Abstract
There is currently little research and development of new compounds with specific anti-human T-cell leukemia virus type 1 (HTLV-1) activity. The few antiretrovirals that have been tested against HTLV-1 in vitro have already been developed into anti-human immunodeficiency virus (HIV) drugs. Here, we show the effects of a newly synthesized family of phosphonated nucleoside compounds, phosphonated carbocyclic 2′-oxa-3′-aza-nucleosides (PCOANs), on HTLV-1 infection in vitro. To ascertain the anti-HTLV-1 activity of PCOANs, peripheral blood mononuclear cells from healthy donors were infected in vitro by coculture with an HTLV-1 donor cell line in the presence of three prototype PCOAN compounds. PCOANs were able to completely inhibit HTLV-1 infection in vitro at a concentration of 1 μM, similar to what has been observed for tenofovir and azidothymidine. Treatment with PCOANs was associated with inhibited growth of HTLV-1-infected cells, and their effects were 100 to 200 times more potent than that of tenofovir. The mechanisms involved in the anti-HTLV-1 effects of PCOANs can mainly be ascribed to their capacity to inhibit HTLV-1 reverse transcriptase activity, as ascertained by means of a cell-free assay. PCOANs caused little reduction in proliferation or induction of apoptotic cell death of uninfected cells, showing toxicity levels similar to tenofovir and lower than azidothymidine. Overall, these results indicate that the family of PCOANs includes potential candidate compounds for long-lasting control of HTLV-1 infection.
The human T-cell leukemia/lymphoma virus type 1 (HTLV-1) is an oncogenic retrovirus endemic in certain areas of the world including Japan and North and South America, where about 5% of the estimated 20 million HTLV-1-infected people develop HTLV-1-associated diseases (10, 11, 43), such as adult T-cell leukemia (ATL), human myelopathy/tropical spastic paraparesis (/TSP), or other minor inflammatory diseases. A number of studies have shown that HTLV-1 infects different types of cells (e.g., lymphocytes, monocytes, and fibroblasts) but preferentially T lymphocytes with a CD4+ phenotype, which become immortalized following infection (26). In HTLV-1 infection viremia is essentially a “cytoviremia,” since the virus is cell associated. In fact, cell-free virions are rarely infectious, and spreading of the virus in vivo does not require the extracellular release of viral particles. The spread of the virus within an individual host is most commonly recognized as being through the mitotic pathway, in which cell divisions and clonal expansion of infected cells ensure a constant level of viral load. However, recently it has been highlighted that the HTLV-1 viral load in vivo is also sustained by cell-to-cell contact, involving intercellular viral spread. In particular, this “horizontal” spread allows the transfer of HTLV-1 infectious viral particles across the cell-cell junction by the generation of “virological synapses” between infected and uninfected cells (19, 21). Transmission of HTLV-1 via cell-to-cell contact involves both integrin (3) and cytoskeleton proteins and participation of Env protein-cell receptor interactions (37). Either way, efficient transmission of HTLV-1 to noninfected cells implies reverse transcriptase (RT) dependency.
In recent years, a number of strategies for therapeutic intervention have been pursued in the treatment of ATL or TSP, based on conventional chemotherapy or innovative approaches. However, until now, limited advances have been achieved in improving the therapy for HTLV-1-associated diseases. Therefore, symptomatic HTLV-1-infected patients still have inadequate treatment options with poor prognoses. Among the novel approaches for treating HTLV-1-related diseases, the use of topoisomerase 1 or 2 inhibitors in a pilot phase II study in refractory ATL did not provide satisfactory results (41). Similarly, treatments with the nucleoside analogue 2′ deoxycoformycin (39) or inhibitors of AMP synthesis resulted in a limited response in ATL patients (42). Interestingly, studies based on a different strategy of chemotherapeutic intervention showed that ATL patients transiently responded to combined therapy with the nucleoside reverse transcriptase inhibitor (NRTI) azidothymidine (AZT) and interferon (IFN) (15) and that AZT treatment was beneficial to TSP patients (33), giving more encouraging results. In addition, a transient response to therapy with the NRTI lamivudine alone (38) or in combination with AZT (25) has been reported for human myelopathy/TSP. Moreover, IFN-α has been shown to have some benefits in ATL patients when used in combination with arsenic trioxide (16, 4). Although in vivo results have highlighted the potential of nucleoside compounds in the treatment of HTLV-1-associated diseases, the number of these compounds that have actually been tested on HTLV-1 infection in vitro still remains limited and is restricted to drugs already developed as anti-human immunodeficiency virus (HIV) agents, such as AZT, lamivudine, and tenofovir (TFV) (1, 2, 17, 24).
Among the molecules capable of antiretroviral activity, NRTIs play an important role. Intracellular phosphorylation by nucleoside kinases is a key step to ensure biological activity to nucleoside analogues by means of the conversion to the triphosphate form. This event is counteracted by nonspecific extracellular phosphohydrolases that destabilize phosphorylated compounds. To overcome the instability of triphosphate nucleoside analogues, several strategies have been proposed such as increasing their resistance toward the phosphohydrolase or ensuring a more efficient phosphorylation within the target cells. These strategies led to the design of a new class of nucleotide prodrugs, described as acyclic nucleoside phosphonates, in which a phosphate group is bound tightly to the acyclic nucleoside moiety (8). In the phosphonated form these compounds bypassed the initial enzymatic phosphorylation step and showed resistance to pirophosphorolysis (13). In addition, acyclic nucleoside phosphonates were found to have a higher affinity for RTs, acting as potent inhibitors of infection by both hepatitis and immunodeficiency viruses (9, 14).
A group of cyclic nucleoside phosphonates, with the furanose ring replaced by an N,O-heterocyclic ring, has recently been developed and described by us (5). Among these, the phosphonated carbocyclic 2′-oxa-3′-aza-nucleosides (PCOANs) are a family of newly synthesized compounds that we have recently shown to exert an inhibitory action towards the enzymatic activity of RTs from different retroviruses, including the RT of HTLV-1 (6, 7). In the present study we have investigated in detail the effects of some prototype compounds belonging to this new family of nucleoside phosphonates on HTLV-1 infection in vitro.
MATERIALS AND METHODS
Assayed compounds.
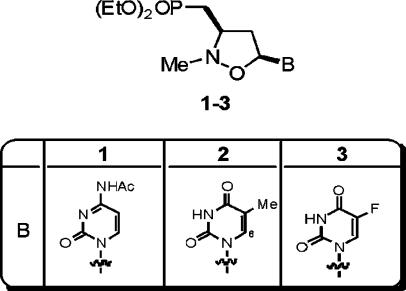
In this study, three prototype PCOANs were used, referred to as PCOAN1, PCOAN2, and PCOAN3 (Fig. 1). The acyclic phosphonate TFV (kindly provided by Jan Balzarini, Rega Institute for Medical Research, Leuven, Belgium) and the NRTI AZT (Wellcome Research Laboratories, Beckenam, England, United Kingdom) were used as reference compounds. The PCOANs were dissolved in dimethyl sulfoxide (DMSO) and maintained at stock concentrations of 200 mM at −20°C. The final concentration of DMSO in cell cultures was never greater than 1%. TFV and AZT were dissolved in culture medium without serum and maintained at stock concentrations of 100 mM at −20°C. The compounds were diluted in culture medium without serum at concentrations appropriate to obtain the final usage concentrations just prior to their use. In at least one of the replicate experiments, two parallel control samples were used: the first consisted of complete medium (CM; see below) alone, and the second consisted of CM containing the highest concentrations of DMSO used in the PCOAN solutions. No differences were seen in comparisons of control samples containing CM plus DMSO with control samples containing CM alone as a control vehicle. For this reason, all control data refer to control samples of CM alone.
FIG. 1.
Structure of PCOANs. Note the direct carbon-phosphorus bond that characterizes phosphonate compounds and renders them resistant to the action of the cellular enzymes that break down phosphorylated compounds. B, pyrimidinic bases; EtO, ethyl group, CH3-CH2.
Cell lines and culture, treatment, and infection of PBMCs.
