Abstract
Catheter-associated urinary tract infections (CAUTIs) represent the most common type of nosocomial infection and are a major health concern due to the complications and frequent recurrence. These infections are often caused by Escherichia coli and Proteus mirabilis. Gram-negative bacterial species that cause CAUTIs express a number of virulence factors associated with adhesion, motility, biofilm formation, immunoavoidance, and nutrient acquisition as well as factors that cause damage to the host. These infections can be reduced by limiting catheter usage and ensuring that health care professionals correctly use closed-system Foley catheters. A number of novel approaches such as condom and suprapubic catheters, intermittent catheterization, new surfaces, catheters with antimicrobial agents, and probiotics have thus far met with limited success. While the diagnosis of symptomatic versus asymptomatic CAUTIs may be a contentious issue, it is generally agreed that once a catheterized patient is believed to have a symptomatic urinary tract infection, the catheter is removed if possible due to the high rate of relapse. Research focusing on the pathogenesis of CAUTIs will lead to a better understanding of the disease process and will subsequently lead to the development of new diagnosis, prevention, and treatment options.
INTRODUCTION
Indwelling urinary catheters are standard medical devices utilized in both hospital and nursing home settings to relieve urinary retention and urinary incontinence. Of the almost 100 million catheters that are sold annually worldwide, one-quarter of them are sold in the United States (50). The most common urinary catheter in use is the Foley indwelling urethral catheter, a closed sterile system that is comprised of a tube inserted through the urethra and held in place by an inflatable balloon to allow urinary drainage of the bladder. Although these devices were originally designed for short-term use in patients, indwelling catheter use is now commonplace in the long-term setting.
Due to the frequent and sometimes unnecessary use of indwelling catheters during hospitalization (21 to 50% of patients) (153), many patients are placed at risk for complications associated with the use of these devices. A study of 1,540 nursing home residents determined that the risk of hospitalization, length of hospitalization, and length of antibiotic therapy were three times higher in catheterized residents than in noncatheterized residents (205). The most notable complication associated with indwelling urinary catheters is the development of nosocomial urinary tract infections (UTIs), known as catheter-associated UTIs (CAUTIs). Infections of the urinary tract associated with catheter use are significant not only due their high incidence and subsequent economic cost but also because of the severe sequelae that can result.
CAUTIs, the most common type of nosocomial infection, account for over 1 million cases annually (401) or over 40% of all nosocomial infections in hospitals and nursing homes (382, 383, 438) and constitute 80% of all nosocomial UTIs (132). Due to this high incidence, the overall cost for medical intervention of nosocomial UTIs is staggering, with an estimated $424 million to $451 million spent annually in the United States to manage these infections (157). Furthermore, catheter-associated bacteremia is estimated to cost approximately $2,900 per episode (339). Costs for treatment of nosocomial UTIs include antimicrobial therapy, increases in length of stay during hospitalization, physician visits, and morbidity (98). These costs will inevitably rise due to advances in preventive medicine that extend life expectancy, increasing the elderly population. This population today (those ≥65 years old) accounts for approximately 12.6% (37,849,672) of the total population of the United States (301,139,947) (422); their care accounts for about one-third (6) of the estimated $1 trillion in U.S. health expenditures (279).
Individuals requiring an indwelling catheter are predisposed to the development of CAUTIs due to the presence of an indwelling catheter device and potentially pathogenic multidrug-resistant organisms in the hospital setting. Despite the imminent threat of infection from these potent opportunistic nosocomial multiresistant strains, most cases of catheter-associated bacteriuria or the presence of bacteria in the urine are asymptomatic.
However, when an episode of CAUTI becomes symptomatic, the resulting sequelae can range from mild (fever, urethritis, and cystitis) to severe (acute pyelonephritis, renal scarring, calculus formation, and bacteremia). Left untreated, these infections can lead to urosepsis and death (284, 438). These complicated infections commonly recur and result in long-term morbidity due to the presence of encrustation and blockage of the catheter by crystalline biofilms that increase resistance to the host immune response and to antibiotics (394). Since the incidence of symptomatic CAUTIs is a major health concern due to the complications and recurrence associated with this type of infection, research directed at understanding the pathogenesis of CAUTIs is warranted and should lead to new and improved diagnosis, prevention, and treatment options.
PATHOGENESIS OF CAUTIs
Despite innate mechanical safeguards against microbial infection of the intact human urinary tract, specific organisms are capable of colonizing and persisting in this environmental niche. Similar to other mucosal pathogens, uropathogens employ specific strategies to infect the urinary tract, including colonization of a urinary catheter and/or mucosal site (uroepithelial cells), evasion of host defenses, replication, and damage to host cells. The insertion of a foreign body such as an indwelling catheter into the bladder increases the susceptibility of a patient to UTIs, as these devices serve as the initiation site of infection by introducing opportunistic organisms into the urinary tract. The majority of these uropathogens are fecal contaminants or skin residents from the patient's own native or transitory microflora that colonize the periurethral area (56, 66, 217, 288, 462). Transitory microflora that originate from hospital personnel or from contact with other patients may represent antibiotic-resistant nosocomial strains, complicating treatment for these infections. Bacterial entry into the bladder can occur at the time of catheter insertion, through the catheter lumen, or along the catheter-urethral interface (439). The preferred mechanism of bladder entry during CAUTIs is extraluminal (66%), where organisms ascend from the urethral meatus along the catheter urethral interface. Organisms can also enter the bladder intraluminally (34%), where the bacteria migrate into the bladder as a result of manipulation of the catheter system (400, 440).
Indwelling urinary catheters further favor the colonization of uropathogens by providing a surface for the attachment of host cell binding receptors that are recognized by bacterial adhesins, thus enhancing microbial adhesion. Upon insertion, urinary catheters may damage the protective uroepithelial mucosa, which leads to the exposure of new binding sites for bacterial adhesins (108). Lastly, the presence of the indwelling catheter in the urinary tract disrupts normal host mechanical defenses, resulting in an overdistension of the bladder and incomplete voiding that leaves residual urine for microbial growth (133).
Organisms capable of infecting the urinary tract during catheterization use approaches to establish infection that are similar to those used by organisms that cause uncomplicated UTIs. However, due to the introduction of a foreign body, organisms causing CAUTIs require fewer recognized virulence factors to colonize and establish infection than those required by pathogens to infect a fully functional urinary tract.
Bacterial adhesins initiate attachment by recognizing host cell receptors located on surfaces of the host cell or catheter. Adhesins initiate adherence by overcoming the electrostatic repulsion observed between bacterial cell membranes and surfaces to allow intimate interactions to occur (61). These factors are differentially expressed during the course of infection not only for the recognition of different surfaces and cell types that the uropathogen encounters (e.g., in bladder versus the kidney) but also to evade the host immune response. These bacterial cell surface structures recognize specific host cell surface and extracellular matrix components such as proteins, glycoproteins, glycolipids, and carbohydrates. Gram-negative uropathogens produce an assortment of adhesins including those attached to the tip of hair-like projections, known as fimbriae or pili, as well as adhesins anchored directly within bacterial cell membranes, known as nonfimbrial adhesins.
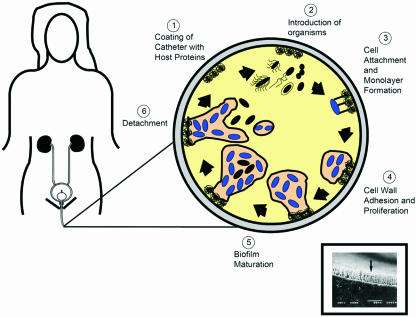
Once firmly attached on the catheter surface or the uroepithelium, bacteria begin to phenotypically change, producing exopolysaccharides that entrap and protect bacteria. These attached bacteria replicate and form microcolonies that eventually mature into biofilms (Fig. 1). During biofilm development, intracellular communication by quorum sensing regulates formation and detachment from biofilms through the collective expression of genes after cellular populations reach a threshold concentration. The rate of genetic material exchange occurring within the biofilm is greater than that between planktonic cells (134, 326), thereby allowing the potential spread of antibiotic resistance genes and other traits. Once established, biofilms inherently protect uropathogens from antibiotics and the host immune response (63). The shedding of daughter cells from actively growing cells and the shearing of biofilm aggregates from the mature biofilm seed other sections of the catheter and bladder.
FIG. 1.
Pathogenesis of biofilm formation on urinary catheters during CAUTIs. The inset (reprinted from reference 393) shows a scanning electron micrograph of a urinary catheter encrusted with P. aeruginosa.
Many uropathogens use flagellum-mediated motility and type IV pilus-mediated (twitching) motility to facilitate the spread of infection from the initial colonization site to the urinary tract. Twitching motility via type IV pili cycles through periods of extension, attachment, and retraction in gram-negative bacteria (33, 256) and is thought to play a significant role in virulence (128).
Once colonized on the catheter and uroepithelium, uropathogens must adapt to the urinary tract environment and acquire nutrients. The production and secretion of degradative enzymes and toxins into the local environment may lead to a breakdown of tissue, releasing nutrients. As iron is a limiting nutrient in the human host (447), uropathogens have developed complex iron acquisition systems such as heme transporters, ferric and ferrous iron transport systems, and siderophore iron uptake systems to circumvent host iron-sequestering mechanisms. Certain uropathogens are capable of using urea, found in high concentrations in human urine (up to 500 mM) (35, 170), as a nitrogen source due to the expression of urease. As a consequence of urease-mediated hydrolysis of urea to ammonia and carbon dioxide, the local environment becomes alkalinized, which leads to the precipitation of polyvalent ions that become enmeshed in the biofilms on catheters and urinary epithelial surfaces (118). These crystalline biofilms must be removed from the host to completely resolve the infection, since antimicrobial agents may be ineffective at eliminating biofilm-associated bacterial populations.
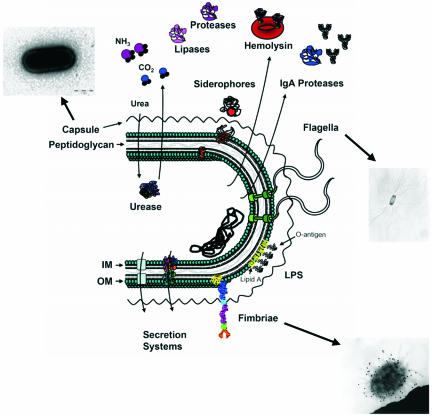
To maintain an infection in the human urinary tract, pathogens must be capable of evading the host immune response. Gram-negative uropathogens enact a number of mechanisms of host immune evasion, including the production of capsules, immunoglobulin A (IgA) proteases, and lipopolysaccharides (LPSs). Capsules, comprised of repeating units of polysaccharides, play a role in evading the immune system by resisting phagocytosis, antimicrobial peptides, and the bactericidal effects of human serum (46, 311, 454). Capsular structures elicit a poor immunogenic response due to their structural similarities to polysialic acid residues found on human cells (415). Additionally, this barrier plays a role in late biofilm development (346) and protects against desiccation and phage attack. It also assists in accelerating the urinary stone crystallization process via electrostatic interactions that accumulate urinary ions at the bacterial surface (55, 87, 408). During the course of UTIs, antibodies that recognize antigenic components of uropathogens are produced. However, proteases targeting immunoglobins and other host defense components such as complement (C1q and C3) and antimicrobial peptides (human beta-defensin 1 [hBD1] and human cathelicidin LL-37) protect uropathogens from the host response (24). LPS, a requisite constituent of gram-negative bacterial outer membranes, is composed of three components: a lipid A molecule that anchors LPS to the membrane, a core consisting of polysaccharides, and a variable O antigen. This macromolecule elicits a potent inflammatory response that initiates the development of septic shock in systemic infections. Various components of LPS have been demonstrated to be important for resistance to antimicrobial peptides (95) and complement-mediated lysis. A summary of virulence factors expressed by gram-negative bacteria is shown in Fig. 2.
FIG. 2.
Virulence factors of the gram-negative uropathogens E. coli and P. mirabilis. IM, inner membrane; OM, outer membrane. (The micrographs are reprinted from references 172, 219, and 346 with permission.)
During the course of researching literature for this review, it was surprising how little research has been directed specifically towards virulence associated with CAUTIs given the staggering number of patients that develop this type of infection annually. As a reflection of this finding, the majority of this review will discuss the virulence factors that are involved in the pathogenesis of UTIs caused by two gram-negative bacterial etiologic agents associated with CAUTIs, Escherichia coli and Proteus mirabilis, and how these factors may contribute to infections associated with indwelling catheters. When applicable, known virulence factors that are associated with the pathogenesis of CAUTIs will be described. Lastly, the review will conclude with methods used for the prevention and treatment of patients who develop these infections.
CAUTIs DUE TO E. COLI
E. coli, undoubtedly the most researched microorganism, is a facultative anaerobe that is a member of the family Enterobacteriaceae. While both commensal and uropathogenic E. coli (UPEC) strains colonize the large intestines of humans, only UPEC strains are primarily selected for growth in the urinary tract. Virulence factors that differentiate these avirulent commensals from virulent strains of E. coli were acquired on mobile genetic elements by horizontal gene transfer; examples of such transfer can be found on the E. coli chromosome in the form of pathogenicity islands (125). These virulence factors enable E. coli strains to colonize and persist in the human host despite highly effective host defenses (278). E. coli strains have evolved to cause a variety of human diseases including sepsis, meningitis, diarrhea, and UTIs (276). These organisms are serotypically diverse, spanning over 250 serotypes based on O, H, and K antigens (292). Strains of E. coli associated with infections of the urinary tract are referred to as UPEC strains and are a subset of strains called extraintestinal pathogenic E. coli strains, which cause UTI, sepsis, and meningitis.
UPEC strains are the most commonly isolated organisms in community-acquired UTIs (70 to 90%) and among the most commonly isolated in nosocomially acquired UTIs (50%) including CAUTIs (202). E. coli has been identified as the causative agent in 90% of all case of UTI in ambulatory patients (167). UPEC strains can be classified into four phylogenetic groups, designated A, B1, B2, and D, with strains classified as B2 and D usually causing the most extraintestinal infections including UTIs (287). Since these organisms are capable of colonizing the intestinal and vaginal tracts as well, these sites can serve as potential reservoirs for UTIs and CAUTIs (83, 160).
As with other organisms, UPEC strains possess an arsenal of virulence factors that specifically contribute to their ability to cause disease in the human urinary tract. Genes encoding hemolysin, P fimbriae, S fimbriae, and cytotoxic necrotizing factor 1 (CNF1), for example, have been identified on various pathogenicity islands in different UPEC strains (125). This genetically heterogenous group of organisms varies in its capacity to colonize and persist in the urinary tract (99, 158). DNA microarray analysis of E. coli CFT073, a pyelonephritis strain, compared transcriptional profiles of this strain grown in LB, in human urine, and in the murine bladder cystitis model of infection (126, 376) and verified the in vivo expression of type 1 pili, iron acquisition proteins, and capsule (15, 335, 336, 345). In a prevalence study conducted by Kanamaru et al. (180), who compared 427 E. coli strains (377 UTI isolates and 50 fecal isolates) using PCR assays, the putative virulence factors iroN, iha, kpsMT, ompT, and usp were found 2.0 to 4.3 times more frequently in UTI isolates than in fecal isolates and were strongly associated with a specific anatomical site of infection (i.e., kidney or bladder).
Since UPEC strains are more commonly associated with infections of the intact urinary tract, it is thought that less-virulent organisms are capable of causing complicated UTIs such as CAUTIs. These bacteria may express less and perhaps different virulence factors during this process compared to organisms that are able to infect structurally and functionally normal urinary tracts (261). It has been implied that UPEC strains that infect the catheterized urinary tract have a reduction in the expression of P fimbriae and possibly other factors such as hemolysin, serum resistance, colicin production, and certain H, O, and K serotypes (261). An analysis of 70 clinical urinary strains of E. coli isolated from patients with spinal injuries undergoing long-term bladder catheterization identified that these strains rarely possess a complete arsenal of virulence factors possessed by strains isolated from cases of uncomplicated UTI (26). Among 70 urinary isolates, the prevalences of virulence factors were as follows: mannose-resistant hemagglutinins, 30%; P fimbriae, 17%; hemolysin, 27%; K antigens, 28%; and aerobactin, 33% by bioassay and 39% by gene probe (26). These findings indicate that the presence of a urinary catheter and a neuropathic bladder increases susceptibility to colonization of the urinary tract (26). Despite its prominent role in CAUTIs, limited research specifically addressing UPEC and its ability to cause these types of infections has been performed. Because of this, we will focus on the most recent developments in the research on UPEC and its role in UTIs and, when applicable, any research that is devoted to the field of bacterial virulence during CAUTIs.
Adhesins
UPEC strains and other uropathogens must attach to uroepithelial cells and the catheter surface to colonize and initiate CAUTI and may express a variety of adhesins to assist in this initial attachment. These adhesins also contribute to the direct triggering of host and bacterial signaling pathways, assisting in the delivery of bacterial products to host tissues, and promoting bacterial invasion into host cells (271). A study by Reid et al. (321) suggested that nonspecific adhesins, not specific fimbriae, expressed by UPEC are responsible for attachment to urinary catheter material. It is unknown which specific adhesin molecules are involved in the colonization of UPEC on catheter surfaces. However, potential adhesins associated with UTIs, including type 1, P, S, FC1, and F9 fimbriae and Iha and Dr adhesins, could possibly play a role during CAUTIs. The most extensively studied adherence factors of UPEC are type 1 and P fimbriae (271); an in-depth description of these structures has been reported previously (96, 271).
Type 1 fimbriae, the most frequently expressed virulence factor of UPEC (80 to 100% of strains), are composite helical cell surface structures consisting of repeating major pilin FimA subunits, tip fibrillum (FimF and FimG), and tip adhesin FimH assembled via the chaperone (FimC)-usher (FimD) pathway (344). These pili undergo phase variation and are regulated by the recombinases FimB and FimE.
In a study by Mobley et al. (261) examining urine cultures of 51 long-term catheterization patients over a year, type 1 fimbriae were expressed by a significantly higher number of UPEC isolates causing the most persistent infections than by strains causing transient infections. These pili are thought to be critical for the interaction of UPEC with uroepithelial cells during colonization of the bladder (59, 211, 272, 403). FimH of type 1 pili is thought to be involved in the adherence of these organisms to the bladder epithelium through the recognition and binding of the mannosylated integral membrane glycoproteins uroplakin Ia (467) and uroplakin Ib located on superficial epithelial cells. This tip adhesin also recognizes extracellular matrix proteins including collagen (types I and IV), fibronectin, and laminin as well as Tamm-Horsfall protein. Therefore, these bacterial adhesive structures are able to recognize epithelia (bladder and kidney), immune cells (macrophages, neutrophils, and mast cells), erythrocytes, and extracellular matrix proteins. This tip adhesin may also mediate bacterial autoaggregation and biofilm formation (313, 347, 348).
In addition, type 1 pili are believed to induce an inflammatory response associated with UPEC attachment and invasion (202) through the binding of FimH to specific mast cell receptors that initiate this response by the secretion of inflammatory mediators (1). Lastly, Snyder et al. (377) demonstrated that the expression of type 1 fimbriae coordinately affects the expression of P fimbriae in an inverse manner that may coordinate sequential events during colonization during UTIs in vitro and in vivo as examined by CFT073-specific DNA microarray analysis and mutagenesis studies using a mouse model. This adhesin has been associated with the invasion process as will be discussed later in the review. As these pili have been suggested to be expressed for the initial interactions between UPEC and various surfaces, it is speculated that type 1 pili could be involved in the initial interactions with the catheter surface or in interactions with uroepithelial cells during CAUTIs associated with UPEC.
P fimbriae or pyelonephritis-associated pili (pap) are the second most common virulence factors associated with UPEC uropathogenesis. The genetic determinants responsible for the production of these fimbriae are encoded on the UPEC chromosome by the papABCDEFGHIJK operon. P fimbriae are composed of heteropolymeric fibers consisting of different protein subunits (148), including proteins involved in the structure of the pilus (major pilin PapA, pilus anchor PapH, tip fibrillum PapKEF, and tip adhesin PapG), pilus assembly (periplasmic chaperone PapD and outer membrane usher PapC), and pilus regulation (PapB and PapI). Some studies have shown that P pili are needed by UPEC strains during UTIs; others have failed to show this requirement. UPEC strains expressing P fimbriae attach to globoside residues present on human kidney epithelial cells, which is suggested to play a role in the virulence associated with pyelonephritis (present in 80% of pyelonephritic E. coli strains) as well as ascending UTI (79, 310). Attachment to uroepithelial cell digalactoside receptors mediated by these fimbriae has been shown to induce cytokine secretion (interleukin-6 [IL-6] and IL-8) by this cell type in vitro (137). Studies have proposed that P pili may be important in establishing a bacterial reservoir in the intestinal mucosa (113, 233). However, experimental evidence suggests that these adhesins appear to have a less important role in colonizing abnormal or obstructed urinary tracts (156, 416). Based on these findings, it is thought that P pili may have either no role or a limited role during CAUTIs caused by UPEC.
UPEC is capable of expressing other surface adhesins including S pili (271), F1C pili, F9 fimbriae, IrgA adhesin, and Dr adhesins (271). S pili, consisting of the major subunit SfaA and the minor subunits SfaG, SfaH, and SfaS, recognize and bind sialyl galactosides on human kidney epithelial cells (191) and have been shown to play a role in UTIs caused by UPEC in rats (238). F1C pili, encoded by 14% of UPEC isolates, recognize and attach to kidney epithelial (distal tubules and collecting ducts) and endothelial (bladder and kidney) cells (183). Recently, Ulett et al. described a novel fimbria for UPEC strain CFT073 known as F9 fimbriae (420). These fimbriae were suggested to play a role during biofilm formation and are found in other UPEC and other pathogenic E. coli strains. The precise role of these surface structures during infection is currently unknown. UPEC expresses an iron-regulated gene homologue adhesin IrgA, designated Iha, during UTIs. This outer membrane protein is prevalent among clinical UPEC strains (38 to 74%) (16, 166) compared to fecal E. coli isolates (14 to 22%). Recombinant Iha from the pyelonephritogenic E. coli isolate CFT073 conferred adherence to cultured T-24 human uroepithelial cells to nonadherent E. coli strain ORN172 (163). In addition, a mutant in iha was more attenuated in a mouse model of ascending UTI than wild-type strain CFT073 and UPEC76 (CFT073 pap mutant) (163).
The Dr adhesin family of UPEC includes the uropathogen-associated fimbrial adhesin Dr and nonfimbrial adhesins (AFA-I, AFA-II, AFA-IV, Nfa-I, and Dr-II) (271) and has been associated with cystitis (30 to 50%) in children (114). The Dr operon consists of six genes encoding the main structural subunit DraA, the chaperone DraB (308), the usher DraC (308), the potential invasin DraD (106), DraP, and the adhesin DraE (465). The structural adhesin DraE determines the receptor-binding specificity of Dr adhesins (285). This adhesin is believed to be important in bacterial persistence in the urinary tract through the invasion of bladder and kidney epithelia via the interaction of these fimbriae with decay-accelerating factor (CD55) (286), a regulatory protein that protects tissues from autologous complement-mediated damage. The Dr adhesin also binds type IV collagen (286) and integrins, thus promoting recognition and the adherence of these organisms to interstitial compartments of the kidney, neutrophils, and erythrocytes. Strains of UPEC expressing Dr adhesin were capable of causing chronic experimental pyelonephritis in C3H/HeJ mice (114). They were also capable of long-term survival in human epithelial cells and persisting in the kidneys of experimental animals for months (215). It is unknown if this family of adhesins is expressed during CAUTIs caused by UPEC.
In summary, UPEC is known to express a number of adherence factors that assist in its ability to persist in the urinary tract. However, there is limited research on how this organism adheres to catheter surfaces. It can be speculated that some of the known adhesins that UPEC uses during UTIs could be expressed during CAUTIs caused by this organism as host cell components attach to the catheter surface to provide binding sites. However, more extensive research on the adherence of UPEC during CAUTIs is warranted and necessary to better understand the pathogenesis of this infection.
Motility
It is postulated that once UPEC is established on the catheter surface, flagellum-mediated motility is important for the ascent of this uropathogen from the catheter to the bladder and subsequently to the upper urinary tract (ureter and kidney). The synthesis of the flagellar structure is coordinated in a complex regulon consisting of several operons arranged in a hierarchical system (discussed in detail in a review by Fernández and Berenguer [96]). The filament of flagella consists of flagellin, the major filament subunit encoded by the fliC gene, that extends into the extracellular milieu from the outer membrane. The filament is connected to the flagellar hook FlgE through its attachment to the junction proteins FlgK and FlgL and the filament scaffolding protein FlgD (96). Two recent mutagenesis studies by Lane et al. (208) and Wright et al. (459) demonstrated that flagella, while not absolutely required for virulence during UTIs, greatly enhanced the persistence and fitness of UPEC during this type of infection. Therefore, flagellum-mediated motility should likely be considered to be important for the movement of UPEC on the catheter surface and from the catheter surface to the uroepithelium. This, however, has not been directly demonstrated.
Invasion and Biofilm Formation
Once initial attachment and permanent adherence commence on either the surface of catheters or uroepithelial cells, the establishment of UPEC infection occurs through the colonization of the bladder by the invasion of host cells and the subsequent formation of biofilms. As demonstrated in murine models, UPEC has developed mechanisms to invade host cells, and several reviews discussed this phenomenon in detail (32, 271). UPEC strains have been observed in vitro and in vivo to be internalized by bladder epithelial cells (103, 241, 251) and renal epithelial cells (82, 294, 380, 442). Several adhesins and toxins have been implicated to be involved in the process of invasion, including type 1 fimbriae, the Afa/Dr adhesin family (Dr, Dr-II, F1845, Afa-1, and Afa-3), S pili, P pili, and CNF1. Type 1 fimbria-mediated invasion is dependent upon FimH expression (241). E. coli strains that express Dr adhesins have been observed to invade epithelial cells including Caco-2 intestinal cells (115, 286). Dr adhesin-mediated invasion of uroepithelial cells is dependent upon the presence of the decay-accelerating factor receptor on host cells (117) and may contribute to persistence within the upper urinary tract (271). Research into the roles of S and P pili (241) during bacterial invasion of epithelial cells has not been studied in depth. However, it has been proposed that these pili, in conjunction with toxins, may facilitate the invasion of host tissues (115, 116).
CNF1, discussed later in the review, has been implicated in the invasion of UPEC into uroepithelial cells. This secreted toxin enters the host via the low-pH-mediated endocytotic pathway (54) and then constitutively activates key Rho GTPases that signal the reorganization of the actin cytoskeleton in the host cell. CNF1 has been shown to induce apoptosis in bladder epithelial cells via terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling. Thus, this toxin may play a role in bladder cell shedding in vivo and exposing the underlying tissue for bacterial invasion (257).
The ability of UPEC strains to persist in the urinary tract has been demonstrated by Justice et al. to reside within the superficial umbrella cells of C3H and BALB/c murine bladders due to the formation of biofilm structures known as intracellular bacterial communities (IBCs) (175). These IBCs are formed in a sequential manner. First, during murine cystitis, UPEC cells are attached to the cell surface by type 1 fimbriae and then invade the uroepithelium (240, 241) 1 to 3 h after the initial inoculation. Localized actin rearrangements occur and engulf the bound organism via zipper-like phagocytosis (240). After being internalized in the murine superficial bladder cell, UPEC replicates rapidly, forming clusters known as early IBCs (175). Recently, type 1 fimbriae have been shown to have an additional intracellular role during this stage of IBC formation (460). As IBCs mature, around 6 to 8 h after inoculation of the murine bladder (175), they more closely resemble classical biofilm structures (88), where the bacterial doubling time is increased (from approximately 30 to 60 min) and the bacterial cell length is shortened (0.7 μm versus 3 μm). At this stage, pod-like protrusions are observed on the surface of murine bladder epithelial cells (8). Around 12 h postinoculation, bacterial detachment is observed (175) as either a whole community or individual highly motile cells that burst out of the murine bladder lumen in a process referred to as fluxing (7). It is postulated that IBCs and biofilms contribute to the persistence of these organisms due to the increase in resistance to antibiotics and the host immune response. IBC formation has not yet been substantiated in humans.
There are several factors that are known to contribute to the formation of biofilms by E. coli. These include fimbriae, curli, flagella, antigen 43, and extracellular matrix molecules including cellulose, colanic acid, and poly-β-1,6-N-acetyl-d-glucosamine (67, 68, 74, 81, 437, 469). Specifically, biofilm formation mediated by type 1 fimbriae may assist in the colonization of urinary catheter surfaces (271).
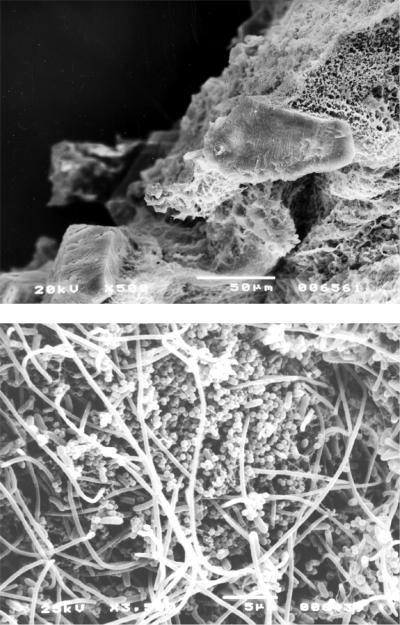
There have been recent studies examining biofilm formation on catheters and UPEC. Ferrières et al. (97) showed that certain catheter materials such as silicone and silicone-latex actually select for and promote biofilm formation for the most virulent UPEC strains, whereas asymptomatic bacteriuria strains form better biofilms on polystyrene and glass (Fig. 3). Koseoglu et al. (194) revealed that UPEC type O4 had formed mature biofilms after 12 to 24 h and developed biofilms completely in almost all latex/silicone balloon catheter samples after 4 to 7 days as examined by scanning electron microscopy.
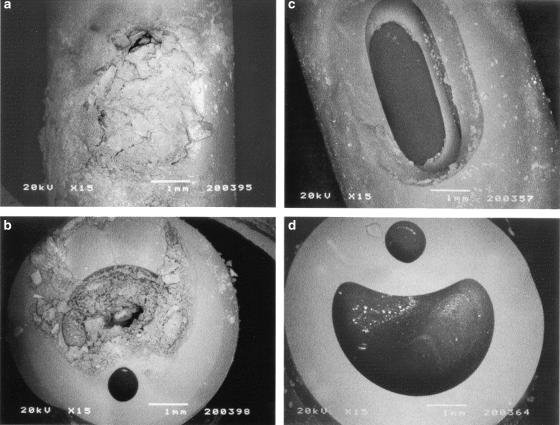
FIG. 3.
Cross section of a silicone catheter removed from a patient after blockage. Crystalline material can be seen completely occluding the catheter lumen. (Reprinted from reference 387a) with permission of the publisher.)
Biofilm formation is critical for initiating and maintaining CAUTIs. Therefore, any factors involved in this process are likely important virulence factors during CAUTIs caused by UPEC. However, there are currently very few studies that have examined biofilm formation during these infections.
