Abstract
Small unilamellar amphotericin B liposomes can reduce the toxicity of amphotericin B. In this study, we compared the physical, antifungal, pharmocokinetic, and toxic properties of two liposomal amphotericin B products, AmBisome and Anfogen, that have the same chemical composition but are manufactured differently. In vitro tests included determinations of the MICs and the concentrations causing the release of 50% of the intracellular potassium from red blood cells (K50 values) to assess toxicity. The 50% lethal dose (LD50) was evaluated by using uninfected C57BL/6 mice and single intravenous (i.v.) doses of 1 to 100 mg/kg of body weight. Multiple i.v. dosing over 18 days was performed with 0.5, 1.0, or 5.0 mg of Anfogen/kg or 1.0, 5.0, or 25 mg of AmBisome/kg to evaluate chronic toxicity. DBA/2 mice were infected intranasally with 2.5 × 106 Aspergillus fumigatus conidia, treated for 3 or 4 days with 3.0, 5.0, or 7.5 mg of Anfogen/kg or 3, 5, 7.5, or 15 mg of AmBisome/kg, and evaluated to assess the toxicity of the drugs to the kidneys (by measurement of blood urea nitrogen and creatinine levels and histopathology) and the drug efficacy. The median particle size was 77.8 nm for AmBisome and 111.5 nm for Anfogen. In vitro K50 values were significantly lower for Anfogen (0.9 μg/ml) than for AmBisome (20 μg/ml), and the LD50 of AmBisome was >100 mg/kg, versus 10 mg of Anfogen/kg. There was significant renal tubular necrosis in uninfected and infected mice given Anfogen but no tubular necrosis in AmBisome-treated mice. AmBisome at 7.5 or 15 mg/kg was also more efficacious than 7.5 mg of Anfogen/kg for the treatment of pulmonary aspergillosis, based on survival and weight loss data and numbers of CFU per gram of lung. In conclusion, the efficacy and toxicity of these two liposomal amphotericin B products were significantly different, and thus, the products were not comparable.
The use of the broad-spectrum fungicidal agent amphotericin B (37) has been limited to 1 mg/kg of body weight/day with a maximum cumulative dose of 2 to 4 g (19) because of its well-documented acute infusion-related toxicities and chronic renal toxicity. Three lipid-amphotericin B formulations approved in the United States and Europe have been developed to reduce these toxicities: Amphotec, Abelcet, and AmBisome (hereinafter referred to as AmBi). Each formulation demonstrates significant reduction in toxicities, the extent of which varies among the products. Amphotec (Three Rivers Pharmaceuticals, LLC, Cranberry Township, PA) consists of amphotericin B in a complex with cholesteryl sulfate at a 1:1 molar ratio to form stable colloidal discoidal structures with diameters of 100 ± 22 nm (21), and the single-administration 50% lethal dose (LD50) for mice is 36 mg/kg (21). Abelcet (Enzon Pharmaceuticals, Inc., Bridgewater, NJ), composed of amphotericin B, dimyristoyl phosphatidylcholine, and dimyristoyl phosphatidylglycerol in a 1:1 drug-to-lipid molar ratio, forms ribbon-like complexes (1.6 to 11 μm), with 90% of the particles being smaller than 6 μm in diameter, and the single-administration LD50 for mice is >40 mg/kg (7). AmBi (Gilead Sciences, Inc., Foster City, CA.), a unilamellar liposomal formulation of amphotericin B in which particle diameters are approximately 80 nm, is composed of hydrogenated soy phosphatidylcholine, cholesterol, distearoylphosphatidylglycerol, and amphotericin B in a 2:1:0.8:0.4 molar ratio, and the single-administration LD50 for mice is >175 mg/kg (35).
These different commercialized amphotericin B-lipid formulations vary in their pharmacokinetic profiles (4), although their efficacies in vivo at doses of 5 to 10 mg/kg are similar in preclinical models of candidiasis (17, 20, 34), cryptococcosis (10), aspergillosis (3, 9, 16, 33), and coccidioidomycosis (13, 18). The significantly lower toxicity of AmBi compared to the other formulations, however, has allowed this agent to be effectively used in immunosuppressed and nonimmunosuppressed animals at doses of 10 to 30 mg/kg for the treatment of severe fungal infections including mucormycosis (22), fusariosis (32), cryptococcal meningitis (2), coccidioidal meningitis (8), paracoccidioidomycosis (11), blastomycosis (12), and pulmonary aspergillosis (31).
In formulating amphotericin B liposomal preparations, the association of the amphotericin B with the lipid is critical and must be carefully controlled to ensure that the decreased toxicity of the amphotericin B in the liposome can be maintained from batch to batch, since this control will have a significant impact on the therapeutic index of the drug. Previous reports have demonstrated that for liposomal amphotericin B formulations, even minor alterations in the molar ratios of the drug to phosphatidylcholine and phosphatidylglycerol or variations in the length of the fatty acid chain of the phosphatidylglycerol can significantly alter the single-administration LD50 for mice (1). The process used for making the liposomes can also affect the product's toxicity. An example of this effect comes from the early development of AmBi, when it was reported that liposomes formed by sonication were more toxic in mice than those produced by homogenization even though the liposomes had the same drug-to-lipid molar ratios (1). Jensen et al. (24) reported that other stresses on a liposomal product, such as sterile filtration during production, storage, and lyophilization procedures, also have to be controlled to produce batches that are reproducible from lot to lot and that sensitive assays have to be developed to monitor any changes. Another liposomal amphotericin B preparation, Anfogen (hereinafter referred to as Anfo; Genpharma, S.A., Argentina), was recently licensed in Argentina and is reported to have the same chemical composition as AmBi, but it is manufactured differently (Administración Nacional de Medicamentos, Alimentos y Tecnología Médica [Argentina] certificate of approval no. 51701; Anfo package insert). Given the limited experimental data reported for this new liposomal amphotericin B preparation, the present study was done to compare the physiochemical, toxicity, and efficacy parameters of the two liposomal amphotericin B products AmBi and Anfo to determine if these products have comparable activities in vitro and in vivo.
MATERIALS AND METHODS
Animals.
Female C57BL/6 mice, 6 weeks old at the start of treatment (Harlan, Indianapolis, IN), were used in the studies of uninfected animals. Female DBA/2 mice, 7 weeks old at the start of treatment (Harlan, Indianapolis, IN), were used for the studies with Aspergillus fumigatus-infected animals. Animals were maintained in microisolater cages on a standard rodent diet (Teklad Laboratory rodent diet no. 2918 [18% protein]; Harlan/Teklad, Madison, WI) with water ad libitum. All animal research procedures were approved by the Institutional Animal Care and Use Committee of California State Polytechnic University, Pomona.
Test substances.
Lyophilized AmBi (Gilead Sciences, Inc., San Dimas, CA) and Anfo (Genpharma, S.A., Argentina) were reconstituted according to the manufacturers' instructions to provide final concentrations of 4 mg of AmBi/ml and 5 mg of Anfo/ml. The AmBi and Anfo were diluted in sterile 5% dextrose (D5W) for intravenous (i.v.) injection. These products and amphotericin B deoxycholate (D-AMB; GIBCO [Invitrogen Corp., Grand Island, NY]) were diluted in RPMI 1640 medium containing 0.165 M morpholinepropanesulfonic acid (RPMI-MOPS), pH 7.0, for in vitro MIC testing. D-AMB for the in vitro assay of potassium release from red blood cells (RBCs) to evaluate toxicity was purchased from Priority Healthcare Corporation (Altamonte Springs, FL).
Particle size determination.
