Abstract
Antibodies in the sera of patients with lacaziosis recognized an ∼193-kDa antigen and other Lacazia loboi antigens. Paracoccidioides brasiliensis gp43 antigen was detected by all evaluated sera, but they failed to detect a protein with the same molecular mass in L. loboi extracts. This study is the first to examine the humoral response to L. loboi antigens by using multiple host sera.
Lacazia loboi is an uncultivated fungal pathogen predominantly restricted to Latin America. Lacaziosis has been recorded among humans dwelling in this geographical area, tourists visiting areas of endemicity (4, 10), and workers handling infected dolphins (19, 24). Lacaziosis has also been diagnosed in dolphins inhabiting the coastal areas of the United States (9, 12, 19), Brazil (16), and France (25). Using molecular methods, the phylogenetic features of L. loboi were only recently deciphered (13, 18, 31). These studies placed L. loboi as a sister group to Paracoccidioides brasiliensis and, in turn, linked to the other dimorphic members of the Onygenales in the family Ajellomycetaceae.
The clinical features of Lacazia loboi and P. brasiliensis infections are different, but both fungi develop yeast cells, which are difficult to differentiate microscopically, in the host's infected tissues (7). This resemblance was used by some investigators to classify this pathogen in the genus Paracoccidioides (1, 6, 8). Thus, due to L. loboi's inability to grow in culture, immunological studies have traditionally been carried out with antigens extracted from mycelial cultures of P. brasiliensis (15, 17, 21, 23-27, 30). The objective of our study was to characterize the immunogenic proteins extracted from L. loboi yeast-like cells in Western blotting analyses, using sera from humans and dolphins with lacaziosis and sera from experimentally infected mice.
Sera from four human patients with lacaziosis from Acre State, Brazil, and from one Brazilian patient with proven paracoccidioidomycosis and four sera from experimentally L. loboi-infected mice were collected and stored at −80°C until use (2). Sera from three bottlenose dolphins (Tursiops truncatus) infected with L. loboi were evaluated: one wild dolphin infected with L. loboi was captured in the Indian River Lagoon, FL, one was a stranded Indian River Lagoon bottlenose dolphin, and one was a captive bottlenose dolphin from SeaWorld in San Diego, CA. In addition, two sera from apparently healthy humans, two sera from healthy dolphins, and two sera from healthy laboratory mice were used as negative controls.
Lacazia loboi yeast-like cell extracts were obtained from the footpads of a previously infected BALB/c mouse 6 months after inoculation, as described by Belone et al. (2). Briefly, L. loboi yeast-like cells were then centrifuged for 10 min at 3,000 rpm, and the supernatant was discarded. Cells were washed twice with saline, and the pellet was suspended in 1 ml of saline. Fungal cells were macerated to a powder with liquid nitrogen inside a mortar and used in the experiments. The gp43 protein of P. brasiliensis was extracted and purified per the method of Taborda et al. (28). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blot analyses were done per the methods of Laemmli (14) and Towbin et al. (29).
