Abstract
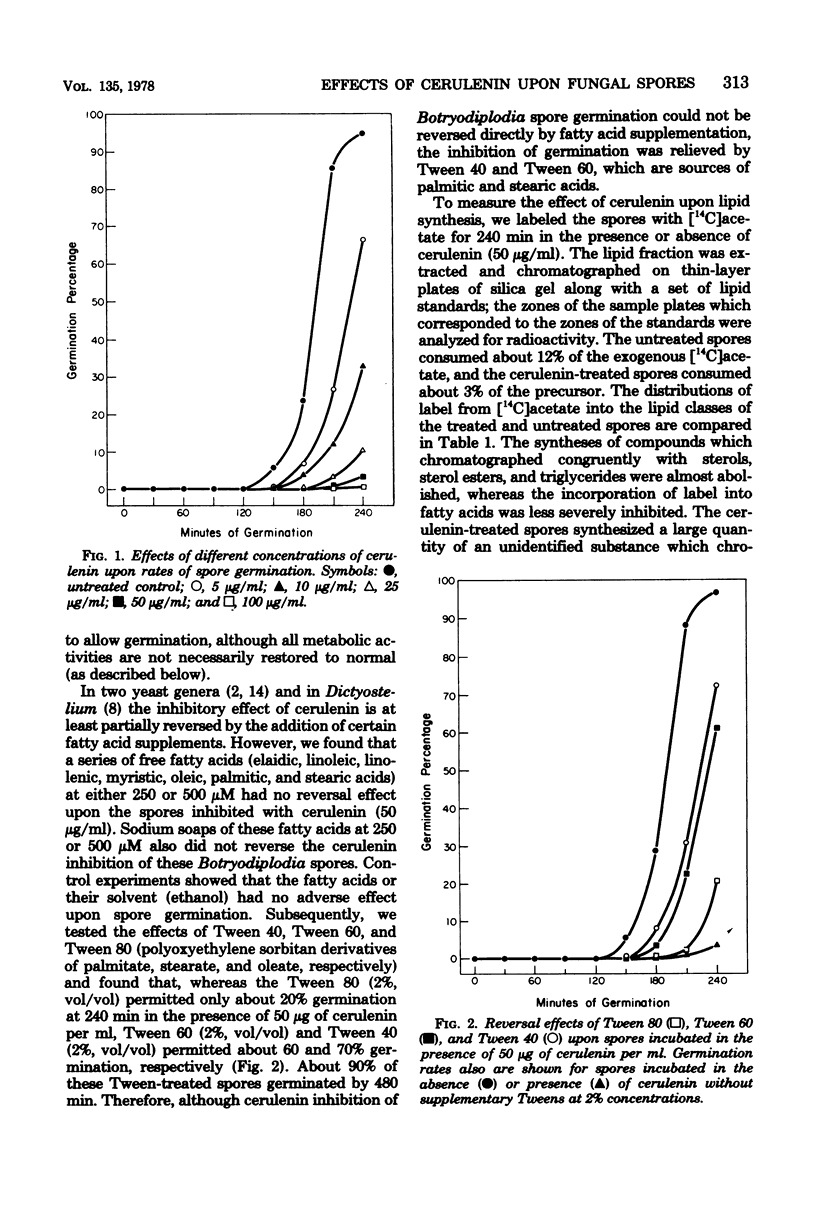
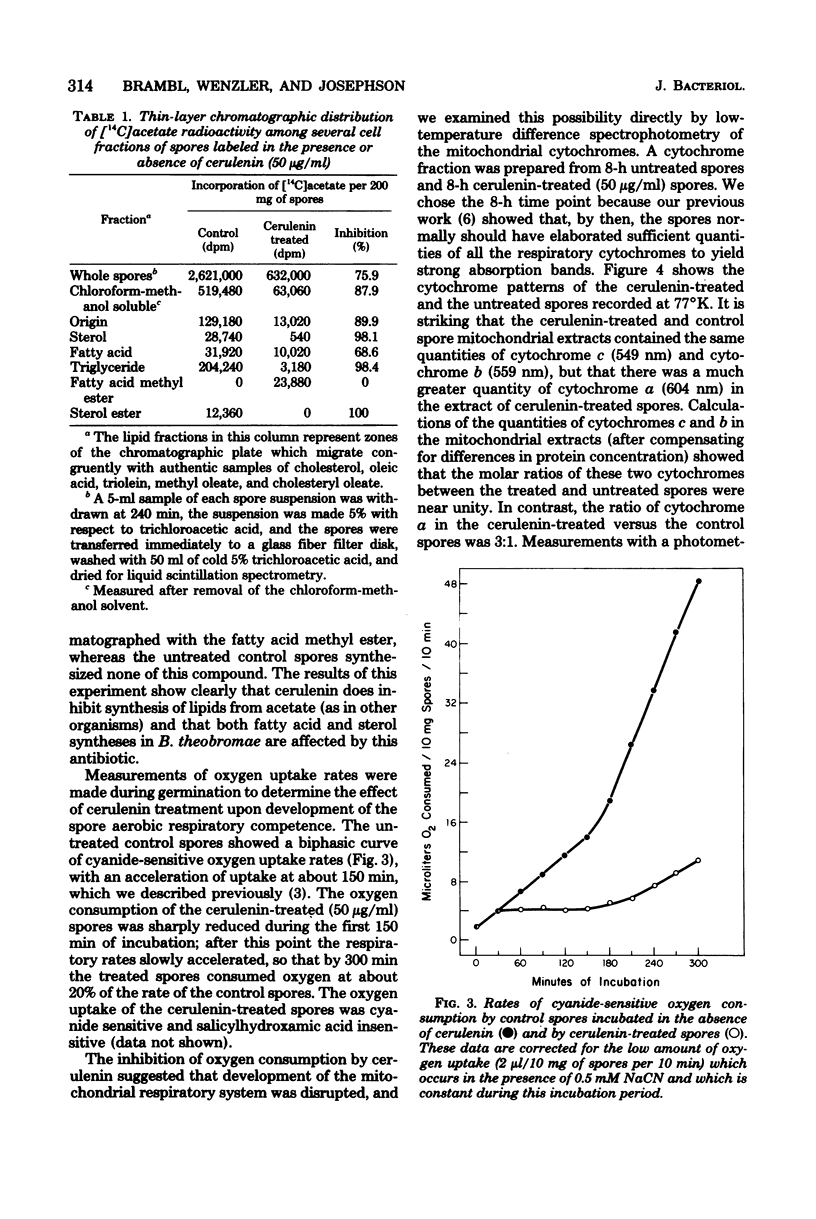
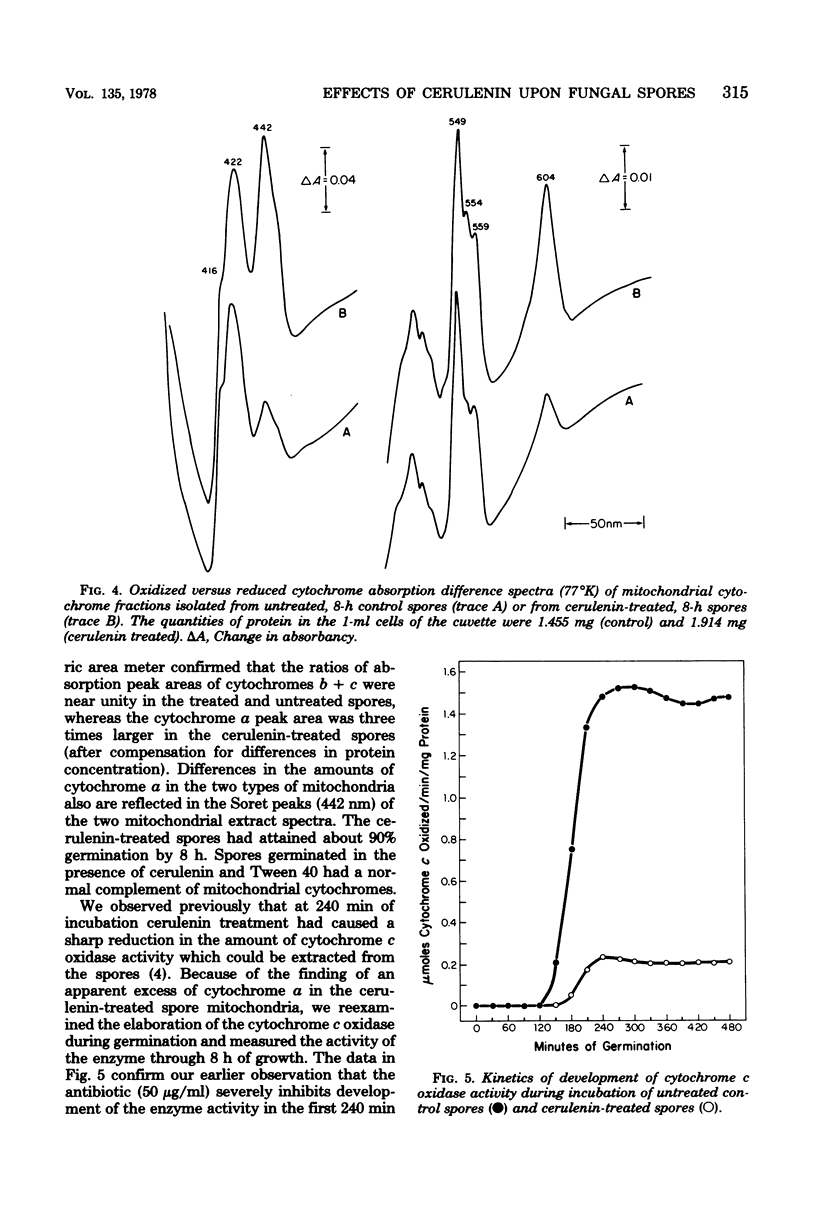
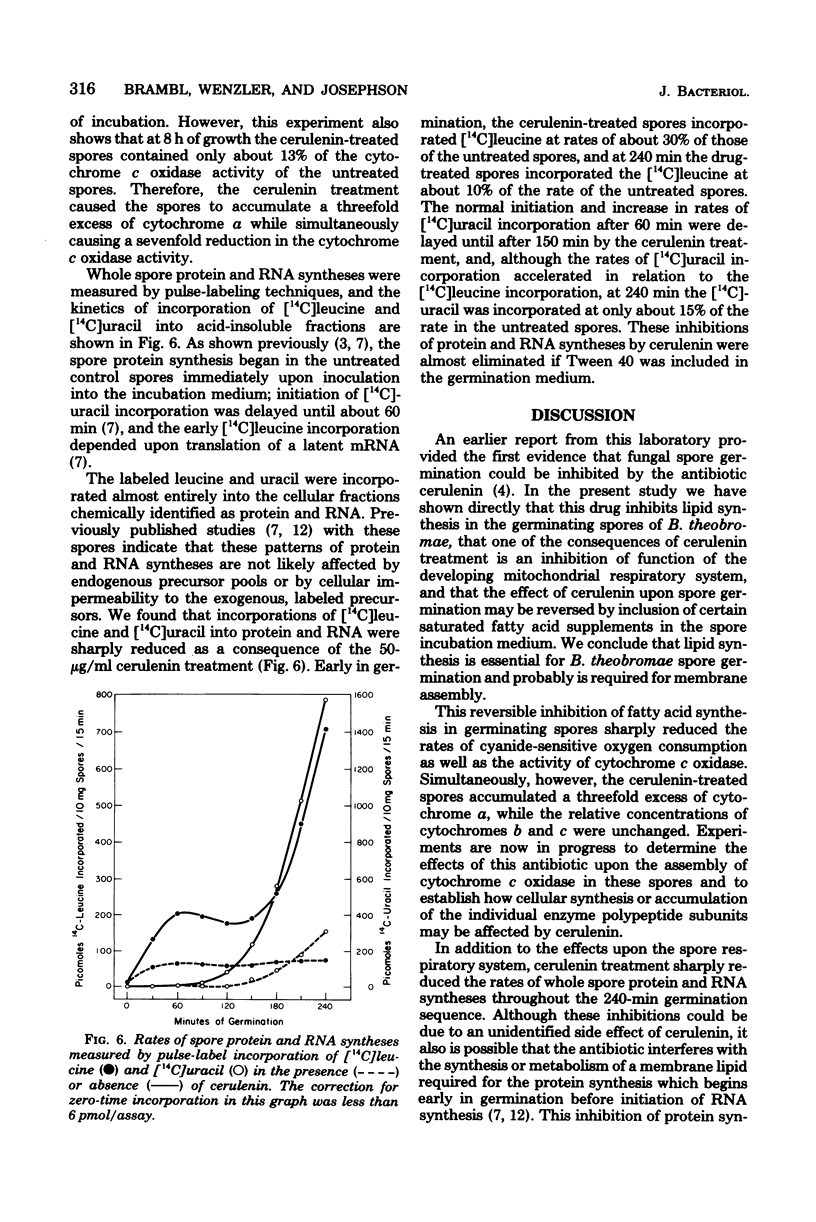
Germination of spores of the fungus Botryodiplodia theobromae was inhibited by the antilipogenic antibiotic cerulenin. The spores remained viable in the presence of the antibiotic, however, and after prolonged incubation they were able to overcome the inhibition. Cerulenin inhibition of germination was reversed by Tween 40 and Tween 60 (derivatives of palmitate and stearate, respectively), but not by representatives of a range of free fatty acids or their soaps. Cerulenin abolished incorporation of [14C]acetate into sterols and triglycerides and reduced its incorporation into fatty acids by 69%. Cyanide-sensitive oxygen consumption by spores incubated in the presence of cerulenin was greatly reduced throughout germination, and the activity of cytochrome c oxidase was no more than 13% of the activity in untreated spores, even after prolonged incubation. However, low-temperature difference spectra of mitochondrial extracts showed that