Abstract
Herpes simplex virus type 2 (HSV-2) is a common human pathogen that can cause a variety of clinical manifestations in humans. In order to provide near-patient results to allow for faster counseling and treatment, a rapid point-of-care test that is accurate and simple to use is desirable. Here, we describe the development and evaluation of an HSV-2 immunoglobulin G (IgG)-specific antibody lateral-flow immunochromatographic assay (LFIA) based on colloidal gold nanoparticles. A total of 359 serum samples and 100 whole-blood samples were tested in the newly developed HSV-2 LFIA. Serum results were compared to those from the HerpeSelect HSV-2 enzyme-linked immunosorbent assay (ELISA), and whole-blood sample results were compared to those of both ELISA and HerpeSelect HSV-1 and -2 immunoblotting (IB). The sensitivity of the HSV-2 LFIA compared to that of the HerpeSelect ELISA was 100% (89/89), and the specificity was 97.3% (257/264). Cross-reactivity with HSV-1 IgG-positive serum samples was observed in 2.6% (5/196) of samples, 2.9% (1/34) for rubella virus, and 6.2% (1/16) for Epstein-Barr virus. No cross-reactivity in varicella-zoster virus or cytomegalovirus IgG-positive serum samples was observed. No interference was observed from bilirubin-, triglyceride-, albumin-, or hemoglobin-spiked samples. The concordance of the LFIA results between capillary whole blood, EDTA-treated venous whole blood, heparin-treated venous whole blood, and serum was 99% (99/100). In conclusion, the LFIA for HSV-2 IgG-specific antibodies demonstrated excellent sensitivity, specificity, and concordance for both serum and whole-blood samples compared to the sensitivity, specificity, and concordance of both HSV-2 ELISA and IB.
Herpes simplex virus (HSV) is a common human pathogen found worldwide that causes a variety of diseases (6). HSV has been characterized into two different serotypes: HSV type 1 (HSV-1) generally is associated with infections in the tongue, mouth, lips, pharynx, and eyes, whereas HSV-2 primarily is associated with genital and neonatal infections (6). HSV infects neonates, children, and adults; by the age of 40, more than 90% of the adult population demonstrates antibodies to HSV-1 (6). In the United States, most young sexually active adults with genital ulcers have genital herpes (8).
Primary HSV-2 infections usually are transmitted through sexual contact. HSV transmission can result from direct contact with infected secretions from symptomatic or asymptomatic individuals (19). The classic presentation is herpes genitalis, an infection characterized by lesions in the genital area, and it may be accompanied by fever, inguinal lymphadenopathy, and dysuria. Previous studies demonstrated that HSV-2 causes approximately 85% of symptomatic primary genital HSV cases, with HSV-1 infections causing the remainder (1). However, more recently it has been shown that approximately 30% of primary genital herpes infections presently are associated with HSV-1 (10, 13, 22). Despite the increase in genital herpes cases due to HSV-1, the HSV-1 recurrence rate for genital herpes has been shown to be 20% of that seen with HSV-2 during the first year after infection, and the rate of recurrence for HSV-1 genital herpes in subsequent years following primary infection decreases at a much faster pace than that of HSV-2 recurrences. Therefore, the majority of genital herpes recurrences are due to HSV-2 rather than HSV-1 infections (11).
Because of the high prevalence of asymptomatic cases, serological diagnosis and the differentiation between HSV-1 and HSV-2 infection continues to be important in the management of HSV (21). The two viruses share considerable antigenicity; however, the G glycoproteins gG-1 (HSV-1) and gG-2 (HSV-2) are divergent between the two viruses and evoke a type-specific antibody response that has been used to develop several commercially available diagnostic products (2, 23). Type-specific serological testing for HSV-1 and HSV-2 has been shown to be an accurate method for the diagnosis of HSV-1 and HSV-2 infection compared to the accuracy of Western blotting (4, 15, 17, 20).
