Abstract
Alveolar echinococcosis, which is due to the massive growth of larval Echinococcus multilocularis, is a life-threatening parasitic zoonosis distributed widely across the northern hemisphere. Commercially available chemotherapeutic compounds have parasitostatic but not parasitocidal effects. Parasitic organisms use various energy metabolic pathways that differ greatly from those of their hosts and therefore could be promising targets for chemotherapy. The aim of this study was to characterize the mitochondrial respiratory chain of E. multilocularis, with the eventual goal of developing novel antiechinococcal compounds. Enzymatic analyses using enriched mitochondrial fractions from E. multilocularis protoscoleces revealed that the mitochondria exhibited NADH-fumarate reductase activity as the predominant enzyme activity, suggesting that the mitochondrial respiratory system of the parasite is highly adapted to anaerobic environments. High-performance liquid chromatography-mass spectrometry revealed that the primary quinone of the parasite mitochondria was rhodoquinone-10, which is commonly used as an electron mediator in anaerobic respiration by the NADH-fumarate reductase system of other eukaryotes. This also suggests that the mitochondria of E. multilocularis protoscoleces possess an anaerobic respiratory chain in which complex II of the parasite functions as a rhodoquinol-fumarate reductase. Furthermore, in vitro treatment assays using respiratory chain inhibitors against the NADH-quinone reductase activity of mitochondrial complex I demonstrated that they had a potent ability to kill protoscoleces. These results suggest that the mitochondrial respiratory chain of the parasite is a promising target for chemotherapy of alveolar echinococcosis.
Echinococcosis is a near-cosmopolitan zoonosis caused by helminthic parasites belonging to the genus Echinococcus (family Taeniidae) (18). The life cycle of Echinococcus spp. includes an egg-producing adult stage in the definitive hosts and a larval stage in intermediate hosts including humans. The larval stage of the parasite produces a large number of infective protoscoleces that develop to adult worms after being ingested by the definitive host, or they produce a new parasite mass when liberated inside the intermediate host, causing metastases of the parasite lesions. The two major species of medical and public health importance are Echinococcus granulosus and E. multilocularis, which cause cystic echinococcosis and alveolar echinococcosis (AE), respectively.
Human AE is a life-threatening disease, and without careful clinical management, it has a high fatality rate and poor prognosis. Humans acquire AE infection by ingesting eggs from adult parasitic worms. Early diagnosis and treatment (mainly by radical surgery) of human AE are difficult because the disease progresses slowly and usually takes more than several years before clinical symptoms become apparent. An efficient chemotherapeutic compound is still not available. The first choice for the chemotherapy of AE is benzimidazole derivatives (18), but they are parasitostatic rather than parasitocidal against larval E. multilocularis. Therefore, the development of highly effective antiechinococcal drugs is urgently needed.
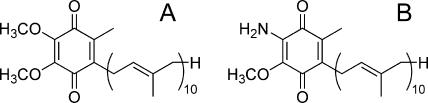
Biological systems for energy metabolism are essential for the survival, continued growth, and reproduction of all living organisms. “Typical” mitochondria are usually considered to be oxygen-consuming, ATP-producing organelles. In fact, typical mitochondria, such as those found in mammalian cells, require oxygen to function. They use pyruvate dehydrogenase for oxidative decarboxylation of pyruvate to acetyl coenzyme A, which is then completely oxidized to CO2 through the Krebs cycle. Most of the energy is produced by oxidative phosphorylation: the electrons from NADH and succinate are transferred to oxygen by the proton-pumping electron transfer respiratory chain in which ubiquinone (UQ) (Fig. 1A) is commonly used as an electron mediator. The backflow of the protons results in ATP formation by the mitochondrial ATP synthase.
FIG. 1.
Chemical structure of ubiquinone-10 (UQ10) (Em′ = +110 mV) (A) and rhodoquinone-10 (RQ10) (Em′ = −63 mV) (B).
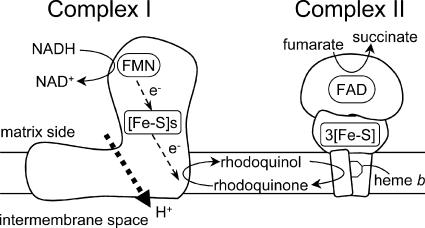
In parasitic organisms, on the other hand, the carbohydrate and energy metabolic pathways of adult parasitic helminths differ greatly from those of their vertebrate hosts. The most important factors in this respect are the nutrient and oxygen supply (reviewed in references 4, 12, and 13). Parasitic helminths have exploited a variety of energy-transducing systems during their adaptation to habitats in their hosts (7, 28). The parasitic nematode Ascaris suum, for example, resides in the host small intestine, where oxygen tensions are low, and exploits a unique anaerobic respiratory chain, called the NADH-fumarate reductase system, to adapt to its microaerobic habitat (Fig. 2) (2, 3, 14, 22; reviewed in reference 10). The NADH-fumarate reductase system is part of the unique respiratory system for parasitic helminthes and is the terminal step in the phosphoenolpyruvate carboxykinase-succinate pathway, which is found in many anaerobic organisms. Electrons from NADH are accepted by rhodoquinone (RQ) (Fig. 1B) via the NADH-RQ reductase activity of mitochondrial complex I and then transferred to fumarate through the rhodoquinol-fumarate reductase activity of mitochondrial complex II. The anaerobic electron transfer in complex I couples with proton transport across the mitochondrial inner membrane, providing ATP even in the absence of oxygen. This system, which does not normally function in mammalian mitochondria, is considered to be a good target for the development of novel anthelminthics (8, 9, 21). With regard to Echinococcus spp., the presence of both aerobic and anaerobic respiratory systems was previously suggested by a series of intensive studies (1, 16, 17), although the respiratory systems in this group of parasites are to be characterized in more detail.
