Lyme disease is the most common arthropod-borne infection in the United States, affecting approximately 23,000 individuals bitten by Borrelia burgdorferi-infected Ixodes ticks in 2005 (30). Infection with B. burgdorferi results in a multisystem disorder, manifesting as early as a week following infection and increasing in severity that includes progression to a chronic disease state in some patients (135). Among the most common hallmarks of Lyme disease are erythema migrans (EM), disruption of electrical conduction of cardiac muscle, the development of neurological abnormalities (135), and episodes of arthritis. Some of these clinical features, especially arthritis, subside and recur throughout the course of infection (135), presumably corresponding to a reemergence of the immune response to the spirochete. The recurrence of symptoms is a reflection of the unique nature of Lyme disease, as its development requires a complex interaction of vector, bacterial, and host factors that mediates the establishment of initial infection, bacterial dissemination to specific body sites, and the development of pathology, respectively. Of particular interest is the role of these interactions in the inflammatory events leading to the establishment of Lyme arthritis.
Arthritis is a leading cause of Lyme disease-associated morbidity in the United States, affecting approximately 60% of individuals infected with B. burgdorferi (139). Intermittent episodes of arthritis develop several weeks or months after infection and, despite adequate antimicrobial therapy, symptoms persist in 10% of patients with arthritis (62, 78, 137, 139). In severe cases, the highly inflammatory aspects of Lyme arthritis can lead to cartilage and bone erosion with permanent joint dysfunction (139). The fact that not all patients with Lyme borreliosis develop arthritis may be a reflection of the genetic diversity among the species comprising B. burgdorferi sensu lato (Borrelia garinii and Borrelia afzelii, common in Europe, where arthritis is not frequently observed, and B. burgdorferi sensu stricto, predominantly observed in North America) (146) as well as within the B. burgdorferi sensu stricto species (148, 149).
The mechanism(s) by which B. burgdorferi interacts with the host immune system to induce arthritis is not fully understood. Initially, effectors of the innate immune system play a significant role in the control of B. burgdorferi infection. While the exact sequence of the initial spirochetal interaction with innate host factors is not completely known, it is known that a variety of host mechanisms work together to limit the dissemination of B. burgdorferi and initiate an adaptive immune response to the organisms. This review provides a synopsis of data from studies of Borrelia-infected humans and animals of currently known mechanisms responsible for the induction of Lyme arthritis following infection with B. burgdorferi.
MODELS OF LYME ARTHRITIS
Animal models of diseases are extremely important for elucidating the mechanisms of pathogenesis and defining targets for therapeutic approaches for treatment of immune-driven disorders such as Lyme arthritis. These animal models also increase knowledge and understanding of the basic infectious process and assist in developing strategies to prevent infection and disease in humans. Unfortunately, no single animal model mimics all the immunological, pathological, and clinical manifestations associated with Lyme borreliosis in humans. Although the inflammatory events leading to the development of arthritis in animals provide a fairly accurate model by which to study Lyme arthritis in humans, these findings need confirmation in humans. Currently, there exist two major models of Lyme arthritis: one of mild arthritis with a short duration of pathology, based on infection of naïve mice with B. burgdorferi, and one of progressive arthritis that is sustained for several months, based on challenge of B. burgdorferi-vaccinated mice. It is likely that the immunological mechanisms that lead to arthritis in these animal models of Lyme arthritis share many pathways. Therefore, the use of these two models provides a more complete picture of the immunological events associated with Lyme arthritis in mice. Hopefully, these immunological events that occur in Borrelia-infected or Borrelia-vaccinated and challenged mice are predictive of events that occur in humans.
Infection model of arthritis.
Intradermal inoculation of susceptible strains of young mice, particularly C3H mice, with B. burgdorferi has been shown to result in the migration of spirochetes to the connective tissues surrounding joints as soon as 5 days after infection (8). Concomitant with this early presence of spirochetes is the development of mild inflammation, characterized by the influx of neutrophils and other leukocytes, including lymphocytes, to the joint capsule. Within several days, the joint rapidly develops a more pronounced inflammation of the synovial lining, tendon sheaths, ligaments, and bursae (8). By approximately 2 weeks after infection, a significant increase in neutrophilic infiltration and fibrin deposition, as well as increased synovial hyperplasia, affect the joint (8). The severity of this arthritis generally peaks around 3 weeks after infection, after which the pathological changes rapidly subside. Long-term examination of these mice demonstrates a recurrence of arthritic symptoms, with subsequent bouts of arthritis decreasing in severity (8). In addition, edematous changes of the joint typically accompany the histological changes. In the infection model of Lyme arthritis, this swelling is observable approximately 2 weeks after infection and gradually diminishes. Consequently, the course of swelling does not correspond directly to the course of actual histopathological changes in the joint (8). Moreover, periarticular edema can develop in the absence of arthritis (8). Therefore, the immunologic mechanisms responsible for the induction and resolution of edema may be different from those that induce arthritis. Observing the degree of swelling has been questioned as a means to measure arthritic severity with this animal model of arthritis (152). Most importantly, the major advantage of the Borrelia-infection model of arthritis is that it may closely predict initial events associated with natural infection of humans with B. burgdorferi. Our knowledge of the immune response to Borrelia infection has been greatly increased with use of this model. However, these findings need confirmation in humans.
Vaccination-challenge model of arthritis.
The infection model of Lyme arthritis has been useful in understanding the basic immunological events leading to the development of B. burgdorferi-induced inflammation (8). Depending upon the experimentation, however, investigators can utilize another model of Lyme arthritis. Arthritis is induced in hamsters (40, 89) and mice (28, 35, 70, 109-111) following Borrelia-vaccination and challenge. The histopathology of the tibiotarsal joint induced in Borrelia-vaccinated and -challenged hamsters or mice is similar to the histopathology induced in Borrelia-infected mice; however, the histopathology is enhanced. Furthermore, the histopathology is sustained for many months, and the severity facilitates objective evaluation of results among treatment groups of Borrelia-vaccinated and -challenged mice. Mice are now used exclusively in the vaccination-challenge model (28, 35, 70, 109-111) due to the commercial availability of murine immunological reagents.
In the vaccination-challenge model of arthritis, C57BL/6 mice (no restriction on age or gender) are vaccinated with formalin-fixed B. burgdorferi organisms in aluminum hydroxide and infected with approximately 106 viable, heterologous Borrelia organisms in the hind paws approximately 3 weeks later. It is necessary to infect with a genetically different isolate because vaccination induces protective antibodies that prevent the homologous infection from eliciting arthritis (40, 89). Swelling of the hind paws is exhibited approximately 4 days after challenge, peaking 8 to 10 days after infection before decreasing gradually (28, 35, 70, 109-111). In contrast to the infection model of arthritis, the severity of swelling of the hind paws does correlate with the severity of histopathologic changes occurring in the tibiotarsal joint. More importantly, these mice develop inflammatory changes of the tibiotarsal joint, characterized by mild synovial hyperplasia and hypertrophy and moderate infiltration of neutrophils, other leukocytes, and lymphocytes into the synovial lining, approximately 1 week after infection (35, 70, 109-111). By approximately 3 weeks after infection, the joints of vaccinated and challenged mice exhibit massive infiltration of lymphocytes and inflammatory cells into the synovium, severe hyperplasia of the synovial and subsynovial tissues, pannus formation, erosion of cartilage, and destruction of bone, which gradually decreases after several months (28, 35, 70, 109-111). Arthritis can still be detected in these mice 1 year after infection. Borrelia-vaccinated mice also develop arthritis after infection with inocula (≤104 organisms) similar to those used in the Borrelia infection model. However, the pathology is sustained and consistently reproduced with a higher inoculum of B. burgdorferi. Therefore, both early and late immunological events responsible for the induction and resolution of arthritis, respectively, can be evaluated. Finally, the manifestations of mild arthritis (day 8 after infection) and severe, destructive arthritis (day 20 after challenge) in the Borrelia vaccination and challenge mouse model provide a basis upon which to examine a broad spectrum of inflammatory severities, including chronic arthritis, in humans.
DEVELOPMENT OF ARTHRITIS UPON INFECTION WITH B. BURGDORFERI
Infection.
What knowledge have we gained using these animal models of arthritis along with observations in humans? Borrelia organisms (generally 1 to 100 spirochetes) are transferred to the skin of the host during the bloodmeal of infected Ixodes ticks. The characteristic EM lesion is caused by an inflammatory response that aids in containing spirochete replication and preventing the migration of spirochetes from the initial site of deposition in the skin. The occurrence of EM lesions in infected humans has been estimated at approximately 60% (139). However, it is likely that this figure is grossly underestimated, owing in part to the concealment of the lesion by skin tone or the presence of hair, in addition to a lack of recognition on the part of the patient or physician. It is unknown why mice fail to develop EM (55), despite the presence of spirochetes in the skin. During the feeding process, saliva from the tick accompanies the spirochete into the host tissue. Recent findings have provided clear evidence of roles for various tick salivary factors, such as B cell inhibitory protein (64) and sialostatin L (84), among others (98, 106, 121-123), in the localized disruption of host tissues and immune responses. The activity of these salivary factors supports the successful transmission of B. burgdorferi organisms to the host as well as providing a localized environment by which the spirochetes can evade immune clearance. Once established in the host, B. burgdorferi is able to modulate the expression of various surface antigens, especially outer surface proteins OspA and OspC (128), providing an additional mechanism by which to evade the immune response. Therefore, dissemination of B. burgdorferi organisms depends on a combination of tick and bacterial factors to evade the innate immune system such that a sufficient number of spirochetes survive in and escape from the site of infection. This initial evasion of the immune response plays a significant role in establishing conditions by which B. burgdorferi organisms are able to disseminate from the site of inoculation, establish residence in joint tissues, and induce arthritis.
Cells of the innate immune system.
There are some individuals exposed to B. burgdorferi that fail to develop clinical disease. These individuals likely possess an efficient innate immune response that eradicates the spirochetes. In other individuals, the innate immune system fails to completely eliminate the infectious agent, but programs the adaptive immune system to mount a strong T helper 1 (Th1) and Th2 response for final elimination of the spirochetes. The extent to which the innate immune factors are effective in their antimicrobial responses is important in determining the likelihood that concurrent damage to the host, including the induction of arthritis, will develop.
The innate immune system is primarily composed of natural killer (NK) cells, neutrophils and monocytes/macrophages, and dendritic cells. Each of these cell types has been considered important because the cells may affect the response to Borrelia infection. However, the role of NK cells in the development of Lyme arthritis has yet to be fully elucidated. B. burgdorferi-infected arthritis-susceptible C3H/HeJ mice and arthritis-resistant C57BL/6 mice genetically deficient in granulocytes and NK cells develop less severe arthritis than their wild-type counterparts (9), suggesting a possible role for NK cells in the induction of Lyme arthritis. In addition, NK cells from arthritis-susceptible C3H/HeJ mice infected with B. burgdorferi produce greater levels of gamma interferon (IFN-γ) than do NK cells from infected arthritis-resistant DBA/2J mice (22). However, the additional IFN-γ provided by NK cells does not appear to contribute significantly to the development of arthritis (22), as depletion of NK cells did not affect disease progression. Moreover, removal of NK cells in infected C57BL/6 mice failed to affect the severity of arthritis (9). These findings suggest that, while NK cells may play a role in the development of Lyme arthritis, they (and the IFN-γ they produce) may not be absolutely required.
Are there other NK cell-mediated factors that may play a role in the immune events leading to the development of arthritis upon infection with B. burgdorferi? The presence of Toll-like receptor 2 (TLR2) on NK cells (145) suggests that interaction with borrelial lipoproteins may induce an inflammatory response mediated by NK cells independently of IFN-γ. In addition, OspA is able to augment the activation of NK cells (97), which can lead to the induction of other inflammatory mediators, such as tumor necrosis factor alpha (TNF-α) (94), interleukin-8 (IL-8), macrophage inflammatory protein 1, and RANTES (regulated on activation normal T cell expressed and secreted) protein (160). Moreover, following activation of dendritic cells (61) or stimulation of the adaptive response, the production of IL-2 can stimulate NK cells to indirectly assist in the stimulation of an overaggressive, pathogenic response to infection, culminating in the induction of Lyme arthritis.
There is more information on the roles of neutrophils in the establishment of infection and induction of arthritis by B. burgdorferi. Although neutrophils are typically among the initial immune cells recruited to sites of infection, their abundant presence in the synovia of humans (136) and animals (22, 28, 35, 70, 109-111) with Lyme arthritis suggests that neutrophils play a pathogenic role in the induction of arthritis as well as an antimicrobial one against the spirochete. Neutrophils can kill B. burgdorferi in the absence of antibody (96, 118) and release specific granules that also augment killing of spirochetes and local tissue damage (57, 96).