The chronically HTLV-1-infected human cell lines MT-2 (30), C91/PL, C5/MJ, and HUT 102 (originally donated by Genoveffa Franchini, National Cancer Institute, NIH, Bethesda, MD) were maintained in RPMI 1640 medium supplemented with 12% fetal bovine serum (FBS), glutamine, and penicillin-streptomycin (CM; Gibco-Invitrogen, Paisley, Scotland, United Kingdom). Peripheral blood mononuclear cells (PBMCs) were harvested from healthy adult donors seronegative for HIV and hepatitis B and C viruses. Mononuclear cells from heparinized blood were separated using a Ficoll-Hypaque density gradient (Cederlane, Hornby, Ontario, Canada). The cells were then washed twice in RPMI 1640 medium (Gibco-Invitrogen). HTLV-1 infection was performed by coculturing PBMCs together with lethally irradiated MT-2 cells (120 Cy, using a cesium gamma cell irradiator 1000; AECL, Ontario, Canada) at a ratio of 5:1. Infected and uninfected PBMC cultures were maintained at 0.8 × 106 cells/ml in 25-cm2 flasks in 10 ml of CM containing 20 U/ml of recombinant interleukin 2 (IL-2; Proleukin, Chiron, Amsterdam, The Netherlands). A compound's ability to protect PBMCs from infection was assayed by adding the compounds to the cultures at final concentrations of 0.1, 1, 5, and 25 μM at 3 to 5 min prior to infection, except for the reference compound TFV, which was added 16 h before infection since we have shown that time is required for its anti-HTLV-1 activity to be detected (2). The compounds were again added at 3, 7, and 10 days postinfection (p.i.), when half the volume of the conditioned medium was replaced by fresh medium. The final concentration of each compound in the added fresh medium was half of that which was used initially. The control uninfected cultures and all the infected cultures, whether treated with the different compounds or with control vehicle, were split weekly. Cell growth of infected and uninfected cultures was monitored weekly by evaluating living cells using a trypan blue dye exclusion test. The number of living cells was calculated as the mean of three independent counts. After the counting step, cell concentration was readjusted to 0.8 × 106 cells/ml. Results of cell growth were expressed as total cell number (TCN) calculated from living cell counts as follows: (i) for the first week, TCN was the actual number of cells, expressed as millions of viable cells, detected before the first adjustment; (ii) in successive weeks, TCN was calculated theoretically as the TCN of the previous week multiplied by the cell concentration, expressed as millions of viable cells per milliliter, detected before weekly adjustment. Aliquots of irradiated MT-2 cells, used as donor cells for infection and kept separately in culture for the duration of the experiments, showed no evidence of growth.
Genomic DNA extraction and PCR analysis for evaluation of HTLV-1 proviral DNA.
At 4 weeks p.i., cells from cultures were harvested and centrifuged on a density gradient to eliminate debris and dead cells. Cells were then incubated with proteinase K at 37°C, and DNA was extracted in phenol-chloroform-isoamylalcohol, according to standard procedures. One microgram of DNA was used as a template and was amplified in a standard PCR mixture: 1× PCR buffer, a 0.2 mM concentration of each deoxynucleoside triphosphate (dNTP), 0.5 μM primer pair specific for the pol region of HTLV-1 (forward, SK54; reverse, SK55) (Perkin Elmer, Boston, MA), and 1.25 U of Taq Gold polymerase (Promega, Madison, WI). As an internal control, the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was amplified using the same PCR mixture with a specific primer pair (forward primer, 5′-CCATGGAAAAAGGCTGGGG-3′; reverse primer, 5′-CAAAGTTGTCATGGATGACC-3′). Samples were subjected to 30 cycles of PCR amplification, each cycle consisting of 30 s at 94°C, 30 s at 55°C, and 45 s at 72°C on a DNA thermal cycler (Eppendorf, Hamburg, Germany). Following the final cycle, samples were incubated at 72°C for 7 min to ensure the completion of the final extension step.
RNA extraction and RT-PCR analysis for evaluation of HTLV-1 expression.
RNA isolation was performed using RNAzol (Life Technologies, Grand Island, NY), according to the manufacturer's instructions. Before the assay for the presence of viral RNA, cells from cultures were subjected to centrifugation on a density gradient to eliminate debris and dead cells. Total RNA from infected and uninfected cells was reverse transcribed into cDNA in a 25-μl reaction mixture as follows: 1 μg of RNA was incubated with a mixture containing a final dilution of 1× RT buffer, a 1 mM concentration of each dNTP (Promega), 1.5 μg of oligo(dT) (New England Biolabs, Beverly, MA), 50 U of recombinant RNase inhibitor (Roche), 10 mM dithiothreitol (DTT; Sigma, St. Louis, MO), and 25 U of Moloney murine leukemia virus RT (New England Biolabs) for 1 h at 37°C. The reaction mixture was incubated at 95°C for 5 min in order to inactivate RT and then chilled on ice. Five microliters of cDNA was amplified by PCR in a total volume of 50 μl. Amplifications with a 0.5 μM concentration of primer pairs specific for the Tax/Rex region of HTLV-1 (22) or, as an internal control, with primers specific for GAPDH (forward primer, 5′-TGGTATCGTGAAAGGACT-3′; reverse primer, 5′-ATGCAAGTGAGCTTCCCGTTC-3′) were performed for 30 cycles and amplified using the program described above for DNA-PCR.
Liquid hybridization.
Amplified DNA was analyzed by liquid hybridization as previously described (1). Briefly, the PCR product was probed using the following specific 32P-labeled oligonucleotides: SK56 (Perkin Elmer) for HTLV-1 pol region, RPXPR-1 for the HTLV-1 Tax/Rex region (22), 5′-CTAAGCAGTTGGTGGTGCA-3′ for the GAPDH DNA-PCR product, and 5′-GAAACTGTGGCGTGATGGC-3′ for the GAPDH RT-PCR product. The samples were then loaded onto an 8% agarose gel to detect the amplified products, and the gel was dried and exposed to Kodak XAR-5 film (Kodak Company, Rochester, NY) for autoradiography.
Western blot analysis for evaluation of HTLV-1 Tax.
Five million cells from treated or control samples were subjected to centrifugation on a density gradient to eliminate debris and dead cells, solubilized at 4°C in lysis buffer (50 mM Tris-HCl pH 7.4, 1 mM EDTA, 1 mM EGTA [pH 7.4], 1% Triton-X, 150 mM NaCl, 0.25% sodium deoxycholate, and 1% NP-40 with freshly added 1 mM phenylmethylsulfonyl fluoride, 5 μM DTT, 1 μg/ml leupeptin, 1 μg/ml pepstatin, 2 μg/ml aprotinin, 1 mM Na3VO4, and 20 mM NaF [all from Sigma]) and centrifuged at 10,000 × g. Aliquots of the supernatants were saved to determine protein concentration, and the rest was boiled in sodium dodecyl sulfate (SDS) sample buffer (50 mM Tris-HCl [pH 6.8], 100 mM DTT, 2% SDS, 0.1% bromophenol blue, 10% glycerol). A total of 30 μg of protein for each sample was loaded onto a 10% SDS-polyacrylamide gel, subjected to electrophoresis, and transferred to a nitrocellulose membrane (Bio-Rad, Hercules, CA), which was subsequently stained with 0.2% Ponceau red to ensure equal protein loading and transfer. After the membrane was blocked in 10% nonfat dried milk and 3% bovine serum albumin in TTBS buffer (20 mM Tris-HCl [pH 8.0], 0.9% NaCl, 0.03% Tween 20), the blots were incubated overnight at 4°C with a 1:1,000 dilution of anti-Tax monoclonal antibody (a generous gift from John Brady, National Cancer Institute, NIH, Bethesda, MD) or with a 1:5,000 dilution of anti-β-tubulin monoclonal antibody (ICN Biomedicals, Aurora, OH). Subsequently, blots were washed and then incubated with goat anti-mouse immunoglobulin G (κ and λ chains) conjugated to peroxidase (Bio-Rad, Hercules, CA). Binding of antibodies was detected by chemiluminescence staining using an ECL detection kit (Amersham Biosciences United Kingdom, Little Chalfont, England, United Kingdom).
Detection of p19 through antigen capture assay.
Supernatants from infected cultures, whether treated with the compounds or with control vehicle, were assayed for production of HTLV-1 p19 using an HTLV-1 p19 Gag antigen capture enzyme-linked immunosorbent assay purchased from ZeptoMetrix (Buffalo, NY). Supernatants from uninfected cultures were used as a negative control. The assays were performed according to the manufacturer's protocols.
Determination of the inhibitory effects of the compounds on HTLV-1 RT.
