Abstract
Scedosporium spp. are increasingly recognized as causes of resistant life-threatening infections in immunocompromised patients. Scedosporium spp. also cause a wide spectrum of conditions, including mycetoma, saprobic involvement and colonization of the airways, sinopulmonary infections, extrapulmonary localized infections, and disseminated infections. Invasive scedosporium infections are also associated with central nervous infection following near-drowning accidents. The most common sites of infection are the lungs, sinuses, bones, joints, eyes, and brain. Scedosporium apiospermum and Scedosporium prolificans are the two principal medically important species of this genus. Pseudallescheria boydii, the teleomorph of S. apiospermum, is recognized by the presence of cleistothecia. Recent advances in molecular taxonomy have advanced the understanding of the genus Scedosporium and have demonstrated a wider range of species than heretofore recognized. Studies of the pathogenesis of and immune response to Scedosporium spp. underscore the importance of innate host defenses in protection against these organisms. Microbiological diagnosis of Scedosporium spp. currently depends upon culture and morphological characterization. Molecular tools for clinical microbiological detection of Scedosporium spp. are currently investigational. Infections caused by S. apiospermum and P. boydii in patients and animals may respond to antifungal triazoles. By comparison, infections caused by S. prolificans seldom respond to medical therapy alone. Surgery and reversal of immunosuppression may be the only effective therapeutic options for infections caused by S. prolificans.
INTRODUCTION
The genus Scedosporium consists of two medically important species: Scedosporium apiospermum (and its teleomorph or sexual state Pseudallescheria boydii) and Scedosporium prolificans (formerly S. inflatum). S. apiospermum/ P. boydii and S. prolificans are ubiquitous filamentous fungi present in soil, sewage, and polluted waters. Scedosporiosis represents a broad spectrum of clinical diseases caused by the agents of the genus Scedosporium. These fungi can be colonizers of previously damaged bronchopulmonary trees (as in old pulmonary tuberculosis cases, cystic fibrosis, or bronchiectatic lungs of any etiology). Infections caused by these organisms can be localized, extend to the surrounding tissues (deep extension), or disseminate (hematogenously) to distant organs. The range of diseases caused by these fungi is broad, ranging from transient colonization of the respiratory tract to saprophytic involvement of abnormal airways, allergic bronchopulmonary reaction, invasive localized disease, and at times disseminated disease. These infections include skin and soft tissue infections with extension to tendons, ligaments, and bone (mycetoma); septic arthritis; osteomyelitis; lymphocutaneous syndrome; pneumonia; endocarditis; peritonitis; meningoencephalitis; meningitis; brain abscess; parotitis; thyroid abscess; otomycosis; sinusitis; keratitis; chorioretinitis; and endophthalmitis. The disseminated form of the disease is mostly seen among immunocompromised patients; however, even in immunocompetent individuals, cases of disseminated disease have been reported. In patients suffering near-drowning events in particular, P. boydii/S. apiospermum should be considered in the differential diagnosis as potential causes of infections, especially if pneumonia or brain abscess ensues. Treatment of scedosporium infections is especially challenging because of their resistance to many antifungal agents.
DEFINITIONS
A number of definitions are introduced in this section to familiarize the reader with the relevant terminology. These definitions are a consensus adapted from multiple authoritative sources (92, 278).
Aleuroconidium (plural, aleuroconidia).
A thallic conidium that develops as an expanded end of an undifferentiated hypha or on a short pedicel and is released by rupture of the supporting cell. This term is not recommended for describing conidia because it has been applied to a number of different structures.
Anamorph.
The asexual form of the fungus that is recognized based only on its anatomic morphology, also applied to asexually reproducing structures.
Annellation.
The formation of ring-like structures at the conidiogenous end of a conidiophore.
Annellide.
A conidiogenous cell that produces conidia in a basipetal way. The apex of an annellide becomes longer and narrower as each subsequent conidium is formed and released. An apical ring composed of outer cell wall remains as each conidium is released.
Annelloconidium.
A conidium formed by an annellide.
Ascospore.
A haploid sexual spore formed in an ascus following meiosis.
Ascus (plural, asci).
A sac-like cell in which the ascospores are formed. Asci are characteristic of ascomycetes.
Basipetal.
The youngest conidium is at the base of a chain.
Chlamydospore.
A holothallic conidium with a thickened cell wall that may be terminal or intercalary and serves the function of survival.
Cleistothecium (plural, cleistothecia).
An enclosed fruiting body that contains randomly dispersed asci.
Conidiogenous cell.
A cell that produces conidia.
Conidiophore.
A specialized hypha on which conidia are formed singly or in clusters.
Conidium (plural, conidia).
A nonmotile deciduous propagule resulting from fungal mitosis; may be unicellular (microconidium) or multicellular (macroconidium).
Coremium (plural, coremia).
See synnema.
Graphium.
Anamorph of ascomycetes that is characterized by having as its fruiting structure a synnema.
Holomorph.
Whole fungus, that is, the anamorph(s) plus the teleomorph state of the fungus.
Holothallic.
Involving all the cell wall layers of the conidiogenous or sporogenous cell in thallic development.
Homothallic.
Sexual reproduction can take place with one thallus.
Intercalary.
Occurring within a hypha.
Ostiolate.
Having an ostiole.
Ostiole.
An opening through which spores or conidia can escape.
Phenetic.
A method in biology that groups or classifies species according to their observable characteristics, or phenotype. No relevance is given to evolution (in contrast with the phylogenetic principle).
Plectenchyma.
A thick tissue formed by hyphae becoming twisted and fused together. Fungal plectenchyma may become very complex and appear almost like true tissues (parenchyma).
Propagule.
A reproductive unit that gives rise to an organism.
Saprobe (or saprotroph).
An organism that obtains its nutrients by absorption of soluble organic compounds from nonliving or decaying organic matter, plant or animal. The term “saprobic” is used throughout this review instead of saprophytic, which is an older term that is now considered obsolete. A saprobe or saprotroph obtains its nutrients from nonviable or decaying organic matter through absorption of soluble molecules. The suffix “phyte” of the former term “saprophytic” means “plant”. As fungi belong to their own kingdom and not to the kingdom Plantae, the term “saprobic” is more appropriate. An alternative term, “saprotrophic,” was recently introduced to replace “saprophytic” (469). However, as the term “saprobic” is better established, this review will refer to the “saprobic” state to replace “saprophytic” state.
Saprobic.
Related to saprobe or saprothroph.
Scedosporium.
Anamorph of an ascomycete (Microascaceae) that does not have a synnema as the reproductive structure.
Sclerotium (plural, sclerotia).
An organized mass of hyphae that remains dormant during unfavorable conditions (also called “grain”).
Synnema (plural, synnemata).
Erect macroscopic structure formed by fused conidiophores that bear conidia terminally, laterally, or in both ways, sometimes forming the appearance of a “sheath of wheat”.
Taxonomy.
Systematic classification of organisms.
Teleomorph.
A form based on a sexual state; also can be applied to sexually reproducing structures.
Thallic.
Involving a conidium, in which the young conidium initially does not begin to develop until after it has been delimited by a septum. The conidium originates from the entire parent cell.
Thallus (plural, thalli).
The vegetative growth of a fungus.
Thermophillic.
Molds that require high temperatures (40°C or higher) to grow and sporulate.
HISTORY
An understanding of the history of P. boydii begins with a discussion of the history of mycetoma. Mycetoma was first described in ancient Sanskrit writings, although it was caused by other fungi, such as Madurella mycetomatis, Madurella grisea, Exophiala jeanselmi, P. boydii, etc., in India. In the Atharva Veda there is mention of mycetoma as “pada valmikan,” meaning anthill foot, which was differentiated, from “Sliptham” or elephant foot, a filarial disease. By late 17th century, Engelbert Kaempfer, a German physician in southern India, described examples of the disease (5). In the 18th century, French missionaries in Pondicherri, India, recorded the disease. In 1842, while working at a Madura dispensary in southern India, John Gill made some vague descriptions of mycetoma (148). However, Godfrey, in Madras (in southern India), recorded the earliest description of mycetoma in 1846. Godfrey reported a collection of four cases (seen between 1844 and 1845), concluding that this was a new entity, and named it “morbus tuberculosis pedis” (159). In 1859 one of his colleagues, Eyre, described 40 patients treated between 1844 and 1848 (118).
The infection was usually caused by a transcutaneous trauma, and the disease usually affected the feet of barefooted native workers and was distinctively characterized by progressive swelling, multiple fistulas, and draining sinuses. The pus that drained from the sinuses displayed various colored grains with different consistencies, from soft to hard. In 1855, Ballingali described two cases of bone destruction associated with Madura foot (24). In 1860, Carter introduced the term “mycetoma” and referred to the causative fungal grains as “fungus particles” (70). By then, there were a number of names for the disease; however, “Madura foot” was the term most commonly used (72). In 1874, Carter produced a monograph on mycetoma, entitled “On Mycetoma or the Fungus Disease of India.” In this work Carter gave a full account of his early case records as well as of the work of his contemporaries on mycetoma, where he provided detailed, illustrated descriptions of the disease, emphasizing its fungal nature (71). Laveran described Streptothrix mycetomi as the agent of black-grain mycetoma in 1902 (5). In 1905, Brumpt reclassified Streptothrix mycetomatis in the genus Madurella (= Madurella mycetomatis). Brumpt also stressed that mycetoma was a clinical syndrome and that several fungi were therefore capable of eliciting the same clinical response. Brumpt also described Indiella spp. (= Streptomyces somaliensis) as the cause of white-grain mycetoma (55). In 1909, Tarozzi reported a rapidly growing hyphomycete isolated from a white-grain mycetoma (437).
In the following years, various fungi as well as actinomycetales were isolated from the different-colored grains contained in the drained pus from patients with Madura foot. In 1911, Radaeli isolated a fungus from the white-grain mycetoma in a butcher in Ibono, Sardinia, and Saccardo finally named it Monosporium apiospermum (now, P. boydii, teleomorph name) (371). In 1913 Pinoy reported the results of his studies on actinomycetoma agents and suggested a classification of the disease in two categories based on the causative agents, actinomycetes or eumycetes (338). Subsequently, in 1916, Chalmers and Archibald redefined the disease once again (77a). In 1962, Lavalle used the terms “actinomycosis” and “maduramycosis” to differentiate between the disease caused by actinomycetes or eumycetes (237a).
In 1973, Mahgoub and Murray published a book entitled Mycetoma (267), in which the history of this fascinating disease was compiled. To this day this book is still considered a valuable resource on the subject. In 1977, the British Medical Research Council changed the name of Madurella mycetomi to Madurella mycetomatis. For recent comprehensive reviews on the subject, refer to the articles by Fahal (119) and Lichon and Khachemoune (249). Thus, P. boydii was first described in human disease as one of the agents of mycetoma.
The history of human disease caused by Scedosporium is as remote as that of disease caused by P. boydii, which was first discovered in 1889 as the cause of a human otitis (171). Saccardo later identified the scedosporium anamorph of the fungus in a case of human mycetoma (371). Ever since, the clinical disease attributed to the fungus has expanded, from mainly subcutaneous infection in apparently immunocompetent hosts to the early 1980s, when the disease started to be seen among the increasing population of immunocompromised patients (171).
While in 1982 Fisher et al. first described scedosporium causing the near-drowning syndrome (131), it was Berenguer et al. who in 1989 pointed to the neurotropic nature of the fungus (34). Creitz and Harris (84) noted the saprobic involvement of previously diseased lungs in a report in 1955; however, the clinical significance of scedosporium in lungs of patients with cystic fibrosis was reported only in 2000 (81).
In the medical mycology literature, clinical diseases have been named after previous synonyms of the fungus. The variety of names includes allescheriasis (22), monosporiosis (491), petriellidiosis (452), pseudallescherioma (380), pseudallescheriosis (212), pseudallescheriasis (356), and scedosporiosis (392). Guarro et al. (171) have underscored the problems associated with naming disease entities after the fungus, as follows. (i) The nomenclature of the fungus has changed several times as a direct consequence of development in taxonomy. In particular for S. apiospermum, which has genetic heterogeneity, subclassification of the species is expected. (ii) Polymorphism of Scedosporium spp. and expression of alternate synanamorphs with different names have given different names to the same clinical entity, making the medical literature even more confusing. (iii) Because scedosporium infections are caused by opportunistic pathogens, their clinical presentations are dependent on host immune status, and thus very different clinical entities will have the same disease name (171).
TAXONOMY OF GENUS SCEDOSPORIUM
The genus Scedosporium includes S. apiospermum/P. boydii and S. prolificans. The agent of pseudallescheriasis is Pseudallescheria boydii (formerly Petriellidium boydii and Allescheria boydii). The anamorph (asexual state) of Pseudallescheria boydii is Scedosporium apiospermum (formerly Monosporium apiospermum). Some reports of pseudallescheriasis have attributed the disease to S. apiospermum, the anamorph name. Many isolates of Pseudallescheria boydii do not form cleistothecia, the characteristic sexual structures, unless grown on special media such as cornmeal agar or potato dextrose agar.
Other media used by most clinical laboratories, such as Sabouraud agar, brain heart infusion agar, and blood agar, promote growth of the fungus; however, these media may not support formation of the Pseudallescheria state. “Perfect” fungi are those that can reproduce by sexual and asexual reproduction. As the perfect form, P. boydii is a homothallic fungus, where cleistothecia are expected to form by a single culture unless the fungus had lost the ability to undergo the sexual life cycle. By convention, the name of the teleomorph, “P. boydii,” has priority over the name of the anamorph, “S. apiospermum.” Scedosporium prolificans is an imperfect fungus because there is no known sexual state or teleomorph (230).
The taxonomy of this genus is rather complex (Table 1) and has changed since the early 1910s, when the first isolate of the genus was described. Saccardo isolated a new fungus from a patient with mycetoma in Italy in 1911. He called it Monosporium apiospermum (371). The isolate developed only the asexual state and was classified as a deuteromycete. Years later, Saccardo suggested the name Scedosporium for this fungus; however, he did not describe the fungus or formally propose it as a new genus. Although by 1919, Castellani and Chalmers (72a) validated the name “Scedosporium” and made the new combination S. apiospermum, this term was not widely accepted by mycologists for many years. In 1922, Shear described the life cycle of a new ascomycete, Allescheria boydii, isolated from a mycetoma patient in Texas (397). Boyd and Crutchfield examined a mycetoma of the foot and isolated the same fungus (54). Shear described the fungus as an ascomycetous teleomorph with simple and coremial conidiophores in 1922 (397).
TABLE 1.
Comparison of taxonomic features of Pseudallescheria boydii and Scedosporium prolificans
| Taxonomists (yr) | Kingdom | Phylum | Class | Order | Family | Genus | Species | Synonymy |
|---|---|---|---|---|---|---|---|---|
| Saccardo (1911), Castellani and Chalmers (1919) | Fungi | Ascomycota | Euascomycetes | Microascales | Microascaceae | Scedosporium | Scedosporium apiospermum (anamorph of P. boydii) | Monosporium apiospermum (Saccardo 1911), Monosporium sclerotiale (Pepere 1914), Indiella americana (Delamere et Gatti 1929), Acremoniella lutzi (Leao et Lobo 1940), Polycytella hominis (Borman 2006) |
| Negroni and Fischer (1943); McGinnis, Padhye, and Ajello (1981) | Fungi | Ascomycota | Euascomycetes | Microascales | Microascaceae | Pseudallescheria | Pseudallescheria boydii (teleomorph of S. apiospermum) | Allescheria boydii (Shear 1922), Pseudallescheria sheari (Negroni et Fischer 1943), Petriellidium malloch (McGinnis 1970), Petriellidium boydii [(Shear) Malloch, 1970]; from http://www.Mycobank.org/MycoTaxo.aspx, Allescheria boydii (Shear), Glenospora graphii (Vuillemin), Petriellidium boydii (Shear) Malloch, Pseudallescheria shearii (Negroni et Fischer), Raffaelea castellanii (Pinoy in Castellani) de Hoog, Sporocybe chartoikoon (Beyerinck) |
| Malloch and Salkin (1984), Gueho and de Hoog (1991) | Fungi | Ascomycota | Euascomycetes | Microascales | Microascaceae | Microascaceae | Scedosporium prolificans | Scedosporium inflatum (Malloch et Salkin 1984) |
The isolate Allescheria boydii was a homothallic fungus that produced brown cleistothecia, annelloconidia, and conidiophores that were single or in coremia (synnema, or conidiophores fused in parallel). Monosporium apiospermum and Allescheria boydii were considered different causative agents of mycetoma until 1944, when Emmons demonstrated that one fungus was the anamorph of the other species (113). The nomenclature for the anamorph and teleomorph of this fungus has undergone several changes over time. In 1970, Malloch reclassified the teleomorph as Petriellidium boydii (269), and later the genus Petriellidium was recognized to be a synonym of the genus Pseudallescheria (269). Hughes considered Monosporium apiospermum“a nomem illegitimum” (198), and Scedosporium apiospermum (Saccardo, Castellani et Chalmers) is now accepted as the correct name for the anamorph of P. boydii.
Currently P. boydii is recognized as one of the medically important opportunistic fungi causing life-threatening infections in immunosuppressed patients. Its clinical relevance (other than as cause of mycetoma) was not recognized until 1948, when Benham and George reported a case of meningitis caused by P. boydii in an otherwise immunocompetent individual (32). Subsequently, Creitz and Harris reported the first case of pulmonary infection resulting from P. boydii (84). That same year, Drouhet reported another case of P. boydii pneumonia (107). Subsequently, numerous case reports of colonization or infection involving various organs were described in the literature. In 1984, Malloch and Salkin described a new species of Scedosporium (S. inflatum) (270) isolated from a bone biopsy specimen from an immunocompetent child. In 1991, Gueho and De Hoog (172) suggested synonomy between S. inflatum and Lomentospora prolificans on the basis of their similar morphological and molecular characteristics. Subsequently, Lennon et al. investigated the ribosomal DNA internal transcribed spacers (ITS), i.e., ITS1 and ITS2, of several isolates of Scedosporium inflatum and Lomentospora prolificans (243). Identical ITS restriction fragment length polymorphisms were found in eight isolates of S. inflatum and L. prolificans. These findings resulted in the proposal to combine S. inflatum and L. prolificans into the new binomial Scedosporium prolificans, the currently accepted term (243).
MOLECULAR PHYLOGENY, BIODIVERSITY, AND RECENTLY DESCRIBED NEW SPECIES
Pseudallescheria is a genus of the ascomycete order Microascales. Genera producing ascomata with preformed openings (perithecia) and without openings (cleisthothecia) were shown to belong to a single family, the Microascaceae, within the Microascales. Petriella produces ascomata with preformed openings, which therefore should be considered perithecia. However, true perithecia have an ordered centrum with asci in star-like arrangement, which is not the case in Petriella (171). Von Arx considers Petriella and Pseudallescheria to be closely related and hypothesized that in Microascaceae fruiting bodies with or without an ostiole could be influenced by the growth conditions (454). Using the ribosomal ITS1 and ITS2 regions to analyze the molecular phylogeny of multiple isolates selected to represent the molecular diversity of Pseudallescheria boydii and its close relatives, Rainer and de Hoog recognized two major groups that match the teleomorph genera Petriella and Pseudallescheria, whereas Scedosporium prolificans represents a completely separate entity (349). These findings confirm the generic phylogeny based on the large-subunit rRNA and the small-subunit rRNA gene sequences (205, 206). The teleomorph genera Petriella and Pseudallescheria are also phenetically different by having ostiolate versus nonostiolate ascomata. Therefore, the family Microascaceae comprises ostiolate as well as nonostiolate members. These authors hypothesize a secondary loss of the ostiole in Pseudallescheria spp. due to its ecological preference for moist, poorly aereated habitats (sediments of polluted ditches, manure, and sewage), where the release of ascospores might be more efficient by deliquescence of the ascoma (cleistothecium) wall after maturation within the fruiting body. By comparison, Petriella spp. have primarily been found growing superficially on dung and on dead plant materials. The production of a mass of slimy ascospores extending on a neck and their exposure for further spread (via arthropod vectors) is likely the most efficient strategy. Therefore, the combination of sequence data and ecological characteristics supports the suggestion that the production of an ostiolum in Microascaceae is determined phylogenetically and is not the mere result of inadequate growth conditions (349). Thus, Scedosporium prolificans has been demonstrated to be a different species with a relation to, but distinct from, the genus Petriella.
Until recently the genus Pseudallescheria was considered to be composed of the following seven species: Pseudallescheria africana, P. angusta, P. boydii, P. desertorum, P. ellipsoidea, P. fimeti, and P. fusoidea. All of the species are morphologically similar, and the main distinction among them is based on the size of the cleistothecia and ascospores (454). Gueho and de Hoog described three specific ecological and clinical groups (172). Rainer et al., using restriction fragment length polymorphism analysis of the 18S ribosomal DNA (also known as the small subunit) sequences within P. boydii, found large infraspecific variability within the P. boydii species; however, P. angusta, P. ellipsoidea, and P. fusoidea were likely synonyms of P. boydii (350). McGinnis et al. already had made this observation on the basis of morphological studies (279). Morphological as well as molecular studies have been performed on numerous strains of clinical and environmental origins and from different countries to establish the basis of such variation (147). Analysis of the partial DNA sequences of four loci, i.e., the β-tubulin gene (two loci), the calmodulin gene (one locus), and the ITS region (one locus), demonstrate that P. boydii is a species complex (147). The combined analysis of the four loci from 60 different strains demonstrated the existence of 44 haplotypes within the group. It was possible to clearly differentiate from the P. boydii sensu stricto the three morphologically related species that were previously considered synonyms of P. boydii: P. angusta, P. ellipsoidea, and P. fusoidea. P. boydii was considered the only pathogenic species of the genus Pseudallescheria until recently; however, the study by Gilgado et al. demonstrated that other phylogenetic species of the P. boydii complex are also clinical isolates (147). That study reported the results of a combination of phenotypic and phylogenetic studies of numerous environmental and clinical isolates. In the same study, the species Pseudallescheria minutispora, named in reference to the small size of the ascospores (i.e., 5 to 7 by 3 to 4 μm) and Scedosporium aurantiacum, named in reference to the yellow coloration of the diffusible pigment of the colonies, were clearly different phylogenetically and therefore were proposed as two new species. All the strains included in S. aurantiacum species have a clinical origin, whereas those included in the P. minutispora species have an environmental origin (147). The natural habitat of P. boydii is unknown; however, the fact that this fungus has emerged and has successfully adapted to the human-dominated environment suggests competition among genotypes for survival of the fit to the new environment. The phenomenon is reflected in the predominance of strains of clinical significance (172) that also may have a higher degree of thermotolerance (349). The phylogenetic position of P. africana is still unclear, because it falls in the Petriella clade on the basis of large-subunit sequence but in the Pseudallescheria branch in the ITS tree. Based on its morphology with nonostiolate ascomata, it should be considered to belong to the genus Pseudallescheria. Based on the ITS tree and large-subunit sequence, P. fimeti is rather removed from pseudallescheria. P. desertorum is found in the Pseudallescheria clade; however, its relatedness is more remote, with a bootstrap value of below 50 by ITS tree (349).
Using PCR amplification and sequencing of two separate regions of the nuclear ribosomal repeat region, Borman et al. have shown that Polycytella hominis is genetically indistinguishable from Scedosporium apiospermum. Moreover, to further complicate this already intricate taxonomy, those authors believe that Polycytella hominis is a mutant of S. apiospermum showing abnormal sporulation and therefore suggest that Polycytella hominis should be regarded as a synonym of Scedosporium apiospermum (48). Therefore, the current line of thought is that P. boydii is a species complex with considerable variability; however, it is distinguishable from the genus Petriella. Scedosporium apiospermum is the anamorph of P. boydii, and S. prolificans is a totally different species within the genus.
GROWTH CHARACTERISTICS AND MICROBIOLOGY
Pseudallescheria boydii/Scedosporium apiospermum
Macroscopic features.
Colonies of P. boydii grow rapidly at 25°C on Sabouraud glucose agar. However, the fungus can tolerate growing at 37°C and even 42°C. The fungus can grow in low oxygen tension and even in strict anaerobism. The fungus can assimilate urea, asparagine, potassium nitrate, and ammonium nitrate. Most isolates tolerate magnesium chloride (5%) better than sodium chloride. Species are proteolytic and amylolytic. Glucose but not lactose or maltose is assimilated (93). However, studies of carbohydrate nutrition and sporulation of P. boydii/S. apiospermum previously had shown that these organisms are capable of assimilating mannitol, maltose, and lactose and grow in media containing up to 8 mg/ml of cycloheximide (actidione) (75, 76).
P. boydii produces floccose colonies that look different from the obverse (upper surface) and from the reverse. From the obverse, the color is initially white and later becomes dark gray or smoky brown. From the reverse, it is pale with brownish black zones (94, 237, 418, 427). Although the cultures are darkly colored due to pigments or production of brown conidia, the hyphae are hyaline. The Fontana-Masson staining for melanin also is negative (219). The hyphae also are hyaline in histopathological sections, and the grains produced in cases of mycetoma are white. The colonies become lighter in color during maintenance on agar media. If maintained for years, the cultures eventually turn a dirty white color and the colony acquires a low cottony, fur-like surface, having lost any conidial production.
There are various interpretations in the medical mycology literature as to whether Scedosporium spp. are hyaline or dematiaceous (pigmented) molds. Favoring the interpretation of a hyaline mold is the absence of discernible pigment in the hyphae of Scedosporium spp. by histological staining and the appearance of nonpigmented grains in cases of scedosporium mycetoma. Favoring interpretation of a dematiaceous mold is the presence of the diffusible melanin-like pigment observed on colonial morphology. Closer examination of this pigment reveals the pigmented conidia as the likely source of the diffusible melanin-like pigment.
Microscopic features.
The microscopic features of P. boydii and S. prolificans are well described in detail in several key sources (94, 171, 237, 418, 427).
(i) Pseudallescheria: the teleomorph.
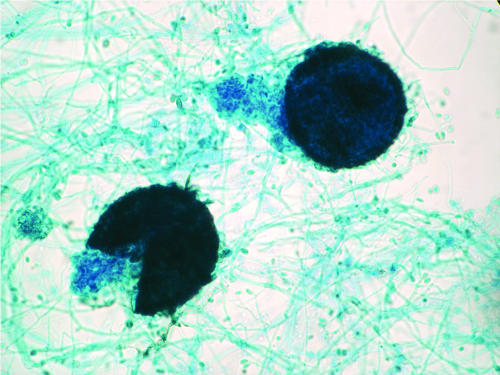
The fungus is homothallic. Many isolates produce brown cleistothecia (100 to 300 μm in diameter) more avidly on nutritionally poor media such as cornmeal, potato dextrose agar, pea agar, potato-carrot agar, or plain water agar. Strains isolated from clinical samples rarely produce the sexual reproductive structures, and an incubation of 2 to 3 weeks is required for formation of cleistothecia. The cleistothecial (ascocarp) formations may be more abundant toward the periphery of the culture plate or at the edge of an agar slant. The formation of cleistothecia is initiated with coiled ascogonia, which develop into mature fruiting bodies within 10 days (Fig. 1). The ascocarp wall is composed of a single layer of thin, flat, polygonal jigsaw-shaped brown cells. At maturation, the cleistothecium bursts and releases the asci, which are filled with ascospores. Asci are subglobose to globose and bear eight ascospores inside. Ascus walls readily dissolve to release the ascospores. Ascospores are unicellular, ovoid to ellipsoidal, smooth, and pale yellow brown to copper. They measure approximately 4 to 5 by 7 to 9 μm, and many of them carry a droplet of oil inside. The presence of an internal oil droplet and absence of a truncated base can help distinguish sexual ascospores from asexually generated conidia. The cleistothecium of Pseudallescheria boydii does not have appendages or ostioles.
FIG. 1.
Pseudallescheria boydii in vitro, depicting a fully developed and ruptured cleistothecium, the hallmark of the sexual stage (teleomorph) of this fungus. Oblong ascospores are liberated in this culture. Magnification, ×100.
(ii) Scedosporium and Graphium: the anamorphs.
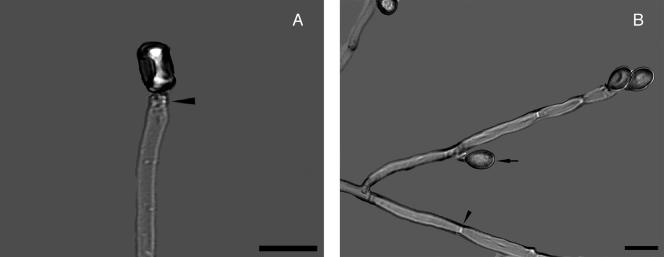
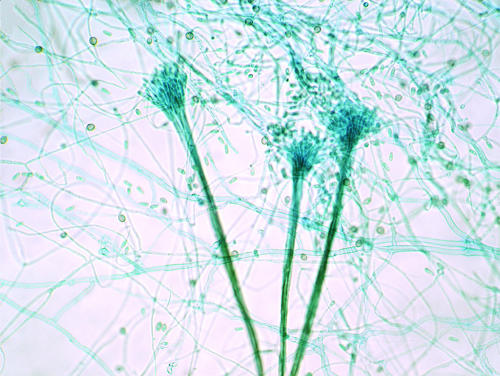
Several types of asexual reproduction are known. A Scedosporium anamorph is almost always present. This type is characterized by septate hyaline cylindrical hyphae (2 to 4 μm in diameter) from which conidiogenous cells emerge. Conidiogenesis is anellidic, producing oval, brown, sticky conidia (4 to 9 by 6 to 10 μm) (Fig. 2). A graphium synanamorph may be produced at the edge of the colony in later stages. This anamorph type is characterized by erect, stiff, olive-brown bundles of hyphae, terminating in a brush of slender conidiogenous cells (Fig. 3). Conidiogenesis is similar to that of the scedosporium type; however, the cells are smaller and the conidia more slender and less pigmented. The scedosporium type is the predominant form, and some isolates may totally lack the graphium type. However, scedosporium, graphium, or both forms may be present in the same isolate. The scedosporium type is characterized by solitary annelloconidia (Fig. 2A). The conidiophores of Scedosporium apiospermum are single, whereas those of Graphium eumorphum are long, erect, narrow, and cemented together, forming synnemata (the erect structure consisting of united conidiophores) (Fig. 3). Conidia (4 to 7 by 5 to 12 μm) of both Scedosporium apiospermum and Graphium eumorphum are unicellular and oval. They are typically truncated at their base. The conidia of Scedosporium apiospermum are often formed singly on the conidiophores, while those of Graphium eumorphum are arranged in clusters at the apices of each synnema.
FIG. 2.
(A) Scedosporium apiospermum conidiophore with annellation (arrowhead). Note the solitary oval to pyriform conidium. (B) Acute-angle branching septate hyaline hyphae. Note the septum (arrowhead) and lateral conidiation (arrow). A KOH preparation using differential interference contrast with polarized light photographic technique is shown, Magnification, ×1,000; bar, 10 μm.
FIG. 3.
Synnemata (coremia) of the Graphium synanamorph of P. boydii bearing terminal conidia. Lactophenol cotton blue stain was used. Magnification, ×100.
Scedosporium prolificans
Macroscopic features.
Colonies of S. prolificans grow moderately to rapidly 25°C on Sabouraud agar and mature within 5 days. The colonies can measure up to 3 cm within a week. The colony is flat and spreading and has a suede-like to downy and moist surface texture with a white color that later becomes brownish olive-gray to black. The reverse turns pale dark brown. S. prolificans also displays a slower development on nutrient agar media and does not grow on media containing cycloheximide (actidione). Unlike S. apiospermum, S. prolificans produces conidiophores with distinctly swollen bases, and the conidial mass forms apical aggregates of conidia and displays positive growth at 45°C. Additionally, S. prolificans lacks the graphium type of conidial state and has not been found to produce a teleomorph (237, 349, 427).
Microscopic features.
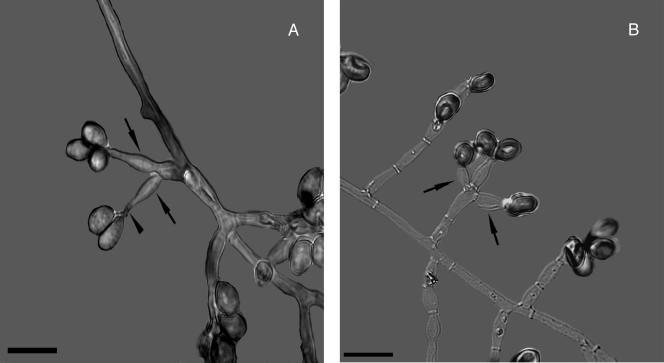
S. prolificans was first described in 1984 by Malloch and Salkin (270) in a pediatric patient with osteomyelitis. It was then called S. inflatum. Identification and differentiation from S. apiospermum are based on the morphological characteristics of the conidiogenous cells of the fungus in culture (376). S. prolificans displays septate hyaline hyphae and has basally swollen (inflated), flask-shaped conidiophores from which a small cluster of single-cell conidia emerges (Fig. 4). The conidia are hyaline to pale brown and ovoid to pyriform, measuring 2 to 5 by 3 to 13 μm (average, 3.4 to 5.3 μm), and have a narrowed, truncated base. In addition, some isolates may produce round, thick-walled conidia which arise directly from the hyphae (237, 427).
FIG. 4.
Scedosporium prolificans (formerly Scedosporium inflatum). (A) The arrowhead points to annellations. The arrows point to the inflated shape of the conidiophores. (B) The arrows point to the inflated conidiophores generating pyriform conidia. A KOH preparation using differential interference contrast with polarized light photographic technique is shown. Bar, 10 μm.
EPIDEMIOLOGY
The recognition of Scedosporium spp. as emergent opportunistic pathogens among the ever-increasing population of immunocompromised individuals is translated in the increasing number of reports and publications in the field of medical mycology in the last few years.
Environmental Epidemiology
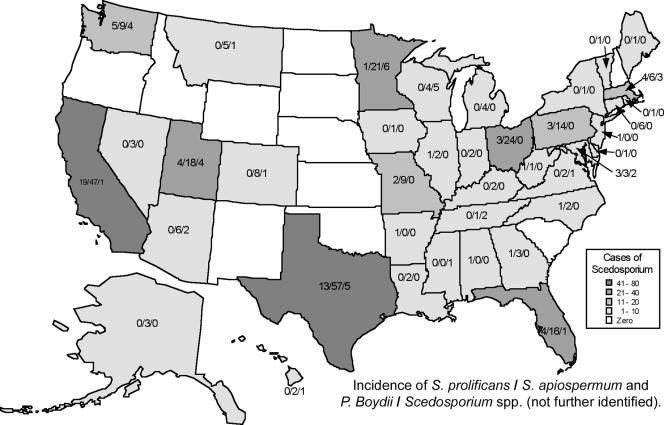
Pseudallescheria boydii/S. apiospermum are found commonly in temperate climates but less frequently in tropical climates. Although infections occur in temperate climates, the species are thermotolerant and have the ability to survive at very low oxygen pressures (http://www.scedosporium-ecmm.com/index.htm). The fungus tolerates a high saline content (5%), and therefore it can survive in polluted environments, where there is poor aeration and high osmotic pressures. The fungus has been recovered from brackish water and saltwater, sewage, soil, swamps, coastal tidelands, manure of poultry (chicken coop, bird guano) and cattle, and bat feces (103, 171, 230, 426). The frequency of Pseudallescheria boydii in the environment is directly related to organic pollution originating from humans, where nitrogen-containing compounds are ubiquitous. The fungus is able to use natural gas, aromatic compounds with potential use in bioremediation of polluted sites (171). In unpolluted environments the recovery of the species is rare. There are only uncommon reports of isolation of the fungus from the intestinal tracts of amphibians (http://www.scedosporium-ecmm.com/index.htm). By comparison, S. prolificans has been isolated from soil and animals (31, 482), such as cats, and horses, but it seems that its ecosystem may be more restricted to soil and potted plants (93, 426). While S. apiospermum has a more uniform geographic worldwide distribution, S. prolificans seems to be restricted to the northern part of the Iberic peninsula and Australia (35, 406), as well as California and the southern United States (204). Specifically, localized osteoarticular infections caused by S. prolificans seem to be more common in the southern United States and California (204). the population-based rate of Pseudallescheria boydii infections was reported for the San Francisco Bay area in 1992 to 1993 to be approximately one case per million population; three cases of infection were reported in a population of 2.94 million (352a).
