Abstract
RWJ-54428 (also known as MC-02,479) is a new cephalosporin with promising activity against gram-positive bacteria. The pharmacodynamics (PDs) of RWJ-54428 against Staphylococcus aureus, Streptococcus pneumoniae, and Enterococcus faecalis were studied in a neutropenic mouse thigh infection model. The RWJ-54428 MICs ranged from 0.25 to 1 mg/liter. Mice with ca. 106 CFU/thigh at the initiation of therapy were treated intraperitoneally with RWJ-54428 at doses that ranged from 3 to 1,200 mg/kg of body weight/day (in 2, 3, 4, 6, or 12 divided doses) for 24 h. The maximal reductions in bacterial counts in thigh tissues at 24 h for the methicillin-resistant S. aureus, penicillin-resistant S. pneumoniae, and E. faecalis strains were −2.8, −3.8, and −1.7 log10 CFU/thigh, respectively. The percentage of a 24-h dosing interval that the unbound serum RWJ-54428 concentrations exceeded the MIC (fT>MIC) was the pharmacokinetic (PK)-PD parameter that best described the efficacy of RWJ-54428. The fT>MICs for a bacteriostatic effect (no net change in the numbers of CFU/thigh over 24 h) ranged from 14 to 20% for staphylococci and streptococci; for maximal reductions in the numbers of CFU/thigh, the fT>MICs ranged from 22 to 36% for these strains. For E. faecalis, the ranges of fT>MICs for static and maximal effects were 30 to 46% and 55 to 60%, respectively. These data show that treatment with RWJ-54428 results in marked antibacterial effects in vivo, with the PK-PD parameters for efficacy being comparable to those for the efficacy of penicillins and carbapenems active against staphylococci and pneumococci.
RWJ-54428 is a novel cephalosporin with good potency in vitro and in vivo against Staphylococcus aureus (including methicillin-resistant S. aureus [MRSA] and glycopeptide-intermediate isolates), Streptococcus pneumoniae (including penicillin-resistant S. pneumoniae isolates), and Enterococcus faecalis (3). Pharmacokinetic (PK)-pharmacodynamic (PD) studies with animal models of infection are useful for targeting the exposures associated with optimal activity. The PK-PD relationships determined in animal models of infection can be used to establish the dosage regimens for testing with humans (4, 5). Previous studies with β-lactams have established that the percentage of a 24-h dosing interval that the free drug concentrations exceed the MIC (fT>MICs) is the PK-PD parameter best linked with efficacy (10, 13). For these studies, we wanted to confirm that the PK-PD parameter that best describes the efficacy of RWJ-54428 was indeed fT>MIC and determine the magnitude of that parameter required to achieve the maximal bactericidal effects against gram-positive pathogens.
(The work described in this paper was conducted as part of a research collaboration with R. W. Johnson Pharmaceutical Research and Development. This work was presented in part at the 39th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA, September 1999.)
MATERIALS AND METHODS
Antimicrobial agents.
RWJ-54428 was synthesized at Microcide Pharmaceuticals, Inc., Mountain View, CA.
Bacterial strains.
MRSA COL and MRSA 076 were provided by Chip Chambers. S. pneumoniae ATCC 49619 and E. faecalis ATCC 29212 are ATCC reference strains. The following clinical isolates were also used: MRSA 602, 604, and 609; S. pneumoniae SP 019; and E. faecalis EFS 007.
Susceptibility testing.
MICs were determined by a broth microdilution assay, according to CLSI (formerly NCCLS) reference methods (11). MICs were determined in triplicate for the isolates used for inoculation and for the isolates recovered from mouse thighs.
Assays were performed by using a final volume of 100 μl. The inocula were adjusted to yield a final cell density of 5 × 105 CFU/ml. The antibiotics were prepared at a concentration equivalent to twofold the highest desired final concentration in culture medium and were then diluted directly into 96-well microtiter plates by serial twofold dilution. The microtiter plates were incubated for 24 h at 35°C and were read with a plate reader (Molecular Devices, Sunnyvale, CA) at 650 nm as well as by visual observation with a reading mirror.
All procedures were approved by the Microcide Pharmaceuticals Institutional Animal Care and Use Committee, as outlined by the Animal Welfare Act.
Neutropenic mouse thigh model.