FIG. 2.
Schematic representation of the NADH-fumarate reductase system in adult A. suum, which catalyzes the final step of the phosphoenolpyruvate carboxykinase-succinate pathway. In this system, the reducing equivalent of NADH is transferred to the low-potential RQ by the NADH-RQ reductase activity of mitochondrial complex I. This pathway ends with the production of succinate by the rhodoquinol-fumarate reductase activity of complex II. Electron transfer from NADH to fumarate is coupled to the site I phosphorylation of complex I via the generation of a proton-motive force. FMN, flavin mononucleotide; FAD, flavin adenine dinucleotide; [Fe-S]s and 3[Fe-S], iron-sulfur clusters.
In the present study, we prepared an enriched mitochondrial fraction from E. multilocularis protoscoleces and characterized the specific enzyme activities involved in mitochondrial energy metabolism as well as the quinone profile in the parasite's respiratory chain. Furthermore, based on findings reported previously by Yamashita et al. that quinazoline derivatives can inhibit the NADH-quinone reductase of mitochondria from A. suum (35), we tested several quinazoline-type compounds, with a view to developing novel antiechinococcal compounds.
MATERIALS AND METHODS
Isolation of E. multilocularis protoscoleces.
We used the Nemuro strain of E. multilocularis, which is maintained at the Hokkaido Institute of Public Health (Sapporo, Japan). Mature larval parasites with protoscolex formation were obtained from cotton rats (Sigmodon hispidus) more than 4 months after oral infection with 50 parasite eggs. To isolate protoscoleces, the mature larval parasites were minced with scissors, pushed through a metal mesh, and washed repeatedly with physiological saline until host materials were thoroughly removed.
Preparation of enriched mitochondrial fractions.
The enriched mitochondrial fractions of E. multilocularis protoscoleces were prepared essentially according to methods described previously for isolating adult Ascaris mitochondria (25, 26). Briefly, the isolated protoscolex sediment was suspended in 5 volumes of mitochondrial preparation buffer (210 mM mannitol, 10 mM sucrose, 1 mM disodium EDTA, and 50 mM Tris-HCl [pH 7.5]) supplemented with 10 mM sodium malonate. The parasite materials were homogenized with a motor-driven glass/glass homogenizer (six passes three to four times). The homogenate was diluted with the mitochondrial preparation buffer to 10 times the volume of the original protoscolex sediment and then centrifuged at 800 × g for 10 min to precipitate cell debris and nuclei. The supernatant was then centrifuged at 8,000 × g for 10 min to obtain the mitochondrial pellet. The pellet was resuspended in mitochondrial preparation buffer (without malonate) and centrifuged at 12,000 × g for 10 min. The resulting enriched mitochondrial fraction was suspended in mitochondrial preparation buffer (without malonate). The protein concentration was determined according to the method of Lowry et al. by using bovine serum albumin as a standard (15).
Western blotting.
An enriched mitochondrial fraction prepared from E. multilocularis protoscoleces and that from the liver of a cotton rat (used as the host animal for the parasite) were analyzed by Western blotting. Reactions were performed according to a method described previously by Towbin et al. (30). The proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on a 10% or 15% acrylamide gel and electrophoretically transferred onto a nitrocellulose membrane. The membrane was soaked in 1:5,000 anti-cytochrome c oxidase subunit IV antibody (component of the ApoAlert cell fractionation kit; Clontech Laboratories) in phosphate-buffered saline containing 0.05% (wt/vol) Tween 20 and 2% (wt/vol) skim milk. The membrane was incubated for 60 min at room temperature and then washed three times for 10 min with washing buffer, which consisted of 0.05% (wt/vol) Tween 20 in phosphate-buffered saline. Alkaline phosphatase-conjugated goat anti-mouse immunoglobulin G was then added as a secondary antibody, and the mixture was incubated for 30 min. After another wash with washing buffer, the membrane was soaked in reaction buffer (100 mM Tris-HCl [pH 9.5], 100 mM NaCl, 5 mM MgCl2, 500 μg/ml of 4-nitroblue tetrazolium chloride, and 165 μg/ml of 5-bromo-4-chloro-3-indolylphosphate) to initiate the development of a colored product. Finally, the membrane was washed with distilled water to stop the reaction. For Western blotting, the amounts of parasite and cotton rat mitochondrial samples were normalized by the total protein amount or cytochrome c oxidase activity (see below).
Enzyme assays.
