Abstract
Weak T-cell reactivity to hepatitis B virus (HBV) is thought to be the dominant cause for chronic HBV infection. Treatment with adefovir dipivoxil (ADV) increases the rate of HBV e antigen (HBeAg) loss; however, the immune mechanisms associated with this treatment response are not understood. Serial analysis of HBV-specific CD4+ T-cell reactivity was performed during 48 weeks of therapy with ADV and correlated with treatment outcome for 19 HBeAg-positive patients receiving ADV (n = 13) or the placebo (n = 6). We tested T-cell reactivity to HBV at seven protocol time points by proliferation, cytokine production, and enzyme-linked immunospot assays. A panel of serum cytokines was quantitated by cytokine bead array. ADV-treated patients showed increased CD4+ T-cell responses to HBV and lower serum levels of cytokines compared to those of placebo-treated patients. Enhanced CD4+ T-cell reactivity to HBV, which peaked at treatment week 16, was confined to a subgroup of ADV-treated patients who achieved greater viral suppression (5.3 ± 0.3 log10 copies/ml [mean ± standard error of the mean {SEM}] serum HBV DNA reduction from baseline) and HBeAg loss, but not to ADV-treated patients with moderate (3.4 ± 0.2 log10 copies/ml [mean ± SEM]) viremia reduction who remained HBeAg positive or to patients receiving the placebo. In conclusion, T-cell reactivity to HBV increases in a proportion of ADV-treated patients and is associated with greater suppression of HBV replication and HBeAg loss.
Hepatitis B virus (HBV) is a noncytopathic virus, and the clinical outcome of HBV infection is determined by the quality and strength of the host immune response to the virus. T-cell responses are weak or undetectable in patients with chronic hepatitis B, which is believed to be the dominant cause for persistent HBV replication (3, 23). During the course of HBV e antigen-positive (HBeAg+) chronic hepatitis B, there may be spontaneous suppression of viral replication with seroconversion from HBeAg+ to anti-HBe+ (5, 12). This is a favorable outcome associated with the normalization of serum aminotransferases, the resolution of liver inflammation (7), and improved patient survival (20). HBeAg seroconversion can be accelerated by treatment with alpha interferon (IFN-α) or oral antivirals (9, 13, 27). However, there is no sterilizing immunity after exposure to HBV, as a low level of viral replication, accompanied by strong HBV-specific T-cell reactivity, is detectable even in subjects with spontaneously resolved hepatitis B who are seronegative for HBsAg (15, 21, 22). Thus, the HBV genome usually persists with a very low replication rate, which is kept under an effective host immune control.
Based on this understanding of HBV-host interactions, the treatment strategy for chronic hepatitis B is direct suppression of HBV replication and enhancement of the host's immunoreactivity to the virus to achieve sustained viral control and remission of liver disease. Currently available treatments with IFN-α or oral antivirals are effective for only a small proportion of patients (9, 12, 27). The underlying mechanisms that differentiate treatment responders from nonresponders, particularly the role of antiviral immune responses, are poorly understood. Adefovir dipivoxil (ADV) is an oral nucleotide analogue of AMP, which blocks HBV DNA synthesis of wild-type (HBeAg+) and precore mutant HBV (HBeAg−) as well as lamivudine-resistant HBV mutants (6, 26). A randomized, double-blind, placebo-controlled trial established that 48 weeks of treatment with ADV significantly reduces HBV DNA, normalizes liver transaminases, and increases the rate of HBeAg seroconversion in patients with HBeAg+ chronic hepatitis B (13).
The aims of this investigation were to prospectively analyze the changes in HBV-specific CD4+ T-cell reactivity during ADV treatment in patients with HBeAg+ chronic hepatitis B and to correlate these changes with treatment outcome.
MATERIALS AND METHODS
Patients and study design.
The investigation of T-cell reactivity was conducted as a substudy to a phase III trial comparing treatment with ADV versus that with a placebo in HBeAg+ patients with chronic hepatitis B (study number GS-98-437, sponsored by Gilead Sciences, Foster City, CA). All 19 patients involved in this substudy were HBeAg+, as this was an inclusion criterion for the trial, and were recruited in six European centers (Lyon, France; Hanover, Germany; Bologna and Turin, Italy; and London and Manchester, United Kingdom) participating in the phase III trial. After completion of the HBV-specific T-cell analyses, subsequent unblinding revealed that 6 patients had received the placebo and 13 had received ADV (either 30 mg [n = 7] or 10 mg [n = 6]) daily for 48 weeks (Table 1). During the study period, patients were monitored at individual centers according to the trial protocol (13). For the analysis of T-cell reactivity, heparinized venous blood was taken at seven study visits: baseline and treatment weeks (TWs) 4, 8, 16, 24, 40, and 48. Additional blood samples were taken in the event of alanine aminotransferase (ALT) flares (an elevation of more than three times the patient's baseline ALT level). The T-cell substudy was approved by the Ethics Committee at each site, separately from the parent GS-98-437 protocol, and informed consent was obtained from all patients prior to study entry.
TABLE 1.
Patient characteristics at baseline
| Characteristic | Value for group given:
|
|
|---|---|---|
| Adefovir (n = 13) | Placebo (n = 6) | |
| Age (yr)a | 39.2 ± 12.8 | 43.3 ± 12.8 |
| Gender (male/female ratio) | 11:2 | 6:0 |
| Racial group (no. of patients) | ||
| White | 10 | 6 |
| Black | 1 | 0 |
| Asian | 2 | 0 |
| ALT (IU/ml)a | 128.3 ± 63.4 | 73.8 ± 16.3b |
| HBV DNA (log10 copies/ml)a | 8.3 ± 0.6 | 8.7 ± 0.7 |
| HBV genotype (no. of patients) | ||
| A | 5 | 4 |
| C | 1 | 0 |
| D | 5 | 2 |
| E | 1 | 0 |
| G | 1 | 0 |
| Received previous IFN-α treatment (no. of patients) | 3 | 2 |
The data are shown as means ± standard deviations.
