Abstract
Using an indicator cell assay that directly quantifies viral replication, we show that human immunodeficiency virus types 1 and 2 (HIV-1 and HIV-2, respectively) exhibit similar sensitivities to 3′-azido-3′-deoxythymidine (zidovudine) as well as other nucleoside analog inhibitors of reverse transcriptase. These data support the use of nucleoside analogs for antiviral therapy of HIV-2 infection.
Two distinct types of human immunodeficiency virus (HIV) are recognized as the causative agents of AIDS. HIV-1 is responsible for the global pandemic that currently involves ∼40 million individuals (24). HIV-2 has achieved only a limited spread in the human population (1 million to 2 million people presently infected) (24) but remains endemic in West Africa and is also found in portions of southern Africa, India, Brazil, and western Europe (21). Although HIV-2 is generally less pathogenic than HIV-1, some HIV-2 patients exhibit relatively high viral loads, low CD4 counts, and other clinical signs of immunodeficiency disease (6, 13). These individuals experience an increased risk of AIDS-associated mortality unless therapeutic suppression of HIV-2 replication is achieved (11).
In contrast to the wealth of data available for HIV-1, there is a scarcity of studies comparing the efficacies of different drug regimens against HIV-2. HIV-2 is resistant to nonnucleoside reverse transcriptase (RT) inhibitors as well as the fusion inhibitor T-20 (enfuvirtide) (27), and some strains also exhibit reduced sensitivity to specific protease inhibitors (16, 20). With respect to nucleoside analogs, studies of zidovudine (AZT) susceptibility in HIV-2 have produced contradictory findings. Some reports suggest that HIV-2 may be resistant to AZT in cell cultures (18, 19) and that, relative to HIV-1 RT, HIV-2 RT is less sensitive to AZT-5′-triphosphate (3). Other studies assert that HIV-1 and HIV-2 are equally sensitive to the inhibitor in vitro (5, 14, 27) and in cell-free assays (9, 26). From a clinical perspective, the efficacy of AZT in HIV-2-infected patients is uncertain due to its concurrent use with other nucleoside and protease inhibitors (1, 25). The potential for intrinsic AZT resistance in HIV-2 is of particular concern, since AZT is widely used in western Africa and other resource-limited areas where HIV-2 is endemic.
Here we revisit this important question by applying a well-established method for quantifying drug sensitivity in vitro. Previous comparisons of HIV-1 and HIV-2 utilized assays that permit multiple cycles of viral replication and, in most cases, used measurements of cytopathic effects to monitor viral spread (5, 14, 18, 19, 27). The outcomes of these experiments are potentially influenced by strain-to-strain differences in viral replication rates, cytopathicities, and multiplicities of infection and therefore may not accurately reflect intrinsic drug susceptibility. To circumvent these potential confounders, we compared the sensitivities of HIV-1 and HIV-2 to AZT and other nucleoside analogs using a HeLa-CD4 indicator cell assay (4). This approach quantifies the level of HIV infection in a single cycle of replication and enables a head-to-head comparison of HIV-1 and HIV-2 drug sensitivities in the same cell type. Our analysis includes wild-type viruses derived from infectious molecular clones of HIV-1NL4-3 and HIV-2ROD (7, 23) as well as isolates obtained from drug-naive patients.
Virus production and drug sensitivity assays.