Avoidance of Host Immune Response
Once established, bacteria must express factors to avoid the host immune response in order to persist in the urinary tract; these factors include fimbriae that are subject to phase variation, capsules, and LPS. As mentioned above, type 1 fimbriae are subject to phase variation to evade the host immune response. UPEC strains, as with other uropathogens, have been shown to produce an exopolysaccharide capsule as a means of avoiding the immune response and thereby contributing to serum resistance. There are over 80 types of these capsular polysaccharides (CPSs) (K antigens), with K1 capsules most frequently observed among urinary and clinical isolates (160, 291). These organisms produce group II or group III capsules, compared to commensals that express group I polysaccharide capsules (161). These capsules are thin, highly anionic structures that tend to aggregate spontaneously (174). The gene locus encoding the group II capsule has been described for UPEC strain NU149 and is organized into three regions. The genes involved in the assembly and transport of capsules are located at conserved regions designated regions 1 (kpsFEDUCS) and 3 (kpsMT). In contrast, region 2 (kfiABCD) is unique to each serotype and is involved in capsular biosynthesis (451). Schwan et al. (355) described the down-regulation of the kpsFEDUCS operon, possessing the genes of capsule assembly region 1 responsible for K-antigen expression (451), in UPEC upon type 1 fimbria attachment to mannose-coated Sepharose beads as a possible initiating event of UTIs. These acidic polysaccharide capsules assist in the avoidance of phagocytosis and complement activation (161). There are no studies that have investigated the role of these structures during CAUTIs caused by UPEC.
Upon entry into the bladder, UPEC contacts uroepithelial cells and initiates a robust innate immune response through the activation of various signaling pathways. Cell activation is accomplished by interactions between bacterial surface molecules, such as fimbriae and LPS, and uroepithelial cell receptors. LPS, a critical constituent of the gram-negative cell wall, was shown to be important for resistance to antimicrobial peptides (95) and complement-mediated lysis. However, the compound activates cells of innate immunity via its interaction with Toll-like receptor 4 (TLR4) and CD14 located on the host cell membrane (349, 350). These TLR molecules (TLR1 to TLR10) are pattern recognition receptors expressed on the cell surfaces of leukocytes and epithelial cells that recognize preserved molecular motifs located on various pathogens (202). TLR4 is important in these events since mice deficient in this surface receptor are incapable of efficiently clearing UPEC from the bladder (127). These initial interactions activate signal pathways that subsequently lead to the release of cytokines (tumor necrosis factors, gamma interferon, IL-8, and IL-1) that guide neutrophil infiltration, stimulate complement and coagulation (130, 351), and trigger the shedding of superficial bladder facet cells (273). Prior to expulsion from the host, intracellular UPEC emerges from these exfoliated bladder epithelial cells to invade the exposed underlying tissues to persist in the host. Type 1 fimbriae, as described by Blomgran et al. (30), interact with neutrophils in a mannose- and LPS-dependent manner, which leads to subsequent neutrophil apoptosis. It is suggested that LPS plays an important role in the persistence of UPEC during UTIs and CAUTIs. However, there is currently no experimental evidence that substantiates these claims.
Damage to the Host and Acquisition of Nutrients
Once established on catheter and uroepithelial surfaces, uropathogens such as UPEC strains must adapt to the urinary tract environment and acquire nutrients. This is accomplished in part by the production of degradative enzymes, toxins, and nutrient acquisition systems. These factors include toxins such as hemolysin, CNF1, and autotransporters such as secreted autotransporter toxin (Sat), Pic (139, 295), and Tsh (139) to break down host peptides and iron acquisition proteins to acquire iron sequestered by the host.
UPEC strains expressing toxins such as hemolysins, CNF, and Sat have been isolated (121, 144, 202). Of the 91 UTI isolates examined by Caprioli et al. (47), 37% produced both the hemolysin HlyA and CNF1, whereas only 1 of 114 fecal isolates produced CNF1. The two common forms of hemolysin expressed by UPEC are the alpha-hemolysin HlyA (384), and cell-associated beta-hemolysin. The presence of hlyA was found to be 31 to 48% for UTI isolates, compared to only 15% for fecal isolates (239). The genes encoding these heat-labile secreted pore-forming proteins are found on either plasmids or the chromosome (252). Hemolysin has been shown to lyse human kidney proximal tubule cells (263). The effect of these toxins is the induction of cell lysis due to an increase in intracellular osmotic pressure caused by the dissipation of calcium ion gradients. A proposed function of these toxins is to cause the release of host cell iron into the extracellular environment that can then be captured by the bacterium.
The toxins classified as CNF (CNF1 or CNF2) are known to constitutively activate the Rho GTPases RhoA, Rac1, and Cdc42, which regulate the actin cytoskeleton (75). Cells exposed to this toxin form enlarged multinucleated cells, as exhibited by membrane ruffling, the formation of focal adhesions and actin stress fibers, and DNA replication in the absence of cell division. The existence of the CNF1 gene was found in 27 to 41% of UTI isolates but in only 9% of fecal isolates (239). CNF1 has been shown to modulate polymorphonuclear leukocyte function through the down-regulation of phagocytosis and altering the distribution of the complement receptor CR3 (CD11b/CD18). A study by Falzano et al. (94) supplied evidence that CNF1 of UPEC is capable of blocking the cell cycle G2/M transition in the T24 uroepithelial cell line.
UPEC has been shown to secrete a number of autotransporters during UTIs. The autotransporter toxin known as Sat (121) is a vacuolating cytotoxin that damages kidney epithelial cells during acute pyelonephritis in the mouse model (122). UPEC strains have also been found to express two other autotransporters, Pic and Tsh, that possess serine protease activity (139). These proteins are expressed in vivo during pyelonephritis in the mouse model (139). Since these proteins have been shown to cause host damage during UTIs in an animal model, it is plausible that these autotransporters could be expressed by UPEC during CAUTIs. However, research to address this hypothesis has not been undertaken.
Although nutrient acquisition systems are important for the survival of UPEC during growth in the urinary tract, this review focuses on the nutrient acquisition systems for iron, as these are the best-studied systems. Iron acquisition systems are important virulence factors produced during UTIs and CAUTIs, as this nutrient is limiting in urine (363). Since iron acquisition is important for the viability of UPEC during infection, these organisms produce multiple systems including siderophore-siderophore receptor and heme uptake systems (334). As stated above, E. coli strains likely use alpha-hemolysin as another means of retrieving iron from the host (290). The known siderophore systems of UPEC include the catechol enterobactin and the hydroxamate aerobactin along with their corresponding receptors, such as the IreA, IroN, and Iha receptors (161, 378). Most E. coli strains produce the siderophore enterobactin. However, the genes encoding aerobactin are found significantly more often in strains isolated from the urinary tract (69.4% of 124 isolates [P = 0.001]) than in fecal E. coli samples (41.2% of 51 isolates) (77). UPEC strains have been shown by hydrolysis fluorescence detection to produce novel siderophores including salmochelins, C-glucosylated enterobactins that are dependent upon the biosynthesis of enterobactin and the iroBCDEN operon (468), and yersiniabactin (423).
Iron acquisition systems have been shown to be important during UTIs caused by UPEC. Snyder et al. demonstrated that five iron acquisitions were upregulated in UPEC strain CFT073 during infection in the CBA mouse model of ascending UTI compared to static in vitro growth in LB (376). Recently, Reigstad et al. (322) revealed that heme- and siderophore-associated iron acquisition systems play key roles in IBC development in female C3H/HeJ mice. Further research is needed to determine if these acquisition systems are important during CAUTIs caused by these organisms.
CAUTIs DUE TO PROTEUS MIRABILIS
Proteus species, members of the family Enterobacteriaceae (301), are distinguishable from most other genera by their ability to swarm across an agar surface. These organisms are widely distributed in the environment, including polluted water, soil, and manure, where Proteus plays a role in the decomposition of organic matter from animals, and in the intestinal tract of mammals. Proteus species are the causative agent of a variety of opportunistic nosocomial infections including those of the respiratory tract, eye, ear, nose, skin, burns, throat, and wounds; it also may cause gastroenteritis (302, 331). Antibodies specific for Proteus have been isolated from patients with active rheumatoid arthritis (76, 90), suggesting some association with this malady. Proteus bacilli are more commonly associated with UTIs in those individuals with structural or functional abnormalities, especially ascending infections in patients undergoing urinary catheterization (441, 444). Colonization of the intestinal tract allows Proteus to establish reservoirs for transmission into the urinary tract by intermittent colonization of the periurethral region. This intermittent colonization can lead to the subsequent contamination of the catheter, thus allowing nosocomial infections to develop (53).
Proteus-associated UTIs may be difficult to treat, and the bacterium persists due to complications associated with this type of infection, including bladder and kidney stone formation (urolithiasis) that can lead to the obstruction of catheters and the urinary tract (200, 356, 444). The three species of Proteus associated with UTIs are Proteus mirabilis, Proteus vulgaris, and Proteus penneri. While UTIs caused by P. vulgaris (361) and P. penneri (199, 200) have been identified, P. mirabilis is the third most common cause of complicated UTI (12%) and the second most common cause of catheter-associated bacteriuria in patients catheterized long term (15%) (439).
P. mirabilis is a common cause of CAUTIs. It was observed by Roberts et al. (327) that P. mirabilis has the greatest ability to attach to catheters out of all gram-negative organisms. As monitored by a low-light optical imaging system, catheter sections colonized with bioluminescent P. mirabilis were inserted into murine bladder lumen. These animals developed severe cystitis that persisted significantly longer than that in mice challenged with bacterial suspensions alone and required prolonged antibiotic treatment to reduce the infection (178). To establish and maintain infections of the urinary tract and colonization of catheters, Proteus species must adapt to the catheterized urinary tract and produce an arsenal of strictly regulated virulence factors.
Adhesins
Indwelling urinary catheters serve as the initiation site of CAUTIs by introducing uropathogens such as Proteus spp. into the urinary tract and providing a surface for coating by host cell debris and protein that may be recognized by bacterial adhesins. P. mirabilis strains tend to attach to catheters with a greater propensity than other gram-negative bacteria (327). Studies have demonstrated that P. mirabilis strains are capable of attaching to a number of catheter polymers including ethylene, propylene, polystyrene, sulfonated polystyrene, silicone, and red rubber (135, 327).
To facilitate binding to these different surfaces, P. mirabilis must be capable of producing a variety of adherence factors, such as fimbriae and hemagglutinins, that are thought to play an important role in the establishment of CAUTIs. Proteus species have been shown to produce various fimbriae and hemagglutinins involved in the colonization of the urinary tract and possibly catheter surfaces, including MR/P fimbriae (289), MR/K hemagglutinin (289), uroepithelial cell adhesin (UCA)/nonagglutinating fimbriae (NAF) (456), P. mirabilis fimbriae (PMF) (10), and ambient-temperature fimbriae (ATF) (243). Others have also been predicted for the genome sequence of strain HI4320 (M. Pearson, J. Parkhill, and H. L. Mobley, unpublished data).
MR/P fimbriae are perhaps the best-understood fimbriae expressed by P. mirabilis strains during UTIs. These fimbriae are thick channeled (7 to 8 nm) and are classified as mannose-resistant fimbriae (36, 371). These fimbriae assemble through the chaperone-usher pathway (379). The genes required for the expression of MR/P fimbriae on the cell surface are encoded on the Proteus chromosome on two divergent transcripts, mrpABCDEFGHJ (designated the mrp operon) and mrpI (14). Some of the proteins encoded by the mrp operon include the fimbrial structural subunit MrpA (13); the terminator for fimbrial assembly, MrpB (222); the minor fimbrial subunit MrpG (224); the tip adhesin MrpH (219); and the repressor of flagellin synthesis, MrpJ (223).
Expression of MR/P fimbriae is subject to phase variation (13, 14). The mrpI gene encodes a site-specific recombinase that reverses the orientation of the 251-bp invertible element that precedes the mrp operon. Expression of MR/P fimbriae correlates with the orientation of this invertible element (466). MrpI orients the invertible element in either an “on” position, allowing the expression of the MR/P fimbria, or an “off” position, in which the promoter is in the opposite orientation and is thus unable to drive transcription.
Many studies have suggested that MR/P fimbriae play a role in the virulence observed during UTIs caused by uropathogenic P. mirabilis strains. In the CBA model of ascending UTI, infection with P. mirabilis elicited a strong immune response to MrpA, the major structural subunit of MR/P fimbria, indicating that MR/P fimbriae were expressed in vivo (11). Isogenic mutants incapable of expressing MR/P fimbriae were attenuated when examined in this mouse model (12, 219, 221, 224). A mutant constitutively expressing MR/P fimbriae outcompeted the wild-type strain in the murine bladder but not the kidneys in a cochallenge experiment, thereby establishing MR/P fimbriae as being an important bladder colonization factor for P. mirabilis (221). Tissue binding studies by Sareneva et al. (343) revealed the propensity of this fimbrial type to adhere specifically to the human renal tubular epithelial cells and to the exfoliated uroepithelial cells of urinary sediment.
Experiments conducted by Jansen et al. (155) suggest that MR/P fimbriae dictate the localization of bacteria in the bladder and contribute to biofilm formation, a process essential for the establishment of CAUTIs. A P. mirabilis HI4320 construct with an invertible element locked in the “on” position colonized the luminal surfaces of murine bladder umbrella cells and formed significantly more biofilms after 2 days of growth in urine (P = 0.05) compared to a construct with the invertible element in the “off” position. The off-position mutant colonized the lamina propria underlying exfoliated uroepithelium. Although studies have associated the expression of MR/P fimbriae with virulence during UTIs caused by P. mirabilis, there is no direct evidence substantiating a role of these fimbriae in CAUTIs.
On the other hand, MR/K fimbriae have been linked with the attachment of organisms to catheter surfaces and with the persistence of catheter-associated bacteriuria (262, 331, 461). These fimbriae have also been detected during Providencia stuartii infections in catheterized elderly patients (262). Expression of these thin (4- to 5-nm) nonchanneled type 1 mannose-resistant fimbriae (36, 371) enables P. mirabilis to attach tightly to the Bowman's capsule of the host kidney glomeruli and to the tubular basement membranes (343). Although more associated with P. penneri strains (461), it is speculated that MR/K fimbriae play a possible role in the initial adherence to catheter biomaterials during P. mirabilis CAUTIs.
Besides MR/P and MR/K fimbriae, the other fimbriae produced by P. mirabilis during UTIs may contribute to attachment to the catheter surface. Surface adhesins determined not to be involved in the hemagglutination caused by MR/P and MR/K fimbriae have been identified in P. mirabilis, including UCA/NAF (10, 29, 60, 406, 456), PMF (244), and ATF (242). Wray et al. (456) characterized UCA, a nonagglutinating fimbria from P. mirabilis HU1069 that was demonstrated to weakly attach to exfoliated human desquamated uroepithelial cells. The 540-bp ucaA gene, which encodes the major fimbrial subunit of UCA, has nucleic acid homology to the F17A gene of E. coli F17 pilin (58%) (60) and was identified in all 26 P. mirabilis strains tested (29). Due to its homology to the F17 pilin of E. coli (60), it has been suggested that these fimbriae might be involved in the colonization of the intestines by these organisms (58). Based on studies conducted by Bahrani et al. (10, 11, 13), there was some ambiguity as to which fimbrial types were identified as UCA (456) since thin (4-nm) and thick (6-nm) fimbrial filaments were observed by electron microscopy, and multiple bands were isolated on sodium dodecyl sulfate-polyacrylamide gels (406). These fimbrial subunits were isolated and characterized from P. mirabilis strain 7570 from a patient with struvite urolithiasis and renamed NAF by Tolson et al. (406). The N-terminal sequence of this fimbrial subunit was confirmed to be identical to the N-terminal sequence from P. mirabilis strain HU1069 of the study reported by Wray et al. and not homologous to the N termini of MR/P, ATF, or PMF fimbrial subunits (406). Bacteria expressing NAF adhered strongly to a number of cell lines in vitro, including uroepithelial cells (407) and MDCK (Madin-Darby canine kidney) (5, 216) and EJ/28 urinary tract tumor (214) cell lines. Purified NAF from P. mirabilis binds to a number of glycolipids such as asialo-GM1, asialo-GM2, and lactosyl ceramide, as demonstrated by thin-layer chromatography overlay assays and solid-phase binding assays (216). Because of its homology to fimbriae that assist in intestinal tract colonization, it is possible that these fimbriae may play a role in the initiation of CAUTIs by allowing P. mirabilis to attach and establish in the intestines and thus form a reservoir of organisms that can potentially cause CAUTIs. However, there have been no definitive studies examining this possibility.
PMF are encoded by genes located in the pmf gene cluster and consist of five polypeptides: PmfA, the 18.9-kDa major subunit of PMF (10); PmfC (93.1 kDa); PmfD (28.2 kDa); PmfE (38.9 kDa); and PmfF (19.7 kDa). The pmf gene cluster has >25% amino acid sequence identity with the pap, mrp, and sfa fimbrial gene clusters. However, pmfE has been identified as being unique to this gene cluster. Thus far, no regulatory elements for the production of these fimbriae have been identified (245). There are conflicting results as to the function these fimbriae during UTI. In a study by Massad et al. (244), PMF were demonstrated to play a role during the colonization of the bladder since an isogenic mutant in the pmfA gene of P. mirabilis HI4320 was 83-fold more attenuated than the wild-type strain during independent challenge in the CBA mouse model of ascending UTI (244). However, in this same study, PMF could not be shown to be involved with attachment to human uroepithelial cells since attachment to this cell type is similar in both the wild type and the pmfA mutant. Its role in the colonization of the kidney is also questionable since no significant difference between numbers of the wild type and pmfA was observed in kidney tissue (244). Contrary to this, a study by Zunino et al. (472) showed that there was significant attenuation observed in the kidney and bladder by the isogenic pmfA mutant compared to the parent strain Pr2921 during cochallenge in a model of ascending UTI in female CD-1 mice. Furthermore, attachment of this isogenic pmfA mutant to T24/83 human-derived bladder carcinoma cells and human uroepithelial cells was significantly less than that of the wild type. These conflicting results require resolution.
ATF were classified as a new fimbrial type, as examined by electron microscopy and immunogold labeling (243), and were identified in all eight P. mirabilis strains analyzed. The genes responsible for the production of ATF are organized in the atf gene cluster and encode a 19-kDa major-subunit AtfA (243), the chaperonin-like protein AtfB, and the outer membrane usher AtfC (243). AtfA has significant amino acid sequence identity to type 1 major fimbrial subunits of several enteric species (38% to 41%) (243). An allelic-replacement atf mutant colonized the murine urinary tract at a gene comparable to that of the wild type in independent challenge and outcompeted the wild type in cochallenge experiments in the murine model of ascending UTI (243). As nonclinical strains of Proteus were shown to express AtfA, as observed by Western blot analysis (470), it is suggested that these fimbriae are involved in the colonization of P. mirabilis in the environment and are most likely not involved in CAUTIs.
Currently, only MR/K fimbriae are known to be associated with the process of attachment during CAUTIs. Clearly, additional studies must ascertain whether known factors or currently uncharacterized factors are involved in adherence, as this process is essential for these types of infections. The identification of novel adherence factors as well as other virulence factors will be facilitated by the recent annotation of the P. mirabilis HI4320 genome by the Sanger Centre in conjunction with the Mobley Laboratory.
Motility
In general, flagella on the surface of bacterial pathogens are thought to assist in host colonization and dissemination, initial attachment, and sensing of the extracellular environment (25). For Proteus species, these surface structures are important in the process known as swarming, a distinct characteristic of these organisms. Therefore, it is speculated that flagellar motility and, potentially, swarming are important during CAUTIs, as the ability of P. mirabilis to disseminate from the initial site of colonization on the catheter surface to the uroepithelial cells of the urinary tract is critical for the establishment of these types of infections.
Swarming is a surface-induced multicellular differentiation process that allows organisms to move in a coordinated manner and expand the population to new locations over solid surfaces (258, 318, 453). During growth in liquid medium, Proteus species assume the form of an infectious single-cell, motile, 1.0- to 2.0-μm-long bacillus that displays a distinct phenotype including the presence of peritrichous flagella on its cell surface and swimming behavior. However, when transferred onto solid medium, these swimmer cells differentiate into hyperflagellated, multinucleated, nonseptated elongated swarmer forms measuring 20 to 80 μm in length. These differentiated swarmer cells migrate out from the original inoculation site in a rapid and highly coordinated manner that is dependent upon multicellular interactions and cell-to-cell signaling (18).
Swarmer cells align themselves in multicellular rafts and are enveloped in the extracellular slime material of the colony migration factor Cmf that is required for and facilitates translocation through a reduction in surface friction (124, 172, 381). The swarming process continues until the cell number is reduced by cell loss or when the bacterial mass changes the direction of motion (18). The cessation of movement, known as consolidation, is accompanied by the dedifferentiation and replication of swarmer cells into vegetative swimmer cells. This periodicity distinguishes P. mirabilis swarming from other swarming processes. For a more in-depth description on the process of Proteus swarming, refer to reviews by Rather (318) and Rozalski et al. (331).
Since swarming is such a dominant characteristic of this genus, any factors that affect or regulate this phenomenon would likely affect the fitness of the organism. The swarming phenomenon is a metabolically complicated and demanding process that must genetically coordinate the expression of over 50 genes (21), including those involved in the production, assembly, and operation of flagella and virulence factors such as flagellin, urease, hemolysin, and the ZapA metalloprotease (4, 24).
To identify potential genes that may be involved in the process of swarming, transposon mutagenesis studies of P. mirabilis using Tn5 transposons were performed (20, 21, 23, 41). Those studies identified over 50 genes that were involved in the swarming process and included genes that encoded proteins involved in flagellar biosynthesis (20), flagellar rotation (20), surface elongation (20), control and coordination of multicellular motility (20), production of LPS and peptidoglycan (23), and cell division (23). Burall et al. (41) identified a mini-Tn5 transposon mutant in dsbA of P. mirabilis HI4320 that was defective in the colonization of the murine urinary tract and in swarming. This gene encodes an oxidoreductase that forms disulfide bonds in periplasm proteins. However, no definitive role of this gene during swarming has been defined. The utilization of signature-tagged mutagenesis has greatly assisted in the identification of genes not previously associated with the swarming process, and any of these genes could play an essential part in the pathogenesis of CAUTIs caused by P. mirabilis.
The definitive roles of flagella and swarmer cell differentiation in the virulence of P. mirabilis during UTIs remain controversial, but some of these suggested roles include dissemination of P. mirabilis from the initial site of infection to other sections of the catheter or to the urinary tract and avoidance of the host immune response. Flagella are believed to contribute to the virulence of swimmer cells by allowing motility from the catheter to the bladder epithelium and onward, ascending into the ureters and kidneys. An isogenic, nonpolar, nonmotile, flagellum-negative mutant in flaD of P. mirabilis WPM111 was attenuated 100-fold compared to the wild type in the CBA model of ascending UTIs, indicating the importance of flagella in murine UTIs (259).
The biosynthesis of flagella is a key process in both motility and swarming and involves numerous genes on the Proteus chromosome (22). Flagellin is encoded by flaC, and the flaD gene encodes the flagellar filament capping protein (19). Studies suggest that the major flagellin protein for P. mirabilis is subject to antigenic variation through homologous recombination as three copies of flagellin-determinant gene (flaA, flaB, and flaC) that reside on the P. mirabilis genome with only one copy that is actively expressed (19, 275). It was proposed that flagellin gene rearrangement is a mechanism for host immune system evasion by P. mirabilis and is extremely relevant for Proteus infections since flagella are highly immunogenic. As a result, any antigenic change could increase the survival of Proteus species in the urinary tract through the evasion of secretory IgA directed toward flagella during colonization in the bladder (19). Due to its relevance during UTIs, it is probable that antigenic variation via flagellin gene rearrangement is a method of host immune response evasion by P. mirabilis during CAUTIs.
Swarming cell differentiation is thought by some to be important for the virulence of P. mirabilis during UTIs since several virulence factors, including flagellin, urease, the hemolysin HmpA, and the IgA metalloprotease ZapA, are upregulated in the differentiated swarmer cell compared to swimmer cells (4, 101). Mutants in FlhA synthesis, proteins required for flagellar synthesis, are nonmotile due to the loss of fliC transcription but also have reduced transcription of hpmA hemolysin (123). Therefore, it has been suggested, based in part on evidence of the coordinate expression of virulence factors during swarming cell differentiation, that factors involved in the swarming process are critical for pathogenesis and that a similar signal must be regulating both swarming and virulence (3, 4, 52, 258). Interestingly, however, swarmer cells are rarely observed in the murine model of ascending UTI, bringing into question the relevance of this morphotype in the absence of a catheter (154).
Swarming may play a role in the migration of Proteus strains on catheter materials; however, swarmer cells of Proteus species are capable of migrating across 1-cm-long sections of Foley catheters consisting of either hydrogel-coated latex, hydrogel/silver-coated latex, silicone-coated latex, and all silicone in vitro (337). Swarmer cells of P. mirabilis have been observed to migrate through populations of E. coli, Klebsiella pneumoniae, Staphylococcus aureus, and Enterococcus faecalis and then continue to migrate with little or no reduction over hydrogel-coated latex catheter sections (337). Nonswarming mutants were shown to have lost the ability to migrate over these catheter sections (172). However, upon their introduction into models of the catheterized bladder, these mutants were just as capable of encrusting and blocking catheters as the wild type (171). It seems that while swarming might have a role in the initiation of infection, facilitating the passage of the cells from the urethral meatus to the bladder, it is not required for the rapid formation of crystalline biofilm once bacteria have colonized the residual urine in the bladder.
There is conflicting experimental evidence about the significant of swarming during the ascension of P. mirabilis into the urinary tract and during pyelonephritis. Swarming-defective mutants and motile, nonswarming mutants of P. mirabilis were significantly attenuated in the colonization of the kidney compared to the wild type upon intravesical (bladder) inoculation of mice (2). Furthermore, the swarming-defective mutant was still able to colonize the murine bladder albeit at a lower rate than that of the wild-type strain. In contrast, the nonswarming mutant was incapable of colonization of the murine kidney. These findings suggest that swarming is important for P. mirabilis-associated ascending UTIs and pyelonephritis. Histological analysis of murine renal tissue has supported these results, as swarmer cells were found to be the predominant cell type (2). Other studies suggested that flagella and/or swarming is not involved in the process of ascension into the upper urinary tract by P. mirabilis. A clinical strain of P. mirabilis that lacks flagella has been isolated (471). In addition, confocal microscopy studies by Jansen et al. (154) demonstrated that the predominant P. mirabilis morphotype was the short swimmer cell, not the swarmer cell, in the mouse model of ascending UTI. However, the differences observed between these experimental results are most likely due to differences in experimental parameters for each study (318). More conclusive studies are required to resolve these conflicts.
Biofilm Formation
After the initial colonization of the catheter surface, Proteus species, as with other uropathogens, form distinctive crystalline biofilm structures during CAUTIs. These structures assist in the persistence of P. mirabilis in the urinary tract by protecting these organisms from antibiotics and the host immune response and obviously contribute to adhesion to surfaces (146). Urinary stone formation during Proteus-mediated UTI is characteristic of this type of infection and is critical for the development of crystalline biofilms. Bacterially derived stones account for up to 30% of all urinary tract stones worldwide and account for approximately 75% of the urinary stones classified as staghorn calculi (141). Upper urinary tract stones are classified as staghorn calculi if stone formation occurs in the renal pelvis and extends out into at least two calyces (253). Crystalline biofilms are especially problematic during CAUTIs since catheters become blocked due to encrustration caused by the formation of these structures.
It should be recognized that there are powerful physical and chemical factors involved in the initiation and development of the crystalline biofilms that block catheters. Experiments in parallel-plate flow cells showed that when urine cultures flow over polymer surfaces, the pH of the urine can be a major factor in determining bacterial adhesion. For example, some polymers with strongly-electron-donating surfaces will resist colonization by cells until the pH of the urine rises above the pH at which calcium and magnesium phosphates precipitate out of solution. In alkaline urine, macroscopic aggregates of cells and crystals form in the urine, settle on the polymer surface, and initiate crystalline biofilm formation (391). These observations indicate that to stop biofilm formation on devices in the urinary tracts of patients infected with P. mirabilis, it is essential to prevent the rise in urinary pH and the crystallization of apatite and struvite (Fig. 4).
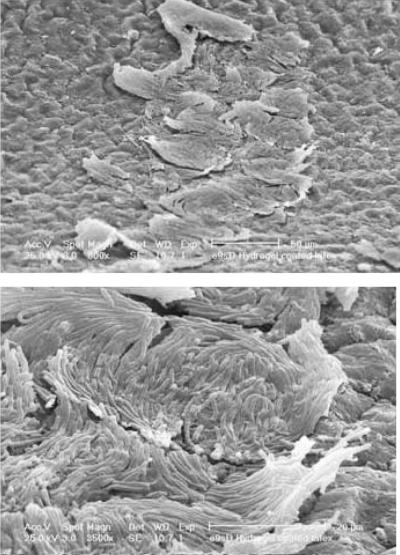
FIG. 4.
Crystalline material that blocked a patient's catheter after just 4 days. The large coffin-shaped crystals were shown by X-ray microanalysis to be a form of magnesium ammonium phosphate (struvite), and the microcrystalline aggregates were shown to be calcium phosphate (apatite). A four-membered bacterial community was isolated from this crystalline biofilm composed of E. coli, P. aeruginosa, E. faecalis, and P. mirabilis. (Modified from reference 390a with permission from Elsevier.)
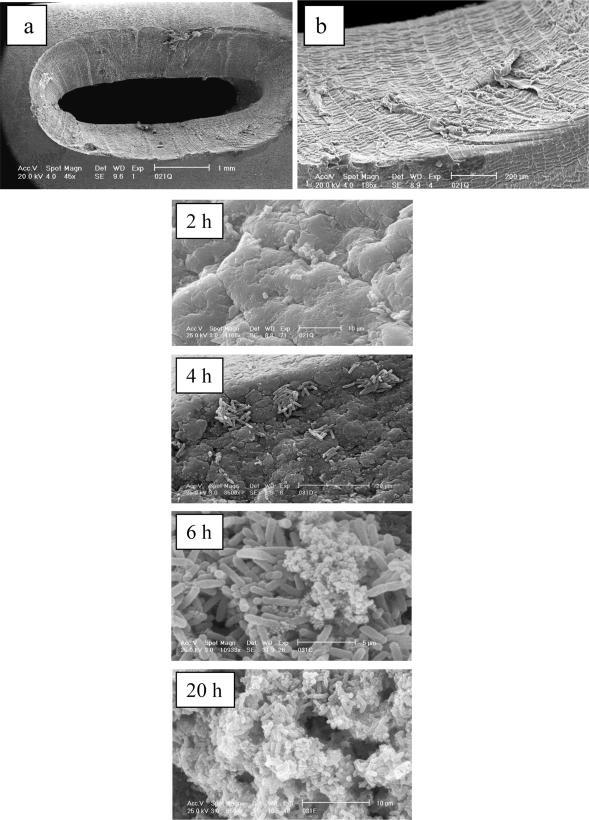
The lumenal surfaces of catheters, particularly those of latex-based catheters, are extremely irregular in nature (64). The engineering techniques used in catheter manufacture produce especially rough surfaces on the rims of the catheter eyeholes. Experiments in physical models of the catheterized bladder in which catheters were removed at various time intervals after infection with P. mirabilis to monitor the initial stages of encrustation demonstrated the vulnerability of the eyeholes to colonization (386). Scanning electron microscopy revealed that within 2 h, bacterial cells were trapped in the crevices in the uneven surfaces of the eyelets. At 4 h, microcolonies of cells had developed in the surface depressions, and by 6 h, with the rise in urinary pH, crystals had started to form in the biofilm. Extensive crystalline biofilm was obvious at 20 h and was spreading down the catheter lumen. Blockage generally occurred at the eyehole or in the balloon region of the lumen.