the cerulenin-treated spores accumulated a threefold excess of cytochrome a, whereas the cellular concentrations of cytochroms c and b were identical to those of untreated spores. Cerulenin treatment sharply reduced the rates of whole spore protein and RNA synthesis. Cerulenin had no effects upon mitochondrial morphology which could be discerned with an electron microscope.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Awaya J., Ohno T., Ohno H., Omura S. Substitution of cellular fatty acids in yeast cells by the antibiotic cerulenin and exogenous fatty acids. Biochim Biophys Acta. 1975 Dec 17;409(3):267–273. doi: 10.1016/0005-2760(75)90022-3. [DOI] [PubMed] [Google Scholar]
- Brambl R. M., Van Etten J. L. Protein synthesis during fungal spore germination. V. Evidence that the ungerminated conidiospores of Botryodiplodia theobromae contain messenger ribonucleic acid. Arch Biochem Biophys. 1970 Apr;137(2):442–452. doi: 10.1016/0003-9861(70)90461-3. [DOI] [PubMed] [Google Scholar]
- Brambl R. Characteristics of developing mitochondrial genetic and respiratory functions in germinating fungal spores. Biochim Biophys Acta. 1975 Aug 11;396(2):175–186. doi: 10.1016/0005-2728(75)90032-8. [DOI] [PubMed] [Google Scholar]