Rapid, point-of-care serology for sexually transmitted diseases such as human immunodeficiency virus type 1 (HIV-1) has substantially reduced the turnaround time for the delivery of results to the patient and increased the likelihood of counseling and treatment (9). Rapid testing has been shown to be more cost-effective with HIV serology testing, since it allows for the reporting of a result and counseling of the patient in a single visit (12). Today, the majority of HSV-2-specific serological testing is from laboratory-based methods such as Western blotting, immunoblotting (IB), and enzyme-linked immunosorbent assay (ELISA). Here, we describe the development and evaluation of a new rapid, sensitive, specific, easy-to-use HSV-2 immunoglobulin G (IgG) serological test that is based on the native gG-2 antigen and lateral-flow technology.
MATERIALS AND METHODS
Human serum and blood samples.
A total of 359 serum samples sequentially submitted for HSV-1 and -2 testing were collected, deidentified, and used to determine the diagnostic sensitivity and specificity of the device with serum. Reference data on serum samples were generated using the HerpeSelect HSV-2 ELISA (Focus Diagnostics, Inc., Cypress, CA). For cross-reactivity studies, a total of 271 IgG-positive serum samples for cytomegalovirus (CMV) (n = 11), varicella-zoster virus (VZV) (n = 14), Epstein-Barr virus (EBV) (n = 16), rubella virus (n = 34), and HSV-1 (n = 196) but negative for HSV-2 IgG antibodies as defined by the HerpeSelect HSV-2 ELISA were collected, deidentified, and tested. For interference testing, one HSV-2-negative and one HSV-2-positive serum sample (as defined by the HerpeSelect HSV-2 ELISA) were split and individually spiked with 10 mg/ml triglycerides, 60 mg/ml albumin, 0.2 mg/ml bilirubin, or 220 mg/ml hemoglobin (all reagents were from Sigma, St. Louis, MO). Samples were run on lateral-flow immunochromatographic assay (LFIA) devices, and results from spiked samples were compared to those from nonspiked whole-blood samples.
To demonstrate equivalent device performance in serum, venous whole blood, and capillary whole blood, 100 volunteer subjects were recruited according to a protocol reviewed by an external institutional review board. The 100 donors were taken from an unscreened, volunteer population of urban and suburban adults. Written informed consent to participate in the study was obtained from each subject in advance. Clinical information was not collected from donors. The study was double blinded; the identities of volunteer subjects were kept anonymous, and HSV-2 results were not provided to the donors. Capillary fingerstick whole-blood, EDTA-treated whole-blood, heparinized whole-blood, and serum samples were collected from each subject. Reference HSV-2 ELISA results, for comparison to the LFIA results, were determined by testing the serum sample.
LFIA device.
The LFIA device for the detection of IgG antibodies to HSV-2 was developed as a dual-antigen direct sandwich assay. The device consists of a plastic support to which a nitrocellulose membrane striped with the test reagents is mounted. The device can analyze 15 to 20 μl of serum or whole blood (venous or capillary). After collection, the sample is added to the sample port, filtered through a blood separation membrane, and absorbed into the test strip. Next, the sample addition portion of the test housing is opened, exposing the chase buffer port. The addition of chase buffer causes the conjugate and sample to migrate up the test strip. Assay (chase) buffer (buffered saline with nonionic detergent and preservative) was provided in dropper bottles. Mylar-wrapped, lithium heparin-coated capillary tubes calibrated to 20 μl (Drummond Scientific, Broomall, PA) are used to collect capillary fingertip whole blood.
Direct antibody sandwich format.
