Abstract
For the first time, mosaic tetracycline resistance genes were identified in Lactobacillus johnsonii and in Bifidobacterium thermophilum strains. The L. johnsonii strain investigated contains a complex hybrid gene, tet(O/W/32/O/W/O), whereas the five bifidobacterial strains possess two different mosaic tet genes: i.e., tet(W/32/O) and tet(O/W). As reported by others, the crossover points of the mosaic tet gene segments were found at similar positions within the genes, suggesting a hot spot for recombination. Analysis of the sequences flanking these genes revealed that the upstream part corresponds to the 5′ end of the mosaic open reading frame. In contrast, the downstream region was shown to be more variable. Surprisingly, in one of the B. thermophilum strains a third tet determinant was identified, coding for the efflux pump Tet(L).
Tetracycline is an antimicrobial agent that is active against a wide range of gram-positive as well as gram-negative bacteria. Consequently, it is used therapeutically in the treatment of various infections in both humans and animals. Furthermore, it was also often applied as a growth promoter in several countries inside and outside Europe (7). However, since the European Union ban on antibiotics as growth promoters, tetracycline has been used primarily as a therapeutic antimicrobial. In comparison to other antibiotics like trimethoprim/sulfonamides and macrolides, sales of tetracycline have increased the most since the prohibition in 1999 (5). Therefore, it is not surprising that tetracycline-resistant bacteria are very prevalent in all kinds of habitats: e.g., oral cavities, soils, and intestinal tracts of humans and animals (1, 7). Resistance to tetracycline can be mediated by different mechanisms: the most common are efflux pumps, ribosome protection proteins, and enzymatic inactivation (23).
New tet genes keep appearing in literature: for example, very recently tet(41) was reported (27). Currently 40 different tetracycline resistance genes (including oxytetracycline-resistant determinants) have been identified (7, 22, 23). Furthermore, during the last decade also mosaic tet genes, primarily encoding for ribosomal protection proteins (RPP), have been characterized in different bacterial species. For instance, intraclass mosaic structures have been described in tet(M) (10, 21). Moreover, multiple interclass hybrid genes originating from tet(O) and tet(W) have been discovered in Megasphaera elsdenii (24, 25) and a tet(O/32/O) gene was identified in Clostridium strain K10, although initially described as tet(32) (18, 25). It remains unclear what the actual role of these interclass RPP mosaic genes is. It has been suggested that these hybrid genes might be restricted to only a very small group of (anaerobic) bacteria (23); however, recently they have been shown to be widespread and abundant (22).
In a recent study, tetracycline-resistant Bifidobacterium thermophilum strains from animal sources have been described (17). PCR analysis revealed the possible presence of both tet(O) and tet(W) in some of the strains analyzed. In this study, these strains, together with Lactobacillus johnsonii L0077, a strain from human intestine which was also suspected to harbor a mosaic gene in a previous survey (2), were analyzed in more detail to investigate the potential presence of hybrid genes composed of tet(O) and tet(W) segments.
MATERIALS AND METHODS
Bacterial strains and DNA isolation.
The strains used in this study are indicated in Table 1. The bifidobacteria were grown in brain heart infusion broth containing 0.05% cysteine-HCl. The Lactobacillus johnsonii L0077 strain was grown in MRS broth supplemented with 0.03% cysteine-HCl. Both were incubated in an anaerobic chamber at 37°C for 48 h. DNA was isolated using the Wizard Genomic DNA isolation kit according to the manufacturer's protocol for gram-positive bacteria (Promega Benelux, Leiden, The Netherlands).
TABLE 1.
Bacterial strains analyzed in this study
| ACE-ART strain no. | Original no. | Species | Origin | Yr of isolation | Phenotypic tetracycline MIC (μg/ml) |
|---|---|---|---|---|---|
| B0219 | B173 | B. thermophilum | Environmental sample from pig slaughterhouse | 2001 | 128a |
| B0241 | B187 | B. thermophilum | Pig feces | 2002 | 128a |
| B0242 | B290 | B. thermophilum | Pig feces | 2002 | 256a |
| B0253 | B226 | B. thermophilum | Pig feces | 2002 | 128a |
| B0256 | B315 | B. thermophilum | Pig feces | 2002 | 128a |
| L0077 | G41 | L. johnsonii | Human feces | 2001 | >256b |
The B. thermophilum strains were differentiated by the BOX primer PCR fingerprinting technique as described by Masco et al. (15).