Figure 5 displays the number of isolates of Scedosporium spp. by state of origin from cases submitted to the Fungus Testing Laboratory at the University of Texas Health Science System at San Antonio from January 2000 to May 2007. As the data presented in Fig. 5 are derived from only one reference laboratory, the figure is not intended as comprehensive representation of the geographic epidemiology of Scedosporium infections. Nevertheless, the data depicted in Fig. 5 confirm the previous observation that S. prolificans is prevalent in California and the southern United States. It also reveals that S. prolificans is the cause of human disease in the northern United States. However, S. prolificans is seldom reported from the Great Plains states or the Rocky Mountain states. While there have been well-described case series of S. prolificans infection from countries such as Spain and Australia, these reports may not necessarily reflect environmental niches for this organism. Instead, the case series may also reflect careful documentation and analysis of the cases by investigators from these countries. As these uncommon infections are not reported nationally, our efforts are limited to those reported to reference centers, in case reports, and in case series. The study by Rees et al. (352a), a population-based assessment, is one approach, but even this type of study has limitations to its extrapolation to more geographically diverse regions throughout the United States.
FIG. 5.
Geographic distribution of cases which Scedosporium spp. were isolated in the United States from specimens submitted to the Fungus Testing Laboratory of the University of Texas Health Science System at San Antonio from January 2000 to May 2007. The white and gray tones represent the total incidences of Scedosporium cases reported by state. The numbers within each state indicate the incidence of Scedosporium prolificans/Scedosporium apiospermum and Pseudallescheria boydii/Scedosporium spp. (not further identified).
In areas of endemicity, thorny trees such as Acacia are abundant. Presumably Scedosporium spp. grow saprobically on the thorns of the trees, and when penetrating trauma occurs, the thorns serve to inoculate the fungus in the tissue. Indeed, thorns have been found embedded in the mycetoma lesions. The disease is not transmitted from person to person or from animals to humans. There is no evidence of particular racial/ethnic predominance.
The overall frequency of Scedosporium infections is relatively low in most geographic areas; however, hospital-based clusters in patients with hematological malignancies have been described (11, 173, 368, 474). Although there have been several nosocomial outbreaks, hospital environmental sampling has been less than helpful in determining a specific source of infection despite the use of selective media for isolation (11, 35). However, Idigoras et al. reported the isolation of S. prolificans from sampled air obtained from a positive-pressure room lodging a neutropenic patient who had disseminated scedosporiosis, using a selective medium with amphotericin B (203). With nonselective media, isolation of S. prolificans was not possible due to overwhelming growth of Aspergillus spp. in the sample. This report laid the foundations for recommending environmental studies using selective media with and without antifungal agents active against Aspergillus spp. Due to the overwhelming amount and more rapid growth of Aspergillus spp. over Scedosporium spp., it is not surprising that the latter were not detected in previous environmental studies (203).
Molecular Epidemiology
Multilocus enzyme electrophoresis, random amplification of polymorphic DNA, and PCR are some of the molecular tools available for epidemiological evaluation of isolates. A high degree of polymorphism has been noted, allowing genotyping differentiation among isolates in cystic fibrosis patients (91, 368, 377, 493). Rainer et al. reported with M-13 fingerprinting that most strains analyzed belonged to another genotype, and several genotypes were recovered from a single sampling site (350).
Epidemiology of Human Infections
Diseases in humans are caused predominantly by S. apiospermum and S. prolificans. Disease states produced by these organisms range from cutaneous and subcutaneous tissue infections to disseminated infections in immunocompromised hosts. Members of this genus have been described as “emerging” fungal pathogens because serious infections caused by these agents have been reported with increasing frequency in more recent years (233, 415, 457).
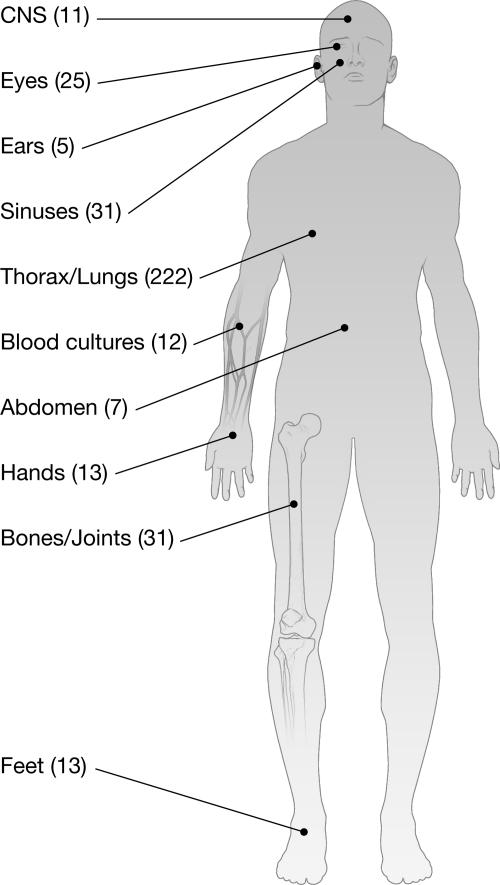
The anatomical locations of human infections caused by Scedosporium spp. have been tallied for 370 isolates submitted to the Fungus Testing Laboratory at the University of Texas Health Science Center at San Antonio (Fig. 6).
FIG. 6.
Anatomical origins (sites of infection) of 370 isolates submitted to the Fungus Testing Laboratory at the University of Texas Health Science System at San Antonio from January 2000 to May 2007.
Comprehensive review of the literature.
In a review of the medical literature from 1940 to the present, we reviewed 435 cases of infections caused by either Pseudallescheria or Scedosporium spp., applying strict case definition criteria (1, 3, 6-17, 22, 23, 25, 26, 29, 30, 32-37, 40, 42-44, 46, 47, 50-53, 56, 57, 60, 66, 67, 73, 74, 78, 79, 82, 84, 88-90, 95-98, 100-102, 104, 108-112, 114, 115, 122-125, 127-134, 137-140, 143, 145, 146, 154, 155, 157, 158, 160, 161, 164, 166-169, 173, 175-179, 181-183, 185-187, 189-197, 199, 202, 204, 207, 210, 211, 213, 215-218, 220-223, 225-229, 231, 232, 234, 238-241, 245-247, 250, 252-256, 258-265, 268, 272, 274-276, 281-283, 289-306, 308-314, 316, 318-320, 323, 324, 327-333, 335-337, 340, 342-344, 346, 348, 351-353, 355, 357, 358, 362-364, 369, 370, 372-375, 380-384, 386, 387, 389-395, 398-400, 402, 404, 405, 407, 408, 410-414, 416, 417, 419-424, 428, 432-436, 438-441, 443, 444, 446-451, 453, 455, 456, 461, 463-466, 468, 470, 472, 473, 475, 476, 478, 480-487, 491). We aimed to review the medical literature for all cases attributed to Pseudallescheria boydii/S. apiospermum and S. prolificans and to these species under their alternative names (Allescheria boydii, Pseudallescheria sheari, Petriellidium boydii, Monosporium apiospermum, Monosporium sclerotiale, Indiella americana, Acremoniella lutzi, and Scedosporium inflatum). An analysis of the demographic features, possible risk factors, and outcome among these 435 patients with scedosporium infections is reported in a separate study (83a).
Mycetoma.
Mycetoma is a clinical syndrome involving cutaneous and subcutaneous tissues, fascia, joints, and bones and is caused by soil-inhabiting bacteria (actinomycetoma) or fungi (eumycetoma). There are at least two dozen species of fungi causing eumycetoma throughout the world. The most prevalent species is Madurella mycetomatis, the etiologic agent of approximately 70% of the reported cases. These agents cause black-grain mycetomas and are usually observed in tropical regions such as India, Sudan, and Madagascar. Pseudallescheria boydii mycetomas are observed mostly in temperate zones, produce white grains in tissues, and are responsible for approximately 10% of the reported cases (280, 356).
In the United States, Green and Adams reviewed 63 cases reported from 23 states, finding that Pseudallescheria boydii is the most common fungal etiologic agent of mycetoma. Pseudallescheriasis causing mycetoma is widely distributed in temperate and subtropical areas. Approximately one-half of the cases seen came from Texas (23 patients) and California (6 patients) (167). In a series of 21 cases of mycetoma observed in the State of Parana, south region of Brazil, 67% (14) were actinomycetoma and 33% (8) were eumycetoma. The principal etiologic agent in these cases was P. boydii. (345).
Mycetoma is more common in males than in females, presumably because of the greater outdoor activities of men. The ratio of males to females varies from 3:1 to 5:1, depending on the observations of different authors. No age group is exempted; the disease is most common in persons between the ages of 20 to 45 years. The population most likely affected is nonimmunocompromised hosts (usually farmers and herdsmen) who live in rural areas and are frequently exposed to accidental, minor penetrating trauma or wounds caused by thorns or splinters to bare feet or other exposed body parts (upper extremities, skull, face, and even the conjunctiva).
Opportunistic infections.
Patients with advanced human immunodeficiency virus (HIV) infection, primary immunodeficiencies (mainly chronic granulomatous disease [CGD] and Job's syndrome), or hematological malignancies, as well as stem cell transplantation recipients and those undergoing antineoplasic or immunosuppressive therapy, are especially susceptible to infections with these filamentous fungi. In advanced HIV infection, patients may develop infections with Scedosporium spp. during neutropenia. Unlike cryptococcosis and histoplasmosis, scedosporiosis may not occur early in the course of HIV disease (359). The majority of the infections caused by the genus Scedosporium in CGD patients have been associated exclusively with S. apiospermum (161, 207, 336, 341, 378). However, in a recent study by Bhat et al., S. prolificans was found to be the etiologic agent of a brain abscess in a CGD patient (38). The most common sites of infection are the lungs and soft tissues, with occasional extension to the bone. Although they are infrequent, infections caused by Scedosporium spp. have been reported in patients with hyper-immunoglobulin E (hyper-IgE) syndrome (135). In most reviewed series, cases of scedosporiosis were seen either in neutropenic patients with acute leukemia undergoing chemotherapy and who had received previous antibiotic and antifungal therapy or in heavily immunosuppressed recipients of a solid organ or hematopoietic stem cell transplantation (HSCT) undergoing treatment for graft-versus-host disease (GVHD) for years (73, 201, 209, 233, 257, 273, 326, 354, 415).
In a retrospective review of the literature, Castiglioni et al. (73) reported that among recipients of solid organ transplantation (SOT) between 1976 and 1999 in Pittsburgh, PA, there were 23 cases of S. apiospermum infections (4 in liver, 8 in kidney, 8 in heart, 2 in lung, and 1 in heart-lung transplant). The overall incidence was 1 per 1,000 patients, with a trend toward higher occurrence in recipients of lung transplants. The male/female ratio was approximately 5:1. The median time to diagnosis of infection was 4 months (range, 0.4 to 156 months) following transplant. In this cohort, 16 of 22 (72.7%) of patients died. In a study from the Fred Hutchinson Cancer Research Center in Seattle, WA, that looked at the frequency of mold infections, nine recipients of an HSCT developed invasive disease due to the Scedosporium spp. over 15 years from 1985 to 1999 (273). Scedosporium infections typically occurred during the first 30 days posttransplantation in the preengraftment period and were more common among those patients who had undergone multiple transplantation procedures. The outcome was typically poor; all nine patients died within 1 month following the diagnosis, accounting for 14% of all nonaspergillus mold infections in HSCT recipients (273). However, in more recent series the number of infections caused by these fungi accounted for approximately 25% of all nonaspergillus mold infections in SOT recipients (200) and 29% of those in HSCT recipients (201). More recently, Husain et al. (201) published a large series of cases of Scedosporium spp. infections in transplant recipients and reported that 75% of the infections in HSCT recipients and 61% of the infections in SOT recipients occurred within 6 months after transplantation. These data are consistent with the changing epidemiology of mycoses from an event that occurs early in the peri-transplant period to a complication of immunosuppressive therapy for GVHD. Disseminated infection was found in 69 and 46% of recipients of HSCT and SOT, respectively. Fungemia was present in 33% of HSCT recipients and in 11% of SOT recipients (P = 0.04). Among the SOT recipients, those with S. prolificans infections were more likely to have fungemia (40%) than those with S. apiospermum infections (5%). The mortality rate for all transplant recipients with scedosporiosis was 58%. The mortality among SOT recipients was 54% (77.8% for patients with S. prolificans infections and 54.5% for patients with S. apiospermum infections). Among the HSCT recipients, the overall mortality rate was 68% (77.8% for patients with S. prolificans infections and 61.5% for patients with S. apiospermum infections).
A recent report from a single institution reviewed the cases of Scedosporium infection from 1989 to 2006 (233). The authors found that the incidence per 100,000 patient-inpatient days increased from 0.82 case between 1993 and 1998 to 1.33 cases in 1999 to 2005. Twenty-five out of 51 patients with positive cultures for scedosporium met criteria for probable or definite Scedosporium infection. All 25 had a diagnosis of hematologic malignancy, and 12 were recipients of bone marrow transplantation. While S. apiospermum was the etiologic agent in 21 patients, S. prolificans was the cause in 4 patients. The other 26 patients were colonized with Scedosporium spp. (18 patients had solid tumors and 8 had hematologic malignancies; S. apiospermum was the etiologic agent in 24, whereas S. prolificans was the agent in 2 patients). Risk factors associated with Scedosporium infections were lymphopenia (88%), steroid treatment (80%), serum albumin level of <3 mg/dl (88%), breakthrough infection (76%) with 74% of the patients receiving amphotericin B, neutropenia (52%) (however, 100% of cases of S. prolificans infections were associated with neutropenia at diagnosis, whereas 43% of the cases of S. apiospermum infections were diagnosed at the time of neutropenia), diabetes (56%), and cytomegalovirus reactivation (24%). Disseminated infection was found in 67% of patients with S. apiospermum and 50% of patients with S. prolificans. Pneumonia was seen in 88% of all patients with disseminated infection. Fungemia was noted in 69% of infections with Scedosporium. Mortality due to S. apiospermum infection was associated with dissemination, fungemia, intensive care unit admission, APACHE (acute physiology and chronic health evaluation) score of >11, prolonged and persistent neutropenia, and breakthrough Scedosporium infection (233).
In the series published by Castiglioni et al., of 23 SOT recipients with S. apiospermum infections, 13 (57%) of the patients presented with sinopulmonary disease and 11 (48%) with invasive pneumonia (73). Of these 11 patients with pulmonary scedosporiosis, 6 (54.5%) developed brain abscesses and 10 (91%) succumbed to the infection. In this series, three lung transplant recipients had S. apiospermum persistently isolated from their respiratory secretions. However, none experienced progression to disease while receiving itraconazole prophylaxis.
In another review of lung and heart-lung transplant recipients between 1986 and 1999 at an Australian center, 7 of 330 (2.3%) had pulmonary scedosporiosis (433). S. apiospermum was documented in the bronchoalveolar lavage (BAL) fluid of all seven and S. prolificans in the BAL fluid of four of these patients. Scedosporium was isolated 9 to 58 months after transplantation. Five of the seven patients had been treated for several months with itraconazole because of previous detection of aspergillus in BAL fluid. All seven patients with Scedosporium infection had abnormal airways, including early ischemic airway stenosis in one and bronchiolitis obliterans in the remaining other six patients. Four of the seven patients died with advanced bronchiolitis obliterans 3 to 35 months after the diagnosis of pulmonary Scedosporium infection. Three patients survived 3, 6, and 7 years after transplantation, showing persistent Scedosporium infection at the time of the report. With the exception of a case report where pneumonia developed after a sternal wound surgical infection after cardiac transplant (432), inhalation seems to be the most likely source of sinopulmonary disease.
In contrast to the late development of pulmonary scedosporiosis in the SOT population, the disease has been reported to occur soon after HSCT, generally during the preengraftment period (21, 140, 374, 410, 411). However, as HSCT practices change, we are witnessing a changing epidemiology of opportunistic fungal infections (i.e., aspergillosis, zygomycosis, scedosporiosis, etc). Although neutropenia following the conditioning regimen remains an important risk factor for opportunistic fungal infections, most cases of invasive mold infection in allogeneic HSCT recipients occur after neutrophil recovery in the setting of potent immunosuppressive therapy for GVHD (39, 67, 208, 273, 315, 372, 477).
Nonopportunistic infections.
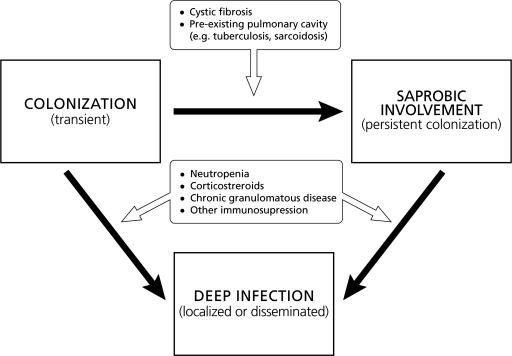
The lung and upper respiratory tract are the most commonly encountered sites of nonopportunistic involvement by P. boydii besides the pedal mycetoma. These conditions fall into several categories: transient local colonization, bronchopulmonary saprobic involvement, fungus ball formation (pseudallescherioma/scedosporioma), and invasive pseudallescheriasis (pseudallescheria pneumonia). We propose a model for host-pathogen interaction in scedosporiosis of the lower respiratory tract (Fig. 7).
FIG. 7.
Model of the host-pathogen interaction in pulmonary scedosporiosis. Pulmonary involvement begins with colonization of the respiratory tract. This colonization appears to be transient in immunocompetent hosts with anatomically normal respiratory tracts. However, colonization may become persistent in certain patient with anatomically altered respiratory tracts, leading to saprobic involvement. Such conditions occur in patients with cystic fibrosis, cavitary tuberculosis or sarcoidosis, and bronchiectasis. Conditions that alter the innate host defense mechanisms of a patient with colonization or saprobic involvement of the respiratory tract may lead to invasive disease manifesting as localized or disseminated infection. Conditions that may predispose to invasive pulmonary scedosporiosis include neutropenia, corticosteroid therapy, and CGD.
The exact prevalence of Pseudallescheria spp. or Scedosporium spp. as constituents of the normal human flora is unknown. Scedosporium spp. are isolated in <1% of dwellings and do not appear to be frequent colonizers of humans (28).
The term “colonization” usually refers to the state in which organisms that are part of the normal flora are found in their habitat. The term “transient colonization” refers to the situation in which microorganisms not usually part of the normal flora may be found on the surface of a mucocutaneous surface without causing disease. It is a transient situation likely to reverse when the host is removed from the exposure in the environment. It is possible that P. boydii or Scedosporium spp. may transiently colonize the respiratory tract of a person exposed to a high environmental inoculum (e.g., in an agricultural setting). However, in the absence of anatomic abnormalities of the respiratory tract, this colonization state would most likely be transient once the patient was removed from the environmental source.
The bronchopulmonary saprobic state appears to be the most common manifestation of pseudallescheriasis of the lung. The first report to describe this condition was published by Creitz and Harris in 1955 (84). In that report, the authors described a patient who had a cavity subsequent to a pyogenic abscess, which was secondarily colonized by P. boydii. Several years later the patient died, and at autopsy the organism was recovered as “strands and clumps” from bilateral upper lobe cavities (444).
In reviews by Lutwick et al., Reddy et al., and Jung et al., the authors identified preformed cavities in 13 out of 14 cases (211, 260, 352). Filaments of mycelium (plectenchyma) and conidia were found in large residual cavities in a resolved case of tuberculosis (10). Fungus ball formation has been reported multiple times but documented in a few cases (490). In pulmonary colonization the predisposing condition is usually the existence of a preformed cavity or cyst. Hence, the patients are not severely debilitated and treatment is often successful. However, in a recent report, Symoens et al. described a fatality due to disseminated Scedosporium apiospermum infection in a cystic fibrosis patient after double-lung transplantation (429), underscoring that in immunocompromised patients, colonization can lead to fatal dissemination.
P. boydii can grow well saprobically inside poorly draining bronchi or paranasal sinuses without causing invasive disease. S. apiospermum may saprobically involve the respiratory tracts of individuals with cystic fibrosis. A prospective study involving 128 cystic fibrosis patients demonstrated that S. apiospermum was isolated in respiratory cultures of 11 (8.6%) of the patients, ranking second to Aspergillus fumigatus as the most common mold found in the airways (81). A number of patients with an underlying diagnosis of asthma or cystic fibrosis may develop clinical symptoms similar to those of allergic bronchopulmonary aspergillosis, the most common type of allergic bronchopulmonary mycosis (ABPM). Pseudallescheria/scedosporium has been implicated as the etiologic agent of ABPM in only three cases of allergic bronchopulmonary pseudallescheriosis. The first case was in a patient with mild asthma, who presented with an occasionally productive cough and whose symptoms resolved after expectoration of a mucous plug. The second case was in a patient with recurrent allergic bronchopulmonary aspergillosis with an exacerbation of ABPM caused by Pseudallescheria boydii. The third case was in a patient on chronic corticosteroid therapy for rheumatoid arthritis who intermittently expectorated P. boydii while on corticosteroid therapy (232, 294, 357).
Other infections caused by P. boydii/S. apiospermum and S. prolificans are sinusitis, meningitis, arthritis and osteomyelitis, endocarditis, cutaneous and subcutaneous infection (nonmycetoma), keratitis, endophthalmitis, and disseminated disease. In most cases, inoculation of spores in skin or soft tissue is due to penetrating trauma or surgery. Following ocular surgery, such as laser-assisted in situ keratomileusis (LASIK) and pterygium excision with and without adjuvant radiation therapy, cases of keratitis anterior and posterior scleritis, corneoscleritis, and sclerokeratitis caused by S. apiospermum and S. prolificans have been reported (227, 302, 303, 424, 436). Other possible routes of entry for these fungi are inhalation (203), Hickman catheters (482), and lumbar puncture (32, 264).
In a nosocomial outbreak of Scedosporium infections, the source was thought to be a construction site at the hospital (11). However, in a particular case, the fungus was isolated from the air in the patient's hospital room (203).
While there is a relatively large body of literature on P. boydii, there is considerably less written on S. prolificans. S. prolificans was associated with subcutaneous soft tissue infections with predilections for cartilage and joint areas (93, 165, 431). Most clinical cases are sporadic and appear in both immunocompetent and immunocompromised individuals. The main risk factors for the former are surgery and trauma. In the immunocompromised population, the most important risk factors are prolonged, profound neutropenia and corticosteroid therapy. In disseminated disease, 90% of the patients had persistent neutropenia (acute leukemia, lymphoma, and peripheral stem cell/bone marrow transplantation). Corticosteroid therapy in cases of lymphoma, autoimmune diseases, organ transplants, and bone marrow transplants (particularly those with GVHD) has been identified as another important risk factor in the development of disseminated scedosporiosis. Other underlying conditions associated with disseminated disease with S. prolificans have been lung transplant, presence of a prosthetic heart valve, and HIV infection. S. prolificans may also be found in the saprobic state among patients with cystic fibrosis. Small outbreaks have been reported (11, 368).
Near drowning.
A distinctive clinical syndrome of sinopulmonary and central nervous system (CNS) infections in immunocompetent individuals has been associated with near drowning in polluted waters and P. boydii as the etiologic agent. Near drowning in polluted water has been reported to result in pulmonary infection, with dissemination to the CNS (58, 77, 83, 87, 110, 216, 225, 291, 306, 367, 473).
Over the last 2 decades at least 21 cases of Pseudallescheria infection associated with near drowning in polluted waters have been reported; two of these cases involved survivors of the tsunami of southeast Asia in December 2004 (58, 77, 78, 108-110, 131, 143, 144, 179, 216, 225, 283, 291, 306, 448, 473). Currently, P. boydii/S. apiospermum is recognized as the fungus most commonly implicated in invasive disease after near drowning. Other fungi, such as Aspergillus spp., also have been reported but to a much lesser extent (244). Notably, S. prolificans has not been associated with infection in near-drowning victims.
Pseudallescheria boydii infection after near drowning usually affects previously healthy, immunocompetent individuals. Several authors, however, have suggested that the hypoxic state following submersion or the use of corticosteroids for treatment of aspiration pneumonia may compromise host immune responses, facilitating the penetration and spreading of the organism (108, 109, 283). The mode of fungal invasion in submersion victims is not always obvious. In an early report by Fisher et al., the presence of a solitary brain fungal abscess, the transient nature of aspiration pneumonitis, and the failure to isolate P. boydii from respiratory secretions led to the hypothesis that the organism could have been introduced into the brain through an unapparent hairline fracture in the cranial vault or paranasal sinuses under increased subsurface barometric pressures (131). An alternative hypothesis may have been forced injection of contaminated water through the cribiform plate and into the cranial cavity. In subsequent reports, however, the occurrence of multiple brain abscesses and/or lesions in different organs, such as the eye, skin, lungs, liver, kidney, and bone, suggested hematogenous dissemination of the infection following massive inoculation of the fungus in the lungs during aspiration of infested water (110, 143, 473).
P. boydii infection associated with near drowning tends to present within a few days to several weeks after the incident, often after temporary improvement of the patient's condition. Notably, a latent period of as long as 4 1/2 months has been reported (131, 216, 473). The CNS has been the most common extrapulmonary site affected (in 20 out of 21 cases reported). CNS infection may present as single or, most commonly, multiple brain abscesses, meningitis, encephalitis, ventriculitis, vasculitis of cerebral vessels and occasionally true mycotic aneurysms and intracerebral hemorrhage. Clinical manifestations may vary and include fever, headache, altered mental status, seizures, and pyramidal signs. Hydrocephalus and brain edema may aggravate the clinical picture and lead to herniation and brain death (58, 77, 78, 108-110, 131, 143, 144, 179, 216, 225, 283, 291, 306, 367, 448, 473).
Infectious lesions in organs other than the CNS have been reported in 13 of the 21 cases, including in the lung (7 of 21, usually manifested as bronchopneumonia) (78, 109, 110, 179, 216, 283, 448), kidney (3 of 21) (108, 110, 283), eye (3 of 21, manifested as endophthalmitis or chorioretinitis) (110, 283, 473), musculoskeletal system (2 of 21, manifested as knee joint synovitis, femoral and tibial osteomyelitis, or spondylodiscitis) (110, 144), heart (2 of 21) (108, 110), liver (283), skin (erythematous lesions with purplish-black necrotic centers in arms and abdomen) (110), and thyroid gland (110).
The diagnosis of P. boydii infection in near-drowning victims was delayed, as the fungus was isolated from respiratory secretions in only 6 of 21 cases (78, 109, 110, 216, 283, 448) and from cerebrospinal fluid (CSF) obtained by lumbar puncture in only 3 patients (109, 131, 225). In the majority of cases the organism was isolated from material obtained by aspiration or surgical drainage of brain abscesses. The delay in diagnosis together with the previous lack of antifungal drugs with potent activity against P. boydii and good penetration into the CNS may explain the poor outcome of these infections, with a survival rate of 33% (seven cases) among those reviewed. Among the patients who survived, two were treated with high doses of the older azole miconazole (110) and five with voriconazole, alone or in combination with other agents, together with surgical intervention (abscess drainage) when needed (77, 78, 144, 306). The role of voriconazole and newer antifungal agents in the treatment of pseudallescheriasis is discussed more extensively in Treatment and Outcomes of Scedosporiosis below.
Given the potential fatal outcome and relatively long latent period of P. boydii infection in near-drowning victims, such patients should be followed closely for several weeks after the episode, and even subtle neurologic symptoms should prompt imaging of the CNS. In the presence of abnormal imaging findings, microbiological diagnosis should be aggressively pursued, and empirical treatment with voriconazole at appropriate doses should be initiated while waiting for definite microbiological results.
PATHOGENESIS AND HOST DEFENSE IN SCEDOSPORIOSIS
Host Defenses and Scedosporium Infections
The study of the immune response and host defenses to and pathogenesis of Scedosporium infections has been a recent event, fuelled by the increasing medical importance of these pathogens. Innate and adaptive immune responses to Scedosporium spp. have been studied to a lesser extent than those to A. fumigatus. In vitro studies have shown that S. apiospermum and S. prolificans conidia and hyphae are susceptible to phagocytes in a manner comparable to those of A. fumigatus, with minor differences (149, 150). Specifically, monocyte-derived macrophages are able to phagocytose scedosporium conidia similarly to aspergillus conidia, despite the larger size of S. prolificans conidia. Additionally, monocyte-derived macrophages have been found to inhibit germination of S. prolificans conidia less efficiently than that of A. fumigatus (149). In vitro studies have demonstrated that phagocytes are capable of exhibiting a sufficient oxidative burst to control S. prolificans strains in the presence of serum. In the absence of serum, however, the production of superoxide anion (O2−) appears to be lessened (149). The way that the opsonization status affects the oxidative burst in response to S. prolificans remains unclear and merits further investigation. Isolates of S. prolificans tested in vitro have been damaged in an effector cell/target ratio-dependent manner when challenged with both kinds of phagocytes. Moreover, phagocytes may induce more damage to S. prolificans than to A. fumigatus (149).
Immunosuppression constitutes a significant risk factor for the surge of invasive fungal infections. In this regard, a number of studies have aimed to assess the immunomodulatory utility of cytokines in confronting emerging fungal pathogens. S. prolificans has been shown to induce significantly more tumor necrosis factor alpha and interleukin-6 (IL-6) release by human monocytes than do Aspergillus spp. This could be attributed to the specific composition of the S. prolificans cell wall (although its exact composition is not known), which may yield more potent stimulatory molecules. Speculatively, this could be associated with the virulence of the specific fungus (462).
It also has been shown that the presence of IL-15 significantly enhances polymorphonuclear leukocyte (PMN)-induced hyphal damage and oxidative respiratory burst of S. prolificans but not S. apiospermum (216). Additionally, IL-15 increases IL-8 release from PMNs challenged by S. prolificans, whereas release of tumor necrosis factor alpha is not affected. The failure of IL-15 to exhibit enhanced damage of S. apiospermum hyphae is in concordance with its greatest intrinsic virulence in humans. These findings suggest that IL-15 has species-specific enhancing effects on antifungal activities of PMNs against Scedosporium spp. Further, some of the cytokine-induced effects have been shown to be the result of direct actions on effector activities of PMNs, while others, related to the increased release of IL-8 acting in an autocrine way on PMNs, result in enhanced indirect antifungal actions (479).
Further insight into the immunopathogenesis of Scedosporium infection has been gained through in vitro studies of the phagocytic cell responses to two S. apiospermum isolates, one amphotericin B resistant and another amphotericin B susceptible. As Scedosporium apiospermum may display variable susceptibilities to fungal agents, such variations in MIC are to be expected. Accordingly, it has been found that macrophages are able to phagocytose S. apiospermum conidia, damage hyphae in an effector cell/target ratio-dependent manner, and release O2− in response to serum-opsonized hyphae of both isolates. It has also been observed that hyphae of the two strains with the different amphotericin B susceptibility patterns exhibit different levels of susceptibility to myeloperoxidase products. This phenomenon, although not fully elucidated, may be related to the various levels of pathogenicity and antifungal drug resistance of S. apiospermum (150).
In the last few years, substantial progress has been achieved in understanding the molecular events of the innate immune response to P. boydii (41). Bittencourt et al. (41) reported the isolation and structural characterization of a α-glucan from the P. boydii cell wall and evaluated its role in the induction of the innate immune response. The soluble α-glucan, but not the β-glucan, leads to a dose-dependent inhibition of conidial phagocytosis. Moreover, a reduction of the phagocytic index was noted when α-glucan from the conidial surface was removed by enzymatic treatment with α-amyloglucosidase, demonstrating a role of glucan in P. boydii internalization by macrophages. Finally, α-glucans induce cytokine secretion by cells of the innate immune system (macrophages and dendritic cells) through Toll-like receptor 2, CD14, and MyD88. By comparison, β-glucans of A. fumigatus, through dectin-1 of macrophages, induce production of tumor necrosis factor alpha, IL-1α, IL-6, and other proinflammatory cytokines (146a, 413a). Similar to P. boydii, A. fumigatus also interacts with Toll-like receptor 2 and MyD88 of macrophages to induce the release of cytokines.
Mycetoma and Local Disease
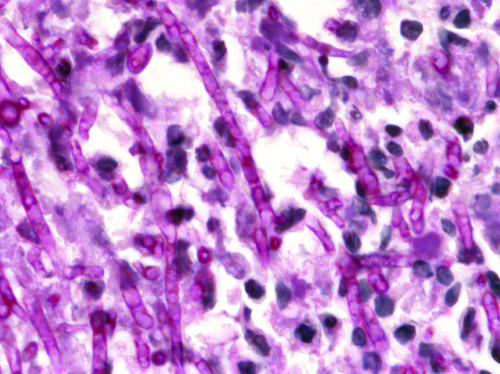
Infection leading to mycetoma occurs following traumatic inoculation of the etiologic agents into the subcutaneous tissue. Once implanted, these usually nonpathogenic organisms adapt to the host tissue environment through a dimorphic mechanism. In order to escape from the host defenses, they grow and survive within grains (also named granules or sclerotia), which are aggregates of the organism and a matrix component or cement. The matrix component has been shown to be host derived with some pathogens. In P. boydii grains are white, soft, and oval to lobed in shape, measuring less than 2 mm in diameter. The hyaline hyphae are approximately 5 μm in diameter but may appear as large swollen cells measuring up to 20 μm in diameter. The grains have no cement; however, toward the periphery, the hyphal elements have thickened cell walls presumably conferring protection against the host immune response (471). Mycetomas are in essence granulomatous tumors, and both types, actinomycetomas and eumycetomas, may present a similar tissue response. Histopathological examination of draining abscess material shows the grains or clusters of grains, true fungal colonies, and granulocytes, surrounded by PMN inflammatory infiltrate (Fig. 8 and 9).
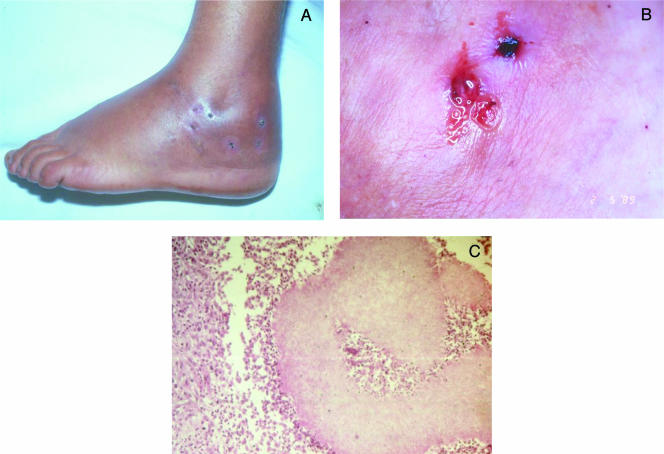
FIG. 8.
