Abstract
Mannose-binding lectin (MBL) deficiency due to variations in the MBL gene is associated with increased susceptibility to infections. In this study, the association between MBL deficiency and the occurrence of abdominal yeast infection (AYI) in peritonitis patients was examined. Eighty-eight patients with secondary peritonitis requiring emergency laparotomy were included. MBL genotype (wild type [WT] versus patients with variant genotypes), MBL plasma concentrations, and Candida risk factors were examined in patients with and those without AYI (positive abdominal yeast cultures during [re]laparotomy). A variant MBL genotype was found in 53% of patients with AYI and 38% of those without AYI (P = 0.18). A significantly higher proportion of variant patients had an AYI during early peritonitis (during first laparotomy) than WT patients (39% versus 16%, respectively; P = 0.012). Patients with AYI had lower MBL levels than did patients without AYI (0.16 μg/ml [0.0 to 0.65 μg/ml] versus 0.65 μg/ml (0.19 to 1.95 μg/ml); P = 0.007). Intensity of colonization (odds ratio [OR], 1.1; 95% confidence interval [CI], 1.0 to 1.1), MBL plasma concentrations of <0.5 μg/ml (OR, 4.5; 95% CI, 1.2 to 16.3), and numbers of relaparotomies (OR, 1.7; 95% CI, 1.0 to 2.8) were independently associated with AYI. In summary, deficient MBL plasma levels were independently associated with the development of AYI in patients with secondary peritonitis and seemed to facilitate early infection.
Secondary peritonitis is a clinical condition frequently observed in surgical wards and intensive care units (ICUs) (15). In spite of major advances in (intensive) care and antimicrobial therapy, mortality in peritonitis has remained approximately 25% for decennia (25, 34). Morbidity in patients with peritonitis also tends to be extensive, with long hospital and ICU stays and long periods of mechanical ventilation (25). Especially when peritonitis persists or recurs, notwithstanding optimal management, and tertiary peritonitis develops, mortality increases to >50% (29). When Candida species are recovered from abdominal isolates from cases of peritonitis, mortality is 60 to 70% when untreated (32). Up to 40% of peritonitis patients have positive abdominal yeast cultures (10, 12, 38), and depending on the body site, Candida species are among the most commonly cultured microorganisms in surgical infections (39). In a recent study, the intraoperative detection of yeast was one of the few independent risk factors for mortality and increased morbidity in cases of secondary peritonitis (38). Yeast is the fourth leading cause of all nosocomial bloodstream infections in the United States, taking third place in bloodstream infections in ICUs (46). Moreover, the incidence of fungal infections with a vast majority of Candida species is increasing (19), increasing numbers of strains of which are becoming resistant to antifungal agents (15, 22).
Recently, the complement protein mannose-binding lectin (MBL) has been shown to play a role in the first line of defense against Candida albicans (20, 26). MBL binds to a wide variety of microorganisms through a carbohydrate recognition domain, exhibiting strong binding to Candida and other yeast species (21, 26, 33). The complement system is activated via this lectin pathway, causing opsonization and direct lysis of microorganisms (21).
Deficiency of MBL is due to variations in the MBL gene, which are present in 20 to 40% of the population. These polymorphisms have been associated with increased susceptibility to a multitude of clinical infections in various conditions (14) such as infectious complications following surgery (40, 47) but also fungal infections, i.e., recurrent vulvovaginal candidiasis and necrotizing pulmonary aspergillosis (2, 7).
The increased burden of infection caused by MBL deficiency seems to weigh particularly on patients with primary or secondary depression of immune systems (14). For instance, critically ill patients with genetically variant MBL alleles or low serum levels have an increased risk of developing and succumbing to sepsis (16, 17). Peritonitis patients are often critically ill and usually require intensive care treatment. In the present study, the role of MBL plasma levels (phenotype) and genetic variants of MBL (genotype) in the development of abdominal yeast infection (AYI) in patients with secondary peritonitis, at index laparotomy, and during the course of disease is examined.
MATERIALS AND METHODS
Patients and controls.
In this prospective cohort study, patients with peritonitis caused by the perforation or infection of a visceral organ, by necrosis of part of the gastrointestinal (GI) tract, or by postoperative peritoneal infection were included. Exclusion criteria were patients aged <18 and >80 years and patients with acute pancreatitis. Patients were included when they underwent emergency laparotomy, referred to as the “index laparotomy,” in which peritonitis was confirmed macroscopically and microbiologically. Operative management of peritonitis was comprised of elimination of the infectious focus and abdominal lavage with saline (0.9%). Relaparotomy was performed in a planned setting or on demand (indicated by clinical deterioration or failure to improve).
Controls for genotyping and plasma MBL concentrations were recruited from a population of healthy blood bank donors (n = 97). The study was approved by the medical ethical committee, and written informed consent was obtained from all patients or their legal representatives when appropriate.
Definitions.
AYI was defined as a positive yeast culture from abdominal fluid sampled during (re)laparotomy with subsequent features of infection/systemic inflammatory response syndrome (see below). Yeast sepsis was defined as a positive culture from a sterile site that could not have been contaminated directly, such as blood or noncontiguous organs. Yeast colonization was defined as one or more positive yeast cultures from sputum, rectum, urine, or wound surface. Intensity of colonization was calculated by dividing the total number of positive yeast cultures by the total number of cultures performed (excluding blood cultures), as described previously by Pittet et al. (36).
