Abstract
Plasmacytoid dendritic cells (pDCs), one of two types of bone marrow (BM)-derived blood DCs, play an important role in linking innate and adaptive immune responses. However, little is known about the nature of pDCs that reside in the BM. Because the simian immunodeficiency virus-macaque model closely mimics human immunodeficiency virus disease in humans, with both infections inducing a decrease in pDCs, we characterized and compared pDCs in the BM with those in peripheral blood (PB) of healthy pig-tailed macaques. The results revealed that pDCs from both compartments had the same CD123++ HLA-DR+ Lin− phenotype and were similar in size. Although BM-derived pDCs (BM-pDCs) were 3-fold greater in frequency and 10-fold greater in number, they had lower cell surface expression of both HLA-DR and the costimulatory molecule CD86 than did PB-pDCs. Both BM- and PB-pDCs responded ex vivo to synthetic CpG oligodeoxynucleotides and inactivated influenza virus by upregulating HLA-DR and CD86 and secreting cytokines; however, stimulated BM-pDCs secreted less alpha interferon and tumor necrosis factor alpha per cell than did PB-pDCs. These results suggest that while BM-pDCs appear to be phenotypically less mature than PB-pDCs, they do respond to pathogens. Thus, during acute infections, these cells could initiate immune responses either in the BM or after rapidly migrating from the BM into the periphery. A better characterization of pDCs in blood and tissues will be beneficial for future studies of macaques that focus on either pathogenesis or vaccine development.
Control of invading pathogens in vertebrates is dependent on the two major arms of the immune system: nonspecific innate immunity and antigen (Ag)-specific adaptive immunity. Components of the innate immune system mobilized during the acute phase of microbial infections include complement, granulocytes, natural killer (NK) cells, alpha/beta interferon (IFN-α/β), and other proinflammatory cytokines. In contrast, mediators of adaptive responses, primarily T and B lymphocytes, specifically recognize an unlimited number of Ags by diversification of T-cell receptors and immunoglobulin genes. The principal links between these two protective components are cytokines and Ag-presenting cells (APCs), of which dendritic cells (DCs) are the most critical. In humans, blood DCs characteristically express high levels of major histocompatibility complex (MHC) class II molecules; lack common lineage markers (Lin−) such as CD3 (T cells), CD14 (monocytes), CD56 and/or CD16 (NK cells), and CD20 (B cells); and are subdivided into CD11c+ myeloid DCs (mDCs) and CD123+ plasmacytoid DCs (pDCs). Although pDCs are generally less efficient as APCs than mDCs, pDC-mediated Ag presentation can be augmented in vitro by the addition to cultured cells of interleukin-3 (IL-3) and tumor necrosis factor alpha (TNF-α) (27). Interestingly, activated pDCs secrete more IFN-α per cell than any other cell type in the body and therefore have been designated natural IFN-producing cells (44).
Both mDCs and pDCs are activated and secrete cytokines after the recognition of diverse configurations that are characteristic of microbes; these motifs are known as pathogen-associated molecular patterns and include, among others, lipopolysaccharide, lipoproteins, peptidoglycan, and unmethylated CpG dinucleotides. Pathogen-associated molecular pattern recognition is a property of three families of receptors: Toll-like receptors (TLRs), NOD-like receptors, and RIG-I-like receptors, all of which overlap functionally, with TLRs having the broadest reactivity (12). TLRs are differentially expressed by human mDCs and pDCs; mDCs express primarily TLR-1 through TLR-6, while pDCs express TLR-7, TLR-9, and TLR-10 (26). TLR-7 and TLR-9 recognize and bind to single-stranded viral RNA and hypomethylated CpG motifs in viral or bacterial DNA, respectively, resulting in the activation of pathways that induce cellular maturation (2, 31, 32). Synthetic oligodeoxynucleotides containing one or more unmethylated CpG motifs (CpG ODNs), which are immunostimulatory in primates, are generally classified into one of three categories: CpG-A ODNs, which induce activation and maturation of pDCs; CpG-B ODNs, which potently activate B cells, which also express TLR-9; and CpG-C ODNs, which stimulate both pDCs and B cells (34). Following TLR ligation, pDCs not only produce and secrete high levels of IFN-α, TNF-α, and, to a lesser extent, IL-6 and -12 but also upregulate the expression of MHC class II proteins and the cell surface-costimulatory molecules CD80 and CD86 (2, 31, 32, 36, 49). IFN-α and TNF-α secreted by pDCs enhance NK cell-mediated killing of susceptible target cells and NK cell secretion of IFN-γ that synergizes with IL-12 to polarize CD4+ T cells for a Th1 response (23, 42); stimulated pDCs can also augment virus-specific memory T-cell activation (18, 22, 28, 49).
pDCs, which are commonly described as being sentinels in peripheral blood (PB), have been identified in various tissues including tonsils, thymus, lungs, spleen, lymph nodes, Peyer's patches, and colon (4, 6, 8, 35, 37, 45, 51). During microbial infections, pDCs can accumulate in inflamed tissues such as the nasal mucosae during acute respiratory viral infections and the central nervous system during bacterial meningitis (24, 25, 39, 53). In mice, pDCs can also infiltrate the vaginal mucosa, where they limit virus replication during acute infection such as herpes simplex virus infection (33). Furthermore, cells with a phenotype identical to that of pDCs have been identified in both murine and human bone marrow (BM) (5, 13, 15, 30, 40, 46). Although BM is considered to be a primary lymphoid organ because it is a major site of hematopoiesis, it can harbor bacterial, parasitic, and viral pathogens, including simian immunodeficiency virus (SIV) and human immunodeficiency virus (HIV), thus making it a site for immunosurveillance (14, 29, 43, 52). For example, memory and regulatory T cells migrate to and from the BM, where they proliferate extensively, suggesting that the BM serves as a central organ for long-term memory responses (3, 16, 54), and in mice, resident BM-DCs present Ag to naïve T cells (21).