Aliquots from each of four vials of AmBi and Anfo were analyzed for volume-weighted median particle size and size distribution parameters as determined by controlled reference dynamic light scattering using a Microtrac ultrafine particle analyzer (Honeywell, Morris Township, NJ).
In vitro RBC potassium release toxicity assay.
The method of Jensen et al. (23) was used for the assay of potassium release from RBCs. A serial dilution of each drug was prepared with D5W as the diluent to provide a range of amphotericin B concentrations from 0.006% (0.24 μg of AmBi/ml, 0.31 μg of Anfo/ml, and 0.31 μg of D-AMB/ml) to 12.5% (500 μg of AmBi/ml, 625 μg of Anfo/ml, and 625 μg of D-AMB/ml) of the original suspension. For each dilution, 50 μl was mixed with 450 μl of washed rat RBCs (Bioreclamation Inc., Hicksville, NY), and the mixture was incubated at 37°C for 4 h. The baseline level (0% release) was then defined as the amount of potassium released from the RBCs after incubation with buffer (147 mM NaCl, 3 mM KCl, 10 mM dibasic sodium phosphate, pH 7.4) (negative control); 100% release was defined as the amount of potassium released from the RBCs after incubation with 10 μM valinomycin (CalBiochem, La Jolla, CA) (positive control). For each agent (AmBi, Anfo, and D-AMB), the concentration of amphotericin B that produced 50% release of the potassium from the RBCs (the K50 value) was then calculated.
Single- and multiple-dose in vivo toxicity studies.
Uninfected (nonimmunosuppressed) C57BL/6 female mice, 6 weeks old (n, 5 per group), were given a single i.v. dose of 1.0, 5.0, 10, or 20 mg of Anfo/kg or 5.0, 10, 50, or 100 mg of AmBi/kg. The animals were monitored daily over a period of 14 days for survival, weight gain or loss, grooming, and activity level. In another study, uninfected C57BL/6 female mice, 6 weeks old (n, 5 per group), were treated i.v. every day for 18 days with 0.5, 1.0, or 5.0 mg of Anfo/kg or 1.0, 5.0, or 25.0 mg of AmBi/kg. The animals were again monitored as described above. Blood was collected by cardiac puncture from the uninfected multidose-receiving animals 12 h after the last dose of the drug, and the sera were analyzed for blood urea nitrogen (BUN) and creatinine levels. Histopathological evaluation was also done on mouse kidneys and lungs collected at the same time point. At necropsy, kidneys and lungs were fixed in 10% neutral buffered formalin. Fixed tissues were processed routinely and stained with hematoxylin and eosin (H&E) for evaluation. Kidney tissues were examined for evidence of treatment-related changes, and severity scores were assigned as follows: minimal (fewer than 25% of tubules affected), mild (25 to 50% of tubules affected), moderate (50 to 75% of tubules affected), and severe (more than 75% of tubules affected). Arterial lesions in the lungs were evaluated semiquantitatively to determine the number of animals per group with vascular changes.
In vitro testing for drug MICs.
A microtiter dilution assay (15) was used to determine the MICs for the yeasts Candida albicans (CP 620), Candida glabrata (ATCC 90030), and Candida parapsilosis (CP 806) and the molds A. fumigatus (ATCC 13073), Aspergillus flavus (ATCC MYA1004), and Aspergillus terreus (ATCC 46941). The yeast cells were prepared by daily subculturing in Sabouraud's dextrose broth for 2 days, pelleting, and rinsing twice with 0.01 M phosphate-buffered saline (PBS), pH 7.2. The final pellets were resuspended in PBS, cells were counted with a hemacytometer, and blastospore suspensions were adjusted with RPMI-MOPS to give 2 × 104 blastospores/ml. The Aspergillus species were cultured on plates with inhibitory mold agar, available as a premixed powder (BBL Microbiology Systems), at 35°C for 9 to 10 days. Conidia were dislodged from the hyphal mats by dispersal in 0.9% saline with 0.05% Tween 80 (Sigma, St. Louis, MO) and stored at 4°C. The conidial count for each species was determined with a hemacytometer, and the conidial suspension was adjusted to 2 × 104 conidia/ml of RPMI-MOPS. The viability of the blastospores or conidia was assessed by plating 200 μl of a given suspension onto inhibitory mold agar plates, followed by incubation at 35°C for 24 to 48 h. Series of twofold dilutions of each drug (0.156 to 80 μg/ml) in RPMI-MOPS were prepared, and 100-μl aliquots of each drug dilution were dispensed into triplicate wells of a 96-well flat-bottom microtiter plate. Final drug concentrations (i.e., concentrations of AmBi, Anfo, or D-AMB) in the wells ranged from 0.078 to 40 μg/ml. Aliquots (100 μl/well) of each test organism were then dispensed into the appropriate wells. Alamar blue (Serotec Ltd., Oxford, United Kingdom; 20 μl/well) was added to all wells, and the plate was incubated at 35°C for 48 h. Negative control wells contained 100 μl of RPMI-MOPS and 100 μl of the drug at 80 μg/ml; positive control wells were made up of 100 μl of RPMI-MOPS and 100 μl of the test organism. The MIC was defined as the lowest concentration of the drug preventing the development of a red color.
Efficacy and toxicity testing with Aspergillus-infected mice.
DBA/2 female mice, 7 weeks old, were immunosuppressed intraperitoneally with triamcinolone at 2 mg/kg (Kenalog-10; Bristol-Myers Squibb Company, Princeton, NJ) on day −2, day 0, and day +2 relative to challenge. The mice were sedated on day 0 with an intraperitoneal injection of 16 mg of xylazine/kg and 80 mg of ketamine/kg and challenged intranasally with 2.5 × 106 conidia of A. fumigatus (ATCC 13073), prepared as described above. Early drug treatment was initiated 2 h postchallenge, with seven groups of mice (n, 17 per group) receiving 3.0, 5.0, or 7.5 mg of i.v. Anfo/kg or 3.0, 5.0, 7.5, or 15 mg of i.v. AmBi/kg. The control group of mice was given i.v. D5W. Seven animals in each group were monitored for survival, weight gain or loss, and disease signs for 14 days, with additional Anfo or AmBi treatments at 24, 48, and 72 h postchallenge. The clinical signs of infection were scored by three independent animal technicians on different days based on the level of grooming, tremors, and inactivity of each mouse in each group. The remaining 10 animals in each group received additional treatments at only 24 and 48 h postchallenge and were sacrificed at 72 h for fungal burden determination, serum chemistry analysis, and histopathology. For fungal burden determination, the lungs of 5 of the remaining 10 mice per group were collected aseptically, weighed, homogenized in 1 ml of PBS, and diluted in PBS, 200-μl aliquots of each dilution were plated in duplicate onto Sabouraud's dextrose agar with 0.05% chloramphenicol, and the plates were incubated at 30°C for 48 h to determine the number of CFU per gram of lung tissue. Blood from these same five animals was taken by cardiac puncture, and their sera were analyzed for BUN and creatinine levels. For histopathological analysis, the kidneys and lungs were collected from the other five mice per group and processed for histopathology as described above. Kidney tissues were examined for evidence of treatment-related changes, and severity scores of minimal, mild, moderate, and severe were assigned. Scoring of the lungs reflected both the severity and the extent of distribution of the changes. Each of the following categories was given a score of 0 (none), 1 (mild), or 2 (moderate to severe): (i) mixed neutrophilic, histiocytic alveolar infiltrate (alveolar exudate), (ii) neutrophilic exudates in airway lumens, (iii) presence of tissue necrosis, and (iv) alveolar hemorrhage and edema. The sum of these scores yielded a total lesion severity score with a possible maximum of 8.
Statistical analysis.