Systemic inflammatory response syndrome, sepsis, severe sepsis, and septic shock (developing within 24 h of enrollment in the study) were defined in accordance with the recommendations of the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference (4). Risk factors associated with Candida infection (36), Candida peritonitis (10, 11), and candidemia (3) in multivariate analysis, as mentioned in recent literature, were surveyed.
Sampling and measurements.
During surgery, cultures of abdominal fluid as well as blood were performed using a collection system for aerobic and anaerobic organisms (BacT/Alert; Biomérieux, Durham, NC). Postoperative cultures were performed routinely and at the discretion of the physician in charge of the patient. Standard empirical antibiotic treatment for severe peritonitis, received by all patients, consisted of a combination of amoxicillin, gentamicin, and metronidazole. This regimen was adjusted according to culture results. When yeast was cultured, fluconazole was the first antifungal agent of choice. All patients received selective bowel decontamination drugs orally as described previously by De Jonge et al. (0.5 g of an oral paste [containing 2% polymyxin E, 2% tobramycin, and 2% amphotericin B] and 100 mg polymyxin E, 80 mg tobramycin, and 500 mg amphotericin B administered through gastric tubes four times daily) (9).
Blood was sampled during or directly following surgery. MBL concentrations were measured in citrated (0.109 M) plasma. DNA was extracted from the cell pellet.
MBL was measured in an enzyme-linked immunosorbent assay as described previously by Tacx et al. (41). The detection limit was 0.02 μg/ml. Nucleic acids were isolated according to a solid-phase extraction method (Boom method) as previously described (5).
The genetic variants associated with deficient MBL production are nucleotide polymorphisms situated in the exon 1 region of the structural gene on codons 52, 54, and 57 (variants D, B, and C, respectively) and in the promoter region flanking exon 1 at positions +4, −70, −336, −349, and −427 and the deletion of nucleotides −329 to −324. According to the positions of nucleotide substitutions, these promoter polymorphisms are called H/L, X/Y, and P/Q variants (28). Exon 1 wild-type individuals (WT) are referred to as AA, heterozygous individuals are referred to as AO, and homozygous or compound individuals are referred to as OO. The L and the X promoter gene variants also display very low levels of MBL production (23). In this study, WT patients are compared with AO/OO and LXA/LXA (“variant”) patients. A cutoff of 0.25 μg/ml may identify MBL deficiency due to genetic variations (13). The cutoff of 0.5 μg/ml also showed high sensitivity and specificity for the identification of structural gene variants (43). Both cutoff levels (0.25 and 0.5 μg/ml) will be examined in relation to the development of AYI.
PCRs were performed in 100-μl volumes using 200 nM of specific primers, 4 mM MgCl2, 0.4 mM of deoxynucleotide triphosphates, and 2.5 U/100 μl Taq DNA polymerase (Invitrogen) with 2 μl of DNA. Separate primer pairs were used for the MBL promoter region (forward primer 5′-TTAGCACTCTGCCAGGGCCAACGT-3′ and reverse primer 5′-GTCTAGGCACAGATGAACCCCTC-3′) and exon 1 (forward primer 5′-TAGTCACGCAGTGTCACAAGGAATGT-3′ and reverse primer 5′-CTTCCAGAGGAAACTGCCTGGGGAT-3′). PCRs were initiated by a 5-min denaturation step at 95°C and completed by a 7-min extension step at 72°C. The temperature cycles were as follows: 40 cycles of 30 s at 95°C, 30 s at 67°C, and 45 s at 72°C. PCR products were electrophoresed on an ethidium bromide-containing agarose gel to examine amplified products.
Sequence reactions.
The genomic PCR products were sequenced in both orientations after clean-up with the Qiaquick PCR purification kit (Qiagen GmbH, Hilden, Germany). Five microliters of purified PCR product was used as a template together with the sense or the antisense primer (250 nM) used to obtain this PCR product. Four microliters of reaction mixture from the Big Dye Terminator cycle sequencing ready reaction kit version 1.1 (PE Applied Biosystems, Warrington, United Kingdom) was mixed with 2 μl of Big Dye Terminator dilution buffer in a total volume of 20 μl. Cycle conditions were 10 s at 95°C and 4 min at 60°C for 50 cycles in a 96-well polycarbonate plate in an Omnigene thermocycler (Hybaid, Teddington, Middlesex, United Kingdom) with simulated tube control and calibration factor 200. The sequencing samples were purified in Multiscreen plates (Millipore, Molsheim, France) according to the DyeTerminator removal protocol (Millipore) and were subsequently loaded onto an ABI 377XL automated DNA sequencer (PE Applied Biosystems).
Statistical analysis.
Known yeast risk factors and MBL genotype and phenotype of peritonitis patients with AYI, initially or during the course of disease, were compared to those of peritonitis patients without AYI. Furthermore, the proportion of patients with a positive culture at index laparotomy or at relaparotomy (during the course of disease) was determined in relation to genotype. Results are presented as medians with interquartile ranges (IQRs) or as quantities with percentages. The nonparametric Mann-Whitney U test was used to evaluate statistical differences between two independent groups of data. Fisher's exact test was used to compare proportions between groups. Univariate and multivariate analyses were performed by means of binary logistic regression. Factors associated with AYI (P < 0.15) were then entered in a multivariate model unless predictive factors were strongly correlated with each other, and only one factor with the strongest association (based on the one-variable-model chi-square test) was then chosen. With respect to the MBL level cutoffs, the two cutoffs most commonly used in the literature (13, 43) were tested as described above. All P values were two sided, with P values of less than 0.05 considered to indicate statistical significance. Statistical analysis was performed using SPSS (Chicago, IL) 12.0.1.
