Abstract
Immunogenicity evaluations in human papillomavirus (HPV) vaccine trials have relied on serological samples, yet cervical antibodies are likely to be most relevant for protection against infection. In order to assess functional antibody levels at the cervix, the secreted-alkaline-phosphatase neutralization assay (SEAPNA) was used to measure HPV-neutralizing activity. We assessed the variability of the SEAPNA with serum samples after vaccination with an HPV type 16 (HPV16) L1 virus-like particle vaccine and whether the SEAPNA can be used to monitor neutralizing activity at the cervix. The SEAPNA has an overall coefficient of variation of 29.3%. Recovery from ophthalmic sponges was assessed by spiking V5 (mouse anti-HPV16) antibody onto and extracting it from sterile Merocel and Ultracell sponges and sponges used to collect specimens from participants. V5 recovery from sterile Merocel sponges was complete, yet that from Ultracell sponges was null. The mean V5 recoveries from participant Ultracell and Merocel sponges were 61.2% and 93.5%, respectively, suggesting that Merocel sponges are more appropriate for specimen collection. The SEAPNA can be applied to determine the surrogates of protection and to examine the durability of protection at the cervix.
Prophylactic human papillomavirus (HPV) L1-based virus-like particle (VLP) vaccines have been shown to be safe, immunogenic, and protective against cervical infection and the associated lesions by homologous HPV types (7-9, 11, 17, 18). In addition, HPV VLP vaccinations induce neutralizing antibodies not only in the periphery but also at the cervix (6, 12). Several assays for monitoring HPV type-specific antibody responses have been developed; however, validation efforts for these assays have focused on serum rather than the cervix, where protection is executed.
HPV VLP enzyme-linked immunosorbent assays (ELISAs) have been used to determine serum antibody titers in epidemiological studies of HPV infection and in HPV vaccine trials (8, 9, 15, 16, 19). Enzyme-linked immunosorbent-based assays have the advantage of being applicable for large-scale studies. However, these assays lack the ability to discern between neutralizing and nonneutralizing antibodies and may have the disadvantage of detecting antibodies to yeast and baculovirus-derived proteins, compromising their specificity. It is therefore still unclear whether ELISAs are optimal for the evaluation of the levels of protective antibodies at the cervix.
Recently, a pseudovirion (PsV) neutralization assay (a secreted-alkaline-phosphatase neutralization assay [SEAPNA]) was developed by Pastrana et al. to evaluate the neutralizing potential against HPV (13). This assay has the advantage of specifically measuring the biological activity believed to be relevant for protection (i.e., neutralization).
Here, we explored whether the SEAPNA could be used to monitor neutralizing antibody levels at the cervix by the use of two different ophthalmic sponges (Merocel and Ultracell) commonly used in large-scale studies for cervical mucus sampling. We evaluated the effect of a standard extraction buffer (EB) previously used for cervical secretion extraction and examined the recovery levels of V5 (mouse anti-HPV type 16 [HPV16] monoclonal type-specific neutralizing antibody) (3) from sterile, unused Merocel and Ultracell sponges and from Merocel and Ultracell sponges used to collect specimens from participants via the SEAPNA. Finally, we evaluated the use of a mouse monoclonal immunogobulin G (IgG) for spiking and recovery from study participant specimens as a means of controlling for recovery efficiency in future efforts and attempted to improve recovery from collection devices.
MATERIALS AND METHODS
SEAPNA with serum. (i) Participant specimens.
Sera collected 1 month after the first vaccination or 1 month after the second vaccination from 12 participants enrolled in a phase I trial of a VLP HPV16 vaccine were used (9). This study was conducted according to the guidelines established by the Joint Committee for Clinical Investigation of the Johns Hopkins University School of Medicine and its institutional review board for human experimentation.
(ii) Cell culture.
293TT cells were expanded and cultured as previously described (13).
(iii) SEAPNA.