Bacterial urease and capsule polysaccharides are two major factors known to be involved in urinary crystal formation and, hence, crystalline biofilm formation in P. mirabilis (277). Since urea is present in concentrations of up to 500 mM in human urine (35, 170), it is not surprising that bacterial ureases play a pivotal role in Proteus-associated UTI. Urease contributes to the development of urinary stones due to urease-mediated hydrolysis of urea to ammonia and carbon dioxide that alkalinizes the local environment. This increase in urinary pH causes the local supersaturation and precipitation of calcium phosphate and magnesium-ammonium phosphate from urine to form crystals of carbonate apatite [Ca10(PO4)6CO3] and struvite (MgNH4PO4·6H2O), respectively (118). These crystals accumulate in the biofilms of catheters and urinary epithelial surfaces and eventually obstruct the flow of urine through the catheter and from the bladder or kidney. Incontinence can develop due to urine leakage around the catheter or retention of urine in the bladder that can seriously complicate the care of patients undergoing long-term bladder catheterization (392). Urease is produced by Proteus species known to cause clinical infections (P. mirabilis, P. vulgaris, and P. penneri) (266), and the urease produced by P. mirabilis is the best characterized one.
The P. mirabilis urease is a 250-kDa multimeric nickel metalloenzyme that is produced in the cytoplasm (266). As this enzyme is inducible in urea, it is assumed to be constitutively expressed during growth in urine (58). This urease is homologous to the urease of Klebsiella aerogenes (266), and the urease operon of P. mirabilis has homology to the urease operon of Providencia stuartii (169, 265, 435) and likely all urease genes. This operon possesses seven genes, ureDABCEFG, that encode proteins involved in the production of urease. The urease apoenzyme is composed of a trimer of trimers consisting of the polypeptides UreA, UreB, and UreC [(UreABC)3] (264) and is activated upon the insertion of the divalent nickel metallocenter into each of the UreC subunits. The insertion and assembly of the nickel ions into the metallocenter are accomplished by the urease accessory proteins UreD chaperone (258), UreE (nickel ion donator), UreF, and UreG (264). The exact mechanism for the assembly of urease is not fully understood (331).
The urease gene cluster is regulated by UreR and the histone-like nucleoid structuring protein (H-NS). The 33-kDa polypeptide UreR, a member of the AraC/XylS family of transcriptional activators (281), initiates transcription of the genes encoding the urease subunits and accessory proteins and of its own gene in a urea-inducible manner through binding to the intergenic region between ureR and ureD (84, 150, 405). UreR binds the promoters of the ureR and ureD genes in the absence of urea, albeit with less affinity than in the presence of urea, suggesting that the organism is prepared for the rapid induction of urease (84, 150, 405). The transcriptional repressor H-NS recognizes and binds to the poly(A) tracts of the intergenic region between the ure genes and bends the DNA to repress the transcription of ureR in the absence of urea induction (57), as shown in an E. coli model system (312). Both UreR and H-NS regulators were able to displace each other from the ureR-ureD intergenic region in a gel shift assay (312).
The importance of urease as a virulence factor of Proteus-associated UTIs as well as CAUTIs has been demonstrated in the CBA mouse model. An insertion mutation in the ureC gene abolished urease activity and was attenuated in the murine model (168). This urease-negative construct colonized the bladder and kidneys in 100-fold-fewer bacteria than the urease-positive strain after 2 days postinoculation and caused no urolithiasis during infection (159). A study by Li et al. (225) revealed that catheterized CBA mice were more susceptible to infection by the wild-type P. mirabilis strain after 7 days than uncatheterized CBA mice. However, although catheterized CBA mice were more susceptible to bladder colonization by the ureC insertion mutant than uncatheterized mice, the mutant was unable to colonize the kidneys under any circumstances (225). These results suggest that even though urease is important during colonization of the urinary tract during uncatheterized infection, it is not necessary for the initial colonization of the bladder during CAUTIs in the mouse model.
Stone formation is the primary role of urease during UTI caused by urease-producing organisms. A secondary role of urease during Proteus UTI is the accumulation of toxic levels of ammonia from urease-mediated hydrolysis of urea that damages tissue including renal epithelia (277). Ammonia has been demonstrated to be toxic to the protective uroepithelial glycosaminoglycan layer (119) present at the bladder surface to effectively block the adherence of bacteria to the uroepithelium. However, the cytotoxic effects observed for urease are not as severe as those observed for HpmA hemolysin (260).
Besides bacterial urease, capsular structures assist in crystalline stone formation observed during UTIs and CAUTIs associated with P. mirabilis. These structures are believed to accelerate struvite crystal growth (55, 249) by aggregating precipitated components of urine into stones (250). Proteus CPSs tend to be acidic due to the presence of uronic acid, pyruvate, or phosphate groups, thus enabling this structure to bind to metal cations such as Ca2+ and Mg2+ (331). Purified partially anionic CPS of P. mirabilis ATCC 49565 added to artificial urine at a pH of 7.5 to 8.0 induced more struvite formation than other CPS types, as examined by particle counting (Coulter counter) and by phase-contrast microscopy (87). With the exception of one polymer (curdlan) that did not bind Mg2+ ions, the enhancement of struvite crystallization by CPS polymers was inversely proportional to their Mg2+ binding ability. Therefore, it is suggested that the weak binding of the Mg2+ ion by the partial anionic structure of P. mirabilis CPS enhances struvite crystallization by enabling the weakly concentrated Mg2+ ions to be readily released from LPS for crystal formation (87, 331).
Once a mature biofilm develops on the surface of uroepithelial cells or catheters, organisms within the mushroom-shaped structure communicate with each other, utilizing diffusible chemical signals that regulate a variety of cellular functions including glutamine (3), autoinducer-2 (AI-2) (354), cyclic dipeptides, and putrescine (396). However, the role of these signaling molecules in the process of biofilm formation or swarming during P. mirabilis UTIs or CAUTIs is unclear.
In short, crystalline biofilms are known to form during CAUTIs associated with P. mirabilis and are responsible for some of the more severe sequelae experienced. Only a few of the proteins involved with this process in P. mirabilis, including bacterial urease, have been identified and studied. Therefore, more extensive studies need to be completed, including the identification of these potential proteins.
Avoidance of Host Immune Response
Besides the formation of crystalline biofilms, P. mirabilis uses several immunoavoidance factors to persist in the urinary tract, including antigenic variation, capsules, IgA proteases, and LPS. As mentioned above, the flagellin protein of P. mirabilis may undergo antigenic variation as a means of avoiding an antibody response. CPS, also referred to as slime material or glycocalyx, is a highly hydrated polymer present on the bacterial cell surface. These structures have several known functions during UTIs and CAUTIs caused by P. mirabilis, such as a role in crystalline stone formation (250), which is discussed above. Other known functions of CPS include protection against the host immune response and antibiotics, attachment to surfaces (146), and, potentially, swarming. Little is known about these structures in Proteus species. Studies of certain O antigens of P. mirabilis (O6 and O57) and P. vulgaris (O19) demonstrated that capsular antigen structures that are identical to the O-specific chains of their LPS are produced (27, 307, 419). One capsular structure of P. mirabilis (ATCC 49565) has been identified as being an acidic CPS consisting of a high-molecular-weight polymer of branched trisaccharide units composed of 2-acetamido-2-deoxy-d-glucose (N-acetyl-d-glucosamine), 2-acetamido-2,6-dideoxy-l-galactose (N-acetyl-l-fucosamine), and d-glucuronic acid (27).
The colony migration factor Cmf, an extracellular slime material, from wild-type P. mirabilis WT19 is an acidic CPS composed of a tetrasaccharide repeating unit. This substance is rich in galacturonic acid and N-acetylgalactosamine, as determined by glycosyl composition and linkage analyses and by one- and two-dimensional nuclear magnetic resonance spectroscopy (316). A mutant in cmfA was attenuated in a model of experimental uropathogenicity compared to the wild type, thereby demonstrating a reduced ability to colonize the urinary tract (2). As described above, this factor facilitates surface colony expansion of the swarm cell population through a reduction in surface friction (124). Therefore, it is proposed that this factor contributes to the uropathogenicity of P. mirabilis during CAUTIs by facilitating the translocation of differentiated cell populations on catheter surfaces.
To combat invading microbes, during the course of UTIs, secretory IgA, the dominant immunoglobulin form in mucus secretion, is produced against antigenic components of uropathogens by the host immune system. This immunoglobulin is important for the protection of the mucous membrane and underlying tissue of the urinary tract during infection and is fairly resistant to proteolytic cleavage. However, proteases targeting immunoglobins and other host defense components such as complement (C1q and C3) and antimicrobial peptides (hBD1 and human cathelicidin LL-37) protect uropathogens from the host response (24). Only a few bacterial species produce proteases against IgA (e.g., Neisseria spp., Haemophilus spp., and Streptococcus spp.) (270, 309), including an IgA protease activity that has been identified for Proteus species.
While not produced by Providencia spp. and Morganella spp., all clinical strains of P. mirabilis and P. penneri and many strains of P. vulgaris examined produced an EDTA-sensitive protease (229, 358, 359). The purified metalloprotease appeared to be a composite of a single band and a double band (53 and 50 kDa, respectively), as visualized by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and all three bands were proteolytically active (229). The optimal pH for these enzymes was pH 8, which would coincide with the action of urease that alkalinizes urine (229). The production and activity of the P. mirabilis metalloprotease appear to occur during UTIs since breakdown fragments detected in urine specimens from patients with clinical evidence of upper UTIs are not detected in urine specimens from patients infected with a nonproteolytic strain. Also, fragments were identical to the breakdown fragments observed when purified IgA was degraded by purified metalloprotease, as examined by immunoblotting (362).
The EDTA-sensitive IgA extracellular metalloprotease produced by a chronic urinary tract isolate of P. mirabilis cleaves the heavy chain of the serum immunoglobulin IgA into two fragments (359). The cleavage sites for this protease differed from those of other microbial IgA1 proteases in that it cleaves outside the hinge region (228, 358). The substrate specificity of the protease isolated from a chronic UTI isolate, P. mirabilis 64676, was expanded to include the cleavage of subclasses of IgA (secretory IgA1 and IgA2) and IgG, both present in urine (331), and the nonimmunoglobulin substrates such as gelatin, secretory component, and casein (228). Cleavage of IgG occurs at the hinge region as a two-stage process that involves a pepsin-like activity and then a papain-like activity to yield Fab and Fc fragments (228).
Wassif et al. (445) isolated and characterized this protease from P. mirabilis BB2000 and designated it ZapA. ZapA is a 55-kDa recombinant extracellular metalloprotein that is a member of the serralysin family of zinc metalloproteases and a member of the ABC transporter family (433). It is produced during swarmer cell differentiation and is stimulated by divalent cations (Ca2+ > Mg2+) (433). This protease was found to be important during Proteus-mediated UTIs, as mutants in the zapA gene fail to degrade IgA and are attenuated compared to the wild type during infection in a CBA mouse model of ascending UTI (433). The genes for ZapA production and secretion are organized into the zapEABCD operon, and based on homologies to other known systems, the system consists of the ZapA-specific ABC transporter and the polypeptides ZapB, which possesses an ATP-binding cassette; ZapC, the membrane-spanning fusion protein; ZapD, which is involved in outer membrane transport; and ZapE, a second metalloprotease (433). ZapE was homologous to other extracellular proteases, including ZapA (42% identity) from P. mirabilis (445), PrtC (45% identity) and PrtA (38%) from Erwinia chrysanthemi (112), serralysin (44% identity) from Serratia marcescens, and AprA (36% identity) from Pseudomonas aeruginosa (89). The substrate specificity of ZapA includes both human and mouse serum and secretory forms of IgA (IgA1 and IgA2) and IgG (445). Additional substrates include many urinary tract proteins such as complement (C1q and C3), cell matrix (collagen, fibronectin, and laminin), cytoskeletal proteins (actin and tubulin), and the innate immune system antimicrobial peptides hBD1, a component of the human renal tubule innate immune response, and human cathelicidin LL-37, as determined by in vitro assays (24).
Because of its broad specificity, there are several probable roles for IgA protease production during Proteus-associated UTI. Its primary role may be to evade the host immune response, including antibody-mediated opsonization and degradation of immune system components. The secretion of the protease by P. mirabilis during UTI or during CAUTIs could serve to cleave IgG and IgA into fragments with defective immune function, leading to decreased respiratory bursts in neutrophils (227). These proteases appear to inactivate effectors of the innate immune system, such as the antibacterial peptides hBD1and LL-37, that have critical roles in preventing infection at the uroepithelial surface (24, 353). As this protease has such a broad specificity, one of its main functions during UTIs and CAUTIs could be to degrade host cell proteins for amino acid and peptide acquisition. Since the production of this enzyme and the ability to invade human uroepithelial cells coincide with swarming cell differentiation, IgA protease also plays a role in invasion during infection.
Lastly, to evade the host immune response, P. mirabilis, as with other gram-negative organisms, possesses LPS. It is well reported that Proteus is an antigenically heterogeneous genus due to structural differences among the O-specific polysaccharide chain of LPS. Currently, there are 60 O serogroups (212, 303), including 49 numbered serogroups (O1 to O49) (212) of P. mirabilis and P. vulgaris and 19 specific H-antigen (flagellum) serotypes as classified previously (195, 304). Immunochemical studies of LPS have also established a number of additional serogroups for P. penneri strains (364, 473, 474). The common O serotypes isolated from clinical strains of Proteus species are O3, O27, O10, and O28 serotypes (212).
LPS is the main structural component of the gram-negative outer membrane and is one of the major virulence factors associated with these organisms. LPS is composed of three components: a lipid A molecule that anchors LPS to the membrane, a core consisting of polysaccharides, and a variable O antigen. Chemically, the LPS of Proteus species has been classified into 16 total chemotypes for P. vulgaris and P. mirabilis (366) and 7 total chemotypes for P. penneri (474). The composition and structure of several O-specific polysaccharides of Proteus species have been described (27, 48, 49, 102, 176, 185, 186, 188-190, 195, 304-307, 365, 367-370, 419, 425-432). In most Proteus strains, the general structure of the O-specific polysaccharides has been found to be acidic due to the presence of uronic acids and various noncarbohydrate acidic components, including phosphate groups (187, 331). For a more detailed description of these structures and LPS, refer to the review by Rozalski et al. (331).
LPS is known to induce a number of effects on its host, including fever, hypotension, disseminated intravascular coagulation, and subsequent shock (324). Various components of LPS have been demonstrated to be important for resistance to antimicrobial peptides (95) and to complement-mediated lysis. However, their role in the pathogenesis of UTIs is unclear.
Certain studies suggested that despite the presence of incomplete LPS in certain rough mutants, P. mirabilis strains are still pathogenic in the urinary tract (201, 213, 331). Other studies have indicated, however, that LPS is a virulence factor of Proteus-associated UTIs. In particular, nonpolar mutants of the waaE gene, a gene involved in the biosynthesis of the inner core of LPS through the substitution of alpha-l-glycero-d-manno-heptopyranose I at the O-4 position by a beta-d-glucopyranose residue (152), of P. mirabilis showed reductions in adhesion to uroepithelial cells. In addition, pathogenicity in female Wister rats, swarming motility, and the ability to form biofilms in vitro were also reduced in these mutants (152).
Due to the acidic, negatively charged residues on its surface, a possible function of LPS during UTIs and CAUTIs is its ability to attach and bind cations such as Ca2+ and Mg2+, leading to the accumulation of these ions around bacterial cells and the acceleration of the crystallization process (408). Studies by Torzewska et al. (408) indicated that the level of Ca2+ and Mg2+ cation binding on the surface of Proteus organisms is dependent upon the sugar composition of the Proteus LPS as determined by in vitro crystallization in artificial urine.
Certain P. mirabilis LPS structures have played a role in this organism's resistance to antimicrobial peptides (120, 248, 395) and defensin molecules. Defensins are a major component the of innate host defense against bacteria, including the potent molecule polycationic hBD3 found on epithelia (31).
In summary, P. mirabilis strains can persist in the urinary tract during UTIs or CAUTIs by evading the host immune response and are known to express several immunoavoidance factors. Currently, there is no direct evidence to confirm that many of these factors are produced during CAUTIs and are important during these infections. Biofilms have been shown to protect organisms against the host immune response (146) and are assumed to protect P. mirabilis against this response during CAUTIs. As P. mirabilis is known to produce a capsule and capsules are one mechanism of evading the host immune response, it has been suggested that these structures protect these uropathogens against the immune system during UTIs and CAUTIs. The colony migration factor Cmf, an acidic CPS, was also proposed to assist in swarming across catheter surfaces. As the IgA protease ZapA has been demonstrated to be expressed during swarming and as swarming has been shown to occur during the migration of P. mirabilis across catheter sections, it can be suggested that this protease is expressed during CAUTIs caused by these organisms. The LPS of P. mirabilis is thought to function in the avoidance of the immune response and the accumulation and acceleration of the crystallization process.
Damage to the Host and Acquisition of Nutrients
Once colonized on the catheter and the uroepithelium, uropathogens must adapt to the urinary tract environment and acquire nutrients. This is accomplished by the production of degradative enzymes, toxins, and nutrient acquisition systems. Uropathogens such as P. mirabilis can express and secrete degradative enzymes and toxins into the local environment to break down tissue, releasing nutrients and/or assisting in the spreading of the infection. Degradative enzymes target substrates located in the host for use as energy sources, including nutrients located in host fluids (e.g., urea from urine) and host cell surface and extracellular matrix components such as collagen, fibronectin, elastin, and hyaluronan. Proteus species produce several of these factors, and these factors include proteases to break down host polypeptides and peptides (e.g., ZapA) and ureases to obtain carbon and nitrogen sources, hemolysin to permeabilize host cell membranes, and iron acquisition systems to acquire iron sequestered by the host.
Hemolysis of human and sheep erythrocytes has been demonstrated to be mediated by 126 different urinary and soil isolates of Proteus species (P. mirabilis and P. vulgaris) (196, 197, 201), and the level of hemolytic activity is significantly higher in P. mirabilis strains than in P. vulgaris strains (296, 297). Hemolysins identified in Proteus species include HlyA and HpmA. HlyA, an extracellular calcium-dependent pore-forming cytotoxin (448, 450), is produced by strains of P. penneri (330, 360) and by 40% of P. vulgaris strains tested but not by P. mirabilis strains (192). The hly genes from P. vulgaris are homologous to the hly genes of E. coli (192) and represent the Morganella, E. coli, Proteus, and Pasteurella family of hemolysins (192, 193, 399, 449). In addition to this hemolysin, P. vulgaris, P. penneri, and P. mirabilis strains produce an unrelated hemolysin known as HpmA, as identified by immunoblot and DNA-DNA hybridization. These 166-kDa hemolysins, or cell-associated hemolysins (177, 192, 196, 197, 360, 449), are calcium-independent (177, 196, 360) pore-forming toxins that insert into target eukaryotic membranes, causing the efflux of Na+ ions and subsequent cell lysis (34). The production of HpmA is observed only in actively growing and replicating organisms in the presence of erythrocytes (297, 299), with maximum production occurring during the late logarithmic phase in P. mirabilis strains (398). This hemolysin and its secretory protein HlyB have significant nucleotide identity (52.1%) to the shlA and shlB hemolysin genes of S. marcescens (230). Cleavage of the 29-amino-acid N-terminal leader sequence is required for the activation and secretion of the HpmA hemolysin of P. mirabilis 477-12 (421) into the periplasm. In addition, the amino-terminal region of HpmA is required for hemolytic activity (398). The secretion and activation of HpmA are assisted by the 63-kDa potential outer membrane protein HpmB (230).
The HpmA hemolysin is thought to play a role in the virulence of Proteus UTIs and CAUTIs since the production of the HpmA hemolysin is upregulated coordinately with other virulence factors during swarming differentiation (4, 101). It was suggested that hemolysin assists in the spread of Proteus into the kidneys via tissue damage (260) and in tissue invasiveness (298, 299, 329, 330). HpmA cytotoxic activity against a number of cell culture lines in vitro including Vero, human renal proximal tubular epithelial (52, 260), Daudi, Raji, and U937 cells by a chromium release assay (260, 398) has been observed. The cytotoxicity of the HpmA hemolysin observed is dependent upon the in vivo condition examined. For example, the lethal dose of a hemolysin-negative mutant was six times higher than that of the wild-type strain upon intravenous challenge (398). However, a mutant in the hpmA gene of P. mirabilis WPM111 showed no significant difference in colonization compared to the wild type in the murine model of ascending UTI (398). While hemolytic activity observed in P. mirabilis strains has been correlated to cell invasiveness (298, 329), the nonhemolytic P. mirabilis strain WPM111 was shown to be 10- to 100-fold more invasive in human renal proximal tubular epithelial cells than hemolytic wild-type strain BA6163 based on a gentamicin protection assay. This result could be misleading, however, since hemolysin-mediated pores could allow the entry of gentamicin into cells (52). Therefore, the definitive role of Proteus hemolysin during UTIs has not been firmly established.
Extracting essential nutrients from host tissues and the surrounding extracellular milieu is critical for any microbe to establish a UTI or CAUTI. The composition of the host environment directly affects which acquisition systems are expressed during infection. Although the acquisition of various metals and carbon and energy sources as well as the presence or absence of oxygen are important in determining what metabolic pathways are enacted by pathogens and are critical for the fitness of any organism, we will focus primarily on the acquisition of iron, as its role in virulence in different organisms is well established.
Iron is an essential element for the production of cytochromes and as an enzyme cofactor. Proteins involved in iron acquisition are critical virulence factors produced during a UTI (9, 42, 45, 457) and, potentially, CAUTIs, since this nutrient is limiting in urine (363). The addition of exogenous iron reduces the susceptibility of animals to the development of P. mirabilis pyelonephritis (131). While most enterobacteria produce phenolate (enterobactin)- and/or hydroxamine (aerobactin)-type siderophores during iron-limiting conditions (42, 45, 296), none of these traditional siderophores have been demonstrated to be produced in Proteus, Providencia, or Morganella species (92, 296, 323). It has been proposed that these bacterial genera utilize α-keto acids derived from the deamination of amino acid by amino acid deaminase to bind iron and act as potential siderophores (85). The 51-kDa amino acid deaminase of P. mirabilis is encoded by the aad gene (246), and attempts to generate a mutant deficient in this gene have been unsuccessful, suggesting that mutations in this gene are lethal (246). Traditionally, the expression of most siderophores is repressed by the binding of the Fur protein on the promoters of iron-regulating genes. However, no Fur binding site has been identified upstream of the aad gene (246). Since deaminase activity was not affected by low-iron conditions, Aad may have other functions aside from the acquisition of iron (58). A signature-tagged mutagenesis study of P. mirabilis HI4320 conducted by Burall et al. (41) identified potential iron acquisition protein homologues such as the Pasteurella multocida membrane-bound heme receptor HasR, the phosphopantetheinyl transferase NrpG, the Yersinia pestis putative outer membrane protein IrgA, and a Y. pestis putative iron transport permease. However, the mechanism of iron acquisition for P. mirabilis is currently unknown. With the accessibility of the genomic sequence of P. mirabilis HI4320 made available by the Sanger Centre, the identification of more potential iron acquisition genes will be facilitated, and perhaps a better understanding of this process will emerge.
In summary, P. mirabilis, as with other uropathogens, must be capable of adapting to the urinary tract environment and acquiring nutrients. This is thought to be accomplished by the production of degradative enzymes such as urease and proteases, toxins such as the hemolysin HpmA, and iron nutrient acquisition proteins.
PREVENTION OF CAUTIs
Over 5 million patients per year receive urinary catheterizations (236), and all are at risk for developing CAUTIs. Current effective infection control measures against CAUTIs are ones that only delay the onset of bacteriuria, since no methods that prevent these types of infections have been developed (410). This section will describe the different practices that have been proposed for reducing the risk of developing CAUTIs. These include avoiding unnecessary catheterization, selecting alternative catheterization procedures, maintaining a closed drainage system, and eliminating bacterial colonization of the meatus. The development of new biomaterials that reduce biofilm formation on the surface of catheters, the addition or impregnation of antimicrobial agents on catheter materials, and the use of probiotics will be described as potential prevention measures. Lastly, future promising technologies for the prevention of CAUTIs will be discussed.
Limiting Catheter Usage
Selective and limited catheter use is critical to reduce the numbers of patients at risk for developing CAUTIs. Similar to the phenomenon observed for antibiotic use, urinary catheters have been overly used in the nosocomial and institutional settings because of their relative ease of application. Studies suggest that this is the case, as 21 to 38% of initial catheterizations had no justifiable indication (153, 274, 342). One of the causes of overuse has been due to “forgotten” catheters, where the physicians or students have not removed catheters after their use is no longer needed. In fact, one survey determined that 28% of inpatient physicians and students did not know which of their patients had received catheterization (341). A reduction in CAUTI due to the presence of forgotten catheters could be achieved through the utilization of a computerized reminder system (340). However, the restriction of urinary catheters to only those patients that truly require catheterization, such as to relieve urinary tract blockage, to allow drainage of neurogenic bladders and urinary retention, to facilitate healing of the genitourinary tract after surgery, or to measure the urine output in postoperative or critically ill patients with accuracy (455), is a key preventive measure for CAUTIs. These devices should be promptly removed after their required use as to reduce the risk of infection (236).
Once it is determined that a patient requires urinary catheterization, the duration of the catheterization affects the risk of developing infections associated with this device. With the risk of bacteriuria associated with catheter insertion being 3 to 10% per day (282), it is estimated that 10 to 50% of patients undergoing short-term catheterization (up to 7 days) and nearly 100% of patients that require long-term catheterization (>28 days) will develop a CAUTI (129, 269, 387). During the course of catheterization, it has been recommended that urinary catheters should be changed every 8 to 10 days (332) to reduce the risk of infection. Drainage bags should be emptied at a minimum of every 4 to 6 h to avoid bacteria entering the catheter lumen (280).
Condom and Suprapubic Catheters and Intermittent Catheterization
Besides their selective and limited use, alternative methods of catheterization that potentially reduce the risk of CAUTIs, including condom and suprapubic catheters and intermittent catheterization, are available. Condom catheters, while useful for male patients that lack bladder outlet obstruction, require meticulous care to avoid complications such as skin maceration and have been associated with an increase risk of UTIs (140, 455). The use of suprapubic catheters, indwelling catheters inserted above the pubic bone directly into the bladder, shows promise in terms of risk of infection (142, 237). However, no controlled clinical studies have been conducted to confirm its benefits (455). Those individuals with spinal cord injuries or individuals with dysfunctional emptying of the bladder commonly utilize intermittent catheterization or temporary insertion of a catheter. As with suprapubic catheters, there is a lack of studies examining the effectiveness of intermittent catheterization in the reduction of the number of CAUTIs compared to indwelling catheters.
Closed-System Foley Catheters and Proper Use by Health Care Professionals
Since its inception, there have been relatively few changes in the original design of the standard Foley indwelling catheter. However, minor modifications to this system, the size of catheter used, and proper catheter care play crucial roles in reducing the risk of CAUTIs. The introduction of the closed catheter system, one in which the collection tube is fused to the drainage bag, was critical for the reduction of CAUTIs, and variations to this drainage system have been attempted to further decrease the rate of infection. Some of these changes include the addition of a urine-sampling port in the drainage tubing and preconnected catheter/collecting tube systems (109). Infections associated with indwelling urinary catheters have dramatically reduced from 100% to less than 25% for up to 2 week of catheterization due to the usage of closed drainage systems versus ones that were open (203).
Choosing the correct catheter size assists in decreasing the risk of developing a CAUTI. It has been suggested that smaller catheters (14 French or 16 French) and 5-ml balloons should be utilized (65), as larger catheters have been shown to be a risk factor for the development of UTI (455). These larger catheters tend to increase the amount of residual urine (91) that can lead to the reinoculation of the bladder (93) and increase the risk of blockage of the periurethral glands that leads to UTI, urethral irritation, and erosion (91). Long-term catheterization using a 30-ml balloon has been associated with urinary leakage, bladder wall irritation, and bladder spasms. However, larger catheters have been shown to be of benefit for short-term use after genitourinary surgery to decrease bleeding (409).
Proper aseptic instillation of the catheter by hospital and institutional personnel is critical for the reduction of CAUTIs as well as the avoidance of collection bag contamination upon emptying (17). Cross-contamination is responsible for many outbreaks of nosocomial UTI, as patients with asymptomatic UTI and hospital personnel can become reservoirs of infections (455). The separation of catheterized patients (234) and increased handwashing have been recommended to reduce cross-contamination. Routine bacterial monitoring has been suggested as a control measure to reduce the number of carriers and subsequently cross-contamination (203).
Prevention of Bacterial Colonization of the Urinary Meatus and Urinary Tract
Studies have been performed to determine if the prevention of bacteria colonized on the meatus from entering the bladder is a plausible method to reduce instances of CAUTIs. Although studies have shown that catheterized patients are at a higher risk for infection if their meatus is colonized with various microorganisms (107, 108), two prospective studies concluded that the current method of meatus care (either twice-a-day cleansing with povidone-iodine solution followed by povidone-iodine ointment or daily cleansing with soap and water) was ineffective in reducing CAUTIs in patients with closed drainage systems (37, 43). Once organisms have entered the urinary tract, methods to eradicate these organisms prior to replication have been implemented. These methods include irrigation of the bladder and the use of prophylactic systemic antibiotics. Continuous irrigation of the bladder with nonabsorbable antibiotics such as neomycin-polymyxin was shown to be ineffective in reducing the rate of CAUTIs and was in fact associated with frequent disruptions of the close drainage system (443). Although treatment with prophylactic antibiotics does delay infection (38, 109), this method is not recommended since the effect is only temporary, with a tendency to be selective for those strains that are antibiotic resistant.