Susceptibility to B. burgdorferi infection may be related to a diminished development of a robust neutrophilic response to the spirochete. Indeed, tick saliva contains factors that impair the actions of neutrophils at the site of infection (106, 122), and EM lesions are typically devoid of neutrophilic involvement (155). In addition, an increase in the recruitment of neutrophils to the infection site reduces the dissemination of B. burgdorferi to the joint, as well as the experimental dose of spirochetes required to establish residence there (155). Therefore, it seems apparent that a strong influx of neutrophils upon infection would assist in the clearance of spirochetes, greatly limiting their dissemination and ability to induce arthritis. However, such a strong neutrophil-mediated response is not observed in the skin (155), although neutrophil infiltration is prominent in the tibiotarsal joints of Borrelia-infected and Borrelia-vaccinated and -challenged mice. Conceivably, antigens of B. burgdorferi in the skin stimulate other cells of the innate immune system, especially dendritic cells, to release immune mediators that prevent the infiltration of neutrophils. In contrast, borrelial antigens, like OspC, would be absent or less likely to be expressed on B. burgdorferi found in the joints. Therefore, these antigens would not stimulate immune mediators, like transforming growth factor β (TGF-β) and IL-10, which could prevent a prominent neutrophilic infiltration into joint tissues.
Some studies suggest that arthritis develops independently of the presence of neutrophils. For example, depletion of granulocytes, including neutrophils, in B. burgdorferi-infected C3H mice led to an increase in arthritic development (9). Although this finding suggests a possible role for neutrophils in the protection against arthritis, it cannot be attributed to the removal of neutrophils alone. B. burgdorferi-infected mice depleted of neutrophils by means of monoclonal antibody increased the presence of spirochetes in the joint tissues and hastened the onset of arthritis (25). While the absence of neutrophils in the joints of these arthritic mice was insufficient to prevent the development of arthritis upon B. burgdorferi infection, the presence of granulocyte precursors may be responsible for the initiation of inflammation (25). These findings demonstrate that the actions of neutrophils may not be required for the development of Lyme arthritis.
Other studies, however, suggest that neutrophils likely play a significant role in the development of Lyme arthritis. B. burgdorferi-infected arthritis-susceptible C3H/HeJ mice express significantly greater levels of the neutrophil-attracting keratinocyte-derived chemokine (KC) in the joint than do infected arthritis-resistant C57BL/6 mice (24). The infiltration of neutrophils via attraction to KC into synovial tissues plays an important role in the development of arthritis, as infected mice deficient in the KC receptor CXCR2 developed significantly milder arthritic changes than infected wild-type mice and lacked the presence of neutrophils in the synovial tissues (24). In addition, it was shown that OspA of B. burgdorferi, the up-regulation of which correlates to the development of arthritis (2), induces the adherence of neutrophils to proteins of the extracellular matrix and stimulates neutrophils in the production of IL-8 (108), which serves to attract other neutrophils. These findings demonstrate that the presence of neutrophils in the tissues of the joint plays a significant role in the development of Lyme arthritis.
How might the actions of neutrophils contribute to the induction of Lyme arthritis? The reemergence of spirochetes at synovial sites may serve as a stimulus for the expression of inflammatory cytokines via binding of borrelial lipoprotein to TLR2 on neutrophils (115). In addition, neutrophils can destroy B. burgdorferi through release of lytic enzymes or phagocytosis (96, 144). Moreover, stimulated neutrophils produce cytokines central to the induction of inflammation, such as IL-1, TNF-α, and IL-8 (12, 48, 143), as well as cytokines involved in the adaptive response to infection, such as IL-15 (76). Depletion of IL-15 in Borrelia-vaccinated and -challenged mice prevents the development of arthritis observed in untreated controls (4). In support of a role for IL-15 in the development of pathology, histopathologic examination showed that anti-IL-15 antibody or recombinant IL-15 receptor alpha also decreased the infiltration of neutrophils at the tibiotarsal joint compared to untreated controls (4). These findings suggest that neutrophils contribute to the induction of arthritis by release of proinflammatory cytokines.
The ability of B. burgdorferi to avoid clearance by the immune system may be attributed in part to an inadequate innate immune response upon infection. However, innate cells such as monocytes, macrophages, and dendritic cells display a vigorous response against borrelial infection and play a major role in the activation of the adaptive immune response against the spirochete. Despite these efforts, though, arthritis still develops in a significant number of patients infected with B. burgdorferi. It appears that the robust response elicited by monocytes, macrophages, and dendritic cells against the spirochete may be viewed as insufficient in early infection but excessive in the later stages. Paradoxically, in mounting such a strong defense against the organism, these cells may inadvertently be contributing to the induction of Lyme arthritis.
Unlike neutrophils, large populations of monocytes, activated macrophages, and mature dendritic cells are found in EM lesions (125), the sites of initial B. burgdorferi infection. The presence of TLR2, which binds borrelial lipoprotein (72), on these cell types (125) may induce the expression of inflammatory cytokines, chemokines, and mediators such as monocyte chemoattractant protein 1 (MCP-1) (161) in an attempt to eradicate the spirochetes within the lesion. In addition, interaction of macrophages with borrelial antigens induces the production of proinflammatory mediators such as nitric oxide, IL-1, TNF-α, IL-6, and IL-12 (97), as well as mediators of tissue destruction such as matrix metalloproteinase 9 (MMP-9) (162). Moreover, the spread of B. burgdorferi is hindered by the ability of macrophages and dendritic cells to readily bind and phagocytize B. burgdorferi by a variety of means (36, 53, 91, 103-105, 107). These findings demonstrate that the presence of B. burgdorferi in the skin upon initial infection establishes a rapid, robust innate response by monocytes, macrophages, and dendritic cells in an attempt to stem the dissemination of spirochetes. However, despite this strong initial response, it is not sufficient to prevent chronic disease in all infected patients.
Are macrophages required for the induction of Lyme arthritis? The synovial tissues of B. burgdorferi-infected, arthritis-susceptible C3H/HeJ mice contain higher levels of MCP-1 than infected arthritis-resistant C57BL/6 mice (24), suggesting a role for macrophages in the induction of arthritis. A deficiency in the primary MCP-1 receptor CCR2 (21) in infected mice did not prevent the influx of macrophages into the synovial tissues, nor did it prevent the development of arthritis (24), demonstrating that the inflammatory response against B. burgdorferi employs various mechanisms to ensure the migration of macrophages to locations harboring numerous organisms. These findings, however, do not directly address whether macrophages are required for the development of arthritis. Viewing the actions of macrophages from the perspective of antigen presentation and T-cell activation may better address the issue.
Direct evidence of the role of macrophages in the induction of arthritis was reported by DuChateau et al. (43-45). They demonstrated in a series of reports that macrophages play a direct role in the induction of arthritis following infection with Borrelia. Transfer of macrophages previously exposed to B. burgdorferi into hamsters infected with the spirochete resulted in the development of severe, destructive arthritis of the tibiotarsal joint to a degree correlating with the number of primed macrophages administered (43). By contrast, transfer of unprimed macrophages to Borrelia-infected mice failed to induce arthritis (43). These findings were extended to show that the transfer of a combination of Borrelia-primed macrophages with naïve or immune (44, 45) T cells to Borrelia-infected mice hastened the onset of destructive arthritis compared to the administration of primed macrophages or T cells alone to Borrelia-infected mice. We now know that cells capable of ingesting and processing Borrelia and presenting its antigens to T cells are required for the development of Borrelia-associated arthritis.
The dissemination of B. burgdorferi following infection, and the frequent development of arthritis subsequently, suggest that the efforts of the innate immune system against B. burgdorferi infection are often insufficient for preventing chronic disease. However, while the lack of an initial coordinated response by macrophages, dendritic cells, and neutrophils against infection permits the dissemination of the spirochetes, these cells eventually recover to mount a highly effective attack against the organisms. Unfortunately for the host, the early failings of these innate cells require the use of immune mechanisms which directly aid in arthritic development in order to fight persistent borrelial infection. Neutrophils, which are absent in EM lesions following infection, appear to be required in the synovial tissues for the induction of arthritis (24). They likely release enzymes that degrade host tissue while killing B. burgdorferi. In addition, antigen-presenting cells such as macrophages and dendritic cells, which work diligently (but inadequately) to clear initial borrelial infection, activate T cells to specifically kill B. burgdorferi and, in doing so, initiate significant inflammatory events that may result in destructive arthritis. These shortcomings in the innate immune response to B. burgdorferi infection are magnified when factors such as differential surface antigen expression in the host (87, 88, 128) elicits additional, potentially damaging immune responses against an organism that escaped innate immune surveillance.
Attachment of spirochetes to host tissues.
B. burgdorferi organisms contain several surface molecules that facilitate binding to proteoglycan components of the extracellular matrix of synovial tissues. Indeed, B. burgdorferi organisms have been shown to persist in the connective tissues of chronically infected mice and humans (10, 11, 67). The presence of spirochetes at these sites is likely required for the inflammatory response leading to arthritis in individuals not receiving antimicrobial therapy. Recent studies have demonstrated that B. burgdorferi can bind to fibronectin via the borrelial BBK32 protein (54, 120) and that BBK32 may be a suitable antigen for serodiagnosis of Lyme arthritis (69). In addition, B. burgdorferi can bind to type I collagen (159), to glycosaminoglycans by the Borrelia glycosaminoglycans-binding protein (116, 117), and to integrins (38, 39) by the p66 protein (27, 113). Whether these molecules are absolutely needed for establishing infectivity and development of the immune response is unclear.
By contrast, the role of B. burgdorferi decorin-binding protein (Dbp) in mediating host attachment and assisting infectivity is more defined. B. burgdorferi organisms have been shown to bind decorin, a proteoglycan molecule found on the surface of collagen fibers (63). Liang et al. (87) demonstrated that the presence of spirochetes was related to the amount of decorin found in the host tissues. It was also shown that the degree of protection for B. burgdorferi in the joints was correlated to the expression of the DbpA gene (dbpA) by B. burgdorferi (87). In addition, the presence of decorin in the host appears to be required for the development of arthritis in mice (26), and immunization of mice with DbpA may be protective against infection (65). Furthermore, it was shown that DbpA may be a suitable antigen for the serodiagnosis of Lyme arthritis (68). By contrast, it was demonstrated that an increase in the amount of DbpA on the surface of B. burgdorferi diminished colonization of the joint and prevented the development of arthritis (156). In this case, it is likely that the adherence of spirochetes to decorin in the skin was increased to a degree such that dissemination to the joints was greatly reduced. The development of Lyme arthritis, therefore, is likely mediated by the binding of B. burgdorferi to decorin located within the tissues of the joints.
Facilitating attachment following infection with B. burgdorferi is the expression of various host molecules in the synovial tissues. The increased expression of adhesion molecules such as intracellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), E-selectin, and P-selectin has been demonstrated upon in vitro interaction of B. burgdorferi and host endothelial tissues (19). The joint tissues of B. burgdorferi-infected mice also have been shown to significantly up-regulate the expression of these adhesion molecules (127). In addition, the expression of ICAM-2 and VCAM-1 was observed in human synovial cells infected with B. burgdorferi (134) as well as in the synovial tissues of patients with Lyme arthritis (3). It is believed that these molecules are responsible for homing of inflammatory cells to the site of infection and maintaining the sustained interaction between T cells and antigen-processing cells. Furthermore, infection with B. burgdorferi has been shown to increase the expression of the neutrophil-attracting KC (24). The infiltration of neutrophils via attraction to KC into synovial tissues plays an important role in the development of arthritis, as infected mice deficient in the KC receptor CXCR2 developed significantly less severe arthritis than wild-type mice and lacked the presence of neutrophils in the synovial tissues (24). Cinco et al. (37) further demonstrated that B. burgdorferi organisms bind to neutrophils via the αmβ2 integrin, adding a novel pathway for the innate immune system to recognize infection without implementing phagocytosis and antigen processing. Taken together, these findings suggest that adhesion molecule induction facilitates Borrelia attachment to neutrophils localized in the tissue and promotes the rapid migration and accumulation of leukocytes at the site of B. burgdorferi infection. Subsequently, neutrophils release inflammatory cytokines before they phagocytize B. burgdorferi organisms, initiating antigen processing for adaptive T-cell responses.
The role of TLRs in B. burgdorferi-induced arthritis.
A robust inflammatory response to B. burgdorferi is the hallmark of both early-stage (EM) and middle and late-stage (arthritis) manifestations of Lyme disease. These inflammatory responses are likely initiated by the expression of genes activated by the binding of borrelial components to Toll-like receptors (TLRs). TLRs are host cell receptors which recognize conserved molecular patterns on microbial components such as lipoproteins, lipopolysaccharide, proteoglycans, flagellin, and nucleic acids. Binding of these components to TLRs initiates a signaling cascade that results in the NF-κB-mediated expression of various proinflammatory cytokines and chemokines. Most TLR signaling is directed through the adapter molecule myeloid differentiation factor 88 (MyD88). Recent studies have illuminated the role of TLR signaling in the control of borrelial load and the development of arthritis.
