Abstract
Many studies have shown that the pharmacological effects of resveratrol, a phytoalexin polyphenolic compound, include protective effects against cancer and inflammation as well as enhancement of stress resistance. In this study, we examined whether resveratrol affected the phagocytosis of bacteria by macrophages and the activation of the transcription factor NF-κB after stimulation with or without the ligand FSL-1 for Toll-like receptor 2 (TLR2). Phagocytosis of Escherichia coli and of Staphylococcus aureus by THP-1 cells and RAW264.7 cells was inhibited by resveratrol in a dose-dependent manner regardless of stimulation with FSL-1. The NF-κB activity in HEK293 cells stably expressing TLR2 was also inhibited by resveratrol after stimulation with FSL-1. Resveratrol also inhibited both the translocation of p65 of NF-κB into nuclei in the transfectant and tumor necrosis factor alpha production by THP-1 cells or RAW264.7 cells. It has recently been reported that TLR-mediated signaling pathways lead to the upregulation of mRNAs of phagocytic receptors, including scavenger receptors and C-type lectin receptors. This study also demonstrated that FSL-1 induced the upregulation of mRNAs of phagocytic receptors such as macrophage scavenger receptor-1, CD36, DC-SIGN, and Dectin-1 and that the FSL-1-induced upregulation of their mRNAs was inhibited by resveratrol. In addition, it was found that the expression of DC-SIGN in HEK293 cells stably expressing DC-SIGN was reduced by resveratrol and that the phagocytic activity was significantly inhibited by resveratrol. Thus, this study suggests that resveratrol inhibited bacterial phagocytosis by macrophages by downregulating the expression of phagocytic receptors and NF-κB activity.
Resveratrol (trans-3,4′,-5-trihydroxystilebene) is a phytoalexin polyphenolic compound found in various plants, including grapes, berries, and peanuts (2, 4). Dozens of studies have shown that the pharmacological effects of resveratrol include protective effects against cancer, cardiovascular diseases, and ischemic injuries as well as enhancement of stress resistance and extension of the life spans of various organisms from yeast to vertebrates (2). To account for the diverse effects of resveratrol, it has been suggested that its biological activities involve downregulation of the expression of proinflammatory markers, including inducible nitric oxide synthase and cyclooxygenase-2, by reducing the activities of nuclear factor κB (NF-κB) or the activator protein-1 (4). Although the inhibitory effects of resveratrol on NF-κB activity have been clearly demonstrated (4), there are few reports about the effects of resveratrol on Toll-like receptor (TLR) signaling, which plays important roles in the recognition of bacterial invasion and in bridging between innate and acquired immunity (19). Moreover, little is known about the effects of resveratrol on bacterial phagocytosis, which is also essential in activating signal transduction pathways leading to the killing and clearance of pathogens after the detection of bacterial invasion (22). Therefore, there is great interest in the effects of resveratrol on bacterial phagocytosis, because phagocytosis plays a vital role in host antibacterial responses.
Phagocytosis is an evolutionarily ancient host cell endocytic response to stimulation by microbes in both innate and acquired immunity (1, 22). Phagocytes, such as monocytes, macrophages, and neutrophils, detect bacterial invasion through various germ line-encoded pattern recognition receptors such as TLRs before internalizing and killing bacteria (19, 21, 22). Thus, the recognition of bacterial invasion by TLRs and bacterial clearance by phagocytosis play key roles in innate immunity. Nevertheless, little is known about the cross talk between TLRs and phagocytic receptors. Recently, several studies have shown that TLR-mediated signaling upregulates bacterial phagocytosis by macrophages and dendritic cells (5, 7). We have also found that the diacylated lipopeptide FSL-1 promotes the phagocytosis of bacteria, possibly through the upregulation of a phagocytic gene subset (13).
The present study, therefore, was designed to determine the effects of resveratrol on bacterial phagocytosis and NF-κB activity, which are mediated by TLRs, especially TLR2. We demonstrated that resveratrol downregulates MyD88-mediated bacterial phagocytosis as well as NF-κB activity in macrophages.
MATERIALS AND METHODS
Reagents and antibodies.
Resveratrol was purchased from Sigma-Aldrich (St. Louis, MO) and was dissolved in dimethyl sulfoxide (DMSO). FSL-1 derived from Mycoplasma salivarium was synthesized according to the method described previously (17). pUNO-DC-SIGN1a (human dendritic cell-specific intercellular adhesion molecule-grabbing nonintegrin-1a) was purchased from InvivoGen (San Diego, CA). A rabbit polyclonal antibody (pAb) against human p65 of NF-κB was obtained from Immuno-Biological Laboratories Co., Ltd. (Gunma, Japan). Alexa Fluor 594-conjugated anti-rabbit immunoglobulin G (IgG) Ab was purchased from Molecular Probes (Eugene, OR). A mouse monoclonal Ab (mAb) against human DC-SIGN1 (MAB161) was purchased from R&D Systems, Inc. (Minneapolis, MN). A mouse mAb against human β-actin (AC-15) was purchased from Abcam (Stockholm, Sweden). A horseradish peroxidase-conjugated anti-mouse IgG Ab was purchased from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA). All other reagents were purchased from commercial sources and were of analytical or reagent grade.