Because pDCs are an integral component of the innate immune system, the observation that the numbers of circulating pDCs are reduced in many viral infections, including primary and chronic HIV-1 infections, is of concern (17). Decreased numbers or impaired functions of pDCs could facilitate the establishment of opportunistic infections. We and others recently showed a similar loss of pDCs in blood and tissues of SIV-infected macaques, indicating that the SIV macaque model is appropriate for use in studying pDC-lentivirus interactions (7, 41). Since both cell-free virus and HIV- and SIV-infected cells, as well as pDCs, are found in the BM (29, 43), the purpose of our investigation was to characterize and compare the phenotypes and functions of BM- and PB-derived pDCs (PB-pDCs) in healthy macaques. SIV infection of macaques is the most reliable model to study immune responses to and the pathogenesis of HIV; therefore, a better understanding of the basic functional properties of macaque pDCs will provide a foundation for exploiting these cells in the design and development of novel vaccines with enhanced immunogenicity and for evaluating the role of pDCs in lentivirus infections.
MATERIALS AND METHODS
Animals, tissue collection, and processing.
Adult pig-tailed macaques (Macaca nemestrina) of either sex were used in this study. Before collecting blood or BM samples, macaques were anesthetized with an intramuscular injection of ketamine HCl (10 mg/kg) and weighed; their physical conditions were also evaluated. Macaques were housed at the University of Alabama at Birmingham in biosafety level 2 facilities in accordance with institutional and Animal Welfare Act guidelines.
Peripheral blood mononuclear cells (PBMC) were isolated from heparinized whole blood by density gradient centrifugation through lymphocyte separation medium (ICN Biomedicals, Inc.). After shaving and cleaning the skin above the proximal humerus of each animal, BM aspirates were obtained with 15-gauge aspiration needles (Medical Device Technologies, Gainesville, FL) that had been flushed with heparin to prevent clotting. Fat layers (yellow BM) were removed from BM in EDTA-treated tubes, followed by isolation of BM mononuclear cells (BMMC) from red BM by density centrifugation through lymphocyte separation medium. Contaminating red blood cells were lysed hypotonically using an ammonium chloride solution. BMMC were washed and initially resuspended in RPMI 1640 supplemented with 10% fetal bovine serum for subsequent assays.
Flow cytometry.
For cell surface staining to determine percentages and to characterize macaque pDCs in EDTA-treated whole blood or in PBMC and BMMC, three- and four-color flow cytometry was used. Mononuclear cells were first gated based on forward- and side-scatter characteristics, and CD123++ HLA-DR+ Lin− pDCs were then identified using phycoerythrin-labeled anti-CD123 (7G3), peridinin chlorophyll protein-cyanin 5.5-labeled anti-HLA-DR (G46-6), and a cocktail of fluorescein isothiocyanate-conjugated antibodies to the Lin markers CD3ɛ (SP34), CD14 (MφP9), CD16 (3G8), and CD20 (2H7). Expression of CD86 was evaluated using an allophycocyanin-conjugated antibody (2331). To calculate percentages of positive cells, background fluorescence was determined with isotype-matched control antibodies conjugated to each fluorochrome. All antibodies and reagents for fluorescence-activated cell sorter analysis were purchased from BD Biosciences Pharmingen (San Diego, CA); acquisitions were performed using a BD LSRII flow cytometer (BD Biosciences).
Mononuclear cell cultures and stimulation assays.
Approximately 1.0 × 106 PBMC or BMMC were cultured in 24-well plates in an enriched medium (RPMI 1640 supplemented with 10% fetal bovine serum, 10% normal macaque serum, and 20 ng of IL-3/ml) and stimulated with either 10 μg/ml CpG-C274 ODN (Dynavax Technologies, Berkeley, CA) or 1 μg/ml formalin-inactivated influenza virus (A/Puerto Rico/8/34; Novavax, Rockville, MD); cells in medium only served as controls. After 18 h at 37°C, culture supernatants were removed; the cells were then washed and analyzed for CD86 expression on CD123++ HLA-DR+ Lin− cells by flow cytometry. Concentrations of IFN-α and TNF-α in culture supernatants were determined using cross-reactive human IFN-α and rhesus monkey TNF-α enzyme-linked immunosorbent assay kits (both from Biosource, Camarillo, CA) according to the manufacturer's protocols; the lower limits of detection were 10 and 2 pg/ml for IFN-α and TNF-α, respectively.
Detection of intracellular cytokines.
Using the same culture conditions as those described above, mononuclear cells were stimulated for 4 h with CpG-C274 ODN or inactivated influenza virus; GolgiPlug (1 μl/ml) (BD Biosciences) was added, and the cells were cultured for an additional 2 h before being harvested, washed, and stained for the extracellular expression of CD123, HLA-DR, and Lin markers. To detect intracellular cytokines in pDCs, a Cytofix/CytoPerm kit (BD Biosciences) was used, according to the manufacturer's protocol. For detection of IFN-α, polyclonal antibodies reactive to multiple isoforms were biotinylated with EZ-Link (Pierce, Rockford, IL) and then used with a streptavidin-allophycocyanin conjugate to stain mononuclear cells. Nonbiotinylated forms of the same polyclonal antibodies served as controls. Intracellular TNF-α was detected using a cross-reactive antibody (MAb11) directly conjugated to allophycocyanin (BD Biosciences). The percentages of cytokine-positive pDCs were determined as the fraction of CD123++ HLA-DR+ Lin− cells in cultured mononuclear cell populations.
Statistical analyses.
