Abstract
To help mitigate the expanding global impact of malaria, with its associated increasing drug resistance, implementation of prompt and accurate diagnosis is needed. Malaria is diagnosed predominantly by using clinical criteria, with microscopy as the current gold standard for detecting parasitemia, even though it is clearly inadequate in many health care settings. Rapid diagnostic tests (RDTs) have been recognized as an ideal method for diagnosing infectious diseases, including malaria, in recent years. There have been a number of RDTs developed and evaluated widely for malaria diagnosis, but a number of issues related to these products have arisen. This review highlights RDTs, including challenges in assessing their performance, internationally available RDTs, their effectiveness in various health care settings, and the selection of RDTs for different health care systems.
INTRODUCTION
A key to effective management of malaria is prompt and accurate diagnosis. The global impact of malaria has spurred interest in developing diagnostic strategies that will be effective not only in resource-limited areas, where malaria has a substantial burden on society, but also in developed countries, where expertise in malaria diagnosis is often lacking (7, 78). Accurate diagnosis of malaria is necessary to prevent morbidity and mortality while avoiding the unnecessary use of antimalarial agents. New rapid diagnostic techniques have been developed and evaluated widely in recent years, but the rapid introduction, withdrawal, and modification of commercially available products, variable quality control in manufacturing, and potential decrements in test performance related to the stability of stored test kits have rendered these reviews largely obsolete (54, 62, 66).
Characteristics required of a rapid malaria diagnostic test (MRDT) vary based on regional malaria epidemiology and the goals of a malaria control program (6). The critical characteristics needed for a diagnostic method in order to reduce mortality from malaria in sub-Saharan Africa include high sensitivity for detecting Plasmodium falciparum and rapid availability of test results. However, overdiagnosis, i.e., test performance characterized by a poor positive predictive value, can produce overestimates of malaria morbidity, inflate treatment costs, create misperceptions of therapeutic failures when fever is due to other illnesses, lead to avoidable drug-related adverse events, and contribute to unnecessary drug pressure, thereby enhancing selection for drug resistance. In contrast, in low-incidence environments where the pretest probability of malaria is low, high specificity and high sensitivity for detection of non-P. falciparum Plasmodium species assume greater importance, and a repeat testing paradigm may be practical. Regardless of the setting, MRDTs should require minimal operator training and yield highly reproducible test interpretations. The results of the test should be rapidly available while the physician is actively managing patients, typically in less than 1 hour. The ideal test should be able to detect a response to therapy, including detection of recrudescence or relapse. The stability of MRDT components should be such that refrigeration and a cold chain are not needed (110). Storage shelf life should be of sufficiently long duration that the logistical burden of resupply is minimized.
TRADITIONAL MALARIA DIAGNOSIS
Historical strategies to diagnose malaria range from basic empirical clinical diagnostic algorithms to examination of stained blood smears by light microscopy. Although empirical clinical diagnosis remains the most common method to diagnose malaria in many regions, the accuracy of this strategy is poor, even in countries of endemicity with high malaria incidence rates. The symptom complex of malaria overlaps with those of many other tropical diseases, and coinfections can occur (9, 14, 34, 51, 73, 83, 112). In high-transmission areas of sub-Saharan Africa, the distinction between the clinical disease of malaria and malaria parasitemia is especially difficult. Persons may present with a wide variety of other fever-inducing diseases accompanied by a parasitemia that is not related to the presenting symptoms (101). In such settings, no testing paradigm currently exists to actually confirm that a given illness is caused by malaria parasitemia. For decades, the wide availability, safety, effectiveness, and minimal cost of chloroquine for the treatment of all malaria cases made the routine empirical use of the drug accepted throughout the world. With the emergence of chloroquine-resistant P. falciparum malaria, the empirical use of chloroquine to treat suspected malaria is no longer safe. The introduction of artemisinin combination therapies (ACTs) in Thailand in the mid-1990s and the adoption of ACTs as first-line treatment by most African countries by the early 2000s have changed the cost-benefit ratio of empirical treatment of fever. The much higher cost of ACTs makes a specific diagnosis of malaria more cost-effective and demands a more accurate diagnostic paradigm.
Microscopic examination of stained blood smears remains the “gold standard” for detection of malaria parasitemia. The sensitivity of this method can be excellent, with detection of malaria parasite densities as low as 5 to 10 parasites/μl of blood (approximately 0.0001% parasitemia) (Table 1) (62). Microscopy permits determination of the infecting species as well as the stage of the circulating parasites. In addition, circulating parasite density can be determined, which may aid in prognosis, and serial examinations can monitor the parasitological response to chemotherapy. Examining an individual sample is relatively inexpensive, but this cost may not incorporate the health care system's cost of equipment and training. The microscope can also be used for other diagnoses, such as tuberculosis and sexually transmitted diseases, affording additional economies. Finally, malaria smears provide a permanent record for quality assessment of the microscopy diagnosis. Despite these strengths, microscopy possesses a number of limitations (4, 19, 25, 42, 43, 45, 56, 72, 97, 113, 114). The procedure is labor-intensive and time-consuming. Variability in stains and in techniques used to collect and process blood affects slide interpretation (61, 107). Importantly, accurate microscopic diagnosis is a skill still learned with extended training and experience, whether in countries where malaria is endemic or in countries with imported malaria. An individual microscopist's interpretative expertise can diminish over time. Routine clinical microscopy cannot reliably detect very low parasitemias (<5 to 10 parasites/μl) or sequestered parasites. Examination of serially obtained smears helps to overcome the challenges posed by parasite sequestration and initially low parasite densities (Table 1) (35). Microscopy diagnostic errors are noted more commonly for low-density parasitemias (10 to 100 parasites/μl of blood), but errors of quantification also occur with densities of >5,000/μl and especially >20,000/μl of blood (45). In addition, mixed infections are often missed, especially when P. malariae or P. ovale parasites are present, as their densities are often low in comparison to that of P. falciparum (45). Finally, even when the test is ordered, clinicians may choose not to wait for the test results or may lack confidence in the test result and treat the patient despite negative microscopy (34, 78). These problems are exacerbated in regions where malaria is not endemic, as malaria microscopy is performed infrequently. Illustrating this problem, a study of 100 patients in Canada conducted by Kain et al. reported that diagnosis at presentation was missed in 59% of malaria cases and the infecting species was identified incorrectly 64% of the time, resulting in therapeutic delays of 5.1 to 7.6 days (43). Many of these errors were associated with infections with P. vivax.
TABLE 1.
Relative levels of parasitemia in different patient groupsa
| Group | Parasitemia (%) | No. of parasites/μl of blood |
|---|---|---|
| Patients with positive thick film | 0.0001-0.0004 | 5-20 |
| Naïve patients with symptoms (below this level) | 0.0002 | 100 |
| Emergency room patients and travelers | 0.2 | 10,000 |
| Immune patients exhibiting symptoms (above this level) | 0.05-0.7 | 2,500 (infants) to 30,000 (immune adults) |
| Patients with maximum parasitemia of P. vivax and P. ovale | 2-5 (rarely exceeds 2) | 100,000-250,000 |
| Patients with hyperparasitemia and severe malaria with increased mortality | 10 | 500,000 |
ALTERNATIVE MALARIA DIAGNOSIS
In recent years, new technological methods have been evaluated as alternatives to microscopy. These methods have included malaria antigen detection using tagged monoclonal antibodies, fluorescence microscopy, flow cytometry, automated analysis of blood cells, quantitative buffy coat inspection, acridine orange staining, serology-antibody detection, molecular amplification methods, and laser desorption mass spectrometry (1, 17, 21, 24, 31, 40, 62, 68, 82, 95). Remote microscopic diagnosis via telemedicine has also been evaluated, but imaging quality and infrastructure requirements pose challenges (67). These methods have various strengths and weaknesses but, overall, are limited by the need for specialized equipment, continuing supplies, operator expertise, cost, assay run time, applicability in the setting of acute infection, and/or availability.
MRDTs utilizing immunochromatographic lateral-flow-strip technology were introduced in the early 1990s. Immunochromatographic technology remains the common basis for all practical MRDTs under consideration at this time. From their introduction, these products suffered from rapid introduction, withdrawal, and modification by their manufacturers, inconsistency in manufacturing standards, quality control problems, and variable product stability (55, 62, 66, 86). The Special Programme for Research and Training in Tropical Diseases recently published principles for the development and evaluation of diagnostic tests for infectious diseases (3). The goals of this organization encompass the development of standards and guidelines to assist diagnostic assay development and manufacture, regulatory approval processes, and policy development to support public health programs.
REQUIREMENTS FOR MRDTS
Immunochromatographic MRDTs represent an evolving technology that can be applicable in a spectrum of settings extending from diagnosis of imported malaria in tertiary hospitals in regions where malaria is not endemic to remote health care clinics without clinical laboratories. However, different test requirements for an MRDT product might be necessary based upon the epidemiological setting where the product will be introduced. These settings can be classified into the following three categories: sub-Saharan Africa, other areas of malaria endemicity, and areas where the disease is not endemic (traveler's malaria) (Table 2). Specific factors that need to be considered in introducing an MRDT can be broken down broadly into performance characteristics, operational characteristics, and cost (6, 108).
TABLE 2.
Ideal requirements for MRDTsa
| Criterionb | Requirement for region
|
||
|---|---|---|---|
| Sub-Saharan Africa | Other areas of malaria endemicity | Malaria-free countries | |
| Assay performance characteristics | |||
| Detects only P. falciparum | Yes | No | No |
| Detects all human malarias | Low priority | Yes | Yes |
| Distinguishes Plasmodium species | Low priority | Ideal | Ideal |
| Able to detect mixed infections | Low priority | Ideal | Ideal |
| Lower limit of detection of <50 parasites/μl | Not necessary | Ideal | Ideal |
| High specificity | Not necessary | Ideal | Yes |
| Semiquantitative | Not necessary | Not necessary | Ideal |
| Able to monitor response to therapy | Low priority | Ideal (for drug-resistant P. falciparum) | Low priority |
| Assay specifications | |||
| ICH GMP | Yes | Yes | Yes |
| Stable to 40°C | Yes | Yes | No (room temp) |
| Long shelf life | Ideal | Ideal | Yes |
| Point-of-care use (CLIA waived) | Not necessary | Not necessary | Ideal |
| Cost ($ per test) | <1 | Few | 20 (less than cost of microscopy) |
All regions share a need for tests to be rapid (<20 min); easy to use, with minimal training requirements and minimal steps and reagents; reproducible, with quality manufacturing; and able to detect parasite densities of <100 parasites/μl.
ICH, International Conference on Harmonization; GMP, good manufacturing practices; CLIA, clinical laboratory improvement amendments.