Cell cultures.
THP-1 cells (TIB-202; ATCC) and RAW264.7 cells (TIB-71; ATCC) were grown at 37°C and in 5% CO2 in RPMI 1640 medium (Sigma) supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum (Gibco BRL, Rockville, MD), 100 units/ml penicillin (Sigma), and 100 μg/ml streptomycin (Sigma) (complete medium). Human embryonic kidney (HEK293) cells (CRL-1573; ATCC) were grown in Dulbecco's modified Eagle's medium (DMEM) (Sigma) complete medium.
Stable transfectants.
The cDNA of human TLR2 obtained by reverse transcriptase-PCR (RT-PCR) of total RNA isolated from THP-1 cells was cloned into a pEF6/V5-His TOPO vector (Invitrogen Co., Carlsbad, CA) (hereafter referred to as pEF-TLR2). pEF-TLR2 or pUNO-hDC-SIGN1a was transfected into HEK293 cells by use of Metafectene transfection reagent (Biontex Laboratories GmbH, München, Germany) according to the manufacturer's instructions. The transfectants were selected in the presence of 50 μg/ml blasticidin S (Invitrogen). The expression of TLR2 or DC-SIGN was confirmed by immunoblot analysis using Abs to TLR2 or DC-SIGN.
FITC-conjugated bacteria.
Escherichia coli K-12 and Staphylococcus aureus 209P were cultured in brain heart infusion medium (Eiken, Tokyo, Japan) at 37°C to reach a concentration of approximately 1 × 109/ml. Bacteria were washed and resuspended in phosphate-buffered saline (PBS) and then inactivated at 95°C for 5 min. Heat-killed bacteria were incubated at 37°C for 1 h with a 0.5 mg/ml solution of fluorescein isothiocyanate (FITC) (Sigma) in 0.1 M carbonate buffer (pH 9.5). The FITC-conjugated bacteria or heat-killed bacteria were washed three times with PBS and resuspended with PBS at a concentration of 1 × 1010/ml.
Phagocytosis assay.
A 0.5-ml suspension of THP-1 cells (1 × 106/ml) or RAW264.7 cells (1 × 106/ml) was added to each well of a 24-well plate and incubated at 37°C for 24 h with various concentrations (0, 1, 10, 100 nM) of FSL-1. In the case of HEK293 transfectant expressing DC-SIGN (293/DC-SIGN cells), a 1.0-ml suspension of the cells (5 × 105/ml) was added to each well of a 12-well plate and then incubated at 37°C on the day before the assay. After the cells had been washed three times with base medium warmed at 37°C, they were treated at 37°C for 1 h with various concentrations (10, 50, 100 μM) of resveratrol. The cells were then incubated for 1 h with 5 × 107 particles of FITC-conjugated E. coli or S. aureus. After the cells had been washed three times with cold PBS, they were suspended in PBS containing 0.2% (wt/vol) trypan blue to quench fluorescence caused by the binding of bacteria to the surface of the cells and 1% (wt/vol) paraformaldehyde to fix the cells. Flow cytometry (FCM) was conducted using a FACSCalibur machine (BD Biosciences, San Diego, CA) and CellQuest software (BD Biosciences). Phagocytic activity was expressed as the mean fluorescence intensity (MFI) obtained by CellQuest software.
For the phagocytosis assay by confocal laser scanning microscopy (CLSM), a 1.0-ml suspension of THP-1 cells (1 × 106/ml) was added to each well of a 24-well plate and incubated at 37°C for 24 h with or without 100 nM FSL-1. After the cells had been washed three times with RPMI 1640 base medium warmed at 37°C, they were treated for 1 h with 100 μM resveratrol or 0.1% (vol/vol) DMSO and then incubated for 1 h with 1 × 108 particles of FITC-conjugated E. coli or S. aureus. The cells were then washed with PBS and reacted for 20 min with 50 μg/ml Alexa Fluor 594-conjugated concanavalin A (Molecular Probes) in PBS, followed by fixation with PBS containing 3% (wt/vol) paraformaldehyde for 20 min. An LSM510 inverted laser scanning microscope (Carl Zeiss, Tokyo, Japan) using a 63× objective (Leica Microsystems, Tokyo, Japan) was used for image acquisition.
Luciferase reporter gene assay.
HEK293 cells or HEK293 transfectant cells expressing TLR2 (293/TLR2 cells) were plated at 1 × 105 cells per well in a 24-well plate on the day before transfection. The cells were transiently transfected by Metafectene transfection reagent with 50 ng of an NF-κB reporter plasmid (pNF-κB-Luc; Stratagene, San Diego, CA) and 5 ng of a construct directing the expression of Renilla luciferase under the control of a constitutively active thymidine kinase promoter (pRL-TK; Promega, Madison, WI) together with 445 ng of pcDNA3 empty vector (Invitrogen). After a 24-h incubation, the cells were stimulated at 37°C for 6 h with FSL-1 or heat-killed bacteria in DMEM base medium, and luciferase activity was measured using a dual luciferase reporter assay system (Promega) according to the manufacturer's instructions.