The SEAPNA was performed as previously described with a few modifications (13). Serum samples were serially diluted in fourfold increments with neutralization buffer (NB). Controls, set up in triplicate, included (i) NB alone, (ii) NB plus HPV16 PsV, (iii) V5 (mouse anti-HPV16) (3) plus HPV16 PsV, and (iv) 5B6 (mouse anti-bovine papillomavirus 1) (14) plus HPV16 PsV. Diluted serum was incubated with HPV16 PsV at 4°C for 1 h in duplicate wells at a 1:5 ratio. Then, the samples were transferred to the 293TT cells and incubated for 72 h. Following incubation, supernatants were transferred to 96-well V-bottom plates, clarified by centrifugation, and frozen at −80°C before SEAP analysis. SEAP was detected using the Great EscAPe SEAP assay kit, according to the manufacturer's protocol (BD Biosciences-Clontech Laboratories, Inc., Mountain View, CA). Samples were read on a chemiluminescence plate reader (SpectraMax M5; Molecular Devices, Sunnyvale, CA). Serum neutralization titers were defined as the reciprocal of the calculated dilution that caused a 50% reduction in SEAP activity based on linear interpolation.
(iv) Testing format.
To evaluate within-plate, between-day, and between-technician variability for the SEAPNA, specimens from the 12 participants described above were each tested twice by two technicians, each on two different days.
(v) Statistical analysis.
For each technician, a nested, within-person analysis of variance was used to test for assay reproducibility across plates. Data were analyzed on the natural logarithmic scale.
SEAPNA with cervical secretions. (i) Collection sponges.
The cellulose-based Ultracell ophthalmic sponge (Ultracell Medical Technologies, Inc., North Stonington, CT) and the polyvinyl acetate-based Merocel ophthalmic sponge (Medtronic Ophthalmics, Jacksonville, FL) were used in this study.
(ii) Participant specimens.
Cervical secretion specimens were taken from participants in a natural history study of HPV and cervical neoplasia in Costa Rica (1) using either Ultracell (n = 35) or Merocel (n = 20) sponges. The Ultracell or Merocel sponge was gently placed in the cervical os for 30 seconds to allow for passive absorption of cervical secretions (2, 10) prior to Pap smear sampling. Sponges were then stored in cryovials, frozen, and shipped to the United States in liquid nitrogen. Upon arrival in the United States, the samples were stored at −80°C until the extraction procedure. Specimens obtained from participants in the natural history study were used for spike-recovery experiments, because they were presumed to have low/undetectable levels of neutralizing antibodies. Prior to the extraction process, the sponges were thawed on ice and participant sponge weights were recorded. All participants provided informed consent, and the study was approved by the National Cancer Institute (NCI) and the local institutional review board in Costa Rica.
(iii) Effect of cervical sponge extraction buffer on SEAPNA.
Fourfold serial dilutions (1:40 to 1:40,960) of the EB (containing phosphate-buffered saline [Invitrogen, Grand Island, NY], 256 mM NaCl [Sigma-Aldrich, St. Louis, MO], and 100 μg/ml aprotinin [Sigma-Aldrich, St. Louis, MO]) (2) were evaluated in triplicate in the SEAPNA. Results were expressed as follows: % of the control (NB plus HPV16 PsV) = (mean number of RLU for diluted EB plus HPV16 PsV/mean number of RLU for NB plus HPV16 PsV) × 100, where RLU is relative light units.
(iv) Recovery of antibodies from unused Ultracell and Merocel sponges.
To assess the recovery of neutralizing antibodies from ophthalmic sponges, V5 was spiked onto sterile unused sponges at 1/1,000 and 1/2,000 dilutions. The mean neutralization values at the 1/1,000 and 1/2,000 dilutions were 93.5% and 69.3%, respectively.
Sponges were extracted by use of Spin-X tubes (Corning Inc., Corning, NY). Ultracell sponges were spiked with 30 μl (average volume collected in participant sponges [12]) of V5 at a dilution of 1/1,000 (n = 8 sponges) or 1/2,000 (n = 8). Merocel sponges were spiked with V5 at a dilution of 1/1,000 (n = 16 sponges) or 1/2,000 (n = 16). In addition, the following controls were set up and spiked with V5 at dilutions of 1/1,000 and 1/2,000: (i) a Spin-X tube without a sponge, (ii) a microcentrifuge tube, and (iii) a microcentrifuge tube extracted with neutralization buffer.