MyD88 appears to play a significant role in the control of B. burgdorferi organisms. The ability of Ixodes scapularis ticks to obtain B. burgdorferi from infected mice was increased in the absence of MyD88 in the mice, as was the ability of the ticks to deliver spirochetes to uninfected mice (18). The importance of MyD88 has also been demonstrated following intradermal infection of mice (15, 20, 92). Mice deficient in MyD88 harbor a significantly higher bacterial load in various tissues, including the joints, than MyD88+/+ and MyD88+/− mice. For example, levels of B. burgdorferi DNA in the joints of MyD88−/− mice were approximately 71 times and 166 times greater than those found in MyD88+/− and MyD88+/+ mice, respectively, 2 weeks after infection (20). A significantly higher bacterial load was still observed in MyD88−/− mice 8 weeks following infection. Similarly, the joints of MyD88−/− mice contained more B. burgdorferi organisms (on the order of 2.5 logs) than infected wild-type mice 3 weeks after infection (15). The role of MyD88 in controlling infection with B. burgdorferi has been supported by findings demonstrating that the organisms are less commonly found in blood cultures of wild-type mice (92). Collectively, these findings suggest that the presence of intact TLR signaling, through the adaptor molecule MyD88, is necessary for a competent innate immune response against B. burgdorferi.
The absence of MyD88-mediated TLR-mediated inflammatory cytokine production in MyD88−/− mice suggests that these mice would fail to develop significant histopathological changes of the joints, despite the presence of high bacterial loads. However, histopathological examination of the joints of these mice has shown differing degrees of arthritic severity. Other studies have reported no significant differences in inflammation and composition of cellular infiltrate in the joints between B. burgdorferi-infected MyD88−/− and wild-type mice (15, 92). Analysis of cytokines and chemokines in the joints of B. burgdorferi-infected MyD88−/− mice showed no significant increase in the levels of IFN-γ, TNF-α, IL-12, CXCL-1, or CXCL-2 compared to infected wild-type mice (15). By contrast, others studies have shown that B. burgdorferi-infected MyD88−/− mice developed arthritis with increased levels of neutrophil and mononuclear cell infiltration relative to both MyD88+/− and MyD88−/− mice (20). Bolz et al. (20) hypothesized that immune events independent of MyD88 signaling may be responsible for the development of arthritis and influx of inflammatory cells in MyD88−/− mice infected with B. burgdorferi.
Research on the role of specific TLRs in Borrelia infection has centered on TLR2, as Borrelia organisms present multiple lipoproteins on the cell surface. Various investigators have demonstrated that B. burgdorferi-infected TLR2-deficient mice harbor significantly more spirochetes in various tissues, including the joints, than TLR2+/+ and TLR2+/− mice (20, 150, 151, 154). For example, TLR2−/− mice harbor approximately sixfold more Borrelia organisms in the joint tissues than wild-type mice 21 days after infection (150). Even greater effects of TLR2 deficiency on borrelial load were reported by Bolz et al. (20), who demonstrated that the presence of spirochetes in the joint of infected TLR2-deficient mice is approximately 15 times greater than that found in infected controls. These findings were extended to show that significantly higher borrelial loads were present in the ankle joints of TLR2-deficient mice up to 8 weeks after infection (154). These findings provide clear evidence that early immune recognition of B. burgdorferi is mediated by TLR2 signaling events. An intact TLR2 response induces the production of inflammatory cytokines and chemokines necessary to provide more efficient clearance of infection.
While it has been shown that the production of inflammatory molecules through TLR2 signaling following interaction with borrelial lipoproteins has the capability to effectively limit infection, these initial inflammatory events may also set the course for development of arthritis. It would be reasonable to infer that absence of immune mediators leading to the increased borrelial load in TLR2-deficient mice would result in a decrease of arthritic pathology in these mice. However, recent findings have provided conflicting results. One study found no significant difference in swelling of the ankles between Borrelia-infected wild-type and TLR2-deficient mice, despite the higher borrelial load in the joints of the latter (150). In addition, the degree of arthritis, as determined by the degree of synovitis and capsular inflammation, was indistinguishable between the groups. By contrast, increases of both ankle swelling (20, 151) and arthritis (20) have been reported in Borrelia-infected TLR2-deficient mice compared to infected wild-type controls. Another study (154) found that infected TLR2-deficient mice experienced greater ankle swelling and slightly increased, but insignificantly different, arthritis. Taken together, these results suggest that mechanisms other than TLR2 signaling may be responsible for the induction of arthritis.
Host proteins responsible for tissue degradation.
In addition to inducing inflammatory mediators from cells of the innate immune system, the presence of B. burgdorferi in the synovial tissues leads to the induction of tissue-degrading factors from nonimmune host cells. B. burgdorferi is not known to export proteases capable of causing degradation of cartilage and erosion of bone. However, elevated levels of host MMPs have been found in synovial fluid of patients with Lyme arthritis (74, 90). MMPs (13, 74) have been induced from chondrocytes following culture with B. burgdorferi. Recently, Behera et al. (14) showed that B. burgdorferi also induced, in human chondrocytes, aggrecanase 1, which exposes the collagen matrix for processing by MMPs (14, 74, 90). The means by which MMPs are induced following infection with B. burgdorferi remain unclear, as TLR-dependent (58) and TLR-independent (16) mechanisms have been reported.
In summary, the presence of B. burgdorferi in the joints plays a major role in the development of Lyme arthritis. These organisms have developed mechanisms to adhere to the connective tissue of the synovium, induce the expression of leukocyte adhesion molecules and chemoattractants from synovial cells, and stimulate the production of extracellular matrix-degrading proteases from cartilage cells and inflammatory cytokines from immune cells via multiple cell-signaling pathways. The various routes by which arthritis may be induced by the presence of B. burgdorferi signify the complexity and intensity of the immune response to the spirochete.
ARTHRITIS DEVELOPMENT IN THE ABSENCE OF B. BURGDORFERI
The presence of B. burgdorferi in connective tissues of the joints of infected individuals likely plays an important role in establishing the course of Lyme arthritis. Indeed, it has been demonstrated that B. burgdorferi organisms and DNA in the joints of infected individuals are not detected following antimicrobial treatment (112) and that arthritic symptoms typically subside shortly thereafter. However, approximately 10% of patients with Lyme arthritis maintain disease despite adequate antimicrobial therapy (137, 139). High levels of various proinflammatory mediators are observed in the joints of these individuals (132), but the levels of these mediators differ among patients prior to and following treatment (90). In addition, the synovial tissues of antibiotic-treated Lyme arthritis patients express high levels of leukocyte adhesion molecules such as ICAM-1 and VCAM-1 (3), which would likely lead to the development of inflammation at the site. The progression of chronic arthritis appears to be independent of spirochetes at the disease site. However, significant controversy about this topic still exists within the field of Lyme disease (7, 142).
Recently, Steere and Glickstein (136) proposed a series of hypotheses to elucidate the mechanism(s) by which arthritis persists despite adequate antimicrobial treatment. The first hypothesis explored the possibility of a sustained presence of B. burgdorferi (136) in the host, possibly by establishing residence in sites inaccessible to antibiotics or by mechanisms of immune evasion (99). Contrary to this hypothesis, the presence of B. burgdorferi DNA has not been observed in chronically arthritic patients following multiple antibiotic regimens (112). These antibiotic-resistant arthritis patients are usually administered anti-inflammatory drugs following antimicrobial therapy to resolve the arthritis (136). However, a recent study demonstrates that B. burgdorferi DNA may be detected in infected mice treated with a combination of ceftriaxone and antibodies to the inflammatory cytokine TNF-α, while treatment with ceftriaxone alone yielded no observation of borrelial DNA (157). The authors claim that their findings support the hypothesis of borrelial persistence despite antibiotic treatment. Moreover, they suggest that the inflammatory response may contribute to both the persistence of arthritis and the suppression of B. burgdorferi to levels below the detection capability of PCR but not the immune components responsible for maintaining arthritis. As a relatively low percentage of mice were positive for B. burgdorferi by culture or PCR (22 to 30%) following treatment with anti-TNF-α antibody and ceftriaxone and as the results were reflective of one individual trial (157), additional studies are needed to clarify whether Borrelia organisms can survive in the host after treatment with antimicrobial agents.
A second hypothesis proposed to explain the occurrence of antibiotic-resistant arthritis pertains to the possibility of borrelial antigens' being retained in the host following treatment (136). While some studies may possibly support this hypothesis (60), the evidence, especially in humans, is lacking. Although Gondolf et al. (60) showed that outer surface proteins of B. burgdorferi are retained on the surface of cartilage and synovial membranes, Carlson et al. (29) could not confirm these findings among 26 patients with treatment-resistant Lyme arthritis. More evidence, however, supports the hypothesis by Steere and Glickstein that antibiotic treatment-resistant Lyme arthritis is mediated by an autoimmune mechanism (136), possibly involving host molecular mimicry to OspA.
Various studies have implicated an autoimmune response to OspA peptides in the development of antibiotic-resistant Lyme arthritis. Among patients with this arthritis, there is an increased frequency in the possession of the major histocompatibility complex class II molecule histocompatibility leukocyte antigen (HLA)-DR4, and this association correlated to prolonged antibody reactivity to OspA (78). In addition, these patients experienced increased T-cell reactivity to OspA peptides (34). It was further shown that certain HLA-DRB1 alleles were also associated with a predisposition to this chronic arthritis (141) and that these molecules were able to bind a peptide of OspA (138) with homology to a peptide of human leukocyte function-associated antigen 1 (hLFA-1) (62). Furthermore, the production of immunoglobulin G antibodies to OspA and OspB correlates with the severity and duration of chronic, destructive arthritis in humans, while the immunoglobulin G antibody response to all other borrelial proteins correlates with earlier disease stages (2).
Additional evidence for the role of autoimmunity induced by B. burgdorferi OspA in the maintenance of arthritis has pertained to the development of a Lyme disease vaccine. Several reports have demonstrated that the immune response against OspA is protective against infection; however, the possibility that this antigen may possess epitopes likely to induce cross-reactivity to host tissues (62) has created a significant amount of controversy in the field of vaccination against Lyme disease.
Fikrig et al. demonstrated that mice immunized with Escherichia coli organisms transformed with OspA from B. burgdorferi provided greater protection from the development of arthritis than immunization with nontransformed E. coli (52). In addition, immunization with a recombinant OspA fusion protein completely prevented the development of arthritis in mice (52). These findings were supported and extended to suggest that antibodies elicited against OspA in the host are capable of clearing B. burgdorferi from the feeding ticks (50). In addition, it was shown that vaccination with OspA prevented the development of arthritis several months after challenge and that the protective potential of OspA vaccination was sufficient to prevent arthritis when challenge was administered months after vaccination (51). These results suggested the feasibility of OspA as a potential Lyme disease vaccine candidate, and independent clinical trials by Steere et al. (140) and Sigal et al. (133) supported the use of a recombinant OspA vaccine in humans. As a result, such a vaccine was approved and marketed for use in humans in 1998.
Concerns about side effects, particularly arthritis (124), following vaccination of humans with the OspA vaccine arose soon after the vaccine was approved by the Food and Drug Administration. Prior to the approval of this vaccine, studies implicated OspA (2, 78) or a peptide of OspA (62) in the development of severe inflammation or autoimmunity. It was shown that the antibody response to OspA correlated with the development of severe arthritis in untreated, B. burgdorferi-infected patients (2) and in genetically susceptible (HLA-DR4), antibiotic-resistant Lyme arthritis patients (78). In addition, the demonstration of structural homology between peptides of OspA and hLFA-1 led to the possibility that vaccination with OspA might lead to an autoimmune response. Following the approval of the OspA vaccine for human use, follow-up experimental studies showed that OspA-vaccinated hamsters challenged with different strains of whole B. burgdorferi organisms exhibited a destructive osteoarthropathy (40). Additional support for the role of auto-reactive OspA peptides in the development of antibiotic-resistant Lyme arthritis was provided (138, 141). However, an investigation of vaccine recipients reported no significant evidence of adverse effects due to the vaccine (86). Despite this, the recombinant OspA vaccine was withdrawn from the market in 2002, citing a lack of demand (73). Its presumed role in arthritis was not mentioned.