Immunostaining for p65 elements of NF-κB.
293/TLR2 cells were seeded on poly-l-lysine-coated coverslips in wells of a six-well plate on the day before transfection. The next day, the cells were washed three times with DMEM base medium warmed at 37°C and incubated for 1 h with 100 μM resveratrol or 0.1% (vol/vol) DMSO. After a 6-h stimulation with E. coli or S. aureus, the cells were incubated with PBS containing Alexa Fluor 594-conjugated concanavalin A (50 μg/ml), followed by methanol fixation for 5 min at −20°C. After the cells had been washed twice with PBS, they were incubated at room temperature for 45 min with a rabbit pAb against p65 of NF-κB (1 μg/ml of PBS) and then for another 45 min with Alexa Fluor 594-conjugated anti-rabbit IgG Ab. The cells were washed three times with PBS and observed using an LSM410 microscope.
ELISA.
THP-1 cells or RAW264.7 cells were plated at 1 × 106 cells per well of a 24-well plate in RPMI 1640 complete medium and incubated at 37°C for 16 h. The cells were washed three times with RPMI 1640 base medium and treated with various concentrations (1, 10, 100 nM) of resveratrol. The cells were stimulated with heat-inactivated E. coli or S. aureus at a cell:bacterium ratio of 1:100, and the culture supernatant was collected by centrifugation at 400 × g for 10 min. The amount of tumor necrosis factor alpha (TNF-α) in the supernatant was determined by using an enzyme-linked immunosorbent assay (ELISA) development kit human TNF-α (PeproTech, Rocky Hill, NJ) for THP-1 cells or an OptEIA set mouse TNF-α (BD Pharmingen, San Diego, CA) for RAW264.7 cells.
RT-PCR.
THP-1 cells were incubated at 37°C for 24 h with 100 nM FSL-1 in a well of a six-well plate and were then incubated for 1 h with 100 μM resveratrol or 0.1% (vol/vol) DMSO. Total RNA isolated from 5 × 106 of the cells was prepared by using an RNeasy kit (Qiagen Inc., Chatsworth, CA) according to the manufacturer's instructions. The RNA was reverse transcribed to cDNA in a 20-μl reaction volume containing 2.5 μM of each of anchored oligo(dT)18 primers. The PCRs were performed in 40-μl final volumes containing 10 μl of cDNA, 2.5 μM MgCl2, and 20 pmol of each sense primer of macrophage scavenger receptor 1 (MSR1), CD36, DC-SIGN, Dectin-1, and β-actin, sequences of which have been described previously (13). After initial denaturation at 94°C for 30 s, amplifications were carried out with 25 cycles for β-actin or 30 cycles for the others. The PCR products were separated of a 3% gel of NuSieve 3:1 agarose in Tris-acetate-EDTA buffer containing ethidium bromide (5 μg/ml).
Western blotting.
293/DC-SIGN cells were plated at 1 × 107 cells in a 10-cm dish in DMEM complete medium and incubated at 37°C for 16 h. After the cells had been washed three times with DMEM base medium, they were incubated for 0, 30, 60, 90, or 120 min with 100 μM resveratrol. The cells were washed twice with ice-cold PBS and then lysed by 62.5 mM Tris-HCl (pH 6.8) containing 2% sodium dodecyl sulfate (SDS), 10% glycerol, and 50 mM dithiothreitol (an SDS sample buffer) in the presence of inhibitor cocktails of proteases (Roche) and boiled for 10 min. The lysates were centrifuged at 14,000 rpm for 10 min, and the resulting supernatants containing cytosolic and membrane proteins were collected. Proteins in the supernatant were separated by electrophoresis on 10% SDS-polyacrylamide gels and transferred to nitrocellulose membranes. After the membranes had been incubated at 4°C overnight with an anti-DC-SIGN mAb or an anti-β-actin mAb, they were incubated with a horseradish peroxidase-conjugated anti-mouse IgG. Immunoreactive proteins were detected by using ECL detection reagents (GE Healthcare, Piscataway, NJ).
RESULTS
Inhibition of bacterial phagocytosis by resveratrol.
To examine whether resveratrol affects phagocytosis, human monocyte-like THP-1 cells and murine macrophage-like RAW264.7 cells were used as phagocytes, and heat-killed gram-positive bacteria (S. aureus) and gram-negative bacteria (E. coli) were used as target bacteria after being conjugated with FITC. Phagocytosis assays were performed with various concentrations of resveratrol in serum-free medium in order to rule out the possibility of involvement of Fc- and complement-mediated opsonization of bacteria. FCM analysis showed that phagocytic activities of both THP-1 cells and RAW264.7 cells increased as the incubation time increased and that resveratrol significantly inhibited the activities in a dose-dependent manner at all incubation times (Fig. 1A and B). In addition, it was found that the activities increased as the ratios of the number of bacterial cells to that of phagocytes increased and that the activities at almost all ratios were suppressed by resveratrol (Fig. 1C and D). The activity level of the monocyte-like THP-1 cells was significantly lower than that of the macrophage-like RAW264.7 cells.