Novel Surfaces
Once a uropathogen gains access to the urinary tract, adhesion to the catheter material and the uroepithelium is imperative to the initial establishment of biofilms in CAUTIs. Bacterial attachment to catheter surfaces is dependent upon the hydrophobicity of the organisms and the biocompatible surface of the catheter. Following urine application in the catheter lumen, a conditioning layer is formed from the deposition of proteins, minerals, polysaccharides, and other host-derived factors in the urine (80). This also occurs on the outside surface of the catheter due to the interaction with the host urogenital surfaces. The resulting attachment of host factors provides binding sites for uropathogens. Therefore, the development of surfaces that are resistant to organism colonization is important for the prevention of CAUTI and is ongoing. Although several control measures for the prevention of biofilm formation on catheters have been examined, no single biosurface, including silicone, polyurethane, composite biomaterials, or hydrogel-coated materials has been shown to be effective in preventing colonization due to the development of the conditioning layer (80, 236, 417) (Fig. 5).
FIG. 5.
Scanning electron micrographs of a section of a hydrogel-coated latex catheter over which swarmer cells of P. mirabilis are migrating. The sections have been removed from the laboratory model described by Sabbuba et al. (337). Multicellular rafts of typical swarmer cells are visible on the irregular surface of the catheter, migrating from left to right. The micrographs were kindly provided by Rob Broomfield of Cardiff School of Biosciences, Cardiff University.
Catheters Containing Antimicrobial Agents
Preventive measures that have been examined but that have not yielded any proven benefit in the prevention of bacteriuria in catheterized patients include the application of antimicrobial solutions and lubricants on the catheter surface prior to catheter insertion and the addition of antimicrobial agents in the collection bag (17). Conversely, in separate prospective, randomized trials, antimicrobial-impregnated catheters containing either nitrofurazone (235) or the combination of the broad-spectrum antibiotics minocycline and rifampin (72) demonstrated significant reductions in bacterial CAUTIs. However, these trials were limited, and the emergence of resistant strains was not resolved (236).
Besides antibiotics, impregnation of catheter material with antiseptics, such as silver compounds, has been studied as a possible preventive measure, with conflicting results on the efficacy of these silver-coated urinary catheters in the prevention of bacteriuria. Clinical trials have shown conflicting results as to the efficacy of silver oxide-coated catheters compared with uncoated catheters. In a prospective clinical trial involving 482 acutely hospitalized patients, silver oxide-coated catheters reduced the incidence of UTI only among women not receiving antimicrobial agents (19% for control catheter versus 0 for silver catheter) (P = 0.04) compared with a control silicone catheter (165). A randomized study of 1,309 patients catheterized longer than 24 h failed to demonstrate the effectiveness of a silicone catheter coated externally with 5% silver oxide compared to a standard silicone elastomer-coated latex catheter. However, these silver oxide catheters did show a significantly increased incidence of bacteriuria in male patients and a significantly increased occurrence of staphylococcal bacteriuria (325).
Similarly, there are conflicting studies regarding the efficacy of silver hydrogel urinary catheters in the prevention of nosocomial UTIs (206, 404). Adherence of several strains that cause CAUTIs (E. coli, P. mirabilis, P. aeruginosa, E. faecalis, and K. pneumoniae) was reduced on silver hydrogel-treated silicone or latex catheters compared with latex or silicone catheters, as shown by radiolabeled-cell assays (104) (Fig. 6).
FIG. 6.
Early stages in the formation of a crystalline P. mirabilis biofilm on a hydrogel-coated latex catheter. Catheters were removed for examination by scanning electron microscopy after incubation for various times in a laboratory model of the catheterized bladder. The irregular nature of the surface of the eyelet is shown in a and b. After 2 h, in the model, cells can be seen trapped in crevices. At 4 h, microcolonies have formed in surface depressions. At 6 h, microcrystalline material accumulated in the developing biofilm as the pH of the urine rose. At 20 h, extensive crystalline biofilm formed at the eyehole. (Reproduced from reference 386 with kind permission from Springer Science and Business Media.)
The silver catheter that is being increasingly used is a hydrogel silver alloy latex-based device. This catheter has metallic silver in a gold and platinum coating linked to a latex base on both the external and internal surfaces. Silver ions are apparently released into the surrounding fluids to exert their antibacterial activity. The outer hydrogel layer gives the catheter its lubricity (255). Despite the fact that this catheter has undergone extensive clinical trials, it is still the subject of considerable controversy. Perhaps the most impressive evidence of its efficacy comes from a study reported by Karchmer et al. (182), who compared the infection rates in hospitalized patients given the hydrogel/silver alloy-coated or silicone-coated latex catheters. In this trial, wards rather than patients were randomized into two groups. During the first 6 months of the study, group 1 wards were supplied with the silver-coated catheters, and group 2 wards were supplied with the silicone-coated catheters. After 1 month, “washout-period” group 1 wards received the silicone-coated catheters, and group 2 received the silver catheters. Surveillance for nosocomial infections was performed by infection control practitioners using CDC criteria (110). These definitions register both symptomatic UTI and asymptomatic bacteriuria. Analysis of the data showed that 154 infections were recorded among the 13,945 (1.1%) patients fitted with the silver catheter, compared to 189 of the 13,933 (1.36%) patients who had the silicone-coated catheter (P = 0.07). When the data were expressed as infection rates per patient days, infections on the wards using silver catheters were calculated to be 2.66 infections per 1,000 patients days, compared to 3.35 per 1,000 days in the control wards (P = 0.04). Secondary bloodstream infections complicated the care of 14 of the 343 patients with UTI. The rate of these infections (0.04%) in patients on units supplied with the silver catheters was not significantly different (P = 0.42) from that (0.07%) when the patients were receiving the silicone-coated catheters. Several other studies reported no significant reductions in infection rates with this catheter. A meta-analysis of trials involving a total of 2,355 patients determined that silver alloy catheters (odds ratio, 0.24; 95% confidence interval, 0.11 to 0.52) were significantly more protective against bacteriuria than silver oxide catheters (odds ratio, 0.79; 95% confidence interval, 0.56 to 1.10) (363). This conclusion was reached even though those authors referred to the difficulties in comparing efficacies from studies that used different criteria of infection (usually bacteriuria at levels ranging from 102 to 105 CFU/ml). They also recognized that methodologically poor studies showed a benefit and that the more rigorous studies did not. Niel-Weise et al. (283) were critical of that review, expressing the opinion that meta-analysis should be based on clinical studies of high methodological quality. They concluded from their own review of the literature that because of the fundamental problems with many of the clinical trials, there was insufficient evidence to recommend the use of the silver alloy catheters.
The subsequent literature on the silver alloy latex catheter has failed to resolve the controversy over its efficacy. For example, Rupp et al. (333) based their conclusion that the silver catheter reduces catheter-associated UTI on a study that used historical control data to assess its effect. They reported that over a 2-year period during which the silver catheter was used, the incidence of catheter-associated UTI was 2.6/1,000 catheter days, compared to the rate in the previous 2 years of 6.13/1,000 catheter days (P = 0.002). Their definition of infection included both symptomatic UTI and asymptomatic bacteriuria, with 43% of the infections recorded as being asymptomatic. Although the trial was not properly controlled, they calculated that the annual savings that might ensue from the use of the silver catheter would be from $5,811 to $535,452. Gentry and Cope (111) also used a definition of infection that included bacteriuria and symptomatic infection. They reported a CAUTI rate of 5.1% when the silver catheters were used, compared to a rate of 7.7% in a baseline period. However, analysis of their raw data suggests that the rate in the baseline period was in fact 7.3%. There are, of course, fundamental problems with this type of uncontrolled study that make it difficult to accept the conclusions that the use of the silver catheter produced a 33.5% (or even 30%) drop in infections. In both periods, there were only four cases of CAUTI recorded. No statistical analysis of the data was performed to test whether these rates were in fact significantly different.
The data available from the clinical trials on antimicrobial catheters have convinced some authors that coating with silver or impregnating with nitrofurazone has “engineered out” the risk of infection in catheterized patients (73, 223). Others are more skeptical, as a recent comprehensive review by Brosnahan et al. (39), for example, concluded that while data from clinical trials on hospitalized adults undergoing short-term catheterization suggest that the use of silver alloy catheters might reduce the risk of infection, the evidence is not strong, and the trials are generally of poor quality. Trautner et al. (412) pointed out that there is no evidence that antimicrobial catheters prevent bacteriuria in patients undergoing long-term catheterization (>28 days). They also expressed the view that clinical trials using bacteriuria (at whatever level) as the criterion for infection are fundamentally flawed. They argued that it is important to appreciate the distinction between UTI and asymptomatic bacteriuria in order to attempt a rational assessment of the evidence in the literature concerning the prevention of catheter-associated UTI. Most cases of catheter-associated bacteriuria did not progress to symptomatic UTI. It would clearly be more impressive if clinical trials showed that the various antimicrobial catheters could significantly reduce the incidence of pyelonephritis or bloodstream infections. Johnson et al. (164) concluded that while both nitrofurazone and silver alloy-coated catheters seem to reduce the development of asymptomatic bacteriuria in comparison to latex or silicone control catheters, during short-term use, there is little or no data on the effect of these devices on symptomatic UTI, morbidity, secondary bloodstream infection, or mortality rates. They expressed the view that the lack of data on these clinically more meaningful end points means that it is difficult to make definitive recommendations to decision makers. The clinical benefit and cost savings have yet to be demonstrated directly in a randomized, properly controlled trial with any of these devices in any patient population.
It is surprising, given the clinical significance of catheter encrustation and blockage, that clinical trials to test the ability of antimicrobial catheters to prevent this complication have not been reported in the literature. Laboratory studies in models of the catheterized bladder have shown that both the silver alloy-coated catheter and the nitrofurazone catheters are rapidly blocked by crystalline P. mirabilis biofilm (267, 268). It is not difficult to explain these observations, as the amounts of silver that diffuse into the urine from the catheter are not sufficient to control the rise in pH, and nitrofurazone has limited activity against P. mirabilis (162).
The application of electric current to catheters fitted with silver electrodes releases ions into urine that inhibit bacterial growth and was shown to significantly reduce the rate of P. mirabilis catheter encrustration (51). This principle may have applications to prevent encrustation during long-term catheterization (51).
Besides silver, other antiseptics have been examined for their efficacy against bacterial colonization. Catheters impregnated with synergistic combinations of chlorhexidine, silver sulfadiazine, and triclosan prevented the adherence of a broad spectrum of extraluminal bacteria on their outer surfaces compared to silver hydrogel latex- and nitrofurazone-treated silicone catheters in an in vitro urinary tract model (105).
Catheter encrustation is a problem of long-term bladder management, and to prevent its development, it is necessary that any antibacterial agent coated onto or incorporated into catheters should diffuse into the urine and prevent the rise in urinary pH and the crystallization of the calcium and magnesium phosphates. Maintaining the release of effective concentrations of antibacterials for the lifetime of long-term catheters is a challenge. Bibby et al. (28) suggested that the catheter balloon could be used as a reservoir for substantial quantities of antibacterial chemicals and that the membrane of the balloon might ensure its controlled release into the residual urine over extended periods. They found that mandelic acid diffused through the catheter balloon, achieving concentrations of around 0.1 mg/ml in urine. Unfortunately, mandelic acid is not very active against P. mirabilis or other urinary pathogens, being bactericidal in urine at concentrations of around 5 mg/ml (328). The biocide triclosan, however, is extremely active against P. mirabilis. The MIC of this agent for P. mirabilis isolates from encrusted catheters was found to be 0.2 μg/ml (173). In experiments in laboratory models supplied with artificial urine and infected with P. mirabilis, triclosan was shown to diffuse through the balloons of all-silicone catheters into the residual urine. The rise in urinary pH and crystalline biofilm formation on the catheters was inhibited. Catheters with their retention balloons inflated with water were blocked within 24 h, while catheters inflated with triclosan (10 mg/ml in 5% [wt/vol] polyethylene glycol) drained freely and showed minimal encrustation at the end of a 7-day experimental period (Fig. 7) (388). The strategy was also effective when artificial urine was replaced with pooled human urine and when latex-based catheters were tested. Triclosan was found to have impregnated the all-silicone catheters but not the latex-based devices (173). These experiments were all performed under conditions in which a catheter is introduced into residual urine that is heavily colonized by P. mirabilis (108 CFU/ml) and has a pH of above 8.0. A low flow rate (0.5 ml/min) of concentrated urine was used to simulate the low fluid intake characteristic of many elderly patients undergoing long-term catheterization. As the triclosan strategy inhibits encrustation under these severe experimental conditions, it may well extend the lifespan of catheters in patients infected with P. mirabilis.
FIG. 7.
Scanning electron micrographs showing the extent of encrustation at the eyeholes and in the central channels of control (a and b) and triclosan-treated (c and d) silicone catheters. The control catheter was removed from the P. mirabilis-infected bladder model when it was blocked at 30 h. The triclosan-treated catheter drained freely for the experimental period and was removed from the model at 7 days. (Reprinted from reference 388 with permission from Elsevier.)
Probiotics
Bacterial interference or the instillation of nonpathogenic strains into the bladder has the potential for the prevention of symptomatic infection through the hindrance of uropathogen colonization (319). In vitro (411, 414) and in vivo (70) studies have shown that nonpathogenic strains of E. coli 83972 reduce the colonization of catheters by a variety of uropathogens. In one study, 21 patients inoculated with E. coli 83972 experienced no symptoms of bacteriuria, while this same patient group experienced a mean of 3.1 symptomatic UTIs per year prior to colonization (147). On this same line of investigation, Trautner et al. (413) colonized a urinary catheter surface with a colicin-producing avirulent strain of E. coli that subsequently prevented the colonization of a uropathogenic clinical isolate of E. coli in vitro.
Future Promising Technologies
Some approaches that offer promise in the reduction of bacterial adherence to and biofilm formation in the catheter and the urinary tract include the sensing of bacterial encrustration, utilization of biofilm inhibitors on catheter surfaces, development of hydrophilic and nutrient-scavenging biomaterials, and use of low-energy surface acoustic waves. A simple sensor has been developed to detect early stages of urinary catheter encrustation and to avoid the clinical crises induced by catheter blockage. In laboratory models of colonization by P. mirabilis, the sensor signaled encrustation at an average time of 43 h before catheters were blocked with crystalline biofilm (389). A subsequent clinical study confirmed that sensors located in urine collection bags could detect infection by P. mirabilis and the early stages of catheter encrustation in patients. The mean time interval between the sensor giving the signal and the catheter being blocked was 12 days, thus allowing catheter replacement in ample time to avoid emergency referrals caused by catheter blockage (390).
Identifying the bacterial factors involved in biofilm formation on catheters and developing and/or determining inhibitors of these factors will be critical in preventing bacteriuria. One such factor is the protein GlmU (N-acetyl-d-glucosamine-1-phosphate acetyltransferase). This protein is involved in the biosynthesis of the activated nucleotide sugar UDP-GlcNAc, which is an essential precursor of peptidoglycans and LPSs in gram-positive and gram-negative bacteria, respectively (315). It also has a role in the synthesis of the β-1,6-N-acetyl-d-glucosamine polysaccharide adhesin required for biofilm formation in E. coli and Staphylococcus epidermidis (151). Therefore, inhibitors of GlmU in conjunction with protamine sulfate show promise for use in anti-infective coatings for urinary catheters (44). These compounds have antibiofilm activity against clinical isolates such as E. coli, P. aeruginosa, K. pneumoniae, S. epidermidis, and E. faecalis, and N,N′-(1,2- phenylene)dimaleimide-plus-protamine sulfate-coated silicone catheters have a reduced bacterial colonization rate for P. aeruginosa and S. epidermidis than catheters coated with silver hydrogel in vitro (44).
Signal molecules of quorum sensing that are critical for biofilm formation for their role in coordinating population behavior during host invasion and colonization are produced by uropathogens (393). Therefore, determining factors that inhibit quorum sensing may be important for the inhibition of biofilm formation and subsequent bacteriuria. Furanones, compounds isolated from a marine red macroalga, Delisea pulchra, that interfere with quorum sensing (184), are of limited use due to toxicity, and studies examining the efficacy of these compounds for clinical use were found to be variable (410). Therefore, other less toxic alternatives need to be discovered.
The development of new biomaterials for the manufacture of catheters that are not suitable for bacterial colonization and are nontoxic to patients is a logical progression in the prevention of bacteriuria during catheterization. The use of LoFric catheters (catheters with a hydrophilic coating such as polyvinyl pyrrolidone and salt) was associated with less hematuria and a significant decrease in the incidence of UTIs (424). This is believed to be due to the difference in water binding ability to reduce friction and urethral trauma (231), and, as such, these catheters were preferred by patients for intermittent self-catheterization due to higher comfort levels (78).
A promising method of bacteriuria prevention is the development of biomaterials that scavenge essential nutrients such as iron. Preliminary studies showed that catecholamine inotropes encourage biofilm formation by S. epidermidis by transferring iron to the bacteria from the host iron binding protein transferrin (232). The addition of exogenous lactoferrin to P. aeruginosa prevents biofilm formation through the stimulation of twitching motility (373). However, catheters composed of biomaterials that scavenge iron have yet to be developed for use in clinical trials (385).
Recently, Hazan et al. (136) examined the effect of low-energy surface acoustic waves on biofilm formation. It was revealed that Foley urinary catheters attached with elastic wave-generating actuators inserted into the urinary tracts of male rabbits maintained urine sterility for up to 9 days, compared to 2 days in control catheterized animals (136). Those findings were substantiated by the presence of diminished biofilm development on these catheters, as determined by scanning electron microscopy and bioburden analyses (136).
DIAGNOSIS AND TREATMENT OF CAUTIs
Even with appropriate preventive measures in place, catheterized patients, and in particular those with long-term catheterization, will experience bacteriuria due to cross-contamination. As mentioned above, most cases (90%) of bacteriuria during catheterization are asymptomatic (401). However, treatment of asymptomatic bacteriuria remains controversial among physicians, with striking a balance between the prevention of UTI-related morbidity and the emergence of antimicrobial resistance. Treatment of catheter-associated bacteriuria is not recommended, since asymptomatic bacteriuria is inevitable in catheterized patients, and in healthy individuals, these infections clear up spontaneously after catheter removal. Also, antibiotic therapy has little benefit for long-term catheterization (434). Because bacteriuria is a common occurrence, the treatment of catheterized patients may lead to the emergence and selection of antibiotic-resistant strains and increase the risk for the development of multiresistant nosocomial strains. Most physicians believe that asymptomatic bacteriuria should be not treated unless the patient is either immunosuppressed (e.g., after organ transplantation), at risk for bacterial endocarditis, about to undergo urinary tract instrumentation, or pregnant (438). However, the presence of bacteriuria serves as a bacterial reservoir for the potential progression of these organisms into symptomatic infection such as cystitis. They may also subsequently lead to significant sequelae such pyelonephritis, bacteremia, and, possibly, mortality. Therefore, this condition is a major health risk factor for those individuals who are utilizing indwelling urinary catheters.
The CDC definition of a nosocomial UTI (110) requires a symptomatic infection with the presence of fever, urgency, increased frequency of urination, dysuria, and a urine culture of >105 CFU/ml (40) with no more than two species of organisms (133). However, CAUTIs may represent a more unique diagnostic situation since the typical symptoms and signs of infection (bacteriuria [401], pyuria [69], and suprapubic pain) are unreliable due to the presence of the catheter. The presence of bacteriuria cannot be used as the sole reliable diagnostic indicator of symptomatic infection, and as mentioned above, most cases of bacteriuria are asymptomatic (401) and spontaneously resolve after catheter removal.
As with bacteriuria, pyuria (the presence of white blood cells [WBCs] in urine) is utilized as an indicator of infection. However, Tambyah and Maki determined that there is a poor correlation of pyuria with CAUTIs (402). WBC counts for catheterized patients are apparently skewed since pyuria can occur in the absence of infection due to irritation of the host uroepithelium caused by the catheter (402). Also, urease-producing uropathogens such as Proteus species often convert normally acidic urine into an alkaline state that can lyse WBCs, thereby reducing the WBC count during an infection (402). Suprapubic pain cannot be used as an accurate indicator of infection, as catheters themselves can cause this type of pain. Therefore, catheterized patients must exhibit other symptoms and signs prior to being diagnosed with and treated for a CAUTI.
We suggest that for preliminary diagnosis, once the patient exhibits the symptoms described by the CDC mentioned above, urinalysis and urine culture should be performed using a urine sample collected from a newly inserted sterile catheter (71) or suprapubic catheter. These steps reduce the incorrect identification of the causative agents responsible for the infection so that an appropriate treatment can be administered.
Urinalysis examines the physical, chemical, and microscopic properties of urine. In a standard urinalysis, the odor, color, and clarity of urine are first evaluated for the possible presence of urine with a strong odor, hematuria (red urine), pyuria (cloudy urine), or phosphate crystal deposits (cloudy urine) (372). After a quick visual examination, chemical analysis of the urine is conducted by dipstick urinalysis. These dry reagent sticks measure a variety of characteristics of urine such as specific gravity, urinary pH, and the presence of nitrites, leukocyte esterase, protein, and peroxidase. The detection of the protein leukocyte esterase in urine is indicative of the presence of neutrophils and thus pyuria (372). The detection of nitrites in urine suggests the presence of bacterial infection since many gram-negative and some gram-positive bacteria are capable of reducing nitrates to nitrite (372). However, non-nitrate-reducing organisms such as enterococci, Staphylococcus saprophyticus, and Acinetobacter spp. will appear as false negatives. Normally, urine is slightly acidic but can range from pH 4.5 to 8 (372). The presence of alkaline urine is suggestive of an infection with a urease-producing organism such as Proteus spp. The detection of peroxidase in urine can indicate hematuria, myoglobinuria, and hemoglobinuria (372).
If the dipstick analysis results suggest that an infection is present, the presence of bacteriuria can be established using several methods that determine bacterial cell count. In one method, the patient's wet-mount spun urine sample is examined microscopically for the presence of crystals, urinary casts, epithelial cells, blood cells, and bacteria. The quantitative loop method determines bacterial number by inoculating plates using calibrated platinum loops and counting colonies directly (293). Several automated methods of ascertaining bacterial counts are currently available, including staining bacterial cells with safranin (Bac-T-Screen) or acridine orange (Autotrak) and measuring the amount of bacterial ATP by bioluminescence (Monolight and LUMAC systems) (86, 375, 458).
Once these initial tests are completed, the presence of a CAUTI is confirmed by the determination of bacterial cell and blood cell counts. Two urine cultures with repeated isolation of the same uropathogen with >100 colonies (100 CFU per ml of urine) or the isolation of a single uropathogen with <105 colonies per ml for a patient being treated with antimicrobial agents is indicative of these types of infections (110). In conjunction with bacterial cell counts, a urine culture containing an abnormal number of red blood cells (hematuria, >5 red blood cells per high-power field) and leukocytes (pyuria, >10 WBCs per ml3) signifies the presence of CAUTIs (452).
Once a catheterized patient is believed to have a symptomatic UTI, the catheter is removed if possible due to the high rate of relapse (383), and empirical therapy is administered. Specific effective treatment of symptomatic UTI is a challenge due to the often polymicrobial nature of CAUTIs in patients catheterized long term (438), antibiotic resistance, and biofilm formation (62). Therefore, empirical therapy consists of broad-spectrum antibiotics and is dependent upon the condition of the patient and the site of infection as well as previous culture and drug sensitivity determinations at individual institutions (179). After the administration of empirical therapy, drug sensitivities of suspected uropathogens are determined to tailor the antimicrobial regimen to a narrow-spectrum, more potent drug (446). Most patients are administered antimicrobial therapy for at least 10 to 14 days. However, parenteral therapy is usually initiated in severely ill patients and then switched to an oral treatment after improvement (383). Parenteral antibiotic therapy is utilized during febrile infections or for those patients unable to tolerate oral medications during the course of treatment and lasts for 14 to 21 days (438). Parenteral antibiotics that are used to treat CAUTIs include certain broad-spectrum and recently developed cephalosporins (ceftriaxone), ticarcillin-clavulanate, and piperacillin-tazobactam (434). If a patient either has been on short-term catheterization, has not been critically ill, or is believed not to have a polymicrobial infection, treatment consists of either trimethoprim-sulfamethoxazole (TMP-SMX) (Bactrim, Cotrim, Septra), fluoroquinolones, or nitrofurantoin (Furadantin or Macrobid) (65). TMP-SMX, taken orally or parenterally, disrupts folate metabolism and is effective against most uropathogens other than E. coli except for Pseudomonas and Enterococcus spp. (374). Fluoroquinolones inhibit DNA gyrase (145), reduce biofilm formation (320, 463), and can reach high urinary concentrations. These drugs are effective in treating Pseudomonas, Proteus, and other resistant gram-negative organisms (143, 374). The UTI-specific antibiotic nitrofurantoin is effective against most uropathogens except Pseudomonas and Proteus spp. (149).
Unlike the drug therapies administered for patients catheterized short term, treatment for those individuals who are undergoing long-term catheterization differs, as these infections are more likely to be polymicrobial in nature. Those individuals undergoing long-term catheterization or suffering from polymicrobial infections and who are noncritical are treated with TMP-SMX or an expanded-spectrum cephalosporin such as cefuroxime (Ceftin) (65, 383). Catheterized patients that are seriously ill require a two-drug regimen that includes ampicillin with either the monobactam aztreonam (Azactam), the expanded-spectrum cephalosporin cefprozil (Cefzil), the broad-spectrum cephalosporin ceftriaxone (Rocephin), gentamicin or another aminoglycoside or ciprofloxacin (Cipro), or other fluoroquinolones (65, 464). Once antibiotic therapy has been administered, the resolution of symptoms and not the absence of bacteriuria indicates that the infection has been cleared.
If a patient does not respond to treatment and/or is suspected of having complicated or recurrent pyelonephritis or a blockage due to urinary stone formation, the patient will undergo diagnostic imaging. During an emergency, an ultrasound can be used for patients with loin pain and fever (100) for a risk-free assessment of the presence of abscesses, stones, and urine flow blockage. Nuclear scans, employing injections of small amounts of radioactive tracers, are used to detect kidney scarring, including dimerceptosuccinic acid scintigraphy. To rule out kidney stones or obstructions, magnetic resonance imaging and computed tomography are performed. X rays, in conjunction with contrast agents, can be used to examine different parts of the urinary tract to determine the existence of structural abnormalities, urethral narrowing, and incomplete emptying. Two examples are an intravenous pyelogram (an X ray for kidney obstructions or abnormalities) and a voiding cystourethrogram (an X ray of the bladder and urethra).
Lastly, cystoscopy can be used to detect structural abnormalities and interstitial cystitis in those individuals with recurrent UTI. A study using cystoscopy has indicated that 38 of 61 (62%) patients suffering from recurrent encrustation and blockage of their catheters had bladder stones (338). It was revealed that pairs of P. mirabilis isolates from the stones and catheter biofilms of six of these patients were identical, as determined by restriction enzyme digest profiles of bacterial DNA (338). This finding indicates that bladder stones harbor strains of P. mirabilis that can rapidly recolonize replacement catheters with crystalline biofilm. It is suggested that the detection and removal of these stones for those patients diagnosed with CAUTIs could be accomplished using flexible cystoscopy to assist in resolving the problem of chronic catheter encrustation and blockage.
The formation of crystalline urinary stones is a serious complication associated with CAUTIs, and as such, the removal of these stones is critical for full recovery from catheter-associated infection, since any remaining stone fragments can serve as a nidus for infections and the formation of new stones (253). Multimodality therapy is the key to a more successful removal of struvite stones, and the combination of open surgery and medical therapy is synergistic (436). Open surgery, while recommended for the complete removal of urinary stones, does not ensure complete removal, as the incidence of residual stone fragments is 12 to 36% (226). However, surgical removal of residual stones has been demonstrated to be important, as 41% of patients with untreated unilateral struvite stones have a 5-year survival rate (314).
The minimally invasive percutaneous nephrolithotomy, with or without extracorporeal shockwave lithotripsy (ESWL), is the preferred nonsurgical alternative (254, 436), with stone-free rates that range from 23 to 86% (207). Percutaneous nephrolithotomy, performed under general anesthesia, involves an incision into the back and the insertion of an endoscope to remove stones from the kidney. Monotherapy with ESWL should be used for those patients with smaller staghorn stones (<500-mm2 surface area) (357). ESWL effectively fragments urinary crystals through the utilization of sound waves to the allow passage of these fragments out of the urinary tract. After either procedure, the insertion of an indwelling ureteral stent may be required to allow the proper drainage of urine. Medical therapy, such as culture-specific antibiotics, urease inhibitors, and urine acidification, complements these above-described treatments to reduce the morbidity associated with persistent UTIs and stone recurrence (436). Acetohydroxamic acid (Lithostat) is the most commonly used urease inhibitor. Chemolysis by urinary acidification is possible by the administration of oral ammonium chloride, glucono-delta-lactone, and magnesium salt carbonate or a citric acid-based solution such as hemiacidrin or Suby solution G (397). However, there are conflicting reports about the efficacy of citric acid for use in chemolysis (138, 247). Once administration of the appropriate treatment is accomplished, therapeutic success for urinary stones is measured by the alleviation of patient symptoms and stone-associated morbidity, such as infection.
PROGNOSIS OF CAUTIs
The prognosis of catheter-associated infection is complicated due to the occurrence of chronic or recurrent UTIs, complicated UTI, and pyelonephritis. If left untreated, these infections can lead to abscess formation, renal obstruction, and scarring and eventually will lead to bacteremia, sepsis, and, possibly, death. These infections are difficult to treat due to the presence of biofilms and crystals that protect uropathogens from proper treatment. Only after the complete eradication of biofilm and crystals in the urinary tract can a catheter-associated infection be eliminated.
To decrease morbidity and mortality associated with infection of urinary catheters, new methods of prevention and treatment need to be continually developed. The development of affordable catheters constructed with anti-infective surfaces may be key to the success in preventing infection (236). The identification and development of agents that prevent attachment and subsequent biofilm formation are also feasible as preventative measures. Inhibitors of other known bacterial virulence factors such as siderophores and the continued identification of new virulence factors associated with CAUTIs will aid in understanding the pathogenicity as well as identifying new targets for the development of potential preventive and/or treatment options for these infections. The development of catheters coated with antiseptics and new catheter surface technologies that can release greater quantities of ionic silver or other anti-infective agents into the aqueous environment may assist in the prevention of CAUTIs caused by intraluminal organisms (236). Alternative catheterization methods such as conformable catheters with a collapsible intraurethral segment can reduce the amount of urethral tissue trauma. Such catheters have been developed but have not been clinically evaluated in randomized controlled trials and are not commercially available (236). Other preventable measures include the development of vaccines against uropathogens (236), including potential vaccines (198, 418) or vaccine candidates for E. coli (181, 209, 210, 336, 352) and P. mirabilis (218, 220, 300). The continuing development of technological advancements of minimally invasive instruments, novel urinary acidification agents, less toxic urease inhibitors, and chemicals to enhance the protective glycosaminoglycan layer can lessen the morbidity associated with catheter-associated stone formation. Advancements might also provide potential alternative treatment options for the clearance of urinary stones (317). As the number of individuals at risk for catheter-associated infections continues to rise due to advances in medicine, continued research efforts for better prevention and treatment options to reduce morbidity and mortality associated with these infections will be critical to keep CAUTIs at a minimum.