Serum ALT levels were higher in the group of patients receiving ADV than in the patients receiving placebo (P = 0.05).
The analyses of T-cell reactivity, performed in a blinded fashion, were coordinated by the Institute of Hepatology, University College London (UCL). To standardize the procedures, the centers were supplied with the same reagents and laboratory protocols and the personnel were trained at the coordinating center. Freshly isolated peripheral blood mononuclear cells (PBMC) were used on site in France, Germany, and Italy to assess T-cell proliferation and in vitro cytokine production in response to HBV antigens. Part of the PBMC were cryopreserved and shipped to the coordinating center, together with the supernatants from PBMC cultures. The heparinized blood samples from the two United Kingdom centers were processed at the Institute of Hepatology, UCL, with T-cell proliferation and in vitro cytokine production tested with fresh cells, and the remaining PBMC were cryopreserved.
Procedures.
PBMC were isolated from heparinized blood by density gradient centrifugation with Lymphoprep (Nygaard, Oslo, Norway). Part of the PBMC were cryopreserved at 5 × 106 cells/ml in heat-inactivated fetal bovine serum (Invitrogen, Paisley, United Kingdom) containing 10% dimethyl sulfoxide (Sigma, Poole, United Kingdom). The aliquots were placed overnight at −80°C in a cryogenic vessel (Merck, Poole, United Kingdom) containing isopentane and subsequently transferred into liquid nitrogen.
T-cell proliferation assay.
All T-cell proliferation tests were performed with freshly isolated PBMC as described previously (10, 24). The cells were resuspended at a concentration of 2 × 106/ml in buffered RPMI, supplemented with 10% human AB serum (Gemini Bio-Products, Sacramento, CA), and 100 μl was added per well in a 96-well, flat-bottom plate (Merck, Poole, United Kingdom). The antigens added to quadruplicate wells were the following: recombinant HBcAg (rHBcAg) (1 μg/ml; American Research Products, Belmont, MA); tetanus toxoid, a positive control as a recall antigen (0.5 μg/ml; Connaught Laboratories, Ontario, Canada); and phytohemagglutinin (1 μg/ml; Sigma-Aldrich, Poole, United Kingdom). After 6 days, the cells were pulsed with 0.5 μCi/well [3H]thymidine and its incorporation into DNA was measured by a beta counter. A stimulation index was calculated by dividing the mean counts per minute of the replicates of antigen-stimulated PBMC by those of PBMC incubated in medium only. A stimulation index greater than the mean plus 3 standard deviations of control subjects was considered a significant response. As the proliferation assays were performed at each study site, the cutoff was determined separately at each center by testing PBMC from 15 to 20 control subjects.
In vitro cytokine production and enzyme-linked immunospot (ELISPOT) assay.
To analyze HBV-specific cytokine production, PBMC (3 × 106/ml) were resuspended in RPMI plus 10% AB serum, and 100 μl was added per well in a 96-well tissue culture plate. PBMC were incubated in quadruplicate for 6 days in the presence or absence of rHBcAg (1 μg/ml; American Research Products, Belmont, MA). The cell-free supernatants were collected and kept at −20°C. IFN-γ and interleukin-10 (IL-10) levels in the supernatants were quantitated by enzyme-linked immunosorbent assay (R&D Systems, Abingdon, United Kingdom) at the coordinating center.
The frequencies of HBV-specific, IFN-γ-producing CD4+ T cells were determined by an ELISPOT assay using cryopreserved PBMC as described previously (24). The cells were thawed, washed, and resuspended in RPMI 1640 with 10% AB serum. The cell viability, by trypan blue exclusion, was always greater than 95%. Sterile MultiScreen 96-well plates (Millipore, Bedford, MA) were coated with 10 μg/ml of a primary antibody to human IFN-γ by incubation at 4°C for 6 h (1-DIK; Mabtech, Nacka, Sweden). In parallel, 2 × 105 PBMC were cultured in triplicate in RPMI-10% AB serum for the CD4+ T-cell assay, with 2 μg/ml recombinant HBcAg (American Research Products, Belmont, MA) or 0.5 μg/ml tetanus toxoid (Connaught Laboratories) as a positive control. Phytohemagglutinin (2 μg/ml) or medium alone was used as an assay control. After 24 h, the cells were transferred to the coated plates for a further 20 h. Subsequently, the plates were washed and 100 μl biotin-conjugated anticytokine antibody (Mabtech, Nacka, Sweden) was added for 2 h. After being washed, the plates were incubated with 100 μl streptavidin-alkaline phosphatase conjugate (Mabtech). The enzyme reaction was developed with freshly prepared nitroblue tetrazolium chloride-bromo-chloro-indolyl-phosphate toluidine salt (Roche Diagnostics, Lewes, England). The spots were counted with an ELISPOT reader (AID, Strassberg, Germany). The number of specific spot-forming cells per 1 × 106 PBMC was determined as the mean number of spots in the presence of an antigen minus the mean number of spots in the wells with medium only. The cutoff for significant response was defined as the mean plus 2 standard deviations from testing 10 subjects without HBV exposure. Repeat testing of positive samples with T-cell proliferation and ELISPOT assays, using immunomagnetic depletion of CD4+ T cells, confirmed that the responses to rHBcAg and tetanus represent CD4+ T-cell reactivity.