FIG. 1.
Inhibitory effect of resveratrol on phagocytosis of FITC-conjugated E. coli and S. aureus by macrophages. FCM data are presented as MFI of each population. RAW264.7 cells (A) and THP-1 cells (B) were treated with the indicated doses of resveratrol for 1 h in a serum-free condition, and they were incubated for 0, 20, 40, 60, or 80 min with heat-killed FITC-conjugated E. coli at a cell:bacterium ratio of 1:100. RAW264.7 cells (C) and THP-1 cells (D) were treated with the indicated doses of resveratrol and were given FITC-conjugated E. coli and FITC-conjugated S. aureus. MFIs of internalized heat-killed FITC-conjugated bacteria were plotted on the y axis versus the dose of bacteria on the x axis. The mean values and standard deviations (SD) from triplicate experiments are shown. The statistically significant difference from the vehicle data was assessed by Student's t test: *, P < 0.05; **, P < 0.01. Representative data are from more than three independent experiments. Veh, vehicle.
Inhibition of the FSL-1-induced enhancement of bacterial phagocytosis by resveratrol.
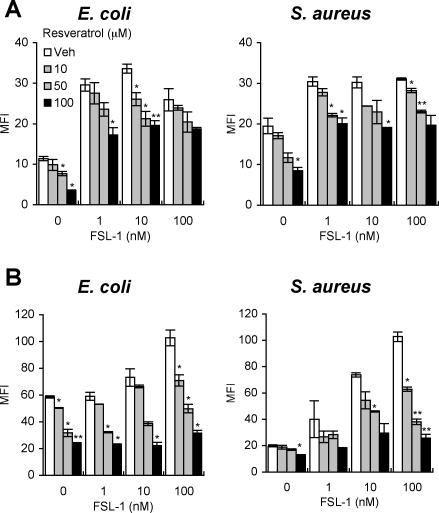
Recently, we found that the TLR2 ligand FSL-1 enhances the phagocytosis of bacteria by macrophages (13). Therefore, we next examined whether resveratrol affected the FSL-1-induced enhancement of phagocytosis. It was found that FSL-1 significantly enhanced the phagocytosis of E. coli and S. aureus by RAW264.7 cells (Fig. 2A) and THP-1 cells (Fig. 2B) and that the enhancement was significantly inhibited by resveratrol in a dose-dependent manner (Fig. 2A and B).
FIG. 2.
Inhibitory effect of resveratrol on enhancement of bacterial phagocytosis by FSL-1 as revealed by FCM. FCM data are presented as MFI of each population. After pretreatment with the indicated dose of FSL-1 for 24 h, RAW264.7 cells (A) and THP-1 cells (B) were incubated with various concentrations of resveratrol and then given heat-killed FITC-conjugated E. coli or FITC-conjugated S. aureus at a cell:bacterium ratio of 1:10 or 1:100, respectively. The mean values and SD of triplicate experiments are shown. The statistically significant difference from the vehicle data was assessed by Student's t test: *, P < 0.05; **, P < 0.01. Representative data are from more than three independent experiments. Veh, vehicle.
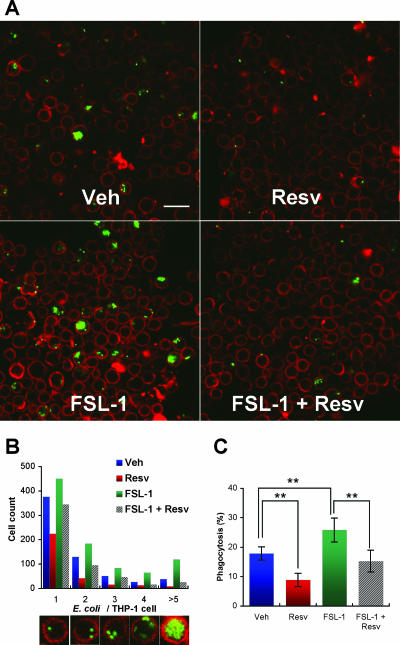
In the following experiments, we tried to evaluate phagocytosis by another assay method using CLSM, which enables the number of bacterial particles phagocytosed per cell to be counted (Fig. 3A). The analysis demonstrated that FSL-1 treatment caused a significant increase in the number of THP-1 cells phagocytosing multiple E. coli particles and that resveratrol treatment reduced the number of cells phagocytosing E. coli particles (Fig. 3B) as well as the percentage of the cells phagocytosing E. coli particles relative to the total cell number analyzed (Fig. 3C). Thus, resveratrol has the activity to inhibit the phagocytic activity of macrophages toward bacteria regardless of TLR stimulation.
FIG. 3.