Some 20 years ago, in an important editorial, Calvin Kunin (204) posed the question, “Can we build a better catheter?” He commented that at a time when impressive technological advances have taken place in many areas of medical care, it is difficult to understand why we are still not able to solve the apparently simple problem of draining urine from a disabled bladder without causing infection and all the associated complications. Building better catheters should certainly be possible, as there is plenty of scope for improving their design, and it is disappointing that little has changed in the meantime. There have been some advances in our understanding of the pathogenesis of CAUTI, including a better appreciation of the central role of bacterial biofilms in these infections. The current level of morbidity associated with the use of catheters is unacceptable, as it undermines the quality of life for so many elderly and disabled people. It is clear that if we are to design effective strategies to avoid these problems, there is a pressing need for more information about the factors that initiate and control the development of catheter biofilms by urinary tract pathogens such as E. coli and P. mirabilis.
REFERENCES
- 1.Abraham, S., J. Shin, and R. Malaviya. 2001. Type 1 fimbriated Escherichia coli-mast cell interactions in cystitis. J. Infect. Dis. 183(Suppl. 1):S51-S55. [DOI] [PubMed] [Google Scholar]
- 2.Allison, C., L. Emody, N. Coleman, and C. Hughes. 1994. The role of swarm cell differentiation and multicellular migration in the uropathogenicity of Proteus mirabilis. J. Infect. Dis. 169:1155-1158. [DOI] [PubMed] [Google Scholar]
- 3.Allison, C., H. C. Lai, D. Gygi, and C. Hughes. 1993. Cell differentiation of Proteus mirabilis is initiated by glutamine, a specific chemoattractant for swarming cells. Mol. Microbiol. 8:53-60. [DOI] [PubMed] [Google Scholar]
- 4.Allison, C., H. C. Lai, and C. Hughes. 1992. Co-ordinate expression of virulence genes during swarm-cell differentiation and population migration of Proteus mirabilis. Mol. Microbiol. 6:1583-1591. [DOI] [PubMed] [Google Scholar]
- 5.Altman, E., B. A. Harrison, R. K. Latta, K. K. Lee, J. F. Kelly, and P. Thibault. 2001. Galectin-3-mediated adherence of Proteus mirabilis to Madin-Darby canine kidney cells. Biochem. Cell Biol. 79:783-788. [PubMed] [Google Scholar]
- 6.American Federation of Aging Research and the Alliance for Aging Research. 1995. Putting aging on hold: delaying the disease of old age. Official report to the White House Conference on Aging. American Federation of Aging Research and the Alliance for Aging Research, Washington, DC.
- 7.Anderson, G. G., K. W. Dodson, T. M. Hooton, and S. J. Hultgren. 2004. Intracellular bacterial communities of uropathogenic Escherichia coli in urinary tract pathogenesis. Trends Microbiol. 12:424-430. [DOI] [PubMed] [Google Scholar]
- 8.Anderson, G. G., J. J. Palermo, J. D. Schilling, R. Roth, J. Heuser, and S. J. Hultgren. 2003. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301:105-107. [DOI] [PubMed] [Google Scholar]
- 9.Bagg, A., and J. B. Neilands. 1987. Molecular mechanism of regulation of siderophore-mediated iron assimilation. Microbiol. Rev. 51:509-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bahrani, F. K., S. Cook, R. A. Hull, G. Massad, and H. L. Mobley. 1993. Proteus mirabilis fimbriae: N-terminal amino acid sequence of a major fimbrial subunit and nucleotide sequences of the genes from two strains. Infect. Immun. 61:884-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bahrani, F. K., D. E. Johnson, D. Robbins, and H. L. Mobley. 1991. Proteus mirabilis flagella and MR/P fimbriae: isolation, purification, N-terminal analysis, and serum antibody response following experimental urinary tract infection. Infect. Immun. 59:3574-3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bahrani, F. K., G. Massad, C. V. Lockatell, D. E. Johnson, R. G. Russell, J. W. Warren, and H. L. Mobley. 1994. Construction of an MR/P fimbrial mutant of Proteus mirabilis: role in virulence in a mouse model of ascending urinary tract infection. Infect. Immun. 62:3363-3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bahrani, F. K., and H. L. Mobley. 1993. Proteus mirabilis MR/P fimbriae: molecular cloning, expression, and nucleotide sequence of the major fimbrial subunit gene. J. Bacteriol. 175:457-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bahrani, F. K., and H. L. Mobley. 1994. Proteus mirabilis MR/P fimbrial operon: genetic organization, nucleotide sequence, and conditions for expression. J. Bacteriol. 176:3412-3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bahrani-Mougeot, F. K., E. L. Buckles, C. V. Lockatell, J. R. Hebel, D. E. Johnson, C. M. Tang, and M. S. Donnenberg. 2002. Type 1 fimbriae and extracellular polysaccharides are preeminent uropathogenic Escherichia coli virulence determinants in the murine urinary tract. Mol. Microbiol. 45:1079-1093. [DOI] [PubMed] [Google Scholar]
- 16.Bauer, R. J., L. Zhang, B. Foxman, A. Siitonen, M. E. Jantunen, H. Saxen, and C. F. Marrs. 2002. Molecular epidemiology of 3 putative virulence genes for Escherichia coli urinary tract infection—usp, iha, and iroN(E. coli). J. Infect. Dis. 185:1521-1524. [DOI] [PubMed] [Google Scholar]
- 17.Beizer, J. L. 1996. Urinary incontinence in women: a review for the pharmacist. J. Am. Pharm. Assoc. 36:196-202. [DOI] [PubMed] [Google Scholar]
- 18.Belas, R. 1992. The swarming phenomenon of Proteus mirabilis. ASM News 58:15-22. [Google Scholar]
- 19.Belas, R. 1994. Expression of multiple flagellin-encoding genes of Proteus mirabilis. J. Bacteriol. 176:7169-7181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belas, R., D. Erskine, and D. Flaherty. 1991. Transposon mutagenesis in Proteus mirabilis. J. Bacteriol. 173:6289-6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belas, R., D. Erskine, and D. Flaherty. 1991. Proteus mirabilis mutants defective in swarmer cell differentiation and multicellular behavior. J. Bacteriol. 173:6279-6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belas, R., and D. Flaherty. 1994. Sequence and genetic analysis of multiple flagellin-encoding genes from Proteus mirabilis. Gene 148:33-41. [DOI] [PubMed] [Google Scholar]
- 23.Belas, R., M. Goldman, and K. Ashliman. 1995. Genetic analysis of Proteus mirabilis mutants defective in swarmer cell elongation. J. Bacteriol. 177:823-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belas, R., J. Manos, and R. Suvanasuthi. 2004. Proteus mirabilis ZapA metalloprotease degrades a broad spectrum of substrates, including antimicrobial peptides. Infect. Immun. 72:5159-5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belas, R., and R. Suvanasuthi. 2005. The ability of Proteus mirabilis to sense surfaces and regulate virulence gene expression involves FliL, a flagellar basal body protein. J. Bacteriol. 187:6789-6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benton, J., J. Chawla, S. Parry, and D. Stickler. 1992. Virulence factors in Escherichia coli from urinary tract infections in patients with spinal injuries. J. Hosp. Infect. 22:117-127. [DOI] [PubMed] [Google Scholar]
- 27.Beynon, L. M., A. J. Dumanski, R. J. McLean, L. L. MacLean, J. C. Richards, and M. B. Perry. 1992. Capsule structure of Proteus mirabilis (ATCC 49565). J. Bacteriol. 174:2172-2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bibby, J. M., A. J. Cox, and D. W. L. Hukins. 1995. Feasibility of preventing encrustation on urinary catheters. Cells Mater. 2:183-195. [Google Scholar]
- 29.Bijlsma, I. G., L. van Dijk, J. G. Kusters, and W. Gaastra. 1995. Nucleotide sequences of two fimbrial major subunit genes, pmpA and ucaA, from canine-uropathogenic Proteus mirabilis strains. Microbiology 141:1349-1357. [DOI] [PubMed] [Google Scholar]
- 30.Blomgran, R., L. Zheng, and O. Stendahl. 2004. Uropathogenic Escherichia coli triggers oxygen-dependent apoptosis in human neutrophils through the cooperative effect of type 1 fimbriae and lipopolysaccharide. Infect. Immun. 72:4570-4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bohling, A., S. O. Hagge, S. Roes, R. Podschun, H. Sahly, J. Harder, J. M. Schroder, J. Grotzinger, U. Seydel, and T. Gutsmann. 2006. Lipid-specific membrane activity of human beta-defensin-3. Biochemistry 45:5663-5670. [DOI] [PubMed] [Google Scholar]
- 32.Bower, J. M., D. S. Eto, and M. A. Mulvey. 2005. Covert operations of uropathogenic Escherichia coli within the urinary tract. Traffic 6:18-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bradley, D. E. 1980. A function of Pseudomonas aeruginosa PAO polar pili: twitching motility. Can. J. Microbiol. 26:146-154. [DOI] [PubMed] [Google Scholar]
- 34.Braun, V., and T. Focareta. 1991. Pore-forming bacterial protein hemolysins (cytolysins). Crit. Rev. Microbiol. 18:115-158. [DOI] [PubMed] [Google Scholar]
- 35.Breitenbach, J. M., and R. P. Hausinger. 1988. Proteus mirabilis urease. Partial purification and inhibition by boric acid and boronic acids. Biochem. J. 250:917-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brinton, C. C., Jr. 1965. The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans. N. Y. Acad. Sci. 27:1003-1054. [DOI] [PubMed] [Google Scholar]
- 37.Britt, M. R., J. P. Burke, W. A. Miller, P. Steinmiller, and R. A. Garibaldi. 1976. The non-effectiveness of daily meatal care in the prevention of catheter-associated bacteriuria, p. 141. In Proceedings of the 16th Interscience Conference on Antimicrobial Agents and Chemotherapy. American Society for Microbiology, Washington, DC.
- 38.Britt, M. R., R. A. Garibaldi, W. A. Miller, R. M. Hebertson, and J. P. Burke. 1977. Antimicrobial prophylaxis for catheter-associated bacteriuria. Antimicrob. Agents Chemother. 11:240-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brosnahan, J., A. Jull, and C. Tracy. 2004. Types of urethral catheters for management of short-term voiding problems in hospitalised adults. Cochrane Database Syst. Rev. 2004:CD004013. [DOI] [PubMed] [Google Scholar]
- 40.Bryan, C. S., and K. L. Reynolds. 1984. Hospital-acquired bacteremic urinary tract infection: epidemiology and outcome. J. Urol. 132:494-498. [DOI] [PubMed] [Google Scholar]
- 41.Burall, L. S., J. M. Harro, X. Li, C. V. Lockatell, S. D. Himpsl, J. R. Hebel, D. E. Johnson, and H. L. Mobley. 2004. Proteus mirabilis genes that contribute to pathogenesis of urinary tract infection: identification of 25 signature-tagged mutants attenuated at least 100-fold. Infect. Immun. 72:2922-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buren, J. J. 1973. The significance of iron in infection. Rev. Infect. Dis. 3:1127-1138. [DOI] [PubMed] [Google Scholar]
- 43.Burke, J. P., R. A. Garibaldi, M. R. Britt, J. A. Jacobson, M. Conti, and D. W. Alling. 1981. Prevention of catheter-associated urinary tract infections. Efficacy of daily meatal care regimens. Am. J. Med. 70:655-658. [DOI] [PubMed] [Google Scholar]
- 44.Burton, E., P. V. Gawande, N. Yakandawala, K. LoVetri, G. G. Zhanel, T. Romeo, A. D. Friesen, and S. Madhyastha. 2006. Antibiofilm activity of GlmU enzyme inhibitors against catheter-associated uropathogens. Antimicrob. Agents Chemother. 50:1835-1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Byers, B. 1987. Pathogenic iron acquisition. Life Chem. Rep. 4:143-159. [Google Scholar]
- 46.Campos, M. A., M. A. Vargas, V. Regueiro, C. M. Llompart, S. Alberti, and J. A. Bengoechea. 2004. Capsule polysaccharide mediates bacterial resistance to antimicrobial peptides. Infect. Immun. 72:7107-7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caprioli, A., V. Falbo, F. M. Ruggeri, L. Baldassarri, R. Bisicchia, G. Ippolito, E. Romoli, and G. Donelli. 1987. Cytotoxic necrotizing factor production by hemolytic strains of Escherichia coli causing extraintestinal infections. J. Clin. Microbiol. 25:146-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cedzynski, M., Y. A. Knirel, A. Rozalski, A. S. Shashkov, E. V. Vinogradov, and W. Kaca. 1995. The structure and serological specificity of Proteus mirabilis O43 O antigen. Eur. J. Biochem. 232:558-562. [DOI] [PubMed] [Google Scholar]
- 49.Cedzynski, M., A. Rozalski, K. Kotelko, W. Kaca, E. V. Vinogradov, Y. A. Knirel, and N. K. Kochetkov. 1993. Structural and immunologic studies of Proteus mirabilis 033 O-specific polysaccharide. Med. Dosw. Mikrobiol. 45:93-97. (In Polish.) [PubMed] [Google Scholar]
- 50.Centers for Disease Control and Prevention. 1992. Public health focus: surveillance, prevention, and control of nosocomial infections. Morb. Mortal. Wkly. Rep. 41:783-787. [PubMed] [Google Scholar]
- 51.Chakravarti, A., S. Gangodawila, M. J. Long, N. S. Morris, A. R. Blacklock, and D. J. Stickler. 2005. An electrified catheter to resist encrustation by Proteus mirabilis biofilm. J. Urol. 174:1129-1132. [DOI] [PubMed] [Google Scholar]
- 52.Chippendale, G. R., J. W. Warren, A. L. Trifillis, and H. L. Mobley. 1994. Internalization of Proteus mirabilis by human renal epithelial cells. Infect. Immun. 62:3115-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chow, A. W., P. R. Taylor, T. T. Yoshikawa, and L. B. Guze. 1979. A nosocomial outbreak of infections due to multiply resistant Proteus mirabilis: role of intestinal colonization as a major reservoir. J. Infect. Dis. 139:621-627. [DOI] [PubMed] [Google Scholar]
- 54.Chung, J. W., S. J. Hong, K. J. Kim, D. Goti, M. F. Stins, S. Shin, V. L. Dawson, T. M. Dawson, and K. S. Kim. 2003. 37-kDa laminin receptor precursor modulates cytotoxic necrotizing factor 1-mediated RhoA activation and bacterial uptake. J. Biol. Chem. 278:16857-16862. [DOI] [PubMed] [Google Scholar]
- 55.Clapham, L. R., J. C. McLean, J. C. Nickel, J. Downey, and J. W. Costerton. 1990. The influence of bacteria on struvite crystal habit and its importance in urinary stone formation. J. Crystal Growth 104:475-484. [Google Scholar]
- 56.Clegg, S., and G. F. Gerlach. 1987. Enterobacterial fimbriae. J. Bacteriol. 169:934-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coker, C., O. O. Bakare, and H. L. Mobley. 2000. H-NS is a repressor of the Proteus mirabilis urease transcriptional activator gene ureR. J. Bacteriol. 182:2649-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Coker, C., C. A. Poore, X. Li, and H. L. Mobley. 2000. Pathogenesis of Proteus mirabilis urinary tract infection. Microbes Infect. 2:1497-1505. [DOI] [PubMed] [Google Scholar]
- 59.Connell, I., W. Agace, P. Klemm, M. Schembri, S. Marild, and C. Svanborg. 1996. Type 1 fimbrial expression enhances Escherichia coli virulence for the urinary tract. Proc. Natl. Acad. Sci. USA 93:9827-9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cook, S. W., N. Mody, J. Valle, and R. Hull. 1995. Molecular cloning of Proteus mirabilis uroepithelial cell adherence (uca) genes. Infect. Immun. 63:2082-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Corpe, W. 1980. Microbial surface components involved in adsorption of microorganisms onto surfaces, p. 105-144. In G. Bitton and K. C. Marshall (ed.), Adsorption of microorganisms to surfaces. John Wiley & Sons, New York, NY.
- 62.Costerton, J. W. 1999. Introduction to biofilm. Int. J. Antimicrob. Agents 11:217-221. [DOI] [PubMed] [Google Scholar]
- 63.Costerton, J. W., Z. Lewandowski, D. E. Caldwell, D. R. Korber, and H. M. Lappin-Scott. 1995. Microbial biofilms. Annu. Rev. Microbiol. 49:711-745. [DOI] [PubMed] [Google Scholar]
- 64.Cox, A. J. 1990. Comparison of catheter surface morphologies. Br. J. Urol. 65:55-60. [DOI] [PubMed] [Google Scholar]
- 65.Cravens, D. D., and S. Zweig. 2000. Urinary catheter management. Am. Fam. Physician 61:369-376. [PubMed] [Google Scholar]
- 66.Daifuku, R., and W. E. Stamm. 1986. Bacterial adherence to bladder uroepithelial cells in catheter-associated urinary tract infection. N. Engl. J. Med. 314:1208-1213. [DOI] [PubMed] [Google Scholar]
- 67.Danese, P. N., L. A. Pratt, and R. Kolter. 2000. Exopolysaccharide production is required for development of Escherichia coli K-12 biofilm architecture. J. Bacteriol. 182:3593-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Danese, P. N., L. A. Pratt, and R. Kolter. 2001. Biofilm formation as a developmental process. Methods Enzymol. 336:19-26. [DOI] [PubMed] [Google Scholar]
- 69.Darouiche, R. O., R. M. Cadle, G. J. Zenon III, J. Markowski, M. Rodriguez, and D. M. Musher. 1993. Progression from asymptomatic to symptomatic urinary tract infection in patients with SCI: a preliminary study. J. Am. Paraplegia Soc. 16:219-224. [DOI] [PubMed] [Google Scholar]
- 70.Darouiche, R. O., W. H. Donovan, T. M. Del, J. I. Thornby, D. C. Rudy, and R. A. Hull. 2001. Pilot trial of bacterial interference for preventing urinary tract infection. Urology 58:339-344. [DOI] [PubMed] [Google Scholar]
- 71.Darouiche, R. O., M. Priebe, and J. E. Clarridge. 1997. Limited vs full microbiological investigation for the management of symptomatic polymicrobial urinary tract infection in adult spinal cord-injured patients. Spinal Cord 35:534-539. [DOI] [PubMed] [Google Scholar]
- 72.Darouiche, R. O., J. A. Smith, Jr., H. Hanna, C. B. Dhabuwala, M. S. Steiner, R. J. Babaian, T. B. Boone, P. T. Scardino, J. I. Thornby, and I. I. Raad. 1999. Efficacy of antimicrobial-impregnated bladder catheters in reducing catheter-associated bacteriuria: a prospective, randomized, multicenter clinical trial. Urology 54:976-981. [DOI] [PubMed] [Google Scholar]
- 73.Davenport, K., and F. X. Keeley. 2005. Evidence for the use of silver-alloy-coated urethral catheters. J. Hosp. Infect. 60:298-303. [DOI] [PubMed] [Google Scholar]
- 74.Davey, M. E., and G. A. O'Toole. 2000. Microbial biofilms: from ecology to molecular genetics. Microbiol. Mol. Biol. Rev. 64:847-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Davis, J. M., S. B. Rasmussen, and A. D. O'Brien. 2005. Cytotoxic necrotizing factor type 1 production by uropathogenic Escherichia coli modulates polymorphonuclear leukocyte function. Infect. Immun. 73:5301-5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Deighton, C. M., J. W. Gray, A. J. Bint, and D. J. Walker. 1992. Anti-Proteus antibodies in rheumatoid arthritis same-sexed sibships. Br. J. Rheumatol. 31:241-245. [DOI] [PubMed] [Google Scholar]
- 77.Demir, M., and I. Kaleli. 2004. Production by Escherichia coli isolates of siderophore and other virulence factors and their pathogenic role in a cutaneous infection model. Clin. Microbiol. Infect. 10:1011-1014. [DOI] [PubMed] [Google Scholar]
- 78.Diokno, A. C., B. A. Mitchell, A. J. Nash, and J. A. Kimbrough. 1995. Patient satisfaction and the LoFric catheter for clean intermittent catheterization. J. Urol. 153:349-351. [DOI] [PubMed] [Google Scholar]
- 79.Dodson, K. W., J. S. Pinkner, T. Rose, G. Magnusson, S. J. Hultgren, and G. Waksman. 2001. Structural basis of the interaction of the pyelonephritic E. coli adhesin to its human kidney receptor. Cell 105:733-743. [DOI] [PubMed] [Google Scholar]
- 80.Donlan, R. M. 2001. Biofilms and device-associated infections. Emerg. Infect. Dis. 7:277-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Donlan, R. M., and J. W. Costerton. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15:167-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Donnenberg, M. S., B. Newman, S. J. Utsalo, A. L. Trifillis, J. R. Hebel, and J. W. Warren. 1994. Internalization of Escherichia coli into human kidney epithelial cells: comparison of fecal and pyelonephritis-associated strains. J. Infect. Dis. 169:831-838. [DOI] [PubMed] [Google Scholar]
- 83.Donnenberg, M. S., and R. A. Welch. 1996. Virulence determinants of uropathogenic Escherichia coli, p. 135-174. In H. L. T. Mobley and J. W. Warren (ed.), Urinary tract infections: molecular pathogenesis and clinical management. ASM Press, Washington, DC.
- 84.D'Orazio, S. E., V. Thomas, and C. M. Collins. 1996. Activation of transcription at divergent urea-dependent promoters by the urease gene regulator UreR. Mol. Microbiol. 21:643-655. [DOI] [PubMed] [Google Scholar]
- 85.Drechsel, H., A. Thieken, R. Reissbrodt, G. Jung, and G. Winkelmann. 1993. α-Keto acids are novel siderophores in the genera Proteus, Providencia, and Morganella and are produced by amino acid deaminases. J. Bacteriol. 175:2727-2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Drow, D. L., C. H. Baum, and G. Hirschfield. 1984. Comparison of the Lumac and Monolight systems for detection of bacteriuria by bioluminescence. J. Clin. Microbiol. 20:797-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dumanski, A. J., H. Hedelin, A. Edin-Liljegren, D. Beauchemin, and R. J. McLean. 1994. Unique ability of the Proteus mirabilis capsule to enhance mineral growth in infectious urinary calculi. Infect. Immun. 62:2998-3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dunne, W. M., Jr. 2002. Bacterial adhesion: seen any good biofilms lately? Clin. Microbiol. Rev. 15:155-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Duong, F., A. Lazdunski, B. Cami, and M. Murgier. 1992. Sequence of a cluster of genes controlling synthesis and secretion of alkaline protease in Pseudomonas aeruginosa: relationships to other secretory pathways. Gene 121:47-54. [DOI] [PubMed] [Google Scholar]
- 90.Ebringer, A., S. Khalafpour, and C. Wilson. 1989. Rheumatoid arthritis and Proteus: a possible aetiological association. Rheumatol. Int. 9:223-228. [DOI] [PubMed] [Google Scholar]
- 91.Evans, E. 1999. Indwelling catheter care: dispelling the misconceptions. Geriatr. Nurs. 20:85-88. [DOI] [PubMed] [Google Scholar]
- 92.Evanylo, L. P., S. Kadis, and J. R. Maudsley. 1984. Siderophore production by Proteus mirabilis. Can. J. Microbiol. 30:1046-1051. [DOI] [PubMed] [Google Scholar]
- 93.Falkiner, F. R. 1993. The insertion and management of indwelling urethral catheters—minimizing the risk of infection. J. Hosp. Infect. 25:79-90. [DOI] [PubMed] [Google Scholar]
- 94.Falzano, L., P. Filippini, S. Travaglione, A. G. Miraglia, A. Fabbri, and C. Fiorentini. 2006. Escherichia coli cytotoxic necrotizing factor 1 blocks cell cycle G2/M transition in uroepithelial cells. Infect. Immun. 74:3765-3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Farnaud, S., C. Spiller, L. C. Moriarty, A. Patel, V. Gant, E. W. Odell, and R. W. Evans. 2004. Interactions of lactoferricin-derived peptides with LPS and antimicrobial activity. FEMS Microbiol. Lett. 233:193-199. [DOI] [PubMed] [Google Scholar]
- 96.Fernández, L. A., and J. Berenguer. 2000. Secretion and assembly of regular surface structures in gram-negative bacteria. FEMS Microbiol. Rev. 24: 21-44. [DOI] [PubMed] [Google Scholar]
- 97.Ferrières, L., V. Hancock, and P. Klemm. 2007. Specific selection for virulent urinary tract infectious Escherichia coli strains during catheter-associated biofilm formation. FEMS Immunol. Med. Microbiol. 51:212-219. [DOI] [PubMed] [Google Scholar]
- 98.Foxman, B. 2002. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am. J. Med. 113(Suppl. 1A):5S-13S. [DOI] [PubMed] [Google Scholar]
- 99.Foxman, B., L. Zhang, P. Tallman, K. Palin, C. Rode, C. Bloch, B. Gillespie, and C. F. Marrs. 1995. Virulence characteristics of Escherichia coli causing first urinary tract infection predict risk of second infection. J. Infect. Dis. 172:1536-1541. [DOI] [PubMed] [Google Scholar]
- 100.Franz, M., and W. H. Horl. 1999. Common errors in diagnosis and management of urinary tract infection. II. Clinical management. Nephrol. Dial. Transplant. 14:2754-2762. [DOI] [PubMed] [Google Scholar]
- 101.Fraser, G. M., L. Claret, R. Furness, S. Gupta, and C. Hughes. 2002. Swarming-coupled expression of the Proteus mirabilis hpmBA haemolysin operon. Microbiology 148:2191-2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fudala, R., A. N. Kondakova, K. Bednarska, S. N. Senchenkova, A. S. Shashkov, Y. A. Knirel, U. Zahringer, and W. Kaca. 2003. Structure and serological characterization of the O-antigen of Proteus mirabilis O18 with a phosphocholine-containing oligosaccharide phosphate repeating unit. Carbohydr. Res. 338:1835-1842. [DOI] [PubMed] [Google Scholar]
- 103.Fukushi, Y., S. Orikasa, and M. Kagayama. 1979. An electron microscopic study of the interaction between vesical epitherlium and E. coli. Investig. Urol. 17:61-68. [PubMed] [Google Scholar]
- 104.Gabriel, M. M., A. D. Sawant, R. B. Simmons, and D. G. Ahearn. 1995. Effects of silver on adherence of bacteria to urinary catheters: in vitro studies. Curr. Microbiol. 30:17-22. [DOI] [PubMed] [Google Scholar]
- 105.Gaonkar, T. A., L. A. Sampath, and S. M. Modak. 2003. Evaluation of the antimicrobial efficacy of urinary catheters impregnated with antiseptics in an in vitro urinary tract model. Infect. Control Hosp. Epidemiol. 24:506-513. [DOI] [PubMed] [Google Scholar]
- 106.Garcia, M. I., M. Jouve, J. P. Nataro, P. Gounon, and C. Le Bouguenec. 2000. Characterization of the AfaD-like family of invasins encoded by pathogenic Escherichia coli associated with intestinal and extra-intestinal infections. FEBS Lett. 479:111-117. [DOI] [PubMed] [Google Scholar]
- 107.Garibaldi, R. A., M. R. Britt, W. A. Miller, P. Steinmuller, and J. P. Burke. 1976. Evaluation of periurethral colonization as a risk factor for catheter-associated bacteriuria, p. 142. In Proceedings of the 16th Interscience Conference on Antimicrobial Agents and Chemotherapy. American Society for Microbiology, Washington, DC.
