Abstract
Staphylococci are important causes of nosocomial and medical-device-related infections. Their virulence is attributed to the elaboration of biofilms that protect the organisms from immune system clearance and to increased resistance to phagocytosis and antibiotics. Photodynamic treatment (PDT) has been proposed as an alternative approach for the inactivation of bacteria in biofilms. In this study, we have investigated the effect of the photodynamic action of toluidine blue O (TBO) on the viability and structure of biofilms of Staphylococcus epidermidis and of a methicillin-resistant Staphylococcus aureus strain. Significant inactivation of cells was observed when staphylococcal biofilms were exposed to TBO and laser simultaneously. The effect was found to be light dose dependent. Confocal laser scanning microscopic study suggested damage to bacterial cell membranes in photodynamically treated biofilms. In addition, scanning electron microscopy provided direct evidence for the disruption of biofilm structure and a decrease in cell numbers in photodynamically treated biofilms. Furthermore, the treatment of biofilms with tetrasodium EDTA followed by PDT enhanced the photodynamic efficacy of TBO in S. epidermidis, but not in S. aureus, biofilms. The results suggest that photodynamic treatment may be a useful approach for the inactivation of staphylococcal biofilms adhering to solid surfaces of medical implants.
Staphylococcus aureus and Staphylococcus epidermidis are the most common causes of osteomyelitis, endocarditis, and catheter- and orthopedic-implant-associated infections in hospitalized patients (7, 8, 13, 19, 32). These bacteria grow as biofilms, which are sessile microbial communities embedded in a self-produced extracellular polymer matrix composed mainly of a large polysaccharide referred to as polysaccharide intercellular adhesion (PIA) (5). The management of medical-device-associated infections is becoming increasingly difficult due to their inherent resistance to antibiotic treatments (11, 18). Although it is not yet clear how biofilms resist antibiotics, several mechanisms have been suggested, such as decreased penetration of the antibiotics, slow growth of cells within the biofilms, the activation of stress responses, and the emergence of biofilm-specific phenotypes (7, 11, 18). To overcome antimicrobial resistance, several new approaches, such as the use of analogues of quorum-sensing signal molecules (7) and enzymatic disruption of biofilms (29), are being investigated. However, more-comprehensive research is required for the effective application of these techniques.
Photodynamic treatment (PDT) is a process in which microorganisms are treated with a photosensitizing drug and then irradiated with low-intensity visible light of the appropriate wavelength (16). The resulting photochemical reactions generate cytotoxic reactive oxygen species, such as singlet oxygen and free radicals, which are able to exert bactericidal effect. Several studies have reported on the photodynamic inactivation of gram-positive and gram-negative bacteria in planktonic cultures using different photosensitizing drugs (16, 22, 23, 25). Although PDT is being actively investigated for the eradication of bacterial biofilms growing in dental plaques and on oral implants (26, 27, 28), there are very few studies on the effect of PDT on staphylococcal biofilms. These studies have shown that the photodynamic efficacies of the photosensitizers for the inactivation of biofilms differ from those of planktonic cultures. Specifically, Lin et al. (17) demonstrated that the light doses required to inactivate S. aureus biofilms using merocyanine 540 are much higher than those used to inactivate planktonic cultures, due to hindrance of the penetration of light within the biofilms. Using confocal laser scanning microscopy (CLSM), Zanin et al. (30) demonstrated that photosensitization in Streptococcus mutans biofilms occurs only in the outer layers due to the inability of the photosensitizer to diffuse into inner regions. Likewise, it has been reported that the presence of PIA/extracellular polymeric substance (EPS) in the biofilm affects the penetration of the photosensitizer and thereby decreases the effect of the photosensitization process (14, 31). Therefore, approaches that increase the permeability of the drugs in the biofilms may be beneficial for enhancement of the photodynamic efficacy of the drugs.
Recent studies have shown that metal chelators, such as disodium and tetrasodium EDTA (TEDTA) cause loss of viability and enhance the sensitivity of both gram-positive and gram-negative bacteria embedded in biofilms to antimicrobial agents, possibly by disrupting biofilm structure (1, 20). EDTA has also been used for increasing the permeability of some anionic photosensitizing drugs in gram-negative microorganisms (3). Thus, we hypothesized that the treatment of biofilms with metal chelators could enhance the photosensitizer penetration inside biofilms and thereby increase the efficacy of the photodynamic treatment.
Toluidine blue O (TBO) is a cationic phenothiazine dye that has been well studied as an antibacterial photosensitizer (10, 24, 25, 30, 31). To the best of our knowledge, the photosensitizing effect of this drug on the viability and structure of staphylococcal biofilms has not been investigated. Thus, in the present study, we examined the photodynamic effects of TBO on the viability and architecture of staphylococcal biofilms. Further, we investigated the effect of the pretreatment of biofilms with TEDTA on the efficacy of the photodynamic inactivation of staphylococci.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The microorganisms used in this study were S. epidermidis 1457 and methicillin-resistant S. aureus LP. S. epidermidis 1457, a standard biofilm-producing strain, was a gift from Tim Foster (Department of Microbiology, Dublin, Ireland). S. aureus LP was a clinical isolate provided by the Department of Microbiology, University of Pavia, Pavia, Italy. Bacteria were routinely grown overnight in tryptic soy broth (TSB) (Difco, Detroit, MI) under aerobic conditions at 37°C using a shaker incubator (New Brunswick Scientific Co., Edison, NJ).