To obtain virus stocks from the infectious molecular clones, purified preparations of each wild-type or mutant plasmid were transfected into 293T-17 cells (293tsA1609neo) (17) using our previously published protocol (22). Culture supernatants were harvested 42 h after transfection, centrifuged at 1,500 × g to remove residual cells, and stored in aliquots at −150°C. For the patient-derived isolates, samples of each virus were obtained through the National Institutes of Health AIDS Research and Reference Reagent Program (http://www.aidsreagent.org/) and were expanded in MT-2 lymphocyte cultures (15) for 6 to 8 days prior to phenotypic testing. Drug sensitivities were measured by quantifying the dose-dependent reduction of β-galactosidase-positive (Lac+) foci in 48-well plates containing microcultures of MAGIC-5A cells (CD4+/CCR5E+ Hela cells that express Lac under the control of an HIV-1 promoter; kindly provided by Michael Emerman, Fred Hutchinson Cancer Research Center) (8). The MAGIC-5A cultures were infected at a multiplicity of infection of 300 focus-forming units (FFU) per 5 × 104 cells and were fixed and stained 40 h after inoculation as previously described to ensure that the majority of the Lac+ foci arose from a single cycle of viral replication (22).
Susceptibility to AZT.
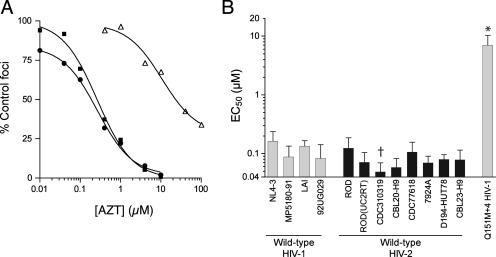
Wild-type HIV-1NL4-3 and wild-type HIV-2ROD yielded similar dose-response profiles when tested against AZT (Fig. 1A), resulting in mean 50% effective concentrations (EC50s) that were comparable for these two strains (Fig. 1B and Table 1). To confirm that HIV-2ROD was representative of other HIV-2 isolates, we replaced the RT-encoding region of pROD9 (codons 14 to 542) with the corresponding region from an independently derived molecular clone of HIV-2UC2 (2). The resulting recombinant strain [HIV-2ROD(UC2RT)] did not significantly differ from wild-type HIV-2ROD and wild-type HIV-1NL4-3 with regard to AZT sensitivity (EC50 of 0.071 ± 0.03 μM) (Fig. 1B). In addition, all wild-type HIV-2 strains from drug-naive patients were statistically equivalent to HIV-1NL4-3 except for HIV-2CDC310319, which was slightly hypersusceptible to AZT (Fig. 1B). These outcomes were not attributable to an inability to detect AZT resistance, since the mean EC50 for a multidrug-resistant A62V V75I F77L F116Y Q151M variant (the Q151M+4 variant) of HIV-1NL4-3 (10) was 43-fold higher than that of the wild type (Fig. 1 and Table 1). Taken together, these data show that HIV-1 and HIV-2 are similarly sensitive to AZT in the MAGIC-5A indicator cell assay.
FIG. 1.
AZT sensitivities of wild-type HIV-1 and HIV-2. (A) Representative data from a single dose-response experiment using strains derived from infectious molecular clones. Wild-type HIV-1NL4-3 (filled squares) and wild-type HIV-2ROD (filled circles) were produced from the full-length plasmids pR9ΔApa (23) and pROD9 (7), respectively. The Q151M+4 variant of HIV-1NL4-3 (open triangles) was produced from a mutated version of pR9ΔApa that encoded the Q151M A62V V75I F77L F116Y complex of mutations in RT (23). Data points are the percentages of Lac+ foci in AZT-treated MAGIC-5A cultures relative to those in solvent-only controls. Each point represents the mean of results from three cultures that were maintained in parallel. The curves were generated using a sigmoidal regression equation (GraphPad Prism 4 software). (B) Summary of the wild-type HIV-1 and HIV-2 isolates, as labeled below the abscissa. Bars represent the EC50s of AZT for inhibiting the formation of Lac+ foci in MAGIC-5A cells and are the means ± the standard deviations from three or more independent dose-response experiments. Wild-type HIV-1NL4-3, wild-type HIV-2ROD, and the Q151M+4 variant of HIV-1NL4-3 were produced from molecular clones as described above. HIV-2ROD(UC2RT) was produced from a molecular clone of HIV-2ROD in which the RT-encoding region was replaced with the equivalent region of HIV-2UC2 (see the text for details). The remaining strains were originally isolated from drug-naive patients and were obtained through the National Institutes of Health AIDS Research and Reference Reagent Program (www.aidsreagent.org). †, significantly less than the value for HIV-1NL4-3; *, significantly greater than the values for all wild-type strains (P < 0.05; analysis of variance of log[EC50] values by use of Tukey's multiple-comparison test). The remaining wild-type HIV-1 and HIV-2 isolates were statistically equivalent (P > 0.05).
