Abstract
Toll-like receptors (TLRs) form a major group of transmembrane receptors that are involved in the detection of invading pathogens. Double-stranded RNA is a marker for viral infection that is recognized by TLR3. TLR3 triggering activates specific signaling pathways that culminate in the activation of NF-κB and IRF3 transcription factors, as well as apoptosis, enabling the host to mount an effective innate immune response through the induction of cytokines, chemokines, and other proinflammatory mediators. In this review, we describe the paradoxical role of TLR3 in innate immunity against different viruses and in viral pathogenesis but also the evidence for TLR3 as a “danger” receptor in nonviral diseases. We also discuss the structure and cellular localization of TLR3, as well as the complex signaling and regulatory events that contribute to TLR3-mediated immune responses.
INTRODUCTION
Antiviral innate immunity depends on different sensor systems that detect viral-pathogen-associated molecular patterns (PAMPs) and initiate specific signaling pathways, including those leading to the activation of the transcription factors nuclear factor-κB (NF-κB) and interferon regulatory factor 3 (IRF3). NF-κB mediates the production of several proinflammatory cytokines and antiapoptotic proteins (86), whereas IRF3 regulates the expression of beta interferon (IFN-β). IFN-β itself activates several other genes, including 2′-5′-oligoadenylate synthetases, protein kinase R, Mx GTPase, and P56, which contribute to an antiviral effect via the inhibition of protein synthesis and viral replication. Viral double-stranded RNA (dsRNA) is a PAMP that is recognized by Toll-like receptor 3 (TLR3) and several cytosolic sensors, such as protein kinase R, 2′-5′-oligoadenylate synthetases, and the recently identified RNA helicases RIG-I (retinoic acid-inducible gene I) and MDA5 (melanoma differentiation-associated gene 5) (26, 43, 70, 113). TLR3 and the RIG-I/MDA5 RNA helicases differ in their cellular localizations, ligand specificities, and downstream signaling pathways, which suggests that host cells have multiple defense mechanisms against viral infection. During viral replication, dsRNA is produced either as an intermediate of the replication cycle or as part of the viral RNA genome (50). Moreover, based on the observation that macrophages lacking the TLR3 adaptor protein TRIF (Toll/interleukin-1 [IL-1] receptor [TIR] domain-containing adaptor-inducing IFN-β) are more susceptible to vaccinia virus (41), it has been suggested that DNA viruses might produce RNA transcripts that engage TLR3. In addition to dsRNA from viral origin, endogenous dsRNA that is released from dying cells activates TLR3 (55). Polyriboinosinic:polyribocytidylic acid [poly(I:C)] is a stable synthetic dsRNA analogue that is frequently used as a TLR3 ligand to mimic viral infection. In contrast to the recognition of dsRNA by intracellular molecules, TLR3 preferentially recognizes synthetic poly(I:C) rather than virus-derived dsRNA, suggesting that TLR3 recognizes a unique dsRNA structure that largely differs from the one recognized by other dsRNA-binding proteins (77). The crucial role of TLR3 in poly(I:C) recognition is reflected in the observation that TLR3-deficient mice show reduced responses to poly(I:C), resistance to the lethal effect of poly(I:C) when sensitized with d-galactosamine, and reduced production of inflammatory cytokines (4). Poly(I:C) in a cell-associated form is even more efficient in triggering TLR3 than soluble dsRNA (67, 93), suggesting that dsRNA from dying cells is most likely a more potent and physiologically relevant TLR3 ligand than dsRNA from live cells. Many TLR3 effects rely on cells of the innate immune system that either express TLR3 or respond to inflammatory mediators that are produced upon TLR3 signaling. Immune cells that express TLR3 and contribute to an innate immune response are dendritic cells, macrophages, natural killer cells, and mast cells (37, 64, 78, 104). Recent work demonstrates that TLR3 is also present in cells that directly participate in the adaptive immune response (100, 109). In this context, TLR3 ligation was shown to directly increase IFN-γ production by antigen-primed CD8+ T cells. Altogether, this indicates that TLR3 is a “danger” receptor with a pleiotropic potential in innate and adaptive immunity.
PARADOXICAL ROLES OF TLR3 IN VIRAL PATHOLOGY
The exact role of TLR3 in viral infection is still controversial (21, 99). Several reports show that TLR3 contributes to the elimination of specific viruses, but others demonstrate that some viruses can benefit from TLR3 stimulation (Table 1). The general outcome is probably dependent on several factors, such as the type of virus, the viral load, its mode of infection (endoplasmic versus cytoplasmic), the cell type that is infected, and the stage of infection.
TABLE 1.
Role of TLR3 in viral infections
| Virusa | Target system, organ, tissue, and/or cells expressing TLR3 | Role of TLR3b | Reference(s) |
|---|---|---|---|
| Influenza A virus | Respiratory tract | − | 62 |
| CNS | + | 40 | |
| Respiratory syncytial virus | Respiratory tract | + | 30 |
| Rabies virus | CNS (neuronal cells) | −? | 49 |
| Herpes simplex virus 1 | CNS (neuronal cells) | −? | 49, 81 |
| West Nile virus | CNS | − | 105 |
| Theiler's murine encephalomyelitis virus | CNS (astrocytes) | +? | 95 |
| Encephalomyocarditis virus | Heart | + | 35 |
| Human immunodeficiency virus | Skeletal muscle | −? | 92 |
| Punta Toro virus | Liver | − | 28 |
| Hepatitis C virus | Liver | +/−? | 63, 73 |
| Kidney | −? | 110 | |
| Herpes simplex virus 2 | Female genital tract | +? | 8, 25, 39 |
Viruses are listed in the order of their appearance in the text.
+, protective; −, detrimental; +/−, protective or detrimental.
TLR3 has been implicated in viral infections of the respiratory tract, which constitutively expresses TLR3. Influenza A virus infection markedly upregulates the pulmonary expression of TLR3 and causes acute pneumonia (31, 62). Despite higher influenza A virus production in the lungs of TLR3-deficient mice than in those of wild-type mice, TLR3-deficient mice have an unexpected survival advantage. Due to the absence of TLR3-mediated inflammatory signaling, influenza A virus-infected mice display significantly lower levels of inflammatory mediators and a lower number of CD8+ T cells that contribute to the clearance of infected cells from the lung than uninfected mice (62). These findings demonstrate that although TLR3 moderates virus production in the lung, it also contributes to the debilitating effects of a detrimental host inflammatory response. Respiratory syncytial virus is another virus that augments TLR3 expression in lung epithelial cells (30, 83). Unlike the role of TLR3 in influenza A virus infection, TLR3 is not required for viral clearance but is necessary to maintain the proper immune environment in the lung to avoid pathological development of the infection. In the absence of TLR3, respiratory syncytial virus-infected mice produce a significantly increased number of T helper 2 (TH2) cytokines, which cause mucus overproduction, a pathological feature of respiratory syncytial virus infection.
Several studies show that TLR3 is also involved in central nervous system (CNS) diseases. The CNS broadly comprises two cell types: glial cells and neuronal cells. Glial cells are further divided into microglial cells, which are CNS-resident innate immune cells, and macroglial cells, such as astrocytes and Schwann cells, which have an ectodermal origin. All these different neuronal cell types have been shown to express TLR3 and initiate signaling upon being triggered with dsRNA or viruses, such as rabies virus and herpes simplex virus type 1 (23, 48, 49, 60, 81). This is particularly surprising since it shows that neurons have the intrinsic machinery to initiate inflammatory and antiviral responses (57, 81). In contrast to the destructive role of TLR3 in influenza A virus infection of the respiratory tract, TLR3 was proposed to have a protective function during influenza A virus-induced encephalopathy. With the latter virus, a specific loss-of-function missense mutation (F303S) encoded by the TLR3 gene was found in one of three patients with influenza-associated encephalopathy (40). Upon peripheral infection of mice with West Nile virus, TLR3-dependent inflammatory signaling was shown to facilitate viral entry into the brain, causing lethal encephalitis (105). Once inside the brain, the immune response leading to encephalitis is independent of TLR3, since wild-type and TLR3-compromised mice are equally susceptible upon intracerebroventricular administration of the virus (105). In vitro infection of astrocytes with Theiler's murine encephalomyelitis virus leads to the TLR3-mediated induction of several IRF- and NF-κB-dependent chemokines and cytokines (95), suggesting that TLR3 signaling is responsible for the initial inflammatory cytokine responses defining the outcome of Theiler's murine encephalomyelitis virus-induced encephalitis. How the TLR3 signaling pathway influences the outcome of Theiler's murine encephalomyelitis virus infection in vivo is not known.
TLR3 positively contributes to the immune response to invading encephalomyocarditis virus. Schulz et al. demonstrated that TLR3 engagement by dsRNA that is released from dying encephalomyocarditis virus-infected cells leads to the cross-priming of myeloid dendritic cells, followed by the cross-presentation and activation of cytotoxic T cells (93). This function is proposed to be important in the clearance of viruses that have no tropism for dendritic cells. Apart from the release of dsRNA, virus-infected cells can release type I IFNs, which also promote cross-priming (59). More recently, TLR3-deficient mice were shown to be more susceptible to encephalomyocarditis virus infection and to have a significantly higher viral load in the heart than wild-type mice (35). Although encephalomyocarditis virus-induced expression of several cytokines and chemokines was impaired in TLR3-deficient mice, IFN-β production was not. The latter finding might reflect a redundant role of TLR3 and other receptors in the signaling toward IFN-β production.
Surprisingly, TLR3 has also been implicated in the immunobiology of skeletal muscle. TLR3 is expressed in muscle cells both in vitro and in vivo and is upregulated by dsRNA and IFN-γ. Furthermore, TLR3 levels are elevated in muscle biopsy specimens from patients with inflammatory and human immunodeficiency virus-associated myopathies (92), suggesting a deleterious role for TLR3 in inflammatory muscle disease.
A detrimental role for TLR3 was established in the viral etiology of liver and kidney disease. TLR3-deficient mice demonstrate reduced liver disease and increased resistance to lethal infection with Punta Toro virus, a hepatotropic phlebovirus. The most dramatic difference upon infection of wild-type and TLR3-deficient mice with Punta Toro virus was the exaggerated release of IL-6 found systemically and in the livers of infected wild-type animals. Although IL-6 is critical to establishing antiviral defense, excessive IL-6 release is detrimental to the liver and thus contributes to viral pathogenesis (28). Hepatitis C virus is a major cause of liver hepatitis, liver cirrhosis, and hepatocellular carcinoma (56). Although several reports describe a role for TLR3 in hepatitis C virus infection, the physiological function of TLR3 in hepatitis remains unclear. Evidence for the physiological relevance of a hepatitis C virus-TLR3 interaction has come from biopsy specimens of patients with hepatitis C virus-positive kidney disease, a frequent complication in hepatitis C virus infections (110). TLR3 mRNA expression was significantly increased in hepatitis C virus-positive glomerulonephritis and was associated with enhanced mRNA levels for the chemokines RANTES and monocyte chemotactic protein 1 (MCP1). TLR3 expression on renal cells may therefore establish a link between viral infections and glomerular diseases.