Biofilm growth.
For screening biofilm formation by the staphylococci, bacteria were grown on Congo red agar as described by Freeman et al. (12). Briefly, brain heart infusion broth (Difco) (37 g/liter), sucrose (50 g/liter), and agar (10 g/liter) were autoclaved, and a separately autoclaved Congo red solution (Sigma, St. Louis, MO) was added at 55°C to give a final concentration of 0.8 g/liter. Colony morphology was examined after 24 h at 37°C. For biofilm assays, overnight cultures of S. epidermidis and S. aureus were diluted at 1:200 and 1:50, respectively, in TSB containing 0.25% glucose. Aliquots (200 μl) of the diluted bacterial suspensions were inoculated into 96-well flat-bottomed sterile polystyrene microplates (Costar; Corning, Inc., NY) and incubated for 16 h at 37°C. For enhancing S. aureus biofilm formation in all the assays, microplates were coated with 20% (vol/vol) human plasma in carbonate buffer (50 mM sodium carbonate, pH 9.5) (2). Biofilm formation by bacteria was detected by the method described by Christensen et al. (6). Briefly, biofilms formed on the plates were washed twice with phosphate-buffered saline (PBS) (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, pH 7.4) to remove the planktonic cells. Then, the cells were fixed with 95% ethanol for 10 min and stained with 0.1% crystal violet for 15 min, and after several washings, the wells were air dried. For a quantitative estimation of biofilms, crystal violet was solubilized with 10% glacial acetic acid and absorbance of the solubilized dye was determined at 590 nm in a microplate reader (model 680; Bio-Rad Laboratories, Inc., Hercules, CA).
Photosensitizer.
A stock solution of 1 mM TBO (Sigma) was prepared in distilled water, filter sterilized, and stored at 4°C in the dark. When used, the stock solution was appropriately diluted in PBS to obtain the desired concentration.
Photodynamic inactivation studies.
To investigate the effect of PDT, 100 μl of diluted TBO (10 to 80 μM) was added to each well and the plates were incubated in the dark for 30 min at 37°C. Wells used as controls were incubated with PBS only. TBO-treated biofilms were irradiated with a 640-nm wavelength of light using a model HL6364DG diode laser (Optnext Lasers Japan, Inc., Nagano-Ken, Japan) at different light doses. Laser light was focused to a spot with a 1-cm diameter using an appropriate lens. The intensity of the light source at the position of the bacterial cells was 42 mW/cm2. The power output was measured by using a power meter (Thorlabs GmbH, Munich, Germany). Following irradiation, TBO was carefully removed from the microwells and the biofilms were washed once with fresh PBS. The biofilms were scraped carefully, sonicated, and then vortexed for 20 s to homogenize the samples. Treated and untreated samples were serially diluted, plated on the TSB agar plates, and incubated for 24 h at 37°C in the dark. For each set of measurements, controls consisting of biofilms treated with TBO but not exposed to light (S+L−), exposed to light only (S−L+), and treated with neither TBO nor light (S−L−) were included. S and L stand for “sensitizer” and “light” respectively. Cell survival was expressed as the ratio of the numbers of CFU of bacteria treated with TBO and light to the numbers of CFU treated with TBO alone.
Effect of TEDTA.
To assess the effect of TEDTA on the viability of cells in biofilms, growth medium was aspirated from biofilm-containing wells; 100-μl amounts of PBS, including different concentrations of TEDTA, were added; and the microplates were incubated at 37°C for increasing periods of time. Subsequently, the wells were washed once with PBS and adherent bacteria were scraped from the wells and serially diluted and plated.
The effect of TEDTA on the photodynamic action of TBO was analyzed by treating biofilms with 20 mM TEDTA for 1 h prior to exposure to TBO and light. Controls consisted of biofilms treated with TEDTA and TBO but not exposed to light (TEDTA+S+L−), biofilms treated with TEDTA only (TEDTA+S−L−), or biofilms treated with neither TBO/light nor TEDTA (TEDTA−S−L−). For both treated and untreated biofilms, colony assays were performed as described previously.
CLSM studies.