Tissue drug concentrations (micrograms per gram) and tissue fungal burdens (CFU per gram) were analyzed by using GraphPad Prism, version 4.0 (GraphPad Software, Inc., San Diego, CA). A Kruskal-Wallis nonparametric analysis of variance was applied to compare the control to all groups in each experiment, and where differences occurred, a two-tailed Mann-Whitney U test was used for paired-group comparisons. Survival curves were compared using the log rank test. Histological parameters were compared using the two-sided Fisher's exact test. A P value of ≤0.05 was considered significant.
RESULTS
Particle size determination.
Reconstituted AmBi consistently appeared as a clear dispersion with slight opalescence, while reconstituted Anfo appeared as a clear dispersion but with notable haze, and in some vials there were visible particulates. The reconstituted materials in each vial were examined for median particle size and for the upper limit of the range of diameters corresponding to 90% of the particles. The latter measure is referred to as the 90% passing diameter and is an indicator of the presence of particles larger than 100 nm in the small unilamellar vesicle dispersion (24). The results for four vials of each product are presented in Table 1. For AmBi, the median particle size ± the standard deviation was 77.8 ± 2.2 nm. The 90% passing diameter averaged 122.0 ± 4.8 nm. For Anfo, the median diameter was 111.5 ± 96.2 nm, and the 90% passing diameter averaged 273.6 ± 158.5 nm. Even excluding the one vial of Anfo with a very large median particle diameter, the average 90% passing diameter for Anfo was 60% greater than that for AmBi, indicating a substantial presence of particles larger than 100 nm in Anfo compared to AmBi.
TABLE 1.
Median particle diameters and 90% passing diameters as determined by dynamic light scattering for four individual vials of AmBi and Anfo
| Vial no. | Diam (nm) of particles in AmBi:
|
Diam (nm) of particles in Anfo:
|
||
|---|---|---|---|---|
| Median | 90% Passing | Median | 90% Passing | |
| 1 | 75.9 | 115.3 | 68.7 | 184.4 |
| 2 | 76.9 | 126.5 | 256 | 511.1 |
| 3 | 80.9 | 123.1 | 63.2 | 199.1 |
| 4 | 77.5 | 123.2 | 58.4 | 199.7 |
| Avg (SD) | 77.8 (2.2) | 122.0 (4.8) | 111.5 (96.2) | 273.6 (158.5) |
Determination of RBC toxicity based on potassium release.
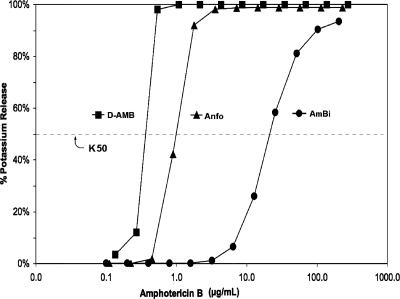
The RBC K50 measurement for AmBi was 20 μg/ml, which was 20- and 50-fold greater than the K50 values for Anfo (K50 = 0.9 μg/ml) and D-AMB (K50 = 0.4 μg/ml), respectively (Fig. 1). The significantly lower toxicity of AmBi for RBCs as reflected in its higher K50 value indicates a higher affinity of amphotericin B for the lipid bilayer of AmBi than for the lipid bilayer of Anfo. D-AMB was the formulation most toxic for the RBCs.
FIG. 1.
Potassium release from rat RBCs measured after 4 h of incubation at 37°C with serial dilutions of AmBi, Anfo, or D-AMB. K50 values were determined for AmBi (K50 = 20 μg/ml), Anfo (K50 = 0.9 μg/ml), and D-AMB (K50 = 0.4 μg/ml).
In vivo single-dose toxicity (LD50) in uninfected mice.
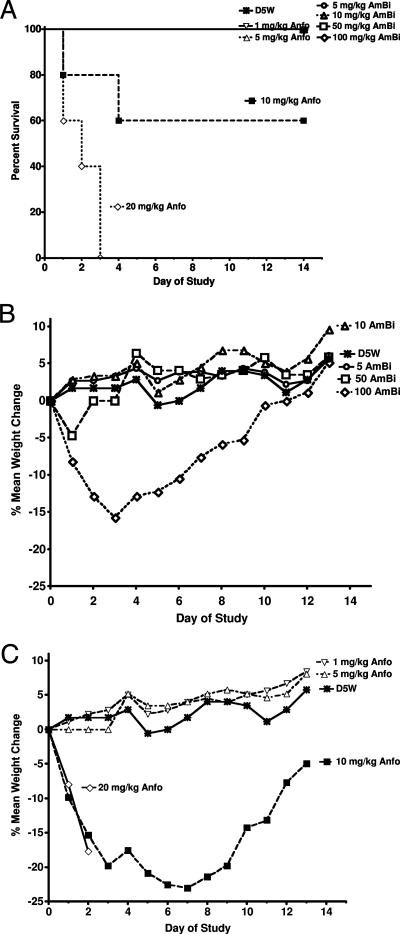
The i.v. delivery of single increasing doses of AmBi or Anfo produced significant differences in toxicity. All of the mice that were given a single dose of 5.0, 10, 50, or 100 mg of AmBi/kg survived (Fig. 2A), and AmBi doses of 5.0, 10, or 50 mg/kg (days 1, 3, 7, and 13) produced no significant weight loss compared to weight changes in mice receiving D5W (P > 0.05) (Fig. 2B). At 100 mg of AmBi/kg (days 1, 3, and 7), significant weight loss compared to the weight changes in mice receiving D5W (P < 0.02) occurred, followed by the recovery of the weight by day 13 (P = 0.54 compared to D5W-treated mice). In comparison, all mice given a single dose of 20 mg of Anfo/kg died by day 3 (0% survival), and there was 40% mortality in the group given 10 mg of Anfo/kg (Fig. 2A). The surviving mice in the 10-mg/kg-Anfo group had significant weight loss compared to the weight changes in D5W-treated mice on day 3 (P = 0.01) and day 7 (P = 0.02), with recovery of the weight by day 13 (P = 0.1 compared to D5W) (Fig. 2C). Those animals given 1 or 5 mg of Anfo/kg, like the D5W-treated control mice, showed no weight loss and continued to gain weight throughout the study period.
FIG. 2.
Single-dose toxicity of Anfo and AmBi for uninfected female C57BL/6 mice. (A) Survival of C57BL/6 female mice (n, 5 per group) given a single i.v. injection of D5W; AmBi at 5.0, 10.0, 50.0, or 100 mg/kg; or Anfo at 1.0, 5.0, 10.0, or 20.0 mg/kg (P = 0.134 for D5W versus Anfo at 10 mg/kg; P < 0.003 for D5W versus Anfo at 20 mg/kg). (B) Mean percent weight changes in groups receiving a single i.v. injection of D5W or AmBi at 5.0, 10.0, 50.0, or 100 mg/kg. (C) Mean percent weight changes in groups receiving a single i.v. injection of D5W or Anfo at 1.0, 5.0, 10.0, or 20.0 mg/kg.
In vivo multiple-dose toxicity in uninfected mice.