Performance characteristics include the ability (or its absence) to distinguish between malaria species; the sensitivity and specificity for detection of each of those species; the parasitemic or antigenemic concentration threshold at which the test is able to identify its targets (lower limit of detection); and the ability to identify mixed infections. For patients with positive test results, the time to parasite antigen clearance after parasites are removed is important to the determination of whether the test may be used to monitor therapy. The ability of the test to distinguish between species is important primarily in regions with high incidences of relapsing malarias because these infections would warrant initial selection of additional drugs for treatment. The World Health Organization (WHO) has recommended a minimal standard of 95% sensitivity for P. falciparum densities of 100/μl and a specificity of 95% (Table 1) (6, 111). Furthermore, if posttreatment monitoring of patients is a requirement and microscopy is unavailable, an MRDT should revert to a negative result within a few days after clearance of viable parasites to permit monitoring of the response to therapy.
Operational characteristics to be considered include the technical simplicity of the test, training requirements, ease of interpretation, reproducibility of results, user acceptability of the test, and absence of any need for electricity to operate the assay. The time required to perform and interpret the test is also important if the results are to be available to the physician during the clinical encounter. Rapid availability of results allows directed instead of empirical therapy. The product's storage requirements and the stability of the test during storage, ideally under ambient temperature/humidity conditions, are important (111). Finally, the cost of assay deployment and use is important in most regions of malaria endemicity in the world.
MRDT TECHNOLOGY
Current MRDTs employ lateral-flow immunochromatographic technology. This technology has been employed for a variety of other diagnostic assays, including pregnancy tests. In these assays, the clinical sample migrates as a liquid across the surface of a nitrocellulose membrane by means of capillary action (Fig. 1) (6, 108). For a targeted parasite antigen, two sets of antibodies, a capture antibody and a detection antibody, are used. Either of these antibodies can be monoclonal or polyclonal. Monoclonal antibodies can be very specific but less sensitive, while polyclonal antibodies can be more sensitive but less specific. Also, the source of antigen used to induce the MRDT antibodies (purified native protein, recombinant proteins, or peptides) can make significant differences in the performance characteristics of the final assay. Even monoclonal antibodies directed against the same antigen, if they target different epitopes of that antigen, may exhibit quite different sensitivities and specificities (48). The capture antibodies are sprayed as a stripe by machine onto the nitrocellulose membrane and bind to the membrane in an immobile phase. These fixed antibodies serve to extract and bind parasite antigen from the migrating liquid sample. The second set of antibodies is conjugated to an indicator, typically gold particles, in a mobile phase. These antibody-indicator complexes bind to the parasite antigen that has been captured by the immobile antibody on the membrane, producing a visible line if the targeted antigen is present in the clinical sample.
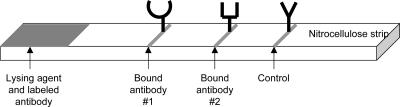
FIG. 1.
Schematic of an MRDT. On one end of the nitrocellulose strip, one or two indicator-labeled antibodies, one specific for each target antigen, are placed. A second antibody specific for a different epitope of each of the target antigens is bound to the strip in a thin line. Another antibody specific for the indicator-labeled antibody complex is bound at the control line. Blood and buffer are added to the strip where the lysing agent and labeled antibody are located and are drawn up the strip. If antigen is present, some indicator-labeled antibody-antigen complexes will be trapped on the test line and become visible. Additional indicator-labeled antibody is trapped on the control line and becomes visible.
The characteristics of the malaria antigen target and the detection antibodies are paramount to understanding the assay performance. Malaria antigens currently used as diagnostic targets are either specific to a Plasmodium species or conserved across the human malarias. P. falciparum-specific monoclonal antibodies have been developed for histidine-rich protein 2 (HRP-2) and P. falciparum lactate dehydrogenase, while P. vivax-specific monoclonal antibodies have undergone limited evaluation (3, 6, 27). Targets conserved across all human malarias (pan-Plasmodium antigens) have been identified on Plasmodium lactate dehydrogenase (PLDH) and aldolase enzymes (11, 27, 52, 53).
The first antigen used in a commercial assay was HRP-2, a water-soluble protein unique to Plasmodium falciparum, which is localized in the parasite cytoplasm and on the parasitized erythrocyte membrane (37, 86). The HRP-2 concentration increases as the parasite develops from the ring stage to the late trophozoite. This antigen readily diffuses into the plasma (37, 80). It is found predominantly in the asexual stages but is also found in young P. falciparum gametocytes (36, 104). It can be detected at lower levels of parasitemia than the panmalarial antigens such as aldolase (28, 79). Many different monoclonal antibodies, both immunoglobulin G (IgG) and IgM, have been raised against this antigen and have been employed on different MRDTs. Variants that escape monoclonal recognition have been identified now and may be responsible for false-negative tests (2, 48). In an assessment of HRP-2 in parasites obtained from 19 countries, an extensive level of diversity of HRP-2 sequences was observed, prompting a prediction that only 84% of P. falciparum infections with low parasite densities (<250/μl) could be detected in the Asia-Pacific region (48).
Parasite enzymes comprise the other primary antigen diagnostic targets. PLDH, the terminal enzyme in the malaria parasite's glycolytic pathway, is also an antigen target for detection of sexual and asexual malaria parasites. Monoclonal antibodies have now been developed that can target a conserved element of PLDH on all human malaria species (panmalarial) or specific regions unique to P. falciparum or P. vivax (53). Aldolase, a key enzyme in the glycosis pathway in malaria parasites, is also well conserved across all human-specific species of Plasmodium and is used as a panmalarial antigen target (32, 49). Other antigens have been recognized as possible components of future diagnostic tests, but no evaluations of P. ovale- or P. malariae-specific antigens have been published (27, 69, 98).
CHALLENGES IN ASSESSING THE PERFORMANCE OF MRDTs
Although a large number of studies assessing MRDTs have been published, many of these studies are characterized by numerous flaws that limit their reproducibility or broader applicability of their results (Table 3). These problems led to the introduction of guidelines outlined by the WHO to assess the utility of RDTs (6, 111). Limited analytical evaluations of MRDTs, conducted by testing the assays against panels of malaria parasite-infected blood samples, may be available from manufacturers. If laboratory-based studies use cultured parasites, qualitative results of antigen activity may be useful, but quantitative results will not reflect the pathophysiological conditions of total parasite biomass sequestration in the host and the stage of parasite in the patient. Laboratory-based studies also do not reproduce the complexities and physical stresses to which a diagnostic test is subjected under field conditions. Unfortunately, peer-reviewed articles reporting independent evaluations do not exist for most commercially available MRDTs today (www.wpro.who.int/sites/rdt/documents).
TABLE 3.
Factors associated with assessment of MRDTsa
| Assessment factor |
|---|
| Study populations |
| Parasite factors |
| Sensitivity is affected by concentration of parasite |
| No. of infections in the community affects predictive value |
| Patient factors |
| Host-parasite interaction in patients with partial immunity |
| Prior therapy and efficacy of prior therapy |
| Comorbidities, such as rheumatoid factor |
| Parasite and antigen issues |
| Lysis of red blood cells occurs during blood storage, which is associated with decrement of antigen activity over time |
| Sequestration of parasites in the host influences the concentration of antigen and parasite density in the peripheral blood |
| Structures of the antigens of parasites vary between and within parasite species and strains |
| Production of antigen varies during the individual stages of the parasite life cycle as well as by parasite species and parasite strains |
| Parasites recovered directly from patients might reflect different antigen activity from that of parasites recovered from laboratory cultures |
| Lysis of red blood cells and aggregation of parasitized red blood cells can reduce consistency of flow |
| Kit issues |
| Manufacturing |
| Quality assurance |
| Transportation and storage-environmental conditions |
| Humidity degrades MRDTs |
| Higher temperatures degrade MRDTs through altering flow characteristics of the nitrocellulose wick and modifying the capture and signaling antibodies on the wick |
| Drastic temperature changes can degrade MRDTs |
| Interpretation issues |
| Standardized control samples affect the sensitivity and specificity of MRDTs |
| Faint lines at low parasite densities might be difficult to see in poor light or if a reader has poor visual acuity |
| Inadequate blood volume reduces antigen, while excess blood volume inhibits clearance of blood stain |
| Well-trained vs field workers |
| Instructions must be understandable to end users |
Data are from reference 7.
Published studies assessing the utility of MRDTs are typically hospital laboratory-based studies, field evaluations of assay performance characteristics, or field effectiveness studies. Errors associated with field trial-based study design include extrapolation of results from a specific study population to all other populations, inadequate reference comparator method validation or blinding, absence of rigorous standardization or validation when microscopy is used as the comparator, failure to control exogenous factors affecting assay performance, and deficient or absent quality assurance. The epidemiology of malaria in the study population can influence the results of field trials (8, 100). Semi-immune individuals may develop symptoms of clinical malaria at higher parasite densities than nonimmune individuals. Parasite density is associated with antigenemia, so the apparent sensitivity of the test may vary when it is used for symptomatic persons with differing levels of immunity. This problem can be mitigated by stratifying calculations of sensitivity according to measured parasite densities. Even this precaution has limitations, particularly when applied to blood samples containing P. falciparum. Due to the effect of sequestration of blood-stage falciparum parasites, observed parasitemia may not directly correlate with parasite antigen or biomass (20, 23). In addition, parasite prevalence in the community affects the positive and negative predictive values of the test. Prior therapy and effectiveness of therapy vary between patients and can affect trial results because some antigens persist in blood for weeks after therapy, while others clear within a few days (38, 100). Polymorphisms in the target antigen(s) from the parasite can also influence the results obtained from the MRDT (2, 41, 49). Also, antigen production can vary between stages of the parasite life cycle and among parasite strains, and circulating gametocytes may produce the target antigen, even after therapy that has eradicated the asexual blood-stage parasites (23, 104). Patient comorbidities may also influence the results, as rheumatoid factor heterophilic antibodies can result in false-positive results for some patients (20, 63).
Clinical history from study subjects should be elicited, as pretreatment with antimalarials impacts parasitemia but may be associated with lingering antigen. Aldolase and PLDH are rapidly cleared after effective therapy, while HRP-2 antigenemia may persist for longer than a month. All current antigen assays may revert to positive if gametocytes subsequently appear in the bloodstream (59, 64, 100). This is important because not all therapeutic regimens, including chloroquine and quinine, are effective at eradicating gametocytes, and some, especially sulfa-containing regimens, may actually induce gametocytemia (74, 94). Persistent low-level parasitemia, including levels below the threshold of microscopic detection (Table 1), may also produce positive antigen test results (8). The impact of the blood source (mixed-capillary sample obtained by finger stick versus venous sample obtained by phlebotomy) on the ability to detect circulating parasites and antigen has been evaluated rigorously, with no significant impact on HRP-2-based and aldolase-based test performance observed (60).