Determination of serum cytokine profiles.
The serum levels of IFN-γ, IL-2, IL-5, IL-8, IL-10, IL-12p70, Fas ligand, and IP-10 were quantitated for each patient at baseline, TW16, and TW48 by using the cytometric bead array assay (BD Biosciences, Oxford, United Kingdom) in accordance with the manufacturer's instructions. This assay employs beads with distinct fluorescence intensities and specificities for a single cytokine. The beads were analyzed using a flow cytometer (FACS array; BD Biosciences) to measure the tested cytokines in a single sample. The concentration of all eight cytokines in each well was calculated using BD FCAP Array software (BD Biosciences, Oxford, United Kingdom). All ELISPOT assays and cytokine measurements were performed at the Institute of Hepatology, UCL, without knowledge of patient treatment assignments and ALT and serum HBV DNA levels.
Hepatitis serology.
Serum HBV markers (HBsAg, anti-HBs, HBeAg, and anti-HBe), anti-HCV, anti-HDV, and antibody to human immunodeficiency virus were tested by enzyme immunoassays (Abbott Laboratories, DiaSorin, and Covance Laboratories). Serum HBV DNA levels were quantitated by Amplicor Monitor PCR (Roche Molecular Systems), with a lower limit of detection (LLD) of 400 copies/ml.
Statistical analyses.
Differences in T-cell responses between patient groups or time points were tested by the Fisher exact test or the Wilcoxon signed-rank test. Correlations were tested by the nonparametric Spearman's rank test. We normalized the cytokine levels for each cytokine and time point, i.e., the cytokine level relative to the average level of that cytokine at that time point. The hierarchical clustering method of the normalized values was used to find whether different cytokine profiles cluster together. This method allowed combining the normalized values of clustered cytokines and analyzing them together at baseline and during therapy. The statistical significance of differences in normalized cytokine levels, HBV DNA, and ALT levels between patient groups was tested by the nonparametric Mann-Whitney U test.
RESULTS
The 13 patients randomized to receive ADV completed 48 weeks of treatment, and serum HBV DNA levels decreased by 4.34 ± 0.36 log10 copies/ml (mean ± standard errors of the mean [SEM]) in comparison to the baseline value. The six patients receiving the placebo had minor changes in serum HBV DNA levels: a decrease of 0.72 ± 0.44 log10 copies/ml (mean ± SEM) at TW48 compared with the baseline value. The loss of HBeAg was observed in 7 of 13 (54%) ADV-treated patients, and 4 of these 7 seroconverted to anti-HBe (Table 2).
TABLE 2.
Comparison of virological parameters in all patients at baseline with those at TW48
| Group | No. of patients who were treated with:
|
Values for parameter at the indicated time point
|
No. of patients with indicated status at TW48
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HBV DNA (log10 copies/ml)a
|
ALT (IU/ml)a
|
|||||||||
| ADV, 30 mg/dayb | ADV, 10 mg/dayb | Placebo | Baseline | TW48 | Δc | Baselined | TW48e | HbeAg− | Anti-Hbe+ | |
| 1 (n = 7) | 4 | 3 | 0 | 8.2 ± 0.7 | 3.0 ± 0.8 | −5.3 ± 1.3 | 133 ± 67 | 35 ± 13 | 7 | 4 |
| 2 (n = 6) | 3 | 3 | 0 | 8.5 ± 0.6 | 5.2 ± 1.0 | −3.4 ± 0.6 | 123 ± 65 | 43 ± 16 | 0 | 0 |
| 3 (n = 6) | 0 | 0 | 6 | 8.7 ± 0.7 | 8.0 ± 1.8 | −0.7 ± 1.1 | 74 ± 16 | 72 ± 42 | 0 | 0 |
The data are shown as means ± standard deviations.
According to the phase III trial protocol, patients receiving ADV were randomized to receive either a 30-mg or a 10-mg dose and the two columns reflect the ADV treatment groups per study protocol. In the present T-cell study, there was no statistical difference between the subgroups of patients receiving 10 versus 30 mg of ADV/day.
Δ, mean log10 copies/ml decrease in HBV DNA levels from baseline to TW48.
There is no significant difference in ALT at baseline between groups. P values were 0.78 for group 1 versus group 2, 0.063 for group 1 versus group 3, and 0.128 for group 2 versus group 3.
There is a significant difference in ALT at TW48 between group 1 and group 3 (P = 0.032), but not group 1 and group 2 (P = 0.317) or group 2 and group 3 (P = 0.11).
Antiviral CD4+ T-cell reactivity may increase during adefovir treatment.
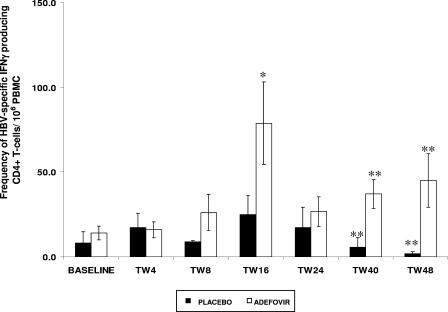
During the study period, T-cell proliferative responses to HBcAg were detected more frequently in ADV-treated patients (30 of 95 tests [32%]) than in placebo-treated patients (5 of 48 tests [10%]; P = 0.02). At baseline, there was no significant difference for HBV core-specific CD4+ T cells (producing IFN-γ) in ADV-treated patients and in those receiving the placebo (Fig. 1). The frequency of HBV-specific CD4+ T cells increased significantly in the ADV group at TW16 in comparison with the baseline value (P < 0.05). There were no significant changes in the placebo group (P = 0.35) (Fig. 1). Furthermore, at TW40 and TW48, ADV-treated patients maintained significantly greater frequencies of virus-specific T cells than did placebo-treated patients (P = 0.03).