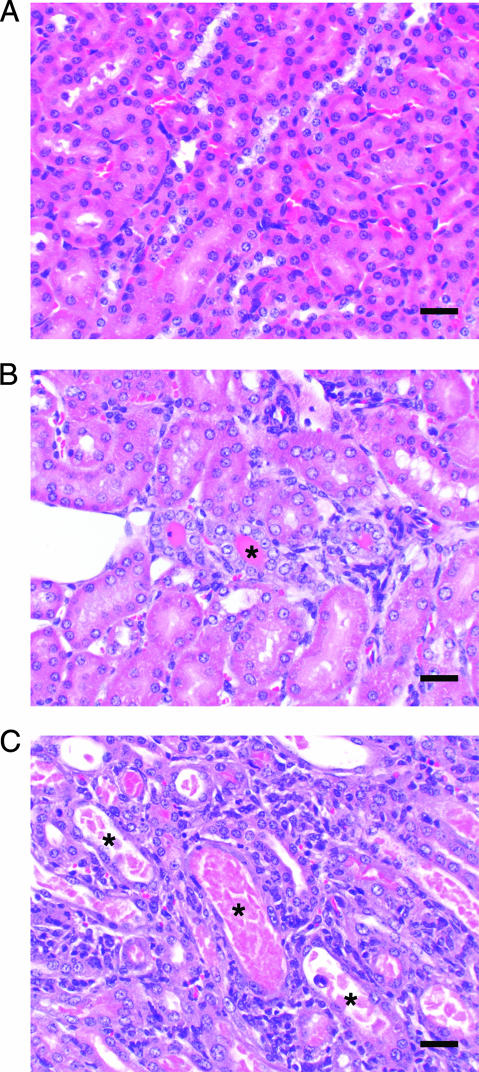
Since the recommended clinical treatment for liposomal amphotericin B is dosing for a period of 2 to 4 weeks, depending upon the patient and the type of fungal infection (26, 29, 39), we compared the toxicities of these two liposome products in a multiple-dose setting. Doses of each drug were selected based on the weight loss data from uninfected mice in the single-dose toxicity testing and included 0.5, 1.0, and 5.0 mg of Anfo/kg and 1.0, 5.0, and 25 mg of AmBi/kg. In the multiple-dose testing, mice in all test groups showed consistent weight gain throughout the 18-day study, similar to that of the control D5W mice, with an overall weight gain of about 6% for all groups, except the 1.0-mg/kg-AmBi group, which gained 8% (data not shown). The BUN and creatinine levels measured 12 h after the last drug treatment were all comparable to those for the D5W-treated control mice. However, the examination of the kidneys and lungs from the 5.0-mg/kg-Anfo group revealed significantly more histopathological alterations in the kidneys and lungs of these mice than in those of the AmBi mice (Table 2 and Fig. 3). In the kidneys of mice given 5.0 mg of Anfo/kg (Fig. 3C), there was significantly more tubular damage, manifested as tubular regeneration with concurrent degeneration and necrosis, than was seen in the kidneys of D5W-treated mice (Fig. 3A) and mice treated with 5.0 mg of AmBi/kg (Table 2; Fig. 3B). Pulmonary arterial changes including intimal and medial proliferation with concurrent transmural and perivascular inflammation were also more frequently observed in mice treated with 5.0 mg of Anfo/kg than in the mice treated with 25 mg of AmBi/kg (P = 0.048) (Table 2). In Anfo-treated mice, both the kidney and lung findings increased in severity in a dose-dependent fashion.
TABLE 2.
Histopathological evaluation of uninfected mice treated with AmBi or Anfo
| Treatment group | No. of mice with minimal renal tubular regeneration/ no. of mice in treatment group | No. of mice with renal tubular regeneration with concurrent degeneration and/or necrosis/no. of mice in treatment group | No. of mice with renal tubular regeneration with concurrent degeneration and/or necrosis classified as:
|
No. of mice with pulmonary arteriopathy/no. of mice in treatment group | |
|---|---|---|---|---|---|
| Minimal | Mild | ||||
| Control D5W | 0/6 | 0/6 | 0/6 | ||
| Anfo at: | |||||
| 0.5 mg/kg | 0/5 | 1/5 | 1 | 0/5 | |
| 1.0 mg/kg | 2/5 | 2/5 | 2 | 1/5 | |
| 5.0 mg/kg | 0/5a | 5/5b | 0 | 5c | 4/5d,e |
| AmBi at: | |||||
| 1.0 mg/kg | 1/5 | 2/5 | 2 | 1/5 | |
| 5.0 mg/kg | 2/5a | 2/5b | 2 | 0c | 1/5d |
| 25 mg/kg | 3/5 | 1/5 | 1 | 0/5e | |
P = 0.444 for Anfo at 5.0 mg/kg versus AmBi at 5.0 mg/kg for minimal tubular regeneration; two-tailed Fisher's exact test.
P = 0.167 for Anfo at 5.0 mg/kg versus AmBi at 5.0 mg/kg for tubular regeneration with concurrent degeneration; two-tailed Fisher's exact test.
P = 0.008 for Anfo at 5.0 mg/kg versus AmBi at 5.0 mg/kg for tubular regeneration with concurrent degeneration and/or mild necrosis; two-tailed Fisher's exact test.
P = 0.206 for Anfo at 5.0 mg/kg versus AmBi at 5.0 mg/kg for pulmonary arteriopathy; two-tailed Fisher's exact test.
P = 0.048 or Anfo at 5.0 mg/kg versus AmBi at 25.0 mg/kg for pulmonary arteriopathy; two-tailed Fisher's exact test.
FIG. 3.
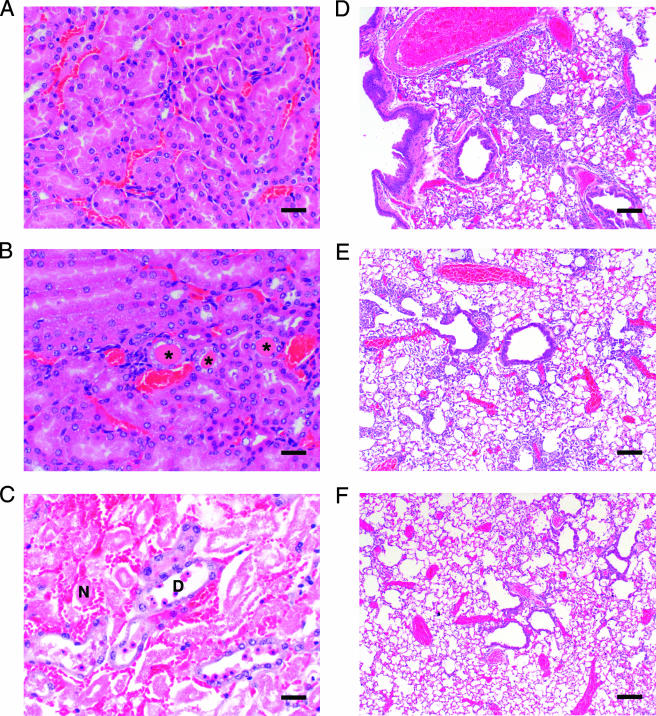
Kidney histopathology of Anfo- and AmBi-treated mice. (A) Appearance of normal renal tubules in a kidney from a representative uninfected female C57BL/6 mouse treated i.v. with 18 daily doses of D5W (control; H&E-stained sample shown at a magnification of ×200). Bar, 30 μm. (B) H&E-stained kidney from a representative uninfected female C57BL/6 mouse treated i.v. with 18 daily doses of 5 mg of AmBi/kg. Rare tubules lined by regenerative epithelium with intraluminal protein casts (asterisk) are visible. Bar, 30 μm. (C) H&E-stained kidney from a representative uninfected female C57BL/6 mouse treated i.v. with 18 daily doses of 5 mg of Anfo/kg. Shown are clusters of dilated renal tubules lined by attenuated epithelium, often filled with cellular debris or granular casts (asterisks) and surrounded by small numbers of inflammatory cells. Bar, 30 μm.
In vitro testing for drug MICs.
