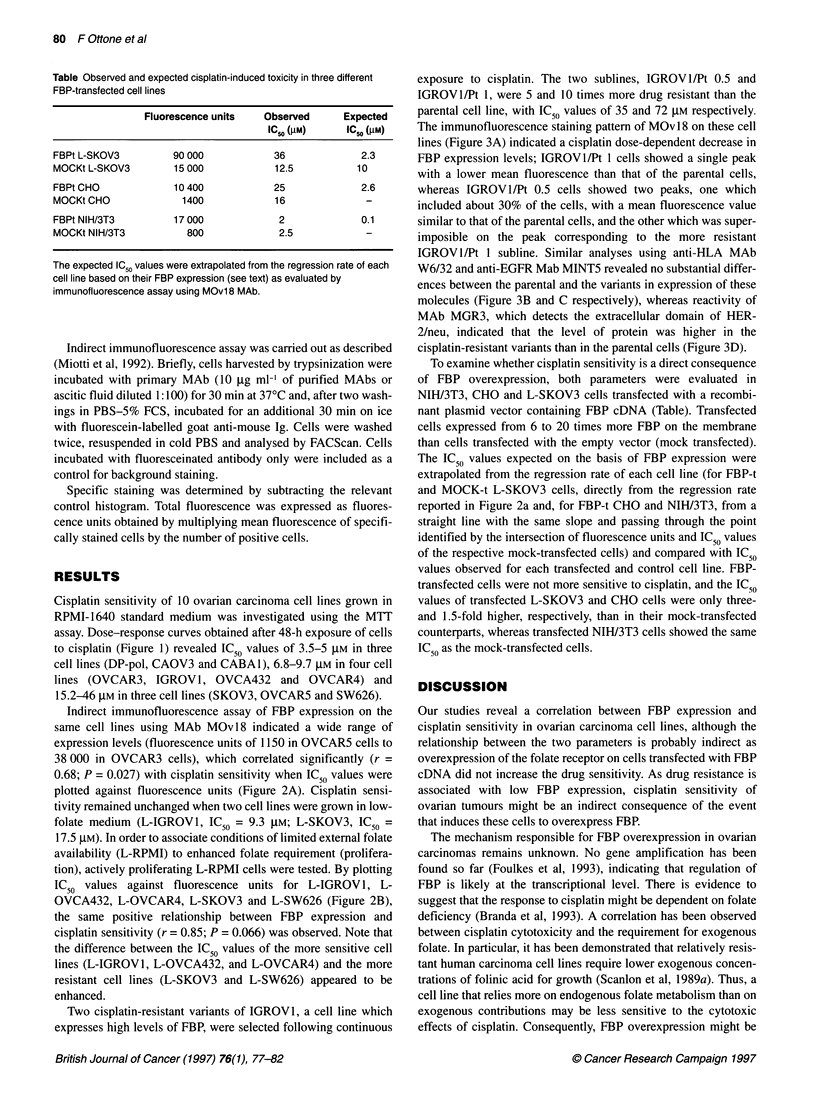


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. A., Howell S. B. Cellular pharmacology of cisplatin: perspectives on mechanisms of acquired resistance. Cancer Cells. 1990 Feb;2(2):35–43. [PubMed] [Google Scholar]
- Andrews P. A., Jones J. A., Varki N. M., Howell S. B. Rapid emergence of acquired cis-diamminedichloroplatinum(II) resistance in an in vivo model of human ovarian carcinoma. Cancer Commun. 1990;2(2):93–100. doi: 10.3727/095535490820874641. [DOI] [PubMed] [Google Scholar]
- Andrews P. A., Murphy M. P., Howell S. B. Metallothionein-mediated cisplatin resistance in human ovarian carcinoma cells. Cancer Chemother Pharmacol. 1987;19(2):149–154. doi: 10.1007/BF00254568. [DOI] [PubMed] [Google Scholar]
- Antony A. C., Kane M. A., Krishnan S. R., Kincade R. S., Verma R. S. Folate (pteroylglutamate) uptake in human red blood cells, erythroid precursors and KB cells at high extracellular folate concentrations. Evidence against a role for specific folate-binding and transport proteins. Biochem J. 1989 Jun 1;260(2):401–411. doi: 10.1042/bj2600401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antony A. C. The biological chemistry of folate receptors. Blood. 1992 Jun 1;79(11):2807–2820. [PubMed] [Google Scholar]
- Berchuck A., Kamel A., Whitaker R., Kerns B., Olt G., Kinney R., Soper J. T., Dodge R., Clarke-Pearson D. L., Marks P. Overexpression of HER-2/neu is associated with poor survival in advanced epithelial ovarian cancer. Cancer Res. 1990 Jul 1;50(13):4087–4091. [PubMed] [Google Scholar]
- Bottero F., Tomassetti A., Canevari S., Miotti S., Ménard S., Colnaghi M. I. Gene transfection and expression of the ovarian carcinoma marker folate binding protein on NIH/3T3 cells increases cell growth in vitro and in vivo. Cancer Res. 1993 Dec 1;53(23):5791–5796. [PubMed] [Google Scholar]
- Branda R. F., Blickensderfer D. B. Folate deficiency increases genetic damage caused by alkylating agents and gamma-irradiation in Chinese hamster ovary cells. Cancer Res. 1993 Nov 15;53(22):5401–5408. [PubMed] [Google Scholar]
- Campbell I. G., Jones T. A., Foulkes W. D., Trowsdale J. Folate-binding protein is a marker for ovarian cancer. Cancer Res. 1991 Oct 1;51(19):5329–5338. [PubMed] [Google Scholar]
- Coney L. R., Tomassetti A., Carayannopoulos L., Frasca V., Kamen B. A., Colnaghi M. I., Zurawski V. R., Jr Cloning of a tumor-associated antigen: MOv18 and MOv19 antibodies recognize a folate-binding protein. Cancer Res. 1991 Nov 15;51(22):6125–6132. [PubMed] [Google Scholar]
- Eastman A., Schulte N. Enhanced DNA repair as a mechanism of resistance to cis-diamminedichloroplatinum(II). Biochemistry. 1988 Jun 28;27(13):4730–4734. doi: 10.1021/bi00413a022. [DOI] [PubMed] [Google Scholar]
- Foulkes W. D., Campbell I. G., Stamp G. W., Trowsdale J. Loss of heterozygosity and amplification on chromosome 11q in human ovarian cancer. Br J Cancer. 1993 Feb;67(2):268–273. doi: 10.1038/bjc.1993.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross R. B., Scanlon K. J. Amino acid membrane transport properties of L1210 cells resistant to cisplatin. Chemioterapia. 1986 Feb;5(1):37–43. [PubMed] [Google Scholar]
- Henderson G. B. Folate-binding proteins. Annu Rev Nutr. 1990;10:319–335. doi: 10.1146/annurev.nu.10.070190.001535. [DOI] [PubMed] [Google Scholar]
- Jekunen A. P., Hom D. K., Alcaraz J. E., Eastman A., Howell S. B. Cellular pharmacology of dichloro(ethylenediamine)platinum(II) in cisplatin-sensitive and resistant human ovarian carcinoma cells. Cancer Res. 1994 May 15;54(10):2680–2687. [PubMed] [Google Scholar]
