Abstract
Recent studies indicate that innate immunity in influenza virus infection is an area of substantial importance for our understanding of influenza virus pathogenesis, yet our knowledge of the mechanisms controlling innate immunity remains limited. Further delineation of the roles of NK cells and innate immunity in viral infection may have important implications for the development of improved influenza virus vaccines. In this study, we evaluated the phenotype and function of NK and T lymphocytes, as well as influenza virus-specific immunoglobulin G production, prior to and following vaccination with the routinely administered trivalent influenza virus vaccine. We demonstrate influenza virus antigen-specific innate and adaptive cellular responses and evaluate changes in NK cell receptor expression over time. Our results demonstrate increased innate and adaptive cellular immune responses and show that NK cells are a significant source of gamma interferon (IFN-γ) following influenza virus vaccination. An increase in the frequency of IFN-γ-producing NK cells was observed in many subjects postvaccination. The subset distribution with respect to CD56dim and CD56bright NK cell subsets remained stable, as did the NK cell phenotype with respect to expression of cell surface activating and inhibitory receptors. These results may form the basis for further investigations of the role of NK cells in immunity to influenza.
Over the past millennium, influenza virus infections have caused substantial human morbidity and mortality. Most notably, the 1918-1919 pandemic caused by influenza virus type A H1N1 was responsible for an estimated 50 million deaths. Until recently, most of those deaths were thought to have been due to secondary bacterial infection, though a recent histopathological analysis of autopsy samples from the 1918 pandemic showed significant damage to the lungs, with bronchitis and alveolitis compounded by massive pulmonary edema, hemorrhage, and a rapid destruction of the respiratory epithelium. The extent of this pathology suggests that enhanced inflammation and cell death might have been contributors to the severe immunopathology (16, 32). How innate immune responses may have contributed to the immunopathology or protection in survivors of the pandemic is unknown. Consequently, an understanding of innate and adaptive responses to influenza virus and vaccines may be an essential step toward providing a broader perspective on influenza virus pathology. The potential for an influenza virus pandemic mediated by the currently circulating avian influenza H5N1 viruses highlights the need for effective vaccines against these highly pathogenic influenza virus strains and for further studies on immunity to current influenza virus vaccines (26).
Influenza viruses are enveloped single-stranded RNA viruses of the orthomyxovirus family whose genome consists of separate segments covered by the nucleocapsid protein. The viral envelope contains the highly variable hemagglutinin (HA) and neuraminidase proteins, which are used to categorize influenza virus strains. In humans, influenza virus types A and B can cause disease, while type C appears to have low pathogenicity. Two influenza virus vaccines are currently licensed for use in adults: the trivalent inactivated influenza virus vaccine (TIV) and the live attenuated influenza virus vaccine. The composition of the vaccine is chosen each year based upon epidemiological evidence of circulating strains (11). Although some form of these vaccines has been in use for much of the past 25 years, a great deal remains to be discovered about what type of immunity the vaccine elicits, and indeed, what types of immunity are needed for protection against highly pathogenic influenza virus strains. Vaccination with TIV elicits secretory immunoglobulin A (IgA) and circulating IgG antibodies, thought to be the primary means by which protection is elicited (8, 27). In addition, several recent studies demonstrate the effectiveness of cellular immunity for providing lasting protection from influenza virus, particularly against H5N1 strains (6, 13, 14). These studies emphasize the importance of understanding the factors that promote innate and adaptive cellular immune responses to influenza virus.
Natural killer (NK) cells may play an important early role in the development of the immune response to influenza virus through the interaction of activating receptors on the cell surface with influenza virus HA protein and stress-induced proteins from infected cells (30, 31). NK cells are large granular lymphocytes that constitute a significant portion of the innate immune response and are capable of recognizing both virus-infected and transformed cells (17). Unlike B and T lymphocytes, NK cells stochastically express multiple germ line-encoded receptors in a variegated manner. These receptors can be segregated into several molecular groups, including (i) killer cell immunoglobulin-like receptors (KIR); (ii) the C-type lectins NKG2A, NKG2C, and NKG2D; and (iii) the natural cytotoxicity receptors NKp30, NKp44, and NKp46. Many of these receptors may be either stimulatory or inhibitory, and the balance of signals they deliver ultimately determines NK cell function. The physiologic role of NK cells in influenza virus infection is highlighted by recent findings that NK cells are capable of lysing influenza virus-infected cells. Targeting of infected cells is mediated predominantly through recognition of influenza virus HA protein by NKp46 (7, 19, 20). This specificity is likely to be influenced by additional NK cell receptors, yet disruption of the mouse NKp46 gene (Ncr1) is sufficient to render influenza virus infection entirely lethal in transgenic mice (7). Adaptive cellular immune responses against influenza virus rely heavily upon the innate immune response and in turn enhance NK cell cytotoxicity and the production of antiviral cytokines. Production of gamma interferon (IFN-γ) by NK cells stimulated ex vivo with influenza A virus is dependent upon the presence of T cells and is mediated largely by interleukin 2 (12). Consequently, both innate and adaptive cellular responses are needed for effective protection. Further, animal models have demonstrated that cellular immune responses targeting influenza virus antigens confer long-lasting and cross-reactive protection against a variety of serotypes, including avian H5N1 (5, 29). At present, little is known about innate immunity to influenza virus and the impact of vaccination on influenza virus-specific cellular immune responses. In humans, there are limited longitudinal studies on T-cell responses to influenza virus vaccine and even more limited longitudinal studies on innate and NK cell responses.