Among the major issues with MRDT trial design are the methodology and accuracy of the “gold standard” comparator during the trial. Although microscopy is typically the appropriate comparator for field trials, detailed methods of microscopic evaluation and microscopist assessment and certification have rarely been included in published papers. Given the demonstrated potential for inconsistency among microscopists (25, 42, 43), all smears should be interpreted by more than one microscopist blinded to both the MRDT results and the findings of the other microscopist(s), with a defined system to evaluate discordant results between the microscopists. Experts should be selected based upon predetermined qualification standards (70). In laboratory-based testing, a liquid-phase enzyme-linked immunosorbent assay using antibodies targeted to the same antigen as the MRDT may be the best method to assess the lateral-flow device (68). PCR may be used as a reference standard, but it is also subject to technical limitations, is expensive, and requires standardized protocols and validation of the specific protocol used in a given trial. For these reasons, PCR is not generally accepted as the “gold standard” for malaria diagnosis.
Historical experience shows that commercially distributed diagnostic products commonly undergo manufacturing and/or material modifications after their initial introduction. These modifications can affect test properties, creating the anomaly that two products, bearing the same name and produced by the same manufacturer, may behave differently in both operational and performance characteristics. In order to clarify precisely which product has been evaluated, all publications reporting assay performance should specify the manufacturer, assay name, manufacturing lot, and kit numbers. Test performance characteristics are influenced by transport and storage of the diagnostic kits. These factors should be reported, particularly for evaluations of MRDTs conducted under field conditions (15, 50). Humidity or windy conditions rapidly degrade nitrocellulose capillary flow action. While proper packaging can mitigate the degrading effects of these factors during storage, once the test kit has been removed from its packaging, it becomes rapidly vulnerable to them. Temperature and time can independently degrade MRDTs by deconjugation of the signal antibody-indicator complex, detachment of the capture antibody from the nitrocellulose strip, and unfolding of binding sites of antibodies. Positive test lines can be faint, especially at low parasite densities; recognizing them requires adequate lighting and good visual acuity of the technician. Guidelines for selection and training of test operators should be suggested by MRDT manufacturers. Product evaluation studies typically use well-trained laboratory personnel, but in clinical settings, the typical end users of these kits may be health care workers with limited training. Manufacturers should also include assay instructions that can be understood readily by end users.
INTERNATIONALLY AVAILABLE MRDTs
The WHO currently has a list of MRDT manufacturers and distributors published online (www.wpro.who.int/sites/rdt/documents). The WHO sets criteria for inclusion on this site, which include evidence of good manufacturing practices, as documented by either compliance with ISO 13485:2003 or 21 CFR 820 from the U.S. Food and Drug Administration (U.S. FDA). In addition, evidence of testing product storage temperature and shelf life must be available to the WHO. Twenty-three different products meeting these criteria are currently available in the international marketplace. Of these, most have had little or no independent assessment of their performance or operational characteristics.
Currently, there is one U.S. FDA-approved MRDT product, namely, BinaxNOW Malaria (Binax, Inc., Inverness Medical Professional Diagnostics, Scarborough, ME) (Fig. 2). This assay is based on detection of the antigens HRP-2 (for P. falciparum) and aldolase (for generic Plasmodium). Rigorous field trials using duplicate mutually blinded microscopic blood smear interpretations and including assessments of multiple manufacturing lots were performed in Thailand and Peru by the authors and their colleagues using this product (28, 60). The larger trial revealed an overall sensitivity of 82% for the detection of any Plasmodium species (28). The overall sensitivity for detection of P. falciparum was 95%, with a sensitivity of 99% for parasitemia in excess of 1,000 parasites/μl, dropping to 89% for parasitemias of 100 to 500 parasites/μl of blood. The overall specificity for P. falciparum was 94% (28). The second trial primarily assessed the utility of finger stick versus venous acquisition of blood for diagnosis (60). When the finger stick technique was used, the assay revealed an overall sensitivity of 100% for P. falciparum and 83% for P. vivax. The specificity of the test was 89%. Per the package insert, the overall sensitivity and specificity are 95.3% and 94.2%, respectively, for P. falciparum and 68.9% and 99.8%, respectively, for P. vivax (Table 4). The BinaxNOW Malaria assay is capable of detecting P. malariae and P. ovale, but insufficient numbers of samples with these parasites have been tested to provide any conclusions about its sensitivity, specificity, or parasitemic thresholds of detection for them. Assay results for the detection of P. falciparum and P. vivax were equivalent whether the blood sample used was obtained by phlebotomy or by the finger stick method (Table 4). This assay was subjected to extensive testing to detect possible cross-reactions with other infections associated with fevers, especially those in tropical regions of endemicity. No such cross-reactions were observed (BinaxNOW Malaria package insert [http://binax.com/uploads/binaxnowmalaria-productinsert6_2007.pdf]).
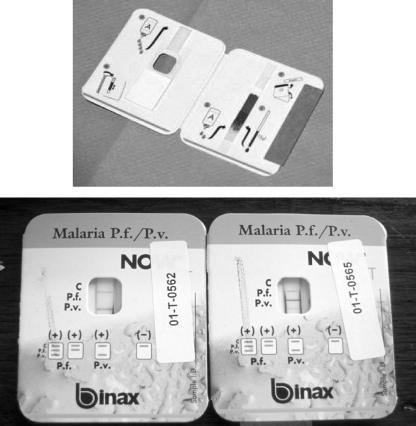
FIG. 2.
Example of the only U.S. FDA-approved MRDT product, BinaxNOW Malaria (Binax, Inc., Inverness Medical Professional Diagnostics, Scarborough, ME (note that the package design has changed from the pictured version)). This assay is based on detection of the antigens HRP-2 (for P. falciparum) and aldolase (for generic Plasmodium). Per the package insert, the overall sensitivity and specificity are 95.3% and 94.2%, respectively, for P. falciparum, and 68.9% and 99.8%, respectively, for P. vivax. (Top) Card undergoing sample wicking up the nitrocellulose strip after application of the blood and reagent. (Bottom) Example of two cards. The left card has a positive control line and a positive generic Plasmodium line, while the right card has a positive control line, a positive P. falciparum line, and a positive generic Plasmodium line.
TABLE 4.
Performance characteristics of BinaxNOW Malaria kit for Plasmodium falciparum and P. vivaxa
| Characteristic |
Plasmodium falciparum
|
Plasmodium vivax
|
||
|---|---|---|---|---|
| % Sensitivity | 95% confidence interval | % Sensitivity | 95% confidence interval | |
| Detection of parasitemia level (per μl) | ||||
| >5,000 | 99.7 | 98-100 | 93.5 | 91-96 |
| 1,000-5,000 | 99.2 | 96-100 | 81.0 | 76-85 |
| 500-1,000 | 92.6 | 76-99 | 47.4 | 36-59 |
| 100-500 | 89.2 | 75-97 | 23.6 | 17-31 |
| 0-100 | 53.9 | 37-70 | 6.2 | 3-12 |
| Overall | 95.3 | 93-97 | 68.9 | 66-72 |
| Specificity | 94.2 | 93-95 | 99.8 | 99-100 |
| Detection in venous vs finger stick samples | 100 vs 98.8 | 96-100 vs 94-100 | 81.6 vs 80.6 | 74-87 vs 73-87 |
| Specificity | 94.7 vs 90.4 | 93-96 vs 88-92 | 99.7 vs 99.5 | 99-100 vs 99-100 |
The BinaxNOW Malaria test is U.S. FDA approved for use only in laboratories that have or can acquire blood samples containing P. falciparum for use as a positive control. This test is intended for use only in the evaluation of symptomatic patients, and negative results should be confirmed by microscopic examination of thick and thin smears. The package insert notes, correctly, that the assay cannot distinguish a single-species malaria infection from a mixed infection. The kits are stable when stored between 2 and 37°C prior to the imprinted expiration date.
The limitations of the BinaxNOW Malaria test include decreased sensitivity at lower levels of parasitemia, potentially yielding false-negative results in nonimmune patients with low levels of parasitemia, “false”-positive test results when gametocytes are present but asexual-stage parasites have been eradicated by therapy, and “false”-positive test results after therapy due to detection of persistent HRP-2 antigenemia despite parasite clearance. The assay's detection of persistent antigenemia after parasite clearance precludes using the test to monitor the response to therapy, particularly in cases of malaria due to P. falciparum. The assay detects the overall antigen load qualitatively and, unlike microscopic examination of blood smears, cannot offer a quantitative assessment of the level of parasitemia, a factor relevant to management decisions. False-positive test results may occur in blood samples containing rheumatoid factor (4 of 50 rheumatoid factor-positive samples yielded false-positive BinaxNOW Malaria test results). Another limitation is that the test is not currently approved for point-of-care use by individual clinicians or patients themselves, a limitation that might hinder the ability to obtain rapid results to guide immediate therapy. Many of these limitations affect other MRDTs which are under development or currently available, especially those that detect HRP-2.
Performance characteristics of other selected MRDTs, segregated by antigen target, are listed in Table 5. Listed studies included a well-described process for comparator testing, either microscopy or PCR, and specific product information. Overall, it appears that MRDTs using HRP-2 are generally more sensitive than MRDTs detecting P. falciparum-specific PLDH. However, the diagnostic accuracies of assays detecting PLDH and aldolase for non-P. falciparum infections appear comparable, based on the limited data available.
TABLE 5.