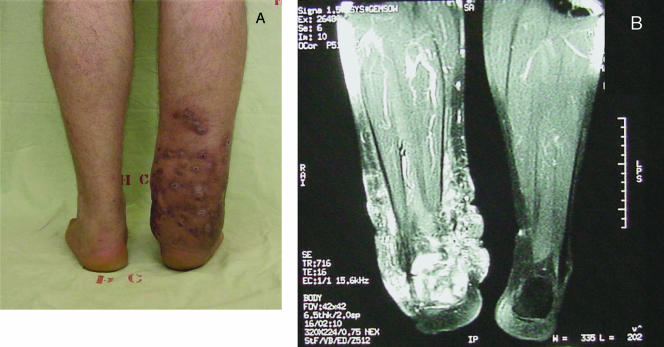
Scedosporium apiospermum pedal mycetoma of 18 years of evolution. Several sinus tracts in different evolution stages on the left foot (A) and draining white yellow grains resembling fig seeds at the openings of three fistulae (B) are shown. A transversal section of a fistula shows several lobed pale grains and an inflammatory infiltrate on the fistula lumen (C). H&E staining was used; magnification, ×400. (Reprinted from reference 345 with permission from Elsevier.)
FIG. 9.
(A) Multifistulous right-lower-limb Scedosporium apiospermum mycetoma of 8 years of evolution. (B) MRI of the same patient, showing extensive inflammatory changes in the medial and lateral aspects of the lower leg and calcaneous osteomyelitis.
The innate immune system interacts at complex levels with the fungal agents of mycetoma. Cells of the innate immune system attempt to phagocytose and inactivate the organisms, but in the disease state, they can be overwhelmed. Complement activation with consequent dependent chemotaxis of granulocytes to mycetoma has been shown in both fungal and actinomycotic antigens in vitro (488).
The tissue reaction in mycetoma is that of a granuloma. Fungal grains are located within the abscess. Immediately surrounding them, and sometimes within the grains, are the PMNs. This zone of PMNs is narrow in cases of mycetoma due to fungal agents. In the natural history of mycetoma, a more mature granuloma develops, consisting of an orderly array of lymphocytes, macrophages, plasma cells, small mononuclear cells, and large mononuclear cells. Occasionally, Russel-Fuchs eosinophilic bodies are seen. Especially among eumycetomas, giant cells containing fragments of fungal material are often seen. In blood vessels contained in the affected area, there may be evidence of endarteritis or periarteritis.
Three types of tissue reactions in response to the grains of mycetoma have been described (120). A type I reaction is seen as PMNs degranulate and adhere to the surface of the grain, leading to a slow disintegration of the grain. Outside this layer there is granulation tissue with macrophages, lymphocytes, plasma cells, and a few neutrophils. Russell's bodies can be observed, and macrophages have multiple cytoplasmic vacuolations. Concentric layers of fibrin giving an onion-like appearance surround capillaries and venules. The outermost layer is fibrous tissue. The walls of arterioles show edema and hypertrophy of the muscularis, thickening of the intima, and overall narrowing of the lumen. The nerves show edema and mononuclear cell infiltration. Sweat glands are hypertrophic or hyperplastic. A type II reaction is characterized by the disappearance of PMNs and arrival of macrophages and multinucleated giant cells. The giant cells are to clear the grains and PMN debris, consisting of pigmented cement substance and some hyphae at times. A type III reaction is marked by the formation of epithelioid granulomas. While this host response may be unable to control infection, it may be responsible for the apparent partial spontaneous healing seen in this disease. Whether subjects who develop mycetoma have subtle predisposing immune deficiencies is not clear. Mycetoma does not appear to be more common in immunocompromised hosts, and early study of the immune function in persons with mycetoma does not point toward a common immune deficit (31, 266).
Pulmonary and Disseminated Disease
Conidia of Scedosporium spp. enter the respiratory tract via inhalation. Germination of conidia results in hyphal invasion of the lower respiratory tract. In a process similar to that of pulmonary aspergillosis, conidia of Scedosporium spp. may be cleared mechanically by mucociliary escalator or by pulmonary alveolar macrophages. If macrophages are unable to destroy these conidia, germination ensues, and PMNs are then necessary to control hyphae and conidia. Such pyogranulomas may control the infection. However, immunocompromised hosts may be unable to mount such a response due to quantitative or qualitative PMN defects. Unchecked proliferation of hyphae in neutropenic hosts may lead to invasion of blood vessels and potential hematogenous dissemination. Characteristic adventitious sporulation of this fungus in vivo may also favor hematogenous dissemination (325, 355).
Other potential mediators of pathogenesis of S. apiospermum include a 33-kDa extracellular serine protease peptidase that is capable of degrading human fibrinogen, suggesting its role as a mediator of tissue injury and inflammation (235). Silva et al. have reported two extracellular peptidases of 28 and 35 kDa that are released from mycelia of P. boydii (401). Such peptidases were also found to be of the metallo-type peptidases, probably zinc dependent, that showed their highest activity in acidic pH (5.5). These peptidases were found to break down portions of fibronectin and laminin through metallopeptidase activity, which may constitute an escape mechanism from host effector cells such as fibronectin-activated macrophages and monocytes. Moreover, the degradation of the matrix proteins could help invasive fungal cells migrate into deeper adjacent tissues and extend into the circulation.
S. prolificans is thought to be more virulent in vivo than S. apiospermum (325). Consistent with this observation, in a literature review of 435 cases, Cortez et al. found that there was a significantly greater mortality among cases due to S. prolificans than among those caused by S. apiospermum or P. boydii as determined by univariate analysis (P < 0.001) (83a).
Some putative virulence factors for scedosporium have been suggested for S. prolificans. Among these factors are melanins, which are dark brown and black pigments of high molecular weight formed from the oxidative polymerization of phenolic compounds (1,8-dihydroxynaphthalene via a polyketide pathway). Melanin may be an important virulence factor contributing to fungal tolerance to environmental stress and in vivo protection against host defense mechanisms (361). The current line of thought is that melanin may serve as a scavenger for oxygen and nitrogen free radicals produced by phagocytic cells during the oxidative burst. Melanins also may confer resistance to heat by sequestering host defense proteins or by cross-linking or shielding the cell wall constituents against hydrolytic enzymes (105, 427). These mechanisms of host evasion and the capacity of in vivo adventitious sporulation may explain the high degree of fungemia in patients with S. prolificans infections.
S. apiospermum, on the other hand, produces an extracellular serine protease (33 kDa) similar to the alkaline protease of A. fumigatus. Both enzymes can degrade human fibrinogen, potentially acting as a mediator of chronic bronchopulmonary inflammation and playing a role in host tissue invasion (235). Patients such as those with cystic fibrosis or primary immunodeficiencies who have saprobic involvement of airways by microbial agents that produce such proteinases may suffer pulmonary damage from degradation of host proteins, such as fibrinogen and basement membrane laminin, or indirectly by hypersensitivity mechanisms. Finally, S. apiospermum and S. prolificans contain siderophore activity, making them iron dependent, which may also explain their neurotropism since the CNS contains free iron, in contrast to serum (93). P. boydii may get access to the CNS in at least four different ways: by direct inoculation from trauma (329); by hematogenous dissemination from a pulmonary source, including aspiration into the lungs, after a near-drowning experience (131); via an intravenous (i.v.) catheter (487); and via direct extension from infected paranasal sinuses (56).
CLINICAL MANIFESTATIONS
As a way of introduction to this section, a brief classification of the clinical manifestations of the interaction of host and pathogen may be illustrative (Table 2). Four very different clinical conditions are associated with infections caused by P. boydii/S. apiospermum as well as S. prolificans: (i) mycetoma, a very characteristic and most common subcutaneous infection caused by these fungi; (ii) saprobic involvement of the airways and respiratory tract, particularly in patients with bronchiectasis (due to cystic fibrosis, Job's syndrome, healed tuberculosis, etc.); and deep infections, which may be classified further as (iii) localized sinopulmonary or extrapulmonary or (iv) disseminated infections. The fact that the reported literature does not include cases of colonization in the immunocompromised population does not necessarily mean that the colonization state does not occur. Due to publication bias and the absence of large studies to determine the predictive value of colonization leading to disease, little is known about this aspect of host-pathogen interaction.
TABLE 2.
Clinical classification of infections caused by Scedosporium spp. and selected examples of other mycoses
| Host-pathogen interaction | Localization | Conventional mycological examples | Pseudallescheria/Scedosporium |
|---|---|---|---|
| Colonization | Airways | Candida spp. | Probably transient; if persistent, suggests abnormal airways |
| Saprobic involvement of airways | Abnormal airways (chronic sinusitis; preexisting cavitary lesions, i.e., pulmonary tuberculosis, pulmonary sarcoidosis) | Aspergilloma, bronchiectatic involvement of Aspergillus, otomycosis, chronic Aspergillus sinusitis | Fungal balls (pseudallescherioma/scedosporioma, bronchiectatic involvement of P. boydii/ Scedosporium spp.) |
| Infection | Superficial | Malassezia spp. | Not available |
| Cutaneous | Dermatophytes | Dermal infections (nodules)/sporotrichoid | |
| Primary | |||
| Secondary | Fusarium solani (hematogenous) | Secondary disseminationa | |
| Subcutaneous | Chromoblastomycosis | Mycetoma | |
| Phaeomycotic cysts | Subcutaneous nodules | ||
| Sporotrichosis | Sporotrichoid-like presentation | ||
| Invasive | |||
| Localized | Invasive pulmonary aspergillosis | Invasive visceral pseudallescheriasis/ scedosporiosis | |
| Disseminated | Disseminated aspergillosis, fusariosis, filamentous fungi |
Cutaneous lesions (papules, nodular) are well-recognized manifestations of hematogenous dissemination of Scedosporium infection.
Mycetoma
Mycetoma is chronic, progressive, indolent, destructive implantation mycosis, usually involving the foot or leg. For the purposes of this review, mycetoma refers to eumycotic mycetoma specifically caused by P. boydii/S. apiospermum or S. prolificans. As is the case for other subcutaneous mycoses, these fungi gain entrance to the host environment through a penetrating transcutaneous trauma, including puncture wounds (such as from thorns, wood splinters, or speculated seeds), abrasions, or any contact with sharp objects such as agricultural tools (345).
The foot and the lower extremities are the most frequently affected areas; however upper extremities and even the skull or face have been also involved. At the site of trauma, the lesion grows slowly and indolently with well-defined margins, remaining localized for long periods. Usually the lesion is painless, and it may be nodular in appearance and have a firm consistency. Sometimes, however, it may be surrounded by soft, even cystic and mobile tissue (Fig. 8). Multiple nodules can appear and spontaneously drain purulent material mixed with soft, white to yellowish grains resembling fig seeds (Fig. 8). The sinus tracts rarely appear before the first 3 months, but are usually present by the end of the first year. The draining sinuses may close and heal completely, while new ones may open. Usually there are all interconnected. Involvement of ligaments, joint cartilage, and even bone may occur with time. Bone involvement is characterized by osteolytic lesions and remodeling of bone tissue. Mycetoma can produce profound disability, distortion, and deformity (Fig. 9). Constitutional symptoms are rarely seen in cases of mycetoma unless a concomitant infection also occurs (249).
Clinically and radiologically, S. apiospermum eumycetomas are similar to other eumycetomas caused by other fungi. Eumycetomas differ from actinomycetomas by their long time of evolution and by the appearance of a drier and less exudative lesion in the former (Table 3). Other differential diagnoses include botryomycosis, actinomycosis, dermatophyte pseudomycetomas, and chronic osteomyelitis. Depending on the evolutive phase, other disease may mimic mycetomas, such as chromoblastomycosis, verrucous sporotrichosis, tuberculosis, mycobacteriosis, filariasis, lymphoma, and neoplasic diseases (249).
TABLE 3.
Main features of eumycotic and actinomycotic grainsa
| Infections and species | Findings by:
|
|
|---|---|---|
| Fresh examination | Histology (H&E) | |
| Eumycetomataa | ||
| Scedosporium apiospermum | <2 mm, yellowish or white, soft, oval to lobed, “fig seed like” | Compact, no cement, interwoven hyaline hyphae <5μm and swollen cells <20μm, eosinophilic border |
| Acremonium kiliense | <1.5 mm, white, soft, irregular shape | Compact, no cement, hyaline hyphae <4 μm, swollen cells <12 μm |
| Aspergillus nidulans | <2 mm, white, soft, oval to lobed | Compact, no cement, interwoven hyaline hyphae <5 μm, eosinophilic border |
| Neotestudina rosatii | White to brownish, soft, <1mm, fragmented angulated mass | Cement and swollen cells at periphery embedded in cement and some central vesicles |
| Madurella mycetomatis | <2 mm, black, firm to brittle (coal consistency), oval to lobed | Compact type, with brown-staining cement; vesicular type, with hyaline center and brown-staining cement and prominent <15 μm at edge |
| Madurella grisea | <1 mm, black, soft to firm, oval to lobed | Little brownish cement and polygonal cells at the periphery and central hyaline hyphae |
| Exophiala jeanselmei | <0.5 mm, black, soft, irregular to vermicular | No cement, hollow center, with melanin-pigmented vesicular cells <10 μm associated with short hyphae <4 μm |
| Leptosphaeria senegalensis | 1 mm, black, soft, irregular shape | Cement in outer zone, dark periphery with hyaline vesicular center |
| Pyrenochaeta romeroi | <2 mm, black, firm to brittle, oval to lobed | Brownish cement at periphery, no vesicles |
| Actinomycetomatab | ||
| Nocardia brasiliensis | <0.5 mm, white, soft, irregular | Small, basophilic stained fringe in layers, homogenous loose clumps of bacterial filaments and rare clubs, positive Gram and Kinyoun stains |
| Actinomadura madurae | 5 mm, yellowish to pink, oval to lobed | Anamorphous empty center with a dense basophilic or pink border associated with loose fringe and clubs, gram positive |
| Actinomadura pelletieri | <1 mm, red, hard, oval to lobed | Homogenous dark staining with light periphery and no clubs, easily fractured, gram positive |
| Streptomyces somaliensis | <2mm, yellow, hard, round to oval | Amorphous center amorphous center with basophilic layers associated with pink patches and dark bacterial filaments at the edge and no clubs, gram positive |
Adapted from reference 356 with permission from Elsevier.
Other eumycetoma agents: Acremonium falciforme, Acremonium recifei, Aspergillus flavus, Leptosphaeria tompkinsii, Pyrenochaeta. mackinnonii, Curvularia geniculata, Curvularia lunata, Fusarium solani, Fusarium oxysporum, Exserohilum rostrata, etc.
cOther actinomycetoma agents: Nocardia asteroides, Nocardia caviae, Nocardia farcinica, Nocardia dassonvillei, etc.
Saprobic Involvement/Colonization of Airways
The epidemiology of colonization and saprobic involvement of the respiratory tract was described above. In the presence of dysfunction of the mucosa or if the respiratory tract is afflicted by preformed cavities (bronchiectasis, chronic obstructive bronchopulmonary disease, tuberculosis, cystic fibrosis, Job's syndrome, etc.), colonization of the airways may be permanent and the saprobic state of scedosporium may ensue. The most common predisposition factors for both colonization and fungus ball formation are tuberculosis, sarcoidosis, and previous bacterial infections that result in cysts and cavities (27). Fungus balls in preexisting cavities can be seen in otherwise normal hosts, but invasive disease is usually limited to immunosuppressed patients (8, 68, 277, 376, 448, 482, 485). In the saprobic state, although some patients may have no or minimal symptoms, some may develop pulmonary infiltrates and even fungus balls with or without symptoms (483), and others develop an allergic reaction to the presence of the fungus (232, 294, 357).
P. boydii and Scedosporium spp. can form “fungus balls” (pseudallescherioma/scedosporioma) that are radiologically indistinguishable from the more common aspergilloma (385). Although they most commonly involve the lungs and sinuses, fungus balls can be found in other organs, and their histological features can vary between involved organs. Those in the lung demonstrate well-defined layers of peripheral mycelial hypocellularity and hypercellularity consisting of hyphae, conidiophores, and conidia. In a report by Schwartz, the author states that fungus balls in all organs were derived from necrotic host tissue, which resulted from nodular infarction due to fungal invasion and thrombosis of blood vessels in the lungs. In those cases, patients presented with cough and occasionally with hemoptysis (385).
Fungus balls caused by P. boydii and Scedosporium spp. (pseudallescherioma/scedosporioma) may also grow within poorly draining paranasal sinuses (379), resulting from recurrent or chronic sinusitis. In such cases, an impaired mucosal lining, debris from previous infections, and compromised drainage set the stage for persistent colonization by Scedosporium spp. and even fungus ball formation. The clinical picture may be that of an acute (although sometimes subacute) sinusitis, with facial pain or headache depending on the involved sinus, and dark gray thick nasal discharge. Usually there is no invasion of respiratory mucosa or by erosions, and surgical debridement is usually curative (44, 174).
Allergic bronchopulmonary pseudallescheriasis has been associated with Pseudallescheria boydii infection (232, 294, 357). The clinical features of ABPM include asthma (paroxistic dyspnea, cyanosis, prolonged expiratory phase, ronchi, expiratory wheezes, and persistent cough with adherent sputum), pulmonary inflammation, systemic or pulmonary eosinophilia, and elevated serum IgE and IgG1 levels. Other features include a Th2 profile response, the presence of precipitating antibodies to the fungus, production of mucus, and globlet cell metaplasia. Pulmonary fibrosis and tissue remodeling can also be seen in the late phases of the disease (18, 19). Such characteristics are common to all forms of ABPM regardless of the fungal organism that triggers the disease. Only three cases of ABPM due to Pseudallescheria/Scedosporium spp. have been reported in the literature (232, 294, 357).
Although uncommon, recent reports have implicated P. boydii/S. apiospermum as a cause of otitis externa; however, the organism may have been a saprobe of the external auditory canal (37, 486). Saprobic involvement of the auditory canal occurs when constant drainage keeps the canal moistened, as occurs in the setting of chronic otitis media with perforated tympanic membranes or when the canal is filled with desquamating debris from chronic seborreic or psoriatic dermatitis. The saprobic states of P. boydii follow closely the patterns of Aspergillus spp. (230). The saprobic states of S. prolificans also involve the external auditory canal, skin, and respiratory tract, particularly in patients with cystic fibrosis, liver transplant, or HIV/AIDS (97).
Nonmycetoma Infections
Localized infections.
Localized nonmycetoma infections can be subclassified into sinopulmonary and extrapulmonary infections.
(i) Sinopulmonary infections.
Following the most common route of entry, the respiratory tract, and uncontrolled by a suppressed immune response, scedosporium conidia may germinate and hyphae invade, typically producing a necrotizing pneumonia. Other than in near-drowning victims, this is a very rare event in the immunocompetent host (217, 370).
Among the clinical features of sinopulmonary infection caused by Scedosporium spp., fever is the single most common clinical sign and symptom in most series, fluctuating between 76 and 100% (73). Other common clinical symptoms were dyspnea and pleuritic chest pain. Chest radiographic findings varied from focal unilateral to bilateral diffused infiltrates and from nodules to bronchopneumonia. Numerous publications address the clinical manifestations of pulmonary P. boydii (16, 160, 258, 259, 327, 387, 407, 455), Scedosporium apiospermum (73, 126, 233, 304, 351, 390, 432), and S. prolificans (35, 170, 204, 233, 257, 265, 354, 470) infections. The symptoms include fever, productive cough, hemopthysis, rales, and rhonchi. Already by the time of diagnosis of scedosporium pneumonia, there may be skin manifestations of disseminated disease in many patients. Such manifestations can appear as a maculopapular rash or as nodular lesions that can enlarge and become necrotic. Myalgias and focal CNS signs are other common manifestations (208), underlying the potential rapid hematogenous dissemination of scedosporium from a pulmonary site.
Sinusitis may present among immunocompetent as well as in immunocompromised patients (174). Such presentation varies widely, with localized mucosal involvement that is relatively easy to treat versus invasive, destructive infection that requires prolonged and combinations of therapies. A case in point is that of a 77-year-old woman from Maryland with a history of recurrent sinusitis for 30 years who noted persistent pain over the right maxillary sinus. Although treatment with nasal decongestant and trimethoprim-sulfamethoxazole was instituted, a naso-antral window was created 6 months after the onset of symptoms. A needle aspirate of the right maxillary sinus contained greenish material with branching septate hyphae. No mucosal involvement was appreciated. No culture was performed. Due to persistence of pain, a computed tomography (CT) scan evaluation was done 3 months later, and a Caldwell-Luc procedure allowed removal of a large volume of greenish material. Again the histopathological evaluation showed a mass of entangled septate hyphae with 45° angle branching and normal respiratory mucosa. A culture grew P. boydii. Seven months after the operation a follow-up CT scan demonstrated that the right maxillary sinus was clear. Symptoms resolved with surgical drainage (463). Outcomes are a direct function of the host immune status (80). A brain abscess caused by S. apiospermum was diagnosed postmortem in a female child with chronic suppurative otitis media and no other immune deficiency (2).
(ii) Extrapulmonary infections.
(a) Cutaneous and subcutaneous infections. Pseudallescheria boydii/S. apiospermum and S. prolificans can be etiologic agents of other localized infections such as cutaneous infections. These infections most likely result from local inoculation of the fungus in the cutaneous and subcutaneous tissues, usually in individuals with some degree of immune deficiency (e.g., from steroid therapy or immunosuppressant therapy) (79, 154, 156, 240, 253, 293, 296, 323, 353, 448, 475, 482). Cutaneous infections by Scedosporium spp. can mimic infections caused by Aspergillus spp., as each can present with ecchymosis, necrotic papules, and hemorrhagic bullae (53). They may also present clinically as solitary ulcers, infiltrative erythematous plaques and nodules, or suppurative nodules and ulcers in a sporotrichoid (lymphangitic) pattern (297, 445). Localized cutaneous disease due S. apiospermum/P. boydii in immunocompromised individuals has been reported (36, 49, 53, 79, 114, 156, 299, 432, 455).
Scedosporium prolificans also has been reported to cause local disease (154). In the immunocompromised host (people receiving chronic steroid therapy for chronic obstructive pulmonary disease or pulmonary fibrosis or receiving immunosuppressive therapy for rheumatoid arthritis), soft tissue infections due to S. prolificans are on the rise (35, 124, 312, 423). A syndrome of nodular lymphangitis or lymphocutaneous syndrome due Scedosporium apiospermum has been reported (61, 180, 221, 229, 445).
Infections in the soft tissues may be the initial presentation of the disease or a sign of hematogenous dissemination. Numerous other skin manifestations, such as cutaneous ulcer, nodules, subcutaneous abscesses, folliculitis, and bullous necrotic purpura, have been described in Scedosporium infections (297). As with other mold infections, the increasing number of highly immunosuppressed individuals and the more frequent use of invasive procedures may explain the increasing number of cutaneous scedosporium infections. The clinical picture may be misleading, as in a case of a 25-year-old immunocompetent woman who was misdiagnosed with tuberculous lymphadenitis when she developed cervical and submandibular lymphoadenopathy that failed to respond to antituberculous therapy. Only after a repeat biopsy of a lymph node with careful histologic examination of the specimen stained with Grocott-Gomori methenamine silver showed branched septate hyphae, and the fungal culture grew S. apiospermum, was the correct diagnosis made (221).
Distinguishing between cutaneous and subcutaneous involvement can be difficult, as the adjacent skin may have some degree of involvement in a clearly subcutaneous infectious process (6, 22, 35, 36, 46, 47, 52, 53, 57, 79, 82, 114, 124, 129, 154, 156, 166, 167, 202, 218, 222, 228, 229, 253, 255, 260, 293, 296, 297, 299, 314, 323, 337, 343, 353, 381, 392, 398, 416, 419, 440, 445, 448-450, 461, 475, 482).
(b) Bone, muscle, and joint infections. Osteomyelitis, septic arthritis, or wound infections usually occur when barriers are breached secondary to trauma or surgery (253, 297). There have been numerous reports of infections in immunocompetent individuals, including osteomyelitis, discitis, and arthritis (95, 145, 155, 245, 268, 409, 443, 476), usually following deep extension of local disease. Among immunocompromised hosts, bone and articular involvement is more likely to be secondary to hematogenous dissemination of the fungus (242). Most patients with joint infection have a history of local trauma, local surgery, or intra-articular steroid use (428). The clinical presentation of localized infection affecting the joint is that of an acute septic arthritis with the five signs of phlogosis: edema, erythema, pain, loss of function, and localized increased of temperature. The classical description of septic arthritis with bone involvement with P. boydii or Scedosporium spp. is in a young male with history of a penetrating traumatic injury to soft tissues around the knee joint. Following severe immunosuppression (in the immunocompromised patient) or penetrating trauma, local surgery, or corticosteroid injections (in the immunocompetent individual), tenderness, erythema, and inflammation ensue. Plain radiography may be normal in the earlier stages of the infection, but magnetic resonance imaging (MRI) may reveal joint effusion and enhancing synovitis and sometimes subcondral bone enhancing, possibly signifying early osteomyelitis. Symptoms may be present for as short a time as a few days to weeks, and the organism may not be identified until culture of the articular fluid or synovial biopsy is performed. A case of Scedosporium apiospermum pyomyositis developed 1 year after a patient with acute myeloblastic leukemia had discitis, lumbar vertebrae osteomyelitis, and sternoclavicular joint arthritis during fever and neutropenia (320).
(c) CNS. In the immunocompromised host, infections with P. boydii/Scedosporium spp. occur in the same clinical setting as aspergillus infections: patients with hematological malignancies and those receiving immunosuppressive agents, including cyclosporine, azathioprine, tacrolimus, and corticosteroids. The presence of signs and symptoms of a space-occupying lesion, such as headache, fever, and focal neurologic deficits, suggest the presence of brain abscess (7, 9, 110, 137, 216, 310, 329, 375, 465). Patients who develop P. boydii/Scedosporium apiospermum infections after a near-drowning experience tend to develop clinical signs and symptoms from a few days to several weeks after the noxious event. Often these patients will have survived an initial acute lung injury and bacteremia or sepsis episode, which was diagnosed and treated after hospital admission. Metastatic skin lesions may herald fungemia. Single or multiple brain abscesses can be found.
Meningitis due to P. boydii is an uncommon event and is usually associated with immunosuppression or near drowning, CSF drainage devices, and rachianesthesia. The prognosis is poor (the mortality rate is >75%). The meningitis normally follows an acute and fatal course and coexists with cerebral infarcts and/or abscesses (34). Chronic presentation is even more uncommon, usually appearing in immunocompetent individuals; however, the prognosis does not change (17, 216). In immunocompetent individuals rachianesthesia and wounds near the CNS have been identified as risk factors for developing CNS infection with P. boydii. In the immunocompromised patient, meningitis and meningoencephalitis have a more rapid course despite timely treatment (264). The clinical presentation is typically that of headache and lower back pain. Physical examination may show papilledema, stiff neck, sixth nerve paralysis, and diminished deep tendon reflexes, and possibly bilateral Lasegue's sign can be present. The CSF may show hypercellularity, an elevated protein level, and a low-normal glucose level. Culture of the CSF may be negative. Cranial CT may be normal. Spinal MRI may show lumbosacral arachnoiditis (344).
(d) Ocular infections. Localized infection secondary to traumatic inoculation of the fungus, surgery, or contiguous spread from an adjacent site is the usual mechanism by which immunocompetent individuals acquire Scedosporium infection in the eye (98, 102, 303, 412, 484). Ocular manifestations of dissemination of disease in immunocompromised individuals are more compatible with endophthalmitis (130, 281).
Keratitis is another localized infection caused by these fungi that has significant associated morbidity and threatens eyesight. Although these infections may be uncommon, there are numerous reports in the medical literature supporting the devastating consequences of infections involving the cornea and sometimes deeper tissues in the ocular globe (40, 43, 98, 101, 102, 112, 115, 187, 226, 231, 241, 246, 252, 295, 303, 316, 317, 328, 331, 364, 369, 394, 404, 412, 424, 484, 491). Keratitis is perhaps the most common clinical manifestation of Scedosporium infection among immunocompetent hosts. Keratitis presents with local pain, photophobia, decrease visual acuity, and lacrimation. The clinical presentation of keratitis usually follows local trauma to the eye, in particular to the cornea, perhaps with a small abrasion or ulceration. Coinfection of scedosporium and acanthamoeba was reported in a 45-year-old woman with contact lenses and a 27-year-old man with previous injury due to a metallic thread contaminated with sewage (136, 369).
The most common clinical manifestations of culture-proven mycotic keratitis are a gray to off-white surface, anterior chamber cellular reaction, irregular margins, elevated borders, dry rough texture, satellite lesions, Descemet's folds, hypopyon, ring infiltrate, endothelial plaque, and keratitic precipitates (360). Keratitis caused by Scedosporium spp. resembles other types of keratitis in predisposing factors and clinical presentation but may not respond satisfactorily to medical therapy alone (442).
Even if infection is suspected, the lack of clinical suspicion for fungal etiology may delay the accurate diagnosis and treatment. Moreover, clinicians frequently prescribe antibiotics and/or steroids without prior culturing of the secretions, and in this matter the diagnosis is delayed even further, allowing spread of the infection to deeper tissues. Inflammation is typically treated with topical steroids, which may further impair local host defenses. Even when treated appropriately, keratitis can leave residual leukomas that can be very incapacitating. (484, 491). Sometimes penetrating keratoplasty with removal of traumatic cataract and intraocular lens implantation are required. Moreover, enucleation of the globe has been necessary in certain aggressive cases that were unresponsive to therapy (231, 303, 404, 424). Other cases of Scedosporium keratitis have had better outcomes (187). A 35-year-old woman had local ocular trauma at a glass factory. Two days later upon evaluation, her visual acuity was 20/40 and a small, superficial corneal abrasion without infiltrates was noted. Bacitracin was prescribed; however, 2 days later, progression of the infiltrates led to corneal scraping, which showed fungal elements on the wet mount. Fungal cultures yielded S. apiospermum. The final best-corrected visual acuity was 20/50.
Infectious scleritis due to Scedosporium spp. following pterygium excision surgery with adjunctive beta-radiation and mitomycin C has been reported and represents one of the most difficult complications seen after pterygium excision (302, 303, 404, 424). The use of these adjuvant therapies may destroy episcleral and conjunctival vessels, inhibit adequate wound healing, and therefore leave the sclera with a diminished ability to resist infection. It is also believed that the use of the bare sclera technique and excessive use of cautery may also contribute to infectious scleritis (284). Clinical manifestation of scleritis, episcleritis, and sclerokeratitis are similar, with ocular pain, lacrimation, purulent ocular discharge, and chemosis.
Endophthalmitis due to P. boydii/Scedosporium spp. has been reported extensively (112, 130, 236, 261, 283, 335, 417). Endophthalmitis can be classified depending on how the microorganism reaches the deeper ocular tissues, as endogenous (via hematogenous dissemination to the eye) or exogenous (via direct introduction of the fungus during eye trauma or surgery or from preexisting scleritis or keratitis). Endogenous endophthalmitis is the result of metastatic spread of the infection from a distant site. The eye becomes the target of multiple microabscesses. This type of endophthalmitis is more common among immunocompromised hosts, patients receiving chemotherapy or total parenteral nutrition, and i.v. drug users (67, 130, 133, 259, 283, 335, 451, 482). P. boydii has been identified as the etiologic agent in a case of endogenous endophthalmitis from an infected porcine allograft of the aortic valve (417) and even in a patient without recognizable risk factors (324).
The clinical presentation of the endophthalmitis is usually ocular pain and photophobia and sometimes blurred vision or spots in the patient's visual fields. On fundoscopic examination, endophthalmitis is recognized by the presence of one or more creamy-white, well-circumscribed lesions of the choroids and retina, often accompanied by an inflammatory infiltrate in the vitreous chamber. Often there also is inflammation of the anterior chamber, presenting itself as a hypopyon. Patients with endogenous endophthalmitis may have positive blood cultures.
Exogenous endophthalmitis occurs by introduction of microorganisms into the eye. Patients with exogenous endophthalmitis are rarely immunocompromised (50, 66, 157, 438). Cataract removal and placement of intraocular lens and corneal transplantation are the surgical procedures most commonly associated with postoperative fungal exogenous endophthalmitis (224). Posttraumatic endophthalmitis due to penetrating wood chip trauma in the cornea rendered enucleation necessary to control the infection (438).
Other ocular infections due to P. boydii/Scedosporium spp. that have been reported include corneal ulcerations, conjunctival mycetoma, keratouveitis, retinitis, corioretinitis, endophthalmitis, and orbital infections (20, 43, 210, 220, 226, 227, 281, 295, 364, 424, 438). Other infections of the eye, such as chorioretinitis, have been reported to have originated in local lymph nodes infected with S. apiospermum (220).
More rarely, local invasion from a local fungal pansinusitis has resulted in orbital infection (210). Orbital infection also has been associated with facial penetrating trauma, as in the case of a 10-year-old male who was struck under the right eye by a wooden stick, leading to the formation of a fistulous tract where P. boydii was isolated (318).
(e) Bloodstream infections and endocarditis. Bloodstream infection is found in two-thirds of patients with disseminated S. prolificans and S. apiospermum infection (326). In a review of scedosporiosis in transplant recipients, Husain et al. (201) reported fungemia in 40% (4/10) of patients with S. prolificans, compared with 4.7% (2/43) of those with S. apiospermum infections. Fungemia was present in 7 (33%) of 21 HSCT recipients, compared to 6 (11%) of 56 organ transplant recipients (P = 0.04).
The high frequency of bloodstream infections may be related to the ability of these organisms to propagate in vivo and possibly to adventitious conidiation. Mycotic aneurysm and large mural vegetations due to S. apiospermum and native and prosthetic valve endocarditis due to both S. apiospermum and S. prolificans have been reported to occur in transplant recipients as well as in i.v. drug users and even immunocompetent individuals. S. apiospermum was isolated from the mitral valve vegetations removed by surgery and blood cultures in an immunocompetent woman who had suffered a multiple severe injuries in a car accident. The same fungus was isolated from the patient's left-side scalp lacerations immediately after the accident, approximately 2 months earlier. Due to persistent fungemia, the valve was replaced; however, hemodynamic instability continued. The patient died after a CT scan showed a large parieto-occipital brain abscess. S. apiospermum was isolated from the brain abscess at autopsy (408). This case illustrates the embolization capacity of very small microvegetations as well as the relevant information that can be gained by blood cultures in infections caused by Scedosporium spp. The clinical implication of this finding is that a significant percentage of patients could be diagnosed by blood culture.
(f) Other infections. Other clinical syndromes due to Scedosporium spp. include otomycosis, onychomycosis, chronic prostatitis, peritonitis, esophagitis, renal infection, and hepatosplenic abscesses. Onychomycosis also has been reported in the literature. In a study conducted in Spain by Garcia-Matos et al., Scedosporium spp. were found in 3% of cases of onychomycosis in toenails (142).
Disseminated infections.
Disseminated infections are usually seen among immunocompromised patients; however, they have also been reported in immunocompetent patients. Scedosporium spp. can be isolated from blood cultures, and embolization to distal organs (i.e., brain, thyroid, heart, kidneys, or eyes) can occur even in the absence of clinical endocarditis. In the immunocompromised patient the pulmonary route of infection is the most common; however, in multiple instances skin lesions will be the harbinger of hematogenous dissemination of the fungus. Dissemination carries a bad prognosis, especially in the presence of persistent profound neutropenia (402).
DIAGNOSIS
A systematic diagnostic approach is necessary to identify the etiologic agent in an accurate and timely manner. Delays in diagnosis would most likely delay appropriate therapy, with potentially fatal consequences, especially in the immunocompromised patient. A diagnosis of Scedosporium infection can be based on cytology and histopathology with isolation of the fungus in culture. Several diagnostic techniques can be applied to Scedosporium infections: microbiology (direct staining and culture), histopathology, radiology, and serological and molecular biology. However, the type of infection (mycetoma or nonmycetoma) may determine the importance of some of these diagnostic techniques.