We set out to determine the effects of the currently administered TIV on NK and T-cell responses in healthy subjects. Blood samples were obtained prior to, and for up to 8 weeks following, vaccination from eight subjects and two unimmunized controls. These subjects were evaluated longitudinally for changes in both NK and T-cell responses to inactivated whole influenza virus and pooled influenza virus HA and M1 peptides. Our results demonstrate influenza virus antigen-specific NK and T-cell responses in vaccinated subjects, in whom the largest increase in IFN-γ-producing cells occurred in the NK cell compartment. The frequencies of NK cells expressing particular activating and inhibitory receptors remained stable over the observed time period, suggesting there was no change in the overall composition of NK cells in peripheral blood.
MATERIALS AND METHODS
Study subjects and influenza virus vaccine.
Ten healthy subjects (two female, eight male; ages, 25 to 45 years; median age, 32 years), eight of whom received influenza virus vaccination, were recruited into this study after informed consent was obtained according to University of California, San Francisco (UCSF), guidelines. Two subjects (subjects 9 and 10) served as unvaccinated controls. Of the 10 subjects, 9 reported having received the influenza virus vaccine previously. Only subject 6 in the vaccinated group reported no previous history of vaccination. Of the eight subjects who received vaccine, four were immunized in the fall of 2003 (subjects 1, 2, 3, and 4) and received the 2003-2004 vaccine composition while four subjects were immunized in the fall of 2005 (subjects 5, 6, 7, and 8) and received the 2005-2006 vaccine composition. Subject 1 was vaccinated on both occasions and provided samples in each instance. Peripheral blood mononuclear cells (PBMC) and plasma samples were obtained from whole blood. PBMC were isolated by lympholyte-M separation and were cryopreserved prior to vaccination and at roughly 1-week intervals following vaccination for up to 8 weeks for each subject. Some subjects were not available precisely at the 1-week time point, and the next nearest time point was sampled. Lympholyte-M-separated PBMC were resuspended at 1.0 × 107 cells/ml in freezing medium (fetal calf serum-10% dimethyl sulfoxide) and frozen to −80°C in 1-ml aliquots in an isopropyl alcohol-filled freezing container. Frozen PBMC were transferred to liquid nitrogen for long-term cryopreservation.
Cell culture and antigenic stimulation.
Cryopreserved PBMC from each subject in longitudinal series were batch processed to reduce the coefficient of variation attributable to antibody staining and flow cytometric evaluation. Cryopreserved specimens were thawed and used for measurements of NK cell frequency, number, and receptor expression. The thawed cells were washed with RPMI medium supplemented with 15% fetal bovine serum before being stained or stimulated. NK and T-cell functions were assessed by cytokine flow cytometry (CFC). The 2003-2004 influenza virus vaccine was composed of A/New Caledonia/20/99-like (H1N1), A/Moscow/10/99-like (H3N2), and B/Hong Kong/330/01-like viruses (3), while the 2005-2006 vaccine was composed of A/New Caledonia/20/99-like (H1N1), A/California/7/2004-like (H3N2), and B/Shanghai/361/20002-like viruses (11). Common to both vaccine compositions was the A/New Caledonia/20/99-like (H1N1) virus, subsequently used to detect antigen-specific responses from subjects vaccinated in 2003 and/or 2005. To measure NK cell function, PBMC were cultured in medium alone or stimulated with 1.0 μg/ml thimerosal-inactivated whole influenza virus (A/New Caledonia/20/99; BioDesign International, Saco, ME) or 1.0 μg/ml influenza virus HA and M1 peptide pool (kindly proved by H. Maecker, BD Biosciences-Immunocytometry Systems, San Jose, CA). The HA and M1 peptide mixes consisted of 79 and 61 15-mer peptides, respectively, each overlapping by 11 amino acids with the HA sequence derived from the A/New Caledonia/20/99 strain and the M1 sequence derived from A/Wisconsin/4754/94. The PBMC cultured in medium alone were taken as a measure of “spontaneous” NK cell function. Briefly, 100 μl thawed PBMC was cultured at 5 × 106 cells/ml in 96-well plates with the respective stimulant for 24 h; during the last 6 h of culture, monensin and brefeldin A were added to block trans-Golgi transport and to allow intracellular accumulation of cytokines. The cells were then harvested, washed in fluorescence-activated cell sorter (FACS) buffer, and prepared for antibody staining and flow cytometry.
Cell staining and flow cytometric analysis.
Thawed cells were washed with phosphate-buffered saline supplemented with 1% bovine serum albumin and 2 mM EDTA (FACS buffer) before being stained. For staining, 5 × 105 cells were incubated with purified human IgG (100 μg/ml) to block nonspecific binding. To define NK cells, we stained the cells with anti-CD3-ECD, anti-CD4-Alexa 700, anti-CD56-phycoerythrin-Cy7, and anti-CD16-Pacific blue. Anti-CD14 and anti-CD19 were used to exclude monocytes and B cells, respectively. We used the commercially available anti-KIR antibodies DX9 and DX27 and antibodies against NKp44, NKp46, CD94, NKG2A, NKG2C, NKG2D, and CD161. Freshly thawed or stimulated cells were stained for cell surface antigens (CD3, CD4, CD14, CD19, CD56, and CD16) and stained with the DNA intercalater ethidium monoazide to exclude dead cells. The cells were fixed in 2% paraformaldehyde and permeabilized with FACS-perm (BD Pharmingen, San Jose, CA). The permeabilized cells were stained for intracellular levels of the cytokine IFN-γ. Fluorescence minus one samples were prepared for each fluorochrome to facilitate gating. All cells were fixed with 2% paraformaldehyde and analyzed by flow cytometry using a four-laser LSR-II instrument modified from the standard configuration by the addition of a 159-mW green (532-nm) diode laser and the upgrade of the blue and red lasers to 100 mW and 25 mW, respectively (BD Biosciences, Mountain View, CA). Anti-mouse IgG-coated beads were stained with each fluorochrome separately and used for software-based compensation. Final analysis was carried out using FlowJo flow cytometric analysis software (Tree Star, Ashland, OR). For the gating strategy, doublets were excluded based on forward scatter (FSC) height and FSC area. A broad PBMC gate was then used based on FSC height and side light scatter. Monocytes and B-cell wells were excluded based on CD14 and CD19 gating, respectively (Fig. 1). CD4-positive (CD4+) T cells and CD3+ CD4-negative (CD4−) (comprised mostly of CD8+ T cells and hereafter referred to as CD8+) and CD3− cells were gated from the CD14 CD19− lymphocyte population, and NK cells were derived from the CD3− gate based on the expression of CD16 and CD56. NK cells were subdivided into CD56bright and CD56dim and analyzed for the expression of particular NK cell-specific markers and IFN-γ expression. CD4+ and CD4− (CD8) T cells were analyzed in a similar manner. Statistical analysis was performed using GraphPad Prism statistical software (GraphPad Software, San Diego, CA). Where indicated, a threshold of two times the standard deviation (SD) over the prevaccine mean was used to evaluate changes postvaccine. This method was arrived at following analysis of the data to set a consistent standard by which values had to exceed some measure of standard variation to be counted as an event.