To test the inhibitory activity of the compounds toward HTLV-1 RT activity, a cell-free HTLV-1 RT inhibitory assay recently described by us (2, 6) was utilized. Briefly, as a template for reverse transcription, we utilized RNA extracted from transfectant cells ectopically expressing the glycoprotein D of herpes simplex virus type 1 (HSV-1) (28) and treated with RNase-free DNase (100 U/μg; Roche). Compounds to be assayed were activated through preincubation with a crude extract prepared from phytohemagglutinin- and IL-2-stimulated PBMCs from healthy donors negative for HIV and hepatitis B and C viruses. For preparation of the crude extract, 1 × 106 PBMCs, previously stimulated with phytohemagglutinin (2 μg/ml) and IL-2 (20 U/ml) for 72 h in RPMI medium plus 20% FBS, were rinsed three times in cold phosphate-buffered saline and then solubilized in lysis buffer (50 mM Tris-HCl [pH 7.4], 1 mM EDTA, 1 mM EGTA [pH 7.4], 0.05% Triton-X, 150 mM NaCl, 0.25% sodium deoxycholate, and 0.1% NP-40 with freshly added 1 mM phenylmethylsulfonyl fluoride, 15 μM DTT, 5 μg/ml leupeptin, 5 μg/ml pepstatin, 5 μg/ml aprotinin, 1 mM Na3VO4, and 20 mM Na3F [all from Sigma]) on ice and centrifuged at 10,000 × g. Lysed extracts were incubated with the compounds at different concentrations for 15 min on ice and subsequently for 45 min at 30°C. After incubation the crude extract-compound mixture was inactivated for 5 min at 95°C. As a source of HTLV-1 RT, usually a viral lysate was prepared from 1 ml of supernatant from chronically HTLV-1-infected cells, as previously described (2). The amount of RT in the viral lysates was indirectly assessed through the antigen capture assay by measuring the amount of p19 in the supernatants from chronically HTLV-1-infected cells used for HTLV-1 RT preparations. Total DNase-treated RNA (1 μg) was specifically reverse transcribed using a 0.5 μM reverse primer specific for the glycoprotein D gene US6 (5′-TGTCGTCATAGTGGGCCTCCAT-3′) in a reaction mixture containing 1× RT buffer, 100 U of RNase inhibitor, 1 mM (each) dNTP, and 10 mM DTT (all from Promega, Madison, WI) plus 10 μl of HTLV-1 RT preparation, equivalent to a standardized amount of 32 pg of p19, as optimized previously. The reactions were performed in the presence or absence of activated substances for 1 h at 37°C. After incubation at 95°C for 5 min, 5 μl of RT reaction mixture was used for DNA PCR in a reaction mixture containing 1× Taq Gold buffer (Promega), 0.5 μM primer pair (US6 reverse [see above] and US6 forward, 5′-AGACTTGTTGTAGGAGCATTCG-3′), 0.2 mM (each) dNTP, 5 mM MgCl2, and 1.25 U of Taq Gold Polymerase (Promega). To investigate the dose response in experiments using only lysates from MT-2 cells as the HTLV-1 RT source, the assayed compounds were added at concentrations ranging from 0.1 to 1 nM, and the DNA PCR was performed for 20 cycles. To compare the inhibitory activity of the compounds toward HTLV-1 RTs from C5/MJ, C91/PL, HUT 102, and MT-2 cell lines, HTLV-1 RT preparations were obtained, and the assay was performed as described above, but PCOAN2 and TFV were added at concentrations of 0.1, 1, 10, and 100 nM, and DNA-PCR was carried out for 35 cycles. Amplified DNA (350 bp) was visualized on a 1% agarose gel containing 10 μg/ml ethidium bromide in 1× Tris-acetate-EDTA buffer.
Determination of cytotoxicity of the compounds.
The cytotoxicity of the compounds on uninfected cells was evaluated by means of different assays using human PBMCs and the human lymphoid cell line MOLT-3.
Apoptosis.
Fresh PBMCs from healthy donors were treated with control vehicle or with the compounds to be assayed at concentrations of 8, 32, 128, 512, and 2,048 μM for 72 h in CM in the presence of 20 U/ml of IL-2. Cell death by apoptosis was then evaluated using flow cytometry analysis of isolated nuclei following lysis of the cells and DNA staining with a solution containing 2% Triton X-100, 25 μg/ml propidium iodide, and 0.05% sodium citrate (all from Sigma), as previously described (27). Analysis was performed using a FACScan flow cytometer (BD Bioscience, Immunocytometry Systems, Mountain View, CA).
MTS assay.
Inhibition of cell metabolic activity revealed by reduction of the oxidative burst was detected through formazan product formation, using a commercial colorimetric kit (MTS [3,4-(5-dimethylthiazol-2-yl)-5-(3-carboxymethoxy phenyl)-2-(4-sulfophenyl)-2H-tetrazolium salt]) (Cell Titer 96 Aqueous One Solution; Promega). The assay was performed by seeding 1 × 104 MOLT-3 cells in 100 μl in the presence or absence of PCOANs or the reference compounds at seven different concentrations ranging from 10 nM to 1 mM in RPMI medium supplemented with 5% FBS, glutamine, and penicillin-streptomycin. Twenty microliters of Cell Titer 96 Aqueous One Solution reagent was added directly to culture wells at the end of the culture period and incubated for 1 to 4 h; then, absorbance was read at 490 nm.
Proliferation.
To assay the levels of proliferation of uninfected PBMCs from healthy donors, cells were set up in CM in the presence of IL-2 (20 U/ml). The control solutions or compounds to be tested were added at the beginning of the culture period at concentrations of 6.25, 25, 100, 200, 400, 600, and 800 μM. The compounds were added again at half the initial concentration on day 3 p.i. After 1 week in culture, [3H]thymidine (Amersham Biosciences United Kingdom) was added at 1 μCi/well. The cultures were harvested after a further 16-h incubation at 37°C for successive evaluation of incorporated 3H by assaying radioactivity.
Calculation of effective and cytotoxic concentrations.
Results from at least three independent determinations were used to calculate the following: (i) the lowest initial compound concentration at which no viral RNA was detected in infected PBMCs by reverse transcription-PCR (RT-PCR) (RNA 100% effective concentration [rnaEC100]), as revealed by values of ≤0 following subtraction of corresponding uninfected controls through densitometry analysis of the appropriate bands; (ii) initial compound concentration effective in inhibiting cell growth of infected cultures by 50% after 4 weeks of culture (50% growth effective concentration [gEC50]); (iii) initial compound concentration required to inhibit proliferation of uninfected PBMCs by 20% or 50% after 1 week of culture (20% and 50% proliferation cytotoxic concentration [pCC20 and pCC50, respectively]); (iv) compound concentration required to inhibit metabolic activity of MOLT-3 cells by 20% or 50% in MTS assays (20% and 50% metabolic activity cytotoxic concentration [maCC20 and maCC50, respectively]). Cumulative results from two determinations were used to calculate the initial compound concentration effective in inhibiting by 50% p19 release in supernatants of infected cultures (p19 50% effective concentration [p19EC50]). The p19EC50, gEC50, pCC20, pCC50, maCC20, and maCC50 values were calculated according to the best-fit curve, y value versus log x, where y is the value of the examined function and x is the drug concentration. The 20% inhibitory concentration in addition to the 50% inhibitory concentration was calculated for pCC and maCC values because of the low toxicity of the compounds.
RESULTS
Protection of PCOANs against cell-to-cell transmission of HTLV-1 in vitro.
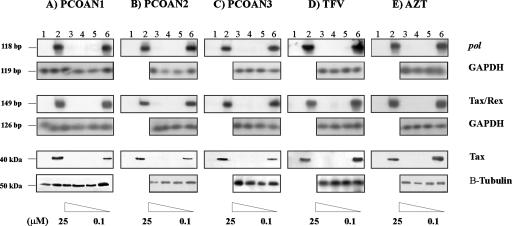
To assay the possible antiviral activity of PCOANs against HTLV-1 infection, three prototype compounds were assayed for their capacity to protect against cell-to-cell transmission of HTLV-1 in vitro. To this purpose, PBMCs from healthy donors were exposed to the virus by cocultivation with lethally irradiated MT-2 cells in the presence of IL-2. Preliminary experiments showed no difference in the antiretroviral activity of PCOANs and AZT regardless of whether they were added to the PBMC cultures overnight prior to infection or immediately before infection. For this reason all experiments shown here refer to PCOANs or AZT added immediately before infection. Conversely, previous studies from our laboratory demonstrated that TFV had to be added to PBMCs some hours prior to contact with MT-2 cells in order to protect against HTLV-1 infection. For this reason, this reference compound was added to PBMCs 16 h prior to infection in all experiments shown. Four weeks p.i., the anti-HTLV-1 protective activity of the compounds was assayed by testing for the presence of HTLV-1 proviral DNA, the expression of viral RNA, and the production of Tax viral protein. Figure 2 shows one representative experiment of the three performed. The results indicate that treatments with concentrations of PCOAN1, PCOAN2, PCOAN3, TFV, or AZT between 25 and 1 μM completely inhibited the presence of proviral DNA, indicated by detection of the pol region by DNA-PCR (Fig. 2, lanes 3 to 5), as well as the expression of viral RNA, evaluated as Tax/Rex expression by RT-PCR (Fig. 2, lanes 3 to 5), and of Tax protein production, measured by Western blotting (Fig. 2, lanes 3 to 5). The uninfected control cells were negative for pol (Fig. 2, lanes 1) while no inhibition was detected in infected cells treated with control vehicle (Fig. 2, lanes 2). When PCOANs or the reference compounds TFV and AZT were added at concentrations of 0.1 μM (Fig. 2, lanes 6), no viral inhibition was observed. The antiviral effects of PCOANs as detected using these assays were thus similar to those exerted by TFV and AZT against HTLV-1 in vitro. The equal presence in the different samples of GAPDH-amplified gene products (Fig. 2A) and of β-tubulin (Fig. 2B to E, lanes 3 to 6) as housekeeping gene controls confirmed that equal amounts of DNA, RNA, and proteins were utilized. The antiretroviral activity of all the compounds was more accurately compared by calculating mean rnaEC100 values through evaluation by densitometry analysis of the appropriate bands in samples from three independent experiments. The limit of detection of our assay is 0.02 pg of total RNA initially extracted from PBMCs efficiently infected by coculture with irradiated MT-2 cells at 4 weeks following infection. This limit of detection corresponds to RNA extracted from 0.005 infected cells. Results confirmed that the inhibitory activities of PCOANs and of the reference compounds TFV and AZT against HTLV-1 infection were comparable (Table 1). However, this assay should not be considered a quantitative one. Thus, inhibition of virus production was also assessed quantitatively through the measurement of HTLV-1 p19 released in the supernatants of infected cells treated with different concentrations of the compounds. The results shown in Table 1 indicate that PCOAN1 inhibited p19 production at a higher level than PCOAN2 and PCOAN3, which were capable of similar levels of anti-HTLV-1 inhibitory activity. In this assay, TFV showed a lower level of anti-HTLV-1 activity than PCOANs.