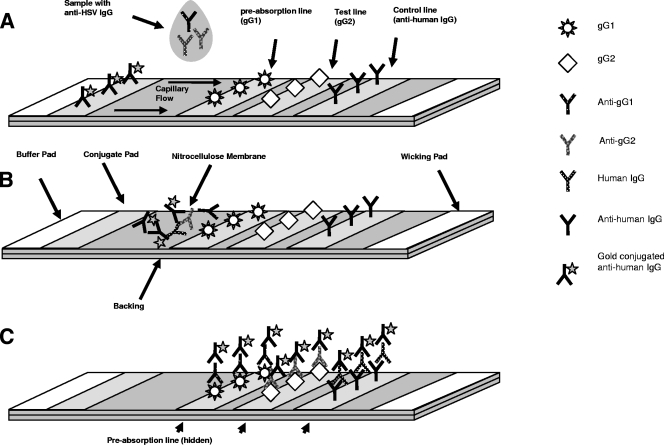
Figure 1 shows a diagram of the test strip. The 40-nm colloidal gold-conjugated goat anti-human IgG is sprayed onto a conjugate pad located between the buffer pad and the sample deposition area of the test strip (Fig. 1A). Three separate reagents (recombinant gG-1 antigen, purified native gG-2 antigen, and goat anti-human IgG antibody) are bound to a solid-phase nitrocellulose membrane. gG-1 antigen is striped in a test line nearest to the sample and serves as a preabsorbent for antibodies to HSV-1 present in the sample. Purified native gG-2 is striped in the test line (T) position, while a goat anti-human IgG is striped in the control line (C) position. The addition of a chase buffer causes the conjugate and sample to migrate across the test strip. As the sample migrates, it sequentially contacts the HSV-1 preabsorption line, the HSV-2 test line, and finally the control line, which binds the human IgG present in the sample (Fig. 1B). The anti-human IgG-gold conjugate migrates through the membrane in the aqueous phase until it is bound by human IgG that is present on the HSV-1 preabsorption and HSV-2 test lines and by the control line (Fig. 1C). The concentration of antibody-gold complexes captured by the test and control lines causes pink lines to form. The color formation is complete after 15 to 20 min. The formation of control and test lines indicates an HSV-2 positive result, while the formation of a control line only indicates an HSV-2-negative result. The absence of a control line is indicative of no sample being added to the test. Representative devices run with samples show results with chase buffer only (Fig. 2A), negative serum (Fig. 2B), and positive whole blood (Fig. 2C).
FIG. 1.
Diagram of the mechanism of the assay. (A) The sample is deposited on the test strip between the gold conjugate and test lines. (B) The addition of chase buffer causes the conjugate and sample to migrate, contacting the test lines sequentially and resulting in the capture of type-specific antibodies to HSV-2. (C) Completed test.
FIG. 2.
Assay procedure and results. (A) Device run with buffer only. (B) Device run with an HSV-2-negative serum. (C) Device run with HSV-2-positive whole blood.
Reference methods.
Data from the LFIA were compared to data from HerpeSelect HSV-1 and HSV-2 indirect ELISAs and HerpeSelect HSV-1 and -2 IBs (Focus Diagnostics, Inc., Cypress, CA), which are FDA-cleared devices for the determination of serum antibodies to gG-1 and gG-2 proteins. All serum samples used to determine the sensitivity and specificity of the LFIA were tested on the HerpeSelect HSV-1 and HSV-2 ELISAs. Additionally, the paired serum samples collected during the whole-blood study were run on the HerpeSelect HSV IB. Data from the latter studies were analyzed using both HSV-2 ELISA and HSV IB as reference methods. Both assays were performed according to the instructions of the manufacturer. Discordant samples positive in the reference ELISA but negative in the rapid test were tested by a modified HSV-2 inhibition ELISA (18). The HSV-2 inhibition ELISA is a validated in-house modification of the HerpeSelect ELISA. The modification is as follows. Serum samples are subjected to preincubation with an equal volume of HSV-2 viral lysate (Virusys, Sykesville, MD) and then further diluted and run in the HSV-2 ELISA per the product insert instructions. The preincubation step with the HSV-2 viral lysate is intended to absorb HSV-2 antibodies from the serum sample prior to exposure to the gG-2 antigen coated onto the ELISA well. As a control for nonspecific binding to proteins in the viral lysate, a second aliquot of the serum is preincubated with HSV-1 viral lysate (Virusys, Sykesville, MD) and then run per the HSV-2 ELISA kit insert instructions. After completion of the ELISA kit procedure, the optical density at 450 nm (OD450) of the samples in the presence of HSV-2 lysate and the HSV-1 lysate is read. The ratio of the OD450 value of the HSV-2 preincubation sample is divided by the OD450 value of the HSV-1 preincubation sample, and this ratio then is converted to a percentage. This measures the percent inhibition of the specific reactivity to gG-2 in the sample. Inhibition of binding of the sample in the ELISA by greater than 60% was validated as the cutoff for a true-positive sample (18).