FIG. 1.
Nine-color flow cytometry for phenotypic and functional analysis of NK and T cells. (A) Lymphocytes were defined as side scatter low and CD14 negative (upper left image). CD19− lymphocytes were separated into CD3−, CD3+ CD4+, and CD3+ CD4− (CD8+ T cells). CD4+ and CD8+ T cells were evaluated for the production of intracellular IFN-γ following overnight stimulation (shown) and for expression of surface receptors on NK and CD8+ T cells. CD3− cells were evaluated for expression of CD56 and CD16 to define NK cells. NK and T cells were then evaluated for IFN-γ production and expression of NK cell phenotypic markers and surface receptors. (B) Longitudinal analysis of all subjects showing the frequency of NK, CD4+, and CD8+ T cells as a percentage of the lymphocyte population (defined in the upper left image in panel A). The prevaccine time point for subject 4 was unavailable for analysis.
Influenza virus antibody ELISA.
Plasma from seven healthy adults (five vaccinated and two unvaccinated) was measured by enzyme-linked immunosorbent assay (ELISA) for influenza virus A-specific IgG according to the manufacturer's instructions (IBL-America; catalog no. IB79251). All samples and controls were run in duplicate. Samples prior to vaccination and at the highest postvaccination time point within a month after vaccination were compared to an internal standard. The results were reported as units/ml.
RESULTS
Increased frequency of NK and T cells producing IFN-γ in response to influenza virus vaccination.
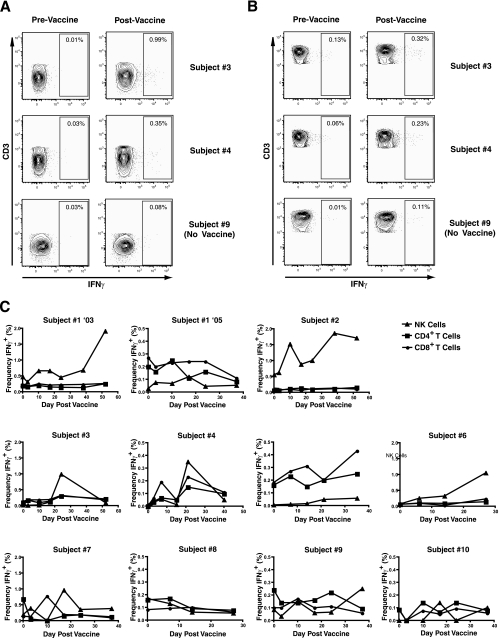
Since no single marker is available, NK cells are commonly defined by a combination of cell surface markers. Mature NK cells are routinely defined as CD3− CD56+ lymphocytes and may be further grouped into subpopulations by the expression of cell surface proteins as assessed by flow cytometric analysis. We have developed nine-color flow cytometry techniques to study NK cell subpopulations from patient PBMC (Fig. 1A). We measured the frequency (percentage), receptor expression, and function of NK cells concurrently with that of T cells by flow cytometry on cryopreserved PBMC over time following influenza virus vaccination. We were unable to detect any notable changes in the frequencies of NK or T cells as a proportion of the lymphocyte population following vaccination (Fig. 1B), though in some subjects, the frequency of NK cells decreased relative to the frequency of CD4+ T cells. This was most pronounced in subject 1 (2005), with the bulk of the change accruing at the final time point (day 38). Overall, lymphocyte frequencies remained relatively stable postvaccine in this longitudinal series. PBMC stimulated with inactivated whole influenza virus, influenza virus HA and M1 peptide pools, or K-562 cells were evaluated for IFN-γ production using intracellular CFC. Stimulated PBMC were separated into CD4+ T cells, CD8 cells (CD3+ CD4−), and NK cells and evaluated for the frequency of producing IFN-γ (Fig. 2A and B). Despite the overall stability of lymphocyte frequencies postvaccine, we were able to detect increases in the percentage of IFN-γ-producing cells. Influenza virus antigen-specific cellular responses were detected in the majority of subjects in response to stimulation with inactivated whole influenza virus and were most dramatically observed in the NK cell population (Fig. 2C). Stimulation with influenza virus HA and M1 peptides did not result in any notable increase in IFN-γ production by either NK or T cells (data not shown).
FIG. 2.