All enzyme assays using the enriched mitochondrial fractions were performed in a 0.7- or 1-ml reaction mixture at 25°C. The reagents used in each assay were mixed with reaction buffer containing 30 mM potassium phosphate (pH 7.4) and 1 mM MgCl2. The final mitochondrial protein concentration was 80 μg per ml of reaction mixture. For all reactions performed under anaerobic conditions, the reaction medium was supplemented with 100 μg/ml glucose oxidase, 2 μg/ml catalase, and 10 mM β-d-glucose and left for 3 min to achieve anaerobiosis. NADH oxidase activity in the isolated mitochondrial fraction was determined in the presence or absence of 2 mM KCN, 100 mM malonate, or both by measuring the absorbance of NADH at 340 nm (ɛ = 6.2 mM−1 cm−1). The reaction was initiated by the addition of 100 μM of NADH to the mixture. Succinate dehydrogenase (SDH) activity was determined by monitoring the absorbance change of 2-(4,5-dimethyl-2-thiazolyl)-3,5-diphenyl-2H-tetrazolium bromide (MTT; 60 μg/ml) at 570 nm in the presence of 120 μg/ml phenazine methosulfate and 2 mM KCN. The reaction was initiated by the addition 10 mM of succinate to the mixture. Succinate-quinone reductase activity was assayed under aerobic or anaerobic conditions in the presence of 0.1%(wt/vol) sucrose monolaurate by determining the amount of decyl UQ (dUQ) or decyl RQ (dRQ) from the absorbance change at 278 nm (ɛ = 12.7 mM−1 cm−1) or 287 nm (ɛ = 9.2 mM−1 cm−1), respectively. Decyl rhodoquinol-fumarate reductase activity was measured under anaerobic conditions in a reaction mixture containing 0.1% (wt/vol) sucrose monolaurate. In this reaction, 60 μM dRQ was reduced to decyl rhodoquinol in the cuvette by adding 200 μM NaBH4. The reaction was started by adding 5 mM fumarate to the mixture, and the oxidation of decyl rhodoquinol was monitored at 287 nm. NADH-fumarate reductase activity was determined by monitoring the oxidation of NADH (100 μM) at 340 nm under anaerobic conditions. The reaction was initiated by the addition of 5 mM fumarate as an electron acceptor. NADH-quinone reductase activity assays were carried out under anaerobic conditions using the same reaction mixture as that used for the NADH-fumarate reductase activity assay except that 60 μM dUQ or dRQ was used as an electron acceptor instead of fumarate. The enzyme activity was determined by monitoring the absorbance change of NADH at 340 nm. Ubiquinol oxidase activity was determined by monitoring the absorbance change of ubiquinol-1 (150 μM) at 278 nm (ɛ = 12.7 mM−1 cm−1) in the presence or absence of 2 mM KCN. The activity of cytochrome c oxidase was determined as N,N,N′,N′-tetramethyl-p-phenylenediamine dihydrochloride (TMPD) oxidase activity, which was measured by monitoring the absorbance change of TMPD (500 μM) at 610 nm (ɛ = 11.0 mM−1 cm−1) in the presence or absence of 2 mM KCN.
Enzyme inhibition assays.
Based on the findings of Yamashita et al. showing that that quinazoline-type compounds inhibit the NADH-quinone reductase activity of A. suum complex I (35), we determined 50% inhibitory concentration (IC50) values of the quionazoline-type compounds against NADH-fumarate reductase activity of the parasite mitochondria and the NADH oxidase activity of bovine heart mitochondria (see “Enzyme assays”). The compounds used in the assays included quinazoline and its derivatives 6-NH2, 6-NHCO(CH=CH2), 7-NH2, 8-OH, 8-OCH3, 8-OCH2CH3, and 8-OCH(CH3)2.
Analysis of the quinone profile of isolated mitochondria.
Quinones were extracted from lyophilized mitochondria essentially according to a method described previously by Takada et al. (24). A lyophilized mitochondrial sample (2.9 mg protein) was crushed into powder before extraction, vortexed in 2:5 (vol/vol) ethanol/n-hexane for 10 min, and centrifuged at 20,000 × g for 5 min at room temperature. The supernatants were pooled, and the extraction of quinones was repeated twice. Pooled extracts were evaporated to dryness, dissolved in ethanol, and kept in the dark until high-performance liquid chromatography (HPLC) analysis. Quinones were applied to a reverse-phase HPLC column (Inertsil ODS-3 [5 μm and 4.6 by 250 mm]; GL Science) and eluted under isocratic conditions (1 ml/min) with 1:4 (vol/vol) diisopropyl ether-methanol at 25°C. The molecular species of the eluted quinones were identified by their retention times and by their spectral characteristics as measured with a UV-visible photodiode array (Shimadzu SPD-10-A). The concentration of quinones was determined spectrophotometrically. The major quinone detected was confirmed by mass spectrometry (MS) using an Applied Biosystems API-165 LC/MS system with electrospray ionization.
In vitro treatment of E. multilocularis protoscoleces.
E. multilocularis protoscoleces were obtained as described above (see “Isolation of E. multilocularis protoscoleces”). The parasite materials were placed into culture medium suitable for the long-term maintenance of the protoscoleces in vitro (27). The parasite cultures were kept in a six-well plate at a density of approximately 500 protoscoleces per ml of culture medium, and half of the medium was replaced twice a week. This culture condition was also applied during in vitro treatment of the parasite. To examine the efficacy of chemical compounds against living E. multilocularis protoscoleces, the parasites were kept in the culture medium supplemented with 5 or 50 μM of each compound, including quinazoline and its 8-OH derivative, rotenone (a specific inhibitor of mitochondrial complex I) (19) and nitazoxanide (a compound with strong protoscolicidal action) (32). One control group was supplemented with 0.5% (vol/vol) dimethyl sulfoxide (vehicle) alone, and all conditions were assayed in triplicate. The viability of protoscoleces was determined by microscopic analysis of more than 170 protoscoleces per well for motile behavior and the ability to exclude trypan blue (32).
RESULTS
Preparation of enriched mitochondrial fractions.