PCR.
PCRs with specific primers for tet(32), tet(O), and tet(W) were performed in a total volume of 50 μl containing approximately 40 ng of bacterial DNA, 10 pmol of each primer (Table 2), 1× PCR buffer, 3 mM MgCl2, 0.2 mM of each deoxynucleoside triphosphate (dNTP), and 2.5 U Taq DNA polymerase recombinant (Invitrogen BV, Breda, The Netherlands). The following PCR program was used: 95°C for 3 min and then 35 cycles of 95°C for 30 s, 58 or 60°C for 30 s, and 72°C for 30 or 60 s, ending with 72°C for 10 min. The annealing temperature depends on the melting temperature (Tm) of the primer pair, whereas the extension time was determined by the expected product length: i.e., fragments shorter than 1,400 bp had an extension period of 30 s and longer products had an extension period of 60 s. The obtained PCR fragments were analyzed by electrophoresis on a 1 to 2% agarose gel, depending on the product sizes, stained with ethidium bromide, and visualized with UV light.
TABLE 2.
Primers used in this study
| Primera | Sequence (5′→3′) |
|---|---|
| BOXb | CTACGGCAAGGCGACGCTGACG |
| DIc | GAYACICCIGGICAYRTIGAYTT |
| DIIc | GCCCATWAIGGRTTIGGIGGIACYTC |
| tet32 1107F | TGATACAGACCCTCTTTTGC |
| tet32 1254R* | AACCGAAGGCTCTTTCATAG |
| tetO −372F | ACAACCGATTAGTGGCAGG |
| tetO −204F | AAGTAGCAGTCCCGTTTCAC |
| tetO 14F | ACTTAGGCATTCTGGCTCAC |
| tetO 144F | GAGCGTCAAAGGGGAATC |
| tetO 161R | ATTCCCCTTTGACGCTCC |
| tetO 1368F* | CGGAGTGCAGTATGAAAGC |
| tetO 1798F* | CAGGGAGTCTGCTTGACAG |
| tetO 1917R | GCTAACTTGTGGAACATATGC |
| tetW −609F | CGCCAGCACTACACTATTC |
| tetW −207F | ATAGCTCCTTTTGTAGGGGC |
| tetW-Fwd | GAGAGCCTGCTATATGCCAGC |
| tetW 61R* | CCGTCAAGGTCGTCTTTCC |
| tetW 384F* | CAAGATCGACCAGGCTGGCG |
| tetW 589R | GGCTGATTGGTTCTCCTGCG |
| tetW 1278F | AGCAGCCAGCCACACCATC |
| tetW 1757R | ATACAGCGGGCGGGAATCTC |
| tetW 1890R | TTGTCCAGGCGGTTGTTTGG |
PCR with the DI-DII primers was performed according to Clermont et al. (8) in a total volume of 50 μl containing approximately 40 ng of bacterial DNA.
PCR-RFLP analysis.
PCR-restriction fragment length polymorphism (RFLP) was performed on 2 μl of DI-DII PCR product using 10 U of the chosen restriction enzyme (New England Biolabs) and the recommended buffer at the appropriate temperature for 2 h. The restriction fragments were separated on a 1.5% agarose gel. The size of DNA fragments was estimated in comparison with two markers: a 100-bp DNA ladder (New England Biolabs) and a 500-bp DNA ladder (Invitrogen BV, Breda, The Netherlands).
Inverse PCR.