The in vitro activities of Anfo, AmBi, and D-AMB against both pathogenic yeasts and molds were determined. The MICs of all three drugs were very similar for C. albicans, C. glabrata, and C. parapsilosis (0.313 to 1.25 μg/ml), as well as for A. fumigatus and A. terreus (0.625 to 2.5 μg/ml). For A. flavus, the MIC of D-AMB was 2.5 μg/ml and that of Anfo was 20 μg/ml, and unlike the other two polyene formulations, AmBi had no in vitro activity at 40 μg/ml against this strain of A. flavus.
Efficacy of AmBi and Anfo in Aspergillus-infected mice.
With comparable in vitro activities against A. fumigatus for the two liposome products, the efficacies of the drugs were compared in a murine model of early treatment of pulmonary A. fumigatus infection. Doses for this study (3, 5, and 7.5 mg of Anfo/kg and 3, 5, 7.5, and 15 mg of AmBi/kg) were selected based on the survival of the mice in the single- and multiple-dose studies. A 15-mg/kg dose of Anfo was not included because even 10 mg of Anfo/kg caused deaths in uninfected mice. Aspergillus-infected animals were given their first drug treatment beginning 2 h post-intranasal fungal challenge, and the treatments were repeated at 24, 48, and 72 h postinfection for the survival studies and at 24 and 48 h for the fungal burden and toxicity studies.
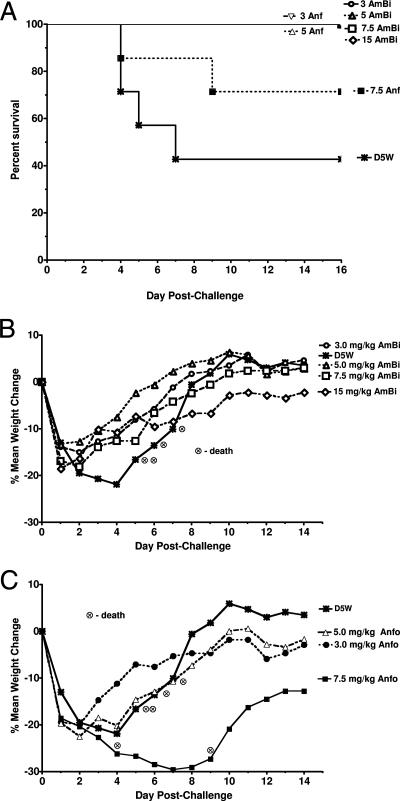
There were no deaths among any of the mice given 3.0- or 5.0-mg/kg doses of either drug, although there were two deaths (19% mortality) in the 7.5-mg/kg-Anfo group and four deaths (57% mortality) in the D5W control group (Fig. 4A). Mice in the control group died between days 4 and 7, with a maximum weight loss on day 4 and survivors regaining their initial weights by day 8 (Fig. 4B and C). In the 3.0-, 5.0-, 7.5-, and 15-mg/kg-AmBi groups, there was marked weight loss by day 2, but mice in all the groups began to regain their weight on day 3, with complete recovery of their original weights by days 6 to 9 except for the mice in the 15-mg/kg-AmBi group (Fig. 4B). Similarly, those mice given 3.0 or 5.0 mg of Anfo/kg also had marked weight loss by day 2 and recovery of their initial weights by day 10 (Fig. 4C). In contrast, the animals given 7.5 mg of Anfo/kg continued to lose weight, with a nadir of 30% weight loss on day 7. Survivors in the 7.5-mg/kg-Anfo group had the lowest weights at the end of the study and did not recover their initial weights. In a separate study of uninfected mice (data not shown), we compared the weights of non-triamcinolone-treated DBA/2 female mice with those of triamcinolone-treated mice (7 weeks old; n, 10 per group) 24 h after the last steroid treatment. We used the same triamcinolone regimen that was used in the efficacy study. Although the triamcinolone treatment was associated with slight weight loss (1.8%) and the non-triamcinolone-treated mice had slight weight gain (1.7%), the difference in weight changes between the triamcinolone-treated mice and the non-triamcinolone-treated mice was not significant (P = 0.12). Thus, the marked changes in weight in the infected animals were due primarily to the infection, not the triamcinolone treatment. The clinical signs paralleled the weight changes, with the control group and the 7.5-mg/kg-Anfo group having the most severe and sustained signs, the 3.0- and 5.0-mg/kg-Anfo groups having moderate clinical signs from days 1 to 3, and the AmBi groups having only minimal signs (data not shown).
FIG. 4.
Comparative efficacy of multidose AmBi and Anfo in triamcinolone-immunosuppressed C57BL/6 female mice (n, 7 per group) challenged intranasally with A. fumigatus (2.6 × 106 spores/mouse). (A) Survival of mice given four i.v. doses of D5W; AmBi at 3.0, 5.0, 7.5, or 15 mg/kg; or Anfo at 3.0, 5.0, or 7.5 mg/kg. P, 0.022 for D5W versus 3.0 and 5.0 mg of Anfo/kg and 3.0, 5.0, 7.5, and 15 mg of AmBi/kg; D5W versus 7.5 mg of Anfo/kg, not significant. (B) Percent mean weight changes for mice given four i.v. doses of D5W or AmBi at 3.0, 5.0, 7.5, or 15 mg/kg. (C) Percent mean weight changes for mice given four i.v. doses of D5W or Anfo at 3.0, 5.0, or 7.5 mg/kg. Observed deaths are indicated, and data for mice who died were not included in weight change calculations.
Toxicity of AmBi and Anfo in Aspergillus-infected mice.
Animals infected with A. fumigatus as described above were evaluated for drug toxicity 24 h after the third drug treatment. This evaluation was done by measuring BUN and creatinine levels and examining histopathological changes in the kidneys and lungs. There was significant elevation in BUN levels in the 7.5-mg/kg-Anfo group compared to the levels in controls (Table 3) (P = 0.008), and the BUN levels were also elevated in mice given 5 mg of Anfo/kg, although the increase was not significant. The BUN levels in all AmBi-treated mice (those receiving 3.0, 5.0, 7.5, and 15 mg/kg) were comparable to those in controls. The creatinine levels in the 7.5-mg/kg-Anfo group were also significantly higher than those in controls (P = 0.008) (Table 3B), and none of the other Anfo or AmBi groups had significantly elevated creatinine levels (Table 3B).
TABLE 3.
BUN levels and blood creatinine levels (means ± standard errors) for groups of mice infected with A. fumigatus and treated three times with the indicated doses of D5W, Anfo, or AmBi
| Measurement | Treatment group | Level (mg/dl) in samples from mice receiving 3 doses of:
|
||||
|---|---|---|---|---|---|---|
| D5W | 3 mg/kg | 5 mg/kg | 7.5 mg/kg | 15 mg/kg | ||
| BUN | Control (D5W) | 12.80 ± 1.50 | ||||
| Anfo | 14.00 ± 1.30 | 22.20 ± 6.73 | 78.20a ± 8.09 | |||
| AmBi | 12.60 ± 0.81 | 13.60 ± 1.03 | 12.00 ± 0.45 | 17.20 ± 0.92 | ||
| Creatinine | Control (D5W) | 0.34 ± 0.02 | ||||
| Anfo | 0.36 ± 0.02 | 0.42 ± 0.02 | 0.64a ± 0.05 | |||
| AmBi | 0.34 ± 0.02 | 0.38 ± 0.02 | 0.36 ± 0.02 | 0.38 ± 0.02 | ||
P = 0.008 versus D5W; Mann-Whitney test.