To characterize the mitochondrial respiratory chain of E. multilocularis protoscoleces, we prepared enriched mitochondrial fractions from the parasite. Approximately 80 g of larval E. multilocularis (containing approximately 105 protoscoleces per gram) was obtained from each cotton rat more than 4 months after oral infection with 50 parasite eggs. Approximately 20 g of the larval parasite was used per isolation of protoscoleces, yielding 2 ml of cleaned protoscolex sediment (Fig. 3). The enriched mitochondrial fractions were prepared from the protoscolex sediment as described in Materials and Methods. Each 1 ml of protoscolex sediment (containing 4.5 × 105 protoscoleces) yielded approximately 4 mg of mitochondria. Western blotting using an antibody to mammalian cytochrome c oxidase detected a specific band in the mitochondria from the liver of a cotton rat but not in mitochondria from E. multilocularis protoscoleces even when the amounts of both mitochondrial samples were normalized according to cytochrome c oxidase activity (data not shown). These results demonstrated that the enriched mitochondrial fractions from the parasite were sufficiently free of host components for use in enzyme assays and quinone analyses. In order to assess the quality of mitochondria, intactness was examined by the reactivity of NADH, which is a non-membrane-permeable substrate. NADH oxidase activity was not detected in the isotonic buffer, whereas it was fully activated in hypotonic buffer after a freeze-thaw treatment of the enriched mitochondrial fraction. Based on the results obtained, the method applied here for mitochondrial preparation seemed to be appropriate.
FIG. 3.
Protoscoleces of E. multilocularis (Nemuro strain) used for the preparation of enriched mitochondrial fractions of the parasite and subsequent analyses. Bar, 500 μm.
Enzyme activities of E. multilocularis mitochondria.
The specific enzyme activities involved in the mitochondrial respiratory chain of E. multilocularis protoscoleces are shown in Table 1. Parasite complex II exhibited an SDH activity of 103 nmol/min/mg. The specific activity of succinate-dUQ reductase was comparable to that of SDH activity (98.9 nmol/min/mg), whereas the succinate-dRQ reductase activity was lower (16.6 nmol/min/mg). The specific activity of decyl rhodoquinol-fumarate reductase, which is the reverse reaction of the succinate-RQ reductase activity of complex II, was determined to be 60.2 nmol/min/mg. The mitochondria of E. multilocularis protoscoleces exhibited NADH oxidase activity of 9.1 nmol/min/mg, which was almost eliminated by 2 mM KCN and 100 mM malonate. Ubiquinol-1 oxidase and TMPD oxidase activities were determined to be 4.4 nmol/min/mg and 12.6 nmol/min/mg, respectively. These activities were completely inhibited by 2 mM KCN. Under anaerobic conditions, the specific activity of NADH-fumarate reductase was 45 nmol/min/mg, which was much higher than the NADH oxidase activity. The specific activity of NADH-dUQ reductase and NADH-dRQ reductase of complex I were determined to be 32.1 and 61.3 nmol/min/mg, respectively.
TABLE 1.
Specific activities of mitochondrial respiratory enzymes in E. multilocularis protoscoleces
| Assay | Sp acta (nmol/min/mg of protein) (mean ± SD) |
|---|---|
| SDH | 103 ± 16 |
| Succinate-quinone reductase | |
| dUQ (anaerobic) | 98.9 ± 12 |
| dRQ (anaerobic) | 16.6 ± 3.5 |
| Quinol-fumarate reductase (decyl rhodoquinol) (anaerobic) | 60.2 ± 18 |
| NADH oxidase | 9.1 ± 2.1 |
| NADH oxidase with: | |
| 2 mM KCN | 7.3 ± 1.5 |
| 100 mM malonate | 4.4 ± 0.4 |
| 2 mM KCN and 100 mM malonate | 1.7 ± 0.7 |
| Ubiquinol-1 oxidase | 4.4 ± 0.6 |
| TMPD oxidase | 12.6 ± 6.3 |
| NADH-fumarate reductase (anaerobic) | 45.0 ± 8.1 |
| NADH-quinone reductase | |
| dUQ (anaerobic) | 32.1 ± 2.7 |
| dRQ (anaerobic) | 61.3 ± 4.3 |
Specific activities were obtained from at least three independently isolated mitochondria.
Quinone components in E. multilocularis mitochondria.
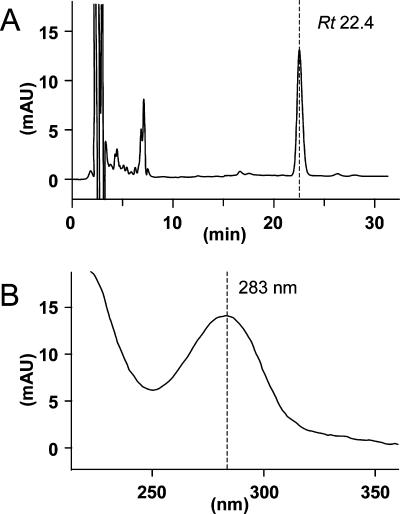
To determine which quinones act as physiological electron mediators in the mitochondrial respiratory system of E. multilocularis protoscoleces, HPLC analyses were performed. As shown in Fig. 4A, the enriched mitochondrial fractions contained only one major quinone component at a retention time (Rt) of 22.4 min. The peak fraction exhibited a characteristic absorption maximum for RQs at 283 nm (Fig. 4B) (20). Subsequent MS analysis confirmed that the primary quinone of the parasite was RQ10 (electrospray ionization-MS m/z 848.8 [M + H]+). The concentration of RQ10 was determined to be 0.73 nmol/mg of mitochondrial protein.
FIG. 4.
(A) HPLC analysis of quinones extracted from the enriched mitochondrial fraction of E. multilocularis protoscoleces. Detailed experimental conditions are described in Materials and Methods. The highest peak had a retention time of 22.4 min (arrow). (B) Absorption of this peak was 283 nm, suggesting that it contained an RQ. mAU, milli-absorbance units.
Effects of inhibitors on NADH-fumarate reductase in E. multilocularis mitochondria.
