Abstract
Shigella spp. are gram-negative pathogenic bacteria that evolved from harmless enterobacterial relatives and may cause devastating diarrhea upon ingestion. Research performed over the last 25 years revealed that a type III secretion system (T3SS) encoded on a large plasmid is a key virulence factor of Shigella flexneri. The T3SS determines the interactions of S. flexneri with intestinal cells by consecutively translocating two sets of effector proteins into the target cells. Thus, S. flexneri controls invasion into EC, intra- and intercellular spread, macrophage cell death, as well as host inflammatory responses. Some of the translocated effector proteins show novel biochemical activities by which they intercept host cell signal transduction pathways. An understanding of the molecular mechanisms underlying Shigella pathogenesis will foster the development of a safe and efficient vaccine, which, in parallel with improved hygiene, should curb infections by this widespread pathogen.
INTRODUCTION
Diarrheal diseases caused by bacterial, viral, or parasitic pathogens are a major public health problem. Estimations by the World Health Organization (WHO) indicate that the world population suffered from 4.5 billion incidences of diarrhea causing 1.8 million deaths in the year 2002 (334). Approximately 99% of the cases occurred in developing countries, where poor hygiene and limited access to clean drinking water promote the spread of enteric diseases. Malnutrition and the lack of appropriate medical intervention contribute to the high mortality rate, especially for young children.
Species of the genus Shigella are among the bacterial pathogens most frequently isolated from patients with diarrhea. Five to fifteen percent of all diarrheal episodes worldwide can be attributed to an infection with Shigella, including 1.1 million fatal cases (136). Two-thirds of all episodes and deaths occur in children under 5 years. A recent multicenter study of the epidemiology and microbiology of shigellosis in Asia revealed that the incidence of this disease might even exceed previous estimations, as Shigella DNA could also be detected in up to one-third of culture-negative specimens (323). A general trend toward less severe clinical manifestations of shigellosis and fewer fatal cases was observed in this study. Despite these encouraging observations, the emergence of multidrug-resistant Shigella strains and a continuous high disease incidence imply that shigellosis is an unsolved global health problem (248).
Shigellosis is an acute intestinal infection, the symptoms of which can range from mild watery diarrhea to severe inflammatory bacillary dysentery characterized by strong abdominal cramps, fever, and stools containing blood and mucus. The disease is usually self-limiting but may become life-threatening if patients are immunocompromised or if adequate medical care is not available. A combination of oral rehydration and antibiotics leads to the rapid resolution of infection. Currently, there is no protective Shigella vaccine available, but several vaccines using bacterial components or killed or live-attenuated bacteria for immunization are under development and are being tested in different clinical phases (335). Excellent recent reviews provide further information on the clinical symptoms, diagnostics, and treatment of shigellosis and give an overview on the different approaches currently employed to develop a protective Shigella vaccine (123, 189, 321). Here, we cover recent progress in our understanding of shigellosis on a cellular level and on a molecular level. Specifically, we focus on the type III secretion system (T3SS) of Shigella flexneri, secreted effector proteins, and the responses that the effectors elicit from host cells.
CELLULAR PATHOGENESIS OF SHIGELLOSIS
Shigella spp. are transmitted by the fecal-oral route and enter the human body via the ingestion of contaminated food or water. The bacteria are highly infectious, since as few as 10 to 100 microorganisms are sufficient to cause disease (61). This low infectious dose can at least partially be attributed to the presence of effective acid resistance systems, which enable S. flexneri to survive the acidic environment in the stomach (88). Furthermore, it was shown that Shigella spp. are able to downregulate the expression of antimicrobial peptides, which are important antibacterial effectors constantly released from the mucosal surfaces of the intestinal tract (118). After passage through the stomach and small intestine, the bacteria reach the large intestine, where they establish an infection.
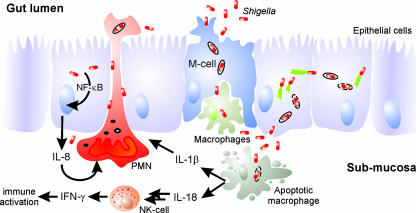
Most of the current knowledge on mechanisms underlying Shigella pathogenesis is derived from studies of S. flexneri. Infection with the invasive pathogen S. flexneri is a multistep process (Fig. 1). To gain access to the intestinal mucosa, S. flexneri crosses the intestinal epithelium, which evolved as a physical and functional barrier to protect the body against the invasion of commensal and pathogenic bacteria (249). In the initial phase of infection, S. flexneri apparently does not invade the epithelial barrier from the apical side but instead triggers its uptake into microfold cells (M cells) and is transcytosed across the epithelial layer (250, 325). M cells are specialized epithelial cells (EC), which continuously sample particles from the gut lumen and deliver them to the underlying mucosal lymphoid tissue, where immune responses can be initiated (157). The use of M cells as an entry port is consistent with the in vitro observation that S. flexneri barely interacts with the apical surface of polarized EC and enters these cells preferentially through the basolateral pole (179).
FIG. 1.
Cellular pathogenesis of Shigella spp. S. flexneri passes the EC barrier by transcytosis through M cells and encounters resident macrophages. The bacteria evade degradation in macrophages by inducing an apoptosis-like cell death, which is accompanied by proinflammatory signaling. Free bacteria invade the EC from the basolateral side, move into the cytoplasm by vectorial actin polymerization, and spread to adjacent cells. Proinflammatory signaling by macrophages and EC further activates the innate immune response involving NK cells and attracts PMN. The influx of PMN disintegrates the EC lining, which initially exacerbates the infection and tissue destruction by facilitating the invasion of more bacteria. Ultimately, PMN phagocytose and kill Shigella, thus contributing to the resolution of the infection.
After transcytosis, S. flexneri is released into an intraepithelial pocket, where the bacteria encounter resident macrophages that engulf and degrade incoming material. S. flexneri ensures its survival in macrophages by rapidly inducing apoptosis (119, 347, 348). Macrophage cell death is accompanied by the release of the proinflammatory cytokines interleukin-1β (IL-1β) and IL-18 (256, 345). Both cytokines are critical mediators of the acute and massive inflammatory response elicited by S. flexneri (Fig. 1). While IL-1β signaling triggers the strong intestinal inflammation characteristic of shigellosis, IL-18 is involved in the generation of an effective antibacterial response (251, 256). IL-18 activates natural killer (NK) cells and promotes the production of gamma interferon (IFN-γ), thus amplifying innate immune responses (148, 328). However, the role of this cytokine in shigellosis has not been completely elucidated.
Once released from the dying macrophage, S. flexneri is able to invade EC from the basolateral side, escapes from the phagosome, and replicates in the cytoplasm (Fig. 1) (257). Cytoplasmic S. flexneri moves by directed polymerization of actin, which allows the bacteria to spread to adjacent EC, avoiding exposure to extracellular components of the host immune defense (22, 174, 291). Nevertheless, this strategy is a double-edged sword, as the invasion of EC also elicits a strong inflammatory response. The Nod1-mediated intracellular surveillance system senses bacterial peptidoglycan fragments released by S. flexneri and activates nuclear factor κB (NF-κB), which triggers the up-regulation and secretion of the chemokine IL-8 (83, 211, 217, 252). IL-8 mediates a massive recruitment of polymorphonuclear neutrophil leukocytes (PMN) to the site of infection (252, 286). Furthermore, recent findings indicate that S. flexneri secretes effector proteins that actively shape the transcriptional response of infected EC to promote PMN migration (169, 344).
Infiltrating PMN destroy the integrity of the epithelial lining, thus enabling more luminal bacteria to reach the submucosa without the need of M cells (214, 215) (Fig. 1). In addition, S. flexneri seems to weaken the sealing of the EC tight junctions by altering the tight-junction protein composition (242). Thus, macrophage killing, destruction of the epithelial layer, and the massive influx of PMN exacerbate the bacterial infection and tissue lesion. These processes are essential for the development of diarrhea and the characteristic pathology of shigellosis. Ultimately, however, the PMN recruited to the site of infection entrap and kill the bacteria, thereby resolving the infection (29, 158, 342). In addition, IFN-γ plays a crucial role for the innate resistance to S. flexneri infection (328). The activation of macrophages and their protection from S. flexneri-triggered cell death likely account for the effects of IFN-γ at least partially (108).
The severe tissue destruction caused by Shigella spp. results in an impaired adsorption of water, nutrients, and solutes, which might cause the watery diarrhea as well as the blood and mucus in stools characteristic of shigellosis. A disturbance of electrolyte homeostasis and changes in membrane transport processes, such as uncontrolled ion and fluid secretion, are typical of diarrheal diseases (147). However, the exact mechanism underlying the onset of diarrhea during shigellosis is still poorly defined. Notably, Shigella enterotoxin 1 (ShET1) and ShET2, which are produced by several Shigella strains, were found to induce fluid secretion into the intestine, thus accounting for the watery phase of diarrhea (70, 183). Moreover, Shiga toxin, which is produced only by Shigella dysenteriae serotype 1, is cytotoxic for a variety of cell types and is responsible for the development of vascular lesions in the colon, the kidney, and the central nervous system (37). Due to the high toxicity of Shiga toxin, infections with S. dysenteriae serotype 1 are frequently associated with life-threatening complications (37, 199).
Even though massive inflammation promotes the initial infection by S. flexneri, recent reports provide evidence that bacteria secrete effectors that actively downregulate proinflammatory signals, perhaps to balance the severity of the inflammation on a level beneficial for the bacteria (14, 117, 131, 197). It is becoming increasingly clear that S. flexneri skillfully exploits and evades the potentially harmful responses of the immune system (216).
EVOLUTION OF SHIGELLA VIRULENCE
Shigella spp. are gram-negative, nonsporulating, rod-shaped bacteria that belong to the family Enterobacteriaceae. The bacteria are facultative intracellular pathogens that show a high specificity for human or primate hosts. The first report on the isolation and characterization of bacteria causing bacillary dysentery, later named Shigella, was published by Kiyoshi Shiga at the end of the 19th century (280). Descriptions of numerous differing strains followed over the next decades, with all of them being closely related to the nonpathogenic bacterium Escherichia coli. To distinguish the pathogenic strains of high clinical relevance from less-pathogenic or nonpathogenic strains, the genus Shigella was defined based on biochemical, serological, and clinical phenotypes (69). The genus Shigella includes the four species S. dysenteriae (serogroup A), S. flexneri (serogroup B), S. boydii (serogroup C), and S. sonnei (serogroup D). According to variations in their O antigens, the species were further divided into several serotypes. S. flexneri and, to a lesser extent, S. sonnei are endemic and cause the majority of all infections (136). S. dysenteriae accounts for epidemic disease outbreaks and the most severe form of dysentery, which causes the majority of fatal shigellosis cases.
Today, this traditional classification is challenged from results obtained by comparative genomics. Several studies using different technical approaches clearly prove that Shigella spp. belong to the species E. coli, rather than forming a separate genus (79, 192, 225, 240). The sequence divergence between S. flexneri and E. coli K-12 is about 1.5% (146). This is marginal compared to, e.g., 15% in the case of pathogenic Salmonella enterica, which is also closely related to E. coli. Moreover, diarrheagenic enteroinvasive E. coli (EIEC) strains share biochemical characteristics, essential virulence factors, and clinical symptoms with Shigella spp. While EIEC does not completely fulfill the definition of the genus Shigella, genome analysis revealed a closer relationship to Shigella spp. than to commensal E. coli strains (144, 337).
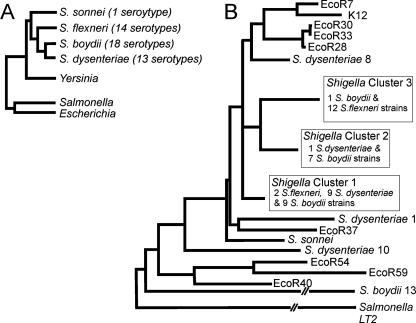
Comparative genomics clearly indicates that Shigella spp. and EIEC evolved from multiple E. coli strains by convergent evolution (226, 337). The two different phylogenetic trees of Shigella deduced either from numerical and phenotypic taxonomy or from comparative genomics are shown in Fig. 2. In the latter, three main Shigella clusters, each containing strains from the traditionally defined species, are identified. S. sonnei and some S. dysenteriae strains are more distantly related to these main clusters but still group with E. coli. The three main Shigella clusters started to diverge from E. coli 35,000 to 270,000 years ago (146). S. sonnei is of more recent origin and separated from the other strains about 10,000 years ago (277). Since EIEC retained more characteristics of commensal E. coli than Shigella spp., these strains apparently acquired the virulence machinery more recently and might reflect an earlier stage of the evolutionary process undergone by Shigella spp. (144, 336). As there is currently no new nomenclature for Shigella that reflects the evolutionary as well as the phenotypic classifications, the traditional nomenclature for Shigella is used in this review.
FIG. 2.
Phylogenetic trees of Shigella and related enterobacteria. (A) Classical taxonomy of Shigella spp. based on phenotypic and numerical classification. (B) Evolutionary tree of Shigella and nonpathogenic E. coli strains (EcoR strains and K-12) based on comparative genomics. Shigella spp. fall into three main clusters, wherein the traditionally classified species are intermingled. The distribution of several E. coli EcoR strains between the Shigella strains indicates that the Shigella strains evolved from multiple ancestral origins. (Adapted from references 57a and 146 with permission from the Society for General Microbiology [United Kingdom] and Elsevier, respectively.)
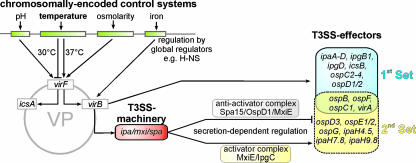
In addition to a reassignment of the phylogenetic relationships between Shigella strains, comparative genomics provides insight into the genetic basis of Shigella virulence. The genetic information constituting the phenotypes of Shigella spp. is encoded on the bacterial chromosome and on a large virulence plasmid. The virulence plasmid is an essential virulence determinant of all Shigella spp. and encodes the molecular machinery necessary for tissue invasion and the intracellular lifestyle (253, 254, 259). The central element of this machinery is a T3SS. The T3SS enables the bacteria to translocate a set of approximately 25 proteins from the bacterial cytoplasm directly into the eukaryotic host cell, where these “effector” proteins interfere with various host cell processes (193, 206, 310).
The complete sequences of the virulence plasmids and chromosomes of several Shigella strains including all four species are currently available (30, 124, 125, 186, 320, 329, 336). Furthermore, the genomes of a strain collection representing all serotypes of Shigella were characterized by comparative genomic hybridization (213). This vast amount of genetic information allows the identification of the successive genetic events that led to the evolution of pathogenic Shigella from nonpathogenic E. coli and provides insight into how variations in the virulence traits of different Shigella strains developed. Figure 3 illustrates the events that are considered to be most important for the evolution of pathogenic Shigella spp.
FIG. 3.
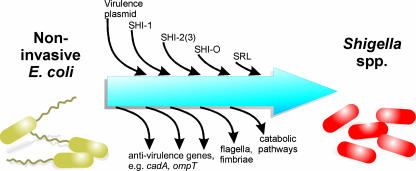
Genetic events contributing to the evolution of Shigella spp. from nonpathogenic E. coli ancestors. Shigella spp. evolved from nonpathogenic E. coli through the acquisition of a large virulence plasmid and chromosomal pathogenicity islands as well as through the loss of genetic loci, which are not functional intracellularly or impede virulence. SRL, Shigella resistance locus. (Modified from reference 191 with permission from Macmillan Publishers Ltd.)
Bacterial evolution is a dynamic process that is accelerated by the gain of phenotypic traits through the acquisition of genes by horizontal gene transfer between distantly related microorganisms as well as through the loss of genes by deletion (191). The transfer and chromosomal incorporation of large mobile genetic entities carrying one or more virulence-associated genes, termed pathogenicity islands (PAI), are important steps for the rapid evolution of pathogenic bacteria from nonpathogenic progenitors (57, 266). Besides the presence of virulence genes, PAI are characterized by nucleotide composition and codon usage differing from those of the rest of the genome, a mosaic-like structure, association with mobile genetic elements, and genetic instability, which is reflected by an increased deletion frequency. Both the Shigella chromosome and the virulence plasmid reflect these dynamics by containing numerous insertion sequences (IS) and markers of genomic rearrangements (30, 125). The distribution of IS types and the phylogenetic grouping of chromosome- and plasmid-encoded genes indicate that the Shigella chromosome and the virulence plasmid coevolved (67, 145, 337). Moreover, these findings suggest that the acquisition and transfer of plasmids between the strains were early steps in the diversification from E. coli ancestors.
In addition to pathogenicity islands on the virulence plasmid, Shigella pathogenicity islands (SHI) were identified in the chromosome (115, 266). The presence and genomic localization of SHI differ between Shigella strains and may contribute to the variation of virulence phenotypes (213, 336). A specific virulence function has been determined for only some of these genes. The immunoglobulin A-like cytotoxic protease SigA and the enterotoxin ShET1, encoded in SHI-1, were found to induce intestinal fluid accumulation in the rabbit ileal loop model of shigellosis (5, 70, 71), and a role for the serine protease Pic in mucus degradation and tissue invasion was postulated based on in vitro studies (105). Moreover, the SHI-2-encoded aerobactin iron acquisition system was found to contribute to virulence in vivo, and shiA attenuates Shigella-induced inflammation by suppression of T-cell signaling (116, 117, 182). Table 1 summarizes the SHI virulence factors that were identified and further characterized.
TABLE 1.
Virulence factors encoded by chromosomal Shigella PAIs
| PAI | Gene(s) | Biochemical activity | Virulence function(s) | Reference(s) |
|---|---|---|---|---|
| SHI-1 | sigA | Immunoglobulin A-like cytopathic protease | Intestinal fluid accumulation, cytopathic toxin | 5 |
| pic | Serine protease, mucinase | Mucus permeabilization, serum resistance, hemagglutination | 105 | |
| set1A, set1B | Enterotoxin ShET1 | Intestinal fluid accumulation, development of watery diarrhea | 70, 71 | |
| SHI-2 | iucA to iucD, iutA | Siderophore (aerobactin) production and transport | Iron acquisition | 177, 182, 322 |
| shiD | Unknown | Colicin I and colicin V immunity | ||
| shiA | Unknown | Downregulation of inflammation by suppression of T-cell signaling | 116, 117 | |
| SHI-3 | iucA to iucD, iutA | Aerobactin production and transport | Iron acquisition (found only in S. boydii) | 227 |
| SHI-O | gtrA, gtrB, gtrV | Modification of O antigens, serotype conversion | Evasion of host immune response | 113, 115 |
| SRL | fecA to fecE, fecI, fecR | Ferric dicitrate uptake | Iron acquisition | 154, 314 |
| tetA to tetD, tetR | Efflux system | Tetracycline resistance | ||
| cat | Acetyltransferase | Chloramphenicol resistance | ||
| oxa-1 | β-Lactamase | Ampicillin resistance | ||
| aadA1 | Adenylyltransferase | Streptomycin resistance |
A late but important event in the diversification of Shigella strains was the acquisition of SHI-O. This PAI harbors genes modifying the structure of the bacterial lipopolysaccharide (LPS) O antigens, thus accounting for the large variety of Shigella serotypes (186). LPS is a crucial virulence factor, and the host immune response to Shigella is serotype specific (153, 216a, 343). The most recent acquisition of genes with new functions is reflected by the emergence of multidrug-resistant Shigella strains. A PAI, designated the Shigella resistance locus, confers resistance to streptomycin, ampicillin, chloramphenicol, and tetracycline (154, 313, 314).
Besides the extensive gain of virulence factors, the evolution of Shigella was shaped by a substantial loss of gene function due to deletion or gene inactivation (Fig. 3). Compared to E. coli K-12, an average of 726 genes are missing, and over 200 pseudogenes are found per Shigella strain (213, 336). Genome reduction due to the loss of functional genes is characteristic of the adaptation of bacteria to an intracellular pathogenic lifestyle (164, 175). Different factors drive this reductive evolution. The arrival of new virulence factors does not enhance bacterial virulence per se, as their expression and function are modulated by the existing genomic background. To be beneficial and therefore retained, virulence factors might require a specific genomic background (66). Consequently, genes that attenuate or interfere with virulence are prone to be inactivated.
Shigella prominently exemplifies the inactivation of so-called “antivirulence genes” (Fig. 3). The outer membrane protease OmpT, which is present in various nonpathogenic E. coli strains but deleted in all Shigella strains, was found to inhibit the spread and virulence of Shigella by interfering with the polar localization of the actin nucleator protein IcsA (VirG) (180, 213). Virulence attenuation was also observed after the introduction of the cadA gene into Shigella. cadA encodes a lysine decarboxylase, which catalyzes the production of the polyamine cadaverine (166). Cadaverine inhibits the activity of the Shigella enterotoxins, thus causing a less severe disease. Genome analyses confirmed that cadA, present in ancestral E. coli strains, was deleted in most of the Shigella strains (46, 213).