IFN-γ production pre- and postvaccination by NK and T cells. NK cells (A) and CD8+ T cells (B) were stained intracellularly for IFN-γ following stimulation with inactivated whole influenza virus (influenza virus A/New Caledonia/20/99 H1N1). The postvaccine plots represent the peak responses for individual subjects, day 24 for subject 3, day 21 for subject 4, and day 38 for subject 9. (C) Longitudinal analysis of IFN-γ production by NK, CD4+, and CD8+ T cells in response to stimulation with inactivated whole influenza virus. The frequencies of IFN-γ-producing cells are shown as percentages of total NK, CD4+, and CD8+ T cells. The y axis scale varies among subjects, with ranges of 0.0% to 0.5% or 0.0% to 2.0% to illustrate minor fluctuations.
The frequencies of cells producing IFN-γ in response to whole influenza virus antigen varied substantially among subjects and were normalized by calculating the change in the frequency of IFN-γ-producing cells postvaccine. Following analysis of the data, a change greater than 2 SD from the prevaccination mean was considered noteworthy. This criterion was used to set a consistent standard by which values must exceed some threshold measure of standard variation to be counted as an event. We detected a change greater than 2 SD above the mean of IFN-γ production by NK cells postvaccine in seven of eight vaccinated subjects (Table 1) and in neither of the two control subjects. The change in IFN-γ-producing NK cells ranged from 3.38 to 70.71, with the peak day of the response (shown parenthetically in Table 1) ranging from day 17 to day 52 in responding subjects.
TABLE 1.
Frequencies of NK cells producing IFN-γ in response to stimulation with inactivated whole influenza virus
| Subject | Influenza vaccine | Frequency of IFN-γ+ NK cells
|
Fold change | Notablea | |
|---|---|---|---|---|---|
| Prevaccine | Postvaccine (peak day) | ||||
| 1 (2003)b | Yes | 0.470 | 1.910 (52) | 4.06 | Yes |
| 1 (2005)b | Yes | 0.026 | 0.120 (17) | 4.62 | Yes |
| 2 | Yes | 0.550 | 1.860 (38) | 3.38 | Yes |
| 3 | Yes | 0.014 | 0.990 (24) | 70.71 | Yes |
| 4 | Yes | 0.028 | 0.350 (21) | 12.50 | Yes |
| 5 | Yes | 0.003 | 0.057 (35) | 21.03 | Yes |
| 6 | Yes | 0.062 | 1.050 (27) | 16.94 | Yes |
| 7 | Yes | 0.190 | 0.960 (17) | 5.05 | Yes |
| 8 | Yes | 0.170 | 0.130 (7) | 0.76 | No |
| 9 | No | 0.085 | 0.150 (NA)c | 1.76 | No |
| 10 | No | 0.100 | 0.140 (NA)c | 1.40 | No |
A change of more than 2 SD greater than the mean prevaccination was considered notable. Prevaccine mean = 0.143; SD = 0.194; {(mean + 2 × SD mean)/mean} = 3.73.
Subject 1 participated in the study in both 2003 and 2005.
The maximum experimental value obtained is shown for control subjects, who did not receive vaccine. NA, not applicable.
Surprisingly, we observed no increase in the frequency of IFN-γ-producing CD4+ T cells following stimulation with whole influenza virus antigen. However, two of eight vaccinated subjects displayed an increase in the frequency of CD8+ T cells producing IFN-γ (Table 2). In these two subjects, the observed peak in the frequency of IFN-γ-producing CD8+ T cells either preceded or coincided with the peak frequency of IFN-γ-producing NK cells (Fig. 2C and Tables 1 and 2). The data for these two subjects is in agreement with previously reported results suggesting that the NK cell response to influenza virus is dependent upon cytokine secretion by T cells (12). However, five of seven subjects displayed increases in the frequency of IFN-γ-producing NK cells with no substantive increase in responding CD8+ T cells. Nevertheless, cross talk between NK and T cells may be important for developing effective cellular immunity against influenza virus infection and may be indicative of a more general mechanism of viral immunity.
TABLE 2.
Frequencies of CD8 T cells producing IFN-γ in response to stimulation with inactivated whole influenza virus
| Subject | Influenza vaccine | Frequency of IFN-γ+ CD8 T cells
|
Fold change | Notablea | |
|---|---|---|---|---|---|
| Prevaccine | Postvaccine (peak day) | ||||
| 1 (2003)b | Yes | 0.220 | 0.260 (52) | 1.18 | No |
| 1 (2005)b | Yes | 0.270 | 0.240 (17) | 0.89 | No |
| 2 | Yes | 0.086 | 0.130 (38) | 1.51 | No |
| 3 | Yes | 0.130 | 0.320 (24) | 2.46 | No |
| 4 | Yes | 0.061 | 0.230 (21) | 3.77 | Yes |
| 5 | Yes | 0.400 | 0.430 (35) | 1.08 | No |
| 6 | Yes | 0.059 | 0.090 (6) | 1.53 | No |
| 7 | Yes | 0.140 | 0.770 (10) | 5.50 | Yes |
| 8 | Yes | 0.081 | 0.099 (14) | 1.22 | No |
| 9 | No | 0.099 | 0.170 (NA)c | 1.72 | No |
| 10 | No | 0.088 | 0.091 (NA)c | 1.03 | No |
A change of more than 2 SD greater than the mean prevaccination was considered notable. Prevaccine mean = 0.143; SD = 0.113; {(mean + 2 × SD mean)/mean} = 2.58.
Subject 1 participated in the study in both 2003 and 2005.
The maximum experimental value obtained is shown for control subjects, who did not receive vaccine. NA, not applicable.
NK cells make up a significant portion of IFN-γ-producing lymphocytes following influenza virus vaccination.