FIG. 2.
Antiviral effect of PCOAN1, PCOAN2, PCOAN3, TFV, and AZT. The antiviral effect was evaluated 4 weeks after in vitro HTLV-1 infection of human PBMCs from healthy donors and is denoted as detection of proviral DNA (pol), viral RNA (Tax/Rex), and protein expression (Tax). Lanes for all panels of this figure are as follows: lanes 1, uninfected control; lanes 2, HTLV-1-infected human PBMCs treated with control vehicle; lanes 3 to 6, human PBMCs infected in vitro with HTLV-1 through cocultivation with the MT-2 cell line and treated with 25 μM, 5 μM, 1 μM, and 0.1 μM concentrations, respectively, of the antiviral agents, as indicated. The presence of equal levels of DNA or RNA within the samples was demonstrated by the presence of the 119-bp and the 126-bp amplified products of the GAPDH housekeeping gene, respectively (panel A, lanes 1 to 6; panels B to E, lanes 3 to 6). The expression of β-tubulin (20 kDa) as a housekeeping gene product indicated that equal amounts of proteins were assayed (panel A, lanes 1 to 6; panels B to E, lanes 3 to 6). The results refer to one representative experiment of three experiments performed on three different donors with similar results.
TABLE 1.
Antiviral activity and growth inhibition of infected cells
| Compound | rnaEC100 (μM [mean ± SD])a | p19EC50 (μM [Pearson's])b | gEC50 (μM [mean ± SD])c |
|---|---|---|---|
| PCOAN1 | 3.7 ± 2.3 | 8.4 × 10−2 (0.99) | 0.3 ± 0.1 |
| PCOAN2 | 1 ± 0 | 0.23 (0.99) | 0.47 ± 0.2 |
| PCOAN3 | 2.3 ± 2.3 | 0.33 (0.97) | 0.61 ± 0.3 |
| TFV | 2.3 ± 2.3 | 0.73 (1) | 66.5 ± 0.7 |
| AZT | 0.7 ± 0.5 | ND | <0.1 |
The values are derived from the analysis of data from three independent experiments performed with PBMCs from three different donors.
Supernatants were harvested from HTLV-1-infected cultures at 4 weeks p.i. and p19 was detected through an antigen capture assay. Values and Pearson's coefficient (Pearson's) were derived from duplicate samples of one experiment. ND, not determined.
The values were derived after 4 weeks of culture in the presence of IL-2 from the analysis of data from three independent experiments performed with PBMCs from three different donors.
Effects of POCANs on the growth of HTLV-1-infected cells.
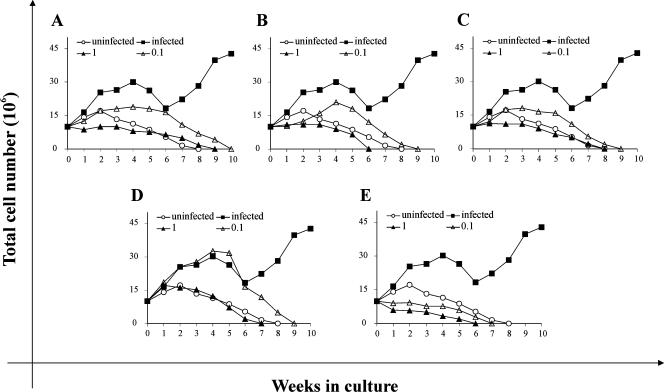
As well as directly assessing various antiviral parameters, we also assayed the effects of PCOANs on cell growth of HTLV-1-infected cells by long-term culturing of cells exposed to the virus. In fact, this is an important parameter to examine for an oncogenic virus such as HTLV-1, since long-term growth is dependent upon continued presence of the virus. Infected cells treated with different concentrations of the compounds or with control vehicle were cultured in the presence of IL-2 and assayed weekly for viable cell number by means of the trypan blue dye exclusion test. The experiment shown in Fig. 3 is representative of the three performed. In the range of 25 μM to 5 μM, the effects of all the compounds tested were similar to those obtained at 1 μM. For this reason Fig. 3 shows only the results obtained with the lower concentrations tested (i.e., 1 μM and 0.1 μM). Infected cells treated with PCOAN1 and PCOAN3 at 1 μM survived until 8 to 9 weeks p.i. (Fig. 3A and C, filled triangles), while cells treated with the same amount of PCOAN2 or TFV and AZT were maintained in culture for no more than 6 to 7 weeks p.i. (Fig. 3B, D, and E, filled triangles). The growth curve of the uninfected control cultures (Fig. 3, open circles) shows that they survived until 8 weeks p.i.; i.e., survival was similar to cultures treated with PCOAN1 and PCOAN3 in the range of 25 μM to 1 μM. Infected cultures treated with PCOANs or TFV at 0.1 μM (Fig. 3A to D, open triangles), i.e., at a concentration that did not fully protect from infection as shown in Fig. 2, survived for no more than 9 to 10 weeks p.i. This indicates that even suboptimal nontoxic concentrations of the phosphonate compounds were able to affect the uncontrolled growth caused by the virus in infected cultures. In fact, infected cultures treated with the control vehicle, after an evident reduction in their growth 4 to 6 weeks p.i., grew rapidly, showing the typical characteristics of cells immortalized by HTLV-1 (Fig. 3, filled squares). Note that compared to PCOANs and TFV, AZT even at 0.1 μM remarkably inhibited the growth of infected cells (Fig. 3E) as well as of uninfected controls, suggesting a higher cytotoxic potential for this compound (see below). The ability of PCOANs, TFV, and AZT to inhibit the growth of infected cells was quantitatively compared by calculating the concentrations of the compounds able to cause 50% growth inhibition in infected cells at 4 weeks p.i., i.e., at the time when the cell number in infected cultures began to decline following treatment. Results are shown in Table 1. There was only a slight difference in the ability of the three PCOANs to inhibit growth of infected cells, as shown by the gEC50 values (Table 1). Conversely, PCOANs were about 100 to 200 times more potent than TFV in inhibiting the growth of infected cells. Moreover, the gEC50 values for PCOANs and TFV were higher than the value for AZT, which exhibited the highest ability to inhibit the growth of infected cells (gEC50 of <0.1).
FIG. 3.
Effects of PCOAN1, PCOAN2, PCOAN3, TFV, and AZT on the growth of HTLV-1-infected cultures. Growth curves of uninfected stimulated PBMCs kept in IL-2 (open circle) are shown for comparison. HTLV-1-infected cultures treated with control vehicle (filled squares) or treated with various concentrations of antiviral agents (filled triangles, 1 μM; open triangles, 0.1 μM): PCOAN1 (A), PCOAN2 (B), PCOAN3 (C), TFV (D), and AZT (E). Each point of the curve represents the mean values of three counts; standard deviations were less than 10%. The results refer to one representative experiment of three experiments performed on three different donors with similar results.
Effect of PCOANs on HTLV-1 RT activity.
