Abstract
Anthrax vaccine adsorbed (AVA; BioThrax), the current FDA-licensed human anthrax vaccine, contains various amounts of the three anthrax toxin components, protective antigen (PA), lethal factor (LF), and edema factor (EF). While antibody to PA is sufficient to mediate protection against anthrax in animal models, it is not known if antibodies to LF or EF contribute to protection in humans. Toxin-neutralizing activity was evaluated in sera from AVA-vaccinated volunteers, all of whom had antibody responses to LF and EF, as well as PA. The contribution of antibodies to LF and EF was assessed using mouse macrophage J774A.1 cells by examining neutralization of LF-induced lysis using alamarBlue reduction and neutralization of EF-induced cyclic AMP increases by enzyme-linked immunosorbent assay. Antibody responses to LF and EF were low compared to those to PA, and the amount of LF or EF in the assay could exceed the amount of antibodies to LF or EF. Higher titers were seen for most individuals when the LF or EF concentration was limiting compared to when LF or EF was in excess, initially suggesting that antibody to LF or EF augmented protection. However, depletion of LF and EF antibodies in sera did not result in a significant decrease in toxin neutralization. Overall, this study suggests that AVA-induced LF and EF antibodies do not significantly contribute to anthrax toxin neutralization in humans and that antibodies to PA are sufficient to neutralize toxin activity.
The Centers for Disease Control and Prevention high-priority biological threat agent Bacillus anthracis has two major virulence factors, an antiphagocytic capsule and a tripartite exotoxin, consisting of protective antigen (PA), lethal factor (LF), and edema factor (EF) (4). PA binds to cellular receptors and mediates entry of LF and EF into the cytosol (1, 34). LF is a zinc protease that cleaves mitogen-activated protein kinase kinases, while EF is an adenylate cyclase that converts ATP to cyclic AMP (cAMP) (5, 13). LF and EF inhibit the acquired and innate immune responses, allowing the bacteria to replicate unchecked in the host. While the poly-d-glutamic acid capsule is nonimmunogenic (20), the PA component of anthrax toxin has been shown to induce a protective antibody response in numerous studies using animal models of infection (11, 16, 18, 19, 23, 27) and is included in anthrax vaccines.
The current FDA-licensed human anthrax vaccine, anthrax vaccine adsorbed (AVA; BioThrax), has been used in the United States for over 30 years. AVA has been shown to protect animals from both cutaneous and inhalational anthrax challenges (for reviews, see references 14, 23, and 27). While AVA has been shown to protect occupationally exposed workers from cutaneous disease (2), the ability of AVA to protect humans from inhalation anthrax is unknown. In addition, there are several problems associated with the AVA vaccine. The immunization schedule is prolonged and consists of the initial inoculation; inoculations at 2 weeks, 4 weeks, 6 months, 12 months, and 18 months; and then a yearly booster. Furthermore, the AVA vaccine is extremely reactogenic, and previous studies have reported numerous adverse reactions to the anthrax vaccine (6-8, 21, 24, 30, 33). It would be advantageous to improve or replace the AVA vaccine if efficacy could be ensured.
AVA is formulated from an aluminum hydroxide-adsorbed, cell-free, formalin-treated filtrate culture of B. anthracis strain V770-NP1-R, a toxigenic, noncapsulated, and nonproteolytic mutant (25). The filtrate utilized for AVA preparation contains predominantly PA but also minute quantities of both LF and EF (35). While PA has been shown to induce a protective antibody response, a role for antibodies to LF and EF in mediating protection is less clear. An early study by Stanley and Smith reported that EF increased the immunizing activity of PA in guinea pigs; however, adding LF to the PA-plus-EF mixture decreased protection (29). Other studies have shown that both LF and EF have an additive effect on the immunizing capability of PA in rats, mice, and guinea pigs (12, 19, 22, 26). Mahlandt et al. further reported that LF was as protective as PA in rats (19). In contrast, a study by Little and Knudson demonstrated that, although PA-plus-LF/EF vaccines induced high LF and EF antibody titers, the vaccines did not increase protection of guinea pigs during B. anthracis spore challenge compared to the PA-alone vaccine (17).