The extraction process has previously been described (2). Briefly, 300 μl of cervical EB was slowly added to the top of the sponge. Following a 30-min incubation at 4°C, the sponges were centrifuged at 13,000 × g for 15 min at 4°C. An additional 300 μl of EB was added to the sponge and immediately centrifuged. Following the second extraction, 4 μl of fetal calf serum was added to each sample, and the total volume recovered from each sponge was ∼560 μl. The samples were aliquoted and frozen at −80°C until further testing.
Aliquots were tested in triplicate. The SEAPNA was performed as described above. Calculations were as follows: % neutralization = 1 − [(mean RLU value of sample X/mean RLU value for NB plus HPV16 PsV) × 100]; % recovery = (% neutralization value of sample X/% neutralization value of V5 spiked into NB) × 100. All values below 0% were arbitrarily given a value of 0 in order to calculate the mean recovery.
Recovery of antibodies from cervical secretions collected from participants by use of Ultracell and Merocel sponges.
As described above, participant Ultracell and Merocel sponges were spiked with 30 μl of V5 at a dilution of 1/1,000 (n = 15 and n = 10, respectively) or 1/2,000 (n = 20 and n = 10, respectively).
The extraction process, SEAP assay, and calculations were performed as described above.
Effect of detergent use on SEAPNA.
The effect of three detergents (Empigen BB [Calbiochem, La Jolla, CA]; Triton X-100 [Sigma, St. Louis, MO], and Tween 20 [Sigma, St. Louis, MO]; concentrations of 0.05% to 0.000005%) on 293TT cell viability was tested using a cell proliferation kit (XTT; Roche Diagnostics, Indianapolis, IN), according to the manufacturer's recommendations, in three separate experiments.
The three detergents were incubated with HPV16 PsV as described for SEAPNA above at several concentrations (0.025% to 0.000005%) in five separate experiments. The results were calculated based on the results for the control wells (EB plus HPV16 PsV).
Effect of mouse monoclonal IgG on SEAPNA.
Mouse IgG1-trinitrophenol (IgG1-TNP; 31.25 to 1,000 ng/ml) (BD Biosciences, San Jose, CA) was incubated with HPV16 PsV as described for SEAPNA above. Calculations were performed as described for SEAPNA above, and tests were performed in duplicate wells. The effects of the mouse IgG1-TNP on V5 neutralization in the SEAPNA were evaluated in triplicate. The variation between the recovery values for V5 alone and V5 and mouse IgG1-TNP was calculated as the percent change between these two groups.
Use of Empigen BB and bovine serum albumin (BSA) to improve antibody extractions from unused Ultracell sponges. (i) Recovery of mouse IgG1-TNP from detergent-extracted sponges.
Thirty microliters of mouse IgG1-TNP (1,000 ng/ml) was spiked onto each of two Ultracell sponges and eluted in EB or EB containing Empigen BB (0.5% to 0.005%). In addition, 30 μl of mouse IgG1-TNP (1,000 ng/ml) was spiked into EB and ELISA buffer as controls. The extraction process was performed as described above, and the amount of mouse IgG1-TNP eluted was evaluated by testing each sample in duplicate wells with a mouse IgG1 ELISA detection kit (Bethyl Laboratories, Inc., Montgomery, TX). The recovery was calculated as follows: % recovery = (quantitative value of sample X/quantitative value of control) × 100.
(ii) Effect of BSA on recovery of mouse IgG1-TNP from Ultracell sponges.
BSA was tested in three formats as follows. (i) Two sponges were incubated with BSA (300 μl each, 0.5% to 0.00005%), diluted in EB for 1 h at 4°C, and centrifuged in a Spin-X tube for 15 min at 13,000 × g at 4°C. Then, 30 μl of mouse IgG1-TNP (1,000 ng/ml) was spiked onto each sponge and eluted in EB. (ii) Mouse IgG1-TNP diluted in BSA (0.5% to 0.00005%) was spiked (30 μl; 1,000 ng/ml) onto each of two sponges and eluted in EB. (iii) Mouse IgG1-TNP (30 μl; 1,000 ng/ml) was spiked onto each of two sponges and eluted with EB containing BSA (10% to 0.5%). Two separate controls, EB and ELISA buffer, were spiked with mouse IgG1-TNP to calculate the recovery. The extraction process was performed as described above, and the mouse IgG1-TNP eluted was quantified by an ELISA as described above.