Colonizing a host exerts strong selective pressure on the optimization of virulence traits. In parallel, continuous interaction with a host reduces the pressure to be able to respond to environmental changes encountered by free-living bacteria. Nutrients are abundant within hosts, which renders many bacterial metabolic pathways dispensable. Hence, catabolic pathways, e.g., lactose fermentation, represent a substantial proportion of genes inactivated or eliminated from the Shigella genome (120, 336). Furthermore, recent reports suggest that de novo synthesis of NAD is inactivated in Shigella spp. and EIEC, because the NAD precursor quinolinate strongly inhibits bacterial virulence (223, 224). These and other intermediates of antivirulence pathways might hold a potential for novel virulence inhibitors.
Shigella spp. have also lost the ability to synthesize functional flagella. Comparisons of different Shigella strains revealed that the genetic basis of this motility defect varies, indicating that the loss of motility is a result of convergent evolution (11, 307). It is tempting to speculate that flagellar motility was abandoned because it was not suitable as a mode of intracellular movement and therefore was substituted by actin-based motility, which exploits host resources. In addition, flagella and other surface structures like fimbriae, which are also degenerated in Shigella, are potent activators of the host immune system (21, 232, 244). The loss of these surface structures might therefore help to minimize the exposure to the host defense system.
MOLECULAR DETERMINANTS OF SHIGELLA PATHOGENESIS
The Shigella Virulence Plasmid
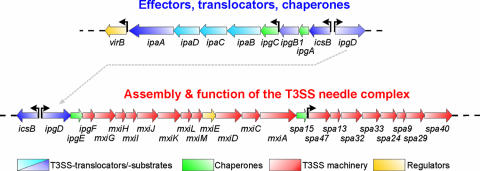
The cellular pathogenesis and clinical presentation of shigellosis are the sum of the complex action of a large number of bacterial virulence factors. The essential part of the molecular machinery required for bacterial invasion and intracellular survival is encoded on the large S. flexneri virulence plasmid (247, 253, 254, 259). Sequencing of virulence plasmids from different Shigella strains revealed that these plasmids of approximately 200 kb contain a mosaic of around 100 genes and a comparable number of IS (30, 124, 320, 336). The core of the plasmid is the conserved 31-kb entry region, which was shown to be necessary and sufficient for EC invasion and macrophage killing (129, 165, 260-262). The PAI-like region consists of 34 genes that are organized into two clusters transcribed in opposite directions (Fig. 4). Based on their functions, these genes can be divided into four different groups.
FIG. 4.
Map of the 31-kb “entry region” on the S. flexneri virulence plasmid pWR100. The genes indicated encode structural components of the Mxi-Spa T3SS, secreted translocator and effector proteins, chaperones, and regulatory proteins. The region shown is essential and sufficient to invade EC and induce macrophage cell death.
The first group consists of proteins secreted by the S. flexneri T3SS that act as effectors manipulating host cell processes in favor of the bacteria (see Table 2 and sections below). Among these proteins are the dominant immunogenic antigens of S. flexneri, the invasion plasmid antigens IpaA to IpaD (32, 190). Three of them, IpaB to IpaD, are the key virulence factors of S. flexneri. Apart from having effector functions that are essential for host cell invasion and intracellular survival, these proteins also control the secretion and translocation of other effector proteins into eukaryotic host cells (23, 170, 171).
TABLE 2.
Virulence factors encoded on the S. flexneri virulence plasmid
| Effector | Biochemical activity | Host cell target(s) | Virulence function and/or phenotype | Reference(s)b |
|---|---|---|---|---|
| IpaA | Vinculin activation | Vinculin, β1-integrins, Rho signaling | Efficient invasion, actin cytoskeleton rearrangements, disassembly of cell-matrix adherence | 52, 96, 121, 311 |
| IpaB | Membrane fusion | Cholesterol, CD44, caspase-1 | Control of type III secretion, translocon formation, phagosome escape, macrophage apoptosis | 23, 36, 103, 109, 114, 170 |
| IpaC | Actin polymerization | Actin, β-catenin | Translocon formation, filopodium formation, phagosome escape, disruption of EC tight junctions | 23, 100, 171, 312 |
| IpaD | Control of type III secretion, membrane insertion of translocon | 68, 170, 317 | ||
| IpaH7.8 | Efficient phagosome escape | 73 | ||
| IpaH9.8 | E3 ubiquitin ligase | Splicing factor U2AF, MAPK kinase | Host cell transcriptome modulation, reduction of inflammation | 197, 236, 309 |
| IcsAa (VirG) | N-WASP, vinculin | Recruitment of actin-nucleating complex required for actin-based motility and intercellular spread | 22, 84, 296, 298 | |
| IcsB | Camouflage of IcsA to prevent autophagic recognition | 7, 194, 195 | ||
| IcsPa | Serine protease | Cleavage of IcsA, modulation of actin-based motility | 63, 278 | |
| IpgB1 | RhoG mimicry | ELMO protein | Induction of Rac1-dependent membrane ruffling | 12, 99, 196 |
| IpgB2 | RhoA mimicry | RhoA ligands | Induction of actin stress fiber-dependent membrane ruffling | 12 |
| IpgD | Phosphoinositide 4-phosphatase | Phosphatidylinositol 4,5-bisphosphate | Facilitation of entry, promotion of host cell survival | 187, 188, 212 |
| OspB | T3SS substrate, unknown function | 258 | ||
| OspC1 | Nucleus and cytoplasm | Induction of PMN migration | 344 | |
| OspD1 | T3SS substrate, unknown function in host cells, antiactivator of MxiE | 207 | ||
| OspE2 | Focal contacts | Maintenance of EC morphology, efficient intercellular spread | 173 | |
| OspF | Phosphothreonine lyase | MAPKs Erk and p38 | Inhibition of histone phosphorylation and NF-κB-dependent gene expression, reduction of PMN recruitment | 14, 137, 151, 344 |
| OspG | Protein kinase, ubiquitination inhibitor | Ubiquitin-conjugating enzymes | Downregulation of NF-κB activation, reduction of inflammation | 131 |
| PhoN2a | Apyrase | Unipolar localization of IcsA | 258 | |
| SepAa | Serine protease | Promotion of intestinal tissue invasion and destruction | 20 | |
| VirA | Cysteine protease | α-Tubulin | Facilitation of entry and intracellular motility by degradation of microtubules | 338, 339 |
These proteins are not substrates of the Mxi-Spa T3SS.
For the sake of clarity, only the most relevant references are cited.
Genes of the second group comprise more than half of the entry region and are required for the secretion of the Ipa proteins and other effector proteins. These genes were designated membrane expression of ipa (mxi) and surface presentation of ipa (spa) antigens (112, 319). The mxi-spa locus encodes the components needed for the assembly and function of a T3SS, which, together with IpaB, IpaC, and IpaD, allows the direct translocation of effector proteins from the bacterial cytoplasm into the host cell (23). Approximately 25 proteins encoded in different locations on the virulence plasmid are secreted via this system (30).
In addition to the fundamental bacterial weaponry, the entry region contains the two transcriptional activators VirB and MxiE, representing group 3, which regulate T3SS-associated genes located in the entry region or scattered throughout the remainder of the virulence plasmid (2, 59, 128, 167). Finally, four genes, classified as group 4, encode chaperones (ipgA, ipgC, ipgE, and spa15) and are also located within the entry region. These chaperones stabilize T3SS substrates in the bacterial cytoplasm, and at least two of them, IpgC and Spa15, participate in the transcriptional regulation of T3SS effector genes located outside of the entry region (167, 201, 207, 208, 316).
Most of the T3SS effector proteins encoded outside of the entry region adopt postinvasion virulence functions. Several members of the outer Shigella protein (Osp) family shape the transcriptional response of EC invaded by S. flexneri, thus modulating the immune response in favor of the bacteria (14, 30, 131, 344). A role in the modulation of the immune response was also established for one member of the multigene IpaH family (102, 197). Members of the IpaH family share a conserved C-terminal domain and a variable N-terminal leucine-rich repeat (LRR) domain (318). LRR domains are involved in the sensing of pathogen-associated molecular patterns (PAMPs) by host cells and the subsequent induction of immune responses (19, 78, 163). Five IpaH genes have been identified on the virulence plasmid, and, depending on the Shigella strain, up to seven genes have been identified in the chromosome (125). The chromosomal IpaH genes seem to be regulated by MxiE/IpgC and are the only chromosomally encoded T3SS substrates (15, 168).
The dissemination of S. flexneri in the epithelial layer crucially depends on the ability to move by directed actin polymerization. The essential mediators of efficient movement by actin nucleation at one pole of the bacterium are IcsA/VirG, SopA/IscP, VirA, and PhoN2 (apyrase). The corresponding genes are also located outside of the entry region on the virulence plasmid (22, 63, 150, 156, 258, 338, 339). Among these proteins, only the secretion of VirA depends on the T3SS. In contrast, IcsA and SepA belong to the family of autotransporter proteins, which possess a C-terminal domain mediating secretion (20, 56, 185). The secreted serine protease SepA was found to enhance tissue destruction and inflammation in a rabbit ligated ileal loop model.
Finally, besides the large repertoire of genes implicated in bacterial virulence, the virulence plasmid encodes systems to ensure its replication and propagation. These systems include a postsegregational killing system, ensuring plasmid maintenance, particularly at 37°C, the temperature of infection (228, 263, 264).
Regulation of Virulence Plasmid Gene Expression
The function of the plasmid-encoded virulence machinery requires the complex interaction of several dozen proteins, the permanent expression of which would be energetically costly. Consequently, all virulence genes are under the tight control of a regulatory network, which responds to environmental changes encountered upon the entry of the bacteria into the host (58, 59, 222, 308) (Fig. 5). The major trigger inducing the expression of the virulence plasmid entry region genes is a temperature shift to 37°C after uptake by the host (305). Additional factors that influence virulence plasmid gene expression are pH, osmolarity, and iron concentration (181, 210, 221).
FIG. 5.
Regulatory elements controlling the expression of the T3SS and its substrates on the S. flexneri virulence plasmid. The major virulence plasmid (VP) activator VirF is induced by environmental stimuli and triggers the expression of the central transcriptional activator VirB. VirB promotes the expression of the entry region genes and some additional effector genes scattered on the virulence plasmid. The secretion of the “first-set” T3SS effectors stored in the bacterial cytoplasm enables MxiE/IpgC-controlled induction of a discrete set of already-induced effector proteins and expression of the “second-set” effectors.
The primary elements of the regulatory cascade, which sense and react to environmental changes, are encoded on the S. flexneri chromosome. Global regulators control the expression of chromosomal as well as virulence plasmid genes (58, 222). The virulence plasmid-encoded transcriptional activator VirF is induced at 37°C (305). VirF activates the second essential virulence plasmid regulator VirB and the actin nucleator protein IcsA (2, 220, 243, 306). VirB in turn induces the transcription of the entry region operons and some additional genes of the Osp family (149). Thus, the bacteria are equipped with the T3SS and a first set of effector proteins, allowing the invasion of host cells (Fig. 5).
The secretion of these “first-set” effector proteins leads to the increased transcription of a subset of already-induced proteins and a “second set” of additional T3SS effectors (53, 149, 167). The expression of the “second-set” effectors is controlled by the transcriptional activator MxiE, the activity of which is blocked by an antiactivator complex consisting of OspD1 and the chaperone Spa15 (207). Upon the induction of type III secretion, the “first-set” effector OspD1 is secreted along with other substrates like IpaB and IpaC, thus liberating the cognate chaperone IpgC. Consequently, MxiE associates with the coactivator IpgC (167), and the MxiE-IpgC complex activates the transcription of the “second-set” effectors (Fig. 5), which are secreted by intracellular bacteria.
The Mxi-Spa T3SS
S. flexneri, as well as other gram-negative pathogenic or symbiotic bacteria, manipulates host cell processes to establish a successful infection. The bacteria directly target intracellular host cell signaling pathways by transporting effector proteins across three membranes: the bacterial inner membrane (IM) and outer membrane (OM) as well as the host plasma membrane. The S. flexneri Mxi-Spa T3SS is a representative of sophisticated molecular export machineries that translocate proteins into the host cell cytoplasm in one step (43, 82). T3SSs are found in more than 25 bacterial species interacting with animal or plant hosts. It is noteworthy that several of these species encode two T3SSs, which usually belong to different subfamilies of T3SSs. As an example, Salmonella enterica serovar Typhimurium harbors two T3SSs encoded in the Salmonella pathogenicity island 1 (SPI-1) or SPI-2. More than 100 different T3SS substrates with versatile biological activities are currently known (1, 90, 265, 340). The architecture of T3SSs from different bacterial species shows some adaptation to the specific hosts but shares a conserved main structure, which is closely related to those of the flagellar T3SS (25, 43, 301). Based on this conserved core structure, T3SSs can be distinguished from type IV secretion systems (T4SSs), which also permit the secretion and direct translocation of proteins from the bacteria into the host cell cytoplasm. T4SSs are found in many gram-negative bacteria, e.g., Legionella spp., and are related to bacterial DNA conjugation systems (16, 38, 275).
Architecture of the Mxi-Spa T3SS.
The visualization of major parts of the closely related T3SSs encoded by S. flexneri and Salmonella SPI-1 by transmission electron microscopy revealed the ultrastructure of this multiprotein complex and allowed the determination of its dimensions (23, 24, 138, 245, 299). The observed macromolecular structure, termed the needle complex, is comprised of a seven-ringed basal body with an average length of 32 nm and a protruding needle with a length of 45 to 60 nm. The rings of the basal body have a diameter of 20 to 40 nm, whereas the outer diameter of the needle is approximately 7 nm. Consistent with a model of a one-step shuttling system, the needle complex contains a central 2- to 3-nm-wide channel. The narrow channel size does not permit the transport of fully folded proteins. Indeed, it was shown that the Salmonella serovar Typhimurium T3SS substrate SptP is separated from its cognate chaperone and largely unfolded prior to its export via the T3SS (3). However, structural models of the T3SS needle and T3SS substrates suggest that some secondary structures such as alpha helices, which are retained after partial unfolding, might be able to pass the needle (47, 65).
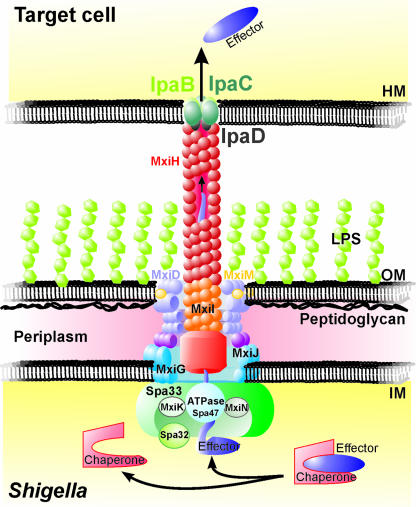
The molecular architecture, assembly, and function of the S. flexneri Mxi-Spa T3SS involves more than 25 proteins encoded in the entry region of the virulence plasmid (43) (Fig. 6). The first step of the assembly of the T3SS is the formation of the seven-ringed basal body, which spans the bacteria IM, periplasm, peptidoglycan layer, and OM. The major constituent of the OM rings of the basal body is the secretin protein MxiD, which is transported to the periplasm by the Sec-dependent secretion pathway (9, 24, 299). Membrane insertion and stabilization of the homo-oligomeric MxiD ring is essentially mediated by the lipoprotein MxiM, also referred to as pilot protein, which is anchored in the periplasmic leaflet of the OM by a lipid moiety (270, 271). The formation of the OM rings requires the penetration of the peptidoglycan meshwork underlying the OM. The efficient assembly and function of T3SSs or T4SSs of different bacterial species, e.g., Citrobacter rodentium or Helicobacter pylori, depends on the peptidoglycan-degrading activity of specialized lytic transglycosylases (54, 77). Interestingly, the protein IpgF encoded next to the mxi-spa locus belongs to this enzyme family and exhibits peptidoglycan-degrading activity in vitro, yet a deletion of this gene does not attenuate virulence in vivo (6, 341). This might indicate that IpgF is not required or functionally redundant for efficient T3SS formation in Shigella.
FIG. 6.
Architecture of the S. flexneri Mxi-Spa T3SS. The S. flexneri Mxi-Spa T3SS consists of four main parts. The seven-ringed basal body spans the bacterial IM, the periplasm, and the OM. The hollow needle is attached to a socket and protrudes from the basal body to the bacterial surface. Contact with host cell membranes (HM) triggers the IpaD-guided membrane insertion of the IpaB-IpaC translocon at the needle tip. The T3SS is completed by the cytoplasmic C ring, which is comprised of proteins that energize the transport process and mediate the recognition of substrates, chaperone release, and substrate unfolding.
Periplasmic and IM rings consist of the lipoprotein MxiJ and the membrane protein MxiG (Fig. 6). These proteins are anchored or inserted into the IM and interact with MxiD and MxiM through periplasmic domains (8, 10, 245, 271). Furthermore, based on sequence similarities with components of the flagellar apparatus, the Mxi-Spa proteins MxiA, Spa9, Spa24, Spa29, and Spa40 are proposed to be associated with the IM ring of the basal body (25, 43). Once the basal body is completed, the T3SS needle is assembled. The needle is composed of the major subunit MxiH and the minor component MxiI, which form a helical polymer extruding into the extracellular space (24, 42, 299). The assembly of the T3SS needle is controlled by cytoplasmic proteins associated with the IM ring of the basal body. The backbone of this cytoplasmic interface of the secretion apparatus, also called the C ring, is the Spa33 protein, which interacts with several T3SS effectors and a set of essential T3SS components (176, 272). This set includes the MxiN and MxiK proteins, which are required for the externalization of the needle subunits but not effector proteins, and Spa32, which determines the length of the T3SS needle (127, 155, 300). The control of the needle length seems to be of high importance for S. flexneri, as single isolated needles are of a strikingly uniform length (24, 299). In fact, it was shown that the needle length coevolved with the length of the S. flexneri LPS O antigens and is adjusted to allow maximal infectivity along with optimal protection against innate immune effectors (330). It is not fully understood how Spa32 regulates needle length, but the protein is proposed to switch substrate specificity of the growing T3SS from the major needle subunit MxiH to the Ipa proteins (155).
Substrate recognition and energetics of type III secretion.
The assembly of the T3SS needle and the subsequent effector secretion also depend on the C-ring ATPase Spa47 (299, 319). T3SS-associated ATPases, such as Spa47, are believed to provide the energy for the unfolding of T3SS substrates, chaperone release, and transmembrane transport (3, 331). The transport of substrates across biological membranes is a thermodynamically unfavorable process and has to be energized by the hydrolysis of ATP as well as the transmembrane proton motive force (4). Accordingly, type III secretion of Yersinia enterocolitica effector proteins consumes ATP and requires the proton motive force (332). In agreement with these findings, the protonophore carbonyl cyanide m-chlorophenylhydrazone blocked S. flexneri type III secretion in infected macrophages as well as in vitro, triggered by the dye Congo red (269).
T3SS ATPases occupy a central position in the secretion process and, together with other C-ring proteins, are also involved in T3SS effector recognition (155, 288). An N-terminal secretion signal targets T3SS effectors to the secretion system, yet it is currently not clear if there is a hierarchy in the release of effector proteins and how intrinsic properties of the effectors contribute to controlling their export (93, 101, 218). Evidence from several bacterial T3SSs indicates an important role for the chaperones of T3SS effectors in orchestrating secretion (31, 72, 202). The S. flexneri T3SS chaperone Spa15 is necessary for the secretion of IpaA stored in the bacterial cytoplasm (203).
Host cell membrane insertion of the translocon.
The complex formed by the C ring, basal body, and needle is sufficient to release proteins into the extracellular space, but a controlled secretion and translocation of effector proteins require the transport of the translocator proteins IpaB, IpaC, and IpaD to the tip of the T3SS needle (170, 317). The translocator proteins are expressed alongside the T3SS components and stored in the cytoplasm. To avoid premature association, IpaB and IpaC are bound by the chaperone IpgC (172), and in addition, IpaD has self-chaperoning activity (126). The exact mechanism of how secretion is controlled by the translocator proteins is not understood. Several reports indicate that in the absence of a host cell, the hydrophilic protein IpaD localizes to the tip of the T3SS needle and blocks secretion through interactions with IpaB (68, 198, 245, 246, 317). In the absence of a secretion trigger, IpaD seems to maintain a conformation and position that retain the hydrophobic protein IpaB inside the needle channel. Upon host cell contact, the T3SS is activated (326). The contact might trigger a conformational change in the “plug” protein IpaD, which also leads to the repositioning of IpaB and allows its passage and membrane insertion along with the second hydrophobic translocator protein IpaC, thus forming the multimeric translocation pore (23, 68, 317). Even though IpaB and IpaC interact and insert into artificial membranes in vitro, the efficient formation of the T3SS translocation pore in vivo strictly depends on IpaD, which might act as a scaffold for the correct translocon assembly (45, 49, 114, 172, 219).
Recent structural and mutational analyses further suggested that, in addition to the translocator proteins IpaB and IpaD, the needle protein MxiH actively participates in the regulation of secretion and host cell sensing (49, 130, 317). As proposed for the tip protein IpaD, MxiH might undergo a subtle conformational change upon host cell contact, which contributes to the correct formation of the needle tip and is relayed through the needle to open the T3SS channel for additional effector proteins. However, structural analysis of MxiH mutants, which display different levels of secretion activity, did not reveal changes in the needle structure, indicating that theses changes are too small to be detected or that MxiH controls secretion through a novel mechanism (41). Once the translocation pore is established and the needle is in the “open” conformation, additional Ipa proteins and other effector proteins can directly pass the T3SS channel and reach their targets inside the host cell.