The presence of LF- and EF-mediated toxic activity in AVA could contribute to the development of adverse reactions to vaccination, and concerns about the safety and efficacy of AVA have led to the development of new recombinant PA vaccines containing only PA (15). However, it is also possible that antibodies to LF and EF could contribute to the development of a protective immune response. In this study, human sera from individuals vaccinated with AVA were evaluated for the presence of toxin-neutralizing activity in two different cellular assays designed to measure LF-induced lysis or EF-mediated cAMP increases. AVA-vaccinated individuals developed antibody responses to LF and EF. However, antibodies to LF and EF did not appear to contribute to toxin neutralization.
MATERIALS AND METHODS
Serum sample collection.
Serum samples were obtained from AVA-vaccinated military personnel and researchers. Sera used for the negative control were drawn from five individuals, presumed to have had no exposure to B. anthracis or the vaccine. Samples were collected and processed as previously described (10).
Proteins and cell line.
PA, LF, and EF were obtained from List Biological Laboratories (Campbell, CA). J774A.1 cells (ATCC TIB-67), obtained from the American Type Culture Collection (Manassas, VA), were grown in high-glucose Dulbecco's modified Eagle medium containing l-glutamine and supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin (10,000 U/ml penicillin G sodium and 10,000 μg/ml streptomycin sulfate) (supplemented DMEM; Invitrogen, Grand Island, NY).
Western blots of serum.
PA, LF, or EF (20 μg/ml) was mixed with an equal volume of sample buffer (62.5 mM Tris-HCl, pH 6.8, 20% glycerol, 2% sodium dodecyl sulfate, 5% β-mercaptoethanol), boiled for 10 minutes, and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis using 10% Tris-glycine precast gels (Lonza, Portsmouth, NH). The proteins were transferred to nitrocellulose membranes (Millipore Corp., Bedford, MA) using a semidry transfer device (Fisher Biotech, Fisher Scientific, Pittsburgh, PA). The membranes were probed with sera as previously described (10). Serum samples were diluted 1:500 in phosphate-buffered saline (PBS) wash buffer (1.25 g low-fat dairy milk, 2.5 ml Tween 20 in 500 ml PBS [0.08 M Na2HPO4, 0.025 M NaH2PO4, 0.1 M NaCl]). The blots were probed with secondary-peroxidase-conjugated goat anti-human immunoglobulin (ICN/Cappel, Aurora, OH) added at a 1:2,000 dilution, and the bands were visualized using Western Lightning Chemiluminescence Reagent Plus (PerkinElmer Life Sciences, Boston, MA). Bands were analyzed and quantified by ImageQuant software (GE Healthcare, Piscataway, NJ).
Antibody-mediated neutralization of LF-induced lysis.
For neutralization assays, twofold dilutions of human sera (starting at 1 to 32) were prepared in supplemented DMEM. PA (1.0 μg/ml) and LF (0.1 μg/ml, 0.5 μg/ml, or 1.0 μg/ml) were added to the serum dilutions, and the mixtures were incubated for 10 minutes at 37°C with shaking. J774A.1 cells were harvested to 2 × 106 cells/ml, and 100 μl was added to a 96-well flat-bottomed tissue culture plate for 2 hours. The medium was removed and replaced with 100 μl of the serum dilutions containing PA plus LF, and the plates were incubated for 4 hours at 37°C in 5% CO2. alamarBlue (80% solution in Hanks balanced salt solution; Trek Diagnostic Systems Inc., Westlake, OH) was added at 10% of the well volume, and the cells were incubated for 20 h at 37°C in 5% CO2. Absorbance at 570 nm (to detect oxidized alamarBlue) and 595 nm (to detect reduced alamarBlue) was measured using a Bio-Tek (Winooski, VT) Elx800 plate reader, and the conversion of oxidized alamarBlue to its reduced form was used to determine metabolic activity. Cells lysed by the addition of 10 μl of Triton X-100 were used as negative controls. Assays were performed in triplicate and were repeated as indicated in the figure legends.
Antibody-mediated neutralization of EF-induced cAMP increases.
