Abstract
The detection of antibody to the Borrelia burgdorferi C6 peptide by use of enzyme-linked immunoassays is a widely accepted method for the diagnosis of Lyme disease spirochete infection in dogs and in humans. Antibody to the C6 peptide is highly specific for B. burgdorferi and declines following treatment of dogs and humans exposed to B. burgdorferi. A quantitative assay for determining C6 antibody levels was developed and used to measure changes in antibody levels following antibiotic treatment of B. burgdorferi antibody-positive nonclinical dogs. One hundred thirty-two client-owned dogs were used in the study; 64 were negative, 53 of 68 positive animals received treatment, and 15 were untreated controls. Test sera were collected at 3, 6, and 12 months from seropositive dogs receiving treatment and untreated controls. Dogs in the treated group were assigned to moderate-to-high (≥29 U/ml)- and low (<29 U/ml)-C6-level groups because the change in the C6 level after treatment was dependent on the level prior to treatment. There were significant declines in the 30 dogs with moderate-to-high initial C6 levels that exceeded the maximal declines of the untreated control dogs in all cases at 6 months (16 data points) and 12 months (29 data points) posttreatment. There was little change in C6 level following antibiotic therapy in the 23 dogs with low initial C6 levels. The quantitative C6 antibody test can be used to measure changes in C6 antibody levels following treatment of antibody-positive nonclinical dogs.
Borrelia burgdorferi, the tick-transmitted bacterium that is the etiological agent of Lyme disease, can infect a wide range of mammals, including humans and dogs, and is the most common vector-borne-disease-causing agent in the United States (3). The organism contains several outer membrane proteins, one of which, an outer membrane lipoprotein referred to as VlsE (VMP-like sequence, expressed), contains both antigenically variable and invariable regions (5). Detection of antibody to the sixth invariable region of the VlsE protein, a 25-amino-acid peptide termed C6, has been shown to be an accurate method for the serological diagnosis of Lyme disease spirochete infection in humans and dogs (7, 8, 9, 10, 11). Studies using samples from clinically defined human cases have shown that the sensitivities and specificities of C6 peptide-based enzyme-linked immunosorbent assays (ELISAs) were equivalent or superior to those of Western blot analysis alone (14) or a standard two-tiered serological procedure consisting of a combination of whole-cell-based ELISA and Western blot analysis (1, 17, 18).
Production of antibody to the C6 sequence appears to depend on the presence of a viable organism and has been used to accurately detect natural exposure of dogs to B. burgdorferi regardless of vaccination history (7, 15, 16). Decreased C6 and VlsE antibody titers have been found in humans and experimentally infected dogs and primates treated for B. burgdorferi infection (13, 16, 17, 18). In a study using clinically defined temporal samples from humans receiving treatment whose clinical symptoms were all subsequently resolved, sera from 36 of 45 patients (80%) had >4-fold declines in C6 titer 6 months or more following treatment (17). In a related follow-up study, using clinically defined samples from patients manifesting clinical signs of disease that were resolved following treatment, 96 of 105 (91.4%) exhibited fourfold or greater declines in C6 titer 6 to 12 months following treatment (18).
Lyme disease in dogs has been primarily associated with limb/joint abnormalities, renal disease, and idiopathic conditions (6, 12, 19, 23). While treatment of dogs with Lyme arthritis may cause clinical signs to diminish in a predictable fashion, Lyme disease spirochete-infected dogs frequently present without clinical signs, thus preventing clinical evaluation of response to treatment. On the basis of the reported declines in C6 antibody levels following antibiotic administration (13, 16, 17, 18), we hypothesized that a serologic response to treatment could be evaluated, provided that we could demonstrate a significant difference in the declines in C6 antibody levels in treated versus untreated dogs. In an effort to test this hypothesis, we developed a quantitative assay to measure the level of C6-specific antibody and initiated a study to evaluate the effects of antibiotic treatment on the C6 antibody levels in B. burgdorferi antibody-positive client-owned dogs presenting to a veterinary practice for routine care. We were able to confirm a response to treatment effect in antibody-positive dogs by comparing changes in C6 levels in dogs receiving treatment to changes in untreated dogs.
MATERIALS AND METHODS
Animals.