- 108.Garibaldi, R. A., J. P. Burke, M. R. Britt, M. A. Miller, and C. B. Smith. 1980. Meatal colonization and catheter-associated bacteriuria. N. Engl. J. Med. 303:316-318. [DOI] [PubMed] [Google Scholar]
- 109.Garibaldi, R. A., J. P. Burke, M. L. Dickman, and C. B. Smith. 1974. Factors predisposing to bacteriuria during indwelling urethral catheterization. N. Engl. J. Med. 291:215-219. [DOI] [PubMed] [Google Scholar]
- 110.Garner, J. S., W. R. Jarvis, T. G. Emori, T. C. Horan, and J. M. Hughes. 1988. CDC definitions for nosocomial infections, 1988. Am. J. Infect. Control 16:128-140. [DOI] [PubMed] [Google Scholar]
- 111.Gentry, H., and S. Cope. 2005. Using silver to reduce catheter-associated urinary tract infections. Nurs. Stand. 19:51-54. [DOI] [PubMed] [Google Scholar]
- 112.Ghigo, J. M., and C. Wandersman. 1992. Cloning, nucleotide sequence and characterization of the gene encoding the Erwinia chrysanthemi B374 PrtA metalloprotease: a third metalloprotease secreted via a C-terminal secretion signal. Mol. Gen. Genet. 236:135-144. [DOI] [PubMed] [Google Scholar]
- 113.Goetz, G. S., A. Mahmood, S. J. Hultgren, M. J. Engle, K. Dodson, and D. H. Alpers. 1999. Binding of pili from uropathogenic Escherichia coli to membranes secreted by human colonocytes and enterocytes. Infect. Immun. 67:6161-6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Goluszko, P., S. L. Moseley, L. D. Truong, A. Kaul, J. R. Williford, R. Selvarangan, S. Nowicki, and B. Nowicki. 1997. Development of experimental model of chronic pyelonephritis with Escherichia coli O75:K5:H-bearing Dr fimbriae: mutation in the dra region prevented tubulointerstitial nephritis. J. Clin. Investig. 99:1662-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Goluszko, P., D. Niesel, B. Nowicki, R. Selvarangan, S. Nowicki, A. Hart, E. Pawelczyk, M. Das, P. Urvil, and R. Hasan. 2001. Dr operon-associated invasiveness of Escherichia coli from pregnant patients with pyelonephritis. Infect. Immun. 69:4678-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Goluszko, P., V. Popov, R. Selvarangan, S. Nowicki, T. Pham, and B. J. Nowicki. 1997. Dr fimbriae operon of uropathogenic Escherichia coli mediate microtubule-dependent invasion to the HeLa epithelial cell line. J. Infect. Dis. 176:158-167. [DOI] [PubMed] [Google Scholar]
- 117.Goluszko, P., R. Selvarangan, V. Popov, T. Pham, J. W. Wen, and J. Singhal. 1999. Decay-accelerating factor and cytoskeleton redistribution pattern in HeLa cells infected with recombinant Escherichia coli strains expressing Dr family of adhesins. Infect. Immun. 67:3989-3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Griffith, D. P., D. M. Musher, and C. Itin. 1976. Urease. The primary cause of infection-induced urinary stones. Investig. Urol. 13:346-350. [PubMed] [Google Scholar]
- 119.Griffith, D. P., and C. A. Osborne. 1987. Infection (urease) stones. Miner. Electrolyte Metab. 13:278-285. [PubMed] [Google Scholar]
- 120.Gunn, J. S. 2001. Bacterial modification of LPS and resistance to antimicrobial peptides. J. Endotox. Res. 7:57-62. [PubMed] [Google Scholar]
- 121.Guyer, D. M., I. R. Henderson, J. P. Nataro, and H. L. Mobley. 2000. Identification of Sat, an autotransporter toxin produced by uropathogenic Escherichia coli. Mol. Microbiol. 38:53-66. [DOI] [PubMed] [Google Scholar]
- 122.Guyer, D. M., S. Radulovic, F. E. Jones, and H. L. Mobley. 2002. Sat, the secreted autotransporter toxin of uropathogenic Escherichia coli, is a vacuolating cytotoxin for bladder and kidney epithelial cells. Infect. Immun. 70:4539-4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gygi, D., M. J. Bailey, C. Allison, and C. Hughes. 1995. Requirement for FlhA in flagella assembly and swarm-cell differentiation by Proteus mirabilis. Mol. Microbiol. 15:761-769. [DOI] [PubMed] [Google Scholar]
- 124.Gygi, D., M. M. Rahman, H. C. Lai, R. Carlson, J. Guard-Petter, and C. Hughes. 1995. A cell-surface polysaccharide that facilitates rapid population migration by differentiated swarm cells of Proteus mirabilis. Mol. Microbiol. 17:1167-1175. [DOI] [PubMed] [Google Scholar]
- 125.Hacker, J., and J. B. Kaper. 2000. Pathogenicity islands and the evolution of microbes. Annu. Rev. Microbiol. 54:641-679. [DOI] [PubMed] [Google Scholar]
- 126.Hagberg, L., I. Engberg, R. Freter, J. Lam, S. Olling, and C. S. Eden. 1983. Ascending, unobstructed urinary tract infection in mice caused by pyelonephritogenic Escherichia coli of human origin. Infect. Immun. 40:273-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hagberg, L., R. Hull, S. Hull, J. R. McGhee, S. M. Michalek, and E. C. Svanborg. 1984. Difference in susceptibility to gram-negative urinary tract infection between C3H/HeJ and C3H/HeN mice. Infect. Immun. 46:839-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hahn, H. P. 1997. The type-4 pilus is the major virulence-associated adhesin of Pseudomonas aeruginosa—a review. Gene 192:99-108. [DOI] [PubMed] [Google Scholar]
- 129.Haley, R. W., T. M. Hooton, D. H. Culver, R. C. Stanley, T. G. Emori, C. D. Hardison, D. Quade, R. H. Shachtman, D. R. Schaberg, B. V. Shah, and G. D. Schatz. 1981. Nosocomial infections in U.S. hospitals, 1975-1976: estimated frequency by selected characteristics of patients. Am. J. Med. 70:947-959. [DOI] [PubMed] [Google Scholar]
- 130.Haraoka, M., L. Hang, B. Frendeus, G. Godaly, M. Burdick, R. Strieter, and C. Svanborg. 1999. Neutrophil recruitment and resistance to urinary tract infection. J. Infect. Dis. 180:1220-1229. [DOI] [PubMed] [Google Scholar]
- 131.Hart, R. C., S. Kadis, and W. L. Chapman, Jr. 1982. Nutritional iron status and susceptibility to Proteus mirabilis pyelonephritis in the rat. Can. J. Microbiol. 28:713-717. [DOI] [PubMed] [Google Scholar]
- 132.Hartstein, A. I., S. B. Garber, T. T. Ward, S. R. Jones, and V. H. Morthland. 1981. Nosocomial urinary tract infection: a prospective evaluation of 108 catheterized patients. Infect. Control 2:380-386. [DOI] [PubMed] [Google Scholar]
- 133.Hashmi, S., E. Kelly, S. O. Rogers, and J. Gates. 2003. Urinary tract infection in surgical patients. Am. J. Surg. 186:53-56. [DOI] [PubMed] [Google Scholar]
- 134.Hausner, M., and S. Wuertz. 1999. High rates of conjugation in bacterial biofilms as determined by quantitative in situ analysis. Appl. Environ. Microbiol. 65:3710-3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hawthorn, L., and G. Reid. 1990. The effect of protein and urine on uropathogen adhesion to polymer substrata. J. Biomed. Mater. Res. 24:1325-1332. [DOI] [PubMed] [Google Scholar]
- 136.Hazan, Z., J. Zumeris, H. Jacob, H. Raskin, G. Kratysh, M. Vishnia, N. Dror, T. Barliya, M. Mandel, and G. Lavie. 2006. Effective prevention of microbial biofilm formation on medical devices by low-energy surface acoustic waves. Antimicrob. Agents Chemother. 50:4144-4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hedges, S. R., W. W. Agace, and C. Svanborg. 1995. Epithelial cytokine responses and mucosal cytokine networks. Trends Microbiol. 3:266-270. [DOI] [PubMed] [Google Scholar]
- 138.Heimbach, D., D. Jacobs, S. C. Muller, and A. Hesse. 2002. Chemolitholysis and lithotripsy of infectious urinary stones—an in vitro study. Urol. Int. 69:212-218. [DOI] [PubMed] [Google Scholar]
- 139.Heimer, S. R., D. A. Rasko, C. V. Lockatell, D. E. Johnson, and H. L. Mobley. 2004. Autotransporter genes pic and tsh are associated with Escherichia coli strains that cause acute pyelonephritis and are expressed during urinary tract infection. Infect. Immun. 72:593-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hirsh, D. D., V. Fainstein, and D. M. Musher. 1979. Do condom catheter collecting systems cause urinary tract infection? JAMA 242:340-341. [PubMed] [Google Scholar]
- 141.Hochreiter, W., T. Knoll, and B. Hess. 2003. Pathophysiology, diagnosis and conservative therapy of non-calcium kidney calculi. Ther. Umsch. 60:89-97. (In German.) [DOI] [PubMed] [Google Scholar]
- 142.Hodgkinson, C. P., and A. A. Hodari. 1966. Trocar suprapubic cystostomy for postoperative bladder drainage in the female. Am. J. Obstet. Gynecol. 96:773-783. [DOI] [PubMed] [Google Scholar]
- 143.Hoffken, G., H. Lode, C. Prinzing, K. Borner, and P. Koeppe. 1985. Pharmacokinetics of ciprofloxacin after oral and parenteral administration. Antimicrob. Agents Chemother. 27:375-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hofman, P., G. Le Negrate, B. Mograbi, V. Hofman, P. Brest, A. Liana-Schmid, G. Flatau, P. Boquet, and B. Rossi. 2000. Escherichia coli cytotoxic necrotizing factor-1 (CNF-1) increases the adherence to epithelia and the oxidative burst of human polymorphonuclear leukocytes but decreases bacteria phagocytosis. J. Leukoc. Biol. 68:522-528. [PubMed] [Google Scholar]
- 145.Hooper, D. C., and J. S. Wolfson. 1991. Fluoroquinolone antimicrobial agents. N. Engl. J. Med. 324:384-394. [DOI] [PubMed] [Google Scholar]
- 146.Hoyle, B. D., J. Alcantara, and J. W. Costerton. 1992. Pseudomonas aeruginosa biofilm as a diffusion barrier to piperacillin. Antimicrob. Agents Chemother. 36:2054-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hull, R., D. Rudy, W. Donovan, C. Svanborg, I. Wieser, C. Stewart, and R. Darouiche. 2000. Urinary tract infection prophylaxis using Escherichia coli 83972 in spinal cord injured patients. J. Urol. 163:872-877. [PubMed] [Google Scholar]
- 148.Hull, R. A., R. E. Gill, P. Hsu, B. H. Minshew, and S. Falkow. 1981. Construction and expression of recombinant plasmids encoding type 1 or d-mannose-resistant pili from a urinary tract infection Escherichia coli isolate. Infect. Immun. 33:933-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Iravani, A. 1991. Advances in the understanding and treatment of urinary tract infections in young women. Urology 37:503-511. [DOI] [PubMed] [Google Scholar]
- 150.Island, M. D., and H. L. Mobley. 1995. Proteus mirabilis urease: operon fusion and linker insertion analysis of ure gene organization, regulation, and function. J. Bacteriol. 177:5653-5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Itoh, Y., X. Wang, B. J. Hinnebusch, J. F. Preston III, and T. Romeo. 2005. Depolymerization of β-1,6-N-acetyl-d-glucosamine disrupts the integrity of diverse bacterial biofilms. J. Bacteriol. 187:382-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Izquierdo, L., N. Abitiu, N. Coderch, B. Hita, S. Merino, R. Gavin, J. M. Tomas, and M. Regue. 2002. The inner-core lipopolysaccharide biosynthetic waaE gene: function and genetic distribution among some Enterobacteriaceae. Microbiology 148:3485-3496. [DOI] [PubMed] [Google Scholar]
- 153.Jain, P., J. P. Parada, A. David, and L. G. Smith. 1995. Overuse of the indwelling urinary tract catheter in hospitalized medical patients. Arch. Intern. Med. 155:1425-1429. [PubMed] [Google Scholar]
- 154.Jansen, A. M., C. V. Lockatell, D. E. Johnson, and H. L. Mobley. 2003. Visualization of Proteus mirabilis morphotypes in the urinary tract: the elongated swarmer cell is rarely observed in ascending urinary tract infection. Infect. Immun. 71:3607-3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jansen, A. M., V. Lockatell, D. E. Johnson, and H. L. Mobley. 2004. Mannose-resistant Proteus-like fimbriae are produced by most Proteus mirabilis strains infecting the urinary tract, dictate the in vivo localization of bacteria, and contribute to biofilm formation. Infect. Immun. 72:7294-7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jantunen, M. E., A. Siitonen, O. Koskimies, S. Wikstrom, U. Karkkainen, E. Salo, and H. Saxen. 2000. Predominance of class II papG allele of Escherichia coli in pyelonephritis in infants with normal urinary tract anatomy. J. Infect. Dis. 181:1822-1824. [DOI] [PubMed] [Google Scholar]
- 157.Jarvis, W. R. 1996. Selected aspects of the socioeconomic impact of nosocomial infections: morbidity, mortality, cost, and prevention. Infect. Control Hosp. Epidemiol. 17:552-557. [DOI] [PubMed] [Google Scholar]
- 158.Johnson, D. E., C. V. Lockatell, R. G. Russell, J. R. Hebel, M. D. Island, A. Stapleton, W. E. Stamm, and J. W. Warren. 1998. Comparison of Escherichia coli strains recovered from human cystitis and pyelonephritis infections in transurethrally challenged mice. Infect. Immun. 66:3059-3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Johnson, D. E., R. G. Russell, C. V. Lockatell, J. C. Zulty, J. W. Warren, and H. L. Mobley. 1993. Contribution of Proteus mirabilis urease to persistence, urolithiasis, and acute pyelonephritis in a mouse model of ascending urinary tract infection. Infect. Immun. 61:2748-2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Johnson, J. R. 1991. Virulence factors in Escherichia coli urinary tract infection. Clin. Microbiol. Rev. 4:80-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Johnson, J. R. 2003. Microbial virulence determinants and the pathogenesis of urinary tract infection. Infect. Dis. Clin. N. Am. 17:261-278. [DOI] [PubMed] [Google Scholar]
- 162.Johnson, J. R., T. Berggren, and A. J. Conway. 1993. Activity of a nitrofurazone matrix urinary catheter against catheter-associated uropathogens. Antimicrob. Agents Chemother. 37:2033-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Johnson, J. R., S. Jelacic, L. M. Schoening, C. Clabots, N. Shaikh, H. L. Mobley, and P. I. Tarr. 2005. The IrgA homologue adhesin Iha is an Escherichia coli virulence factor in murine urinary tract infection. Infect. Immun. 73:965-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Johnson, J. R., M. A. Kuskowski, and T. J. Wilt. 2006. Systematic review: antimicrobial urinary catheters to prevent catheter-associated urinary tract infection in hospitalized patients. Ann. Intern. Med. 144:116-126. [DOI] [PubMed] [Google Scholar]
- 165.Johnson, J. R., P. L. Roberts, R. J. Olsen, K. A. Moyer, and W. E. Stamm. 1990. Prevention of catheter-associated urinary tract infection with a silver oxide-coated urinary catheter: clinical and microbiologic correlates. J. Infect. Dis. 162:1145-1150. [DOI] [PubMed] [Google Scholar]
- 166.Johnson, J. R., T. A. Russo, P. I. Tarr, U. Carlino, S. S. Bilge, J. C. Vary, Jr., and A. L. Stell. 2000. Molecular epidemiological and phylogenetic associations of two novel putative virulence genes, iha and iroNE. coli, among Escherichia coli isolates from patients with urosepsis. Infect. Immun. 68:3040-3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Johnson, J. R., and W. E. Stamm. 1989. Urinary tract infections in women: diagnosis and treatment. Ann. Intern. Med. 111:906-917. [DOI] [PubMed] [Google Scholar]
- 168.Jones, B. D., C. V. Lockatell, D. E. Johnson, J. W. Warren, and H. L. Mobley. 1990. Construction of a urease-negative mutant of Proteus mirabilis: analysis of virulence in a mouse model of ascending urinary tract infection. Infect. Immun. 58:1120-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jones, B. D., and H. L. Mobley. 1988. Proteus mirabilis urease: genetic organization, regulation, and expression of structural genes. J. Bacteriol. 170:3342-3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jones, B. D., and H. L. Mobley. 1987. Genetic and biochemical diversity of ureases of Proteus, Providencia, and Morganella species isolated from urinary tract infection. Infect. Immun. 55:2198-2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Jones, B. V., E. Mahenthiralingam, N. A. Sabbuba, and D. J. Stickler. 2005. Role of swarming in the formation of crystalline Proteus mirabilis biofilms on urinary catheters. J. Med. Microbiol. 54:807-813. [DOI] [PubMed] [Google Scholar]
- 172.Jones, B. V., R. Young, E. Mahenthiralingam, and D. J. Stickler. 2004. Ultrastructure of Proteus mirabilis swarmer cell rafts and role of swarming in catheter-associated urinary tract infection. Infect. Immun. 72:3941-3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Jones, G. L., A. D. Russell, Z. Caliskan, and D. J. Stickler. 2005. A strategy for the control of catheter blockage by crystalline Proteus mirabilis biofilm using the antibacterial agent triclosan. Eur. Urol. 48:838-845. [DOI] [PubMed] [Google Scholar]
- 174.Jorgensen, S. E., E. C. Short, Jr., H. J. Kurtz, H. K. Mussen, and G. K. Wu. 1976. Studies on the origin of the alpha-haemolysin produced by Escherichia coli. J. Med. Microbiol. 9:173-189. [DOI] [PubMed] [Google Scholar]
- 175.Justice, S. S., C. Hung, J. A. Theriot, D. A. Fletcher, G. G. Anderson, M. J. Footer, and S. J. Hultgren. 2004. Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc. Natl. Acad. Sci. USA 101:1333-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kaca, W., Y. A. Knirel, E. V. Vinogradov, and K. Kotelko. 1987. Structure of the O-specific polysaccharide of Proteus mirabilis S 1959. Arch. Immunol. Ther. Exp. 35:431-437. [PubMed] [Google Scholar]
- 177.Kaca, W., and A. Rozalski. 1991. Characterization of cell-bound and cell-free hemolytic activity of Proteus strains. Eur. J. Epidemiol. 7:159-165. [DOI] [PubMed] [Google Scholar]
- 178.Kadurugamuwa, J. L., K. Modi, J. Yu, K. P. Francis, T. Purchio, and P. R. Contag. 2005. Noninvasive biophotonic imaging for monitoring of catheter-associated urinary tract infections and therapy in mice. Infect. Immun. 73:3878-3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kamel, H. K. 2004. Managing urinary tract infections in the nursing home: myths, mysteries and realities. Internet J. Geriatr. Gerontol. 1. http://www.ispub.com/ostia/index.php?xmlFilePath=journals/ijgg/vol1n2/uri.xml.
- 180.Kanamaru, S., H. Kurazono, S. Ishitoya, A. Terai, T. Habuchi, M. Nakano, O. Ogawa, and S. Yamamoto. 2003. Distribution and genetic association of putative uropathogenic virulence factors iroN, iha, kpsMT, ompT and usp in Escherichia coli isolated from urinary tract infections in Japan. J. Urol. 170:2490-2493. [DOI] [PubMed] [Google Scholar]
- 181.Kantele, A., T. Mottonen, K. Ala-Kaila, and H. S. Arvilommi. 2003. P fimbria-specific B cell responses in patients with urinary tract infection. J. Infect. Dis. 188:1885-1891. [DOI] [PubMed] [Google Scholar]
- 182.Karchmer, T. B., E. T. Giannetta, C. A. Muto, B. A. Strain, and B. M. Farr. 2000. A randomized crossover study of silver-coated urinary catheters in hospitalized patients. Arch. Intern. Med. 160:3294-3298. [DOI] [PubMed] [Google Scholar]
- 183.Khan, A. S., B. Kniep, T. A. Oelschlaeger, I. Van Die, T. Korhonen, and J. Hacker. 2000. Receptor structure for F1C fimbriae of uropathogenic Escherichia coli. Infect. Immun. 68:3541-3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kjelleberg, S., P. D. Steinberg, M. Givskov, L. Gram, M. Manefield, and R. de Nys. 1997. Do marine products interfere with prokaryotic AHL regulatory systems? Aquat. Microb. Ecol. 13:85-93. [Google Scholar]
- 185.Knirel, Y. A., N. A. Paramonov, E. V. Vinogradov, N. K. Kochetkov, Z. Sidorczyk, and K. Zych. 1994. 2-Acetamido-4-O-[(S)-1-carboxyethyl]-2-deoxy-D-glucose: a new natural isomer of N-acetylmuramic acid from the O-specific polysaccharide of Proteus penneri 35. Carbohydr. Res. 259: C1-C3. [DOI] [PubMed] [Google Scholar]
- 186.Knirel, Y. A., N. A. Paramonov, E. V. Vinogradov, A. S. Shashkov, N. K. Kochetkov, Z. Sidorczyk, and A. Swierzko. 1992. Structure of the O-specific polysaccharide of Proteus penneri 62 containing 2-acetamido-3-O-[(S)-1-carboxyethyl]-2-deoxy-D-glucose (N-acetylisomuramic acid). Carbohydr. Res. 235:C19-C23. [DOI] [PubMed] [Google Scholar]
- 187.Knirel, Y. A., E. V. Vinogradov, A. S. Shashkov, Z. Sidorczyk, A. Rózalski, J. Radziejewska-Lebrecht, and W. Kaca. 1993. Structural study of O-specific polysaccharides of Proteus. J. Carbohydr. Chem. 12:379-414. [Google Scholar]
- 188.Kolodziejska, K., A. N. Kondakova, K. Zych, S. N. Senchenkova, A. S. Shashkov, Y. A. Knirel, and Z. Sidorczyk. 2003. Structure of the O-polysaccharide of a serologically separate strain of Proteus mirabilis, TG 332, from a new proposed Proteus serogroup O50. Carbohydr. Res. 338:2105-2109. [DOI] [PubMed] [Google Scholar]
- 189.Kondakova, A. N., R. Fudala, K. Bednarska, S. N. Senchenkova, Y. A. Knirel, and W. Kaca. 2004. Structure of the neutral O-polysaccharide and biological activities of the lipopolysaccharide of Proteus mirabilis O20. Carbohydr. Res. 339:623-628. [DOI] [PubMed] [Google Scholar]
- 190.Kondakova, A. N., R. Fudala, S. N. Senchenkova, A. S. Shashkov, Y. A. Knirel, and W. Kaca. 2003. Structural and serological studies of the O-antigen of Proteus mirabilis O-9. Carbohydr. Res. 338:1191-1196. [DOI] [PubMed] [Google Scholar]
- 191.Korhonen, T. K., J. Parkkinen, J. Hacker, J. Finne, A. Pere, M. Rhen, and H. Holthofer. 1986. Binding of Escherichia coli S fimbriae to human kidney epithelium. Infect. Immun. 54:322-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Koronakis, V., M. Cross, B. Senior, E. Koronakis, and C. Hughes. 1987. The secreted hemolysins of Proteus mirabilis, Proteus vulgaris, and Morganella morganii are genetically related to each other and to the alpha-hemolysin of Escherichia coli. J. Bacteriol. 169:1509-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Koronakis, V., and C. Hughes. 1988. Identification of the promoters directing in vivo expression of hemolysin genes in Proteus vulgaris and Escherichia coli. Mol. Gen. Genet. 213:99-104. [DOI] [PubMed] [Google Scholar]
- 194.Koseoglu, H., G. Aslan, N. Esen, B. H. Sen, and H. Coban. 2006. Ultrastructural stages of biofilm development of Escherichia coli on urethral catheters and effects of antibiotics on biofilm formation. Urology 68:942-946. [DOI] [PubMed] [Google Scholar]
- 195.Kotelko, K. 1986. Proteus mirabilis: taxonomic position, peculiarities of growth, components of the cell envelope. Curr. Top. Microbiol. Immunol. 129:181-215. [DOI] [PubMed] [Google Scholar]
- 196.Kotelko, K., W. Kaca, A. Rozalski, and M. Deka. 1983. Some biological features of Proteus bacilli. 2. Haemolytic activities of Proteus mirabilis and Proteus vulgaris strains. Acta Microbiol. Pol. 32:345-351. [PubMed] [Google Scholar]
- 197.Kotelko, K., A. Rozalski, M. Deka, W. Kaca, Z. Sidorczyk, W. Gromska, and K. Zych. 1983. Some biological features of Proteus bacilli. 1. Comparison of Proteus mirabilis strains provided from various sources. Acta Microbiol. Pol. 32:339-344. [PubMed] [Google Scholar]
- 198.Koukalova, D., V. Hajek, and R. Kodusek. 1999. Development of a vaccine for treatment of urinary tract infections. Bratisl. Lek. Listy 100:92-95. [PubMed] [Google Scholar]
- 199.Krajden, S., M. Fuksa, W. Lizewski, L. Barton, and A. Lee. 1984. Proteus penneri and urinary calculi formation. J. Clin. Microbiol. 19:541-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Krajden, S., M. Fuksa, C. Petrea, L. J. Crisp, and J. L. Penner. 1987. Expanded clinical spectrum of infections caused by Proteus penneri. J. Clin. Microbiol. 25:578-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Krajewska-Pietrasik, D., A. Rozalski, B. Bartodziejska, J. Radziejewska-Lebrecht, H. Mayer, and K. Kotelko. 1991. Properties of a deep Proteus R mutant isolated from clinical material. APMIS 99:499-506. [PubMed] [Google Scholar]
- 202.Kucheria, R., P. Dasgupta, S. H. Sacks, M. S. Khan, and N. S. Sheerin. 2005. Urinary tract infections: new insights into a common problem. Postgrad. Med. J. 81:83-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kunin, C. M. 1979. Detection, prevention, and management of urinary tract infections. Lea and Feniger, Philadelphia, PA.
- 204.Kunin, C. M. 1988. Can we build a better urinary catheter? N. Engl. J. Med. 319:365-366. [DOI] [PubMed] [Google Scholar]
- 205.Kunin, C. M., S. Douthitt, J. Dancing, J. Anderson, and M. Moeschberger. 1992. The association between the use of urinary catheters and morbidity and mortality among elderly patients in nursing homes. Am. J. Epidemiol. 135:291-301. [DOI] [PubMed] [Google Scholar]
- 206.Lai, K. K., and S. A. Fontecchio. 2002. Use of silver-hydrogel urinary catheters on the incidence of catheter-associated urinary tract infections in hospitalized patients. Am. J. Infect. Control 30:221-225. [DOI] [PubMed] [Google Scholar]
- 207.Lam, H. S., J. E. Lingeman, P. G. Mosbaugh, R. E. Steele, P. M. Knapp, J. W. Scott, and D. M. Newman. 1992. Evolution of the technique of combination therapy for staghorn calculi: a decreasing role for extracorporeal shock wave lithotripsy. J. Urol. 148:1058-1062. [DOI] [PubMed] [Google Scholar]
- 208.Lane, M. C., V. Lockatell, G. Monterosso, D. Lamphier, J. Weinert, J. R. Hebel, D. E. Johnson, and H. L. Mobley. 2005. Role of motility in the colonization of uropathogenic Escherichia coli in the urinary tract. Infect. Immun. 73:7644-7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Langermann, S. and W. R. Ballou, Jr. 2001. Vaccination utilizing the FimCH complex as a strategy to prevent Escherichia coli urinary tract infections. J. Infect. Dis. 183(Suppl. 1):S84-S86. [DOI] [PubMed] [Google Scholar]
- 210.Langermann, S., R. Mollby, J. E. Burlein, S. R. Palaszynski, C. G. Auguste, A. DeFusco, R. Strouse, M. A. Schenerman, S. J. Hultgren, J. S. Pinkner, J. Winberg, L. Guldevall, M. Soderhall, K. Ishikawa, S. Normark, and S. Koenig. 2000. Vaccination with FimH adhesin protects cynomolgus monkeys from colonization and infection by uropathogenic Escherichia coli. J. Infect. Dis. 181:774-778. [DOI] [PubMed] [Google Scholar]
- 211.Langermann, S., S. Palaszynski, M. Barnhart, G. Auguste, J. S. Pinkner, J. Burlein, P. Barren, S. Koenig, S. Leath, C. H. Jones, and S. J. Hultgren. 1997. Prevention of mucosal Escherichia coli infection by FimH-adhesin-based systemic vaccination. Science 276:607-611. [DOI] [PubMed] [Google Scholar]
- 212.Larsson, P. 1984. Serology of Proteus mirabilis and Proteus vulgaris. Methods Microbiol. 14:187-214. [Google Scholar]
- 213.Larsson, P., and S. Olling. 1977. O antigen distribution and sensitivity to the bactericidal effect of normal human serum of Proteus strains from clinical specimens. Med. Microbiol. Immunol. (Berlin) 163:77-82. [DOI] [PubMed] [Google Scholar]
- 214.Latta, R. K., M. J. Schur, D. L. Tolson, and E. Altman. 1998. The effect of growth conditions on in vitro adherence, invasion, and NAF expression by Proteus mirabilis 7570. Can. J. Microbiol. 44:896-904. [DOI] [PubMed] [Google Scholar]
- 215.Lea, S., R. Powell, and D. Evans. 1999. Crystallization and preliminary X-ray diffraction analysis of a biologically active fragment of CD55. Acta Crystallogr. D Biol. Crystallogr. 55:1198-1200. [DOI] [PubMed] [Google Scholar]
- 216.Lee, K. K., B. A. Harrison, R. Latta, and E. Altman. 2000. The binding of Proteus mirabilis nonagglutinating fimbriae to ganglio-series asialoglycolipids and lactosyl ceramide. Can. J. Microbiol. 46:961-966. [PubMed] [Google Scholar]
- 217.Leranoz, S., P. Orus, M. Berlanga, F. Dalet, and M. Vinas. 1997. New fimbrial adhesins of Serratia marcescens isolated from urinary tract infections: description and properties. J. Urol. 157:694-698. [PubMed] [Google Scholar]
- 218.Li, X., J. L. Erbe, C. V. Lockatell, D. E. Johnson, M. G. Jobling, R. K. Holmes, and H. L. Mobley. 2004. Use of translational fusion of the MrpH fimbrial adhesin-binding domain with the cholera toxin A2 domain, coexpressed with the cholera toxin B subunit, as an intranasal vaccine to prevent experimental urinary tract infection by Proteus mirabilis. Infect. Immun. 72:7306-7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Li, X., D. E. Johnson, and H. L. Mobley. 1999. Requirement of MrpH for mannose-resistant Proteus-like fimbria-mediated hemagglutination by Proteus mirabilis. Infect. Immun. 67:2822-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Li, X., C. V. Lockatell, D. E. Johnson, M. C. Lane, J. W. Warren, and H. L. Mobley. 2004. Development of an intranasal vaccine to prevent urinary tract infection by Proteus mirabilis. Infect. Immun. 72:66-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Li, X., C. V. Lockatell, D. E. Johnson, and H. L. Mobley. 2002. Identification of MrpI as the sole recombinase that regulates the phase variation of MR/P fimbria, a bladder colonization factor of uropathogenic Proteus mirabilis. Mol. Microbiol. 45:865-874. [DOI] [PubMed] [Google Scholar]
- 222.Li, X., and H. L. Mobley. 1998. MrpB functions as the terminator for assembly of Proteus mirabilis mannose-resistant Proteus-like fimbriae. Infect. Immun. 66:1759-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Li, X., D. A. Rasko, C. V. Lockatell, D. E. Johnson, and H. L. Mobley. 2001. Repression of bacterial motility by a novel fimbrial gene product. EMBO J. 20:4854-4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Li, X., H. Zhao, L. Geymonat, F. Bahrani, D. E. Johnson, and H. L. Mobley. 1997. Proteus mirabilis mannose-resistant, Proteus-like fimbriae: MrpG is located at the fimbrial tip and is required for fimbrial assembly. Infect. Immun. 65:1327-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Li, X., H. Zhao, C. V. Lockatell, C. B. Drachenberg, D. E. Johnson, and H. L. Mobley. 2002. Visualization of Proteus mirabilis within the matrix of urease-induced bladder stones during experimental urinary tract infection. Infect. Immun. 70:389-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Lingeman, J. E., D. A. Lifshitz, and A. P. Evan. 2002. Surgical management of urinary lithiasis, p. 3366-3370. In P. C. Walsh, A. B. Retik, E. D. Vaughan, and A. J. Wein (ed.), Campbell's urology. W. B. Saunders, Philadelphia, PA.