Recently, an OspA vaccine containing a mutated auto-reactive epitope was shown to decrease the occurrence of paw swelling in mice compared to a wild-type OspA vaccine while providing similar protection against infection (153). Despite this, however, further studies will be required to determine the safety, efficacy, and necessity for a Lyme disease vaccine, especially one comprised of OspA peptides, for humans. The major concern about OspA, besides induction of arthritis, is that OspA expression is rapidly down-regulated upon tick feeding (128), and therefore vaccination with OspA offered little or no protection for humans. Although this argument was sidestepped with claims that blood from the vaccinated host killed the OspA-expressing Borrelia in the midgut of the ticks (51), we now know that the saliva of ticks can inactivate complement (101, 121), eliminating the threat of complement-dependent OspA bactericidal antibody. The present focus on vaccination, however, has turned to OspC (46, 47, 93) and other antigens because they are expressed on Borrelia when it enters the human host. Again, epitopes that elicit broad protection without inducing arthritis are prime targets for a vaccine.
The final hypothesis posed by Steere and Glickstein for explaining the development of antibiotic-resistant arthritis is a dysfunction in the immunoregulation of innate or adaptive responses, leading to the perpetuation of inflammation induced by B. burgdorferi in the synovium (“bystander activation”) (136). Although little evidence is available to directly support this hypothesis (136), it is intriguing nonetheless. A role for regulatory T cells in the control of a sustained inflammatory response to B. burgdorferi was posed (136). In support of this hypothesis, CD4+ CD25+ T cells were shown to mediate the development of arthritis in Borrelia-vaccinated and -challenged mice (109, 111). An increase in the population of these cells corresponded to a significant reduction of arthritic severity (109). In addition, removal of these cells with a monoclonal antibody induced a severe, destructive osteoarthropathy (109). Moreover, adoptive transfer of enriched populations of CD4+ CD25+ T cells into Borrelia-vaccinated and -challenged mice prevents the development of arthritis (111). These findings support a role for cells with immunoregulatory function in the control of an excessive immune response leading to Borrelia-associated arthritis. However, these findings do not directly address a dysfunction of specific components of innate or adaptive immunity and, as such, are limited in their support of this bystander activation hypothesis.
Finally, other B. burgdorferi antigens, besides OspA, may be responsible for autoimmune effects. It has been demonstrated that B. burgdorferi 50772, which lacks the B. burgdorferi ospA/B operon, is able to induce severe, destructive arthritis in mice (unpublished data). In addition, antibodies to a borrelial 37-kDa protein (arthritis-related protein; Arp) was able to hasten the resolution of established arthritis in severe combined immunodeficient mice (49); however, immunization with recombinant Arp or anti-Arp serum prior to infection with B. burgdorferi failed to prevent the induction of arthritis. Furthermore, administration of inactivated or heat-killed whole B. burgdorferi is unable to elicit arthritis upon challenge or in a vaccination-challenge model of Lyme arthritis (unpublished data). These findings demonstrate that borrelial antigens in addition to OspA may be responsible for the autoimmune effects of B. burgdorferi infection. The mechanisms responsible for their induction of arthritis may be the same or different from that of OspA.
LYME ARTHRITIS: A Th1-MEDIATED RESPONSE?
An early investigation demonstrated that, upon stimulation with borrelial antigens, synovial fluid cells from patients with chronic Lyme arthritis produced IL-2, TNF-α, and IFN-γ but not IL-3, IL-4, or IL-5 (158). Following this finding, various studies implicated IFN-γ as a key modulator of arthritic development. As a result, the inflammation characterizing Lyme arthritis has traditionally been assigned as a Th1-mediated response. However, recent findings have questioned the absolute requirement for IFN-γ in the development of Lyme arthritis (22, 23, 35, 59, 130). These findings have generated considerable research interest, as their implications would lead to an amendment of the established paradigm of Lyme arthritis as solely a Th1 cytokine-driven inflammatory response.
Evidence for lyme arthritis as a Th1 cytokine-driven inflammatory response.
It has been demonstrated that the host genetics of experimental animals play a significant role in determining the severity of arthritis upon infection with the Lyme spirochete (8). For example, C3H/HeJ mice have been characterized as “arthritis-susceptible,” as they have been shown to develop relatively severe inflammation of the paws upon infection with B. burgdorferi, while “arthritis-resistant” BALB/c mice develop only mild inflammation of the paws (8). This difference in pathology led to the investigation of a corresponding difference in the Th cell-mediated response to Borrelia infection. Specifically, Borrelia-stimulated popliteal lymph node cells from infected C3H (arthritis susceptible) showed a characteristic Th1 cytokine response (high production of IFN-γ and low production of IL-4), while those of BALB/c mice showed a Th2 cytokine-mediated response (low production of IFN-γ and high production of IL-4) (102). In addition, it was demonstrated that administration of anti-IFN-γ antibodies to Borrelia-infected C3H and BALB/c mice reduced the degree of paw swelling (80, 102) and reduced the spirochete load in the joints (80), while administration of anti-IL-4 antibodies to these mice increased both the severity of paw swelling (80, 102) and the number of spirochetes found in the joints (80). Furthermore, administration of recombinant IL-4 to arthritis-susceptible C3H mice reduced swelling and the number of spirochetes found in the joints (81). Overall, these findings suggest that Th1 cytokines, such as IFN-γ, play a significant role in the development of Lyme arthritis, while Th2 cytokines serve to influence protection from arthritis.
Moreover, the role of IL-4 as a Lyme arthritis-preventing cytokine was challenged by Kang et al. (79), who demonstrated that arthritis-resistant BALB/c mice and arthritis-susceptible C3H mice develop similar degrees of arthritis during early infection and that lymph node cells from BALB/c mice actually produce greater amounts of IFN-γ than C3H mice 2 days after stimulation with borrelial antigens. It was also shown that BALB/c mice recover from arthritis more rapidly than C3H mice, and the observation from previous studies of high levels of IL-4 detected in Borrelia-infected BALB/c mice may be a reflection of immune events leading to the resolution, rather than the prevention, of arthritis (79). In support of this finding, it was shown (42) that the ratio of IL-4-producing CD4+ T cells to IFN-γ-producing-CD4+ T cells increased with time in cultures of Borrelia-stimulated lymph node cells from infected BALB/c mice. These findings provide further evidence of an initial Th1 cytokine-mediated response in the development of Lyme arthritis and suggest that arthritis resolution, but not prevention, is associated with Th2 cytokine production.
Other studies have provided additional evidence of a role for Th1 cytokines in the development of Lyme arthritis. Administration of antibodies to IL-12, an IFN-γ-inducing cytokine, to Borrelia-infected C3H mice decreased the production of IFN-γ and reduced the severity of arthritis (5). Interestingly, treatment of infected SCID mice with anti-IL-12 antibodies increased the severity of arthritis (6), indicating a role for innate immunity in determining the degree of arthritic pathology. In addition, activation of endothelial cells with B. burgdorferi and IFN-γ led to the increased expression of various mediators involved in leukocyte and T-cell recruitment (41); these cells would likely function to contribute to an inflammatory response leading to arthritis (41). In support of this hypothesis, it was shown that the synovial fluid of patients with antibiotic-resistant Lyme arthritis contains significant levels of IFN-γ and chemokines responsible for the recruitment of inflammatory cells and lymphocytes (132). Collectively, many studies demonstrate roles for Th1 and Th2 cytokines in the development and resolution of Lyme arthritis.
Evidence that Th1 cytokines are not required to induce Lyme arthritis.
Recent studies examining the roles of IFN-γ and IL-4 in the development of Lyme arthritis have reached conclusions at odds with the established Th1/Th2-cytokine model. Shanafelt et al. (130) demonstrated that, although disrupting B7/CD28-mediated costimulation of T cells with antibodies to CD80 and CD86 increased levels of IFN-γ, this antibody treatment resulted in a trend of decreased severity of arthritis. In addition, since blocking costimulation with anti-CD86 antibodies also prevented the production of IL-4 (130), it was determined that a mediator(s) other than this Th2 cytokine is responsible for the resolution of Lyme arthritis. Furthermore, it was shown that disruption of the genes encoding IL-4 and the IL-4 receptor alpha failed to affect joint swelling and arthritis in Borrelia-infected mice (119).
Brown and Reiner (22, 23) also showed that IFN-γ is not required for the induction of Lyme arthritis. They demonstrated that depletion of IFN-γ-producing NK cells in arthritis-susceptible C3H mice did not affect the development of arthritis in challenged mice (22). It was also shown that Borrelia-infected IFN-γ-deficient mice developed arthritis to the same degree as wild-type mice (23). In addition, infected mice deficient in the IFN-γ receptor displayed pathology to a similar extent as the wild-type parental strain (59). Furthermore, vaccinated and challenged IFN-γ-deficient C57BL/6 mice developed a chronic, severe, destructive osteoarthropathy characterized by destruction of cartilage and erosion of bone (35). These results suggest that Lyme arthritis is induced by cytokines other than IFN-γ.
Interleukin-17 as a possible mediator of Lyme arthritis.
The findings that arthritis can develop in the absence of IFN-γ indicate that the currently accepted model of Lyme arthritis as a Th1 cytokine-driven inflammatory response is incomplete. That the prototypical Th1 cytokine is not absolutely required for the induction of arthritis following B. burgdorferi infection warrants a modification of the paradigm to include additional proinflammatory cytokines, chemokines, or immune modulators.
What other immune factors could contribute to the paradigm of Lyme arthritis as a Th1-mediated response? A recently discovered subset of helper T cells, Th17 cells, which are distinct from Th1 and Th2 cells (66, 85, 114) and are characterized by the production of the inflammatory cytokine IL-17, has been shown to play a role in the development of arthritis (71, 131) and the erosion of bone (126). In addition, high levels of IL-17 are present in the synovial fluid of patients with rheumatoid arthritis (32, 163). IL-17 induces the production of proinflammatory cytokines from stromal cells, synoviocytes, chondrocytes, and macrophages (31, 56, 129) and shows synergy with other cytokines for the induction of bone resorption (83, 95) and stimulation of osteoclast differentiation (83). Furthermore, neutralization of IL-17 causes substantial reduction of collagenase activity (33), osteoclast formation (83), and production of proinflammatory cytokines (1, 32, 77). Moreover, B. burgdorferi (82) or its lipoproteins (75) have been shown to induce the production of IL-17. These properties make the Th17 cell subset a strong candidate for an additional route by which Lyme arthritis may be induced.
What evidence is available for an amendment of the current paradigm of Lyme arthritis as a solely Th1-mediated inflammatory response to include IL-17-producing Th17 cells? IFN-γ-deficient Borrelia-vaccinated and -infected mice administered antibodies to IL-17 (28, 109) or to the IL-17 receptor (28) fail to develop the destructive arthritis observed in untreated control mice. The induction of destructive arthritis has also been observed in wild-type C57BL/6 Borrelia-vaccinated and -challenged mice (70) and has been prevented by administration of anti-IL-17 antibodies (unpublished data). These findings suggest that IL-17 and presumably the subset of activated T cells that produce it (1) play a major role in the development of Borrelia-induced arthritis, even in the presence of an intact Th1 cytokine response.
Additional evidence implies that Th17 cells may play a role in the modulation of arthritis induced upon Borrelia infection. Neutralization of IL-17 in IFN-γ-deficient Borrelia-vaccinated and -challenged mice induces the production of CD4+ CD25+ T cells (109) with the ability to prevent the induction of arthritis upon adoptive transfer into Borrelia-vaccinated and -challenged mice (111). These findings were the first to demonstrate a relationship between the absence of IL-17 and the development of T cells with immunoregulatory function. In support of these findings, the local inflammatory cytokine environment has been shown to modulate the development of Th17 cells and CD4+ CD25+ Foxp3+ T regulatory cells from a common precursor in vitro (17, 100, 147), and evidence indicates that development of these cells is polar (17). Furthermore, alterations of this cytokine environment dictate the induction of helper T cells into either a Th1 or Th17 phenotype (17, 100, 147). These parallel findings suggest that the IL-17 responsible for the development of Borrelia-induced arthritis may be derived from a subset of helper T cells distinct from Th1 cells, implying the necessity to modify the current paradigm of Lyme arthritis as solely a Th1-mediated response.
CONCLUSION
Arthritis is a well-documented complication following infection with the tick-borne spirochete B. burgdorferi. The severity of arthritis can range from mild to moderate inflammation of the joints and tendons months after infection, to a chronic, debilitating osteoarthropathy complete with destruction of cartilage and erosion of bone in a subset of these individuals within a few years. Host morbidity following infection with B. burgdorferi is an unintended consequence of a robust immune response against the bacteria. The innate response against B. burgdorferi has been implicated in the release of cytokines, chemokines, and other immune mediators responsible for the development of an inflammatory response that inflicts damage to host tissues while attempting to eliminate the spirochetes. In addition, processing and presentation of B. burgdorferi antigens by macrophages and dendritic cells initiate an adaptive immune response, characterized by the release of Th1 cytokines, which further exacerbates the inflammatory response.