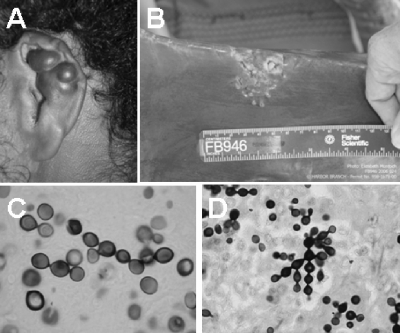
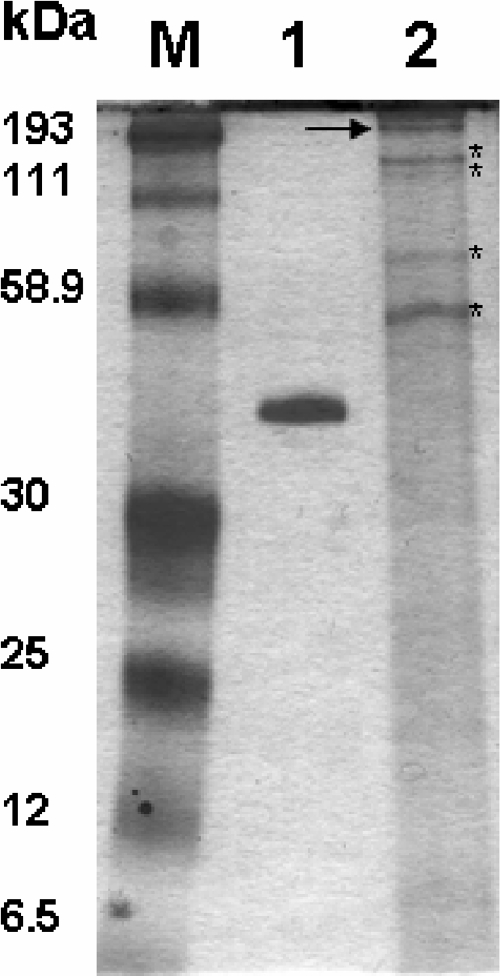
The diagnosis of lacaziosis in the investigated humans and dolphins was confirmed by clinical and histopathological methods (Fig. 1). The stained gels of L. loboi protein extracts showed at least 10 bands, ranging from ∼193 kDa to 9.0 kDa (Fig. 2). The L. loboi protein extract lacked a visible protein band at 43 kDa. The purified gp43 protein of P. brasiliensis showed a strong band at the expected molecular mass and did not contain other proteins (Fig. 2). Normal mouse skin tissue samples processed as described above did not show visible bands (data not shown).
FIG. 1.
Human parakeloidal (A) and dolphin verrucous (B) lesions from two of the hosts with lacaziosis used in this study. The histopathology of these lesions is shown in panels C (human) (silver stain; magnification, ×100) and D (dolphin) (silver stain; magnification, ×40).
FIG. 2.
Coomassie blue-stained sodium dodecyl sulfate-polyacrylamide gel showing purified Paracoccidioides brasiliensis gp43 (lane 1) and the profile of an Lacazia loboi protein extract (lane 2). The figure shows the ∼193-kDa immunodominant antigen (arrow) as well as other proteins in the L. loboi protein extract. The asterisks indicate common proteins detected by the sera tested in this study. Lane M, molecular mass marker.
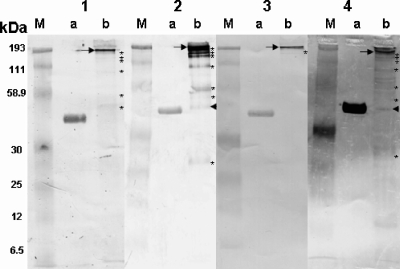
The immunoglobulin G (IgG) antibodies in the sera of all tested hosts with lacaziosis detected the gp43 protein of P. brasiliensis fairly well (Fig. 3). The tested dolphin, human, and experimentally infected mouse sera detected a major, immunodominant, ∼193-kDa antigen in the L. loboi protein extract (Fig. 3) and also reacted weakly with other immunogenic antigens (Fig. 3). The experimentally infected mouse antibodies mildly detected five other immunogens, including an ∼48-kDa protein not detected by the other sera (Fig. 3, panel 2, lane b, arrowhead). The IgG antibodies of the dolphins detected only two high-molecular-mass antigens in the L. loboi protein extract, including the immunodominant, ∼193-kDa antigen (Fig. 3, panel 3, lane b). The antibodies in the serum from a patient with paracoccidioidomycosis weakly detected at least 13 other antigenic components in the L. loboi protein extract, ranging from ∼193 kDa to 9.0 kDa. Some of these antigens were not detected by the antibodies in the sera from humans and dolphins with lacaziosis or the antibodies in the sera from experimentally infected mice (Fig. 3, panel 4, lane b). Only the antibodies in the serum from the patient with paracoccidioidomycosis weakly detected a molecular antigen of 43 kDa in the L. loboi protein extract (Fig. 3, panel 4, lane b). The healthy control human, dolphin, and mouse sera did not react with the antigens used in this study (data not shown). One of the two control dolphin sera possessed antibodies against the 43-kDa purified protein of P. brasiliensis and the major antigens of L. loboi normally detected by the dolphins with lacaziosis. Putative residual normal mouse skin cells were not detected by the tested sera (data not shown).
FIG. 3.
