Abstract
The purine nucleoside 5′-deoxy-5′-(hydroxyethylthio)-adenosine (HETA) is an analog of the polyamine pathway metabolite 5′-deoxy-5′-(methylthio)-adenosine (MTA). HETA is a lead structure for the ongoing development of selectively targeted trypanocidal agents. Thirteen novel HETA analogs were synthesized and examined for their in vitro trypanocidal activities against bloodstream forms of Trypanosoma brucei brucei LAB 110 EATRO and at least one drug-resistant Trypanosoma brucei rhodesiense clinical isolate. New compounds were also assessed in a cell-free assay for their activities as substrates of trypanosome MTA phosphorylase. The most potent analog in this group was 5′-deoxy-5′-(hydroxyethylthio)-tubercidin, whose in vitro cytotoxicity (50% inhibitory concentration [IC50], 10 nM) is 45 times greater than that of HETA (IC50, 450 nM) against pentamidine-resistant clinical isolate KETRI 269. Structure-activity analyses indicate that the enzymatic cleavage of HETA analogs by trypanosome MTA phosphorylase is not an absolute requirement for trypanocidal activity. This suggests that additional biochemical mechanisms are associated with the trypanocidal effects of HETA and its analogs.
African sleeping sickness is endemic to vast areas of sub-Saharan Africa. The World Health Organization estimates that 55 million people in 35 African countries are at risk for contracting the disease, which is invariably fatal to untreated individuals (29). An alarming resurgence of African sleeping sickness in Sudan and other parts of Central Africa has created great concern among African governments and international health care agencies. Pentamidine and the organoarsenicals (e.g., melarsoprol and melarsen), which have been used to treat African sleeping sickness for many years, are highly toxic and have led to drug-resistant clinical strains that are refractory to chemotherapy (9, 29). The drug α-difluoromethylornithine (ornidyl, eflornithine), which was approved by the FDA in 1990 for treatment of the disease, is costly to prepare, cumbersome to administer, and ineffective against the disease forms prevalent in East Africa (29). At present, DB289, a prodrug of the pentamidine analog 2,5-bis(4-amidinophenyl)furan, is the only potential new treatment undergoing clinical trials for early-stage human African sleeping sickness (41). New agents for the treatment of African sleeping sickness are urgently needed (9, 14, 29). Ideally, these should be highly effective in eradicating host-dwelling parasites, nontoxic to the host, inexpensive to produce, and easy to administer to patients in rudimentary medical care settings.
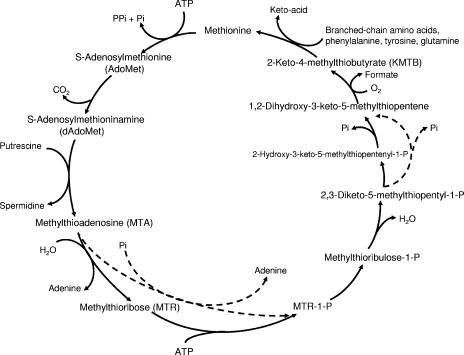
Polyamine biosynthetic pathways in trypanosomes have provided effective targets for the development of trypanocidal agents (4). Difluoromethylornithine, the agent used to treat late-stage African sleeping sickness, is an inhibitor of ornithine decarboxylase (5, 6). Another polyamine pathway enzyme, 5′-deoxy-5′-(methylthio)-adenosine phosphorylase (MTA-Pase), has been studied by our laboratories as a potential trypanocidal target. 5′-Deoxy-5′-(methylthio)-adenosine MTA, the nucleoside by-product of spermidine synthesis in trypanosomes, is cleaved by MTA-Pase to generate methylthioribose-1-phosphate (MTRP) and adenine (Fig. 1). MTRP is recycled to methionine, while adenine is redirected to nucleoside synthesis. Trypanosomes lack de novo purine biosynthetic pathways (11). In addition, they have continuously high demands for S-adenosylmethionine (AdoMet), the methionine metabolite that serves as a polyamine precursor (22). MTA-Pase plays a critical role in these parasites by enabling the salvage of adenine and methionine, both of which are essential for trypanosome survival (11, 22). Moreover, MTA-Pase presents a selective chemotherapeutic target in African trypanosomes, based on a significant difference in the substrate activities of the MTA analog, 5′-(hydroxyethylthio)-adenosine (HETA), toward mammalian and trypanosome forms of MTA-Pase: the trypanososme form of MTA-Pase (Tryp-MTA-Pase) rapidly metabolizes HETA, whereas the mammalian enzyme is comparably ineffective (8, 44). Although the mechanism of HETA's toxicity is not known, its rapid breakdown, once it is inside trypanosomes, is an indication that these effects are not caused by the inhibition of Tryp-MTA-Pase. More likely, HETA's toxicity is related to its ability to interfere with methionine recycling. This is supported by the finding that HETA's in vitro trypanocidal effects can be reversed by the concomitant administration of methionine or 2-keto-4-methylthio-butyrate, the immediate precursor of methionine salvage from MTA (8). HETA's toxicity may also be associated, in part, with the generation of a toxic methionine analog, as was observed in Klebsiella pneumonia (32). Other key biochemical properties that contribute to HETA's trypanocidal potency include its rapid, carrier-mediated accumulation inside trypanosomes via the P2 purine transporter and a novel AdoMet transporter discovered in these parasites (19, 20, 22). HETA was observed to induce elevations in AdoMet and MTA levels in trypanosomes (3) and to inhibit trypanosome protein methylation reactions (21).
FIG. 1.
Pathways of methionine salvage from MTA. (Adapted from reference 40 with permission.)
The preferential metabolism of HETA by MTA-Pase in trypanosomes, which provided the initial basis for its potential selectivity, was supported by results from in vivo studies demonstrating its curative effects in mice infected with Trypanosoma brucei brucei and T. brucei rhodesiense strains and the absence of host toxicity (7, 8). These in vivo studies strongly validated the designation of HETA as a lead compound for further analog development. Our continuing efforts to identify more potent structural analogs of our lead compound, described in this study, have centered on the synthesis and in vitro antitrypanosomal screening of novel purine- and ribose-modified analogs of HETA.