To compare results and to determine statistical significance, the Welch alternate t test or the Mann-Whitney U test was used. Spearman's correlation test was used to determine whether changes in two parameters were linked. All analyses were performed with InStat 2.0 software (GraphPad, San Diego, CA).
RESULTS AND DISCUSSION
Phenotype and frequency of macaque pDCs in blood and BM.
Using flow cytometry, PB-pDCs were found in an expanded lymphocyte/monocyte gate (Fig. 1A, R1) and were phenotypically CD123++ HLA-DR+ Lin− (Fig. 1A, R2 and R3), as are human PB-pDCs. Since there are DC precursors and resident DCs in the BM, we determined whether cells with a phenotype similar to that of PB-pDCs could be identified in macaque BM. BM aspirates were collected from six adult pig-tailed macaques; these aspirates contained a median of 2.9 × 107 mononuclear cells per ml (range, 4.7 × 106 to 8.0 × 107 mononuclear cells per ml). A homogeneous CD123++ HLA-DR+ Lin− cell population was identified among BMMCs using the same gating strategy as that used for PB-pDCs (Fig. 1B, R2 and R3). However, both the frequencies and absolute numbers of BM-pDCs were significantly greater than those of PB-pDCs (Table 1). This result was not unexpected, since Szabolcs et al. (46) identified pDCs in human BM and found them to be fivefold greater in number than those in blood. In addition, we found no correlations between the frequencies or the absolute numbers of macaque pDCs in the BM with those in PB (data not shown). Furthermore, while pDCs from both sites were similar in size, BM-pDCs consistently had lower levels HLA-DR cell surface expression, measured as mean fluorescence intensity (MFI), than PB-pDCs (Fig. 1 and Table 1), suggesting that BM-pDCs were less mature than PB-pDCs (19).
FIG. 1.
Phenotypic identification of pDCs in pig-tailed macaque PB (A) and BM (B). Mononuclear cells were gated based on forward-scatter (FSC) and side-scatter (SSC) characteristics (R1) and then as Lin− (R2) and CD123++ HLA-DR+ (R3). Back-gating of the R3 population showed that BM- and PB-pDCs were similar in size (forward scatter) and granularity (side scatter) (rightmost panels). The data are representative of three-color flow cytometric analyses of PB and BM from all animals.
TABLE 1.
Quantification and characterization of macaque BM- and PB-pDCsa
| pDC type | % Frequency among mononuclear cells (range)b | Absolute no. of pDCs per ml (range)c | HLA-DR MFI (range)d | Relative size (range)e | No. of animals |
|---|---|---|---|---|---|
| PB | 0.245 (0.147-0.34) | 10,140 (4,858-14,161) | 655 (104-1230) | 526 (445-676) | 10f |
| BM | 0.764 (0.301-1.19) | 101,495 (20,088-272,000) | 229 (81.5-480) | 514 (320-785) | 6 |
Statistical differences between BM- and PB-pDCs were calculated using the Mann-Whitney U test; P values of <0.05 were assumed to be significant. The P value for frequency among MCs between BM- and PB-pDCs was <0.001, that for the absolute number per milliliter was <0.01, that for HLA-DR MFI was 0.09, and that for relative size was 0.64.
Frequency of CD123++ HLA-DR+ Lin− pDCs among total BMMC or PBMC (based on forward- versus side-scatter characteristics) as determined by flow cytometry (Fig. 1).
Absolute numbers (medians) of BM-pDCs were calculated by multiplying the percentages of CD123+ HLA-DR+ Lin− pDCs by the number of BMMCs per ml of BM collected and of PB-pDCs by the total number of lymphocytes and monocytes per ml of blood calculated from complete blood counts and differentials.
Median MFI of HLA-DR expression on BM- and PB-pDCs.
Median forward-scatter measurements of BM- and PB-pDCs (Fig. 1, rightmost panels).
All parameters were evaluated for PB-pDCs from 10 animals, except for the absolute numbers, which reflect data for 6 animals.
Activation of pDCs.
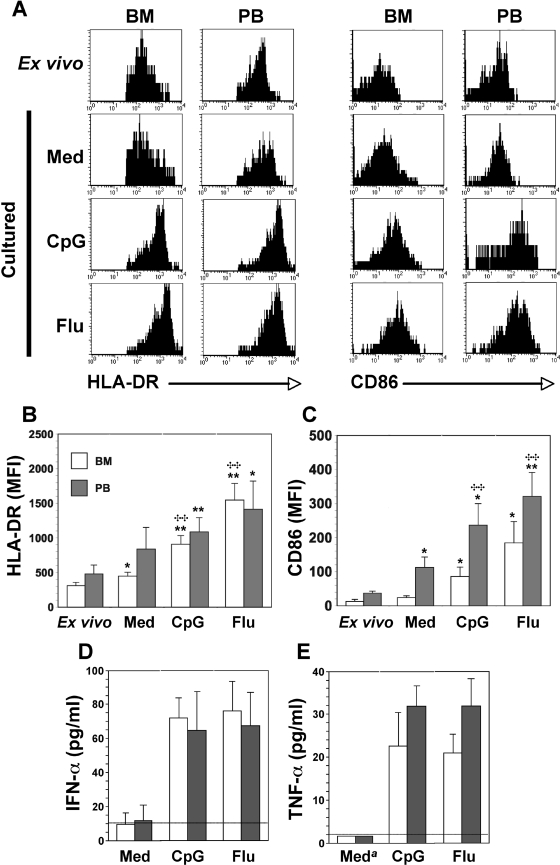
The low frequencies of macaque pDCs in peripheral blood and tissues and the lack of sufficient cross-reactivity of human pDC-specific antibodies such as BDCA-2 and BDCA-4 (our unpublished data) that are used for isolation of these cells preclude the thorough characterization of purified macaque pDCs (1, 9, 11). Thus, we analyzed BM-pDCs and PB-pDCs in mononuclear cells (BMMC and PBMC) ex vivo either directly or after 18 h of culture with traditional pDC stimuli. As described above, in freshly isolated mononuclear cells, HLA-DR expression (MFI) was generally lower on BM-pDCs than on PB-pDCs and was upregulated minimally on both cell types after 18 h of culture in enriched medium (Fig. 2A and B). Furthermore, upon stimulation with CpG-C274 ODN or inactivated influenza virus, HLA-DR expression on BM-pDCs increased three- to fivefold above MFI levels detected ex vivo. While the mean MFI of HLA-DR on the surface of PB-pDCs increased to similar levels in response to CpG-C274 ODN or influenza virus, the increase was lower