RESULTS
Patients.
Eighty-eight consecutive peritonitis patients were included in this study. The etiology of peritonitis was perforation in 39 patients (44%), necrosis in 16 patients (18%), and anastomotic leakage in 28 patients (32%). Peritonitis originated from either the upper (stomach, duodenum, and jejunum) or lower (ileum, colon, sigmoid, and rectum) GI tract in 14 and 74 patients, respectively. All patients had polymicrobial peritonitis with or without yeast. The antibiotic treatment was started empirically (cefuroxime [or amoxicillin], gentamicin, and metronidazole) in all patients. Antibiotic regimens were adapted if necessary after culture results became known.
Short-term mortality (n = 11 [13%]) was all due to septic complications. With regard to long-term mortality (n = 24 [27%]), 18 patients died from the results of sepsis, 4 died due to malignancy, and 2 died due to cardiovascular complications. A total of 223 laparotomies were performed in 88 patients, 55 of which were performed prior to the index laparotomy, 88 of which were index laparotomies, and 80 of which were relaparotomies (32 planned and 18 on demand).
Frequencies of exon 1 variants in the peritonitis group were comparable to frequencies in the regional population (Table 1). All four exon 1 variant alleles (A, B, C, and D) were encountered at a frequency comparable to that of the control group and the general European population as described in the literature (23, 27, 28). Exon 1 AA patients and controls produced significantly higher levels of MBL (Table 1) than did AO (P < 0.001) and OO (P = 0.001) individuals. Notably, MBL plasma levels in AA controls were significantly higher than those in AA patients (P = 0.005) (Table 1).
TABLE 1.
Structural exon 1 MBL genotypes and plasma concentrations of patients with secondary peritonitis and healthy controls
| Genotype | Peritonitis patients (n = 88)
|
Controls (n = 97)
|
||
|---|---|---|---|---|
| No. (%) | MBL level (μg/ml) (IQR) | No. (%) | MBL level (μg/ml) (IQR)a | |
| AA | 53 (60) | 1.2 (0.6-2.3) | 52 (54) | 2.2 (1.3-3.1)* |
| AO | 30 (34) | 0.1 (0.0-0.3) | 41 (42) | 0.4 (0.0-0.5) |
| OO | 5 (6) | 0.0 (0.0-0.0) | 4 (4) | 0.0 (0.0-0.1) |
*, the P value was 0.005 in comparison to patients.
The frequencies of H, L, Y, and X alleles and HL-XY combinations, which are known to be associated with various levels of MBL, were similar to previously reported values (23). Three LXA/LXA patients who produced low MBL levels (0.08, 0.12, and 0.22 μg/ml) were encountered. For analysis, 50 WT (AA) (n = 50) patients were compared with 38 variant (AO/OO [n = 35] and LXA/LXA [n = 3]) peritonitis patients.
Yeast.
AYI was observed in 28 (32%) patients (Table 2). Twenty of these 28 patients (71%) were also colonized with yeast, compared to 30 of the 60 patients without AYI (P = 0.062). Fifty-seven percent (n = 50) of all patients were colonized with yeast during their hospital stays. A total of 1,620 cultures (excluding blood cultures) were obtained. In patients with AYI, a median of 13 (range, 5 to 26) cultures were performed, and in non-AYI patients, seven (range, 2 to 23) cultures were taken (P = 0.20 between groups). Of these cultures, 223 were positive for yeasts in sputum (122 cultures in 40 patients), rectum (58 cultures in 27 patients), urine (seven cultures in six patients), abdominal wound (26 cultures in 17 patients), and abdominal drain (10 cultures in 9 patients). MBL levels in patients with yeast colonization were not different from levels in patients without yeast colonization (0.55 μg/ml [IQR, 0.11 to 1.95 μg/ml] and 0.36 μg/ml [IQR, 0.09 to 1.21 μg/ml], respectively; P = 0.29). Five patients (6%) suffered from candidemia (two variant patients and three WT patients). Four of these patients had AYI (P = 0.046 compared with patients without AYI). One of the total 88 patients was treated preemptively with antifungal agents for suspected yeast infection during peritonitis. Others received treatment with antifungal agents only when culture results for abdominal yeast came back positive.
TABLE 2.