TABLE 1.
Susceptibilities of HIV-1NL4-3 and HIV-2ROD to nucleoside analogs
| Straina | EC50 (μM)b
|
||||||
|---|---|---|---|---|---|---|---|
| AZT | ddI | d4T | PMPA | 3TC | FTC | ABC | |
| HIV-1NL4-3 (wt) | 0.16 ± 0.07 (1) | 4.7 ± 2.9 (1) | 5.5 ± 1.6 (1) | 7.2 ± 2.0 (1) | 0.88 ± 0.29 (1) | 0.27 ± 0.11 (1) | 7.6 ± 3.0 (1) |
| HIV-1NL4-3 Q151M+4 variantc | 7.0 ± 3.4 (43) | 45 ± 17 (10) | 79 ± 42 (14) | 26 ± 11 (4) | 3.7 ± 3.0 (4) | 0.79 ± 0.10 (3) | 40 ± 24 (5) |
| HIV-2ROD (wt) | 0.12 ± 0.06 (1) | 8.0 ± 5.5 (2) | 7.1 ± 3.7 (1) | 7.2 ± 3.8 (1) | 2.0 ± 1.2 (2) | 0.50 ± 0.14 (2) | 7.2 ± 2.1 (1) |
Viruses produced with full-length plasmid clones of HIV-1NL4-3 (pR9ΔApa) (23) or HIV-2ROD (pROD9) (7). Infectivities of wild-type (wt) HIV-1NL4-3 and the Q151M+4 variant of HIV-1NL4-3 were 6,300 ± 2,000 and 4,500 ± 2,000 FFU/ng HIV-1 capsid p24, respectively (means ± standard errors of the means). The infectivity of wild-type HIV-2ROD was 15 ± 9 FFU/ng HIV-2 capsid p26.
EC50s were obtained with MAGIC-5A cells as previously described (22). The values shown in bold are significantly different from values for wild-type HIV-1NL4-3 (P < 0.05; analysis of variance of log[EC50] values by use of Tukey's multiple-comparison test). The numbers in parentheses indicate n-fold changes in the EC50s relative to that for wild-type HIV-1NL4-3. EC50s are the means ± the standard deviations from four or more independent experiments. ddI, didanosine; d4T, stavudine; PMPA, tenofovir; ABC, abacavir.
Multinucleoside-resistant mutant (10).
Susceptibility to other nucleoside analogs.
In addition to AZT, wild-type HIV-1NL4-3 and HIV-2ROD showed comparable sensitivities to 2′,3′-didehydro-3′-deoxythymidine (stavudine [d4T]), (R)-9-(2-phosphonylmethoxypropyl)adenine (tenofovir [PMPA]), and (1S,4R)-4-[2-amino-6-(cyclopropylamino)-9H-purin-9-yl]-2-cyclopentene-1-methanol (abacavir [ABC]) (Table 1). Although the mean EC50 for (−)-β-2′,3′-dideoxy-5-fluoro-3′-thiacytidine (emtricitabine [FTC]) was statistically greater for HIV-2ROD than for HIV-1NL4-3 by a factor of 2, this difference was at the threshold of reliable detection in our assay, as the twofold differences in the sensitivities of these strains to 2′,3′-dideoxyinosine (didanosine [ddI]) and (−)-β-2′,3′-dideoxy-3′-thiacytidine (lamivudine [3TC]) were not statistically significant. In contrast to the wild type, the Q151M+4 variant of HIV-1NL4-3 was 3- to 43-fold more resistant to each of the inhibitors (Table 1), and an M184V variant of HIV-1NL4-3 exhibited >100-fold-higher resistance to both FTC and 3TC (data not shown). Thus, wild-type HIV-2ROD did not substantially differ from wild-type HIV-1NL4-3 in its sensitivity to any of the nucleoside analogs tested.