Dogs were selected following agreement of owners to participate in this study and categorized as clinical or nonclinical on the basis of clinical signs consistent with Lyme disease. All initial samples included in this study were obtained from nonclinical dogs during routine physicals, annual vaccination, and vector-borne-disease surveillance testing. At the time of initial examination, the dogs were categorized as B. burgdorferi antibody positive or negative on the basis of reaction of whole blood as determined using a qualitative in-office C6 ELISA kit (SNAP 3Dx test; IDEXX Laboratories, Inc., Westbrook, ME). Additional samples from dogs were obtained at various times during the subsequent 12 months. Serum or plasma samples from blood samples were stored frozen at −15°C and sent to IDEXX Laboratories for testing using a quantitative C6 ELISA. One hundred twenty-five dogs were patients at the Durham Veterinary Hospital in Durham, CT (samples obtained between June 2001 and August 2003), and 7 dogs were patients at Lakeland Veterinary Clinic in Baxter, MN (samples obtained between July 2004 and October 2005).
Fifteen B. burgdorferi antibody-positive dogs (8 from the Durham Veterinary Hospital and 7 from the Lakeland Veterinary Clinic) served as controls and did not receive treatment. All control dogs, with the exception of dog C6 (Table 1), which was not vaccinated, received whole-cell B. burgdorferi vaccine (Lymevax; Fort Dodge Animal Health, Overland Park, KS). Control dogs from the Durham Veterinary Clinic (C1, C3, C7, C10, C11, C13, C14, and C15) received two doses of whole-cell B. burgdorferi vaccine at the start of the study. Control dogs from the Lakeland Clinic (C2, C4, C5, C8, C9, and C12) were vaccinated one or more times on a yearly basis prior to the study. Dogs C1 to C11 were controls for dogs with initial C6 levels of ≥29 U/ml; dogs C12 to C15 were controls for dogs with initial C6 levels of <29 U/ml. The B. burgdorferi antibody-positive dogs that received treatment (all from the Durham Veterinary Hospital) were put on an antibiotic treatment regime for Lyme disease and received two doses of a whole-cell B. burgdorferi vaccine (Lymevax; Fort Dodge Animal Health, Overland Park, KS). Two C6 ELISA-positive dogs (from the Durham Veterinary Hospital) had clinical signs consistent with Lyme disease (lameness, polyarthritis, and general malaise) and were not included in this study.
TABLE 1.
C6 levels in untreated Lyme-positive dogs at time points between 0 and 12 months for dogs with initial C6 levels in the moderate-to-high-C6-level group (C6 levels of 46 to 278 U/ml)
| Dog no. | C6 level (U/ml) at indicated no. of mos posttreatment
|
% changea | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 3 | 4 | 5 | 6 | 7 | 8 | 12 | ||
| C1 | 46 | 106 | +130.4 | |||||||
| C2 | 49 | 49 | 48 | −2.0 | ||||||
| C3 | 81 | 131 | 119 | NA | ||||||
| C4 | 100 | 110 | 98 | −2.0 | ||||||
| C5 | 131 | 123 | 119 | −9.2 | ||||||
| C6 | 211 | 212 | +0.5 | |||||||
| C7 | 225 | 137 | −39.1 | |||||||
| C8 | 249 | 264 | 181 | −27.3 | ||||||
| C9 | 256 | 294 | 204 | −20.3 | ||||||
| C10 | 273 | 116 | 139 | −49.1 | ||||||
| C11 | 278 | 232 | −16.5 | |||||||
Percent change was calculated using the C6 value of the initial time point and the C6 value of the final time point past 6 months.
Therapy.
Dogs were treated for 28 days with either amoxicillin or doxycycline. Dosages for doxycycline were at either a lower dose range (5 mg per kilogram of body weight, orally [p.o.], twice a day) or a higher dose range (10 mg per kilogram, p.o., twice a day). Amoxicillin was administered at a dose of 5 mg per kilogram, p.o., twice a day. It was recommended that the antibiotic be given with a meal.
C6 peptide.
The C6 B. burgdorferi synthetic peptide contained 25 amino acids from the IR6 sequence found within the variable surface antigen (VlsE) of B. burgdorferi sensu lato (9). The peptide was synthesized as a multiple antigenic peptide with a four-branch lysine core (Applied Biosystems, Foster City, CA) by using standard 9-fluorenylmethoxycarbonyl chemistry as previously described (15).
SNAP 3Dx test.