Mycetoma
The diagnosis of mycetoma is based on the presence of the following triad of clinical symptoms plus the typical location of the lesion on a foot (particularly in areas of endemicity): (i) tumefaction (subcutaneous tissues become edematous), (ii) multiple draining sinuses, and (iii) extrusion of grains. Pus, exudate, bandage gauze, and biopsy tissue should be macroscopically examined for the presence of grains. If found, pale P. boydii grains should be mounted on a microscope slide for fresh examination and cultured. Histologic sections can be stained with hematoxylin and eosin (H&E), periodic acid-Schiff (PAS) stain, or Grocott-Gomori methenamine silver. Grains may be difficult to locate in a histopathological section, and this may require multiple cuts through the paraffin block. Organisms are usually not seen outside the grain. The H&E stain can be helpful in detecting the grains. Tissue Gram stain detects fine, branching hyphae in the actinomycetoma grain. Grocott-Gomori methenamine silver or PAS stain, in the case of pale grains, detects the larger hyphae of eumycetoma grains. A more specific diagnosis is based on isolation of the organism.
Nonmycetoma Infections
Pseudallescheria boydii/S. apiospermum and S. prolificans are not pathogens commonly seen in the clinical laboratory. Therefore, when they are isolated it is always important to take into account the source of the specimen, the number of times the fungus was isolated (especially if isolated from sputum), and the underlying condition of the patient from whom the specimen was collected.
Direct Microscopy
Histochemical staining techniques include immersion of samples in 20% KOH followed by fluorescence microscopy using the Uvitek 2B, Blankophor, or calcofluor white M2R (365, 366), which is a general stain that does not discriminate among the various filamentous ascomycetes. Kaufman et al. reported the use of a polyclonal fluorescent antibody that successfully identified S. apiospermum (214). Clinical samples from mycetoma are extruded serosanguinous fluid containing grains or pieces of a biopsy specimen. Characteristics of shape, texture, and color can help identify grains to the genus level. Grains of scedosporium are white and large (1 to 2 mm), with a lobed surface. A significant eosinophilic zone usually surrounds them, which is not present around grains of other agents of white mycetoma (184).
Culture
Obtaining a good, deep-seated biopsy specimen will be key for providing both histological and microbiological diagnoses. An alternative strategy for diagnosis of mycetoma is the aspiration of grains directly from an unopened sinus tract for microscopic examination and culture. However, if extruded grains are used, most experts recommend that the grains be rinsed in 70% ethanol and washed several times in sterile saline to decreased bacterial contamination before inoculation in culture media. In cases of nonmycetoma infections, samples from the respiratory tract (tracheal aspirates, BAL fluid, induced sputum ear or sinus samples, or tissue) and biopsy specimens from other sites should be carefully handled to avoid contamination. The fungus grows well on routine fungal media such as Sabouraud glucose agar, blood agar, and chocolate agar. The fungus also grows in selective media such as modified Leonian agar supplemented with 10 μg/ml benomyl (425), medium containing cycloheximide (8 mg/ml), and medium with amphotericin B (203). These media have allowed the selective growth of Scedosporium over other filamentous fungi such as Aspergillus spp. in clinical samples. Such media (benomyl agar or cycloheximide agar) are recommended for isolation of Scedosporium spp. in particular when dealing with BAL fluid or other respiratory tenacious secretions, which often contain more rapidly growing Aspergillus spp. and Candida spp. (81, 425). Isolation of Scedosporium spp. from CSF and from brain, meninges, and other deep tissues has been possible. However, even when infected, CSF culture for scedosporium may be negative or delayed by several weeks (7, 34, 108, 399, 487). The incubation temperature for growth for all strains is 25 to 35°C; not all strains will grow at 37°C. However, some will grow at 42°C. The growth response of S. apiospermum and S. prolificans is a useful feature for distinction between the two species (106, 376).
Scedosporium spp. can be isolated from blood cultures with a much higher frequency than Aspergillus spp. In a series in Spain from 1990 to 1999 reported by Idigoras et al., the most common filamentous fungus isolated in blood cultures was S. prolificans (204). For a series of 16 patients, Berenguer et al. reported that 75% (12/16) had a positive blood culture (35). Therefore, a simple and high-yield specimen for culture is whole blood.
Histopathology
The clinical presentation and even the findings on cytopathology and/or histopathology of Scedosporium spp., Aspergillus spp., Fusarium spp., Petriella spp., and other hyalohyphomycotic organisms are very similar (Fig. 10). All of these organisms produce hyaline (nonpigmented) hyphal structures that display septation at regular intervals, have dichotomous branching, and may show angioinvasion. However, Scedosporium and Aspergillus may have some differences in histopathological preparations of tissue and fluids. Aspergillus displays a regular, dichotomous branching pattern in cytology and histopathological sections, while Scedosporium may present a more irregular branching (460). Similar to the case for Aspergillus fumigatus, branching in Scedosporium spp. may be at acute angles and dichotomous (127, 179). Scedosporium spp. may also present terminal or intercalary chlamydospores (460) that can be confused with yeasts.
FIG. 10.
Scedosporium apiospermum infection in human tissue. The main host response is a mixed neutrophilic and monocytic infiltrate. PAS staining of brain tissue in a patient with CNS scedosporiosis was performed. Magnification, ×100.
Tissue invasion and angioinvasion have been reported in brain parenchyma in cases of brain abscesses (7, 308). In cases of meningitis, CSF may be frankly purulent, revealing an elevated white blood cell count, with neutrophil predominance, but also multinucleate giant cells.
Radiology
The role of radiology in the diagnosis of mycetoma is limited to the assessment of the extent of the disease and involvement of bone and possibly follow-up of disease progression or regression. Standard X-ray studies may reveal periosteal reaction, secondary to osteomyelitis, or osteoporosis lytic lesions. The role of ultrasonography has been evaluated in a study including 100 patients with foot swelling prior to surgical excision (121). In that study, eumycetomas were found to have single or multiple thick-walled cavities without acoustic enhancement, and the grains were represented as hyperreflective echoes. Actinomycetomas produced similar radiological findings; however, their grains were represented as fine echoes at the bottom of the cavities. MRI and CT in the diagnosis of mycetoma can provide an accurate assessment of the extent of disease and soft tissue involvement (396).
In pulmonary or brain infections, the radiologic lesions are very similar to those caused by other infections. Pulmonary infection simulates aspergillosis, with fungus balls in preexisting cavities and abscesses with central cavitation (217). Scedosporium can cause necrotizing pneumonia, especially among immunocompromised hosts. However, the “air crescent” sign seem to be more frequent in cases of pulmonary aspergillosis or Pseudomonas infection in the neutropenic patient (59). CNS involvement can also occur via direct extension of the disease or hematogenous dissemination of the fungus. Standard X-ray, CT, and MRI are used in the assessment of disease; however, there are no pathognomonic radiologic findings. Radiographic findings of pulmonary infections due to Scedosporium spp. are variable and can include solitary or multiple nodular lesions with or without cavitation, focal infiltrates, lobar infiltrates, and bilateral diffused infiltrates.
Serology
Infections due to scedosporium can be detected using antigen detection by counterimmunoelectrophoresis, but cross-reactions with antigens from other fungi such as Aspergillus spp. may occur (81). Pinto et al. (339) isolated a peptidopolysaccharide, a peptidorhamnomannan antigen from mycelial forms of P. boydii. The authors also provided evidence that although these antigens are similar to Sporothrix schenckii peptidosaccharides they do not cross-react with these peptidosaccharides and that they differ from the major aspergillus glyconjugates. It is therefore possible that peptidorhamnomannan may be developed into a diagnostic antigen test for P. boydii in the near future (339).
Molecular Diagnostics
PCR-based identification of invasive scedosporiosis/pseudallescheriasis is another promising tool. Wedde et al. in 1998 reported on a species-specific PCR that could differentiate P. boydii/S. apiospermum from S. prolificans (467). Direct sequencing of DNA was critical in the timely diagnosis of mycotic keratitis in a patient with Scedosporium apiospermum infection (271). Several authors have suggested that molecular methods of detection may very well parallel classical techniques (271). Despite the obvious limitation of molecular diagnostic in mycology, with a lack of standardized techniques and complete and well-referenced sequence databases, it complements rather than substitutes for clinical skill and conventional diagnostic mycology.
TREATMENT AND OUTCOMES OF SCEDOSPORIOSIS
In Vitro Susceptibilities to Single Antifungal Agents
The data on the in vitro susceptibilities of S. prolificans and S. apiospermum isolates to various members of different classes of antifungal agents (azoles, polyenes, allylamines, pyrimidines, and echinocandins) have been reviewed, and the results are summarized in Table 4. Although different MIC endpoints and incubation periods have been used, there are no validated interpretive breakpoints for determining resistance to any antifungal agent. One of the most common in vitro methods used to assess the susceptibility of scedosporium isolates is broth micro- and macrodilution, a methodology of the M38-A protocol of the Clinical and Laboratory Standards Institute (CLSI) (307). However, it is important to underscore that no interpretive breakpoints exist for either of these organisms.
TABLE 4.
In vitro drug susceptibilities of Scedosporium spp.
| Species and drug | Reference(s) | No. of strains | Methoda | Endpointsb | Incubation time (h) | MIC, μg/ml
|
|||
|---|---|---|---|---|---|---|---|---|---|
| Range | Median geometric mean (range) | 50% (range) | 90% (range) | ||||||
| S. prolificans | |||||||||
| Miconazole | 86, 286 | 98 | M38-A-like | MIC-0, -1 | 48-72 | 4->64 | 9.85->16 | 8->16 | >16-64 |
| Itraconazole | 68, 86, 286 | 131 | M38-A-like | MIC-0, -1, -2 | 48-72 | 8->32 | 15.36 (16->32) | >16 (>16->32) | >16 (>16->32) |
| 117, 430 | 5 | Etest | No growth | 48 | >32 | NAc | NA | NA | |
| 347 | 6 | Agar dilution | No growth | NA | >64 | NA | NA | NA | |
| 69 | 18 | Yeast one | Red well | 72-120 | ≥16 | NA | NA | ≥16 | |
| Albaconazole | 68, 286 | 98 | M38-A | MIC-1, -2 | 72 | 0.01-8 | 0.35-1.97 | 0.5-2 | 2-4 |
| Voriconazole | 68, 86, 286 | 131 | M38-A-like | MIC-0, -1, -2 | 48-72 | 0.06-32 | 3.29 (1.83-16) | 4 (2-16) | 4 (4-16) |
| 347 | 6 | Agar dilution | No growth | NA | 4 | NA | NA | NA | |
| Posaconazole | 68, 286 | 88 | M38-A | MIC-1, -2 | 72 | 0.25->16 | >8-10.55 | >8-16 | >8-16 |
| Ravuconazole | 68, 85 | 68 | M38-A-like | MIC-0, -2 | 48-72 | 0.5->16 | 8.9->8 | >8-16 | >8-16 |
| Fluconazole | 86 | 43 | M38-A-like | MIC-0 | 48 | >16 | >16 | >16 | >16 |
| 69 | 18 | Yeast one | Red well | 72-120 | 32-≥256 | NA | NA | ≥256 | |
| Ketoconazole | 68, 86 | 78 | M38-A-like | MIC-0, -2 | 48-72 | 0.5->16 | 11.52->16 | >16 | >16 |
| 69 | 18 | Yeast one | Red well | 72-120 | ≥16 | NA | NA | ≥16 | |
| Terbinafine | 141, 286 | 75 | M38-A-like | MIC-0, -1 | 48-72 | 2->32 | 16.5-19.16 | 16 | 16-32 |
| Amphotericin B | 68, 86, 286 | 131 | M38-A-like | MIC-0 | 48-72 | 0.125->16 | >16 (6.48->16) | >16 (8->16) | >16 (16->16) |
| 117, 430 | 5 | Etest | No growth | 48 | >32 | NA | NA | NA | |
| 69 | 18 | Yeast one | Red well | 72-120 | 2->16 | NA | NA | >16 | |
| Nystatin | 68, 286 | 88 | M38-A | MIC-0 | 72 | 4->32 | 4.13-32 | >16-32 | >16->32 |
| l-Nystatin | 286 | 55 | M38-A | MIC-0 | 72 | 16-32 | 32 | 32 | 32 |
| Flucytosine | 86 | 43 | M38-A-like | MIC-0 | 48 | >16 | >16 | >16 | >16 |
| 69 | 18 | Yeast one | Red well | 72-120 | ≥64 | NA | NA | ≥64 | |
| Caspofungin | 116 | 2 | M38-A | MIC-2 | 72 | 4-8 | NA | NA | NA |
| 99 | 2 | Broth macrodilution | 80% | 72 | 6.25-12.5 | 8.83 | NA | NA | |
| Anidulafungin | 116, 321 | 7 | M38-A | MIC-2 | 72 | 4->16 | 8 | NA | NA |
| Micafungin | 489 | 17 | M38-A | MIC-2 | 48 | >32 | >32 | >32 | >32 |
| 188 | 3 | XTT color | MIC-2 | 24 | >128 | NA | >128 | NA | |
| S. apiospermum | |||||||||
| Miconazole | 86, 286, 492 | 57 | M38-A-like | MIC-0, -1 | 48-72 | 0.06-4 | 0.5-0.58 | 0.5 (0.25-0.5) | 1 (0.5-2) |
| Itraconazole | 68, 86, 162, 286, 492 | 96 | M38-A-like | MIC-0, -1, -2 | 48-72 | 0.03->16 | 1.56 (0.78-8) | 2 (0.5->16) | 4 (1->16) |
| 117, 430 | 5 | Etest | No growth | 48 | 0.5-32 | NA | 1.5 | >32 | |
| 347 | 6 | Agar dilution | No growth | NA | 0.12->64 | NA | NA | NA | |
| 69 | 10 | Yeast one | Red well | 72-120 | 0.5-≥16 | NA | NA | ≥16 | |
| Albaconazole | 68, 286 | 24 | M38-A-like | MIC-1,-2 | 72 | 0.06-2 | 0.41-1 | 0.5-1 | 1-2 |
| Voriconazole | 68, 86, 162, 286, 492 | 96 | M38-A-like | MIC-0, -1, -2 | 48-72 | 0.01-2 | 0.17 (0.06-1.13) | 0.25 (0.06-2) | 0.5 (0.125-2) |
| 347 | 6 | Agar dilution | No growth | NA | 0.12-0.5 | NA | NA | NA | |
| 251 | 6 | Yeast one | Purple well | 72 | 1 | NA | 0.5 | 1 | |
| Posaconazole | 68, 162, 286 | 52 | M38-A-like | MIC-1, -2 | 72 | 0.03-2 | 0.79 (0.08-1.05) | 1 (0.03-1) | 2 (0.25-2) |
| Ravuconazole | 68, 162, 286 | 93 | M38-A-like | MIC-0, -2 | 48-72 | 0.125-8 | 2.21 (0.125-3.5) | 2 (0.125-4) | 4 (0.125->8) |
| Fluconazole | 86, 162, 492 | 72 | M38-A-like | MIC-0, -1, -2 | 48-72 | 4-64 | 16 (12.16->16) | 16 (16->16) | 32 (>16->64) |
| 69 | 10 | Yeast one | Red well | 72-120 | 16-≥256 | NA | NA | ≥256 | |
| Ketoconazole | 68, 86 | 38 | M38-A-like | MIC-0, -2 | 48-72 | 0.06->16 | 2-10.07 | 2-16 | <16 |
| 69 | 10 | Yeast one | Red well | 72-120 | 0.25-≥16 | NA | NA | ≥16 | |
| Terbinafine | 141, 286 | 44 | M38-A-like | MIC-0, -1 | 48-72 | 8->32 | 17.1->32 | >32 | >32->16 |
| Amphotericin B | 86, 286, 492 | 68 | M38-A-like | MIC-0 | 48-72 | 1->16 | 4 (2.97-4) | 4 (4-4) | 16 (16->16) |
| 117, 430 | 5 | Etest | No growth | 48 | 2->32 | NA | >32 | >32 | |
| 69 | 10 | Yeast one | Red well | 72-120 | 2->16 | NA | NA | 4 | |
| Nystatin | 68, 286 | 24 | M38-A | MIC-0 | 72 | 4-32 | 12.7-13.24 | 16 | >16-32 |
| l-Nystatin | 286 | 13 | M38-A | MIC-0 | 72 | 4-16 | 11.99 | 16 | 16 |
| Flucytosine | 85, 492 | 44 | M38-A-like | MIC-0, -1 | 48-72 | 8->64 | >16->64 | >16->64 | >16 |
| 69 | 10 | Yeast one | Red well | 72-120 | 4-≥64 | NA | NA | ≥64 | |
| Caspofungin | 116, 334 | 11 | M38-A | MIC-1, -2 | 72 | 0.25-4 | 1.3 | 0.5 | NA |
| 99 | 4 | Broth macrodilution | 80% | 72 | 0.19-0.78 | 0.38 | NA | NA | |
| Anidulafungin | 116, 321, 334 | 17 | M38-A | MIC-1, -2 | 72 | 1->16 | 2.5-4 | 1 | NA |
| Micafungin | 489, 492 | 36 | M38-A | MIC-1, -2 | 72 | 1->32 | 5.25 | 2->16 | >16 |
| 188 | 3 | XTT color | MIC-2 | 24 | 0.25-128 | NA | 32 | NA | |
M38-A, CLSI M38-A reference method for broth dilution antifungal susceptibility testing of filamentous fungi (307). M38-A-like, method which modifies the M38-A reference method.
”Red well” and “purple well,” endpoints used in colorimetric assays. MIC-0, -1, and -2, endpoints by visual inspection, where MIC-0 is optical clearance, MIC-1 is +1 turbidity, and MIC-2 is +2 turbidity.
NA, not available.
All in vitro studies have shown high MICs for S. prolificans, with the median MICs being higher than 8 μg/ml for most of the antifungal drugs. Among the available triazoles, the best in vitro activity was found with voriconazole (median MIC50s of 4 μg/ml). However, this value exceeds the free drug concentration of voriconazole in most patients. None of the other azoles or the polyenes amphotericin B and nystatin, the allylamine terbinafine, and the pyrimidine 5-flucytosine showed appreciable in vitro activity against S. prolificans, as the MICs for most of the isolates were off scale. However, it is noteworthy that for some isolates low MICs were observed, particularly when a less stringent endpoint was used, indicating the presence of some in vitro antifungal activity that could potentially be enhanced if combined with other agents. Among the echinocandins, no activity was found for anidulafungin and micafungin, whereas caspofungin could inhibit the growth by 50% at on-scale concentrations (4 to 8 μg/ml). These results were also confirmed with other methodologies such as agar dilution tests, agar diffusion-dilution tests (e.g., Etest), and colorimetric assays (e.g., Yeastone, using XTT [2,3-bis{2-methoxy-4-nitro-5-[(sulfenylamino)carbon-yl]-2H-tetrazolium-hydroxide}]), indicating the in vitro multiresistance of S. prolificans.
S. apiospermum seems to be more susceptible to systemic antifungal agents than S. prolificans. Most in vitro studies showed significant antifungal activity for all azoles except fluconazole and ketoconazole; however, low MICs were observed for some isolates (Table 4). The most potent in vitro activity was observed with voriconazole (median MIC50 of 0.25 μg/ml), followed by miconazole (median MIC50 of 0.5 μg/ml) and albaconazole (median MIC50s of 0.5 to 1 μg/ml). Terbinafine and 5-flucytosine did not show any antifungal activity for almost all of the isolates tested. Among the polyenes, amphotericin B showed limited in vitro antifungal activity (median MIC50 of 4 μg/ml). Echinocandins showed antifungal activity, with caspofungin being the most active (median MIC50 of 0.5 μg/ml), followed by anidulafungin (median MIC50 of 1 μg/ml). This susceptibility profile also was confirmed with other in vitro methodologies (Table 4).
In Vitro Susceptibilities to Combinations of Antifungal Agents
In search of new strategies against scedosporium isolates and given the observation that despite the high median MICs, antifungal activity was found for some isolates, combinations of antifungal agents have been studied. The results of these studies are summarized in Table 5. Despite the high in vitro MICs or absence of in vitro antifungal activity of terbinafine and azoles against S. prolificans, the combination of terbinafine with itraconazole, miconazole, and voriconazole resulted in synergy for more than 85% of the isolates tested, reducing the MICs by more than 16 times at clinically achievable concentrations (287, 288). This synergistic interaction was explained based on the mechanisms of action of azoles and terbinafine, which block different steps of the same pathway of fungal ergosterol biosynthesis. Synergy also was found for a large collection of S. prolificans isolates for the combination of amphotericin B with the protein synthesis inhibitor antiparasitic drug pentamidine, which reduced high amphotericin B MICs at 1 to 4 μg/ml (4). This interaction may be explained in fashion similar to that for amphotericin B-rifampin, where amphotericin B may increase intracellular levels of rifampin (285). The combination of voriconazole and amphotericin B with micafungin was synergistic against S. prolificans and S. apiospermum isolates, although at lower frequencies (31% to 75% of the tested isolates). For those isolates for which the interactions were not classified as synergistic, a significant reduction of the MICs were observed and no antagonism was found (188, 489). This synergistic interaction could be explained by the different mechanisms of action of these drugs, where the action of one agent reduces the resistance to the second agent. Finally, amphotericin B was found to act additively or synergistically with miconazole (76%), fluconazole (88%), and itraconazole (38%) against S. apiospermum isolates (458). No synergy was found between nikomycin Z and itraconazole or fluconazole against two scedosporium isolates (248).
TABLE 5.
In vitro studies of drug combinations against Scedosporium spp.
| Species | No. of strains | Drug combination (drug A + drug B) | Median MIC (range), μg/ml
|
Interactionb (% of strains) | Reference | |||
|---|---|---|---|---|---|---|---|---|
| Drugs alone
|
Drugs in combination
|
|||||||
| Drug A | Drug B | Drug A | Drug B | |||||
| S. prolificans | 20 | Terbinafine + itraconazole | 32 (4->64) | >32 (>32) | 1 (0.5-4) | 1 (0.5-16) | SYN (85), ADD (15) | 288 |
| 5 | Terbinafine + voriconazole | >64 (>64) | 8 (4-16) | 9.51 (2-32) | 0.71 (0.03-2) | SYN (100) | 287 | |
| 5 | Terbinafine + miconazole | >64 (>64) | 64 (8->64) | 2 (1-4) | 2 (0.13-8) | SYN (100) | 287 | |
| 30 | Amphotericin B + pentamidine | 32 (4-32) | 64 (8-128) | 2 (1-4) | 8 (2-32) | SYN (93), ADD (7) | 4 | |
| 4 | Voriconazole + micafungin | 4 (2-4) | 256 (256) | 1 (1-2) | 0.5 (13-64) | SYN (75), ADD (15) | 188 | |
| 17 | Amphotericin B + micafungin | >8 (1->8) | >32 (>32) | 4 (0.5-8) | 16 (0.06-16) | SYN (84), ADD (16) | 489 | |
| S. apiospermum | 3 | Voriconazole + micafungin | 0.13 (0.13-0.25) | 32 (0.25-128) | 0.06 (0.06) | 0.25 (0.13-0.5) | SYN (66), ADD (33) | 188 |
| 19 | Amphotericin B + micafungin | 8 (0.5->8) | 2 (1->32) | 1 (0.12-8) | 1 (0.12-8) | SYN (31), ADD (69) | 489 | |
| 8 | Amphotericin B + miconazole | 1 (0.25-2) | 0.25 (0.13-0.5) | NAa | NA | SYN (13), ADD (63) | 458 | |
| 8 | Amphotericin B + itraconazole | 1 (0.25-2) | 0.25 (0.03-0.5) | NA | NA | SYN (25), ADD (13) | 458 | |
| 8 | Amphotericin B + fluconazole | 1 (0.25-2) | 16 (4-32) | NA | NA | SYN (38), ADD (50) | 458 | |
NA, not available.
Synergy (SYN) was defined as being when fractional inhibitory indices were ≤0.5. Additivity (ADD) was defined as being when fractional inhibitory indices were >0.5 and ≤1. The remaining isolates showed indifferent interactions (fractional inhibitory indices of >1 and <4). No antagonism was observed (fractional inhibitory index, >4).
In Vivo Antifungal Therapy
The in vivo data on chemotherapeutic approaches with antifungal agents studied in different models of experimental scedosporiosis are summarized in Table 6. These data are in concordance with the in vitro resistance of Scedosporium spp. Treatment of experimental scedosporiosis with antifungal agents at the doses used to treat other mold infections was inefficacious; only by delivering higher doses of antifungals was an effect observed.
TABLE 6.
In vivo data for treatment of experimental Scedosporium infection
| Species | Animal model | Infection, CFU/animal | Immunosuppression | Chemotherapy | Dosage (mg/kg)d | Prolonged survival (%) | Fungal burden reduction | Reference |
|---|---|---|---|---|---|---|---|---|
| S. prolificans | Mouse | i.v., 5 × 104 | Cyclophosphamide | Liposomal amphotericin B | 10, 20; q.d. i.v. | 60c | Yes | 45 |
| Caspofungin | 10, 20; q.d. i.v. | 30 | No | |||||
| Rabbit | i.v., 107 | No | Amphotericin B | 0.8; q.d. i.v. | 50 | Yes | ||
| Albaconazole (UR-9825) | 25, 50; p.o. | 50-100 | Yes (eradication) | 65 | ||||
| S. apiospermum | Mouse | i.v., 4 × 106a | Cyclophosphamide + | Amphotericin B | 0.31-2.5; q.d. i.p. | 0 | No eradication | 322 |
| mechlorethamine | Itraconazole | 2.5-20; q.d. i.p. | 0 | No eradication | ||||
| i.v., 2 × 106a | Cyclophosphamide | Amphotericin B | 1.25; q.d. i.p. | 40-50 | No eradication | |||
| Itraconazole | 2.5; q.d. i.p. | 10-40b | No eradication | |||||
| Mouse | i.v., 105 | Cyclophosphamide | Posaconazole | 25; b.i.d., 30-50; q.d. p.o. | 70-75 | Yes | 163 | |
| Fluconazole | 20; b.i.d. p.o. | 55 | Yes | |||||
| Itraconazole | 30; t.i.d. p.o. | No significant | No | |||||
| Mouse | i.v., 104 | Cyclophosphamide + | Amphotericin B | 0.8-1.5; q.d. i.p. | 0 | No | ||
| fluorouracil | Voriconazole | 40; q.d. p.o. | 50 | Yes | 64 | |||
| Mouse | i.v. or i.c., 5 | Cyclophosphamide + | Liposomal amphotericin B | 40; q.d. i.v. | 0 | NAe | 63 | |
| × 104 | fluorouracil | Amphotericin B | 0.8; q.d. i.v. | 0 | NA | |||
| Guinea pig | i.v., 7 × 105 | Cyclophosphamide | Amphotericin B | 1.5; q.d. i.p. | 0 | No | 62 | |
| Voriconazole | 5, 10, 20; q.d. p.o. | 30-100 | Yes |
The inoculum is expressed as CFU/kg.
The same outcome was observed with higher inoculums and no immunosuppression.
Using a higher inoculum of 2.3 × 106 CFU/animal, liposomal amphotericin B was ineffective.
q.d., once daily; p.o., orally; i.p., intraperitoneally; b.i.d., twice a day; t.i.d., three times a day.
NA, not available.
Experimental S. prolificans infections.
In a mouse model of experimental scedosporiosis caused by S. prolificans, treatment with liposomal amphotericin B at 20 mg/kg of body weight/day i.v. prolonged mouse survival by 60%. In the same mouse model, caspofungin prolonged mouse survival by 30% at a dosage of 10 mg/kg/day. However, fungal burden reduction was observed only with liposomal amphotericin B (45). The MIC of amphotericin B and the minimal effective concentration of caspofungin were 8 μg/ml for the isolates of S. prolificans used in these studies. In a nonimmunocompromised rabbit model of scedosporiosis, similar results were found using 0.8 mg/kg of deoxycholate amphotericin B (50% survival) (65). Thus, experimental scedosporiosis caused by S. prolificans may be treated with high dosages of antifungal agents.
Experimental S. apiospermum infections.
Multiple studies have shown the lack of efficacy of conventional and liposomal amphotericin B and itraconazole (63, 65, 163, 322). However, in an immunocompromised murine model of scedosporiosis using a low inoculum (2 × 106 CFU/kg) of S. apiospermum, a small effect, with a prolongation of mouse survival of 10 to 40%, was found with amphotericin B and itraconazole (322). Treatment with itraconazole showed a similar effect in a nonimmunocompromised murine model of scedosporiosis using 5 × 107 CFU/kg (322).
In these studies, the amphotericin B MIC was 2 to 8 μg/ml and the itraconazole MIC was 0.5 to 4 μg/ml. In an immunocompromised murine model, posaconazole prolonged survival by 70 to 75% at dosages of >30 mg/kg/day. Surprisingly, in the same model, 40 mg/kg/day of fluconazole resulted in 55% survival and a reduced fungal burden (163). The posaconazole and fluconazole MICs in the latter study were 0.125 to 1 μg/ml and 32 to >64 μg/ml, respectively. Voriconazole treatment with high dosages (>10 mg/kg/day) in experimental scedosporiosis using an S. apiospermum isolate (MIC, 0.5 to 1 μg/ml) had similar effects in mice (50% survival) (63) and guinea pigs (30 to 100% survival) (62). Interestingly, when an S. apiospermum isolate with a higher MIC (8 μg/ml) was used to establish experimental scedosporiosis in guinea pigs, voriconazole was unable to prolong survival and reduce the fungal burden (62). Thus, high dosages of posaconazole and voriconazole could be used to treat infections caused by S. apiospermum, whereas the in vivo effect of fluconazole warrants further investigation.
The antifungal susceptibility data on 403 clinical isolates in the United States submitted to the Fungal Testing Laboratory from January 2000 to May 2007 are depicted in Table 7. A few points can be made from these data. (i) Given the wide range of MIC50 that these organism display, antifungal susceptibilities seem to be helpful in the treatment of infections caused by Scedosporium spp. (ii) Itraconazole and voriconazole are more active against S. prolificans than heretofore appreciated. (iii) The echinocandins show a marked difference in in vitro activity against Scedosporium spp.
TABLE 7.
Antifungal susceptibility data for 403 clinical isolates in the United States (January 2000 to May 2007)a
| Species | Antifungal agent | No. of isolates | MIC, μg/mlb
|
||
|---|---|---|---|---|---|
| Range | 50% | 90% | |||
| S. prolificans | Amphotericin B | 24 | 0.5->16 | >16 | >16 |
| 5-Flucytosine | 2 | >64 | >64 | >64 | |
| Fluconazole | 13 | 8->64 | 32 | >64 | |
| Itraconazole | 20 | 0.125->8 | 1 | >8 | |
| Ketoconazole | 4 | 1-2 | NDc | ND | |
| Voriconazole | 30 | 0.25->8 | 0.5 | >8 | |
| Posaconazole | 11 | 0.5->8 | 4 | >8 | |
| Caspofungin | 18 | 0.25->16 | 8 | >16 | |
| Micafungin | 1 | 0.5 | ND | ND | |
| Anidulafungin | 0 | ND | ND | ND | |
| S. apiospermum | Amphotericin B | 133 | 0.5->16 | >16 | >16 |
| 5-Flucytosine | 13 | >64 | >64 | >64 | |
| Fluconazole | 61 | 4->64 | 32 | >64 | |
| Itraconazole | 131 | 0.125->8 | 1 | 4 | |
| Ketoconazole | 28 | 1-2 | 2 | 2 | |
| Voriconazole | 178 | 0.25->8 | 0.5 | 1 | |
| Posaconazole | 55 | 0.25-4 | 1 | 2 | |
| Caspofungin | 105 | 0.25->16 | >16 | >16 | |
| Micafungin | 6 | 0.125-0.25 | 0.25 | ND | |
| Anidulafungin | 2 | 0.25-0.5 | ND | ND | |
| Scedosporium sp. | Amphotericin B | 31 | >16 | >16 | >16 |
| 5-Flucytosine | 3 | >64 | >64 | >64 | |
| Fluconazole | 5 | >64 | >64 | >64 | |
| Itraconazole | 20 | >8 | >8 | >8 | |
| Ketoconazole | 3 | >16 | >16 | >16 | |
| Voriconazole | 49 | >8 | >8 | >8 | |
| Posaconazole | 32 | >8 | >8 | >8 | |
| Caspofungin | 36 | 4->16 | 8 | >16 | |
| Micafungin | 2 | >16 | >16 | >16 | |
| Anidulafungin | 1 | >16 | >16 | >16 | |
Testing was performed by the Fungus Testing Laboratory of the University of Texas Health Science Center at San Antonio.
MICs were determined as described in CLSI document M38-A for filamentous fungi (307). MICs were read at 48 h. Amphotericin B, caspofungin, micafungin, and anidulafungin were tested in antibiotic medium 3, whereas all azoles and flucytosine were tested in RPMI 1640.
ND, not determined.
Clinical Outcomes
Clinical infections due to Scedosporium spp. are difficult to treat and frequently fatal. Scedosporium infections usually present in patients with hematological malignancies during periods of prolonged persistent neutropenia or in otherwise severely immunocompromised patients. Surgical excision of the disease has been a component of the standard of care and should be considered whenever possible. Even among immunocompetent individuals, infections caused by these agents usually require extensive debridement and sometimes amputation to achieve cure. Newer agents such as voriconazole have shown variable results, and echinocandins seem to be noneffective. As outlined above in “In Vitro Susceptibilities to Combinations of Antifungal Agents,” various reports have underscored the potential of combination antifungal therapy (287).
Gosbell et al. reported successful treatment of a case of an orthopedic infection with S. prolificans in a nonimmunocompromised host, using the synergistic combination of voriconazole and terbinafine (166). The synergy between voriconazole and terbinafine was once again tested in a patient with disseminated infection due to S. prolificans in the setting of bone marrow transplantation. In this case a disseminated infection was kept under control using the combination therapy; however, aggressive surgical debridement, supportive therapy with granulocyte-macrophage colony-stimulating factor (GM-CSF), and autologous recovery of the patient's counts together with cessation of immunosuppressive therapy may have been as important in this outcome (196). In the case reported by Gosbell et al. (166), the combination of terbinafine and voriconazole may have worked in taking care of the somewhat localized infection, where part of the disease was cutaneous or subcutaneous. In this regard, terbinafine, by reaching high levels in the teguments, may have been helpful in treating the infection. In the second case, the particular role that terbinafine had in keeping the infection under control is less apparent. Whyte et al. reported a case of disseminated S. prolificans infection in a patient with acute lymphocytic leukemia that responded to a combination of voriconazole and terbinafine together with local surgical debridement (472). Singh and McClusdey also used the combination of voriconazole and terbinafine in a case of sclerokeratitis in a patient who had pterygion excision and adjuvant mitomycin C; however, enucleation also took place (404). Most recently, Bhat et al. (38) reported a case of brain abscess caused by S. prolificans in a patient with CGD. In vitro susceptibility testing guided the combination antifungal therapy with voriconazole and terbinafine, resulting in total resolution of the brain abscess. This is the first reported case of cure of a CNS infection caused by S. prolificans. Apart from all other therapeutic modalities (GM-CSF, aggressive surgical debridement, recovery from neutropenia, granulocyte transfusions, etc.), it could be argued that voriconazole at the correct dose as a single antifungal agent would have been enough in treating these infections. So far, from the data generated by multiple investigators, it remains unclear (although promising) whether the in vitro synergism seen with terbinafine and voriconazole translates into in vivo synergy. Systematic evaluation of this particular combination of antifungals in a well-established animal model of scedosporiosis is warranted.