Determination of the visual limit of detection.
During assay development, test lines were evaluated semiquantitatively by using a colorimetric scale (delineated in OD units) of striped lines of colloidal gold at various concentrations. A series of five pink/red lines sprayed with a colloidal gold solution of decreasing OD450 values from 10 (maximum line intensity) to 2 (weakest visible line) are striped on a card that is used to evaluate the intensity of test and control lines. HerpeSelect HSV-2 ELISA and HerpeSelect HSV-1 and -2 IB cutoff calibrators, or a venous whole-blood sample spiked with HSV-2-positive serum to be equivalent to an HSV-2 ELISA cutoff value, were used to set the visual limit of detection. Both ELISA and IB cutoff calibrators were set during the development of these products by using the gold standard HSV-2 Western blot test as a reference method (14). The visual limit of detection was set by titration of the gG-2 test line to produce a test line score of 2 (the weakest line intensity that still can be detected) with these samples.
Data evaluation.
The presence of a visible test line in the presence of a visible control line was used to define a sample as LFIA positive. The presence of a visible control line and the absence of a visible test line was used to define a sample as LFIA negative. All LFIA results were verified by a second reader. Receiver operating characteristic (ROC) analysis, a measure of the sensitivity and specificity of a diagnostic test; likelihood ratios; and positive and negative predictive values (PPV and NPV, respectively) were calculated using the MedCalc for Windows statistical package, version 9.3.0.0 (MedCalc Software, Mariakerke, Belgium). ROC analysis also was used to determine the area under the curve (AUC). AUC values approaching 1 indicate a test with a high degree of diagnostic accuracy. The diagnostic sensitivity and specificity were calculated using the following definitions: TP (a true-positive result), TN (a true-negative result), FN (a false-negative result), and FP (a false-positive result). The diagnostic sensitivity was calculated as {[TP/(TP + FN)] × 100}, and the diagnostic specificity was calculated as {[TN/(TN + FP)] × 100}. PPV, the proportion of specimens with positive tests that show evidence of infection, and NPV, the proportion of specimens with negative tests that do not show evidence of infection, were calculated with the following equations: PPV = {[TP/(TP +FP)] × 100} and NPV = {[TN/(TN +FN)] × 100}. The PPV and NPV are highly dependent on the prevalence of the infection in the study population. The positive likelihood ratio (LR+), the ratio between the probability of a positive test result given the presence of the disease and the probability of a positive test result given the absence of the disease, was calculated as follows: LR+ = sensitivity/(1 − specificity). The negative likelihood ratio (LR−), the ratio between the probability of a negative test result given the presence of the disease and the probability of a negative test result given the absence of the disease, was calculated as follows: LR− = (1 − sensitivity)/(specificity). LR+ and LR− are used in medicine to rule in disease (e.g., if the LR+ is >10) or to rule out disease (e.g., if the LR− is <0.1) (16).
RESULTS
Performance of the LFIA with serum.