MATERIALS AND METHODS
MTA analogs.
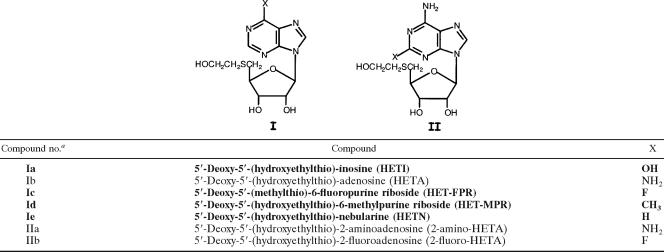
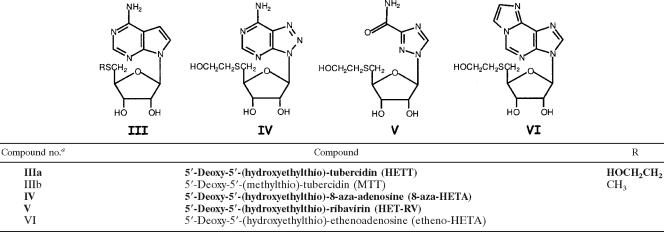
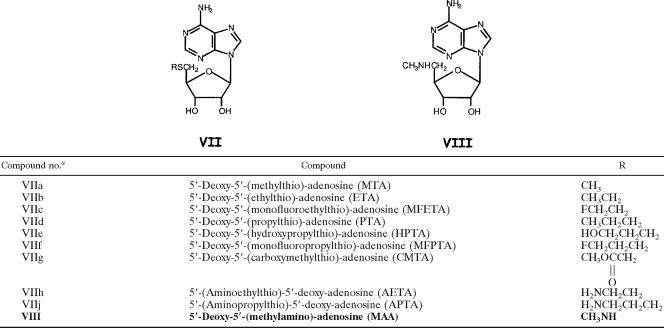
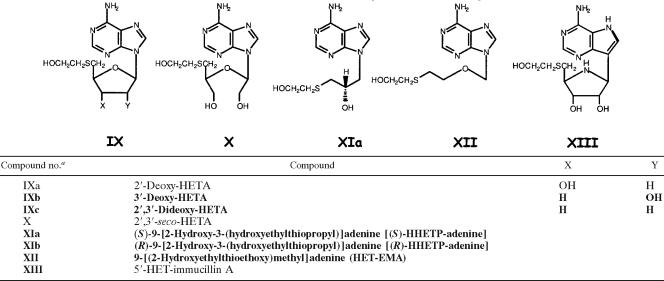
The structures of the MTA analogs that were evaluated for trypanocidal activity in this study, as well as in previous studies (3, 8, 43), are depicted in Tables 1 to 4. The structures of the 13 newly synthesized analogs are highlighted by boldface type in all tables; the methods developed for their chemical synthesis appear in the supplemental material (1, 10, 23, 25, 31, 33, 34, 35, 37, 47). Also included in the supplemental material are detailed methods for the synthesis of 2-amino-HETA, 2-fluoro-HETA, and 2′-deoxy-HETA, analogs whose trypanocidal effects were reported previously (3, 43). Confirmation of the structures of the newly synthesized analogs was accomplished by 1H nuclear magnetic resonance (NMR) spectroscopy. The purity of the compounds was assessed by thin-layer chromatography and NMR spectroscopy. Other nucleosides shown in Tables 1 to 4, i.e., 5′-(ethylthio)-adenosine (ETA), 5′-(propylthio)-adenosine (PTA), HETA, 2′,3′-seco-HETA, 5′-(hydroxypropylthio)-adenosine (HPTA), 5′-(monofluoroethylthio)-adenosine (MFETA), 5′-(monofluoropropylthio)-adenosine (MFPTA), 5′-(carboxymethylthio)-adenosine (CMTA), 5′-(aminoethylthio)-adenosine (AETA), 5′-(aminopropylthio)-adenosine (APTA), 5′-(methylamino)-adenosine (MAA), 5′-(methylthio)-7-deaza-adenosine [5′-(methylthio)-tubercidin (MTT)], and 5′-(hydroxyethylthio)-immucillin A (HET-immucillin A), were synthesized by previously published procedures (8, 13, 16, 26, 27, 30, 38, 39, 43-45).
TABLE 1.
Structures of exocyclic purine-modified MTA analogs
Novel HETA analogs are distinguished in boldface.
TABLE 2.
Structures of heterocyclic ring-modified MTA analogs
Novel HETA analogs are distinguished in boldface.
TABLE 3.
Structures of exocyclic (5′)-ribose-modified MTA analogs
Novel HETA analogs are distinguished in boldface.
TABLE 4.
Structures of ribofuranose ring-modified MTA analogs
Novel HETA analogs are distinguished in boldface.
Trypanosome strains.
Trypanosoma brucei brucei LAB 110 EATRO was obtained from the late William Trager of the Rockefeller University (46). Clinical isolates of T. brucei rhodesiense (KETRI 243 and KETRI 269) were obtained from A. R. Njogu of the Kenya Trypanosomiasis Research Institute (KETRI). KETRI 243 is resistant to pentamidine and melarsoprol, and KETRI 269 is resistant to pentamidine (2). KETRI 243-As10-3 is a highly melarsen- and diamidine-resistant clone of KETRI 243 (2). The bloodstream forms of the strains were adapted to grow under axenic conditions in Iscove's modified Dulbecco medium with hypoxanthine at 1 μM and 20% horse serum instead of synthetic serum (HMI-18 medium) (24).
MTA phosphorylase enzyme assays.