Histopathological examination of the kidneys of all AmBi-treated mice showed only minimal tubular damage, rare dilated degenerative tubules, and no evidence of more severe, acute tubular necrosis (Table 4; Fig. 5B). In comparison, acute tubular necrosis could be detected in animals that received 5.0 or 7.5 mg of Anfo/kg (P = 0.02 for 7.5 mg of Anfo/kg versus D5W; P = 0.008 for 7.5 mg of Anfo/kg versus 7.5 or 15 mg of AmBi/kg) (Table 4; Fig. 5C). Some tubular degeneration in the kidneys of mice in all test groups, except the 3-mg/kg-AmBi group, was observed. In the lungs, pneumonia was seen in all animals, although lesion severity was greater in the controls than in the 5-mg/kg- and 15-mg/kg-AmBi groups and the 3-mg/kg-, 5-mg/kg-, and 7.5-mg/kg-Anfo groups (Table 4). Lesion severity was also greater in the 3-mg/kg-AmBi group than in the 3-mg/kg-Anfo group. Lesion severity decreased in a dose-dependent fashion with AmBi treatment. All groups had spherical as well as rare elongating fungal elements admixed with inflammatory cells in the lung tissue. The inflammatory cell infiltrate was composed primarily of neutrophils and histiocytes, with lesser numbers of perivascular lymphocytes (Table 4; Fig. 5D to F).
TABLE 4.
Histopathological incidence and severity of kidney and lung lesions in mice infected with A. fumigatus and treated with AmBi or Anfo
| Treatment group | No. of mice with acute tubular necrosis and degeneration in kidneys/no of mice in treatment group | No. of mice with acute tubular necrosis and degeneration classified as:
|
No. of mice with histiocytic, neutrophilic, multifocal bronchopneumonia in lungs/no. of mice in treatment group | Mean lesion severity score | ||
|---|---|---|---|---|---|---|
| Minimal | Mild | Moderate | ||||
| Control (D5W) | 0/3a,b | 3/3 | 5.7f,g | |||
| Anfo at: | ||||||
| 3.0 mg/kg | 0/5 | 5/5 | 2.4f,h | |||
| 5.0 mg/kg | 2/5a,c | 1 | 1 | 5/5 | 1.8f | |
| 7.5 mg/kg | 5/5b,d,e | 2 | 3 | 5/5 | 2.2f | |
| AmBi at: | ||||||
| 3.0 mg/kg | 0/5 | 5/5 | 4.6h | |||
| 5.0 mg/kg | 0/5c | 5/5 | 3.2g | |||
| 7.5 mg/kg | 0/5d | 5/5 | 2.6 | |||
| 15.0 mg/kg | 0/5e | 5/5 | 2.0g | |||
P = 0.464 for Anfo at 5.0 mg/kg versus control; two-tailed Fisher's exact test.
P = 0.018 for Anfo at 7.5 mg/kg versus control; two-tailed Fisher's exact test.
P = 0.444 for Anfo at 5.0 mg/kg versus AmBi at 5.0 mg/kg; two-tailed Fisher's exact test.
P = 0.008 for Anfo at 7.5 mg/kg versus AmBi at 7.5 mg/kg; two-tailed Fisher's exact test.
P = 0.008 for Anfo at 7.5 mg/kg versus AmBi at 15 mg/kg; two-tailed Fisher's exact test.
P = 0.017, 0.034, and 0.023, respectively, for control versus Anfo at 3.0, 5.0, and 7.5 mg/kg; Mann-Whitney U test.
P = 0.041 for control versus AmBi at 5.0 and 15.0 mg/kg; Mann-Whitney U test.
P = 0.021 for Anfo at 3.0 mg/kg versus AmBi at 3.0 mg/kg; Mann-Whitney U test.
FIG. 5.
Kidney and lung histopathology of Anfo- and AmBi-treated mice. (A) Appearance of normal renal tubules in an H&E-stained kidney from a representative A. fumigatus-infected female DBA/2 mouse treated i.v. with three daily doses of D5W (control). Bar, 30 μm. (B) H&E-stained kidney from a representative A. fumigatus-infected female DBA/2 mouse treated i.v. with three daily doses of 7.5 mg of AmBi/kg. Rare tubules lined by attenuated epithelium with intraluminal protein casts (asterisks) are visible. Bar, 30 μm. (C) H&E-stained kidney from a representative A. fumigatus-infected female DBA/2 mouse treated i.v. with three daily doses of 7.5 mg of Anfo/kg. Shown are clusters of dilated degenerative tubules (D) containing exfoliated necrotic epithelium admixed with necrotic tubules (N) completely devoid of epithelial-lining cells containing intraluminal granular casts surrounded by congested capillaries and interstitial hemorrhage. Bar, 30 μm. (D) H&E-stained lung from a representative A. fumigatus-infected female DBA/2 mouse treated i.v. with three daily doses of D5W (control). The sample shows evidence of pneumonia characterized by histiocytic and neutrophilic inflammation within terminal airways and alveoli. Necrosis is limited, and angioinvasion is absent. Bar, 150 μm. (E) H&E-stained lung from a representative A. fumigatus-infected female DBA/2 mouse treated i.v. with three daily doses of 7.5 mg of AmBi/kg. The sample shows evidence of pneumonia similar in character to that seen in D5W-treated mice but decreased in severity. Bar, 150 μm. (F) H&E-stained lung from a representative A. fumigatus-infected female DBA/2 mouse treated i.v. with three daily doses of 7.5 mg of Anfo/kg. The sample shows evidence of pneumonia similar in character to that seen in D5W-treated mice but decreased in severity. Bar, 150 μm.
Fungal burden (CFU per gram) in the lungs of Aspergillus-infected mice.
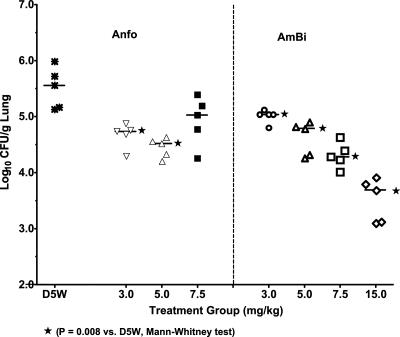
The degree of fungal clearance from the infected lungs was evaluated by assessing the number of CFU per gram of lung 24 h after the third drug treatment. Anfo at 3.0 or 5.0 mg/kg reduced the fungal burden significantly compared to the burden in controls (P = 0.008), although Anfo at 7.5 mg/kg did not (Fig. 6). In comparison, all four AmBi dose regimens (3.0, 5.0, 7.5, and 15.0 mg/kg) reduced the number of CFU per gram of lung significantly compared to that in the controls (P = 0.008), and there was a dose-dependent reduction in the lung fungal burden. Comparisons of numbers of CFU per gram of lung between Anfo and AmBi treatment groups showed that 3.0 mg of Anfo/kg was better than 3.0 mg of AmBi/kg (P = 0.02) but that 5.0 mg of Anfo/kg and 5.0 mg of AmBi/kg were comparable. The highest tested dose of AmBi (i.e., 15 mg/kg) was the most effective, reducing the fungal burden by about 100-fold.
FIG. 6.
CFU per gram of lung from triamcinolone-immunosuppressed C57BL/6 female mice (n, 5 per group) infected intranasally with A. fumigatus (2.6 × 106 spores/mouse). Three i.v. treatments with D5W (control); Anfo at 3.0, 5.0, or 7.5 mg/kg; or AmBi at 3.0, 5.0, 7.5, or 15 mg/kg were given, and lungs were collected from mice sacrificed 24 h after the third treatment. Bars indicate median values for each group. (P < 0.01 for D5W versus 3.0 or 5.0 mg of Anfo/kg; P = 0.095 for D5W versus 7.5 mg of Anfo/kg; P < 0.01 for D5W versus all AmBi doses; P = 0.056 for 7.5 mg of Anfo/kg versus 7.5 mg of AmBi/kg; P = 0.016 for 3.0 mg of Anfo/kg versus 3.0 mg of AmBi/kg; P < 0.008 for 15 mg of AmBi/kg versus 3.0, 5.0, and 7.5 mg of AmBi/kg and 3.0, 5.0, and 7.5 mg of Anfo/kg).