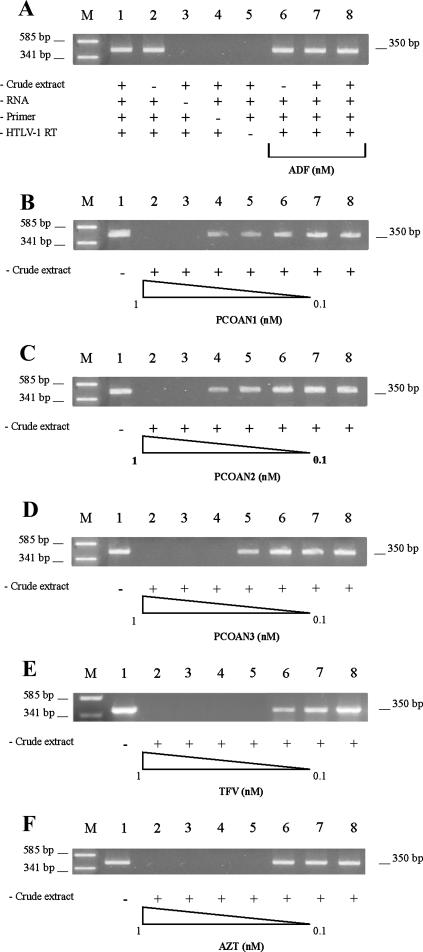
Having demonstrated that PCOANs were able to exert an anti-HTLV-1 antiviral effect in vitro which was at least comparable with that of TFV and AZT, we wanted to obtain further information about their mechanism of action. In fact, based on their structure, the potential target of PCOANs would appear to be reverse transcription of the virus by the HTLV-1-specific RT enzyme. To test this hypothesis specifically, PCOANs, TFV, and AZT were assayed by means of a cell-free RT inhibition assay that we have recently developed. For the general study, viral lysate prepared from the MT-2 supernatant was used as a source of HTLV-1 RT. The tested compounds were first activated through incubation with a cell extract from mitogen-stimulated PBMCs as a kinase source. To obtain information on the phosphorylation efficiency of the adopted system, we compared the inhibitory effect of AZT activated through incubation with the cell extract from mitogen-stimulated PBMCs with that of a commercially available triphosphate-AZT. The inhibitory activity toward HTLV-1 RT of AZT subjected to PBMC extract activation was about 10 times lower than that of an equal quantity of triphosphate-AZT (data not shown). Figure 4A shows a series of control samples used in order to validate our assay. First, we demonstrated that preincubation with the cell-free crude extract from mitogen-stimulated PBMCs did not affect cDNA formation and detection (Fig. 4A, lanes 1 and 2). In addition, as expected, subtraction of template RNA (lane 3), the primer pair (lane 4), or HTLV-1 RT (lane 5) prevented cDNA generation, as shown by the absence of the amplified product. Moreover, as a negative control, we assayed the activity of adefovir (ADF), a nucleoside compound lacking the hydroxymethyl group in the furanose ring (5) and consequently unable to act as an RT inhibitor chain terminator. In fact, (5′S)-5-fluoro-1-isoxazolidin-5-yl-1H-pyrimidine-2,4-dione was completely unable to inhibit cDNA generation either at 100 nM without crude extract activation (Fig. 4A, lane 6) or at 100 or 10 nM following crude extract activation (Fig. 4A, lanes 7 and 8). To investigate in detail the dose response of HTLV-1 RT inhibition for each compound, we used concentrations ranging from 1 to 0.1 nM following crude extract activation, based on previous experience with TFV and AZT and preliminary experiments with PCOANs. Results are shown in Fig. 4. Panels B to F show that nonactivated (in absence of crude extract) compounds, even at a concentration of 10 nM (lanes 1), were not able to inhibit cDNA generation. In fact, the 350-bp amplification product in lane 1 is similar to that detected in a complete reaction mixture control, with the addition of control vehicle (Fig. 4B to F, lanes 8). Conversely, preactivated PCOAN1 and PCOAN2 at 1 and 0.8 nM inhibited cDNA elongation completely (Fig. 4B and C, lanes 2 and 3), while at concentrations ranging from 0.6 nM to 0.1 nM (Fig. B and C, lanes 4 to 7), neither was inhibitory. PCOAN3 completely inhibited cDNA generation at 1, 0.8, and 0.6 nM (Fig. 4D, lanes 2 to 4), while it did not exert any effect at lower concentrations (lanes 5 to 7). Both activated TFV and AZT inhibited cDNA at concentrations of 1, 0.8, 0.6, and 0.4 nM (Fig. 4E and F, lanes 2 to 5), while they were not effective at 0.2 and 0.1 nM (lanes 6 and 7). No compound tested in this present study was able to directly inhibit Taq polymerase enzymatic activity when it was added to the PCR mixture immediately before the amplification reaction at concentrations as high as 10 μM (data not shown), confirming that the action of PCOANs was directed toward HTLV-1 RT activity and not toward the ensuing amplification phase.
FIG. 4.
Effects of PCOAN1, PCOAN2, PCOAN3, TFV, and AZT on HTLV-1 RT activity evaluated in vitro by means of a cell-free assay. (A) Panel of controls. RT-PCR assay visualized on agarose gel on control samples without the addition of the compounds (lanes 1 to 5) or with ADF (lanes 6 to 8) as an RT inhibition-negative reference compound. As indicated below the lanes, PCRs were performed with (+) or without (−) crude extract, RNA template of HSV-1, specific primer pairs, and HTLV-1 RT. In addition, lanes 6 to 8 included ADF in concentrations of 100 nM (lanes 6 and 7) and 10 nM (lane 8). (B to F) Dose-response assay. RT-PCR products of samples from cultures treated with control vehicle or different concentrations of PCOAN1, PCOAN2, PCOAN3, TFV, or AZT with or without activation follow ing exposure to crude extract. Lanes 1, no crude extract, RNA template of HSV-1, specific primer pairs, HTLV-1 RT, and a 100 nM concentration of the respective antiviral agent, as indicated on the figure. Lanes 2 to 7 contain crude extract, RNA template of HSV-1, specific primer pairs, HTLV-1 RT, and various concentrations of the respective antiviral agent (indicated on the figure) as follows: 1 nM (lanes 2), 0.8 nM (lanes 3), 0.6 nM (lanes 4), 0.4 nM (lanes 5), 0.2 nM (lanes 6), and 0.1 nM (lanes 7). Lanes 8, crude extract, RNA template of HSV-1, specific primer pairs, HTLV-1 RT, and control vehicle; lanes M, plasmid pUC18 DNA DpnI digest. The results refer to one representative experiment of three independent experiments with similar results.
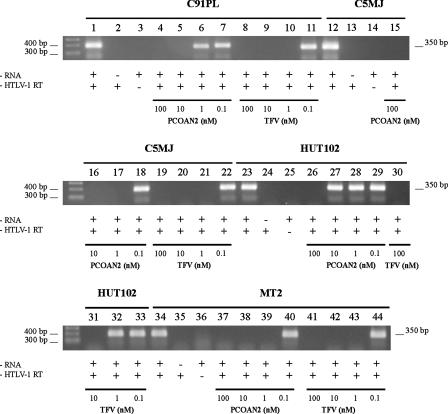
Having demonstrated that PCOANs were able to inhibit the enzymatic activity of HTLV-1 RT from the MT-2 cell line, we next wanted to investigate whether RTs from other HTLV-1-transformed cell lines, established following HTLV-1 infection in vitro or from HTLV-1-infected individuals, would be sensitive to the treatment with these compounds. To this purpose we decided to carry on a comparative study using chronically infected C91/PL, C5/MJ, HUT 102, and MT-2 HTLV-1-producing cell lines as a source of RT supernatants. In these experiments, PCOAN2 and TFV were added to the RT inhibition assay at concentrations ranging from 100 nM to 0.1 nM. The results shown in Fig. 5, which refer to the gels as they were originally run, show that RTs from the different HTLV-1-transformed cell lines were sensitive to PCOAN2 and to TFV to various degrees. In fact, PCOAN2 inhibited the RT activity in supernatants from C91/PL at the concentrations of 100 nM and 10 nM (lanes 4 and 5), while it shut down C5/MJ RT activity and also that of MT-2 at 1 nM (lanes 17 and 39). Conversely, RT from HUT102 cells was inhibited by PCOAN2 only at a concentration of 100 nM (lane 26). TFV inhibited RT from C91/PL, C5/MJ, and MT-2 at 100 nM, 10 nM, and 1 nM (lanes 8, 9, 10, 19, 20, 21, 41, 42, and 43), while RT from HUT102 was inhibited at 100 nM and 10 nM (lanes 30 and 31). Conversely, both PCOAN2 and TFV were unable to inhibit the RTs from the various cell lines at a concentration of 0.1 nM (lanes 7, 11, 18, 22, 29, 33, 40, and 44). Thus, even if RTs from different cell lines showed different thresholds of sensitivity to inhibition by both PCOAN2 and TFV, the results obtained overall reflect those obtained with RT from MT-2 cells.
FIG. 5.
Effects of PCOAN2 and TFV on the enzymatic activity of HTLV-1-RT from different HTLV-1-producing cell lines, evaluated by means of a cell-free assay. RT-PCRs were carried out in the presence of an RNA template, control vehicle, and HTLV-1 RT (+RNA and +HTLV-1 RT; lanes 1, 12, 23, and 34) from various cell lines, as indicated above the lanes. RT-PCRs were carried out in the absence of RNA template (−RNA; lanes 2, 13, 24, and 35) or in the absence of RTs (−HTLV-1 RT; lanes 3, 14, 25, and 36). RT-PCRs were carried out in the presence of RTs from the indicated cell lines with various concentrations of PCOAN2 and TFV. Concentrations and antiviral agent are identified below the lanes. The results refer to one of two experiments performed with similar results.
Toxicity of the compounds on uninfected cells.