Cell-free enzyme preparations were obtained from bloodstream trypanosomes by a previously published procedure (8). Briefly, bloodstream trypanosomes were harvested from rats. Cell lysates were frozen-thawed in dry ice-methanol for three cycles and centrifuged at 5,000 × g for 10 min, and endogenous unbound phosphate was removed by dialysis for 1 h (0.05 M Tris-HCl, 0.1 mM disodium EDTA, 1 mM 2-mercaptoethanol, pH 7.4). Trypanosome extracts were frozen and stored at −70°C. MTA analogs were assayed for their ability to act as substrates for Tryp-MTA-Pase with and without added phosphate in the reaction mixture. Enzyme assays were based upon the phosphorolysis of MTA to yield adenine. The conversion of adenine to 2,8-dihydroxyadenine in the presence of commercial xanthine oxidase was measured at 305 nm (change in E = 15.5 × 10 3 M−1 cm−1). The reaction mixtures (1-ml final volume in quartz cuvettes) contained 0 or 50 mM potassium phosphate (pH 7.4), xanthine oxidase (1,000 U; type III; Sigma Chemical Co., St. Louis, MO), and 100 μl of trypanosomal extract. MTA or its substrate analog (25 to 500 μM) was added to start the reaction. Normally, 200 μM MTA or its analog was saturating. The increase in absorbance over 1 h was measured in a recording spectrophotometer (DU7; Beckman) with a temperature-controlled cuvette holder (37°C). The rates were linear for the 1-h period (18).
Determination of in vitro antitrypanosomal activity.
Drug studies were done in duplicate in 24-well plates (1 ml per well) with final inhibitor concentrations of 0.1, 1, 10, 25, and 100 μM. After 48 h, the number of parasites was determined in a Z1 Coulter counter, and the approximate range of activity was determined. The 50% inhibitory concentrations (IC50s) were then determined from additional studies with a narrower range of inhibitor concentrations. Inhibitors with <50% inhibition at 100 μM were considered inactive and their IC50 values were not further determined. Analogs were dissolved in water or dimethyl sulfoxide. Dilutions were made with HMI-18 medium, such that the dimethyl sulfoxide concentration never exceeded a noninhibitory concentration of 0.3%.
RESULTS
MTA phosphorylase assays.
MTA analogs, whose structures are depicted in Tables 1 to 4, were assayed for their abilities to act as substrates for Tryp-MTA-Pase with and without phosphate added to the reaction mixture. Inorganic phosphate is an absolute requirement for MPA-Pase activity. Thus, the MTA substrate analogs display differential activities in the presence and absence of phosphate: MTA substrate activity is 20.86 nmol/mg protein/h with added phosphate, and with no added phosphate, the baseline activity is 7.67 nmol/mg protein/h, likely contributed by the action of nonspecific nucleosidases (Table 5). As seen in Table 5, the analogs demonstrated various abilities to act as substrates. The substrate activity of 2-fluoro-HETA (compound IIb) was significantly greater than that of MTA (compound VIIa). The substrate activities of HETA (compound Ib) and 2′-deoxy-HETA (compound IXa) were comparable to the activity of MTA (compound VIIa). ETA (compound VIIb), MFETA (compound VIIc), HPTA (compound VIIe), and CMTA (compound VIIg) were also effective substrates, with relative activities in the range of 72 to 84%. Five analogs, 5′-deoxy-5′-(hydroxyethylthio)-inosine (HETI; compound Ia), 5′-deoxy-5′-(hydroxyethylthio)-6-methylpurine riboside (HET-MPR; compound Id), 5′-deoxy-5′-(hydroxyethylthio)-tubercidin (HETT; compound IIIa), MTT (compound IIIb), and MAA (compound VIII), were devoid of substrate activity. The 7-deaza analog, HETT (compound IIIa), which was also assayed for its inhibitory effects on enzyme activity in the presence of MTA, was neither a substrate nor an inhibitor of the trypanosome enzyme.
TABLE 5.
Activities of MTA analogs as substrates for Trypanosoma brucei brucei MTA-Pase
| Compound group and no.a | Substrate | % of control activity
|
|
|---|---|---|---|
| With 50 mM PO4− | Without PO4− | ||
| Purine-modified analogs | |||
| IIb | 2-Fluoro-HETA | 148b | 106b |
| VIIa | MTA | 100c | 100d |
| Ib | HETA | 96 | 100 |
| V | HET-RV | 61 | 70 |
| VI | Etheno-HETA | 56 | 62 |
| Ic | HET-FPR | 42 | 60 |
| IIa | 2-Amino-HETA | 29b | 18b |
| Ie | HETN | 11 | 4 |
| Id | HET-MPR | 2 | 25 |
| Ia | HETI | 0 | 0 |
| IIIb | MTT | 0 | 0 |
| IIIa | HETT | 0 | 0 |
| HETT + MTAe | 99 | 2 | |
| Ribose-modified analogs | |||
| IXa | 2′-Deoxy-HETA | 100 | 81 |
| VIIg | CMTA | 84f | 34f |
| VIIb | ETA | 76.2f | 26.9f |
| VIIc | MFETA | 75.3f | 29.3f |
| VIIe | HPTA | 72.5f | 27.4f |
| VIId | PTA | 57.5f | 26.0f |
| XII | HET-EMA | 56 | 78 |
| VIIf | MFPTA | 47.5f | 31.3f |
| VIIj | APTA | 46 | 60 |
| X | 2′, 3′-seco-HETA | 44 | 0 |
| IXb | 3′-Deoxy-HETA | 39.4 | 37.8 |
| IXc | 2′, 3′-Dideoxy-HETA | 17 | 18 |
| VIII | MAA | 4 | 0 |
In vitro growth inhibitory activities of MTA analogs.