For confocal studies, 500 μl of diluted cell suspensions were dispensed into 24-well microplates (Costar) containing glass coverslips. To examine biofilm formation by S. aureus, the coverslips were coated with human plasma, as mentioned previously. Coverslips containing cell suspensions were incubated for 16 h at 37°C. To determine the viability of bacteria within the biofilms after photodynamic treatment, a BacLight Live/Dead viability kit (Molecular Probes, Eugene, OR) was used. The kit includes two fluorescent nucleic acid stains: SYTO9 and propidium iodide. SYTO9 penetrates both viable and nonviable bacteria, while propidium iodide penetrates bacteria with damaged membranes and quenches SYTO9 fluorescence. Dead cells, which take up propidium iodide, fluoresce red, and cells fluorescing green are deemed viable. For assessing viability, 1 μl of the stock solution of each stain was added to 3 ml of PBS and, after being mixed, 500 μl of the solution was dispensed into 24-well microplates containing treated and untreated biofilms and incubated at 22°C for 15 min in the dark. Stained biofilms were examined under a Leica CLSM (model TCS SPII; Leica, Heidelberg, Germany) using a 40× oil immersion objective. The excitation and emission wavelengths used for detecting SYTO9 were 488 and 525 nm, respectively. Propidium iodide was excited at 520 nm, and its emission was monitored at 620 nm. The optical sections of 0.9 μm were collected over the complete thickness of the biofilm, and for each sample, images from three randomly selected positions were acquired. The resulting stacks of images were analyzed using Leica confocal software and subsequently processed using ImageJ software (Wayne Rasband, National Institutes of Health).
SEM.
S. epidermidis was grown in TSB on sterile Thermanox plastic coverslips (polyolefin) (Nalge Nunc International, Rochester, NY). S. aureus cells were grown as described above except that the coverslips were precoated with 20% human plasma. Following photodynamic treatment, samples were washed several times and then fixed for scanning electron microscopy (SEM) with 2.5% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.2, for 1 h at 4°C. After additional washing, the samples were incubated using increasing concentrations of ethanol (25, 50, 75, and 96%) for 10 min, dried to the critical point using an Emitech K-850 apparatus, and placed on a mounting base. Finally, the specimens were coated with gold and examined under an SEM (model EVO 50 EP; Zeiss-Leica, Cambridge, United Kingdom) (9).
Statistical methods.
An unpaired, two-sided Student t test was used for evaluating the differences between the means of the results for the control and treated samples. P values of less than 0.05 were considered significant.
RESULTS
Biofilm formation.
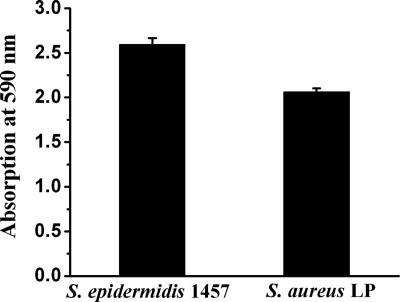
As indicated by the results of the crystal violet assay, both the S. epidermidis 1457 and S. aureus LP strains produced biofilms (Fig. 1). However, biofilm formation was substantially higher for S. epidermidis 1457 than for the clinical isolate S. aureus LP. In confirmation of this, the colony morphology of S. epidermidis 1457 on Congo red agar was a black coloration with a dry crystalline consistency, while for S. aureus, the colonies were pink (data not shown).
FIG. 1.
Biofilm assay for staphylococcal strains. Biofilms were grown in 96-well microtiter plates for 16 h at 37°C, and adherent cells were stained with crystal violet as described in Materials and Methods. The values represented are the means ± standard errors of the means of the results of two experiments done in duplicate plates. In each plate, four wells were used for the assay.
Photodynamic inactivation.
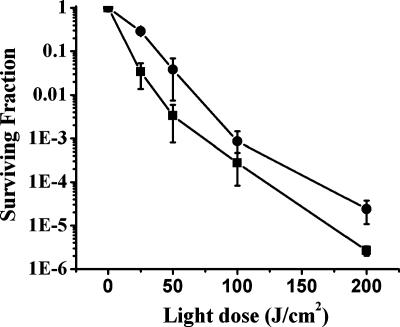
Our preliminary experiments showed that the treatment of biofilms with 40 μM TBO in the dark did not exhibit any toxicity against the bacterial cells. Similarly, the exposure of cells to 640 nm of light alone did not cause any change in cell survival compared to the survival rates of untreated controls. In Fig. 2, the surviving fraction as a function of light dose of cells in biofilms treated with 40 μM TBO is presented. The survival of both S. aureus and S. epidermidis in biofilms decreased gradually with increasing light doses; however, at a given light dose, a more remarkable decrease was observed for S. epidermidis. At a lower light dose (25 J/cm2), the cell survival of S. epidermidis was almost 10-fold lower than that of S. aureus. This difference was highly significant (P < 0.05). Of note, the use of TBO concentrations higher than 40 μM did not lead to a remarkable change in phototoxicity (data not shown).
FIG. 2.
Light dose-dependent killing of staphylococcal cells treated with TBO. Biofilms were incubated with TBO (40 μM) for 30 min in the dark and subsequently irradiated with different light doses. ▪, S. epidermidis 1457; •, S. aureus LP. Surviving fractions of cells are expressed as the ratios of numbers of CFU from bacteria treated with TBO and light over numbers of CFU of bacteria treated with TBO alone. The values represented are the means of the results for duplicate biofilms and three separate experiments. Error bars indicate standard errors of the means.