SUBVERSION OF EPITHELIAL CELL SIGNALING
Adherence and Induction of Type III Secretion
While recent insight in the molecular architecture of the Mxi-Spa T3SS led to a model on how conformational changes in the translocator and needle tip open the transport channel (47, 68, 130), how host cell contact triggers translocation under natural conditions (327) or how the dye Congo red induces secretion in vitro (17) is not well defined.
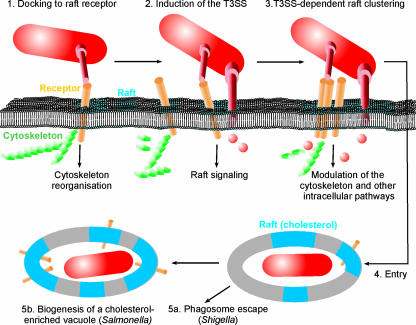
The initial interactions of S. flexneri and its close relative Salmonella with a host cell occur at cholesterol-rich microdomains in the plasma membrane (80, 142) (Fig. 7). Cholesterol is a ubiquitous component of eukaryotic membranes and strongly influences their biophysical properties (283, 285). The majority of cholesterol is found in the plasma membrane of the eukaryotic cell, where it is not distributed evenly but rather accumulates in microdomains enriched in sphingolipids and proteins, so-called lipid rafts (98, 282, 285). A consequence of the unique raft lipid composition is the preferential recruitment or exclusion of membrane proteins, thus creating clusters of specific proteins and an asymmetric distribution of these clusters on the cell surface (238). Raft protein clusters are intimately connected to the actin cytoskeleton and to proteins localizing at the cytoplasmic leaflet of the plasma membrane (152). These exceptional properties predispose rafts as versatile signaling platforms, controlling signal transduction pathways such as endocytosis, intracellular vesicle trafficking, and activation of immune responses and apoptosis (86, 87, 104, 209, 230, 284). The central role of lipid rafts in the signaling networks of eukaryotic cells renders them a prime target for bacterial pathogens. Specifically, the unique protein and lipid composition, especially the high cholesterol content, might be a trigger to release bacterial effectors in close proximity to these signaling hot spots (Fig. 7).
FIG. 7.
Cholesterol and lipid rafts as triggers of type III secretion and targets of effectors. (1) The initial contact of bacteria with cholesterol-rich membrane microdomains is mediated by receptors, which partition to rafts. Receptor binding might already induce raft signaling, e.g., trigger the reorganization of the cytoskeleton. (2) Raft contact is followed by the cholesterol-dependent induction of effector protein secretion. (3) Receptor binding and effector secretion lead to the clustering of rafts and the subsequent uptake of the bacteria as well as the activation of other signaling pathways. (4) After uptake, the bacteria reside in a raft-containing vacuole, which is lysed (5a) or modified to create a replication-permissive niche (5b). Modification of the vacuole leads to a further enrichment of rafts (cholesterol), thus permitting bacterial manipulation of raft-associated signaling pathways.
The T3SS-mediated effector translocation by Shigella, Salmonella, and enteropathogenic E. coli requires the presence of membrane cholesterol (103). Interestingly, purified lipid rafts or liposomes, which mimic raft lipid composition but do not contain proteins, trigger effector secretion by the Mxi-Spa T3SS (315). A biochemical analysis revealed that the translocator protein IpaB and its Salmonella homologue SipB directly bind cholesterol with high affinity and insert into membranes in a cholesterol-dependent manner. The translocon protein IpaC also integrates into cholesterol-containing membranes, but a high cholesterol content strongly reduces its membrane disruption activity (100). However, the in vivo significance of this observation and whether the needle tip protein IpaD also interacts with cholesterol remain unclear. It is noteworthy that a large number of secreted bacterial pore-forming or enzymatically active toxins, such as perfringolysin O and Shiga toxin, also target lipid rafts and require cholesterol for membrane insertion, oligomerization, and activity (204, 235).
The induction of the S. flexneri Mxi-Spa T3SS or Salmonella SPI-1 T3SS at cholesterol-rich membrane microdomains triggers the accumulation of cholesterol and lipid raft markers at the bacterial entry sites (80, 142). This observation indicates that the bacteria promote the aggregation and fusion of rafts at the entry site, thus creating an extended raft signaling platform with a high density of receptors and putative signaling molecules. Several other pathogens, e.g., Mycobacterium spp., Brucella spp., or Chlamydia spp., enter their host cells via rafts (143, 159). S. flexneri adheres to host cells by binding to two receptors, the hyaluronan receptor CD44 and α5β1 integrin, which both partition to lipid rafts and accumulate at the bacterial entry site (287, 326). Binding to CD44 apparently occurs through IpaB, while the α5β1 integrin receptor is bound by a IpaBCD complex. These interactions might precede the induction of the T3SS and direct the bacteria to lipid rafts. Finally, binding to either receptor induces actin cytoskeleton reorganization and promotes invasion by S. flexneri (142, 326).
Entry via lipid rafts not only remodels the actin cytoskeleton but also critically influences the fate of the intracellular bacteria (Fig. 7). Lipid rafts modulate intracellular vesicle trafficking, and raft-derived endosomes do not enter the lysosomal degradation pathway (104). Accordingly, uptake via lipid rafts protects some bacteria, including Mycobacterium spp., against lysosomal degradation (48, 75). Along the same line, the formation of replication-permissive vacuoles by invading bacteria, e.g., the Salmonella-containing vacuole or the parasitophorous vacuole of the obligate intracellular pathogen Coxiella burnetii, coincides with an enrichment of cholesterol and raft proteins on the vacuolar membranes (33, 111). In Salmonella infections, cholesterol acquisition correlates with bacterial replication and seems to depend on the SPI-2 T3SS, which is activated in the intracellular environment. It is tempting to speculate that S. flexneri, which escapes from the phagosome into the host cell cytoplasm, uses the signaling potential of lipid rafts for the manipulation of host cell processes other than the initial steps of invasion. Recently, cholesterol depletion by the compound methyl-β-cyclodextrin after macrophage invasion by S. flexneri was found to inhibit macrophage cell death and the activation of caspase-1 (268). Furthermore, the amount of membrane-bound IpaB in S. flexneri-infected macrophages was reduced under these conditions. These findings indicate that cholesterol-dependent signaling might be required for the proapoptotic cascade induced by intracellular S. flexneri.
Pathogen-Triggered Invasion
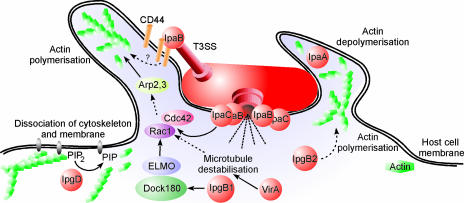
The uptake of S. flexneri depends on complex rearrangements of membranes and the actin cytoskeleton, which are mediated by the controlled temporal and spatial actions of a multitude of bacterial and host cell factors (44, 310) (Fig. 8). As described above, the initial contact between Shigella and host cells takes place at lipid raft membrane domains and is mediated by the receptors CD44 and α5β1 integrin. Receptor binding induces early actin cytoskeleton rearrangements and primes the cell for uptake, but the efficient and complete engulfment of the bacteria is triggered by the translocation of at least six T3SS effector proteins.
FIG. 8.
Subversion of host cell signaling by S. flexneri type III-secreted effectors. Injection of the Mxi-Spa-secreted effectors IpaC, IpgB1, and VirA by S. flexneri induces Rac1/Cdc42-dependent actin polymerization and the formation of large membrane ruffles. The binding of IpaB to the CD44 receptor and the activity of IpgB2 might also trigger cytoskeleton remodeling or membrane ruffling, respectively. The phosphoinositide 4-phosphatase IpgD promotes the disconnection of the actin cytoskeleton from the cytoplasmic membrane, thus facilitating the structural reorganization of the entry site. IpaA mediates the localized depolymerization of actin, which is required to close the phagocytic cup. PIP2, phosphatidylinositol-4,5-biphosphate; PIP, phosphatidylinositol-5-phosphate.
The reorganization of the eukaryotic cytoskeleton is governed predominantly by small Rho GTPases and tyrosine kinases, which renders these enzymes targets for manipulation by pathogenic bacteria (27, 107a, 141). Indeed, S. flexneri, as well as many other bacterial pathogens, requires and manipulates these Rho GTPase pathways to promote its uptake (27, 178). At the entry site, S. flexneri induces massive actin polymerization, leading to the formation of large membrane protrusions, which form a macropinocytic pocket enclosing the bacteria. This process requires the activation of the small GTPases Rac1 and Cdc42, which recruit the actin-nucleating complex Arp2/Arp3 (312). Several bacterial effectors have been implicated in the induction of actin polymerization (Table 2). The translocator protein IpaC was shown to be able to initiate actin nucleation and the formation of filopodial and lamellipodial membrane extensions, yet how IpaC stimulates Rac1 and Cdc42 remains obscure (312).
Recent findings suggest a pivotal role of the T3SS effector IpgB1 in triggering invasion (196). IpgB1 mimics the active small GTPase RhoG and elicits Rac1 activation and membrane ruffling through the ELMO-Dock180 pathway (99). Rac1 is further activated in response to the destabilization of the microtubule network by the S. flexneri cysteine protease VirA (339). Mxi-Spa effectors, which induce actin polymerization at the entry site, might also include IpgB2. The IpgB1 homologue IpgB2 was shown to mimic the GTP-bound form of RhoA, and its expression in eukaryotic cells triggered the formation of actin stress fibers and membrane ruffling (12). However, the specific contribution of IpgB2 to the signaling induced in the course of an infection of EC with S. flexneri remains to be established.
Two additional T3SS effector proteins are necessary for the full invasiveness of S. flexneri (Table 2). The phosphoinositide 4-phosphatase IpgD hydrolyzes phosphatidylinositol-4,5-biphosphate [PtdIns(4,5)P2] to yield phosphatidylinositol-5-phosphate [PtdIns(5)P] (107, 187). Hydrolysis of PtdIns(4,5)P2 leads to the dissociation of the actin cytoskeleton from the plasma membrane, which facilitates the remodeling of membranes and actin. Finally, the translocated effector IpaA binds vinculin and enhances its association to actin filaments, thus mediating localized actin depolymerization and a reduction of the adhesion between cells and the extracellular matrix. This process might promote the closure of the phagocytic cup around the bacteria (28, 52, 231).
Phagosome Escape
After triggering its uptake into host cells, S. flexneri is entrapped in the phagosome. In contrast to other bacterial pathogens like Salmonella serovar Typhimurium, which modify the membrane-bound compartment to create a replication-permissive vacuole (134), S. flexneri lyses the surrounding membranes and escapes into the cytoplasm in less than 15 min (40, 278). Membrane lysis does not depend on the acidification of the phagosome and is mediated by the Mxi-Spa T3SS and the translocator proteins IpaB, IpaC, and IpaD (18, 76, 106, 219). Although all three translocator proteins are needed for the escape from the phagosome, there is evidence that IpaC is the decisive factor mediating membrane lysis. In vitro, purified IpaC disrupts the integrity of phospholipid vesicles after membrane insertion, whereas IpaB forms trimers in artificial membranes without disturbing the membrane bilayer integrity (100, 114). These findings are supported by the observation that the heterologous expression of IpaC in a mutant strain lacking the closely related IpaC homologue SipC not only complements the SipC deletion phenotype but also leads to the lysis of the Salmonella-containing vacuole (200).
In addition to the translocator proteins, the T3SS effector IpaH7.8 was found to promote bacterial phagosome escape in macrophages by an unknown mechanism (73). IpaH7.8 belongs to the “second-set” T3SS effectors, which are expressed after the activation of the T3SS and the translocation of “first-set” effectors (Fig. 5).
Intracellular Motility and Intercellular Dissemination
The cytoplasm of EC is the main replicative niche for S. flexneri. Here, the bacteria are protected from the immune system components present in the extracellular environment. Nevertheless, bacteria have to evade intracellular host defense mechanisms and prepare to spread to new replicative niches. The intracellular survival and intercellular spread of S. flexneri are linked to the machinery allowing the bacteria to move by directed actin polymerization.
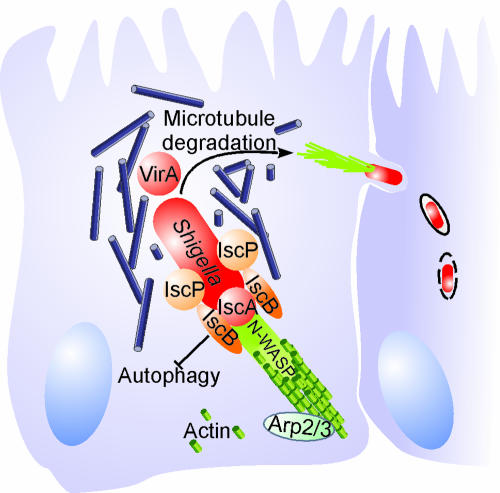
The molecular mechanisms mediating the actin-based motility of S. flexneri and other intracellular pathogens have been studied extensively (89, 291) (Fig. 9). The central bacterial mediator of actin polymerization is the IcsA (intracellular spread) protein, which localizes to one pole of the bacterium (22, 84, 85) (Table 2). The unipolar localization and activity of IscA are controlled by the serine protease SopA/IscP and the apyrase PhoN2 (63, 239, 258, 278, 290). Surface-bound IscA recruits and activates host cell factors, including the neuronal Wiskott-Aldrich syndrome protein (N-WASP) and the Arp2/Arp3 complex (64, 97, 294-296). The complex thus formed serves as an actin nucleator and catalyzes the directed elongation of an actin tail, which propels S. flexneri through the cytoplasm. In addition to the actin-nucleating complex, the T3SS-secreted cysteine protease VirA was recently identified to be pivotal for efficient intracellular movement and subsequent intercellular spread (338). VirA degrades α-tubulin, thus creating a “tunnel” in the dense intracellular microtubule network.
FIG. 9.
Intracellular movement of S. flexneri by directed actin polymerization. Due to the activity of the serine protease SopA/IcsP, S. flexneri IcsA localizes to one pole of the bacterium, where it interacts with the host cell N-WASP protein. The IcsA/N-WASP complex recruits and activates the Arp2/Arp3 complex, thereby mediating actin nucleation. Elongation of the actin tail pushes S. flexneri through the cytoplasm. The movement is facilitated by VirA, which opens a path by degradation of the microtubule network. To avoid sequestration by the autophagy defense system, an autophagy recognition site on IcsA is masked by the protein IcsB.
Intracellular motility, replication, and survival further depend on the T3SS substrate IscB (194), which protects the bacteria from being recognized and entrapped by the host cell autophagy machinery (195). Autophagy is a host cell recycling and defense system, which engulfs and sequesters cytoplasmic components in double-membrane-bound compartments (55, 132). Interestingly, IscA contains an autophagy-inducing recognition site, which has to be masked by IscB to prevent engulfment by autophagic vacuoles and to ensure the intracellular survival of S. flexneri (195).
Due to propulsion through the host cell cytoplasm by actin polymerization, moving S. flexneri eventually impinges on the plasma membrane at the tight junctions of adjacent EC. The arising protrusions can be endocytosed by the neighboring cell in a process requiring host cell factors like myosin light-chain kinase (233). After lysis of the surrounding double membrane, which depends on the T3SS and the translocator proteins IpaB, IpaC, and IpaD, S. flexneri is free in the cytoplasm, and a new cycle of replication and cell-to-cell spread can start (201, 219, 234, 273).
Modulation of Host Cell Survival and Immune Signaling
The intracellular lifestyle protects Shigella spp. from immune system effector cells present in the subepithelial layer. S. flexneri secretes at least two different type III effectors, which promote the survival of the invaded host cells. In addition to being involved in the uptake of the bacteria, IpgD triggers the activation of the phosphatidylinositol 3-kinase/Akt antiapoptotic signaling pathway. The activation of this pathway requires the formation of PtdIns(5)P and involves host cell protein phosphorylation events (212). Moreover, intracellular S. flexneri secretes an MxiE-activated effector protein, which blocks cell death triggered by the apoptosis inducer staurosporine (39). The identity of this antiapoptotic activity and its intracellular target currently remain unknown.
While intracellular S. flexneri is protected from patrolling immune cells, in EC, the bacteria are detected by several intracellular surveillance systems that recognize microbial components and activate the host immune defense. S. flexneri secretes T3SS effectors, which interfere with these host cell signaling cascades on different levels (Table 2). The effector protein OspF interferes with mitogen-activated protein kinase (MAPK) signaling pathways (14, 137, 151). OspF is the first member of a new family of phosphothreonine lyases, which irreversibly remove phosphate groups from MAPKs such as p38. Interestingly, the inhibition of MAPK signaling specifically blocks histone phosphorylation and thus impairs the access of the transcription factor NF-κB to its chromatin target sites (14). Therefore, genes involved in immune signaling are transcribed at a lower level. It is noteworthy that the production of the chemokine IL-8 (a potent chemoattractant for PMN) is reduced, which in turn results in a less pronounced inflammatory response. OspG is another Mxi-Spa-secreted effector protein, which blocks NF-κB-dependent immune signaling, thus dampening intestinal inflammation. OspG is a protein kinase that targets and inhibits ubiquitinylated ubiquitin-conjugating enzymes such as the stem cell factor SCFβ-TrCP, which is required to activate the degradation of the phosphorylated inhibitor of NF-κB type α (phospho-IκBα) by the proteasome (131).
Recently, the interference of effector proteins with ubiquitination-dependent protein degradation was established as a virulence strategy of S. flexneri (236). The Mxi-Spa-secreted effector IpaH9.8 functions as an E3 ubiquitin ligase and inhibits MAPK kinase-dependent signaling by targeting components of this pathway for degradation by the proteasome. IpaH9.8 belongs to the IpaH multiprotein family, suggesting that other S. flexneri proteins also possess ubiquitin ligase activity. However, their specific intracellular protein targets have yet to be identified. Interestingly, the chromosome-encoded IpaH family protein members reduce Shigella-induced inflammation in a mouse pulmonary infection model (15). Several IpaH proteins might be functionally redundant, as only an S. flexneri strain deficient in all chromosomal ipaH genes displayed a detectable phenotype. Finally, apart from their ubiquitin ligase activities, the IpaH proteins might have additional functions, as IpaH9.8 localizes to the nucleus and interacts with the splicing factor U2AF35 (197, 309). The reduction in the U2AF35 levels resulted in a decreased expression of proinflammatory cytokines such as IL-8 or IL-1β, thus strongly decreasing the inflammatory response upon infection with S. flexneri. In summary, these findings indicate that S. flexneri modulates the survival and the inflammatory responses of invaded EC in intricate ways.
INDUCTION OF MACROPHAGE DEATH
Caspase-1-Dependent Macrophage Death
Macrophages are professional phagocytes that take up and degrade invading pathogens, and thus, these cells represent a fundamental component of the innate immune response (122, 229, 302). Bacterial pathogens have developed various strategies to avoid recognition and killing by macrophages. Whereas some bacteria inject effectors to prevent phagocytosis or interfere with vacuolar degradation, S. flexneri and other bacterial species avoid being killed by inducing macrophage cell death (34, 51, 107a, 133, 184, 241). The evasion of killing by macrophages is a crucial step of the pathogenesis of S. flexneri.
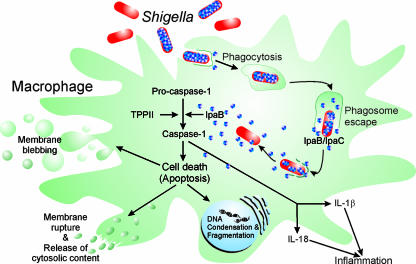
Macrophages are among the first cells that S. flexneri encounters after transcytosis through M cells from the lumen to the subepithelial dome (Fig. 10). The professional phagocytes engulf S. flexneri, yet the bacteria further enhance their uptake by secreting effectors via the Mxi-Spa T3SS, in particular IpaC (140). After uptake, S. flexneri rapidly disrupts the phagosomal membrane, thereby avoiding phagosome-lysosome fusion and degradation. Instead, the bacteria kill the macrophage (40) but only after they escaped from the phagosome (36, 108, 109, 347). Macrophage death is mediated by the Mxi-Spa-secreted translocator/effector protein IpaB (36, 109, 346). S. flexneri triggers an apoptotic cell death pathway characterized by chromatin condensation, DNA fragmentation, cytoplasmic blebbing and vacuolization, as well as a loss of plasma membrane integrity (40, 347). Moreover, macrophage death is paralleled by a rapid decrease of intracellular ATP and the loss of the mitochondrial membrane potential (135, 255). In S. flexneri-infected macrophages, caspase-1 (IL-1β-converting enzyme [ICE]) is activated (36, 108, 109, 345) (Fig. 10). Caspase-1 is required for this cell death pathway, and purified IpaB specifically binds to this caspase-1 (109). The inhibition of type III secretion throughout an infection of macrophages strongly reduces the cytotoxicity of S. flexneri, and consequently, the maximum activation of caspase-1 and macrophage cell death appear to require prolonged intracellular type III secretion of IpaB by cytosolic bacteria (269). Furthermore, cholesterol depletion after invasion and phagosome escape of S. flexneri inhibits caspase-1 activation as well as cell death and coincides with a reduced membrane association of IpaB (268). These findings suggest a functional role for host cell membranes, in particular cholesterol, in caspase-1 activation.