Risk factors for Candida infection, peritonitis, and bloodstream infections in the total group of peritonitis patients, patients with AYI, and patients without AYIa
| Parameter | Value for group
|
P valueb | ||
|---|---|---|---|---|
| Total (n = 88) | AYI+ (n = 28) | AYI− (n = 60) | ||
| Patient characteristic | ||||
| Age (yr) (IQR) | 62 (43-74) | 62 (40-72) | 62 (48-78) | 0.30 |
| No. (%) of patients with: | ||||
| Yeast colonization | 50 (57) | 20 (71) | 30 (50) | 0.062 |
| Candidemia | 5 (6) | 4 (14) | 1 (2) | 0.046 |
| Sepsis | 74 (84) | 22 (79) | 52 (87) | 0.34 |
| Severe sepsis | 58 (66) | 16 (57) | 42 (70) | 0.24 |
| Septic shock | 12 (14) | 2 (7) | 10 (16) | 0.24 |
| 28 days mortality | 11 (13) | 4 (14) | 7 (12) | 0.73 |
| 180 days mortality | 25 (28) | 10 (36) | 15 (25) | 0.30 |
| No. of days (IQR) of: | ||||
| ICU stay | 7 (2-17) | 6 (2-22) | 8 (1-15) | 0.68 |
| Ventilation | 5 (1-14) | 6 (1-11) | 5 (1-14) | 0.97 |
| Hospital stay | 29 (17-56) | 30 (19-66) | 28 (16-53) | 0.96 |
| Risk factors | ||||
| % Colonization intensity (IQR) | 17 (0-33) | 36 (22-57) | 0 (0-21) | <0.001 |
| APACHE-II score (IQR) | 15 (12-18) | 16 (12-18) | 15 (12-19) | 0.66 |
| No. (%) of patients with: | ||||
| APACHE-II score >17 | 37 (42) | 11 (39) | 26 (43) | 0.72 |
| Respiratory failure | 18 (21) | 5 (18) | 13 (22) | 0.68 |
| Cardiovascular failure | 27 (31) | 7 (25) | 20 (33) | 0.43 |
| Upper GI tract infection | 14 (16) | 8 (29) | 6 (10) | 0.033 |
| >48 h AB | 19 (22) | 7 (25) | 12 (20) | 0.60 |
| Female gender | 44 (50) | 12 (43) | 32 (53) | 0.36 |
| Renal failure/hemodialysis | 19 (22) | 4 (14) | 15 (25) | 0.26 |
| TPN | 46 (52) | 18 (64) | 28 (47) | 0.13 |
| Central venous catheter | 56 (64) | 19 (69) | 37 (62) | 0.57 |
| No. of abdominal operations (IQR) | ||||
| Prior to index | 1 (0-1) | 1 (0-1) | 1 (0-1) | 0.86 |
| Relaparotomies | 1 (0-1) | 1 (1-2) | 0 (0-1) | 0.025 |
| MBL | ||||
| No. (%) of patients with variant MBL genotype | 38 (43) | 15 (54) | 23 (38) | 0.18 |
| MBL plasma concn (μg/ml) (IQR) | 0.41 (0.10-1.40) | 0.16 (0.0-0.65) | 0.65 (0.19-1.95) | 0.007 |
| No. (%) of patients with MBL level of: | ||||
| <0.25 μg/ml | 34 (39) | 16 (57) | 18 (30) | 0.026 |
| <0.50 μg/ml | 45 (51) | 20 (71) | 25 (42) | 0.019 |
Variant MBL genotypes are AO/OO and LXA/LXA. >48 h AB, more than 48 h of antibiotic treatment before positive yeast culture; TPN, total parenteral nutrition; AYI+, patients with AYI; AYI−, patients without AYI.
P value for the comparison between those with AYI and those without AYI.
In univariate models, none of the clinical characteristics were different between patients with AYI and those without AYI (Table 2). Of the known yeast risk factors, colonization intensity, upper GI tract source of peritonitis, and number of relaparotomies were significantly increased in patients with AYI (Table 2).
MBL.
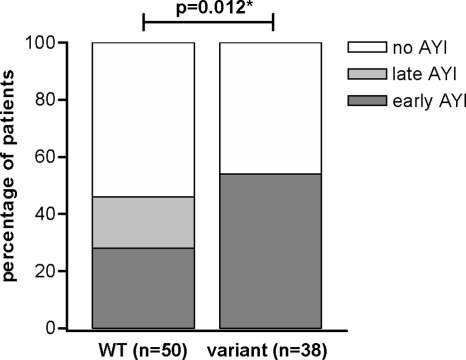
Levels of MBL in WT patients were higher than levels in variant patients (1.30 μg/ml [IQR, 0.63 to 2.29 μg/ml] versus 0.10 μg/ml [IQR, 0.00 to 0.28 μg/ml]; P < 0.001). A variant MBL genotype was found in 54% of patients who developed AYI and in 38% of patients without AYI (P = 0.18) (Table 2). However, the timing of AYI for patients with the WT genotype was different from that for patients with the variant genotype. The proportions of early (during index laparotomy) AYI, late (during relaparotomy) AYI, or no AYI were significantly different between WT and variant patients, as shown in Fig. 1. Thirty-nine percent of variant patients (15/38 patients) had early AYI, compared to 16% in WT patients (8/50 patients), while late AYI was not encountered in patients with variant genotypes (P = 0.012). Note that the same percentage of WT (30/50 patients) and variant (19/38 patients) patients underwent a relaparotomy (P = 0.39).
FIG. 1.
Proportional differences of early (during index laparotomy), late (during relaparotomy), or no AYI in patients with WT or variant MBL genotypes. *, P value of the comparison of proportions of patients with no, early, or late AYI between WT and variant groups in a chi-square test.
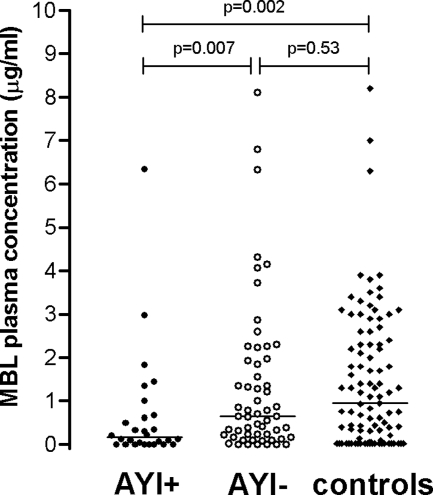
Overall, MBL levels were significantly lower in patients with AYI than those in patients without AYI (median of 0.16 μg/ml [IQR, 0.0 to 0.65 μg/ml] versus 0.65 μg/ml [IQR, 0.19 to 1.95 μg/ml]; P = 0.007) (Fig. 2 and Table 2) and controls (median of 0.95 μg/ml [IQR, 0.05 to 2.35 μg/ml]; P = 0.002) (Fig. 2).