Male Swiss mice were made neutropenic by the intraperitoneal injection of 150 mg/kg of body weight cyclophosphamide (Cytoxan, Mead Johnson, Princeton, NJ) on days 1 and 4. On day 5, the mice were infected by the intramuscular injection of 0.1 ml of inoculum in each thigh (four thighs per group per time point). The bacteria were grown overnight at 35°C in brain heart infusion broth (BHIB) or Todd-Hewitt broth (THB). On the following morning they were subcultured into fresh BHIB or THB and incubated for 4 h at 35°C. The inocula were adjusted to a final concentration of ∼5.0 × 106 CFU/ml. RWJ-54428 was administered intraperitoneally at various time points beginning 2 h postinfection. RWJ-54428 was administered at total daily doses of 3 to 1,200 mg/kg/day divided into 2, 3, 6, or 12 doses. The individual doses ranged from 1.5 to 200 mg/kg. All mice were euthanized after 24 h of therapy by using CO2, and both thighs were removed aseptically and homogenized in 4 ml of ice-cold phosphate-buffered saline. Serial 10-fold dilutions of the homogenized material were plated on Mueller-Hinton or blood agar, and the colonies were counted. The reduction in bacterial counts was determined by inspection of the log number of CFU/thigh at the start of therapy and comparison to the numbers recovered after 24 h of therapy.
PKs.
Male Swiss mice were administered a single intraperitoneal dose of RWJ-54428 at 20 and 100 mg/kg on the fifth day after the initiation of cyclophosphamide treatment (as described above for the thigh infection model). Groups of three mice each were killed at 0.08, 0.16, 0.25, 0.5, 0.75, 1.0, and 2.0 h after dosing. Blood samples (one sample from each animal) were collected by cardiac puncture. Serum concentrations were fit by using the WinNonlin program (Pharsight, Mountain View, CA).
Protein binding.
The level of protein binding of RWJ-54428 when it was administered at doses of 25, 50, and 100 mg/liter was determined by ultrafiltration. The serum was prewarmed to 37°C and adjusted to pH 7.4 ± 0.2 with CO2. Aliquots were loaded into the upper reservoir of Centrifree disposable micropartition units (YM-30; Millipore, Bedford, MA) and centrifuged at 1,900 × g for 10 min at 25°C. A solution of 0.2 N acetic acid was mixed with aliquots of the ultrafiltrate prior to high-performance liquid chromatography (HPLC) analysis or, in the case of mouse serum, was added to the bottom reservoir of the ultrafiltration devices prior to centrifugation to prevent drug degradation in the alkaline ultrafiltrate. The protein binding was controlled by the use of a standard curve (1 to 100 μg/ml) prepared with a control sample (25 μg/ml) generated with spiked serum ultrafiltrates and a standard curve prepared with a sample generated by refiltering the spiked ultrafiltrates. The double filtration ensured the same filter membrane recovery of drug as that for the actual serum-binding sample. The percent recovery of RWJ-54428 from the ultrafiltration units was determined by comparison of the peak areas of these nonultrafiltered standards with those of the ultrafiltered standards used to prepare the standard curves (AN and AU, respectively), as follows: (AU/AN) × 100. The percent serum protein binding was determined as [1 − (RWJ-54428 concentration in serum after ultrafiltration/spiked RWJ-54428 concentration in serum)] × 100.
Bioanalytical assay.
The serum and serum ultrafiltrate samples were analyzed for RWJ-54428 by reverse-phase HPLC with UV detection at 254 and 280 nm. The HPLC system used a 5-μm Phenomenex Luna C18 column (2.5 mm by 250 mm). The mobile phase consisted of 0.1 M ammonium acetate buffer, pH 6, and acetonitrile; the flow rate was 1.0 ml/min of 90% 0.1 M ammonium acetate, pH 6, and 10% acetonitrile for 5 min, and then 79% 0.1 M ammonium acetate, pH 6, and 21% acetonitrile for 15 min. Serum and serum ultrafiltrate samples were prepared for analysis by using an acid-protein precipitation method. The extraction efficiencies ranged from 80 to 100%. The range of detection of the assay was from 0.5 to 100 mg/liter, and the intraday and interday precisions (coefficients of variation) were in the ranges of 1 to 30 and 1 to 46%, respectively.
PD modeling.
The relationship between each of the PK and PD parameters (i.e., fT>MIC, the free area under the concentration-time curve [fAUC/MIC], and the free maximum concentration in serum/MIC [fCmax/MIC]) and the reduction in the log number of CFU/thigh between time zero and 24 h after the start of treatment were analyzed by using the sigmoid maximum-effect (Emax) PD model, as follows: change in log CFU/thigh = [Emax·Xg/(EC50g + Xg)] + E0, where Emax is the maximum reduction in the log number of CFU/thigh, X is the PK-PD parameter being examined (e.g., 24-h AUC/MIC), EC50 is the X value corresponding to 50% of the Emax, E0 is the effect when X is equal to 0 (i.e., for the untreated control animals), and g is a sigmoidicity factor which controls the steepness of the curve.