Serum dilutions were prepared, incubated with PA (1.0 μg/ml) and EF (0.5 μg/ml or 1.0 μg/ml), and added to J774A.1 cells as described above. The plates were incubated for 4 h at 37°C in 5% CO2. Intracellular cAMP was measured utilizing the BioTrak enzyme-linked immunosorbent assay (ELISA) kit according to the manufacturer's protocol (Amersham Biosciences/GE Healthcare, United Kingdom). The medium was removed, and the cells were lysed with 200 μl of the manufacturer's lysis buffer 1B. Assays were performed in duplicate and were repeated as indicated in the figure legends.
Serum depletion of LF and EF antibodies.
Tosyl-activated M-280 Dynabeads (Dynal Biotech Invitrogen) were used to deplete sera of antibodies to either LF or EF. Purified LF or EF (5 μg) was conjugated to 107 beads, according to the manufacturer's instructions. To block unbound sites on the beads, protein-conjugated beads were washed extensively with PBS (pH 7.4) containing 0.1% (wt/vol) bovine serum albumin. Control beads were not conjugated to protein and only washed with PBS plus bovine serum albumin. Serum was incubated with the protein-conjugated beads for 2 hours at 4°C with rotation. The beads were magnetically removed, and the process was repeated six times. Antibody depletion was verified by Western blotting.
Depleted serum was evaluated in both the LF and EF antibody-mediated neutralization assays described above. Each experiment with the depleted serum samples was performed in duplicate. Sample S4 was assayed in three independent trials, while sample S8 was tested only once. Data from sample S4 were analyzed using Student's paired t test.
RESULTS
AVA vaccination histories of the human volunteers.
The AVA vaccination histories of the subjects in this study are described in Table 1. Only two of the eight volunteers received all six of the recommended inoculations; however, neither received the recommended annual boosters (samples S1 and S2; Table 1). Two subjects received their primary immunization series during the study (samples S5 and S7). The remaining volunteers received five of the six recommended inoculations, with 9 to 12 months since the last inoculation (samples S3, S4, S6, and S8; Table 1). Sera were also drawn from five individuals presumed to have had no exposure to anthrax or the vaccine and pooled to serve as a negative control (pool; Table 1).
TABLE 1.
AVA vaccination histories of the human subjects and LF-to-EF ratios of antibody responses and neutralization titers
| Sample | Total no. of inoculations | No. of mos since last inoculation | Antibody response ratio, LF/EFa | Neutralization ratio, LF/EFb |
|---|---|---|---|---|
| S1 | 6 | 30 | 0.7 | 2.0 |
| S2 | 6 | 16 | 0.6 | 1.0 |
| S3 | 5 | 12 | 2.3 | 0.5 |
| S4 | 5 | 12 | 0.3 | 2.0 |
| S5 | 4 | 6 | 0.3 | 1.0 |
| S6 | 5 | 9 | 11.8 | 1.0 |
| S7 | 5 | 1 | 3.6 | 1.0 |
| S8 | 5 | 12 | 1.3 | 0.5 |
| Pool | 0 | NAc | NRd | NA |
Serum sample reactivity to PA, LF, or EF proteins was detected by Western blotting. Band intensity was quantified, and the ratio of LF antibody response to EF antibody response was calculated.
Ratio of titers determined by comparing antibody-mediated neutralization of LF-induced lysis to neutralization of EF-induced cAMP increases.
NA, not applicable.
NR, not reactive.
AVA induction of LF and EF antibodies.
The serum samples from the volunteers were evaluated for the presence of PA, LF, and EF antibodies by Western blotting. All samples from AVA-vaccinated individuals possessed antibodies to all three anthrax toxin components. High antibody responses to PA were observed for serum from all vaccinated individuals. Compared to those to PA, lower antibody responses to LF and EF were observed. Furthermore, LF and EF antibody responses were variable; for example, sample S4 had a higher antibody response to EF than LF. The ratio of LF to EF antibody responses was calculated by quantifying the Western blots (Table 1). Samples S6 and S7 displayed the highest relative antibody response to LF, while samples S4 and S5 displayed the highest relative antibody response to EF. These results confirm previous studies demonstrating that LF and EF are antigenic and present in the AVA vaccine (11, 16, 19, 35).