Effect of TEDTA treatment on biofilm stability.
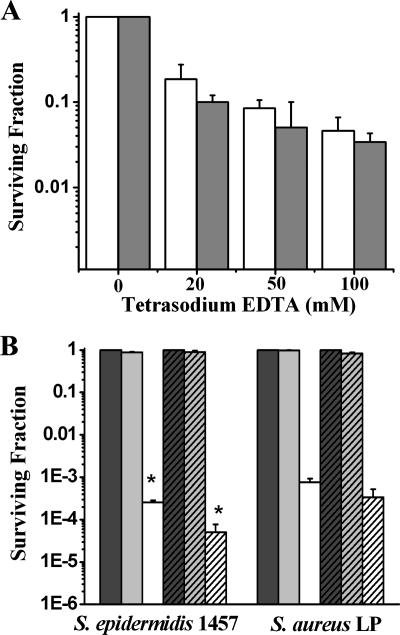
TEDTA at high concentrations (40 mg/ml, 89 mM) has been shown to affect the structure and viability of many biofilm-forming bacteria, including staphylococci, when incubated with them for long periods of time (>20 h) (20). In order to determine the potential role of TEDTA as an enhancer of the photodynamic action of TBO, the effects of different concentrations and incubation times on staphylococcal survival with TEDTA were determined. As shown in Fig. 3A, cell survival was inversely related to the addition of increasing amounts of TEDTA to the wells. More specifically, the numbers of bacteria treated with 20 mM TEDTA for 1 h were reduced by almost 0.8 log and 1.0 log for S. epidermidis and S. aureus, respectively, compared to the survival rates of untreated controls. Incubation time also affected the survival of cells treated with 20 mM TEDTA (data not shown).
FIG. 3.
Effect of pretreatment with TEDTA on photodynamic action of TBO on biofilms. Biofilms grown for 16 h were treated with TEDTA for 1 h. (A) Effects of different concentrations of TEDTA on the viability of cells in biofilms. White bar, S. epidermidis 1457; gray bar, S. aureus LP. (B) Comparison of surviving fractions of PDT-subjected biofilms with and without TEDTA pretreatment. Biofilms pretreated with TEDTA (20 mM, 1 h) were incubated with TBO (40 μM) for 30 min and then irradiated with light (100 J/cm2). Dark-gray bar, not treated with TBO or exposed to light (S−L−); light-gray bar, treated with TBO in the absence of light (S+L−); white bar, treated with TBO and light (S+L+); dark-gray hatched bar, treated with TEDTA in the absence of TBO and light (TEDTA+S−L−); light-gray hatched bar, treated with TEDTA and TBO in the absence of light (TEDTA+S+L−); white hatched bar, pretreated with TEDTA and exposed to TBO and light (TEDTA+S+L+). *, P < 0.05, showing the statistical difference between PDT-subjected biofilms with or without TEDTA. The surviving fraction of pretreated biofilm is expressed as the ratio of numbers of CFU of bacteria treated with TEDTA+S+L+ over numbers of CFU of bacteria treated with TEDTA+S−L−. The surviving fraction of untreated biofilms subjected to PDT is expressed as the ratio of numbers of CFU of bacteria treated with TBO and light (S+L+) over numbers of CFU of bacteria treated with neither TBO nor light (S−L−). The values represented are the means of the results for duplicate biofilms in three separate experiments. Error bars indicate standard errors of the means.
In Fig. 3B, we show the effect of pretreatment of biofilms with TEDTA on the photodynamic action of TBO at a light dose of 100 J/cm2. Under these conditions, the survival of S. epidermidis cells was reduced by almost 1.0 log with respect to the survival of cells treated with the combination of TBO and light alone (P < 0.05). Surprisingly, TEDTA pretreatment did not induce a significant cell death enhancement in the photodynamically treated S. aureus biofilm compared to cell death in biofilm not treated with TEDTA (P > 0.05).
CLSM of photodynamically treated biofilms.
To better understand the effect of PDT on the viability of bacteria with or without pretreatment with TEDTA, a CLSM study was carried out.
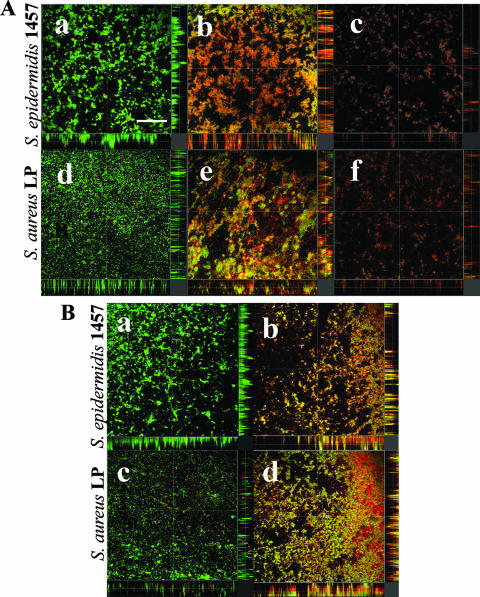
In biofilms incubated with TBO in the absence of light (Fig. 4A, panels a and d), CLSM images of S. epidermidis and S. aureus showed multilayered clumps of bacteria and the majority of the cells showed green fluorescence. The images for both the species were similar to those acquired in the absence of TBO (data not shown). Biofilms treated with TBO and then irradiated at 100 J/cm2 showed cells fluorescing both green (live) and red (dead) (Fig. 4A, panels b and e). Upon irradiation at 200 J/cm2, predominantly red-fluorescing cells were observed (Fig. 4A, panels c and f). In addition, these biofilms were less dense than the controls.