TLR3 has also been implicated in the protection against herpes simplex virus type 2 infection of the female genital tract (8, 25, 39). In this context, it was shown that cells of the reproductive tract express functional TLR3 (6, 74) and that treatment with dsRNA protects against genital herpes infection in mice (8). Although an interaction between herpes simplex virus type 2 RNA and TLR3 has not yet been shown, it is very likely that herpes simplex virus produces dsRNA intermediates that trigger TLR3 (38).
Finally, a role for TLR3 in virus-induced tumor formation has also been proposed. For example, the Moloney murine and feline leukemia viruses activate NF-κB via a specific RNA transcript from their long terminal repeat region, which is capable of stimulating TLR3. Since the antiapoptotic and growth-promoting activities of NF-κB have been implicated in leukemogenesis, these data suggest a role for TLR3 in the promotion of tumor formation under certain conditions (1).
Viruses have evolved multiple ways to modulate TLR3 signaling. Different viruses, including hepatitis C virus, influenza A virus, respiratory syncytial virus, human immunodeficiency virus, simian immunodeficiency virus, and measles virus, augment TLR3 expression, which is in some cases associated with an increase in its membrane localization (30, 85, 102). Since upregulation of TLR3 sensitizes the cells to subsequent viral or dsRNA exposure, we hypothesize that this will contribute to pathological inflammatory signaling if the host does not cope appropriately with the increased susceptibility resulting from increased TLR3 expression. Interestingly, some viruses directly interfere with intracellular signaling leading to NF-κB or IRF3 activation as a means of escaping the host immune response (33, 86). For example, the vaccinia virus A52R protein inhibits TLR3-induced NF-κB activation by sequestering key signaling proteins (tumor necrosis factor [TNF] receptor-associated factor 6 [TRAF6] and IL-1 receptor-associated kinase 2 [IRAK2]) (36). Another vaccinia virus protein, the A46R protein, inhibits both NF-κB and IRF3 activation via its interaction with TRIF and other TLR adaptor proteins (96). Li et al. showed that the hepatitis C virus nonstructural protein 3/4A (NS3/4A) protease can cleave the TLR3 adaptor protein TRIF (also known as TICAM-1 or Lps2), thereby inhibiting the TLR3 signaling pathway that leads to NF-κB and IRF3 activation and subsequent IFN-β production (see also below) (63). On the other hand, the hepatitis C virus-encoded RNA-dependent RNA polymerase NS5B induces IFN-β production in a TLR3-dependent manner, probably through the synthesis of dsRNA, using cellular RNA as a template (73). A possible explanation for the contrasting effects of NS5B and NS3/4A on IFN-β production might be the maintenance of a low, nonlethal level of hepatitis C virus, which may promote distraction of the host defense system and enable persistent infection.
A FUNCTION FOR TLR3 BEYOND ITS ROLE IN VIRAL INFECTION?
In addition to mediating an antiviral host immune response, TLR3 has been implicated in the protection of immune-privileged sites, including the CNS and the liver. Bsibsi et al. showed that TLR3 is induced on human astrocytes upon inflammation and, when activated, mediates a comprehensive neuroprotective response rather than a polarized proinflammatory reaction (13). TLR3 stimulation also suppresses experimental autoimmune encephalomyelitis by inducing endogenous IFN-β (103). TLR3 stimulation was also shown to protect against lipopolysaccharide-induced liver injury by downregulating TLR4 expression on macrophages (51). Rather than having this hepatoprotective effect, TLR3 causes the breakdown of hepatic tolerance (58), leading to TLR3-mediated autoimmune liver disease. In this case, the TLR3 triggering of myeloid dendritic cells and macrophages leads to the production of IFN-α and TNF, which induce the secretion of CXC chemokine ligand 9 by hepatocytes or other cells. CXC chemokine ligand 9 serves as an effective chemo-attractant for autoimmune CD8+ T cells into the liver, where they cause autoimmune liver damage (16, 58). TLR3 expression is also associated with lupus nephritis, an autoimmune disease affecting the kidney. Exposure to poly(I:C) can aggravate lupus nephritis, and this is probably mediated through TLR3, which is present on both antigen-presenting cells and glomerular mesangial cells (80). Finally, the observation that poly(I:C)-induced TLR3 signaling results in pancreatic β-cell death and (unlike other PAMPs, such as single-stranded RNA, lipopolysaccharide, or peptidoglycan) the development of diabetes in mice (19, 108) suggests a role for TLR3 in autoimmune diabetes.
All together, these data demonstrate that TLR3 is a crucial “danger” signaling receptor that, through its presence on both immune and nonimmune cells, is involved in controlling the delicate balance between tolerance and inflammation on the one hand and inflammation and disease on the other hand. Whether viral RNA is responsible for all TLR3-mediated responses that have been reported to date or whether there is also a role for cellular RNA or other molecules that function as endogenous TLR3 ligands remains to be investigated.
TLR3 STRUCTURE, LIGAND BINDING, AND SPECIFICITY
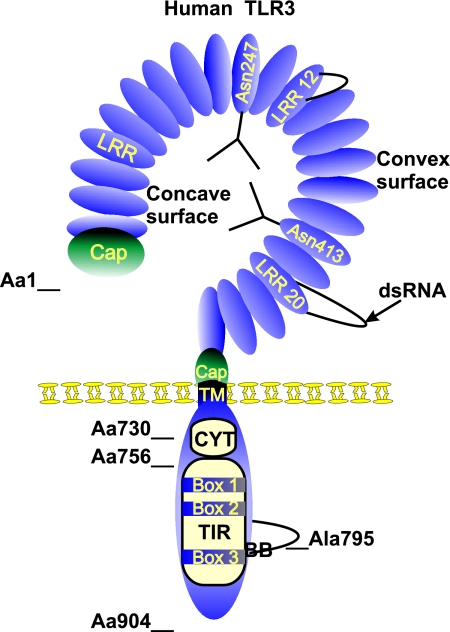
Like all other members of the TLR family, TLR3 is a type I transmembrane receptor protein composed of an extracellular domain containing multiple leucine-rich repeats (LRRs), a transmembrane region, and a cytoplasmic tail containing the conserved TIR domain (Fig. 1). The transmembrane domain consists of a single α-helix spanning the membrane, while the TIR domain is made up of a five-stranded β-sheet surrounded by five α-helices. These two secondary-structure elements are connected by loops of which the BB loop (connecting β-strand B with α-helix B) is described to interact with the TLR adaptor molecules. This BB loop contains in all TLRs a conserved proline residue, except in TLR3, where the proline is replaced with an alanine (Fig. 1). The importance of this residue is demonstrated by the failure of the TLR3-Ala795His mutant to bind the adaptor protein TRIF (79). TLR3 is the sole TLR that interacts directly with TRIF, whereas other TLRs physically interact with the adaptor proteins MyD88 adaptor-like, MyD88, and TRIF-related adaptor molecule, an activity which is probably related to the divergence in the BB loop. In addition to the conserved BB loop, three particular boxes that are highly conserved among TLR family members define the TIR domain and are involved in TLR3 signaling (20, 64).
FIG. 1.
Schematic structure of human TLR3. TLR3 is a type I integral membrane protein of 904 amino acids. The TLR3 extracellular domain is a horseshoe-shaped solenoid in which LRR forms one turn of the solenoid. The LRRs are at the N-terminal and C-terminal regions, flanked by a cysteine-rich Cap domain. The concave surface is rich in potential N-glycosylation sites and probably heavily glycosylated. Here we represent two N-glycan structures on Asn247 and Asn413, two residues which are implicated in glycosylation. LRR12 and LRR20 are atypical LRR motifs containing large insertions which protrude from the solenoid. According to the symmetrical assembly model, ligand binding occurs at the glycan-free surface involving LRR20. The transmembrane domain (TM) is made up of one single α-helix. The cytoplasmic domain comprises the cytoplasmic linker region (CYT) (amino acid [Aa] 730 to Aa756) and the TIR domain, from which the adaptor-binding BB loop protrudes. Ala795 is a conserved residue residing at the top of the BB loop and is involved in the binding of TRIF. The three conserved boxes that define the TIR domain are also indicated.
The extracellular domain of human TLR3, which supports ligand binding, consists of 23 tandemly arranged LRRs. Seventeen of the 23 LRRs follow the consensus motif of a 24-residue repeat, consisting of XL2XXL5XL7XXN10XL12XXXXF20XXL23X, where L represents an obligate hydrophobic residue of which leucine is most prevalent, F is a conserved phenylalanine, and N is a conserved asparagine. The remaining noncanonical LRRs contain insertions that form large, protruding loops in LRR12 and LRR20 (Fig. 1) (11, 15). Recently, Bell et al. (11) and Choe et al. (15) described independently from each other the crystal structure of the TLR3 extracellular domain. Their studies showed that the LRRs adopt a horseshoe-shaped solenoid structure that is heavily glycosylated. One face of the extracellular domain, residing at the convex (outer) surface, is glycan free and is predicted to accommodate nucleotide binding due to the presence of many positively charged residues (15). Alternatively and in agreement with the assumed binding mechanism for LRR-containing proteins, Bell and colleagues proposed that the nucleotide binding site may reside at the concave surface, which harbors four potential Asn (N)-linked glycosylation sites and has a predominant negative charge. It has been suggested that glycosylation of the concave region could provide a mechanism for controlling dsRNA binding by TLR3 (11). In addition, the large insertions in LRR12 and LRR20, which are unique to TLR3 and conserved in all known mammalian orthologues, might also play an important role in ligand binding (11, 15). More recently, mutational analysis of TLR3 located the dsRNA binding site on the glycan-free surface of the extracellular domain at the position of LRR20 (Fig. 1) (10). Bell and colleagues proposed a symmetrical model of receptor chain assembly that takes advantage of the inherent twofold symmetry of dsRNA. In this model, two TLR3 molecules dimerize in such a way that the extracellular domain of the second TLR3 rotates by 180° in order to bind identically to the opposite strand of the RNA duplex (79). In consideration of these findings together, TLR3 might provide several areas that accommodate ligand binding, depending on the glycosylation status of the extracellular domain and the structural characteristics of the ligand (e.g., modifications and duplex length). Studies using different lengths of the preferential TLR3 ligand poly(I:C) indicated that longer duplexes are more potent inducers of TLR3 signaling (18, 77). Differences in potency might be proportional to the ability of different ligands to mediate TLR3 multimerization. In line with the symmetrical assembly model, long dsRNA helices are likely to bind several TLR3 molecules in similar manners (10). Although TLR3 is monomeric in solution, it can readily form a stable or transient dimer when embedded in the membrane and undergo ligand-binding-promoted multimerization (10, 11). Two lines of experimental evidence corroborate this hypothesis. Inactive TLR3 mutant molecules block the activity of wild-type endogenous TLR3, which is a dominant negative characteristic of di- or multimerization-induced signaling events (18). Additional evidence was obtained from a myeloid U937 cell line stably transfected with a chimeric TLR3-CD32 protein. In this case, the cross-linking of CD32 causes a rapid rise in intracellular calcium levels, which is mediated by the cytosolic portion of CD32. Treatment with an anti-TLR3 antibody also induced a calcium flux but only when TLR3 was cross-linked by a secondary antibody, confirming that multimerization is required for signaling (18). The overall dimensions of TLR3, based on crystal structure, roughly match the shape of a CD14 dimer (15). This suggests that TLR3, like TLR4, might associate with CD14. This was experimentally confirmed by Lee and coworkers, who showed that TLR3 can be coimmunoprecipitated with CD14 independently of poly(I:C). They also observed the intracellular colocalization of TLR3 and CD14 in multiple compartments, including the endoplasmic reticulum and the Golgi apparatus. Once poly(I:C) is internalized, TLR3 and CD14 colocalize in the endosomal and lysosomal compartments. CD14 is essential for the uptake of poly(I:C) in these compartments and enhances TLR3 signaling (61).