The SNAP 3Dx test is an in-clinic ELISA for simultaneous detection of canine heartworm antigen, antibodies to Ehrlichia canis, and antibodies to Borrelia burgdorferi in canine serum, plasma, or whole-blood samples. The B. burgdorferi antibody test is specific for antibody to the C6 peptide. The SNAP 3Dx test does not react to antibody elicited following vaccination (7, 15). The specificity of the SNAP 3Dx test was greater than 99.5% when the test was used with field samples from 987 dogs in North Carolina (4). The SNAP test sensitivity was 94.4% (238 of 252) compared to that of a combination of an indirect fluorescent-antibody assay and Western blot analysis (15). The SNAP test was performed using the instructions supplied in the package insert.
Quantitative immunoassay for antibody to the C6 peptide.
The quantitative C6 ELISA was designed to measure the levels of C6-specific antibody in the sera of SNAP 3Dx-positive dogs. Quantitative measurement of C6 antibody was performed using a microtiter plate format ELISA kit containing C6 multiple-antigenic-peptide-coated microtiter plates and a rabbit anti-canine immunoglobulin G horseradish peroxidase conjugate (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). Test samples were diluted 1:1,000 in 0.2 M phosphate saline containing 0.05% Tween 20, nonspecific proteins, and detergent. A volume of 100 μl was added to four individual microtiter wells for each sample and incubated for 30 min. The contents of the microtiter wells were aspirated, washed with phosphate saline containing 0.05% Tween 20, and incubated with rabbit anti-canine immunoglobulin G horseradish peroxidase conjugate (30 min) in a conjugate diluent containing nonspecific proteins and detergent. The microtiter plate wells were aspirated, washed, and incubated with a substrate solution containing 3,3′,5,5′-tetramethylbenzidine; optical density values were determined spectrophotometrically at 650 nm. Relative antibody levels for samples were reported in units/ml (U/ml), which was the quantity of canine antibody that gave a reaction equivalent to that for 1 μg/ml of affinity-purified rabbit antibody prepared against the C6 peptide. Antibody levels were calculated using proprietary software developed at IDEXX by constructing a standard curve produced using optical density values of five calibrated controls. The average optical density values produced by samples and controls in four microtiter wells were used in all calculations. In validated assays, the interwell variability levels for individual samples and controls were required to be less than 27% and 30%, respectively, and were typically observed to be less than 10%. The dynamic range of the assay was 10 U/ml to >400 U/ml. Levels less than 10 U/ml or greater than the value of the high calibrator control were reported as <10 U/ml or exceeding the reportable range, respectively. The Quant C6 test was optimized to be quantitative in nature and was not intended to provide qualitative results. Antibody-positive samples with very low levels of C6 antibody (<10 U/ml) and true negative samples would be expected to yield the same result (i.e., <10 U/ml). Samples yielding C6 antibody levels exceeding the dynamic range of the assay were diluted 1:2 and retested.
Assay specificity was verified by testing 146 sera obtained over the course of 12 months from 64 SNAP 3Dx-negative dogs (Durham Veterinary Hospital). One hundred forty-five of 146 samples (99.3%) gave C6 antibody levels of <10 U/ml. The SNAP-negative sample with a C6 antibody level of >10 U/ml may have come from a recently infected animal; a follow-up sample (obtained 12 months later) tested positive with the SNAP assay and had a C6 level of 227 U/ml (data not shown). Assay precision was measured by testing five samples (with a range of anti-C6 values from 25 to 276 U/ml) a total of 12 times each on three separate days. The percents variation were 3.3, 5.7, 6.3, 6.7, and 7.2 for samples with C6 levels of 25, 40, 56, 180, and 276 U/ml, respectively.
Classification scheme: assignment of dogs to low- and moderate-to-high-C6-level groups.
Analysis of results for serial samples obtained between 0 and 6 months revealed a biased change in C6 level after treatment that was dependent on C6 level prior to treatment. Dogs with initial values of 29 U/ml or greater showed statistically significant declines in C6 antibody level at 6 months posttreatment, while dogs with C6 values of less than 29 U/ml had small, inconsistent changes in C6 antibody levels. In this study, the demarcation point at or above which one would consistently expect a significant response to treatment was 29 U/ml. As a result of this bias in posttreatment response, dogs were retrospectively classified in two separate groups (≥29 U/ml and <29 U/ml) in an effort to characterize the samples as an aid in the evaluation of response to treatment data.
Data analysis.