because of the inherently higher MFI before stimulation (Fig. 2A and B). The level of the activation/costimulatory molecule CD86 was also significantly (P = 0.01) lower on BM-pDCs ex vivo than on their peripheral blood counterparts (mean MFIs, 13.2 and 37.4, respectively) (Fig. 2A and C). After cultures were grown overnight in medium without one of the TLR agonists, CD86 was upregulated on pDCs from both sources, but the increase in MFIs on PB-pDCs was greater than that on BM-pDCs, as was reported previously for macaque pDCs cultured similarly (47). In response to CpG-C274 or influenza virus, mean MFIs associated with CD86 expression on BM-pDCs increased 6- and 14-fold, respectively, above those of uncultured cells, whereas mean increases in CD86 MFIs for PB-pDCs were six- and ninefold, respectively (Fig. 2A and C). The upregulation of HLA-DR and CD86 in response to CpG-C274 and an RNA virus, such as influenza virus, is consistent with data from previous reports for macaque and human PB-pDCs (2, 22, 48, 49); this result also suggests that BM-pDCs, like PB-pDCs, express TLR-7 and TLR-9, the cognate receptors for single-stranded viral RNA and bacterial DNA with hypomethylated CpG motifs. It is interesting, however, that CD86 levels were consistently lower on BM-pDCs than on PB-pDCs in identically treated cultures. Our experiments monitored pDCs after only 18 h of stimulation; therefore, it is possible that longer incubation times would overcome this difference. Also, the increases in MFIs of HLA-DR and CD86 (for all cultures) were directly correlated (R = 0.979 [P < 0.001] for BM-pDCs; R = 0.916 [P < 0.001] for PB-pDCs) (data not shown). This result was not unexpected since in murine cells, MHC class II and costimulatory molecules cluster together intracellularly, and the activation of APCs induces the coaggregation of MHC class II and costimulatory molecules on the cell surface, thereby enhancing Ag presentation to T cells (10). Although pDCs are generally poor stimulators of naïve T cells, the upregulation of both surface Ags might make pDCs more effective APCs. Feuerer et al. (21) previously showed that murine CD11c+ BM-DCs form clusters with CD3+ T cells in situ and process and present Ags by both MHC class I and class II pathways to prime local T cells; activated macaque BM-pDCs might behave in a similar manner.
FIG. 2.
Activation of BM- and PB-pDCs in response to CpG-C274 (CpG) or influenza virus (Flu). BMMC and PBMC were cultured for 18 h in medium (Med) or in the presence of CpG-C274 or formalin-inactivated influenza virus. (A) Representative histograms of BM- and PB-pDCs (Fig. 1, R3 gate) before and after culture with the indicated stimuli are shown. Before culture, immediately after isolation (ex vivo), and then after culture, cell aliquots were stained with anti-Lin, anti-HLA-DR, anti-CD123, and anti-CD86 antibodies before flow cytometric analysis. HLA-DR (B) and CD86 (C) expression levels were evaluated as mean MFI plus 1 standard deviation using results from three experiments, each of which was done with cells from a different macaque. Significant differences between values for ex vivo cultures and stimulated cultures are indicated by asterisks. *, P < 0.05; **, P < 0.02; ✣✣, significant differences between values for “Med” cultures and stimulated cultures (P < 0.02) (Welch alternate t test). Induction and secretion of IFN-α (D) and TNF-α (E) in cell-free supernatants after 18 h of culture were assessed by comparing the mean concentration (pg/ml) plus 1 standard deviation of results from three experiments. The lower limits of detection for IFN-α and TNF-α were 10 and 2 pg/ml, respectively. a, undetectable in all experiments.
One consequence of pDC activation is the production of IFN-α and TNF-α. After macaque BMMC and PBMC were cultured with CpG-C274 or influenza virus for 18 h, the concentrations of IFN-α and TNF-α in the medium were quantified. In control cultures, the amounts of these two cytokines were generally at or below the limits of detection (Fig. 2D and E). In contrast, when CpG-C274 or influenza virus was added at the time that the BMMC and PBMC cultures were established, the secretion of both cytokines was upregulated, and regardless of the stimulus, there was no statistically significant difference in the amount of either IFN-α or TNF-α that accumulated in the medium. Encabo et al. (20) recently reported that DCs from umbilical cord blood were phenotypically less mature and secreted less TNF-α than PB-DCs. This difference might be true for pDCs in the BM, consistent with the more immature phenotype of these cells. Since IFN-α and TNF-α augment NK cell killing and IFN-γ secretion (23, 34), it appears that both BM- and PB-pDCs can support innate effector functions ex vivo and therefore might behave similarly in vivo.
Frequency of cytokine-producing cells.