The endosomal and lysosomal localization of TLR3 is thought to be crucial for providing self- versus non-self-discrimination of dsRNA. Under normal conditions, “self-RNA” is present in the cytoplasm and cannot enter the membrane-bound vesicles in which the extracellular domain of TLR3 is present. When present in cellular debris from dying cells, it can, however, be taken up and delivered to the endosomes and elicit a potentially hazardous danger response. The immunostimulatory potential of RNA is also modulated by nucleoside modification (54, 77). In this context, suppression of RNA recognition is proportional to the number of modified nucleosides present in the RNA (54), explaining why mitochondrial RNA, which is the least modified fraction of mammalian RNA, is a better TLR3 ligand than mRNA or total RNA. The TLR3-stimulatory potential of RNA from dying cells is likely a result of the presence of this mitochondrial RNA (54, 55, 77). The U1 small nuclear RNA is also capable of TLR3 activation. U1 small nuclear RNA is the endogenous ligand of the 70-kDa protein subunit of U1 ribonucleoprotein, which is an auto-antigen frequently associated with rheumatoid arthritis or systemic lupus erythematosus. The potential involvement of TLR3 may help account for the prominence of antiribonucleoprotein responses observed in autoimmune diseases (42). Additionally, a specific motif of the endogenous tRNA(Ala)(UGC) that induces TH1 and cytotoxic-T-lymphocyte immune responses was shown to be effectively recognized by TLR3 (107). In addition to nucleoside modification and the endosomal localization of TLR3, glycosylation of the TLR3 extracellular domain might provide a mechanism for discriminating between host and foreign RNA. Since the mutation of two potential N-glycosylation sites, Asn247 and Asn413 (Fig. 1), or the use of glycosylation inhibitors abrogates TLR3 signaling (18, 98), we hypothesize that the glycosylation of TLR3 is inhibited in the absence of foreign intruders, thus contributing to the distinction between host and foreign RNA by keeping TLR3 in an inactive state in the absence of infection.
For viral RNA sensing in the cytoplasm, discrimination between self-RNA and viral foreign RNA cannot be explained by a spatial barrier mechanism, as described above for TLR3. The RNA helicase RIG-I was recently shown to recognize the 5′ ends of certain viral RNA genomes, rather than the dsRNA structure (113). More specifically, RIG-I recognizes and binds the 5′-triphosphate group of cytoplasmic viral RNA that appears after viral infection or replication. Such 5′-triphosphates are generally removed from, or masked on, host RNA species, thereby remaining silent to innate immunity and providing a structural basis for the distinction between self- and non-self-RNA. Despite structural and functional similarity between RIG-I and MDA5, RNA sensing by MDA5 does not involve a 5′-triphosphate moiety (e.g., in the case of picornaviruses) but seems to involve the sensing of a dsRNA structure by a still-unknown mechanism.
TLR3 LOCALIZATION AND SIGNALING
Localization Holds the Key to TLR3 Activity
In contrast to the viral RNA sensors protein kinase R, RIG-I, and MDA5, whose localization is exclusively cytoplasmic (113), TLR3 has been found in endosomal compartments or at the cell surface. The localization of TLR3 is cell type dependent, which may reflect the participation of cell-type-specific pathways in antiviral IFN induction via TLR3. Human fibroblasts (e.g., the MRC-5 cell line) express TLR3 on the cell surface, and anti-TLR3 monoclonal antibodies inhibit dsRNA-induced IFN-β secretion by fibroblasts, suggesting that in these cells TLR3 acts on the cell surface to sense viral infection. However, in most cell types, including dendritic cells, macrophages, and TLR3-transfected HEK293 cells, TLR3 is detected predominantly in intracellular compartments (24, 65, 66, 75). The cytoplasmic linker region (Fig. 1) contains a sequence motif that is important for intracellular targeting of TLR3, since deleting the linker region interferes with normal intracellular targeting and causes cell surface expression of TLR3 (24, 75). In the case of human TLR3, Arg740 and Val741 residues were identified as crucial determinants for intracellular expression (24), while in murine TLR3, no crucial residues were found in the linker region (Glu727 to Asp749). Yet, this sequence was shown to be sufficient for targeting the plasma membrane protein CD25 to an intracellular location, indicating that it is responsible for the intracellular targeting of TLR3 (75). The intracellular targeting sequence of TLR3 leads this receptor to the same cytoplasmic membranes where TLR7 is localized and adjacent to phagosomes containing apoptotic cell particles. The fusion of such phagosomes to TLR3-containing membranes might enhance the access of TLR3 to dsRNA that is derived from apoptotic cells (75). In addition to having a targeting function, the cytoplasmic linker region contains, at least in human TLR3, several residues that are required for TLR3-induced NF-κB and IFN-β-promoter activation (24). Phe732, Tyr733, Leu742, and Gly743 are conserved across human, mouse, and other species, indicating their importance for TLR3 biology.
Although dendritic cells do not express TLR3 on their surfaces, exogenously added dsRNA activates the cells to produce IFN-α/β and IL-12p70, suggesting that after internalization, dsRNA encounters intracellular TLR3 present in the subcellular compartments and activates TLR3 signals inside the cells. Supporting this suggestion is the fact that treatment of dendritic cells, peripheral blood mononuclear cells, and TLR3-transfected HEK293 cells with the lysosome maturation and acidification inhibitors chloroquine and bafilomycin inhibits the response to poly(I:C) (18, 24, 65). Moreover, the need for internalization of poly(I:C) also fits with the fact that long-term incubation with poly(I:C) is required for IFN-β induction in dendritic cells but not in fibroblasts expressing cell surface TLR3 (65). Extracellularly delivered dsRNA is internalized by clathrin-mediated endocytosis, since a dominant negative version of Eps15, an essential scaffolding molecule in clathrin-mediated coat assembly and endocytosis, impairs dsRNA-induced NF-κB and IFN-β activation (52). The localization of TLR3 in subcellular compartments of the endocytic trafficking pathway is also in harmony with the observation that the interaction between TLR3 and dsRNA and subsequent TLR3 signaling require an acidic pH ranging from 5.7 to 6.5 (18).
TRIF Functions as a TLR3 Adaptor Molecule
The TIR domain of TLR3 binds the TIR domain-containing adaptor protein TRIF, which indirectly activates several transcription factors, including NF-κB, IRF3, and activating protein 1 (AP-1). TRIF knockout mice show defective responses to poly(I:C), indicating that TRIF is essential for TLR3-mediated signaling pathways (79, 112). The mechanisms by which TRIF activates NF-κB and IRF3 have been reviewed extensively (71). We will therefore focus mainly on the most recently identified signaling components of the TLR3/TRIF pathway.
To mediate IRF3 activation, the N-terminal domain of TRIF was originally proposed to engage with two kinases, IκB kinase ɛ (IKKɛ) (also known as IKKi, where “i” means “inducible”) and TANK-binding kinase 1 (TBK1) (also known as T2K or NF-κB-activating kinase [NAK]), enabling them to phosphorylate IRF3, which then forms a dimer that translocates to the nucleus to induce the expression of IFN-β (68). However, it is now thought that TRIF associates with TBK-IKKɛ through the adaptor protein NAK-associated protein 1 (NAP1). RNA interference of NAP1 results in a failure of poly(I:C)-mediated IRF3 activation and IFN-β production, indicating that NAP1 is a TBK1/IKKɛ kinase subunit that participates in TRIF-induced IRF3 activation (Fig. 2) (90). In addition to NAP1, TRAF3 is part of the TBK/IKKɛ kinase complex that coprecipitates with TRIF. Moreover, TLR3 stimulation no longer induces IFN-β in TRAF3-deficient cells, suggesting that TRAF3 is a critical link between TRIF and the kinases required for IRF3 activation (Fig. 2) (32, 76). Interestingly, both TRAF3 and NAP1 are also critical in the TLR-independent RIG-I/MDA5 cytoplasmic signaling pathway leading to IRF3 activation (76, 90).
FIG. 2.
TLR3 signaling pathways. Binding of dsRNA to the TLR3-CD14 complex induces the activation of several intracellular signaling pathways. The activation of NF-κB and IRF3 is achieved by two different signaling branches emanating from the TLR3 adaptor molecule TRIF, which binds to the BB loop of the TLR3 TIR domain. Distinct regions of TRIF bind the ubiquitin ligase TRAF6 and the kinase RIP1. Analogously with the ubiquitin ligase activity of TRAF2 in the TNF receptor pathway, the activity of TRIF-associated TRAF6 might be responsible for the Ub of RIP1 in the TLR3 pathway. RIP1 ubiquitination is recognized by the ubiquitin receptor proteins TAB2 and TAB3, leading to the activation of the kinase TAK1, which is part of the same complex. TAK1 phosphorylates and activates IKKα and IKKβ, which are part of a bigger IKK complex with the IKK adaptor protein IKKγ. IKKβ is known to be the crucial IKK in TLR signaling and phosphorylates IκBα, which binds and keeps NF-κB (here depicted as a p65/p50 dimer) in an inactive state in the cytoplasm. IκBα phosphorylation leads to its recognition and degradation by the proteasome, thus allowing NF-κB to translocate to the nucleus, where it binds and activates specific gene promoters (e.g., A20). TRIF also binds TRAF3 and NAP1. Whereas the role of TRAF3 is still largely unclear, NAP1 functions as an adaptor for the IKK-related kinases IKKɛ and TBK1, which have largely redundant functions. Both kinases phosphorylate IRF3, leading to its dimerization and translocation to the nucleus, where it binds and activates specific gene promoters (e.g., IFN-β). Whereas these TRIF-mediated signaling pathways result in the activation of NF-κB and IRF3, the phosphorylation of NF-κB and IRF3 is involved in acquiring the fully activated status of both transcription factors (see the text for more details). Signaling leading to these events is still largely unclear, but IRF3 phosphorylation is dependent on the kinase Akt, which is activated by the lipid kinase PI3K, which binds phospho-Tyr759 of TLR3. Interestingly, PI3K also seems to have an inhibitory function on NF-κB activation, whereas the phosphorylation of TLR3 on Tyr858 enhances NF-κB activation by an unknown mechanism. TLR3 also induces apoptosis via a TRIF- and RIP1-dependent mechanism. The binding of RIP1 to TRIF not only activates NF-κB but also recruits the DD-containing adaptor protein FADD via a homotypic DD-DD interaction. FADD in turn interacts with the cysteine protease procaspase-8 through the death effector domain (DED) present in both proteins. This is believed to result in the proteolytic auto-activation of procaspase-8 and the initiation of cell death. CYT, cytoplasmic linker.