FIG. 2.
Scatter plots of MBL plasma concentrations in patients with and those without AYI (AYI+ and AYI−, respectively) and controls from the general population (lines represent median concentrations). P values were derived by comparisons between those with AYI and those without AYI and between those with and without AYI and controls (Mann-Whitney U test).
Low-MBL-producing patients, identified by cutoff levels of 0.25 or 0.5 μg/ml, had significantly more AYIs. At a cutoff level of 0.25 μg/ml, 16 of 34 (47%) low producers versus 12 of 54 (22%) high producers had AYI (P = 0.026); at a cutoff level of 0.5 μg/ml, 20 of 45 (44%) low producers versus 8 of 43 (19%) high producers had AYI (P = 0.019).
Multivariate analysis.
An MBL cutoff value of <0.25 μg/ml was a weaker predictor than a cutoff value of <0.50 μg/ml (one-variable-model chi-square value of 5,072 versus 5,881, respectively) and, hence, was not entered into the multivariate model. The factors that were independently associated with the development of AYI were intensity of colonization (odds ration [OR], 1.08; 95% confidence interval [CI], 1.04 to 1.11 per percentage of increase; P < 0.001), MBL plasma concentrations of <0.5 μg/ml (OR, 4.47; 95% CI, 1.22 to 16.28; P = 0.023), and number of relaparotomies (OR, 1.70; 95% CI, 1.03 to 2.81 per relaparotomy; P = 0.038).
DISCUSSION
Peritonitis patients who encountered an AYI had lower plasma MBL levels than did patients who did not develop AYI. The plasma MBL level was measured in blood sampled during or shortly after emergency surgery. MBL levels start to rise only about 5 days after the initiation of an inflammatory response (43), so MBL levels in the current study were likely to approximate baseline levels and were probably not yet influenced by the abdominal infection and/or operation. MBL plasma levels below 0.5 μg/ml were independently associated with the development of AYIs, as were intensity of colonization and the number of relaparotomies. Increased intensity of colonization may lead to an increased burden of the fungal microbial load and thus an increased incidence of infection. The importance of colonization is illustrated by the fact that Candida colonization or infection with an identical strain frequently preceded (bloodstream) infection in nonneutropenic patients (44, 45). The extent (intensity) of Candida colonization was shown to be an independent factor for Candida infection (30, 36). Furthermore, positive peritoneal yeast cultures were associated with mortality in peritonitis patients (11, 31). The number of relaparotomies is likely a reflection of disease severity and protracted abdominal infection with an increased risk of AYI.
Patients with MBL levels of <0.5 μg/ml had a risk of developing AYI that was 4.5 times greater than that for patients with higher MBL plasma levels. The pathophysiology of the relation between MBL deficiency and the early appearance of yeast cultured from the abdominal fluid is possibly the reduced clearance capacity of the complement system of abdominal yeast in individuals with an impaired MBL pathway. WT patients may be able to clear yeast more efficiently, postponing the development of a yeast infection to a state of generally depressed immunity later in the disease. Peritonitis patients may develop immunosuppression as a result of initially up-regulated responses during intra-abdominal infection combined with the effects of surgery. Surgery itself can suppress the immune response (42), as was confirmed in experimental studies reported previously (1, 37). Conditional immunosuppression plus low functional MBL levels may reduce the inhibition of yeast proliferation. In the current study, MBL plasma levels were lower in peritonitis patients with the WT structural genotype (AA) than in AA controls, which may indicate MBL consumption.
Patients with variant MBL genes also have significantly lower peritoneal MBL levels than do WT AA patients (24), but in both WT and variant patients, these levels are very low compared to plasma levels. MBL-deficient patients may thus have relatively less biologically functional MBL available in plasma as well as in the abdomen to counteract yeast proliferation. These patients may benefit from early MBL suppletion therapy, which was previously shown to reduce mortality in experimental candidemia (26).
The 0.5-μg/ml and not the 0.25-μg/ml MBL level cutoff was independently related with AYI in two separate models. This cutoff point has also been associated with other clinical conditions. An association between adverse outcome in ICU patients and severe MBL deficiency using the 0.5-μg/ml cutoff was found (18). There was an increased susceptibility to infections in chemotherapy patients with MBL levels of 0.5 μg/ml or less (35). Also, sepsis patients with baseline MBL deficiencies failed to produce MBL at levels greater than 0.5 μg/ml (8).
The baseline cutoff level of <0.5 μg/ml in plasma reliably identifies individuals with variant exon 1 alleles (sensitivity of 100% and specificity of 83%) (43). However, the genotype of the patient does not ultimately reflect MBL production. Not all AO individuals are deficient, while some AA individuals with normal promoter haplotypes are indeed deficient, as was observed in British Caucasians (6). Thus, MBL deficiency can be adequately detected by measuring plasma levels by a simple enzyme-linked immunosorbent assay.
Abdominal yeast infections were encountered with an incidence comparable to those reported in previous studies of secondary peritonitis (10, 12, 38). A significantly higher proportion of MBL variant patients had an early AYI (at index laparotomy) than WT patients. Thus, MBL genetic variation may amount for the early emergence of AYI.