- Kamen B. A., Capdevila A. Receptor-mediated folate accumulation is regulated by the cellular folate content. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5983–5987. doi: 10.1073/pnas.83.16.5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane M. A., Elwood P. C., Portillo R. M., Antony A. C., Najfeld V., Finley A., Waxman S., Kolhouse J. F. Influence on immunoreactive folate-binding proteins of extracellular folate concentration in cultured human cells. J Clin Invest. 1988 May;81(5):1398–1406. doi: 10.1172/JCI113469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh S. Y., Mistry P., Kelland L. R., Abel G., Harrap K. R. Reduced drug accumulation as a major mechanism of acquired resistance to cisplatin in a human ovarian carcinoma cell line: circumvention studies using novel platinum (II) and (IV) ammine/amine complexes. Br J Cancer. 1992 Dec;66(6):1109–1115. doi: 10.1038/bjc.1992.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Han J., Scanlon K. J. Biochemical and molecular properties of cisplatin-resistant A2780 cells grown in folinic acid. J Biol Chem. 1988 Apr 5;263(10):4891–4894. [PubMed] [Google Scholar]
- Masuda H., Ozols R. F., Lai G. M., Fojo A., Rothenberg M., Hamilton T. C. Increased DNA repair as a mechanism of acquired resistance to cis-diamminedichloroplatinum (II) in human ovarian cancer cell lines. Cancer Res. 1988 Oct 15;48(20):5713–5716. [PubMed] [Google Scholar]
- Matsue H., Rothberg K. G., Takashima A., Kamen B. A., Anderson R. G., Lacey S. W. Folate receptor allows cells to grow in low concentrations of 5-methyltetrahydrofolate. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6006–6009. doi: 10.1073/pnas.89.13.6006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miotti S., Alberti S., Facheris P., Mantovani L., Fornaro M., Stella M., Ménard S., Canevari S., Colnaghi M. I. Membrane association and shedding of the GPI-anchored Ca-MOv18 antigen in human ovary carcinoma cells. Int J Cancer. 1992 May 28;51(3):499–505. doi: 10.1002/ijc.2910510326. [DOI] [PubMed] [Google Scholar]
- Miotti S., Canevari S., Ménard S., Mezzanzanica D., Porro G., Pupa S. M., Regazzoni M., Tagliabue E., Colnaghi M. I. Characterization of human ovarian carcinoma-associated antigens defined by novel monoclonal antibodies with tumor-restricted specificity. Int J Cancer. 1987 Mar 15;39(3):297–303. doi: 10.1002/ijc.2910390306. [DOI] [PubMed] [Google Scholar]
- Miotti S., Facheris P., Tomassetti A., Bottero F., Bottini C., Ottone F., Colnaghi M. I., Bunni M. A., Priest D. G., Canevari S. Growth of ovarian-carcinoma cell lines at physiological folate concentration: effect on folate-binding protein expression in vitro and in vivo. Int J Cancer. 1995 Nov 3;63(3):395–401. doi: 10.1002/ijc.2910630316. [DOI] [PubMed] [Google Scholar]
- Misawa T., Kikkawa F., Maeda O., Obata N. H., Higashide K., Suganuma N., Tomoda Y. Establishment and characterization of acquired resistance to platinum anticancer drugs in human ovarian carcinoma cells. Jpn J Cancer Res. 1995 Jan;86(1):88–94. doi: 10.1111/j.1349-7006.1995.tb02992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry P., Kelland L. R., Abel G., Sidhar S., Harrap K. R. The relationships between glutathione, glutathione-S-transferase and cytotoxicity of platinum drugs and melphalan in eight human ovarian carcinoma cell lines. Br J Cancer. 1991 Aug;64(2):215–220. doi: 10.1038/bjc.1991.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow C. S., Cowan K. H. Glutathione S-transferases and drug resistance. Cancer Cells. 1990 Jan;2(1):15–22. [PubMed] [Google Scholar]