The inverse PCR was carried out on the B. thermophilum strains according to the principle described by Ochman et al. (20). In total, 12 different restriction enzymes were used: i.e., BclI, ClaI, HindIII, KpnI, NcoI, NheI, NsiI, PvuI, SalI, TaqI, XbaI, and XmnI. In the digestion, 20 ng of genomic DNA was used together with 10 U of the endonuclease in the buffer specified by the supplier (New England Biolabs) in a total volume of 20 μl. Intramolecular ligation was performed using 5 μl digested DNA, 1× ligation buffer, and 200 U T4 DNA ligase (New England Biolabs) in a total volume of 200 μl at 4°C for at least 16 h. The ligated DNA was precipitated, collected by centrifugation, and dissolved in 100 μl sterile water. The inverse PCR was carried out in a total volume of 50 μl using 2 μl of ligated DNA, 10 pmol of each primer (various divergent primer pairs were used, as indicated in Table 2), 1× PCR buffer, 3 mM MgCl2, 0.2 mM of each dNTP, and 2.5 U Taq DNA polymerase recombinant (Invitrogen BV, Breda, The Netherlands). The following PCR program was used: 95°C for 3 min and 35 cycles of 95°C for 30 s, 58°C for 30 s, 72°C for 3 min, and 72°C for 10 min.
Sequence analysis.
The various (inverse) PCR fragments were cloned in the pGEM-T Easy vector (Promega Benelux BV, Leiden, The Netherlands) and transformed into Escherichia coli XL2-Blue ultracompetent cells (Stratagene Europe, Amsterdam, The Netherlands). Plasmid DNA was isolated with a QIAprep Spin Miniprep kit (QIAGEN Benelux B.V., Venlo, The Netherlands). DNA sequencing was carried out with the GenomeLab methods development kit dye terminator cycle sequencing chemistry protocol and analyzed on a CEQ 2000 DNA analysis system (Beckman Coulter [Nederland] B.V., Mijdrecht, The Netherlands). Multiple clones were analyzed for each strain and PCR fragment. The Seaview software program (9) freely available by anonymous FTP at http://pbil.univ-lyon1.fr/software/seaview.html was used to align the various sequenced fragments.
Nucleotide sequence accession number.
The nucleotide sequences of the tet(O/W) and tet(W/32/O) genes and their flanking regions from the five B. thermophilum strains have been deposited in the EMBL nucleotide sequence database under accession no. AM889118 to AM889122 and AM710601 to AM710605, respectively. The sequence of the tet(O/W/32/O/W/O) gene of L. johnsonii L0077 has also been submitted (DQ525023).
RESULTS
Identification of mosaic genes.
The mosaic tetracycline resistance genes identified in the first instance by PCR in five B. thermophilum strains and one L. johnsonii strain were sequenced. A complete open reading frame (ORF) was found for these mosaic genes, and they contained parts of tet(O), tet(W), and/or tet(32). In the B. thermophilum strains, the mosaic structure was tet(W/32/O). In contrast, the gene identified in the L. johnsonii L0077 strain was far more complex, tet(O/W/32/O/W/O). Homology analysis revealed that the tet(W/32/O) genes are very similar (>99.8%) or even identical (B0242 and B0253). BOX-PCR, a DNA fingerprinting technique targeting repetitive genomic elements, was used to exclude clonality of the strains B0242 and B0253. The obtained patterns clearly demonstrated that these two strains are different, as are the other three B. thermophilum strains (results not shown). The few dissimilarities between the tet(W/32/O) of the five B. thermophilum bacteria seem to be randomly distributed within the mosaic gene, with one exception: the stop codon of strain B0241 is TGA, whereas the others end with TAA, as do all other known tet(O)/tet(W) hybrids.
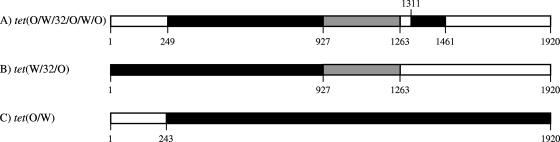
A schematic representation of the identified mosaic tet genes clearly demonstrates that the tet(W/32/O) and tet(O/W/32/O/W/O) have similar crossover points (Fig. 1).
FIG. 1.