For NF-κB activation, two separate pathways mediated by, respectively, receptor-interacting protein 1 (RIP1) and TRAF6 seem to bifurcate from TRIF. In murine embryonic fibroblasts deficient in RIP1, poly(I:C)-induced NF-κB is completely blocked in deficient mice, indicating that RIP1 is an essential mediator of the TRIF pathway leading to NF-κB activation (69). The interaction of TRIF and RIP1 is mediated through the RIP homotypic interaction motif (RHIM) present in both proteins, in the C-terminal part of TRIF and the intermediary domain of RIP (Fig. 2). TRAF6 is recruited to the N-terminal domain of TRIF, but the role of TRAF6 is somewhat controversial and probably cell type specific (27, 91). At least in mouse embryonic fibroblasts, TRAF6 is recruited to TRIF along with RIP1, followed by polyubiquitination (Ub) of RIP1 (Fig. 2) (17). In a manner similar to what occurs in the TNF receptor pathway, Ub RIP1 then recruits the ubiquitin receptor protein transforming growth factor β-activating kinase (TAK) binding protein 2 (TAB2) and TAK1. TAK1 phosphorylates IKKα and IKKβ, which in turn phosphorylate the NF-κB inhibitor IκB, eventually leading to its degradation and the nuclear translocation of NF-κB (Fig. 2). Poly(I:C)-induced NF-κB activation, but not IRF3 activation, is decreased in TAK1-deficient mouse embryonic fibroblasts, showing that TAK1 is specifically needed for TLR3-induced NF-κB activation (94). TAK1 also activates the mitogen-activated protein kinases c-jun N-terminal kinase, p38, and extracellular signal-regulated kinase, leading to the phosphorylation and activation of members of the AP-1 family of transcription factors.
TRIF is the sole TLR adaptor that is able to engage mammalian cell death signaling pathways. TRIF-induced cell death requires caspase activity initiated by the Fas-associated death domain protein (FADD)/caspase-8 axis and is unaffected by inhibitors of the intrinsic mitochondrial apoptotic machinery. The proapoptotic potential of TRIF maps to the C-terminal RHIM motif that physically interacts with RIP1. Deletion and mutational analyses revealed that the RHIM in TRIF is essential not only for TRIF-induced NF-κB activation but also for TRIF-induced apoptosis. Yet the activation of NF-κB can be blocked by the superrepressor IκBα without blocking apoptosis, indicating that the ability of TRIF to induce apoptosis is NF-κB independent (34, 53, 82). All together, these data demonstrate that TLR3 is able to induce apoptosis through a TRIF/RIP1/FADD/caspase-8-dependent pathway (Fig. 2), which is supposed to represent an important host defense for limiting the spread of a viral infection. Interestingly, FADD-deficient as well as caspase-8-deficient B cells were shown to be defective in proliferative responses induced by dsRNA. Therefore, in addition to having an apoptotic function, FADD and caspase-8 also play a role in TLR3-induced proliferative responses in B cells (9, 46).
Tyrosine Phosphorylation of TLR3
The tyrosine phosphorylation of TLR2, TLR3, and TLR5 has been shown to play a role in the initiation or regulation of downstream TLR signaling (7, 47, 87, 89). TLR3 harbors five tyrosine residues in its cytoplasmic tail, two of which (Tyr759 and Tyr858) were shown to be phosphorylated and to contribute to the full activation of IRF3 and NF-κB-dependent gene expression (Fig. 2) (87-89). The phosphorylation of Tyr858 is presumably involved in TBK1 activation, which induces the partial phosphorylation and activation of IRF3, accompanied by IRF3 dimerization and translocation to the nucleus, but which still needs a second phosphorylation-dependent signal from the receptor to promote IRF3-dependent reporter gene induction (88). In this context, phospho-Tyr759 leads to the recruitment of phosphatidylinositol-3-kinase (PI3K) and activation of the downstream kinase Akt, which is required to obtain the full phosphorylation and activation of IRF3 in the nucleus. In this two-step model of IRF3 activation, both arms of phosphorylation, one via TRIF and TBK1 and the other via PI3K, are thus needed to obtain fully active IRF3 (Fig. 2). A similar two-step model depending on Tyr858 and Tyr759 was established for TLR3-mediated NF-κB activation (87). One signal leads to the phosphorylation of the inhibitory protein IκB, which is followed by the release and nuclear translocation of NF-κB. The other signal leads to the phosphorylation of the p65 (also known as RelA) subunit of NF-κB, leading to its transactivation (Fig. 2). In this model, the role of TLR3 tyrosine phosphorylation has been illustrated for the TLR3-induced expression of A20 mRNA, which is known to be NF-κB dependent. Mutation of Tyr759 inhibited A20 gene induction, although NF-κB was still activated and translocated to the nucleus. However, NF-κB failed to bind to the κB site of the target A20 gene promoter. This defect could be attributed to incomplete phosphorylation of the p65 subunit of NF-κB (87). Although PI3K has an essential role in TLR3-induced IRF3 activation, it is dispensable for NF-κB activation, as illustrated by the insensitivity of A20 mRNA expression to the PI3K inhibitor LY294002 (87, 88). In contrast, PI3K has been shown to impair NF-κB-dependent proinflammatory signaling by interacting with TRIF and interfering with its ability to channel optimal NF-κB, but not IRF3, transcriptional activity (Fig. 2 and 3) (3). Altogether, this indicates that PI3K biases the TLR3 pathway toward IRF3 and the induction of IFN-stimulated genes while impairing NF-κB-dependent proinflammatory signaling.
FIG. 3.
Endogenous and viral (green) inhibitors of TLR3-mediated NF-κB or IRF3 activation. Most known inhibitors interfere with the function of TRIF, either by interacting with TRIF (PIASy, TRAF1, SARM, A20, TRAF4, and the vaccinia virus protein A46R) or by degrading TRIF (hepatitis C virus protease NS3/4A). Other inhibitors interact with TRAF6 (TRAF4, A20, and vaccinia virus protein A52R), RIP1 (RIP3), TBK1/IKKɛ (A20, SIKE, and SHP-2), or IRF3 (PIASy). See the text for more details.
The phosphorylated tyrosine residues of TLR3 can also be expected to bind SH2 domain-containing proteins other than PI3K. With respect to this, the tyrosine kinase c-Src was recently shown to bind TLR3 and to be necessary for the TLR3-induced activation of IRF3 through a TRIF-dependent mechanism (52).
In conclusion, to initiate intracellular signaling events, TLR3 utilizes, in addition to its conserved boxes and BB loop, at least two phospho-acceptor tyrosine residues that recruit PI3K, c-Src, and most likely other unidentified signaling molecules. Upon TLR3 engagement, these tyrosine residues are phosphorylated by as-yet-unidentified tyrosine kinases. Whichever route is followed by the phospho-tyrosine signal, it always converges with the “classical” TRIF-dependent signaling pathway, leading to the full activation of IRF3- or NF-κB-dependent gene expression.
Negative Regulation of TLR3 Signaling
In the section on viral pathologies, we already discussed viral immune evasion strategies that target the TLR3 pathway. However, as described above, strong or sustained TLR3 signaling is also potentially harmful or even fatal for the host cell. It is thus not surprising that mammalian cells have also evolved several mechanisms for modulating TLR3-mediated responses (Fig. 3).
We already described above the negative regulatory effect of the binding of PI3K with TRIF on TLR3-induced NF-κB activation. Similarly, the kinase RIP3 specifically inhibits TLR3-induced NF-κB activation by competing with RIP1 for TRIF binding (69). Other endogenous negative regulators that interact with TRIF include protein inhibitor of activated signal transducers and activators of transcription (PIASy), TRAF1, sterile alpha and TIR motif-containing protein (SARM), A20, and TRAF4 (12, 14, 84, 97, 101, 106, 115). However, these proteins inhibit NF-κB as well as IRF3 activation. PIASy is a member of the SUMO-ligase family that also interacts with IRF3 and IRF7. Although this protein inhibits TRIF-induced NF-κB and IRF3 activation, it has no effect on TRIF-induced apoptosis (115). TRAF1 is an inducible protein that binds with the TIR domain of TRIF and is cleaved by a TRIF-activated caspase. Because caspase inhibition or the expression of a noncleavable TRAF1 mutant abolishes the inhibitory effect of TRAF1, it has been suggested that TRIF-induced cleavage of TRAF1 is essential for the inhibition of TRIF signaling (97). The TIR-containing protein SARM also associates with the TIR domain of TRIF and is a broad inhibitor of TRIF-induced cytokine and chemokine production (14). A20 is a deubiquitinating enzyme that is induced by several stimuli, including dsRNA and Sendai virus infection. A20 has been shown to coprecipitate with TRIF and to inhibit TLR3-mediated NF-κB and IRF3 activation. However, its deubiquitinating activity does not seem to be required for the inhibition of TRIF signaling (106). Additionally, A20 has been shown to deubiquitinate RIP1, TRAF6, and IKKγ in the TNF and TLR4 signaling pathway to NF-κB (111), suggesting that these signaling proteins might also be targeted in the TLR3 signaling pathway to NF-κB. Furthermore, A20 also coprecipitates with TBK1 and IKKɛ and inhibits IRF3 phosphorylation and dimerization following the engagement of TLR3 (84). Finally, TRAF4 is another inducible protein that also physically interacts with TRIF and TRAF6 and counteracts their function (101).
In contrast to the above-described inhibitors that inhibit both NF-κB and IRF3 activation, suppressor of IKKɛ (SIKE) interferes uniquely with TLR3-triggered IRF3 activation. Under physiological conditions, SIKE is associated with TBK1 and dissociates upon TLR3 stimulation. The overexpression of SIKE disrupts the interactions of IKKɛ or TBK1 with TRIF and IRF3, without affecting the interactions of TRIF with TRAF6 and RIP1. Consistently, the overexpression of SIKE inhibits virus- and TLR3-triggered IRF3 but not NF-κB activation (44).