FIG. 1.
Comparison of CD4+ T-cell reactivity to HBcAg in patients receiving ADV or the placebo. *, there was a significant increase in the frequencies of IFN-γ-producing CD4+ T cells at TW16 compared to baseline values for ADV-treated patients (P = 0.03). **, at TW40 and TW48, patients receiving ADV had significantly higher frequencies of IFN-γ-producing CD4+ T cells than did patients receiving placebos (P = 0.03). The bars represent means ± SEMs.
Relationship between viral suppression and T-cell responses.
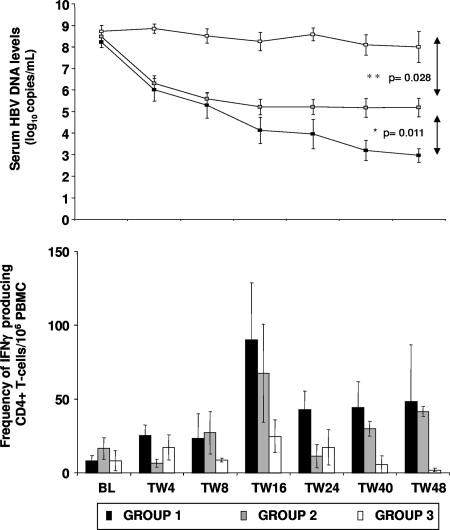
Based on HBeAg status and serum HBV DNA levels at TW48, three subgroups of patients were identified (Table 2). Seven ADV-treated patients lost HBeAg and showed a reduction in viral load of 5.3 ± 0.4 log10 copies/ml (mean ± SEM); in five of seven patients, serum HBV DNA was below the LLD (group 1). The other 12 patients remained HBeAg+; of these, 6 ADV-treated patients had HBV DNA reductions of 3.4 ± 0.2 log10 copies/ml (mean ± SEM) (only 1 of 6 patients had HBV DNA level below the LLD [group 2]), while 6 patients who received the placebo showed smaller changes in HBV-DNA level (0.7 ± 0.4 log10 copies/ml [mean ± SEM] [group 3]). There were no significant differences in serum HBV DNA levels at baseline between these three groups of patients (the P value was >0.1) (Table 2); however, there were significant differences in serum HBV DNA levels at TW48 (the P values were 0.011 for group 1 versus group 2 and 0.028 for group 2 versus group 3) (Fig. 2).
FIG. 2.
Relationship between serum HBV DNA levels and the frequency of virus-specific CD4+ T cells during the study period for the three groups of patients presented in Table 2. *, patients in group 1 had significantly lower HBV DNA levels at TW48 than did patients in groups 2 (P = 0.011) and 3 (P = 0.003). **, patients in group 2 had significantly lower HBV DNA levels at TW48 than did patients in group 3 (P = 0.028). The bars represent the means ± SEMs for the frequency of HBV-specific CD4+ T cells. Error bars indicate standard deviations.
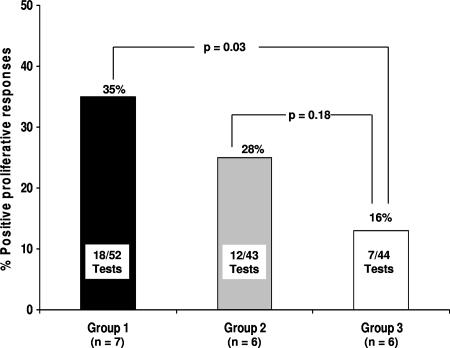
The analysis of T-cell proliferative responses in these three subgroups revealed that T-cell proliferation to HBcAg occurred more frequently in patients with a greater viral load reduction and HBeAg loss, i.e., more frequently in group 1 (18 of 52 tests [35%]) than in group 3 (7 of 44 tests [16%]; P = 0.03) (Fig. 3). There was no significant difference in T-cell proliferative responses between HBeAg+ patients with lower viral suppression levels (group 2, 12 of 43 tests [28%]) in comparison with those of group 3 (P = 0.18). The frequency of HBcAg-specific, IFN-γ-producing CD4+ T cells reached the highest level at TW16, particularly in group 1, compared with the baseline values (P = 0.06 [Fig. 2]). The peak increase of virus-specific T cells at TW16 in group 1 patients was followed by a further reduction in viremia (particularly at TW40 and TW48), which was not observed in group 2 (Fig. 2). PBMC from all patients showed good proliferative responses in the control assays (data not shown).
FIG. 3.
HBcAg-specific T-cell proliferation according to virological response. Patients in group 1 had a higher number of positive tests results for T-cell proliferation to HBcAg than did patients in group 3 (P = 0.03).
There were marked differences in the in vitro PBMC production of IFN-γ in response to HBcAg between the three groups. The IFN-γ levels in the supernatants of PBMC cultured with HBcAg of group 1 patients were significantly higher than those of group 3 at baseline (P = 0.05) (Table 3). IFN-γ production was also higher in group 1 patients at TW4, TW8, and TW16 than in group 2 or group 3 patients, although these differences were not significant. The quantitation of the production of IL-10 in the same supernatants showed no significant differences between patients in any of the three groups or between those receiving ADV or the placebo.
TABLE 3.