Schematic depiction of the different mosaic tet genes identified in L. johnsonii (A) and B. thermophilum (B and C). Black bars indicate regions with very high sequence identity to known tet(W) genes. Gray sections represent parts with high homology to tet(32), whereas open bars symbolize regions with high sequence identity to known tet(O) genes. The different crossover positions are shown.
Upstream region of the tet(O/W/32/O/W/O) gene.
The 5′-end flanking sequence of the L. johnsonii strain was investigated by various PCR tests. Two PCR tests using primers directed against sequences at approximately 400 and 200 nucleotides (nt), respectively, upstream of known tet(O) genes in combination with a reverse primer binding to sequences located within the mosaic gene resulted in the amplification of the correct PCR fragments (Table 3, PCR tests 1 to 4 and 17). In contrast, PCRs identifying approximately 600 bp and 200 bp of the upstream region of tet(W) did not result in any amplicons (results not shown).
TABLE 3.
PCR results with various tet(32), tet(O), and tet(W) primers
| PCR test | Forward primer | Reverse primer | Annealing Tm (°C) | Product length (bp) | Presence/absence of strain(s)
|
|||
|---|---|---|---|---|---|---|---|---|
| B. thermophilum | L. johnsonii | tet(W) | tet(O) | |||||
| 1 | tetO −372F | tetO 161R | 58 | 533 | + | + | − | + |
| 2 | tetO −204F | 58 | 365 | + | + | − | + | |
| 3 | tetO −372F | tetW 589R | 58 | 961 | + | + | − | − |
| 4 | tetO −204F | 58 | 793 | + | + | − | − | |
| 5 | tetO 144F | 58 | 445 | + | + | − | − | |
| 6 | tetO −372F | tetW 1757R | 58 | 2,129 | + | − | − | − |
| 7 | tetO −204F | 58 | 1,961 | + | − | − | − | |
| 8 | tetW-Fw | 60 | 1,695 | − | − | + | − | |
| 9 | tetO 144F | 58 | 1,613 | + | − | − | − | |
| 10 | tetW 384F | 60 | 1,373 | + | − | + | − | |
| 11 | tetW 1278F | 60 | 479 | + | − | + | − | |
| 12 | tetO −204F | tetW 1890R | 58 | 2,094 | + | − | − | − |
| 13 | tetO 14F | 60 | 1,876 | + | − | − | − | |
| 14 | tetO 144F | 58 | 1,746 | + | − | − | − | |
| 15 | tetW 384F | 60 | 1,506 | + | − | + | − | |
| 16 | tetW 1278F | 60 | 612 | + | − | + | − | |
| 17 | tetO −204F | tetO 1917R | 58 | 2,121 | − | + | − | + |
| 18 | tetO 14F | 60 | 1,903 | − | + | − | + | |
| 19 | tetW-Fw | 60 | 1,855 | + | − | − | − | |
| 20 | tetO 144F | 58 | 1,773 | − | + | − | + | |
| 21 | tetW 384F | 60 | 1,533 | + | + | − | − | |
| 22 | tet32 1107F | 58 | 810 | + | + | − | − | |
| 23 | tetW 1278F | 60 | 612 | − | − | − | − | |
Flanking regions of the tet(W/32/O) genes.
Inverse PCR was used to determine up- and downstream sequences of tet(W/32/O) in the five B. thermophilum strains. From the 12 different restriction enzymes tested only with HindIII and TaqI, fragments of the flanking regions were retrieved. The DNA sequences obtained are schematically represented in Fig. 2. The upstream regions of four tet(W/32/O) genes are identical and very similar to the approximately 600-bp region commonly found in front of various tet(W) genes (12). Unfortunately, inverse PCR did not give sequence data of this upstream part in the B0241 strain. Moreover, in contrast to the other four strains, PCR tests amplifying 600 bp and 200 bp, respectively, of the 5′ flanking region of tet(W) genes did not result in the amplification of the expected PCR fragments (data not shown), indicating that this part is different in B0241.
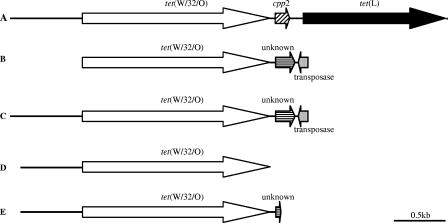
FIG. 2.