Due to the need for phospho-tyrosine residues in the TIR domain of TLR3, one might expect that an alternative way of interfering with TLR3 signaling is dephosphorylation by a tyrosine phosphatase. In this context, SH2-containing protein tyrosine phosphatase 2 (SHP-2) was recently reported to inhibit TLR3-activated IFN-β production. However, this seems to occur by a phosphatase activity-independent mechanism, in which SHP-2 interacts with the kinase domain of TBK1 to inhibit its activity (5).
Although it is astounding how many different proteins and mechanisms have evolved to negatively regulate TLR3 signaling, this complexity also underscores the importance of this process. The diversity of NF-κB and IRF inhibitory proteins may have evolved to establish a redundant system in which one negative-feedback regulator can compensate for the loss or failure of others. Moreover, specific regulatory proteins might change the balance between NF-κB and IRF3 activation. Most likely, the role of specific negative regulatory proteins also depends on the cell type or the cell context. For instance, a restricted expression pattern could confine the effects of the inhibitory proteins to specific organs or cells. Conditional gene-targeting studies of negative regulatory proteins will surely provide the answer to these unresolved questions in the near future.
CLINICAL RELEVANCE
As our understanding of innate immunity and TLR biology has developed, so has an interest in applying that understanding to clinical problems. Vaccine adjuvants are perhaps the most extensively explored applications for TLR agonists. The rational design of specific TLR agonists with reduced toxicity but increased potency, compared to those of adjuvant candidates from only a decade ago, offers the opportunity to meet the stringent safety criteria required for prophylactic vaccines. At present, two improved adult hepatitis B virus vaccines and a papillomavirus vaccine that use TLR4 agonists as the adjuvant have been approved, and there is considerable research and early development activity exploring the adjuvant activities of ligands for most other TLRs. Also, the TLR3 ligand poly(I:C) has already proven to be beneficial as a mucosal adjuvant for influenza virus vaccine in a murine infection model (45). In those studies, coadministration of antigen and poly(I:C) was shown to upregulate the expression of TLR3 and IFN-α/β. Preclinical studies suggest that TLR3 and other TLR agonists also have the potential to enhance therapeutic vaccination for cancer. In this context, immunization with the melanoma peptide trp2 and adjuvants consisting of cationic liposomes complexed with TLR3 and TLR9 agonists has been shown to control the growth of established B16 melanoma tumors in a therapeutic tumor vaccine model (114). Agonists for TLR3 (as well as for TLR7, TLR8, and TLR9) have shown promise as a treatment for viral infections. The synthetic nontoxic poly(I:C) analog poly(I:C12U) (Ampligen) is a mismatched dsRNA helix in which cytosine is replaced by uridine, statistically at each 13th residue (2). It has a rapid half-life compared to that of poly(I:C), which enabled its development as a clinically useful drug. Unlike poly(I:C), poly(I:C12U) is specifically recognized by TLR3 but not MDA5, which might account for its reduced toxicity and safe use in clinical trials in which it has shown anti-human immunodeficiency virus effects. Poly(I:C12U) has been shown to also have various degrees of antiviral activity against hepatitis B virus, several flaviviruses, coxsackie B3 virus, and Punta Toro virus (2, 22, 29). Moreover, a large phase III clinical trial for the treatment of chronic fatigue syndrome with Ampligen has successfully been completed (72).
Sustained TLR3 activation is associated with the overproduction of proinflammatory cytokines and can result in systemic inflammatory response syndrome. In addition, excessive TLR3 expression or triggering is associated with several inflammatory diseases, such as inflammation-associated myopathies, lupus nephritis, West Nile virus-driven CNS inflammation, and viral or autoimmune liver disease (see above for more-detailed information). TLR3 antagonists might therefore be quite promising for a number of infectious and inflammatory diseases. Antagonists for TLR3 and several other TLRs currently under development are structural analogs of agonists that bind the receptor but fail to signal. Other possibilities include anti-TLR antibodies and small-molecule antagonists selected from compound libraries. In addition to direct therapeutic targeting of TLR3 by specific TLR3 antagonists, targeting the intracellular TLR3 signaling molecules is becoming a realistic possibility. This might involve targeting the enzymes that modulate IRF3 or NF-κB activation (e.g., TBK1). Moreover, the insights gained into the regions of signaling proteins involved in protein-protein interactions might allow for the development of specific agents to disrupt these interactions and thereby limit their signaling capacity. Altogether, we can conclude that the manipulation of TLR3 responses harbors therapeutic value for the treatment of a wide range of diseases, including both infectious and autoimmune disorders in which TLR3 has been shown to have a role. Moreover, the list of disease states for which one or more TLRs represent a reasonable target is growing rapidly. This will surely continue to be a productive field for drug development in the future.
CONCLUSIONS AND FUTURE PERSPECTIVES
The past couple of years have witnessed tremendous progress in our understanding of the molecular mechanisms of TLR signaling. Here we have summarized current knowledge regarding the function and regulation of TLR3 as a sentinel for dsRNA-induced responses. Although it is clear that several viruses stimulate TLR3-dependent signaling, the importance of TLR3 signaling in antiviral responses or viral pathogenesis is still far from clear. In many cases, the lack of a clear effect of TLR3 deficiency on the outcome of a viral infection in mice is most likely due to redundancy with other dsRNA sensors, such as RIG-I and MDA5. The use of better and more physiologically relevant virus infection models, as well as the use of other readout systems, might clarify the specific role of each receptor during a viral infection. Moreover, while TLR3 is well known to activate inflammatory responses to viruses, evidence that TLR3 fulfils additional roles in the absence of infection is growing.
Another complication in our current understanding of TLR3 function is that the optimal and physiological ligand for TLR3 is not yet known. Most studies have been done with synthetic poly(I:C), but the identities of the viral RNA sequences that trigger TLR3 are still poorly known and depend on the physiological conditions. In this regard, the potential of endogenous RNA (e.g., from dying cells) in mediating TLR3 signaling and subsequent inflammation or inflammatory disease needs further attention. In addition, the possibility of the existence of other still-unidentified TLR3 ligands different from dsRNA cannot be excluded.
Although our knowledge of TLR3 signaling is already substantial, there are as yet many outstanding questions that need to be addressed. It will be important to clarify the place in the cell from which the TLR3 signals. Although there is ample evidence that TLR3 signals from within endosomes, the identities of these vesicular structures are still unclear. Moreover, in some cell types, TLR3 might also be triggered from the outside of the cell membrane. It is not unlikely that signaling pathways initiated from the cell surface or from an intracellular location are at least partially different. It should also be noted that a large part of our current knowledge on TLR3 signaling and the protein-protein complexes that are involved is still based on overexpression studies. Although knockout mouse studies confirmed the essential role of most of these signaling proteins in TLR3 responses, the exact stoichiometry of the signaling complexes that are formed encourages their characterization at endogenous expression levels.
In conclusion, although the identification of TLR3 and other mammalian TLRs has truly revolutionized the field of microbial pathogenesis and human immunology, we are just beginning to understand the complexities of this evolutionarily conserved system and the essential role that it plays in innate and adaptive immunity. As the basic understanding of microbially induced TLR signaling reaches a critical level, novel therapies that can effectively improve the outcomes of infectious and other inflammatory diseases may arise.
Acknowledgments
Work in our laboratory is supported in part by grants from the Interuniversitaire Attractiepolen (IAP6/18), the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen (FWO; grant 3G010505), and the Geconcerteerde Onderzoeksacties of the University of Ghent (GOA; grant 01G06B6). E.V. was supported as a predoctoral research fellow by the FWO.
REFERENCES
- 1.Abujamra, A. L., R. A. Spanjaard, I. Akinsheye, X. Zhao, D. V. Faller, and S. K. Ghosh. 2006. Leukemia virus long terminal repeat activates NFκB pathway by a TLR3-dependent mechanism. Virology 345:390-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adis, R. D. P. 2004. Mismatched double-stranded RNA: polyI:polyC12U. Drugs R D 5:297-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aksoy, E., W. Vanden Berghe, S. Detienne, Z. Amraoui, K. A. Fitzgerald, G. Haegeman, M. Goldman, and F. Willems. 2005. Inhibition of phosphoinositide 3-kinase enhances TRIF-dependent NF-κB activation and IFN-β synthesis downstream of Toll-like receptor 3 and 4. Eur. J. Immunol. 35:2200-2209. [DOI] [PubMed] [Google Scholar]