During the course of our study, we noticed that several HIV-2 isolates were able to induce extensive cell-to-cell fusion in MT-2 cultures, leading to complete lysis of the cells within 3 to 4 days after the initial appearance of syncitia (data not shown). In contrast, the MT-2 cultures infected with HIV-1 contained far fewer syncitia at comparable time points and multiplicities of infection. This difference in cytopathicity may explain why HIV-2 occasionally appears to be resistant to AZT in assays that measure virus-mediated cell killing (18, 19). Rapid syncytium formation by HIV-2 can potentially destroy cultures that are maintained at low drug concentrations, thereby saturating the assay and falsely inflating the resultant EC50. In contrast, assays that constrain viral infections to a single cycle are unaffected by strain-to-strain differences in replication capacity and cytopathic potential (12) and therefore provide a more accurate comparison of the drug sensitivities of divergent HIV isolates.
Implications for HIV-2 antiviral therapy.
In summary, our analysis demonstrates that HIV-1 and HIV-2 exhibit comparable susceptibilities to AZT and other nucleoside analogs in culture. These findings are consistent with the similar sensitivities of HIV-1 and HIV-2 RT to chain-terminating nucleoside-5′-triphosphate inhibitors (9, 26). Although the efficiency of AZT-5′-monophosphate incorporation may be slightly lower for HIV-2 RT under certain conditions, this difference is apparently offset by a diminished level of primer-unblocking activity relative to that of the HIV-1 enzyme (3). As a result, HIV-1 and HIV-2 exhibit comparable sensitivities to AZT during viral replication (Fig. 1 and Table 1). Our analysis also agrees with clinical studies showing that specific combinations of nucleoside analogs and protease inhibitors can suppress viral loads in HIV-2-infected individuals (1, 25). Taken together, these findings support initiatives to provide a broad array of nucleoside analog inhibitors to HIV-2 patients in western Africa and other developing regions.
Acknowledgments
This work was supported by Public Health Service grants R01 AI060466 to G.S.G. and R01 AI34834 to B.D.P. Additional support was provided by an undergraduate research grant from the Mary Gates Endowment to C.L.P. and the University of Washington Center for AIDS Research.
We thank Salif Sow (University of Dakar, Dakar, Senegal), James Mullins, and Nancy Kiviat (University of Washington, Seattle) for helpful discussions.
Footnotes
Published ahead of print on 29 October 2007.