Western blot analyses of transferred proteins on nylon membranes. The antibodies in the serum from a human with lacaziosis weakly detected Paracoccidioides brasiliensis gp43 (lane 1a). This serum strongly detected an ∼193-kDa immunodominant antigen (arrow) as well as six other weak bands (asterisks) (lane 1b). The IgG in the serum from the experimentally infected mouse weakly detected P. brasiliensis gp43 (lane 2a). The mouse antibodies strongly detected the ∼193-kDa immunodominant antigen (arrow) as well as seven other proteins (asterisks) and an ∼48-kDa protein not detected by the other sera (lane 2b, arrowhead). The antibodies in the dolphin with lacaziosis slightly detected P. brasiliensis gp43 (lane 3a). The antibodies strongly recognized the ∼193-kDa immunodominant antigen (arrow) and only one of the minor antigens previously detected in the L. loboi extract (lane 3b). The antibodies in the serum from the patient with paracoccidioidomycosis strongly detected gp43 (lane 4a). The antibodies of this patient also detected the immunodominant ∼193-kDa antigen (arrow) in the L. loboi extract as well as other antigens (asterisks), including a 43-kDa antigen (arrowhead) (lane 4b). The molecular mass marker (lanes M) appears in each of the panels.
With the exception of three previous studies (1, 23, 30), in the past 75 years L. loboi immunological research has been carried out using antigens of P. brasiliensis (6, 8, 15, 17, 21, 24, 26, 27, 32). Our study showed that patients with lacaziosis possessed antibodies not only against the gp43 antigen of P. brasiliensis but mainly against an ∼193-kDa immunodominant L. loboi antigen. The possibility that the ∼193-kDa antigen comprises several compressed L. loboi immunogens is currently under investigation in our laboratory. The IgGs in the sera from patients with lacaziosis failed to detect the gp43-like antigen in the protein extract of L. loboi. However, the serum from the patient with paracoccidioidomycosis did detect an antigen with a similar molecular mass. The cross-reactive antigens reported in this study, including purified gp43 and those reported by other investigators (1, 6, 15, 21, 24, 26, 30), were not unexpected, since recent molecular studies have shown that both L. loboi and P. brasiliensis share the same ancestor (13, 18, 31).
The many reports of lacaziosis in dolphins have puzzled investigators for decades (3, 5, 9, 12, 19, 22, 25, 32). However, studies to elucidate its etiology, epidemiology, and immunology have been equally challenging (5, 22, 25). Our study showed that dolphins develop IgG antibodies against similar prominent antigens to those recognized by humans with lacaziosis and by experimentally infected mice. Interestingly, one of the negative control dolphins detected the gp43 antigen of P. brasiliensis and the other major antigens of L. loboi identified by dolphins with lacaziosis. This unexpected result suggests a previous exposure to L. loboi without disease, as it is frequent among the other dimorphic members of the Onygenales (15, 17, 20). Thus, Western blotting analyses could be a useful tool for epidemiological studies of L. loboi. Although some investigators have reported morphological differences between the yeast-like cells of L. loboi in both species (11), our study is the first to suggest that dolphins are infected with similar L. loboi strains to those infecting humans.
In contrast to previous serological reports (1, 23, 30), this study suggests that during infection, L. loboi presents immunogens to the immune system which are different from that in P. brasiliensis infection. The molecular characterization of these antigens, especially the ∼193-kDa immunodominant antigen, could generate valuable information to better understand the immunology and serology of L. loboi infection and possibly aid in the development of new therapies for infections caused by this resilient fungal pathogen.
Acknowledgments
Lesions from the free-ranging dolphin were collected under National Marine Fisheries Service scientific research permit no. 998-1678, issued to Gregory D. Bossart as part of the Bottlenose Dolphin Health and Risk Assessment (HERA) Project conducted in the Indian River Lagoon, FL, and the estuarine waters of Charleston, SC. Samples from the stranded dolphin were collected under a National Marine Fisheries Service letter of agreement, and this collection was supported in part under award NA06NMF4390138 from the National Oceanic and Atmospheric Administration, U.S. Department of Commerce. This research was supported in part by the Biomedical Laboratory Diagnosis Program, Michigan State University, MI.
Footnotes
Published ahead of print on 24 October 2007.