While the exact sequential mechanism(s) responsible for the development of Lyme arthritis is unknown, various studies implicate an intricate network of vector, bacterial, and host factors responsible for aiding initial spirochetal infection, dissemination to and establishment within joint tissues, and the recruitment of immune and nonimmune cells which release inflammatory and tissue-degrading mediators within the joint. Complicating the prevention of Lyme arthritis is a genetic predisposition in certain individuals to autoimmunity after vaccination. Therefore, the current prevention of morbidity associated with B. burgdorferi infection may have to rely on treatment of established infection rather than vaccination.
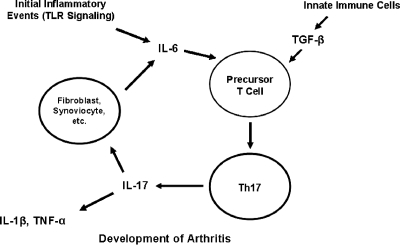
The development of Lyme arthritis has traditionally been attributed to Th1 cytokines, such as IFN-γ. However, recent studies demonstrate that IFN-γ is not absolutely required for the induction of Lyme arthritis. This suggests that the established model of Lyme arthritis as solely a Th1-mediated response may have to be modified to include other inflammatory cytokines or mediators. One possible candidate for the updating of this paradigm is the Th17 cell subset, a recently discovered helper T-cell subset distinct from Th1 and Th2 cells. The prototypical Th17 cytokine, IL-17, has been shown to play a major role in the development of arthritis in Borrelia-vaccinated and challenged mice (28, 109). How might Th17 cells play a role in the development of arthritis following infection with B. burgdorferi? Interaction of host cells with B. burgdorferi may lead to the production of proinflammatory cytokines, including IL-6. Transforming growth factor-β (100), in combination with IL-6 (17, 147), may induce the production of IL-17-producing Th17 cells. IL-17 may then induce the downstream production of inflammatory cytokines such as IL-1β and TNF-α. Moreover, IL-17 may stimulate cells such as fibroblasts and synoviocytes to produce inflammatory cytokines, including IL-6. This IL-6 may serve to contribute to additional TGF-β-mediated Th17 cell production until the spirochete burden is eventually reduced to levels inefficient for inducing further inflammation (Fig. 1). In addition to significantly altering the release of IL-1β and TNF-α, blocking IL-17 may prevent the release of additional IL-6 required for the development of Th17 cells.
FIG. 1.
Proposed mechanism for the development of Th17 cells and arthritis following infection with B. burgdorferi. Infection with B. burgdorferi stimulates the TLR-mediated release of proinflammatory cytokines, such as IL-6. IL-6, in combination with endogenous TGF-β, induces the development of Th17 cells from T-cell precursors. Th17 cells produce IL-17, which stimulates the production of other proinflammatory cytokines, such as IL-1β and TNF-α. IL-17 also induces from fibroblasts and synovial cells the production of IL-6, which serves to continue the inflammatory feedback loop. This process may occur until the borrelial load is reduced such that TLR-mediated production of IL-6 is insufficient to influence Th17 cell production.
Further investigation of IL-17 and Th17 cells in the development of arthritis in the Borrelia infection and Borrelia vaccination and challenge models will provide a more complete picture of the inflammatory events leading to the development of arthritis in mice. The development of novel targets for the treatment and prevention of Borrelia-associated arthritis in humans may include expansion of the current paradigm that Th1 cells are solely responsible for the arthritis.
Footnotes
Published ahead of print on 14 November 2007.
REFERENCES
- 1.Aggarwal, S., and A. L. Gurney. 2002. IL-17: prototype member of an emerging cytokine family. J. Leukoc. Biol. 71:1-8. [PubMed] [Google Scholar]
- 2.Akin, E., G. McHugh, R. Flavell, E. Fikrig, and A. Steere. 1999. The immunoglobulin (IgG) antibody response to OspA and OspB correlates with severe and prolonged Lyme arthritis and the IgG response to P35 correlates with mild brief arthritis. Infect. Immun. 67:173-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akin, E., J. Aversa, and A. C. Steere. 2001. Expression of adhesion molecules in synovia of patients with treatment-resistant Lyme arthritis. Infect. Immun. 69:1774-1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amlong, C. A., D. T. Nardelli, S. Heil Peterson, T. F. Warner, S. M. Callister, and R. F. Schell. 2006. Anti-interleukin-15 prevents arthritis in Borrelia-vaccinated and -infected mice. Clin. Vaccine Immunol. 13:289-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anguita, J., D. H. Persing, M. Rincón, S. W. Barthold, and E. Fikrig. 1996. Effect of anti-interleukin 12 treatment of murine Lyme borreliosis. J. Clin. Investig. 97:1028-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anguita, J., S. Samanta, S. W. Barthold, and E. Fikrig. 1997. Ablation of interleukin-12 exacerbates Lyme arthritis in SCID mice. Infect. Immun. 65:4334-4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Auwaerter, P. G. 2007. Point: antibiotic therapy is not the answer for patients with persisting symptoms attributable to Lyme disease. Clin. Infect. Dis. 45:143-148. [DOI] [PubMed] [Google Scholar]
- 8.Barthold, S. W. 1996. Lyme borreliosis in the laboratory mouse. J. Spirochet. Tick-borne Dis. 3:22-44. [Google Scholar]
- 9.Barthold, S. W., and M. de Souza 1995. Exacerbation of Lyme arthritis in beige mice. J. Infect. Dis. 172:778-784. [DOI] [PubMed] [Google Scholar]
- 10.Barthold, S. W., D. H. Persing, A. L. Armstrong, and R. A. Peeples. 1991. Kinetics of Borrelia burgdorferi dissemination and evolution of disease after intradermal inoculation of mice. Am. J. Pathol. 139:263-273. [PMC free article] [PubMed] [Google Scholar]
- 11.Barthold, S. W., M. S. de Souza, J. L. Janotka, A. L. Smith, and D. H. Persing. 1993. Animal model: chronic Lyme borreliosis in the laboratory mouse. Am. J. Pathol. 143:959-971. [PMC free article] [PubMed] [Google Scholar]
- 12.Bazzoni, F., M. A. Cassatella, F. Rossi, M. Ceska, B. Dewald, and M. Baggiolini. 1991. Phagocytizing neutrophils produce and release high amounts of the neutrophil activating peptide-1/interleukin-8. J. Exp. Med. 173:771-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Behera, A. K., E. Hildebrand, J. Scagliotti, A. C. Steere, and L. T. Hu. 2005. Induction of host matrix metalloproteinases by Borrelia burgdorferi differs in human and murine Lyme disease. Infect. Immun. 73:126-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Behera, A. K., E. Hildebrand, J. Szafranski, H. H. Hung, A. J. Grodzinsky, R. Lafyatis, A. E. Koch, R. Kalish, G. Perides, A. C. Steere, and L. T. Hu. 2006. Role of aggrecanase 1 in Lyme arthritis. Arthritis Rheum. 54:3319-3329. [DOI] [PubMed] [Google Scholar]
- 15.Behera, A. K., E. Hildebrand, R. T. Bronson, G. Perides, S. Uematsu, S. Akira, and L. T. Hu. 2006. MyD88 deficiency results in tissue-specific changes in cytokine induction and inflammation in interleukin-18-independent mice infected with Borrelia burgdorferi. Infect. Immun. 74:1462-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Behera, A. K., E. Hildebrand, S. Uematsu, S. Akira, J. Coburn, and L. T. Hu. 2006. Identification of a TLR-independent pathway for Borrelia burgdorferi-induced expression of matrix metalloproteinases and inflammatory mediators through binding to integrin α3β1. J. Immunol. 177:657-664. [DOI] [PubMed] [Google Scholar]
- 17.Bettelli, E., Y. Carrier, W. Gao, T. Korn, T. B. Strom, M. Oukka, H. L. Weiner, and V. K. Kuchroo. 2006. Reciprocal development pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441:235-238. [DOI] [PubMed] [Google Scholar]
- 18.Bockenstedt, L. K., N. Liu, I. Schwartz, and D. Fish. 2006. MyD88 deficiency enhances acquisition and transmission of Borrelia burgdorferi by Ixodes scapularis ticks. Infect. Immun. 74:2154-2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Böggemeyer, E., T. Stehle, U. E. Schaible, M. Hahne, D. Vestweber, and M. M. Simon. 1994. Borrelia burgdorferi upregulates the adhesion molecules E-selectin, P-selectin, ICAM-1 and VCAM-1 on mouse endothelioma cells in vitro. Cell. Adhes. Commun. 2:145-157. [DOI] [PubMed] [Google Scholar]
- 20.Bolz, D. D., R. S. Sundsbak, Y. Ma, S. Akira, C. J. Kirschning, J. F. Zachary, J. H. Weis, and J. J. Weis. 2004. MyD88 plays a unique role in host defense but not arthritis development in Lyme disease. J. Immunol. 173:2003-2010. [DOI] [PubMed] [Google Scholar]
- 21.Boring, L., J. Gosling, S. W. Chensue, S. L. Kunkel, R. V. Farese, Jr., H. E. Broxmeyer, and I. F. Charo. 1997. Impaired monocyte migration and reduced type I (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J. Clin. Investig. 100:2552-2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown, C. R., and S. L. Reiner. 1998. Activation of natural killer cells in arthritis-susceptible but not arthritis-resistant mouse strains following Borrelia burgdorferi infection. Infect. Immun. 66:5208-5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown, C. R., and S. L. Reiner. 1999. Experimental Lyme arthritis in the absence of interleukin-4 or gamma interferon. Infect. Immun. 67:3329-3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown, C. R., V. A. Blaho, and C. M. Loiacono. 2003. Susceptibility to experimental Lyme arthritis correlates with KC and monocyte chemoattractant protein-1 production in joints and requires neutrophil recruitment via CXCR2. J. Immunol. 171:893-901. [DOI] [PubMed] [Google Scholar]
- 25.Brown, C. R., V. A. Blaho, and C. M. Loiacono. 2004. Treatment of mice with the neutrophil-depleting antibody RB6-8C5 results in early development of experimental Lyme arthritis via the recruitment of GR-1-polymorphonuclear leukocyte-like cells. Infect. Immun. 72:4956-4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown, E. L., R. M. Wooten, B. J. B. Johnson, R. V. Iozzo, A. Smith, M. C. Dolan, B. P. Guo, J. J. Weis, and M. Höök. 2001. Resistance to Lyme disease in decorin-deficient mice. J. Clin. Investig. 107:845-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bunikis, J., L. Noppa, and S. Bergstrom. 1995. Molecular analysis of a 66-kDa protein associated with the outer membrane of Lyme disease Borrelia. FEMS Microbiol. Lett. 131:139-145. [DOI] [PubMed] [Google Scholar]
- 28.Burchill, M. A., D. T. Nardelli, D. M. England, D. J. DeCoster, J. A. Christopherson, S. M. Callister, and R. F. Schell. 2003. Inhibition of interleukin-17 prevents the development of arthritis in vaccinated mice challenged with Borrelia burgdorferi. Infect. Immun. 71:3437-3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carlson, D., J. Hernandez, B. J. Bloom, J. Coburn, J. M. Aversa, and A. C. Steere. 1999. Lack of Borrelia burgdorferi DNA in synovial samples from patients with antibiotic treatment-resistant Lyme arthritis. Arthritis Rheum. 42:2705-2709. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention. 2007. Lyme disease—United States, 2003-2005. Morb. Mortal. Wkly. Rep. 56:573-576. [PubMed] [Google Scholar]
- 31.Chabaud, M., F. Fossiez, J. L. Taupin, and P. Miossec. 1998. Enhancing effect of IL-17 on IL-1-induced IL-6 and leukemia inhibitory factor production by rheumatoid arthritis synoviocytes and its regulation of Th2 cytokines. J. Immunol. 161:409-414. [PubMed] [Google Scholar]
- 32.Chabaud, M., J. M. Durand, N. Bush, F. Fossiez, G. Page, L. Frapport, and P. Miossec. 1999. Human interleukin-17: a T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum. 42:963-970. [DOI] [PubMed] [Google Scholar]