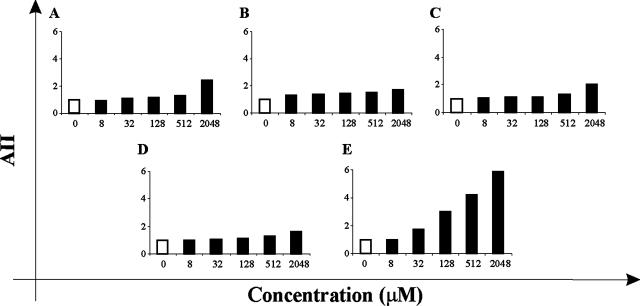
In order to assess levels of toxicity of PCOANs toward uninfected cells, their effects on apoptotic cell death, on cell proliferation, and on cell metabolic activity were tested in uninfected PBMCs and lymphoid MOLT-3 cells. For detection of apoptosis, PBMCs from healthy donors were either untreated or treated with the different compounds at concentrations ranging from 8 μM to 2,048 μM in the presence of IL-2. After 72 h, apoptosis was evaluated by flow cytometry analysis and expressed as a percentage of hypodiploid nuclei. The results are shown in Fig. 6 and are represented as a mean apoptosis induction index (AII) calculated from the cumulative results obtained in three experiments. AII for each sample was calculated as the relative increase (n-fold) in the percentage of hypodiploid nuclei with respect to the mean percentage hypodiploid nuclei from untreated control samples. Both PCOAN1 (Fig. 6A) and PCOAN3 (Fig. 6C) were very slightly apoptotic, since only at the concentration of 2,048 μM were their AII values twice as high as those of the untreated control. PCOAN2 (Fig. 6B) induced an even lower apoptotic response with respect to that detected following treatments with PCOAN1 and PCOAN3. TFV induced an apoptotic response similar to that of PCOAN2, with values of apoptosis slightly higher than that of PCOAN2 at low concentrations (Fig. 6D). Conversely, AZT was found to induce a significantly higher level of apoptosis in a dose-response fashion, in comparison to both PCOANs and TFV, at concentrations ranging from 32 to 2,048 μM (Fig. 6E). PCOANs were also investigated for their effects on the proliferation of uninfected cells by means of a [3H]thymidine incorporation assay. Uninfected PBMCs were treated with the compounds at concentrations ranging from 6.25 to 800 μM in the presence of IL-2, and after 7 days the [3H]thymidine incorporation was assayed. Results are shown in Table 2. The ability to inhibit cell proliferation of uninfected PBMCs was calculated and expressed as pCC20 or pCC50. Values were calculated from data obtained in three independent experiments using PBMCs from three different donors. PCOAN1 showed a pCC20 slightly, but not significantly, lower than that of TFV (44.9 ± 27 versus 63.2 ± 16.3), while PCOAN2 showed pCC20 values four to five times higher than those of both PCOAN1 and TFV (298.7 ± 46.8). Of the PCOANs, PCOAN3 showed the highest proliferation inhibitory activity on uninfected cells, with the lowest pCC20 value (17.2 ± 9.6). Moreover, compared to PCOANs and TFV, AZT showed the highest antiproliferative activity (pCC20 value of 3.62 ± 2) on uninfected cells. Due to their low antiproliferative effects, PCOAN1, PCOAN2, and TFV had pCC50 values above the highest concentration assayed, and the pCC50 of PCOAN3 was much above its antiviral concentration; AZT showed the more potent antiproliferative action also using this comparative parameter (Table 2). The cytotoxic effect of the PCOANs on uninfected cells was also measured by assaying inhibition of oxygen burst using the MTS assay in the lymphoid cell line MOLT-3. The results in Table 2 are the means of three experiments and show that the inhibitory effect of PCOANs on cell metabolic activity in MOLT-3 cells was greater than that of TFV but, in any case, extremely low, with maCC50 values about 1,000 times higher than the anti-HTLV-1 EC50 (p19EC50 and gEC50) (Table 1) values. Also in this case, AZT showed the highest cytotoxic effect in comparison with phosphonate compounds.
FIG. 6.
Effect of PCOANs on apoptosis in uninfected human PBMCs. Apoptosis was evaluated as a percentage hypodyploid nuclei by flow cytometry analysis and expressed as the AII (relative increase in apoptosis with respect to control) in PBMCs cultured with control vehicle (open bars; AII of 1) or treated with the indicated concentrations (solid bars) of PCOAN1 (A), PCOAN2 (B), PCOAN3 (C), TFV (D), and AZT (E). The results represent the mean values of three experiments performed using PBMCs from three different healthy donors (standard deviations were <10%).
TABLE 2.
Cytotoxicity of the compounds on uninfected cells
| Compound | pCC20 (μM [mean ± SD])a | pCC50 (μM [mean ± SD])a | maCC20 (μM [mean ± SD])b | maCC50 (μM [mean ± SD])b |
|---|---|---|---|---|
| PCOAN1 | 44.9 ± 27 | >800 | 480 ± 110 | >103 |
| PCOAN2 | 298.7 ± 46.8 | >800 | 470 ± 112 | >103 |
| PCOAN3 | 17.2 ± 9.6 | 303.8 ± 13.4 | 220 ± 13 | 875 ± 125 |
| TFV | 63.2 ± 16.3 | >800 | >103 | >103 |
| AZT | 3.62 ± 2 | 51.6 ± 9.7 | 10 ± 8.1 | 76.2 ± 15.3 |
Values were determined after 7 days in cultures with IL-2 from [H]thymidine incorporation assay. The values were derived from three independent experiments performed with PBMCs from three different donors.
Values were derived from three independent MTS assays in MOLT-3 cells.
DISCUSSION
In the past, lack of clear evidence that horizontal transmission of HTLV-1 contributes to maintaining or, indeed, increasing the viral burden in infected patients discouraged the use of antiviral agents for the control of HTLV-1-associated diseases. In fact, the principle that HTLV-1 survived and spread within the host exclusively through proliferation of infected cells negated a rationale for such a therapeutic approach. However, recent advances in HTLV-1 biology, particularly those demonstrating mechanisms involved in direct cell-to-cell transmission of the virus, together with partial results obtained in clinical studies involving antiviral agents for the treatment of HTLV-1-associated diseases, give new impulse to studies aimed at identifying compounds able to efficiently inhibit the HTLV-1 replication cycle. In fact, the overall number of potential antiviral compounds that have been tested on HTLV-1-infection in vitro remains limited. Of the few compounds that have actually been tested, IFN, AZT, some anti-HIV RT nucleoside analogues, and TFV were found by us and other authors to be active toward HTLV-1 infection in vitro (2, 15, 17, 24). Conversely, at least two studies have shown that a nucleoside analogue, such as lamivudine, known for its ability to control HIV infection, was not as effective toward HTLV-1 infection in vitro, probably due to some natural resistance (12, 40). Similarly, none of the inhibitors designed to act against the HIV-1 proteinases was able to efficiently inhibit Gag processing in HTLV-1-infected cells (31).
Lessons learned from studies on anti-HIV chemotherapy suggest that the development of an optimal antiretroviral therapeutic intervention should rely on a wide range of potentially active agents. This would allow us to select and utilize the antiviral agents in ideal combinations that are most efficient in counteracting the specific infectious strain affecting each individual patient. Thus, considering that only a few antiretroviral compounds have been tested in vitro against HTLV-1 and that some results indicate that compounds able to control HIV infection could be inefficient in controlling HTLV-1 infection, this report contains some interesting novel findings. It is the first time, to our knowledge, that a new family of compounds with RT-inhibitory potential has been assayed for its ability to provide protection from HTLV-1 infection, regardless of whether it showed anti-HIV activity. Whether PCOANs also exert anti-HIV activity is not known and will be a topic of research in future studies. Moreover, HTLV-1 infection of PBMCs, unlike HIV infection, stimulates the growth and eventually immortalizes the infected cells without causing a rapid cytopathic effect. This allowed us to perform an overall evaluation of the efficacy of the compounds, taking into account not only their presumable HTLV-1 RT-inhibitory activity but also their effects on the growth of cells exposed to HTLV-1. In fact, the ideal drug to counteract HTLV-1 infection should be able both to inhibit virus transmission and to act on infected provirus-carrying cells by interfering with mechanisms involved in cell cycle and transcription. The present study shows that PCOANs are able to impair HTLV-1 infection, with a potency comparable to TFV or AZT in protecting cell-to cell viral transmission. The RT-inhibitory activity was not identical for all the PCOANs tested. In fact, PCOAN3 showed higher activity than either PCOAN1 or PCOAN2, as revealed by our cell-free assay. Nevertheless, the ability of all three PCOANs to inhibit growth of infected cells after 4 weeks in culture was 100- to 200-fold greater than that of TFV and eventually caused exhaustion of cell growth in HTLV-1-immortalized cell lines even at low concentrations. This did not appear to be due to a greater ability to protect cells from infection, since PCOANs and TFV inhibited HTLV-1 transmission to PBMCs equally and since the capacity of PCOANs to inhibit HTLV-1-infected cell growth was also shown at a concentration of 0.1 μM, when none of them were able to completely protect PBMCs from HTLV-1 cell-to-cell transmission. In addition, this effect was not directly attributable to the PCOANs' having higher cytotoxicity than TFV, as shown by the lack of correlation between inhibition of HTLV-1-infected cell growth and their capability to inhibit the proliferation or to induce apoptosis of uninfected PBMCs at different concentrations. Conversely, the high capacity of AZT to control the growth of infected cells correlates to its higher cytotoxic potential. Taken all together, these data suggest that, in addition to inhibition of RT activity, unidentified mechanisms could account for the effect of PCOANs on HTLV-1-infected cells. Indeed, PCOANs could act on factors controlling cell proliferation/survival versus cell death that are selectively altered in HTLV-1-infected cells. A number of studies have shown that HTLV-1 infection promotes the expression of different cytokines, their receptors, and factors involved in the cell cycle (18, 20, 23, 34). Among these factors, several authors focused their attention on IL-2/IL-2-receptor and on the role of chronic activation of the inducible transcription factor NF-κB in the process of HTLV-1-driven leukemogenesis (11, 35, 36). Inhibition of IL-2 mRNA transcription by PCOANs, as quantitatively detected by real-time PCR, correlated closely with their antiviral activity (data not shown), suggesting that the anti-HTLV-1 activity of PCOANs cannot be directly ascribed to IL-2 down-modulation. Moreover, preliminary experiments did not show any change in NF-κB activation in uninfected MOLT-3 cells following treatment with PCOANs at antiviral concentrations (data not shown). However, MOLT-3 lymphoid cells do not show constitutive NF-κB activation as HTLV-1-infected cells do. As a consequence, we cannot exclude the possibility that PCOANs reduce the uncontrolled proliferation induced by the virus by acting selectively on HTLV-1 infected cells in a way similar to the negative regulators of the NF-κB system (32). Thus, the effects of PCOANs on the IL-2/IL-2 receptor autocrine loop or on NF-κB activation and the roles of these effects in the anti-HTLV-1 activity of PCOANs need further investigation.