RESULTS
Serum SEAPNA.
To assess the SEAPNA variability, 12 serum samples from vaccine recipients were tested in duplicate per plate with two technicians setting up two plates for each sample, on two different days. The titer range for the samples from the 12 vaccinees was 1/98 to 1/16,731. The overall coefficient of variation (CV) of the assay was 29.3% for the SEAPNA, with most of the variability occurring within the plate (26.3% CV). The between-plate variability was low (7.4% CV), and the intraclass correlation coefficient was high (91.1%), demonstrating that biological variability among women is much larger than the assay variability.
Evaluation of SEAPNA for measurement of antibodies collected using ophthalmic sponges.
The EB had little to no effect on the SEAPNA (95% confidence interval [CI] of 98.3% to 111% of the control in two separate experiments). The total mean recovery of V5 from sterile unused Ultracell sponges (n = 16) was only 2.5% (95% CI, −0.6% to 5.6%) (Table 1). In contrast, Merocel sponges (n = 32) had a mean recovery of 102% (95% CI, 98.1% to 106%) (Table 1).
TABLE 1.
Cumulative recoveries of V5 (anti-HPV16) spiked at 1/1,000 and 1/2,000 onto Merocel and Ultracell spongesa
| Sponge type | No. of sponges | % V5 recovery
|
||
|---|---|---|---|---|
| Mean | SD | 95% CI | ||
| Merocel | ||||
| Sterile | 32 | 102 | 11.4 | 98.1-106 |
| Participant | 20 | 93.5 | 15.2 | 86.4-101 |
| Ultracell | ||||
| Sterile | 16 | 2.5 | 5.8 | −0.64-5.6 |
| Participant | 35 | 61.2 | 49.3 | 44.3-78.2 |
Mean, SD, and 95% CI values were calculated by combining the results obtained from sponges spiked with V5 at dilutions of 1/1,000 and 1/2,000.
Subsequently, we examined the recovery of V5 spiked prior to extraction onto the polyvinyl acetate-based Merocel sponges used in the collection of cervical secretions from 20 participants enrolled in an HPV natural history cohort study in Guanacaste, Costa Rica. The combined results from two separate experiments using spikes with two different dilutions of V5 demonstrate that 18 of 20 (90%) participant Merocel sponges had recoveries of >80%, with a mean recovery of 93.5% (95% CI, 86.4% to 101%) (Table 1). The recovery of V5 from the participant Ultracell sponges was more variable than that observed for Merocel sponges, with 18 of 35 (51.4%) having recoveries of >80%. In four experiments with spikes at two different dilutions of V5, the mean recovery of V5 neutralization was 61.2% (95% CI, 44.3% to 78.2%) from participant Ultracell sponges.
It is interesting to note that the participant Ultracell sponges that gave the poorest recoveries also had the lowest weights, on average. The sponges with greater than 50% recovery were heavier (sponge weights were 0.1280 g), while those with less than 50% recovery had a mean weight of 0.0896 g (P < 0.0001, Mann-Whitney test). The menopausal statuses of participants who provided the Ultracell specimens were also indicative of poor recovery status, a phenomenon likely explained by the lower sponge weights obtained from menopausal participants, who are known to have decreased levels of secretions in their genital tracts.
Strategies to monitor and improve antibody recovery from cervical secretions collected using cellulose-based Ultracell sponges.
The results from Ultracell sponges, either sterile or from participants, were null or very erratic, respectively. Due to the use of the Ultracell sponges in some natural history studies, we focused on improving the recovery of neutralizing antibodies and implementing controls to monitor extraction efficiency. Detergents and BSA were used to improve recovery, while the spiking of mouse IgG1-TNP onto sponges after collection and prior to extraction was tested as an agent to monitor extraction efficiency.