In vitro IFN-γ production after stimulation with rHBcAg
| Group | IFN-γ production indexa at:
|
||||||
|---|---|---|---|---|---|---|---|
| BL | TW4 | TW8 | TW16 | TW24 | TW40 | TW48 | |
| 1 | 99.2 ± 29.3 | 58.9 ± 67.2 | 184.3 ± 308.3 | 63.9 ± 52.7 | 26.3 ± 32.2 | 61.5 ± 73.2 | 18.0 ± 26.7 |
| 2 | 26.2 ± 53.6 | 33.8 ± 58.8 | 7.5 ± 11.9 | 30.0 ± 55.9 | 52.0 ± 48.2 | 15.7 ± 6.8 | 37.8 ± 51.6 |
| 3 | 20.3 ± 5.0 | 2.3 ± 2.2 | 17.0 ± 20.3 | 8.8 ± 8.5 | 22.5 ± 17.6 | 14.5 ± 8.8 | 7.1 ± 7.3 |
The IFN-γ production index was calculated at each time point by dividing the mean pg/ml IFN-γ production of four replicates of HBcAg-stimulated PBMC by that of the control cultures (PBMC incubated in medium only). Group 1 patients had significantly higher IFN-γ production values at baseline compared to those of group 3 (P = 0.05), but not to those of group 2 (P = 0.1). All data are shown as means ± standard deviations.
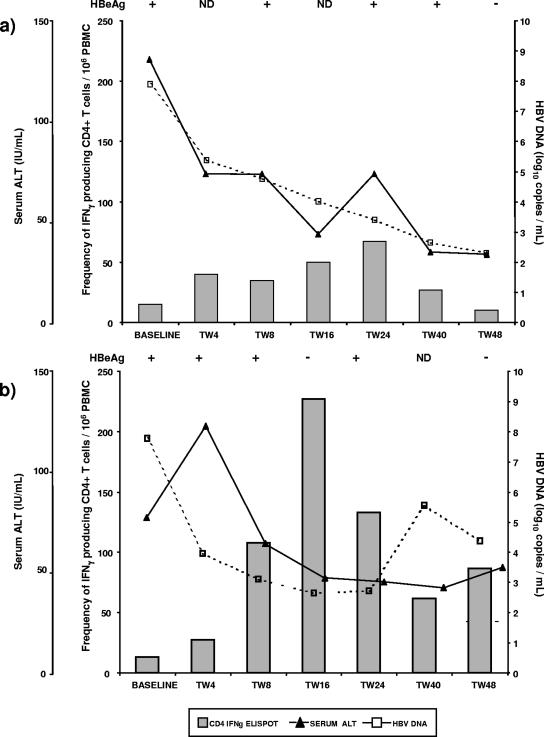
To gain information about the relationship between viral load reduction during ADV treatment, HBeAg loss, and the frequencies of HBV-specific T cells, we analyzed the time course of these events in individual patients. A reduction in serum HBV DNA to around 3 log10 copies/ml was associated with increased IFN-γ-producing CD4+ T cells at different time points in individual cases, with the highest frequency observed usually at TW16 or TW24 (Fig. 4a and b). The increase of IFN-γ-producing CD4+ T cells preceded or coincided with the loss of HBeAg. Importantly, the loss of HBeAg was not associated with ALT flare. Patients in group 2 showed no significant changes in HBV core-specific CD4+ T-cell reactivity during the course of ADV treatment (Fig. 4c).
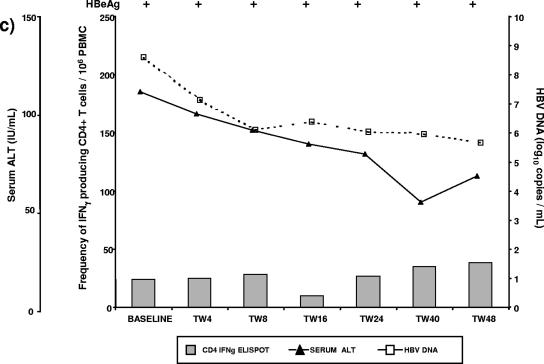
FIG.4.
Time course analysis of serum ALT levels, virological parameters, and the frequency of IFN-γ-producing CD4+ T cells during the study period. (a and b) For two representative patients from group 1, an increased frequency of IFN-γ-producing CD4+ T cells preceded or coincided with HBeAg loss, without ALT flare. (c) A representative patient from group, who received ADV treatment, but had moderate reduction in viremia, remained HBeAg+ and showed no increase in the frequency of virus-specific, IFN-γ-producing T cells. IFNγ, IFN-γ; ND, not determined.
Serum cytokine profiles during adefovir treatment.