Antibody-mediated toxin neutralizing activity.
Two different assays using mouse macrophage J774A.1 cells were developed to evaluate the toxin-neutralizing activity of antibodies to LF and EF. LF induces lysis of J774A.1 cells (9, 28). Antibody-mediated protection from LF-induced lysis was determined by a variation of the standard toxin neutralization assay, monitoring reduction of the indicator alamarBlue instead of reduction of MTT [(4-5-dimethylthiazol-2-yl) 2,5-diphenyltetrazolium bromide] as an indicator of metabolic activity. Oxidized alamarBlue is nonfluorescent and blue. Metabolically active cells can internalize alamarBlue and convert the oxidized form to the reduced form. The reduced form is fluorescent and pink. The amount of reduced alamarBlue can be determined spectrophotometrically and used to assess cellular viability following treatment with LF toxin (10).
To assess antibody-mediated toxin neutralization, J774A.1 cells were treated with PA plus LF with or without serum for 4 hours and metabolic activity was assessed. The LF antibody neutralizing titers were defined as the reciprocals of the largest serum dilutions that conferred at least 50% protection. In initial studies, two scenarios were envisioned. If antibodies to LF do not contribute to neutralization and only antibodies to PA are important, identical neutralization titers would be obtained when the amount of PA was kept constant, even if the amount of LF was varied. In contrast, if antibodies to LF contributed to neutralization, the amount of LF in the assay could exceed the amount of antibody to LF and neutralization mediated by antibodies to LF would be most apparent when LF was limiting.
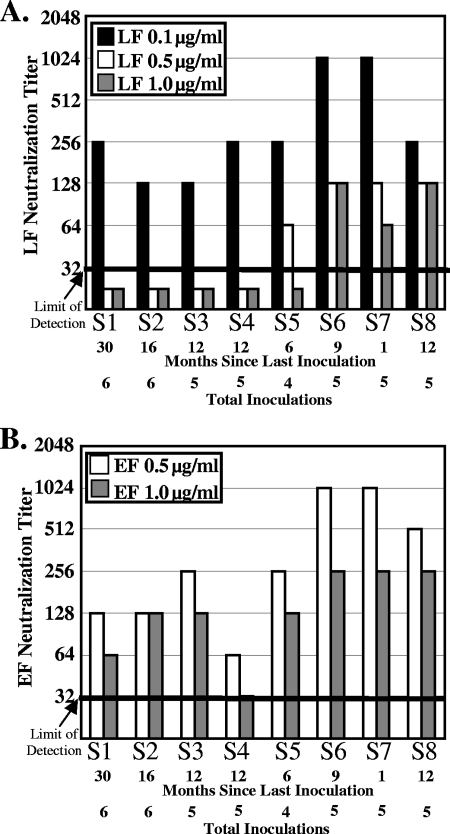
To distinguish between these hypotheses, conditions were established where similar levels of lysis were observed when the concentration of PA was kept constant but the concentration of LF was varied. In the absence of antibody, LF induced lysis of about 75% of the J774A.1 cells when added at 0.1, 0.5, or 1.0 μg/ml in the presence of 1.0 μg/ml PA, but significant lysis was not observed when LF was added at less than 0.1 μg/ml (data not shown). Neutralization assays were performed with PA added at 1.0 μg/ml and LF added at 0.1, 0.5, or 1.0 μg/ml (Fig. 1A). Different titers were obtained when different amounts of LF were present. Titers above the limit of detection (dilutions greater than 1 to 32) were observed for all sera when the cells were treated with the smallest amount of LF, 0.1 μg/ml (Fig. 1A). At higher concentrations of LF, lower neutralization titers were seen. These results suggest that the amount of LF influences antibody-mediated toxin neutralization, even when the amount of PA is kept constant.
FIG. 1.