- 227.Loomes, L. M., M. A. Kerr, and B. W. Senior. 1993. The cleavage of immunoglobulin G in vitro and in vivo by a proteinase secreted by the urinary tract pathogen Proteus mirabilis. J. Med. Microbiol. 39:225-232. [DOI] [PubMed] [Google Scholar]
- 228.Loomes, L. M., B. W. Senior, and M. A. Kerr. 1990. A proteolytic enzyme secreted by Proteus mirabilis degrades immunoglobulins of the immunoglobulin A1 (IgA1), IgA2, and IgG isotypes. Infect. Immun. 58:1979-1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Loomes, L. M., B. W. Senior, and M. A. Kerr. 1992. Proteinases of Proteus spp.: purification, properties, and detection in urine of infected patients. Infect. Immun. 60:2267-2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Lukomski, S., L. Serwecinska, A. Rozalski, J. Dziadek, P. Staczek, and A. Jaworski. 1991. Cell-free and cell-bound hemolytic activities of Proteus penneri determined by different Hly determinants. Can. J. Microbiol. 37:419-424. [DOI] [PubMed] [Google Scholar]
- 231.Lundgren, J., O. Bengtsson, A. Israelsson, A. C. Jonsson, A. S. Lindh, and J. Utas. 2000. The importance of osmolality for intermittent catheterization of the urethra. Spinal Cord 38:45-50. [DOI] [PubMed] [Google Scholar]
- 232.Lyte, M., P. P. Freestone, C. P. Neal, B. A. Olson, R. D. Haigh, R. Bayston, and P. H. Williams. 2003. Stimulation of Staphylococcus epidermidis growth and biofilm formation by catecholamine inotropes. Lancet 361:130-135. [DOI] [PubMed] [Google Scholar]
- 233.Mahmood, A., M. J. Engle, S. J. Hultgren, G. S. Goetz, K. Dodson, and D. H. Alpers. 2000. Role of intestinal surfactant-like particles as a potential reservoir of uropathogenic Escherichia coli. Biochim. Biophys. Acta 1523:49-55. [DOI] [PubMed] [Google Scholar]
- 234.Maki, D. G., C. H. Hennekens, and J. V. Bennett. 1972. Prevention of catheter-associated urinary tract infection. An additional measure. JAMA 221:1270-1271. [PubMed] [Google Scholar]
- 235.Maki, D. G., V. Knasinski, K. T. Halvorson, P. A. Tambyah, and R. G. Holcomb. 1997. A prospective, randomized, investigator-blinded trial of a novel nitrofurazone-impregnated urinary catheter. Infect. Control Hosp. Epidemiol. 18:50. [Google Scholar]
- 236.Maki, D. G., and P. A. Tambyah. 2001. Engineering out the risk for infection with urinary catheters. Emerg. Infect. Dis. 7:342-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Marcus, R. T. 1967. Narrow-bore suprapubic bladder drainage in Uganda. Lancet i:748-750. [DOI] [PubMed] [Google Scholar]
- 238.Marre, R., J. Hacker, W. Henkel, and W. Goebel. 1986. Contribution of cloned virulence factors from uropathogenic Escherichia coli strains to nephropathogenicity in an experimental rat pyelonephritis model. Infect. Immun. 54:761-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Marrs, C. F., L. Zhang, P. Tallman, S. D. Manning, P. Somsel, P. Raz, R. Colodner, M. E. Jantunen, A. Siitonen, H. Saxen, and B. Foxman. 2002. Variations in 10 putative uropathogen virulence genes among urinary, faecal and peri-urethral Escherichia coli. J. Med. Microbiol. 51:138-142. [DOI] [PubMed] [Google Scholar]
- 240.Martinez, J. J., and S. J. Hultgren. 2002. Requirement of Rho-family GTPases in the invasion of type 1-piliated uropathogenic Escherichia coli. Cell. Microbiol. 4:19-28. [DOI] [PubMed] [Google Scholar]
- 241.Martinez, J. J., M. A. Mulvey, J. D. Schilling, J. S. Pinkner, and S. J. Hultgren. 2000. Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. EMBO J. 19:2803-2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Massad, G., F. K. Bahrani, and H. L. Mobley. 1994. Proteus mirabilis fimbriae: identification, isolation, and characterization of a new ambient-temperature fimbria. Infect. Immun. 62:1989-1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Massad, G., J. F. Fulkerson, Jr., D. C. Watson, and H. L. Mobley. 1996. Proteus mirabilis ambient-temperature fimbriae: cloning and nucleotide sequence of the aft gene cluster. Infect. Immun. 64:4390-4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Massad, G., C. V. Lockatell, D. E. Johnson, and H. L. Mobley. 1994. Proteus mirabilis fimbriae: construction of an isogenic pmfA mutant and analysis of virulence in a CBA mouse model of ascending urinary tract infection. Infect. Immun. 62:536-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Massad, G., and H. L. Mobley. 1994. Genetic organization and complete sequence of the Proteus mirabilis pmf fimbrial operon. Gene 150:101-104. [DOI] [PubMed] [Google Scholar]
- 246.Massad, G., H. Zhao, and H. L. Mobley. 1995. Proteus mirabilis amino acid deaminase: cloning, nucleotide sequence, and characterization of aad. J. Bacteriol. 177:5878-5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Mayes, J., J. Bliss, and P. Griffiths. 2003. Preventing blockage of long-term indwelling catheters in adults: are citric acid solutions effective? Br. J. Commun. Nurs. 8:172-175. [DOI] [PubMed] [Google Scholar]
- 248.McCoy, A. J., H. Liu, T. J. Falla, and J. S. Gunn. 2001. Identification of Proteus mirabilis mutants with increased sensitivity to antimicrobial peptides. Antimicrob. Agents Chemother. 45:2030-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.McLean, R. J., J. R. Lawrence, D. R. Korber, and D. E. Caldwell. 1991. Proteus mirabilis biofilm protection against struvite crystal dissolution and its implications in struvite urolithiasis. J. Urol. 146:1138-1142. [DOI] [PubMed] [Google Scholar]
- 250.McLean, R. J., J. C. Nickel, K. J. Cheng, and J. W. Costerton. 1988. The ecology and pathogenicity of urease-producing bacteria in the urinary tract. Crit. Rev. Microbiol. 16:37-79. [DOI] [PubMed] [Google Scholar]
- 251.McTaggart, L. A., R. C. Rigby, and T. S. Elliott. 1990. The pathogenesis of urinary tract infections associated with Escherichia coli, Staphylococcus saprophyticus and S. epidermidis. J. Med. Microbiol. 32:135-141. [DOI] [PubMed] [Google Scholar]
- 252.Menestrina, G., C. Pederzolli, S. M. Dalla, M. Bregante, and F. Gambale. 1996. Permeability increase induced by Escherichia coli hemolysin A in human macrophages is due to the formation of ionic pores: a patch clamp characterization. J. Membr. Biol. 149:113-121. [DOI] [PubMed] [Google Scholar]
- 253.Meng, M., M. L. Stoller, and T. Minor. 2005. Struvite and staghorn calculi. eMedicine. http://www.emedicine.com/med/topic2834.htm.
- 254.Meretyk, S., O. N. Gofrit, O. Gafni, D. Pode, A. Shapiro, A. Verstandig, T. Sasson, G. Katz, and E. H. Landau. 1997. Complete staghorn calculi: random prospective comparison between extracorporeal shock wave lithotripsy monotherapy and combined with percutaneous nephrostolithotomy. J. Urol. 157:780-786. [DOI] [PubMed] [Google Scholar]
- 255.Merrell, D. S., and A. Camilli. 2000. Detection and analysis of gene expression during infection by in vivo expression technology. Philos. Trans. R. Soc. Lond. B Biol. Sci. 355:587-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Merz, A. J., M. So, and M. P. Sheetz. 2000. Pilus retraction powers bacterial twitching motility. Nature 407:98-102. [DOI] [PubMed] [Google Scholar]
- 257.Mills, M., K. C. Meysick, and A. D. O'Brien. 2000. Cytotoxic necrotizing factor type 1 of uropathogenic Escherichia coli kills cultured human uroepithelial 5637 cells by an apoptotic mechanism. Infect. Immun. 68:5869-5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Mobley, H. L., and R. Belas. 1995. Swarming and pathogenicity of Proteus mirabilis in the urinary tract. Trends Microbiol. 3:280-284. [DOI] [PubMed] [Google Scholar]
- 259.Mobley, H. L., R. Belas, V. Lockatell, G. Chippendale, A. L. Trifillis, D. E. Johnson, and J. W. Warren. 1996. Construction of a flagellum-negative mutant of Proteus mirabilis: effect on internalization by human renal epithelial cells and virulence in a mouse model of ascending urinary tract infection. Infect. Immun. 64:5332-5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Mobley, H. L., G. R. Chippendale, K. G. Swihart, and R. A. Welch. 1991. Cytotoxicity of the HpmA hemolysin and urease of Proteus mirabilis and Proteus vulgaris against cultured human renal proximal tubular epithelial cells. Infect. Immun. 59:2036-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Mobley, H. L., G. R. Chippendale, J. H. Tenney, R. A. Hull, and J. W. Warren. 1987. Expression of type 1 fimbriae may be required for persistence of Escherichia coli in the catheterized urinary tract. J. Clin. Microbiol. 25:2253-2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Mobley, H. L., G. R. Chippendale, J. H. Tenney, A. R. Mayrer, L. J. Crisp, J. L. Penner, and J. W. Warren. 1988. MR/K hemagglutination of Providencia stuartii correlates with adherence to catheters and with persistence in catheter-associated bacteriuria. J. Infect. Dis. 157:264-271. [DOI] [PubMed] [Google Scholar]
- 263.Mobley, H. L., D. M. Green, A. L. Trifillis, D. E. Johnson, G. R. Chippendale, C. V. Lockatell, B. D. Jones, and J. W. Warren. 1990. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect. Immun. 58:1281-1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Mobley, H. L., M. D. Island, and R. P. Hausinger. 1995. Molecular biology of microbial ureases. Microbiol. Rev. 59:451-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Mobley, H. L., B. D. Jones, and A. E. Jerse. 1986. Cloning of urease gene sequences from Providencia stuartii. Infect. Immun. 54:161-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Mobley, H. L., B. D. Jones, and J. L. Penner. 1987. Urease activity of Proteus penneri. J. Clin. Microbiol. 25:2302-2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Morris, N. S., and D. J. Stickler. 1998. Encrustation of indwelling urethral catheters by Proteus mirabilis biofilms growing in human urine. J. Hosp. Infect. 39:227-234. [DOI] [PubMed] [Google Scholar]
- 268.Morris, N. S., D. J. Stickler, and C. Winters. 1997. Which indwelling urethral catheters resist encrustation by Proteus mirabilis biofilms? Br. J. Urol. 80:58-63. [DOI] [PubMed] [Google Scholar]
- 269.Mulhall, A. B., R. G. Chapman, and R. A. Crow. 1988. Bacteriuria during indwelling urethral catheterization. J. Hosp. Infect. 11:253-262. [DOI] [PubMed] [Google Scholar]
- 270.Mulks, M. 1985. Microbial IgA proteases, p. 81-104. In I. A. Holder (ed.), Bacterial enzymes and virulence. CRC Press, Inc., Boca Raton, FL.
- 271.Mulvey, M. A. 2002. Adhesion and entry of uropathogenic Escherichia coli. Cell. Microbiol. 4:257-271. [DOI] [PubMed] [Google Scholar]
- 272.Mulvey, M. A., Y. S. Lopez-Boado, C. L. Wilson, R. Roth, W. C. Parks, J. Heuser, and S. J. Hultgren. 1998. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 282:1494-1497. [DOI] [PubMed] [Google Scholar]
- 273.Mulvey, M. A., J. D. Schilling, J. J. Martinez, and S. J. Hultgren. 2000. Bad bugs and beleaguered bladders: interplay between uropathogenic Escherichia coli and innate host defenses. Proc. Natl. Acad. Sci. USA 97:8829-8835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Munasinghe, R. L., H. Yazdani, M. Siddique, and W. Hafeez. 2001. Appropriateness of use of indwelling urinary catheters in patients admitted to the medical service. Infect. Control Hosp. Epidemiol. 22:647-649. [DOI] [PubMed] [Google Scholar]
- 275.Murphy, C. A., and R. Belas. 1999. Genomic rearrangements in the flagellin genes of Proteus mirabilis. Mol. Microbiol. 31:679-690. [DOI] [PubMed] [Google Scholar]
- 276.Murray, P., K. Rosenthal, G. S. Kobayashi, and M. A. Pfaller. 2002. Enterobacteriaceae, p. 266-280. In Medical microbiology. Mosby Publishing, St. Louis, MO.
- 277.Musher, D. M., D. P. Griffith, D. Yawn, and R. D. Rossen. 1975. Role of urease in pyelonephritis resulting from urinary tract infection with Proteus. J. Infect. Dis. 131:177-181. [DOI] [PubMed] [Google Scholar]
- 278.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.National Centers for Health Statistics. 1997. Health, United States, 1996-1997, and injury chartbook. National Centers for Health Statistics, Hyattsville, MD.
- 280.Newman, D. K. 1998. Managing indwelling urethral catheters. Ostomy. Wound Manage. 44:26-2830:32. [PubMed] [Google Scholar]
- 281.Nicholson, E. B., E. A. Concaugh, P. A. Foxall, M. D. Island, and H. L. Mobley. 1993. Proteus mirabilis urease: transcriptional regulation by UreR. J. Bacteriol. 175:465-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Nicolle, L. E. 2001. A practical guide to antimicrobial management of complicated urinary tract infection. Drugs Aging 18:243-254. [DOI] [PubMed] [Google Scholar]
- 283.Niel-Weise, B. S., S. M. Arend, and P. J. van den Broek. 2002. Is there evidence for recommending silver-coated urinary catheters in guidelines? J. Hosp. Infect. 52:81-87. [DOI] [PubMed] [Google Scholar]
- 284.Niel-Weise, B. S., and P. J. van den Broek. 2005. Urinary catheter policies for long-term bladder drainage. Cochrane Database Syst. Rev. 2005:CD004201. [DOI] [PubMed] [Google Scholar]
- 285.Nowicki, B. 2002. Urinary tract infection in pregnant women: old dogmas and current concepts regarding pathogenesis. Curr. Infect. Dis. Rep. 4:529-535. [DOI] [PubMed] [Google Scholar]
- 286.Nowicki, B., R. Selvarangan, and S. Nowicki. 2001. Family of Escherichia coli Dr adhesins: decay-accelerating factor receptor recognition and invasiveness. J. Infect. Dis. 183(Suppl. 1):S24-S27. [DOI] [PubMed] [Google Scholar]
- 287.Nowrouzian, F. L., I. Adlerberth, and A. E. Wold. 2006. Enhanced persistence in the colonic microbiota of Escherichia coli strains belonging to phylogenetic group B2: role of virulence factors and adherence to colonic cells. Microbes Infect. 8:834-840. [DOI] [PubMed] [Google Scholar]
- 288.Old, D. C., R. Adegbola, and S. S. Scott. 1983. Multiple fimbrial haemagglutinins in Serratia species. Med. Microbiol. Immunol. (Berlin) 172:107-115. [DOI] [PubMed] [Google Scholar]
- 289.Old, D. C., and R. A. Adegbola. 1982. Haemagglutinins and fimbriae of Morganella, Proteus and Providencia. J. Med. Microbiol. 15:551-564. [DOI] [PubMed] [Google Scholar]
- 290.Opal, S. M., A. S. Cross, P. Gemski, and L. W. Lyhte. 1990. Aerobactin and alpha-hemolysin as virulence determinants in Escherichia coli isolated from human blood, urine, and stool. J. Infect. Dis. 161:794-796. [DOI] [PubMed] [Google Scholar]
- 291.Orskov, F. 1978. Virulence factors of the bacterial cell surface. J. Infect. Dis. 137:630-633. [DOI] [PubMed] [Google Scholar]
- 292.Orskov, I., F. Orskov, B. Jann, and K. Jann. 1977. Serology, chemistry, and genetics of O and K antigens of Escherichia coli. Bacteriol. Rev. 41:667-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.O'Sullivan, D. J., M. G. Fitzgerald, M. J. Meynell, and J. M. Malins. 1960. A simplified method for the quantitative bacterial culture of urine. J. Clin. Pathol. 13:527-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Palmer, L. M., T. J. Reilly, S. J. Utsalo, and M. S. Donnenberg. 1997. Internalization of Escherichia coli by human renal epithelial cells is associated with tyrosine phosphorylation of specific host cell proteins. Infect. Immun. 65:2570-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Parham, N. J., U. Srinivasan, M. Desvaux, B. Foxman, C. F. Marrs, and I. R. Henderson. 2004. PicU, a second serine protease autotransporter of uropathogenic Escherichia coli. FEMS Microbiol. Lett. 230:73-83. [DOI] [PubMed] [Google Scholar]
- 296.Payne, S. M. 1988. Iron and virulence in the family Enterobacteriaceae. Crit. Rev. Microbiol. 16:81-111. [DOI] [PubMed] [Google Scholar]
- 297.Peerbooms, P. G., A. M. Verweij, and D. M. MacLaren. 1983. Investigation of the haemolytic activity of Proteus mirabilis strains. Antonie Leeuwenhoek 49:1-11. [DOI] [PubMed] [Google Scholar]
- 298.Peerbooms, P. G., A. M. Verweij, and D. M. MacLaren. 1984. Vero cell invasiveness of Proteus mirabilis. Infect. Immun. 43:1068-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Peerbooms, P. G., A. M. Verweij, and D. M. MacLaren. 1985. Uropathogenic properties of Proteus mirabilis and Proteus vulgaris. J. Med. Microbiol. 19:55-60. [DOI] [PubMed] [Google Scholar]
- 300.Pellegrino, R., U. Galvalisi, P. Scavone, V. Sosa, and P. Zunino. 2003. Evaluation of Proteus mirabilis structural fimbrial proteins as antigens against urinary tract infections. FEMS Immunol. Med. Microbiol. 36:103-110. [DOI] [PubMed] [Google Scholar]
- 301.Penner, J. L. 1984. Genus XI Proteus, p. 491-494. In N. R. Krieg and J. G. Holt (ed.), Bergey's manual of systemic bacteriology, vol. 1. Williams & Wilkins Co., Baltimore, MD. [Google Scholar]
- 302.Penner, J. L. 1992. The genera Proteus, Providencia, and Morganella, p. 2849-2853. In I. Balows, G. Truper, W. Harder, and K. Schleifer (ed.), The prokaryotes, vol. III. Springer-Verlag KG, Berlin, Germany. [Google Scholar]
- 303.Penner, J. L., and J. N. Hennessy. 1980. Separate O-grouping schemes for serotyping clinical isolates of Proteus vulgaris and Proteus mirabilis. J. Clin. Microbiol. 12:304-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Perepelov, A. V., A. Rozalski, B. Bartodziejska, S. N. Senchenkova, and Y. A. Knirel. 2004. Structure of the O-polysaccharide of Proteus mirabilis O19 and reclassification of certain Proteus strains that were formerly classified in serogroup O19. Arch. Immunol. Ther. Exp. 52:188-196. [PubMed] [Google Scholar]
- 305.Perepelov, A. V., A. S. Shashkov, S. N. Senchenkova, Y. A. Knirel, E. Literacka, and W. Kaca. 2000. Structure of the O-specific polysaccharide of the bacterium Proteus mirabilis O29. Biochemistry (Moscow) 65:176-179. [PubMed] [Google Scholar]
- 306.Perepelov, A. V., E. Ujazda, S. N. Senchenkova, A. S. Shashkov, W. Kaca, and Y. A. Knirel. 1999. Structural and serological studies on the O-antigen of Proteus mirabilis O14, a new polysaccharide containing 2-[(R)-1-carboxyethylamino]ethyl phosphate. Eur. J. Biochem. 261:347-353. [DOI] [PubMed] [Google Scholar]
- 307.Perry, M. B., and L. L. MacLean. 1994. The structure of the polysaccharide produced by Proteus vulgaris (ATCC 49990). Carbohydr. Res. 253:257-263. [DOI] [PubMed] [Google Scholar]
- 308.Piatek, R., B. Zalewska, O. Kolaj, M. Ferens, B. Nowicki, and J. Kur. 2005. Molecular aspects of biogenesis of Escherichia coli Dr fimbriae: characterization of DraB-DraE complexes. Infect. Immun. 73:135-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Plaut, A. G. 1983. The IgA1 proteases of pathogenic bacteria. Annu. Rev. Microbiol. 37:603-622. [DOI] [PubMed] [Google Scholar]
- 310.Plos, K., H. Connell, U. Jodal, B. I. Marklund, S. Marild, B. Wettergren, and C. Svanborg. 1995. Intestinal carriage of P fimbriated Escherichia coli and the susceptibility to urinary tract infection in young children. J. Infect. Dis. 171:625-631. [DOI] [PubMed] [Google Scholar]
- 311.Podschun, R., and U. Ullmann. 1998. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin. Microbiol. Rev. 11:589-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Poore, C. A., and H. L. Mobley. 2003. Differential regulation of the Proteus mirabilis urease gene cluster by UreR and H-NS. Microbiology 149:3383-3394. [DOI] [PubMed] [Google Scholar]
- 313.Pratt, L. A., and R. Kolter. 1998. Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol. Microbiol. 30:285-293. [DOI] [PubMed] [Google Scholar]
- 314.Priestley, J. T., and J. H. Dunn. 1949. Branched renal calculi. J. Urol. 61:194. [DOI] [PubMed] [Google Scholar]
- 315.Raetz, C. R. H. 1996. Bacterial lipopolysaccharides: a remarkable family of bioactive macroamphiphiles, p. 1035-1063. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology. American Society for Microbiology, Washington, DC.
- 316.Rahman, M. M., J. Guard-Petter, K. Asokan, C. Hughes, and R. W. Carlson. 1999. The structure of the colony migration factor from pathogenic Proteus mirabilis. A capsular polysaccharide that facilitates swarming. J. Biol. Chem. 274:22993-22998. [DOI] [PubMed] [Google Scholar]
- 317.Rahman, N. U., M. V. Meng, and M. L. Stoller. 2003. Infections and urinary stone disease. Curr. Pharm. Des. 9:975-981. [DOI] [PubMed] [Google Scholar]
- 318.Rather, P. N. 2005. Swarmer cell differentiation in Proteus mirabilis. Environ. Microbiol. 7:1065-1073. [DOI] [PubMed] [Google Scholar]
- 319.Reid, G., J. Howard, and B. S. Gan. 2001. Can bacterial interference prevent infection? Trends Microbiol. 9:424-428. [DOI] [PubMed] [Google Scholar]
- 320.Reid, G., S. Sharma, K. Advikolanu, C. Tieszer, R. A. Martin, and A. W. Bruce. 1994. Effects of ciprofloxacin, norfloxacin, and ofloxacin on in vitro adhesion and survival of Pseudomonas aeruginosa AK1 on urinary catheters. Antimicrob. Agents Chemother. 38:1490-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Reid, G., H. C. van der Mei, C. Tieszer, and H. J. Busscher. 1996. Uropathogenic Escherichia coli adhere to urinary catheters without using fimbriae. FEMS Immunol. Med. Microbiol. 16:159-162. [DOI] [PubMed] [Google Scholar]
- 322.Reigstad, C. S., S. J. Hultgren, and J. I. Gordon. 2007. Functional genomic studies of uropathogenic Escherichia coli and host urothelial cells when intracellular bacterial communities are assembled. J. Biol. Chem. 282:21259-21267. [DOI] [PubMed] [Google Scholar]
- 323.Reissbrodt, R., and W. Rabsch. 1988. Further differentiation of Enterobacteriaceae by means of siderophore-pattern analysis. Zentralbl. Bakteriol. Mikrobiol. Hyg. A 268:306-317. [DOI] [PubMed] [Google Scholar]
- 324.Rietschel, E. T., T. Kirikae, F. U. Schade, U. Mamat, G. Schmidt, H. Loppnow, A. J. Ulmer, U. Zahringer, U. Seydel, F. Di Padova, M. Schreier, and H. Brade. 1994. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J. 8:217-225. [DOI] [PubMed] [Google Scholar]
- 325.Riley, D. K., D. C. Classen, L. E. Stevens, and J. P. Burke. 1995. A large randomized clinical trial of a silver-impregnated urinary catheter: lack of efficacy and staphylococcal superinfection. Am. J. Med. 98:349-356. [DOI] [PubMed] [Google Scholar]
- 326.Roberts, A. P., J. Pratten, M. Wilson, and P. Mullany. 1999. Transfer of a conjugative transposon, Tn5397 in a model oral biofilm. FEMS Microbiol. Lett. 177:63-66. [DOI] [PubMed] [Google Scholar]
- 327.Roberts, J. A., E. N. Fussell, and M. B. Kaack. 1990. Bacterial adherence to urethral catheters. J. Urol. 144:264-269. [DOI] [PubMed] [Google Scholar]
- 328.Rosenheim, M. L. 1935. Mandelic acid in the treatment of urinary tract infections. Lancet i:1032?-1035. [Google Scholar]
- 329.Rozalski, A., H. Dlugonska, and K. Kotelko. 1986. Cell invasiveness of Proteus mirabilis and Proteus vulgaris strains. Arch. Immunol. Ther. Exp. 34:505-512. [PubMed] [Google Scholar]
- 330.Rozalski, A., and K. Kotelko. 1987. Hemolytic activity and invasiveness in strains of Proteus penneri. J. Clin. Microbiol. 25:1094-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Rozalski, A., Z. Sidorczyk, and K. Kotelko. 1997. Potential virulence factors of Proteus bacilli. Microbiol. Mol. Biol. Rev. 61:65-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Rudra, A., and P. Rudra. 2002. Nosocomial infections in intensive care unit. Ind. J. Crit. Care Med. 6:127-138. [Google Scholar]
- 333.Rupp, M. E., T. Fitzgerald, N. Marion, V. Helget, S. Puumala, J. R. Anderson, and P. D. Fey. 2004. Effect of silver-coated urinary catheters: efficacy, cost-effectiveness, and antimicrobial resistance. Am. J. Infect. Control 32:445-450. [DOI] [PubMed] [Google Scholar]
- 334.Russo, T. A., U. B. Carlino, and J. R. Johnson. 2001. Identification of a new iron-regulated virulence gene, ireA, in an extraintestinal pathogenic isolate of Escherichia coli. Infect. Immun. 69:6209-6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Russo, T. A., C. D. McFadden, U. B. Carlino-MacDonald, J. M. Beanan, T. J. Barnard, and J. R. Johnson. 2002. IroN functions as a siderophore receptor and is a urovirulence factor in an extraintestinal pathogenic isolate of Escherichia coli. Infect. Immun. 70:7156-7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Russo, T. A., C. D. McFadden, U. B. Carlino-MacDonald, J. M. Beanan, R. Olson, and G. E. Wilding. 2003. The siderophore receptor IroN of extraintestinal pathogenic Escherichia coli is a potential vaccine candidate. Infect. Immun. 71:7164-7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Sabbuba, N., G. Hughes, and D. J. Stickler. 2002. The migration of Proteus mirabilis and other urinary tract pathogens over Foley catheters. BJU Int. 89:55-60. [DOI] [PubMed] [Google Scholar]
- 338.Sabbuba, N. A., D. J. Stickler, E. Mahenthiralingam, D. J. Painter, J. Parkin, and R. C. Feneley. 2004. Genotyping demonstrates that the strains of Proteus mirabilis from bladder stones and catheter encrustations of patients undergoing long-term bladder catheterization are identical. J. Urol. 171:1925-1928. [DOI] [PubMed] [Google Scholar]
- 339.Saint, S. 2000. Clinical and economic consequences of nosocomial catheter-related bacteriuria. Am. J. Infect. Control 28:68-75. [DOI] [PubMed] [Google Scholar]
- 340.Saint, S., and C. E. Chenoweth. 2003. Biofilms and catheter-associated urinary tract infections. Infect. Dis. Clin. N. Am. 17:411-432. [DOI] [PubMed] [Google Scholar]
- 341.Saint, S., J. G. Elmore, S. D. Sullivan, S. S. Emerson, and T. D. Koepsell. 1998. The efficacy of silver alloy-coated urinary catheters in preventing urinary tract infection: a meta-analysis. Am. J. Med. 105:236-241. [DOI] [PubMed] [Google Scholar]
- 342.Saint, S., J. Wiese, J. K. Amory, M. L. Bernstein, U. D. Patel, J. K. Zemencuk, S. J. Bernstein, B. A. Lipsky, and T. P. Hofer. 2000. Are physicians aware of which of their patients have indwelling urinary catheters? Am. J. Med. 109:476-480. [DOI] [PubMed] [Google Scholar]
- 343.Sareneva, T., H. Holthofer, and T. K. Korhonen. 1990. Tissue-binding affinity of Proteus mirabilis fimbriae in the human urinary tract. Infect. Immun. 58:3330-3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Sauer, F. G., M. A. Mulvey, J. D. Schilling, J. J. Martinez, and S. J. Hultgren. 2000. Bacterial pili: molecular mechanisms of pathogenesis. Curr. Opin. Microbiol. 3:65-72. [DOI] [PubMed] [Google Scholar]
- 345.Schaeffer, A. J., W. R. Schwan, S. J. Hultgren, and J. L. Duncan. 1987. Relationship of type 1 pilus expression in Escherichia coli to ascending urinary tract infections in mice. Infect. Immun. 55:373-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Schembri, M. A., J. Blom, K. A. Krogfelt, and P. Klemm. 2005. Capsule and fimbria interaction in Klebsiella pneumoniae. Infect. Immun. 73:4626-4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Schembri, M. A., G. Christiansen, and P. Klemm. 2001. FimH-mediated autoaggregation of Escherichia coli. Mol. Microbiol. 41:1419-1430. [DOI] [PubMed] [Google Scholar]
- 348.Schembri, M. A., and P. Klemm. 2001. Biofilm formation in a hydrodynamic environment by novel FimH variants and ramifications for virulence. Infect. Immun. 69:1322-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Schilling, J. D., S. M. Martin, C. S. Hung, R. G. Lorenz, and S. J. Hultgren. 2003. Toll-like receptor 4 on stromal and hematopoietic cells mediates innate resistance to uropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 100:4203-4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Schilling, J. D., S. M. Martin, D. A. Hunstad, K. P. Patel, M. A. Mulvey, S. S. Justice, R. G. Lorenz, and S. J. Hultgren. 2003. CD14- and Toll-like receptor-dependent activation of bladder epithelial cells by lipopolysaccharide and type 1 piliated Escherichia coli. Infect. Immun. 71:1470-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Schilling, J. D., M. A. Mulvey, C. D. Vincent, R. G. Lorenz, and S. J. Hultgren. 2001. Bacterial invasion augments epithelial cytokine responses to Escherichia coli through a lipopolysaccharide-dependent mechanism. J. Immunol. 166:1148-1155. [DOI] [PubMed] [Google Scholar]
- 352.Schmidhammer, S., R. Ramoner, L. Holtl, G. Bartsch, M. Thurnher, and C. Zelle-Rieser. 2002. An Escherichia coli-based oral vaccine against urinary tract infections potently activates human dendritic cells. Urology 60:521-526. [DOI] [PubMed] [Google Scholar]
- 353.Schmidtchen, A., I. M. Frick, E. Andersson, H. Tapper, and L. Bjorck. 2002. Proteinases of common pathogenic bacteria degrade and inactivate the antibacterial peptide LL-37. Mol. Microbiol. 46:157-168. [DOI] [PubMed] [Google Scholar]
- 354.Schneider, R., C. V. Lockatell, D. Johnson, and R. Belas. 2002. Detection and mutation of a luxS-encoded autoinducer in Proteus mirabilis. Microbiology 148:773-782. [DOI] [PubMed] [Google Scholar]
- 355.Schwan, W. R., M. T. Beck, S. J. Hultgren, J. Pinkner, N. L. Woolever, and T. Larson. 2005. Down-regulation of the kps region 1 capsular assembly operon following attachment of Escherichia coli type 1 fimbriae to d-mannose receptors. Infect. Immun. 73:1226-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Scott, T. G. 1960. The bacteriology of urinary infections in paraplegia. J. Clin. Pathol. 13:54-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Segura, J. W., G. M. Preminger, D. G. Assimos, S. P. Dretler, R. I. Kahn, J. E. Lingeman, J. N. Macaluso, Jr., D. L. McCullough, et al. 1994. Nephrolithiasis Clinical Guidelines Panel summary report on the management of staghorn calculi. J. Urol. 151:1648-1651. [DOI] [PubMed] [Google Scholar]
- 358.Senior, B. W., M. Albrechtsen, and M. A. Kerr. 1988. A survey of IgA protease production among clinical isolates of Proteeae. J. Med. Microbiol. 25:27-31. [DOI] [PubMed] [Google Scholar]
- 359.Senior, B. W., M. Albrechtsen, and M. A. Kerr. 1987. Proteus mirabilis strains of diverse type have IgA protease activity. J. Med. Microbiol. 24:175-180. [DOI] [PubMed] [Google Scholar]
- 360.Senior, B. W., and C. Hughes. 1988. Production and properties of haemolysins from clinical isolates of the Proteeae. J. Med. Microbiol. 25:17-25. [DOI] [PubMed] [Google Scholar]
- 361.Senior, B. W., and D. L. Leslie. 1986. Rare occurrence of Proteus vulgaris in faeces: a reason for its rare association with urinary tract infections. J. Med. Microbiol. 21:139-144. [DOI] [PubMed] [Google Scholar]
- 362.Senior, B. W., L. M. Loomes, and M. A. Kerr. 1991. The production and activity in vivo of Proteus mirabilis IgA protease in infections of the urinary tract. J. Med. Microbiol. 35:203-207. [DOI] [PubMed] [Google Scholar]
- 363.Shand, G. H., H. Anwar, J. Kadurugamuwa, M. R. Brown, S. H. Silverman, and J. Melling. 1985. In vivo evidence that bacteria in urinary tract infection grow under iron-restricted conditions. Infect. Immun. 48:35-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Shashkov, A. S., A. N. Kondakova, S. N. Senchenkova, K. Zych, F. V. Toukach, Y. A. Knirel, and Z. Sidorczyk. 2000. Structure of a 2-aminoethyl phosphate-containing O-specific polysaccharide of Proteus penneri 63 from a new serogroup O68. Eur. J. Biochem. 267:601-605. [DOI] [PubMed] [Google Scholar]
- 365.Shashkov, A. S., S. N. Senchenkova, E. V. Vinogradov, G. V. Zatonsky, Y. A. Knirel, E. Literacka, and W. Kaca. 2000. Full structure of the O-specific polysaccharide of Proteus mirabilis O24 containing 3,4-O-[(S)-1-carboxyethylidene]-D-galactose. Carbohydr. Res. 329:453-457. [DOI] [PubMed] [Google Scholar]
- 366.Sidorczyk, Z., W. Kaca, and K. Kotelko. 1975. Studies on lipopolysaccharides of Proteus vulgaris serogroups. Chemotypes of genus Proteus lipopolysaccharides. Bull. Acad. Pol. Sci. Biol. 23:603-609. [PubMed] [Google Scholar]
- 367.Sidorczyk, Z., A. Swierzko, E. V. Vinogradov, Y. A. Knirel, and A. S. Shashkov. 1994. Structural and immunochemical studies on O-specific polysaccharide of Proteus penneri strain 14. Arch. Immunol. Ther. Exp. 42:209-215. [PubMed] [Google Scholar]
- 368.Sidorczyk, Z., A. Swierzko, K. Zych, A. S. Shashkov, E. V. Vinogradov, and Y. A. Knirel. 1993. Immunochemical studies of O-specific polysaccharide of Proteus penneri 42 lipopolysaccharide. Med. Dosw. Mikrobiol. 45:89-92. (In Polish.) [PubMed] [Google Scholar]
- 369.Sidorczyk, Z., K. Zych, F. V. Toukach, N. P. Arbatsky, A. Zablotni, A. S. Shashkov, and Y. A. Knirel. 2002. Structure of the O-polysaccharide and classification of Proteus mirabilis strain G1 in Proteus serogroup O3. Eur. J. Biochem. 269:1406-1412. [DOI] [PubMed] [Google Scholar]
- 370.Sidroczyk, Z., A. Swierzko, M. Lipinska, E. V. Vinogradov, A. S. Shashkov, and Y. A. Knirel. 1993. Immunochemical studies of O-specific polysaccharide from Proteus penneri 14 lipopolysaccharide. Med. Dosw. Mikrobiol. 45:85-87. (In Polish.) [PubMed] [Google Scholar]
- 371.Silverblatt, F. J., and I. Olek. 1978. Effects of pili on susceptibility of Proteus mirabilis to phagocytosis and on adherence to bladder cells, p. 49-59. In E. Kass and W. Brumfitt (ed.), Infections of the urinary tract. University of Chicago Press, Chicago, IL.