FIG. 4.
CLSM images of PDT-treated staphylococcal biofilms. Biofilms were grown for 16 h and then treated with TEDTA (20 mM, 1 h) before the photodynamic treatment with TBO (40 μm, 30 min) and light. Biofilms were stained with BacLight Live/Dead stain. (A) Effect of light dose on the viability of bacteria. S+L−, 0 J/cm2 (a, d); S+L+, 100 J/cm2 (b, e); S+L+, 200 J/cm2 (c, f). (B) Effect of TEDTA treatment on PDT. TEDTA+S−L− (a, c); TEDTA+S+L+, 100 J/cm2 (b, d). Sagittal sections of the biofilms are shown below and to the right of each panel. Scale bar: 75 μm.
Figure 4B shows the combined effects of TEDTA pretreatment and the photodynamic action of TBO on the viability of S. epidermidis 1457 and S. aureus LP biofilms. Most of the cells showed green fluorescence, and very few cells were stained with propidium iodide in biofilms treated with TEDTA alone (Fig. 4B, panels a and c). This staining profile was similar to those observed with staphylococcal biofilms treated (Fig. 4A, panels a and d) or untreated (data not shown) with TBO in the dark. In contrast, following exposure to TEDTA, biofilms incubated with TBO and irradiated at 100 J/cm2 showed more red-fluorescing cells than those biofilms exposed to PDT without TEDTA pretreatment (Fig. 4B, panels b and d).
Morphological alterations.
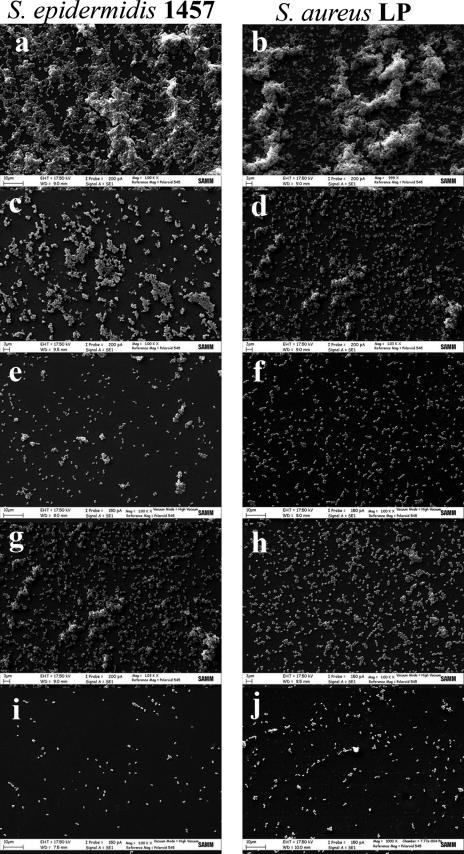
To visualize the effect of PDT on the morphology of the biofilms, SEM studies were carried out. Large cellular aggregates surrounded by extracellular matrix could be observed in the biofilms produced by both S. epidermidis 1457 and S. aureus LP treated with TBO in the dark (Fig. 5, panels a and b). These images were very similar to those of untreated controls (data not shown).
FIG. 5.
SEM images of PDT-subjected staphylococcal biofilms. Biofilms were grown as reported in Materials and Methods. Biofilms treated with 40 μM TBO for 30 min in the dark (a, b); irradiated with a light dose of 100 J/cm2 (c, d); or irradiated with a light dose of 200 J/cm2 (e, f). Biofilms pretreated with 20 mM TEDTA for 1 h without PDT (g, h) or subjected to PDT of 100 J/cm2 (i, j). Magnification, ×1,000.
Irradiation in the presence of 40 μM TBO led to a light dose-dependent disruption of the biofilms. In fact, as the light dose increased, the number of adherent bacteria was reduced and very few aggregated colonies were observed in both the staphylococcal biofilms. Most of the bacteria were single cells or organized in short chains (Fig. 5, panels e and f). The treatment of biofilms with TEDTA also resulted in the dissociation of large colonies of bacteria (Fig. 5, panels g and h). A more remarkable reduction in the cell numbers of both S. epidermidis and S. aureus was observed when biofilms were treated with TEDTA and then subjected to photodynamic treatment (100 J/cm2) (Fig. 5, panels i and j) compared to cell numbers in biofilms treated in the absence of the chelator (Fig. 5, panels c and d). Nevertheless, in contrast to the membrane damage observed by CLSM, no apparent alteration in the morphology of cell surfaces could be noted in PDT-subjected or TEDTA-treated biofilms of S. epidermidis and S. aureus.