Performance assessments of selected MRDTsa
| Product (manufacturer) | Antigen | Sensitivity (%)
|
Specificity (%) | Reference standard | Population | Year of study (reference) | |
|---|---|---|---|---|---|---|---|
| P. falciparum | P. vivax | ||||||
| P. falciparum-only tests | |||||||
| Paracheck Pf (Orchid Biomedical Systems) | HRP-2 | 97.2 (75 for gametocytes) | NA | 88.8 (gametocytes excluded) | Hospital-based blinded single microscopy | Uganda, endemic, 423 positive results, 16 gametocyte-positive results, 303 negative results | NA (33) |
| HRP-2 | 100 | NA | 93 | Field-based blinded single microscopy | India, endemic, 94 positive results, 43 P. vivax/negative results | 2003 (91) | |
| HRP-2 | 93.3 | NA | 84.4 | Field-based blinded single microscopy | India, endemic, placental blood after delivery, 30 positive results, 179 P. vivax/negative results | 2003 (92) | |
| HRP-2 | 90.1 | NA | 99.5 | Field-based blinded single microscopy | Colombia, endemic, 152 positive results, 744 P. vivax/negative results | 2005 (106) | |
| HRP-2 | 100 | NA | 52 | Field-based blinded dual microscopy | Congo, endemic, children of 0 to 5 yr, 235 positive results, 123 negative results | 2004 (100) | |
| ParaHIT f (Span Diagnostic Ltd.) | HRP-2 | 97.6 | NA | 87.7 | Hospital-based blinded single microscopy | Uganda, endemic, 423 positive results, 16 gametocyte-positive results, 303 negative results | NA (33) |
| HRP-2 | 88.1 | NA | 91.2 | Field- and hospital-based blinded single microscopy | India, endemic, 268 positive, 949 P. vivax/negative results | 2004 (90) | |
| HRP-2 | 87.5 | NA | 97 | Field-based blinded single microscopy | India, endemic, placental blood after delivery, 30 positive results, 179 P. vivax/negative results | 2003 (92) | |
| P. falciparum and panmalaria tests | |||||||
| BinaxNOW Malaria (Binax Inc.)b | HRP-2 (P. falciparum), aldolase (panmalarial) | 95 | 69 | 94 | Field-based blinded dual certified microscopy | Thailand, endemic, 565 P. falciparum-positive samples, 1,202 P. vivax-positive samples, 2,315 negative samples | NA (28) |
| HRP-2 (P. falciparum) | 96.0 | 86.7 | 98.7 | Hospital-based blinded single microscopy | Symptomatic travelers, Toronto, 106 P. falciparum-positive samples, 103 non-P. falciparum-positive samples, 47 negative samples | 1999-2003 (26) | |
| Aldolase (panmalarial) | 94.3 | 86.7 | 98.7 | Blinded PCR | Symptomatic travelers, Toronto, 106 P. falciparum-positive samples, 103 non-P. falciparum-positive samples, 47 negative samples | 1999-2003 (26) | |
| HRP-2 (P. falciparum) and aldolase (panmalarial) | 97.5 | 100 | 98.9 | Hospital-based blinded dual microscopy | Symptomatic travelers, France, 80 P. falciparum-positive samples, 13 P. vivax-positive samples, 457 negative samples | 2002 (22) | |
| HRP-2 (P. falciparum) and aldolase (panmalarial) | 100 (venous) | 81.8 (venous) | 94.9 (venous) | Field-based blinded dual certified microscopy | Thailand, endemic, 81 P. falciparum-positive samples, 126 P. vivax-positive samples, 588 negative samples | NA (60) | |
| HRP-2 (P. falciparum) and aldolase (panmalarial) | 100 (finger stick) | 82.5 (finger stick) | 89.8 (finger stick) | Field-based blinded dual certified microscopy | Thailand, endemic, 81 P. falciparum-positive samples, 126 P. vivax-positive samples, 588 negative samples | NA (60) | |
| HRP-2 (P. falciparum) and aldolase (panmalarial) | 94.7 | 81.4 | 89.9 | Field-based blinded single microscopy | Colombia, endemic, 139 P. falciparum-positive samples, 279 P. vivax-positive samples, 465 negative samples | 2005 (106) | |
| HRP-2 (P. falciparum) and aldolase (panmalarial) | 96.7 | NA | 95.6 | Hospital-based expert microscopy | Italy, travelers and migrants, 118 P. falciparum-positive samples, 11 P. vivax-positive samples, 159 negative samples | NA (29) | |
| CareStart Malaria test (AccessBio Inc.) | PfLDH and non-P. falciparum LDH | 95.6 | 92.3 | 94.1 | Field-based blinded single microscopy | Madagascar, endemic, 72 P. falciparum-positive samples, 9 P. vivax-positive samples, 104 negative samples | 2005 (77) |
| OptiMAL strip (DiaMed AG) | PfLDH and PLDH | 96.8 | 100 | 99.4 | Hospital-based blinded single microscopy | U.S. travelers, 32 P. falciparum-positive samples, 11 non-P. falciparum positive samples, 173 negative samples | NA (71) |
| OptiMAL-IT (DiaMed AG) | PfLDH and PLDH | 84.3 | 84.6 | 98.4 | Hospital-based blinded dual microscopy | Symptomatic travelers, France, 80 P. falciparum-positive samples, 13 P. vivax-positive samples, 457 negative samples | 2002 (22) |
| PfLDH and PLDH | 83.6 | 91.0 | 97.8 | Field-based blinded single microscopy | Colombia, endemic, 139 P. falciparum-positive samples, 279 P. vivax-positive samples, 465 negative samples | 2005 (106) | |
| PfLDH and PLDH | 92.6 | 86.7 | 98.1 | Field-based blinded single microscopy | Madagascar, endemic, 72 P. falciparum-positive samples, 9 P. vivax-positive samples, 104 negative samples | 2005 (77) | |
| SD malaria antigen bioline (Standard Diagnostics) | PfLDH and PLDH | 89.4 | 73.3 | 9.1 | Field-based blinded single microscopy | Madagascar, endemic, 72 P. falciparum-positive samples, 9 P. vivax-positive samples, 104 negative samples | 2005 (77) |
Listed studies include a well-described process for comparator testing, either microscopy or PCR diagnosis, and specific product information. NA, not available.
BinaxNOW Malaria kit was previously the NOW ICT Malaria Pf/Pv kit.
CURRENT STATE OF EFFECTIVENESS OF RDTs FOR MALARIA DIAGNOSIS
An estimated 12 million MRDTs were produced globally in 2005 (http://www.wpro.who.int/NR/rdonlyres/A15EBA35-91E1-4D8F-9798-5352CEBC7395/0/20_May_2007_RDT_Forecast_report.pdf [accessed 8 June 2007]). The international community has recognized that MRDTs are being used at almost every level of the health care system. In addition, others, and now the WHO, have recognized that the lack of quality control for diagnostic devices in the marketplace has hampered the assessment of the best implementation practices in the public health infrastructure (7).
Diagnosis of Symptomatic Persons in Health Care Settings
The large majority of published studies have evaluated MRDTs in health care settings where trained personnel performed the assay and microscopy. The tests perform optimally in this setting and in the targeted patient population with febrile illness, as summarized in Table 5. There are far fewer data on test performance for children, but limited studies have shown no effect of age on MRDT performance (28). Among pregnant women with possible P. falciparum malaria, placental sequestration of parasites can reduce the sensitivity of microscopic diagnosis; however, HRP-2 is still detectable in peripheral blood samples (50). HRP-2 MRDTs show a higher sensitivity for this population than does microscopy (50). Notably, placental malaria infections detected by MRDTs using peripheral blood were associated with a lower median birth weight (89). The ability to detect placental infection by antigen detection when microscopy does not identify parasitemia could have a significant impact on material and fetal health care. The implications of persistent HRP-2 antigenemia for up to a month after successful therapy are unclear in this setting.
An unresolved issue concerns the diagnostic testing of young children with clinical illnesses compatible with malaria in regions where P. falciparum greatly (>90%) predominates, such as sub-Saharan Africa. In such falciparum malaria-predominant regions, the WHO currently recommends parasitological confirmation of the diagnosis of malaria as part of malaria case management in all cases except those of children under 5 years old (109, 110). The WHO considers the risk of failing to treat such children with false-negative tests greater than the drawbacks of empirical therapy, even with relatively expensive ACTs. The results of a recently published analysis are in contrast to this WHO position on testing of young children, though. Using a decision tree model to evaluate the impact of introducing MRDTs for the diagnosis of febrile children under the age of 5 years in sub-Saharan Africa, this analysis demonstrated that an MRDT with 95% sensitivity for parasitemia of ≥500/μl and 95% specificity could avert over 100,000 malaria-related deaths and about 400 million unnecessary treatments (75). The key to gaining this improvement in outcomes was the much wider availability of an MRDT leading to a marked increase in access to the population.
The potential for MRDTs to give false-negative results in testing samples with low but clinically relevant levels of parasitemia is noted above. The improved sensitivity of MRDTs as antigenemia and, in rough proportion, parasitemia increase suggests that MRDTs using blood samples obtained serially over 24 to 48 h might permit diagnosis even for patients whose initial test results were negative. This model of serial testing has long been used for microscopic examination of blood smears from patients for whom suspicion of malaria remains after a negative initial examination (102). Even with such serial testing using MRDTs, however, expert microscopy would still be helpful to determine the species and the level of parasitemia.
Diagnosis by Nonlaboratory Personnel
The low complexity of MRDTs invites their use by village workers without formal medical laboratory training, or even by travelers for self-diagnosis and treatment. Few studies using current products report the field effectiveness in these settings, but available evidence suggests that HRP-2-based MRDTs can be employed successfully by village volunteers with minimal training (12). Emergency self-diagnosis of travelers or the application of relative/buddy testing of travelers who do not have access to medical attention within 24 h of symptom onset might seem to be candidates for these strategies. Some products (e.g., OptiMal-IT and DiaMed AG) are packaged for individual use and include a lancet, which is ideal for use in this setting. Data on acceptance and test performance for self-diagnosis by travelers is limited, but as a proof of concept, Behrens and Whitty evaluated the ICT Malaria Pf test (an HRP-2-only test which is no longer available; ICT Diagnostics, Sydney, Australia) on 153 symptomatic volunteers who presented to the Hospital of Tropical Diseases in London, United Kingdom (5). Seventy-five percent of symptomatic volunteers felt that the instructions were easy to follow, and 84% felt that they were easy to interpret. Compared to microscopy, the subject's test results showed a sensitivity and specificity of 97% and 95%, respectively. However, in another study, among travelers presenting with fever in Kenya, only 68% of persons were able to perform and interpret the kit accurately, with 10 of 11 with malaria failing to diagnose themselves correctly (39). Some studies have alluded to difficulties experienced by travelers in following the instructions, interpreting the results, and obtaining blood (105). Training and practice with good visual aids prior to travel were recommended. More data are clearly needed before any recommendations can be made concerning such self- or buddy testing with MRDTs.
Prevalence Surveys of Asymptomatic Persons
Historically, malaria prevalence surveys have been conducted using field microscopy as the diagnostic method. These studies typically suffer from microscopy's lack of sensitivity for detecting the low parasitemias of asymptomatic carriers (18, 57, 85). While MRDTs would reduce logistical challenges in these studies, their parasitemia threshold of detection does not appear to be sufficiently low to be useful for asymptomatic screening (19, 57, 85).
Monitoring Treatment
As mentioned above, the HRP-2 antigen is known to possibly persist at detectable levels for more than 28 days, well after the symptoms have disappeared and the asexual-stage parasites that cause disease have been cleared from the patient's blood (44, 100). Aldolase and PLDH rapidly fall to undetectable levels after initiation of effective therapy, but all of these antigens are expressed in gametocytes, which may appear after the clinical infection is cleared (64). Therefore, none of these assays is useful for monitoring the response to treatment. Microscopy remains the test of choice for this purpose.