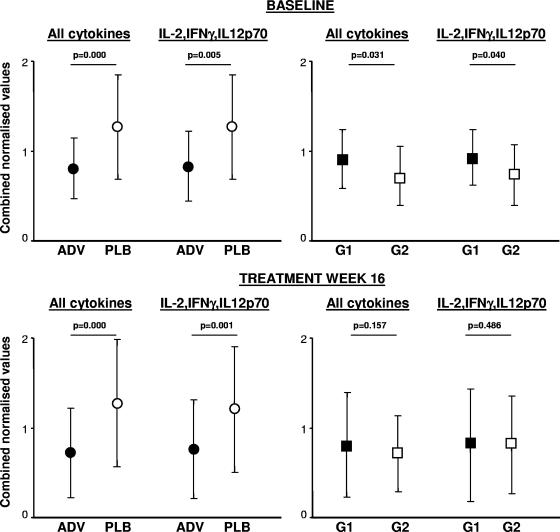
There was no correlation between any of the cytokine levels at baseline (for all patients studied or for ADV and placebo groups separately) and baseline HBV-DNA or ALT levels. Although baseline ALT levels were higher for the ADV group than for the placebo group (Table 1), serum cytokine levels did not differ, except for IL-10, which was significantly higher for patients who received the placebo than for patients who received ADV (51 ± 19 pg/ml versus 32 ± 13 [mean ± SEM]; P = 0.028). A highly significant correlation was found between baseline serum levels for IL-12p70 and IFN-γ (r = 0.801; P < 0.001), IL-12p70 and IL-2 (r = 0.958; P < 0.001), and IFN-γ and IL-2 (r = 0.679; P = 0.005). The hierarchical clustering analysis showed that the normalized levels of six of eight measured cytokines (IFN-γ, IL-2, IL-5, IL-10, IL-12p70, and Fas ligand, but not IP-10 and IL-8) strongly intercorrelated (r > 0.7; P < 0.001). The combined normalized values for the six clustered cytokines were higher for the placebo group than for patients randomized to ADV (P < 0.001) and were also higher when we compared only IL-12p70, IFN-γ, and IL-2 (P = 0.005) (Fig. 5). When we compared the subgroups treated with ADV, baseline cytokine levels showed a trend to be higher for group 1 than for group 2 (Fig. 5).
FIG. 5.
Comparison of serum cytokine profiles using cluster analysis of normalized values. The levels of all cytokines measured, or measured separately for IL-12p70, IFN-γ, and IL-2, were significantly higher in the placebo (PLB)-treated group than in the ADV-treated group. At TW16, the difference was even more apparent between PLB- and ADV-treated groups, while there was no difference between the two ADV-treated subgroups with or without HBeAg loss (group 1 [G1] or group 2 [G2], respectively). Error bars indicate standard deviations.
Interestingly, the difference in cytokine levels between the ADV and placebo groups became more pronounced at TW16, with significantly lower absolute values for IFN-γ, IL-10, IL-12p70, Fas ligand, IL-2, and IL-5 for ADV-treated patients than those for patients in the placebo group. This result was also found when cluster analysis, including all clustered cytokines (except IP-10 and IL-8) or only IL-12p70, IL-2, and IFN-γ, was used (Fig. 5). At TW16, there was no difference between the absolute values, or with the cluster analysis, between patients in groups 1 and 2.
DISCUSSION
This longitudinal, placebo-controlled study demonstrates that the suppression of HBV replication with ADV significantly enhances virus-specific CD4+ T-cell reactivity with IFN-γ production in some patients with HBeAg+ chronic hepatitis B. T-cell reactivity increased only in a proportion of ADV-treated patients and may have contributed to a greater suppression of HBV replication with HBeAg loss in this subgroup. Thus, the present study indicates that overcoming T-cell hyporesponsiveness to HBV, which is characteristic for patients with high viremia levels (4), is an important component for achieving HBe seroconversion with oral antiviral therapy. Studies of adaptive immunity to HBV have established a correlation between strong proliferative responses of CD4+ T cells and spontaneous resolution of HBV infection (4). The critical role of host immune response in the resolution of chronic HBV infection is best demonstrated by HBsAg+ recipients of bone marrow transplantation who received marrow from donors with natural immunity to HBV. The transplantation of a healthy immune system, including T cells reactive to HBV nucleocapsid protein, led to the resolution of chronic hepatitis B with anti-HBs seroconversion (10).
The present study reveals that patients with chronic hepatitis B, who cleared HBeAg within 48 weeks of ADV treatment, are characterized by higher CD4+ T-cell reactivity to HBV. These patients showed higher IFN-γ production at baseline and an increase of the frequency of HBcAg-specific, IFN-γ-producing CD4+ T cells upon treatment. In contrast, the measurement of serum cytokines appeared to reflect the nonspecific immune responses and showed the opposite profile to HBV-specific T-cell reactivity, as detected by proliferation, ELISPOT, and HBcAg-specific IFN-γ production assays. HBV suppression with ADV decreased serum cytokine levels at TW16, in contrast with the increased virus-specific reactivity of CD4+ T cells at the same time point.
HBeAg is believed to induce T-cell tolerance, thus promoting chronic HBV persistence (18). However, the enhanced CD4+ T-cell reactivity in group 1 patients usually preceded HBeAg loss, suggesting that T-cell hyporesponsiveness may be associated with high viral load, while HBeAg loss during treatment may be mainly a result of enhanced T-cell function. Combined monitoring of viral load decline and the T-cell reactivity to HBV during antiviral treatment would provide a better understanding of the mechanisms and likelihood of treatment response. Future studies on adaptive immunity during therapy will need to clarify the interplay between HBV-specific CD4+ and CD8+ T cells (18) and the relative impact of viral suppression for improved dendritic cell function (18) and/or HBV-specific T-cell responses. Importantly, the production of anti-HBe is strictly dependent on CD4+ T cells and anti-HBe titers are higher in patients with raised ALT levels (16, 19). The role of CD4+ T cells in promoting humoral immunity to HBV, in particular anti-HBe production, during antiviral therapy deserves further investigation.
The enhanced frequency of virus-specific, IFN-γ-producing T cells in patients in group 1 was not associated with ALT flares, thus emphasizing the involvement of noncytolytic antiviral mechanisms. Moreover, the serum levels of nonspecific cytokines for these patients were lower than those for patients receiving the placebo. A large body of evidence has established that HBV replication can be controlled efficiently by IFN-γ released from HBV-specific T cells, resulting in intracellular virus inactivation without killing infected hepatocytes (8). In vitro experiments have demonstrated directly that IFN-γ secreted by T cells from patients with chronic hepatitis B effectively inhibits HBV transcription and replication in hepatocytes without cell lysis (25).