- 372.Simerville, J. A., W. C. Maxted, and J. J. Pahira. 2005. Urinalysis: a comprehensive review. Am. Fam. Physician 71:1153-1162. [PubMed] [Google Scholar]
- 373.Singh, P. K., M. R. Parsek, E. P. Greenberg, and M. J. Welsh. 2002. A component of innate immunity prevents bacterial biofilm development. Nature 417:552-555. [DOI] [PubMed] [Google Scholar]
- 374.Siroky, M. B. 2002. Pathogenesis of bacteriuria and infection in the spinal cord injured patient. Am. J. Med. 113(Suppl. 1A):67S-79S. [DOI] [PubMed] [Google Scholar]
- 375.Smith, T. K., A. J. Hudson, and R. C. Spencer. 1988. Evaluation of six screening methods for detecting significant bacteriuria. J. Clin. Pathol. 41:904-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Snyder, J. A., B. J. Haugen, E. L. Buckles, C. V. Lockatell, D. E. Johnson, M. S. Donnenberg, R. A. Welch, and H. L. Mobley. 2004. Transcriptome of uropathogenic Escherichia coli during urinary tract infection. Infect. Immun. 72:6373-6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Snyder, J. A., B. J. Haugen, C. V. Lockatell, N. Maroncle, E. C. Hagan, D. E. Johnson, R. A. Welch, and H. L. Mobley. 2005. Coordinate expression of fimbriae in uropathogenic Escherichia coli. Infect. Immun. 73:7588-7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Sorsa, L. J., S. Dufke, J. Heesemann, and S. Schubert. 2003. Characterization of an iroBCDEN gene cluster on a transmissible plasmid of uropathogenic Escherichia coli: evidence for horizontal transfer of a chromosomal virulence factor. Infect. Immun. 71:3285-3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Soto, G. E., and S. J. Hultgren. 1999. Bacterial adhesins: common themes and variations in architecture and assembly. J. Bacteriol. 181:1059-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Springall, T., N. S. Sheerin, K. Abe, V. M. Holers, H. Wan, and S. H. Sacks. 2001. Epithelial secretion of C3 promotes colonization of the upper urinary tract by Escherichia coli. Nat. Med. 7:801-806. [DOI] [PubMed] [Google Scholar]
- 381.Stahl, S. J., K. R. Stewart, and F. D. Williams. 1983. Extracellular slime associated with Proteus mirabilis during swarming. J. Bacteriol. 154:930-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Stamm, W. E. 1991. Catheter-associated urinary tract infections: epidemiology, pathogenesis, and prevention. Am. J. Med. 91:65S-71S. [DOI] [PubMed] [Google Scholar]
- 383.Stamm, W. E., and T. M. Hooton. 1993. Management of urinary tract infections in adults. N. Engl. J. Med. 329:1328-1334. [DOI] [PubMed] [Google Scholar]
- 384.Stanley, P., V. Koronakis, and C. Hughes. 1998. Acylation of Escherichia coli hemolysin: a unique protein lipidation mechanism underlying toxin function. Microbiol. Mol. Biol. Rev. 62:309-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Stewart, P. S. 2003. New ways to stop biofilm infections. Lancet 361:97. [DOI] [PubMed] [Google Scholar]
- 386.Stickler, D., R. Young, G. Jones, N. Sabbuba, and N. Morris. 2003. Why are Foley catheters so vulnerable to encrustation and blockage by crystalline bacterial biofilm? Urol. Res. 31:306-311. [DOI] [PubMed] [Google Scholar]
- 387.Stickler, D. J. 1996. Bacterial biofilms and the encrustation of urethral catheters. Biofouling 9:293-305. [Google Scholar]
- 387a.Stickler, D. J. 1996. Biofilms, catheters, and urinary tract infections. Eur. Urol. Update Ser. 5:1-8. [Google Scholar]
- 388.Stickler, D. J., G. L. Jones, and A. D. Russell. 2003. Control of encrustation and blockage of Foley catheters. Lancet 361:1435-1437. [DOI] [PubMed] [Google Scholar]
- 389.Stickler, D. J., S. M. Jones, G. O. Adusei, and M. G. Waters. 2006. A sensor to detect the early stages in the development of crystalline Proteus mirabilis biofilm on indwelling bladder catheters. J. Clin. Microbiol. 44:1540-1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Stickler, D. J., S. M. Jones, G. O. Adusei, M. G. Waters, J. Cloete, S. Mathur, and R. C. Feneley. 2006. A clinical assessment of the performance of a sensor to detect crystalline biofilm formation on indwelling bladder catheters. BJU Int. 98:1244-1249. [DOI] [PubMed] [Google Scholar]
- 390a.Stickler, D. J., J. B. King, C. Winters, and S. L. Morris. 1993. Blockage of urethral catheters by bacterial biofilms. J. Infect. 27:133-135. [DOI] [PubMed] [Google Scholar]
- 391.Stickler, D. J., J. C. Lear, N. S. Morris, S. M. Macleod, A. Downer, D. H. Cadd, and W. J. Feast. 2006. Observations on the adherence of Proteus mirabilis onto polymer surfaces. J. Appl. Microbiol. 100:1028-1033. [DOI] [PubMed] [Google Scholar]
- 392.Stickler, D. J., and S. D. Morgan. 2006. Modulation of crystalline Proteus mirabilis biofilm development on urinary catheters. J. Med. Microbiol. 55:489-494. [DOI] [PubMed] [Google Scholar]
- 393.Stickler, D. J., N. S. Morris, R. J. McLean, and C. Fuqua. 1998. Biofilms on indwelling urethral catheters produce quorum-sensing signal molecules in situ and in vitro. Appl. Environ. Microbiol. 64:3486-3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Stickler, D. J., and J. Zimakoff. 1994. Complications of urinary tract infections associated with devices used for long-term bladder management. J. Hosp. Infect. 28:177-194. [DOI] [PubMed] [Google Scholar]
- 395.St. Swierzko, A., T. Kirikae, F. Kirikae, M. Hirata, M. Cedzynski, A. Ziolkowski, Y. Hirai, S. Kusumoto, T. Yokochi, and M. Nakano. 2000. Biological activities of lipopolysaccharides of Proteus spp. and their interactions with polymyxin B and an 18-kDa cationic antimicrobial protein (CAP18)-derived peptide. J. Med. Microbiol. 49:127-138. [DOI] [PubMed] [Google Scholar]
- 396.Sturgill, G., and P. N. Rather. 2004. Evidence that putrescine acts as an extracellular signal required for swarming in Proteus mirabilis. Mol. Microbiol. 51:437-446. [DOI] [PubMed] [Google Scholar]
- 397.Suby, H. I., and F. Albright. 1943. Dissolution of phosphatic urinary calculi by the retrograde introduction of a citrate solution containing magnesium. N. Engl. J. Med. 228:81-91. [Google Scholar]
- 398.Swihart, K. G., and R. A. Welch. 1990. Cytotoxic activity of the Proteus hemolysin HpmA. Infect. Immun. 58:1861-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Swihart, K. G., and R. A. Welch. 1990. The HpmA hemolysin is more common than HlyA among Proteus isolates. Infect. Immun. 58:1853-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Tambyah, P. A., K. T. Halvorson, and D. G. Maki. 1999. A prospective study of pathogenesis of catheter-associated urinary tract infections. Mayo Clin. Proc. 74:131-136. [DOI] [PubMed] [Google Scholar]
- 401.Tambyah, P. A., and D. G. Maki. 2000. Catheter-associated urinary tract infection is rarely symptomatic: a prospective study of 1,497 catheterized patients. Arch. Intern. Med. 160:678-682. [DOI] [PubMed] [Google Scholar]
- 402.Tambyah, P. A., and D. G. Maki. 2000. The relationship between pyuria and infection in patients with indwelling urinary catheters: a prospective study of 761 patients. Arch. Intern. Med. 160:673-677. [DOI] [PubMed] [Google Scholar]
- 403.Thankavel, K., B. Madison, T. Ikeda, R. Malaviya, A. H. Shah, P. M. Arumugam, and S. N. Abraham. 1997. Localization of a domain in the FimH adhesin of Escherichia coli type 1 fimbriae capable of receptor recognition and use of a domain-specific antibody to confer protection against experimental urinary tract infection. J. Clin. Investig. 100:1123-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Thibon, P., C. Le, X. R. Leroyer, and J. Fabry. 2000. Randomized multi-centre trial of the effects of a catheter coated with hydrogel and silver salts on the incidence of hospital-acquired urinary tract infections. J. Hosp. Infect. 45:117-124. [DOI] [PubMed] [Google Scholar]
- 405.Thomas, V. J., and C. M. Collins. 1999. Identification of UreR binding sites in the Enterobacteriaceae plasmid-encoded and Proteus mirabilis urease gene operons. Mol. Microbiol. 31:1417-1428. [DOI] [PubMed] [Google Scholar]
- 406.Tolson, D. L., D. L. Barrigar, R. J. McLean, and E. Altman. 1995. Expression of a nonagglutinating fimbria by Proteus mirabilis. Infect. Immun. 63:1127-1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Tolson, D. L., B. A. Harrison, R. K. Latta, K. K. Lee, and E. Altman. 1997. The expression of nonagglutinating fimbriae and its role in Proteus mirabilis adherence to epithelial cells. Can. J. Microbiol. 43:709-717. [DOI] [PubMed] [Google Scholar]
- 408.Torzewska, A., P. Staczek, and A. Rozalski. 2003. Crystallization of urine mineral components may depend on the chemical nature of Proteus endotoxin polysaccharides. J. Med. Microbiol. 52:471-477. [DOI] [PubMed] [Google Scholar]
- 409.Toughill, E. 2005. Indwelling urinary catheters: common mechanical and pathogenic problems. Am. J. Nurs. 105:35-37. [DOI] [PubMed] [Google Scholar]
- 410.Trautner, B. W., and R. O. Darouiche. 2004. Role of biofilm in catheter-associated urinary tract infection. Am. J. Infect. Control 32:177-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Trautner, B. W., R. O. Darouiche, R. A. Hull, S. Hull, and J. I. Thornby. 2002. Pre-inoculation of urinary catheters with Escherichia coli 83972 inhibits catheter colonization by Enterococcus faecalis. J. Urol. 167:375-379. [PMC free article] [PubMed] [Google Scholar]
- 412.Trautner, B. W., R. A. Hull, and R. O. Darouiche. 2005. Prevention of catheter-associated urinary tract infection. Curr. Opin. Infect. Dis. 18: 37-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Trautner, B. W., R. A. Hull, and R. O. Darouiche. 2005. Colicins prevent colonization of urinary catheters. J. Antimicrob. Chemother. 56:413-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Trautner, B. W., R. A. Hull, and R. O. Darouiche. 2003. Escherichia coli 83972 inhibits catheter adherence by a broad spectrum of uropathogens. Urology 61:1059-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Troy, F. A. 1992. Polysialylation: from bacteria to brains. Glycobiology 2:5-23. [DOI] [PubMed] [Google Scholar]
- 416.Tseng, C. C., J. J. Huang, W. C. Ko, J. J. Yan, and J. J. Wu. 2001. Decreased predominance of papG class II allele in Escherichia coli strains isolated from adults with acute pyelonephritis and urinary tract abnormalities. J. Urol. 166:1643-1646. [PubMed] [Google Scholar]
- 417.Tunney, M. M., D. S. Jones, and S. P. Gorman. 1999. Biofilms and biofilm-related encrustration of urinary tract devices. Methods Enzymol. 310:558-566. [DOI] [PubMed] [Google Scholar]
- 418.Uehling, D. T., W. J. Hopkins, J. E. Elkahwaji, D. M. Schmidt, and G. E. Leverson. 2003. Phase 2 clinical trial of a vaginal mucosal vaccine for urinary tract infections. J. Urol. 170:867-869. [DOI] [PubMed] [Google Scholar]
- 419.Uhrin, D., J. R. Brisson, L. L. MacLean, J. C. Richards, and M. B. Perry. 1994. Application of 1D and 2D NMR techniques to the structure elucidation of the O-polysaccharide from Proteus mirabilis O:57. J. Biomol. NMR 4:615-630. [DOI] [PubMed] [Google Scholar]
- 420.Ulett, G. C., A. N. Mabbett, K. C. Fung, R. I. Webb, and M. A. Schembri. 2007. The role of F9 fimbriae of uropathogenic Escherichia coli in biofilm formation. Microbiology 153:2321-2331. [DOI] [PubMed] [Google Scholar]
- 421.Uphoff, T. S., and R. A. Welch. 1990. Nucleotide sequencing of the Proteus mirabilis calcium-independent hemolysin genes (hpmA and hpmB) reveals sequence similarity with the Serratia marcescens hemolysin genes (shlA and shlB). J. Bacteriol. 172:1206-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.U.S. Census Bureau. 2007. Midyear population, by age and sex. Population Division, U.S. Census Bureau, Washington, DC. http://www.census.gov/cgi-bin/ipc/idbsprd.
- 423.Valdebenito, M., B. Bister, R. Reissbrodt, K. Hantke, and G. Winkelmann. 2005. The detection of salmochelin and yersiniabactin in uropathogenic Escherichia coli strains by a novel hydrolysis-fluorescence-detection (HFD) method. Int. J. Med. Microbiol. 295:99-107. [DOI] [PubMed] [Google Scholar]
- 424.Vapnek, J. M., F. M. Maynard, and J. Kim. 2003. A prospective randomized trial of the LoFric hydrophilic coated catheter versus conventional plastic catheter for clean intermittent catheterization. J. Urol. 169:994-998. [DOI] [PubMed] [Google Scholar]
- 425.Vinogradov, E., and M. B. Perry. 2000. Structural analysis of the core region of lipopolysaccharides from Proteus mirabilis serotypes O6, O48 and O57. Eur. J. Biochem. 267:2439-2446. [DOI] [PubMed] [Google Scholar]
- 426.Vinogradov, E., and J. Radziejewska-Lebrecht. 2000. The structure of the carbohydrate backbone of the core-lipid A region of the lipopolysaccharide from Proteus mirabilis serotype O28. Carbohydr. Res. 329:351-357. [DOI] [PubMed] [Google Scholar]
- 427.Vinogradov, E. V., W. Kaca, Y. A. Knirel, A. Rozalski, and N. K. Kochetkov. 1989. Structural studies on the fucosamine-containing O-specific polysaccharide of Proteus vulgaris O19. Eur. J. Biochem. 180:95-99. [DOI] [PubMed] [Google Scholar]
- 428.Vinogradov, E. V., W. Kaca, A. Rozalski, A. S. Shashkov, M. Cedzynski, Y. A. Knirel, and N. K. Kochetkov. 1991. Structural and immunochemical studies of O-specific polysaccharide of Proteus vulgaris 5/43 belonging to OX19 group (O-variants). Eur. J. Biochem. 200:195-201. [DOI] [PubMed] [Google Scholar]
- 429.Vinogradov, E. V., W. Kaca, A. S. Shashkov, D. Krajewska-Pietrasik, A. Rozalski, Y. A. Knirel, and N. K. Kochetkov. 1990. The structure of Proteus mirabilis O3 O-specific polysaccharide containing N-(2-hydroxyethyl)-D-alanine. Eur. J. Biochem. 188:645-651. [DOI] [PubMed] [Google Scholar]
- 430.Vinogradov, E. V., D. Krajewska-Pietrasik, W. Kaca, A. S. Shashkov, Y. A. Knirel, and N. K. Kochetkov. 1989. Structure of Proteus mirabilis O27 O-specific polysaccharide containing amino acids and phosphoethanolamine. Eur. J. Biochem. 185:645-650. [DOI] [PubMed] [Google Scholar]
- 431.Vinogradov, E. V., A. S. Shashkov, Y. A. Knirel, N. K. Kochetkov, Z. Sidorczyk, and A. Swierzko. 1991. The structure of Proteus penneri strain 14-O-specific polysaccharide containing D- and L-alanine. Carbohydr. Res. 219:c1-c3. [DOI] [PubMed] [Google Scholar]
- 432.Vinogradov, E. V., Z. Sidorczyk, A. Swierzko, A. Rozalski, E. D. Daeva, A. S. Shashkov, Y. A. Knirel, and N. K. Kochetkov. 1991. The structure of the O-specific polysaccharide chain of Proteus penneri strain 16 lipopolysaccharide. Eur. J. Biochem. 197:93-103. [DOI] [PubMed] [Google Scholar]
- 433.Walker, K. E., S. Moghaddame-Jafari, C. V. Lockatell, D. Johnson, and R. Belas. 1999. ZapA, the IgA-degrading metalloprotease of Proteus mirabilis, is a virulence factor expressed specifically in swarmer cells. Mol. Microbiol. 32:825-836. [DOI] [PubMed] [Google Scholar]
- 434.Walker, S., A. McGeer, A. E. Simor, M. Armstrong-Evans, and M. Loeb. 2000. Why are antibiotics prescribed for asymptomatic bacteriuria in institutionalized elderly people? A qualitative study of physicians’ and nurses’ perceptions. Can. Med. Assoc. J. 163:273-277. [PMC free article] [PubMed] [Google Scholar]
- 435.Walz, S. E., S. K. Wray, S. I. Hull, and R. A. Hull. 1988. Multiple proteins encoded within the urease gene complex of Proteus mirabilis. J. Bacteriol. 170:1027-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Wang, L. P., H. Y. Wong, and D. P. Griffith. 1997. Treatment options in struvite stones. Urol. Clin. N. Am. 24:149-162. [DOI] [PubMed] [Google Scholar]
- 437.Wang, X., J. F. Preston III, and T. Romeo. 2004. The pgaABCD locus of Escherichia coli promotes the synthesis of a polysaccharide adhesin required for biofilm formation. J. Bacteriol. 186:2724-2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 438.Warren, J. W. 1997. Catheter-associated urinary tract infections. Infect. Dis. Clin. N. Am. 11:609-622. [DOI] [PubMed] [Google Scholar]
- 439.Warren, J. W. 1996. Clinical presentations and epidemiology of urinary tract infections, In H. L. Mobley and J. W. Warren (ed.), Urinary tract infections: molecular pathogenesis and clinical management. ASM Press, Washington, DC.
- 440.Warren, J. W. 2001. Catheter-associated urinary tract infections. Int. J. Antimicrob. Agents 17:299-303. [DOI] [PubMed] [Google Scholar]
- 441.Warren, J. W., D. Damron, J. H. Tenney, J. M. Hoopes, B. Deforge, and H. L. Muncie, Jr. 1987. Fever, bacteremia, and death as complications of bacteriuria in women with long-term urethral catheters. J. Infect. Dis. 155:1151-1158. [DOI] [PubMed] [Google Scholar]
- 442.Warren, J. W., H. L. Mobley, and A. L. Trifillis. 1988. Internalization of Escherichia coli into human renal tubular epithelial cells. J. Infect. Dis. 158:221-223. [DOI] [PubMed] [Google Scholar]
- 443.Warren, J. W., R. Platt, R. J. Thomas, B. Rosner, and E. H. Kass. 1978. Antibiotic irrigation and catheter-associated urinary-tract infections. N. Engl. J. Med. 299:570-573. [DOI] [PubMed] [Google Scholar]
- 444.Warren, J. W., J. H. Tenney, J. M. Hoopes, H. L. Muncie, and W. C. Anthony. 1982. A prospective microbiologic study of bacteriuria in patients with chronic indwelling urethral catheters. J. Infect. Dis. 146:719-723. [DOI] [PubMed] [Google Scholar]
- 445.Wassif, C., D. Cheek, and R. Belas. 1995. Molecular analysis of a metalloprotease from Proteus mirabilis. J. Bacteriol. 177:5790-5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Wazait, H. D., H. R. Patel, V. Veer, M. Kelsey, J. H. Van Der Meulen, R. A. Miller, and M. Emberton. 2003. Catheter-associated urinary tract infections: prevalence of uropathogens and pattern of antimicrobial resistance in a UK hospital (1996-2001). BJU Int. 91:806-809. [DOI] [PubMed] [Google Scholar]
- 447.Weinberg, E. D. 1984. Iron withholding: a defense against infection and neoplasia. Physiol. Rev. 64:65-102. [DOI] [PubMed] [Google Scholar]
- 448.Welch, R. A. 1991. Pore-forming cytolysins of gram-negative bacteria. Mol. Microbiol. 5:521-528. [DOI] [PubMed] [Google Scholar]
- 449.Welch, R. A. 1987. Identification of two different hemolysin determinants in uropathogenic Proteus isolates. Infect. Immun. 55:2183-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Welch, R. A., C. Forestier, A. Lobo, S. Pellett, W. Thomas, Jr., and G. Rowe. 1992. The synthesis and function of the Escherichia coli hemolysin and related RTX exotoxins. FEMS Microbiol. Immunol. 5:29-36. [DOI] [PubMed] [Google Scholar]
- 451.Whitfield, C., and I. S. Roberts. 1999. Structure, assembly and regulation of expression of capsules in Escherichia coli. Mol. Microbiol. 31:1307-1319. [DOI] [PubMed] [Google Scholar]
- 452.Williams, D. H., and A. J. Schaeffer. 2004. Current concepts in urinary tract infections. Minerva Urol. Nefrol. 56:15-31. [PubMed] [Google Scholar]
- 453.Williams, F. D., and R. H. Schwarzhoff. 1978. Nature of the swarming phenomenon in Proteus. Annu. Rev. Microbiol. 32:101-122. [DOI] [PubMed] [Google Scholar]
- 454.Williams, P., P. A. Lambert, M. R. Brown, and R. J. Jones. 1983. The role of the O and K antigens in determining the resistance of Klebsiella aerogenes to serum killing and phagocytosis. J. Gen. Microbiol. 129:2181-2191. [DOI] [PubMed] [Google Scholar]
- 455.Wong, E. S. 1983. Guideline for prevention of catheter-associated urinary tract infections. Am. J. Infect. Control 11:28-36. [DOI] [PubMed] [Google Scholar]
- 456.Wray, S. K., S. I. Hull, R. G. Cook, J. Barrish, and R. A. Hull. 1986. Identification and characterization of a uroepithelial cell adhesin from a uropathogenic isolate of Proteus mirabilis. Infect. Immun. 54:43-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Wright, A. C., L. M. Simpson, and J. D. Oliver. 1981. Role of iron in the pathogenesis of Vibrio vulnificus infections. Infect. Immun. 34:503-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Wright, D. N., B. Saxon, and J. M. Matsen. 1986. Use of the Bac-T-Screen to predict bacteriuria from urine specimens held at room temperature. J. Clin. Microbiol. 24:214-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Wright, K. J., P. C. Seed, and S. J. Hultgren. 2005. Uropathogenic Escherichia coli flagella aid in efficient urinary tract colonization. Infect. Immun. 73:7657-7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Wright, K. J., P. C. Seed, and S. J. Hultgren. 2007. Development of intracellular bacterial communities of uropathogenic Escherichia coli depends on type 1 pili. Cell. Microbiol. 9:2230-2241. [DOI] [PubMed] [Google Scholar]
- 461.Yakubu, D. E., D. C. Old, and B. W. Senior. 1989. The haemagglutinins and fimbriae of Proteus penneri. J. Med. Microbiol. 30:279-284. [DOI] [PubMed] [Google Scholar]
- 462.Yamamoto, T., A. Ariyoshi, and K. Amako. 1985. Fimbria-mediated adherence of Serratia marcescens strain US5 to human urinary bladder surface. Microbiol. Immunol. 29:677-681. [DOI] [PubMed] [Google Scholar]
- 463.Yassien, M., N. Khardori, A. Ahmedy, and M. Toama. 1995. Modulation of biofilms of Pseudomonas aeruginosa by quinolones. Antimicrob. Agents Chemother. 39:2262-2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Yoshikawa, T. T., L. E. Nicolle, and D. C. Norman. 1996. Management of complicated urinary tract infection in older patients. J. Am. Geriatr. Soc. 44:1235-1241. [DOI] [PubMed] [Google Scholar]
- 465.Zalewska, B., R. Piatek, K. Bury, A. Samet, B. Nowicki, S. Nowicki, and J. Kur. 2005. A surface-exposed DraD protein of uropathogenic Escherichia coli bearing Dr fimbriae may be expressed and secreted independently from DraC usher and DraE adhesin. Microbiology 151:2477-2486. [DOI] [PubMed] [Google Scholar]
- 466.Zhao, H., X. Li, D. E. Johnson, I. Blomfield, and H. L. Mobley. 1997. In vivo phase variation of MR/P fimbrial gene expression in Proteus mirabilis infecting the urinary tract. Mol. Microbiol. 23:1009-1019. [DOI] [PubMed] [Google Scholar]
- 467.Zhou, G., W. J. Mo, P. Sebbel, G. Min, T. A. Neubert, R. Glockshuber, X. R. Wu, T. T. Sun, and X. P. Kong. 2001. Uroplakin Ia is the urothelial receptor for uropathogenic Escherichia coli: evidence from in vitro FimH binding. J. Cell Sci. 114:4095-4103. [DOI] [PubMed] [Google Scholar]
- 468.Zhu, M., M. Valdebenito, G. Winkelmann, and K. Hantke. 2005. Functions of the siderophore esterases IroD and IroE in iron-salmochelin utilization. Microbiology 151:2363-2372. [DOI] [PubMed] [Google Scholar]
- 469.Zogaj, X., M. Nimtz, M. Rohde, W. Bokranz, and U. Romling. 2001. The multicellular morphotypes of Salmonella typhimurium and Escherichia coli produce cellulose as the second component of the extracellular matrix. Mol. Microbiol. 39:1452-1463. [DOI] [PubMed] [Google Scholar]
- 470.Zunino, P., L. Geymonat, A. G. Allen, C. Legnani-Fajardo, and D. J. Maskell. 2000. Virulence of a Proteus mirabilis ATF isogenic mutant is not impaired in a mouse model of ascending urinary tract infection. FEMS Immunol. Med. Microbiol. 29:137-143. [DOI] [PubMed] [Google Scholar]
- 471.Zunino, P., C. Piccini, and C. Legnani-Fajardo. 1994. Flagellate and non-flagellate Proteus mirabilis in the development of experimental urinary tract infection. Microb. Pathog. 16:379-385. [DOI] [PubMed] [Google Scholar]
- 472.Zunino, P., V. Sosa, A. G. Allen, A. Preston, G. Schlapp, and D. J. Maskell. 2003. Proteus mirabilis fimbriae (PMF) are important for both bladder and kidney colonization in mice. Microbiology 149:3231-3237. [DOI] [PubMed] [Google Scholar]
- 473.Zych, K., N. A. Kocharova, M. Kowalczyk, F. V. Toukach, D. Kaminska, A. S. Shashkov, Y. A. Knirel, and Z. Sidorczyk. 2000. Structure of the O-specific polysaccharide of Proteus penneri 71 and classification of cross-reactive P. penneri strains to a new proposed serogroup O64. Eur. J. Biochem. 267:808-814. [DOI] [PubMed] [Google Scholar]
- 474.Zych, K., and Z. Sidorczyk. 1989. Lipopolysaccharides of Proteus penneri species novum. Carbohydr. Res. 188:105-111. [DOI] [PubMed] [Google Scholar]