REFERENCES
- 1.Bahawan, J., R. W. Bain, D. T. Purtilo, N. Gomez, C. Dewan, C. F. Whelan, M. Dolorum, and L. Edelstein. 1976. Lobomycosis. An electron microscopic, histochemical and immunologic study. J. Cutan. Pathol. 3:5-16. [DOI] [PubMed] [Google Scholar]
- 2.Belone, A. F. F., S. Madeira, P. S. Rosa, and D. V. A. Opromolla. 2001. Experimental reproduction of the Jorge Lobo's disease in BALB/c mice inoculated with Lacazia loboi obtained from a previously infected mouse. Mycopathologia 155:191-194. [DOI] [PubMed] [Google Scholar]
- 3.Bossart, G. D. 1984. Suspected acquired immunodeficiency in an Atlantic bottlenosed dolphin with chronic-active hepatitis and lobomycosis. J. Am. Vet. Med. Assoc. 185:1413-1414. [PubMed] [Google Scholar]
- 4.Burns, R. A., J. S. Roy, C. Woods, A. A. Padhye, and D. W. Warnock. 2000. Report of the first human case of lobomycosis in the United States. J. Clin. Microbiol. 38:1283-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caldwell, D. K., M. C. Caldwell, J. C. Woodard, L. Ajello, W. Kaplan, and H. M. McClure. 1975. Lobomycosis as a disease of the Atlantic bottle-nosed dolphin (Tursiops truncatus Montagu, 1821). Am. J. Trop. Med. Hyg. 24:105-114. [DOI] [PubMed] [Google Scholar]
- 6.Camargo, Z. P., R. G. Baruzzi, S. M. Maeda, and M. C. Floriano. 1998. Antigenic relationship between Loboa loboi and Paracoccidioides brasiliensis as shown by serological methods. Med. Mycol. 36:413-417. [PubMed] [Google Scholar]
- 7.Chandler, F. W., W. Kaplan, and L. Ajello. 1980. Color atlas and text of histopathology of mycotic diseases, p. 218-221, 246-252. Year Book Medical Publishers, Inc., Chicago, IL.
- 8.de Fonseca, O. J., and C. S. Lacaz. 1971. Estudo de culturas isoladas de blastomycosis queloidiforme (doença de Jorge Lôbo). Denominação ao seu agente etiológico. Rev. Inst. Med. Trop. Sao Paulo 13:225-252. [PubMed] [Google Scholar]
- 9.De Vries, G. A., and J. J. Laarman. 1973. A case of Lobo's disease in the dolphin Sotalia guianensis. J. Aquat. Mammals 1:26-33. [Google Scholar]
- 10.Elsayed, S., S. M. Kuhn, D. Barber, D. L. Church, S. Adams, and R. Kasper. 2004. Human case of lobomycosis. Emerg. Infect. Dis. 10:715-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haubold, E. M., Jr., C. R. Cooper, J. W. Wen, M. R. McGinnis, and D. F. Cowan. 2000. Comparative morphology of Lacazia loboi (syn. Loboa loboi) in dolphins and humans. Med. Mycol. 38:9-14. [DOI] [PubMed] [Google Scholar]
- 12.Heel, W. H. D. 1977. Successful treatment in a case of lobomycosis (Lobo's disease) in Tursiops truncatus (Mont.) at the Dolphinarium, Harderwijk. Aquat. Mammals 5:8-15. [Google Scholar]
- 13.Herr, R. A., E. J. Tarcha, P. R. Taborda, J. W. Taylor, L. Ajello, and L. Mendoza. 2001. Phylogenetic analysis of Lacazia loboi places this previously uncharacterized pathogen within the dimorphic Onygenales. J. Clin. Microbiol. 39:309-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 227:680-685. [DOI] [PubMed] [Google Scholar]
- 15.Landman, G., M. A. Velludo, J. A. Lopes, and E. Mendes. 1988. Crossed-antigenicity between the etiologic agents of lobomycosis and paracoccidioidomycosis evidenced by an immunoenzymatic method (PAP). Allergol. Immunopathol. (Madrid) 16:215-218. [PubMed] [Google Scholar]
- 16.Lopes, P. C. S., G. S. Paula, M. C. Both, F. M. Xanvier, and A. C. Scaramello. 1993. First case of lobomycosis in a bottlenose dolphin from Southern Brazil. Mar. Mamm. Sci. 9:329-331. [Google Scholar]