As it appeared that the greatest change in the frequency of IFN-γ-producing cells occurred in the NK cell population following influenza virus vaccination, we evaluated the proportions of total IFN-γ production due to NK cells and CD4+and CD8+ T cells. As shown in Fig. 3, NK cells, with a few notable exceptions, comprise a relatively small proportion of the IFN-γ-producing cells in response to whole influenza virus stimulation prevaccination. Here, the majority of IFN-γ-producing cells were either CD4+ or CD8+ T cells. However, following vaccination, there was a large increase in the proportion of IFN-γ-producing NK cells. In five of eight vaccinated subjects, this represented a noticeable change in the NK cell IFN-γ production as a proportion of lymphocytes. Overall, the mean proportion of IFN-γ-producing cells that were NK cells was 16.4% (quartiles, 3.2% and 24.0%) prevaccine and increased to 30.3% (quartiles, 14.3% and 48.1%) postvaccine.
FIG. 3.
Proportion of IFN-γ production by NK, CD4+, and CD8+ T cells pre- and postvaccination. The proportion of NK, CD4+, or CD8+ T cells producing IFN-γ was calculated based on the summed frequencies of the three populations as a percentage of lymphocytes. Pre- and postvaccination values are shown for each subject, with a prevaccine mean value for NK cells of 16.4 (SD, 17.1) and a peak postvaccine mean value of 30.3 (SD, 18.2). Subjects 1 (2005), 3, 5, 6, and 7 had notable changes greater than 2 SD over the prevaccine mean proportion.
Phenotypic stability of NK cells in study subjects.
We evaluated the expression of several cell surface proteins expressed on NK cells in both vaccinated and control subjects. While some markers in individual subjects displayed transient changes in frequency, the overall picture was one of phenotypic stability. Where changes were present, they were inconsistent, but some notable trends appeared. Most subjects displayed a brief increase in KIR-expressing NK cells following vaccination. This was most consistent with the expression of KIR3DL1 (DX9 antibody) (Fig. 4A). Several subjects showed increases in KIR recognized by the DX27 antibody (anti-KIR2DL2, -KIR2DL3, and -KIR2DS2) and KIR2DL3, and only two subjects had minor increases in KIR2DL1. In addition to KIR expression, we assayed for changes in the frequency of NK cells expressing the C-type lectin receptors CD94, NKG2A, NKG2C, and CD161 (NKR-P1A). NKG2A and NKG2C form inhibitory and activating homodimers, respectively, with CD94 at the cell surface (18). CD161 is considered to be an inhibitory receptor on NK cells and binds the ligand lectin-like transcript 1 (2, 28). The frequency of NK cells expressing these receptors remained remarkably unchanged following vaccination in most subjects. Any changes seen were inconsistent and not attributable to influenza virus vaccination. Of note, however, was the range of variation in receptor expression among subjects. This was especially apparent with NKG2C and CD161. The frequency of NK cells expressing NKG2C ranged from a low of 0.8% to a high of 19.6%. Similarly, the frequency of CD161-expressing cells ranged from a low of 5.7% to a high of 75.2%. There was a wide range of expression of these receptors, not only among individuals, but also within one subject, subject 1, whose response to influenza virus vaccination was tested twice with 2 years intervening, from 2003 to 2005 (Fig. 4B). Though receptor expression for KIR and C-type lectins remained stable during the course of each evaluation in subject 1, marked changes were noted from the 2003 study period to the 2005 study period. Most prominent was the increase in the frequency of CD161-expressing NK cells from an average frequency of 4.4% in 2003 to an average frequency of 48.1% in 2005. Similar changes were seen in KIR2DL3 (16.6% to 27.5%) and NKG2A (12.9% to 26.7%). These changes from 2003 to 2005 coincided with a decreased cellular response, with the frequency of IFN-γ-secreting NK cells dropping from 1.9% in 2003 to 0.1% in 2005 (Table 1).
FIG. 4.
Longitudinal evaluation of NK cell receptor expression following influenza virus vaccination. PBMC were evaluated by flow cytometry for the expression of NK cell receptors. (A) The top row shows natural cytotoxicity receptor expression as a percentage of NK cells. The expression of NKp44 was relatively stable over time, whereas inconsistent fluctuations were observed in the frequency of NKp46-positive cells. The frequency of KIR3DL1 (DX9)-expressing cells (second row) increased transiently in vaccinated subjects, but trends in other KIR were inconsistent. The frequencies of CD94, NKG2C, NKG2A, and CD161 (third row) remained stable, but a significant degree of variation was seen across subjects with both NKG2C and CD161. (B) Changes in NK cell receptor expression in PBMC samples separated by 2 years in subject 1. Subject 1 was evaluated in 2003 and again in 2005. Increased expression of NKG2A, KIR2DL3, and CD161 from 2003 to 2005 correlated with decreased IFN-γ production by NK cells over the same period.
The natural cytotoxicity receptors, comprised of NKp30, NKp44, and NKp46, are a group of immunoglobulin superfamily receptors exclusively expressed on NK cells (17, 24). We evaluated the frequency of NK cell subsets expressing NKp46 (Fig. 5A), as NKp46 has been demonstrated to interact directly with the HA protein of influenza virus and represents an important mechanism of influenza virus recognition by NK cells (4, 7). The frequency with which CD56bright or CD56dim NK cells express NKp46, and whether this changes over time, had not been previously described. Longitudinal analysis of NKp46-expressing NK cells following influenza virus vaccination revealed that the frequency of this population remained relatively stable. The CD56bright cells had the highest frequency of NKp46 expression, with a mean prevaccine level of 90.0% (range, 78.6% to 97.2%), whereas the CD56dim cells had a mean frequency of 55.7% (range, 12.7% to 87.2%). Although we have not directly tested whether the NKp46+ population of NK cells secrete IFN-γ in response to influenza virus, the fact that the frequency of IFN-γ-producing NK cells increased postvaccination without concomitant increases in NKp46 frequency might suggest that this population of NK cells may not be directly responsible for the increased IFN-γ output.