The 95% two-sided upper and lower confidence limits (based on the sign test) were calculated for the median percent decline of the control dogs and compared to the median percent decline observed for the treated dogs at 6 and 12 months posttreatment. The upper and lower confidence limits are calculated so that we know the minimal and maximal declines, respectively, expected for the control dogs. The median percent decline in C6 value for the control group was calculated using the last time point for each dog between 6 and 12 months (a total of 10 dogs, as dog C3 did not have a time point past 6 months). The median value was calculated as opposed to the mean value because the control group and the treated dogs in the 12-month group each had single outliers (dog C1 in the control group and dog 15 in the 12-month group). The one-sided Mann-Whitney test was used to show that the declines in C6 antibody for the treated dogs were significantly greater than those for the control dogs at both 6 and 12 months and was used to calculate the minimal expected difference (95% lower confidence limit) in percent decline for the treated dogs compared to the values for control dogs.
RESULTS
Study animals.
A total of 132 client-owned dogs were used in this study. Sixty-eight of these dogs tested positive with the in-clinic C6 ELISA kit (SNAP 3Dx test). Fifty-three of the positive animals were treated, and the remaining 15 were not treated and served as controls. Forty-one dogs (30 treated dogs and 11 untreated controls) were in the moderate-to-high-C6-level group (≥29 U/ml), and 27 dogs (23 treated dogs and 4 untreated controls) were in the low-C6-level group (<29 U/ml). Samples were obtained from each of these dogs at various time points and tested using the quantitative C6 ELISA.
Quantitative C6 antibody test results for control dogs with moderate-to-high C6 levels.
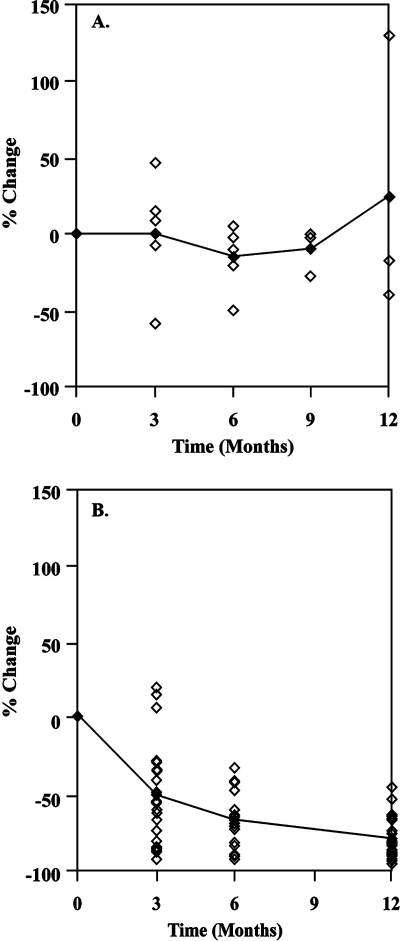
Control samples for the moderate-to-high-C6-level control group were obtained from 11 untreated B. burgdorferi antibody-positive dogs at the time of initial examination and at one or more time points during the succeeding 12 months. Quant C6 assay results (C6 U/ml) are given in Table 1 for individual dogs at various times after enrollment in the study. The percents change in C6 level for individual dogs during the period are shown in Fig. 1. The C6 antibody levels found in these dogs ranged from 46 to 278 U/ml at the start of the evaluation (mean level, 173 U/ml). The median percent drop in C6 value for this group, calculated using the last time point for each dog between 6 and 12 months (a total of 10 dogs) was 12.9%. The calculated expected minimal and maximal declines in C6 U/ml for dogs in this group are 1.1% and 31.3% (95% confidence limits), respectively, indicating that the typical expected drop in C6 value for untreated dogs would be between 1.1% and 31.3%.
FIG. 1.
Percents change in C6 U/ml in sera of 11 untreated dogs (A) and 30 treated dogs (B) 3 to 12 months following enrollment in the study. Data points represent percents decline in C6 value for individual animals with moderate-to-high serum C6 levels prior to treatment (C6 levels of 29 to 614 U/ml). The linear trend line shows the best fits of the average values of data points at 3, 6, 9, and 12 months. Time points that did not occur at 3, 6, 9, or 12 months were adjusted to the nearest value, which was typically within ±1 month.
Quantitative C6 antibody test results for treated positive dogs with moderate-to-high initial C6 levels.