- Nakagawa M., Nomura Y., Kohno K., Ono M., Mizoguchi H., Ogata J., Kuwano M. Reduction of drug accumulation in cisplatin-resistant variants of human prostatic cancer PC-3 cell line. J Urol. 1993 Dec;150(6):1970–1973. doi: 10.1016/s0022-5347(17)35948-7. [DOI] [PubMed] [Google Scholar]
- Newman E. M., Lu Y., Kashani-Sabet M., Kesavan V., Scanlon K. J. Mechanisms of cross-resistance to methotrexate and 5-fluorouracil in an A2780 human ovarian carcinoma cell subline resistant to cisplatin. Biochem Pharmacol. 1988 Feb 1;37(3):443–447. doi: 10.1016/0006-2952(88)90212-2. [DOI] [PubMed] [Google Scholar]
- Ozols R. F. Ovarian cancer, Part II: Treatment. Curr Probl Cancer. 1992 Mar-Apr;16(2):61–126. [PubMed] [Google Scholar]
- Ozols R. F., Young R. C. Chemotherapy of ovarian cancer. Semin Oncol. 1991 Jun;18(3):222–232. [PubMed] [Google Scholar]
- Rosenberg B. Fundamental studies with cisplatin. Cancer. 1985 May 15;55(10):2303–23l6. doi: 10.1002/1097-0142(19850515)55:10<2303::aid-cncr2820551002>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Scanlon K. J., Kashani-Sabet M. Elevated expression of thymidylate synthase cycle genes in cisplatin-resistant human ovarian carcinoma A2780 cells. Proc Natl Acad Sci U S A. 1988 Feb;85(3):650–653. doi: 10.1073/pnas.85.3.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanlon K. J., Kashani-Sabet M., Miyachi H. Differential gene expression in human cancer cells resistant to cisplatin. Cancer Invest. 1989;7(6):581–587. doi: 10.3109/07357908909017533. [DOI] [PubMed] [Google Scholar]
- Scanlon K. J., Kashani-Sabet M., Miyachi H., Sowers L. C., Rossi J. Molecular basis of cisplatin resistance in human carcinomas: model systems and patients. Anticancer Res. 1989 Sep-Oct;9(5):1301–1312. [PubMed] [Google Scholar]
- Scanlon K. J., Newman E. M., Lu Y., Priest D. G. Biochemical basis for cisplatin and 5-fluorouracil synergism in human ovarian carcinoma cells. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8923–8925. doi: 10.1073/pnas.83.23.8923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shionoya S., Lu Y., Scanlon K. J. Properties of amino acid transport systems in K562 cells sensitive and resistant to cis-diamminedichloroplatinum(II). Cancer Res. 1986 Jul;46(7):3445–3448. [PubMed] [Google Scholar]
- Tagliabue E., Centis F., Campiglio M., Mastroianni A., Martignone S., Pellegrini R., Casalini P., Lanzi C., Ménard S., Colnaghi M. I. Selection of monoclonal antibodies which induce internalization and phosphorylation of p185HER2 and growth inhibition of cells with HER2/NEU gene amplification. Int J Cancer. 1991 Apr 1;47(6):933–937. doi: 10.1002/ijc.2910470625. [DOI] [PubMed] [Google Scholar]
- Tedeschi M., Bohm S., Di Re F., Oriana S., Spatti G. B., Tognella S., Zunino F. Glutathione and detoxification. Cancer Treat Rev. 1990 Sep;17(2-3):203–208. doi: 10.1016/0305-7372(90)90048-k. [DOI] [PubMed] [Google Scholar]
- Tosi E., Valota O., Negri D. R., Adobati E., Mazzoni A., Meazza R., Ferrini S., Colnaghi M. I., Canevari S. Anti-tumor efficacy of an anti-epidermal-growth-factor-receptor monoclonal antibody and its F(ab')2 fragment against high- and low-EGFR-expressing carcinomas in nude mice. Int J Cancer. 1995 Sep 4;62(5):643–650. doi: 10.1002/ijc.2910620525. [DOI] [PubMed] [Google Scholar]