Mouse IgG1-TNP had no adverse effects on PsV infectivity and V5 neutralization (data not shown).
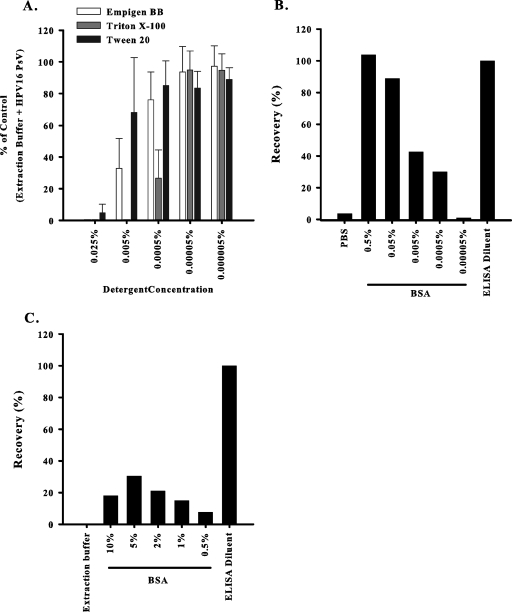
Strategies to improve recovery from Ultracell sponges included treating Ultracell sponges with three different detergents and blocking nonspecific binding to the cellulose-based Ultracell sponge. We discovered the maximum concentrations of Empigen BB (0.0005%), Triton X-100 (0.00005%), and Tween 20 (0.0005%) that could be cultured with the 293TT cells without having an impact on cell viability. Similarly, the maximum concentrations of Empigen BB, Triton X-100, and Tween 20 that could be used in the SEAPNA were 0.00005% (Fig. 1A). Next, we tested whether Empigen BB could improve the recovery of antibodies from unused sterile Ultracell sponges. Even at Empigen BB concentrations as high as 0.5%, only 17.1% of the mouse IgG1-TNP could be recovered. Thus, the three detergents tested in this study do not appear to improve the extraction of neutralizing antibodies from Ultracell sponges.
FIG. 1.
Strategies implemented to improve antibody extraction from Ultracell sponges. (A) Detergents influence the SEAPNA in a concentration-dependent manner. Each condition was set up in triplicate, and the error bars were calculated from the standard deviations of the results of five separate experiments. (B) Recovery of mouse IgG1-TNP from Ultracell sponges incubated with BSA is dependent upon BSA concentration. BSA (0.5% to 0.00005%) was incubated with Ultracell sponges before they were spiked with mouse IgG1-TNP. The extracts were analyzed in a mouse IgG1 ELISA to determine recovery. (C) The addition of BSA to the EB slightly improved the recovery of mouse IgG1-TNP from Ultracell sponges. Mouse IgG1-TNP was spiked onto the sponges at 1,000 ng/ml. The extracts were analyzed in a mouse IgG1 ELISA to determine recovery. The results are representative of two separate experiments.
BSA was used in an attempt to block nonspecific binding of the antibodies to the cellulose-based Ultracell sponges. We compared the recoveries of mouse IgG1-TNP antibodies from the following: (i) Ultracell sponges soaked in BSA prior to spiking with mouse IgG1-TNP, (ii) Ultracell sponges spiked with mouse IgG1-TNP diluted in BSA, and (iii) Ultracell sponges spiked with mouse IgG1-TNP and extracted in BSA. The results indicate that BSA at concentrations of >0.05% added to the sponge (Fig. 1B) or mouse IgG1-TNP prior to spiking (data not shown) was sufficient to effectively elute the mouse IgG1-TNP. In contrast, adding BSA to the EB could not sufficiently extract the purified mouse IgG1-TNP previously spiked onto Ultracell sponges (Fig. 1C).
DISCUSSION
The SEAPNA is a novel assay designed to directly measure HPV type-specific neutralizing activity. SEAPNA variability has not been extensively characterized, and the assay has not been formally validated for use with genital tract specimens. In the present study, we defined the components of variability of the SEAPNA using serum and evaluated the ability of the assay to measure neutralizing activity in cervical secretion specimens.