- 33.Chabaud, M., P. Garnero, J. M. Dayer, P. A. Guerne, F. Fossiez, and P. Miossec. 2000. Contribution of interleukin-17 to synovium matrix destruction in rheumatoid arthritis. Cytokine 12:1092-1099. [DOI] [PubMed] [Google Scholar]
- 34.Chen, J., J. A. Field, L. Glickstein, P. J. Molloy, B. T. Huber, and A. C. Steere. 1999. Association of antibiotic treatment-resistant Lyme arthritis with T cell responses to dominant epitopes of outer surface protein A of Borrelia burgdorferi. Arthritis Rheum. 42:1813-1822. [DOI] [PubMed] [Google Scholar]
- 35.Christopherson, J. A., E. L. Munson, D. M. England, C. L. Croke, M. C. Remington, M. L. Molitor, D. J. DeCoster, S. M. Callister, and R. F. Schell. 2003. Destructive arthritis in vaccinated interferon gamma-deficient mice challenged with Borrelia burgdorferi: modulation by tumor necrosis factor alpha. Clin. Diag. Lab. Immunol. 10:44-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cinco, M., B. Cini, R. Murgia, G. Presani, M. Prodan, and S. Perticarari. 2001. Evidence of involvement of the mannose receptor in adhesion of Borrelia burgdorferi to monocyte/macrophages. Infect. Immun. 69:2743-2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cinco, M., R. Murgia, S. Perticarari, and G. Presani. 1998. Surface receptors of neutrophils towards B. burgdorferi. Wien. Klin. Wochenschr. 110:866-869. [PubMed] [Google Scholar]
- 38.Coburn, J., J. M. Leong, and J. K. Erban. 1993. Integrin αIIbβ3 mediates binding of the Lyme disease agent, Borrelia burgdorferi, to human platelets. Proc. Natl. Acad. Sci. USA 90:7058-7063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coburn, J., L. Magoun, and S. C. Bodary, and J. M. Leong. 1998. Integrins αvβ3 and α5β1 mediate attachment of Lyme disease spirochetes to human cells. Infect. Immun. 66:1946-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Croke, C. L., E. L. Munson, S. D. Lovrich, J. A. Christopherson, M. C. Remington, D. M. England, S. M. Callister, and R. F. Schell. 2000. Occurrence of severe destructive Lyme arthritis in hamsters vaccinated with outer surface protein A and challenged with Borrelia burgdorferi. Infect. Immun. 68:658-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dame, T. M., B. L. Orenzoff, L. E. Palmer, and M. B. Furie. 2007. IFN-γ alters the response of Borrelia burgdorferi-activated endothelium to favor chronic inflammation. J. Immunol. 178:1172-1179. [DOI] [PubMed] [Google Scholar]
- 42.Dong, Z., M. D. Edelstein, and L. J. Glickstein. 1997. CD8+ T cells are activated during the early Th1 and Th2 immune responses in a murine Lyme disease model. Infect. Immun. 65:5334-5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DuChateau, B. K., D. M. England, S. M. Callister, L. C. L. Lim, S. D. Lovrich, and R. F. Schell. 1996. Macrophages exposed to Borrelia burgdorferi induce Lyme arthritis in hamsters. Infect. Immun. 64:2540-2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DuChateau, B. K., E. L. Munson, D. M. England, S. D. Lovrich, S. M. Callister, J. R. Jensen, and R. F. Schell. 1999. Macrophages interact with enriched populations of distinct T lymphocyte subsets for the induction of severe destructive Lyme arthritis. J. Leukoc. Biol. 65:162-170. [DOI] [PubMed] [Google Scholar]
- 45.DuChateau, B. K., J. R. Jensen, D. M. England, S. M. Callister, S. D. Lovrich, and R. F. Schell. 1997. Macrophages and enriched populations of T lymphocytes interact synergistically for the induction of severe, destructive Lyme arthritis. Infect. Immun. 65:2829-2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Earnhart, C. G., and R. T. Marconi. 2007. OspC phylogenetic analyses support the feasibility of a broadly protective polyvalent chimeric Lyme disease vaccine. Clin. Vaccine Immunol. 14:628-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Earnhart, C. G., E. L. Buckles, and R. T. Marconi. 2007. Development of an OspC-based tetravalent, recombinant, chimeric vaccinogen that elicits bactericidal antibody against diverse Lyme disease spirochete strains. Vaccine 25:466-480. [DOI] [PubMed] [Google Scholar]
- 48.Fäldt, J., C. Dahlgren, and M. Ridell. 2002. Difference in neutrophil cytokine production induced by pathogenic and non-pathogenic mycobacteria. APMIS 110:593-600. [DOI] [PubMed] [Google Scholar]
- 49.Feng, S., E. Hodzic, and S. Barthold. 2000. Lyme arthritis resolution to a 37-kilodalton Borrelia burgdorferi protein. Infect. Immun. 68:4169-4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fikrig, E., S. R. Telford III, S. W. Barthold, R. S. Kantor, A. Spielman, and R. A. Flavell. 1992. Elimination of Borrelia burgdorferi from vector ticks feeding on OspA-immunized mice. Proc. Natl. Acad. Sci. USA 89:5418-5421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fikrig, E., S. W. Barthold, R. S. Kantor, and R. A. Flavell. 1992. Long-term protection of mice from Lyme disease by vaccination with OspA. Infect. Immun. 60:773-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fikrig, E., S. W. Barthold, R. S. Kantor, and R. A. Flavell. 1990. Protection of mice against the Lyme disease agent by immunizing with recombinant OspA. Science 250:553-556. [DOI] [PubMed] [Google Scholar]
- 53.Filgueira, L., F. O. Nestlé, M. Rittig, H. I. Joller, and P. Groscurth. 1996. Human dendritic cells phagocytose and process Borrelia burgdorferi. J. Immunol. 157:2998-3005. [PubMed] [Google Scholar]
- 54.Fischer, J. R., K. T. LeBlanc, and J. M. Leong. 2006. Fibronectin binding protein BBK2 of the Lyme disease spirochete promotes bacterial attachment of glycosaminoglycans. Infect. Immun. 74:435-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Foley, D. M., R. J. Gayek, J. T. Skare, E. A. Wagar, C. I. Champion, D. R. Blanco, M. A. Lovett, and J. M. Miller. 1995. Rabbit model of Lyme borreliosis: erythema migrans, infection-derived immunity, and identification of Borrelia burgdorferi proteins associated with virulence and protective immunity. J. Clin. Investig. 96:965-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fossiez, F., O. Djossou, P. Chomarat, L. Flores-Romo, S. Ait-Yahia, C. Maat, J. J. Pin, P. Garrone, E. Garcia, S. Saeland, D. Blanchard, C. Gaillard, B. Das Mahapatra, E. Rouveir, P. Golstein, J. Banchereau, and S. Lebecque. 1996. T-cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J. Exp. Med. 183:2593-2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garcia, R., L. Gusmani, R. Murgia, C. Guarnaccia, M. Cinco, and G. Rottini. 1998. Elastase is the only human neutrophil granule protein that alone is responsible for in vitro killing of Borrelia burgdorferi. Infect. Immun. 66:1408-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gebbia, J. A., J. L. Coleman, and J. L. Benach. 2004. Selective induction of matrix metalloproteinases by Borrelia burgdorferi via toll-like receptor 2 in monocytes. J. Infect. Dis. 189:113-119. [DOI] [PubMed] [Google Scholar]
- 59.Glickstein, L., M. Edelstein, and J. Z. Dong. 2001. Gamma interferon is not required for arthritis resistance in the murine Lyme disease model. Infect. Immun. 69:3737-3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gondolf, K. B., M. Mihatsch, E. Curschellas, J. J. Dunn, and S. R. Batsford. 1994. Induction of experimental allergic arthritis with outer surface proteins of Borrelia burgdorferi. Arthritis Rheum. 37:1070-1077. [DOI] [PubMed] [Google Scholar]
- 61.Granucci, F., I. Zanoni, N. Pavelka, S. L. Van Dommelen, C. E. Andoniou, F. Belardelli, M. A. Degli Esposti, and P. Ricciardi-Castagnoli. 2004. A contribution of mouse dendritic cell-derived IL-2 for NK cell activation. J. Exp. Med. 200:287-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gross, D. M., T. Forsthuber, M. Tary-Lehmann, C. Etling, K. Ito, Z. A. Nagy, J. A. Field, A. C. Steere, and B. Hubner. 1998. Identification of LFA-1 as a candidate autoantigen in treatment-resistant Lyme arthritis. Science 281:703-706. [DOI] [PubMed] [Google Scholar]
- 63.Guo, B. P., S. J. Norris, L. C. Rosenberg, and M. Höök. 1995. Adherence of Borrelia burgdorferi to the proteoglycan decorin. Infect. Immun. 63:3467-3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hannier, S., J. Liversidge, J. M. Sternberg, and A. S. Bowman. 2004. Characterization of the B-cell inhibitory protein factor in Ixodes ricinus tick saliva: a potential role in enhanced Borrelia burgdorferi transmission. Immunology 113:401-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hanson, M. S., D. R. Cassatt, B. P. Guo, N. K. Patel, M. P. McCarthy, D. W. Dorward, and M. Höök. 1998. Active and passive immunity against Borrelia burgdorferi decorin binding A (DbpA) protects against infection. Infect. Immun. 66:2143-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harrington, L. E., P. R. Mangan, and C. T. Weaver. 2006. Expanding the effector CD4 T-cell repertoire: the Th17 lineage. Curr. Opin. Immunol. 18:349-356. [DOI] [PubMed] [Google Scholar]
- 67.Häupl, T., G. Hahn, M. Rittig, A. Krause, C. Schoerner, U. Schönherr, J. R. Kalden, and G. R. Burmester. 1993. Persistence of Borrelia burgdorferi in ligamentous tissue from a patient with chronic Lyme borreliosis. Arthritis Rheum. 36:1621-1626. [DOI] [PubMed] [Google Scholar]
- 68.Heikkilä, T., I. Seppälä, H. Saxén, J. Panelius, H. Yrjänäinen, and P. Lahdenne. 2002. Species-specific serodiagnosis of Lyme arthritis and neuroborreliosis due to Borrelia burgdorferi sensu stricto, B. afzelii, and B. garinii by using decorin binding protein A. J. Clin. Microbiol. 40:453-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Heikkilä, T., I. Seppäalä, H. Saxén, J. Panelius, M. Peltomaa, T. Julin, S.-A. Carlsson, and P. Lahdenne. 2002. Recombinant BBK32 protein in serodiagnosis of early and late Lyme borreliosis. J. Clin. Microbiol. 40:1174-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Heil Peterson, S., D. T. Nardelli, T. F. Warner, S. M. Callister, J. R. Torrealba, and R. F. Schell. 2007. Anti-p19 antibody treatment exacerbates Lyme arthritis and enhances borreliacidal activity. Clin. Vaccine Immunol. 14:510-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hirota, K., M. Hashimoto, H. Yoshitomi, S. Tanaka, T. Nomura, T. Yamaguchi, Y. Iwakura, N. Sakaguchi, and S. Sakaguchi. 2007. T cell self-reactivity forms a cytokine milieu for spontaneous development of IL-17+ Th cells that cause autoimmune arthritis. J. Exp. Med. 204:41-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hirschfeld, M., C. J. Kirschning, R. Schwandner, H. Wesche, J. H. Weis, R. M. Wooten, and J. J. Weis. 1999. Cutting edge: inflammatory signaling by Borrelia burgdorferi lipoproteins is mediated by toll-like receptor 2. J. Immunol. 163:2382-2386. [PubMed] [Google Scholar]
- 73.Hitt, E. 2002. Poor sales trigger vaccine withdrawal. 2002. Nat. Med. 8:311-312. [DOI] [PubMed] [Google Scholar]
- 74.Hu, L. T., M. A. Eskildsen, C. Masgala, A. C. Steere, E. C. Arner, M. A. Pratta, A. J. Grodzinsky, A. Loening, and G. Perides. 2001. Host metalloproteinases in Lyme arthritis. Arthritis Rheum. 44:1401-1410. [DOI] [PubMed] [Google Scholar]
- 75.Infante-Duarte, C., H. F. Horton, M. C. Byrne, and T. Kamradt. 2000. Microbial lipopeptides induce the production of IL-17 in Th cells. J. Immunol. 165:6107-6115. [DOI] [PubMed] [Google Scholar]
- 76.Jablonska, E., M. Marcinczyk, L. Tabarek, S. Pancewicz, T. Hermanowska-Szpakowicz, and J. Jablonski. 2003. IL-15 in the culture supernatants of PMN and PBMC and the serum of patients with Lyme disease. Rocz. Akad. Med. Bialymst. 48:78-81. [In Polish.] [PubMed] [Google Scholar]