Inhibitory effect of resveratrol on enhancement of bacterial phagocytosis by FSL-1 as revealed by CLSM. (A) THP-1 cells were incubated in the absence or presence of 100 nM FSL-1 for 24 h and treated with 100 μM resveratrol for 1 h. The cells were given heat-killed FITC-conjugated E. coli at a ratio of 1:100, stained with Alexa Fluor 488-conjugated concanavalin A to cell surface glycoproteins, and subjected to CLSM. Bar, 25 μm. (B) The numbers of the cells shown in panel A were counted to determine the number of E. coli particles per individual cell. Data are presented as the number of THP-1 cells (out of 3,500) that phagocytosed 1, 2, 3, 4, or >5 E. coli particles per cell. The pictures along the x axis are representative of the cells counted. (C) Phagocytosis (%) in one field containing at least 100 cells is expressed as [the number of cells taking up bacteria]/[total number (>100) of cells in one field] × 100. Each value is the mean and SD of phagocytosis (%) obtained from 25 fields. The statistically significant difference was assessed by Student's t test: **, P < 0.01. Representative data are from at least two independent experiments. Veh, vehicle; Resv, resveratrol.
Inhibition of NF-κB activation by resveratrol.
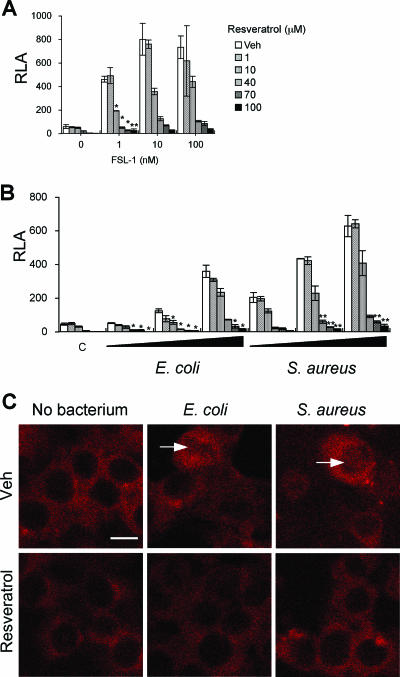
To get an insight into the molecular mechanisms underlying the resveratrol-induced impairment of phagocytosis enhanced by TLR2 stimulation, we examined whether resveratrol suppressed the transcriptional activity of NF-κB, which is known to be activated downstream of the TLR2 signaling pathway. Neither the heat-killed bacteria nor FSL-1 could stimulate NF-κB in wild-type HEK293 cells in the absence or presence of resveratrol (data not shown). In contrast, the NF-κB activity in HEK293 cells stably expressing TLR2 (293/TLR2) was activated by FSL-1 in a dose-dependent manner, and the FSL-1-induced enhancement was inhibited by resveratrol (Fig. 4A). The heat-killed bacteria also had activity to stimulate NF-κB in 293/TLR2 cells, and the activity was also suppressed by resveratrol in a dose-dependent manner (Fig. 4B). Upon activation, the p65 element of NF-κB is translocated from the cytoplasm to the nucleus. Therefore, the intracellular localization of p65 in 293/TLR2 cells in response to these bacterial stimulations was examined. p65 of NF-κB was translocated into nuclei in 293/TLR2 cells, but the translocation was inhibited by resveratrol (Fig. 4C). These results also suggest that E. coli and S. aureus possess TLR2 ligands on the cell surfaces.
FIG. 4.
Effect of resveratrol on the recognition of FSL-1, E. coli, or S. aureus by 293/TLR2 cells. 293/TLR2 cells were plated at 1 × 105 cells per well in 24-well plates on the day before transfection. The cells were transiently transfected with an NF-κB reporter plasmid and a construct directing expression of Renilla luciferase under the control of a constitutively active thymidine kinase promoter. After a 24-h incubation, 293/TLR2 cells were pretreated for 1 h with various concentrations of resveratrol and stimulated for 6 h with FSL-1 (1, 10, 100 nM) (A) or with heat-killed E. coli (cell:bacterium ratios of 1:1, 1:10, 1:100) or heat-killed S. aureus (cell:bacterium ratios of 1:1, 1:10, 1:100) (B) in a serum-free condition. The mean values and SD from triplicate experiments are shown. The statistically significant difference from vehicle was assessed by Student's t test: *, P < 0.05; **, P < 0.01. (C) Representative CLSM images of the p65 element of NF-κB in 293/TLR2 cells are shown. The cells were treated with or without 100 μM resveratrol for 1 h and stimulated with heat-killed E. coli at a ratio of 1:100 or with heat-killed S. aureus at a ratio of 1:100 for 6 h. The nuclear translocation of the p65 element of NF-κB was detected with a pAb against p65 of NF-κB (arrows). Representative data are from at least two independent experiments. Bar, 10 μm. Veh, vehicle.
Inhibition of TNF-α production by resveratrol.