The IC50 values of the parent nucleoside analogs were determined (Table 6). These compounds were grouped according to their relative activities: the highly active analogs (those active at concentrations of ≤1 μM), which consisted of HETA (compound Ib), HETT (compound IIIa), and MFETA (compound VIIc); active analogs (analogs active at concentrations of 2 to 10 μM), which consisted of 2-amino-HETA (compound IIa), 2-fluoro-HETA (compound IIb), and 8-aza-HETA (compound IV); moderately active analogs (analogs active at concentrations of 10 to 50 μM), which consisted of HET-MPR (compound Id), 5′-deoxy-5′-(hydroxyethylthio)-nebularine (HETN; compound Ie), 3′-deoxy-HETA (compound IXb), and 2′,3′-seco-HETA (compound X); active analogs (analogs active at concentrations of 50 to 100 μM), which consisted of MTT (compound IIIb); and inactive analogs (analogs active at concentrations of >100 μM). Most analogs were either slightly growth inhibitory or non-growth inhibitory for T. brucei brucei LAB 110 EATRO. Unexpectedly, three analogs, 5′-deoxy-5′-(hydroxyethylthio)-ribavirin (HET-RV; compound V), AETA (compound VIIh), and 2′-deoxy-HETA (compound IXa), were observed to stimulate the growth of a drug-resistant strain (KETRI 243 or 243-As10-3).
TABLE 6.
In vitro efficacies of MTA analogs and selected O-acetylated derivatives in trypanosome isolates grown as bloodstream formsa
| Compound group and no.a | Compoundb | IC50 (μM)
|
|||
|---|---|---|---|---|---|
| LAB 110 EATRO | KETRI 243 | KETRI 243-As10-3 | KETRI 269 | ||
| Purine-modified analogs | |||||
| Ia | HETI | >100 | >100 | NDc | ND |
| Ib | HETA | 0.54d | 0.44d | 0.19 | 0.45d |
| Tri-OAc-HETA | 0.32d | 0.31d | ND | 0.26d | |
| Ic | HET-FPR | >100 | >100 | ND | ND |
| Tri-OAc-Ic | >100 | >100 | ND | ND | |
| Id | HET-MPR | 24.5 | 29.5 | 29.0 | 19 |
| Tri-OAc-Id | 9.3 | 22 | 22 | 20.5 | |
| Ie | HETN | 34 | 66 | 54 | 72 |
| Tri-OAc-Ie | 3.65 | 2.9 | 6.4 | 6.7 | |
| IIa | 2-Amino-HETA | 3.9 | 1.75 | 3.45 | 5.5 |
| Tri-OAc-IIa | 12.5 | 43 | ND | ND | |
| IIb | 2-Fluoro-HETA | 1.9 | 1.4 | ND | 1.2 |
| Tri-OAc-IIb | 2.75 | 2.2 | 3.0 | 3.0 | |
| IIIa | HETT | 0.042 | 0.015 | 0.09 | 0.010 |
| Tri-OAc-IIIa | 0.145 | 0.155 | 0.10 | 0.150 | |
| IIIb | MTT | 67 | 100 | 26.5 | >100 |
| Tri-OAc-IIIb | 46 | 100 | 66 | >100 | |
| IV | 8-aza-HETA | 7.0 | 7.8 | ND | 6.4 |
| V | HET-RV | 9e | +f | ND | ND |
| Tri-OAc-HET-RV | 26.5 | 39 | 25 | ND | |
| VI | Etheno-HETA | >100 | >100 | ND | ND |
| Tri-OAc-VI | 35 | 19 | 38.5 | ND | |
| Ribose-modified analogs | |||||
| VIIa | MTA | 100d | ND | ND | ND |
| VIIb | ETA | 138g | ND | ND | ND |
| VIIc | MFETA | 0.20d | 0.32d | ND | 0.28d |
| VIIc | Di-OAc-VIIIc | 0.83d | 0.62d | ND | 0.60d |
| VIId | PTA | 46g | ND | ND | ND |
| VIIe | HPTA | 170g | ND | ND | ND |
| VIIf | MFPTA | 120g | ND | ND | ND |
| VIIg | CMTA | 130g | ND | ND | ND |
| VIIh | AETA | >100μM | +f | ND | ND |
| VIIj | APTA | >100 | 72.5 | >100 | 15.75 |
| VIII | MAA | >100 | >100 | ND | ND |
| IXa | 2′-Deoxy-HETA | >100d | >100d | +d,f | >100d |
| Di-OAc-IXa | 22d | 30.6d | 14.9d | ||
| IXb | 3′-Deoxy-HETA | 12.5 | 12.5 | ND | 40.5 |
| Di-OAc-IXb | 0.64 | 0.82 | ND | 0.66 | |
| IXc | 2′, 3′-Dideoxy-HETA | 100 | 71 | ND | ND |
| X | 2′, 3′-seco-HETA | 14d | 15d | ND | 25d |
| Tri-OAc-X | 5.3d | 5.2d | ND | >100d | |
| XIa | (S)-HHETP-adenine | 60 | >100 | ND | ND |
| XIb | (R)-HHETP-adenine | >100 | >100 | ND | ND |
| XII | HET-EMA | 64 | 100 | >100 | >100 |
| XIII | 5′-HET-immucillin A | >10 | ND | ND | ND |
DISCUSSION
The biochemical differences between the mammalian and the microbial pathways of MTA metabolism as selective targets for drug design have been studied extensively (3, 4, 7, 8, 18, 28, 36, 40, 42). Unlike mammalian cells, two distinct pathways of MTA metabolism are known to exist among microbial species (Fig. 1): one operates by the initial phosphorolytic cleavage of MTA by MTA-Pase to produce adenine and MTRP, and the other operates by the initial hydrolytic cleavage of MTA by MTA nucleosidase to produce adenine and 5-methylthioribose (MTR), which is phosphorylated by MTR kinase to produce MTRP. The further metabolism of MTRP, an intermediate common to both pathways, results in the recycling of MTA to methionine. African trypanosomes appear to metabolize MTA exclusively by MTA-Pase cleavage. Ghoda et al. (18) observed differences in the substrate specificities of MTA-Pases from T. brucei brucei and mammalian cells and proposed the development of cytotoxic MTA analogs that could be selectively activated by the MTA-Pase of trypanosomes. We identified one such MTA analog, HETA, and found, as anticipated, that HETA has significantly greater cytotoxicity for trypanosomes than for mammalian cells (8).