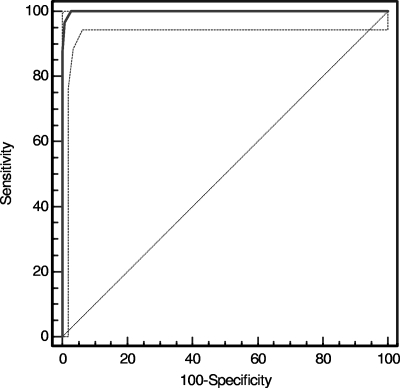
A total of 359 serum samples sequentially submitted for HSV testing were tested by HerpeSelect HSV-2 ELISA and LFIA devices. The seroprevalence of HSV-2 in this population was 25.2%. Table 1 shows the results of this testing. Both tests were positive in 89 cases and were negative in 257 cases. Six samples were equivocal by HSV-2 ELISA; of these, two were negative and four were positive in the LFIA. All six samples were tested by the HSV-2 inhibition ELISA, and all showed >60% inhibition in that test, indicating some level of HSV-2 antibody in the samples. Seven samples exhibited discordant results: all seven were negative on the HerpeSelect HSV-2 ELISA but positive on the HSV-2 LFIA. Although all seven samples had negative HerpeSelect HSV-2 ELISA index values, five had index values of >0.4 that were reduced by >60% in the inhibition ELISA, indicating the presence of some HSV-2 antibody in the sera. Figure 3 shows the ROCs, a measure of the sensitivity and specificity of the assay with serum; the sensitivity was 100%, and the specificity was 97.3%. The AUC was 0.999 (95% confidence interval, 0.988 to 1.000; P = 0.0001). The PPV was 92.7, and the NPV was 100.0 in this population. The LR+, a measure of the likelihood that a given positive test result would be expected in a patient with HSV-2 compared to the likelihood that that same result would be expected in a patient without HSV-2, was 37.7, indicating a strong likelihood of the presence of HSV-2 infection with a positive test result. The LR− was 0.0, indicating a strong likelihood of the absence of HSV-2 infection with a negative test result.
TABLE 1.
Comparison of LFIA and HSV-2 ELISA devices
| Sample type | HSV-2 LFIA result | No. of samples with indicated HSV-2 ELISA result (% agreement)a
|
||
|---|---|---|---|---|
| Positiveb | Equivocalc | Negatived | ||
| Serum | Positive | 89 (100) | 4 | 7 |
| Negative | 0 | 2 | 257 (97.3) | |
| Whole blood | Positive | 19 (86.4) | 0 | 0 |
| (venous or capillary) | Negative | 3 | 2 | 76 (100) |
Data depict the correlation of results of the LFIA with serum samples (n = 359) sequentially submitted for HSV testing and volunteer donor whole-blood samples (n = 100) with results by the HerpeSelect HSV-2 ELISA for paired-donor or sequentially submitted serum samples.
Index, ≥1.1.
Index, 0.9 to 1.09 (equivocal results were not included in the calculations).
Index, <0.9.
FIG. 3.
ROCs of the HSV-2 LFIA performed with serum. ROCs were generated with MedCalc, version 9.3.0.0 (Mariakerke, Belgium), using the HerpeSelect HSV-2 ELISA as the reference method. Visible test lines were scored as positive in the LFIA. The sensitivity of the test was 100%, and the specificity was 97.3%. The AUC was 0.999 (P = 0.0001). Dashed lines indicate the 95% confidence bounds of the curve.
Performance of the LFIA with whole blood.
To evaluate the devices’ performance with fingerstick samples, a total of 100 capillary whole-blood samples were collected along with paired serum, EDTA-whole-blood samples, and heparin-whole-blood samples. Paired serum samples were tested by the HerpeSelect HSV-2 ELISA and HerpeSelect HSV IB. Serum and whole-blood samples were tested by the HSV-2 LFIA. Table 1 shows the results of this study. When whole blood (venous or capillary) was used as a sample, both the HerpeSelect HSV-2 ELISA and HSV-2 LFIA were positive in 19 cases and negative in 76 cases. Two paired serum samples were equivocal in the HerpeSelect HSV-2 ELISA; the matched whole-blood samples both were negative on the HSV-2 LFIA. Three samples were positive by ELISA with serum and negative by LFIA with whole blood. These discordant results were resolved by HSV-2 inhibition ELISA testing; all three samples were confirmed as positive on the HerpeSelect HSV-2 ELISA and negative on the HSV-2 LFIA. Therefore, the sensitivity of the LFIA with whole blood compared to that of the HSV-2 ELISA was 86.4%, and the specificity was 100%.