- 17.Mendes-Giannini, M. J. S., M. E. Camargo, C. S. Lacaz, and A. W. Ferreira. 1984. Immunoenzymatic absorption test for serodiagnosis of paracoccidioidomycosis. J. Clin. Microbiol. 20:103-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mendoza, L., R. Vilela, P. R. Rosa, and A. F. F. Belone. 2005. Lacazia loboi and Rhinosporidium seeberi: a genomic perspective. Rev. Iberoam. Micol. 22:213-216. [DOI] [PubMed] [Google Scholar]
- 19.Migaki, G., M. G. Valerio, B. Irvine, and F. M. Garner. 1971. Lobo's disease in an Atlantic bottle-nosed dolphin. J. Am. Vet. Med. Assoc. 159:578-582. [PubMed] [Google Scholar]
- 20.Norton, S. A. 2006. Dolphin-to-human transmission of lobomycosis? J. Am. Acad. Dermatol. 55:723-724. [DOI] [PubMed] [Google Scholar]
- 21.Puccia, R., and L. R. Travassos. 1991. 43-Kilodalton glycoprotein from immunochemical reactions with sera from patients with Paracoccidioides brasiliensis paracoccidioidomycosis, histoplasmosis, or Jorge Lobo's disease. J. Clin. Microbiol. 29:1610-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reif, J. S., M. S. Mazzoil, S. D. McCulloch, R. A. Varela, J. D. Goldstein, P. A. Fair, and G. D. Bossart. 2006. Lobomycosis in Atlantic bottlenose dolphins from the Indian River Lagoon, Florida. J. Am. Vet. Med. Assoc. 228:104-108. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez, M. C., and C. S. Lacaz. 1984. Provas de contrainmunoelectroforese na doença de Jorge Lôbo, p. 62. In C. S. Lacaz, R. G. Baruzzi, and M. C. B. Rosa (ed.), Doença de Jorge Lôbo. Universidade de São Paulo, São Paulo, Brazil.
- 24.Silva, M. E., W. Kaplan, and J. L. Miranda. 1968. Antigenic relationships between Paracoccidioides loboi and other pathogenic fungi determined by immunofluorescence. Mycopathologia 36:97-106. [DOI] [PubMed] [Google Scholar]
- 25.Symmers, W. 1983. A possible case of Lobo's disease acquired in Europe from a bottle nosed dolphin (Tursiops truncatus). Bull. Soc. Pathol. Exot. 76:777-784. [PubMed] [Google Scholar]
- 26.Taborda, C. P., and Z. P. Camargo. 1993. Diagnosis of paracoccidioidomycosis by passive haemagglutination assay of antibody using a purified and specific antigen—gp43. J. Med. Vet. Mycol. 31:155-160. [DOI] [PubMed] [Google Scholar]
- 27.Taborda, C. P., and Z. P. Camargo. 1994. Diagnosis of paracoccidioidomycosis by dot immunobinding assay for antibody detection using the purified and specific antigen gp43. J. Clin. Microbiol. 32:554-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taborda, C. P., M. A. Juliano, R. Puccia, M. Franco, and L. R. Travassos. 1998. Mapping of the T-cell epitope in the major 43-kilodalton glycoprotein of Paracoccidioides brasiliensis which induces a Th-1 response protective against fungal infection in BALB/c mice. Infect. Immun. 66:786-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vidal, M. S., S. A. Palacios, N. T. de Melo, and C. S. Lacaz. 1997. Reactivity of anti-gp43 antibodies from Paracoccidioides brasiliensis antiserum with extracts from cutaneous lesions of Lobo's disease. Rev. Inst. Med. Trop. Sao Paulo 39:35-37. [DOI] [PubMed] [Google Scholar]
- 31.Vilela, R., L. Mendoza, P. S. Rosa, A. F. Belone, S. Madeira, D. V. A. Opromolla, and M. A. de Resende. 2005. Molecular model for studying the uncultivated fungal pathogen Lacazia loboi. J. Clin. Microbiol. 43:3657-3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woodard, J. C. 1972. Electron microscopic study of lobomycosis (Loboa loboi). Lab. Investig. 27:606-612. [PubMed] [Google Scholar]