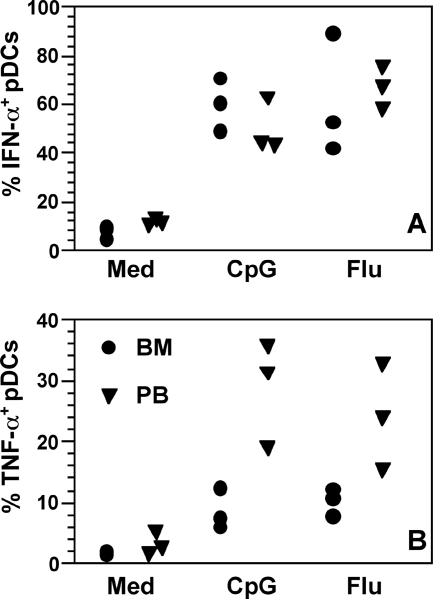
As shown in Table 1, the frequency of pDCs in BMMC is approximately threefold greater than that found in blood; however, comparable amounts of IFN-α and TNF-α accumulated in stimulated BMMC and PBMC cultures (Fig. 2D and E). These results suggest that either fewer BM-pDCs produce cytokines in response to stimuli or they produce lower amounts per cell. To define differences in cytokine secretion by BM- and PB-pDCs more accurately, BMMC and PBMC were stimulated with CpG-C274 or influenza virus for 6 h, and pDCs were then analyzed for intracellular IFN-α and TNF-α by multicolor flow cytometry. The frequencies of activated IFN-α-positive (IFN-α+) BM- and PB-pDCs were consistent with data from previous reports showing that not all human and macaque pDCs secrete IFN-α in response to CpG ODNs or RNA viruses (Fig. 3A) (1, 9). Low frequencies (up to 12%) of IFN-α+ pDCs were detected in unstimulated control cultures, indicating that either pDCs spontaneously produced IFN-α upon culture, pDCs may have been nonspecifically stimulated during mononuclear cell isolation, or pDCs were activated in vivo, perhaps by an infectious agent. Of interest, the frequencies of TNF-α+ pDCs appeared to be lower in BMMC than in PBMC, but this difference was not statistically significant (Fig. 3B). This observation is consistent with the lower concentrations of TNF-α that we found in activated BMMC culture supernatants (Fig. 2E). The frequencies of TNF-α+ pDCs in both BMMC and PBMC were lower than those of IFN-α+ pDCs, which is not unexpected, since pDCs are characterized by their ability to produce large amounts of IFN-α. In addition, our results agree with those that were obtained using macaque cells as described previously (9). However, the reason for comparable levels of IFN-α accumulating in culture supernatants, despite the fact that BMMC cultures contained far greater numbers of pDCs than PBMC cultures, is still unclear. Our intracellular cytokine analyses showed similar frequencies of IFN-α-secreting cells in both cultures, making it unlikely that differences in the relative numbers of IFN-α-secreting cells account for the disparity. Another possibility is that BM-pDCs secrete less IFN-α per cell than do PB-pDCs. Since the cellular and cytokine milieus of BMMC and PBMC are undoubtedly different, it is possible that other cells in the cultures are rapidly taking up IFN-α produced de novo, or some unknown regulatory pressures are being exerted on pDCs in BMMC that are less pronounced in PBMC.
FIG. 3.
Frequencies of pDCs in macaque BMMC and PBMC that produced IFN-α (A) and TNF-α (B) after BMMC and PBMC were cultured in medium (Med) only or with CpG-C274 ODN (CpG) or formalin-inactivated influenza virus (Flu). Four-color flow cytometry was first used to identify pDCs, which were then gated to determine the percentages of these cells expressing intracellular cytokines. Symbols represent the results of three experiments, each using cells from a different animal.
If the BM-pDC population that we identified is a more immature form of pDC, it might be equivalent to the CD123+ CD45RA+ “Pro-pDC” subset in primary lymphoid tissues described previously by Blom et al. (5), a population that also secreted IFN-α in response to viral stimulation. This could imply that BM-pDCs are poised for rapid migration out of the BM into blood and tissues during acute infections. Pelayo et al. (40) also described a distinct and homogeneous pDC population in BM of BALB/c mice that were functionally responsive to CpG ODNs. Although they did not directly compare this subset to PB-pDCs, those authors suggested that murine BM-pDCs are highly differentiated and may be resident DCs.
Regardless of whether macaque BM-pDCs are precursors of PB-pDCs or are resident DCs, it would be interesting to determine if a decrease in BM-pDCs occurs in HIV-infected persons, as was shown for human PB-pDCs (17) and, more recently, was documented for pDCs in macaques (7, 41). Since multilineage hematopoiesis is impaired during SIV/HIV infection (38, 50), pDC dendropoiesis might also be affected, which could contribute to the loss of these cells in blood and tissues. The results presented herein provide a partial characterization of BM-pDCs in macaques and therefore should enhance our understanding of pDC dynamics in future studies of SIV infections of nonhuman primates.
Acknowledgments
We thank Jason Marshall and Gary Van Nest of Dynavax Technologies Corp. for the gift of CpG-C274 ODN, Jacqueline Stallworth for processing blood samples, and Marion Spell for flow cytometry.
This work was supported, in part, by the UAB Center for AIDS Research Flow Cytometry core, NIH grant P30 AI027767, and NIH grant U01 AI028147. R.K.R. was supported by an NIH Basic Mechanisms of Virology training grant, T32 AI007150-29.
Footnotes
Published ahead of print on 7 November 2007.