DISCUSSION
A number of studies have shown that TBO is phototoxic to several planktonic gram-positive and gram-negative bacteria (10, 22, 24). The photodynamic efficacy of TBO has also been demonstrated in several oral biofilm models (30, 31). In this study we investigated the effect of the photodynamic action of TBO on the viability and structure of staphylococcal biofilms, which are common causative agents of medical-device-related infections.
In our study, different surfaces, such as glass and plastic, were used to produce biofilm formation by S. epidermidis and S. aureus. It is not clear whether the biochemical natures and architectures of the biofilms formed on these surfaces are different, as both the physicochemical properties of surfaces and the bacterial surface may influence attachment and colonization (15). In this context, we and others (2) found that S. aureus produced better biofilms on surfaces coated with human plasma. This probably reflects the crucial requirement of plasma proteins for the early attachment by S. aureus to the surfaces.
The results of this study show that a large number of S. aureus and S. epidermidis cells could be inactivated when biofilms were irradiated by using a diode laser in the presence of TBO. The threshold concentration of the TBO required for achieving a significant light dose response was 40 μM for both S. aureus and S. epidermidis biofilms. As reported by others (10, 24), the TBO concentration and the light dose (25 to 200 J/cm2) used for the photoinactivation of biofilms were considerably higher than those required to inactivate S. aureus suspensions. The differences in the photodynamic efficacies of the drug could be due to several reasons. In fact, cells growing in a biofilm differ from their planktonic counterparts in a number of respects, such as the cell wall composition, rate of growth, and presence of PIA, which may hinder the uptake of the photosensitizer and penetration of light and thereby reduce the photosensitizing process (7, 31). Our results also show that the photosensitivity of S. epidermidis 1457 biofilm was higher than that of S. aureus LP biofilm when they were irradiated under similar conditions, and this difference was more significant at lower light doses. In confirmation of this, Gad et al. (14) have reported a higher photokilling of S. epidermidis than of S. aureus using methylene blue, which is a photosensitizer similar to TBO. Abundant production of PIA has been suggested to obstruct the diffusion of the photosensitizer through the matrix, thus reducing the susceptibility of biofilms to photosensitization. In contrast with this observation, we found that, despite the reduced biofilm produced by S. aureus, these microbial cells appeared more resistant to photokilling, suggesting that other factors, such as intrinsic variation in the cell wall thickness, influence the dye uptake (4).
Of note, the results of our study show that the photodynamic efficacy of TBO for the inactivation of staphylococcal biofilms is higher than that reported for oral biofilms (30, 31). These variations could be due to different experimental conditions and/or the diverse nature of the biofilms produced by staphylococci and streptococci.
CLSM images of biofilms subjected to photosensitization showed an increased permeability of bacterial cells to propidium iodide, indicating that the cell membrane could be an important site of damage in TBO-mediated photodamage. This observation is consistent with the results of previous studies on the photodynamic effects of TBO in planktonic cultures of bacteria and yeast (16, 24) and with the high affinity of TBO for polyphosphates, lipids, and membrane proteins (24).
Furthermore, the presence of dead cells throughout the thickness of the biofilm implies that there is no hindrance to photosensitizer diffusion and light penetration. In addition, the reduced cell density observed following photodynamic treatment indicates the loss of cells within the biofilms. Although the reason for these changes is not obvious, damage to bacterial membranes due to PDT may reduce cell-to-cell contact or cell-to-EPS binding, causing a loss of cells within the biofilms. These observations are consistent with the results reported by Wood et al. (27) which show the loss of biofilm mass in oral plaque following PDT using pyridinum zinc(II) phthalocyanine. Our results from CLSM studies were also consistent with the alteration in biofilm morphology observed by using SEM.
In the present communication, we also found that the incubation of staphyloccocal biofilms with high concentrations of TEDTA for a short period of time resulted in a disruption of the large clusters of cells without compromising the viability of cells. This effect is probably due to the sequestration of divalent cations, such as Ca2+ and Mg2+, that are important for maintaining the integrity of EPS in the biofilm (1, 20). A different effect of TEDTA pretreatment was observed when S. epidermidis or S. aureus cells were subjected to PDT. When S. epidermidis cells were exposed to the chelator, a strong disruption of biofilm structure was noticed. This enhancement could be due to the destabilization of the biofilm structure, which makes cells more accessible to drug and light penetration. However, although there was disruption of the S. aureus biofilm exposed to the chelator, no significant enhancement in PDT results was observed. The reason for this lack of effect by PDT is unclear.