Diagnosis of Falciparum Malaria Remote from Acute Infection
In some cases, empirical treatment may have been started or death may have ensued before the diagnosis of falciparum malaria has been established. In this setting, the persistence of HRP-2 and its abundance in the plasma fraction allow MRDTs to make this diagnosis remotely. No formal studies comparing sensitivities in plasma and whole blood have been performed, but the test performances have been similar in our experience (R. S. Miller). The diagnosis of P. falciparum has been made on blood samples obtained 14 to 21 days after resolution of the febrile illness, at postmortem assessments (88), and even from mummified tissue (58).
Blood Donor Screening
In the United States, the American Association of Blood Banks currently (Blood Donor History Questionnaire, version 1.2 [http://www.aabb.org/Documents/Donate_Blood/Donor_History_Questionnaire/udhqfullv1-2.pdf]; accessed 11 June 2007) recommends deferral for 3 years after departure from a malarial country of birth or residency or for 12 months after departure from Iraq or from recent travel to a malarial area, whichever is later. Between 1963 and 1999, 93 cases of transfusion-transmitted malaria were reported to the Centers for Disease Control and Prevention (CDC) (65). Currently, the American Red Cross does not screen blood donations for malaria. Recently, the American Red Cross broadened the screening for new tropical infectious agents and directed the screening of all blood donations for Trypanosoma cruzi on 29 January 2007 (13). There have been evaluations of other techniques for screening blood donor units for malaria (87). For malaria detection, high-throughput detection cannot be achieved with microscopy of Giemsa-stained thick and thin smears or readily with individually packaged MRDTs (46). Current antigen detection and nucleic acid amplification methods have too high of thresholds of detection to detect individuals with very low parasitemias (84). Plate enzyme immunoassay technology for detection of malaria antibodies has shown the most promise for this purpose (46, 47). In summary, MRDTs cannot yet be used safely to exclude parasitemia in prospective blood donors.
SELECTION OF AN MRDT
Although many products are available, selection of a specific MRDT must take into consideration the malaria epidemiology in the region of the world where it will be used, the expected health benefit from use of the device, a plan for how to use the results provided by the device, the ability to monitor the accuracy of the device, whether the environmental conditions in which the test is operated will degrade its performance, supply and distribution, product stability during storage and transport, shelf life, training needed by the test operators, and finally, the cost of use. Health care services will have to develop laboratory strategies that will determine the best device to be used in their clinical settings, based upon expected species of infection, level of parasitemia, and treatment paradigms. The following criteria should be used to choose an MRDT: the malaria epidemiology, species to be detected (P. falciparum only or all species), and sensitivity and specificity requirements for the patient population; product stability based upon the intended conditions of use and storage (shelf life, temperature stability, and humidity stability); ease of use, including the format of the test; requirements for posttreatment testing of patients; and cost, which includes training and quality control. Clearly, more effectiveness studies are needed.
Choosing the correct MRDT for use in malaria control and treatment programs should be influenced by the epidemiology of malaria in the area to be served. In sub-Saharan Africa and lowland Papua New Guinea, where infections occur predominantly or solely with P. falciparum (i.e., >90%), use of an assay that detects P. falciparum alone may be clinically sufficient and more cost-effective. It is of concern that in these P. falciparum-predominant regions, 1 to 10% of malaria patients may be coinfected with another species, possibly including relapsing malarias that might require warranted treatment with primaquine (93). In situations like this, resources and the importance of detecting the other species will influence the MRDT chosen. In areas of endemicity in Asia and the Americas, as well as isolated areas in Africa such as the Ethiopian highlands, where falciparum and non-falciparum malaria parasites cocirculate, typically occurring as single-species infections, or in regions where P. vivax predominates, use of an MRDT that can detect both falciparum and non-falciparum malarias, and distinguish between them, is warranted (109).
Although the burden of malaria is in countries where it is endemic, many persons are exposed to the disease while traveling to these areas but are not diagnosed until returning to their country of origin. There were roughly 842 million international travelers in 2006, with common travel destinations including regions of malaria endemicity (www.world-tourism.org). In the United States in 2005, the CDC received 1,528 reports of malaria cases, of which 7 were fatal (103). On the basis of estimated volume of travel, the highest estimated case rates are among travelers to West Africa. Almost 50% of travel-associated cases were due to P. falciparum, while 24% were caused by P. vivax. In addition, personnel who work or are deployed overseas as part of military service can develop malaria upon return (16, 76). Laboratory personnel testing clinical samples from such patients often lack experience in microscopic diagnosis of malaria, so the use of an MRDT could improve the initial diagnosis of malaria and provide more rapid results (99). This strategy has been used in screening immigrants moving from regions of endemicity to regions where malaria is not endemic (96). A large meta-analysis of rapid tests for malaria in travelers suggested that MRDTs may be effective adjuncts to microscopy in centers without expertise in tropical medicine but that expert microscopy is still needed for species identification and confirmation (54).
CONCLUSIONS
Malaria is a life-threatening infection with a global impact extending from the most developed countries to regions of the world with only the most basic of health care infrastructure. The increased burden of disease, the emergence of resistance to antimalarial agents, and now the deployment of expensive ACTs into regions where malaria is highly endemic are increasing the need for rapid, accurate diagnosis of patients who may be infected with malaria. At this time, selection of a quality manufactured MRDT selected based on the malaria epidemiology and clinical situation will improve malaria diagnosis. Attention to product stability in tropical climates, the logistics of product acquisition, shipment, and storage, to include a “cool” chain protecting the product from exposures to temperature extremes, and the performance and operational limitations of the specific assay are all required. Some products among the current generation of antigen detection diagnostics perform sufficiently well to achieve clinical utility. Given the absence or poor execution of microscopy, especially in some areas where malaria is highly endemic, alternative diagnostic strategies are needed today. If there is no diagnostic support, MRDTs should serve as the only test introduced into the region. Where quality microscopy is available, then microscopy and MRDTs can run in parallel, with MRDTs providing a rapid or screening diagnosis and microscopy reserved for resolution of confusing cases, verification of negative results where the pretest probability of malaria seemed high, and overall quality control of the MRDT program. Although there is now a U.S. FDA-approved MRDT, MRDTs continue to have limitations, notably including an inability to detect mixed infections, inability to distinguish every species of Plasmodium, failure to detect infections with low but clinically relevant concentrations of parasites, and limited ability to monitor responses to therapy. In the case of the U.S. FDA-approved MRDT, the granted regulatory clearance includes the requirement that negative results by the MRDT be confirmed by microscopy (http://www.fda.gov/cdrh/pdf6/K061542.pdf). These various considerations show that MRDTs, although an important advance, have not yet eliminated the need to perform microscopic examination of stained blood smears and to maintain expertise in the varied skills required to do so accurately.
Acknowledgments
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or reflecting the views of the U.S. Department of the Army, the U.S. Department of Defense, or the U.S. government.
The authors are employees of the U.S. government and this work was performed as part of their official duties. As such, there is no copyright to be transferred.
REFERENCES
- 1.Anthony, R. L., M. J. Bangs, J. M. Anthony, and Purnomo. 1992. On-site diagnosis of Plasmodium falciparum, P. vivax, and P. malariae by using the quantitative buffy coat system. J. Parasitol. 78:994-998. [PubMed] [Google Scholar]
- 2.Baker, J., J. McCarthy, M. Gatton, D. E. Kyle, V. Belizario, J. Luchavez, D. Bell, and Q. Cheng. 2005. Genetic diversity of Plasmodium falciparum histidine-rich protein 2 (PfHRP2) and its effect on the performance of PfHRP2-based rapid diagnostic tests. J. Infect. Dis. 192:870-877. [DOI] [PubMed] [Google Scholar]
- 3.Banoo, S., D. Bell, P. Bossuyt, A. Herring, D. Mabey, F. Poole, P. G. Smith, N. Sriram, C. Wongsrichanalai, R. Linke, R. O'Brien, M. Perkins, J. Cunningham, P. Matsoso, C. M. Nathanson, P. Olliaro, R. W. Peeling, and A. Ramsay. 2006. Evaluation of diagnostic tests for infectious diseases: general principles. Nat. Rev. Microbiol. 4:S20-S32. [DOI] [PubMed] [Google Scholar]