Antibody-mediated neutralization of LF-induced lysis and EF-induced cAMP increases. (A) PA (1.0 μg/ml) and LF (0.1 μg/ml, 0.5 μg/ml, or 1.0 μg/ml) were incubated with twofold dilutions of sera from samples S1 to S8 (starting at a dilution of 1 to 32) for 10 minutes and added to J774A.1 cells for 4 hours. alamarBlue was added overnight, and the amount of reduced alamarBlue was measured as an indicator of metabolic activity. The neutralization titer, defined as the highest dilution that conferred 50% protection from LF-mediated lysis, is plotted, and the bar indicates the limit of detection. (B) PA (1.0 μg/ml) and EF (0.5 μg/ml or 0.5 μg/ml) were incubated with twofold dilutions of sera (starting at a dilution of 1 to 32) for 10 minutes and added to J774A.1 cells for 4 hours. The cells were lysed, and intracellular cAMP was monitored by ELISA. The neutralization titer, defined as the highest dilution that conferred 50% protection from EF-mediated elevation of cAMP, is plotted as described above. Identical results were observed in an independent trial.
Similar studies were performed to assess the contribution of neutralizing antibodies to EF. EF catalyzes the conversion of ATP to cAMP (13), and EF-mediated cAMP increases were determined by monitoring cAMP levels by ELISA. EF toxin elevated cAMP levels from about 3,000 to 5,000 fmol/well when EF was added at 0.5 or 1.0 μg/ml in the presence of 1.0 μg/ml PA, but significant increases in cAMP levels were not observed when EF was added at less than 0.5 μg/ml (data not shown). Neutralization assays were repeated as described above with limiting amounts of EF (Fig. 1B). Titers at or above the limit of detection were observed for all sera at both concentrations of EF, and, as with the LF assay, lower neutralization titers were seen when cells were treated with the higher concentration of EF, 1.0 μg/ml (Fig. 1B).
The ratios of the LF titer to the EF titer were compared using the most sensitive assay conditions (LF at 0.1 μg/ml and EF at 0.5 μg/ml), since under these conditions all of the samples possessed a titer that was above the limit of detection (Table 1). A ratio of 1.0 was obtained for four of the eight serum samples and only varied by 1 dilution for the remaining samples. Interestingly, the ratio of LF to EF neutralization did not correlate with the ratio of the antibody responses to LF and EF.
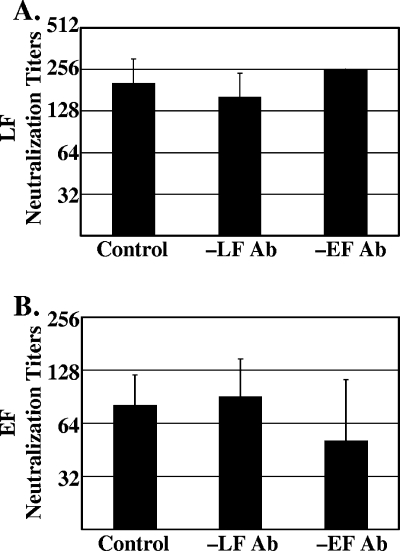
To further elucidate the activity of the LF and EF antibodies, two serum samples, S4 and S8, were depleted of either LF or EF antibodies. The serum samples were incubated with magnetic beads derivatized with purified LF or EF. Unmodified beads served as a negative control. Antibody-mediated neutralization titers from both the LF and EF assays were determined for the depleted sera as described above. Neutralization titers for the control, LF-depleted, and EF-depleted sera were not significantly different for serum sample S4 in either the LF-induced lysis neutralization assay (Fig. 2A) or the EF-mediated cAMP increase neutralization assay (Fig. 2B). Similar results were seen for serum sample S8, which was only tested once.
FIG. 2.
Neutralization titers of serum depleted of LF or EF antibodies. Serum sample S4 was incubated with magnetic beads conjugated to LF (-LF Ab) or EF (-EF Ab) or unconjugated beads (control). Depletion was verified by Western blotting (data not shown). The antibody-mediated neutralization titers were determined for LF at 0.1 μg/ml (A) and EF at 0.5 μg/ml (B). Data are represented as means ± standard deviations based on three independent trials.