Immunotherapy
Immunocompromised hosts facing Scedosporium infections generally do extremely poorly in the absence of immune reconstitution (415). In order to illustrate the relevance of the immune host response, Berenguer et al. reported for a series of 16 patients with S. prolificans infection that despite the use of amphotericin B and fluocytosine, itraconazole, or fluconazole, those patients who did not recover from neutropenia had a fatal outcome (35). In another series of five patients with S. prolificans who were treated with liposomal amphotericin B in addition to itraconazole, three patients were persistently neutropenic and all three died; the other two were not neutropenic and survived the infection (25).
In neutropenic patients, disseminated Scedosporium prolificans is rapidly fatal (265). Seven of 11 immunocompromised patients infected with S. prolificans died despite administration of antifungal therapy (204). Only 2 of 16 patients infected with Scedosporium prolificans survived; both had recovered from neutropenia. Treatment of those patients with amphotericin B and azole, either sequentially or in combination, was inefficacious. At autopsy, dissemination was the rule. A median of five sites per patient were infected. The most common infected sites were lungs, kidneys, brain, and spleen (35). It has been suggested that cytokines (G-CSF) in combination with antifungals may have increased efficacy against Scedosporium infections (52). In vitro studies have suggested that an amphotericin B lipid complex in combination with PMNs has significantly greater antifungal activity against Scedosporium spp. (151).
PMNs appear to exert a more destructive effect on the hyphae of Scedosporium prolificans than on the hyphae of Aspergillus fumigatus (149). Triazoles (itraconazole, voriconazole, and posaconazole) have additive antifungal activities in combination with PMNs against S. apiospermum. Voriconazole plus posaconazole demonstrated synergistic interactions with PMNs against S. prolificans (152). In a murine model of Scedosporium infection, Ortoneda and colleagues (325) demonstrated that liposomal amphotericin B plus G-CSF improved survival over that with liposomal amphotericin B or amphotericin B deoxycholate. It may be possible that the addition of G-CSF to the antifungal therapy based on liposomal amphotericin B may be a useful adjunctive therapy. Consistent with these experimental observations, a neutropenic patient survived Scedosporium prolificans fungemia with amphotericin B plus itraconazole plus G-CSF, when the neutrophil count started to recover (52).
Antifungal agents may collaborate with host defense factors for an improved outcome. In this regard, amphotericin B lipid complex has been found to exert a significant additive effect with PMNs against S. prolificans and S. apiospermum in vitro (151). Similarly, in another in vitro study, the triazoles itraconazole, voriconazole, and posaconazole used in combination with PMNs caused a significant additive increase in the damage of the hyphae of S. prolificans and S. apiospermum. Furthermore, under certain conditions, synergism between voriconazole or posaconazole and PMNs against S. prolificans hyphae has been noted. Of note, the synergistic activity has been observed at low concentrations of the antifungals. This finding may be of particular importance, especially in immunocompromised patients, when a triazole reaches its trough level in plasma where such synergy may prevent fungal regrowth (152). Regardless of the mechanisms behind these collaborative effects, the findings from these studies would support the concomitant administration of antifungals and PMN transfusions to persistently neutropenic patients with invasive scedosporiosis. However, no cases of scedosporiosis treated with PMN transfusions have been reported to date.
Among the clinically available cytokines studied that enhance PMN antifungal activity against Scedosporium spp. are gamma interferon (IFN-γ) and GM-CSF (153). Treatment of PMNs with the combination of IFN-γ and GM-CSF had broader effects on Scedosporium spp., enhancing PMN functions, while cytokines alone had no effect. Despite the poor effect of either cytokine alone on the PMN oxidative burst after 22 h, the combined treatment showed enhancement of the oxidative burst in response to opsonized S. apiospermum hyphae. Similarly, after incubation with cytokines for 2 h, only the combination significantly enhanced the oxidative burst against serum-opsonized and nonopsonized hyphae of Scedosporium spp. Thus, in that study it was demonstrated that IFN-γ and GM-CSF exhibit a significant time- and species-dependent ability to enhance PMN activity against Scedosporium spp. (153).
In an immunocompetent murine model of disseminated S. prolificans infection, posaconazole and GM-CSF had a combined effect in damaging S. prolificans hyphae ex vivo. However, when posaconazole and GM-CSF were administered to mice with invasive infection due to S. prolificans, they had selective beneficial effects on the burdens in certain organs but offered no additional benefit to survival (403).
PREVENTION
The ubiquitous nature of Scedosporium spp. makes it a daunting task to exclude this mold from the hospital environment. Prevention can be attempted by using HEPA filtration in areas where immunocompromised patients are kept. Outbreaks in neutropenic patients in hospitals have been described and have been associated with hospital construction (11, 402).
Observing precautions at times of renovation or construction in the hospital or surrounding areas is important. The installation of physical barriers (dry wall or plastic barriers) to shield the patients from potential contaminants as well as the maintenance of ventilation systems, ensuring mold removal, and limiting dust-generating activities are critical and feasible measures to prevent mold infections in neutropenic patients (459).
Active surveillance for mold, especially during periods of construction and particularly in areas where immunocompromised patients reside, should be performed as suggested by the Healthcare Infection Control Practice Advisory Committee (388). Some authors have suggested antifungal prophylaxis of high-risk patients based on their immune status during health care-associated outbreaks (326). However, this practice will not likely protect against all mold infections (e.g., S. prolificans). Scedosporium is an emergent fungal pathogen associated with high mortality in neutropenic patients. The intrinsic resistance of most isolates to amphotericin B has made the treatment of this fungal infection very challenging. Clinicians and health care professionals need to be aware of the importance and lifesaving nature of early and accurate diagnosis, mainly because these fungi may be confused histologically with Aspergillus spp. and other hyalohyphomycetes. With the expansion of the antifungal armamentarium, treatment may be possible, and effective strategies may include combinations of antifungals, surgery, cytokines, and other disease-modifying agents that may expedite immune reconstitution, which seems to be the most important factor determining the outcome of these otherwise devastating invasive diseases caused by these frequently lethal pathogens.
CONCLUSIONS AND CLOSING REMARKS
Pseudallescheria boydii/Scedosporium apiospermum and Scedosporium prolificans are emerging opportunistic pathogens among the ever-increasing immunocompromised patient population. These organisms are also emerging pathogens among immunocompetent individuals and are the etiologic agents of white-grain mycetoma and keratitis infections, which even when recognized and treated leave individuals with potential severe deficits. These fungal pathogens are distinctively difficult to treat given their inherent resistance to available antifungal agents. Isolation, proper identification, and susceptibility testing of the fungal isolates are important steps in the optimal treatment of these infections. While advances in antifungal therapy have been achieved during the past decade, surgical debridement and augmentation of host defenses remain critical elements in the battle against these organisms.
REFERENCES
- 1.Abzug, M. J., and T. J. Walsh. 2004. Interferon-gamma and colony-stimulating factors as adjuvant therapy for refractory fungal infections in children. Pediatr. Infect. Dis. J. 23:769-773. [DOI] [PubMed] [Google Scholar]
- 2.Acharya, A., A. Ghimire, B. Khanal, S. Bhattacharya, N. Kumari, and R. Kanungo. 2006. Brain abscess due to Scedosporium apiospermum in a non immunocompromised child. Indian J. Med. Microbiol. 24:231-232. [PubMed] [Google Scholar]
- 3.Adelson, H. T., and J. A. Malcolm. 1968. Endocavitary treatment of pulmonary mycetomas. Am. Rev. Respir. Dis. 98:87-92. [DOI] [PubMed] [Google Scholar]
- 4.Afeltra, J., E. Dannaoui, J. F. Meis, J. L. Rodriguez-Tudela, and P. E. Verweij. 2002. In vitro synergistic interaction between amphotericin B and pentamidine against Scedosporium prolificans. Antimicrob. Agents Chemother. 46:3323-3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmed, A. O., W. van Leeuwen, A. Fahal, W. van de Sande, H. Verbrugh, and A. van Belkum. 2004. Mycetoma caused by Madurella mycetomatis: a neglected infectious burden. Lancet Infect. Dis. 4:566-574. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed, J., D. M. Ditmars, T. Sheppard, R. del Busto, K. K. Venkat, and R. Parasuraman. 2004. Recurrence of Scedosporium apiospermum infection following renal re-transplantation. Am. J. Transplant. 4:1720-1724. [DOI] [PubMed] [Google Scholar]
- 7.Albernaz, V., B. Huston, M. Castillo, S. Mukherji, and T. W. Bouldin. 1996. Pseudallescheria boydii infection of the brain: imaging with pathologic confirmation. Am. J. Neuroradiol. 17:589-592. [PMC free article] [PubMed] [Google Scholar]
- 8.Al Refai, M., C. Duhamel, J. P. Le Rochais, and P. Icard. 2002. Lung scedosporiosis: a differential diagnosis of aspergillosis. Eur. J. Cardiothorac. Surg. 21:938-939. [DOI] [PubMed] [Google Scholar]
- 9.Alsip, S. G., and C. G. Cobbs. 1986. Pseudallescheria boydii infection of the central nervous system in a cardiac transplant recipient. South. Med. J. 79:383-384. [DOI] [PubMed] [Google Scholar]
- 10.Alture-Werber, E., S. C. Edberg, and J. M. Singer. 1976. Pulmonary infection with Allescheria boydii. Am. J. Clin. Pathol. 66:1019-1024. [DOI] [PubMed] [Google Scholar]
- 11.Alvarez, M., B. Lopez Ponga, C. Rayon, J. Garcia Gala, M. C. Roson Porto, M. Gonzalez, J. V. Martinez-Suarez, and J. L. Rodriguez-Tudela. 1995. Nosocomial outbreak caused by Scedosporium prolificans (inflatum): four fatal cases in leukemic patients. J. Clin. Microbiol. 33:3290-3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson, R. L., T. F. Carroll, J. T. Harvey, and M. G. Myers. 1984. Petriellidium (Allescheria) boydii orbital and brain abscess treated with intravenous miconazole. Am. J. Ophthalmol. 97:771-775. [DOI] [PubMed] [Google Scholar]
- 13.Ansari, R. A., D. A. Hindson, D. L. Stevens, and J. G. Kloss. 1987. Pseudallescheria boydii arthritis and osteomyelitis in a patient with Cushing's disease. South. Med. J. 80:90-92. [DOI] [PubMed] [Google Scholar]
- 14.Ariewitsch, A. M., S. G. Stepaniszewa, and O. W. Tiufilina. 1969. A case of lung mycetoma caused by Monosporium apiospermum. Mycopathol. Mycol. Appl. 37:171-178. [DOI] [PubMed] [Google Scholar]
- 15.Armin, A. R., V. B. Reddy, and E. Orfei. 1987. Fungal endocarditis caused by Pseudallescheria (Petriellidium) boydii in an intravenous drug abuser. Tex. Heart Inst. J. 14:321-324. [PMC free article] [PubMed] [Google Scholar]
- 16.Arnett, J. C., Jr., and H. B. Hatch, Jr. 1975. Pulmonary allescheriasis. Report of a case and review of the literature. Arch. Intern. Med. 135:1250-1253. [DOI] [PubMed] [Google Scholar]
- 17.Aronson, S. M., R. Benham, and A. Wolf. 1953. Maduromycosis of the central nervous system. J. Neuropathol. Exp. Neurol. 12:158-168. [DOI] [PubMed] [Google Scholar]
- 18.Arora, S., Y. Hernandez, J. R. Erb-Downward, R. A. McDonald, G. B. Toews, and G. B. Huffnagle. 2005. Role of IFN-gamma in regulating T2 immunity and the development of alternatively activated macrophages during allergic bronchopulmonary mycosis. J. Immunol. 174:6346-6356. [DOI] [PubMed] [Google Scholar]
- 19.Arora, S., and G. B. Huffnagle. 2005. Immune regulation during allergic bronchopulmonary mycosis: lessons taught by two fungi. Immunol. Res. 33:53-68. [DOI] [PubMed] [Google Scholar]
- 20.Arthur, S., L. L. Steed, D. J. Apple, Q. Peng, G. Howard, and M. Escobar-Gomez. 2001. Scedosporium prolificans keratouveitis in association with a contact lens retained intraocularly over a long term. J. Clin. Microbiol. 39:4579-4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baddley, J. W., T. P. Stroud, D. Salzman, and P. G. Pappas. 2001. Invasive mold infections in allogeneic bone marrow transplant recipients. Clin. Infect. Dis. 32:1319-1324. [DOI] [PubMed] [Google Scholar]
- 22.Bakerspigel, A., and D. Schaus. 1984. Petriellidosis (pseudallescheriasis) in southwestern Ontario, Canada. Sabouraudia 22:247-249. [DOI] [PubMed] [Google Scholar]
- 23.Bakerspigel, A., T. Wood, and S. Burke. 1977. Pulmonary allescheriasis: report of a case from Ontario, Canada. Am. J. Clin. Pathol. 68:299-303. [DOI] [PubMed] [Google Scholar]
- 24.Ballingalli, G. R. 1855. An account of a tumor affecting the foot. Trans. Med. Phys. Soc. Bombay 2:273-276. [Google Scholar]
- 25.Barbaric, D., and P. J. Shaw. 2001. Scedosporium infection in immunocompromised patients: successful use of liposomal amphotericin B and itraconazole. Med. Pediatr. Oncol. 37:122-125. [DOI] [PubMed] [Google Scholar]
- 26.Bark, C. J., L. J. Zaino, K. Rossmiller, and C. L. Copper. 1978. Petriellidium boydii sinusitis. JAMA 240:1339-1340. [DOI] [PubMed] [Google Scholar]
- 27.Bashir, G., S. Shakeel, T. Wani, and D. K. Kakru. 2004. Pulmonary pseudallescheriasis in a patient with healed tuberculosis. Mycopathologia 158:289-291. [DOI] [PubMed] [Google Scholar]
- 28.Beguin, H., and N. Nolard. 1994. Mould biodiversity in homes. Air and surface analysis of 130 dwellings. Aerobiologia 10:157-166. [Google Scholar]
- 29.Belitsos, N. J., W. G. Merz, D. W. Bowersox, and G. M. Hutchins. 1974. Allescheria boydii mycetoma complicating pulmonary sarcoid. Johns Hopkins Med. J. 135:259-267. [PubMed] [Google Scholar]
- 30.Bell, W. E., and M. G. Myers. 1978. Allescheria (Petriellidium) boydii brain abscess in a child with leukemia. Arch. Neurol. 35:386-388. [DOI] [PubMed] [Google Scholar]
- 31.Bendl, B. J., D. Mackey, F. Al-Saati, K. V. Sheth, S. N. Ofole, and T. M. Bailey. 1987. Mycetoma in Saudi Arabia. J. Trop. Med. Hyg. 90:51-59. [PubMed] [Google Scholar]
- 32.Benham, R. W., and L. K. George. 1948. Allescheria boydii, causative agent in a case report of meningitis. J. Investig. Dermatol. 10:99-110. [DOI] [PubMed] [Google Scholar]
- 33.Ben Hamida, M., J. Bedrossian, A. Pruna, B. Fouqueray, F. Metivier, and J. M. Idatte. 1993. Fungal mycotic aneurysms and visceral infection due to Scedosporium apiospermum in a kidney transplant patient. Transplant Proc. 25:2290-2291. [PubMed] [Google Scholar]
- 34.Berenguer, J., J. Diaz-Mediavilla, D. Urra, and P. Munoz. 1989. Central nervous system infection caused by Pseudallescheria boydii: case report and review. Rev. Infect. Dis. 11:890-896. [DOI] [PubMed] [Google Scholar]
- 35.Berenguer, J., J. L. Rodriguez-Tudela, C. Richard, M. Alvarez, M. A. Sanz, L. Gaztelurrutia, J. Ayats, J. V. Martinez-Suarez, et al. 1997. Deep infections caused by Scedosporium prolificans. A report on 16 cases in Spain and a review of the literature. Medicine (Baltimore) 76:256-265. [DOI] [PubMed] [Google Scholar]
- 36.Bernstein, E. F., M. G. Schuster, D. D. Stieritz, P. C. Heuman, and J. Uitto. 1995. Disseminated cutaneous Pseudallescheria boydii. Br. J. Dermatol. 132:456-460. [DOI] [PubMed] [Google Scholar]
- 37.Bhally, H. S., C. Shields, S. Y. Lin, and W. G. Merz. 2004. Otitis caused by Scedosporium apiospermum in an immunocompetent child. Int. J. Pediatr. Otorhinolaryngol. 68:975-978. [DOI] [PubMed] [Google Scholar]
- 38.Bhat, S. V., D. L. Paterson, M. G. Rinaldi, and P. J. Veldkamp. 2007. Scedosporium prolificans brain abscess in a patient with chronic granulomatous disease: successful combination therapy with voriconazole and terbinafine. Scand. J. Infect. Dis. 39:87-90. [DOI] [PubMed] [Google Scholar]
- 39.Bhatti, Z., A. Shaukat, N. G. Almyroudis, and B. H. Segal. 2006. Review of epidemiology, diagnosis, and treatment of invasive mould infections in allogeneic hematopoietic stem cell transplant recipients. Mycopathologia 162:1-15. [DOI] [PubMed] [Google Scholar]
- 40.Bhermi, G., I. Gillespie, and B. Mathalone. 2000. Scedosporium (Pseudallescheria) fungal infection of a sponge explant. Eye 14:247-249. [DOI] [PubMed] [Google Scholar]
- 41.Bittencourt, V. C., R. T. Figueiredo, R. B. da Silva, D. S. Mourao-Sa, P. L. Fernandez, G. L. Sassaki, B. Mulloy, M. T. Bozza, and E. Barreto-Bergter. 2006. An alpha-glucan of Pseudallescheria boydii is involved in fungal phagocytosis and Toll-like receptor activation. J. Biol. Chem. 281:22614-22623. [DOI] [PubMed] [Google Scholar]
- 42.Blank, F., and E. A. Stuart. 1955. Monosporium apiospermum Sacc., 1911, associated with otomycosis. Can. Med. Assoc. J. 72:601. [PMC free article] [PubMed] [Google Scholar]
- 43.Bloom, P. A., D. A. Laidlaw, D. L. Easty, and D. W. Warnock. 1992. Treatment failure in a case of fungal keratitis caused by Pseudallescheria boydii. Br. J. Ophthalmol. 76:367-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bloom, S. M., R. R. Warner, and I. Weitzman. 1982. Maxillary sinusitis: isolation of Scedosporium (Monosporium) apiospermum, anamorph of Petriellidium (Allescheria) boydii. Mt. Sinai J. Med. 49:492-494. [PubMed] [Google Scholar]
- 45.Bocanegra, R., L. K. Najvar, S. Hernandez, D. I. McCarthy, and J. R. Graybill. 2005. Caspofungin and liposomal amphotericin B therapy of experimental murine scedosporiosis. Antimicrob. Agents Chemother. 49:5139-5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bolland, M. J., W. Bagg, M. G. Thomas, J. A. Lucas, R. Ticehurst, and P. N. Black. 2004. Cushing's syndrome due to interaction between inhaled corticosteroids and itraconazole. Ann. Pharmacother. 38:46-49. [DOI] [PubMed] [Google Scholar]
- 47.Bonduel, M., P. Santos, C. F. Turienzo, G. Chantada, and H. Paganini. 2001. Atypical skin lesions caused by Curvularia spp. and Pseudallescheria boydii in two patients after allogeneic bone marrow transplantation. Bone Marrow Transplant. 27:1311-1313. [DOI] [PubMed] [Google Scholar]
- 48.Borman, A. M., C. K. Campbell, C. J. Linton, P. D. Bridge, and E. M. Johnson. 2006. Polycytella hominis is a mutated form of Scedosporium apiospermum. Med. Mycol. 44:33-39. [DOI] [PubMed] [Google Scholar]
- 49.Bosma, F., A. Voss, H. W. van Hamersvelt, R. G. de Sevaux, J. Biert, B. J. Kullberg, W. G. Melchers, and P. E. Verweij. 2003. Two cases of subcutaneous Scedosporium apiospermum infection treated with voriconazole. Clin. Microbiol. Infect. 9:750-753. [DOI] [PubMed] [Google Scholar]
- 50.Bouchard, C. S., B. Chacko, H. P. Cupples, H. D. Cavanagh, and W. D. Mathers. 1991. Surgical treatment for a case of postoperative Pseudallescheria boydii endophthalmitis. Ophthalmic Surg. 22:98-101. [PubMed] [Google Scholar]
- 51.Bousley, P. H. 1977. Isolation of Allescheria boydii from pleural fluid. J. Clin. Microbiol. 5:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bouza, E., P. Munoz, L. Vega, M. Rodriguez-Creixems, J. Berenguer, and A. Escudero. 1996. Clinical resolution of Scedosporium prolificans fungemia associated with reversal of neutropenia following administration of granulocyte colony-stimulating factor. Clin. Infect. Dis. 23:192-193. [DOI] [PubMed] [Google Scholar]
- 53.Bower, C. P., J. D. Oxley, C. K. Campbell, and C. B. Archer. 1999. Cutaneous Scedosporium apiospermum infection in an immunocompromised patient. J. Clin. Pathol. 52:846-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boyd, M., and E. D. Crutchfield. 1921. A contribution to the study of mycetoma in North America. Am. J. Trop. Med. 1:215-289. [Google Scholar]
- 55.Brumpt, E. 1906. Les mycetomes. Arch. Parasitol. 10:489-564. [Google Scholar]
- 56.Bryan, C. S., A. F. DiSalvo, L. Kaufman, W. Kaplan, A. H. Brill, and D. C. Abbott. 1980. Petriellidium boydii infection of the sphenoid sinus. Am. J. Clin. Pathol. 74:846-851. [DOI] [PubMed] [Google Scholar]
- 57.Buckley, C. E., III, R. S. Mathews, J. A. Grant, W. G. Edwards, and H. S. Neilsen. 1969. Local chemotherapy of maduromycosis caused by Monosporium apiospermum. Arch. Intern. Med. 124:748-753. [PubMed] [Google Scholar]
- 58.Buzina, W., G. Feierl, D. Haas, F. F. Reinthaler, A. Holl, R. Kleinert, B. Reichenpfader, P. Roll, and E. Marth. 2006. Lethal brain abscess due to the fungus Scedosporium apiospermum (teleomorph Pseudallescheria boydii) after a near-drowning incident: case report and review of the literature. Med. Mycol. 44:473-477. [DOI] [PubMed] [Google Scholar]
- 59.Caillot, D., L. Mannone, and F. Kara-Slimane. 2001. Invasive aspergillosis. Rev. Prat. 51:731-737. [PubMed] [Google Scholar]
- 60.Campagnaro, E. L., K. J. Woodside, M. G. Early, K. K. Gugliuzza, M. I. Colome-Grimmer, F. A. Lopez, and J. A. Daller. 2002. Disseminated Pseudallescheria boydii (Scedosporium apiospermum) infection in a renal transplant patient. Transpl. Infect. Dis. 4:207-411. [DOI] [PubMed] [Google Scholar]
- 61.Canet, J. J., X. Pagerols, C. Sanchez, P. Vives, and J. Garau. 2001. Lymphocutaneous syndrome due to Scedosporium apiospermum. Clin. Microbiol. Infect. 7:648-650. [DOI] [PubMed] [Google Scholar]
- 62.Capilla, J., and J. Guarro. 2004. Correlation between in vitro susceptibility of Scedosporium apiospermum to voriconazole and in vivo outcome of scedosporiosis in guinea pigs. Antimicrob. Agents Chemother. 48:4009-4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Capilla, J., E. Mayayo, C. Serena, F. J. Pastor, and J. Guarro. 2004. A novel murine model of cerebral scedosporiosis: lack of efficacy of amphotericin B. J. Antimicrob. Chemother. 54:1092-1095. [DOI] [PubMed] [Google Scholar]
- 64.Capilla, J., C. Serena, F. J. Pastor, M. Ortoneda, and J. Guarro. 2003. Efficacy of voriconazole in treatment of systemic scedosporiosis in neutropenic mice. Antimicrob. Agents Chemother. 47:3976-3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Capilla, J., C. Yustes, E. Mayayo, B. Fernandez, M. Ortoneda, F. J. Pastor, and J. Guarro. 2003. Efficacy of albaconazole (UR-9825) in treatment of disseminated Scedosporium prolificans infection in rabbits. Antimicrob. Agents Chemother. 47:1948-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carney, M. D., A. Tabassian, and R. K. Guerry. 1996. Pseudo-Allescheria boydii endophthalmitis. Retina 16:263-264. [PubMed] [Google Scholar]
- 67.Carreter de Granda, M. E., C. Richard, E. Conde, A. Iriondo, F. Marco de Lucas, R. Salesa, and A. Zubizarreta. 2001. Endocarditis caused by Scedosporium prolificans after autologous peripheral blood stem cell transplantation. Eur. J. Clin. Microbiol. Infect. Dis. 20:215-217. [DOI] [PubMed] [Google Scholar]
- 68.Carrillo, A. J., and J. Guarro. 2001. In vitro activities of four novel triazoles against Scedosporium spp. Antimicrob. Agents Chemother. 45:2151-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carrillo-Munoz, A. J., G. Quindos, M. Ruesga, O. del Valle, J. Peman, E. Canton, J. M. Hernandez-Molina, and P. Santos. 2006. In vitro antifungal susceptibility testing of filamentous fungi with Sensititre Yeast One. Mycoses 49:293-297. [DOI] [PubMed] [Google Scholar]
- 70.Carter, H. 1860. On a new striking form of fungus disease principally affecting the foot and prevailing endemically in many parts of India. Trans. Med. Phys. Soc. Bombay 60:140-142. [Google Scholar]
- 71.Carter, H. V. 1874. On mycetoma or the fungs disease of India. J. &A Churchill, London, United Kingdom.
- 72.Carter, H. V. 1861. On mycetoma or the fungus disease of India including notes of recent cases and new observations of the structure, etc. of the entophytic growth. Trans. Med. Physiol. Soc. Bombay 7:206-221. [Google Scholar]
- 72a.Castellani, A., and A. J. Chalmers. 1919. Manual of tropical medicine, p. 2110-2150. Williams, Wood, and Co., New York, NY.
- 73.Castiglioni, B., D. A. Sutton, M. G. Rinaldi, J. Fung, and S. Kusne. 2002. Pseudallescheria boydii (Anamorph Scedosporium apiospermum). Infection in solid organ transplant recipients in a tertiary medical center and review of the literature. Medicine (Baltimore) 81:333-348. [DOI] [PubMed] [Google Scholar]
- 74.Caya, J. G., S. G. Farmer, G. A. Williams, T. R. Franson, R. A. Komorowski, and J. C. Kies. 1988. Bilateral Pseudallescheria boydii endophthalmitis in an immunocompromised patient. Wis. Med. J. 87:11-14. [PubMed] [Google Scholar]
- 75.Cazin, J., Jr., and D. W. Decker. 1964. Carbohydrate nutrition and sporulation of Allescheria boydii. J. Bacteriol. 88:1624-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cazin, J., Jr., and D. W. Decker. 1965. Growth of Allescheria boydii in anitbiotic-containing media. J. Bacteriol. 90:1308-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chakraborty, A., M. R. Workman, and P. R. Bullock. 2005. Scedosporium apiospermum brain abscess treated with surgery and voriconazole. J. Neurosurg. 103:83-87. [DOI] [PubMed] [Google Scholar]
- 77a.Chalmers, A. J., and R. G. Archibald. 1916. A Sudanese maduromycosis. Ann. Trop. Med. Parasitol. 10:169-229. [Google Scholar]
- 78.Chaney, S., R. Gopalan, and R. E. Berggren. 2004. Pulmonary Pseudallescheria boydii infection with cutaneous zygomycosis after near drowning. South. Med. J. 97:683-687. [DOI] [PubMed] [Google Scholar]
- 79.Chaveiro, M. A., R. Vieira, J. Cardoso, and A. Afonso. 2003. Cutaneous infection due to Scedosporium apiospermum in an immunosuppressed patient. J. Eur. Acad. Dermatol. Venereol. 17:47-49. [DOI] [PubMed] [Google Scholar]
- 80.Chikhani, L., B. Dupont, F. Guilbert, L. Improvisi, A. Corre, and J. C. Bertrand. 1995. Uncommon fungal maxillary sinusitis of dental origin due to Scedosporium prolificans. Rev. Stomatol. Chir. Maxillofac. 96:66-69. [PubMed] [Google Scholar]
- 81.Cimon, B., J. Carrere, J. F. Vinatier, J. P. Chazalette, D. Chabasse, and J. P. Bouchara. 2000. Clinical significance of Scedosporium apiospermum in patients with cystic fibrosis. Eur. J. Clin. Microbiol. Infect. Dis. 19:53-56. [DOI] [PubMed] [Google Scholar]
- 82.Collignon, P. J., C. Macleod, and D. R. Packham. 1985. Miconazole therapy in Pseudallescheria boydii infection. Australas. J. Dermatol. 26:129-132. [DOI] [PubMed] [Google Scholar]
- 83.Cooke, W. B., and P. Kabler. 1955. Isolation of potentially pathogenic fungi from polluted water and sewage. Public Health Rep. 70:689-694. [PMC free article] [PubMed] [Google Scholar]
- 84.Creitz, S., and H. W. Harris. 1955. Isolation of Allescheria boydii from sputum. Am. Rev. Tuberc. 71:126-130. [DOI] [PubMed] [Google Scholar]
- 85.Cuenca-Estrella, M., A. Gomez-Lopez, E. Mellado, G. Garcia-Effron, A. Monzon, and J. L. Rodriguez-Tudela. 2005. In vitro activity of ravuconazole against 923 clinical isolates of nondermatophyte filamentous fungi. Antimicrob. Agents Chemother. 49:5136-5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cuenca-Estrella, M., B. Ruiz-Diez, J. V. Martinez-Suarez, A. Monzon, and J. L. Rodriguez-Tudela. 1999. Comparative in-vitro activity of voriconazole (UK-109496) and six other antifungal agents against clinical isolates of Scedosporium prolificans and Scedosporium apiospermum. J. Antimicrob. Chemother. 43:149-151. [DOI] [PubMed] [Google Scholar]
- 87.Dabrowa, N., J. W. Landau, V. D. Newcomer, and O. A. Plunkett. 1964. A survey of tide-washed coastal areas of southern California for fungi potentially pathogenic to man. Mycopathol. Mycol. Appl. 24:136-150. [DOI] [PubMed] [Google Scholar]
- 88.Danaher, P. J., and E. A. Walter. 2004. Successful treatment of chronic meningitis caused by Scedosporium apiospermum with oral voriconazole. Mayo Clin. Proc. 79:707-708. [DOI] [PubMed] [Google Scholar]
- 89.Davis, W. A., J. M. Isner, A. W. Bracey, W. C. Roberts, and V. F. Garagusi. 1980. Disseminated Petriellidium boydii and pacemaker endocarditis. Am. J. Med. 69:929-932. [DOI] [PubMed] [Google Scholar]
- 90.de Batlle, J., M. Motje, R. Balanza, R. Guardia, and R. Ortiz. 2000. Disseminated infection caused by Scedosporium prolificans in a patient with acute multilineal leukemia. J. Clin. Microbiol. 38:1694-1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Defontaine, A., R. Zouhair, B. Cimon, J. Carrere, E. Bailly, F. Symoens, M. Diouri, J. N. Hallet, and J. P. Bouchara. 2002. Genotyping study of Scedosporium apiospermum isolates from patients with cystic fibrosis. J. Clin. Microbiol. 40:2108-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.de Hoog, G. S., J. Guarro, J. Gene, and M. J. Figueras. 2000. Atlas of clinical fungi, 2nd ed. ASM Press, Washington, DC.
- 93.de Hoog, G. S., F. D. Marvin-Sikkema, G. A. Lahpoor, J. C. Gottschall, R. A. Prins, and E. Gueho. 1994. Ecology and physiology of the emerging opportunistic fungi Pseudallescheria boydii and Scedosporium prolificans. Mycoses 37:71-78. [DOI] [PubMed] [Google Scholar]
- 94.de Hoog, G. S., F. Queiroz-Telles, G. Haase, G. Fernandez-Zeppenfeldt, D. Attili Angelis, A. H. Gerrits Van Den Ende, T. Matos, H. Peltroche-Llacsahuanga, A. A. Pizzirani-Kleiner, J. Rainer, N. Richard-Yegres, V. Vicente, and F. Yegres. 2000. Black fungi: clinical and pathogenic approaches. Med. Mycol. 38(Suppl. 1):243-1250. [PubMed] [Google Scholar]
- 95.Dellestable, F., L. Kures, D. Mainard, P. Pere, and A. Gaucher. 1994. Fungal arthritis due to Pseudallescheria boydii (Scedosporium apiospermum). J. Rheumatol. 21:766-768. [PubMed] [Google Scholar]
- 96.Deloach, E. D., R. J. DiBenedetto, W. S. Hitch, and P. Russell. 1979. Pulmonary infection with Petriellidium boydii. South. Med. J. 72:479-481. [DOI] [PubMed] [Google Scholar]
- 97.del Palacio, A., M. Garau, E. Amor, I. Martinez-Alonso, T. Calvo, A. Carrillo-Munoz, and J. Guarro. 2001. Transient colonization with Scedosporium prolificans. Report of four cases in Madrid. Mycoses 44:321-325. [PubMed] [Google Scholar]
- 98.del Palacio, A., E. Perez-Blazquez, M. S. Cuetara, M. Garcia-Bravo, D. Criado, C. Gimeno, and A. R. Noriega. 1991. Keratomycosis due to Scedosporium apiospermum. Mycoses 34:483-487. [DOI] [PubMed] [Google Scholar]
- 99.del Poeta, M., W. A. Schell, and J. R. Perfect. 1997. In vitro antifungal activity of pneumocandin L-743,872 against a variety of clinically important molds. Antimicrob. Agents Chemother. 41:1835-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.de Ment, S. H., R. R. Smith, J. E. Karp, and W. G. Merz. 1984. Pulmonary, cardiac, and thyroid involvement in disseminated Pseudallescheria boydii. Arch. Pathol. Lab. Med. 108:859-861. [PubMed] [Google Scholar]
- 101.D'Hondt, K., R. Parys-Van Ginderdeuren, and B. Foets. 2000. Fungal keratitis caused by Pseudallescheria boydii (Scedosporium apiospermum). Bull. Soc. Belge Ophtalmol. 277:53-56. [PubMed] [Google Scholar]
- 102.Diaz-Valle, D., J. M. Benitez del Castillo, E. Amor, N. Toledano, M. M. Carretero, and T. Diaz-Valle. 2002. Severe keratomycosis secondary to Scedosporium apiospermum. Cornea 21:516-518. [DOI] [PubMed] [Google Scholar]
- 103.Dignani, M. C., N. Kiwan, and E. J. Anaissie. 2003. Hyalohyphomycoses, p. 309-324. In E. J. Anaissie, M. R. McGinnis, and M. A. Pfaller (ed.), Clinical mycology. Churchill Livingstone, Philadelphia, PA.
- 104.Dinesha, M. Roshan, K. R. Dinesh, R. Shetty, C. S. Jayaprakash, and C. V. Raghuveer. 2001. Pseudallescheriasis—a rare fungal infection of brain and lungs. J. Assoc. Physicians India 49:574-575. [PubMed] [Google Scholar]
- 105.Dixon, D. M., A. Polak, and P. J. Szaniszlo. 1987. Pathogenicity and virulence of wild-type and melanin-deficient Wangiella dermatitidis. J. Med. Vet. Mycol. 25:97-106. [DOI] [PubMed] [Google Scholar]
- 106.Dixon, D. M., and A. Polak-Wyss. 1991. The medically important dematiaceous fungi and their identification. Mycoses 34:1-18. [DOI] [PubMed] [Google Scholar]
- 107.Drouhet, E. 1955. The status of fungus diseases in France, p. 43-53. In T. H. Sternberg and V. D. Newcomer (ed.), Therapy of fungus diseases: an international symposium. Little Brown, Boston, MA.