While the SEAPNA is more variable than the ELISA (5) and the competitive Luminex assay (4), the level of variability seen among women was far greater than that observed for the SEAPNA, as indicated by the high intraclass correlation coefficient observed with serum samples (91.1%). Important conclusions from our methodological work to evaluate the ability of the SEAPNA to measure neutralizing activity at the cervix are as follows. First, we have shown that neutralizing antibodies can be completely recovered from Merocel sponges, while recoveries from Ultracell sponges are low and more variable. This is likely explained by the chemical compositions of Merocel (polyvinyl acetate) and Ultracell (cellulose) sponges. Our poor recovery results from Ultracell sponges appear to contrast with a previous study that analyzed the recovery of cytokines and antibodies from different sponge materials (2). However, the Ultracell sponges evaluated in the study by Castle et al. (2) were made of polyvinyl alcohol, in contrast to the cellulose-based Ultracell sponges used in this study. It is intriguing to note that in the study by Castle et al., the performance of the cellulose-based Weck-Cel sponges was not dramatically different from that of the polyvinyl alcohol-based Ultracell or polyvinyl acetate-based Merocel sponges tested here. Cytokines and antibodies in that study may have been spiked onto the sponges in the presence of a carrier protein, which would have decreased the effect of nonspecific binding with the sponges, as noticed in our study. Second, we have shown that the presence of cervical mucus has little impact on the ability of the SEAPNA to measure neutralizing activity in material extracted from Merocel sponges (90% of the specimens evaluated had recoveries of >80%). This contrasts with the Ultracell results, in which only 51.4% of the sponges tested had recoveries of >80%. It is important to note that Ultracell sponges with the lowest recoveries also had the lowest cervical secretion weights, suggesting that the degree of nonspecific trapping of antibodies onto cellulose sponges was probably dependent on the amount of protein preadsorbed onto the sponge. This was confirmed in additional experiments that demonstrated an increase in antibody recovery from Ultracell sponges that had been presoaked in BSA prior to spiking with V5 compared to those spiked with V5 before the addition of BSA.
Third, we have demonstrated that the spiking of Ultracell or Merocel sponges with mouse IgG1-TNP can be used to assess recovery efficiency during extraction without a negative impact on the SEAPNA. This finding is of particular importance for studies that have used cellulose-based Ultracell sponges to collect cervical secretions, given the variable and incomplete recovery seen with this collection device.
Finally, we explored alternative extraction protocols to increase the recovery of antibodies from Ultracell sponges and demonstrated that the use of detergents and the use of BSA in the EB were not viable options. The levels of detergent required for optimal extraction were cytotoxic in the SEAPNA, and BSA would need to be added prior to specimen collection to block nonspecific binding to the sponge. Additional studies will be required to define a protocol capable of efficiently eluting antibodies from non-BSA-treated Ultracell sponges without interfering with the SEAPNA.
In summary, this study demonstrates that SEAPNA variability is modest relative to the variability observed among vaccinated women and that the SEAPNA can be used to measure neutralizing titers in cervical secretions. Collection devices made of polyvinyl acetate were shown to be superior to cellulose-based sponges. For studies that have used cellulose-based collection devices, a method is described to enable direct assessment of recovery efficiency from individual specimens. Additional evaluation of cervical secretion collection, processing methods, and direct comparisons between alternative testing procedures (e.g., the SEAPNA versus the standard ELISA) are needed to define the best approaches to measure immunogenicity at the cervix in clinical trials.
Acknowledgments
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract N01-CO-12400.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does the mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
Footnotes
Published ahead of print on 21 November 2007.