In seeking to enhance HETA's trypanocidal activity, we synthesized new analogs which were assayed for their susceptibility to cleavage by Tryp-MTA-Pase and for their in vitro trypanocidal activity in T. brucei brucei LAB 110 EATRO and at least one drug-resistant KETRI clinical isolate. The biological results from the present study were compared with relevant data from previous studies (3, 8, 43) in order to determine the distinct effects of each structural modification on enzyme activity and trypanocidal activity, respectively (Tables 5 and 6). The most significant increase in substrate activity occurred when an exocyclic fluoro substituent was added at the C-2 position of the purine moiety, as evidenced by the substrate activity of 2-fluoro-HETA (compound IIb), which was 1.5 times greater than that of MTA and HETA. The greatest enhancement in trypanocidal activity resulted from replacement of the N-7 nitrogen of HETA by a C atom; thus, when HETT (IC50 value, 10 nM) was evaluated with pentamidine-resistant clinical isolate KETRI 269, it was 45 times more potent than HETA (IC50 value, 450 nM).
MTA-Pases from mammalian and microbial sources are known to have a broad tolerance for substrates with various alkyl and aryl substituents as replacements for the terminal 5′-methyl group (3, 8, 12, 15, 18, 28, 44). Accordingly, a library of novel S-alkyl and/or S-aryl analogs of MTA is likely to contain a small subset of MTA analogs that are more susceptible to cleavage by microbial MTA-Pases than by mammalian MTA-Pases. Our initial success in identifying HETA as one such MTA analog was further validated by a detailed comparison of the mammalian and trypanosome MTA-Pase substrate activities of a selected group of S-alkyl-modified MTA analogs. The Tryp-MTA-Pase substrates had decreasing activity in the following order: MTA (100%) = HETA > ETA (76%) ≅ MFETA ≅ HPTA > PTA (57%) > MFPTA (47%) ≅ APTA. The mammalian-MTA-Pase substrates have decreasing activity in the following order (44): MTA (100%) = PTA ≅ ETA > MFPTA (64%) = MFETA > HETA (34%). The noteworthy increase in the Tryp-MTA-Pase substrate activity of HETA compared with that of ETA and the increase in the Tryp-MTA-Pase substrate activity of HPTA compared with that of PTA are a consequence of the addition of a terminal hydroxyl group. By contrast, the addition of a hydroxyl group reduces the substrate activity toward mammalian MTA-Pase by 60% (i.e., for HETA compared with that of ETA).
Our initial in vitro studies of HETA and MFETA pointed to a direct association between their trypanocidal effects and their efficacies as substrates of Tryp-MTA-Pase and implied that their cytotoxicity is mediated through interference with methionine recycling (8). This is supported by the finding that HETA's in vitro trypanocidal effects can be reversed by the concomitant administration of methionine or 2-keto-4-methylthio-butyrate, the immediate precursor of methionine salvage from MTA (8). However, the current studies, which encompass a more extensive series of purine- and ribose-modified MTA analogs, do not substantiate the link between substrate activities and IC50 values for many compounds. We have shown that HETA has many effects on the metabolism of African trypanosomes. These include increases in AdoMet, S-adenosylhomocysteine, and MTA concentrations to unphysiological levels; decreases in spermidine concentrations; and a reduction of the level of methylation of proteins (3). HETA's trypanocidal effects are enhanced by its extremely rapid uptake and concentration by parasites, with its concentration reaching >800 μM in minutes (3). This suggests that additional factors, such as differences in carrier-mediated transport and the involvement of other biochemical targets, also contribute to the trypanocidal effects of HETA and its analogs. To overcome possible barriers to the active transport of these novel HETA analogs, we prepared, as potential prodrugs, their O-acetylated derivatives, which are expected to readily diffuse across the outer membrane. In fact, O acetylation of several analogs, HET-MPR, HETN, HET-RV, etheno-HETA, 2′,3′-deoxy-HETA, and 3′-deoxy-HETA, markedly enhanced their IC50 values (Table 6).
Enzyme and growth inhibition assays have been used throughout our studies as preliminary screens to select compounds as possible candidates for in vivo evaluations. In seeking compounds with IC50 values ≤1 μM, we identified HETA, MFETA, and 2-fluoro-HETA in prior studies (3, 8); and in the present studies, we identified HETT, tri-O-acetyl-HETT, and di-O-acetyl-3′-deoxy-HETA. HETT, the most cytotoxic compound that we have identified in our in vitro screening assays, is neither a substrate nor an inhibitor of Tryp-MTA-Pase. Table 5 indicates that while HETT is not an inhibitor of Tryp-MTA-Pase, it inhibits the nonphosphorolytic cleavage of MTA and, presumably, other nucleosides. Note the difference in enzyme activity without PO4− in the presence of MTA alone and in the presence of MTA and HETT in Table 5. Since trypanosomes and related kinetoplastid protozoa have extensive purine and purine nucleoside salvage pathways, this finding suggests that some nucleoside analogs which are not cleavable by MTA-Pase may be converted to nucleotides by a nonspecific nucleoside phosphotransferase, such as that which happens with allopurinol in the T. brucei brucei group (17). Taken together, these observations indicate that the potent trypanocidal effects of HETT and 3′-deoxy-HETA are elicited by their interactions with a novel, unidentified molecular target(s). Since neither analog is a substrate of Tryp-MTA-Pase, their potential for selective toxicity against trypanosomes may be diminished. This concern needs to be clarified in future studies. We now recognize that the trypanocidal effects of HETA analogs are not always dependent upon their initial phosphorolytic cleavage by Tryp-MTA-Pase. However, we consider the limited ability of mammalian MTA-Pase to cleave HETA to be a common characteristic of HETA analogs, which protects these compounds from metabolism by their host and enhances their bioavailability to bloodstream trypanosomes.