Schematic representation of tet(W/32/O) and flanking regions in different B. thermophilum strains: A, B0219; B, B0241; C, B0242; D, B0253; and E, B0256. The overall sequence lengths determined were as follows: B0219, 4,339 nt; B0241, 2,290 nt; B0242, 2,991 nt; B0253, 2,520 nt; and B0256, 2,623 nt.
The B. thermophilum strain isolated in 2001 (B0219) has clearly different downstream sequences from the isolates from the year 2002. Inverse PCR demonstrated that besides the tet(W/32/O) gene, this strain also seems to possess another tetracycline resistance gene, coding for the efflux pump Tet(L) (Fig. 2). This result was confirmed by microarray analysis (16, 29), PCR (data not shown), and sequence analysis of a complete tet(L) ORF. In between these two resistance genes, an ORF is present which has a high sequence identity (99%) with the cpp2 gene found on the tetracycline resistance plasmids of two different Campylobacter species, where it is localized downstream of tet(O) (3).
In contrast, the tet(W/32/O) gene of the B. thermophilum strains B0241, B0242, and B0256 is followed by two ORFs coding for a so-far-unknown protein and a transposase gene, respectively. Unfortunately, this sequence information was not retrieved for the B0253 strain.
PCR-RFLP.
Amplified DNA fragments of 1.1 kb obtained with the primer pair DI-DII (localized at approximately 219 and 1,328 bp from the start codon of the RPP genes) generated a slightly different pattern after gel electrophoresis for the B. thermophilum strains: i.e., several vague larger fragments (results not shown). This phenomenon was further investigated by RFLP of the generated fragments. Besides the B. thermophilum and L. johnsonii strains, two other bacteria were included: i.e., B. thermophilum LMG 21813T containing tet(W) and Bifidobacterium bifidum B0045 with tet(O) (2, 17). The RFLP of the DI-DII products of the L. johnsonii tet(O/W/32/O/W/O), B. thermophilum tet(W), and B. bifidum tet(O) only generated fragments that were expected based on the analysis of the gene sequence using the REBsites software program (http://tools.neb.com/REBsites; results not shown). In contrast, RFLP of the DI-DII products of the five B. thermophilum strains generated fragments corresponding to the expectations for the tet(W/32/O) gene; however, a tet(W) restriction pattern was also found in these bacteria. To investigate this in more detail, a number of different PCR tests were performed using various different tet(32), tet(O), and tet(W) primers (Table 2). A summary of the PCRs performed is shown in Table 3. The presence of the tet(W/32/O) was indicated by PCR tests 19, 21, 22, and 23, whereas all other PCR tests clearly demonstrated the existence of an additional tetracycline resistance gene in the B. thermophilum strains with a probable mosaicism of tet(O/W) (Fig. 1) preceded by a region similar to 5′ flanking sequences of tet(O) (PCR tests 1 to 4, 6, 7, and 12). The absence of tet(32) sequences in this mosaic gene was shown by RFLP analysis of the fragments obtained with PCR tests 3, 4, and 12 (Table 3) using more than 10 different restriction enzymes. All digestion results corresponded with a pattern expected for a tet(O/W) gene (results not shown). This was confirmed by sequence analysis revealing a crossover point at position 243 (Fig. 1), similar to the previously described tet(O/W)-2 gene in Megasphaera elsdenii (26).