Table 2 shows quantitative C6 antibody levels measured using sera obtained from 30 moderate-to-high-level dogs (no. 1 to 30) immediately prior to treatment and at various time points up to 12 months following treatment. The pretreatment C6 levels ranged from 29 to 614 U/ml (mean value, 174 U/ml). The median percents decline in C6 level relative to the pretreatment values at 6 and 12 months were 68.0% (16 samples; lower 95% confidence limit, 58.3%) and 83.3% (29 samples; 95% lower confidence limit, 76.0%), respectively. The C6 level decreased to an extent greater than the maximal decline expected for the untreated Lyme disease spirochete-infected dogs (31.3%) in each of the samples from 16 dogs at 6 months posttreatment and in each of the samples from 29 dogs at 12 months posttreatment (Fig. 1). The one-sided Mann-Whitney test provides evidence that the declines in C6 antibody for the treated dogs were significantly greater than those for the control dogs at both 6 and 12 months (P value of 0.00 for both time points). The minimal expected differences (95% lower confidence limit) in percent decline for the treated dogs compared to the values for control dogs are 40.4% and 54.5% at 6 and 12 months, respectively (P value of 0.00 for both time points).
TABLE 2.
C6 antibody levels in sera of dogs measured before treatment and 3, 6, and 12 months following treatment for dogs with initial C6 levels in the moderate-to-high-C6-level group (C6 levels of 29 to 614 U/ml)
| Dog no. | C6 antibody level (U/ml) at indicated no. of mos posttreatmenta
|
Treatment groupb | |||
|---|---|---|---|---|---|
| 0 | 3 | 6 | 12 | ||
| 1 | 29 | <10 | <10 | LD | |
| 2 | 31 | 35 | 12 | LD | |
| 3 | 45 | 16 | HD | ||
| 4 | 49 | 18 | 13 | <10 | LD |
| 5 | 56 | <10 | <10 | HD | |
| 6 | 64 | <10 | HD | ||
| 7 | 82 | 26 | 37 | LD | |
| 8 | 86 | 16 | <10 | HD | |
| 9 | 102 | 12 | <10 | <10 | HD |
| 10 | 102 | 66 | 36 | 17 | HD |
| 11 | 118 | 53 | 54 | HD | |
| 12 | 124 | 54 | 12 | HD | |
| 13 | 125 | 80 | 64 | 20 | HD |
| 14 | 137 | 25 | HD | ||
| 15 | 144 | 43 | 18 | HD | |
| 16 | 149 | 10 | 10 | 47 | LD |
| 17 | 154 | 107 | 86 | 82 | AM |
| 18 | 156 | 185 | 103 | 37 | LD |
| 19 | 158 | 166 | 91 | 57 | LD |
| 20 | 159 | 80 | 45 | 40 | HD |
| 21 | 178 | 26 | 10 | HD | |
| 22 | 237 | 138 | 38 | HD | |
| 23 | 251 | 21 | HD | ||
| 24 | 258 | 99 | 84 | HD | |
| 25 | 264 | 17 | HD | ||
| 26 | 289 | 10 | HD | ||
| 27 | 292 | 207 | 100 | 59 | HD |
| 28 | 326 | 45 | 12 | HD | |
| 29 | 440 | 112 | 70 | 45 | HD |
| 30 | 614 | 75 | 51 | 36 | HD |
Time points that did not occur at 3, 6, or 12 months were adjusted to the nearest value, which was typically within ±1 month.
AM, amoxicillin; LD, low dose of doxycycline; HD, high dose of doxycycline.
Follow-up samples were available for 15 treated dogs at time periods of 22 to 30 months. Within normal assay variation, the C6 level in each of these periods either was further reduced or stayed the same as that for the 1-year time point (data not shown).
Dogs with low initial serum C6 levels.
Table 3 shows C6 levels measured in sera from 23 treated dogs whose C6 levels prior to treatment were <29 U/ml. The C6 levels in samples for which actual values were reported (i.e., C6 levels of ≥10 U/ml) showed a small average change (increase of 4.5%) between 0 and 6 months. At time points of 12 months, the C6 levels in samples either were reduced compared to the pretreatment levels or remained at <10 U/ml. Associated with this group were four untreated control dogs (C12 to C15) with initial C6 levels of <29 U/ml (data not shown). The C6 level in the serum of each of the untreated dogs was <10 U/ml at the start of study and remained at <10 U/ml at time points 9 to 14 months later.