Our long-term goal is to develop new HETA analogs with improved in vivo properties compared with those of HETA, a compound which has already been demonstrated to have a broad spectrum of curative effects (summarized in Table 7). HETT is by far the most potent trypanocide that we have identified in vitro; and further studies with this compound are warranted to determine its biochemical properties, to identify its molecular targets, and to compare its in vivo trypanocidal effects with those of HETA.
TABLE 7.
Efficacies of HETA and O-acyl-HETA derivatives against T. brucei rhodesiense infections in mice
| Isolate | Efficacya
|
|||
|---|---|---|---|---|
| HETA | Di-OAcb-HETA | Tri-OAc-HETA | Tri-O-propyl-HETA | |
| LAB 110 EATRO | Curative (5/5) | Curative (9/10) | Curative (5/5) | Curative (3/5) |
| KETRI 243 | No effect | No effect | No effect | No effect |
| KETRI 269 | No effect | No effect | Curative (1/5) | No effect |
| KETRI 1992 | No effect | No effect | No effect | No effect |
| KETRI 2002 | Curative (3/5) | Curative (3/5) | No effect | No effect |
| KETRI 2285 | Curative (5/5) | No effect | Curative (3/5) | Curative (3/5) |
| KETRI 2537 | No effect | No effect | No effect | |
| KETRI 2538 | Curative (5/5) | Curative (4/5) | Curative (3/5) | Curative (2/5) |
| KETRI 2545 | No effect | No effect | No effect | |
| KETRI 2772 | Curative (4/5) | Curative (3/5) | Curative (2/5) | |
| ATCC 30027 (Wellcome CT) | No effect | No effect | No effect | |
| ATCC 30119 (EATRO 105) | Curative (4/5) | Curative (5/5) | Curative (1/5) | |
Data are from reference 7. Numbers in parentheses denote the number of cures/total number of mice tested at the reported optimal daily dose (50, 100, 150, or 200 mg/kg of body weight for seven days) of compound.
OAc, O-acetyl.
Supplementary Material
Acknowledgments
These studies were supported in part by grants 920082 (to J.R.S.) and 95094 (to C.J.B.) from the United Nations Development Programme/World Bank/World Health Organization Special Programme for Research and Training in Tropical Diseases and Public Health Service grant AI32975 (to J.R.S.). NMR and mass spectra were provided by the Roswell Park Cancer Institute NMR and Biopolymer Core Facilities, which are supported in part by NCI core grant CA16056 to the Roswell Park Cancer Institute. Support for the synthesis of HET-immucillin A was from NIH and Science and Technology grants to Industrial Research Ltd., Lower Hutt, New Zealand, and the Albert Einstein College of Medicine.
We thank Pharmacia & Upjohn, Inc. (Kalamazoo, MI), for the generous gift of tubercidin. HET-immucillin A was synthesized by Peter C. Tyler of Industrial Research Ltd. and was provided by Vern L. Schramm of the Albert Einstein College of Medicine.
Footnotes
Published ahead of print on 22 October 2007.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Anisuzzaman, A. K. M., and R. L. Whistler. 1978. Selective replacement of primary hydroxyl groups in carbohydrates: preparation of some carbohydrate derivatives containing halomethyl groups. Carbohydr. Res. 61:511-518. [Google Scholar]
- 2.Bacchi, C. J., J. Garofalo, M. A. Ciminelli, D. Rattendi, B. Goldberg, P. P. McCann, and N. Yarlett. 1993. Resistance to dl-α-difluoromethylornithine by clinical isolates of Trypanosoma brucei rhodesiense: role of S-adenosylmethionine. Biochem. Pharmacol. 46:471-481. [DOI] [PubMed] [Google Scholar]
- 3.Bacchi, C. J., B. Goldberg, D. Rattendi, T. E. Gorrell, A. J. Spiess, and J. R. Sufrin. 1999. Metabolic effects of a methylthioadenosine phosphorylase substrate analog on African trypanosomes. Biochem. Pharmacol. 57:89-96. [DOI] [PubMed] [Google Scholar]
- 4.Bacchi, C. J., P. P. McCann, H. C. Nathan, S. H. Hutner, and A. Sjoerdsma. 1982. Antagonism of polyamine metabolism—a critical factor in chemotherapy of African trypanosomiasis. Adv. Polyamine Res. 4:221-231. [Google Scholar]
- 5.Bacchi, C. J., H. C. Nathan, A. B. Clarkson, E. J. Bienen, A. J. Bitonti, P. P. McCann, and A. Sjoerdsma. 1987. Effects of the ornithine decarboxylase inhibitors dl-alpha-difluoromethyl-ornithine and alpha-monofluoromethyl-dehydroornithine methyl ester alone and in combination with suramin against Trypanosoma-brucei brucei central-nervous-system models. Am. J. Trop. Med. Hyg. 36:46-52. [DOI] [PubMed] [Google Scholar]
- 6.Bacchi, C. J., H. C. Nathan, N. Yarlett, B. Goldberg, P. P. McCann, A. J. Bitonti, and A. Sjoerdsma. 1992. Cure of murine Trypanosoma brucei rhodesiense infections with an S-adenosylmethionine decarboxylase inhibitor. Antimicrob. Agents Chemother. 32:2736-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bacchi, C. J., K. Sanabria, A. J. Spiess, M. Vargas, C. J. Marasco, Jr., L. M. Jiminez, B. Goldberg, and J. R. Sufrin. 1997. In vivo efficacies of 5′-methylthioadenosine analogs as trypanocides. Antimicrob. Agents Chemother. 41:2108-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bacchi, C. J., J. R. Sufrin, H. C. Nathan, A. J. Spiess, T. Hannan, J. Garofalo, K. Alecia, L. Katz, and N. Yarlett. 1991. 5′-Alkyl-substituted analogs of 5′-methylthioadenosine as trypanocides. Antimicrob. Agents Chemother. 35:1315-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balasegaram, M., S. Harris, F. Checchi, S. Ghorashian, C. Hamel, and U. Karunakara. 2006. Melarsoprol versus eflornithine for treating late-stage Gambian trypanosomiasis in the Republic of the Congo. Bull. W. H. O. 84:783-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beachum, L. M. 1979. Convenient preparation of 5′-chloro-2′,5′-dideoxyadenosine. J. Org. Chem. 44:3100-3101. [Google Scholar]
- 11.Berens, R. L., E. C. Krug, and J. J. Marr. 1995. Purine and pyrimidine metabolism, p. 89-117. In J. J. Marr and M. Muller (ed.), Biochemistry and molecular biology of parasites. Academic Press, Inc., New York, NY.