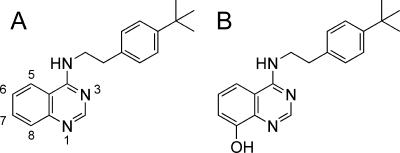
To investigate the inhibitory effect of quinazoline (Fig. 5A) and its derivatives on the enzymatic activities in the anaerobic respiratory system of E. multilocularis mitochondria, we determined IC50 values against the NADH-fumarate reductase activity of the enriched mitochondrial fraction of the parasite. We found that all of the compounds inhibited the NADH-fumarate reductase activity of the parasite to some extent. Quinazoline and its derivatives including 6-NH2, 6-NHCO(CH=CH2), 7-NH2, 8-OH, 8-OCH3, 8-OCH2CH3, and 8-OCH(CH3)2 exhibited IC50 values of 2.3, 2.1, 16, 62, 71, 48, 4,100, and 910 nM, respectively. Of the compounds tested, the 8-OH derivative (Fig. 5B) exhibited relatively selective inhibition against the NADH-fumarate reductase activity of E. multilocularis protoscoleces compared with the NADH oxidase activities of mammalian mitochondria: the IC50 values of quinazoline and its 8-OH derivative for the NADH oxidase activities of mammalian (bovine heart) mitochondria were 0.40 and 230 nM, respectively.
FIG. 5.
Structures of quinazoline (A) and its 8-OH derivative (B) used for the enzyme inhibition assays and in vitro treatment of E. multilocularis protoscoleces.
Effects of inhibitors on living E. multilocularis protoscoleces.
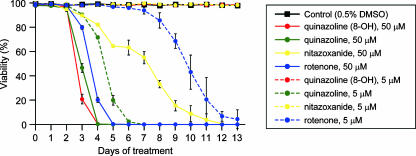
In order to examine the parasite-killing activities of the quinazoline-type compounds with different degrees of inhibitory effects against NADH-fumarate activities of E. multilocularis protoscoleces, we performed in vitro treatment of the parasite using quinazoline and its 8-OH derivative. The viability of the E. multilocularis protoscolex was progressively reduced during in vitro treatment of the parasites with 50 μM of the 8-OH derivative, and by day 5, all the parasites died (Fig. 6). The same compound did not have an obvious antiparasitic effect when used at a concentration of 5 μM. On the other hand, nonsubstituted quinazoline, which showed lower IC50 values with the enzymatic assay, eliminated the parasites on days 5 and 7 of in vitro treatment when used at 50 and 5 μM, respectively. Treatment with rotenone, a specific inhibitor of mitochondrial complex I (19), affected the viability of the parasite in a manner similar to that of the 8-OH derivative. The antiechinococcal effect of nitazoxanide was relatively mild: even in the presence of 50 μM nitazoxanide, the viability decreased, but it did so only gradually, and it took 13 days before all the protoscoleces died. This compound did not affect parasite viability when used at 5 μM.
FIG. 6.
Viability of E. multilocularis protoscoleces during in vitro treatment with quinazoline and its 8-OH derivatives, rotenone and nitazoxanide. Each compound was added to the culture medium at 5 or 50 μM. The results represent the means ± standard deviations of at least triplicate samples. DMSO, dimethyl sulfoxide.
DISCUSSION
The most notable finding of the present study is that E. multilocularis protoscoleces possess a unique mitochondrial respiratory system that is highly adapted to anaerobic conditions. Specifically, the predominant enzymatic activity in the enriched mitochondrial fraction prepared from the parasite protoscoleces is the NADH-fumarate reductase system, which does not normally function in the aerobic respiratory chain of mammals. Thus, we infer that mitochondrial respiratory system of E. multilocularis would be a good target for the development of novel selective antiechinococcal compounds as demonstrated previously for other helminthic diseases (8, 21).
As early as 1957, Agosin found that E. granulosus protoscoleces have both aerobic and anaerobic respiratory systems and that glycolytic inhibitors are effective against both of them, indicating that they both depend on glycolysis (1). Subsequently, McManus and Smyth observed that protoscoleces cultured under anaerobic conditions produce more succinate than parasites kept under aerobic conditions, suggesting that the parasites survive under anaerobic conditions by utilizing the NADH-fumarate reductase system (16). Furthermore, McManus and Smyth reported that the specific activity of fumarate reductase in Echinococcus protoscoleces is lower than those of enzymes involved in the tricarboxylic acid cycle (17). These results, however, did not establish the importance of NADH-fumarate reductase activity in the mitochondrial respiratory system of the parasite because the other enzyme activities were not analyzed.
In the present study, we focused on the enzyme activities of the mitochondrial respiratory system of the parasite to determine whether the system is adapted to anaerobic conditions. Using the enriched mitochondrial fractions prepared from E. multilocularis protoscoleces, we showed that the activity of NADH-fumarate reductase in the respiratory system of the parasite is predominant compared with that of NADH oxidase, an enzyme involved in aerobic respiration in aerobic organisms such as mammals. Furthermore, direct measurements of complex II activities in both directions (i.e., succinate-RQ reductase and rhodoquinol-fumarate reductase activities) indicated that parasite complex II functions more favorably as a rhodoquinol-fumarate reductase in the presence of RQ/rhodoquinol. Thus, our results using isolated mitochondria of E. multilocularis protoscoleces coupled with assay systems for the determination of the parasite's enzyme activities revealed for the first time that the parasite mitochondria are highly adapted to anaerobic environments.