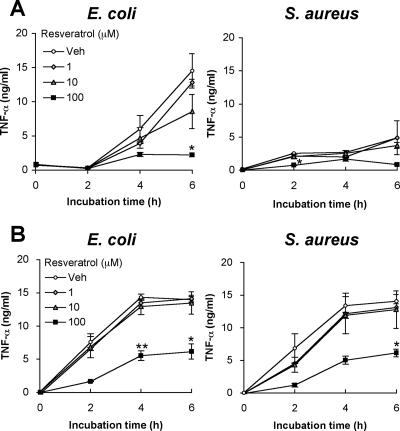
Since the transcription factor NF-κB plays an important role in the expression of a large number of inducible genes, including genes for inflammatory cytokines (14), an experiment was carried out to determine whether resveratrol inhibits TNF-α production by THP-1 cells and RAW264.7 cells. It is thought that the gram-negative bacterium E. coli is mainly recognized by TLR2 and TLR4, whereas the gram-positive bacterium S. aureus is mainly recognized by TLR2 but not by TLR4 (20). Therefore, RAW264.7 cells and THP-1 cells were stimulated by both bacteria, and the amounts of TNF-α produced were measured by ELISA. It was found that S. aureus and E. coli stimulated both types of cells to induce the production of TNF-α in a time-dependent manner (Fig. 5A and B). S. aureus and E. coli stimulated THP-1 cells more strongly than RAW264.7 cells (Fig. 5A and B), and RAW264.7 cells were stimulated more strongly by E. coli than by S. aureus (Fig. 5A). The difference in the profiles of TNF-α production by THP-1 and RAW264.7 cells might be explained by the difference in the expression levels of TLR2 and TLR4, although we cannot rule out other potential reasons for the differences in TNF-α production between these cell types. That is, the expression levels of TLR2 and TLR4 in THP-1 cells might be higher than those in RAW264.7 cells and/or the expression level of TLR4 might be higher than that of TLR2 in RAW264.7 cells.
FIG. 5.
TNF-α production by RAW264.7 cells and THP-1 cells in response to heat-killed E. coli or S. aureus. RAW264.7 cells (A) and THP-1 cells (B) were advance treated for 1 h with various concentrations of resveratrol and incubated for 0, 2, 4, or 6 h in a serum-free condition with heat-killed E. coli and S. aureus at a cell:bacterium ratio of 1:100. The amounts of TNF-α produced in the supernatants were measured by ELISA. See text for details. The mean values and SD of triplicate experiments are shown. The statistically significant difference from vehicle data was assessed by Student's t test: *, P < 0.05; **, P < 0.01. Representative data are from more than three independent experiments. Veh, vehicle.
Inhibition of phagocytic receptor-mediated bacterial phagocytosis by resveratrol.
It has recently been reported that TLR-mediated signaling pathways lead to the upregulation of mRNAs of phagocytic receptors, including scavenger receptors (SRs) and C-type lectin receptors (CLRs) (7). Several lines of evidence have indicated that SRs and CLRs function as pattern recognition receptors and mediate the phagocytosis of microbes by phagocytes (11, 16, 21, 22). We have also found that FSL-1 stimulation is able to induce upregulation of the expression of mRNAs for MSR1, CD36, DC-SIGN, and Dectin-1 in THP-1 cells (Fig. 6A) (13). Therefore, we examined whether resveratrol inhibited the FSL-1-induced upregulation of the expression of their mRNAs and found that resveratrol inhibited the FSL-1-induced upregulation (Fig. 6A). Therefore, the inhibition of the FSL-1-induced enhancement of phagocytosis by resveratrol may be explained by downregulation of the expression of mRNAs for these phagocytic receptors. In order to further confirm this, we established HEK293 cells stably expressing DC-SIGN and examined the phagocytosis activity of the cells toward E. coli and S. aureus. It was found that the expression of DC-SIGN in the transfectant was reduced by resveratrol (Fig. 6B) and that the phagocytosis activity of 293/DC-SIGN cells clearly increased as the ratios of bacteria to cells increased; in addition, resveratrol significantly inhibited the phagocytosis activity in a dose-dependent manner (Fig. 6C).
FIG. 6.
Inhibitory effect of resveratrol on impaired phagocytosis of bacteria mediated by SRs and CLRs. (A) THP-1 cells incubated for 24 h with 100 nM FSL-1 were treated with 100 μM resveratrol for 1 h in a serum-free condition. The expression of mRNAs for MSR1, CD36, DC-SIGN, Dectin-1, and β-actin was confirmed by RT-PCR using total RNA isolated from the cells. Representative data are from more than five independent experiments. (B) 293/DC-SIGN cells were incubated for 0, 30, 60, 90, or 120 min with 100 μM resveratrol. The cells were then lysed in SDS sample buffer, and proteins were separated by electrophoresis on 10% SDS-polyacrylamide gels and transferred to a nitrocellulose membrane. The membrane was reacted with anti-DC-SIGN mAb or anti-β-actin mAb. (C) 293/DC-SIGN cells were treated with the indicated doses of resveratrol for 1 h and given heat-killed FITC-conjugated E. coli or S. aureus at various cell:bacterium ratios. The mean values and SD from triplicate experiments are shown. The statistically significant difference from vehicle was assessed by Student's t test: *, P < 0.05; **, P < 0.01. Representative data are from more than three independent experiments. Veh, vehicle.
On the basis of these results, it was concluded that resveratrol inhibited bacterial phagocytosis by macrophages by downregulating the expression of phagocytic receptors, including SRs and CLRs.