TABLE 3.
C6 antibody levels in sera of dogs measured before treatment and 3, 6, and 12 months following treatment for dogs with low initial C6 levels (<10 to 22 U/ml)a
| Dog no. | C6 antibody level (U/ml) at indicated no. of mos posttreatmentb
|
Treatment groupc | |||
|---|---|---|---|---|---|
| 0 | 3 | 6 | 12 | ||
| L7 | <10 | 10 | 14 | <10 | AM |
| L11 | 10 | 19 | 17 | <10 | AM |
| L12 | 10 | 10 | <10 | <10 | AM |
| L13 | 11 | <10 | <10 | <10 | HD |
| L14 | 12 | 14 | <10 | HD | |
| L15 | 13 | 10 | HD | ||
| L16 | 14 | 12 | <10 | <10 | HD |
| L17 | 15 | 23 | 13 | <10 | LD |
| L18 | 19 | 16 | 17 | HD | |
| L19 | 19 | 19 | 19 | 12 | HD |
| L20 | 21 | <10 | <10 | <10 | AM |
| L21 | 21 | 25 | 25 | LD | |
| L22 | 21 | 21 | 11 | AM | |
| L23 | 22 | 19 | 15 | LD | |
The C6 levels in samples from dogs L1, L2, L3, L4, L5, L6, L8, L9, and L10 were <10 U/ml at the initial time point and remained <10 U/ml at all time points tested.
Time points that did not occur at 3, 6, or 12 months were adjusted to the nearest value, which was typically within ±1 month.
AM, amoxicillin; LD, low dose of doxycycline; HD, high dose of doxycycline.
DISCUSSION
Several previous studies have shown that anti-C6 antibody levels remain stable in the sera of untreated experimentally infected monkeys and dogs for extended periods of time. The levels of C6 antibody in 10 untreated chronically infected monkeys remained essentially unchanged for a period of up to 3 years (9). A similar result was found for four untreated experimentally infected dogs held for a period of 69 weeks (16). In 11 naturally infected, untreated client-owned dogs with moderate-to-high C6 levels, we found that the mean percent decline in C6 antibody level following a period of time up to 12 months was 12.9%. We may have been able to observe a decline in C6 antibody level in the present study as opposed to the previous studies because the quantitative C6 ELISA was optimized to detect and quantify small changes in C6 antibody within this range.
In contrast to those in untreated animals, the C6 antibody levels in experimentally infected monkeys and dogs have been shown to decline rapidly following treatment (16). This observed decline in C6 antibody level in response to treatment was extended to human cases of early Lyme disease and was shown to coincide with a successful clinical response measured using defined physical symptoms (16, 17, 18). We observed substantial declines in C6 level in naturally infected dogs with moderate-to-high C6 levels receiving antibiotic therapy 6 and 12 months following treatment. The median percents decline in C6 antibody level were 68.0% and 83.3%, respectively, in sera obtained from 30 dogs at 6 months (16 samples) and 12 months (29 samples) following treatment. The C6 levels declined to extents greater than the maximal value (31.3%) expected for untreated antibody-positive dogs in 16 of 16 cases at 6 months posttreatment and in 29 of 29 cases at 12 months posttreatment.
There was little change in C6 antibody level following antibiotic therapy in dogs with very low C6 levels (<29 U/ml). A previously infected dog that had eliminated the organism but had maintained a low antibody titer would give results of this type. However, data indicating that antibiotic treatment diminishes but does not eliminate persistent infection would suggest that this is not the case (20, 21, 22). The continued low antibody levels in these dogs may reflect the presence of antigen and the subsequent stimulation of an antibody response. Resistance to antibiotic therapy in dogs has been postulated to be the result of low levels of spirochetes held in privileged locations or a change in the form of the spirochete itself (2, 20, 21, 22).
The samples with low antibody level results (>10 to 29 U/ml) in the Quant C6 test likely represent true positive samples, given that these results are within the dynamic range of the assay (10 to 400 U/ml) and that the samples also tested positive with the SNAP 3Dx test, which has been shown to have a specificity of 99.5% (4). The value of 29 U/ml can be used only as a guideline to predict the expected change in C6 antibody level following treatment. Studies with additional dogs are needed to determine the significance of this value as a guide for predicting changes in C6 level for different populations of dogs and for dogs in different stages of disease.