Analyses of the quinone components of E. multilocularis mitochondria revealed that RQ10 (Fig. 1B), whose redox potential is much more negative (Em′ [midpoint potential] = −63 mV) than that of UQ10 (Em′ = +110 mV) (Fig. 1A), was the primary quinone component of parasite mitochondria. In other parasitic helminths, like A. suum and Hymenolepis diminuta, RQ is an essential component of the NADH-fumarate reductase system (5, 11). In addition, van Hellemond et al. previously demonstrated that for all eukaryotes, the relative amount of RQ compared to the total amount of quinones correlates well with the importance of fumarate reduction in vivo (31). Similarly, during the development of the liver fluke Fasciola hepatica, there is a good correlation between the quinone composition and the importance of fumarate reduction in vivo (31). Therefore, RQ seems to be an essential component of fumarate reduction in eukaryotic respiration. Although menaquinone-related fumarate reduction in prokaryotes is well known (33, 34), there is no evidence that menaquinone serves this function in eukaryotes. In this study, enzyme assays demonstrated that the mitochondria from E. multilocularis possess NADH-fumarate activity as the predominant activity. In addition, the NADH-dRQ reductase activity was much higher than that of NADH-dUQ reductase, indicating that E. multilocularis complex I may interact preferentially with RQ rather than with UQ. Taken together, these results indicate that, as in other metazoan eukaryotes with anaerobic respiratory systems, E. multilocularis protoscoleces have a unique respiratory system that is highly adapted to anaerobic environments and in which RQ10 is used as the primary electron mediator.
Spiliotis et al. recently reported that the in vitro growth of larval E. multilocularis is more active under anaerobic than aerobic conditions (23). Thus, our findings for the respiratory system of E. multilocularis protoscoleces are consistent with the observations reported previously by Spiliotis et al. Larval E. multilocularis containing a large number of protoscoleces lives in host tissues, mainly the liver, surrounded by thick connective tissues containing carbohydrate-rich laminated layers, which probably provide the parasite cells with an extremely-low-oxygen environment. Accordingly, it is not surprising that the parasite survives in the host by utilizing an anaerobic respiratory system.
Many anaerobic parasitic eukaryotes use the NADH-fumarate pathway, which is absent in mammals (2, 3, 10, 14, 22, 29). Therefore, this unique respiratory system is regarded as a promising chemotherapeutic target for the development of novel anthelminthics, as discussed in a recent review (9). In fact, Omura et al. previously found a natural compound, nafuredin, that is a potent inhibitor of the adult A. suum mitochondrial respiratory chain but much weaker against the mammalian mitochondrial respiratory chain (21). Yamashita et al. also found that quinazoline-type inhibitors were highly effective against adult A. suum complex I (35). Kinetic analyses using a series of quinazoline-type inhibitors revealed that A. suum complex I recognizes RQ2 or UQ2 in different ways, suggesting that mitochondrial complex I, which reacts preferably with RQs, could be a good target for chemotherapy. In the present study, we also tested several quinazoline-type compounds for their abilities to inhibit the anaerobic respiratory system of E. multilocularis protoscoleces. We found that all of the quinazoline-type compounds inhibited the NADH-fumarate reductase activity of E. multilocularis mitochondria to different extents. Furthermore, these compounds exhibited potent parasite-killing activities against E. multilocularis protoscoleces under in vitro culture conditions. Importantly, the nonsubstituted quinazoline, which has a higher inhibitory effect against NADH-fumarate oxidoreductase of the parasite mitochondria than the 8-OH derivative does, exhibited the parasite-killing activity even when used at 5 μM, whereas the 8-OH derivative did not do so at the same concentration. Such a correlation between the enzyme inhibition and the parasite-killing activities of these compounds suggests that the anaerobic NADH-fumarate reductase system of the parasite is a promising target for the development of antiechinococcal drugs.
Antiechinococcal drugs for chemotherapy of human AE should target not only protoscoleces but also the germinal layers of the E. multilocularis metacestode. The germinal layers in the larval parasite exhibit extremely unique characteristics. The parasite cells forming the germinal layers can differentiate into various tissues, including brood capsules and protoscoleces, and at the same time, they proliferate asexually as they remain in an undifferentiated state. This causes enlargement and, occasionally, metastasis of the lesions due to the formation of a large parasite mass. Therefore, for chemotherapy of AE, a complete cure cannot be achieved unless the germinal cells of the larval parasite are eliminated. Therefore, the mitochondrial respiratory system of germinal cells should be further characterized to aid in the development of a novel antiechinococcal compound(s) targeting the energy metabolism of larval E. multilocularis. However, it is presently quite difficult to obtain enough metacetode materials with homogeneous quality. Established methodologies for the in vitro cultivation of E. multilocularis metacestodes are now available (6, 23), and they will hopefully be applicable to large-scale preparations of metacestode materials in the near future.
During the life cycle of E. multilocularis, the parasite never undergoes active development and/or energy metabolism under aerobic conditions. The larval parasite lives mainly in the liver of intermediate host animals, whereas the adult worm dwells inside the small intestine of the final host, both of which are microaerobic conditions. Although the eggs of the parasite are exposed to air, they already contain a mature infective larva (oncosphere) waiting to be taken up by the next intermediate host. Therefore, the oncosphere does not develop or move under aerobic conditions. Taken together, these findings suggest that the respiratory system of E. multilocularis protoscoleces, as characterized in the present study, could represent the respiratory system used by the parasite throughout its developmental stages. Based on this speculation, the use of protoscolex materials in the first-step screening of candidate compounds by enzyme inhibition assays and subsequent in vitro parasite-killing assays appears to be reasonable, although it should be confirmed that the respiratory system of the E. multilocularis metacestode shares the same basic characteristics with that of the protoscolex stage of the parasite. We have already done preliminary experiments on the effects of the compounds used in this study, including the quinazoline derivative (8-OH), against in vitro-cultured metacestodes and found that the compounds exhibited high parasite-killing activities as evaluated by a modified MTT assay (data not shown). These results strongly suggest that our strategy is appropriate.