DISCUSSION
Although Anfo and AmBi have the same chemical composition, they are manufactured differently. The present studies demonstrated marked differences in the particle sizes and the toxicity and efficacy profiles for these two preparations, with AmBi being 10-fold less toxic than Anfo for uninfected mice. At the doses at which Anfo was nontoxic (3.0 and 5.0 mg/kg), Anfo and AmBi showed equivalent levels of efficacy in A. fumigatus-infected mice, but AmBi at doses of 7.5 and 15 mg/kg was less toxic and more efficacious than Anfo at 7.5 mg/kg. The results of the comparison of these two liposomal amphotericin B products underscore the importance of controlling not only the chemical composition but also the process parameters and the quality control testing of the liposomes. Various stresses on liposomes, such as sterile filtration during production and filtration prior to use, requirements for refrigeration, lyophilization conditions, and dilution in infusion diluents, can lead to flocculation, aggregation, leakage of the drug, phase separation, and disintegration of the liposomes (24). To adequately monitor these variables, strict product quality control assays that include extensive chemical, physical, and biological testing should be done to ensure uniformity of the final product.
To acquire preliminary data about the acute toxicity of a liposomal amphotericin B product, investigators have used in vitro RBC toxicity tests because of the membrane leakage and disruption associated with exposure to low doses of amphotericin B (25). It should be noted, however, that these tests have limited utility for accurately predicting the acute intravenous in vivo toxicity of liposomal amphotericin B unless side-by-side testing of a product by both in vitro and in vivo standardized assays is done to establish a direct correlation between the results of the in vitro and in vivo assays. This testing has been done with the K50 assay described in these studies (23). In vitro RBC toxicity tests cannot be used to predict in vivo amphotericin B nephrotoxicity, which is associated with chronic drug dosing (28). The chronic toxicity of amphotericin B is of particular concern since life-threatening fungal infections often require several weeks of amphotericin B therapy (19) in patients who may be receiving other nephrotoxic drugs (36). In vivo animal testing to evaluate BUN and creatinine levels, along with histopathological examination of the kidneys, similar to what was done in the present study, is needed to determine if repeated dosing with a particular liposomal amphotericin B preparation will cause chronic toxicity.
The C57BL/6 strain of mice was chosen for toxicity testing in these experiments because this strain of mice is particularly sensitive to the toxic effects of amphotericin B (5) and provides a stringent test for determining tolerance to increasing doses of amphotericin B. In a comparison with Anfo in uninfected C57BL/6 mice, we demonstrated that AmBi was at least 10-fold less toxic based on single-i.v.-administration LD50 testing. The i.v. route of administration was used for these tests because it simulates the drug's clinical use. Although the intraperitoneal administration of amphotericin B is much better tolerated in mice than i.v. delivery (2), we recently reported that the biodistributions of amphotericin B following i.v. and intraperitoneal administration are not comparable (6). In multiple-dose testing of the uninfected mice, the BUN and creatinine levels did not differ between the AmBi and Anfo groups but there was a significant increase in chronic tubular degeneration and necrosis in the kidneys of mice given 5.0 mg of Anfo/kg, emphasizing the value of using histopathology to evaluate nephrotoxicity in mice. Although pulmonary arterial lesions also occurred at increased frequency and severity in the uninfected mice treated with 5.0 mg of Anfo/kg, the pathogenesis of the vascular lesions was uncertain.
To obtain a comprehensive evaluation of a liposomal amphotericin B preparation, tests of infected animals should also be done since there are marked changes in physiology during infection. We selected an A. fumigatus pulmonary infection model for these experiments, as A. fumigatus causes 56% of aspergillosis infections (30) and AmBi is frequently used to treat patients with probable or proven aspergillosis (14). Our MIC data showed that Anfo and AmBi had comparable in vitro activities against the strain of A. fumigatus used to infect the mice. In the present study, dosing for only 3 days produced acute tubular necrosis in Aspergillus-infected animals that were given 5.0 or 7.5 mg of Anfo/kg whereas no mice treated with AmBi (at up to 15 mg/kg) had any evidence of acute tubular necrosis. In addition, BUN and creatinine levels were significantly increased in the infected mice treated with 7.5 mg of Anfo/kg for 3 days, unlike the uninfected mice treated with Anfo for 18 days. None of the AmBi-treated mice had elevated levels. The increased toxicity of an amphotericin B-lipid preparation for infected 7- to 8-week-old DBA/2 triamcinolone-immunosuppressed mice compared to that for uninfected mice seen in the present study was observed previously in a different murine pulmonary aspergillosis model. In the previous study, young DBA/2 mice (3 to 4 weeks old) were immunosuppressed with the cytotoxic drug cyclophosphamide and treated at 2 h post-A. fumigatus challenge with either AmBi or Abelcet. Unlike AmBi, Abelcet treatment was associated with increased toxicity in the infected mice compared to that in the uninfected mice (31).
The optimum treatment for pulmonary aspergillosis in the present study was achieved with 15 mg of AmBi/kg, which reduced the lung fungal burden by 100-fold and caused no significant nephrotoxicity. In comparison, the highest tolerable dose of Anfo (i.e., 7.5 mg/kg) produced significant nephrotoxicity. The group receiving this Anfo dose also had no significant reduction in lung fungal burden compared to controls. One possible explanation for the latter observation may be related to a severe pH imbalance in the lungs of the mice receiving 7.5 mg of Anfo/kg caused by the combined effect of respiratory acidosis and kidney damage, since normal kidneys play a significant role in modulating respiratory acid-base disorders associated with pneumonia (27). The lower pH may increase CO2 in the lungs and decrease the concentration of oxygen, which may lead to a reduction in the oxidative activity of amphotericin B and result in a decrease in fungal lysis (38).
In conclusion, the results presented here from a variety of preclinical tests showed that the AmBi and Anfo were not comparable, despite the fact that the chemical composition of the products was the same. The association between the carrier and the active agent, amphotericin B, can be significantly altered by the processes used to prepare the product, and this association is critical for obtaining the desired therapeutic index of the carrier-drug preparation. By combining particle size determination, in vitro testing, in vivo single- and multiple-dose toxicity testing, measurement of BUN and creatinine levels, and histopathological evaluation of both uninfected and infected animals, a more accurate assessment of the drug's potential performance in the clinic can be achieved than can be provided by any of these tests alone.
Acknowledgments
We thank David Constable, Peter Smith, and Tarquinus Bunch for their excellent technical support.
Support for this research was provided by a research grant from Gilead Sciences, Inc. J.P.A.-M. has received funds for speaking at symposia organized on behalf of Gilead Sciences, Inc. G.M.J. is an employee of Gilead Sciences. J.A.O., J.P.A.-M., J.S., and R.T.P. have received funds for research support from Gilead Sciences, Inc.
Footnotes
Published ahead of print on 29 October 2007.