For an analysis of HSV-2 LFIA data using HSV IB as a reference method, paired serum results by HSV IB were compared to LFIA results with whole blood. The results were similar to those observed with the HerpeSelect HSV-2 ELISA: both tests were positive for 19 samples and negative for 78 samples (not shown). Three samples were discordant before resolution with HSV-2 ELISA testing; of these, one was confirmed positive by HSV-2 inhibition ELISA, and two were negative by HSV-2 ELISA. Therefore, the sensitivity of the HSV-2 LFIA compared to that of the HSV IB is revised to 95% (19/20), while the specificity remains at 100% (80/80). Concordance among the four sample types tested (heparinized whole blood, EDTA-treated whole blood, serum, and capillary fingerstick whole blood) was 99% (99/100). For capillary whole blood and both types of venous whole blood, the concordance of results in the LFIA was 100%. Serum showed a slightly higher sensitivity than whole blood (91 and 86.3%, respectively) to the presence of HSV-2 IgG antibody on the LFIA. This may account for the slightly lower specificity seen with serum (97.3%) compared to that seen with capillary whole blood (100%).
Cross-reactivity and interference.
Table 2 summarizes the results of the antibody reactivity of CMV (n = 11), VZV (n = 14), rubella virus (n = 34), EBV (n = 16), and HSV-1 (n = 196) IgG-positive serum samples tested in the HSV-2 LFIA. The level of cross-reactivity observed was low; only 7 out of 269 serum samples (2.6%) tested positive in the HSV-2 LFIA: one EBV IgG sample, one rubella virus IgG sample, and five HSV-1 IgG serum samples. The HSV-2-negative and the HSV-2-positive serum samples that were spiked with triglycerides, albumin, bilirubin, and hemoglobin remained unchanged in interpretation in the LFIA (data not shown).
TABLE 2.
Cross-reactivity of 269 IgG-positive serum samples tested in the HSV-2 LFIAa
| Virus | No. of sera tested | No. (%) of sera LFIA positive |
|---|---|---|
| CMV | 11 | 0 (0.0) |
| EBV | 15 | 1 (6.7) |
| Rubella virus | 33 | 1 (3.0) |
| VZV | 14 | 0 (0.0) |
| HSV-1 | 196 | 5 (2.8) |
| Total | 269 | 7 (2.6) |
The 269 samples were IgG positive for CMV, VZV, EBV, rubella virus, or HSV-1 but negative for HSV-2 serum antibodies as defined by the HerpeSelect HSV-2-specific ELISA.
DISCUSSION
Many cases of genital herpes are transmitted by persons who are unaware that they are infected or do not recognize the symptoms (4, 24). Type-specific, serological diagnosis of HSV infection is an important method used in the counseling, treatment, and prevention of genital herpes (4). Additionally, infection with HSV-2 has been associated with an increased risk for HIV infections (7, 8). The suppression of HSV reactivation by acyclovir treatment has been correlated with a reduction in HIV-1 RNA (19). Therefore, counseling and treatment for HSV-2 are important parts of HIV-1 management for coinfected individuals as well as for HSV-2 management and prevention efforts (19).