REFERENCES
- 1.Abel, K., Y. Wang, L. Fritts, E. Sanchez, E. Chung, P. Fitzgerald-Bocarsly, A. M. Krieg, and C. J. Miller. 2005. Deoxycytidyl-deoxyguanosine oligonucleotide classes A, B, and C induce distinct cytokine gene expression patterns in rhesus monkey peripheral blood mononuclear cells and distinct alpha interferon responses in TLR9-expressing rhesus monkey plasmacytoid dendritic cells. Clin. Diagn. Lab. Immunol. 12:606-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauer, M., V. Redecke, J. W. Elwart, B. Scherer, J. P. Kremer, H. Wagner, and G. B. Lipford. 2001. Bacterial CpG-DNA triggers activation and maturation of human CD11c−, CD123+ dendritic cells. J. Immunol. 166:5000-5007. [DOI] [PubMed] [Google Scholar]
- 3.Becker, T. C., S. M. Coley, E. J. Wherry, and R. Ahmed. 2005. Bone marrow is a preferred site for homeostatic proliferation of memory CD8 T cells. J. Immunol. 174:1269-1273. [DOI] [PubMed] [Google Scholar]
- 4.Bilsborough, J., T. C. George, A. Norment, and J. L. Viney. 2003. Mucosal CD8α+ DC, with a plasmacytoid phenotype, induce differentiation and support function of T cells with regulatory properties. Immunology 108:481-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blom, B., S. Ho, S. Antonenko, and Y.-J. Liu. 2000. Generation of interferon α-producing predendritic cell (pre-DC)2 from human CD34+ hematopoietic stem cells. J. Exp. Med. 192:1785-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bratke, K., M. Lommatzsch, P. Julius, M. Kuepper, H.-D. Kleine, W. Luttmann, and J. C. Virchow. 2007. Dendritic cell subsets in human bronchoalveolar lavage fluid after segmental allergen challenge. Thorax 62:168-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown, K. N., A. Trichel, and S. M. Barratt-Boyes. 2007. Parallel loss of myeloid and plasmacytoid dendritic cells from blood and lymphoid tissue in simian AIDS. J. Immunol. 178:6958-6967. [DOI] [PubMed] [Google Scholar]
- 8.Cella, M., D. Jarrossay, F. Facchetti, O. Alebardi, H. Nakajima, A. Lanzavecchia, and M. Colonna. 1999. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat. Med. 5:919-923. [DOI] [PubMed] [Google Scholar]
- 9.Chung, E., S. B. Amrute, K. Abel, G. Gupta, Y. Wang, C. J. Miller, and P. Fitzgerald-Bocarsly. 2005. Characterization of virus-responsive plasmacytoid dendritic cells in the rhesus macaque. Clin. Diagn. Lab. Immunol. 12:425-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clatza, A., L. C. Bonifaz, D. A. A. Vignali, and J. Moreno. 2003. CD40-induced aggregation of MHC class II and CD80 on the cell surface leads to an early enhancement in antigen presentation. J. Immunol. 171:6478-6487. [DOI] [PubMed] [Google Scholar]
- 11.Coates, P. T., S. M. Barratt-Boyes, L. Zhang, V. S. Donnenberg, P. J. O'Connell, A. J. Logar, F. J. Duncan, M. Murphey-Corb, A. D. Donnenberg, A. E. Morelli, C. R. Maliszewski, and A. W. Thomson. 2003. Dendritic cell subsets in blood and lymphoid tissue of rhesus monkeys and their mobilization with Flt3 ligand. Blood 102:2513-2521. [DOI] [PubMed] [Google Scholar]
- 12.Creagh, E. M., and L. A. J. O'Neill. 2006. TLRs, NLRs, and RLRs: a trinity of pathogen sensors that co-operate in innate immunity. Trends Immunol. 27:352-357. [DOI] [PubMed] [Google Scholar]
- 13.D'Amico, A., and L. Wu. 2003. The early progenitors of mouse dendritic cells and plasmacytoid predendritic cells are within the bone marrow hematopoietic precursors expressing Flt3. J. Exp. Med. 198:293-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Da Silva, E. S., C. M. F. Gontijo, R. da Silva Pacheco, and R. P. Brazil. 2004. Diagnosis of visceral leishmaniasis by PCR using blood samples spotted on filter paper. Genet. Mol. Res. 3:251-257. [PubMed] [Google Scholar]
- 15.Diao, J., E. Winter, W. Chen, C. Cantin, and M. S. Cattral. 2004. Characterization of distinct conventional and plasmacytoid dendritic cell-committed precursors in murine bone marrow. J. Immunol. 173:1826-1833. [DOI] [PubMed] [Google Scholar]
- 16.Di Rosa, F., and R. Pabst. 2005. The bone marrow: a nest for migratory memory T cells. Trends Immunol. 26:360-366. [DOI] [PubMed] [Google Scholar]
- 17.Donaghy, H., J. Wilkinson, and A. L. Cunningham. 2006. HIV interactions with dendritic cells: has our focus been too narrow? J. Leukoc. Biol. 80:1001-1012. [DOI] [PubMed] [Google Scholar]
- 18.Dong, L., I. Mori, M. J. Hossain, B. Liu, and Y. Kimura. 2003. An immunostimulatory oligodeoxynucleotide containing a cytidine-guanosine motif protects senescence-accelerated mice from lethal influenza virus by augmenting the T helper type 1 response. J. Gen. Virol. 84:1623-1628. [DOI] [PubMed] [Google Scholar]
- 19.Drenou, B., L. Amiot, N. Setterblad, S. Taque, V. Guilloux, D. Charron, R. Fauchet, and N. Mooney. 2005. MHC class II signaling function is regulated during maturation of plasmacytoid dendritic cells. J. Leukoc. Biol. 77:560-567. [DOI] [PubMed] [Google Scholar]