- 77.Jovanovic, D. V., J. A. Di Battista, J. Martel-Pelletier, F. C. Jolicoeur, Y. He., M. Zhang, F. Mineau, and J. P. Pelletier. 1998. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-1β and TNF-α, by human macrophages. J. Immunol. 160:3513-3521. [PubMed] [Google Scholar]
- 78.Kalish, R. A., J. M. Leong, and A. C. Steere. 1993. Association of treatment-resistant chronic Lyme arthritis with HLA-DR4 and antibody reactivity to OspA and OspB of Borrelia burgdorferi. Infect. Immun. 61:2774-2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kang, I., S. W. Barthold, D. H. Persing, and L. K. Bockenstedt. 1997. T-helper-cell cytokines in the early evolution of murine Lyme arthritis. Infect. Immun. 65:3107-3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Keane-Myers, A., and S. P. Nickell. 1995. Role of IL-4 and IFN-γ in modulation of immunity to Borrelia burgdorferi in mice. J. Immunol. 155:2020-2028. [PubMed] [Google Scholar]
- 81.Keane-Myers, A., C. R. Maliszewski, F. D. Finkelman, and S. P. Nickell. 1996. Recombinant IL-4 treatment augments resistence to Borrelia burgdorferi infections in both normal susceptible and antibody-deficient susceptible mice. J. Immunol. 156:2488-2494. [PubMed] [Google Scholar]
- 82.Knauer, J., S. Siegemund, U. Müller, S. Al-Robaiy, R. A. Kastelein, G. Alber, and R. K. Straubinger. 2007. Borrelia burgdorferi potently activates bone marrow-derived conventional dendritic cells for production of IL-23 required for IL-17 release by T cells. FEMS Immunol. Med. Microbiol. 49:353-363. [DOI] [PubMed] [Google Scholar]
- 83.Kotake, S., N. Udagawa, N. Takahashi, K. Matsuzaki, K. Itoh, S. Ishiyama, S. Saito, K. Inoue, N. Kamatani, M. T. Gillespie, T. J. Martin, and T. Suda. 1999. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J. Clin. Investig. 103:1345-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kotsyfakis, M., A. Sá-Nunes, I. M. B. Francischetti, T. N. Mather, J. F. Andersen, and J. M. C. Ribeiro. 2006. Antiinflammatory and immunosuppressive activity of sialostatin L, a salivary cystatin from the tick Ixodes scapularis. J. Biol. Chem. 281:26298-26307. [DOI] [PubMed] [Google Scholar]
- 85.Langrish, C. L., Y. Chen, W. M. Blumenschein, J. Mattson, B. Basham, J. D. Sedgwick, T. McClanahan, R. A. Kastelein, and D. J. Cua. 2005. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201:233-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lathrop, S. L., R. Ball, P. Haber, G. T. Mootrey, M. M. Braun, S. V. Shadomy, S. S. Ellenberg, R. T. Chen, and E. B. Hayes. 2002. Adverse event reports following vaccination for Lyme disease: December 1998-July 2000. Vaccine 20:1603-1608. [DOI] [PubMed] [Google Scholar]
- 87.Liang, F. T., E. L. Brown, T. Wang, R. V. Iozzo, and E. Fikrig. 2004. Protective niche for Borrelia burgdorferi to evade humoral immunity. Am. J. Pathol. 165:977-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liang, F. T., J. Yan, M. L. Mbow, S. L. Sviat, R. D. Gilmore, M. Mamula, and E. Fikrig. 2004. Borrelia burgdorferi changes its surface antigenic expression in response to host immune responses. Infect. Immun. 72:5759-5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lim, L. C. L., D. M. England, B. K. DuChateau, N. J. Glowacki, J. R. Creson, S. D. Lovrich, S. M. Callister, D. A. Jobe, and R. F. Schell. 1994. Development of destructive arthritis in vaccinated hamsters challenged with Borrelia burgdorferi. Infect. Immun. 62:2825-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lin, B., M. Kidder, R. Noring, A. C. Steere, M. S. Klempner, and L. T. Hu. 2001. Differences in synovial fluid levels of matrix metalloproteinases suggest separate mechanisms of pathogenesis in Lyme arthritis before and after antibiotic treatment. J. Infect. Dis. 184:174-180. [DOI] [PubMed] [Google Scholar]
- 91.Linder, S., C. Heimerl, V. Fingerle, M. Aepfelbacher, and B. Wilske. 2001. Coiling phagocytosis of Borrelia burgdorferi by primary macrophages Is controlled by CDC42Hs and Rac1 and involves recruitment of Wiskott-Aldrich syndrome protein and Arp2/3 complex. Infect. Immun. 69:1739-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu, N., R. R. Montgomery, S. W. Barthold, and L. K. Bockenstedt. 2004. Myeloid differentiation antigen 88 deficiency impairs pathogen clearance but does not alter inflammation in Borrelia burgdorferi-infected mice. Infect. Immun. 72:3195-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lovrich, S. D., D. A. Jobe, R. F. Schell, and S. M. Callister. 2005. Borreliacidal OspC antibodies specific for a highly conserved epitope are immunodominant in human Lyme disease and do not occur in mice or hamsters. Clin. Diag. Lab. Immunol. 12:746-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Loza, M. J., and B. Perussia. 2001. Final steps of natural killer cell maturation: a model for type 1-type 2 differentiation? Nat. Immunol. 2:917-924. [DOI] [PubMed] [Google Scholar]
- 95.Lubberts, E., L. A. B. Joosten, B. Oppers, L. Van den Bersselaar, C. J. J. Coenen-de-Roo, J. D. Kolles, P. Schwarzenberger, F. A. J. Van de Loo, and W. B. Van den Berg. 2001. IL-1-independent role of IL-17 in synovial inflammation and joint destruction during collagen-induced arthritis. J. Immunol. 167:1004-1013. [DOI] [PubMed] [Google Scholar]
- 96.Lusitani, D., S. E. Malawista, and R. R. Montgomery. 2002. Borrelia burgdorferi are susceptible to killing by a variety of PMN components. J. Infect. Dis. 185:797-804. [DOI] [PubMed] [Google Scholar]
- 97.Ma, Y., K. Petri Seiler, K.-F. Tai, L. Yang, M. Woods, and J. J. Weis. 1994. Outer surface lipoproteins of Borrelia burgdorferi stimulate nitric oxide production by the cytokine-inducible pathway. Infect. Immun. 62:3663-3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Macháèková, M., M. Oborník, and J. Kopecký. 2006. Effect of salivary gland extract from Ixodes ricinus ticks on the proliferation of Borrelia burgdorferi sensu stricto in vivo. Folia Parasitol. 53:153-158. [PubMed] [Google Scholar]
- 99.Malawista, S. E. 2000. Resolution of Lyme arthritis, acute or prolonged: a new look. Inflammation 24:493-504. [DOI] [PubMed] [Google Scholar]
- 100.Mangan, P. R., L. E. Harrington, D. B. O'Quinn, W. S. Helms, D. C. Bullard, C. O. Elson, R. D. Hatton, S. M. Wahl, T. R. Schoeb, and C. T. Weaver. 2006. Transforming growth factor-β induces development of the TH17 lineage. Nature 441:231-234. [DOI] [PubMed] [Google Scholar]
- 101.Mather, T. N., M. T. Yeh, J. M. C. Ribeiro, A. Scorpio, D. R. Nelson, and R. T. Coughlin. 1996. Ixodes saliva: vector competence for Borrelia burgdorferi and potential vaccine strategies, abstr. A029. Abstr. VII Int. Cong. Lyme Borreliosis, San Francisco, CA.
- 102.Matyniak, J. E., and S. L. Reiner. 1995. T helper phenotype and genetic susceptibility in experimental Lyme disease. J. Exp. Med. 181:1251-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Modolell, M., U. E. Schaible, M. Rittig, and M. M. Simon. 1994. Killing of Borrelia burgdorferi by macrophages is dependent on oxygen radicals and nitric oxide and can be enhanced by antibodies to outer surface proteins of the spirochete. Immunol. Lett. 40:139-146. [DOI] [PubMed] [Google Scholar]
- 104.Montgomery, R. R., and S. E. Malawista. 1996. Entry of Borrelia burgdorferi is end-on and leads to degradation in lysosomes. Infect. Immun. 64:2867-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Montgomery, R. R., D. Lusitani, A. de Boisfleury Chevance, and S. E. Malawista. 2002. Human phagocytic cells in the early innate immune response to Borrelia burgdorferi. J. Infect. Dis. 185:1773-1779. [DOI] [PubMed] [Google Scholar]
- 106.Montgomery, R. R., D. Lusitani, A. de Boisfleury Chevance, and S. E. Malawista. 2004. Tick saliva reduces adherence and area of human neutrophils. Infect. Immun. 72:2989-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Montgomery, R. R., M. H. Nathanson, and S. E. Malawista. 1994. Fc- and non-Fc-mediated phagocytosis of Borrelia burgdorferi by macrophages. J. Infect. Dis. 170:890-893. [DOI] [PubMed] [Google Scholar]
- 108.Morrison, T. B., J. H. Weis, and J. J. Weis. 1997. Borrelia burgdorferi outer surface protein A (OspA) activates and primes human neutrophils. J. Immunol. 158:4838-4845. [PubMed] [Google Scholar]
- 109.Nardelli, D. T., M. A. Burchill, D. M. England, J. Torrealba, S. M. Callister, and R. F. Schell. 2004. Association of CD4+ CD25+ T cells with prevention of severe destructive arthritis in Borrelia burgdorferi-vaccinated and challenged gamma interferon-deficient mice treated with anti-interleukin-17 antibody. Clin. Diagn. Lab. Immun. 11:1075-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nardelli, D. T., T. F. Warner, S. M. Callister, and R. F. Schell. 2006. Anti-CD25 antibody treatment of mice vaccinated and challenged with Borrelia spp. does not exacerbate arthritis but inhibits borreliacidal antibody production. Clin. Vaccine Immunol. 13:884-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nardelli, D. T., J. P. Cloute, K. H. K. Luk, J. Torrealba, T. F. Warner, S. M. Callister, and R. F. Schell. 2005. CD4+CD25+ T cells prevent arthritis associated with Borrelia vaccination and infection. Clin. Diag. Lab. Immunol. 12:786-792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nocton, J. J., F. Dressler, B. J. Rutledge, P. N. Rys, D. H. Persing, and A. C. Steere. 1994. Detection of Borrelia burgdorferi DNA by polymerase chain reaction in synovial fluid from patients with Lyme arthritis. N. Engl. J. Med. 330:229-234. [DOI] [PubMed] [Google Scholar]
- 113.Ntchobo, H., H. Rothermel, W. Chege, A. C. Steere, and J. Coburn. 2001. Recognition of multiple antibody epitopes throughout Borrelia burgdorferi p66, a candidate adhesin, in patients with early or late manifestations of Lyme disease. Infect. Immun. 69:1953-1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Park, H., Z. Li, X. O. Yang, S. H. Chang, R. Nurieva, Y. H. Wang, Y. Wang, L. Hood, Z. Zhu, Q. Tian, and C. Dong. 2005. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 6:1133-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Parker, L. C., M. K. B. Whyte, S. K. Dower, and I. Sabroe. 2005. The expression and roles of Toll-like receptors in the biology of the human neutrophil. J. Leukoc. Biol. 77:886-892. [DOI] [PubMed] [Google Scholar]
- 116.Parveen, N., and J. M. Leong. 2000. Identification of a candidate glycosaminoglycan-binding adhesion of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35:1220-1234. [DOI] [PubMed] [Google Scholar]
- 117.Parveen, N., M. Caimano, J. D. Radolf, and J. M. Leong. 2003. Adaptation of the Lyme disease spirochaete to the mammalian host environment results in enhanced glycosaminoglycan and host cell binding. Mol. Microbiol. 47:1433-1444. [DOI] [PubMed] [Google Scholar]
- 118.Peterson, P. K., C. C. Clawson, D. A. Lee, D. J. Garlich, P. G. Quie, and R. C. Johnson. 1984. Human phagocyte interactions with the Lyme disease spirochete. Infect. Immun. 46:608-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Potter, M. R., N. Noben-Trauth, J. H. Weis, C. Teuscher, and J. J. Weis. 2000. Interleukin-4 (IL-4) and IL-13 signaling pathways do not regulate Borrelia burgdorferi-induced arthritis in mice: IgG1 is not required for host control of tissue spirochetes. Infect. Immun. 68:5603-5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Probert, W. S., J. H. Kim, M. Höök, and B. J. B. Johnson. 2001. Mapping the ligand-binding region of Borrelia burgdorferi fibronectin-binding protein BBK2. Infect. Immun. 69:4129-4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ribeiro, J. M. 1987. Ixodes dammini: salivary anti-complementary activity. Exp. Parasitol. 64:347-353. [DOI] [PubMed] [Google Scholar]