FIG. 5.
Longitudinal analysis of NKp46 and perforin postvaccination. (A) Frequencies of NK cells and NK cell subsets expressing NKp46. (B) Frequencies of NK cells and NK cell subsets expressing perforin. A general trend of increasing perforin-positive CD56bright NK cells was seen in most subjects postvaccination, with only subject 1 (2005) having a change greater than 2 SD above the mean.
We analyzed the frequency of NK cell subsets, CD56dim, CD56bright, and CD56neg, for production of perforin by CFC. Perforin is a pore-forming cytolytic protein found in the granules of cytotoxic T cells and NK cells. Previous studies have revealed that perforin-containing NK cells reside predominantly within the CD56dim population (15). Our longitudinal analysis reflected these previous data (Fig. 5B); however, increases in perforin expression were observed within the CD56bright population in some subjects. This was particularly pronounced in subject 1 (2005), in whom the frequency of perforin-positive NK cells increased from 12.8% prevaccine to a maximum value of 51.5% at day 17 postvaccine. A trend toward an increasing proportion of CD56bright NK cells producing perforin was noted in five of eight vaccinated subjects and one of two control subjects.
Increased influenza virus antigen-specific IgG antibodies in the plasma of vaccinated subjects.
Influenza virus vaccination is well characterized with respect to the induction of B-cell antibody responses, which are thought to provide a large measure of the protection associated with immunization (1, 27). To further characterize the breadth of the immune response we observed in our study, plasma samples were collected at each time point from the subjects vaccinated in 2005. We were able to detect increased production of plasma IgG in vaccinated subjects that was not detected in either control subject (P = 0.03). The vaccinated subjects displayed a mean prevaccine titer of 99.6 units/ml (quartiles, 76.5 and 122.5) versus a mean peak titer postvaccine of 133.2 units/ml (quartiles, 122.0 and 145.5), representing a mean increase of 40.7% (Fig. 6). The largest change in antibody titer was reported in one subject with no prior history of vaccination (subject 6; 107.9% increase).
FIG. 6.
Influenza virus A antigen-specific ELISA for plasma IgG. Plasma was obtained at each time point from subjects vaccinated in 2005. Shown are the titers pre- and postvaccine. The maximum titer obtained postvaccine is shown for vaccinated subjects, and the highest experimental value is shown for controls.
DISCUSSION
A more thorough understanding of the immune response following influenza virus vaccination in humans may prove to be important for the preparation of improved vaccines directed against future pandemic influenza virus infections. In this study, we evaluated the phenotypes and functions of T and NK cells, as well as influenza virus-specific IgG production, following vaccination with the inactivated TIV. PBMC from volunteers were stimulated with both inactivated influenza virus and overlapping pools of HA and M1 peptides. An increased frequency of IFN-γ-expressing NK and CD8+ T cells was observed in response to inactivated whole influenza virus stimulation (seven of eight and two of eight, respectively). Sizeable increases in the frequency of IFN-γ-producing NK cells were observed in many subjects and demonstrate that NK cells are a substantial source of IFN-γ postvaccination. A recent study by Mbawuike et al. evaluated influenza virus vaccine responses in 30 subjects and noted an increase in IFN-γ in the supernatants of restimulated PBMC that did not coincide with cell-mediated cytotoxicity (21). The cytokine-producing cells were not identified, but our results here suggest that IFN-γ production by NK cells may have been largely responsible for this observation. Further studies are needed to determine the effects of NK cells and adaptive cellular immune responses on influenza virus infection, yet these results imply that NK cells may be an important consideration for the design of improved influenza virus vaccines.
There were subtle differences in NK cell phenotypes in subjects receiving the influenza virus vaccine, but these phenotypes remained largely stable over time and were not correlated with either NK or T-cell function. However, a general trend was observed in which subjects with a high frequency of CD161-expressing NK cells (an inhibitory C-type lectin receptor) had lower frequencies of IFN-γ-expressing NK cells. Subject 1, who provided samples twice, 2 years apart, further highlights this observation. Subject 1 (2003) had a low frequency of CD161-expressing NK cells with a high frequency of IFN-γ-producing NK cells in response to whole influenza virus stimulation (Fig. 2C and 5B). The opposite was true 2 years later, when subject 1 (2005) had a high frequency of CD161-expressing NK cells with a lower frequency of IFN-γ-producing NK cells. The reasons for this change are unclear, but they suggest that lower levels of inhibitory NK cell receptors may correlate with improved influenza virus vaccine responses.
The wide variation in the frequency of NK cells expressing the NKG2C receptor may be influenced by the presence of chronic viral infections, such as cytomegalovirus (9, 10) and human immunodeficiency virus. Infection with cytomegalovirus has an “imprinting” effect that alters the repertoire of NK and T cells expressing NKG2C. We and others have noted an increased frequency of NKG2C-expressing cells in human immunodeficiency virus type 1-seropositive individuals (23). Additionally, influenza virus-infected cells upregulate the expression of the class I-like protein MICB, which is a ligand for the activating NK cell receptor NKG2D (30).