- 4.Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413:732-738. [DOI] [PubMed] [Google Scholar]
- 5.An, H., W. Zhao, J. Hou, Y. Zhang, Y. Xie, Y. Zheng, H. Xu, C. Qian, J. Zhou, Y. Yu, S. Liu, G. Feng, and X. Cao. 2006. SHP-2 phosphatase negatively regulates the TRIF adaptor protein-dependent type I interferon and proinflammatory cytokine production. Immunity 25:919-928. [DOI] [PubMed] [Google Scholar]
- 6.Andersen, J. M., D. Al-Khairy, and R. R. Ingalls. 2006. Innate immunity at the mucosal surface: role of Toll-like receptor 3 and Toll-like receptor 9 in cervical epithelial cell responses to microbial pathogens. Biol. Reprod. 74:824-831. [DOI] [PubMed] [Google Scholar]
- 7.Arbibe, L., J. P. Mira, N. Teusch, L. Kline, M. Guha, N. Mackman, P. J. Godowski, R. J. Ulevitch, and U. G. Knaus. 2000. Toll-like receptor 2-mediated NF-κB activation requires a Rac1-dependent pathway. Nat. Immunol. 1:533-540. [DOI] [PubMed] [Google Scholar]
- 8.Ashkar, A. A., X. D. Yao, N. Gill, D. Sajic, A. J. Patrick, and K. L. Rosenthal. 2004. Toll-like receptor (TLR)-3, but not TLR4, agonist protects against genital herpes infection in the absence of inflammation seen with CpG DNA. J. Infect. Dis. 190:1841-1849. [DOI] [PubMed] [Google Scholar]
- 9.Beisner, D. R., I. L. Ch'en, R. V. Kolla, A. Hoffmann, and S. M. Hedrick. 2005. Cutting edge: innate immunity conferred by B cells is regulated by caspase-8. J. Immunol. 175:3469-3473. [DOI] [PubMed] [Google Scholar]
- 10.Bell, J. K., J. Askins, P. R. Hall, D. R. Davies, and D. M. Segal. 2006. The dsRNA binding site of human Toll-like receptor 3. Proc. Natl. Acad. Sci. USA 103:8792-8797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bell, J. K., I. Botos, P. R. Hall, J. Askins, J. Shiloach, D. M. Segal, and D. R. Davies. 2005. The molecular structure of the Toll-like receptor 3 ligand-binding domain. Proc. Natl. Acad. Sci. USA 102:10976-10980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boone, D. L., E. E. Turer, E. G. Lee, R. C. Ahmad, M. T. Wheeler, C. Tsui, P. Hurley, M. Chien, S. Chai, O. Hitotsumatsu, E. McNally, C. Pickart, and A. Ma. 2004. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat. Immunol. 5:1052-1060. [DOI] [PubMed] [Google Scholar]
- 13.Bsibsi, M., C. Persoon-Deen, R. W. Verwer, S. Meeuwsen, R. Ravid, and J. M. Van Noort. 2006. Toll-like receptor 3 on adult human astrocytes triggers production of neuroprotective mediators. Glia 53:688-695. [DOI] [PubMed] [Google Scholar]
- 14.Carty, M., R. Goodbody, M. Schroder, J. Stack, P. N. Moynagh, and A. G. Bowie. 2006. The human adaptor SARM negatively regulates adaptor protein TRIF-dependent Toll-like receptor signaling. Nat. Immunol. 7:1074-1081. [DOI] [PubMed] [Google Scholar]
- 15.Choe, J., M. S. Kelker, and I. A. Wilson. 2005. Crystal structure of human Toll-like receptor 3 (TLR3) ectodomain. Science 309:581-585. [DOI] [PubMed] [Google Scholar]
- 16.Colonna, M. 2006. Toll-like receptors and IFN-α: partners in autoimmunity. J. Clin. Investig. 116:2319-2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cusson-Hermance, N., S. Khurana, T. H. Lee, K. A. Fitzgerald, and M. A. Kelliher. 2005. Rip1 mediates the Trif-dependent Toll-like receptor 3- and 4-induced NF-κB activation but does not contribute to interferon regulatory factor 3 activation. J. Biol. Chem. 280:36560-36566. [DOI] [PubMed] [Google Scholar]
- 18.de Bouteiller, O., E. Merck, U. A. Hasan, S. Hubac, B. Benguigui, G. Trinchieri, E. E. Bates, and C. Caux. 2005. Recognition of double-stranded RNA by human Toll-like receptor 3 and downstream receptor signaling requires multimerization and an acidic pH. J. Biol. Chem. 280:38133-38145. [DOI] [PubMed] [Google Scholar]
- 19.Devendra, D., J. Jasinski, E. Melanitou, M. Nakayama, M. Li, B. Hensley, J. Paronen, H. Moriyama, D. Miao, G. S. Eisenbarth, and E. Liu. 2005. Interferon-α as a mediator of polyinosinic:polycytidylic acid-induced type 1 diabetes. Diabetes 54:2549-2556. [DOI] [PubMed] [Google Scholar]
- 20.Dunne, A., and L. A. O'Neill. 2003. The interleukin-1 receptor/Toll-like receptor superfamily: signal transduction during inflammation and host defense. Sci. STKE 2003:re3. [DOI] [PubMed] [Google Scholar]
- 21.Edelmann, K. H., S. Richardson-Burns, L. Alexopoulou, K. L. Tyler, R. A. Flavell, and M. B. Oldstone. 2004. Does Toll-like receptor 3 play a biological role in virus infections? Virology 322:231-238. [DOI] [PubMed] [Google Scholar]
- 22.Essey, R. J., B. R. McDougall, and W. E. Robinson. 2001. Mismatched double-stranded RNA (polyI-polyC12U) is synergistic with multiple anti-HIV drugs and is active against drug-sensitive and drug-resistant HIV-1 in vitro. Antivir. Res. 51:189-202. [DOI] [PubMed] [Google Scholar]
- 23.Farina, C., M. Krumbholz, T. Giese, G. Hartmann, F. Aloisi, and E. Meinl. 2005. Preferential expression and function of Toll-like receptor 3 in human astrocytes. J. Neuroimmunol. 159:12-19. [DOI] [PubMed] [Google Scholar]
- 24.Funami, K., M. Matsumoto, H. Oshiumi, T. Akazawa, A. Yamamoto, and T. Seya. 2004. The cytoplasmic ‘linker region’ in Toll-like receptor 3 controls receptor localization and signaling. Int. Immunol. 16:1143-1154. [DOI] [PubMed] [Google Scholar]
- 25.Gill, N., P. M. Deacon, B. Lichty, K. L. Mossman, and A. A. Ashkar. 2006. Induction of innate immunity against herpes simplex virus type 2 infection via local delivery of Toll-like receptor ligands correlates with beta interferon production. J. Virol. 80:9943-9950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gitlin, L., W. Barchet, S. Gilfillan, M. Cella, B. Beutler, R. A. Flavell, M. S. Diamond, and M. Colonna. 2006. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc. Natl. Acad. Sci. USA 103:8459-8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gohda, J., T. Matsumura, and J. Inoue. 2004. Cutting edge: TNFR-associated factor (TRAF) 6 is essential for MyD88-dependent pathway but not Toll/IL-1 receptor domain-containing adaptor-inducing IFN-β (TRIF)-dependent pathway in TLR signaling. J. Immunol. 173:2913-2917. [DOI] [PubMed] [Google Scholar]
- 28.Gowen, B. B., J. D. Hoopes, M. H. Wong, K. H. Jung, K. C. Isakson, L. Alexopoulou, R. A. Flavell, and R. W. Sidwell. 2006. TLR3 deletion limits mortality and disease severity due to Phlebovirus infection. J. Immunol. 177:6301-6307. [DOI] [PubMed] [Google Scholar]
- 29.Gowen, B. B., M.-H. Wong, K.-H. Jung, A. B. Sanders, W. M. Mitchell, L. Alexopoulou, R. A. Flavell, and R. W. Sidwell. 2007. TLR3 is essential for the induction of protective immunity against Punta Toro Virus infection by the double-stranded RNA (dsRNA), poly(I:C12U), but not poly(I:C): differential recognition of synthetic dsRNA molecules. J. Immunol. 178:5200-5208. [DOI] [PubMed] [Google Scholar]
- 30.Groskreutz, D. J., M. M. Monick, L. S. Powers, T. O. Yarovinsky, D. C. Look, and G. W. Hunninghake. 2006. Respiratory syncytial virus induces TLR3 protein and protein kinase R, leading to increased double-stranded RNA responsiveness in airway epithelial cells. J. Immunol. 176:1733-1740. [DOI] [PubMed] [Google Scholar]
- 31.Guillot, L., R. Le Goffic, S. Bloch, N. Escriou, S. Akira, M. Chignard, and M. Si-Tahar. 2005. Involvement of Toll-like receptor 3 in the immune response of lung epithelial cells to double-stranded RNA and influenza A virus. J. Biol. Chem. 280:5571-5580. [DOI] [PubMed] [Google Scholar]
- 32.Hacker, H., V. Redecke, B. Blagoev, I. Kratchmarova, L. C. Hsu, G. G. Wang, M. P. Kamps, E. Raz, H. Wagner, G. Hacker, M. Mann, and M. Karin. 2006. Specificity in Toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6. Nature 439:204-207. [DOI] [PubMed] [Google Scholar]
- 33.Haller, O., G. Kochs, and F. Weber. 2006. The interferon response circuit: induction and suppression by pathogenic viruses. Virology 344:119-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Han, K. J., X. Su, L. G. Xu, L. H. Bin, J. Zhang, and H. B. Shu. 2004. Mechanisms of the TRIF-induced interferon-stimulated response element and NF-κB activation and apoptosis pathways. J. Biol. Chem. 279:15652-15661. [DOI] [PubMed] [Google Scholar]
- 35.Hardarson, H. S., J. S. Baker, Z. Yang, E. Purevjav, C. H. Huang, L. Alexopoulou, N. Li, R. A. Flavell, N. E. Bowles, and J. G. Vallejo. 2007. Toll-like receptor 3 is an essential component of the innate stress response in virus-induced cardiac injury. Am. J. Physiol. Heart Circ. Physiol. 292:H251-H258. [DOI] [PubMed] [Google Scholar]
- 36.Harte, M. T., I. R. Haga, G. Maloney, P. Gray, P. C. Reading, N. W. Bartlett, G. L. Smith, A. Bowie, and L. A. O'Neill. 2003. The poxvirus protein A52R targets Toll-like receptor signaling complexes to suppress host defense. J. Exp. Med. 197:343-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heinz, S., V. Haehnel, M. Karaghiosoff, L. Schwarzfischer, M. Muller, S. W. Krause, and M. Rehli. 2003. Species-specific regulation of Toll-like receptor 3 genes in men and mice. J. Biol. Chem. 278:21502-21509. [DOI] [PubMed] [Google Scholar]
- 38.Herbst-Kralovetz, M., and R. Pyles. 2006. Toll-like receptors, innate immunity and HSV pathogenesis. Herpes 13:37-41. [PubMed] [Google Scholar]
- 39.Herbst-Kralovetz, M. M., and R. B. Pyles. 2006. Quantification of poly(I:C)-mediated protection against genital herpes simplex virus type 2 infection. J. Virol. 80:9988-9997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hidaka, F., S. Matsuo, T. Muta, K. Takeshige, T. Mizukami, and H. Nunoi. 2006. A missense mutation of the Toll-like receptor 3 gene in a patient with influenza-associated encephalopathy. Clin. Immunol. 119:188-194. [DOI] [PubMed] [Google Scholar]