The diagnosis and treatment of these yeast infections are challenging and largely empirical. Candida is a part of the normal commensal flora, so the interpretation of a positive yeast culture can be difficult. Furthermore, the classical patient that develops a yeast infection is incapable of mounting a normal host response and thus does not stereotypically present clinical signs of infection. Sedation and mechanical ventilation while in the ICU may also hamper clinical assessment and early diagnosis, which are vital to proper and rapid treatment. Diagnosis is frequently delayed because culture results take a few days to become interpretable as being positive or negative. MBL levels are a promising tool to recognize patients with a high risk of fungal infection. A future risk prediction score may be based upon the present prediction model with colonization intensity, baseline MBL level (at a cutoff of <0.5 μg/ml), and number of relaparotomies as predictive variables. Antifungal treatment may be targeted more appropriately.
In summary, the incidence of abdominal yeast infection is high in secondary peritonitis (32%). MBL plasma levels of <0.5 μg/ml, intensity of colonization, and the number of relaparotomies are independently associated with the development of AYIs in patients with secondary peritonitis. A variant MBL genotype may facilitate the early emergence of AYI during peritonitis.
Acknowledgments
This work was supported by a research grant from the Dutch Digestive Diseases Foundation (grant number WS 00-54).
Footnotes
Published ahead of print on 31 October 2007.
REFERENCES
- 1.Attalah, H. L., E. Azoulay, K. Yang, C. Lasclos, H. Jouault, C. J. Soussy, T. Guillot, L. Brochard, C. Brun-Buisson, A. Harf, and C. Delclaux. 2002. Granulocyte colony-stimulating factor enhances host defenses against bacterial pneumonia following peritonitis in nonneutropenic rats. Crit. Care Med. 30:2107-2114. [DOI] [PubMed] [Google Scholar]
- 2.Babula, O., G. Lazdane, J. Kroica, W. J. Ledger, and S. S. Witkin. 2003. Relation between recurrent vulvovaginal candidiasis, vaginal concentrations of mannose-binding lectin, and a mannose-binding lectin gene polymorphism in Latvian women. Clin. Infect. Dis. 37:733-737. [DOI] [PubMed] [Google Scholar]
- 3.Blumberg, H. M., W. R. Jarvis, J. M. Soucie, J. E. Edwards, J. E. Patterson, M. A. Pfaller, M. S. Rangel-Frausto, M. G. Rinaldi, L. Saiman, R. T. Wiblin, R. P. Wenzel, et al. 2001. Risk factors for candidal bloodstream infections in surgical intensive care unit patients: the NEMIS prospective multicenter study. Clin. Infect. Dis. 33:177-186. [DOI] [PubMed] [Google Scholar]
- 4.Bone, R. C., R. A. Balk, F. B. Cerra, R. P. Dellinger, A. M. Fein, W. A. Knaus, R. M. H. Schein, W. J. Sibbald, J. H. Abrams, G. R. Bernard, J. W. Biondi, J. E. Calvin, R. Demling, P. J. Fahey, C. J. Fisher, C. Franklin, K. J. Gorelick, M. A. Kelley, D. G. Maki, J. C. Marshall, W. W. Merrill, J. P. Pribble, E. C. Rackow, T. C. Rodell, J. N. Sheagren, M. Silver, C. L. Sprung, R. C. Straube, M. J. Tobin, G. M. Trenholme, D. P. Wagner, C. D. Webb, J. C. Wherry, H. P. Wiedemann, and C. H. Wortel. 1992. American College of Chest Physicians Society of Critical Care Medicine Consensus Conference—definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit. Care Med. 20:864-874.1597042 [Google Scholar]
- 5.Boom, R., C. J. A. Sol, M. M. M. Salimans, C. L. Jansen, P. M. E. Wertheim-van Dillen, and J. Van der Noordaa. 1990. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 28:495-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crosdale, D. J., W. E. Ollier, W. Thomson, P. A. Dyer, J. C. Jensenius, R. W. Johnson, and K. V. Poulton. 2000. Mannose binding lectin (MBL) genotype distributions with relation to serum levels in UK Caucasoids. Eur. J. Immunogenet. 27:111-117. [DOI] [PubMed] [Google Scholar]
- 7.Crosdale, D. J., K. V. Poulton, W. E. Ollier, W. Thomson, and D. W. Denning. 2001. Mannose-binding lectin gene polymorphisms as a susceptibility factor for chronic necrotizing pulmonary aspergillosis. J. Infect. Dis. 184:653-656. [DOI] [PubMed] [Google Scholar]
- 8.Dean, M. M., R. M. Minchinton, S. Heatley, and D. P. Eisen. 2005. Mannose binding lectin acute phase activity in patients with severe infection. J. Clin. Immunol. 25:346-352. [DOI] [PubMed] [Google Scholar]
- 9.De Jonge, E., M. J. Schultz, L. Spanjaard, P. M. Bossuyt, M. B. Vroom, J. Dankert, and J. Kesecioglu. 2003. Effects of selective decontamination of digestive tract on mortality and acquisition of resistant bacteria in intensive care: a randomised controlled trial. Lancet 367:1101-1106. [DOI] [PubMed] [Google Scholar]