FIG. 10.
S. flexneri-induced macrophage death. Depending on the Mxi-Spa T3SS, S. flexneri enhances its uptake by macrophages and secretes the translocators/effectors IpaB and IpaC to escape from the phagosome. In the cytoplasm, the remaining bacterial pool of IpaB is gradually released. Secreted IpaB integrates into membranes in a cholesterol-dependent manner and triggers the proteolytic activation of procaspase-1, which might be further promoted by TPPII. Active caspase-1 executes cell death and cleaves the precursors of the proinflammatory cytokines IL-1β and IL-18. The mature cytokines are released from the dying macrophage and elicit the strong inflammation characteristic of shigellosis.
The onset of S. flexneri-induced macrophage death depends on caspase-1 but not other caspases, such as caspase-3, an “executioner” of cell death, or caspase-11, an activator of caspase-1 in response to LPS challenge (50, 109, 324). In addition, the cytoplasmic high-molecular-weight serine protease tripeptidyl peptidase II (TPPII) might participate upstream of caspase-1 in this cell death pathway (110). TPPII is a member of the family of self-compartmentalizing proteases involved particularly in protein degradation during the trimming of major histocompatibility complex class I antigens and cell cycle control (81, 276, 289).
The only cellular targets of activated caspase-1 that have been identified in the course of an infection with S. flexneri are the precursors of the proinflammatory cytokines IL-1β and IL-18 (256, 345). The cleavage of pro-IL-1β and pro-IL-18 generates their biologically active forms, which are released from dying macrophages. Both cytokines are central mediators of inflammatory immune signaling and the key triggers of the massive inflammation characteristic of shigellosis (251, 256). The caspase-1 substrates that execute S. flexneri-induced macrophage death are presently not known.
In addition to the pathway described above, S. flexneri triggers a caspase-1-independent cell death pathway in macrophages and dendritic cells. S. flexneri-induced killing of dendritic cells was found to be only partially prevented by caspase-1 inhibitors (62). This caspase-1-independent cell death is activated by the presence of bacterial LPS in the macrophage cytoplasm (297). Thus, different cell death phenotypes induced by S. flexneri in macrophages may be explained by the complex interplay of multiple cell death pathways (95).
Activation of Caspase-1 in Multiprotein Complexes
Caspase-1 belongs to the family of aspartate-specific cysteine proteases, which are essential mediators of apoptosis and inflammatory signaling (139, 162, 304). Based on their main cellular functions, caspases are defined as apoptotic caspases (e.g., caspase-2, caspase-3, caspase-6, caspase-8, and caspase-10) or inflammatory caspases (caspase-1, caspase-4, caspase-5, caspase-11, and caspase-12) (274, 281). However, there is increasing evidence that several caspases, e.g., caspase-1 and caspase-8, fulfill dual functions in apoptotic and immune signaling. An alternative classification distinguishes initiator caspases (e.g., caspase-1, caspase-2, and caspase-8) and executioner caspases (e.g., caspase-3, caspase-6, and caspase-7) according to their positions in cell death pathways (237). Caspases are synthesized as inactive zymogens comprised of a prodomain and a catalytic domain. Upon extra- or intracellular stimulation, initiator procaspases are recruited into multiprotein complexes, wherein they come in close proximity and become activated by autocatalytic proteolysis (26, 279). Mature initiator caspases in turn activate downstream executioner caspases, which cleave a large number of cellular targets that ultimately kill and dismantle the cell.
The breakthrough in the understanding of how various stimuli converge in the activation of caspase-1 was the discovery of the inflammasome, the caspase-1-activating protein platform (161) (Fig. 11). Extensive research over the last years described the inflammasome as being a dynamic multiprotein complex (160, 162). The central scaffold of this complex is comprised of proteins of the nucleotide-binding oligomerization domain (NOD)-like receptor (NLR) family. NOD proteins and NLR proteins represent a class of intracellular receptors that share a conserved domain structure and sense a wide variety of PAMPs and other danger signals (78, 292). Furthermore, NLRs harbor an ATPase domain that is essential for their signaling function (60). Three major subtypes of NLRs are involved in inflammasome assembly, the NACHT-, LRR-, and pyrin-domain-containing proteins (NALPs), the neuronal apoptosis inhibitor proteins (NAIPs), and the ICE protease-activating factor (IPAF). The presence of 14 different NALPs, IPAF, and NAIP in the human genome and even more homologues in the mouse genome suggests that each NLR functions as a receptor for a specific danger signal or a small number of danger signals. Recently, several caspase-1-activating PAMPs including bacterial flagellin, RNA, and bacterial pore-forming toxins and their cognate NLRs were identified (160, 162).
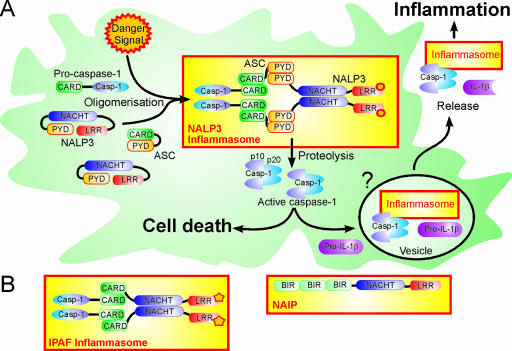
FIG. 11.
Caspase-1 activation in the inflammasome. (A) Caspase-1 activation and IL-1β processing by the NALP3 inflammasome. A danger signal(s) induces a conformational change in NALP3, which leads to oligomerization and exposure of the PYD. Through homotypic PYD-PYD interactions, ASC is bound and recruits procaspase-1 through its CARD domain. Brought into close proximity, procaspase-1 undergoes autocatalytic cleavage into p10 and p20 fragments, which form the active caspase-1 tetramer. Active caspase-1 induces cell death and processes pro-IL-1β. The activation of caspase-1 occurs in or leads to the formation of vesicles through which caspase-1, IL-1β, and the inflammasome are released into the extracellular space. (B) Domain structure of the IPAF inflammasome and the NAIP.
The conserved modular domain structure enables NLRs to detect these multiple danger signals and integrate them in one response, namely, caspase-1 activation (78) (Fig. 11). All of these proteins consist of a mostly C-terminal variable LRR domain sensing the danger signal, a conserved NACHT oligomerization domain, and an N-terminal protein-protein interaction domain, which is involved in caspase-1 recruitment. The N-terminal domain is characteristic of each NLR subtype and consists of three repeats of a baculovirus inhibitor-of-apoptosis repeat domain in NAIPs, one caspase recruitment domain (CARD) in IPAF, and one pyrin domain (PYD) in the case of the NALPs. The CARD, which is also present in the prodomains of several caspases, e.g., caspase-1, caspase-4, and caspase-9, and the PYD belong to the death domain superfamily and fulfill critical roles in apoptotic and inflammatory signaling (205).
How NAIPs mediate caspase-1 activation is poorly understood. NALPs and IPAF become activated upon danger signal recognition by their LRR domains, oligomerize via the central NACHT domain, and expose the protein-protein interaction CARD or PYD. The formation of the inflammasome complex and caspase-1 activation require the ATPase activity of the NLR (60). The PYD of the NALPs engages an adaptor protein, the apoptosis-associated speck-like protein containing a CARD (ASC), through the homotypic binding of the PYD of ASC. Homotypic interactions between the CARDs of IPAF or ASC and the CARD of the caspase-1 zymogen recruit the proenzyme to the inflammasome complex and induce its autocatalytic activation. Finally, the proteolysis of the 45-kDa caspase-1 zymogen removes the prodomain and generates a 10-kDa (p10) subunit and a 20-kDa (p20) subunit, which associate to form the active caspase-1 heterotetramer (92, 333).
The Mxi-Spa-secreted S. flexneri translocator/effector protein IpaB binds caspase-1 directly in vitro (109). However, it is not clear whether IpaB has to be considered a bacterial PAMP, which induces inflammasome formation upon infection, or if IpaB directly recruits caspase-1 into the inflammasome complex. Caspase-1 activation in response to S. flexneri was recently shown to depend on the cytosolic pattern recognition receptors IPAF and ASC (293). While the bacterial component inducing caspase-1 activation remains unidentified, active caspase-1 was found to dampen autophagy triggered by intracellular S. flexneri. IPAF but not ASC is required for autophagy inhibition and the rapid induction of macrophage membrane permeabilization observed. These findings suggest that ASC might have different regulatory functions, which are dependent on the presence or absence of other inflammasome components.
Active caspase-1 cleaves the precursors of the proinflammatory cytokines IL-1β, IL-18, and IL-33 and triggers cell death by an unknown mechanism (35, 91, 267, 303). However, the activation of caspase-1 is not only a trigger of cell death. Rather, this caspase also seems to have vital functions, e.g., the induction of membrane repair in response to bacterial pore-forming toxins (94). The factor(s), that tips the balance between cell survival and death has not been discovered. Several reports showed that mature IL-1β, active caspase-1, and inflammasome components can be released from living cells, possibly by exocytosis of endolysosome-related vesicles or the budding of microvesicles (13, 74, 161). How these vesicles are formed, contribute to caspase-1 activation, and are released is presently unclear. It will be interesting to determine whether and how S. flexneri controls the composition and secretion of vesicles containing inflammasome components to modulate macrophage death.
CONCLUDING REMARKS
More than 100 years after their discovery, Shigella spp. still pose a worldwide major health problem due to the devastating diarrhea that the bacteria may cause. Research performed over the last 25 years has tremendously contributed to our understanding of shigellosis on molecular, cellular, and organismic levels. Thus, we gained profound insight into the evolution of Shigella spp. originating from harmless enterobacterial relatives, the structure and function of the major virulence factors, including a T3SS and cognate effector proteins, as well as the interactions of S. flexneri with various host cells. Specifically, pathogen-triggered invasion of EC and the induction of macrophage death by S. flexneri have been studied in detail. Intricate mechanisms by which S. flexneri modulates inflammatory pathways in the host, thereby launching a successful colonization, were unraveled only recently. An in-depth understanding of the molecular mechanisms underlying these processes is a prerequisite to design a protective, live-attenuated Shigella vaccine strain that is safe and efficient. In concert with ongoing efforts to improve hygiene standards, such a vaccine would offer substantial relief from what is still one of the most dreadful bacterial pathogens.
Acknowledgments
We thank Philippe Sansonetti for critical reading of the manuscript.
This work was supported by grants from the Swiss National Science Foundation (631-065952; PP00A-112592) and the ETH Zürich (TH 17/02-3).
REFERENCES
- 1.Abe, A., T. Matsuzawa, and A. Kuwae. 2005. Type-III effectors: sophisticated bacterial virulence factors. C. R. Biol. 328:413-428. [DOI] [PubMed] [Google Scholar]
- 2.Adler, B., C. Sasakawa, T. Tobe, S. Makino, K. Komatsu, and M. Yoshikawa. 1989. A dual transcriptional activation system for the 230 kb plasmid genes coding for virulence-associated antigens of Shigella flexneri. Mol. Microbiol. 3:627-635. [DOI] [PubMed] [Google Scholar]
- 3.Akeda, Y., and J. E. Galan. 2005. Chaperone release and unfolding of substrates in type III secretion. Nature 437:911-915. [DOI] [PubMed] [Google Scholar]
- 4.Alder, N. N., and S. M. Theg. 2003. Energy use by biological protein transport pathways. Trends Biochem. Sci. 28:442-451. [DOI] [PubMed] [Google Scholar]
- 5.Al-Hasani, K., I. R. Henderson, H. Sakellaris, K. Rajakumar, T. Grant, J. P. Nataro, R. Robins-Browne, and B. Adler. 2000. The sigA gene which is borne on the she pathogenicity island of Shigella flexneri 2a encodes an exported cytopathic protease involved in intestinal fluid accumulation. Infect. Immun. 68:2457-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allaoui, A., R. Ménard, P. J. Sansonetti, and C. Parsot. 1993. Characterization of the Shigella flexneri ipgD and ipgF genes, which are located in the proximal part of the mxi locus. Infect. Immun. 61:1707-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allaoui, A., J. Mournier, M. C. Prévost, P. J. Sansonetti, and C. Parsot. 1992. icsB: a Shigella flexneri virulence gene necessary for the lysis of protrusions during intercellular spread. Mol. Microbiol. 6:1605-1616. [DOI] [PubMed] [Google Scholar]
- 8.Allaoui, A., P. J. Sansonetti, R. Ménard, S. Barzu, J. Mounier, A. Phalipon, and C. Parsot. 1995. MxiG, a membrane protein required for secretion of Shigella spp. Ipa invasins: involvement in entry into epithelial cells and in intercellular dissemination. Mol. Microbiol. 17:461-470. [DOI] [PubMed] [Google Scholar]
- 9.Allaoui, A., P. J. Sansonetti, and C. Parsot. 1993. MxiD: an outer membrane protein necessary for the secretion of the Shigella flexneri Ipa invasins. Mol. Microbiol. 7:59-68. [DOI] [PubMed] [Google Scholar]
- 10.Allaoui, A., P. J. Sansonetti, and C. Parsot. 1992. MxiJ, a lipoprotein involved in secretion of Shigella Ipa invasins, is homologous to YscJ, a secretion factor of the Yersinia Yop proteins. J. Bacteriol. 174:7661-7669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al Mamun, A. A., A. Tominaga, and M. Enomoto. 1996. Detection and characterization of the flagellar master operon in the four Shigella subgroups. J. Bacteriol. 178:3722-3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alto, N. M., F. Shao, C. S. Lazar, R. L. Brost, G. Chua, S. Mattoo, S. A. McMahon, P. Ghosh, T. R. Hughes, C. Boone, and J. E. Dixon. 2006. Identification of a bacterial type III effector family with G protein mimicry functions. Cell 124:133-145. [DOI] [PubMed] [Google Scholar]
- 13.Andrei, C., C. Dazzi, L. Lotti, M. R. Torrisi, G. Chimini, and A. Rubartelli. 1999. The secretory route of the leaderless protein interleukin-1β involves exocytosis of endolysosome-related vesicles. Mol. Biol. Cell 10:1463-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arbibe, L., D. W. Kim, E. Batsche, T. Pedron, B. Mateescu, C. Muchardt, C. Parsot, and P. J. Sansonetti. 2007. An injected bacterial effector targets chromatin access for transcription factor NF-kappaB to alter transcription of host genes involved in immune responses. Nat. Immunol. 8:47-56. [DOI] [PubMed] [Google Scholar]
- 15.Ashida, H., T. Toyotome, T. Nagai, and C. Sasakawa. 2007. Shigella chromosomal IpaH proteins are secreted via the type III secretion system and act as effectors. Mol. Microbiol. 63:680-693. [DOI] [PubMed] [Google Scholar]
- 16.Backert, S., and T. F. Meyer. 2006. Type IV secretion systems and their effectors in bacterial pathogenesis. Curr. Opin. Microbiol. 9:207-217. [DOI] [PubMed] [Google Scholar]
- 17.Bahrani, F. K., P. J. Sansonetti, and C. Parsot. 1997. Secretion of Ipa proteins by Shigella flexneri: inducer molecules and kinetics of activation. Infect. Immun. 65:4005-4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barzu, S., Z. Benjelloun-Touimi, A. Phalipon, P. Sansonetti, and C. Parsot. 1997. Functional analysis of the Shigella flexneri IpaC invasin by insertional mutagenesis. Infect. Immun. 65:1599-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bell, J. K., G. E. Mullen, C. A. Leifer, A. Mazzoni, D. R. Davies, and D. M. Segal. 2003. Leucine-rich repeats and pathogen recognition in Toll-like receptors. Trends Immunol. 24:528-533. [DOI] [PubMed] [Google Scholar]
- 20.Benjelloun-Touimi, Z., P. J. Sansonetti, and C. Parsot. 1995. SepA, the major extracellular protein of Shigella flexneri: autonomous secretion and involvement in tissue invasion. Mol. Microbiol. 17:123-135. [DOI] [PubMed] [Google Scholar]
- 21.Bergsten, G., B. Wullt, and C. Svanborg. 2005. Escherichia coli, fimbriae, bacterial persistence and host response induction in the human urinary tract. Int. J. Med. Microbiol. 295:487-502. [DOI] [PubMed] [Google Scholar]
- 22.Bernardini, M. L., J. Mounier, H. d'Hauteville, M. Coquis-Rondon, and P. J. Sansonetti. 1989. Identification of icsA, a plasmid locus of Shigella flexneri that governs bacterial intra- and intercellular spread through interaction with F-actin. Proc. Natl. Acad. Sci. USA 86:3867-3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blocker, A., P. Gounon, E. Larquet, K. Niebuhr, V. Cabiaux, C. Parsot, and P. Sansonetti. 1999. The tripartite type III secreton of Shigella flexneri inserts IpaB and IpaC into host membranes. J. Cell Biol. 147:683-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blocker, A., N. Jouihri, E. Larquet, P. Gounon, F. Ebel, C. Parsot, P. Sansonetti, and A. Allaoui. 2001. Structure and composition of the Shigella flexneri “needle complex,” a part of its type III secreton. Mol. Microbiol. 39:652-663. [DOI] [PubMed] [Google Scholar]
- 25.Blocker, A., K. Komoriya, and S. Aizawa. 2003. Type III secretion systems and bacterial flagella: insights into their function from structural similarities. Proc. Natl. Acad. Sci. USA 100:3027-3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boatright, K. M., M. Renatus, F. L. Scott, S. Sperandio, H. Shin, I. M. Pedersen, J. E. Ricci, W. A. Edris, D. P. Sutherlin, D. R. Green, and G. S. Salvesen. 2003. A unified model for apical caspase activation. Mol. Cell 11:529-541. [DOI] [PubMed] [Google Scholar]
- 27.Boquet, P., and E. Lemichez. 2003. Bacterial virulence factors targeting Rho GTPases: parasitism or symbiosis? Trends Cell Biol. 13:238-246. [DOI] [PubMed] [Google Scholar]
- 28.Bourdet-Sicard, R., M. Rudiger, B. M. Jockusch, P. Gounon, P. J. Sansonetti, and G. Tran Van Nhieu. 1999. Binding of the Shigella protein IpaA to vinculin induces F-actin depolymerization. EMBO J. 18:5853-5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brinkmann, V., U. Reichard, C. Goosmann, B. Fauler, Y. Uhlemann, D. S. Weiss, Y. Weinrauch, and A. Zychlinsky. 2004. Neutrophil extracellular traps kill bacteria. Science 303:1532-1535. [DOI] [PubMed] [Google Scholar]
- 30.Buchrieser, C., P. Glaser, C. Rusniok, H. Nedjari, H. D'Hauteville, F. Kunst, P. Sansonetti, and C. Parsot. 2000. The virulence plasmid pWR100 and the repertoire of proteins secreted by the type III secretion apparatus of Shigella flexneri. Mol. Microbiol. 38:760-771. [DOI] [PubMed] [Google Scholar]
- 31.Buttner, D., and U. Bonas. 2006. Who comes first? How plant pathogenic bacteria orchestrate type III secretion. Curr. Opin. Microbiol. 9:193-200. [DOI] [PubMed] [Google Scholar]
- 32.Buysse, J. M., C. K. Stover, E. V. Oaks, M. Venkatesan, and D. J. Kopecko. 1987. Molecular cloning of invasion plasmid antigen (ipa) genes from Shigella flexneri: analysis of ipa gene products and genetic mapping. J. Bacteriol. 169:2561-2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Catron, D. M., M. D. Sylvester, Y. Lange, M. Kadekoppala, B. D. Jones, D. M. Monack, S. Falkow, and K. Haldar. 2002. The Salmonella-containing vacuole is a major site of intracellular cholesterol accumulation and recruits the GPI-anchored protein CD55. Cell. Microbiol. 4:315-328. [DOI] [PubMed] [Google Scholar]