The data that we obtained using naturally infected dogs suggest that determining baseline and 6-month C6 antibody levels may be an effective way to monitor antibody levels in dogs infected with Borrelia burgdorferi following treatment. In this pilot study, a reduction in C6 level of greater than 58.3% was the typical result 6 months following treatment for dogs whose initial C6 levels are in the medium-to-high range. Failure of patients to respond to this degree may indicate treatment failure, recrudescence, noncompliance with administering treatment, or reexposure to and infection with B. burgdorferi during or after the treatment period. A second, prospective study is needed to confirm these observations before these criteria can be applied in clinical situations. Studies with additional dogs are needed to determine whether the extent of decline in C6 antibody can be used to predict the outcome of treatment, to determine if C6 antibody levels continue to remain low for extended times following treatment in the absence of recurrent infection, and to determine factors that influence the decline in C6 value after treatment.
In human patients with Lyme disease, antibodies to C6 were more likely to persist following treatment of patients who receive treatment at latter stages in the infection process (18). There were two dogs with clinical signs of Lyme disease that were not included in this study (see Materials and Methods). We did not attempt to categorize changes in C6 levels for these dogs, because these dogs constituted a clinically different subset and there were too few dogs to draw valid conclusions. Additional studies using clinical B. burgdorferi antibody-positive dogs are needed to determine if the response following treatment and following no treatment are similar to the serological response that occurs in nonclinical dogs.
Acknowledgments
We gratefully acknowledge Stacey Huth, Marilyn Strong-Townsend, Georges Mubalamate, and the staff at the IDEXX Reference Laboratory in North Grafton, MA, for their help in optimizing and standardizing the assay and the veterinary technical team at Durham Veterinary Hospital for collecting and organizing samples.
Footnotes
Published ahead of print on 14 November 2007.
REFERENCES
- 1.Bacon, R. M., B. J. Biggerstaff, M. E. Schriefer, R. D. Gilmore, M. T. Philipp, A. S. Steere, G. P. Wormser, A. R. Marques, and B. J. Johnson. 2003. Serodiagnosis of Lyme disease by kinetic enzyme-linked immunosorbent assay using recombinant VlsE1 or peptide antigens of Borrelia burgdorferi compared with 2-tiered testing using whole-cell lysates. J. Infect. Dis. 187:1187-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brorson, O., and S. H. Brorson. 1997. Transformation of cystic forms of Borrelia burgdorferi to normal, mobile spirochetes. Infection 25:240-246. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2002. Lyme disease—United States, 2000. Morb. Mortal. Wkly. Rep. 51:29-31. [PubMed] [Google Scholar]
- 4.Duncan, A. W., M. T. Correa, J. F. Levine, and E. B. Breitschwerdt. 2004. The dog as a sentinel for human infection: prevalence of Borrelia burgdorferi C6 antibodies in dogs from southeastern and mid-Atlantic states. Vector Borne Zoonotic Dis. 4:221-229. [DOI] [PubMed] [Google Scholar]
- 5.Lawrenz, M. B., J. M. Hardham, R. T. Owens, J. Nowakowski, A. C. Steere, G. P. Worsmer, and S. J. Norris. 1999. Human antibody responses to VlsE antigenic variation protein of Borrelia burgdorferi. J. Clin. Microbiol. 37:3997-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levy, S. A., S. W. Barthold, D. M. Dombach, and T. L. Wasmoen. 1993. Canine Lyme borreliosis. Compend. Contin. Educ. Pract. Vet. 15:833-848. [Google Scholar]
- 7.Levy, S. A., T. P. O'Connor, J. L. Hanscom, and P. Shields. 2002. Utility of an in-office C6 ELISA test kit for the determination of infection status of dogs naturally exposed to Borrelia burgdorferi. Vet. Ther. 3:308-315. [PubMed] [Google Scholar]
- 8.Levy, S. A. 2002. Use of C6 ELISA test to evaluate the efficacy of a whole-cell bacterin for the prevention of naturally transmitted Borrelia burgdorferi infection. Vet. Ther. 3:420-424. [PubMed] [Google Scholar]