DISCUSSION
Tetracycline resistance genes have been identified in both gram-positive and gram-negative bacteria. Besides the 40 thoroughly characterized tet genes, mosaic genes also have been reported. For example, more than 10 different tet(O)/tet(W) hybrid genes have been currently recognized (22, 24-26). Most of the bacteria harboring these mosaic genes were isolated from the intestinal tract of pigs. The B. thermophilum strains investigated in this study also originate from pig intestines, while the L. johnsonii isolate came from human feces. The DNA sequence of the mosaic gene determined in L. johnsonii was identified as tet(O/W/32/O/W/O), whereas in five B. thermophilum strains a tet(W/32/O) ORF was characterized. Subsequently and very surprisingly, a second mosaic gene, tet(O/W), was demonstrated in these bifidobacteria. The more complex tet(O/W/32/O/W/O) gene was nearly identical to a mosaic tet gene recently recovered from a tet(O)-based clone library of pig feces by Patterson et al. (22): i.e., 99.9% identity on the DNA sequence level and 99.7% identity on the deduced amino acid level. Phylogenetic analysis of the mosaic tet sequences identified in this study with related RPP genes including other hybrid genes indicated that they are closely related. The nearest nonmosaic RPP relative is tet(W), whereas tet(O) and tet(32) are more distantly related (data not shown).
The investigation of the upstream sequences showed that this region corresponds to the first part of the mosaic ORF. For example, the tet(O/W/32/O/W/O) and tet(O/W) genes are preceded (i.e., nearly 400 bp) by sequences found in front of several tet(O) genes (Table 3). Since Wang and Taylor (30) described that this DNA sequence upstream of the tet(O) gene is required for full expression of tetracycline resistance, it seems likely that the mosaic genes tet(O/W/32/O/W/O) and tet(O/W) are expressed. A similar situation was identified for the tet(W/32/O) genes. In four out of five strains, the gene is flanked at the 5′ end by sequences (up to 600 bp) nearly 100% identical and commonly found upstream of tet(W) in several different bacterial species (4, 12) and required for full expression (18). The upstream region of the tet(W/32/O) gene of the B0241 strain appeared to be different, and furthermore, the stop codon of this gene also differed from the rest (TGA versus TAA). These results could indicate that the other identified hybrid gene, tet(O/W), might be the most active component in B0241; however, this was not supported by the phenotypes of the bifidobacteria investigated (Table 1). Based on the obtained results of the flanking sequences, it is most likely that these hybrid genes arose from the interclass recombination within the coding regions of the RPP genes tet(O) and tet(W). Detailed characterization of the coding sequences confirms this fact and reveals preferential crossover positions (Fig. 1), which was also demonstrated by other studies (22, 25, 26).
Various methods (several PCR tests, PCR-RFLP, and sequencing) demonstrated the unexpected presence of an additional mosaic tetracycline resistance gene in the B. thermophilum strains investigated, showing a mosaicism of tet(O/W) (Fig. 1). To our knowledge, this is the first description of multiple mosaic tet genes within one bacterium, although this could also be the case in the study by Patterson et al. (22), since individual bacteria were not isolated from the pig and human feces, with the exception of the tet(32) gene-containing human oral strain, Streptococcus salivarius. Very surprisingly, one of the B. thermophilum strains (i.e., B0219), besides the two mosaic RPP genes also seems to possess an additional tet determinant, tet(L), coding for an efflux pump (Fig. 2). However, the presence of three tet genes did not result in an extremely high phenotypic tetracycline resistance profile in this isolate (Table 1).
The large diversity of mosaic genes identified in bacteria isolated from the intestinal tract of pigs (references 22 and 24 and this study) and the fact that these strains demonstrate a high level of tetracycline resistance (for resistance levels of the parent genes, see reference 26) clearly suggest the need to have a closer look at the use of tetracycline in pig husbandry as also shown by other studies of swine production facilities and pigs' waste treatment systems (6, 11, 14). Furthermore, since, cooking procedures for meat, even to “well done,” cannot be relied on to completely inactivate even the more heat-sensitive tetracyclines (13, 19, 28), consumption of tetracycline-containing meat and meat products might further drive selection of tetracycline-resistant bacteria in the human intestines.
Acknowledgments
This research was funded by the European Commission under the 6th Framework Program (ACE-ART, project no. CT-2003-506214).
Françoise Gavini and Matthias Upmann are gratefully acknowledged for providing the bifidobacterial strains isolated in the EU project “BIFID” (CT-2000-00805).
Footnotes
Published ahead of print on 29 October 2007.