- 108.Dubeau, F., L. E. Roy, J. Allard, M. Laverdiere, S. Rousseau, F. Duplantis, J. Boileau, and J. Lachapelle. 1984. Brain abscess due to Petriellidium boydii. Can. J. Neurol. Sci. 11:395-398. [DOI] [PubMed] [Google Scholar]
- 109.Durieu, I., M. Parent, F. Ajana, P. Gosset, D. Smadja, X. Leclerc, Y. Fournier, and D. Leys. 1991. Monosporium apiospermum meningoencephalitis: a clinico-pathological case. J. Neurol. Neurosurg. Psychiatry. 54:731-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dworzack, D. L., R. B. Clark, W. J. Borkowski, Jr., D. L. Smith, M. Dykstra, M. P. Pugsley, E. A. Horowitz, T. L. Connolly, D. L. McKinney, M. K. Hostetler, et al. 1989. Pseudallescheria boydii brain abscess: association with near-drowning and efficacy of high-dose, prolonged miconazole therapy in patients with multiple abscesses. Medicine (Baltimore) 68:218-224. [PubMed] [Google Scholar]
- 111.Eckburg, P. B., A. R. Zolopa, and J. G. Montoya. 1999. Invasive fungal sinusitis due to Scedosporium apiospermum in a patient with AIDS. Clin. Infect. Dis. 29:212-213. [DOI] [PubMed] [Google Scholar]
- 112.Elliott, I. D., C. Halde, and J. Shapiro. 1977. Keratitis and endophthalmitis caused by Petriellidium boydii. Am. J. Ophthalmol. 83:16-18. [DOI] [PubMed] [Google Scholar]
- 113.Emmons, C. W. 1944. Allescheria boydii and Monosporium apiospermum. Mycologia 36:188-193. [Google Scholar]
- 114.Enggano, I. L., W. T. Hughes, D. K. Kalwinsky, T. A. Pearson, D. M. Parham, and S. A. Stass. 1984. Pseudallescheria boydii in a patient with acute lymphoblastic leukemia. Arch. Pathol. Lab. Med. 108:619-622. [PubMed] [Google Scholar]
- 115.Ernest, J. T., and J. W. Rippon. 1966. Keratitis due to Allescheria boydii (Monosporium apiospermum). Am. J. Ophthalmol. 62:1202-1204. [DOI] [PubMed] [Google Scholar]
- 116.Espinel-Ingroff, A. 1998. Comparison of in vitro activities of the new triazole SCH56592 and the echinocandins MK-0991 (L-743872) and LY303366 against opportunistic filamentous and dimorphic fungi and yeasts. J. Clin. Microbiol. 36:2950-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Espinel-Ingroff, A. 2001. Comparison of the E-test with the NCCLS M38-P method for antifungal susceptibility testing of common and emerging pathogenic filamentous fungi. J. Clin. Microbiol. 39:1360-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Eyre, E. W. 1859. An account of a peculiar disease “tubercular” of the foot. Ind. Ann. Med. Sci. 6:513-520. [Google Scholar]
- 119.Fahal, A. H. 2004. Mycetoma: a thorn in the flesh. Trans. R. Soc. Trop. Med. Hyg. 98:3-11. [DOI] [PubMed] [Google Scholar]
- 120.Fahal, A. H., E. A. el Toum, A. M. el Hassan, E. S. Mahgoub, and S. A. Gumaa. 1995. The host tissue reaction to Madurella mycetomatis: new classification. J. Med. Vet. Mycol. 33:15-17. [PubMed] [Google Scholar]
- 121.Fahal, A. H., H. E. Sheik, M. M. Homeida, Y. E. Arabi, and E. S. Mahgoub. 1997. Ultrasonographic imaging of mycetoma. Br. J. Surg. 84:1120-1122. [PubMed] [Google Scholar]
- 122.Farag, S. S., F. C. Firkin, J. H. Andrew, C. S. Lee, and D. H. Ellis. 1992. Fatal disseminated Scedosporium inflatum infection in a neutropenic immunocompromised patient. J. Infect. 25:201-204. [DOI] [PubMed] [Google Scholar]
- 123.Farina, C., M. Arosio, G. Marchesi, and M. Amer. 2002. Scedosporium apiospermum post-traumatic cranial infection. Brain Inj. 16:627-631. [DOI] [PubMed] [Google Scholar]
- 124.Feltkamp, M. C., M. J. Kersten, J. van der Lelie, J. D. Burggraaf, G. S. de Hoog, and E. J. Kuijper. 1997. Fatal Scedosporium prolificans infection in a leukemic patient. Eur. J. Clin. Microbiol. Infect. Dis. 16:460-464. [DOI] [PubMed] [Google Scholar]
- 125.Fernandez-Guerrero, M. L., P. Ruiz Barnes, and J. M. Ales. 1987. Postcraniotomy mycetoma of the scalp and osteomyelitis due to Pseudallescheria boydii. J. Infect. Dis. 156:855. [DOI] [PubMed] [Google Scholar]
- 126.Fernando, S. S., P. Jones, and R. Vaz. 2005. Fine needle aspiration of a pulmonary mycetoma. A case report and review of the literature. Pathology 37:322-324. [DOI] [PubMed] [Google Scholar]
- 127.Fessler, R. G., and F. D. Brown. 1989. Superior sagittal sinus infection with Petriellidium boydii: case report. Neurosurgery 24:604-607. [DOI] [PubMed] [Google Scholar]
- 128.Fiero, R. A., M. Groth, A. Hurewitz, M. April, and M. Nuovo. 1995. Chronic hemoptysis: an unusual manifestation of fungal sinusitis. South. Med. J. 88:782-785. [DOI] [PubMed] [Google Scholar]
- 129.Fietz, T., W. Knauf, S. Schwartz, and E. Thiel. 2003. Intramedullary abscess in a patient with disseminated Scedosporium apiospermum infection. Br. J. Haematol. 120:724. [DOI] [PubMed] [Google Scholar]
- 130.Figueroa, M. S., J. Fortun, A. Clement, and B. F. De Arevalo. 2004. Endogenous endophthalmitis caused by Scedosporium apiospermum treated with voriconazole. Retina 24:319-320. [DOI] [PubMed] [Google Scholar]
- 131.Fisher, J. F., S. Shadomy, J. R. Teabeaut, J. Woodward, G. E. Michaels, M. A. Newman, E. White, P. Cook, A. Seagraves, F. Yaghmai, and J. P. Rissing. 1982. Near-drowning complicated by brain abscess due to Petriellidium boydii. Arch. Neurol. 39:511-513. [DOI] [PubMed] [Google Scholar]
- 132.Forno, L. S., and M. E. Billingham. 1972. Allescheria boydii infection of the brain. J. Pathol. 106:195-198. [DOI] [PubMed] [Google Scholar]
- 133.Fortun, J., P. Martin-Davila, M. A. Sanchez, V. Pintado, M. E. Alvarez, A. Sanchez-Sousa, and S. Moreno. 2003. Voriconazole in the treatment of invasive mold infections in transplant recipients. Eur. J. Clin. Microbiol. Infect. Dis. 22:408-413. [DOI] [PubMed] [Google Scholar]
- 134.Foster, M. R., Z. B. Friedenberg, and F. Passero. 1994. Lumbar Petriellidium boydii osteomyelitis with a systemic presentation. J. Spinal Disord. 7:356-360. [PubMed] [Google Scholar]
- 135.Freeman, A. F., D. E. Kleiner, H. Nadiminti, J. Davis, M. Quezado, V. Anderson, J. M. Puck, and S. M. Holland. 2007. Causes of death in hyper-IgE syndrome. J. Allergy Clin. Immunol. 119:1234-1240. [DOI] [PubMed] [Google Scholar]
- 136.Froumis, N. A., B. J. Mondino, and B. J. Glasgow. 2001. Acanthamoeba keratitis associated with fungal keratitis. Am. J. Ophthalmol. 131:508-509. [DOI] [PubMed] [Google Scholar]
- 137.Fry, V. G., and C. N. Young. 1981. A rare fungal brain abscess in an uncompromised host. Surg. Neurol. 15:446-449. [DOI] [PubMed] [Google Scholar]
- 138.Galgiani, J. N., D. A. Stevens, J. R. Graybill, D. L. Stevens, A. J. Tillinghast, and H. B. Levine. 1984. Pseudallescheria boydii infections treated with ketoconazole. Clinical evaluations of seven patients and in vitro susceptibility results. Chest 86:219-224. [DOI] [PubMed] [Google Scholar]
- 139.Garcia, J. A., C. W. Ingram, and D. Granger. 1990. Persistent neutrophilic meningitis due to Pseudallescheria boydii. Rev. Infect. Dis. 12:959-960. [DOI] [PubMed] [Google Scholar]
- 140.Garcia-Arata, M. I., M. J. Otero, M. Zomeno, M. A. de la Figuera, M. C. de las Cuevas, and M. Lopez-Brea. 1996. Scedosporium apiospermum pneumonia after autologous bone marrow transplantation. Eur. J. Clin. Microbiol. Infect. Dis. 15:600-603. [DOI] [PubMed] [Google Scholar]
- 141.Garcia-Effron, G., A. Gomez-Lopez, E. Mellado, A. Monzon, J. L. Rodriguez-Tudela, and M. Cuenca-Estrella. 2004. In vitro activity of terbinafine against medically important non-dermatophyte species of filamentous fungi. J. Antimicrob. Chemother. 53:1086-1089. [DOI] [PubMed] [Google Scholar]
- 142.Garcia-Martos, P., I. Dominguez, P. Marin, M. Linares, J. Mira, and J. Calap. 2000. Onychomycoses caused by non-dermatophytic filamentous fungi in Cadiz. Enferm. Infecc. Microbiol. Clin. 18:319-324. [PubMed] [Google Scholar]
- 143.Gari, M., J. Fruit, P. Rousseaux, J. M. Garnier, C. Trichet, J. C. Baudrillart, P. Comte, P. Feucheres, and J. M. Pinon. 1985. Scedosporium (Monosporium) apiospermum: multiple brain abscesses. Sabouraudia 23:371-376. [DOI] [PubMed] [Google Scholar]
- 144.Garzoni, C., S. Emonet, L. Legout, R. Benedict, P. Hoffmeyer, L. Bernard, and J. Garbino. 2005. Atypical infections in tsunami survivors. Emerg. Infect. Dis. 11:1591-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Gatto, J., D. Paterson, L. Davis, L. Lockwood, and A. Allworth. 1997. Vertebral osteomyelitis due to Pseudallescheria boydii. Pathology 29:238-240. [DOI] [PubMed] [Google Scholar]
- 146.German, J. W., S. M. Kellie, M. P. Pai, and P. T. Turner. 2004. Treatment of a chronic Scedosporium apiospermum vertebral osteomyelitis. Case report. Neurosurg. Focus 17E 9:1-8. [DOI] [PubMed] [Google Scholar]
- 146a.Gersuk, G., D. Underhill, L. Zhu, and K. Marr. 2006. Dectin-1 and TLRs permit macrophages to distinguish between different Aspergillus fumigatus cellular states. J. Immunol. 176:3717-3724. [DOI] [PubMed] [Google Scholar]
- 147.Gilgado, F., J. Cano, J. Gene, and J. Guarro. 2005. Molecular phylogeny of the Pseudallescheria boydii species complex: proposal of two new species. J. Clin. Microbiol. 43:4930-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gill, J. 1842. Indian Naval Medical Reports—quoted by Ghosh, L. M., et al., 1950. Madura foot (mycetoma). Indian Med. Gazette 85:288. [PMC free article] [PubMed] [Google Scholar]
- 149.Gil-Lamaignere, C., A. Maloukou, J. L. Rodriguez-Tudela, and E. Roilides. 2001. Human phagocytic cell responses to Scedosporium prolificans. Med. Mycol. 39:169-175. [DOI] [PubMed] [Google Scholar]
- 150.Gil-Lamaignere, C., E. Roilides, C. A. Lyman, M. Simitsopoulou, T. Stergiopoulou, A. Maloukou, and T. J. Walsh. 2003. Human phagocytic cell responses to Scedosporium apiospermum (Pseudallescheria boydii): variable susceptibility to oxidative injury. Infect. Immun. 71:6472-6478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gil-Lamaignere, C., E. Roilides, A. Maloukou, I. Georgopoulou, G. Petrikkos, and T. J. Walsh. 2002. Amphotericin B lipid complex exerts additive antifungal activity in combination with polymorphonuclear leucocytes against Scedosporium prolificans and Scedosporium apiospermum. J. Antimicrob. Chemother. 50:1027-1030. [DOI] [PubMed] [Google Scholar]
- 152.Gil-Lamaignere, C., E. Roilides, J. Mosquera, A. Maloukou, and T. J. Walsh. 2002. Antifungal triazoles and polymorphonuclear leukocytes synergize to cause increased hyphal damage to Scedosporium prolificans and Scedosporium apiospermum. Antimicrob. Agents Chemother. 46:2234-2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gil-Lamaignere, C., R. M. Winn, M. Simitsopoulou, A. Maloukou, T. J. Walsh, and E. Roilides. 2005. Inteferon gamma and granulocyte-macrophage colony-stimulating factor augment the antifungal activity of human polymorphonuclear leukocytes against Scedosporium spp.: comparison with Aspergillus spp. Med. Mycol. 43:253-260. [DOI] [PubMed] [Google Scholar]
- 154.Gillum, P. S., A. Gurswami, and J. W. Taira. 1997. Localized cutaneous infection by Scedosporium prolificans (inflatum). Int. J. Dermatol. 36:297-299. [DOI] [PubMed] [Google Scholar]
- 155.Ginter, G., G. S. de Hoog, A. Pschaid, M. Fellinger, A. Bogiatzis, C. Berghold, E. M. Reich, and F. C. Odds. 1995. Arthritis without grains caused by Pseudallescheria boydii. Mycoses 38:369-371. [DOI] [PubMed] [Google Scholar]
- 156.Ginter, G., B. Petutschnig, G. Pierer, H. P. Soyer, S. Reischle, T. Kern, and S. de Hoog. 1999. Atypical cutaneous pseudallescheriosis refractory to antifungal agents. Mycoses 42:507-511. [DOI] [PubMed] [Google Scholar]
- 157.Glassman, M. I., P. Henkind, and E. Alture-Werber. 1973. Monosporium apiospermum endophthalmitis. Am. J. Ophthalmol. 76:821-824. [DOI] [PubMed] [Google Scholar]
- 158.Gluckman, S. J., K. Ries, and E. Abrutyn. 1977. Allescheria (Petriellidium) boydii sinusitis in a compromised host. J. Clin. Microbiol. 5:481-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Godfrey, J. 1846. Diseases of the foot not hitherto described. Lancet i:593-594. [Google Scholar]
- 160.Goldberg, S. L., D. J. Geha, W. F. Marshall, D. J. Inwards, and H. C. Hoagland. 1993. Successful treatment of simultaneous pulmonary Pseudallescheria boydii and Aspergillus terreus infection with oral itraconazole. Clin. Infect. Dis. 16:803-805. [DOI] [PubMed] [Google Scholar]
- 161.Gompels, M. M., C. A. Bethune, G. Jackson, and G. P. Spickett. 2002. Scedosporium apiospermum in chronic granulomatous disease treated with an HLA matched bone marrow transplant. J. Clin. Pathol. 55:784-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gonzalez, G. M., A. W. Fothergill, D. A. Sutton, M. G. Rinaldi, and D. Loebenberg. 2005. In vitro activities of new and established triazoles against opportunistic filamentous and dimorphic fungi. Med. Mycol. 43:281-284. [DOI] [PubMed] [Google Scholar]
- 163.Gonzalez, G. M., R. Tijerina, L. K. Najvar, R. Bocanegra, M. G. Rinaldi, D. Loebenberg, and J. R. Graybill. 2003. Activity of posaconazole against Pseudallescheria boydii: in vitro and in vivo assays. Antimicrob. Agents Chemother. 47:1436-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gordon, M. A., W. W. Vallotton, and G. S. Croffead. 1959. Corneal allescheriosis. Arch. Ophthalmol. 62:50-55. [Google Scholar]
- 165.Gosbell, I. B., P. J. Newton, and E. A. Sullivan. 1999. Survey of blood cultures from five community hospitals in south-western Sydney, Australia, 1993-1994. Aust. N. Z. J. Med. 29:684-692. [DOI] [PubMed] [Google Scholar]
- 166.Gosbell, I. B., V. Toumasatos, J. Yong, R. S. Kuo, D. H. Ellis, and R. C. Perrie. 2003. Cure of orthopaedic infection with Scedosporium prolificans, using voriconazole plus terbinafine, without the need for radical surgery. Mycoses 46:233-236. [DOI] [PubMed] [Google Scholar]
- 167.Green, W. O., Jr., and T. E. Adams. 1964. Mycetoma in the United States; a review and report of seven additional cases. Am. J. Clin. Pathol. 42:75-91. [DOI] [PubMed] [Google Scholar]
- 168.Greig, J. R., M. A. Khan, N. S. Hopkinson, B. G. Marshall, P. O. Wilson, and S. U. Rahman. 2001. Pulmonary infection with Scedosporium prolificans in an immunocompetent individual. J. Infect. 43:15-17. [DOI] [PubMed] [Google Scholar]
- 169.Grigg, A. P., P. Phillips, S. Durham, and J. D. Shepherd. 1993. Recurrent Pseudallescheria boydii sinusitis in acute leukemia. Scand. J. Infect. Dis. 25:263-267. [DOI] [PubMed] [Google Scholar]
- 170.Groll, A. H., and T. J. Walsh. 2001. Uncommon opportunistic fungi: new nosocomial threats. Clin. Microbiol. Infect. 7(Suppl. 2):8-24. [DOI] [PubMed] [Google Scholar]
- 171.Guarro, J., A. S. Kantarcioglu, R. Horre, J. L. Rodriguez-Tudela, M. Cuenca Estrella, J. Berenguer, and G. S. de Hoog. 2006. Scedosporium apiospermum: changing clinical spectrum of a therapy-refractory opportunist. Med. Mycol. 44:295-327. [DOI] [PubMed] [Google Scholar]
- 172.Gueho, E., and G. S. de Hoog. 1991. Taxonomy of the medical species of Pseudallescheria and Scedosporium. J. Mycol. Méd. 1:3-9. [Google Scholar]
- 173.Guerrero, A., P. Torres, M. T. Duran, B. Ruiz-Diez, M. Rosales, and J. L. Rodriguez-Tudela. 2001. Airborne outbreak of nosocomial Scedosporium prolificans infection. Lancet 357:1267-1268. [DOI] [PubMed] [Google Scholar]
- 174.Guez, S., V. Calas, B. Couprie, D. Stoll, and G. Cabanieu. 1992. Apropos of 2 cases of Scedosporium apiospermum nasosinusal infection. Rev. Med. Intern. 13:145-148. [DOI] [PubMed] [Google Scholar]
- 175.Gugnani, H. C., B. C. Ezeanolue, M. Khalil, C. D. Amoah, E. U. Ajuiu, and E. A. Oyewo. 1995. Fluconazole in the therapy of tropical deep mycoses. Mycoses 38:485-488. [DOI] [PubMed] [Google Scholar]
- 176.Gumbart, C. H. 1983. Pseudallescheria boydii infection after bone marrow transplantation. Ann. Intern. Med. 99:193-194. [DOI] [PubMed] [Google Scholar]
- 177.Guyotat, D., M. A. Piens, R. Bouvier, and D. Fiere. 1987. A case of disseminated Scedosporium apiospermum infection after bone marrow transplantation. Mykosen 30:151-154. [DOI] [PubMed] [Google Scholar]
- 178.Haapasaari, J., R. V. Essen, A. Kahanpaa, A. A. Kostiala, K. Holmberg, and J. Ahlqvist. 1982. Fungal arthritis simulating juvenile rheumatoid arthritis. Br. Med. J. 285:923-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hachimi-Idrissi, S., M. Willemsen, B. Desprechins, A. Naessens, A. Goossens, L. De Meirleir, and J. Ramet. 1990. Pseudallescheria boydii and brain abscesses. Pediatr. Infect. Dis. J. 9:737-741. [DOI] [PubMed] [Google Scholar]
- 180.Hagari, Y., S. Ishioka, F. Ohyama, and M. Mihara. 2002. Cutaneous infection showing sporotrichoid spread caused by Pseudallescheria boydii (Scedosporium apiospermum): successful detection of fungal DNA in formalin-fixed, paraffin-embedded sections by seminested PCR. Arch. Dermatol. 138:271-272. [DOI] [PubMed] [Google Scholar]
- 181.Hainer, J. W., J. H. Ostrow, and D. W. Mackenzie. 1974. Pulmonary monosporosis: report of a case with precipitating antibody. Chest 66:601-603. [DOI] [PubMed] [Google Scholar]
- 182.Halpern, A. A., D. A. Nagel, and D. J. Schurman. 1977. Allescheria boydii osteomyelitis following multiple steroid injections and surgery. Clin. Orthop. Relat. Res. 182:232-234. [PubMed] [Google Scholar]
- 183.Hay, R. J., and D. W. Mackenzie. 1983. Mycetoma (madura foot) in the United Kingdom—a survey of forty-four cases. Clin. Exp. Dermatol. 8:553-562. [DOI] [PubMed] [Google Scholar]
- 184.Hay, R. J., and D. W. Mackenzie. 1982. The histopathological features of pale grain eumycetoma. Trans. R. Soc. Trop. Med. Hyg. 76:839-844. [DOI] [PubMed] [Google Scholar]
- 185.Hayden, G., C. Lapp, and F. Loda. 1977. Arthritis caused by Monosporium apiospermum treated with intraarticular amphotericin B. Am. J. Dis. Child. 131:927. [DOI] [PubMed] [Google Scholar]
- 186.Hecht, R., and J. Z. Montgomerie. 1978. Maxillary sinus infection with Allescheria boydii (Petriellidium boydii). Johns Hopkins Med. J. 142:107-109. [PubMed] [Google Scholar]
- 187.Hernandez Prats, C., F. Llinares Tello, A. Burgos San Jose, J. Selva Otaolaurruchi, and J. P. Ordovas Baines. 2004. Voriconazole in fungal keratitis caused by Scedosporium apiospermum. Ann. Pharmacother. 38:414-417. [DOI] [PubMed] [Google Scholar]
- 188.Heyn, K., A. Tredup, S. Salvenmoser, and F. M. Muller. 2005. Effect of voriconazole combined with micafungin against Candida, Aspergillus, and Scedosporium spp. and Fusarium solani. Antimicrob. Agents Chemother. 49:5157-5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hofman, P., M. C. Saint-Paul, M. Gari-Toussaint, J. F. Michiels, C. Boissy, P. Jambou, J. Gugenheim, and R. Loubiere. 1993. Disseminated infection due to Scedosporium apiospermum in liver transplantation. Differential diagnosis from invasive aspergillosis. Ann. Pathol. 13:332-335. [PubMed] [Google Scholar]
- 190.Hopwood, V., E. G. Evans, J. Matthews, and D. W. Denning. 1995. Scedosporium prolificans, a multi-resistant fungus, from a U.K. AIDS patient. J. Infect. 30:153-155. [DOI] [PubMed] [Google Scholar]
- 191.Horre, R., E. Feil, A. P. Stangel, H. Zhou, S. Gilges, A. Wohrmann, G. S. de Hoog, J. F. Meis, G. Marklein, and K. P. Schaal. 2000. Scedosporiosis of the brain with fatal outcome after traumatization of the foot. case report. Mycoses 43(Suppl. 2):33-36. [PubMed] [Google Scholar]
- 192.Horre, R., B. Jovanic, G. Marklein, G. Schumacher, N. Friedrichs, T. Neuhaus, G. S. de Hoog, W. H. Becker, S. M. Choi, and K. P. Schaal. 2003. Fatal pulmonary scedosporiosis. Mycoses 46:418-421. [DOI] [PubMed] [Google Scholar]
- 193.Horre, R., G. Schumacher, G. Marklein, H. Stratmann, E. Wardelmann, S. Gilges, G. S. De Hoog, and K. P. Schaal. 2002. Mycetoma due to Pseudallescheria boydii and co-isolation of Nocardia abscessus in a patient injured in road accident. Med. Mycol. 40:525-527. [DOI] [PubMed] [Google Scholar]
- 194.Horton, C. K., L. Huang, and L. Gooze. 1999. Pseudallescheria boydii infection in AIDS. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 20:209-211. [DOI] [PubMed] [Google Scholar]
- 195.Horton, C. M., C. D. Freeman, P. E. Nolan, Jr., and J. G. Copeland, 3rd. 1992. Cyclosporine interactions with miconazole and other azole-antimycotics: a case report and review of the literature. J. Heart Lung Transplant. 11:1127-1132. [PubMed] [Google Scholar]
- 196.Howden, B. P., M. A. Slavin, A. P. Schwarer, and A. M. Mijch. 2003. Successful control of disseminated Scedosporium prolificans infection with a combination of voriconazole and terbinafine. Eur. J. Clin. Microbiol. Infect. Dis. 22:111-113. [DOI] [PubMed] [Google Scholar]
- 197.Huang, H. J., J. Y. Zhu, and Y. H. Zhang. 1990. The first case of Pseudallescheria boydii meningitis in China—electron microscopic study and antigenicity analysis of the agent. J. Tongji. Med. Univ. 10:218-221. [DOI] [PubMed] [Google Scholar]
- 198.Hughes, S. J. 1958. Revisiones hyphomycetum aliquot cum appendice de nominilus rejiciendisi. Can. J. Bot. 36:727-836. [Google Scholar]
- 199.Hung, L. H., and L. A. Norwood. 1993. Osteomyelitis due to Pseudallescheria boydii. South. Med. J. 86:231-234. [DOI] [PubMed] [Google Scholar]
- 200.Husain, S., B. D. Alexander, P. Munoz, R. K. Avery, S. Houston, T. Pruett, R. Jacobs, E. A. Dominguez, J. G. Tollemar, K. Baumgarten, C. M. Yu, M. M. Wagener, P. Linden, S. Kusne, and N. Singh. 2003. Opportunistic mycelial fungal infections in organ transplant recipients: emerging importance of non-Aspergillus mycelial fungi. Clin. Infect. Dis. 37:221-229. [DOI] [PubMed] [Google Scholar]
- 201.Husain, S., P. Munoz, G. Forrest, B. D. Alexander, J. Somani, K. Brennan, M. M. Wagener, and N. Singh. 2005. Infections due to Scedosporium apiospermum and Scedosporium prolificans in transplant recipients: clinical characteristics and impact of antifungal agent therapy on outcome. Clin. Infect. Dis. 40:89-99. [DOI] [PubMed] [Google Scholar]
- 202.Ichikawa, T., M. Saiki, S. Tokunaga, and T. Saida. 1997. Scedosporium apiospermum skin infection in a patient with nephrotic syndrome. Acta Derm. Venereol. 77:172-173. [DOI] [PubMed] [Google Scholar]
- 203.Idigoras, P., J. M. Garcia-Arenzana, J. R. Saenz, L. Pineiro, and J. Marin. 2000. Isolation of Scedosporium prolificans from the air in the room of a patient with leukemia and disseminated infection with this fungus. Enferm. Infecc. Microbiol. Clin. 18:426-427. [PubMed] [Google Scholar]
- 204.Idigoras, P., E. Perez-Trallero, L. Pineiro, J. Larruskain, M. C. Lopez-Lopategui, N. Rodriguez, and J. M. Gonzalez. 2001. Disseminated infection and colonization by Scedosporium prolificans: a review of 18 cases, 1990-1999. Clin. Infect. Dis. 32:E158-E165. [DOI] [PubMed] [Google Scholar]
- 205.Issakainen, J., J. Jalava, E. Eerola, and C. K. Campbell. 1997. Relatedness of Pseudallescheria, Scedosporium and Graphium pro parte based on SSU rDNA sequences. J. Med. Vet. Mycol. 35:389-398. [PubMed] [Google Scholar]
- 206.Issakainen, J., J. Jalava, J. Hyvonen, N. Sahlberg, T. Pirnes, and C. K. Campbell. 2003. Relationships of Scopulariopsis based on LSU rDNA sequences. Med. Mycol. 41:31-42. [DOI] [PubMed] [Google Scholar]
- 207.Jabado, N., J. L. Casanova, E. Haddad, F. Dulieu, J. C. Fournet, B. Dupont, A. Fischer, C. Hennequin, and S. Blanche. 1998. Invasive pulmonary infection due to Scedosporium apiospermum in two children with chronic granulomatous disease. Clin. Infect. Dis. 27:1437-1441. [DOI] [PubMed] [Google Scholar]
- 208.Jahagirdar, B. N., and V. A. Morrison. 2002. Emerging fungal pathogens in patients with hematologic malignancies and marrow/stem-cell transplant recipients. Semin. Respir. Infect. 17:113-120. [DOI] [PubMed] [Google Scholar]
- 209.Jayamohan, Y., and J. A. Ribes. 2006. Pseudallescheriasis: a summary of patients from 1980-2003 in a tertiary care center. Arch. Pathol. Lab. Med. 130:1843-1846. [DOI] [PubMed] [Google Scholar]
- 210.Jones, J., S. E. Katz, and M. Lubow. 1999. Scedosporium apiospermum of the orbit. Arch. Ophthalmol. 117:272-273. [PubMed] [Google Scholar]
- 211.Jung, J. Y., R. Salas, C. H. Almond, S. Saab, and R. Reyna. 1977. The role of surgery in the management of pulmonary monosporosis. A collective review. J. Thorac. Cardiovasc. Surg. 73:139-144. [PubMed] [Google Scholar]
- 212.Kamiya, M., T. Noda, A. Nakatani, K. Yoneda, M. Fujihiro, and S. Udagawa. 1998. A case of cutaneous pseudallescheriosis resembling sporotrichosis. Nippon Ishinkin Gakkai Zasshi 39:33-36. [DOI] [PubMed] [Google Scholar]
- 213.Kanafani, Z. A., Y. Comair, and S. S. Kanj. 2004. Pseudallescheria boydii cranial osteomyelitis and subdural empyema successfully treated with voriconazole: a case report and literature review. Eur. J. Clin. Microbiol. Infect. Dis. 23:836-840. [DOI] [PubMed] [Google Scholar]
- 214.Kaufman, L., P. G. Standard, M. Jalbert, and D. E. Kraft. 1997. Immunohistologic identification of Aspergillus spp. and other hyaline fungi by using polyclonal fluorescent antibodies. J. Clin. Microbiol. 35:2206-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kemp, H. B., A. F. Bedford, and W. J. Fincham. 1982. Petriellidium boydii infection of the knee: a case report. Skeletal Radiol. 9:114-117. [DOI] [PubMed] [Google Scholar]
- 216.Kershaw, P., R. Freeman, D. Templeton, P. C. DeGirolami, U. DeGirolami, D. Tarsy, S. Hoffmann, G. Eliopoulos, and A. W. Karchmer. 1990. Pseudallescheria boydii infection of the central nervous system. Arch. Neurol. 47:468-472. [DOI] [PubMed] [Google Scholar]
- 217.Khurshid, A., V. T. Barnett, M. Sekosan, A. S. Ginzburg, and E. Onal. 1999. Disseminated Pseudallescheria boydii infection in a nonimmunocompromised host. Chest 116:572-574. [DOI] [PubMed] [Google Scholar]
- 218.Kim, H. U., S. C. Kim, and H. S. Lee. 1999. Localized skin infection due to Scedosporium apiospermum: report of two cases. Br. J. Dermatol. 141:605-606. [DOI] [PubMed] [Google Scholar]
- 219.Kimura, M., and M. R. McGinnis. 1998. Fontana-Masson-stained tissue from culture-proven mycoses. Arch. Pathol. Lab. Med. 122:1107-1111. [PubMed] [Google Scholar]
- 220.Kiratli, H., O. Uzun, N. Kiraz, and B. Eldem. 2001. Scedosporium apiospermum chorioretinitis. Acta Ophthalmol. Scand. 79:540-542. [DOI] [PubMed] [Google Scholar]
- 221.Kiraz, N., Z. Gulbas, Y. Akgun, and O. Uzun. 2001. Lymphadenitis caused by Scedosporium apiospermum in an immunocompetent patient. Clin. Infect. Dis. 32:E59-61. [DOI] [PubMed] [Google Scholar]
- 222.Kish, L. S., J. S. Taylor, W. F. Bergfeld, and G. S. Hall. 1983. Petriellidium (Allescheria) boydii mycetoma in an immunosuppressed host. Cleve. Clin. Q. 50:209-211. [DOI] [PubMed] [Google Scholar]
- 223.Klopfenstein, K. J., R. Rosselet, A. Termuhlen, and D. Powell. 2003. Successful treatment of Scedosporium pneumonia with voriconazole during AML therapy and bone marrow transplantation. Med. Pediatr. Oncol. 41:494-495. [DOI] [PubMed] [Google Scholar]
- 224.Klotz, S. A., C. C. Penn, G. J. Negvesky, and S. I. Butrus. 2000. Fungal and parasitic infections of the eye. Clin. Microbiol. Rev. 13:662-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kowacs, P. A., C. E. Soares Silvado, S. Monteiro de Almeida, M. Ramos, K. Abrao, L. E. Madaloso, R. L. Pinheiro, and L. C. Werneck. 2004. Infection of the CNS by Scedosporium apiospermum after near drowning. Report of a fatal case and analysis of its confounding factors. J. Clin. Pathol. 57:205-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ksiazek, S. M., D. A. Morris, S. Mandelbaum, and P. S. Rosenbaum. 1994. Fungal panophthalmitis secondary to Scedosporium apiospermum (Pseudallescheria boydii) keratitis. Am. J. Ophthalmol. 118:531-533. [DOI] [PubMed] [Google Scholar]
- 227.Kumar, B., G. J. Crawford, and G. C. Morlet. 1997. Scedosporium prolificans corneoscleritis: a successful outcome. Aust. N. Z. J. Ophthalmol. 25:169-171. [DOI] [PubMed] [Google Scholar]
- 228.Kusne, S., S. Ariyanayagam-Baksh, D. C. Strollo, and J. Abernethy. 2000. Invasive Scedosporium apiospermum infection in a heart transplant recipient presenting with multiple skin nodules and a pulmonary consolidation. Transplant. Infect. Dis. 2:194-196. [DOI] [PubMed] [Google Scholar]
- 229.Kusuhara, M., and H. Hachisuka. 1997. Lymphocutaneous infection due to Scedosporium apiospermum. Int. J. Dermatol. 36:684-688. [DOI] [PubMed] [Google Scholar]
- 230.Kwon-Chung, K. J., and J. E. Bennett. 1992. Medical mycology. Lea & Febiger, Philadelphia, PA.