The best model for each data set was established by using the Akaike information criterion (1).
RESULTS
Susceptibility studies.
The MICs of RWJ-54428 are shown in Table 1. The MICs for the posttreatment isolates recovered from the mouse thighs did not change after exposure to RWJ-54428 therapy.
TABLE 1.
Relationship between RWJ-54428 MIC and fT>MIC required to achieve various degrees of antibacterial effects in vivo
| Organism | MIC (μg/ml) | % fT>MIC of RWJ-54428 required to achieve:
|
|||
|---|---|---|---|---|---|
| Static effect | 1-log drop | 2-log drop | Emax | ||
| MRSA COL | 1.0 | 14 | 20 | NAa | 36 |
| MRSA 076 | 1.0 | 16 | 23 | 28 | 32 |
| MRSA 602 | 0.5 | 17 | 20 | NA | 24 |
| MRSA 604 | 0.5 | 20 | 23 | 28 | 30 |
| MRSA 609 | 0.5 | 16 | 18 | 21 | 22 |
| S. pneumoniae ATCC 49619 | 0.03 | 18 | 21 | 23 | 28 |
| S. pneumoniae SP 019b | 0.5 | 16 | 22 | 28 | 30 |
| E. faecalis ATCC 29212 | 0.06 | 30 | 48 | NA | 60 |
| E. faecalis EFS 007 | 0.125 | 46 | 56 | NA | 60 |
NA, not applicable.
Penicillin-resistant strain.
Mouse PKs.
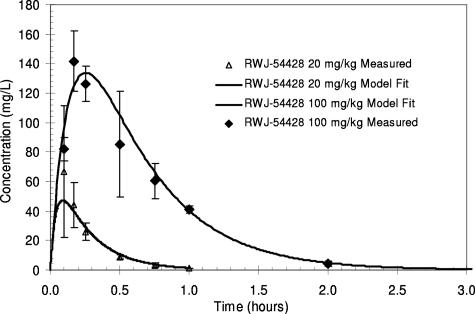
The total drug serum concentrations of RWJ-54428 following a single intraperitoneal dose in neutropenic mice are shown in Fig. 1. RWJ-54428 was absorbed rapidly after intraperitoneal administration, with the times to Cmax achieved at 0.09 and 0.26 h for 20 and 100 mg/kg, respectively. The decline in the concentrations was best described by a one-compartment model with first-order absorption. The values for the PK parameters are shown in Table 2. The range of RWJ-54428 serum clearance values was 0.9 to 1.2 liters/h/kg.
FIG. 1.
Serum RWJ-54428 concentrations in mice after a single intraperitoneal dose.
TABLE 2.
Serum PK parameters of RWJ-54428 following a single intraperitoneal injection in male neutropenic micea
| RWJ-54428 dose (mg/kg) | Mean wt (kg) | V/F (liter/kg) | Cmax (mg/liter) | Tmax (h) | AUC (mg·h/liter) | CL/F (liter/hr/kg) | t1/2 (h) | PB (%) |
|---|---|---|---|---|---|---|---|---|
| 20 | 0.022 | 0.3 | 47.3 | 0.09 | 16.6 | 1.2 | 0.17 | 60 |
| 100 | 0.022 | 0.44 | 133.3 | 0.26 | 108 | 0.9 | 0.33 | 60 |
V/F, volume of distribution; Tmax, time to Cmax; CL/F, clearance; t1/2, half-life; PB, protein binding. The other abbreviations are defined in the text.
PD modeling.