- 9.Liang, F. T., A. C. Steere, A. R. Marques, B. J. Johnson, J. N. Miller, and M. T. Phlipp. 1999. Sensitive and specific serodiagnosis of Lyme disease by enzyme-linked immunosorbent assay with a peptide based on an immunodominant conserved region of Borrelia burgdorferi VlsE. J. Clin. Microbiol. 37:3990-3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang, F. T., and M. T. Philipp. 1999. Analysis of antibody response to invariable regions of VlsE, the variable surface antigen of Borrelia burgdorferi. Infect. Immun. 67:6702-6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang, F. T., R. H. Jacobsen, R. K. Straubinger, A. Grooters, and M. T. Philipp. 2000. Characterization of a Borrelia burgdorferi VlsE invariable region useful in canine Lyme disease serodiagnosis by enzyme-linked immunosorbent assay. J. Clin. Microbiol. 38:4160-4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lissman, B. A., E. M. Bosler, H. Camay, B. G. Ormiston, and J. L. Benach. 1984. Spirochete-associated arthritis (Lyme disease) in a dog. J. Am. Vet. Med. Assoc. 185:210-220. [PubMed] [Google Scholar]
- 13.Marangoni, A. M., V. Sambri, S. Accardo, F. Cavrini, V. Mondardini, A. Moroni, E. Storni, and R. Cevenini. 2006. A decrease in the immunoglobulin G antibody response against the VlsE protein of Borrelia burgdorferi sensu lato correlates with the resolution of clinical signs in antibiotic-treated patients with early Lyme disease. Clin. Vaccine Immunol. 13:525-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mogilyansky, E., C. C. Loa, M. E. Adelson, E. Mordechai, and R. C. Tilton. 2004. Comparison of Western immunoblotting and the C6 Lyme antibody test for laboratory detection of Lyme disease. Clin. Diagn. Lab. Immunol. 11:924-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Connor, T. P., K. J. Esty, J. L. Hanscom, P. Shields, and M. T. Philipp. 2004. Dogs vaccinated with common Lyme disease vaccines do not respond to IR6, the conserved immunodominant region of the VlsE surface protein of Borrelia burgdorferi. Clin. Diagn. Lab. Immunol. 11:458-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Philipp, M. T., L. C. Bowers, P. T. Fawcett, M. B. Jacobs, F. T. Liang, A. R. Marques, P. D. Mitchell, J. E. Purcell, M. S. Ratterree, and R. K. Straubinger. 2001. Antibody response to IR6, a conserved immunodominant region of the VlsE lipoprotein, wanes rapidly after antibiotic treatment of Borrelia burgdorferi infection in experimental animals and in humans. J. Infect. Dis. 184:870-878. [DOI] [PubMed] [Google Scholar]
- 17.Philipp, M. T., A. R. Marques, P. T. Fawcett, L. G. Dally, and D. S. Martin. 2003. C6 test as an indicator of therapy outcome for patients with localized or disseminated Lyme borreliosis. J. Clin. Microbiol. 41:4955-4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Philipp, M. T., G. P. Wormser, A. R. Marques, S. Bittker, D. S. Martin, J. Nowakowski, and L. G. Dally. 2005. A decline in C6 antibody titer occurs in successfully treated patients with culture-confirmed early localized or early disseminated Lyme borreliosis. Clin. Diagn. Lab. Immunol. 12:1069-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rondeau, M. P., R. M. Walton, S. Bissett, K. J. Drobatz, and R. J. Washabau. 2005. Suppurative, nonseptic polyarthropathy in dogs. J. Vet. Intern. Med. 19:654-662. [DOI] [PubMed] [Google Scholar]
- 20.Straubinger, R. K., A. F. Straubinger, B. A. Summers, and R. H. Jacobson. 2000. Status of Borrelia burgdorferi infection after antibiotic treatment and the effects of corticosteroids: an experimental study. J. Vet. Intern. Med. 181:1069-1081. [DOI] [PubMed] [Google Scholar]
- 21.Straubinger, R. K., B. A. Summers, Y. F. Chang, and M. J. Appel. 1997. Persistence of Borrelia burgdorferi in experimentally infected dogs after antibiotic treatment. J. Clin. Microbiol. 35:111-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Straubinger, R. K. 2000. PCR-based quantification of Borrelia burgdorferi organisms in canine tissues over a 500-day postinfection period. J. Clin. Microbiol. 38:2191-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Summers, B. A., A. F. Straubinger, R. H. Jacobson, Y. F. Chang, M. J. Appel, and R. K. Straubinger. 2005. Histopathological studies of experimental Lyme disease in the dog. J. Comp. Pathol. 133:1-13. [DOI] [PubMed] [Google Scholar]