- 34.Celli, J., and B. B. Finlay. 2002. Bacterial avoidance of phagocytosis. Trends Microbiol. 10:232-237. [DOI] [PubMed] [Google Scholar]
- 35.Cerretti, D. P., C. J. Kozlosky, B. Mosley, N. Nelson, K. Van Ness, T. A. Greenstreet, C. J. March, S. R. Kronheim, T. Druck, L. A. Cannizarro, K. Huebner, and R. A. Black. 1992. Molecular cloning of the interleukin-1β converting enzyme. Science 256:97-99. [DOI] [PubMed] [Google Scholar]
- 36.Chen, Y., M. R. Smith, K. Thirumalai, and A. Zychlinsky. 1996. A bacterial invasin induces macrophage apoptosis by directly binding ICE. EMBO J. 15:3853-3860. [PMC free article] [PubMed] [Google Scholar]
- 37.Cherla, R. P., S. Y. Lee, and V. L. Tesh. 2003. Shiga toxins and apoptosis. FEMS Microbiol. Lett. 228:159-166. [DOI] [PubMed] [Google Scholar]
- 38.Christie, P. J., K. Atmakuri, V. Krishnamoorthy, S. Jakubowski, and E. Cascales. 2005. Biogenesis, architecture, and function of bacterial type IV secretion systems. Annu. Rev. Microbiol. 59:451-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clark, C. S., and A. T. Maurelli. 2007. Shigella flexneri inhibits staurosporine-induced apoptosis in epithelial cells. Infect. Immun. 75:2531-2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clerc, P. L., A. Ryter, J. Mounier, and P. J. Sansonetti. 1987. Plasmid-mediated early killing of eukaryotic cells by Shigella flexneri as studied by infection of J774 macrophages. Infect. Immun. 55:521-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cordes, F. S., S. Daniell, R. Kenjale, S. Saurya, W. L. Picking, W. D. Picking, F. Booy, S. M. Lea, and A. Blocker. 2005. Helical packing of needles from functionally altered Shigella type III secretion systems. J. Mol. Biol. 354:206-211. [DOI] [PubMed] [Google Scholar]
- 42.Cordes, F. S., K. Komoriya, E. Larquet, S. Yang, E. H. Egelman, A. Blocker, and S. M. Lea. 2003. Helical structure of the needle of the type III secretion system of Shigella flexneri. J. Biol. Chem. 278:17103-17107. [DOI] [PubMed] [Google Scholar]
- 43.Cornelis, G. R. 2006. The type III secretion injectisome. Nat. Rev. Microbiol. 4:811-825. [DOI] [PubMed] [Google Scholar]
- 44.Cossart, P., and P. J. Sansonetti. 2004. Bacterial invasion: the paradigms of enteroinvasive pathogens. Science 304:242-248. [DOI] [PubMed] [Google Scholar]
- 45.Davis, R., M. E. Marquart, D. Lucius, and W. D. Picking. 1998. Protein-protein interactions in the assembly of Shigella flexneri invasion plasmid antigens IpaB and IpaC into protein complexes. Biochim. Biophys. Acta 1429:45-56. [DOI] [PubMed] [Google Scholar]
- 46.Day, W. A., Jr., R. E. Fernandez, and A. T. Maurelli. 2001. Pathoadaptive mutations that enhance virulence: genetic organization of the cadA regions of Shigella spp. Infect. Immun. 69:7471-7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deane, J. E., P. Roversi, F. S. Cordes, S. Johnson, R. Kenjale, S. Daniell, F. Booy, W. D. Picking, W. L. Picking, A. J. Blocker, and S. M. Lea. 2006. Molecular model of a type III secretion system needle: implications for host-cell sensing. Proc. Natl. Acad. Sci. USA 103:12529-12533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Chastellier, C., and L. Thilo. 2006. Cholesterol depletion in Mycobacterium avium-infected macrophages overcomes the block in phagosome maturation and leads to the reversible sequestration of viable mycobacteria in phagolysosome-derived autophagic vacuoles. Cell. Microbiol. 8:242-256. [DOI] [PubMed] [Google Scholar]
- 49.De Geyter, C., B. Vogt, Z. Benjelloun-Touimi, P. J. Sansonetti, J. M. Ruysschaert, C. Parsot, and V. Cabiaux. 1997. Purification of IpaC, a protein involved in entry of Shigella flexneri into epithelial cells and characterization of its interaction with lipid membranes. FEBS Lett. 400:149-154. [DOI] [PubMed] [Google Scholar]
- 50.Degterev, A., M. Boyce, and J. Yuan. 2003. A decade of caspases. Oncogene 22:8543-8567. [DOI] [PubMed] [Google Scholar]
- 51.DeLeo, F. R. 2004. Modulation of phagocyte apoptosis by bacterial pathogens. Apoptosis 9:399-413. [DOI] [PubMed] [Google Scholar]
- 52.Demali, K. A., A. L. Jue, and K. Burridge. 2006. IpaA targets beta 1 integrins and rho to promote actin cytoskeleton rearrangements necessary for Shigella entry. J. Biol. Chem. 281:39534-39541. [DOI] [PubMed] [Google Scholar]
- 53.Demers, B., P. J. Sansonetti, and C. Parsot. 1998. Induction of type III secretion in Shigella flexneri is associated with differential control of transcription of genes encoding secreted proteins. EMBO J. 17:2894-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deng, W., J. L. Puente, S. Gruenheid, Y. Li, B. A. Vallance, A. Vazquez, J. Barba, J. A. Ibarra, P. O'Donnell, P. Metalnikov, K. Ashman, S. Lee, D. Goode, T. Pawson, and B. B. Finlay. 2004. Dissecting virulence: systematic and functional analyses of a pathogenicity island. Proc. Natl. Acad. Sci. USA 101:3597-3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deretic, V. 2006. Autophagy as an immune defense mechanism. Curr. Opin. Immunol. 18:375-382. [DOI] [PubMed] [Google Scholar]
- 56.Desvaux, M., N. J. Parham, and I. R. Henderson. 2004. The autotransporter secretion system. Res. Microbiol. 155:53-60. [DOI] [PubMed] [Google Scholar]
- 57.Dobrindt, U., B. Hochhut, U. Hentschel, and J. Hacker. 2004. Genomic islands in pathogenic and environmental microorganisms. Nat. Rev. Microbiol. 2:414-424. [DOI] [PubMed] [Google Scholar]
- 57a.Dodd, C. E. R., and D. Jones. 1982. A numerical taxonomic study of the genus Shigella. J. Gen. Microbiol. 128:1933-1957. [DOI] [PubMed] [Google Scholar]
- 58.Dorman, C. J., S. McKenna, and C. Beloin. 2001. Regulation of virulence gene expression in Shigella flexneri, a facultative intracellular pathogen. Int. J. Med. Microbiol. 291:89-96. [DOI] [PubMed] [Google Scholar]
- 59.Dorman, C. J., and M. E. Porter. 1998. The Shigella virulence gene regulatory cascade: a paradigm of bacterial gene control mechanisms. Mol. Microbiol. 29:677-684. [DOI] [PubMed] [Google Scholar]
- 60.Duncan, J. A., D. T. Bergstralh, Y. Wang, S. B. Willingham, Z. Ye, A. G. Zimmermann, and J. P. Ting. 2007. Cryopyrin/NALP3 binds ATP/dATP, is an ATPase, and requires ATP binding to mediate inflammatory signaling. Proc. Natl. Acad. Sci. USA 104:8041-8046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.DuPont, H. L., M. M. Levine, R. B. Hornick, and S. B. Formal. 1989. Inoculum size in shigellosis and implications for expected mode of transmission. J. Infect. Dis. 159:1126-1128. [DOI] [PubMed] [Google Scholar]
- 62.Edgeworth, J. D., J. Spencer, A. Phalipon, G. E. Griffin, and P. J. Sansonetti. 2002. Cytotoxicity and interleukin-1β processing following Shigella flexneri infection of human monocyte-derived dendritic cells. Eur. J. Immunol. 32:1464-1471. [DOI] [PubMed] [Google Scholar]
- 63.Egile, C., H. d'Hauteville, C. Parsot, and P. J. Sansonetti. 1997. SopA, the outer membrane protease responsible for polar localization of IcsA in Shigella flexneri. Mol. Microbiol. 23:1063-1073. [DOI] [PubMed] [Google Scholar]
- 64.Egile, C., T. P. Loisel, V. Laurent, R. Li, D. Pantaloni, P. J. Sansonetti, and M. F. Carlier. 1999. Activation of the CDC42 effector N-WASP by the Shigella flexneri IcsA protein promotes actin nucleation by Arp2/3 complex and bacterial actin-based motility. J. Cell Biol. 146:1319-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Erskine, P. T., M. J. Knight, A. Ruaux, H. Mikolajek, N. W. F. Sang, J. Withers, R. Gill, S. P. Wood, M. Wood, G. C. Fox, and J. B. Cooper. 2006. High resolution structure of BipD: an invasion protein associated with the type III secretion system of Burkholderia pseudomallei. J. Mol. Biol. 363:125-136. [DOI] [PubMed] [Google Scholar]
- 66.Escobar-Paramo, P., O. Clermont, A. B. Blanc-Potard, H. Bui, C. Le Bouguenec, and E. Denamur. 2004. A specific genetic background is required for acquisition and expression of virulence factors in Escherichia coli. Mol. Biol. Evol. 21:1085-1094. [DOI] [PubMed] [Google Scholar]
- 67.Escobar-Paramo, P., C. Giudicelli, C. Parsot, and E. Denamur. 2003. The evolutionary history of Shigella and enteroinvasive Escherichia coli revised. J. Mol. Evol. 57:140-148. [DOI] [PubMed] [Google Scholar]
- 68.Espina, M., A. J. Olive, R. Kenjale, D. S. Moore, S. F. Ausar, R. W. Kaminski, E. V. Oaks, C. R. Middaugh, W. D. Picking, and W. L. Picking. 2006. IpaD localizes to the tip of the type III secretion system needle of Shigella flexneri. Infect. Immun. 74:4391-4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ewing, W. H. 1949. Shigella nomenclature. J. Bacteriol. 57:633-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fasano, A., F. R. Noriega, F. M. Liao, W. Wang, and M. M. Levine. 1997. Effect of Shigella enterotoxin 1 (ShET1) on rabbit intestine in vitro and in vivo. Gut 40:505-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fasano, A., F. R. Noriega, D. R. Maneval, Jr., S. Chanasongcram, R. Russell, S. Guandalini, and M. M. Levine. 1995. Shigella enterotoxin 1: an enterotoxin of Shigella flexneri 2a active in rabbit small intestine in vivo and in vitro. J. Clin. Investig. 95:2853-2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Feldman, M. F., and G. R. Cornelis. 2003. The multitalented type III chaperones: all you can do with 15 kDa. FEMS Microbiol. Lett. 219:151-158. [DOI] [PubMed] [Google Scholar]
- 73.Fernandez-Prada, C. M., D. L. Hoover, B. D. Tall, A. B. Hartman, J. Kopelowitz, and M. M. Venkatesan. 2000. Shigella flexneri IpaH7.8 facilitates escape of virulent bacteria from the endocytic vacuoles of mouse and human macrophages. Infect. Immun. 68:3608-3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ferrari, D., C. Pizzirani, E. Adinolfi, R. M. Lemoli, A. Curti, M. Idzko, E. Panther, and F. Di Virgilio. 2006. The P2X7 receptor: a key player in IL-1 processing and release. J. Immunol. 176:3877-3883. [DOI] [PubMed] [Google Scholar]
- 75.Ferrari, G., H. Langen, M. Naito, and J. Pieters. 1999. A coat protein on phagosomes involved in the intracellular survival of mycobacteria. Cell 97:435-447. [DOI] [PubMed] [Google Scholar]
- 76.Finlay, B. B., and S. Falkow. 1988. Comparison of the invasion strategies used by Salmonella cholera-suis, Shigella flexneri and Yersinia enterocolitica to enter cultured animal cells: endosome acidification is not required for bacterial invasion or intracellular replication. Biochimie 70:1089-1099. [DOI] [PubMed] [Google Scholar]
- 77.Fischer, W., J. Puls, R. Buhrdorf, B. Gebert, S. Odenbreit, and R. Haas. 2001. Systematic mutagenesis of the Helicobacter pylori cag pathogenicity island: essential genes for CagA translocation in host cells and induction of interleukin-8. Mol. Microbiol. 42:1337-1348. [DOI] [PubMed] [Google Scholar]
- 78.Fritz, J. H., R. L. Ferrero, D. J. Philpott, and S. E. Girardin. 2006. Nod-like proteins in immunity, inflammation and disease. Nat. Immunol. 7:1250-1257. [DOI] [PubMed] [Google Scholar]
- 79.Fukushima, M., K. Kakinuma, and R. Kawaguchi. 2002. Phylogenetic analysis of Salmonella, Shigella, and Escherichia coli strains on the basis of the gyrB gene sequence. J. Clin. Microbiol. 40:2779-2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Garner, M. J., R. D. Hayward, and V. Koronakis. 2002. The Salmonella pathogenicity island 1 secretion system directs cellular cholesterol redistribution during mammalian cell entry and intracellular trafficking. Cell. Microbiol. 4:153-165. [DOI] [PubMed] [Google Scholar]
- 81.Geier, E., G. Pfeifer, M. Wilm, M. Lucchiari-Hartz, W. Baumeister, K. Eichmann, and G. Niedermann. 1999. A giant protease with potential to substitute for some functions of the proteasome. Science 283:978-981. [DOI] [PubMed] [Google Scholar]
- 82.Ghosh, P. 2004. Process of protein transport by the type III secretion system. Microbiol. Mol. Biol. Rev. 68:771-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Girardin, S. E., I. G. Boneca, L. A. Carneiro, A. Antignac, M. Jehanno, J. Viala, K. Tedin, M. K. Taha, A. Labigne, U. Zahringer, A. J. Coyle, P. S. DiStefano, J. Bertin, P. J. Sansonetti, and D. J. Philpott. 2003. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science 300:1584-1587. [DOI] [PubMed] [Google Scholar]
- 84.Goldberg, M. B., O. Barzu, C. Parsot, and P. J. Sansonetti. 1993. Unipolar localization and ATPase activity of IcsA, a Shigella flexneri protein involved in intracellular movement. J. Bacteriol. 175:2189-2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Goldberg, M. B., and J. A. Theriot. 1995. Shigella flexneri surface protein IcsA is sufficient to direct actin-based motility. Proc. Natl. Acad. Sci. USA 92:6572-6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Golub, T., S. Wacha, and P. Caroni. 2004. Spatial and temporal control of signaling through lipid rafts. Curr. Opin. Neurobiol. 14:542-550. [DOI] [PubMed] [Google Scholar]
- 87.Gombos, I., E. Kiss, C. Detre, G. Laszlo, and J. Matko. 2006. Cholesterol and sphingolipids as lipid organizers of the immune cells' plasma membrane: their impact on the functions of MHC molecules, effector T-lymphocytes and T-cell death. Immunol. Lett. 104:59-69. [DOI] [PubMed] [Google Scholar]
- 88.Gorden, J., and P. L. Small. 1993. Acid resistance in enteric bacteria. Infect. Immun. 61:364-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gouin, E., M. D. Welch, and P. Cossart. 2005. Actin-based motility of intracellular pathogens. Curr. Opin. Microbiol. 8:35-45. [DOI] [PubMed] [Google Scholar]
- 90.Grant, S. R., E. J. Fisher, J. H. Chang, B. M. Mole, and J. L. Dangl. 2006. Subterfuge and manipulation: type III effector proteins of phytopathogenic bacteria. Annu. Rev. Microbiol. 60:425-449. [DOI] [PubMed] [Google Scholar]
- 91.Gu, Y., K. Kuida, H. Tsutsui, G. Ku, K. Hsiao, M. A. Fleming, N. Hayashi, K. Higashino, H. Okamura, K. Nakanishi, M. Kurimoto, T. Tanimoto, R. A. Flavell, V. Sato, M. W. Harding, D. J. Livingston, and M. S.-S. Su. 1997. Activation of interferon-γ inducing factor mediated by interleukin-1β converting enzyme. Science 275:206-209. [DOI] [PubMed] [Google Scholar]
- 92.Gu, Y., J. Wu, C. Faucheu, J. L. Lalanne, A. Diu, D. J. Livingston, and M. S. Su. 1995. Interleukin-1β converting enzyme requires oligomerization for activity of processed forms in vivo. EMBO J. 14:1923-1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Guichon, A., D. Hersh, M. R. Smith, and A. Zychlinsky. 2001. Structure-function analysis of the Shigella virulence factor IpaB. J. Bacteriol. 183:1269-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gurcel, L., L. Abrami, S. Girardin, J. Tschopp, and F. G. van der Goot. 2006. Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell 126:1135-1145. [DOI] [PubMed] [Google Scholar]
- 95.Haimovich, B., and M. M. Venkatesan. 2006. Shigella and Salmonella: death as a means of survival. Microbes Infect. 8:568-577. [DOI] [PubMed] [Google Scholar]
- 96.Hamiaux, C., A. van Eerde, C. Parsot, J. Broos, and B. W. Dijkstra. 2006. Structural mimicry for vinculin activation by IpaA, a virulence factor of Shigella flexneri. EMBO Rep. 7:794-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hanajima-Ozawa, M., T. Matsuzawa, A. Fukui, S. Kamitani, H. Ohnishi, A. Abe, Y. Horiguchi, and M. Miyake. 2007. Enteropathogenic Escherichia coli, Shigella flexneri, and Listeria monocytogenes recruit a junctional protein, zonula occludens-1, to actin tails and pedestals. Infect. Immun. 75:565-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hancock, J. F. 2006. Lipid rafts: contentious only from simplistic standpoints. Nat. Rev. Mol. Cell Biol. 7:456-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Handa, Y., M. Suzuki, K. Ohya, H. Iwai, N. Ishijima, A. J. Koleske, Y. Fukui, and C. Sasakawa. 2007. Shigella IpgB1 promotes bacterial entry through the ELMO-Dock180 machinery. Nat. Cell Biol. 9:121-128. [DOI] [PubMed] [Google Scholar]
- 100.Harrington, A., N. Darboe, R. Kenjale, W. L. Picking, C. R. Middaugh, S. Birket, and W. D. Picking. 2006. Characterization of the interaction of single tryptophan containing mutants of IpaC from Shigella flexneri with phospholipid membranes. Biochemistry 45:626-636. [DOI] [PubMed] [Google Scholar]
- 101.Harrington, A. T., P. D. Hearn, W. L. Picking, J. R. Barker, A. Wessel, and W. D. Picking. 2003. Structural characterization of the N terminus of IpaC from Shigella flexneri. Infect. Immun. 71:1255-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hartman, A. B., M. Venkatesan, E. V. Oaks, and J. M. Buysse. 1990. Sequence and molecular characterization of a multicopy invasion plasmid antigen gene, ipaH, of Shigella flexneri. J. Bacteriol. 172:1905-1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hayward, R. D., R. J. Cain, E. J. McGhie, N. Phillips, M. J. Garner, and V. Koronakis. 2005. Cholesterol binding by the bacterial type III translocon is essential for virulence effector delivery into mammalian cells. Mol. Microbiol. 56:590-603. [DOI] [PubMed] [Google Scholar]
- 104.Helms, J. B., and C. Zurzolo. 2004. Lipids as targeting signals: lipid rafts and intracellular trafficking. Traffic 5:247-254. [DOI] [PubMed] [Google Scholar]
- 105.Henderson, I. R., J. Czeczulin, C. Eslava, F. Noriega, and J. P. Nataro. 1999. Characterization of Pic, a secreted protease of Shigella flexneri and enteroaggregative Escherichia coli. Infect. Immun. 67:5587-5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.High, N., J. Mounier, M. C. Prevost, and P. J. Sansonetti. 1992. IpaB of Shigella flexneri causes entry into epithelial cells and escape from the phagocytic vacuole. EMBO J. 11:1991-1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hilbi, H. 2006. Modulation of phosphoinositide metabolism by pathogenic bacteria. Cell. Microbiol. 8:1697-1706. [DOI] [PubMed] [Google Scholar]
- 107a.Hilbi, H. 1999. Host responses to secreted Shigella virulence factors. Curr. Opin. Infect. Dis. 12:221-228. [DOI] [PubMed] [Google Scholar]
- 108.Hilbi, H., Y. Chen, K. Thirumalai, and A. Zychlinsky. 1997. The interleukin-1β converting enzyme, caspase-1, is activated during Shigella flexneri-induced apoptosis in human monocyte-derived macrophages. Infect. Immun. 65:5165-5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hilbi, H., J. E. Moss, D. Hersh, Y. Chen, J. Arondel, S. Banerjee, R. A. Flavell, J. Yuan, P. J. Sansonetti, and A. Zychlinsky. 1998. Shigella-induced apoptosis is dependent on caspase-1 which binds to IpaB. J. Biol. Chem. 273:32895-32900. [DOI] [PubMed] [Google Scholar]
- 110.Hilbi, H., R. J. Puro, and A. Zychlinsky. 2000. Tripeptidyl peptidase II promotes maturation of caspase-1 in Shigella flexneri-induced macrophage apoptosis. Infect. Immun. 68:5502-5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Howe, D., and R. A. Heinzen. 2006. Coxiella burnetii inhabits a cholesterol-rich vacuole and influences cellular cholesterol metabolism. Cell. Microbiol. 8:496-507. [DOI] [PubMed] [Google Scholar]