Highly effective chemotherapeutic compounds against human AE are not currently available despite the fact that the disease can be lethal unless the patient is appropriately treated during the early stage of the infection. Based on the findings presented here, it appears that the anaerobic respiratory system of E. multilocularis, which is distinct from that of host mammals, is a good target for the development of highly effective antiechinococcal drugs and, furthermore, that respiratory chain inhibitors (21, 35) are possible lead compounds for the development of antiechinococcal drugs.
Acknowledgments
We thank Andrew Hemphill at the University of Berne for kindly providing us with precious chemical compounds.
This work was supported by grants from the following organizations: the Ministry of Education, Culture, Sports, Science, and Technology of Japan for the 21st Century COE Program, Program of Excellence for Zoonosis Control, and 18073004; the Ministry of Health and Welfare, Japan, for the Control of Emerging and Reemerging Diseases in Japan; the Japan Society of the Promotion of Science (grants 17790274 and 18GS0314); the Northern Advancement Center for Science and Technology; and the Akiyama Foundation.
Footnotes
Published ahead of print on 22 October 2007.
REFERENCES
- 1.Agosin, M. 1957. Studies on the metabolism of Echinococcus granulosus. II. Some observations on the carbohydrate metabolism of hydatid cyst scolices. Exp. Parasitol. 6:586-593. [DOI] [PubMed] [Google Scholar]
- 2.Amino, H., A. Osanai, H. Miyadera, N. Shinjyo, T. Tomitsuka, H. Taka, R. Mineki, K. Murayama, S. Takamiya, T. Aoki, H. Miyoshi, K. Sakamoto, S. Kojima, and K. Kita. 2003. Isolation and characterization of the stage-specific cytochrome b small subunit (CybS) of Ascaris suum complex II from the aerobic respiratory chain of larval mitochondria. Mol. Biochem. Parasitol. 128:175-186. [DOI] [PubMed] [Google Scholar]
- 3.Amino, H., H. Wang, H. Hirawake, F. Saruta, D. Mizuchi, R. Mineki, N. Shindo, K. Murayama, S. Takamiya, T. Aoki, S. Kojima, and K. Kita. 2000. Stage-specific isoforms of Ascaris suum complex. II. The fumarate reductase of the parasitic adult and the succinate dehydrogenase of free-living larvae share a common iron-sulfur subunit. Mol. Biochem. Parasitol. 106:63-76. [DOI] [PubMed] [Google Scholar]
- 4.Bryant, C., and C. Behm. 1989. Energy metabolism, p. 25-69. In C. Bryant and C. Behm (ed.), Biochemical adaptation in parasites. Chapman and Hall, London, United Kingdom.
- 5.Fioravanti, C. F., and Y. Kim. 1988. Rhodoquinone requirement of the Hymenolepis diminuta mitochondrial electron transport system. Mol. Biochem. Parasitol. 28:129-134. [DOI] [PubMed] [Google Scholar]
- 6.Hemphill, A., and B. Gottstein. 1995. Immunology and morphology studies on the proliferation of in vitro cultivated Echinococcus multilocularis metacestodes. Parasitol. Res. 81:605-614. [DOI] [PubMed] [Google Scholar]
- 7.Kita, K., H. Hirawake, and S. Takamiya. 1997. Cytochromes in the respiratory chain of helminth mitochondria. Int. J. Parasitol. 27:617-630. [DOI] [PubMed] [Google Scholar]
- 8.Kita, K., C. Nihei, and E. Tomitsuka. 2003. Parasite mitochondria as drug target: diversity and dynamic changes during the life cycle. Curr. Med. Chem. 10:2535-2548. [DOI] [PubMed] [Google Scholar]
- 9.Kita, K., K. Shiomi, and S. Omura. 2007. Advances in drug discovery and biochemical studies. Trends Parasitol. 23:223-229. [DOI] [PubMed] [Google Scholar]
- 10.Kita, K., and S. Takamiya. 2002. Electron-transfer complexes in Ascaris mitochondria. Adv. Parasitol. 51:95-131. [DOI] [PubMed] [Google Scholar]
- 11.Kita, K., S. Takamiya, R. Furushima, Y. Ma, H. Suzuki, T. Ozawa, and H. Oya. 1988. Electron-transfer complexes of Ascaris suum muscle mitochondria. III. Composition and fumarate reductase activity of complex II. Biochim. Biophys. Acta 935:130-140. [DOI] [PubMed] [Google Scholar]
- 12.Köhler, P. 1991. The pathways of energy generation in filarial parasites. Parasitol. Today 7:21-25. [DOI] [PubMed] [Google Scholar]
- 13.Komuniecki, R., and B. G. Harris. 1995. Carbohydrate and energy metabolism in helminths, p. 49-66. In J. J. Marr and M. Müller (ed.), Biochemistry and molecular biology of parasites. Academic Press, New York, NY.