- 122.Ribeiro, J. M. C., J. J. Weis, and S. R. Telford III. 1990. Saliva of the tick Ixodes dammini inhibits neutrophil function. Exp. Parasitol. 70:382-388. [DOI] [PubMed] [Google Scholar]
- 123.Ribeiro, J. M. C., G. T. Makoul, J. Levine, D. R. Robinson, and A. Spielman. 1985. Antihemostatic, antiinflammatory, and immunosuppressove properties of the saliva of a tick, Ixodes dammini. J. Exp. Med. 161:332-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rosé, C. D., P. T. Fawcett, and K. M. Gibney. 2001. Arthritis following recombinant outer surface protein A vaccination for Lyme disease. J. Rheumatol. 28:2555-2557. [PubMed] [Google Scholar]
- 125.Salazar, J. C., C. D. Pope, T. J. Sellati, H. M. Feder, Jr., T. G. Kiely, K. R. Dardick, R. L. Buckman, M. W. Moore, M. J. Caimano, J. G. Pope, P. J. Krause, J. D. Radolf, and The Lyme Disease Network. 2003. Coevolution of markers of innate and adaptive immunity in skin and peripheral blood of patients with erythema migrans. J. Immunol. 171:2660-2670. [DOI] [PubMed] [Google Scholar]
- 126.Sato, K., A. Suematsu, K. Okamoto, A. Yamaguchi, Y. Morishita, Y. Kadono, T. Kodama, S. Akira, Y. Iwakura, D. J. Cua, and H. Takayanagi. 2006. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J. Exp. Med. 203:2673-2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schaible, U. E., D. Vestweber, E. G. Butcher, T. Stehle, and M. M. Simon. 1994. Expression of endothelial cell adhesion molecules in joints and heart during Borrelia burgdorferi infection of mice. Cell. Adhes. Commun. 2:465-479. [DOI] [PubMed] [Google Scholar]
- 128.Schwan, T. G., and J. Piesman. 2000. Temporal changes in outer surface proteins A and C of the Lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J. Clin. Microbiol. 38:382-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shalom-Barak, T., J. Quach, and M. Lotz. 1998. Interleukin-17 induced gene expression in articular chondrocytes is associated with activation of mitogen-activated protein kinases and NF-κB. J. Biol. Chem. 273:27467-27473. [DOI] [PubMed] [Google Scholar]
- 130.Shanafelt, M.-C., I. Kang, S. W. Barthold, and L. K. Bockenstedt. 1998. Modulation of murine Lyme borreliosis by interruption of the B7/CD28 T-cell costimulatory pathway. Infect. Immun. 66:266-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sheibanie, A. F., T. Khayrullina, T., F. F. Safadi, and D. Ganea. 2007. Prostaglandin E(2) exacerbates collagen-induced arthritis in mice through the inflammatory interleukin-23/interleukin-17 axis. Arthritis Rheum. 56:2608-2619. [DOI] [PubMed] [Google Scholar]
- 132.Shin, J. J., L. J. Glickstein, and A. C. Steere. 2007. High levels of inflammatory chemokines and cytokines in joint fluid and synovial tissue throughout the course of antibiotic-refractory Lyme arthritis. Arthritis Rheum. 56:1325-1335. [DOI] [PubMed] [Google Scholar]
- 133.Sigal, L. H., J. M. Zahradnik, P. Lavin, S. J. Patella, G. Bryant, R. Haselby, E. Hilton, M. Kunkel, D. Adler-Klein, T. Doherty, J. Evans, S. E. Malawista, and The Recombinant Outer-Surface Protein A Lyme Disease Vaccine Study Consortium. 1998. A vaccine consisting of recombinant Borrelia burgdorferi outer surface protein A to prevent Lyme disease. N. Engl. J. Med. 339:216-222. [DOI] [PubMed] [Google Scholar]
- 134.Singh, S. K., V. Baar, H. Morbach, and H. J. Girschick. 2006. Expression of ICAM-1, ICAM-2, NCAM-1 and VCAM-1 by human synovial cells exposed to Borrelia burgdorferi in vitro. Rhematol. Int. 26:818-827. [DOI] [PubMed] [Google Scholar]
- 135.Steere, A. C. 2001. Lyme disease. N. Engl. J. Med. 345:115-125. [DOI] [PubMed] [Google Scholar]
- 136.Steere, A. C., and L. Glickstein. 2004. Elucidation of Lyme arthritis. Nat. Rev. Immunol. 4:143-152. [DOI] [PubMed] [Google Scholar]
- 137.Steere, A. C., A. Grbofsky, M. E. Patarroyo, F. J. Winchester, J. A. Hardin, and S. E. Malawista. 1979. Chronic Lyme arthritis: clinical and immunogenetic differentiation from rheumatoid arthritis. Ann. Intern. Med. 90:896-901. [DOI] [PubMed] [Google Scholar]
- 138.Steere, A. C., B. Falk, E. E. Drouin, L. A. Baxter-Lowe, J. Hammer, and G. T. Nepom. 2003. Binding of outer surface protein A and human lymphocyte function-associated antigen 1 peptides to HLA-DR molecules associated with antibiotic treatment-resistant Lyme arthritis. Arthritis Rheum. 48:534-540. [DOI] [PubMed] [Google Scholar]
- 139.Steere, A. C., R. T. Schoen, and E. Taylor. 1987. The clinical evolution of Lyme arthritis. Ann. Intern. Med. 107:725-731. [DOI] [PubMed] [Google Scholar]
- 140.Steere, A. C., V. K. Sikand, F. Meurice, D. L. Parenti, E. Fikrig, R. T Schoen, J. Nowakowski, C. H. Schmid, S. Laukamp, C. Buscarino, D. S. Krause, and The Lyme Disease Vaccine Study Group. 1998. Vaccination against Lyme disease with recombinant Borrelia burgdorferi outer surface lipoprotein A with adjuvant. N. Engl. J. Med. 339:209-215. [DOI] [PubMed] [Google Scholar]
- 141.Steere, A. C., W. Klitz, E. E. Drouin, B. A. Falk, W. W. Kwok, G. T. Nepom, and L. A. Baxter-Lowe. 2006. Antibiotic-refractory Lyme arthritis is associated with HLA-DR molecules that bind a Borrelia burgdorferi peptide. J. Exp. Med. 203:961-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Stricker, R. B. 2007. Counterpoint: long-term antibiotic therapy improves persistent symptoms associated with Lyme disease. Clin. Infect. Dis. 45:149-157. [DOI] [PubMed] [Google Scholar]
- 143.Strieter, R. M., K. Kasahara, R. Allen, H. J. Showell, T. J. Standiford, and S. L. Kunkel. 1990. Human neutrophils exhibit disparate chemotactic gene expression. Biochem. Biophys. Res. Commun. 173:725-730. [DOI] [PubMed] [Google Scholar]
- 144.Szczepanski, A., and H. B. Fleit. 1988. Interaction between Borrelia burgdorferi and polymorphonuclear leukocytes: phagocytosis and the induction of respiratory burst. Ann. N. Y. Acad. Sci. 539:425-428. [Google Scholar]
- 145.Szomolanyi-Tsuda, E., X. Liang, R. M. Welsh, E. A. Kurt-Jones, and R. W. Finberg. 2006. Role for TLR2 in NK cell-mediated control of murine cytomegalovirus in vivo. J. Virol. 80:4286-4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.van Dam, A. P., H. Kuiper, K. Vos, A. Widjojokusumo, B. M. de Jongh, L. Spanjaard, A. C. Ramselaar, M. D. Kramer, and J. Dankert. 1993. Different genospecies of Borrelia burgdorferi are associated with distinct clinical manifestations of Lyme borreliosis. Clin. Infect. Dis. 17:708-717. [DOI] [PubMed] [Google Scholar]
- 147.Veldhoen, M., R. J. Hocking, C. J. Atkins, R. M. Locksley, and B. Stockinger. 2006. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24:179-189. [DOI] [PubMed] [Google Scholar]
- 148.Wang, G., C. Ojaimi, R. Iyer, V. Saksenberg, S. A. McClain, G. P. Wormser, and I. Schwartz. 2001. Impact of genotypic variation on Borrelia burgdorferi sensu stricto on kinetics of dissemination and severity of disease in C3H/HeJ mice. Infect. Immun. 69:4303-4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang, G., C. Ojaimi, H. Wu, V. Saksenberg, R. Iyer, D. Liveris, S. A. McClain, G. P. Wormser, and I. Schwartz. 2002. Disease severity in a murine model of Lyme borreliosis is associated with the genotype of the infecting Borrelia burgdorferi sensu stricto strain. J. Infect. Dis. 186:782-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang, G., Y. Ma, A. Buyuk, S. McClain, J. J. Weis, and I. Schwartz. 2004. Impaired host defense to infection and Toll-like receptor 2-independent killing of Borrelia burgdorferi clinical isolated in TLR2-deficient C3H/HeJ mice. FEMS Microbiol. Lett. 231:219-225. [DOI] [PubMed] [Google Scholar]
- 151.Wang, X., Y. Ma, J. H. Weis, J. F. Zachary, C. J. Kirschning, and J. J. Weis. 2005. Relative contributions of innate and acquired host responses to bacterial control and arthritis development in Lyme disease. Infect. Immun. 73:657-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Weis, J. J., B. A. McCracken, Y. Ma, D. Fairbairn, R. J. Roper, T. B. Morrison, J. H. Weis, J. F. Zachary, R. W. Doerge, and C. Teuscher. 1999. Identification of quantitative trait loci governing arthritis severity and humoral responses in the murine model of Lyme disease. J. Immunol. 162:948-956. [PubMed] [Google Scholar]
- 153.Willett, T. A., A. L. Meyer, E. L. Brown, and B. T. Huber. 2004. An effective second-generation outer surface protein A-derived Lyme vaccine that eliminates a potentially autoreactive T cell epitope. Proc. Natl. Acad. Sci. USA 101:1303-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wooten, R. M., Y. Ma, R. A. Yoder, J. P. Brown, J. H. Weis, J. F. Zachary, C. J. Kirschning, and J. J. Weis. 2002. Toll-like receptor 2 is required for innate, but not acquired, host defense to Borrelia burgdorferi. J. Immunol. 168:348-355. [DOI] [PubMed] [Google Scholar]
- 155.Xu, Q., S. V. Seemanapalli, K. E. Reif, C. R. Brown, and F. T. Liang. 2007. Increasing the recruitment of neutrophils to the site of infection dramatically attenuates Borrelia burgdorferi infectivity. J. Immunol. 178:5109-5115. [DOI] [PubMed] [Google Scholar]
- 156.Xu, Q., S. V. Seemanaplli, K. McShan, and F. T. Liang. 2007. Increasing the interaction of Borrelia burgdorferi with decorin significantly reduces 50 percent infectious dose and severely impairs dissemination. Infect. Immun. 75:4272-4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yrjänäinen, H., J. Hytönen, X. R. Song, J. Oksi, K. Hariala, and M. K. Viljanen. 2007. Anti-tumor necrosis factor-α treatment activates Borrelia burgdorferi spirochetes 4 weeks after ceftriaxone treatment in C3H/He mice. J. Infect. Dis. 195:1489-1496. [DOI] [PubMed] [Google Scholar]
- 158.Yssel, H., M.-C. Shanafelt, C. Soderbreg, P. V. Schneider, J. Anzola, and G. Peltz. 1991. Borrelia burgdorferi activates a T helper type 1-like T cell subset in Lyme arthritis. J. Exp. Med. 174:593-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zambrano, M. C., A. A. Beklemisheva, A. V. Bryksin, S. A. Newman, and F. C. Cabello. 2004. Borrelia burgdorferi binds to, invades, and colonizes native type I collagen lattices. Infect. Immun. 72:3138-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zanoni, I., F. Granucci, M. Foti, and P. Ricciardi-Castagnoli. 2007. Self-tolerance, dendritic cell (DC)-mediated activation and tissue distribution of natural killer (NK) cells. Immunol. Lett. 110:6-17. [DOI] [PubMed] [Google Scholar]
- 161.Zhao, Z., B. McCloud, R. Fleming, and M. S. Klempner. 2007. Borrelia burgdorferi-induced monocyte chemoattractant protein-1 production in vivo and in vitro. Biochem. Biophys. Res. Commun. 358:528-533. [DOI] [PubMed] [Google Scholar]
- 162.Zhao, Z., H. Chang, R. P. Trevino, K. Whren, J. Bhawan, and M. S. Klempner. 2003. Selective up-regulation of matrix metalloproteinase-9 expression in human erythema migrans skin lesions of acute Lyme disease. J. Infect. Dis. 188:1098-1104. [DOI] [PubMed] [Google Scholar]
- 163.Ziolkowska, M., A. Koc, G. Luszczykiewicz, K. Ksiezopolska-Pietrzak, E. Klimczak, H. Chwalinska-Sadowska, and W. Maslinski. 2000. High levels of IL-17 in rheumatoid arthritis patients: IL-15 triggers in vitro IL-17 production via cyclosporin A-sensitive mechanism. J. Immunol. 164:2832-2838. [DOI] [PubMed] [Google Scholar]