Studies by Banin et al. (1) have shown that combined treatment of chelating agents such as EDTA with the antibiotic gentamicin could enhance the killing of Pseudomonas aeruginosa embedded in the biofilms by dispersing the biofilm structure and increasing the permeability to antibiotics. The combined action of minocycline and EDTA has also been used to increase the killing of antibiotic-resistant S. aureus cells (21). Consistent with these observations, we found that the subjection of biofilms to TEDTA and PDT could enhance killing in S. epidermidis biofilms.
To the best of our knowledge, there are no published reports on the combined use of chelating agents and PDT for the inactivation of biofilms. PDT is proposed as an alternative antimicrobial method for the inactivation of biofilm-related diseases owing to several advantages over the conventional antibacterial treatment. However, the efficacy of PDT for biofilm treatment depends mainly on the penetration of the photosensitizer and light to the deeper layers. Our studies show that pretreatment of biofilms with chelating agents, such as TEDTA, can enhance the efficacy of PDT by dispersing the biofilm structure and thereby enhancing the photosensitizer and light penetration. Thus, the use of combined TEDTA and PDT has the intrinsic advantage of treating and eradicating even thick biofilms and circumventing the disadvantage of using antibiotics, with the consequent generation of antimicrobial resistance.
In summary, TBO may be a potential photosensitizer for the inactivation of staphylococcal biofilms for many device-related infections which are accessible to light. Hence, an experimental approach involving a combination of TEDTA and PDT in treating biofilm-associated medical devices may be worth exploring.
Acknowledgments
This work was partially funded by a grant from the Italian Ministero della Salute (identification code RF-IOR-2006-349032; Opportunistic bacteria in orthopaedic implant infections: new strategies of molecular and gene therapies). M. Sharma is a recipient of a scholarship for Indian researchers under the project BOIND22703, MIUR, Italy.
We are grateful to Patrizia Vaghi and Dario Picenoni for their technical assistance in the confocal and scanning electron microscopic studies.
Footnotes
Published ahead of print on 29 October 2007.
REFERENCES
- 1.Banin, E., K. M. Brady, and E. P. Greenberg. 2006. Chelator-induced dispersal and killing of Pseudomonas aeruginosa cells in a biofilm. Appl. Environ. Microbiol. 72:2064-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beenken, K. E., J. S. Blevins, and M. S. Smeltzer. 2003. Mutation of sarA in Staphylococcus aureus limits biofilm formation. Infect. Immun. 71:4206-4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertoloni, G., F. Rossi, G. Valduga, G. Jori, and J. van Lier. 1990. Photosensitizing activity of water- and lipid-soluble phthalocyanines on Escherichia coli. FEMS Microbiol. Lett. 59:149-155. [DOI] [PubMed] [Google Scholar]
- 4.Boyle-Vavra, S., H. Labischinski, C. C. Ebert, K. Ehlert, and R. S. Daum. 2001. A spectrum of changes occurs in peptidoglycan composition of glycopeptide-intermediate clinical Staphylococcus aureus isolates. Antimicrob. Agents Chemother. 45:280-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chokr, A., D. Watier, H. Eleaume, B. Pangon, J. C. Ghnassia, D. Mack, and S. Jabbouri. 2006. Correlation between biofilm formation and production of polysaccharide intercellular adhesin in clinical isolates of coagulase-negative staphylococci. Int. J. Med. Microbiol. 296:381-388. [DOI] [PubMed] [Google Scholar]
- 6.Christensen, G. D., W. A. Simpson, J. J. Younger, L. M. Baddour, F. F. Barrett, D. M. Melton, and E. H. Beachey. 1985. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J. Clin. Microbiol. 22:996-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 8.Costerton, W., R. Veeh, M. Shirtliff, M. Pasmore, C. Post, and G. Ehrlich. 2003. The application of biofilm science to the study and control of chronic bacterial infections. J. Clin. Investig. 112:1466-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Del Curto, B., M. F. Brunella, C. Giordano, M. P. Pedeferri, V. Valtulina, L. Visai, and A. Cigada. 2005. Decreased bacterial adhesion to surface treated titanium. Int. J. Artif. Organs 28:718-730. [DOI] [PubMed] [Google Scholar]
- 10.Demidova, T. N., and M. R. Hamblin. 2005. Effect of cell-photosensitizer binding and cell density on microbial photoinactivation. Antimicrob. Agents Chemother. 49:2329-2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donlan, R. M., and J. W. Costerton. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15:167-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freeman, D. J., F. R. Falkiner, and C. T. Keane. 1989. New method for detecting slime production by coagulase negative staphylococci. J. Clin. Pathol. 42:872-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fux, C. A., P. Stoodley, L. Hall-Stoodley, and J. W. Costerton. 2003. Bacterial biofilms: a diagnostic and therapeutic challenge. Expert Rev. Anti-Infect. Ther. 1:667-683. [DOI] [PubMed] [Google Scholar]