Interestingly, our data showing the effects of PCOANs and TFV on RTs from different HTLV-1-infected cell lines emphasize the fact that, although HTLV-1, unlike other retroviruses, is characterized by low genetic variability, sensitivity of HTLV-1 RT of different origins to the inhibitory activity of an NRTI compound could vary greatly. However, a recent study on the pol genes and deduced amino acid sequences from primary isolates and proviruses from cell lines revealed some genetic differences in the RT-encoding regions (29). Among these differences, the HUT102 cell line, established from an HTLV-1-infected individual, presented two threonine residue variations at positions 197 and 429, in place of the consensus sequence of MT-2, although viruses from these cell lines are classified as belonging to the same cosmopolitan transcontinental subgroup A. This difference could account for the lower sensitivity of RT from HUT102 cells to the inhibitory activity of both PCOAN2 and TFV, confirming at the same time the high degree of sensitivity and accuracy of our cell-free assay. Nevertheless, this result highlights the importance of a wider choice of RT-inhibitory drugs for the treatment of patients infected with HTLV-1 and, possibly, the benefit of an assay to predict in vitro the sensitivity of the specific virus strain of the patients to the different compounds. Studies are currently in progress in our laboratory to develop such an assay.
In conclusion, the results reported in the present study demonstrate that some prototype PCOANs exert potent anti-HTLV-1 activity in vitro. These results could be the basis for future studies aimed at designing compounds capable of ensuring long-lasting control of HTLV-1 infection by keeping virus transmission at a low level through their effect on RT activity and by interfering with mechanisms involved in cell activation and proliferation. Results of these studies will allow us to understand the feasibility of using PCOANs for the treatment of HTLV-1-associated diseases.
Acknowledgments
This work was supported by grants from the Italian Ministry of University and Research, Research Projects of National Interest (to B. Macchi); from the Istituto Superiore di Sanità (AIDS Project); from the University of Rome Tor Vergata (B. Macchi); and from the University of Messina (A. Mastino).
We thank Alison Inglis for her linguistic assistance.
Footnotes
Published ahead of print on 29 October 2007.
REFERENCES
- 1.Balestrieri, E., G. Forte, C. Matteucci, A. Mastino, and B. Macchi. 2002. Effect of lamivudine on transmission of human T-cell lymphotropic virus type 1 to adult peripheral blood mononuclear cells in vitro. Antimicrob. Agents Chemother. 46:3080-3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balestrieri, E., M. T. Sciortino, A. Mastino, and B. Macchi. 2005. Protective effect of the acyclic nucleoside phosphonate tenofovir toward human T-cell leukemia/lymphotropic virus type 1 infection of human peripheral blood mononuclear cells in vitro. Antiv. Res. 68:154-162. [DOI] [PubMed] [Google Scholar]
- 3.Barnard, A. L., T. Igakura, Y. Tanaka, G. P. Taylor, and C. R. Bangham. 2005. Engagement of specific T-cell surface molecules regulates cytoskeletal polarization in HTLV-1-infected lymphocytes. Blood 106:988-995. [DOI] [PubMed] [Google Scholar]
- 4.Bazarbachi, A., D. Ghez, Y. Lepelletier, R. Nasr, H. de The, M. E. El-Sabban, and O. Hermine. 2004. New therapeutic approaches for adult T-cell leukaemia. Lancet Oncol. 5:664-672. [DOI] [PubMed] [Google Scholar]
- 5.Chiacchio, U., A. Corsaro, D. Iannazzo, A. Piperno, V. Pistarà, A. Rescifina, R. Romeo, V. Valveri, A. Mastino, and G. Romeo. 2003. Enantioselective syntheses and cytotoxicity of N,O-nucleosides. J. Med. Chem. 46:3696-3702. [DOI] [PubMed] [Google Scholar]
- 6.Chiacchio, U., E. Balestrieri, B. Macchi, D. Iannazzo, A. Piperno, A. Rescifina, R. Romeo, M. Saglimbeni, M. T. Sciortino, V. Valveri, A. Mastino, and G. Romeo. 2005. Synthesis of phosphonated carbocyclic 2′-oxa-3′-aza-nucleosides: novel inhibitors of reverse transcriptase. J. Med. Chem. 48:1389-1394. [DOI] [PubMed] [Google Scholar]
- 7.Chiacchio, U., A. Rescifina, D. Iannazzo, A. Piperno, R. Romeo, L. Borrello, M. T. Sciortino, E. Balestrieri, B. Macchi, A. Mastino, and G. Romeo. 2007. Phosphonated carbocyclic 2′-oxa-3′-aza-nucleosides as new antiretroviral agents. J. Med. Chem. 50:3747-3750. [DOI] [PubMed] [Google Scholar]
- 8.De Clercq, E., and A. Holy. 2005. Acyclic nucleoside phosphonates: a key class of antiviral drugs. Nat. Rev. Drug Discov. 4:928-940. [DOI] [PubMed] [Google Scholar]
- 9.Deeks, S. G., P. Barditch-Crovo, P. S. Lietman, F. Hwang, K. C. Cundy, J. F. Rooney, N. S. Hellmann, S. Safrin, and J. O. Kahn. 1998. Safety, pharmacokinetics and antiretroviral activity of intravenous 9-[2-(R)-phosphonomethoxy)propyl] adenine, a novel anti-human immunodeficiency virus (HIV) therapy, in HIV-infected adults. Antimicrob. Agents Chemother. 42:2380-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edlich, R. F., J. A. Arnett, and F. M. Williams. 2000. Global epidemic of human T-cell lymphotropic virus type-I (HTLV-1). J. Emerg. Med. 18:109-119. [DOI] [PubMed] [Google Scholar]
- 11.Franchini, G., C. Nicot, and J. M. Johnson. 2003. Seizing of T cells by human T-cell leukaemia/lymphoma virus type 1. Adv. Cancer Res. 89:69-132. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Lerma, J. G., S. Nidtha, and W. Heneine. 2001. Susceptibility of human T cell leukemia virus type 1 to reverse transcriptase inhibitors: evidence for resistance to lamivudine. J. Infect. Dis. 184:507-510. [DOI] [PubMed] [Google Scholar]
- 13.Goldschmidt, V., and R. Marquet. 2004. Primer unblocking by HIV-1 reverse transcriptase and resistance to nucleoside RT inhibitors (NRTIs). Int. J. Biochem. Cell Biol. 36:1687-1705. [DOI] [PubMed] [Google Scholar]
- 14.Heijtink, R. A., G. A. Kruining, G. A. De Wilde, J. Balzarini, E. De Clerq, and S. W. Schalm. 1994. Inhibitory effects of acyclic nucleoside phosphonates on human hepatitis B virus and duck hepatitis B virus infections in tissue culture. Antimicrob. Agents Chemother. 38:2180-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hermine, O., D. Bouscary, and A. Gessain. 1995. Brief report: treatment of adult T-cell leukemia-lymphoma with zidovudine and interferon alpha. N. Engl. J. Med. 332:1749-1751. [DOI] [PubMed] [Google Scholar]
- 16.Hermine, O., H. Dombret, J. Poupon, B. Arnulf, F. Lefrere, P. Rousselot, and G. Damaj. 2004. Phase III trial of arsenic trioxide and alpha interferon in patients with relapsed/refractory adult T-cell leukemia/lymphoma. Hematol. J. 5:130-134. [DOI] [PubMed] [Google Scholar]
- 17.Hill, S. A., P. A. Lloyd, S. McDonald, J. Wykoff, and D. Derse. 2003. Susceptibility of human T cell leukaemia virus type I to nucleoside reverse transcriptase inhibitors. J. Infect. Dis. 188:424-427. [DOI] [PubMed] [Google Scholar]