- 41.Hoebe, K., X. Du, P. Georgel, E. Janssen, K. Tabeta, S. O. Kim, J. Goode, P. Lin, N. Mann, S. Mudd, K. Crozat, S. Sovath, J. Han, and B. Beutler. 2003. Identification of Lps2 as a key transducer of MyD88-independent TIR signalling. Nature 424:743-748. [DOI] [PubMed] [Google Scholar]
- 42.Hoffman, R. W., T. Gazitt, M. F. Foecking, R. A. Ortmann, M. Misfeldt, R. Jorgenson, S. L. Young, and E. L. Greidinger. 2004. U1 RNA induces innate immunity signaling. Arthritis Rheum. 50:2891-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hornung, V., J. Ellegast, S. Kim, K. Brzozka, A. Jung, H. Kato, H. Poeck, S. Akira, K. K. Conzelmann, M. Schlee, S. Endres, and G. Hartmann. 2006. 5′-Triphosphate RNA is the ligand for RIG-I. Science 314:994-997. [DOI] [PubMed] [Google Scholar]
- 44.Huang, J., T. Liu, L. G. Xu, D. Chen, Z. Zhai, and H. B. Shu. 2005. SIKE is an IKKɛ/TBK1-associated suppressor of TLR3- and virus-triggered IRF-3 activation pathways. EMBO J. 24:4018-4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ichinohe, T., I. Watanabe, S. Ito, H. Fujii, M. Moriyama, S.-I. Tamura, H. Takahashi, H. Sawa, J. Chiba, T. Kurata, T. Sata, and H. Hasegawa. 2005. Synthetic double-stranded RNA poly(I:C) combined with mucosal vaccine protects against influenza virus infection. J. Virol. 79:2910-2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Imtiyaz, H. Z., S. Rosenberg, Y. Zhang, Z. S. Rahman, Y. J. Hou, T. Manser, and J. Zhang. 2006. The Fas-associated death domain protein is required in apoptosis and TLR-induced proliferative responses in B cells. J. Immunol. 176:6852-6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ivison, S. M., M. A. Khan, N. R. Graham, C. Q. Bernales, A. Kaleem, C. O. Tirling, A. Cherkasov, and T. S. Steiner. 2007. A phosphorylation site in the Toll-like receptor 5 TIR domain is required for inflammatory signalling in response to flagellin. Biochem. Biophys. Res. Commun. 352:936-941. [DOI] [PubMed] [Google Scholar]
- 48.Jack, C. S., N. Arbour, J. Manusow, V. Montgrain, M. Blain, E. McCrea, A. Shapiro, and J. P. Antel. 2005. TLR signaling tailors innate immune responses in human microglia and astrocytes. J. Immunol. 175:4320-4330. [DOI] [PubMed] [Google Scholar]
- 49.Jackson, A. C., J. P. Rossiter, and M. Lafon. 2006. Expression of Toll-like receptor 3 in the human cerebellar cortex in rabies, herpes simplex encephalitis, and other neurological diseases. J. Neurovirol. 12:229-234. [DOI] [PubMed] [Google Scholar]
- 50.Jacobs, B. L., and J. O. Langland. 1996. When two strands are better than one: the mediators and modulators of the cellular responses to double-stranded RNA. Virology 219:339-349. [DOI] [PubMed] [Google Scholar]
- 51.Jiang, W., R. Sun, H. Wei, and Z. Tian. 2005. Toll-like receptor 3 ligand attenuates LPS-induced liver injury by down-regulation of toll-like receptor 4 expression on macrophages. Proc. Natl. Acad. Sci. USA 102:17077-17082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnsen, I. B., T. T. Nguyen, M. Ringdal, A. M. Tryggestad, O. Bakke, E. Lien, T. Espevik, and M. W. Anthonsen. 2006. Toll-like receptor 3 associates with c-Src tyrosine kinase on endosomes to initiate antiviral signaling. EMBO J. 25:3335-3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaiser, W. J., and M. K. Offermann. 2005. Apoptosis induced by the Toll-like receptor adaptor TRIF is dependent on its receptor interacting protein homotypic interaction motif. J. Immunol. 174:4942-4952. [DOI] [PubMed] [Google Scholar]
- 54.Kariko, K., M. Buckstein, H. Ni, and D. Weissman. 2005. Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity 23:165-175. [DOI] [PubMed] [Google Scholar]
- 55.Kariko, K., H. Ni, J. Capodici, M. Lamphier, and D. Weissman. 2004. mRNA is an endogenous ligand for Toll-like receptor 3. J. Biol. Chem. 279:12542-12550. [DOI] [PubMed] [Google Scholar]
- 56.Kato, N. 2001. Molecular virology of hepatitis C virus. Acta Med. Okayama 55:133-159. [DOI] [PubMed] [Google Scholar]
- 57.Lafon, M., F. Megret, M. Lafage, and C. Prehaud. 2006. The innate immune facet of brain: human neurons express TLR-3 and sense viral dsRNA. J. Mol. Neurosci. 29:185-194. [DOI] [PubMed] [Google Scholar]
- 58.Lang, K. S., P. Georgiev, M. Recher, A. A. Navarini, A. Bergthaler, M. Heikenwalder, N. L. Harris, T. Junt, B. Odermatt, P. A. Clavien, H. Pircher, S. Akira, H. Hengartner, and R. M. Zinkernagel. 2006. Immunoprivileged status of the liver is controlled by Toll-like receptor 3 signaling. J. Clin. Investig. 116:2456-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Le Bon, A., N. Etchart, C. Rossmann, M. Ashton, S. Hou, D. Gewert, P. Borrow, and D. F. Tough. 2003. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat. Immunol. 4:1009-1015. [DOI] [PubMed] [Google Scholar]
- 60.Lee, H., E. K. Jo, S. Y. Choi, S. B. Oh, K. Park, J. S. Kim, and S. J. Lee. 2006. Necrotic neuronal cells induce inflammatory Schwann cell activation via TLR2 and TLR3: implication in Wallerian degeneration. Biochem. Biophys. Res. Commun. 350:742-747. [DOI] [PubMed] [Google Scholar]
- 61.Lee, H. K., S. Dunzendorfer, K. Soldau, and P. S. Tobias. 2006. Double-stranded RNA-mediated TLR3 activation is enhanced by CD14. Immunity 24:153-163. [DOI] [PubMed] [Google Scholar]
- 62.Le Goffic, R., V. Balloy, M. Lagranderie, L. Alexopoulou, N. Escriou, R. Flavell, M. Chignard, and M. Si-Tahar. 2006. Detrimental contribution of the Toll-like receptor (TLR)3 to influenza A virus-induced acute pneumonia. PLoS Pathog. 2:e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li, K., E. Foy, J. C. Ferreon, M. Nakamura, A. C. Ferreon, M. Ikeda, S. C. Ray, M. Gale, Jr., and S. M. Lemon. 2005. Immune evasion by hepatitis C virus NS3/4A protease-mediated cleavage of the Toll-like receptor 3 adaptor protein TRIF. Proc. Natl. Acad. Sci. USA 102:2992-2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Matsumoto, M., K. Funami, H. Oshiumi, and T. Seya. 2004. Toll-like receptor 3: a link between toll-like receptor, interferon and viruses. Microbiol. Immunol. 48:147-154. [DOI] [PubMed] [Google Scholar]
- 65.Matsumoto, M., K. Funami, M. Tanabe, H. Oshiumi, M. Shingai, Y. Seto, A. Yamamoto, and T. Seya. 2003. Subcellular localization of Toll-like receptor 3 in human dendritic cells. J. Immunol. 171:3154-3162. [DOI] [PubMed] [Google Scholar]
- 66.Matsumoto, M., S. Kikkawa, M. Kohase, K. Miyake, and T. Seya. 2002. Establishment of a monoclonal antibody against human Toll-like receptor 3 that blocks double-stranded RNA-mediated signaling. Biochem. Biophys. Res. Commun. 293:1364-1369. [DOI] [PubMed] [Google Scholar]
- 67.McBride, S., K. Hoebe, P. Georgel, and E. Janssen. 2006. Cell-associated double-stranded RNA enhances antitumor activity through the production of type I IFN. J. Immunol. 177:6122-6128. [DOI] [PubMed] [Google Scholar]
- 68.McWhirter, S. M., K. A. Fitzgerald, J. Rosains, D. C. Rowe, D. T. Golenbock, and T. Maniatis. 2004. IFN-regulatory factor 3-dependent gene expression is defective in Tbk1-deficient mouse embryonic fibroblasts. Proc. Natl. Acad. Sci. USA 101:233-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Meylan, E., K. Burns, K. Hofmann, V. Blancheteau, F. Martinon, M. Kelliher, and J. Tschopp. 2004. RIP1 is an essential mediator of Toll-like receptor 3-induced NF-κB activation. Nat. Immunol. 5:503-507. [DOI] [PubMed] [Google Scholar]
- 70.Meylan, E., and J. Tschopp. 2006. Toll-like receptors and RNA helicases: two parallel ways to trigger antiviral responses. Mol. Cell 22:561-569. [DOI] [PubMed] [Google Scholar]
- 71.Meylan, E., J. Tschopp, and M. Karin. 2006. Intracellular pattern recognition receptors in the host response. Nature 442:39-44. [DOI] [PubMed] [Google Scholar]
- 72.Mitchell, W. 2006. Review of Ampligen clinical trials in chronic fatigue syndrome. J. Clin. Virol. 37:S113. [Google Scholar]
- 73.Naka, K., H. Dansako, N. Kobayashi, M. Ikeda, and N. Kato. 2006. Hepatitis C virus NS5B delays cell cycle progression by inducing interferon-beta via Toll-like receptor 3 signaling pathway without replicating viral genomes. Virology 346:348-362. [DOI] [PubMed] [Google Scholar]
- 74.Nasu, K., H. Itoh, A. Yuge, M. Nishida, and H. Narahara. 2007. Human oviductal epithelial cells express Toll-like receptor 3 and respond to double-stranded RNA: fallopian tube-specific mucosal immunity against viral infection. Hum. Reprod. 22:356-361. [DOI] [PubMed] [Google Scholar]
- 75.Nishiya, T., E. Kajita, S. Miwa, and A. L. Defranco. 2005. TLR3 and TLR7 are targeted to the same intracellular compartments by distinct regulatory elements. J. Biol. Chem. 280:37107-37117. [DOI] [PubMed] [Google Scholar]
- 76.Oganesyan, G., S. K. Saha, B. Guo, J. Q. He, A. Shahangian, B. Zarnegar, A. Perry, and G. Cheng. 2006. Critical role of TRAF3 in the Toll-like receptor-dependent and -independent antiviral response. Nature 439:208-211. [DOI] [PubMed] [Google Scholar]
- 77.Okahira, S., F. Nishikawa, S. Nishikawa, T. Akazawa, T. Seya, and M. Matsumoto. 2005. Interferon-beta induction through Toll-like receptor 3 depends on double-stranded RNA structure. DNA Cell Biol. 24:614-623. [DOI] [PubMed] [Google Scholar]
- 78.Orinska, Z., E. Bulanova, V. Budagian, M. Metz, M. Maurer, and S. Bulfone-Paus. 2005. TLR3-induced activation of mast cells modulates CD8+ T-cell recruitment. Blood 106:978-987. [DOI] [PubMed] [Google Scholar]
- 79.Oshiumi, H., M. Matsumoto, K. Funami, T. Akazawa, and T. Seya. 2003. TICAM-1, an adaptor molecule that participates in Toll-like receptor 3-mediated interferon-beta induction. Nat. Immunol. 4:161-167. [DOI] [PubMed] [Google Scholar]