- 231.Lai, T. F., R. Malhotra, R. Esmail-Zaden, A. Galanopoulas, M. Chehade, and D. Selva. 2003. Use of voriconazole in Scedosporium apiospermum keratitis. Cornea 22:391-392. [DOI] [PubMed] [Google Scholar]
- 232.Lake, F. R., A. E. Tribe, R. McAleer, J. Froudist, and P. J. Thompson. 1990. Mixed allergic bronchopulmonary fungal disease due to Pseudallescheria boydii and Aspergillus. Thorax 45:489-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Lamaris, G. A., G. Chamilos, R. E. Lewis, A. Safdar, I. I. Raad, and D. P. Kontoyiannis. 2006. Scedosporium infection in a tertiary care cancer center: a review of 25 cases from 1989-2006. Clin. Infect. Dis. 43:1580-1584. [DOI] [PubMed] [Google Scholar]
- 234.Lang, A. G., and H. A. Peterson. 1976. Osteomyelitis following puncture wounds of the foot in children. J. Trauma 16:993-999. [DOI] [PubMed] [Google Scholar]
- 235.Larcher, G., B. Cimon, F. Symoens, G. Tronchin, D. Chabasse, and J. P. Bouchara. 1996. A 33 kDa serine proteinase from Scedosporium apiospermum. Biochem. J. 315:119-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Larocco, A., Jr., and J. B. Barron. 2005. Endogenous scedosporium apiospermum endophthalmitis. Retina 25:1090-1093. [DOI] [PubMed] [Google Scholar]
- 237.Larone, D. H. 1995. Medically important fungi: a guide to identification, 3rd ed. ASM Press, Washington, DC.
- 237a.Lavalle, P. 1962. Agents of mycetoma, p. 50-68. In G. Dalldorf (ed.), Fungi and fungous diseases. Charles C. Thomas, Springfield, IL.
- 238.Lavigne, C., F. Maillot, A. de Muret, M. Therizol-Ferly, F. Lamisse, and L. Machet. 1999. Cutaneous infection with Scedosporium apiospermum in a patient treated with corticosteroids. Acta Derm. Venereol. 79:402-403. [DOI] [PubMed] [Google Scholar]
- 239.Lavy, D., O. Morin, G. Venet, Y. Maugars, A. Prost, and J. M. Berthelot. 2001. Pseudallescheria boydii knee arthritis in a young immunocompetent adult two years after a compound patellar fracture. Joint Bone Spine 68:517-520. [DOI] [PubMed] [Google Scholar]
- 240.Lazarus, H. S., J. P. Myers, and R. J. Brocker. 1986. Post-craniotomy wound infection caused by Pseudallescheria boydii. Case report. J. Neurosurg. 64:153-154. [DOI] [PubMed] [Google Scholar]
- 241.Leck, A., M. Matheson, S. Tuft, K. Waheed, and H. Lagonowski. 2003. Scedosporium apiospermum keratomycosis with secondary endophthalmitis. Eye 17:841-843. [DOI] [PubMed] [Google Scholar]
- 242.Le Gouill, S. L., N. Morineau, M. Miegeville, N. Milpied, J. L. Harousseau, and P. Moreau. 1999. Pseudallescheria boydii osteoarthritis in a patient with acute lymphoblastic leukemia: a case report. Rev. Med. Intern. 20:434-438. [DOI] [PubMed] [Google Scholar]
- 243.Lennon, P. A., C. R. Cooper, Jr., I. F. Salkin, and S. B. Lee. 1994. Ribosomal DNA internal transcribed spacer analysis supports synonomy of Scedosporium inflatum and Lomentospora prolificans. J. Clin. Microbiol. 32:2413-2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Leroy, P., A. Smismans, and T. Seute. 2006. Invasive pulmonary and central nervous system aspergillosis after near-drowning of a child: case report and review of the literature. Pediatrics 118:e509-513. [DOI] [PubMed] [Google Scholar]
- 245.Levine, N. B., R. Kurokawa, C. J. Fichtenbaum, J. A. Howington, and C. Kuntz IV. 2002. An immunocompetent patient with primary Scedosporium apiospermum vertebral osteomyelitis. J. Spinal Disord. Tech. 15:425-430. [DOI] [PubMed] [Google Scholar]
- 246.Levitt, J. M., and J. Goldstein. 1971. Keratomycosis due to Allescheria boydii. Am. J. Ophthalmol. 71:1190-1191. [DOI] [PubMed] [Google Scholar]
- 247.Lexier, R., and S. L. Walmsley. 1999. Successful treatment of Madura foot caused by Pseudallescheria boydii with Escherichia coli superinfection: a case report. Can. J. Surg. 42:307-309. [PMC free article] [PubMed] [Google Scholar]
- 248.Li, R. K., and M. G. Rinaldi. 1999. In vitro antifungal activity of nikkomycin Z in combination with fluconazole or itraconazole. Antimicrob. Agents Chemother. 43:1401-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Lichon, V., and A. Khachemoune. 2006. Mycetoma: a review. Am. J. Clin. Dermatol. 7:315-321. [DOI] [PubMed] [Google Scholar]
- 250.Lichtman, D. M., D. C. Johnson, G. R. Mack, and E. E. Lack. 1978. Maduromycosis (Allescheria boydii) infection of the hand. A case report. J. Bone Joint Surg. 60:546-548. [PubMed] [Google Scholar]
- 251.Linares, M. J., G. Charriel, F. Solis, F. Rodriguez, A. Ibarra, and M. Casal. 2005. Susceptibility of filamentous fungi to voriconazole tested by two microdilution methods. J. Clin. Microbiol. 43:250-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Linares Sicilia, M. J., M. Santos Lacomba, F. Solis Cuesta, R. Sanchez Pedraza, T. Nievas Gomez, and M. Casal Roman. 2003. Scedosporium apiospermum keratitis. Rev. Iberoam. Micol. 20:68-70. [PubMed] [Google Scholar]
- 253.Liu, Y. F., X. D. Zhao, C. L. Ma, C. X. Li, T. S. Zhang, and W. J. Liao. 1997. Cutaneous infection by Scedosporium apiospermum and its successful treatment with itraconazole. Clin. Exp. Dermatol. 22:198-200. [PubMed] [Google Scholar]
- 254.Lonser, R. R., D. S. Brodke, and A. T. Dailey. 2001. Vertebral osteomyelitis secondary to Pseudallescheria boydii. J. Spinal Disord. 14:361-364. [DOI] [PubMed] [Google Scholar]
- 255.Lopes, J. O., S. H. Alves, J. P. Benevenga, A. Salla, C. Khmohan, and C. B. Silva. 1994. Subcutaneous pseudallescheriasis in a renal transplant recipient. Mycopathologia 125:153-156. [DOI] [PubMed] [Google Scholar]
- 256.Lopez, F. A., R. S. Crowley, L. Wastila, H. A. Valantine, and J. S. Remington. 1998. Scedosporium apiospermum (Pseudallescheria boydii) infection in a heart transplant recipient: a case of mistaken identity. J. Heart Lung Transplant. 17:321-324. [PubMed] [Google Scholar]
- 257.Lopez, L., L. Gaztelurrutia, M. Cuenca-Estrella, A. Monzon, J. Barron, J. L. Hernandez, and R. Perez. 2001. Infection and colonization by Scedosporium prolificans. Enferm. Infecc. Microbiol. Clin. 19:308-313. [DOI] [PubMed] [Google Scholar]
- 258.Louria, D. B., P. H. Lieberman, H. S. Collins, and A. Blevins. 1966. Pulmonary mycetoma due to Allescheria boydii. Arch. Intern. Med. 117:748-751. [PubMed] [Google Scholar]
- 259.Lutwick, L. I., J. N. Galgiani, R. H. Johnson, and D. A. Stevens. 1976. Visceral fungal infections due to Petriellidium boydii (Allescheria boydii). In vitro drug sensitivity studies. Am. J. Med. 61:632-640. [DOI] [PubMed] [Google Scholar]
- 260.Lutwick, L. I., M. W. Rytel, J. P. Yanez, J. N. Galgiani, and D. A. Stevens. 1979. Deep infections from Petriellidium boydii treated with miconazole. JAMA 241:272-273. [PubMed] [Google Scholar]
- 261.Luu, K. K., I. U. Scott, D. Miller, and J. L. Davis. 2001. Endogenous Pseudallescheria boydii endophthalmitis in a patient with ring-enhancing brain lesions. Ophthalm. Surg. Lasers 32:325-329. [PubMed] [Google Scholar]
- 262.Machado, C. M., M. A. Martins, E. M. Heins-Vaccari, S. Lacaz Cda, M. C. Macedo, J. B. Castelli, R. S. Medeiros, R. L. Silva, and F. L. Dulley. 1998. Scedosporium apiospermum sinusitis after bone marrow transplantation: report of a case. Rev. Inst. Med. Trop. Sao Paulo 40:321-323. [DOI] [PubMed] [Google Scholar]
- 263.Mader, J. T., R. S. Ream, and P. W. Heath. 1978. Petriellidium boydii (Allescheria boydii) sphenoidal sinusitis. JAMA 239:2368-2369. [DOI] [PubMed] [Google Scholar]
- 264.Madrigal, V., J. Alonso, E. Bureo, F. J. Figols, and R. Salesa. 1995. Fatal meningoencephalitis caused by Scedosporium inflatum (Scedosporium prolificans) in a child with lymphoblastic leukemia. Eur. J. Clin. Microbiol. Infect. Dis. 14:601-603. [DOI] [PubMed] [Google Scholar]
- 265.Maertens, J., K. Lagrou, H. Deweerdt, I. Surmont, G. E. Verhoef, J. Verhaegen, and M. A. Boogaerts. 2000. Disseminated infection by Scedosporium prolificans: an emerging fatality among haematology patients. Case report and review. Ann. Hematol. 79:340-344. [DOI] [PubMed] [Google Scholar]
- 266.Mahgoub, E. S., S. A. Gumaa, and A. M. El Hassan. 1977. Immunological status of mycetoma patients. Bull. Soc. Pathol. Exot. Filiales. 70:48-54. [PubMed] [Google Scholar]
- 267.Mahgoub, E. S., and I. G. Murray. 1973. Mycetoma. William Heinemann Medical Book, London, United Kingdom.
- 268.Malekzadeh, M., G. D. Overturf, S. B. Auerbach, L. Wong, and M. Hirsch. 1990. Chronic, recurrent osteomyelitis caused by Scedosporium inflatum. Pediatr. Infect. Dis. J. 9:357-359. [DOI] [PubMed] [Google Scholar]
- 269.Malloch, D. 1970. New concepts in the Microascaceae illustrated by two new species. Mycologia 62:727-739. [Google Scholar]
- 270.Malloch, D., and I. A. Salkin. 1984. A new species of Scedosporium associated with osteomyelitis in humans. Mycotaxon 21:247-255. [Google Scholar]
- 271.Mancini, N., C. M. Ossi, M. Perotti, S. Carletti, C. Gianni, G. Paganoni, S. Matusuka, M. Guglielminetti, A. Cavallero, R. Burioni, P. Rama, and M. Clementi. 2005. Direct sequencing of Scedosporium apiospermum DNA in the diagnosis of a case of keratitis. J. Med. Microbiol. 54:897-900. [DOI] [PubMed] [Google Scholar]
- 272.Marin, J., M. A. Sanz, G. F. Sanz, J. Guarro, M. L. Martinez, M. Prieto, E. Gueho, and J. L. Menezo. 1991. Disseminated Scedosporium inflatum infection in a patient with acute myeloblastic leukemia. Eur. J. Clin. Microbiol. Infect. Dis. 10:759-761. [DOI] [PubMed] [Google Scholar]
- 273.Marr, K. A., R. A. Carter, F. Crippa, A. Wald, and L. Corey. 2002. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 34:909-917. [DOI] [PubMed] [Google Scholar]
- 274.Martino, R., R. Lopez, A. Sureda, S. Brunet, and A. Domingo-Albos. 1997. Risk of reactivation of a recent invasive fungal infection in patients with hematological malignancies undergoing further intensive chemo-radiotherapy. A single-center experience and review of the literature. Haematologica 82:297-304. [PubMed] [Google Scholar]
- 275.Martino, R., J. Nomdedeu, A. Altes, A. Sureda, S. Brunet, C. Martinez, and A. Domingo-Albos. 1994. Successful bone marrow transplantation in patients with previous invasive fungal infections: report of four cases. Bone Marrow Transplant. 13:265-269. [PubMed] [Google Scholar]
- 276.McCall, R. E. 1981. Maduromycosis “Allescheria boydii” septic arthritis of the knee: a case report. Orthopedics 4:1144-1146. [DOI] [PubMed] [Google Scholar]
- 277.McCarthy, D. S., J. L. Longbottom, R. W. Riddell, and J. C. Batten. 1969. Pulmonary mycetoma due to Allescheria boydii. Am. Rev. Respir. Dis. 100:213-216. [DOI] [PubMed] [Google Scholar]
- 278.McGinnis, M. R. 1980. Laboratory handbook of medical mycology, 1st ed. Academic Press, Inc., New York, NY.
- 279.McGinnis, M. R., A. A. Padhye, and L. Ajello. 1982. Pseudallescheria Negroni et Fischer, 1943 and its later synonym Petriellidium Malloch, 1970. Mycotaxon 14:94-102. [Google Scholar]
- 280.McGinnis, M. R., and R. C. Fader. 1988. Mycetoma: a contemporary concept. Infect. Dis. Clin. N. Am. 2:939-954. [PubMed] [Google Scholar]
- 281.McKelvie, P. A., E. Y. Wong, L. P. Chow, and A. J. Hall. 2001. Scedosporium endophthalmitis: two fatal disseminated cases of Scedosporium infection presenting with endophthalmitis. Clin. Exp. Ophthalmol. 29:330-334. [DOI] [PubMed] [Google Scholar]
- 282.McNeil, M. M., J. M. Brown, C. H. Magruder, K. T. Shearlock, R. A. Saul, D. P. Allred, and L. Ajello. 1992. Disseminated Nocardia transvalensis infection: an unusual opportunistic pathogen in severely immunocompromised patients. J. Infect. Dis. 165:175-178. [DOI] [PubMed] [Google Scholar]
- 283.Meadow, W. L., M. A. Tipple, and J. W. Rippon. 1981. Endophthalmitis caused by Petriellidium boydii. Am. J. Dis. Child. 135:378-380. [DOI] [PubMed] [Google Scholar]
- 284.Meallet, M. A. 2006. Subpalpebral lavage antibiotic treatment for severe infectious scleritis and keratitis. Cornea 25:159-163. [DOI] [PubMed] [Google Scholar]
- 285.Medoff, G. 1983. Antifungal action of rifampin. Rev. Infect. Dis. 5(Suppl. 3):S614-S619. [DOI] [PubMed] [Google Scholar]
- 286.Meletiadis, J., J. F. Meis, J. W. Mouton, J. L. Rodriquez-Tudela, J. P. Donnelly, and P. E. Verweij. 2002. In vitro activities of new and conventional antifungal agents against clinical Scedosporium isolates. Antimicrob. Agents Chemother. 46:62-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Meletiadis, J., J. W. Mouton, J. F. Meis, and P. E. Verweij. 2003. In vitro drug interaction modeling of combinations of azoles with terbinafine against clinical Scedosporium prolificans isolates. Antimicrob. Agents Chemother. 47:106-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Meletiadis, J., J. W. Mouton, J. L. Rodriguez-Tudela, J. F. Meis, and P. E. Verweij. 2000. In vitro interaction of terbinafine with itraconazole against clinical isolates of Scedosporium prolificans. Antimicrob. Agents Chemother. 44:470-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Mellinghoff, I. K., D. J. Winston, G. Mukwaya, and G. J. Schiller. 2002. Treatment of Scedosporium apiospermum brain abscesses with posaconazole. Clin. Infect. Dis. 34:1648-1650. [DOI] [PubMed] [Google Scholar]
- 290.Mesnard, R., T. Lamy, C. Dauriac, and P. Y. Le Prise. 1992. Lung abscess due to Pseudallescheria boydii in the course of acute leukaemia. Report of a case and review of the literature. Acta Haematol. 87:78-82. [DOI] [PubMed] [Google Scholar]
- 291.Messori, A., C. Lanza, M. De Nicola, F. Menichelli, T. Capriotti, L. Morabito, and U. Salvolini. 2002. Mycotic aneurysms as lethal complication of brain pseudallescheriasis in a near-drowned child: a CT demonstration. AJNR. Am. J. Neuroradiol. 23:1697-1699. [PMC free article] [PubMed] [Google Scholar]
- 292.Meyer, R. D., C. R. Gaultier, J. T. Yamashita, R. Babapour, H. E. Pitchon, and P. R. Wolfe. 1994. Fungal sinusitis in patients with AIDS: report of 4 cases and review of the literature. Medicine (Baltimore) 73:69-78. [DOI] [PubMed] [Google Scholar]
- 293.Miele, P. S., C. S. Levy, M. A. Smith, E. M. Dugan, R. H. Cooke, J. A. Light, and D. R. Lucey. 2002. Primary cutaneous fungal infections in solid organ transplantation: a case series. Am. J. Transplant. 2:678-683. [DOI] [PubMed] [Google Scholar]
- 294.Miller, M. A., P. A. Greenberger, R. Amerian, J. H. Toogood, G. A. Noskin, M. Roberts, and R. Patterson. 1993. Allergic bronchopulmonary mycosis caused by Pseudallescheria boydii. Am. Rev. Respir. Dis. 148:810-812. [DOI] [PubMed] [Google Scholar]
- 295.Mills, R., and G. Garrett. 1992. Pseudallescheria boydii keratitis. Aust. N. Z. J. Ophthalmol. 20:253-256. [DOI] [PubMed] [Google Scholar]
- 296.Milne, L. J., W. S. McKerrow, W. D. Paterson, G. R. Petrie, and R. Postlethwaite. 1986. Pseudallescheriasis in northern Britain. J. Med. Vet. Mycol. 24:377-382. [DOI] [PubMed] [Google Scholar]
- 297.Miyamoto, T., R. Sasaoka, M. Kawaguchi, S. Ishioka, T. Inoue, N. Yamada, and M. Mihara. 1998. Scedosporium apiospermum skin infection: a case report and review of the literature. J. Am. Acad. Dermatol. 39:498-500. [DOI] [PubMed] [Google Scholar]
- 298.Mohr, J. A., and H. G. Muchmore. 1968. Maduromycosis due to Allescheria boydii. JAMA 204:335-336. [PubMed] [Google Scholar]
- 299.Montejo, M., M. L. Muniz, S. Zarraga, K. Aguirrebengoa, J. J. Amenabar, L. Lopez-Soria, and R. Gonzalez. 2002. Infection due to Scedosporium apiospermum in renal transplant recipients: a report of two cases and literature review of central nervous system and cutaneous infections by Pseudallescheria boydii/Sc. apiospermum. Mycoses 45:418-427. [DOI] [PubMed] [Google Scholar]
- 300.Montero, A., J. E. Cohen, M. A. Fernandez, G. Mazzolini, C. R. Gomez, and J. Perugini. 1998. Cerebral pseudallescheriasis due to Pseudallescheria boydii as the first manifestation of AIDS. Clin. Infect. Dis. 26:1476-1477. [DOI] [PubMed] [Google Scholar]
- 301.Morgan, M. A., W. R. Wilson, H. B. Neel III, and G. D. Roberts. 1984. Fungal sinusitis in healthy and immunocompromised individuals. Am. J. Clin. Pathol. 82:597-601. [DOI] [PubMed] [Google Scholar]
- 302.Moriarty, A. P., G. J. Crawford, I. L. McAllister, and I. J. Constable. 1993. Fungal corneoscleritis complicating beta-irradiation-induced scleral necrosis following pterygium excision. Eye 7:525-528. [DOI] [PubMed] [Google Scholar]
- 303.Moriarty, A. P., G. J. Crawford, I. L. McAllister, and I. J. Constable. 1993. Severe corneoscleral infection. A complication of beta irradiation scleral necrosis following pterygium excision. Arch. Ophthalmol. 111:947-951. [DOI] [PubMed] [Google Scholar]
- 304.Munoz, P., M. Marin, P. Tornero, P. Martin Rabadan, M. Rodriguez-Creixems, and E. Bouza. 2000. Successful outcome of Scedosporium apiospermum disseminated infection treated with voriconazole in a patient receiving corticosteroid therapy. Clin. Infect. Dis. 31:1499-1501. [DOI] [PubMed] [Google Scholar]
- 305.Murayama, T., R. Amitani, K. Tsuyuguchi, I. Watanabe, T. Kimoto, K. Suzuki, E. Tanaka, K. Kamei, and K. Nishimura. 1998. Polypoid bronchial lesions due to Scedosporium apiospermum in a patient with Mycobacterium avium complex pulmonary disease. Eur. Respir. J. 12:745-747. [DOI] [PubMed] [Google Scholar]
- 306.Mursch, K., S. Trnovec, H. Ratz, D. Hammer, R. Horre, A. Klinghammer, S. de Hoog, and J. Behnke-Mursch. 2006. Successful treatment of multiple Pseudallescheria boydii brain abscesses and ventriculitis/ependymitis in a 2-year-old child after a near-drowning episode. Childs Nerv. Syst. 22:189-192. [DOI] [PubMed] [Google Scholar]
- 307.NCCLS. 2002. Reference method for broth dilution antifungals susceptibility testing of filamentous fungi; aproved standard. NCCLS document M38-A. National Commitee for Clinical Laboratory Standards, Wayne, PA.
- 308.Nenoff, P., U. Gutz, K. Tintelnot, A. Bosse-Henck, M. Mierzwa, J. Hofmann, L. C. Horn, and U. F. Haustein. 1996. Disseminated mycosis due to Scedosporium prolificans in an AIDS patient with Burkitt lymphoma. Mycoses 39:461-465. [DOI] [PubMed] [Google Scholar]
- 309.Nenoff, P., L. C. Horn, H. Schwenke, M. Mierzwa, K. Rieske, and U. F. Haustein. 1996. Invasive mold infections in the university clinics of Leipzig in the period from 1992-1994. Mycoses 39(Suppl. 1):107-112. [DOI] [PubMed] [Google Scholar]
- 310.Nesky, M. A., E. C. McDougal, and J. E. Peacock, Jr. 2000. Pseudallescheria boydii brain abscess successfully treated with voriconazole and surgical drainage: case report and literature review of central nervous system pseudallescheriasis. Clin. Infect. Dis. 31:673-677. [DOI] [PubMed] [Google Scholar]
- 311.Nguyen, B. D. 2001. Pseudallescheriasis of the lung and central nervous system: multimodality imaging. Am. J. Roentgenol. 176:257-258. [DOI] [PubMed] [Google Scholar]
- 312.Nielsen, K., H. Lang, A. C. Shum, K. Woodruff, and J. D. Cherry. 1993. Disseminated Scedosporium prolificans infection in an immunocompromised adolescent. Pediatr. Infect. Dis. J. 12:882-884. [DOI] [PubMed] [Google Scholar]
- 313.Nomdedeu, J., S. Brunet, R. Martino, A. Altes, V. Ausina, and A. Domingo-Albos. 1993. Successful treatment of pneumonia due to Scedosporium apiospermum with itraconazole: case report. Clin. Infect. Dis. 16:731-733. [DOI] [PubMed] [Google Scholar]
- 314.Nonaka, D., H. Yfantis, P. Southall, and C. C. Sun. 2002. Pseudallescheriasis as an aggressive opportunistic infection in a bone marrow transplant recipient. Arch. Pathol. Lab. Med. 126:207-209. [DOI] [PubMed] [Google Scholar]
- 315.Nucci, M. 2003. Emerging moulds: Fusarium, Scedosporium and Zygomycetes in transplant recipients. Curr. Opin. Infect. Dis. 16:607-612. [DOI] [PubMed] [Google Scholar]
- 316.Nulens, E., C. Eggink, A. J. Rijs, P. Wesseling, and P. E. Verweij. 2003. Keratitis caused by Scedosporium apiospermum successfully treated with a cornea transplant and voriconazole. J. Clin. Microbiol. 41:2261-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Nulens, E., C. Eggink, and P. E. Verweij. 2003. Combination therapy for keratitis by the fungus Scedosporium. Cornea 22:92. [DOI] [PubMed] [Google Scholar]
- 318.Nunery, W. R., M. G. Welsh, and R. L. Saylor. 1985. Pseudallescheria boydii (Petriellidium boydii) infection of the orbit. Ophthalmic Surg. 16:296-300. [PubMed] [Google Scholar]
- 319.O'Bryan, T. A., F. A. Browne, and J. F. Schonder. 2002. Scedosporium apiospermum (Pseudallescheria boydii) endocarditis. J. Infect. 44:189-192. [DOI] [PubMed] [Google Scholar]
- 320.Ochiai, N., C. Shimazaki, R. Uchida, S. Fuchida, A. Okano, E. Ashihara, T. Inaba, N. Fujita, and M. Nakagawa. 2003. Disseminated infection due to Scedosporium apiospermum in a patient with acute myelogenous leukemia. Leuk. Lymphoma 44:369-372. [DOI] [PubMed] [Google Scholar]
- 321.Odabasi, Z., V. L. Paetznick, J. R. Rodriguez, E. Chen, and L. Ostrosky-Zeichner. 2004. In vitro activity of anidulafungin against selected clinically important mold isolates. Antimicrob. Agents Chemother. 48:1912-1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Odds, F. C., F. Van Gerven, A. Espinel-Ingroff, M. S. Bartlett, M. A. Ghannoum, M. V. Lancaster, M. A. Pfaller, J. H. Rex, M. G. Rinaldi, and T. J. Walsh. 1998. Evaluation of possible correlations between antifungal susceptibilities of filamentous fungi in vitro and antifungal treatment outcomes in animal infection models. Antimicrob. Agents Chemother. 42:282-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Oliveira, J. S., F. R. Kerbauy, A. L. Colombo, D. M. Bahia, G. S. Pinheiro, M. R. Silva, M. S. Ribeiro, G. Raineri, and J. Kerbauy. 2002. Fungal infections in marrow transplant recipients under antifungal prophylaxis with fluconazole. Braz. J. Med. Biol. Res. 35:789-798. [DOI] [PubMed] [Google Scholar]
- 324.Orr, P. H., J. R. Safneck, and L. B. Napier. 1993. Monosporium apiospermum endophthalmitis in a patient without risk factors for infection. Can. J. Ophthalmol. 28:187-190. [PubMed] [Google Scholar]
- 325.Ortoneda, M., F. J. Pastor, E. Mayayo, and J. Guarro. 2002. Comparison of the virulence of Scedosporium prolificans strains from different origins in a murine model. J. Med. Microbiol. 51:924-928. [DOI] [PubMed] [Google Scholar]
- 326.Panackal, A. A., and K. A. Marr. 2004. Scedosporium/Pseudallescheria infections. Semin. Respir. Crit. Care Med. 25:171-181. [DOI] [PubMed] [Google Scholar]
- 327.Patterson, T. F., V. T. Andriole, M. J. Zervos, D. Therasse, and C. A. Kauffman. 1990. The epidemiology of pseudallescheriasis complicating transplantation: nosocomial and community-acquired infection. Mycoses 33:297-302. [PubMed] [Google Scholar]
- 328.Pautler, E. E., R. W. Roberts, and P. R. Beamer. 1955. Mycotic infection of the eye; Monosporium apiospermum associated with corneal ulcer. AMA Arch. Ophthalmol. 53:385-389. [DOI] [PubMed] [Google Scholar]
- 329.Perez, R. E., M. Smith, J. McClendon, J. Kim, and N. Eugenio. 1988. Pseudallescheria boydii brain abscess. Complication of an intravenous catheter. Am. J. Med. 84:359-362. [DOI] [PubMed] [Google Scholar]
- 330.Perlroth, M. G., and J. Miller. 2004. Pseudoallescheria boydii pneumonia and empyema: a rare complication of heart transplantation cured with voriconazole. J. Heart Lung Transplant. 23:647-649. [DOI] [PubMed] [Google Scholar]
- 331.Perry, H. D., S. J. Doshi, E. D. Donnenfeld, and G. S. Bai. 2002. Topical cyclosporin A in the management of therapeutic keratoplasty for mycotic keratitis. Cornea 21:161-163. [DOI] [PubMed] [Google Scholar]
- 332.Persaud, V., and J. B. Holroyd. 1968. Mycetoma of the palpebral conjunctiva caused by Allescheria boydii (Monosporium apiospermum). Br. J. Ophthalmol. 52:857-859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Pether, J. V., W. Jones, F. B. Greatorex, and W. Bunting. 1992. Acute pyogenic Pseudallescheria boydii foot infection sequentially treated with miconazole and itraconazole. J. Infect. 25:335-336. [DOI] [PubMed] [Google Scholar]
- 334.Pfaller, M. A., F. Marco, S. A. Messer, and R. N. Jones. 1998. In vitro activity of two echinocandin derivatives, LY303366 and MK-0991 (L-743792), against clinical isolates of Aspergillus, Fusarium, Rhizopus, and other filamentous fungi. Diagn. Microbiol. Infect. Dis. 30:251-255. [DOI] [PubMed] [Google Scholar]
- 335.Pfeifer, J. D., M. G. Grand, M. A. Thomas, A. R. Berger, M. J. Lucarelli, and M. E. Smith. 1991. Endogenous Pseudallescheria boydii endophthalmitis. Clinicopathologic findings in two cases Arch. Ophthalmol. 109:1714-1717. (Erratum, 110:449, 1992.) [DOI] [PubMed] [Google Scholar]
- 336.Phillips, P., J. C. Forbes, and D. P. Speert. 1991. Disseminated infection with Pseudallescheria boydii in a patient with chronic granulomatous disease: response to gamma-interferon plus antifungal chemotherapy. Pediatr. Infect. Dis. J. 10:536-539. [DOI] [PubMed] [Google Scholar]
- 337.Pickles, R. W., D. E. Pacey, D. B. Muir, and W. H. Merrell. 1996. Experience with infection by Scedosporium prolificans including apparent cure with fluconazole therapy. J. Infect. 33:193-197. [DOI] [PubMed] [Google Scholar]
- 338.Pinoy, E. 1913. Actinomycoses and mycetomas. Borntragen (Leipzig) 11:929-938. [Google Scholar]
- 339.Pinto, M. R., B. Mulloy, R. M. Haido, L. R. Travassos, and E. Barreto Bergter. 2001. A peptidorhamnomannan from the mycelium of Pseudallescheria boydii is a potential diagnostic antigen of this emerging human pathogen. Microbiology 147:1499-1506. [DOI] [PubMed] [Google Scholar]
- 340.Piper, J. P., J. Golden, D. Brown, and J. Broestler. 1990. Successful treatment of Scedosporium apiospermum suppurative arthritis with itraconazole. Pediatr. Infect. Dis. J. 9:674-675. [PubMed] [Google Scholar]
- 341.Pistono, P. G., I. Rapetti, E. Stacchini, and C. Guasco. 1989. Clinical case of mycetoma caused by Scedosporium apiospermum. G. Batteriol. Virol. Immunol. 82:88-91. [PubMed] [Google Scholar]
- 342.Pluss, J. L., and S. M. Opal. 1985. An additional case of pulmonary Pseudallescheria boydii improved with ketoconazole therapy. Chest 87:843. [DOI] [PubMed] [Google Scholar]
- 343.Posteraro, P., C. Frances, B. Didona, R. Dorent, B. Posteraro, and G. Fadda. 2003. Persistent subcutaneous Scedosporium apiospermum infection. Eur. J. Dermatol. 13:603-605. [PubMed] [Google Scholar]
- 344.Poza, G., J. Montoya, C. Redondo, J. Ruiz, N. Vila, J. L. Rodriguez-Tudela, A. Ceron, and E. Simarro. 2000. Meningitis caused by Pseudallescheria boydii treated with voriconazole. Clin. Infect. Dis. 30:981-982. [DOI] [PubMed] [Google Scholar]
- 345.Queiroz-Telles, F., M. R. McGinnis, I. Salkin, and J. R. Graybill. 2003. Subcutaneous mycoses. Infect. Dis. Clin. N. Am. 17:59-85, viii. [DOI] [PubMed] [Google Scholar]
- 346.Rabodonirina, M., S. Paulus, F. Thevenet, R. Loire, E. Gueho, O. Bastien, J. F. Mornex, M. Celard, and M. A. Piens. 1994. Disseminated Scedosporium prolificans (S. inflatum) infection after single-lung transplantation. Clin. Infect. Dis. 19:138-142. [DOI] [PubMed] [Google Scholar]
- 347.Radford, S. A., E. M. Johnson, and D. W. Warnock. 1997. In vitro studies of activity of voriconazole (UK-109,496), a new triazole antifungal agent, against emerging and less-common mold pathogens. Antimicrob. Agents Chemother. 41:841-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Raffanti, S. P., B. Fyfe, S. Carreiro, S. E. Sharp, B. A. Hyma, and K. R. Ratzan. 1990. Native valve endocarditis due to Pseudallescheria boydii in a patient with AIDS: case report and review. Rev. Infect. Dis. 12:993-996. [DOI] [PubMed] [Google Scholar]
- 349.Rainer, J., and G. S. De Hoog. 2006. Molecular taxonomy and ecology of Pseudallescheria, Petriella and Scedosporium prolificans (Microascaceae) containing opportunistic agents on humans. Mycol. Res. 110:151-160. [DOI] [PubMed] [Google Scholar]
- 350.Rainer, J., G. S. de Hoog, M. Wedde, Y. Graser, and S. Gilges. 2000. Molecular variability of Pseudallescheria boydii, a neurotropic opportunist. J. Clin. Microbiol. 38:3267-3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Raj, R., and A. E. Frost. 2002. Scedosporium apiospermum fungemia in a lung transplant recipient. Chest 121:1714-1716. [DOI] [PubMed] [Google Scholar]
- 352.Reddy, P. C., C. S. Christianson, D. F. Gorelick, and H. W. Larsh. 1969. Pulmonary monosporosis: an uncommon pulmonary mycotic infection. Thorax 24:722-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352a.Rees, J., R. Pinner, R. Hajjeh, M. Brandt, and A. Reingold. 1998. The epidemiological features of invasive mycotic infections in the San Francisco Bay area, 1992-1993: results of population-based laboratory active surveillance. Clin. Infect. Dis. 27:1138-1147. [PubMed] [Google Scholar]
- 353.Reimann, D., E. Bussemaker, and P. Gross. 2004. Successful treatment due to vacuum seal technique of a severe Scedosporium apiospermum skin infection in a renal transplant recipient. Nephrol. Dial. Transplant. 19:245-248. [DOI] [PubMed] [Google Scholar]
- 354.Revankar, S. G., J. E. Patterson, D. A. Sutton, R. Pullen, and M. G. Rinaldi. 2002. Disseminated phaeohyphomycosis: review of an emerging mycosis. Clin. Infect. Dis. 34:467-476. [DOI] [PubMed] [Google Scholar]
- 355.Riddell, J. T., C. E. Chenoweth, and C. A. Kauffman. 2004. Disseminated Scedosporium apiospermum infection in a previously healthy woman with HELLP syndrome. Mycoses 47:442-446. [DOI] [PubMed] [Google Scholar]
- 356.Rippon, J. 1988. Medical mycology. The pathogenic fungi and the pathogenic actinomycetes, 3rd ed. W. B. Saunders Co., Philadelphia, PA.