The baseline levels of virus-specific IFN-γ production were significantly higher for group 1 than for group 2 or group 3, indicating that the T cells in patients from group 1 are more “immunoreactive” with the production of IFN-γ, which may exert noncytolytic control of HBV replication in addition to the antiviral effect of adefovir.
Different patterns of T-cell reactivity to HBV antigens have been observed during lamivudine treatment of chronic hepatitis B. In HBeAg+ patients with markedly raised ALT levels, a temporal association was observed between serum HBV DNA decline and increased CD4+ T-cell reactivity (1, 2). Such a marked increase of T-cell reactivity during lamivudine treatment, however, is not universal. We have previously shown that HBeAg+ patients with low ALT levels, who had failed previous interferon therapy, did not show significant changes in CD4+ T-cell proliferation to rHBcAg during lamivudine treatment (14). The latter subset corresponds to patients in group 2 in the present study, who had a moderate decrease in serum HBV DNA but showed no significant increase in T-cell reactivity to HBV and remained HBeAg+ despite undergoing ADV treatment as patients in group 1.
Chronic hepatitis B is a heterogeneous disease, requiring more individualized treatment regimens. For example, a combination of ADV plus DNA vaccine has shown an additive antiviral effect in the duck HBV infection model (11); in chronically infected woodchucks, prolonged antiviral treatment, followed by therapeutic immunization, induced HBsAg clearance (17). The present study suggests that in a subset of ADV-treated patients with enhanced T-cell reactivity upon treatment, therapeutic vaccination may boost T-cell responses and increase HBeAg clearance. Instead, patients with a moderate HBV DNA reduction and T-cell hyporeactivity may require prolonged therapy or additional antiviral compounds for greater suppression of HBV replication and to prevent drug resistance.
Acknowledgments
The study was supported by a research grant from Gilead Sciences, Foster City, CA.
The following persons contributed to the organization or provided technical support for the study: Michael Manns (Hanover, Germany); Carmela Cursaro (Bologna, Italy); Alexander Smith (Manchester, United Kingdom); Mario Rizzetto (Turin, Italy); Alexander Thermet (Lyon, France); and Shelly Xiong, Craig P. James, Anant Jain, and Craig Gibbs (Gilead Sciences, Foster City, CA).
Footnotes
Published ahead of print on 5 November 2007.
REFERENCES
- 1.Boni, C., A. Bertoletti, A. Penna, A. Cavalli, M. Pilli, S. Urbani, P. Scognamiglio, R. Boehme, R. Panebianco, F. Fiaccadori, and C. Ferrari. 1998. Lamivudine treatment can restore T cell responsiveness in chronic hepatitis B. J. Clin. Investig. 102:968-975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boni, C., A. Penna, A. Bertoletti, V. Lamonaca, I. Rapti, G. Missale, M. Pilli, S. Urbani, A. Cavalli, S. Cerioni, R. Panebianco, J. Jenkins, and C. Ferrari. 2003. Transient restoration of anti-viral T cell responses induced by lamivudine therapy in chronic hepatitis B. J. Hepatol. 39:595-605. [DOI] [PubMed] [Google Scholar]
- 3.Chisari, F. V. 2000. Rous-Whipple award lecture. Viruses, immunity, and cancer: lessons from hepatitis B. Am. J. Pathol. 156:1117-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chisari, F. V., and C. Ferrari. 1995. Hepatitis B virus immunopathogenesis. Annu. Rev. Immunol. 13:29-60. [DOI] [PubMed] [Google Scholar]
- 5.de Franchis, R., A. Hadengue, G. Lau, D. Lavanchy, A. Lok, N. McIntyre, A. Mele, G. Paumgartner, A. Pietrangelo, J. Rodes, W. Rosenberg, D. Valla, and the EASL Jury. 2003. EASL Consensus Conference on Hepatitis B: consensus statement. J. Hepatol. 39(Suppl. 1):S3-S25. [PubMed] [Google Scholar]
- 6.Delaney, W. E., S. Locarnini, and T. Shaw. 2001. Resistance of hepatitis B virus to antiviral drugs: current aspects and directions for future investigation. Antivir. Chem. Chemother. 12:1-35. [DOI] [PubMed] [Google Scholar]
- 7.Fattovich, G., M. Rugge, L. Brollo, P. Pontisso, F. Noventa, M. Guido, A. Alberti, and G. Realdi. 1986. Clinical, virologic and histologic outcome following seroconversion from HBeAg to anti-HBe in chronic hepatitis type B. Hepatology 6:167-172. [DOI] [PubMed] [Google Scholar]
- 8.Guidotti, L. G., and F. V. Chisari. 2001. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu. Rev. Immunol. 19:65-91. [DOI] [PubMed] [Google Scholar]
- 9.Lai, C. L., R. N. Chien, N. W. Leung, T. T. Chang, R. Guan, D. I. Tai, K. Y. Ng, P. C. Wu, J. C. Dent, J. Barber, S. L. Stephenson, D. F. Gray, et al. 1998. A one-year trial of lamivudine for chronic hepatitis B. N. Engl. J. Med. 339:61-68. [DOI] [PubMed] [Google Scholar]