- Brambl R., Handschin B. Mitochondrial biogenesis during fungal spore germination: products of mitochondrial protein synthesis in vivo. Arch Biochem Biophys. 1976 Aug;175(2):606–617. doi: 10.1016/0003-9861(76)90551-8. [DOI] [PubMed] [Google Scholar]
- Brambl R., Josephson M. Mitochondrial biogenesis during fungal spore germination: respiratory cytochromes of dormant and germinating spores of Botryodiplodia. J Bacteriol. 1977 Jan;129(1):291–297. doi: 10.1128/jb.129.1.291-297.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambl R. Mitochondrial biogenesis during fungal spore germination. Development of cytochrome c oxidase activity. Arch Biochem Biophys. 1977 Jul;182(1):273–281. doi: 10.1016/0003-9861(77)90308-3. [DOI] [PubMed] [Google Scholar]
- Chance K., Hemmingsen S., Weeks G. Effect of cerulenin on the growth and differentiation of Dictyostelium discoideum. J Bacteriol. 1976 Oct;128(1):21–27. doi: 10.1128/jb.128.1.21-27.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. L., Charalampous F. C. Mechanism of induction of cytochrome oxidase in yeast. I. Kinetics of induction and evidence for accumulation of cytoplasmic and mitochondrial precursors. J Biol Chem. 1969 May 25;244(10):2767–2776. [PubMed] [Google Scholar]
- ESTABROOK R. W., HOLOWINSKY A. Studies on the content and organization of the respiratory enzymes of mitochondria. J Biophys Biochem Cytol. 1961 Jan;9:19–28. doi: 10.1083/jcb.9.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Knight R. H., Van Etten J. L. Synthesis of ribonucleic acids during the germination of Botryodiplodia theobromae pycnidiospores. J Gen Microbiol. 1976 Aug;96(2):257–267. doi: 10.1099/00221287-95-2-257. [DOI] [PubMed] [Google Scholar]
- Marzuki S., Cobon G. S., Haslam J. M., Linnane A. W. Biogenesis of mitochondria. The effects of altered steady-state membrane lipid composition on mitochondrial-energy metabolism in Saccharomyces cerevisiae. Arch Biochem Biophys. 1975 Aug;169(2):577–590. doi: 10.1016/0003-9861(75)90202-7. [DOI] [PubMed] [Google Scholar]