- 14.Gad, F., T. Zahra, T. Hasan, and M. R. Hamblin. 2004. Effects of growth phase and extracellular slime on photodynamic inactivation of gram-positive pathogenic bacteria. Antimicrob. Agents Chemother. 48:2173-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heilmann, C., M. Hussain, G. Peters, and F. Götz. 1997. Evidence for autolysin-mediated primary attachment of Staphylococcus epidermidis to a polystyrene surface. Mol. Microbiol. 24:1013-1024. [DOI] [PubMed] [Google Scholar]
- 16.Jori, G. 2006. Photodynamic therapy of microbial infections: state of the art and perspectives. J. Environ. Pathol. Toxicol. Oncol. 25:505-519. [DOI] [PubMed] [Google Scholar]
- 17.Lin, H. Y., C. T. Chen, and C. T. Huang. 2004. Use of merocyanine 540 for photodynamic inactivation of Staphylococcus aureus planktonic and biofilm cells. Appl. Environ. Microbiol. 70:6453-6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mah, T. F., and G. A. O'Toole. 2001. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 9:34-39. [DOI] [PubMed] [Google Scholar]
- 19.Moreillon, P., and Y. A. Que. 2004. Infective endocarditis. Lancet 363:139-149. [DOI] [PubMed] [Google Scholar]
- 20.Percival, S. L., P. Kite, K. Eastwood, R. Murga, J. Carr, M. J. Arduino, and R. M. Donlan. 2005. Tetrasodium EDTA as a novel central venous catheter lock solution against biofilm. Infect. Control Hosp. Epidemiol. 26:515-519. [DOI] [PubMed] [Google Scholar]
- 21.Raad, I., H. Hanna, T. Dvorak, G. Chaiban, and R. Hachem. 2007. Optimal antimicrobial catheter lock solution using different combinations of minocycline, EDTA, and 25 percent ethanol rapidly eradicates organisms embedded in biofilm. Antimicrob. Agents Chemother. 51:78-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang, H. M., M. R. Hamblin, and C. M. Yow. 2007. A comparative in vitro photoinactivation study of clinical isolates of multidrug-resistant pathogens. J. Infect. Chemother. 13:87-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tegos, G. P., M. Anbe, C. Yang, T. N. Demidova, M. Satti, P. Mroz, S. Janjua, F. Gad, and M. R. Hamblin. 2006. Protease-stable polycationic photosensitizer conjugates between polyethyleneneimine and chlorin (e6) for broad-spectrum antimicrobial photoinactivation. Antimicrob. Agents Chemother. 50:1402-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Usacheva, M. N., M. C. Teichert, and M. A. Biel. 2001. Comparison of the methylene blue and toluidine blue photobactericidal efficacy against gram-positive and gram-negative microorganisms. Lasers Surg. Med. 29:165-173. [DOI] [PubMed] [Google Scholar]
- 25.Wainwright, M. 1998. Photodynamic antimicrobial chemotherapy (PACT). J. Antimicrob. Chemother. 42:13-28. [DOI] [PubMed] [Google Scholar]
- 26.Wilson, M. 2004. Lethal photosensitisation of oral bacteria and its potential application in the photodynamic therapy of oral infections. Photochem. Photobiol. Sci. 3:412-418. [DOI] [PubMed] [Google Scholar]
- 27.Wood, S., B. Nattress, J. Kirkham, R. Shore, S. Brookes, J. Griffiths, and C. Robinson. 1999. An in vitro study of the use of photodynamic therapy for the treatment of natural oral plaque biofilms formed in vivo. J. Photochem. Photobiol. B 50:1-7. [DOI] [PubMed] [Google Scholar]
- 28.Wood, S., D. Metcalf, D. Devine, and C. Robinson. 2006. Erythrosine is a potential photosensitizer for the photodynamic therapy of oral plaque biofilms. J. Antimicrob. Chemother. 57:680-684. [DOI] [PubMed] [Google Scholar]
- 29.Wu, J. A., C. Kusuma, J. J. Mond, and J. F. Kokai-Kun. 2003. Lysostaphin disrupts Staphylococcus aureus and Staphylococcus epidermidis biofilms on artificial surfaces. Antimicrob. Agents Chemother. 47:3407-3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zanin, I. C., R. B. Gonçalves, A. B. Junior, C. K. Hope, and J. Pratten. 2005. Susceptibility of Streptococcus mutans biofilms to photodynamic therapy: an in vitro study. J. Antimicrob. Chemother. 56:324-330. [DOI] [PubMed] [Google Scholar]
- 31.Zanin, I. C., M. M. Lobo, L. K. Rodrigues, L. A. Pimenta, J. F. Höfling, and R. B. Gonçalves. 2006. Photosensitization of in vitro biofilms by toluidine blue O combined with light-emitting diode. Eur. J. Oral Sci. 114:64-69. [DOI] [PubMed] [Google Scholar]
- 32.Zimmerli, W., A. Trampuz, and P. E. Ochsner. 2004. Prosthetic-joint infections. N. Engl. J. Med. 351:1645-1654. [DOI] [PubMed] [Google Scholar]