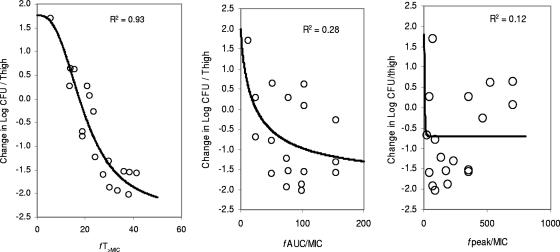
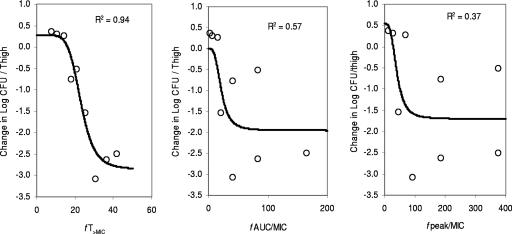
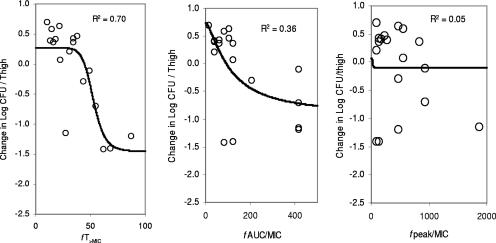
At the start of RWJ-54428 treatment, the bacterial titers in the thighs were 5.8 to 6.8, 5.9 to 6.1, and 5.6 to 6.6 log CFU/thigh for MRSA, E. faecalis, and S. pneumoniae, respectively. The relationships between the antibacterial effects and each of the PD parameters are shown in Fig. 2 for MRSA COL, Fig. 3 for S. pneumoniae SP 019, and Fig. 4 for E. faecalis EFS 007. The best relationships were seen when the results were correlated with fT>MIC, with R2 values of 0.93, 0.94, and 0.70 for MRSA COL, S. pneumoniae SP019, and E. faecalis EFS 007, respectively. The relationships between the RWJ-54428 MICs and the fT>MICs for all strains are shown in Table 1. The maximal bactericidal effects varied between strains and organisms. For MRSA, the maximal bactericidal effects ranged from −1.95 to −2.83 log CFU/thigh, and for S. pneumoniae, the maximal bactericidal effects were −2.79 to −3.75 log CFU/thigh; but for E. faecalis, the maximal effects were only −1.42 to −1.65 log CFU/thigh.
FIG. 2.
Relationships between fT>MIC, fAUC/MIC, and fCmax/MIC (fpeak/MIC) and the change in the log10 number of CFU/thigh of MRSA COL. Each symbol represents the data from four thighs. The lines are the best model fits of the data. R2 is the correlation coefficient.
FIG. 3.
Relationships between fT>MIC, fAUC/MIC, and fCmax/MIC (fpeak/MIC) and the change in the log10 number of CFU/thigh of S. pneumoniae SP 019. Each symbol represents the data from four thighs. The lines are the best model fits of the data. R2 is the correlation coefficient.
FIG. 4.
Relationships between fT>MIC, fAUC/MIC, and fCmax/MIC (fpeak/MIC) and the change in the log10 number of CFU/thigh of E. faecalis EFS 007. Each symbol represents the data from four thighs. The lines are the best model fits of the data. R2 is the correlation coefficient.
DISCUSSION
The increasing resistance of gram-positive organisms to all classes of antibacterial agents has limited the therapeutic options for many infections. The high incidence of methicillin resistance in hospitals (as well as its appearance in the outpatient setting), coupled with the emergence of glycopeptide-intermediate Staphylococcus aureus strains, has further complicated the prevention and treatment of serious infections due to staphylococci (9, 12). Furthermore, resistance to penicillin among strains of Streptococcus pneumoniae has spread worldwide (8), with a recent survey in the United States reporting that over 20% of S. pneumoniae isolates are resistant to penicillin (14). These reports emphasize the need for new antibacterials with activity against resistant gram-positive organisms.
This study describes the PK-PD of RWJ-54428 against MRSA, S. pneumoniae, and E. faecalis. As was determined for other β-lactams (10, 13), T>MIC was the PD parameter that best correlated with the in vivo antibacterial effects of RWJ-54428. As is the case for the β-lactam class of antimicrobials, increasing concentrations did not increase the antimicrobial effect. The 24-h AUC also showed a poor correlation with efficacy, despite the presence of a prolonged in vivo postantibiotic effect against S. aureus (7).
Analysis of the results of the experiments with MRSA and S. pneumoniae revealed that a static effect (no change in the bacterial density over 24 h) is achieved with an fT>MIC of 14 to 20%, and a 1-log drop in bacterial density is achieved with an fT>MIC of 18 to 23%. These data are very similar to those for the activities of other cephalosporins against staphylococci but show a distinct difference from those for the activities of other cephalosporins against pneumococci. Craig found that cefotaxime, ceftriaxone, ceftazidime, and cefpirome achieved static effects against S. aureus with fT>MICs of 19 to 28%, but for the streptococci, these cephalosporins required fT>MICs of 36 to 41% (6). Analysis of the results of the experiments with E. faecalis showed that a static effect was not achieved until the fT>MIC had reached 30 to 46% and that a 1-log drop was not achieved until the fT>MIC reached 48 to 56%. The additional fT>MIC required to achieve a static effect or a 1-log drop with E. faecalis may be due to the slow rate of expansion observed with these organisms in this model (data not shown).