- 80.Patole, P. S., H. J. Grone, S. Segerer, R. Ciubar, E. Belemezova, A. Henger, M. Kretzler, D. Schlondorff, and H. J. Anders. 2005. Viral double-stranded RNA aggravates lupus nephritis through Toll-like receptor 3 on glomerular mesangial cells and antigen-presenting cells. J. Am. Soc. Nephrol. 16:1326-1338. [DOI] [PubMed] [Google Scholar]
- 81.Préhaud, C., F. Mégret, M. Lafage, and M. Lafon. 2005. Virus infection switches TLR-3-positive human neurons to become strong producers of beta interferon. J. Virol. 79:12893-12904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ruckdeschel, K., G. Pfaffinger, R. Haase, A. Sing, H. Weighardt, G. Hacker, B. Holzmann, and J. Heesemann. 2004. Signaling of apoptosis through TLRs critically involves Toll/IL-1 receptor domain-containing adapter inducing IFN-β, but not MyD88, in bacteria-infected murine macrophages. J. Immunol. 173:3320-3328. [DOI] [PubMed] [Google Scholar]
- 83.Rudd, B. D., J. J. Smit, R. A. Flavell, L. Alexopoulou, M. A. Schaller, A. Gruber, A. A. Berlin, and N. W. Lukacs. 2006. Deletion of TLR3 alters the pulmonary immune environment and mucus production during respiratory syncytial virus infection. J. Immunol. 176:1937-1942. [DOI] [PubMed] [Google Scholar]
- 84.Saitoh, T., M. Yamamoto, M. Miyagishi, K. Taira, M. Nakanishi, T. Fujita, S. Akira, N. Yamamoto, and S. Yamaoka. 2005. A20 is a negative regulator of IFN regulatory factor 3 signaling. J. Immunol. 174:1507-1512. [DOI] [PubMed] [Google Scholar]
- 85.Sanghavi, S. K., and T. A. Reinhart. 2005. Increased expression of TLR3 in lymph nodes during simian immunodeficiency virus infection: implications for inflammation and immunodeficiency. J. Immunol. 175:5314-5323. [DOI] [PubMed] [Google Scholar]
- 86.Santoro, M. G., A. Rossi, and C. Amici. 2003. NF-κB and virus infection: who controls whom. EMBO J. 22:2552-2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sarkar, S. N., C. P. Elco, K. L. Peters, S. Chattopadhyay, and G. C. Sen. 2007. Two tyrosine residues of Toll-like receptor 3 trigger different steps of NF-κB activation. J. Biol. Chem. 282:3423-3427. [DOI] [PubMed] [Google Scholar]
- 88.Sarkar, S. N., K. L. Peters, C. P. Elco, S. Sakamoto, S. Pal, and G. C. Sen. 2004. Novel roles of TLR3 tyrosine phosphorylation and PI3 kinase in double-stranded RNA signaling. Nat. Struct. Mol. Biol. 11:1060-1067. [DOI] [PubMed] [Google Scholar]
- 89.Sarkar, S. N., H. L. Smith, T. M. Rowe, and G. C. Sen. 2003. Double-stranded RNA signaling by Toll-like receptor 3 requires specific tyrosine residues in its cytoplasmic domain. J. Biol. Chem. 278:4393-4396. [DOI] [PubMed] [Google Scholar]
- 90.Sasai, M., H. Oshiumi, M. Matsumoto, N. Inoue, F. Fujita, M. Nakanishi, and T. Seya. 2005. Cutting edge: NF-κB-activating kinase-associated protein 1 participates in TLR3/Toll-IL-1 homology domain-containing adapter molecule-1-mediated IFN regulatory factor 3 activation. J. Immunol. 174:27-30. [DOI] [PubMed] [Google Scholar]
- 91.Sato, S., M. Sugiyama, M. Yamamoto, Y. Watanabe, T. Kawai, K. Takeda, and S. Akira. 2003. Toll/IL-1 receptor domain-containing adaptor inducing IFN-β (TRIF) associates with TNF receptor-associated factor 6 and TANK-binding kinase 1, and activates two distinct transcription factors, NF-κB and IFN-regulatory factor-3, in the Toll-like receptor signaling. J. Immunol. 171:4304-4310. [DOI] [PubMed] [Google Scholar]
- 92.Schreiner, B., J. Voss, J. Wischhusen, Y. Dombrowski, A. Steinle, H. Lochmuller, M. Dalakas, A. Melms, and H. Wiendl. 2006. Expression of toll-like receptors by human muscle cells in vitro and in vivo: TLR3 is highly expressed in inflammatory and HIV myopathies, mediates IL-8 release and up-regulation of NKG2D-ligands. FASEB J. 20:118-120. [DOI] [PubMed] [Google Scholar]
- 93.Schulz, O., S. S. Diebold, M. Chen, T. I. Naslund, M. A. Nolte, L. Alexopoulou, Y. T. Azuma, R. A. Flavell, P. Liljestrom, and C. Reis e Sousa. 2005. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature 433:887-892. [DOI] [PubMed] [Google Scholar]
- 94.Shim, J. H., C. Xiao, A. E. Paschal, S. T. Bailey, P. Rao, M. S. Hayden, K. Y. Lee, C. Bussey, M. Steckel, N. Tanaka, G. Yamada, S. Akira, K. Matsumoto, and S. Ghosh. 2005. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 19:2668-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.So, E. Y., M. H. Kang, and B. S. Kim. 2006. Induction of chemokine and cytokine genes in astrocytes following infection with Theiler's murine encephalomyelitis virus is mediated by the Toll-like receptor 3. Glia 53:858-867. [DOI] [PubMed] [Google Scholar]
- 96.Stack, J., I. R. Haga, M. Schroder, N. W. Bartlett, G. Maloney, P. C. Reading, K. A. Fitzgerald, G. L. Smith, and A. G. Bowie. 2005. Vaccinia virus protein A46R targets multiple Toll-like-interleukin-1 receptor adaptors and contributes to virulence. J. Exp. Med. 201:1007-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Su, X., S. Li, M. Meng, W. Qian, W. Xie, D. Chen, Z. Zhai, and H. B. Shu. 2006. TNF receptor-associated factor-1 (TRAF1) negatively regulates Toll/IL-1 receptor domain-containing adaptor inducing IFN-β (TRIF)-mediated signaling. Eur. J. Immunol. 36:199-206. [DOI] [PubMed] [Google Scholar]
- 98.Sun, J., K. E. Duffy, C. T. Ranjith-Kumar, J. Xiong, R. J. Lamb, J. Santos, H. Masarapu, M. Cunningham, A. Holzenburg, R. T. Sarisky, M. L. Mbow, and C. Kao. 2006. Structural and functional analyses of the human Toll-like receptor 3: role of glycosylation. J. Biol. Chem. 281:11144-11151. [DOI] [PubMed] [Google Scholar]
- 99.Tabeta, K., P. Georgel, E. Janssen, X. Du, K. Hoebe, K. Crozat, S. Mudd, L. Shamel, S. Sovath, J. Goode, L. Alexopoulou, R. A. Flavell, and B. Beutler. 2004. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc. Natl. Acad. Sci. USA 101:3516-3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tabiasco, J., E. Devevre, N. Rufer, B. Salaun, J. C. Cerottini, D. Speiser, and P. Romero. 2006. Human effector CD8+ T lymphocytes express TLR3 as a functional coreceptor. J. Immunol. 177:8708-8713. [DOI] [PubMed] [Google Scholar]
- 101.Takeshita, F., K. J. Ishii, K. Kobiyama, Y. Kojima, C. Coban, S. Sasaki, N. Ishii, D. M. Klinman, K. Okuda, S. Akira, and K. Suzuki. 2005. TRAF4 acts as a silencer in TLR-mediated signaling through the association with TRAF6 and TRIF. Eur. J. Immunol. 35:2477-2485. [DOI] [PubMed] [Google Scholar]
- 102.Tanabe, M., M. Kurita-Taniguchi, K. Takeuchi, M. Takeda, M. Ayata, H. Ogura, M. Matsumoto, and T. Seya. 2003. Mechanism of up-regulation of human Toll-like receptor 3 secondary to infection of measles virus-attenuated strains. Biochem. Biophys. Res. Commun. 311:39-48. [DOI] [PubMed] [Google Scholar]
- 103.Touil, T., D. Fitzgerald, G. X. Zhang, A. Rostami, and B. Gran. 2006. Cutting edge: TLR3 stimulation suppresses experimental autoimmune encephalomyelitis by inducing endogenous IFN-β. J. Immunol. 177:7505-7509. [DOI] [PubMed] [Google Scholar]
- 104.Wang, J., R. Sun, H. Wei, Z. Dong, B. Gao, and Z. Tian. 2006. Poly I:C prevents T cell-mediated hepatitis via an NK-dependent mechanism. J. Hepatol. 44:446-454. [DOI] [PubMed] [Google Scholar]
- 105.Wang, T., T. Town, L. Alexopoulou, J. F. Anderson, E. Fikrig, and R. A. Flavell. 2004. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat. Med. 10:1366-1373. [DOI] [PubMed] [Google Scholar]
- 106.Wang, Y. Y., L. Li, K. J. Han, Z. Zhai, and H. B. Shu. 2004. A20 is a potent inhibitor of TLR3- and Sendai virus-induced activation of NF-κB and ISRE and IFN-β promoter. FEBS Lett. 576:86-90. [DOI] [PubMed] [Google Scholar]
- 107.Wang, Z., L. Xiang, J. Shao, and Z. Yuan. 2006. The 3′ CCACCA sequence of tRNAAla(UGC) is the motif that is important in inducing Th1-like immune response, and this motif can be recognized by Toll-like receptor 3. Clin. Vaccine Immunol. 13:733-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wen, L., J. Peng, Z. Li, and F. S. Wong. 2004. The effect of innate immunity on autoimmune diabetes and the expression of Toll-like receptors on pancreatic islets. J. Immunol. 172:3173-3180. [DOI] [PubMed] [Google Scholar]
- 109.Wesch, D., S. Beetz, H. H. Oberg, M. Marget, K. Krengel, and D. Kabelitz. 2006. Direct costimulatory effect of TLR3 ligand poly(I:C) on human γδ T lymphocytes. J. Immunol. 176:1348-1354. [DOI] [PubMed] [Google Scholar]
- 110.Wornle, M., H. Schmid, B. Banas, M. Merkle, A. Henger, M. Roeder, S. Blattner, E. Bock, M. Kretzler, H. J. Grone, and D. Schlondorff. 2006. Novel role of Toll-like receptor 3 in hepatitis C-associated glomerulonephritis. Am. J. Pathol. 168:370-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wullaert, A., K. Heyninck, S. Janssens, and R. Beyaert. 2006. Ubiquitin: tool and target for intracellular NF-κB inhibitors. Trends Immunol. 27:533-540. [DOI] [PubMed] [Google Scholar]
- 112.Yamamoto, M., S. Sato, H. Hemmi, K. Hoshino, T. Kaisho, H. Sanjo, O. Takeuchi, M. Sugiyama, M. Okabe, K. Takeda, and S. Akira. 2003. Role of adaptor TRIF in the MyD88-independent Toll-like receptor signaling pathway. Science 301:640-643. [DOI] [PubMed] [Google Scholar]
- 113.Yoneyama, M., and T. Fujita. 2007. Function of RIG-I-like receptors in antiviral innate immunity. J. Biol. Chem. 282:15315-15318. [DOI] [PubMed] [Google Scholar]
- 114.Zaks, K., M. Jordan, A. Guth, K. Sellins, R. Kedl, A. Izzo, C. Bosio, and S. Dow. 2006. Efficient immunization and cross-priming by vaccine adjuvants containing TLR3 or TLR9 agonists complexed to cationic liposomes. J. Immunol. 176:7335-7345. [DOI] [PubMed] [Google Scholar]
- 115.Zhang, J., L. G. Xu, K. J. Han, X. Wei, and H. B. Shu. 2004. PIASy represses TRIF-induced ISRE and NF-κB activation but not apoptosis. FEBS Lett. 570:97-101. [DOI] [PubMed] [Google Scholar]