DISCUSSION
The current FDA-approved anthrax vaccine, AVA, contains various amounts of PA, LF, and EF depending on the lot preparation (35). Previous studies have demonstrated that the receptor-binding anthrax toxin component, PA, is sufficient to elicit protection against anthrax in animal models of disease (3, 12, 14, 15, 23, 27, 31). However, it is unclear if LF or EF antibodies contribute to protection in humans. This information is especially important since recombinant vaccines containing only PA have been developed as a replacement for the current AVA vaccine. In this study, the contribution of antibodies to LF and EF to toxin neutralization was evaluated using sera from human volunteers immunized with the AVA vaccine.
Results from this study support earlier observations that LF and EF are antigenic and elicit an antibody response in vaccinated humans (11, 16, 19). All of the serum samples from vaccinated individuals demonstrated a strong antibody response to PA. However, the response to LF and EF was variable; for example, sample S6 had a much higher antibody response to LF than to EF (Table 1). Turnbull et al. reported in a study published in 1986 that they were unable to detect an antibody response to LF or EF in individuals vaccinated with AVA (32); however, these differences between the studies could be due to variations in the amounts of LF and EF proteins present in different AVA lots (35) or assay sensitivity.
Two different cellular assays were developed to assess antibody-mediated neutralization of LF and EF. The LF and EF assays used the same J774A.1 cell line, a constant amount of PA, and a constant time of toxin treatment so that results from the two assays could be compared. Interestingly, different neutralization titers were obtained when the amount of LF or EF was varied, and the highest titers were obtained when the concentration of LF or EF was limiting. One might predict that, if the neutralizing antibody response was directed only to PA, then the amount of LF or EF would not matter, and these results would suggest a role for antibodies to LF or EF in mediating toxin neutralization. However, similar results would also be observed if antibody bound to PA interfered with binding of LF or EF, and excess LF or EF could outcompete antibody for binding to PA. Several lines of evidence support the latter explanation. First, the antibody response to LF and EF as determined by Western blotting did not consistently parallel toxin neutralization activity; for example, sample S4, the sample with the strongest response to EF, had the lowest EF titer (Fig. 1B). However, in a previous study, neutralization of LF-mediated toxicity by antibodies from AVA-vaccinated individuals was not found to consistently correlate with antibody titers to PA, suggesting that some individuals can possess very low levels of highly effective neutralizing antibodies (10). Stronger support comes from the observation that, when the serum titer determined from the LF assay was compared to the serum titer determined in the EF assay, the ratio was the same for one-half of the subjects and those for the other subjects varied by only 1 dilution, even though the ratios of antibody levels to LF and EF varied considerably. This result suggests that neutralization was primarily determined by antibodies to the common factor, PA, not antibody to LF or EF. Finally, the strongest evidence that antibodies to LF and EF do not mediate toxin neutralization comes from the depletion studies. Neutralization titers did not change when sera were depleted of antibodies to LF or EF (Fig. 2). Unfortunately, the depletion studies were performed only on a few samples due to limiting amounts of serum.
The current AVA vaccination requires a lengthy dosing schedule and has been associated with numerous adverse reactions (6-8, 21, 24, 30, 33). Since it is possible that the presence of LF and EF, in addition to PA, in AVA could generate intact anthrax toxin and contribute to the development of adverse reactions to vaccination, this study was conducted to determine if removal of these antigens could compromise efficacy. Overall, the results suggest that AVA-induced LF and EF antibodies do not significantly contribute to anthrax toxin neutralization in humans and that antibodies to PA are sufficient to neutralize toxin activity.
Acknowledgments
This work was sponsored by the NIH/NIAID Regional Center of Excellence for Biodefense and Emerging Infectious Disease Research (RCE) Program. We acknowledge membership within and support from the Region V (Great Lakes) RCE (NIH award 1-U54-AI-057153). S.C.T. was supported by NIH/NIAID training grant T32-AI055406 (Training in Biologic Threat Agents) and a University Research Council summer fellowship.
Footnotes
Published ahead of print on 21 November 2007.