- 112.Hromockyj, A. E., and A. T. Maurelli. 1989. Identification of Shigella invasion genes by isolation of temperature-regulated inv::lacZ operon fusions. Infect. Immun. 57:2963-2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Huan, P. T., D. A. Bastin, B. L. Whittle, A. A. Lindberg, and N. K. Verma. 1997. Molecular characterization of the genes involved in O-antigen modification, attachment, integration and excision in Shigella flexneri bacteriophage SfV. Gene 195:217-227. [DOI] [PubMed] [Google Scholar]
- 114.Hume, P. J., E. J. McGhie, R. D. Hayward, and V. Koronakis. 2003. The purified Shigella IpaB and Salmonella SipB translocators share biochemical properties and membrane topology. Mol. Microbiol. 49:425-439. [DOI] [PubMed] [Google Scholar]
- 115.Ingersoll, M., E. A. Groisman, and A. Zychlinsky. 2002. Pathogenicity islands of Shigella. Curr. Top. Microbiol. Immunol. 264:49-65. [PubMed] [Google Scholar]
- 116.Ingersoll, M. A., J. E. Moss, Y. Weinrauch, P. E. Fisher, E. A. Groisman, and A. Zychlinsky. 2003. The ShiA protein encoded by the Shigella flexneri SHI-2 pathogenicity island attenuates inflammation. Cell. Microbiol. 5:797-807. [DOI] [PubMed] [Google Scholar]
- 117.Ingersoll, M. A., and A. Zychlinsky. 2006. ShiA abrogates the innate T-cell response to Shigella flexneri infection. Infect. Immun. 74:2317-2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Islam, D., L. Bandholtz, J. Nilsson, H. Wigzell, B. Christensson, B. Agerberth, and G. Gudmundsson. 2001. Downregulation of bactericidal peptides in enteric infections: a novel immune escape mechanism with bacterial DNA as a potential regulator. Nat. Med. 7:180-185. [DOI] [PubMed] [Google Scholar]
- 119.Islam, D., B. Veress, P. K. Bardhan, A. A. Lindberg, and B. Christensson. 1997. In situ characterization of inflammatory responses in the rectal mucosae of patients with shigellosis. Infect. Immun. 65:739-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ito, H., N. Kido, Y. Arakawa, M. Ohta, T. Sugiyama, and N. Kato. 1991. Possible mechanisms underlying the slow lactose fermentation phenotype in Shigella spp. Appl. Environ. Microbiol. 57:2912-2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Izard, T., G. Tran Van Nhieu, and P. R. Bois. 2006. Shigella applies molecular mimicry to subvert vinculin and invade host cells. J. Cell Biol. 175:465-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Janeway, C. A., Jr., and R. Medzhitov. 2002. Innate immune recognition. Annu. Rev. Immunol. 20:197-216. [DOI] [PubMed] [Google Scholar]
- 123.Jennison, A. V., and N. K. Verma. 2004. Shigella flexneri infection: pathogenesis and vaccine development. FEMS Microbiol. Rev. 28:43-58. [DOI] [PubMed] [Google Scholar]
- 124.Jiang, Y., F. Yang, X. Zhang, J. Yang, L. Chen, Y. Yan, H. Nie, Z. Xiong, J. Wang, J. Dong, Y. Xue, X. Xu, Y. Zhu, S. Chen, and Q. Jin. 2005. The complete sequence and analysis of the large virulence plasmid pSS of Shigella sonnei. Plasmid 54:149-159. [DOI] [PubMed] [Google Scholar]
- 125.Jin, Q., Z. Yuan, J. Xu, Y. Wang, Y. Shen, W. Lu, J. Wang, H. Liu, J. Yang, F. Yang, X. Zhang, J. Zhang, G. Yang, H. Wu, D. Qu, J. Dong, L. Sun, Y. Xue, A. Zhao, Y. Gao, J. Zhu, B. Kan, K. Ding, S. Chen, H. Cheng, Z. Yao, B. He, R. Chen, D. Ma, B. Qiang, Y. Wen, Y. Hou, and J. Yu. 2002. Genome sequence of Shigella flexneri 2a: insights into pathogenicity through comparison with genomes of Escherichia coli K12 and O157. Nucleic Acids Res. 30:4432-4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Johnson, S., P. Roversi, M. Espina, A. Olive, J. E. Deane, S. Birket, T. Field, W. D. Picking, A. J. Blocker, E. E. Galyov, W. L. Picking, and S. M. Lea. 2007. Self-chaperoning of the type III secretion system needle tip proteins IpaD and BipD. J. Biol. Chem. 282:4035-4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jouihri, N., M. P. Sory, A. L. Page, P. Gounon, C. Parsot, and A. Allaoui. 2003. MxiK and MxiN interact with the Spa47 ATPase and are required for transit of the needle components MxiH and MxiI, but not of Ipa proteins, through the type III secretion apparatus of Shigella flexneri. Mol. Microbiol. 49:755-767. [DOI] [PubMed] [Google Scholar]
- 128.Kane, C. D., R. Schuch, W. A. Day, Jr., and A. T. Maurelli. 2002. MxiE regulates intracellular expression of factors secreted by the Shigella flexneri 2a type III secretion system. J. Bacteriol. 184:4409-4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kato, J.-I., K.-I. Ito, A. Nakamura, and H. Watanabe. 1989. Cloning of regions required for contact hemolysis and entry into LLC-MK2 cells from Shigella sonnei form I plasmid: virF is a positive regulator gene for these phenotypes. Infect. Immun. 57:1391-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kenjale, R., J. Wilson, S. F. Zenk, S. Saurya, W. L. Picking, W. D. Picking, and A. Blocker. 2005. The needle component of the type III secreton of Shigella regulates the activity of the secretion apparatus. J. Biol. Chem. 280:42929-42937. [DOI] [PubMed] [Google Scholar]
- 131.Kim, D. W., G. Lenzen, A. L. Page, P. Legrain, P. J. Sansonetti, and C. Parsot. 2005. The Shigella flexneri effector OspG interferes with innate immune responses by targeting ubiquitin-conjugating enzymes. Proc. Natl. Acad. Sci. USA 102:14046-14051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kirkegaard, K., M. P. Taylor, and W. T. Jackson. 2004. Cellular autophagy: surrender, avoidance and subversion by microorganisms. Nat. Rev. Microbiol. 2:301-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kirschnek, S., J. Scheffel, U. Heinzmann, and G. Hacker. 2004. Necrosis-like cell death induced by bacteria in mouse macrophages. Eur. J. Immunol. 34:1461-1471. [DOI] [PubMed] [Google Scholar]
- 134.Knodler, L. A., and O. Steele-Mortimer. 2003. Taking possession: biogenesis of the Salmonella-containing vacuole. Traffic 4:587-599. [DOI] [PubMed] [Google Scholar]
- 135.Koterski, J. F., M. Nahvi, M. M. Venkatesan, and B. Haimovich. 2005. Virulent Shigella flexneri causes damage to mitochondria and triggers necrosis in infected human monocyte-derived macrophages. Infect. Immun. 73:504-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kotloff, K. L., J. P. Winickoff, B. Ivanoff, J. D. Clemens, D. L. Swerdlow, P. J. Sansonetti, G. K. Adak, and M. M. Levine. 1999. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull. W. H. O. 77:651-666. [PMC free article] [PubMed] [Google Scholar]
- 137.Kramer, R. W., N. L. Slagowski, N. A. Eze, K. S. Giddings, M. F. Morrison, K. A. Siggers, M. N. Starnbach, and C. F. Lesser. 2007. Yeast functional genomic screens lead to identification of a role for a bacterial effector in innate immunity regulation. PLoS Pathog. 3:e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kubori, T., Y. Matsushima, D. Nakamura, J. Uralil, M. Lara-Tejero, A. Sukhan, J. E. Galan, and S. I. Aizawa. 1998. Supramolecular structure of the Salmonella typhimurium type III protein secretion system. Science 280:602-605. [DOI] [PubMed] [Google Scholar]
- 139.Kumar, S. 2007. Caspase function in programmed cell death. Cell Death Differ. 14:32-43. [DOI] [PubMed] [Google Scholar]
- 140.Kuwae, A., S. Yoshida, K. Tamano, H. Mimuro, T. Suzuki, and C. Sasakawa. 2001. Shigella invasion of macrophage requires the insertion of IpaC into the host plasma membrane. Functional analysis of IpaC. J. Biol. Chem. 276:32230-32239. [DOI] [PubMed] [Google Scholar]
- 141.Kwiatkowska, K., and A. Sobota. 1999. Signaling pathways in phagocytosis. Bioessays 21:422-431. [DOI] [PubMed] [Google Scholar]
- 142.Lafont, F., G. Tran Van Nhieu, K. Hanada, P. Sansonetti, and F. G. van der Goot. 2002. Initial steps of Shigella infection depend on the cholesterol/sphingolipid raft-mediated CD44-IpaB interaction. EMBO J. 21:4449-4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lafont, F., and F. G. van der Goot. 2005. Bacterial invasion via lipid rafts. Cell. Microbiol. 7:613-620. [DOI] [PubMed] [Google Scholar]
- 144.Lan, R., M. C. Alles, K. Donohoe, M. B. Martinez, and P. R. Reeves. 2004. Molecular evolutionary relationships of enteroinvasive Escherichia coli and Shigella spp. Infect. Immun. 72:5080-5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lan, R., B. Lumb, D. Ryan, and P. R. Reeves. 2001. Molecular evolution of large virulence plasmid in Shigella clones and enteroinvasive Escherichia coli. Infect. Immun. 69:6303-6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lan, R., and P. R. Reeves. 2002. Escherichia coli in disguise: molecular origins of Shigella. Microbes Infect. 4:1125-1132. [DOI] [PubMed] [Google Scholar]
- 147.Laohachai, K. N., R. Bahadi, M. B. Hardo, P. G. Hardo, and J. I. Kourie. 2003. The role of bacterial and non-bacterial toxins in the induction of changes in membrane transport: implications for diarrhea. Toxicon 42:687-707. [DOI] [PubMed] [Google Scholar]
- 148.Le-Barillec, K., J. G. Magalhaes, E. Corcuff, A. Thuizat, P. J. Sansonetti, A. Phalipon, and J. P. Di Santo. 2005. Roles for T and NK cells in the innate immune response to Shigella flexneri. J. Immunol. 175:1735-1740. [DOI] [PubMed] [Google Scholar]
- 149.Le Gall, T., M. Mavris, M. C. Martino, M. L. Bernardini, E. Denamur, and C. Parsot. 2005. Analysis of virulence plasmid gene expression defines three classes of effectors in the type III secretion system of Shigella flexneri. Microbiology 151:951-962. [DOI] [PubMed] [Google Scholar]
- 150.Lett, M.-C., C. Sasakawa, N. Okada, T. Sakai, S. Makino, M. Yamada, K. Komatsu, and M. Yoshikawa. 1989. virG, a plasmid-coded virulence gene of Shigella flexneri: identification of the VirG protein and determination of the complete coding sequence. J. Bacteriol. 171:353-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Li, H., H. Xu, Y. Zhou, J. Zhang, C. Long, S. Li, S. Chen, J. M. Zhou, and F. Shao. 2007. The phosphothreonine lyase activity of a bacterial type III effector family. Science 315:1000-1003. [DOI] [PubMed] [Google Scholar]
- 152.Lillemeier, B. F., J. R. Pfeiffer, Z. Surviladze, B. S. Wilson, and M. M. Davis. 2006. Plasma membrane-associated proteins are clustered into islands attached to the cytoskeleton. Proc. Natl. Acad. Sci. USA 103:18992-18997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lindberg, A. A., A. Karnell, and A. Weintraub. 1991. The lipopolysaccharide of Shigella bacteria as a virulence factor. Rev. Infect. Dis. 13(Suppl. 4):S279-S284. [DOI] [PubMed] [Google Scholar]
- 154.Luck, S. N., S. A. Turner, K. Rajakumar, H. Sakellaris, and B. Adler. 2001. Ferric dicitrate transport system (Fec) of Shigella flexneri 2a YSH6000 is encoded on a novel pathogenicity island carrying multiple antibiotic resistance genes. Infect. Immun. 69:6012-6021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Magdalena, J., A. Hachani, M. Chamekh, N. Jouihri, P. Gounon, A. Blocker, and A. Allaoui. 2002. Spa32 regulates a switch in substrate specificity of the type III secreton of Shigella flexneri from needle components to Ipa proteins. J. Bacteriol. 184:3433-3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Makino, S., C. Sasakawa, K. Kamata, T. Kurata, and M. Yoshikawa. 1986. A genetic determinant required for continuous reinfection of adjacent cells on the large plasmid in S. flexneri 2a. Cell 46:551-555. [DOI] [PubMed] [Google Scholar]
- 157.Man, A. L., M. E. Prieto-Garcia, and C. Nicoletti. 2004. Improving M cell-mediated transport across mucosal barriers: do certain bacteria hold the keys? Immunology 113:15-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mandic-Mulec, I., J. Weiss, and A. Zychlinsky. 1997. Shigella flexneri is trapped in polymorphnuclear leukocyte vacuoles and efficiently killed. Infect. Immun. 65:110-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Manes, S., G. del Real, and A. C. Martinez. 2003. Pathogens: raft hijackers. Nat. Rev. Immunol. 3:557-568. [DOI] [PubMed] [Google Scholar]
- 160.Mariathasan, S., and D. M. Monack. 2007. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat. Rev. Immunol. 7:31-40. [DOI] [PubMed] [Google Scholar]
- 161.Martinon, F., K. Burns, and J. Tschopp. 2002. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-1β. Mol. Cell 10:417-426. [DOI] [PubMed] [Google Scholar]
- 162.Martinon, F., and J. Tschopp. 2007. Inflammatory caspases and inflammasomes: master switches of inflammation. Cell Death Differ. 14:10-22. [DOI] [PubMed] [Google Scholar]
- 163.Martinon, F., and J. Tschopp. 2005. NLRs join TLRs as innate sensors of pathogens. Trends Immunol. 26:447-454. [DOI] [PubMed] [Google Scholar]
- 164.Maurelli, A. T. 2007. Black holes, antivirulence genes, and gene inactivation in the evolution of bacterial pathogens. FEMS Microbiol. Lett. 267:1-8. [DOI] [PubMed] [Google Scholar]
- 165.Maurelli, A. T., B. Baudry, H. d'Hauteville, T. L. Hale, and P. J. Sansonetti. 1985. Cloning of plasmid DNA sequences involved in invasion of HeLa cells by Shigella flexneri. Infect. Immun. 49:164-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Maurelli, A. T., R. E. Fernandez, C. A. Bloch, C. K. Rode, and A. Fasano. 1998. “Black holes” and bacterial pathogenicity: a large genomic deletion that enhances the virulence of Shigella spp. and enteroinvasive Escherichia coli. Proc. Natl. Acad. Sci. USA 95:3943-3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mavris, M., A. L. Page, R. Tournebize, B. Demers, P. Sansonetti, and C. Parsot. 2002. Regulation of transcription by the activity of the Shigella flexneri type III secretion apparatus. Mol. Microbiol. 43:1543-1553. [DOI] [PubMed] [Google Scholar]
- 168.Mavris, M., P. J. Sansonetti, and C. Parsot. 2002. Identification of the cis-acting site involved in activation of promoters regulated by activity of the type III secretion apparatus in Shigella flexneri. J. Bacteriol. 184:6751-6759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.McCormick, B. A., A. M. Siber, and A. T. Maurelli. 1998. Requirement of the Shigella flexneri virulence plasmid in the ability to induce trafficking of neutrophils across polarized monolayers of the intestinal epithelium. Infect. Immun. 66:4237-4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ménard, R., P. Sansonetti, and C. Parsot. 1994. The secretion of the Shigella flexneri Ipa invasins is activated by epithelial cells and controlled by IpaB and IpaD. EMBO J. 13:5293-5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ménard, R., P. J. Sansonetti, and C. Parsot. 1993. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J. Bacteriol. 175:5899-5906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ménard, R., P. J. Sansonetti, C. Parsot, and T. Vasselon. 1994. Extracellular association and cytoplasmic partitioning of the IpaB and IpaC invasins of S. flexneri. Cell 79:515-525. [DOI] [PubMed] [Google Scholar]
- 173.Miura, M., J. Terajima, H. Izumiya, J. Mitobe, T. Komano, and H. Watanabe. 2006. OspE2 of Shigella sonnei is required for the maintenance of cell architecture of bacterium-infected cells. Infect. Immun. 74:2587-2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Monack, D. M., and J. A. Theriot. 2001. Actin-based motility is sufficient for bacterial membrane protrusion formation and host cell uptake. Cell. Microbiol. 3:633-647. [DOI] [PubMed] [Google Scholar]
- 175.Moran, N. A. 2002. Microbial minimalism: genome reduction in bacterial pathogens. Cell 108:583-586. [DOI] [PubMed] [Google Scholar]
- 176.Morita-Ishihara, T., M. Ogawa, H. Sagara, M. Yoshida, E. Katayama, and C. Sasakawa. 2006. Shigella Spa33 is an essential C-ring component of type III secretion machinery. J. Biol. Chem. 281:599-607. [DOI] [PubMed] [Google Scholar]
- 177.Moss, J. E., T. J. Cardozo, A. Zychlinsky, and E. A. Groisman. 1999. The selC-associated SHI-2 pathogenicity island of Shigella flexneri. Mol. Microbiol. 33:74-83. [DOI] [PubMed] [Google Scholar]
- 178.Mounier, J., V. Laurent, A. Hall, P. Fort, M. F. Carlier, P. J. Sansonetti, and C. Egile. 1999. Rho family GTPases control entry of Shigella flexneri into epithelial cells but not intracellular motility. J. Cell Sci. 112:2069-2080. [DOI] [PubMed] [Google Scholar]
- 179.Mounier, J., T. Vasselon, R. Hellio, M. Lesourd, and P. J. Sansonetti. 1992. Shigella flexneri enters human colonic Caco-2 epithelial cells through the basolateral pole. Infect. Immun. 60:237-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Nakata, N., T. Tobe, I. Fukuda, T. Suzuki, K. Komatsu, M. Yoshikawa, and C. Sasakawa. 1993. The absence of a surface protease, OmpT, determines the intercellular spreading ability of Shigella: the relationship between the ompT and kcpA loci. Mol. Microbiol. 9:459-468. [DOI] [PubMed] [Google Scholar]
- 181.Nakayama, S., and H. Watanabe. 1995. Involvement of CpxA, a sensor of a two-component regulatory system, in the pH-dependent regulation of expression of Shigella sonnei virF gene. J. Bacteriol. 177:5062-5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Nassif, X., M. C. Mazert, J. Mounier, and P. J. Sansonetti. 1987. Evaluation with an iuc::Tn10 mutant of the role of aerobactin production in the virulence of Shigella flexneri. Infect. Immun. 55:1963-1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Nataro, J. P., J. Seriwatana, A. Fasano, D. R. Maneval, L. D. Guers, F. Noriega, F. Dubovsky, M. M. Levine, and J. G. Morris, Jr. 1995. Identification and cloning of a novel plasmid-encoded enterotoxin of enteroinvasive Escherichia coli and Shigella strains. Infect. Immun. 63:4721-4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Navarre, W. W., and A. Zychlinsky. 2000. Pathogen-induced apoptosis of macrophages: a common end for different pathogenic strategies. Cell. Microbiol. 2:265-273. [DOI] [PubMed] [Google Scholar]
- 185.Newman, C. L., and C. Stathopoulos. 2004. Autotransporter and two-partner secretion: delivery of large-size virulence factors by gram-negative bacterial pathogens. Crit. Rev. Microbiol. 30:275-286. [DOI] [PubMed] [Google Scholar]
- 186.Nie, H., F. Yang, X. Zhang, J. Yang, L. Chen, J. Wang, Z. Xiong, J. Peng, L. Sun, J. Dong, Y. Xue, X. Xu, S. Chen, Z. Yao, Y. Shen, and Q. Jin. 2006. Complete genome sequence of Shigella flexneri 5b and comparison with Shigella flexneri 2a. BMC Genomics 7:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Niebuhr, K., S. Giuriato, T. Pedron, D. J. Philpott, F. Gaits, J. Sable, M. P. Sheetz, C. Parsot, P. J. Sansonetti, and B. Payrastre. 2002. Conversion of PtdIns(4,5)P(2) into PtdIns(5)P by the S. flexneri effector IpgD reorganizes host cell morphology. EMBO J. 21:5069-5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Niebuhr, K., N. Jouihri, A. Allaoui, P. Gounon, P. J. Sansonetti, and C. Parsot. 2000. IpgD, a protein secreted by the type III secretion machinery of Shigella flexneri, is chaperoned by IpgE and implicated in entry focus formation. Mol. Microbiol. 38:8-19. [DOI] [PubMed] [Google Scholar]
- 189.Niyogi, S. K. 2005. Shigellosis. J. Microbiol. 43:133-143. [PubMed] [Google Scholar]
- 190.Oaks, E. V., T. L. Hale, and S. B. Formal. 1986. Serum immune response to Shigella protein antigens in rhesus monkeys and humans infected with Shigella spp. Infect. Immun. 53:57-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ochman, H., J. G. Lawrence, and E. A. Groisman. 2000. Lateral gene transfer and the nature of bacterial innovation. Nature 405:299-304. [DOI] [PubMed] [Google Scholar]