- 4.Barat, L., J. Chipipa, M. Kolczak, and T. Sukwa. 1999. Does the availability of blood slide microscopy for malaria at health centers improve the management of persons with fever in Zambia? Am. J. Trop. Med. Hyg. 60:1024-1030. [DOI] [PubMed] [Google Scholar]
- 5.Behrens, R. H., and C. J. Whitty. 2000. Self-use of rapid tests for malaria diagnosis. Lancet 355:237. [DOI] [PubMed] [Google Scholar]
- 6.Bell, D., and R. W. Peeling. 2006. Evaluation of rapid diagnostic tests: malaria. Nat. Rev. Microbiol. 4(Suppl. 9):S34-S38. [DOI] [PubMed] [Google Scholar]
- 7.Bell, D., C. Wongsrichanalai, and J. W. Barnwell. 2006. Ensuring quality and access for malaria diagnosis: how can it be achieved? Nat. Rev. Microbiol. 4:S7-S20. [DOI] [PubMed] [Google Scholar]
- 8.Bell, D. R., D. W. Wilson, and L. B. Martin. 2005. False-positive results of a Plasmodium falciparum histidine-rich protein 2-detecting malaria rapid diagnostic test due to high sensitivity in a community with fluctuating low parasite density. Am. J. Trop. Med. Hyg. 73:199-203. [PubMed] [Google Scholar]
- 9.Berkley, J. A., K. Maitland, I. Mwangi, C. Ngetsa, S. Mwarumba, B. S. Lowe, C. R. Newton, K. Marsh, J. A. Scott, and M. English. 2005. Use of clinical syndromes to target antibiotic prescribing in seriously ill children in malaria endemic area: observational study. BMJ 330:995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boisier, P., R. Jambou, L. Raharimalala, and J. Roux. 2002. Relationship between parasite density and fever risk in a community exposed to a low level of malaria transmission in Madagascar highlands. Am. J. Trop. Med. Hyg. 67:137-140. [DOI] [PubMed] [Google Scholar]
- 11.Brown, W. M., C. A. Yowell, A. Hoard, T. A. Vander Jagt, L. A. Hunsaker, L. M. Deck, R. E. Royer, R. C. Piper, J. B. Dame, M. T. Makler, and D. L. Vander Jagt. 2004. Comparative structural analysis and kinetic properties of lactate dehydrogenases from the four species of human malarial parasites. Biochemistry 43:6219-6229. [DOI] [PubMed] [Google Scholar]
- 12.Carrara, V. I., S. Sirilak, J. Thonglairuam, C. Rojanawatsirivet, S. Proux, V. Gilbos, A. Brockman, E. A. Ashley, R. McGready, S. Krudsood, S. Leemingsawat, S. Looareesuwan, P. Singhasivanon, N. White, and F. Nosten. 2006. Deployment of early diagnosis and mefloquine-artesunate treatment of falciparum malaria in Thailand: the Tak Malaria Initiative. PLoS Med. 3:e183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control. 2007. Blood donor screening for Chagas disease—United States, 2006-2007. MMWR Morb. Mortal. Wkly. Rep. 56:141-143. [PubMed] [Google Scholar]
- 14.Chandramohan, D., S. Jaffar, and B. Greenwood. 2002. Use of clinical algorithms for diagnosing malaria. Trop. Med. Int. Health 7:45-52. [DOI] [PubMed] [Google Scholar]
- 15.Chiodini, P. L., K. Bowers, P. Jorgensen, J. W. Barnwell, K. K. Grady, J. Luchavez, A. H. Moody, A. Cenizal, and D. Bell. 2007. The heat stability of Plasmodium lactate dehydrogenase-based and histidine-rich protein 2-based malaria rapid diagnostic tests. Trans. R. Soc. Trop. Med. Hyg. 101:331-337. [DOI] [PubMed] [Google Scholar]
- 16.Ciminera, P., and J. Brundage. 2007. Malaria in U.S. military forces: a description of deployment exposures from 2003 through 2005. Am. J. Trop. Med. Hyg. 76:275-279. [PubMed] [Google Scholar]
- 17.Cobelens, F. G., J. P. Verhave, A. Leentvaar-Kuijpers, and P. A. Kager. 1998. Testing for anti-circumsporozoite and anti-blood-stage antibodies for epidemiologic assessment of Plasmodium falciparum infection in travelers. Am. J. Trop. Med. Hyg. 58:75-80. [DOI] [PubMed] [Google Scholar]
- 18.Coleman, R. E., N. Maneechai, N. Rachaphaew, C. Kumpitak, R. S. Miller, V. Soyseng, K. Thimasarn, and J. Sattabongkot. 2002. Comparison of field and expert laboratory microscopy for active surveillance for asymptomatic Plasmodium falciparum and Plasmodium vivax in western Thailand. Am. J. Trop. Med. Hyg. 67:141-144. [DOI] [PubMed] [Google Scholar]
- 19.Coleman, R. E., J. Sattabongkot, S. Promstaporm, N. Maneechai, B. Tippayachai, A. Kengluecha, N. Rachapaew, G. Zollner, R. S. Miller, J. A. Vaughan, K. Thimasarn, and B. Khuntirat. 2006. Comparison of PCR and microscopy for the detection of asymptomatic malaria in a Plasmodium falciparum/vivax endemic area in Thailand. Malar. J. 5:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Craig, M. H., B. L. Bredenkamp, C. H. Williams, E. J. Rossouw, V. J. Kelly, I. Kleinschmidt, A. Martineau, and G. F. Henry. 2002. Field and laboratory comparative evaluation of ten rapid malaria diagnostic tests. Trans. R. Soc. Trop. Med. Hyg. 96:258-265. [DOI] [PubMed] [Google Scholar]
- 21.Demirev, P. A., A. B. Feldman, D. Kongkasuriyachai, P. Scholl, D. Sullivan, Jr., and N. Kumar. 2002. Detection of malaria parasites in blood by laser desorption mass spectrometry. Anal. Chem. 74:3262-3266. [DOI] [PubMed] [Google Scholar]
- 22.De Monbrison, F., P. Gerome, J. F. Chaulet, M. Wallon, S. Picot, and F. Peyron. 2004. Comparative diagnostic performance of two commercial rapid tests for malaria in a non-endemic area. Eur. J. Clin. Microbiol. Infect. Dis. 23:784-786. [DOI] [PubMed] [Google Scholar]
- 23.Dondorp, A. M., V. Desakorn, W. Pongtavornpinyo, D. Sahassananda, K. Silamut, K. Chotivanich, P. N. Newton, P. Pitisuttithum, A. M. Smithyman, N. J. White, and N. P. Day. 2005. Estimation of the total parasite biomass in acute falciparum malaria from plasma PfHRP2. PLoS Med. 2:e204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Draper, C. C., and S. S. Sirr. 1980. Serological investigations in retrospective diagnosis of malaria. Br. Med. J. 280:1575-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Durrheim, D. N., P. J. Becker, and K. Billinghurst. 1997. Diagnostic disagreement—the lessons learnt from malaria diagnosis in Mpumalanga. S. Afr. Med. J. 87:1016. [PubMed] [Google Scholar]
- 26.Farcas, G. A., K. J. Zhong, F. E. Lovegrove, C. M. Graham, and K. C. Kain. 2003. Evaluation of the Binax NOW ICT test versus polymerase chain reaction and microscopy for the detection of malaria in returned travelers. Am. J. Trop. Med. Hyg. 69:589-592. [PubMed] [Google Scholar]
- 27.Forney, J. R., C. Wongsrichanalai, A. J. Magill, L. G. Craig, J. Sirichaisinthop, C. T. Bautista, R. S. Miller, C. F. Ockenhouse, K. E. Kester, N. E. Aronson, E. M. Andersen, H. A. Quino-Ascurra, C. Vidal, K. A. Moran, C. K. Murray, C. C. DeWitt, D. G. Heppner, K. C. Kain, W. R. Ballou, and R. A. Gasser, Jr. 2003. Devices for rapid diagnosis of malaria: evaluation of prototype assays that detect Plasmodium falciparum histidine-rich protein 2 and a Plasmodium vivax-specific antigen. J. Clin. Microbiol. 41:2358-2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gasser, R. A., Jr., A. J. Magill, T. Ruebush, R. S. Miller, J. Sirichaisinthop, J. R. Forney, C. T. Bautista, I. Arevalo, J. Rhorer, J. Wittes, and C. Wongsrichanalai. 2005. Malaria diagnosis: performance of NOW ICT Malaria in a large scale field trial. Abstr. 54th Annu. Meet. Am. Soc. Trop. Med. Hyg., abstr. 2338.
- 29.Gatti, S., M. Gramegna, Z. Bisoffi, A. Raglio, M. Gulletta, C. Klersy, A. Bruno, R. Maserati, S. Madama, and M. Scaglia. 2007. A comparison of three diagnostic techniques for malaria: a rapid diagnostic test (NOW Malaria), PCR and microscopy. Ann. Trop. Med. Parasitol. 101:195-204. [DOI] [PubMed] [Google Scholar]
- 30.Gatton, M. L., and Q. Cheng. 2002. Evaluation of the pyrogenic threshold for Plasmodium falciparum malaria in naive individuals. Am. J. Trop. Med. Hyg. 66:467-473. [DOI] [PubMed] [Google Scholar]
- 31.Gay, F., B. Traore, J. Zanoni, M. Danis, and A. Fribourg-Blanc. 1996. Direct acridine orange fluorescence examination of blood slides compared to current techniques for malaria diagnosis. Trans. R. Soc. Trop. Med. Hyg. 90:516-518. [DOI] [PubMed] [Google Scholar]
- 32.Genrich, G. L., J. Guarner, C. D. Paddock, W. J. Shieh, P. W. Greer, J. W. Barnwell, and S. R. Zaki. 2007. Fatal malaria infection in travelers: novel immunohistochemical assays for the detection of Plasmodium falciparum in tissues and implications for pathogenesis. Am. J. Trop. Med. Hyg. 76:251-259. [PubMed] [Google Scholar]
- 33.Guthmann, J. P., A. Ruiz, G. Priotto, J. Kiguli, L. Bonte, and D. Legros. 2002. Validity, reliability and ease of use in the field of five rapid tests for the diagnosis of Plasmodium falciparum malaria in Uganda. Trans. R. Soc. Trop. Med. Hyg. 96:254-257. [DOI] [PubMed] [Google Scholar]
- 34.Hamer, D. H., M. Ndhlovu, D. Zurovac, M. Fox, K. Yeboah-Antwi, P. Chanda, N. Sipilinyambe, J. L. Simon, and R. W. Snow. 2007. Improved diagnostic testing and malaria treatment practices in Zambia. JAMA 297:2227-2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanscheid, T. 1999. Diagnosis of malaria: a review of alternatives to conventional microscopy. Clin. Lab. Haematol. 21:235-245. [DOI] [PubMed] [Google Scholar]
- 36.Hayward, R. E., D. J. Sullivan, and K. P. Day. 2000. Plasmodium falciparum: histidine-rich protein II is expressed during gametocyte development. Exp. Parasitol. 96:139-146. [DOI] [PubMed] [Google Scholar]
- 37.Howard, R. J., S. Uni, M. Aikawa, S. B. Aley, J. H. Leech, A. M. Lew, T. E. Wellems, J. Rener, and D. W. Taylor. 1986. Secretion of a malarial histidine-rich protein (Pf HRP II) from Plasmodium falciparum-infected erythrocytes. J. Cell Biol. 103:1269-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iqbal, J., A. Siddique, M. Jameel, and P. R. Hira. 2004. Persistent histidine-rich protein 2, parasite lactate dehydrogenase, and panmalarial antigen reactivity after clearance of Plasmodium falciparum monoinfection. J. Clin. Microbiol. 42:4237-4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jelinek, T., L. Amsler, M. P. Grobusch, and H. D. Nothdurft. 1999. Self-use of rapid tests for malaria diagnosis by tourists. Lancet 354:1609. [DOI] [PubMed] [Google Scholar]