The breadth of the immune response to influenza virus vaccination encompasses innate, cellular, and humoral immunities. Consequently, it is surprising that we did not observe an increase in IFN-γ production by CD4+ T cells. CD4+ T-cell function is generally required for effective stimulation of antibody production and isotype switching by plasma cells. Analysis of plasma samples from vaccinated subjects showed a 40.7% increase in plasma IgG from prevaccination levels, with the largest increase occurring in one subject with no prior history of vaccination. It may be possible that the function of stimulated CD4+ T cells is not reflected by IFN-γ production. Indeed, studies suggest that Th2 cytokines are more important for antibody-mediated humoral responses than IFN-γ, a prototypical TH1 cytokine (25). Although the currently administered influenza virus vaccine has demonstrable clinical utility, those most at risk, including the very young and the elderly, respond most poorly and remain vulnerable to infection. In these patients, cellular immune responses are more critical for long-lasting protective immunity (22).
Our data and previous studies indicate that the influenza virus vaccination should, and does, influence innate immunity. Increased frequencies of IFN-γ-producing NK and T cells were observed in response to vaccination, and this coincided in some cases with fluctuations in NK cell receptor expression. Consequently, an enhancement of influenza virus vaccines may be possible by targeting innate and cellular immunity and may prove especially beneficial for those most at risk of infection. In this regard, more work needs to be done in defining NK cell receptor interactions with influenza virus and virus-infected cells that improve innate and acquired immunity.
Acknowledgments
We thank Holden Maecker of BD Biosciences-Immunocytometry Systems, San Jose, CA, for the kind gift of influenza virus HA and M1 peptides.
Support for this work was provided by the National Institute of Allergy and Infectious Diseases (NIAID P01 AI064520), the Swedish Research Council, and the Swedish Foundation for Strategic Research. Additional support was provided by the UCSF Dean's Fellowship, the Alpha Omega Alpha Medical Honor Society Research Fellowship, the American Pediatric Society, and the UCSF AIDS Research Institute (ARI).
Footnotes
Published ahead of print on 14 November 2007.
REFERENCES
- 1.Ada, G. L., and P. D. Jones. 1986. The immune response to influenza infection. Curr. Top. Microbiol. Immunol. 128:1-54. [DOI] [PubMed] [Google Scholar]
- 2.Aldemir, H., V. Prod'homme, M. J. Dumaurier, C. Retiere, G. Poupon, J. Cazareth, F. Bihl, and V. M. Braud. 2005. Cutting edge: lectin-like transcript 1 is a ligand for the CD161 receptor. J. Immunol. 175:7791-7795. [DOI] [PubMed] [Google Scholar]
- 3.Bridges, C. B., S. A. Harper, K. Fukuda, T. M. Uyeki, N. J. Cox, and J. A. Singleton. 2003. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommend. Rep. 52:1-34. [PubMed] [Google Scholar]
- 4.Draghi, M., A. Pashine, B. Sanjanwala, K. Gendzekhadze, C. Cantoni, D. Cosman, A. Moretta, N. M. Valiante, and P. Parham. 2007. NKp46 and NKG2D recognition of infected dendritic cells is necessary for NK cell activation in the human response to influenza infection. J. Immunol. 178:2688-2698. [DOI] [PubMed] [Google Scholar]
- 5.Epstein, S. L., W. P. Kong, J. A. Misplon, C. Y. Lo, T. M. Tumpey, L. Xu, and G. J. Nabel. 2005. Protection against multiple influenza A subtypes by vaccination with highly conserved nucleoprotein. Vaccine 23:5404-5410. [DOI] [PubMed] [Google Scholar]
- 6.Gao, W., A. C. Soloff, X. Lu, A. Montecalvo, D. C. Nguyen, Y. Matsuoka, P. D. Robbins, D. E. Swayne, R. O. Donis, J. M. Katz, S. M. Barratt-Boyes, and A. Gambotto. 2006. Protection of mice and poultry from lethal H5N1 avian influenza virus through adenovirus-based immunization. J. Virol. 80:1959-1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gazit, R., R. Gruda, M. Elboim, T. I. Arnon, G. Katz, H. Achdout, J. Hanna, U. Qimron, G. Landau, E. Greenbaum, Z. Zakay-Rones, A. Porgador, and O. Mandelboim. 2006. Lethal influenza infection in the absence of the natural killer cell receptor gene Ncr1. Nat. Immunol. 7:517-523. [DOI] [PubMed] [Google Scholar]
- 8.Greenbaum, E., D. Engelhard, R. Levy, M. Schlezinger, A. Morag, and Z. Zakay-Rones. 2004. Mucosal (SIgA) and serum (IgG) immunologic responses in young adults following intranasal administration of one or two doses of inactivated, trivalent anti-influenza vaccine. Vaccine 22:2566-2577. [DOI] [PubMed] [Google Scholar]
- 9.Guma, M., A. Angulo, C. Vilches, N. Gomez-Lozano, N. Malats, and M. Lopez-Botet. 2004. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood 104:3664-3671. [DOI] [PubMed] [Google Scholar]
- 10.Guma, M., C. Cabrera, I. Erkizia, M. Bofill, B. Clotet, L. Ruiz, and M. Lopez-Botet. 2006. Human cytomegalovirus infection is associated with increased proportions of NK cells that express the CD94/NKG2C receptor in aviremic HIV-1-positive patients. J. Infect. Dis. 194:38-41. [DOI] [PubMed] [Google Scholar]