- 12.Cartini-Farina, M., A. Oliva, G. Romeo, G. Napolitano, M. De Rosa, A. Giambacorta, and V. Zappia. 1979. 5′-Methylthioadenosine phosphorylase from Caldariella acidophila. Eur. J. Biochem. 101:317-324. [DOI] [PubMed] [Google Scholar]
- 13.Coward, J. K., N. C. Motola, and J. D. Moyer. 1977. Polyamine biosynthesis in rat prostate. Substrate and inhibitor properties of 7-deaza analogues of decarboxylated S-adenosylmethionine and 5′-methylthioadenosine. J. Med. Chem. 20:500-505. [DOI] [PubMed] [Google Scholar]
- 14.Dardonville, C. 2005. Recent advances in antitrypanosomal chemotherapy: patent literature 2002-2004. Expert Opin. Ther. Patents 15:1-17. [Google Scholar]
- 15.Della Ragione, F., and A. E. Pegg. 1983. Effect of analogues of 5′-methylthioadenosine on cellular metabolism. Biochem. J. 210:429-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans, G. B., R. H. Furneaux, V. L. Schramm, V. Singh, and P. C. Tyler. 2004. Targeting the polyamine pathways with transition-state analogue inhibitors of 5′-methylthioadenosine phosphorylase. J. Med. Chem. 47:3275-3281. [DOI] [PubMed] [Google Scholar]
- 17.Fish, W. R., J. J. Marr, R. L. Berens, D. L. Looker, D. J. Nelson, S. LaFon, and A. E. Balber. 1985. Inosine analogs as chemotherapeutic agents for African trypanosomes: metabolism in trypanosomes and efficacy in tissue culture. Antimicrob. Agents Chemother. 27:33-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghoda, L. Y., T. M. Savarese, C. H. Northup, R. E. Parks, Jr., J. Garofalo, L. Katz, B. B. Ellenbogen, and C. J. Bacchi. 1988. Substrate specificities of 5′-deoxy-5′-methylthioadenosine phosphorylase from Trypanosoma brucei brucei and mammalian cells. Mol. Biochem. Parasitol. 27:109-118. [DOI] [PubMed] [Google Scholar]
- 19.Goldberg, B., D. Rattendi, D. Lloyd, J. R. Sufrin, and C. J. Bacchi. 1998. The effects of intermediates of methionine metabolism and nucleoside analogs on S-adenosylmethionine transport by Trypanosoma brucei brucei and a drug-restistant Trypanosoma brucei rhodesiense. Biochem. Pharmacol. 56:95-103. [DOI] [PubMed] [Google Scholar]
- 20.Goldberg, B., D. Rattendi, D. Lloyd, J. R. Sufrin, and C. J. Bacchi. 2001. In situ kinetic characterization of methylthioadenosine transport by the adenosine transporter (P2) of the African Trypanosoma brucei brucei and Trypanosoma brucei rhodesiense. Biochem. Pharmacol. 61:449-457. [DOI] [PubMed] [Google Scholar]
- 21.Goldberg, B., N. Yarlett, D. Rattendi, D. Lloyd, and C. J. Bacchi. 1997. Rapid methylation of cell proteins and lipids in Trypanosoma brucei. J. Eukaryot. Microbiol. 44:345-351. [DOI] [PubMed] [Google Scholar]
- 22.Goldberg, B., N. Yarlett, J. R. Sufrin, D. Lloyd, and C. J. Bacchi. 1997. A unique transporter of S-adenosylmethionine in African trypanosomes. FASEB J. 11:256-260. [DOI] [PubMed] [Google Scholar]
- 23.Hanna, N. B., K. G. Upadha, C. R. Petrie, R. K. Robins, and G. R. Revankar. 1986. Synthesis of certain 5′-substituted derivatives of ribavirin and tiazofuran. Nucleosides Nucleotides 5:343-362. [Google Scholar]
- 24.Hirumi, H., and K. Hirumi. 1989. Continuous cultivation of Trypanosoma brucei bloodstream forms in a medium containing a low concentration of serum protein without feeder cell layers. J. Parasitol. 75:985-989. [PubMed] [Google Scholar]
- 25.Holy, A. 1975. Aliphatic analogues of nucleosides, nucleotides and olignucleotides. Coll. Czech. Chem. Commun. 40:187-214. [Google Scholar]
- 26.Kikugawa, K., K. K. Izuka, Y. Higuchi, H. Hirayama, and M. Ichino. 1972. Platelet aggregation inhibitors. 2. Inhibition of platelet aggregation by 5′-, 2′-, 6′- and 8′-substituted adenosines. J. Med. Chem. 15:387-390. [DOI] [PubMed] [Google Scholar]
- 27.Kuhn, R., and W. Jahn. 1965. Vom adenosine abgeleitete thioather und S-oxide. Chemistry (Berlin) 98:1699-1704. [DOI] [PubMed] [Google Scholar]
- 28.Kung, P.-P., L. R. Zehnder, J. J. Meng, S. W. Kupchinsky, D. J. Skalitzkt, M. C. Johnson, K. A. Maegley, A. Ekker, L. A. Kuhn, P. W. Rose, and L. A. Bloom. 2005. Design, synthesis and biological evaluation of novel human 5′-deoxy-5′methylthioadenosine phosphorylase (MTAP) substrates. Bioorg. Med. Chem. Lett. 15:2829-2833. [DOI] [PubMed] [Google Scholar]
- 29.Legros, D., G. Ollivier, M. Gastellu-Etchegorry, C. Paquet, C. Burri, J. Jannin, et. al. 2002. Treatment of human African trypanosomiasis—present situation and needs for research and development. Lancet Infect. Dis. 2:437-440. [DOI] [PubMed] [Google Scholar]