- 192.Ochman, H., T. Whittam, D. A. Caugant, and R. K. Selander. 1983. Enzyme polymorphism and genetic population structure in Escherichia coli and Shigella. J. Gen. Microbiol. 129:2715-2726. [DOI] [PubMed] [Google Scholar]
- 193.Ogawa, M., and C. Sasakawa. 2006. Intracellular survival of Shigella. Cell. Microbiol. 8:177-184. [DOI] [PubMed] [Google Scholar]
- 194.Ogawa, M., T. Suzuki, I. Tatsuno, H. Abe, and C. Sasakawa. 2003. IcsB, secreted via the type III secretion system, is chaperoned by IpgA and required at the post-invasion stage of Shigella pathogenicity. Mol. Microbiol. 48:913-931. [DOI] [PubMed] [Google Scholar]
- 195.Ogawa, M., T. Yoshimori, T. Suzuki, H. Sagara, N. Mizushima, and C. Sasakawa. 2005. Escape of intracellular Shigella from autophagy. Science 307:727-731. [DOI] [PubMed] [Google Scholar]
- 196.Ohya, K., Y. Handa, M. Ogawa, M. Suzuki, and C. Sasakawa. 2005. IpgB1 is a novel Shigella effector protein involved in bacterial invasion of host cells. Its activity to promote membrane ruffling via Rac1 and Cdc42 activation. J. Biol. Chem. 280:24022-24034. [DOI] [PubMed] [Google Scholar]
- 197.Okuda, J., T. Toyotome, N. Kataoka, M. Ohno, H. Abe, Y. Shimura, A. Seyedarabi, R. Pickersgill, and C. Sasakawa. 2005. Shigella effector IpaH9.8 binds to a splicing factor U2AF(35) to modulate host immune responses. Biochem. Biophys. Res. Commun. 333:531-539. [DOI] [PubMed] [Google Scholar]
- 198.Olive, A. J., R. Kenjale, M. Espina, D. S. Moore, W. L. Picking, and W. D. Picking. 2007. Bile salts stimulate recruitment of IpaB to the Shigella flexneri surface, where it colocalizes with IpaD at the tip of the type III secretion needle. Infect. Immun. 75:2626-2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.O'Loughlin, E. V., and R. M. Robins-Browne. 2001. Effect of Shiga toxin and Shiga-like toxins on eukaryotic cells. Microbes Infect. 3:493-507. [DOI] [PubMed] [Google Scholar]
- 200.Osiecki, J. C., J. Barker, W. L. Picking, A. B. Serfis, E. Berring, S. Shah, A. Harrington, and W. D. Picking. 2001. IpaC from Shigella and SipC from Salmonella possess similar biochemical properties but are functionally distinct. Mol. Microbiol. 42:469-481. [DOI] [PubMed] [Google Scholar]
- 201.Page, A. L., H. Ohayon, P. J. Sansonetti, and C. Parsot. 1999. The secreted IpaB and IpaC invasins and their cytoplasmic chaperone IpgC are required for intercellular dissemination of Shigella flexneri. Cell. Microbiol. 1:183-193. [DOI] [PubMed] [Google Scholar]
- 202.Page, A. L., and C. Parsot. 2002. Chaperones of the type III secretion pathway: jacks of all trades. Mol. Microbiol. 46:1-11. [DOI] [PubMed] [Google Scholar]
- 203.Page, A. L., P. Sansonetti, and C. Parsot. 2002. Spa15 of Shigella flexneri, a third type of chaperone in the type III secretion pathway. Mol. Microbiol. 43:1533-1542. [DOI] [PubMed] [Google Scholar]
- 204.Palmer, M. 2004. Cholesterol and the activity of bacterial toxins. FEMS Microbiol. Lett. 238:281-289. [DOI] [PubMed] [Google Scholar]
- 205.Park, H. H., Y. C. Lo, S. C. Lin, L. Wang, J. K. Yang, and H. Wu. 2007. The death domain superfamily in intracellular signaling of apoptosis and inflammation. Annu. Rev. Immunol. 25:561-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Parsot, C. 2005. Shigella spp. and enteroinvasive Escherichia coli pathogenicity factors. FEMS Microbiol. Lett. 252:11-18. [DOI] [PubMed] [Google Scholar]
- 207.Parsot, C., E. Ageron, C. Penno, M. Mavris, K. Jamoussi, H. d'Hauteville, P. Sansonetti, and B. Demers. 2005. A secreted anti-activator, OspD1, and its chaperone, Spa15, are involved in the control of transcription by the type III secretion apparatus activity in Shigella flexneri. Mol. Microbiol. 56:1627-1635. [DOI] [PubMed] [Google Scholar]
- 208.Parsot, C., C. Hamiaux, and A. L. Page. 2003. The various and varying roles of specific chaperones in type III secretion systems. Curr. Opin. Microbiol. 6:7-14. [DOI] [PubMed] [Google Scholar]
- 209.Parton, R. G., and A. A. Richards. 2003. Lipid rafts and caveolae as portals for endocytosis: new insights and common mechanisms. Traffic 4:724-738. [DOI] [PubMed] [Google Scholar]
- 210.Payne, S. M., E. E. Wyckoff, E. R. Murphy, A. G. Oglesby, M. L. Boulette, and N. M. Davies. 2006. Iron and pathogenesis of Shigella: iron acquisition in the intracellular environment. Biometals 19:173-180. [DOI] [PubMed] [Google Scholar]
- 211.Pedron, T., C. Thibault, and P. J. Sansonetti. 2003. The invasive phenotype of Shigella flexneri directs a distinct gene expression pattern in the human intestinal epithelial cell line Caco-2. J. Biol. Chem. 278:33878-33886. [DOI] [PubMed] [Google Scholar]
- 212.Pendaries, C., H. Tronchere, L. Arbibe, J. Mounier, O. Gozani, L. Cantley, M. J. Fry, F. Gaits-Iacovoni, P. J. Sansonetti, and B. Payrastre. 2006. PtdIns5P activates the host cell PI3-kinase/Akt pathway during Shigella flexneri infection. EMBO J. 25:1024-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Peng, J., X. Zhang, J. Yang, J. Wang, E. Yang, W. Bin, C. Wei, M. Sun, and Q. Jin. 2006. The use of comparative genomic hybridization to characterize genome dynamics and diversity among the serotypes of Shigella. BMC Genomics 7:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Perdomo, J. J., P. Gounon, and P. J. Sansonetti. 1994. Polymorphonuclear leukocyte transmigration promotes invasion of colonic epithelial monolayer by Shigella flexneri. J. Clin. Investig. 93:633-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Perdomo, O. J., J. M. Cavaillon, M. Huerre, H. Ohayon, P. Gounon, and P. J. Sansonetti. 1994. Acute inflammation causes epithelial invasion and mucosal destruction in experimental shigellosis. J. Exp. Med. 180:1307-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Phalipon, A., and P. J. Sansonetti. 2007. Shigella's ways of manipulating the host intestinal innate and adaptive immune system: a tool box for survival? Immunol. Cell Biol. 85:119-129. [DOI] [PubMed] [Google Scholar]
- 216a.Phalipon, A., M. Kaufmann, J. M. Cavaillon, M. Huerre, P. Sansonetti, and J. P. Kraehenbuhl. 1995. Monoclonal immunoglobulin A antibody directed against serotype-specific epitope of Shigella flexneri lipopolysaccharide protects against murine experimental shigellosis. J. Exp. Med. 182:769-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Philpott, D. J., S. Yamaoka, A. Israel, and P. J. Sansonetti. 2000. Invasive Shigella flexneri activates NF-kappa B through a lipopolysaccharide-dependent innate intracellular response and leads to IL-8 expression in epithelial cells. J. Immunol. 165:903-914. [DOI] [PubMed] [Google Scholar]
- 218.Picking, W. L., L. Coye, J. C. Osiecki, A. B. Serfis, E. Schaper, and W. D. Picking. 2001. Identification of functional regions within invasion plasmid antigen C (IpaC) of Shigella flexneri. Mol. Microbiol. 39:100-111. [DOI] [PubMed] [Google Scholar]
- 219.Picking, W. L., H. Nishioka, P. D. Hearn, M. A. Baxter, A. T. Harrington, A. Blocker, and W. D. Picking. 2005. IpaD of Shigella flexneri is independently required for regulation of Ipa protein secretion and efficient insertion of IpaB and IpaC into host membranes. Infect. Immun. 73:1432-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Porter, M. E., and C. J. Dorman. 1997. Differential regulation of the plasmid-encoded genes in the Shigella flexneri virulence regulon. Mol. Gen. Genet. 256:93-103. [DOI] [PubMed] [Google Scholar]
- 221.Porter, M. E., and C. J. Dorman. 1994. A role for H-NS in the thermo-osmotic regulation of virulence gene expression in Shigella flexneri. J. Bacteriol. 176:4187-4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Prosseda, G., M. Falconi, M. Nicoletti, M. Casalino, G. Micheli, and B. Colonna. 2002. Histone-like proteins and the Shigella invasivity regulon. Res. Microbiol. 153:461-468. [DOI] [PubMed] [Google Scholar]
- 223.Prunier, A.-L., R. Schuch, R. E. Fernández, and A. T. Maurelli. 2007. Genetic structure of the nadA and nadB antivirulence loci in Shigella spp. J. Bacteriol. 189:6482-6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Prunier, A. L., R. Schuch, R. E. Fernandez, K. L. Mumy, H. Kohler, B. A. McCormick, and A. T. Maurelli. 2007. nadA and nadB of Shigella flexneri 5a are antivirulence loci responsible for the synthesis of quinolinate, a small molecule inhibitor of Shigella pathogenicity. Microbiology 153:2363-2372. [DOI] [PubMed] [Google Scholar]
- 225.Pupo, G. M., D. K. Karaolis, R. Lan, and P. R. Reeves. 1997. Evolutionary relationship among pathogenic and nonpathogenic Escherichia coli strains inferred from multilocous enzyme electrophoresis and mdh sequence studies. Infect. Immun. 65:2685-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Pupo, G. M., R. Lan, and P. R. Reeves. 2000. Multiple independent origins of Shigella clones of Escherichia coli and convergent evolution of many of their characteristics. Proc. Natl. Acad. Sci. USA 97:10567-10572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Purdy, G. E., and S. M. Payne. 2001. The SHI-3 iron transport island of Shigella boydii 0-1392 carries the genes for aerobactin synthesis and transport. J. Bacteriol. 183:4176-4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Radnedge, L., M. A. Davis, B. L. Youngren, and S. J. Austin. 1997. Plasmid maintenance functions of the large virulence plasmid of Shigella flexneri. J. Bacteriol. 197:3670-3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Radtke, A. L., and M. X. O'Riordan. 2006. Intracellular innate resistance to bacterial pathogens. Cell. Microbiol. 8:1720-1729. [DOI] [PubMed] [Google Scholar]
- 230.Rajendran, L., and K. Simons. 2005. Lipid rafts and membrane dynamics. J. Cell Sci. 118:1099-1102. [DOI] [PubMed] [Google Scholar]
- 231.Ramarao, N., C. Le Clainche, T. Izard, R. Bourdet-Sicard, E. Ageron, P. J. Sansonetti, M. F. Carlier, and G. Tran Van Nhieu. 2007. Capping of actin filaments by vinculin activated by the Shigella IpaA carboxyl-terminal domain. FEBS Lett. 581:853-857. [DOI] [PubMed] [Google Scholar]
- 232.Ramos, H. C., M. Rumbo, and J. C. Sirard. 2004. Bacterial flagellins: mediators of pathogenicity and host immune responses in mucosa. Trends Microbiol. 12:509-517. [DOI] [PubMed] [Google Scholar]
- 233.Rathman, M., P. de Lanerolle, H. Ohayon, P. Gounon, and P. Sansonetti. 2000. Myosin light chain kinase plays an essential role in S. flexneri dissemination. J. Cell Sci. 113:3375-3386. [DOI] [PubMed] [Google Scholar]
- 234.Rathman, M., N. Jouirhi, A. Allaoui, P. Sansonetti, C. Parsot, and G. Tran Van Nhieu. 2000. The development of a FACS-based strategy for the isolation of Shigella flexneri mutants that are deficient in intercellular spread. Mol. Microbiol. 35:974-990. [DOI] [PubMed] [Google Scholar]
- 235.Reig, N., and F. G. van der Goot. 2006. About lipids and toxins. FEBS Lett. 580:5572-5579. [DOI] [PubMed] [Google Scholar]
- 236.Rhode, J. R., A. Breitkreutz, A. Chenal, P. J. Sansonetti, and C. Parsot. 2007. Type III secretion effectors of the IpaH family are E3 ubiquitin ligases. Cell Host Microbe 1:77-83. [DOI] [PubMed] [Google Scholar]
- 237.Riedl, S. J., and Y. Shi. 2004. Molecular mechanisms of caspase regulation during apoptosis. Nat. Rev. Mol. Cell Biol. 5:897-907. [DOI] [PubMed] [Google Scholar]
- 238.Rietveld, A., and K. Simons. 1998. The differential miscibility of lipids as the basis for the formation of functional membrane rafts. Biochim. Biophys. Acta 1376:467-479. [DOI] [PubMed] [Google Scholar]
- 239.Robbins, J. R., D. Monack, S. J. McCallum, A. Vegas, E. Pham, M. B. Goldberg, and J. A. Theriot. 2001. The making of a gradient: IcsA (VirG) polarity in Shigella flexneri. Mol. Microbiol. 41:861-872. [DOI] [PubMed] [Google Scholar]
- 240.Rolland, K., N. Lambert-Zechovsky, B. Picard, and E. Denamur. 1998. Shigella and enteroinvasive Escherichia coli strains are derived from distinct ancestral strains of E. coli. Microbiology 144:2667-2672. [DOI] [PubMed] [Google Scholar]
- 241.Rosenberger, C. M., and B. B. Finlay. 2003. Phagocyte sabotage: disruption of macrophage signalling by bacterial pathogens. Nat. Rev. Mol. Cell Biol. 4:385-396. [DOI] [PubMed] [Google Scholar]
- 242.Sakaguchi, T., H. Kohler, X. Gu, B. A. McCormick, and H. C. Reinecker. 2002. Shigella flexneri regulates tight junction-associated proteins in human intestinal epithelial cells. Cell. Microbiol. 4:367-381. [DOI] [PubMed] [Google Scholar]
- 243.Sakai, T., C. Sasakawa, and M. Yoshikawa. 1988. Expression of four virulence antigens of Shigella flexneri is positively regulated at the transcriptional level by the 30 kilodalton VirF protein. Mol. Microbiol. 2:589-597. [DOI] [PubMed] [Google Scholar]
- 244.Sakellaris, H., N. K. Hannink, K. Rajakumar, D. Bulach, M. Hunt, C. Sasakawa, and B. Adler. 2000. Curli loci of Shigella spp. Infect. Immun. 68:3780-3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Sani, M., A. Allaoui, F. Fusetti, G. T. Oostergetel, W. Keegstra, and E. J. Boekema. 2007. Structural organization of the needle complex of the type III secretion apparatus of Shigella flexneri. Micron 38:291-301. [DOI] [PubMed] [Google Scholar]
- 246.Sani, M., A. Botteaux, C. Parsot, P. Sansonetti, E. J. Boekema, and A. Allaoui. 2007. IpaD is localized at the tip of the Shigella flexneri type III secretion apparatus. Biochim. Biophys. Acta 1770:307-311. [DOI] [PubMed] [Google Scholar]
- 247.Sansonetti, P. J. 2001. Rupture, invasion and inflammatory destruction of the intestinal barrier by Shigella, making sense of prokaryote-eukaryote cross-talks. FEMS Microbiol. Rev. 25:3-14. [DOI] [PubMed] [Google Scholar]
- 248.Sansonetti, P. J. 2006. Shigellosis: an old disease in new clothes? PLoS Med. 3:e354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Sansonetti, P. J. 2004. War and peace at mucosal surfaces. Nat. Rev. Immunol. 4:953-964. [DOI] [PubMed] [Google Scholar]
- 250.Sansonetti, P. J., J. Arondel, R. Cantey, M. C. Prevost, and M. Huerre. 1996. Infection of rabbit Peyer's patches by Shigella flexneri: effect of adhesive or invasive bacterial phenotypes on follicle-associated epithelium. Infect. Immun. 64:2752-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Sansonetti, P. J., J. Arondel, J.-M. Cavaillon, and M. Huerre. 1995. Role of IL-1 in the pathogenesis of experimental shigellosis. J. Clin. Investig. 96:884-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Sansonetti, P. J., J. Arondel, M. Huerre, A. Harada, and K. Matsushima. 1999. Interleukin-8 controls bacterial transepithelial translocation at the cost of epithelial destruction in experimental shigellosis. Infect. Immun. 67:1471-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Sansonetti, P. J., T. L. Hale, G. J. Dammin, C. Kapfer, H. H. Collins, and S. B. Formal. 1983. Alterations in the pathogenicity of Escherichia coli K-12 after transfer of plasmid and chromosomal genes from Shigella flexneri. Infect. Immun. 39:1392-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Sansonetti, P. J., D. J. Kopecko, and S. B. Formal. 1982. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect. Immun. 35:852-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Sansonetti, P. J., and J. Mounier. 1987. Metabolic events mediating early killing of host cells infected by Shigella flexneri. Microb. Pathog. 3:53-61. [DOI] [PubMed] [Google Scholar]
- 256.Sansonetti, P. J., A. Phalipon, J. Arondel, K. Thirumalai, S. Banerjee, S. Akira, K. Takeda, and A. Zychlinsky. 2000. Caspase-1 activation of IL-1β and IL-18 are essential for Shigella flexneri-induced inflammation. Immunity 12:581-590. [DOI] [PubMed] [Google Scholar]
- 257.Sansonetti, P. J., A. Ryter, P. Clerc, A. T. Maurelli, and J. Mounier. 1986. Multiplication of Shigella flexneri within HeLa cells: lysis of the phagocytic vacuole and plasmid-mediated contact hemolysis. Infect. Immun. 51:461-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Santapaola, D., F. Del Chierico, A. Petrucca, S. Uzzau, M. Casalino, B. Colonna, R. Sessa, F. Berlutti, and M. Nicoletti. 2006. Apyrase, the product of the virulence plasmid-encoded phoN2 (apy) gene of Shigella flexneri, is necessary for proper unipolar IcsA localization and for efficient intercellular spread. J. Bacteriol. 188:1620-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Sasakawa, C., K. Kamata, T. Sakai, S. Y. Murayama, S. Makino, and M. Yoshikawa. 1986. Molecular alteration of the 140-megadalton plasmid associated with loss of virulence and Congo red binding activity in Shigella flexneri. Infect. Immun. 54:470-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Sasakawa, C., K. Kamata, T. Sakai, S. Makino, M. Yamada, N. Okada, and M. Yoshikawa. 1988. Virulence-associated genetic regions comprising 31 kilobases of the 230-kilobase plasmid in Shigella flexneri 2a. J. Bacteriol. 170:2480-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Sasakawa, C., K. Komatsu, T. Tobe, T. Suzuki, and M. Yoshikawa. 1993. Eight genes in region 5 that form an operon are essential for invasion of epithelial cells by Shigella flexneri 2a. J. Bacteriol. 175:2334-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Sasakawa, C., S. Makino, K. Kamato, and M. Yoshikawa. 1986. Isolation, characterization, and mapping of Tn5 insertions into the 140-megadalton invasion plasmid defective in the mouse Sereny test in Shigella flexneri 2a. Infect. Immun. 54:32-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Sayeed, S., T. Brendler, M. Davis, L. Reaves, and S. Austin. 2005. Surprising dependence on postsegregational killing of host cells for maintenance of the large virulence plasmid of Shigella flexneri. J. Bacteriol. 187:2768-2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Sayeed, S., L. Reaves, L. Radnedge, and S. Austin. 2000. The stability region of the large virulence plasmid of Shigella flexneri encodes an efficient postsegregational killing system. J. Bacteriol. 182:2416-2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Schlumberger, M. C., and W. D. Hardt. 2006. Salmonella type III secretion effectors: pulling the host cell's strings. Curr. Opin. Microbiol. 9:46-54. [DOI] [PubMed] [Google Scholar]
- 266.Schmidt, H., and M. Hensel. 2004. Pathogenicity islands in bacterial pathogenesis. Clin. Microbiol. Rev. 17:14-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Schmitz, J., A. Owyang, E. Oldham, Y. Song, E. Murphy, T. K. McClanahan, G. Zurawski, M. Moshrefi, J. Qin, X. Li, D. M. Gorman, J. F. Bazan, and R. A. Kastelein. 2005. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23:479-490. [DOI] [PubMed] [Google Scholar]
- 268.Schroeder, G. N., and H. Hilbi. 2007. Cholesterol is required to trigger caspase-1 activation and macrophage apoptosis after phagosomal escape of Shigella. Cell. Microbiol. 9:265-278. [DOI] [PubMed] [Google Scholar]
- 269.Schroeder, G. N., N. J. Jann, and H. Hilbi. 2007. Intracellular type III secretion by cytosolic Shigella flexneri promotes caspase-1-dependent macrophage cell death. Microbiology 153:2862-2876. [DOI] [PubMed] [Google Scholar]