- 10.Dupont, H., A. Bourichon, C. Paugam-Burtz, J. Mantz, and J. M. Desmonts. 2003. Can yeast isolation in peritoneal fluid be predicted in intensive care unit patients with peritonitis? Crit. Care Med. 31:752-757. [DOI] [PubMed] [Google Scholar]
- 11.Dupont, H., C. Paugam-Burtz, C. Muller-Serieys, L. Fierobe, D. Chosidow, J. P. Marmuse, J. Mantz, and J. M. Desmonts. 2002. Predictive factors of mortality due to polymicrobial peritonitis with Candida isolation in peritoneal fluid in critically ill patients. Arch. Surg. 137:1341-1346. [DOI] [PubMed] [Google Scholar]
- 12.Eggimann, P., P. Francioli, J. Bille, R. Schneider, M. M. Wu, G. Chapuis, R. Chiolero, A. Pannatier, J. Schilling, S. Geroulanos, M. P. Glauser, and T. Calandra. 1999. Fluconazole prophylaxis prevents intra-abdominal candidiasis in high-risk surgical patients. Crit. Care Med. 27:1066-1072. [DOI] [PubMed] [Google Scholar]
- 13.Eisen, D. P., M. M. Dean, P. Thomas, P. Marshall, N. Gerns, S. Heatley, J. Quinn, R. M. Minchinton, and J. Lipman. 2006. Low mannose-binding lectin function is associated with sepsis in adult patients. FEMS Immunol. Med. Microbiol. 48:274-282. [DOI] [PubMed] [Google Scholar]
- 14.Eisen, D. P., and R. M. Minchinton. 2003. Impact of mannose-binding lectin on susceptibility to infectious diseases. Clin. Infect. Dis. 37:1496-1505. [DOI] [PubMed] [Google Scholar]
- 15.Farthmann, E. H., and U. Schoffel. 1998. Epidemiology and pathophysiology of intraabdominal infections (IAI). Infection 26:329-334. [DOI] [PubMed] [Google Scholar]
- 16.Fidler, K. J., P. Wilson, J. C. Davies, M. W. Turner, M. J. Peters, and N. J. Klein. 2004. Increased incidence and severity of the systemic inflammatory response syndrome in patients deficient in mannose-binding lectin. Intensive Care Med. 30:1438-1445. [DOI] [PubMed] [Google Scholar]
- 17.Garred, P., J. J. Strom, L. Quist, E. Taaning, and H. O. Madsen. 2003. Association of mannose-binding lectin polymorphisms with sepsis and fatal outcome, in patients with systemic inflammatory response syndrome. J. Infect. Dis. 188:1394-1403. [DOI] [PubMed] [Google Scholar]
- 18.Hansen, T. K., S. Thiel, P. J. Wouters, J. S. Christiansen, and G. Van den Berghe. 2003. Intensive insulin therapy exerts antiinflammatory effects in critically ill patients and counteracts the adverse effect of low mannose-binding lectin levels. J. Clin. Endocrinol. Metab. 88:1082-1088. [DOI] [PubMed] [Google Scholar]
- 19.Henderson, V. J., and E. R. Hirvela. 1996. Emerging and reemerging microbial threats—nosocomial fungal infections. Arch. Surg. 131:330-337. [DOI] [PubMed] [Google Scholar]
- 20.Ip, W. K., and Y. L. Lau. 2004. Role of mannose-binding lectin in the innate defense against Candida albicans: enhancement of complement activation, but lack of opsonic function, in phagocytosis by human dendritic cells. J. Infect. Dis. 190:632-640. [DOI] [PubMed] [Google Scholar]
- 21.Jack, D. L., and M. W. Turner. 2003. Anti-microbial activities of mannose-binding lectin. Biochem. Soc. Trans. 31:753-757. [DOI] [PubMed] [Google Scholar]
- 22.Jarvis, W. R. 1995. Epidemiology of nosocomial fungal infections, with emphasis on Candida species. Clin. Infect. Dis. 20:1526-1530. [DOI] [PubMed] [Google Scholar]
- 23.Kilpatrick, D. C. 2002. Mannan-binding lectin: clinical significance and applications. Biochim. Biophys. Acta 1572:401-413. [DOI] [PubMed] [Google Scholar]
- 24.Lam, M. F., J. C. Leung, C. C. Tang, W. K. Lo, K. C. Tse, T. P. Yip, S. L. Lui, T. M. Chan, and K. N. Lai. 2005. Mannose binding lectin level and polymorphism in patients on long-term peritoneal dialysis. Nephrol. Dial. Transplant. 20:2489-2496. [DOI] [PubMed] [Google Scholar]
- 25.Lamme, B., M. A. Boermeester, E. J. T. Belt, J. W. O. Van Till, D. J. Gouma, and H. Obertop. 2004. Mortality and morbidity of planned relaparotomy versus relaparotomy on demand for secondary peritonitis. Br. J. Surg. 91:1046-1054. [DOI] [PubMed] [Google Scholar]
- 26.Lillegard, J. B., R. B. Sim, P. Thorkildson, M. A. Gates, and T. R. Kozel. 2006. Recognition of Candida albicans by mannan-binding lectin in vitro and in vivo. J. Infect. Dis. 193:1589-1597. [DOI] [PubMed] [Google Scholar]
- 27.Madsen, H. O., P. Garred, J. A. Kurtzhals, L. U. Lamm, L. P. Ryder, S. Thiel, and A. Svejgaard. 1994. A new frequent allele is the missing link in the structural polymorphism of the human mannan-binding protein. Immunogenetics 40:37-44. [DOI] [PubMed] [Google Scholar]
- 28.Madsen, H. O., M. L. Satz, B. Hogh, A. Svejgaard, and P. Garred. 1998. Different molecular events result in low protein levels of mannan-binding lectin in populations from Southeast Africa and South America. J. Immunol. 161:3169-3175. [PubMed] [Google Scholar]