REFERENCES
- 1.Aminov, R. I., N. Garrigues-Jeanjean, and R. I. Mackie. 2001. Molecular ecology of tetracycline resistance: development and validation of primers for detection of tetracycline resistance genes encoding ribosomal protection proteins. Appl. Environ. Microbiol. 67:22-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ammor, M. S., A. B. Flórez, A. H. A. M. van Hoek, C. G. de los Reyes-Gavilán, H. J. M. Aarts, A. Margolles, and B. Mayo. 2008. Molecular characterization of specific and non-specific antibiotic resistance in lactic acid bacteria and bifidobacteria. J. Mol. Microbiol. Biotechnol. 14:6-15. [DOI] [PubMed] [Google Scholar]
- 3.Batchelor, R. A., B. M. Pearson, L. M. Friis, P. Guerry, and J. M. Wells. 2004. Nucleotide sequences and comparison of two large conjugative plasmids from different Campylobacter species. Microbiology 150:3507-3517. [DOI] [PubMed] [Google Scholar]
- 4.Billington, S. J., and B. H. Jost. 2006. Multiple genetic elements carry the tetracycline resistance gene tet(W) in the animal pathogen Arcanobacterium pyogenes. Antimicrob. Agents Chemother. 50:3580-3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casewell, M., C. Friis, E. Marco, P. McMullin, and I. Phillips. 2003. The European ban on growth-promoting antibiotics and emerging consequences for human and animal health. J. Antimicrob. Chemother. 52:159-161. [DOI] [PubMed] [Google Scholar]
- 6.Chee-Sanford, J. C., R. I. Aminov, I. J. Krapac, N. Garrigues-Jeanjean, and R. I. Mackie. 2001. Occurrence and diversity of tetracycline resistance genes in lagoons and groundwater underlying two swine production facilities. Appl. Environ. Microbiol. 67:1494-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chopra, I., and M. Roberts. 2001. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 65:232-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clermont, D., O. Chesneau, G. De Cespédès, and T. Horaud. 1997. New tetracycline resistance determinants coding for ribosomal protection in streptococci and nucleotide sequence of tet(T) isolated from Streptococcus pyogenes A498. Antimicrob. Agents Chemother. 41:112-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galtier, N., M. Gouy, and C. Gautier. 1996. SEAVIEW and PHYLO_WIN: two graphic tools for sequence alignment and molecular phylogeny. Comput. Appl. Biosci. 12:543-548. [DOI] [PubMed] [Google Scholar]
- 10.Huys, G., K. D'Haene, J.-M. Collard, and J. Swings. 2004. Prevalence and molecular characterization of tetracycline resistance in Enterococcus isolates from food. Appl. Environ. Microbiol. 70:1555-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jindal, A., S. Kocherginskaya, A. Mehboob, M. Robert, R. I. Mackie, L. Raskin, and J. L. Zilles. 2006. Antimicrobial use and resistance in swine waste treatment systems. Appl. Environ. Microbiol. 72:7813-7820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kazimierczak, K. A., H. J. Flint, and K. P. Scott. 2006. Comparative analysis of sequences flanking tet(W) resistance genes in multiple species of gut bacteria. Antimicrob. Agents Chemother. 50:2632-2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kühne, M., U. Körner, and S. Wenzel. 2001. Tetracycline residues in meat and bone meals. Part 2. The effect of heat treatments on bound tetracycline residues. Food Addit. Contam. 18:593-600. [DOI] [PubMed] [Google Scholar]
- 14.Mackie, R. I., S. Koike, I. Krapac, J. Chee-Sanford, S. Maxwell, and R. I. Aminov. 2006. Tetracycline residues and tetracycline resistance genes in groundwater impacted by swine production facilities. Anim. Biotechnol. 17:157-176. [DOI] [PubMed] [Google Scholar]
- 15.Masco, L., G. Huys, D. Gevers, L. Verbrugghen, and J. Swings. 2003. Identification of Bifidobacterium species using rep-PCR fingerprinting. Syst. Appl. Microbiol. 26:557-563. [DOI] [PubMed] [Google Scholar]