- 270.Schuch, R., and A. T. Maurelli. 1999. The Mxi-Spa type III secretory pathway of Shigella flexneri requires an outer membrane lipoprotein, MxiM, for invasin translocation. Infect. Immun. 67:1982-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Schuch, R., and A. T. Maurelli. 2001. MxiM and MxiJ, base elements of the Mxi-Spa type III secretion system of Shigella, interact with and stabilize the MxiD secretin in the cell envelope. J. Bacteriol. 183:6991-6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Schuch, R., and A. T. Maurelli. 2001. Spa33, a cell surface-associated subunit of the Mxi-Spa type III secretory pathway of Shigella flexneri, regulates Ipa protein traffic. Infect. Immun. 69:2180-2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Schuch, R., R. C. Sandlin, and A. T. Maurelli. 1999. A system for identifying post-invasion functions of invasion genes: requirements for the Mxi-Spa type III secretion pathway of Shigella flexneri in intercellular dissemination. Mol. Microbiol. 34:675-689. [DOI] [PubMed] [Google Scholar]
- 274.Scott, A. M., and M. Saleh. 2007. The inflammatory caspases: guardians against infections and sepsis. Cell Death Differ. 14:23-31. [DOI] [PubMed] [Google Scholar]
- 275.Segal, G., M. Feldman, and T. Zusman. 2005. The Icm/Dot type-IV secretion systems of Legionella pneumophila and Coxiella burnetii. FEMS Microbiol. Rev. 29:65-81. [DOI] [PubMed] [Google Scholar]
- 276.Seifert, U., C. Maranon, A. Shmueli, J. F. Desoutter, L. Wesoloski, K. Janek, P. Henklein, S. Diescher, M. Andrieu, H. de la Salle, T. Weinschenk, H. Schild, D. Laderach, A. Galy, G. Haas, P. M. Kloetzel, Y. Reiss, and A. Hosmalin. 2003. An essential role for tripeptidyl peptidase in the generation of an MHC class I epitope. Nat. Immunol. 4:375-379. [DOI] [PubMed] [Google Scholar]
- 277.Shepherd, J. G., L. Wang, and P. R. Reeves. 2000. Comparison of O-antigen gene clusters of Escherichia coli (Shigella) Sonnei and Plesiomonas shigelloides O17: Sonnei gained its current plasmid-borne O-antigen genes from P. shigelloides in a recent event. Infect. Immun. 68:6056-6061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Shere, K. D., S. Sallustio, A. Manessis, T. G. D'Aversa, and M. B. Goldberg. 1997. Disruption of IcsP, the major Shigella protease that cleaves IcsA, accelerates actin-based motility. Mol. Microbiol. 25:451-462. [DOI] [PubMed] [Google Scholar]
- 279.Shi, Y. 2004. Caspase activation: revisiting the induced proximity model. Cell 117:855-858. [DOI] [PubMed] [Google Scholar]
- 280.Shiga, K. 1897. Sekiri byogen kenkyu hokoku dai-ichi (first report on etiologic research on dysentery). Saikingaku Zasshi 25:790-810. [Google Scholar]
- 281.Siegel, R. M. 2006. Caspases at the crossroads of immune-cell life and death. Nat. Rev. Immunol. 6:308-317. [DOI] [PubMed] [Google Scholar]
- 282.Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature 387:569-572. [DOI] [PubMed] [Google Scholar]
- 283.Simons, K., and E. Ikonen. 2000. How cells handle cholesterol. Science 290:1721-1726. [DOI] [PubMed] [Google Scholar]
- 284.Simons, K., and D. Toomre. 2000. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1:31-39. [DOI] [PubMed] [Google Scholar]
- 285.Simons, K., and W. L. Vaz. 2004. Model systems, lipid rafts, and cell membranes. Annu. Rev. Biophys. Biomol. Struct. 33:269-295. [DOI] [PubMed] [Google Scholar]
- 286.Singer, M., and P. J. Sansonetti. 2004. IL-8 is a key chemokine regulating neutrophil recruitment in a new mouse model of Shigella-induced colitis. J. Immunol. 173:4197-4206. [DOI] [PubMed] [Google Scholar]
- 287.Skoudy, A., J. Mounier, A. Aruffo, H. Ohayon, P. Gounon, P. Sansonetti, and G. Tran Van Nhieu. 2000. CD44 binds to the Shigella IpaB protein and participates in bacterial invasion of epithelial cells. Cell. Microbiol. 2:19-33. [DOI] [PubMed] [Google Scholar]
- 288.Sorg, J. A., B. Blaylock, and O. Schneewind. 2006. Secretion signal recognition by YscN, the Yersinia type III secretion ATPase. Proc. Natl. Acad. Sci. USA 103:16490-16495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Stavropoulou, V., V. Vasquez, B. Cereser, E. Freda, and M. G. Masucci. 2006. TPPII promotes genetic instability by allowing the escape from apoptosis of cells with activated mitotic checkpoints. Biochem. Biophys. Res. Commun. 346:415-425. [DOI] [PubMed] [Google Scholar]
- 290.Steinhauer, J., R. Agha, T. Pham, A. W. Varga, and M. B. Goldberg. 1999. The unipolar Shigella surface protein IcsA is targeted directly to the bacterial old pole: IcsP cleavage of IcsA occurs over the entire bacterial surface. Mol. Microbiol. 32:367-377. [DOI] [PubMed] [Google Scholar]
- 291.Stevens, J. M., E. E. Galyov, and M. P. Stevens. 2006. Actin-dependent movement of bacterial pathogens. Nat. Rev. Microbiol. 4:91-101. [DOI] [PubMed] [Google Scholar]
- 292.Strober, W., P. J. Murray, A. Kitani, and T. Watanabe. 2006. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat. Rev. Immunol. 6:9-20. [DOI] [PubMed] [Google Scholar]
- 293.Suzuki, T., L. Franchi, C. Toma, H. Ashida, M. Ogawa, Y. Yoshikawa, H. Mimuro, N. Inohara, C. Sasakawa, and G. Nunez. 2007. Differential regulation of caspase-1 activation, pyroptosis, and autophagy via Ipaf and ASC in Shigella-infected macrophages. PLoS Pathog. 3:e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Suzuki, T., H. Miki, T. Takenawa, and C. Sasakawa. 1998. Neural Wiskott-Aldrich syndrome protein is implicated in the actin-based motility of Shigella flexneri. EMBO J. 17:2767-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Suzuki, T., H. Mimuro, H. Miki, T. Takenawa, T. Sasaki, H. Nakanishi, Y. Takai, and C. Sasakawa. 2000. Rho family GTPase Cdc42 is essential for the actin-based motility of Shigella in mammalian cells. J. Exp. Med. 191:1905-1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Suzuki, T., H. Mimuro, S. Suetsugu, H. Miki, T. Takenawa, and C. Sasakawa. 2002. Neural Wiskott-Aldrich syndrome protein (N-WASP) is the specific ligand for Shigella VirG among the WASP family and determines the host cell type allowing actin-based spreading. Cell. Microbiol. 4:223-233. [DOI] [PubMed] [Google Scholar]
- 297.Suzuki, T., K. Nakanishi, H. Tsutsui, H. Iwai, S. Akira, N. Inohara, M. Chamaillard, G. Nunez, and C. Sasakawa. 2005. A novel caspase-1/Toll-like receptor 4-independent pathway of cell death induced by cytosolic Shigella in infected macrophages. J. Biol. Chem. 280:14042-14050. [DOI] [PubMed] [Google Scholar]
- 298.Suzuki, T., S. Saga, and C. Sasakawa. 1996. Functional analysis of Shigella VirG domains essential for interaction with vinculin and actin-based motility. J. Biol. Chem. 271:21878-21885. [DOI] [PubMed] [Google Scholar]
- 299.Tamano, K., S. Aizawa, E. Katayama, T. Nonaka, S. Imajoh-Ohmi, A. Kuwae, S. Nagai, and C. Sasakawa. 2000. Supramolecular structure of the Shigella type III secretion machinery: the needle part is changeable in length and essential for delivery of effectors. EMBO J. 19:3876-3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Tamano, K., E. Katayama, T. Toyotome, and C. Sasakawa. 2002. Shigella Spa32 is an essential secretory protein for functional type III secretion machinery and uniformity of its needle length. J. Bacteriol. 184:1244-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Tampakaki, A. P., V. E. Fadouloglou, A. D. Gazi, N. J. Panopoulos, and M. Kokkinidis. 2004. Conserved features of type III secretion. Cell. Microbiol. 6:805-816. [DOI] [PubMed] [Google Scholar]
- 302.Taylor, P. R., L. Martinez-Pomares, M. Stacey, H. H. Lin, G. D. Brown, and S. Gordon. 2005. Macrophage receptors and immune recognition. Annu. Rev. Immunol. 23:901-944. [DOI] [PubMed] [Google Scholar]
- 303.Thornberry, N. A., H. G. Bull, J. R. Calaycay, K. T. Chapman, A. D. Howard, M. J. Kostura, D. K. Miller, S. M. Molineaux, J. R. Weidner, J. Aunins, K. O. Elliston, J. M. Ayala, F. J. Casano, J. Chin, J.-F. Ding, L. A. Egger, E. P. Gaffney, G. Limjuco, P. O. C., S. M. Raju, A. M. Rolando, J. P. Salley, T. T. Yamin, T. D. Lee, J. E. Shively, M. MacCross, R. A. Mumford, J. A. Schmidt, and M. J. Tocci. 1992. A novel heterodimeric cysteine protease is required for interleukin-1β-processing in monocytes. Nature 356:768-774. [DOI] [PubMed] [Google Scholar]
- 304.Thornberry, N. A., and Y. Lazebnik. 1998. Caspases: enemies within. Science 281:1312-1316. [DOI] [PubMed] [Google Scholar]
- 305.Tobe, T., S. Nagai, N. Okada, B. Adler, M. Yoshikawa, and C. Sasakawa. 1991. Temperature-regulated expression of invasion genes in Shigella flexneri is controlled through the transcriptional activation of the virB gene on the large plasmid. Mol. Microbiol. 5:887-893. [DOI] [PubMed] [Google Scholar]
- 306.Tobe, T., M. Yoshikawa, T. Mizuno, and C. Sasakawa. 1993. Transcriptional control of the invasion regulatory gene virB of Shigella flexneri: activation by VirF and repression by H-NS. J. Bacteriol. 175:6142-6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Tominaga, A., R. Lan, and P. R. Reeves. 2005. Evolutionary changes of the flhDC flagellar master operon in Shigella strains. J. Bacteriol. 187:4295-4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Torres, A. G. 2004. Current aspects of Shigella pathogenesis. Rev. Latinoam. Microbiol. 46:89-97. [PubMed] [Google Scholar]
- 309.Toyotome, T., T. Suzuki, A. Kuwae, T. Nonaka, H. Fukuda, S. Imajoh-Ohmi, T. Toyofuku, M. Hori, and C. Sasakawa. 2001. Shigella protein IpaH(9.8) is secreted from bacteria within mammalian cells and transported to the nucleus. J. Biol. Chem. 276:32071-32079. [DOI] [PubMed] [Google Scholar]
- 310.Tran Van Nhieu, G., J. Enninga, P. Sansonetti, and G. Grompone. 2005. Tyrosine kinase signaling and type III effectors orchestrating Shigella invasion. Curr. Opin. Microbiol. 8:16-20. [DOI] [PubMed] [Google Scholar]
- 311.Tran Van Nhieu, G., A. Ben-Ze'ev, and P. Sansonetti. 1997. Modulation of bacterial entry into epithelial cells by association between vinculin and the Shigella IpaA invasin. EMBO J. 16:2717-2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Tran Van Nhieu, G., E. Caron, A. Hall, and P. J. Sansonetti. 1999. IpaC induces actin polymerization and filopodia formation during Shigella entry into epithelial cells. EMBO J. 18:3249-3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Turner, S. A., S. N. Luck, H. Sakellaris, K. Rajakumar, and B. Adler. 2003. Molecular epidemiology of the SRL pathogenicity island. Antimicrob. Agents Chemother. 47:727-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Turner, S. A., S. N. Luck, H. Sakellaris, K. Rajakumar, and B. Adler. 2001. Nested deletions of the SRL pathogenicity island of Shigella flexneri 2a. J. Bacteriol. 183:5535-5543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.van der Goot, F. G., G. Tran Van Nhieu, A. Allaoui, P. Sansonetti, and F. Lafont. 2004. Rafts can trigger contact-mediated secretion of bacterial effectors via a lipid-based mechanism. J. Biol. Chem. 279:47792-47798. [DOI] [PubMed] [Google Scholar]
- 316.van Eerde, A., C. Hamiaux, J. Perez, C. Parsot, and B. W. Dijkstra. 2004. Structure of Spa15, a type III secretion chaperone from Shigella flexneri with broad specificity. EMBO Rep. 5:477-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Veenendaal, A. K., J. L. Hodgkinson, L. Schwarzer, D. Stabat, S. F. Zenk, and A. J. Blocker. 2007. The type III secretion system needle tip complex mediates host cell sensing and translocon insertion. Mol. Microbiol. 63:1719-1730. [DOI] [PubMed] [Google Scholar]
- 318.Venkatesan, M. M., J. M. Buysse, and A. B. Hartman. 1991. Sequence variation in two ipaH genes of Shigella flexneri 5 and homology to the LRG-like family of proteins. Mol. Microbiol. 5:2435-2446. [DOI] [PubMed] [Google Scholar]
- 319.Venkatesan, M. M., J. M. Buysse, and E. V. Oaks. 1992. Surface presentation of Shigella flexneri invasion plasmid antigens requires the products of the spa locus. J. Bacteriol. 174:1990-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Venkatesan, M. M., M. B. Goldberg, D. J. Rose, E. J. Grotbeck, V. Burland, and F. R. Blattner. 2001. Complete DNA sequence and analysis of the large virulence plasmid of Shigella flexneri. Infect. Immun. 69:3271-3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Venkatesan, M. M., and R. T. Ranallo. 2006. Live-attenuated Shigella vaccines. Expert Rev. Vaccines 5:669-686. [DOI] [PubMed] [Google Scholar]
- 322.Vokes, S. A., S. A. Reeves, A. G. Torres, and S. M. Payne. 1999. The aerobactin iron transport system genes in Shigella flexneri are present within a pathogenicity island. Mol. Microbiol. 33:63-73. [DOI] [PubMed] [Google Scholar]
- 323.von Seidlein, L., D. R. Kim, M. Ali, H. Lee, X. Wang, V. D. Thiem, D. G. Canh, W. Chaicumpa, M. D. Agtini, A. Hossain, Z. A. Bhutta, C. Mason, O. Sethabutr, K. Talukder, G. B. Nair, J. L. Deen, K. Kotloff, and J. Clemens. 2006. A multicentre study of Shigella diarrhoea in six Asian countries: disease burden, clinical manifestations and microbiology. PLoS Med. 3:e353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Wang, S., M. Miura, Y. K. Jung, H. Zhu, E. Li, and J. Yuan. 1998. Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell 92:501-509. [DOI] [PubMed] [Google Scholar]
- 325.Wassef, J. S., D. F. Keren, and J. L. Mailloux. 1989. Role of M cells in initial antigen uptake and in ulcer formation in rabbit intestinal loop model of shigellosis. Infect. Immun. 57:858-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Watarai, M., S. Funato, and C. Sasakawa. 1996. Interaction of Ipa proteins of Shigella flexneri with α5β1 integrin promotes entry of the bacteria into mammalian cells. J. Exp. Med. 183:991-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Watarai, M., T. Tobe, M. Yoshikawa, and C. Sasakawa. 1995. Contact of Shigella with host cells triggers release of Ipa invasins and is an essential function of invasiveness. EMBO J. 14:2461-2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Way, S. S., A. C. Borczuk, R. Dominitz, and M. B. Goldberg. 1998. An essential role for gamma interferon in innate resistance to Shigella flexneri infection. Infect. Immun. 66:1342-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Wei, J., M. B. Goldberg, V. Burland, M. M. Venkatesan, W. Deng, G. Fournier, G. F. Mayhew, G. Plunkett III, D. J. Rose, A. Darling, B. Mau, N. T. Perna, S. M. Payne, L. J. Runyen-Janecky, S. Zhou, D. C. Schwartz, and F. R. Blattner. 2003. Complete genome sequence and comparative genomics of Shigella flexneri serotype 2a strain 2457T. Infect. Immun. 71:2775-2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.West, N. P., P. Sansonetti, J. Mounier, R. M. Exley, C. Parsot, S. Guadagnini, M. C. Prevost, A. Prochnicka-Chalufour, M. Delepierre, M. Tanguy, and C. M. Tang. 2005. Optimization of virulence functions through glucosylation of Shigella LPS. Science 307:1313-1317. [DOI] [PubMed] [Google Scholar]
- 331.Wilharm, G., S. Dittmann, A. Schmid, and J. Heesemann. 2006. On the role of specific chaperones, the specific ATPase, and the proton motive force in type III secretion. Int. J. Med. Microbiol. 297:27-36. [DOI] [PubMed] [Google Scholar]
- 332.Wilharm, G., V. Lehmann, K. Krauss, B. Lehnert, S. Richter, K. Ruckdeschel, J. Heesemann, and K. Trulzsch. 2004. Yersinia enterocolitica type III secretion depends on the proton motive force but not on the flagellar motor components MotA and MotB. Infect. Immun. 72:4004-4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Wilson, K. P., J. A. Black, J. A. Thomson, E. E. Kim, J. P. Griffith, M. A. Navia, M. A. Murcko, S. P. Chambers, R. A. Aldape, S. A. Raybuck, et al. 1994. Structure and mechanism of interleukin-1β converting enzyme. Nature 370:270-275. [DOI] [PubMed] [Google Scholar]
- 334.World Health Organization. 2004. Global burden of disease (GBD) 2002 estimates. World Health Organization, Geneva, Switzerland. http://www.who.int/topics/global_burden_of_disease/en/.
- 335.World Health Organization. 2006. Initiative for vaccine research (IVR). New vaccines against infectious disesases: research and development status. World Health Organization, Geneva, Switzerland. http://www.who.int/vaccine_research/en/.
- 336.Yang, F., J. Yang, X. Zhang, L. Chen, Y. Jiang, Y. Yan, X. Tang, J. Wang, Z. Xiong, J. Dong, Y. Xue, Y. Zhu, X. Xu, L. Sun, S. Chen, H. Nie, J. Peng, J. Xu, Y. Wang, Z. Yuan, Y. Wen, Z. Yao, Y. Shen, B. Qiang, Y. Hou, J. Yu, and Q. Jin. 2005. Genome dynamics and diversity of Shigella species, the etiologic agents of bacillary dysentery. Nucleic Acids Res. 33:6445-6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Yang, J., H. Nie, L. Chen, X. Zhang, F. Yang, X. Xu, Y. Zhu, J. Yu, and Q. Jin. 2007. Revisiting the molecular evolutionary history of Shigella spp. J. Mol. Evol. 64:71-79. [DOI] [PubMed] [Google Scholar]
- 338.Yoshida, S., Y. Handa, T. Suzuki, M. Ogawa, M. Suzuki, A. Tamai, A. Abe, E. Katayama, and C. Sasakawa. 2006. Microtubule-severing activity of Shigella is pivotal for intercellular spreading. Science 314:985-989. [DOI] [PubMed] [Google Scholar]
- 339.Yoshida, S., E. Katayama, A. Kuwae, H. Mimuro, T. Suzuki, and C. Sasakawa. 2002. Shigella delivers effector proteins to trigger host microtubule destabilization, which promotes Rac1 activity and efficient bacterial internalization. EMBO J. 21:2923-2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Zaharik, M. L., S. Gruenheid, A. J. Perrin, and B. B. Finlay. 2002. Delivery of dangerous goods: type III secretion in enteric pathogens. Int. J. Med. Microbiol. 291:593-603. [DOI] [PubMed] [Google Scholar]
- 341.Zahrl, D., M. Wagner, K. Bischof, M. Bayer, B. Zavecz, A. Beranek, C. Ruckenstuhl, G. E. Zarfel, and G. Koraimann. 2005. Peptidoglycan degradation by specialized lytic transglycosylases associated with type III and type IV secretion systems. Microbiology 151:3455-3467. [DOI] [PubMed] [Google Scholar]
- 342.Zhang, Z., L. Jin, G. Champion, K. B. Seydel, and S. L. Stanley, Jr. 2001. Shigella infection in a SCID mouse-human intestinal xenograft model: role for neutrophils in containing bacterial dissemination in human intestine. Infect. Immun. 69:3240-3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Zhong, Q. P. 1999. Pathogenic effects of O-polysaccharide from Shigella flexneri strain. World J. Gastroenterol. 5:245-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Zurawski, D. V., C. Mitsuhata, K. L. Mumy, B. A. McCormick, and A. T. Maurelli. 2006. OspF and OspC1 are Shigella flexneri type III secretion system effectors that are required for postinvasion aspects of virulence. Infect. Immun. 74:5964-5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Zychlinsky, A., C. Fitting, J. M. Cavaillon, and P. J. Sansonetti. 1994. Interleukin-1 is released by murine macrophages during apoptosis induced by Shigella flexneri. J. Clin. Investig. 94:1328-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Zychlinsky, A., B. Kenny, R. Ménard, M. C. Prévost, I. B. Holland, and P. J. Sansonetti. 1994. IpaB mediates macrophage apoptosis induced by Shigella flexneri. Mol. Microbiol. 11:619-627. [DOI] [PubMed] [Google Scholar]
- 347.Zychlinsky, A., M. C. Prévost, and P. J. Sansonetti. 1992. Shigella flexneri induces apoptosis in infected macrophages. Nature 358:167-168. [DOI] [PubMed] [Google Scholar]
- 348.Zychlinsky, A., K. Thirumalai, J. Arondel, J. R. Cantey, A. Aliprantis, and P. J. Sansonetti. 1996. In vivo apoptosis in Shigella flexneri infections. Infect. Immun. 64:5357-5365. [DOI] [PMC free article] [PubMed] [Google Scholar]