In recent years, the use of recombinant gG-2 proteins for the detection of HSV-2-specific serum antibodies has increased through the availability of commercial gG-based products. The availability of these HSV-2-specific tests offers the opportunity for clinical and peripheral diagnostic laboratories to confirm a clinical HSV-2 infection. However, the majority of the assays are relatively time-consuming and do not provide an immediate result to the patient. Here, we describe the development of an LFIA for the detection of HSV-2-specific antibodies in serum and whole blood. Such a rapid test has the potential to be used outside the routine laboratory and in less sophisticated clinical facilities. This study demonstrates that the newly developed HSV-2 LFIA is suitable for the determination of IgG antibodies to HSV-2 and can be used with serum, venous whole blood, and capillary fingerstick whole blood. A comparison of the results obtained with the HSV-2 LFIA and the HerpeSelect HSV-2-specific ELISA using serum samples demonstrated a high degree of concordance. Discrepancies between the HSV-2 LFIA and the HSV-2 ELISA may be due to the use of native gG-2 antigen in the LFIA versus recombinant gG-2 in the ELISA. In addition, the LFIA is run with undiluted serum and the HSV-2 ELISA is run at a serum dilution of 1:100. Therefore, the LFIA may be slightly more sensitive to the presence of low levels of IgG or lower-avidity IgG in serum. This slightly increased sensitivity to the presence of IgG antibody to HSV-2 is not seen when the test is used with either venous or capillary whole-blood samples. The performance of this device with both serum and whole blood gives diagnostic sensitivity and specificity that are comparable to those of existing FDA-cleared type-specific HSV-2 ELISA and HSV-1 and -2 IB methods. Therefore, this test can be used for the rapid serodiagnosis of HSV-2 infections as well as the laboratory confirmation of serum samples that tested HSV-2 positive by different HSV-2-specific methods, such as Western blotting, ELISA, and IB.
The first FDA-cleared HSV-2 rapid test (POCkit) for whole blood and serum was described in 1999 (3). This product is classified as moderately complex by the Clinical Laboratory Improvement Amendments. The performance of the HSV-2 LFIA reported here shows equivalent performance in both sensitivity and specificity compared to those of the POCkit test (5). In addition, this test is simpler to use than the POCkit test (i.e., the POCkit test has 10 steps, whereas the HSV-2 LFIA has 2), gives a result to the patient in 15 min, and can be used in a non-laboratory environment. Its use would be a valuable addition to the spectrum of tests available for the determination of HSV-2 serostatus and has the potential to allow for more expeditious counseling and treatment of patients.
Acknowledgments
We thank J. Gonzalez for his assistance in the laboratory and K. Laderman and H. Prince for critical reading of the manuscript.
Footnotes
Published ahead of print on 14 November 2007.
REFERENCES
- 1.Arvin, A., and C. Prober. 1995. Herpes simplex viruses, p. 876-883. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 6th ed. ASM Press, Washington, DC.
- 2.Ashley, R. L. 2001. Sorting out the new HSV type specific antibody tests. Sex. Transm. Infect. 77:232-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashley, R. L., M. Eagleton, and N. Pfeiffer. 1999. Ability of a rapid serology test to detect seroconversion to herpes simplex virus type 2 glycoprotein G soon after infection. J. Clin. Microbiol. 37:1632-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashley, R. L., and A. Wald. 1999. Genital herpes: review of the epidemic and potential use of type-specific serology. Clin. Microbiol. Rev. 12:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashley, R. L., A. Wald, and M. Eagleton. 2000. Premarket evaluation of the POCkit HSV-2 type-specific serologic test in culture-documented cases of genital herpes simplex virus type 2. Sex. Transm. Dis. 27:266-269. [DOI] [PubMed] [Google Scholar]
- 6.Aurelian, L. 1992. Herpes simplex viruses, p. 473-497. In S. Specter and G. Lancz (ed.), Clinical virology manual, 2nd ed. Elsevier, New York, NY.