REFERENCES
- 1.Blaustein, R. O., T. M. Koehler, R. J. Collier, and A. Finkelstein. 1989. Anthrax toxin: channel-forming activity of protective antigen in planar phospholipid bilayers. Proc. Natl. Acad. Sci. USA 86:2209-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brachman, P. S., H. Gold, S. A. Plotkin, F. R. Fekety, M. Werrin, and N. R. Ingraham. 1962. Field evaluation of a human anthrax vaccine. Am. J. Public Health 52:632-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brey, R. N. 2005. Molecular basis for improved anthrax vaccines. Adv. Drug Deliv. Rev. 57:1266-1292. [DOI] [PubMed] [Google Scholar]
- 4.Collier, R. J., and J. A. Young. 2003. Anthrax toxin. Annu. Rev. Cell Dev. Biol. 19:45-70. [DOI] [PubMed] [Google Scholar]
- 5.Duesbery, N. S., C. P. Webb, S. H. Leppla, V. M. Gordon, K. R. Klimpel, T. D. Copeland, N. G. Ahn, M. K. Oskarsson, K. Fukasawa, K. D. Paull, and G. F. Vande Woude. 1998. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science 280:734-737. [DOI] [PubMed] [Google Scholar]
- 6.Geier, D. A., and M. R. Geier. 2002. Anthrax vaccination and joint related adverse reactions in light of biological warfare scenarios. Clin. Exp. Rheumatol. 20:217-220. [PubMed] [Google Scholar]
- 7.Geier, M. R., and D. A. Geier. 2004. Gastrointestinal adverse reactions following anthrax vaccination: an analysis of the Vaccine Adverse Events Reporting System (VAERS) database. Hepatogastroenterology 51:762-767. [PubMed] [Google Scholar]
- 8.Greidanus, T. G., and B. A. Honl. 2002. Delayed-type hypersensitivity reaction to anthrax vaccine. Mil. Med. 167:74-75. [PubMed] [Google Scholar]
- 9.Hanna, P. C., S. Kochi, and R. J. Collier. 1992. Biochemical and physiological changes induced by anthrax lethal toxin in J774 macrophage-like cells. Mol. Biol. Cell 3:1269-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanson, J. F., S. C. Taft, and A. A. Weiss. 2006. Neutralizing antibodies and persistence of immunity following anthrax vaccination. Clin. Vaccine Immunol. 13:208-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ivins, B. E., J. W. Ezzell, Jr., J. Jemski, K. W. Hedlund, J. D. Ristroph, and S. H. Leppla. 1986. Immunization studies with attenuated strains of Bacillus anthracis. Infect. Immun. 52:454-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ivins, B. E., and S. L. Welkos. 1988. Recent advances in the development of an improved, human anthrax vaccine. Eur. J. Epidemiol. 4:12-19. [DOI] [PubMed] [Google Scholar]
- 13.Leppla, S. H. 1982. Anthrax toxin edema factor: a bacterial adenylate cyclase that increases cyclic AMP concentrations of eukaryotic cells. Proc. Natl. Acad. Sci. USA 79:3162-3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leppla, S. H., J. B. Robbins, R. Schneerson, and J. Shiloach. 2002. Development of an improved vaccine for anthrax. J. Clin. Investig. 110:141-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Little, S. F. 2005. Anthrax vaccines: a development update. BioDrugs 19:233-245. [DOI] [PubMed] [Google Scholar]
- 16.Little, S. F., B. E. Ivins, P. F. Fellows, and A. M. Friedlander. 1997. Passive protection by polyclonal antibodies against Bacillus anthracis infection in guinea pigs. Infect. Immun. 65:5171-5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Little, S. F., and G. B. Knudson. 1986. Comparative efficacy of Bacillus anthracis live spore vaccine and protective antigen vaccine against anthrax in the guinea pig. Infect. Immun. 52:509-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Little, S. F., S. H. Leppla, and E. Cora. 1988. Production and characterization of monoclonal antibodies to the protective antigen component of Bacillus anthracis toxin. Infect. Immun. 56:1807-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahlandt, B. G., F. Klein, R. E. Lincoln, B. W. Haines, W. I. Jones, Jr., and R. H. Friedman. 1966. Immunologic studies of anthrax. IV. Evaluation of the immunogenicity of three components of anthrax toxin. J. Immunol. 96:727-733. [DOI] [PubMed] [Google Scholar]