- 18.Horiuchi, S., N. Yamamoto, M. Z. Dewan, Y. Takahashi, A. Yamashita, T. Yoshida, M. A. Nowell, P. J. Richards, S. A. Jones, and N. Yamamoto. 2006. Shedding of soluble IL-6R and activation of STAT3 signalling. Int. J. Cancer 119:823-830. [DOI] [PubMed] [Google Scholar]
- 19.Igakura, T., J. C. Stinchcombe, P. K. Goon, G. P. Taylor, J. N. Weber, G. M. Griffiths, Y. Tanaka, M. Osame, and C. R. Bangham. 2003. Spread of HTLV-I between lymphocytes by virus-induced polarization of the cytoskeleton. Science 299:1713-1716. [DOI] [PubMed] [Google Scholar]
- 20.Jeang, K. T., C. Z. Giam, F. Majone, and M. Aboud. 2004. Life, death, and tax: role of HTLV-1 oncoprotein in genetic instability and cellular transformation. J. Biol. Chem. 279:31991-31994. [DOI] [PubMed] [Google Scholar]
- 21.Jolly, C., and Q. J. Sattentau. 2004. Retroviral spread by induction of virological synapses. Traffic 5:643-650. [DOI] [PubMed] [Google Scholar]
- 22.Kinoshita, T., S. Masanori, K. Tobinai, M. Ito, S. Ito, S. Ikeda, K. Tajima, K. Shimotohno, and T. Sugimura. 1989. Detection of mRNA for the tax1/Rex1 gene of human T-cell leukemia virus type I in fresh peripheral blood mononuclear cells of adult T-cell leukemia patients and viral carriers by using polymerase chain reaction. Proc. Natl. Acad. Sci. USA 86:5620-5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Macchi, B., S. Grelli, C. Matteucci, M. M. D' Elios, M. De Carli, C. Favalli, G. Del Prete, and A. Mastino. 1998. Human Th1 and Th2 cell clones are equally susceptible to infection and immortalization by human T-lymphotropic virus type 1. J. Gen. Virol. 79:2469-2474. [DOI] [PubMed] [Google Scholar]
- 24.Macchi, B., I. Faraoni, J. Zhang, S. Grelli, C. Favalli, A. Mastino, and E. Bonmassar. 1997. AZT inhibits the transmission of human T cell leukaemia/lymphoma virus type I to adult peripheral blood mononuclear cells in vitro. J. Gen. Virol. 78:1007-1016. [DOI] [PubMed] [Google Scholar]
- 25.Machuca, A., and V. Soriano. 2000. In vivo fluctuation of HTLV-1 and HTLV-1I proviral load in patients receiving antiretroviral drugs. J. Acquir. Immune. Defic. Syndr. 24:189-193. [DOI] [PubMed] [Google Scholar]
- 26.Matsuoka, M., and K. T. Jeang. 2007. Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nat. Rev. Cancer 7:270-280. [DOI] [PubMed] [Google Scholar]
- 27.Matteucci, C., S. Grelli, E. De Smaele, C. Fontana, and A. Mastino. 1999. Identification of nuclei from apoptotic, necrotic and viable lymphoid cells by using multiparameter flow cytometry. Cytometry 35:145-153. [DOI] [PubMed] [Google Scholar]
- 28.Medici, M. A., M. T. Sciortino, D. Perri, C. Amici, E. Avitabile, M. Ciotti, E. Balestrieri, E. De Smaele, G. Franzoso, and A. Mastino. 2003. Protection by herpes simplex virus glycoprotein D against Fas-mediated apoptosis: role of nuclear factor kappa-B. J. Biol. Chem. 278:36059-36067. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell, M. S., E. T. Bodine, S. Hill, G. Princler, P. Lloyd, H. Mitsuya, M. Matsuoka, and D. Derse. 2007. Phenotypic and genotypic comparisons of human T-cell leukemia virus type 1 reverse transcriptases from infected T-cell lines and patient samples. J. Virol. 81:4422-4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miyoshi, I., I. Kubonishi, S. Yoshimoto, T. Akagi, Y. Ohtsuki, Y. Shirashi, K. Nagata, and Y. Hinuma. 1981. Type C virus particles in a cord T-cell line derived by cocultivating normal human cord leucocytes and human leukemic cells. Nature 294:770-771. [DOI] [PubMed] [Google Scholar]
- 31.Pettit, S. C., R. Sanchez, T. Smith, R. Wehbie, D. Derse, and R. Swanstrom. 1998. HIV type 1 protease inhibitors fail to inhibit HTLV-I Gag processing in infected cells. AIDS Res. Hum. Retrovir. 14:1007-1014. [DOI] [PubMed] [Google Scholar]
- 32.Sanda, T., K. Asamitsu, H. Ogura, S. Ita, A. Utsunomiya, R. Ueda, and T. Okamoto. 2006. Induction of cell death in adult T-cell leukemia cells by a novel IκB kinase inhibitor. Leukemia 20:590-598. [DOI] [PubMed] [Google Scholar]
- 33.Sheremata, W. A., D. Benedict, D. C. Squilacote, A. Sazant, and E. DeFreitas. 1993. High-dose zidovudine induction in HTLV-I-associated myelopathy: safety and possible efficacy. Neurology 43:2125-2129. [DOI] [PubMed] [Google Scholar]
- 34.Siekevitz, M. M. Feinberg, M. Holbrook, F. Wong-Staal, and W. C. Greene. 1987. Activation of Interleukin 2 and interleukin 2-receptor (tac) promoter expression by transactivation (tat) gene product of human T-cell leukemia virus type 1. Proc. Natl. Acad. Sci. USA 84:5389-5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun, S. C., and D. W. Ballard. 1999. Persistent activation of NF-kappaB by the tax transforming protein of HTLV-1: hijacking cellular IκB kinases. Oncogene 18:6948-6958. [DOI] [PubMed] [Google Scholar]
- 36.Sun, S. C., and S. Yamaoka. 2005. Activation of NF-κB by HTLV-1 and implications for cell transformation. Oncogene 24:5952-5964. [DOI] [PubMed] [Google Scholar]
- 37.Takenouchi, N., K. S. Jones, I. Lisinski K. Fugo, K. Yao, S. W. Cushman, F. W. Ruscetti, and S. Jacobson. 2007. GLUT1 is not the primary binding receptor but is associated with cell-to-cell transmission of human T-cell leukemia virus type 1. J. Virol. 81:1506-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor, G. P., S. E. Hall, S. Navarrete, C. A. Michie, R. Davis, A. D. Witkover, M. Rossor, M. A. Nowak, P. Rudge, E. Matutes, C. R. Bangham, and J. N. Weber. 1999. Effect of lamivudine on human T-cell leukemia virus type I (HTLV-1) DNA copy number, T-cell phenotype, and anti-Tax cytotoxic T-cell frequency in patients with HTLV-1-associated myelopathy. J. Virol. 73:10289-10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tobinai, K., M. Shimoyama, S. Inoue, S. Takayasu, C. Mikuni, M. Kozuru, S. Oda, H. Nakajima, et al. 1992. Phase I study of YK-176 (2′-deoxycoformycin) in patients with adult T-cell leukemia-lymphoma. Jpn. J. Clin. Oncol. 22:164-171. [PubMed] [Google Scholar]
- 40.Toro, C., B. Rodes, C. de Mendoza, and V. Soriano. 2003. Lamivudine resistance in human T-cell leukemia virus type 1 may be due to a polymorphism at codon 118 (V-I) of the reverse transcriptase. Antimicrob. Agents Chemother. 47:1774-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsuda, H., K. Takatsuki, R. Ohno, T. Masaoka, K. Okada, S. Shirakawa, Y. Ohashi, and K. Ota. 1994. Treatment of adult T-cell leukaemia-limphoma with irinotecano hydrochloride (CP11). Br. J. Cancer 70:771-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uike, N., I. Choi, A. Tokoro, T. Goto, Y. Yufu, M. Kozuru, and K. Tobinai. 1998. Adult T-cell leukemia-lymphoma successfully treated with 2-chlorodeoxyadenosine. Intern. Med. 37:411-413. [DOI] [PubMed] [Google Scholar]
- 43.Yoshida, M. 2005. Discovery of HTLV-1, the first human retrovirus, its unique regulatory mechanisms, and insight into pathogenesis. Oncogene 24:5931-5937. [DOI] [PubMed] [Google Scholar]