- 14.Kuramochi, T., H. Hirawake, S. Kojima, S. Takamiya, R. Furushima, T. Aoki, R. Komuniecki, and K. Kita. 1994. Sequence comparison between the flavoprotein subunit of the fumarate reductase (complex II) of the anaerobic parasitic nematode, Ascaris suum and the succinate dehydrogenase of the aerobic, free-living nematode, Caenorhabditis elegans. Mol. Biochem. Parasitol. 68:177-187. [DOI] [PubMed] [Google Scholar]
- 15.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 16.McManus, D. P., and J. D. Smyth. 1978. Differences in the chemical composition and carbohydrate metabolism of Echinococcus granulosus (horse and sheep strains) and E. multilocularis. Parasitology 77:103-109. [DOI] [PubMed] [Google Scholar]
- 17.McManus, D. P., and J. D. Smyth. 1982. Intermediary carbohydrate metabolism in protoscoleces of Echinococcus granulosus (horse and sheep strains) and E. multilocularis. Parasitology 84:351-366. [DOI] [PubMed] [Google Scholar]
- 18.McManus, D. P., W. Zhang, J. Li, and P. B. Bartley. 2003. Echinococcosis. Lancet 362:1295-1304. [DOI] [PubMed] [Google Scholar]
- 19.Miyoshi, H. 1998. Structure-activity relationships of some complex I inhibitors. Biochim. Biophys. Acta 1364:236-244. [DOI] [PubMed] [Google Scholar]
- 20.Moore, H. W., and K. Folkers. 1965. Coenzyme Q. LXII. Structure and synthesis of rhodoquinone, a natural aminoquinone of the coenzyme Q group. J. Am. Chem. Soc. 87:1409-1410. [DOI] [PubMed] [Google Scholar]
- 21.Omura, S., H. Miyadera, H. Ui, K. Shiomi, Y. Yamaguchi, R. Masuma, T. Nagamitsu, D. Takano, T. Sunazuka, A. Harder, H. Kölbl, M. Namikoshi, H. Miyoshi, K. Sakamoto, and K. Kita. 2001. An anthelmintic compound, nafuredin, shows selective inhibition of complex I in helminth mitochondria. Proc. Natl. Acad. Sci. USA 98:60-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saruta, F., T. Kuramochi, K. Nakamura, S. Takamiya, Y. Yu, T. Aoki, K. Sekimizu, S. Kojima, and K. Kita. 1995. Stage-specific isoforms of complex II (succinate-ubiquinone oxidoreductase) in mitochondria from the parasitic nematode, Ascaris suum. J. Biol. Chem. 270:928-932. [DOI] [PubMed] [Google Scholar]
- 23.Spiliotis, M., D. Tappe, L. Sesterhenn, and K. Brehm. 2004. Long-term in vitro cultivation of Echinococcus multilocularis metacestodes under axenic conditions. Parasitol. Res. 92:430-432. [DOI] [PubMed] [Google Scholar]
- 24.Takada, M., S. Ikenoya, T. Yuzuriha, and K. Katayama. 1982. Studies on reduced and oxidized coenzyme Q (ubiquinones). II. The determination of oxidation-reduction levels of coenzyme Q in mitochondria, microsomes and plasma by high-performance liquid chromatography. Biochim. Biophys. Acta 679:308-314. [DOI] [PubMed] [Google Scholar]
- 25.Takamiya, S., R. Furushima, and H. Oya. 1984. Electron transfer complexes of Ascaris suum muscle mitochondria. I. Characterization of NADH-cytochrome c reductase (complex I-III), with special reference to cytochrome localization. Mol. Biochem. Parasitol. 13:121-134. [DOI] [PubMed] [Google Scholar]
- 26.Takamiya, S., K. Kita, H. Wang, P. P. Weinstein, A. Hiraishi, H. Oya, and T. Aoki. 1993. Developmental changes in the respiratory chain of Ascaris mitochondria. Biochim. Biophys. Acta 1141:65-74. [DOI] [PubMed] [Google Scholar]
- 27.Thompson, R. C., P. Deplazes, and J. Eckert. 1990. Uniform strobilar development of Echinococcus multilocularis in vitro from protoscolex to immature stages. J. Parasitol. 76:240-247. [PubMed] [Google Scholar]
- 28.Tielens, A. G. M., C. Rotte, J. J. van Hellemond, and W. Martin. 2002. Mitochondria as we don't know them. Trends Biochem. Sci. 27:564-572. [DOI] [PubMed] [Google Scholar]
- 29.Tielens, A. G. M., and J. J. van Hellemond. 1998. The electron transport chain in anaerobically functioning eukaryotes. Biochim. Biophys. Acta 1365:71-78. [DOI] [PubMed] [Google Scholar]
- 30.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Hellemond, J. J., M. Klockiewicz, C. P. Gaasenbeek, M. H. Roos, and A. G. M. Tielens. 1995. Rhodoquinone and complex II of the electron transport chain in anaerobically functioning eukaryotes. J. Biol. Chem. 270:31065-31070. [DOI] [PubMed] [Google Scholar]
- 32.Walker, M., J. F. Rossignol, P. Torgerson, and A. Hemphill. 2004. In vitro effects of nitazoxanide on Echinococcus granulosus protoscoleces and metacestodes. J. Antimicrob. Chemother. 54:609-616. [DOI] [PubMed] [Google Scholar]
- 33.Wissenbach, U., A. Kroger, and G. Unden. 1990. The specific functions of menaquinone and demethylmenaquinone in anaerobic respiration with fumarate, dimethylsulfoxide, trimethylamine N-oxide and nitrate by Escherichia coli. Arch. Microbiol. 154:60-66. [DOI] [PubMed] [Google Scholar]
- 34.Wissenbach, U., D. Ternes, and G. Unden. 1992. An Escherichia coli mutant containing only demethylmenaquinone, but no menaquinone: effects on fumarate, dimethylsulfoxide, trimethylamine N-oxide and nitrate respiration. Arch. Microbiol. 158:68-73. [DOI] [PubMed] [Google Scholar]
- 35.Yamashita, T., T. Ino, H. Miyoshi, K. Sakamoto, A. Osanai, E. Nakamaru-Ogiso, and K. Kita. 2004. Rhodoquinone reaction site of mitochondrial complex I, in parasitic helminth, Ascaris suum. Biochim. Biophys. Acta 1608:97-103. [DOI] [PubMed] [Google Scholar]