- Nomura S., Horiuchi T., Omura S., Hata T. The action mechanism of cerulenin. I. Effect of cerulenin on sterol and fatty acid biosynthesis in yeast. J Biochem. 1972 May;71(5):783–796. doi: 10.1093/oxfordjournals.jbchem.a129827. [DOI] [PubMed] [Google Scholar]
- Omura S. The antibiotic cerulenin, a novel tool for biochemistry as an inhibitor of fatty acid synthesis. Bacteriol Rev. 1976 Sep;40(3):681–697. doi: 10.1128/br.40.3.681-697.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono T., Kesado T., Awaya J., Omura S. Target of inhibition by the anti-lipogenic antibiotic cerulenin of sterol synthesis in yeast. Biochem Biophys Res Commun. 1974 Apr 23;57(4):1119–1124. doi: 10.1016/0006-291x(74)90812-2. [DOI] [PubMed] [Google Scholar]
- Tzagoloff A., MacLennan D. H. Studies of the electron-transfer system. LXIV. Role of phospholipid in cytochrome oxidase. Biochim Biophys Acta. 1965 Jun 22;99(3):476–485. doi: 10.1016/s0926-6593(65)80201-6. [DOI] [PubMed] [Google Scholar]
- van Gelder B. F. On cytochrome c oxidase. I. The extinction coefficients of cytochrome a and cytochrome a3. Biochim Biophys Acta. 1966 Apr 12;118(1):36–46. doi: 10.1016/s0926-6593(66)80142-x. [DOI] [PubMed] [Google Scholar]