REFERENCES
- 1.Bratti, M. C., A. C. Rodriguez, M. Schiffman, A. Hildesheim, J. Morales, M. Alfaro, D. Guillen, M. Hutchinson, M. E. Sherman, C. Eklund, J. Schussler, J. Buckland, L. A. Morera, F. Cardenas, M. Barrantes, E. Perez, T. J. Cox, R. D. Burk, and R. Herrero. 2004. Description of a seven-year prospective study of human papillomavirus infection and cervical neoplasia among 10000 women in Guanacaste, Costa Rica. Rev. Panam. Salud Publica 15:75-89. [DOI] [PubMed] [Google Scholar]
- 2.Castle, P. E., A.-C. Rodriguez, F. P. Bowman, R. Herrero, M. Schiffman, M. C. Bratti, L. A. Morera, D. Schust, P. Crowley-Nowick, and A. Hildesheim. 2004. Comparison of ophthalmic sponges for measurements of immune markers from cervical secretions. Clin. Diagn. Lab. Immunol. 11:399-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Christensen, N. D., J. Dillner, C. Eklund, J. J. Carter, G. C. Wipf, C. A. Reed, N. M. Cladel, and D. A. Galloway. 1996. Surface conformational and linear epitopes on HPV-16 and HPV-18 L1 virus-like particles as defined by monoclonal antibodies. Virology 223:174-184. [DOI] [PubMed] [Google Scholar]
- 4.Dias, D., J. Van Doren, S. Schlottmann, S. Kelly, D. Puchalski, W. Ruiz, P. Boerckel, J. Kessler, J. M. Antonello, T. Green, M. Brown, J. Smith, N. Chirmule, E. Barr, K. U. Jansen, and M. T. Esser. 2005. Optimization and validation of a multiplexed luminex assay to quantify antibodies to neutralizing epitopes on human papillomaviruses 6, 11, 16, and 18. Clin. Diagn. Lab Immunol. 12:959-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dillner, J. 1999. The serological response to papillomaviruses. Semin. Cancer Biol. 9:423-430. [DOI] [PubMed] [Google Scholar]
- 6.Fife, K. H., C. M. Wheeler, L. A. Koutsky, E. Barr, D. R. Brown, M. A. Schiff, N. B. Kiviat, K. U. Jansen, H. Barber, J. F. Smith, A. Tadesse, K. Giacoletti, P. R. Smith, G. Suhr, and D. A. Johnson. 2004. Dose-ranging studies of the safety and immunogenicity of human papillomavirus type 11 and type 16 virus-like particle candidate vaccines in young healthy women. Vaccine 22:2943-2952. [DOI] [PubMed] [Google Scholar]
- 7.Harper, D. M., E. L. Franco, C. Wheeler, D. G. Ferris, D. Jenkins, A. Schuind, T. Zahaf, B. Innis, P. Naud, N. S. De Carvalho, C. M. Roteli-Martins, J. Teixeira, M. M. Blatter, A. P. Korn, W. Quint, and G. Dubin. 2004. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet 364:1757-1765. [DOI] [PubMed] [Google Scholar]
- 8.Harper, D. M., E. L. Franco, C. M. Wheeler, A. B. Moscicki, B. Romanowski, C. M. Roteli-Martins, D. Jenkins, A. Schuind, S. A. Costa Clemens, and G. Dubin. 2006. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 367:1247-1255. [DOI] [PubMed] [Google Scholar]
- 9.Harro, C. D., Y. Y. Pang, R. B. Roden, A. Hildesheim, Z. Wang, M. J. Reynolds, T. C. Mast, R. Robinson, B. R. Murphy, R. A. Karron, J. Dillner, J. T. Schiller, and D. R. Lowy. 2001. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J. Natl. Cancer Inst. 93:284-292. [DOI] [PubMed] [Google Scholar]
- 10.Hildesheim, A., L. M. McShane, M. Schiffman, M. C. Bratti, A. C. Rodriguez, R. Herrero, L. A. Morera, F. Cardenas, L. Saxon, F. P. Bowman, and P. A. Crowley-Nowick. 1999. Cytokine and immunoglobulin concentrations in cervical secretions: reproducibility of the Weck-cel collection instrument and correlates of immune measures. J. Immunol. Methods 225:131-143. [DOI] [PubMed] [Google Scholar]