DISCUSSION
This study demonstrates that both the transcriptional activity and translocation into nuclei of NF-κB functioning downstream of TLR2, activated by bacteria as well as TLR2 agonist FSL-1, are inhibited by resveratrol (Fig. 4 and 5). The first evidence of resveratrol affecting NF-κB was obtained by Draczynska-Lusiak et al. (8). They demonstrated that oxidized low-density lipoprotein treatment activates NF-κB in PC12 cells and that resveratrol attenuates the activation (8). Thereafter, there were many reports of resveratrol suppressing NF-κB activation in a variety of cell lines, including U-937, Jurkat, and HeLa cells, induced by several agents, including 12-O-tetradecanoylphorbol-13-acetate, lipopolysaccharide, H2O2, and ceramide (4). Therefore, there seems to be no doubt that resveratrol inhibits NF-κB activation. However, Youn et al. reported that resveratrol suppresses NF-κB activation in RAW264.7 cells downstream of TLR3 and TLR4 signaling pathways but not TLR2 or TLR9 signaling pathways (23). That is, they concluded that resveratrol inhibits NF-κB activation induced by TRIF but not by MyD88. Our results obtained in this study are in contrast to their findings. Even in their study, weak but not significant inhibition of NF-κB activation by the TLR2 agonist MALP2 was observed (23). Therefore, we think that the discrepancy can be explained by the level of inhibitory activity of resveratrol against MyD88- and TRIF-mediated NF-κB activation.
Recently, we and others have reported that TLR-mediated signals leading to NF-κB activation upregulate the phagocytosis of bacteria by macrophages and dendritic cells (7, 9, 13, 15, 18). Therefore, we examined whether resveratrol affected bacterial phagocytosis by macrophages with or without TLR2 stimulation. This study demonstrated that phagocytic activities of both THP-1 and RAW264.7 cells toward bacteria were dose-dependently inhibited by resveratrol regardless of TLR-mediated signals (Fig. 1, 2, and 3). When macrophages were stimulated by the TLR2 agonist FSL-1, resveratrol inhibited bacterial phagocytosis by macrophages by downregulating the expression of phagocytic receptors including SRs and CLRs. This finding is supported by the finding of Leiro et al. (12) that the phagocytosis of Kluyveromyces lactis by macrophages is inhibited by resveratrol, although they did not clarify the mechanism by which resveratrol inhibits bacterial phagocytosis of macrophages. However, resveratrol at low concentrations (1 to 10 μM) enhanced the phagocytosis of Candida albicans in human macrophage-like cells (3). In the present study, resveratrol even at 10 μM significantly attenuated the phagocytosis of E. coli or S. aureus by a human monocytic cell line, THP-1 cells. This discrepancy may be explained by the difference in cell surface components of the target microbes, i.e., the eukaryotic microbe Candida albicans and the prokaryotic microbial bacteria. The C-type lectin Dectin-1 functions not only as a recognition receptor of Candida albicans but also as a phagocytic receptor (6, 10). Therefore, it is thought that the enhancement of phagocytosis of Candida albicans is mediated by Dectin-1. In addition, we have recently reported that HEK293 cells expressing Dectin-1 do not ingest E. coli or S. aureus (13). Thus, phagocytic receptors for yeast seem to be different from those for bacteria. This may explain the discrepancy described above, although the exact mechanism remains unknown.
Thus, the present study demonstrated that resveratrol not only inhibits the activation of NF-κB induced by TLR2-mediated signals but also inhibits the phagocytosis of macrophages regardless of TLR stimulation. Judging from our results and the immunomodulatory effects that have been reported previously (2, 4), there is no doubt that resveratrol potentially has anti-inflammatory properties against bacterial infection by repressing TLR-mediated recognition and/or subsequent phagocytosis.
Acknowledgments
This work was supported by Grants-in-Aid for Science Research (B17390498 and B19390477) provided by the Japan Society for the Promotion of Science.
Footnotes
Published ahead of print on 15 October 2007.