- 357.Rippon, J. W., and J. W. Carmichael. 1976. Petriellidiosis (allescheriosis): four unusual cases and review of literature. Mycopathologia 58:117-124. [DOI] [PubMed] [Google Scholar]
- 358.Roberts, F. J., P. Allen, and T. K. Maybee. 1977. Petriellidium boydii (Allescheria boydii) endocarditis associated with porcine valve replacement. Can. Med. Assoc. J. 117:1250-1252. [PMC free article] [PubMed] [Google Scholar]
- 359.Rollot, F., P. Blanche, B. Richaud-Thiriez, F. Le Pimpec-Barthes, M. Riquet, D. Dusser, D. Salmon, and D. Sicard. 2000. Pneumonia due to Scedosporium apiospermum in a patient with HIV infection. Scand. J. Infect. Dis. 32:439. [DOI] [PubMed] [Google Scholar]
- 360.Rosa, R. H., Jr., D. Miller, and E. C. Alfonso. 1994. The changing spectrum of fungal keratitis in south Florida. Ophthalmology 101:1005-1013. [DOI] [PubMed] [Google Scholar]
- 361.Rosas, A. L., R. S. MacGill, J. D. Nosanchuk, T. R. Kozel, and A. Casadevall. 2002. Activation of the alternative complement pathway by fungal melanins. Clin. Diagn. Lab. Immunol. 9:144-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Rosen, F., J. H. Deck, and N. B. Rewcastle. 1965. Allescheria boydii—unique systemic dissemination to thyroid and brain. Can. Med. Assoc. J. 93:1125-1127. [PMC free article] [PubMed] [Google Scholar]
- 363.Rosen, P., H. T. Adelson, and E. Burleigh. 1969. Bronchiectasis complicated by the presence of Monosporium apiospermum and Aspergillus fumigatus. Am. J. Clin. Pathol. 52:182-187. [DOI] [PubMed] [Google Scholar]
- 364.Ruben, S. 1991. Pseudallescheria boydii keratitis. Acta Ophthalmol. (Copenhagen) 69:684-686. [DOI] [PubMed] [Google Scholar]
- 365.Ruchel, R., and S. Margraf. 1993. Rapid microscopical diagnosis of deep-seated mycoses following maceration of fresh specimens and staining with optical brighteners. Mycoses 36:239-242. [DOI] [PubMed] [Google Scholar]
- 366.Ruchel, R., M. Schaffrinski, K. R. Seshan, and G. T. Cole. 2000. Vital staining of fungal elements in deep-seated mycotic lesions during experimental murine mycoses using the parenterally applied optical brightener Blankophor. Med. Mycol. 38:231-237. [DOI] [PubMed] [Google Scholar]
- 367.Ruchel, R., and E. Wilichowski. 1995. Cerebral Pseudallescheria mycosis after near-drowning. Mycoses 38:473-475. [DOI] [PubMed] [Google Scholar]
- 368.Ruiz-Diez, B., F. Martin-Diez, J. L. Rodriguez-Tudela, M. Alvarez, and J. V. Martinez-Suarez. 1997. Use of random amplification of polymorphic DNA (RAPD) and PCR-fingerprinting for genotyping a Scedosporium prolificans (inflatum) outbreak in four leukemic patients. Curr. Microbiol. 35:186-190. [DOI] [PubMed] [Google Scholar]
- 369.Rumelt, S., I. Cohen, E. Lefler, and U. Rehany. 2001. Corneal co-infection with Scedosporium apiospermum and Acanthamoeba after sewage-contaminated ocular injury. Cornea 20:112-116. [DOI] [PubMed] [Google Scholar]
- 370.Saadah, H. A., and T. Dixon. 1981. Petriellidium boydii (Allescheria boydii). Necrotizing pneumonia in a normal host. JAMA 245:605-606. [DOI] [PubMed] [Google Scholar]
- 371.Saccardo, P. A. 1911. Sylloge fungorum ommium hucusque cognitorum. Borntragen (Leipzig) 4:282. [Google Scholar]
- 372.Safdar, A., E. B. Papadopoulos, and J. W. Young. 2002. Breakthrough Scedosporium apiospermum (Pseudallescheria boydii) brain abscess during therapy for invasive pulmonary aspergillosis following high-risk allogeneic hematopoietic stem cell transplantation. Scedosporiasis and recent advances in antifungal therapy. Transpl. Infect. Dis. 4:212-217. [DOI] [PubMed] [Google Scholar]
- 373.Saito, Y., M. Mikami, S. Nakamura, N. Hashimoto, Y. Abe, M. Baba, J. Takizawa, M. Kawakami, and K. Kamei. 1998. Pulmonary pseudallescheriasis in a patient with diabetes mellitus and alcoholic liver cirrhosis. Nihon Kokyuki Gakkai Zasshi 36:498-502. [PubMed] [Google Scholar]
- 374.Salesa, R., A. Burgos, R. Ondiviela, C. Richard, G. Quindos, and J. Ponton. 1993. Fatal disseminated infection by Scedosporium inflatum after bone marrow transplantation. Scand. J. Infect. Dis. 25:389-393. [DOI] [PubMed] [Google Scholar]
- 375.Salitan, M. L., W. Lawson, P. M. Som, E. J. Bottone, and H. F. Biller. 1990. Pseudallescheria sinusitis with intracranial extension in a nonimmunocompromised host. Otolaryngol. Head Neck Surg. 102:745-750. [DOI] [PubMed] [Google Scholar]
- 376.Salkin, I. F., M. R. McGinnis, M. J. Dykstra, and M. G. Rinaldi. 1988. Scedosporium inflatum, an emerging pathogen. J. Clin. Microbiol. 26:498-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.San Millan, R., G. Quindos, J. Garaizar, R. Salesa, J. Guarro, and J. Ponton. 1997. Characterization of Scedosporium prolificans clinical isolates by randomly amplified polymorphic DNA analysis. J. Clin. Microbiol. 35:2270-2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Santos, P. E., M. Oleastro, M. Galicchio, and M. Zelazko. 2000. Fungal infections in paediatric patients with chronic granulomatous disease. Rev. Iberoam. Micol. 17:6-9. [PubMed] [Google Scholar]
- 379.Sateesh, C. S., and J. H. Makannavar. 2001. Chronic sino-naso-orbital fungal infection due to Pseudallescheria boydii in a nonimmunocompromised host—a case report. Indian J. Pathol. Microbiol. 44:359-361. [PubMed] [Google Scholar]
- 380.Sawada, M., S. Isogai, S. Miyake, T. Kubota, and Y. Yoshizawa. 1998. Pulmonary pseudallescherioma associated with systemic lupus erythematosus. Intern. Med. 37:1046-1049. [DOI] [PubMed] [Google Scholar]
- 381.Schaenman, J. M., D. B. Digiulio, L. F. Mirels, N. M. McClenny, G. J. Berry, A. W. Fothergill, M. G. Rinaldi, and J. G. Montoya. 2005. Scedosporium apiospermum soft tissue infection successfully treated with voriconazole: potential pitfalls in the transition from intravenous to oral therapy. J. Clin. Microbiol. 43:973-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Scharyj, M., N. Levene, and H. Gordon. 1960. Primary pulmonary infection with Monosporium apiospermum. Report of a case with clinical, pathologic and mycologic data. J. Infect. Dis. 106:141-148. [DOI] [PubMed] [Google Scholar]
- 383.Scherr, G. R., S. G. Evans, M. T. Kiyabu, and E. C. Klatt. 1992. Pseudallescheria boydii infection in the acquired immunodeficiency syndrome. Arch. Pathol. Lab. Med. 116:535-536. [PubMed] [Google Scholar]
- 384.Schiess, R. J., M. F. Coscia, and G. A. McClellan. 1984. Petriellidium boydii pachymeningitis treated with miconazole and ketoconazole. Neurosurgery 14:220-224. [DOI] [PubMed] [Google Scholar]
- 385.Schwartz, D. A. 1989. Organ-specific variation in the morphology of the fungomas (fungus balls) of Pseudallescheria boydii. Development within necrotic host tissue. Arch. Pathol. Lab. Med. 113:476-480. [PubMed] [Google Scholar]
- 386.Schwartz, D. A., P. S. Amenta, and S. D. Finkelstein. 1989. Cerebral Pseudallescheria boydii infection: unique occurrence of fungus ball formation in the brain. Clin. Neurol. Neurosurg. 91:79-84. [DOI] [PubMed] [Google Scholar]
- 387.Seale, J. P., and J. A. Hudson. 1985. Successful medical treatment of pulmonary petriellidiosis. South. Med. J. 78:473-476. [DOI] [PubMed] [Google Scholar]
- 388.Sehulster, L., and R. Y. Chinn. 2003. Guidelines for environmental infection control in health-care facilities. Recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). Morb. Mortal. Wkly. Rep. Recomm. Rep. 52:1-42. [PubMed] [Google Scholar]
- 389.Selby, R. 1972. Pachymeningitis secondary to Allescheria boydii. Case report. J. Neurosurg. 36:225-227. [DOI] [PubMed] [Google Scholar]
- 390.Severo, L. C., F. Oliveira, C. D. Garcia, A. Uhlmann, and A. T. Londero. 1999. Peritonitis by Scedosporium apiospermum in a patient undergoing continuous ambulatory peritoneal dialysis. Rev. Inst. Med. Trop. Sao Paulo 41:263-264. [DOI] [PubMed] [Google Scholar]
- 391.Severo, L. C., M. Oliveira Fde, and K. Irion. 2004. Respiratory tract intracavitary colonization due to Scedosporium apiospermum: report of four cases. Rev. Inst. Med. Trop. Sao Paulo 46:43-46. [DOI] [PubMed] [Google Scholar]
- 392.Severo, L. C., M. Oliveira Fde, and A. T. Londero. 1997. Subcutaneous scedosporiosis. Report of two cases and review of the literature. Rev. Inst. Med. Trop. Sao Paulo 39:227-230. [DOI] [PubMed] [Google Scholar]
- 393.Severo, L. C., S. Porto Nda, and A. T. Londero. 1998. Pulmonary scedosporiosis. Rev. Inst. Med. Trop. Sao Paulo 40:241-243. [DOI] [PubMed] [Google Scholar]
- 394.Shah, K. B., T. G. Wu, K. R. Wilhelmus, and D. B. Jones. 2003. Activity of voriconazole against corneal isolates of Scedosporium apiospermum. Cornea 22:33-36. [DOI] [PubMed] [Google Scholar]
- 395.Shand, J. M., R. M. Albrecht, H. F. Burnett III, and A. Miyake. 2004. Invasive fungal infection of the midfacial and orbital complex due to Scedosporium apiospermum and mucormycosis. J. Oral Maxillofac. Surg. 62:231-234. [DOI] [PubMed] [Google Scholar]
- 396.Sharif, H. S., D. C. Clark, M. Y. Aabed, O. A. Aideyan, T. A. Mattsson, M. C. Haddad, S. O. Ohman, R. K. Joshi, H. A. Hasan, and A. Haleem. 1991. Mycetoma: comparison of MR imaging with CT. Radiology 178:865-870. [DOI] [PubMed] [Google Scholar]
- 397.Shear, C. L. 1922. Life history of an undescribed ascomycete isolated from a granular mycetoma of man. Mycologia 14:239-243. [Google Scholar]
- 398.Sheftel, T. G., J. T. Mader, and G. Cierny. 1987. Pseudoallescheria boydii soft tissue abscess. Clin. Orthop. Relat. Res. 215:212-216. [PubMed] [Google Scholar]
- 399.Shih, L. Y., and N. Lee. 1984. Disseminated petriellidiosis (allescheriasis) in a patient with refractory acute lymphoblastic leukaemia. J. Clin. Pathol. 37:78-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Shu, T., J. M. Green, and E. Orihuela. 2004. Testicular involvement in disseminated fungal infection by Pseudallescheria boydii. Urology 63:981-982. [DOI] [PubMed] [Google Scholar]
- 401.Silva, B. A., M. R. Pinto, R. M. Soares, E. Barreto-Bergter, and A. L. Santos. 2006. Pseudallescheria boydii releases metallopeptidases capable of cleaving several proteinaceous compounds. Res. Microbiol. 157:425-432. [DOI] [PubMed] [Google Scholar]
- 402.Simarro, E., F. Marin, A. Morales, E. Sanz, J. Perez, and J. Ruiz. 2001. Fungemia due to Scedosporium prolificans: a description of two cases with fatal outcome. Clin. Microbiol. Infect. 7:645-647. [DOI] [PubMed] [Google Scholar]
- 403.Simitsopoulou, M., C. Gil-Lamaignere, N. Avramidis, A. Maloukou, S. Lekkas, E. Havlova, L. Kourounaki, D. Loebenberg, and E. Roilides. 2004. Antifungal activities of posaconazole and granulocyte-macrophage colony-stimulating factor ex vivo and in mice with disseminated infection due to Scedosporium prolificans. Antimicrob. Agents Chemother. 48:3801-3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Singh, R. P., and P. McCluskey. 2005. Scedosporium prolificans sclerokeratitis 10 years after pterygium excision with adjunctive mitomycin C. Clin. Exp. Ophthalmol. 33:433-434. [DOI] [PubMed] [Google Scholar]
- 405.Slack, C. L., D. W. Watson, M. J. Abzug, C. Shaw, and K. H. Chan. 1999. Fungal mastoiditis in immunocompromised children. Arch. Otolaryngol. Head Neck Surg. 125:73-75. [DOI] [PubMed] [Google Scholar]
- 406.Slavin, M. A. 2002. The epidemiology of candidaemia and mould infections in Australia. J. Antimicrob. Chemother. 49(Suppl. 1):3-6. [DOI] [PubMed] [Google Scholar]
- 407.Smith, A. G., S. M. Crain, C. Dejongh, G. M. Thomas, and R. D. Vigorito. 1985. Systemic pseudallescheriasis in a patient with acute myelocytic leukemia. Mycopathologia 90:85-89. [DOI] [PubMed] [Google Scholar]
- 408.Sobottka, I., J. Deneke, W. Pothmann, A. Heinemann, and D. Mack. 1999. Fatal native valve endocarditis due to Scedosporium apiospermum (Pseudallescheria boydii) following trauma. Eur. J. Clin. Microbiol. Infect. Dis. 18:387-389. [DOI] [PubMed] [Google Scholar]
- 409.Sopta, J., M. Atanackovic, V. Raspopovic, L. Markovic, I. Kranjcic-Zec, and A. Lesic. 2005. Pseudoallescheria boydii (Scedosporium apiospermum), cause of mycotic granulomatous osteomyelitis—case diagnosis. Srp. Arh. Celok. Lek. 133:366-369. [DOI] [PubMed] [Google Scholar]
- 410.Sparrow, S. A., L. A. Hallam, B. E. Wild, and D. L. Baker. 1992. Scedosporium inflatum: first case report of disseminated infection and review of the literature. Pediatr. Hematol. Oncol. 9:293-295. [DOI] [PubMed] [Google Scholar]
- 411.Spielberger, R. T., B. R. Tegtmeier, M. R. O'Donnell, and J. I. Ito. 1995. Fatal Scedosporium prolificans (S. inflatum) fungemia following allogeneic bone marrow transplantation: report of a case in the United States. Clin. Infect. Dis. 21:1067. [DOI] [PubMed] [Google Scholar]
- 412.Sridhar, M. S., P. Garg, A. K. Bansal, and S. Sharma. 2000. Fungal keratitis after laser in situ keratomileusis. J. Cataract Refract. Surg. 26:613-615. [DOI] [PubMed] [Google Scholar]
- 413.Stamm, M. A., and M. A. Frable. 1992. Invasive sinusitis due to Pseudallescheria boydii in an immunocompetent host. South. Med. J. 85:439-441. [DOI] [PubMed] [Google Scholar]
- 413a.Steele, C., R. Rapaka, A. Metz, S. Pop, D. Williams, S. Gordon, J. Kolls, and G. Brown. 2005. The beta-glucan receptor dectin-1 recognizes specific morphologies of Aspergillus fumigatus. PLoS Pathog. 1:e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Steens, R. D., Q. A. Summers, and R. A. Tarala. 1992. Pulmonary alveolar proteinosis in association with Fanconi's anemia and psoriasis. A possible common pathogenetic mechanism. Chest 102:637-638. [DOI] [PubMed] [Google Scholar]
- 415.Steinbach, W. J., and J. R. Perfect. 2003. Scedosporium species infections and treatments. J. Chemother. 15(Suppl. 2):16-27. [DOI] [PubMed] [Google Scholar]
- 416.Steinbach, W. J., W. A. Schell, J. L. Miller, and J. R. Perfect. 2003. Scedosporium prolificans osteomyelitis in an immunocompetent child treated with voriconazole and caspofungin, as well as locally applied polyhexamethylene biguanide. J. Clin. Microbiol. 41:3981-3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Stern, R. M., Z. N. Zakov, D. M. Meisler, G. S. Hall, and A. Martin. 1986. Endogenous Pseudoallescheria boydii endophthalmitis. A clinicopathologic report. Cleve. Clin. Q. 53:197-203. [DOI] [PubMed] [Google Scholar]
- 418.St. Germain, G., and R. C. Summerbell. 1996. Scedosporium, identifying filamentous fungi, 2nd ed. Blackwell Science, Belmont, CA.
- 419.Stierstorfer, M. B., B. K. Schwartz, J. B. McGuire, and A. C. Miller. 1988. Pseudallescheria boydii mycetoma in northern New England. Int. J. Dermatol. 27:383-387. [DOI] [PubMed] [Google Scholar]
- 420.Stoeckel, H., and C. Ermer. 1960. A case of Monosporium mycetoma of the lung. Beitr. Klin. Tuberk. Spezif. Tuberkuloseforsch. 122:30-38. [PubMed] [Google Scholar]
- 421.Stolk-Engelaar, M. V., and N. J. Cox. 1993. Successful treatment of pulmonary pseudallescheriasis with itraconazole. Eur. J. Clin. Microbiol. Infect. Dis. 12:142. [DOI] [PubMed] [Google Scholar]
- 422.Strickland, L. B., J. N. Greene, R. L. Sandin, and J. H. Hiemenz. 1998. Scedosporium species infections in cancer patietns: three cases and review of the literature. Infect. Dis. Clin. Pract. 7:4-11. [Google Scholar]
- 423.Studahl, M., T. Backteman, F. Stalhammar, E. Chryssanthou, and B. Petrini. 2003. Bone and joint infection after traumatic implantation of Scedosporium prolificans treated with voriconazole and surgery. Acta Paediatr. 92:980-982. [DOI] [PubMed] [Google Scholar]
- 424.Sullivan, L. J., G. Snibson, C. Joseph, and H. R. Taylor. 1994. Scedosporium prolificans sclerokeratitis. Aust. N. Z. J. Ophthalmol. 22:207-209. [DOI] [PubMed] [Google Scholar]
- 425.Summerbell, R. C. 1993. The benomyl test as a fundamental diagnostic method for medical mycology. J. Clin. Microbiol. 31:572-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Summerbell, R. C., S. Krajden, and J. Kane. 1989. Potted plants in hospitals as reservoirs of pathogenic fungi. Mycopathologia 106:13-22. [DOI] [PubMed] [Google Scholar]
- 427.Sutton, D. A., A. W. Fothergill, and M. G. Rinaldi. 1998. Guide to clinically significant fungi, 1st ed. Williams & Wilkins, Baltimore, MD.
- 428.Sydnor, M. K., S. Kaushik, T. E. Knight, Jr., C. L. Bridges, and J. M. McCarty. 2003. Mycotic osteomylitis due to Scedosporium apiospermum: MR imaging-pathologic correlation. Skeletal Radiol. 32:656-660. [DOI] [PubMed] [Google Scholar]
- 429.Symoens, F., C. Knoop, M. Schrooyen, O. Denis, M. Estenne, N. Nolard, and F. Jacobs. 2006. Disseminated Scedosporium apiospermum infection in a cystic fibrosis patient after double-lung transplantation. J. Heart Lung Transplant. 25:603-607. [DOI] [PubMed] [Google Scholar]
- 430.Szekely, A., E. M. Johnson, and D. W. Warnock. 1999. Comparison of E-test and broth microdilution methods for antifungal drug susceptibility testing of molds. J. Clin. Microbiol. 37:1480-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Tadros, T. S., K. A. Workowski, R. J. Siegel, S. Hunter, and D. A. Schwartz. 1998. Pathology of hyalohyphomycosis caused by Scedosporium apiospermum (Pseudallescheria boydii): an emerging mycosis. Hum. Pathol. 29:1266-1272. [DOI] [PubMed] [Google Scholar]
- 432.Talbot, T. R., J. Hatcher, S. F. Davis, R. N. Pierson III, R. Barton, and S. Dummer. 2002. Scedosporium apiospermum pneumonia and sternal wound infection in a heart transplant recipient. Transplantation 74:1645-1647. [DOI] [PubMed] [Google Scholar]
- 433.Tamm, M., M. Malouf, and A. Glanville. 2001. Pulmonary scedosporium infection following lung transplantation. Transpl. Infect. Dis. 3:189-194. [DOI] [PubMed] [Google Scholar]
- 434.Tan, T. Y., C. W. Liou, and L. C. Kung. 2004. Meningitis caused by Pseudallescheria boydii. Chang Gung Med. J. 27:228-232. [PubMed] [Google Scholar]
- 435.Tapia, M., C. Richard, J. Baro, R. Salesa, J. Figols, F. Zurbano, and A. Zubizarreta. 1994. Scedosporium inflatum infection in immunocompromised haematological patients. Br. J. Haematol. 87:212-214. [DOI] [PubMed] [Google Scholar]
- 436.Taravella, M. J., D. W. Johnson, J. G. Petty, R. B. Keyser, C. S. Foster, and B. E. Lundberg. 1997. Infectious posterior scleritis caused by Pseudallescheria boydii. Clinicopathologic findings. Ophthalmology 104:1312-1316. [DOI] [PubMed] [Google Scholar]
- 437.Tarozzi, G. 1909. Ricerche anatomo-patologiehe, baceriologiehet e sperimentali sopra un caso di actinomicosi del piede. Arch. Perle Sc. Med. 33:553-632. [Google Scholar]
- 438.Taylor, A., S. J. Wiffen, and C. J. Kennedy. 2002. Post-traumatic Scedosporium inflatum endophthalmitis. Clin. Exp. Ophthalmol. 30:47-48. [DOI] [PubMed] [Google Scholar]
- 439.Tekavec, J., E. Mlinaric-Missoni, and V. Babic-Vazic. 1997. Pulmonary tuberculosis associated with invasive pseudallescheriasis. Chest 111:508-511. [DOI] [PubMed] [Google Scholar]
- 440.Thammayya, A., and M. Sanyal. 1973. Monosporium apiospermum causing mycetoma pedis in India. Indian J. Med. Res. 61:1289-1291. [PubMed] [Google Scholar]
- 441.Thiagalingam, S., G. T. Fernando, K. Tan, B. A. O'Donnell, K. Weeks, and M. Branley. 2004. Orbital apex syndrome secondary to Pseudallescheria boydii fungal sinusitis in an immunocompetent patient. Clin. Exp. Ophthalmol. 32:545-547. [DOI] [PubMed] [Google Scholar]
- 442.Thomas, P. A. 2003. Current perspectives on ophthalmic mycoses. Clin. Microbiol. Rev. 16:730-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Tirado-Miranda, R., J. Solera-Santos, J. C. Brasero, M. Haro-Estarriol, P. Cascales-Sanchez, and J. B. Igualada. 2001. Septic arthritis due to Scedosporium apiospermum: case report and review. J. Infect. 43:210-212. [DOI] [PubMed] [Google Scholar]
- 444.Tong, J. L., E. H. Valentine, J. R. Durrance, G. M. Wilson, and D. A. Fischer. 1958. Pulmonary infection with Allescheria boydii; report of a fatal case. Am. Rev. Tuberc. 78:604-609. [DOI] [PubMed] [Google Scholar]
- 445.Torok, L., G. Simon, A. Csornai, M. Tapai, and I. Torok. 1995. Scedosporium apiospermum infection imitating lymphocutaneous sporotrichosis in a patient with myeloblastic-monocytic leukaemia. Br. J. Dermatol. 133:805-809. [DOI] [PubMed] [Google Scholar]
- 446.Torok, L., G. Simon, A. Csornai, M. Tapai, and I. Torok. 1994. Scedosporium apiospermum infection in a patient with myelomonocytic leukemia presenting as lympho-cutaneous sporotrichosis. Orv. Hetil. 135:2377-2380. [PubMed] [Google Scholar]
- 447.Toy, E. C., M. G. Rinaldi, C. B. Savitch, and E. R. Leibovitch. 1990. Endocarditis and hip arthritis associated with Scedosporium inflatum. South. Med. J. 83:957-960. [DOI] [PubMed] [Google Scholar]
- 448.Travis, L. B., G. D. Roberts, and W. R. Wilson. 1985. Clinical significance of Pseudallescheria boydii: a review of 10 years' experience. Mayo Clin. Proc. 60:531-537. [DOI] [PubMed] [Google Scholar]
- 449.Turner, P. G. 1989. Madura foot or plantar fibromatosis. J. Bone Joint Surg. Br. 71:531. [DOI] [PubMed] [Google Scholar]
- 450.Uenotsuchi, T., Y. Moroi, K. Urabe, G. Tsuji, T. Koga, T. Matsuda, and M. Furue. 2005. Cutaneous Scedosporium apiospermum infection in an immunocompromised patient and a review of the literature. Acta Derm. Venereol. 85:156-159. [DOI] [PubMed] [Google Scholar]
- 451.Vagefi, M. R., E. T. Kim, R. G. Alvarado, J. L. Duncan, E. L. Howes, and J. B. Crawford. 2005. Bilateral endogenous Scedosporium prolificans endophthalmitis after lung transplantation. Am. J. Ophthalmol. 139:370-373. [DOI] [PubMed] [Google Scholar]
- 452.van Hecke, E., M. L. Geerts, and D. den Dooven. 1983. Petriellidiosis of the skin. Mykosen 26:17-21. [DOI] [PubMed] [Google Scholar]
- 453.Venugopal, P. V., and T. V. Venugopal. 1995. Pale grain eumycetomas in Madras. Australas. J. Dermatol. 36:149-151. [DOI] [PubMed] [Google Scholar]
- 454.von Arx, J. 1988. Sordariaceous ascomycetes without ascospore ejaculation. J. Cramer, Berlin, Germany.
- 455.Walker, D. H., T. Adamec, and M. Krigman. 1978. Disseminated petriellidosis (allescheriosis). Arch. Pathol. Lab. Med. 102:158-160. [PubMed] [Google Scholar]
- 456.Walsh, M., L. White, K. Atkinson, and A. Enno. 1992. Fungal Pseudoallescheria boydii lung infiltrates unresponsive to amphotericin B in leukaemic patients. Aust. N. Z. J. Med. 22:265-268. [DOI] [PubMed] [Google Scholar]
- 457.Walsh, T. J., and A. H. Groll. 1999. Emerging fungal pathogens: evolving challenges to immunocompromised patients for the twenty-first century. Transpl. Infect. Dis. 1:247-261. [DOI] [PubMed] [Google Scholar]
- 458.Walsh, T. J., J. Peter, D. A. McGough, A. W. Fothergill, M. G. Rinaldi, and P. A. Pizzo. 1995. Activities of amphotericin B and antifungal azoles alone and in combination against Pseudallescheria boydii. Antimicrob. Agents Chemother. 39:1361-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Walsh, T. J., and P. A. Pizzo. 1988. Nosocomial fungal infections: a classification for hospital-acquired fungal infections and mycoses arising from endogenous flora or reactivation. Annu. Rev. Microbiol. 42:517-545. [DOI] [PubMed] [Google Scholar]
- 460.Walts, A. E. 2001. Pseudallescheria: an underdiagnosed fungus? Diagn. Cytopathol. 25:153-157. [DOI] [PubMed] [Google Scholar]
- 461.Warintarawej, A., W. G. Winter, Jr., and N. L. Goodman. 1975. Maduromycosis (Madura foot) in Kentucky. South. Med. J. 68:1570-1575. [DOI] [PubMed] [Google Scholar]
- 462.Warris, A., M. G. Netea, P. E. Verweij, P. Gaustad, B. J. Kullberg, C. M. Weemaes, and T. G. Abrahamsen. 2005. Cytokine responses and regulation of interferon-gamma release by human mononuclear cells to Aspergillus fumigatus and other filamentous fungi. Med. Mycol. 43:613-621. [DOI] [PubMed] [Google Scholar]
- 463.Washburn, R. G., D. W. Kennedy, M. G. Begley, D. K. Henderson, and J. E. Bennett. 1988. Chronic fungal sinusitis in apparently normal hosts. Medicine (Baltimore) 67:231-247. [DOI] [PubMed] [Google Scholar]
- 464.Watanabe, S., and M. Hironaga. 1981. An atypical isolate of Scedosporium apiospermum from a purulent meningitis in man. Sabouraudia 19:209-215. [PubMed] [Google Scholar]
- 465.Watson, J. C., J. S. Myseros, and M. R. Bullock. 1999. True fungal mycotic aneurysm of the basilar artery: a clinical and surgical dilemma. Cerebrovasc. Dis. 9:50-53. [DOI] [PubMed] [Google Scholar]
- 466.Watters, G. W., and C. A. Milford. 1993. Isolated sphenoid sinusitis due to Pseudallescheria boydii. J. Laryngol. Otol. 107:344-346. [DOI] [PubMed] [Google Scholar]
- 467.Wedde, M., D. Muller, K. Tintelnot, G. S. De Hoog, and U. Stahl. 1998. PCR-based identification of clinically relevant Pseudallescheria/Scedosporium strains. Med. Mycol. 36:61-67. [PubMed] [Google Scholar]
- 468.Welty, F. K., G. X. McLeod, C. Ezratty, R. W. Healy, and A. W. Karchmer. 1992. Pseudallescheria boydii endocarditis of the pulmonic valve in a liver transplant recipient. Clin. Infect. Dis. 15:858-860. [DOI] [PubMed] [Google Scholar]
- 469.Werner, P. G. 2006. Myco-heterotrophs: hacking the mycorrhizal network. Mycena News 57:8. [Google Scholar]
- 470.Westerman, D. A., B. R. Speed, and H. M. Prince. 1999. Fatal disseminated infection by Scedosporium prolificans during induction therapy for acute leukemia: a case report and literature review. Pathology 31:393-394. [DOI] [PubMed] [Google Scholar]
- 471.Wethered, D. B., M. A. Markey, R. J. Hay, E. S. Mahgoub, and S. A. Gumaa. 1987. Ultrastructural and immunogenic changes in the formation of mycetoma grains. J. Med. Vet. Mycol. 25:39-46. [DOI] [PubMed] [Google Scholar]
- 472.Whyte, M., H. Irving, P. O'Regan, M. Nissen, D. Siebert, and R. Labrom. 2005. Disseminated Scedosporium prolificans infection and survival of a child with acute lymphoblastic leukemia. Pediatr. Infect. Dis. J. 24:375-377. [DOI] [PubMed] [Google Scholar]
- 473.Wilichowski, E., H. J. Christen, H. Schiffmann, W. Schulz-Schaeffer, and W. Behrens-Baumann. 1996. Fatal Pseudallescheria boydii panencephalitis in a child after near-drowning. Pediatr. Infect. Dis. J. 15:365-370. [DOI] [PubMed] [Google Scholar]
- 474.Williamson, E. C., D. Speers, I. H. Arthur, G. Harnett, G. Ryan, and T. J. Inglis. 2001. Molecular epidemiology of Scedosporium apiospermum infection determined by PCR amplification of ribosomal intergenic spacer sequences in patients with chronic lung disease. J. Clin. Microbiol. 39:47-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Willsteed, E., and W. Regan. 1987. Treatment of Pseudallescheria boydii infection with oral ketoconazole and topical miconazole. Australas. J. Dermatol. 28:21-23. [DOI] [PubMed] [Google Scholar]
- 476.Wilson, C. M., E. J. O'Rourke, M. R. McGinnis, and I. F. Salkin. 1990. Scedosporium inflatum: clinical spectrum of a newly recognized pathogen. J. Infect. Dis. 161:102-107. [DOI] [PubMed] [Google Scholar]
- 477.Wingard, J. R. 2005. The changing face of invasive fungal infections in hematopoietic cell transplant recipients. Curr. Opin. Oncol. 17:89-92. [DOI] [PubMed] [Google Scholar]
- 478.Winn, R. E., P. D. Ramsey, J. C. McDonald, and K. J. Dunlop. 1983. Maxillary sinusitis from Pseudallescheria boydii. Efficacy of surgical therapy. Arch. Otolaryngol. 109:123-125. [DOI] [PubMed] [Google Scholar]
- 479.Winn, R. M., C. Gil-Lamaignere, E. Roilides, M. Simitsopoulou, C. A. Lyman, A. Maloukou, and T. J. Walsh. 2005. Effects of interleukin-15 on antifungal responses of human polymorphonuclear leukocytes against Fusarium spp. and Scedosporium spp. Cytokine 31:1-8. [DOI] [PubMed] [Google Scholar]
- 480.Winston, D. J., M. C. Jordan, and J. Rhodes. 1977. Allescheria boydii infections in the immunosuppressed host. Am. J. Med. 63:830-835. [DOI] [PubMed] [Google Scholar]
- 481.Wise, K. A., B. R. Speed, D. H. Ellis, and J. H. Andrew. 1993. Two fatal infections in immunocompromised patients caused by Scedosporium inflatum. Pathology 25:187-189. [DOI] [PubMed] [Google Scholar]
- 482.Wood, G. M., J. G. McCormack, D. B. Muir, D. H. Ellis, M. F. Ridley, R. Pritchard, and M. Harrison. 1992. Clinical features of human infection with Scedosporium inflatum. Clin. Infect. Dis. 14:1027-1033. [DOI] [PubMed] [Google Scholar]
- 483.Woodard, B. H. 1982. Asymptomatic pulmonary coin lesion with Petriellidium boydii. South. Med. J. 75:229-230. [DOI] [PubMed] [Google Scholar]
- 484.Wu, Z., H. Ying, S. Yiu, J. Irvine, and R. Smith. 2002. Fungal keratitis caused by Scedosporium apiospermum: report of two cases and review of treatment. Cornea 21:519-523. [DOI] [PubMed] [Google Scholar]
- 485.Yano, S., S. Shishido, T. Toritani, K. Yoshida, and H. Nakano. 1997. Intrabronchial pseudallescheriasis in an immunocompetent woman. Clin. Infect. Dis. 24:735-736. [DOI] [PubMed] [Google Scholar]
- 486.Yao, M., and A. H. Messner. 2001. Fungal malignant otitis externa due to Scedosporium apiospermum. Ann. Otol. Rhinol. Laryngol. 110:377-380. [DOI] [PubMed] [Google Scholar]
- 487.Yoo, D., W. H. Lee, and K. J. Kwon-Chung. 1985. Brain abscesses due to Pseudallescheria boydii associated with primary non-Hodgkin's lymphoma of the central nervous system: a case report and literature review. Rev. Infect. Dis. 7:272-277. [DOI] [PubMed] [Google Scholar]
- 488.Yousif, M. A., and R. J. Hay. 1987. Leucocyte chemotaxis to mycetoma agents—the effect of the antifungal drugs griseofulvin and ketoconazole. Trans. R. Soc. Trop. Med. Hyg. 81:319-321. [DOI] [PubMed] [Google Scholar]
- 489.Yustes, C., and J. Guarro. 2005. In vitro synergistic interaction between amphotericin B and micafungin against Scedosporium spp. Antimicrob. Agents Chemother. 49:3498-3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 490.Zaas, D. 2002. Scedosporium apiospermum mycetoma of the lung. Am. J. Med. 113:760-762. [DOI] [PubMed] [Google Scholar]
- 491.Zapater, R. C., and E. J. Albesi. 1979. Corneal monosporiosis. A review and report of 1 case. Ophthalmologica 178:142-147. [DOI] [PubMed] [Google Scholar]
- 492.Zeng, J., K. Kamei, Y. Zheng, and K. Nishimura. 2004. Susceptibility of Pseudallescheria boydii and Scedosporium apiospermum to new antifungal agents. Nippon Ishinkin Gakkai Zasshi 45:101-104. [DOI] [PubMed] [Google Scholar]
- 493.Zouhair, R., A. Defontaine, C. Ollivier, B. Cimon, F. Symoens, J. N. Halle, J. Deunff, and J. P. Bouchara. 2001. Typing of Scedosporium apiospermum by multilocus enzyme electrophoresis and random amplification of polymorphic DNA. J. Med. Microbiol. 50:925-932. [DOI] [PubMed] [Google Scholar]