- 29.Marshall, J. C., and M. Innes. 2003. Intensive care unit management of intra-abdominal infection. Crit. Care Med. 31:2228-2237. [DOI] [PubMed] [Google Scholar]
- 30.McKinnon, P. S., D. A. Goff, J. W. Kern, J. W. Devlin, J. F. Barletta, S. J. Sierawski, A. C. Mosenthal, P. Gore, A. J. Ambegaonkar, and T. J. Lubowski. 2001. Temporal assessment of Candida risk factors in the surgical intensive care unit. Arch. Surg. 136:1401-1408. [DOI] [PubMed] [Google Scholar]
- 31.Montravers, P., H. Dupont, R. Gauzit, B. Veber, C. Auboyer, P. Blin, C. Hennequin, and C. Martin. 2006. Candida as a risk factor for mortality in peritonitis. Crit. Care Med. 34:646-652. [DOI] [PubMed] [Google Scholar]
- 32.Nathens, A. B., O. D. Rotstein, and J. C. Marshall. 1998. Tertiary peritonitis: clinical features of a complex nosocomial infection. World J. Surg. 22:158-163. [DOI] [PubMed] [Google Scholar]
- 33.Neth, O., D. L. Jack, A. W. Dodds, H. Holzel, N. J. Klein, and M. W. Turner. 2000. Mannose-binding lectin binds to a range of clinically relevant microorganisms and promotes complement deposition. Infect. Immun. 68:688-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pacelli, F., G. B. Doglietto, S. Alfieri, E. Piccioni, A. Sgadari, D. Gui, and F. Crucitti. 1996. Prognosis in intra-abdominal infections—multivariate analysis on 604 patients. Arch. Surg. 131:641-645. [DOI] [PubMed] [Google Scholar]
- 35.Peterslund, N. A., C. Koch, J. C. Jensenius, and S. Thiel. 2001. Association between deficiency of mannose-binding lectin and severe infections after chemotherapy. Lancet 358:637-638. [DOI] [PubMed] [Google Scholar]
- 36.Pittet, D., M. Monod, P. M. Suter, E. Frenk, and R. Auckenthaler. 1994. Candida colonization and subsequent infections in critically ill surgical patients. Ann. Surg. 220:751-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reddy, R. C., G. H. Chen, M. W. Newstead, T. Moore, X. Y. Zeng, K. Tateda, and T. J. Standiford. 2001. Alveolar macrophage deactivation in murine septic peritonitis: role of interleukin 10. Infect. Immun. 69:1394-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sandven, P., H. Qvist, E. Skovlund, and K. E. Giercksky. 2002. Significance of Candida recovered from intraoperative specimens in patients with intra-abdominal perforations. Crit. Care Med. 30:541-547. [DOI] [PubMed] [Google Scholar]
- 39.Sawyer, R. G., D. P. Raymond, S. J. Pelletier, T. D. Crabtree, T. G. Gleason, and T. L. Pruett. 2001. Implications of 2,457 consecutive surgical infections entering year 2000. Ann. Surg. 233:867-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Siassi, M., W. Hohenberger, and J. Riese. 2003. Mannan-binding lectin (MBL) serum levels and post-operative infections. Biochem. Soc. Trans. 31:774-775. [DOI] [PubMed] [Google Scholar]
- 41.Tacx, A. N., A. B. Groeneveld, M. H. Hart, L. A. Aarden, and C. E. Hack. 2003. Mannan binding lectin in febrile adults: no correlation with microbial infection and complement activation. J. Clin. Pathol. 56:956-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Sandick, J. W., S. S. Gisbertz, I. J. M. ten Berge, M. A. Boermeester, T. C. T. M. Kraan, T. A. Out, H. Obertop, and J. J. B. van Lanschot. 2003. Immune responses and prediction of major infection in patients undergoing transhiatal or transthoracic esophagectomy for cancer. Ann. Surg. 237:35-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Till, J. W. O., M. A. Boermeester, P. W. Modderman, J. W. van Sandick, M. H. Hart, S. S. Gisbertz, J. J. Van Lanschot, and L. A. Aarden. 2006. Variable mannose-binding lectin expression during the postoperative acute-phase response. Surg. Infect. 7:443-452. [DOI] [PubMed] [Google Scholar]
- 44.Voss, A., R. J. Hollis, M. A. Pfaller, R. P. Wenzel, and B. N. Doebbeling. 1994. Investigation of the sequence of colonization and candidemia in nonneutropenic patients. J. Clin. Microbiol. 32:975-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wey, S. B., M. Mori, M. A. Pfaller, R. F. Woolson, and R. P. Wenzel. 1989. Risk factors for hospital-acquired candidemia. A matched case-control study. Arch. Intern. Med. 149:2349-2353. [PubMed] [Google Scholar]
- 46.Wisplinghoff, H., T. Bischoff, S. M. Tallent, H. Seifert, R. P. Wenzel, and M. B. Edmond. 2004. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 39:309-317. [DOI] [PubMed] [Google Scholar]
- 47.Ytting, H., I. J. Christensen, J. C. Jensenius, S. Thiel, and H. J. Nielsen. 2005. Preoperative mannan-binding lectin pathway and prognosis in colorectal cancer. Cancer Immunol. Immunother. 54:265-272. [DOI] [PMC free article] [PubMed] [Google Scholar]