- 40.Johnston, S. P., N. J. Pieniazek, M. V. Xayavong, S. B. Slemenda, P. P. Wilkins, and A. J. da Silva. 2006. PCR as a confirmatory technique for laboratory diagnosis of malaria. J. Clin. Microbiol. 44:1087-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joshi, H. 2003. Markers for population genetic analysis of human plasmodia species, P. falciparum and P. vivax. J. Vector Borne Dis. 40:78-83. [PubMed] [Google Scholar]
- 42.Kachur, S. P., E. Nicolas, V. Jean-Francois, A. Benitez, P. B. Bloland, Y. Saint Jean, D. L. Mount, T. K. Ruebush II, and P. Nguyen-Dinh. 1998. Prevalence of malaria parasitemia and accuracy of microscopic diagnosis in Haiti, October 1995. Rev. Panama Salud Publica 3:35-39. [DOI] [PubMed] [Google Scholar]
- 43.Kain, K. C., M. A. Harrington, S. Tennyson, and J. S. Keystone. 1998. Imported malaria: prospective analysis of problems in diagnosis and management. Clin. Infect. Dis. 27:142-149. [DOI] [PubMed] [Google Scholar]
- 44.Karbwang, J., O. Tasanor, T. Kanda, Y. Wattanagoon, M. Ibrahim, K. Na-Bangchang, A. Thanavibul, and W. Rooney. 1996. ParaSight-F test for the detection of treatment failure in multidrug resistant Plasmodium falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 90:513-515. [DOI] [PubMed] [Google Scholar]
- 45.Kilian, A. H., W. G. Metzger, E. J. Mutschelknauss, G. Kabagambe, P. Langi, R. Korte, and F. von Sonnenburg. 2000. Reliability of malaria microscopy in epidemiological studies: results of quality control. Trop. Med. Int. Health 5:3-8. [DOI] [PubMed] [Google Scholar]
- 46.Kitchen, A. D., and P. L. Chiodini. 2006. Malaria and blood transfusion. Vox Sang. 90:77-84. [DOI] [PubMed] [Google Scholar]
- 47.Kitchen, A. D., P. H. Lowe, K. Lalloo, and P. L. Chiodini. 2004. Evaluation of a malarial antibody assay for use in the screening of blood and tissue products for clinical use. Vox Sang. 87:150-155. [DOI] [PubMed] [Google Scholar]
- 48.Lee, N., J. Baker, K. T. Andrews, M. L. Gatton, D. Bell, Q. Cheng, and J. McCarthy. 2006. Effect of sequence variation in Plasmodium falciparum histidine-rich protein 2 on binding of specific monoclonal antibodies: implications for rapid diagnostic tests for malaria. J. Clin. Microbiol. 44:2773-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee, N., J. Baker, D. Bell, J. McCarthy, and Q. Cheng. 2006. Assessing the genetic diversity of the aldolase genes of Plasmodium falciparum and Plasmodium vivax and its potential effect on performance of aldolase-detecting rapid diagnostic tests. J. Clin. Microbiol. 44:4547-4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leke, R. F., R. R. Djokam, R. Mbu, R. J. Leke, J. Fogako, R. Megnekou, S. Metenou, G. Sama, Y. Zhou, T. Cadigan, M. Parra, and D. W. Taylor. 1999. Detection of the Plasmodium falciparum antigen histidine-rich protein 2 in blood of pregnant women: implications for diagnosing placental malaria. J. Clin. Microbiol. 37:2992-2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luxemburger, C., F. Nosten, D. E. Kyle, L. Kiricharoen, T. Chongsuphajaisiddhi, and N. J. White. 1998. Clinical features cannot predict a diagnosis of malaria or differentiate the infecting species in children living in an area of low transmission. Trans. R. Soc. Trop. Med. Hyg. 92:45-49. [DOI] [PubMed] [Google Scholar]
- 52.Makler, M. T., and D. J. Hinrichs. 1993. Measurement of the lactate dehydrogenase activity of Plasmodium falciparum as an assessment of parasitemia. Am. J. Trop. Med. Hyg. 48:205-210. [DOI] [PubMed] [Google Scholar]
- 53.Makler, M. T., R. C. Piper, and W. K. Milhous. 1998. Lactate dehydrogenase and the diagnosis of malaria. Parasitol. Today 14:376-377. [DOI] [PubMed] [Google Scholar]
- 54.Marx, A., D. Pewsner, M. Egger, R. Nuesch, H. C. Bucher, B. Genton, C. Hatz, and P. Juni. 2005. Meta-analysis: accuracy of rapid tests for malaria in travelers returning from endemic areas. Ann. Intern. Med. 142:836-846. [DOI] [PubMed] [Google Scholar]
- 55.Mason, D. P., F. Kawamoto, K. Lin, A. Laoboonchai, and C. Wongsrichanalai. 2002. A comparison of two rapid field immunochromatographic tests to expert microscopy in the diagnosis of malaria. Acta Trop. 82:51-59. [DOI] [PubMed] [Google Scholar]
- 56.McKenzie, F. E., J. Sirichaisinthop, R. S. Miller, R. A. Gasser, Jr., and C. Wongsrichanalai. 2003. Dependence of malaria detection and species diagnosis by microscopy on parasite density. Am. J. Trop. Med. Hyg. 69:372-376. [PMC free article] [PubMed] [Google Scholar]
- 57.Mens, P., N. Spieker, S. Omar, M. Heijnen, H. Schallig, and P. A. Kager. 2007. Is molecular biology the best alternative for diagnosis of malaria to microscopy? A comparison between microscopy, antigen detection and molecular tests in rural Kenya and urban Tanzania. Trop. Med. Int. Health 12:238-244. [DOI] [PubMed] [Google Scholar]
- 58.Miller, R. L., S. Ikram, G. J. Armelagos, R. Walker, W. B. Harer, C. J. Shiff, D. Baggett, M. Carrigan, and S. M. Maret. 1994. Diagnosis of Plasmodium falciparum infections in mummies using the rapid manual ParaSight-F test. Trans. R. Soc. Trop. Med. Hyg. 88:31-32. [DOI] [PubMed] [Google Scholar]
- 59.Miller, R. S., P. McDaniel, and C. Wongsrichanalai. 2001. Following the course of malaria treatment by detecting parasite lactate dehydrogenase enzyme. Br. J. Haematol. 113:558-559. [DOI] [PubMed] [Google Scholar]
- 60.Miller, R. S. 2006. Comparison of performance characteristics of the Binax NOW Malaria test using venous and fingerstick samples abstr. Abstr. 55th Annu. Meet. Am. Soc. Trop. Med. Hyg., abstr. 533.
- 61.Milne, L. M., M. S. Kyi, P. L. Chiodini, and D. C. Warhurst. 1994. Accuracy of routine laboratory diagnosis of malaria in the United Kingdom. J. Clin. Pathol. 47:740-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moody, A. 2002. Rapid diagnostic tests for malaria parasites. Clin. Microbiol. Rev. 15:66-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moody, A. H., and P. L. Chiodini. 2002. Non-microscopic method for malaria diagnosis using OptiMAL IT, a second-generation dipstick for malaria pLDH antigen detection. Br. J. Biomed. Sci. 59:228-231. [DOI] [PubMed] [Google Scholar]
- 64.Mueller, I., I. Betuela, M. Ginny, J. C. Reeder, and B. Genton. 2007. The sensitivity of the OptiMAL rapid diagnostic test to the presence of Plasmodium falciparum gametocytes compromises its ability to monitor treatment outcomes in an area of Papua New Guinea in which malaria is endemic. J. Clin. Microbiol. 45:627-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mungai, M., G. Tegtmeier, M. Chamberland, and M. Parise. 2001. Transfusion-transmitted malaria in the United States from 1963 through 1999. N. Engl. J. Med. 344:1973-1978. [DOI] [PubMed] [Google Scholar]
- 66.Murray, C. K., D. Bell, R. A. Gasser, and C. Wongsrichanalai. 2003. Rapid diagnostic testing for malaria. Trop. Med. Int. Health 8:876-883. [DOI] [PubMed] [Google Scholar]
- 67.Murray, C. K., R. M. Mody, D. P. Dooley, D. R. Hospenthal, L. L. Horvath, K. A. Moran, and R. W. Muntz. 2006. The remote diagnosis of malaria using telemedicine or e-mailed images. Mil. Med. 171:1167-1171. [DOI] [PubMed] [Google Scholar]
- 68.Noedl, H., K. Yingyuen, A. Laoboonchai, M. Fukuda, J. Sirichaisinthop, and R. S. Miller. 2006. Sensitivity and specificity of an antigen detection ELISA for malaria diagnosis. Am. J. Trop. Med. Hyg. 75:1205-1208. [PubMed] [Google Scholar]
- 69.Nyame, A. K., Z. S. Kawar, and R. D. Cummings. 2004. Antigenic glycans in parasitic infections: implications for vaccines and diagnostics. Arch. Biochem. Biophys. 426:182-200. [DOI] [PubMed] [Google Scholar]
- 70.Ohrt, C., P. Obare, A. Nanakorn, C. Adhiambo, K. Awuondo, W. P. O'Meara, S. Remich, K. Martin, E. Cook, J. P. Chretien, C. Lucas, J. Osoga, P. McEvoy, M. L. Owaga, J. S. Odera, and B. Ogutu. 2007. Establishing a malaria diagnostics centre of excellence in Kisumu, Kenya. Malar. J. 6:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Palmer, C. J., J. A. Bonilla, D. A. Bruckner, E. D. Barnett, N. S. Miller, M. A. Haseeb, J. R. Masci, and W. M. Stauffer. 2003. Multicenter study to evaluate the OptiMAL test for rapid diagnosis of malaria in U.S. hospitals. J. Clin. Microbiol. 41:5178-5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Payne, D. 1988. Use and limitations of light microscopy for diagnosing malaria at the primary health care level. Bull. W. H. O. 66:621-626. [PMC free article] [PubMed] [Google Scholar]
- 73.Peters, R. P., E. E. Zijlstra, M. J. Schijffelen, A. L. Walsh, G. Joaki, J. J. Kumwenda, J. G. Kublin, M. E. Molyneux, and D. K. Lewis. 2004. A prospective study of bloodstream infections as cause of fever in Malawi: clinical predictors and implications for management. Trop. Med. Int. Health 9:928-934. [DOI] [PubMed] [Google Scholar]
- 74.Puta, C., and C. Manyando. 1997. Enhanced gametocyte production in Fansidar-treated Plasmodium falciparum malaria patients: implications for malaria transmission control programmes. Trop. Med. Int. Health 2:227-229. [DOI] [PubMed] [Google Scholar]
- 75.Rafael, M. E., T. Taylor, A. Magill, Y. W. Lim, F. Girosi, and R. Allan. 2006. Reducing the burden of childhood malaria in Africa: the role of improved diagnostics. Nature 444(Suppl. 1):39-48. [DOI] [PubMed] [Google Scholar]
- 76.Rathnam, P. J., J. P. Bryan, and M. Wolfe. 2007. Epidemiology of malaria among United States government personnel assigned to diplomatic posts. Am. J. Trop. Med. Hyg. 76:260-266. [PubMed] [Google Scholar]
- 77.Ratsimbasoa, A., A. Randriamanantena, R. Raherinjafy, N. Rasoarilalao, and D. Menard. 2007. Which malaria rapid test for Madagascar? Field and laboratory evaluation of three tests and expert microscopy of samples from suspected malaria patients in Madagascar. Am. J. Trop. Med. Hyg. 76:481-485. [PubMed] [Google Scholar]