REFERENCES
- 1.Aderem, A., and D. M. Underhill. 1999. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 17:593-623. [DOI] [PubMed] [Google Scholar]
- 2.Baur, J. A., and D. A. Sinclair. 2006. Therapeutic potential of resveratrol: the in vivo evidence. Nat. Rev. Drug Discov. 5:493-506. [DOI] [PubMed] [Google Scholar]
- 3.Bertelli, A. A., F. Ferrara, G. Diana, A. Fulgenzi, M. Corsi, W. Ponti, M. E. Ferrero, and A. Bertelli. 1999. Resveratrol, a natural stilbene in grapes and wine, enhances intraphagocytosis in human promonocytes: a co-factor in antiinflammatory and anticancer chemopreventive activity. Int. J. Tissue React. 21:93-104. [PubMed] [Google Scholar]
- 4.Bhat, K. P., and J. M. Pezzuto. 2002. Cancer chemopreventive activity of resveratrol. Ann. N. Y. Acad. Sci. 957:210-229. [DOI] [PubMed] [Google Scholar]
- 5.Blander, J. M., and R. Medzhitov. 2004. Regulation of phagosome maturation by signals from toll-like receptors. Science 304:1014-1018. [DOI] [PubMed] [Google Scholar]
- 6.Brown, G. D. 2005. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat. Rev. Immunol. 6:33-43. [DOI] [PubMed] [Google Scholar]
- 7.Doyle, S. E., R. M. O'Connell, G. A. Miranda, S. A. Vaidya, E. K. Chow, P. T. Liu, S. Suzuki, N. Suzuki, R. L. Modlin, W. C. Yeh, T. F. Lane, and G. Cheng. 2004. Toll-like receptors induce a phagocytic gene program through p38. J. Exp. Med. 199:81-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Draczynska-Lusiak, B., Y. M. Chen, and A. Y. Sun. 1998. Oxidized lipoproteins activate NF-kappaB binding activity and apoptosis in PC12 cells. Neuroreport 9:527-532. [DOI] [PubMed] [Google Scholar]
- 9.Fujita, M., T. Into, M. Yasuda, T. Okusawa, S. Hamahira, Y. Kuroki, A. Eto, T. Nisizawa, M. Morita, and K. Shibata. 2003. Involvement of leucine residues at positions 107, 112, and 115 in a leucine-rich repeat motif of human Toll-like receptor 2 in the recognition of diacylated lipoproteins and lipopeptides and Staphylococcus aureus peptidoglycans. J. Immunol. 171:3675-3683. [DOI] [PubMed] [Google Scholar]
- 10.Gantner, B. N., R. M. Simmons, and D. M. Underhill. 2005. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. EMBO J. 24:1277-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geijtenbeek, T. B., S. J. van Vliet, A. Engering, B. A. t Hart, and Y. van Kooyk. 2004. Self- and nonself-recognition by C-type lectins on dendritic cells. Annu. Rev. Immunol. 22:33-54. [DOI] [PubMed] [Google Scholar]
- 12.Leiro, J., E. Alvarez, D. Garcia, and F. Orallo. 2002. Resveratrol modulates rat macrophage functions. Int. Immunopharmacol. 2:767-774. [DOI] [PubMed] [Google Scholar]
- 13.Mae, M., M. Iyori, H. Shamsul, H. Kataoka, K. Kiura, Y. Totsuka, and K. Shibata. 2007. The diacylated lipopeptide FSL-1 enhances phagocytosis of bacteria by macrophages through Toll-like receptor 2-mediated signaling pathway. FEMS Immunol. Med. Microbiol. 49:398-409. [DOI] [PubMed] [Google Scholar]
- 14.May, M. J., and S. Ghosh. 1998. Signal transduction through NF-kappa B. Immunol. Today 19:80-88. [DOI] [PubMed] [Google Scholar]
- 15.Okusawa, T., M. Fujita, J. Nakamura, T. Into, M. Yasuda, A. Yoshimura, Y. Hara, A. Hasebe, D. T. Golenbock, M. Morita, Y. Kuroki, T. Ogawa, and K. Shibata. 2004. Relationship between structures and biological activities of mycoplasmal diacylated lipopeptides and their recognition by Toll-like receptors 2 and 6. Infect. Immun. 72:1657-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peiser, L., S. Mukhopadhyay, and S. Gordon. 2002. Scavenger receptors in innate immunity. Curr. Opin. Immunol. 14:123-128. [DOI] [PubMed] [Google Scholar]
- 17.Shibata, K., A. Hasebe, T. Into, M. Yamada, and T. Watanabe. 2000. The N-terminal lipopeptide of a 44-kDa membrane-bound lipoprotein of Mycoplasma salivarium is responsible for the expression of intercellular adhesion molecule-1 on the cell surface of normal human gingival fibroblasts. J. Immunol. 165:6538-6544. [DOI] [PubMed] [Google Scholar]
- 18.Suzuki, E., and K. Umezawa. 2006. Inhibition of macrophage activation and phagocytosis by a novel NF-kappaB inhibitor, dehydroxymethylepoxyquinomicin. Biomed. Pharmacother. 60:578-586. [DOI] [PubMed] [Google Scholar]
- 19.Takeda, K., and S. Akira. 2005. Toll-like receptors in innate immunity. Int. Immunol. 17:1-14. [DOI] [PubMed] [Google Scholar]
- 20.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]
- 21.Taylor, P. R., L. Martinez-Pomares, M. Stacey, H. H. Lin, G. D. Brown, and S. Gordon. 2005. Macrophage receptors and immune recognition. Annu. Rev. Immunol. 23:901-944. [DOI] [PubMed] [Google Scholar]
- 22.Underhill, D. M., and A. Ozinsky. 2002. Phagocytosis of microbes: complexity in action. Annu. Rev. Immunol. 20:825-852. [DOI] [PubMed] [Google Scholar]
- 23.Youn, H. S., J. Y. Lee, K. A. Fitzgerald, H. A. Young, S. Akira, and D. H. Hwang. 2005. Specific inhibition of MyD88-independent signaling pathways of TLR3 and TLR4 by resveratrol: molecular targets are TBK1 and RIP1 in TRIF complex. J. Immunol. 175:3339-3346. [DOI] [PubMed] [Google Scholar]