REFERENCES
- 1.Adje-Toure, C. A., R. Cheingsong, J. G. Garcia-Lerma, S. Eholie, M. Y. Borget, J. M. Bouchez, R. A. Otten, C. Maurice, M. Sassan-Morokro, R. E. Ekpini, M. Nolan, T. Chorba, W. Heneine, and J. N. Nkengasong. 2003. Antiretroviral therapy in HIV-2-infected patients: changes in plasma viral load, CD4+ cell counts, and drug resistance profiles of patients treated in Abidjan, Cote d'Ivoire. AIDS 17(Suppl. 3):S49-S54. [PubMed] [Google Scholar]
- 2.Barnett, S. W., H. S. Legg, Y. Sun, J. Klinger, D. J. Blackbourn, C. P. Locher, and J. A. Levy. 1996. Molecular cloning of the human immunodeficiency virus subtype 2 strain HIV-2UC2. Virology 222:257-261. [DOI] [PubMed] [Google Scholar]
- 3.Boyer, P. L., S. G. Sarafianos, P. K. Clark, E. Arnold, and S. H. Hughes. 2006. Why do HIV-1 and HIV-2 use different pathways to develop AZT resistance? PLoS Pathog. 2:101-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charneau, P., G. Mirambeau, P. Roux, S. Paulous, H. Buc, and F. Clavel. 1994. HIV-1 reverse transcription. A termination step at the center of the genome. J. Mol. Biol. 241:651-662. [DOI] [PubMed] [Google Scholar]
- 5.Cox, S. W., K. Aperia, J. Albert, and B. Wahren. 1994. Comparison of the sensitivities of primary isolates of HIV type 2 and HIV type 1 to antiviral drugs and drug combinations. AIDS Res. Hum. Retrovir. 10:1725-1729. [DOI] [PubMed] [Google Scholar]
- 6.Gottlieb, G. S., P. S. Sow, S. E. Hawes, I. Ndoye, M. Redman, A. M. Coll-Seck, M. A. Faye-Niang, A. Diop, J. M. Kuypers, C. W. Critchlow, R. Respess, J. I. Mullins, and N. B. Kiviat. 2002. Equal plasma viral loads predict a similar rate of CD4+ T cell decline in human immunodeficiency virus (HIV) type 1- and HIV-2-infected individuals from Senegal, West Africa. J. Infect. Dis. 185:905-914. [DOI] [PubMed] [Google Scholar]
- 7.Guyader, M., M. Emerman, L. Montagnier, and K. Peden. 1989. VPX mutants of HIV-2 are infectious in established cell lines but display a severe defect in peripheral blood lymphocytes. EMBO J. 8:1169-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hachiya, A., S. Aizawa-Matsuoka, M. Tanaka, Y. Takahashi, S. Ida, H. Gatanaga, Y. Hirabayashi, A. Kojima, M. Tatsumi, and S. Oka. 2001. Rapid and simple phenotypic assay for drug susceptibility of human immunodeficiency virus type 1 using CCR5-expressing HeLa/CD4+ cell clone 1-10 (MAGIC-5). Antimicrob. Agents Chemother. 45:495-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hizi, A., R. Tal, M. Shaharabany, and S. Loya. 1991. Catalytic properties of the reverse transcriptases of human immunodeficiency viruses type 1 and type 2. J. Biol. Chem. 266:6230-6239. [PubMed] [Google Scholar]
- 10.Iversen, A. K. N., R. W. Shafer, K. Wehrly, M. A. Winters, J. I. Mullins, B. Chesebro, and T. C. Merigan. 1996. Multidrug-resistant human immunodeficiency virus type 1 strains resulting from combination antiretroviral therapy. J. Virol. 70:1086-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaffar, S., A. D. Grant, J. Whitworth, P. G. Smith, and H. Whittle. 2004. The natural history of HIV-1 and HIV-2 infections in adults in Africa: a literature review. Bull. W. H. O. 82:462-469. [PMC free article] [PubMed] [Google Scholar]
- 12.Larder, B. A., B. Chesebro, and D. D. Richman. 1990. Susceptibilities of zidovudine-susceptible and -resistant human immunodeficiency virus isolates to antiviral agents determined by using a quantitative plaque reduction assay. Antimicrob. Agents Chemother. 34:436-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marlink, R., P. Kanki, I. Thior, K. Travers, G. Eisen, T. Siby, I. Traore, C. C. Hsieh, M. C. Dia, E. H. Gueye, J. Hellinger, A. Gueye-Ndiaye, J. L. Sankale, I. Ndoye, S. Mboup, and M. Essex. 1994. Reduced rate of disease development after HIV-2 infection as compared to HIV-1. Science 265:1587-1590. [DOI] [PubMed] [Google Scholar]