- 20.Encabo, A., P. Solves, F. Carbonell-Uberos, and M. D. Minana. 2007. The functional immaturity of dendritic cells can be relevant to increased tolerance associated with cord blood transplantation. Transfusion 47:272-279. [DOI] [PubMed] [Google Scholar]
- 21.Feuerer, M., P. Beckhove, N. Garbi, Y. Mahnke, A. Limmer, M. Hommel, G. J. Hammerling, B. Kyewski, A. Hamman, V. Umansky, and V. Schirrmacher. 2003. Bone marrow as a priming site for T-cell responses to blood-borne antigen. Nat. Med. 9:1151-1157. [DOI] [PubMed] [Google Scholar]
- 22.Fonteneau, J. F., M. Gilliet, M. Larsson, I. Dashilva, C. Munz, Y. J. Liu, and N. Bhardwaj. 2003. Activation of influenza virus-specific CD4+ and CD8+ T cells: a new role for plasmacytoid dendritic cells in adaptive immunity. Blood 101:3520-3526. [DOI] [PubMed] [Google Scholar]
- 23.Gerosa, F., A. Gobbi, P. Zorzi, S. Burg, F. Briere, G. Carra, and G. Trinchieri. 2005. The reciprocal interaction of NK cells with plasmacytoid or myeloid dendritic cells profoundly affects innate resistance functions. J. Immunol. 174:727-734. [DOI] [PubMed] [Google Scholar]
- 24.Gill, M. A., K. Palucka, T. Barton, F. Ghaffar, H. Jafri, J. Banchereau, and O. Ramilo. 2005. Mobilization of plasmacytoid and myeloid dendritic cells to mucosal sites in children with respiratory syncytial virus and other viral respiratory infections. J. Infect. Dis. 191:1105-1115. [DOI] [PubMed] [Google Scholar]
- 25.Hartmann, E., H. Graefe, A. Hopert, R. Pries, S. Rothenfusser, H. Poeck, B. Mack, S. Endres, G. Hartmann, and B. Wollenberg. 2006. Analysis of plasmacytoid and myeloid dendritic cells in nasal epithelium. Clin. Vaccine Immunol. 13:1278-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hornung, V., S. Rothenfusser, S. Britsch, A. Krug, B. Jahrsdorfer, T. Giese, S. Endres, and G. Hartmann. 2002. Quantitative expression of Toll-like receptor 1-10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol. 168:4531-4537. [DOI] [PubMed] [Google Scholar]
- 27.Kohrgruber, N., N. Halanek, M. Groger, D. Winter, K. Rappersberger, M. Schmitt-Egenolf, G. Stingl, and D. Maurer. 1999. Survival, maturation, and function of CD11c− and CD11c+ peripheral blood dendritic cells are differentially regulated by cytokines. J. Immunol. 163:3250-3259. [PubMed] [Google Scholar]
- 28.Kvale, E. O., J. Dalgaard, F. Lund-Johansen, H. Rollag, L. Farkas, K. Midtvedt, F. L. Jahsen, J. E. Brinchmann, and J. Olweus. 2006. CD11c+ dendritic cells and plasmacytoid DCs are activated by human cytomegalovirus and retain efficient T cell-stimulatory capability upon infection. Blood 107:2022-2029. [DOI] [PubMed] [Google Scholar]
- 29.Lee, C. I., M. J. Cowan, D. B. Kohn, and A. F. Tarantal. 2004. Simian immunodeficiency virus infection of hematopoietic stem cells and bone marrow stromal cells. J. Acquir. Immune Defic. Syndr. 36:553-561. [DOI] [PubMed] [Google Scholar]
- 30.Lian, Z.-X., K. Kilkuchi, G.-X. Yang, A. A. Ansari, S. Ikehara, and M. E. Gershwin. 2004. Expansion of bone marrow IFN-α-producing dendritic cells in New Zealand black (NZB) mice: high level expression of TLR9 and secretion of IFN-α in NZB bone marrow. J. Immunol. 173:5283-5289. [DOI] [PubMed] [Google Scholar]
- 31.Lund, J., A. Sato, S. Akira, R. Medzhitov, and A. Iwasaki. 2003. Toll-like receptor 9-mediated recognition of herpes simplex virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 198:513-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lund, J. M., L. Alexopoulou, A. Sato, M. Karow, N. C. Adams, N. W. Gale, A. Iwasaki, and R. A. Flavell. 2004. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. USA 101:5598-5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lund, J. M., M. M. Linehan, N. Iijima, and A. Iwasaki. 2006. Plasmacytoid dendritic cells provide innate immune protection against mucosal viral infection in situ. J. Immunol. 177:7510-7514. [DOI] [PubMed] [Google Scholar]
- 34.Marshall, J. D., K. Fearon, C. Abbate, S. Subramanian, P. Yee, J. Gregorio, R. L. Coffman, and G. Van Nest. 2003. Identification of a novel CpG DNA class and motif that optimally stimulate B cell and plasmacytoid dendritic cell functions. J. Leukoc. Biol. 73:38-46. [DOI] [PubMed] [Google Scholar]
- 35.Masten, B. J., G. K. Olson, C. A. Tarleton, C. Rund, M. Schuyler, R. Mehran, T. Archibeque, and M. F. Lipscomb. 2006. Characterization of myeloid and plasmacytoid dendritic cells in human lung. J. Immunol. 177:7784-7793. [DOI] [PubMed] [Google Scholar]
- 36.Megjugorac, N. J., H. A. Young, S. B. Amrute, S. L. Olshalsky, and P. Fitzgerald-Bocarsly. 2004. Virally stimulated plasmacytoid dendritic cells produce chemokines and induce migration of T and NK cells. J. Leukoc. Biol. 75:504-514. [DOI] [PubMed] [Google Scholar]
- 37.Middel, P., D. Raddatz, B. Gunawan, F. Haller, and H.-J. Radzun. 2006. Increased number of mature dendritic cells in Crohn's disease: evidence for a chemokine mediated retention mechanism. Gut 55:220-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moses, A., J. Nelson, and G. C. Bagby. 1998. The influence of human immunodeficiency virus-1 on hematopoiesis. Blood 91:1479-1495. [PubMed] [Google Scholar]