PD studies in animal models of infection have proved effective in the study of potential human dosage regimens and in the development of in vitro susceptibility breakpoints (2, 4, 5). The data for RWJ-54428 in the mouse models suggest that human dosage regimens should supply fT>MICs of RWJ-54428 for 20 to 30% of the interval for staphylococci and pneumococci. For enterococci, effective regimens would need fT>MICs of RWJ-54428 for ∼50% of the dosing interval, which may be more challenging, given the MICs of many of these strains to RWJ-54428 (3).
Acknowledgments
We gratefully acknowledge the assistance of Johanne Blais and Craig Park with the microbiology; Doug Clark, Keith Huie, and Vrushali Tembe with the bioanalytical analysis; and Scott Hecker and Tomasz Glinka with compound synthesis.
Footnotes
Published ahead of print on 22 October 2007.
REFERENCES
- 1.Akaike, A. C. 1978. Posterior probabilities for choosing a regression model. Ann. Inst. Math. Stat. 30A:9-14. [Google Scholar]
- 2.Andes, D., and W. A. Craig. 1998. In vivo activities of amoxicillin and amoxicillin-clavulanate against Streptococcus pneumoniae: application to breakpoint determinations. Antimicrob. Agents Chemother. 42:2375-2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chamberland, S., J. Blais, M. Hoang, C. Dinh, D. Cotter, E. Bond, C. Gannon, C. Park, F. Malouin, and M. N. Dudley. 2001. In vitro activity of RWJ-54428 (MC-02,479) against multiresistant gram-positive bacteria. Antimicrob. Agents Chemother. 45:1422-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Craig, W. 1993. Relevance of animal models for clinical treatment. Eur. J. Clin. Microbiol. Infect. Dis. 12(Suppl. 1):55-57. [DOI] [PubMed] [Google Scholar]
- 5.Craig, W. A. 1998. Pharmacokinetics and pharmacodynamics of antibiotics in mice and men. Clin. Infect. Dis. 26:1-12. [DOI] [PubMed] [Google Scholar]
- 6.Craig, W. A. 1995. Interrelationship between pharmacokinetics and pharmacodynamics in determining dosage regimens for broad-spectrum cephalosporins. Diagn. Microbiol. Infect. Dis. 22:89-96. [DOI] [PubMed] [Google Scholar]
- 7.Griffith, D. C., L. Harford, R. Williams, V. J. Lee, and M. N. Dudley. 2003. In vivo antibacterial activity of RWJ-54428, a new cephalosporin with activity against gram-positive bactera. Antimicrob. Agents Chemother. 47:43-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heffelfinger, J. D., S. F. Dowell, J. H. Jorgensen, K. P. Klugman, L. R. Mabry, D. M. Musher, J. F. Plouffe, A. Rakowsky, A. Schuchat, and C. G. Whitney. 2000. Management of community-acquired pneumonia in the era of pneumococcal resistance: a report from the Drug-Resistant Streptococcus pneumoniae Therapeutic Working Group. Arch. Intern. Med. 160:1399-1408. [DOI] [PubMed] [Google Scholar]
- 9.Herold, B. C., L. C. Immergluck, M. C. Maranam, et al. 1998. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA 279:593-598. [DOI] [PubMed] [Google Scholar]
- 10.Leggett, J. E., B. Fantin, S. Ebert, K. Totsuka, B. Vogelman, W. Calame, H. Mattie, and W. A. Craig. 1989. Comparative antibiotic dose-effect relationships at several dosing intervals in murine pneumonitis and thigh-infection models. J. Infect. Dis. 159:281-292. [DOI] [PubMed] [Google Scholar]
- 11.National Committee for Clinical Laboratory Standards. 2004. Methods for dilution of antimicrobial susceptibility tests for bacteria that grow aerobically, 4th ed. Approved standard M7-A4, vol. 17, no. 2. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 12.Tenover, F. C. 1999. Implications of vancomycin resistant Staphylococcus aureus. J. Hosp. Infect. 43(Suppl.):S3-S7. [DOI] [PubMed] [Google Scholar]
- 13.Vogelman, B., S. Gudmundsson, J. Leggett, J. Turnidge, S. Ebert, and W. A. Craig. 1988. Correlation of antimicrobial pharmacokinetic parameters with therapeutic efficacy in an animal model. J. Infect. Dis. 158:831-847. [DOI] [PubMed] [Google Scholar]
- 14.Whitney, C. G., M. M. Farley, J. Hadler, L.H. Harrison, C. Lexau, A. Reingold, L. Lefkowitz, P. R. Cieslak, M. Cetron, E. R. Zell, J. H. Jorgensen, and A. Schuchat. 2000. Increasing prevalence of multidrug-resistant Streptococcus pneumoniae in the United States. N. Engl. J. Med. 343:1917-1924. [DOI] [PubMed] [Google Scholar]