- 78.Reyburn, H., H. Mbakilwa, R. Mwangi, O. Mwerinde, R. Olomi, C. Drakeley, and C. J. Whitty. 2007. Rapid diagnostic tests compared with malaria microscopy for guiding outpatient treatment of febrile illness in Tanzania: randomised trial. BMJ 334:403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Richter, J., K. Gobels, I. Muller-Stover, B. Hoppenheit, and D. Haussinger. 2004. Co-reactivity of plasmodial histidine-rich protein 2 and aldolase on a combined immuno-chromographic-malaria dipstick (ICT) as a potential semi-quantitative marker of high Plasmodium falciparum parasitaemia. Parasitol. Res. 94:384-385. [DOI] [PubMed] [Google Scholar]
- 80.Rock, E. P., K. Marsh, A. J. Saul, T. E. Wellems, D. W. Taylor, W. L. Maloy, and R. J. Howard. 1987. Comparative analysis of the Plasmodium falciparum histidine-rich proteins HRP-I, HRP-II and HRP-III in malaria parasites of diverse origin. Parasitology 95:209-227. [DOI] [PubMed] [Google Scholar]
- 81.Rogier, C., D. Commenges, and J. F. Trape. 1996. Evidence for an age-dependent pyrogenic threshold of Plasmodium falciparum parasitemia in highly endemic populations. Am. J. Trop. Med. Hyg. 54:613-619. [DOI] [PubMed] [Google Scholar]
- 82.Saito-Ito, A., Y. Akai, S. He, M. Kimura, and M. Kawabata. 2001. A rapid, simple and sensitive flow cytometric system for detection of Plasmodium falciparum. Parasitol. Int. 50:249-257. [DOI] [PubMed] [Google Scholar]
- 83.Schellenberg, J. R., T. Smith, P. L. Alonso, and R. J. Hayes. 1994. What is clinical malaria? Finding case definitions for field research in highly endemic areas. Parasitol. Today 10:439-442. [DOI] [PubMed] [Google Scholar]
- 84.Seed, C. R., A. Kitchen, and T. M. Davis. 2005. The current status and potential role of laboratory testing to prevent transfusion-transmitted malaria. Transfus. Med. Rev. 19:229-240. [DOI] [PubMed] [Google Scholar]
- 85.Shekalaghe, S. A., J. T. Bousema, K. K. Kunei, P. Lushino, A. Masokoto, L. R. Wolters, S. Mwakalinga, F. W. Mosha, R. W. Sauerwein, and C. J. Drakeley. 2007. Submicroscopic Plasmodium falciparum gametocyte carriage is common in an area of low and seasonal transmission in Tanzania. Trop. Med. Int. Health 12:547-553. [DOI] [PubMed] [Google Scholar]
- 86.Shiff, C. J., Z. Premji, and J. N. Minjas. 1993. The rapid manual ParaSight-F test. A new diagnostic tool for Plasmodium falciparum infection. Trans. R. Soc. Trop. Med. Hyg. 87:646-648. [DOI] [PubMed] [Google Scholar]
- 87.Silvie, O., M. Thellier, M. Rosenheim, A. Datry, P. Lavigne, M. Danis, and D. Mazier. 2002. Potential value of Plasmodium falciparum-associated antigen and antibody detection for screening of blood donors to prevent transfusion-transmitted malaria. Transfusion 42:357-362. [DOI] [PubMed] [Google Scholar]
- 88.Sing, A., E. Rauch, A. Roggenkamp, I. B. Autenrieth, and J. Heesemann. 2000. Evaluation of the ICT malaria Pf test for rapid post-mortem diagnosis of Plasmodium falciparum malaria in corpses examined for forensic reasons. Int. J. Legal Med. 113:251-252. [DOI] [PubMed] [Google Scholar]
- 89.Singer, L. M., R. D. Newman, A. Diarra, A. C. Moran, C. S. Huber, G. Stennies, S. B. Sirima, A. Konate, M. Yameogo, R. Sawadogo, J. W. Barnwell, and M. E. Parise. 2004. Evaluation of a malaria rapid diagnostic test for assessing the burden of malaria during pregnancy. Am. J. Trop. Med. Hyg. 70:481-485. [PubMed] [Google Scholar]
- 90.Singh, N., A. K. Mishra, M. M. Shukla, S. K. Chand, and P. K. Bharti. 2005. Diagnostic and prognostic utility of an inexpensive rapid on site malaria diagnostic test (ParaHIT f) among ethnic tribal population in areas of high, low and no transmission in central India. BMC Infect. Dis. 5:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Singh, N., and A. Saxena. 2005. Usefulness of a rapid on-site Plasmodium falciparum diagnosis (Paracheck PF) in forest migrants and among the indigenous population at the site of their occupational activities in central India. Am. J. Trop. Med. Hyg. 72:26-29. [PubMed] [Google Scholar]
- 92.Singh, N., A. Saxena, S. B. Awadhia, R. Shrivastava, and M. P. Singh. 2005. Evaluation of a rapid diagnostic test for assessing the burden of malaria at delivery in India. Am. J. Trop. Med. Hyg. 73:855-858. [PubMed] [Google Scholar]
- 93.Snounou, G., and N. J. White. 2004. The co-existence of Plasmodium: sidelights from falciparum and vivax malaria in Thailand. Trends Parasitol. 20:333-339. [DOI] [PubMed] [Google Scholar]
- 94.Sokhna, C. S., J. F. Trape, and V. Robert. 2001. Gametocytaemia in Senegalese children with uncomplicated falciparum malaria treated with chloroquine, amodiaquine or sulfadoxine + pyrimethamine. Parasite 8:243-250. [DOI] [PubMed] [Google Scholar]
- 95.Spielman, A., J. B. Perrone, A. Teklehaimanot, F. Balcha, S. C. Wardlaw, and R. A. Levine. 1988. Malaria diagnosis by direct observation of centrifuged samples of blood. Am. J. Trop. Med. Hyg. 39:337-342. [DOI] [PubMed] [Google Scholar]
- 96.Stauffer, W. M., A. M. Newberry, C. P. Cartwright, J. E. Rosenblatt, K. L. Hanson, L. Sloan, D. T. Tsukayama, C. Taylor, and B. A. Juni. 2006. Evaluation of malaria screening in newly arrived refugees to the United States by microscopy and rapid antigen capture enzyme assay. Pediatr. Infect. Dis. J. 25:948-950. [DOI] [PubMed] [Google Scholar]
- 97.Stow, N. W., J. K. Torrens, and J. Walker. 1999. An assessment of the accuracy of clinical diagnosis, local microscopy and a rapid immunochromatographic card test in comparison with expert microscopy in the diagnosis of malaria in rural Kenya. Trans. R. Soc. Trop. Med. Hyg. 93:519-520. [DOI] [PubMed] [Google Scholar]
- 98.Suh, I. B., H. K. Choi, S. W. Lee, S. K. Woo, H. Y. Kang, Y. D. Won, M. Cho, and C. S. Lim. 2003. Reactivity of sera from cases of Plasmodium vivax malaria towards three recombinant antigens based on the surface proteins of the parasite. Ann. Trop. Med. Parasitol. 97:481-487. [DOI] [PubMed] [Google Scholar]
- 99.Susi, B., T. Whitman, D. L. Blazes, T. H. Burgess, G. J. Martin, and D. Freilich. 2005. Rapid diagnostic test for Plasmodium falciparum in 32 Marines medically evacuated from Liberia with a febrile illness. Ann. Intern. Med. 142:476-477. [DOI] [PubMed] [Google Scholar]
- 100.Swarthout, T. D., H. Counihan, R. K. Senga, and I. van den Broek. 2007. Paracheck-Pf accuracy and recently treated Plasmodium falciparum infections: is there a risk of over-diagnosis? Malar. J. 6:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Taylor, T. E., W. J. Fu, R. A. Carr, R. O. Whitten, J. S. Mueller, N. G. Fosiko, S. Lewallen, N. G. Liomba, and M. E. Molyneux. 2004. Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat. Med. 10:143-145. [DOI] [PubMed] [Google Scholar]
- 102.The Malaria Working Party of The General Haematology Task Force of the British Committee for Standards in Haematology. 1997. The laboratory diagnosis of malaria. Clin. Lab. Haematol. 19:165-170. [PubMed] [Google Scholar]
- 103.Thwing, J., J. Skarbinski, R. D. Newman, A. M. Barber, S. Mali, J. M. Roberts, L. Slutsker, and P. M. Arguin. 2007. Malaria surveillance—United States, 2005. MMWR Surveill. Summ. 56:23-40. [PubMed] [Google Scholar]
- 104.Tjitra, E., S. Suprianto, J. McBroom, B. J. Currie, and N. M. Anstey. 2001. Persistent ICT malaria P.f/P.v panmalarial and HRP2 antigen reactivity after treatment of Plasmodium falciparum malaria is associated with gametocytemia and results in false-positive diagnoses of Plasmodium vivax in convalescence. J. Clin. Microbiol. 39:1025-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Trachsler, M., P. Schlagenhauf, and R. Steffen. 1999. Feasibility of a rapid dipstick antigen-capture assay for self-testing of travellers' malaria. Trop. Med. Int. Health 4:442-447. [DOI] [PubMed] [Google Scholar]
- 106.van den Broek, I., O. Hill, F. Gordillo, B. Angarita, P. Hamade, H. Counihan, and J. P. Guthmann. 2006. Evaluation of three rapid tests for diagnosis of P. falciparum and P. vivax malaria in Colombia. Am. J. Trop. Med. Hyg. 75:1209-1215. [PubMed] [Google Scholar]
- 107.Warhurst, D. C., and J. E. Williams. 1996. ACP broadsheet no. 148. Laboratory diagnosis of malaria. J. Clin. Pathol. 49:533-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.WHO. 2000. New perspectives. Malaria. Diagnosis. Report of a joint WHO/USAID informal consultation, 25-27 October 1999. WHO, Geneva, Switzerland.
- 109.WHO. 2006. The role of laboratory diagnosis to support malaria disease management: focus on the use of rapid diagnostic tests in areas of high transmission. WHO, Geneva, Switzerland.
- 110.WHO. 2006. The use of malaria rapid diagnostic tests. WHO, Geneva, Switzerland.
- 111.WHO, Western Pacific Region. 2006. Towards quality testing of malaria rapid diagnostic tests: evidence and methods. WHO, Western Pacific Region, Manila, Philippines.
- 112.Wongsrichanalai, C., C. K. Murray, M. Gray, R. S. Miller, P. McDaniel, W. J. Liao, A. L. Pickard, and A. J. Magill. 2003. Co-infection with malaria and leptospirosis. Am. J. Trop. Med. Hyg. 68:583-585. [DOI] [PubMed] [Google Scholar]
- 113.Zhou, M., Q. Liu, C. Wongsrichanalai, W. Suwonkerd, K. Panart, S. Prajakwong, A. Pensiri, M. Kimura, H. Matsuoka, M. U. Ferreira, S. Isomura, and F. Kawamoto. 1998. High prevalence of Plasmodium malariae and Plasmodium ovale in malaria patients along the Thai-Myanmar border, as revealed by acridine orange staining and PCR-based diagnoses. Trop. Med. Int. Health 3:304-312. [DOI] [PubMed] [Google Scholar]
- 114.Zurovac, D., B. Midia, S. A. Ochola, M. English, and R. W. Snow. 2006. Microscopy and outpatient malaria case management among older children and adults in Kenya. Trop. Med. Int. Health 11:432-440. [DOI] [PubMed] [Google Scholar]