- 14.Mitsuya, H., and S. Broder. 1988. Inhibition of infectivity and replication of HIV-2 and SIV in helper T-cells by 2′,3′-dideoxynucleosides in vitro. AIDS Res. Hum. Retrovir. 4:107-113. [DOI] [PubMed] [Google Scholar]
- 15.Miyoshi, I., I. Kubonishi, S. Yoshimoto, T. Akagi, Y. Ohtsuki, Y. Shiraishi, K. Nagata, and Y. Hinuma. 1981. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature 294:770-771. [DOI] [PubMed] [Google Scholar]
- 16.Ntemgwa, M., B. G. Brenner, M. Oliveira, D. Moisi, and M. A. Wainberg. 2007. Natural polymorphisms in the human immunodeficiency virus type 2 protease can accelerate time to development of resistance to protease inhibitors. Antimicrob. Agents Chemother. 51:604-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pear, W. S., G. P. Nolan, M. L. Scott, and D. Baltimore. 1993. Production of high titer helper free retroviruses by transient transfection. Proc. Natl. Acad. Sci. USA 90:8392-8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reid, P., H. MacInnes, M. E. Cong, W. Heneine, and J. G. Garcia-Lerma. 2005. Natural resistance of human immunodeficiency virus type 2 to zidovudine. Virology 336:251-264. [DOI] [PubMed] [Google Scholar]
- 19.Richman, D. D. 1987. Dideoxynucleosides are less inhibitory in vitro against human immunodeficiency virus type 2 (HIV-2) than against HIV-1. Antimicrob. Agents Chemother. 31:1879-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodes, B., J. Sheldon, C. Toro, V. Jimenez, M. A. Alvarez, and V. Soriano. 2006. Susceptibility to protease inhibitors in HIV-2 primary isolates from patients failing antiretroviral therapy. J. Antimicrob. Chemother. 57:709-713. [DOI] [PubMed] [Google Scholar]
- 21.Schim van der Loeff, M. F., and P. Aaby. 1999. Towards a better understanding of the epidemiology of HIV-2. AIDS 13(Suppl. A):S69-S84. [PubMed] [Google Scholar]
- 22.Smith, R. A., D. J. Anderson, and B. D. Preston. 2006. Hypersusceptibility to substrate analogs conferred by mutations in human immunodeficiency virus type 1 reverse transcriptase. J. Virol. 80:7169-7178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith, R. A., D. J. Anderson, and B. D. Preston. 2004. Purifying selection masks the mutational flexibility of HIV-1 reverse transcriptase. J. Biol. Chem. 279:26726-26734. [DOI] [PubMed] [Google Scholar]
- 24.UNAIDS/WHO. 2006. AIDS epidemic update: December 2006. Joint United Nations Programme on HIV/AIDS (UNAIDS), Geneva, Switzerland.
- 25.van der Ende, M. E., J. M. Prins, K. Brinkman, M. Keuter, J. Veenstra, S. A. Danner, H. G. Niesters, A. D. Osterhaus, and M. Schutten. 2003. Clinical, immunological and virological response to different antiretroviral regimens in a cohort of HIV-2-infected patients. AIDS 17(Suppl. 3):S55-S61. [DOI] [PubMed] [Google Scholar]
- 26.Vrang, L., B. Öberg, J. Löwer, and R. Kurth. 1988. Reverse transcriptases from human immunodeficiency virus type 1 (HIV-1), HIV-2, and simian immunodeficiency virus (SIVMAC) are susceptible to inhibition by foscarnet and 3′-azido-3′-deoxythymidine triphosphate. Antimicrob. Agents Chemother. 32:1733-1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Witvrouw, M., C. Pannecouque, W. M. Switzer, T. M. Folks, E. De Clercq, and W. Heneine. 2004. Susceptibility of HIV-2, SIV and SHIV to various anti-HIV-1 compounds: implications for treatment and postexposure prophylaxis. Antivir. Ther. 9:57-65. [PubMed] [Google Scholar]