- 7.Bünzli, D., V. Wietlisbach, F. Barazzoni, R. Sahli, and P. R. Meylan. 2004. Seroepidemiology of herpes simplex virus type 1 and 2 in Western and Southern Switzerland in adults aged 25-74 in 1992-93: a population-based study. BMC Infect. Dis. 4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. 2002. Sexually transmitted diseases treatment guidelines. Morb. Mortal. Wkly. Rep. 51:1-80. [Google Scholar]
- 9.Cohen, M. H., Y. Olszewski, M. Robey, and F. Love. 2003. Rapid point-of-care testing for HIV-1 during labor and delivery. Morb. Mortal. Wkly. Rep. 52:866-868. [PubMed] [Google Scholar]
- 10.Craig, R. M., J. R. Pfister, and S. J. Spear. 2003. Increasing proportion of herpes simplex virus type 1 as a cause of genital herpes infection in college students. Sex. Transm. Dis. 30:797-800. [DOI] [PubMed] [Google Scholar]
- 11.Engelberg, R., D. Carrell, E. Krantz, L. Corey, and A. Wald. 2003. Natural history of genital herpes simplex virus type 1 infection. Sex. Transm. Dis. 30:174-177. [DOI] [PubMed] [Google Scholar]
- 12.Farnham, P. G., R. D. Gorsky, D. R. Holtgrave, W. K. Jones, and M. E. Guinan. 1996. Counseling and testing for HIV prevention: costs, effects, and cost-effectiveness of more rapid screening tests. Public Health Rep. 111: 44-53. [PMC free article] [PubMed] [Google Scholar]
- 13.Haddow, L. J., B. Dave, A. Mindel, K. A. McPhie, C. Chung, C. Marks, and D. E. Dwyer. 2006. Increase in rates of herpes simplex virus type 1 as a cause of anogenital herpes in western Sydney, Australia, between 1979 and 2003. Sex. Transm. Infect. 82:255-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hogrefe, W., X. Su, J. Song, R. Ashley, and L. J. Kong. 2002. Detection of herpes simplex virus type 2-specific immunoglobulin G antibodies in African sera by using recombinant gG2, Western blotting, and gG2 inhibition. J. Clin. Microbiol. 40:3635-3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malkin, J. E. 2004. Epidemiology of genital herpes simplex virus infection in developed countries. Herpes 11(Suppl. 1):2A. [PubMed] [Google Scholar]
- 16.McGee, S. 2002. Simplifying likelihood ratios. J. Gen. Int. Med. 17:646-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morrow, R. A., and D. Friedrich. 2003. Inaccuracy of certain commercial enzyme immunoassays in diagnosing genital infections with herpes simplex virus types 1 or 2. Am. J. Clin. Pathol. 120:839-844. [DOI] [PubMed] [Google Scholar]
- 18.Prince, H. E., C. E. Ernst, and W. R. Hogrefe. 2000. Evaluation of an enzyme immunoassay system for measuring herpes simplex virus (HSV) type 1-specific and HSV type 2-specific IgG antibodies. J. Clin. Lab. Anal. 14:13-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schacker, T., J. Zeh, H. Hu, M. Shaughnessy, and L. Corey. 2002. Changes in plasma human immunodeficiency virus type 1 RNA associated with herpes simplex virus reactivation and suppression. J. Infect. Dis. 186:1718-1725. [DOI] [PubMed] [Google Scholar]
- 20.Slomka, M. J. 1996. Seroepidemiology and control of genital herpes: the value of type specific antibodies to herpes simplex virus. Commun. Dis. Rep. CDR Rev. 6:R41-R45. [PubMed] [Google Scholar]
- 21.Strick, L. B., and A. Wald. 2006. Diagnostics for herpes simplex virus: is PCR the new gold standard? Mol. Diagn. Ther. 10:17-28. [DOI] [PubMed] [Google Scholar]
- 22.Tran, T., J. D. Druce, M. C. Catton, H. Kelly, and C. J. Birch. 2004. Changing epidemiology of genital herpes simplex virus infection in Melbourne, Australia, between 1980 and 2003. Sex. Transm. Infect. 80:277-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wald, A., and R. Ashley-Morrow. 2002. Serological testing for herpes simplex virus (HSV)-1 and HSV-2 infection. Clin. Infect. Dis. 35(Suppl. 2):S173. [DOI] [PubMed] [Google Scholar]
- 24.Wald, A., J. Zeh, S. Selke, T. Warren, R. Ashley, and L. Corey. 2002. Genital shedding of herpes simplex virus among men. J. Infect. Dis. 186(Suppl. 1):S34. [DOI] [PubMed] [Google Scholar]