- 16.Mättö, J., A. H. A. M. van Hoek, K. J. Domig, M. Saarela, A. B. Floréz, E. Brockmann, E. Amtmann, B. Mayo, H. J. M. Aarts, and M. Danielsen. 2007. Susceptibility of human and probiotic Bifidobacterium spp. to selected antibiotics as determined by the Etest method. Int. Dairy J. 17:1123-1131. [Google Scholar]
- 17.Mayrhofer, S., K. J. Domig, E. Amtmann, A. H. A. M. van Hoek, A. Petersson, C. Mair, H. K. Mayer, and W. Kneifel. 2007. Antibiotic susceptibility of Bifidobacterium thermophilum and Bifidobacterium pseudolongum isolates from animal sources. J. Food Prot. 70:119-124. [DOI] [PubMed] [Google Scholar]
- 18.Melville, C. M., K. P. Scott, D. K. Mercer, and H. J. Flint. 2001. Novel tetracycline resistance gene, tet(32), in the Clostridium-related human colonic anaerobe K10 and its transmission in vitro to the rumen anaerobe Butyrivibrio fibrisolvens. Antimicrob. Agents Chemother. 45:3246-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moats, W. A. 1999. The effect of processing on veterinary residues in foods. Adv. Exp. Med. Biol. 459:233-241. [DOI] [PubMed] [Google Scholar]
- 20.Ochman, H., A. S. Gerber, and D. L. Hartl. 1988. Genetic applications of an inverse polymerase chain reaction. Genetics 120:621-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oggioni, M. R., C. G. Dowson, J. M. Smith, R. Provvedi, and G. Pozzi. 1996. The tetracycline resistance gene tet(M) exhibits mosaic structure. Plasmid 35:156-163. [DOI] [PubMed] [Google Scholar]
- 22.Patterson, A. J., M. T. Rincon, H. J. Flint, and K. P. Scott. 2007. Mosaic tetracycline resistance genes are widespread in human and animal fecal samples. Antimicrob. Agents Chemother. 51:1115-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts, M. C. 2005. Update on acquired tetracycline resistance genes. FEMS Microbiol. Lett. 245:195-203. [DOI] [PubMed] [Google Scholar]
- 24.Stanton, T. B., and S. B. Humphrey. 2003. Isolation of tetracycline-resistant Megasphaera elsdenii strains with novel mosaic gene combinations of tet(O) and tet(W) from swine. Appl. Environ. Microbiol. 69:3874-3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stanton, T. B., S. B. Humphrey, K. P. Scott, and H. J. Flint. 2005. Hybrid tet genes and tet gene nomenclature: request for opinions. Antimicrob. Agents Chemother. 49:1265-1266. (Letter.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stanton, T. B., J. S. McDowall, and M. A. Rasmussen. 2004. Diverse tetracycline resistance genotypes of Megasphaera elsdenii strains selectively cultured from swine feces. Appl. Environ. Microbiol. 70:3754-3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson, S. A., E. V. Maani, A. H. Lindell, C. J. King, and J. V. McArthur. 2007. Novel tetracycline resistance determinant isolated from an environmental strain of Serratia marcescens. Appl. Environ. Microbiol. 73:2199-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Egmond, H. J., J. F. M. Nouws, R. Schilt, W. D. M. van Lankveld-Driessen, E. P. M. Streutjens-van Neer, and F. G. H. Simons. 2000. Stability of antibiotics in meat during a simulated high temperature destruction process. EuroResidue IV:430-437. [Google Scholar]
- 29.van Hoek, A. H. A. M., I. M. J. Scholtens, A. Cloeckaert, and H. J. M. Aarts. 2005. Detection of antibiotic resistance genes in different Salmonella serovars by oligonucleotide microarray analysis. J. Microbiol. Methods 62:13-23. [DOI] [PubMed] [Google Scholar]
- 30.Wang, Y., and D. E. Taylor. 1991. A DNA sequence upstream of the tet(O) gene is required for full expression of tetracycline resistance. Antimicrob. Agents Chemother. 35:2020-2025. [DOI] [PMC free article] [PubMed] [Google Scholar]