- 30.Marasco, C. J., Jr., D. L. Kramer, J. Miller, C. W. Porter, C. J. Bacchi, D. Rattendi, L. Kucera, N. Iyer, R. Bernacki, P. Pera, and J. R. Sufrin. 2002. Synthesis and evaluation of analogues of 5′-([(Z)-4-amino-2-butenyl]methylamino)-5′-deoxyadenosine (MDL73811) as inhibitors of tumor cell growth, trypanosomal growth and HIV-1 infectivity. J. Med. Chem. 45:5112-5122. [DOI] [PubMed] [Google Scholar]
- 31.Marasco, C. J., Jr., P. J. Pera, A. J. Spiess, R. J. Bernacki, and J. R. Sufrin. 2005. Improved synthesis of β-d-6-methylpurine riboside and antitumor effects of the β-d- and α-d-anomers. Molecules 10:1015-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meyers, R. W., and R. H. Abeles. 1989. Conversion of 5-S-ethyl-5-thio-d-ribose to ethionine in Klebsiella pneumoniae. Basis for the selective toxicity of 5-S-ethyl-5-thio-d-ribose. J. Biol. Chem. 264:10547-10551. [PubMed] [Google Scholar]
- 33.Montgomery, J. A., and K. Hewson. 1968. A convenient method for the synthesis of 2-fluoroadenosine. J. Org. Chem. 33:432-434. [DOI] [PubMed] [Google Scholar]
- 34.Montgomery, J. A., and K. Hewson. 1969. The preparation of 6-fluoropurines by the modified Schiemann reaction. J. Org. Chem. 34:1396-1399. [Google Scholar]
- 35.Novotny, L., and J. Beranek. 1991. 5′-Chloro-5′-deoxyinosine. 5′-halogeno purine nucleosides in the synthesis of anomalous nucleosides. Nucleic Acids Chem. 4:189-193. [Google Scholar]
- 36.Riscoe, M. K., A. J. Ferro, and J. H. Fitchen. 1989. Methionine recycling as a target for antiprotozoal drug development. Parasitol. Today 5:330-333. [DOI] [PubMed] [Google Scholar]
- 37.Robins, M. J., and P. W. Hatfield. 1982. Nucleic acid related compounds. 37. Convenient and high-yield syntheses of N-[(2-hydroxyethoxy)methyl] heterocycles as “acyclic nucleoside” analogues. Can. J. Chem. 60:547-553. [Google Scholar]
- 38.Robins, M. J., V. Neschadimenko, B.-O. Ro, C.-S. Yuan, R. T. Borchardt, and S. F. Wnuk. 1998. Nucleic acid related compounds. 101. S-adenosyl-l-homocysteine hydrolase does not hydrate (5′-fluoro)vinyl or (6′-halo)homovinyl analogs derived from 3′-deoxyadenosine or 3′-(chloro or fluoro)-3′-deoxyadenosine. J. Org. Chem. 63:1205-1211. [Google Scholar]
- 39.Samejima, K., Y. Nakazawa, and I. Matsusaga. 1978. Improved synthesis of decarboxylated S-adenosylmethionine and related sulfonium compounds. Chem. Pharm. Bull. 26:1480-1485. [Google Scholar]
- 40.Sekowska, A., V. Denervaud, H. Ashida, K. Michoud, D. Haas, A. Yokota, and A. Danchin. 2004. Bacterial variations on the methionine salvage pathway. BMC Microbiol. 4:9. http://www.biomedcentral.com/1471-2180/4/9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sturk, L. M., J. L. Brock, C. R. Bagnell, J. E. Hall, and R. R. Tidwell. 2004. Distribution and quantitation of the anti-trypanosomal diamidine 2,5-bis(4-amidinophenyl)furan (DB75 and its N-methoxy prodrug DB289 in murine brain tissue. Acta Trop. 9:131-143. [DOI] [PubMed] [Google Scholar]
- 42.Sufrin, J. R., S. R. Meshnick, A. J. Spiess, J. Garofalo-Hannan, X.-Q. Pan, and C. J. Bacchi. 1995. Methionine recycling and antimalarial drug design. Antimicrob. Agents Chemother. 39:2511-2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sufrin, J. R., D. Rattendi, A. J. Spiess, S. Lane, C. J. Marasco, and C. J. Bacchi. 1996. Antitrypanosomal activity of purine nucleosides can be enhanced by their conversion to O-acetylated derivatives. Antimicrob. Agents Chemother. 40:2567-2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sufrin, J. R., A. J. Spiess, D. L. Kramer, P. L. Libby, J. T. Miller, R. J. Bernacki, R. T. Borchardt, Y. Lee, and C. W. Porter. 1991. Targeting 5′-deoxy-5′-(methylthio)-adenosine phosphorylase by 5′-haloalkyl analogues of 5′-deoxy-5′-(methylthio)-adenosine. J. Med. Chem. 34:2600-2606. [DOI] [PubMed] [Google Scholar]
- 45.Sufrin, J. R., A. J. Spiess, C. J. Marasco, S. L. Croft, D. Snowdon, V. Yardley, and C. J. Bacchi. 1995. Purine 2′,3′-acyclonucleosides: improved synthesis and antiparasitic activity. Bioorg. Med. Chem. Lett. 5:1961-1964. [Google Scholar]
- 46.Trager, W. 1978. Cultivation of parasites in vitro. Am. J. Trop. Med. Hyg. 27:216-222. [DOI] [PubMed] [Google Scholar]
- 47.Vorbruggen, H., and B. Bennua. 1981. A new simplified nucleoside synthesis. Chemistry (Berlin) 114:1279-1286. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.