- 39.Pashenkov, M., N. Teleshova, M. Kouwenhoven, T. Smirnova, Y.-P. Jin, V. Kostulas, Y.-M. Huang, B. Pinegin, A. Boiko, and H. Link. 2002. Recruitment of dendritic cells to the cerebrospinal fluid in bacterial neuroinfections. J. Neuroimmunol. 122:106-116. [DOI] [PubMed] [Google Scholar]
- 40.Pelayo, R., J. Hirose, J. Huang, K. P. Garrett, A. Delogu, M. Busslinger, and P. W. Kincade. 2005. Derivation of 2 categories of plasmacytoid dendritic cells in murine bone marrow. Blood 105:4407-4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reeves, R. K., and P. N. Fultz. 2007. Disparate effects of acute and chronic infection with SIVmac239 or SHIV-89.6P on macaque plasmacytoid dendritic cells. Virology 365:356-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Romagnani, C., M. D. Chiesa, S. Kohler, B. Moewes, A. Radbruch, L. Moretta, A. Moretta, and A. Thiel. 2005. Activation of human NK cells by plasmacytoid dendritic cells and its modulation by CD4+ T helper cells and CD4+ CD25hi T regulatory cells. Eur. J. Immunol. 35:2452-2458. [DOI] [PubMed] [Google Scholar]
- 43.Salahuddin, S. Z., P. D. Markham, M. Popovic, M. G. Sarngadharan, S. Orndoff, A. Fladagar, A. Patel, J. Gold, and R. C. Gallo. 1985. Isolation of infectious human T-cell leukemia/lymphotropic virus type III (HTLV-III) from patients with acquired immunodeficiency syndrome (AIDS) or AIDS-related complex (ARC) and from healthy carriers: a study of risk groups and tissue sources. Proc. Natl. Acad. Sci. USA 82:5530-5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siegal, F. P., N. Kadowaki, M. Shodell, P. A. Fitzgerald-Bocarsly, K. Shah, S. Ho, S. Antonenko, and Y. J. Liu. 1999. The nature of the principal type 1 interferon-producing cells in human blood. Science 284:1835-1837. [DOI] [PubMed] [Google Scholar]
- 45.Summers, K. L., B. D. Hock, J. L. McKenzie, and D. N. J. Hart. 2001. Phenotypic characterization of five dendritic cell subsets in human tonsils. Am. J. Pathol. 159:285-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Szabolcs, P., K.-D. Park, M. Reese, L. Marti, G. Broadwater, and J. Kurtzberg. 2003. Absolute values of dendritic cell subsets in bone marrow, cord blood, and peripheral blood enumerated by a novel method. Stem Cells 21:296-303. [DOI] [PubMed] [Google Scholar]
- 47.Teleshova, N., J. Jones, J. Kenney, J. Purcell, R. Bohm, A. Gettie, and M. Pope. 2004. Short-term Flt3L treatment effectively mobilizes functional macaque dendritic cells. J. Leukoc. Biol. 75:1102-1110. [DOI] [PubMed] [Google Scholar]
- 48.Teleshova, N., J. Kenney, G. Van Nest, J. Marshall, J. D. Lifson, I. Sivin, J. Dufour, R. Bohm, A. Gettie, and M. Robbiani. 2006. Local and systemic effects of intranodally injected CpG-C immunostimulatory-oligodeoxyribonucleotides in macaques. J. Immunol. 177:8531-8541. [DOI] [PubMed] [Google Scholar]
- 49.Teleshova, N., J. Kenney, J. Jones, J. Marshall, G. Van Nest, J. Dufour, R. Bohm, J. D. Lifson, A. Gettie, and M. Pope. 2004. CpG-C immunostimulatory oligodeoxyribonucleotide activation of plasmacytoid dendritic cells in rhesus macaques to augment the activation of IFN-γ-secreting simian immunodeficiency virus-specific T cells. J. Immunol. 173:1647-1657. [DOI] [PubMed] [Google Scholar]
- 50.Thiebot, H., F. Louache, B. Vaslin, T. de Revel, O. Neildez, J. Larghero, W. Vainchenker, D. Dormont, and R. Le Grand. 2001. Early and persistent bone marrow hematopoiesis defect in simian/human immunodeficiency virus-infected macaques despite efficient reduction of viremia by highly active antiretroviral therapy during primary infection. J. Virol. 75:11594-11602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vandenabeele, S., H. Hochrein, N. Mavaddat, K. Winkel, and K. Shortman. 2001. Human thymus contains 2 distinct dendritic cell populations. Blood 97:1733-1741. [DOI] [PubMed] [Google Scholar]
- 52.Wain, J., V. B. Pham, V. Ha, N. M. Nguyen, S. D. To, A. L. Walsh, C. M. Parry, R. P. Hasserjian, V. A. Ho, T. H. Tran, J. Farrar, N. J. White, and N. P. Day. 2001. Quantitation of bacteria in bone marrow from patients with typhoid fever: relationship between counts and clinical features. J. Clin. Microbiol. 39:1571-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoneyama, H., K. Matsuno, Y. Zhang, T. Nishiwaki, M. Kitabatake, S. Ueha, S. Narumi, S. Morikawa, T. Ezaki, B. Lu, C. Gerard, S. Ishikawa, and K. Matsushima. 2004. Evidence for recruitment of plasmacytoid dendritic cell precursors to inflamed lymph nodes through high endothelial venules. Int. Immunol. 16:915-928. [DOI] [PubMed] [Google Scholar]
- 54.Zou, L., B. Barnett, H. Safah, V. F. LaRussa, M. Evdemon-Hogan, P. Mottram, S. Wei, O. David, T. J. Curiel, and W. Zou. 2004. Bone marrow is a reservoir for CD4+CD25+ regulatory T cells that traffic through CXCL12/CXCR4 signals. Cancer Res. 64:8451-8455. [DOI] [PubMed] [Google Scholar]