- 10.Lau, G. K., D. Suri, R. Liang, E. I. Rigopoulou, M. G. Thomas, I. Mullerova, A. Nanji, S. T. Yuen, R. Williams, and N. V. Naoumov. 2002. Resolution of chronic hepatitis B and anti-HBs seroconversion in humans by adoptive transfer of immunity to hepatitis B core antigen. Gastroenterology 122:614-624. [DOI] [PubMed] [Google Scholar]
- 11.Le Guerhier, F., A. Thermet, S. Guerret, M. Chevallier, C. Jamard, C. S. Gibbs, C. Trepo, L. Cova, and F. Zoulim. 2003. Antiviral effect of adefovir in combination with a DNA vaccine in the duck hepatitis B virus infection model. J. Hepatol. 38:328-334. [DOI] [PubMed] [Google Scholar]
- 12.Lok, A. S., and B. J. McMahon. 2001. AASLD practice guidelines: chronic hepatitis B. Hepatology 34:1225-1241. [DOI] [PubMed] [Google Scholar]
- 13.Marcellin, P., T. T. Chang, S. G. Lim, M. J. Tong, W. Sievert, M. L. Shiffman, L. Jeffers, Z. Goodman, M. S. Wulfsohn, S. Xiong, J. Fry, and C. L. Brosgart. 2003. Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. N. Engl. J. Med. 348:808-816. [DOI] [PubMed] [Google Scholar]
- 14.Marinos, G., N. V. Naoumov, and R. Williams. 1996. Impact of complete inhibition of viral replication on the cellular immune response in chronic hepatitis B virus infection. Hepatology 24:991-995. [DOI] [PubMed] [Google Scholar]
- 15.Marusawa, H., S. Uemoto, M. Hijikata, Y. Ueda, K. Tanaka, K. Shimotohno, and T. Chiba. 2000. Latent hepatitis B virus infection in healthy individuals with antibodies to hepatitis B core antigen. Hepatology 31:488-495. [DOI] [PubMed] [Google Scholar]
- 16.Maruyama, T., S. Iino, K. Koike, K. Yasuda, and D. R. Milich. 1993. Serology of acute exacerbation in chronic hepatitis B virus infection. Gastroenterology 105:1141-1151. [DOI] [PubMed] [Google Scholar]
- 17.Menne, S., C. A. Roneker, B. E. Korba, J. L. Gerin, B. C. Tennant, and P. J. Cote. 2002. Immunization with surface antigen vaccine alone and after treatment with 1-(2-fluoro-5-methyl-β-l-arabinofuranosyl)-uracil (l-FMAU) breaks humoral and cell-mediated immune tolerance in chronic woodchuck hepatitis virus infection. J. Virol. 76:5305-5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milich, D. R., M. K. Chen, J. L. Hughes, and J. E. Jones. 1998. The secreted hepatitis B precore antigen can modulate the immune response to the nucleocapsid: a mechanism for persistence. J. Immunol. 160:2013-2021. [PubMed] [Google Scholar]
- 19.Milich, D. R., and A. McLachlan. 1986. The nucleocapsid of hepatitis B virus is both a T-cell-independent and a T-cell-dependent antigen. Science 234:1398-1401. [DOI] [PubMed] [Google Scholar]
- 20.Niederau, C., T. Heintges, S. Lange, G. Goldmann, C. M. Niederau, L. Mohr, and D. Haussinger. 1996. Long-term follow-up of HBeAg-positive patients treated with interferon alfa for chronic hepatitis B. N. Engl. J. Med. 334:1422-1427. [DOI] [PubMed] [Google Scholar]
- 21.Penna, A., M. Artini, A. Cavalli, M. Levrero, A. Bertoletti, M. Pilli, F. V. Chisari, B. Rehermann, P. G. Del, F. Fiaccadori, and C. Ferrari. 1996. Long-lasting memory T cell responses following self-limited acute hepatitis B. J. Clin. Investig. 98:1185-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rehermann, B., C. Ferrari, C. Pasquinelli, and F. V. Chisari. 1996. The hepatitis B virus persists for decades after patients' recovery from acute viral hepatitis despite active maintenance of a cytotoxic T-lymphocyte response. Nat. Med. 2:1104-1108. [DOI] [PubMed] [Google Scholar]
- 23.Rehermann, B., and M. Nascimbeni. 2005. Immunology of hepatitis B virus and hepatitis C virus infection. Nat. Rev. Immunol. 5:215-229. [DOI] [PubMed] [Google Scholar]
- 24.Rigopoulou, E. I., D. Suri, S. Chokshi, I. Mullerova, S. Rice, R. S. Tedder, R. Williams, and N. V. Naoumov. 2005. Lamivudine plus interleukin-12 combination therapy in chronic hepatitis B: antiviral and immunological activity. Hepatology 42:1028-1036. [DOI] [PubMed] [Google Scholar]
- 25.Suri, D., R. Schilling, A. R. Lopes, I. Mullerova, G. Colucci, R. Williams, and N. V. Naoumov. 2001. Non-cytolytic inhibition of hepatitis B virus replication in human hepatocytes. J. Hepatol. 35:790-797. [DOI] [PubMed] [Google Scholar]
- 26.Tsiang, M., J. F. Rooney, J. J. Toole, and C. S. Gibbs. 1999. Biphasic clearance kinetics of hepatitis B virus from patients during adefovir dipivoxil therapy. Hepatology 29:1863-1869. [DOI] [PubMed] [Google Scholar]
- 27.Wong, D. K., A. M. Cheung, K. O'Rourke, C. D. Naylor, A. S. Detsky, and J. Heathcote. 1993. Effect of alpha-interferon treatment in patients with hepatitis B e antigen-positive chronic hepatitis B. A meta-analysis. Ann. Intern. Med. 119:312-323. [DOI] [PubMed] [Google Scholar]