- 20.Makino, S., I. Uchida, N. Terakado, C. Sasakawa, and M. Yoshikawa. 1989. Molecular characterization and protein analysis of the cap region, which is essential for encapsulation in Bacillus anthracis. J. Bacteriol. 171:722-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muniz, A. E. 2003. Lymphocytic vasculitis associated with the anthrax vaccine: case report and review of anthrax vaccination. J. Emerg. Med. 25:271-276. [DOI] [PubMed] [Google Scholar]
- 22.Pezard, C., M. Weber, J. C. Sirard, P. Berche, and M. Mock. 1995. Protective immunity induced by Bacillus anthracis toxin-deficient strains. Infect. Immun. 63:1369-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phipps, A. J., C. Premanandan, R. E. Barnewall, and M. D. Lairmore. 2004. Rabbit and nonhuman primate models of toxin-targeting human anthrax vaccines. Microbiol. Mol. Biol Rev. 68:617-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pittman, P. R., P. H. Gibbs, T. L. Cannon, and A. M. Friedlander. 2001. Anthrax vaccine: short-term safety experience in humans. Vaccine 20:972-978. [DOI] [PubMed] [Google Scholar]
- 25.Puziss, M., L. C. Manning, J. W. Lynch, E. Barclay, I. Abelow, and G. G. Wright. 1963. Large-scale production of protective antigen of Bacillus anthracis in anaerobic cultures. Appl. Microbiol. 11:330-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quesnel-Hellmann, A., A. Cleret, D. R. Vidal, and J. N. Tournier. 2006. Evidence for adjuvanticity of anthrax edema toxin. Vaccine 24:699-702. [DOI] [PubMed] [Google Scholar]
- 27.Scorpio, A., T. E. Blank, W. A. Day, and D. J. Chabot. 2006. Anthrax vaccines: Pasteur to the present. Cell. Mol. Life Sci. 63:2237-2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh, Y., S. H. Leppla, R. Bhatnagar, and A. M. Friedlander. 1989. Internalization and processing of Bacillus anthracis lethal toxin by toxin-sensitive and -resistant cells. J. Biol. Chem. 264:11099-11102. [PubMed] [Google Scholar]
- 29.Stanley, J. L., and H. Smith. 1963. The three factors of anthrax toxin: their immunogenicity and lack of demonstrable enzymic activity. J. Gen. Microbiol. 31:329-337. [DOI] [PubMed] [Google Scholar]
- 30.Swanson-Biearman, B., and E. P. Krenzelok. 2001. Delayed life-threatening reaction to anthrax vaccine. J. Toxicol. Clin. Toxicol. 39:81-84. [DOI] [PubMed] [Google Scholar]
- 31.Turnbull, P. C. 1991. Anthrax vaccines: past, present and future. Vaccine 9:533-539. [DOI] [PubMed] [Google Scholar]
- 32.Turnbull, P. C., M. G. Broster, J. A. Carman, R. J. Manchee, and J. Melling. 1986. Development of antibodies to protective antigen and lethal factor components of anthrax toxin in humans and guinea pigs and their relevance to protective immunity. Infect. Immun. 52:356-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vasudev, M., and M. C. Zacharisen. 2006. New-onset rheumatoid arthritis after anthrax vaccination. Ann. Allergy Asthma Immunol. 97:110-112. [DOI] [PubMed] [Google Scholar]
- 34.Wesche, J., J. L. Elliott, P. O. Falnes, S. Olsnes, and R. J. Collier. 1998. Characterization of membrane translocation by anthrax protective antigen. Biochemistry 37:15737-15746. [DOI] [PubMed] [Google Scholar]
- 35.Whiting, G. C., S. Rijpkema, T. Adams, and M. J. Corbel. 2004. Characterisation of adsorbed anthrax vaccine by two-dimensional gel electrophoresis. Vaccine 22:4245-4251. [DOI] [PubMed] [Google Scholar]