- 11.Mao, C., L. A. Koutsky, K. A. Ault, C. M. Wheeler, D. R. Brown, D. J. Wiley, F. B. Alvarez, O. M. Bautista, K. U. Jansen, and E. Barr. 2006. Efficacy of human papillomavirus-16 vaccine to prevent cervical intraepithelial neoplasia: a randomized controlled trial. Obstet. Gynecol. 107:18-27. [DOI] [PubMed] [Google Scholar]
- 12.Nardelli-Haefliger, D., D. Wirthner, J. T. Schiller, D. R. Lowy, A. Hildesheim, F. Ponci, and P. De Grandi. 2003. Specific antibody levels at the cervix during the menstrual cycle of women vaccinated with human papillomavirus 16 virus-like particles. J. Natl. Cancer Inst. 95:1128-1137. [DOI] [PubMed] [Google Scholar]
- 13.Pastrana, D. V., C. B. Buck, Y. Y. Pang, C. D. Thompson, P. E. Castle, P. C. FitzGerald, S. Kruger Kjaer, D. R. Lowy, and J. T. Schiller. 2004. Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology 321:205-216. [DOI] [PubMed] [Google Scholar]
- 14.Roden, R. B. S., E. M. Weissinger, D. W. Henderson, F. Booy, R. Kirnbauer, J. F. Mushinski, D. R. Lowy, and J. T. Schiller. 1994. Neutralization of bovine papillomavirus by antibodies to L1 and L2 capsid proteins. J. Virol. 68:7570-7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shin, H. R., D. H. Lee, R. Herrero, J. S. Smith, S. Vaccarella, S. H. Hong, K. Y. Jung, H. H. Kim, U. D. Park, H. S. Cha, S. Park, A. Touze, N. Munoz, P. J. Snijders, C. J. Meijer, P. Coursaget, and S. Franceschi. 2003. Prevalence of human papillomavirus infection in women in Busan, South Korea. Int. J. Cancer 103:413-421. [DOI] [PubMed] [Google Scholar]
- 16.Silverberg, M. J., M. F. Schneider, B. Silver, K. M. Anastos, R. D. Burk, H. Minkoff, J. Palefsky, A. M. Levine, and R. P. Viscidi. 2006. Serological detection of human papillomavirus type 16 infection in human immunodeficiency virus (HIV)-positive and high-risk HIV-negative women. Clin. Vaccine Immunol. 13:511-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Villa, L. L., K. A. Ault, A. R. Giuliano, R. L. Costa, C. A. Petta, R. P. Andrade, D. R. Brown, A. Ferenczy, D. M. Harper, L. A. Koutsky, R. J. Kurman, M. Lehtinen, C. Malm, S. E. Olsson, B. M. Ronnett, F. E. Skjeldestad, M. Steinwall, M. H. Stoler, C. M. Wheeler, F. J. Taddeo, J. Yu, L. Lupinacci, R. Railkar, R. Marchese, M. T. Esser, J. Bryan, K. U. Jansen, H. L. Sings, G. M. Tamms, A. J. Saah, and E. Barr. 2006. Immunologic responses following administration of a vaccine targeting human papillomavirus types 6, 11, 16, and 18. Vaccine 24:5571-5583. [DOI] [PubMed] [Google Scholar]
- 18.Villa, L. L., R. L. Costa, C. A. Petta, R. P. Andrade, K. A. Ault, A. R. Giuliano, C. M. Wheeler, L. A. Koutsky, C. Malm, M. Lehtinen, F. E. Skjeldestad, S. E. Olsson, M. Steinwall, D. R. Brown, R. J. Kurman, B. M. Ronnett, M. H. Stoler, A. Ferenczy, D. M. Harper, G. M. Tamms, J. Yu, L. Lupinacci, R. Railkar, F. J. Taddeo, K. U. Jansen, M. T. Esser, H. L. Sings, A. J. Saah, and E. Barr. 2005. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 6:271-278. [DOI] [PubMed] [Google Scholar]
- 19.Wang, S. S., M. Schiffman, T. S. Shields, R. Herrero, A. Hildesheim, M. C. Bratti, M. E. Sherman, A. C. Rodriguez, P. E. Castle, J. Morales, M. Alfaro, T. Wright, S. Chen, B. Clayman, R. D. Burk, and R. P. Viscidi. 2003. Seroprevalence of human papillomavirus-16, -18, -31, and -45 in a population-based cohort of 10000 women in Costa Rica. Br. J. Cancer 89:1248-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]