- 11.Harper, S. A., K. Fukuda, T. M. Uyeki, N. J. Cox, and C. B. Bridges. 2005. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommend. Rep. 54:1-40. [PubMed] [Google Scholar]
- 12.He, X. S., M. Draghi, K. Mahmood, T. H. Holmes, G. W. Kemble, C. L. Dekker, A. M. Arvin, P. Parham, and H. B. Greenberg. 2004. T cell-dependent production of IFN-gamma by NK cells in response to influenza A virus. J. Clin. Investig. 114:1812-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He, X. S., T. H. Holmes, C. Zhang, K. Mahmood, G. W. Kemble, D. B. Lewis, C. L. Dekker, H. B. Greenberg, and A. M. Arvin. 2006. Cellular immune responses in children and adults receiving inactivated or live attenuated influenza vaccines. J. Virol. 80:11756-11766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoelscher, M. A., S. Garg, D. S. Bangari, J. A. Belser, X. Lu, I. Stephenson, R. A. Bright, J. M. Katz, S. K. Mittal, and S. Sambhara. 2006. Development of adenoviral-vector-based pandemic influenza vaccine against antigenically distinct human H5N1 strains in mice. Lancet 367:475-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobs, R., G. Hintzen, A. Kemper, K. Beul, S. Kempf, G. Behrens, K. W. Sykora, and R. E. Schmidt. 2001. CD56bright cells differ in their KIR repertoire and cytotoxic features from CD56dim NK cells. Eur. J. Immunol. 31:3121-3127. [DOI] [PubMed] [Google Scholar]
- 16.Kash, J. C., T. M. Tumpey, S. C. Proll, V. Carter, O. Perwitasari, M. J. Thomas, C. F. Basler, P. Palese, J. K. Taubenberger, A. Garcia-Sastre, D. E. Swayne, and M. G. Katze. 2006. Genomic analysis of increased host immune and cell death responses induced by 1918 influenza virus. Nature 443:578-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lanier, L. L. 2005. NK cell recognition. Annu. Rev. Immunol. 23:225-274. [DOI] [PubMed] [Google Scholar]
- 18.Lopez-Botet, M., T. Bellon, M. Llano, F. Navarro, P. Garcia, and M. de Miguel. 2000. Paired inhibitory and triggering NK cell receptors for HLA class I molecules. Hum. Immunol. 61:7-17. [DOI] [PubMed] [Google Scholar]
- 19.Mandelboim, O., N. Lieberman, M. Lev, L. Paul, T. I. Arnon, Y. Bushkin, D. M. Davis, J. L. Strominger, J. W. Yewdell, and A. Porgador. 2001. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature 409:1055-1060. [DOI] [PubMed] [Google Scholar]
- 20.Mandelboim, O., and A. Porgador. 2001. NKp46. Int. J. Biochem. Cell. Biol. 33:1147-1150. [DOI] [PubMed] [Google Scholar]
- 21.Mbawuike, I., Y. Zang, and R. B. Couch. 2007. Humoral and cell-mediated immune responses of humans to inactivated influenza vaccine with or without QS21 adjuvant. Vaccine 25:3263-3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McElhaney, J. E., D. Xie, W. D. Hager, M. B. Barry, Y. Wang, A. Kleppinger, C. Ewen, K. P. Kane, and R. C. Bleackley. 2006. T cell responses are better correlates of vaccine protection in the elderly. J. Immunol. 176:6333-6339. [DOI] [PubMed] [Google Scholar]
- 23.Mela, C. M., C. T. Burton, N. Imami, M. Nelson, A. Steel, B. G. Gazzard, F. M. Gotch, and M. R. Goodier. 2005. Switch from inhibitory to activating NKG2 receptor expression in HIV-1 infection: lack of reversion with highly active antiretroviral therapy. AIDS 19:1761-1769. [DOI] [PubMed] [Google Scholar]
- 24.Moretta, A., C. Bottino, M. Vitale, D. Pende, C. Cantoni, M. C. Mingari, R. Biassoni, and L. Moretta. 2001. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu. Rev. Immunol. 19:197-223. [DOI] [PubMed] [Google Scholar]
- 25.Mosmann, T. R., and S. Sad. 1996. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol. Today 17:138-146. [DOI] [PubMed] [Google Scholar]
- 26.Palese, P. 2004. Influenza: old and new threats. Nat. Med. 10:S82-S87. [DOI] [PubMed] [Google Scholar]
- 27.Potter, C. W., and J. S. Oxford. 1979. Determinants of immunity to influenza infection in man. Br. Med. Bull. 35:69-75. [DOI] [PubMed] [Google Scholar]
- 28.Rosen, D. B., J. Bettadapura, M. Alsharifi, P. A. Mathew, H. S. Warren, and L. L. Lanier. 2005. Cutting edge: lectin-like transcript-1 is a ligand for the inhibitory human NKR-P1A receptor. J. Immunol. 175:7796-7799. [DOI] [PubMed] [Google Scholar]
- 29.Sambhara, S., A. Kurichh, R. Miranda, T. Tumpey, T. Rowe, M. Renshaw, R. Arpino, A. Tamane, A. Kandil, O. James, B. Underdown, M. Klein, J. Katz, and D. Burt. 2001. Heterosubtypic immunity against human influenza A viruses, including recently emerged avian H5 and H9 viruses, induced by FLU-ISCOM vaccine in mice requires both cytotoxic T-lymphocyte and macrophage function. Cell Immunol. 211:143-153. [DOI] [PubMed] [Google Scholar]
- 30.Siren, J., T. Sareneva, J. Pirhonen, M. Strengell, V. Veckman, I. Julkunen, and S. Matikainen. 2004. Cytokine and contact-dependent activation of natural killer cells by influenza A or Sendai virus-infected macrophages. J. Gen. Virol. 85:2357-2364. [DOI] [PubMed] [Google Scholar]
- 31.Spies, T., and V. Groh. 2006. Natural cytotoxicity receptors: influenza virus in the spotlight. Nat. Immunol. 7:443-444. [DOI] [PubMed] [Google Scholar]
- 32.Taubenberger, J. K., and D. M. Morens. 2006. 1918 influenza: the mother of all pandemics. Emerg. Infect. Dis. 12:15-22. [DOI] [PMC free article] [PubMed] [Google Scholar]