REFERENCES
- 1.Adler-Moore, J. P., and R. T. Proffitt. 1993. Development, characterization, efficacy and mode of action of AmBisome, a unilamellar liposomal formulation of amphotericin B. J. Liposome Res. 3:429-450. [Google Scholar]
- 2.Albert, M. M., L. Stahl-Carroll, M. F. Luther, and J. R. Graybill. 1995. Comparison of liposomal amphotericin B to amphotericin B for treatment of murine cryptococcal meningitis. J. Mycol. Med. 5:1-6. [Google Scholar]
- 3.Allende, M. C., J. W. Lee, P. Francis, K. Garrett, H. Dollenberg, J. Berenguer, C. A. Lyman, P. A. Pizzo, and T. J. Walsh. 1994. Dose-dependent antifungal activity and nephrotoxicity of amphotericin B colloidal dispersion in experimental pulmonary aspergillosis. Antimicrob. Agents Chemother. 38:518-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boswell, G. W., D. Buell, and I. Bekersky. 1998. AmBisome (liposomal amphotericin B): a comparative review. J. Clin. Pharmacol. 38:583-592. [DOI] [PubMed] [Google Scholar]
- 5.Brajtburg, J., S. Elberg, G. S. Kobayashi, and G. Medoff. 1986. Toxicity and induction of resistance to Listeria monocytogenes infection by amphotericin B in inbred strains of mice. Infect. Immun. 54:303-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang, T., J. Olson, and J. Adler-Moore. 2007. Tissue concentrations following IV or IP amphotericin B (Amp) or liposomal amphotericin B (L-AmB) treatment, abstr. P-0011, p. 209. Focus on Fungal Infections 17, San Diego, CA.
- 7.Clark, J. M., R. R. Whitney, S. J. Olsen, R. J. George, M. R. Swerdel, L. Kunselman, and D. P. Bonner. 1991. Amphotericin B lipid complex therapy of experimental fungal infections in mice. Antimicrob. Agents Chemother. 35:615-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clemons, K. V., R. A. Sobel, P. L. Williams, D. Pappagianis, and D. A. Stevens. 2002. Efficacy of intravenous liposomal amphotericin B (AmBisome) against coccidioidal meningitis in rabbits. Antimicrob. Agents Chemother. 46:2420-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clemons, K. V., and D. A. Stevens. 2004. Comparative efficacies of four amphotericin B formulations—Fungizone, Amphotec (Amphocil), AmBisome, and Abelcet—against systemic murine aspergillosis. Antimicrob. Agents Chemother. 48:1047-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clemons, K. V., and D. A. Stevens. 1998. Comparison of Fungizone, Amphotec, AmBisome, and Abelcet for treatment of systemic murine cryptococcosis. Antimicrob. Agents Chemother. 42:899-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clemons, K. V., and D. A. Stevens. 1993. Comparison of a liposomal amphotericin B formulation (AmBisome) and deoxycholate amphotericin B (Fungizone) for the treatment of murine paracoccidioidomycosis. J. Med. Vet. Mycol. 31:387-394. [Google Scholar]
- 12.Clemons, K. V., and D. A. Stevens. 1993. Therapeutic efficacy of a liposomal formulation of amphotericin B (AmBisome) against murine blastomycosis. J. Antimicrob. Chemother. 32:465-472. [DOI] [PubMed] [Google Scholar]
- 13.Clemons, K. V., and D. A. Stevens. 1991. Comparative efficacy of amphotericin B colloidal dispersion and amphotericin B deoxycholate suspension in treatment of murine coccidioidomycosis. Antimicrob. Agents Chemother. 35:1829-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cornely, O. A., J. Maertens, M. Bresnik, R. Ebrahimi, A. J. Ullmann, E. Bouza, C. P. Heussel, O. Lortholary, C. Rieger, A. Boehme, M. Aoun, H. A. Horst, A. Thiebaut, M. Ruhnke, D. Reichert, N. Vianelli, S. W. Krause, E. Olavarria, R. Herbrecht, and the AmBiLoad Trial Study Group. 2007. Liposomal amphotericin B as initial therapy for invasive mold infection: a randomized trial comparing a high-loading dose regimen with standard dosing (AmBiLoad trial). Clin. Infect. Dis. 44:1289-1297. [DOI] [PubMed] [Google Scholar]
- 15.Espinel-Ingroff, A., J. L. Rodriguez-Tudela, and J. V. Martinez-Suarez. 1995. Comparison of two alternative microdilution procedures with the National Committee for Clinical Laboratory Standards reference macrodilution method M27-P for in vitro testing of fluconazole-resistant and -susceptible isolates of Candida albicans. J. Clin. Microbiol. 33:3154-3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Francis, P., J. W. Lee, A. Hoffman, J. Peter, A. Francesconi, J. Bacher, J. Shelhamer, P. A. Pizzo, and T. J. Walsh. 1994. Efficacy of unilamellar liposomal amphotericin B in treatment of pulmonary aspergillosis in persistently granulocytopenic rabbits: the potential role of bronchoalveolar d-mannitol and serum galactomannan as markers of infection. J. Infect. Dis. 169:356-368. [DOI] [PubMed] [Google Scholar]
- 17.Gondal, J. A., R. P. Swartz, and A. Rahman. 1989. Therapeutic evaluation of free and liposome-encapsulated amphotericin B in the treatment of systemic candidiasis in mice. Antimicrob. Agents Chemother. 33:1544-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzalez, G. M., R. Tijerina, L. K. Najvar, R. Bocanegra, M. G. Rinaldi, and J. R. Graybill. 2004. Efficacies of amphotericin B (AMB) lipid complex, AMB colloidal dispersion, liposomal AMB, and conventional AMB in treatment of murine coccidioidomycosis. Antimicrob. Agents Chemother. 48:2140-2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodman, J. S., and M. G. Koenig. 1970. Amphotericin B—specifics of administration. Mod. Treat. 7:581-595. [PubMed] [Google Scholar]
- 20.Groll, A. H., N. Giri, V. Petraitis, R. Petraitiene, M. Candelario, J. S. Bacher, S. C. Piscitelli, and T. J. Walsh. 2000. Comparative efficacy and distribution of lipid formulations of amphotericin B in experimental Candida albicans infection of the central nervous system. J. Infect. Dis. 182:274-282. [DOI] [PubMed] [Google Scholar]
- 21.Guo, L. S., R. M. Fielding, D. D. Lasic, R. L. Hamilton, and D. Mufson. 1991. Novel antifungal drug delivery: stable amphotericin B-cholesteryl sulfate discs. Int. J. Pharmacol. 75:45-54. [Google Scholar]
- 22.Ibraham, A. S., V. Avanessian, B. Spellberg, and J. E. Edwards, Jr. 2003. Liposomal amphotericin B, and not amphotericin B deoxycholate, improves survival of diabetic mice infected with Rhizopus oryzae. Antimicrob. Agents Chemother. 47:3343-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen, G. M., C. R. Skenes, T. H. Bunch, C. A. Weissman, N. Amirghahari, A. Satorius, K. Moynihan, and C. G. S. Eley. 1999. Determination of the relative toxicity of amphotericin B formulations: a red blood cell potassium release assay. Drug Deliv. 6:81-88. [Google Scholar]
- 24.Jensen, G. M., T. H. Bunch, N. Hu, and C. G. S. Eley. 2006. Process development and quality control of injectable liposome therapeutics, p. 297-310. In G. Gregoriadis (ed.), Liposome technology, 3rd ed., vol. I. Liposome preparation and related techniques. Informa Healthcare, New York, NY. [Google Scholar]
- 25.Kotler-Brajtburg, J., G. Medoff, G. S. Kobayashi, S. Boggs, D. Schlessinger, R. C. Pandey, and K. L. Rinehart, Jr. 1979. Classification of polyene antibiotics according to chemical structure and biological effects. Antimicrob. Agents Chemother. 15:716-722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leenders, A. C., S. Daenen, R. L. Jansen, W. C. Hop, B. Lowenberg, P. W. Wijermans, J. Cornelissen, R. Herbrecht, H. van der Lelie, H. C. Hoogsteden, H. A. Verbrugh, and S. de Marie. 1998. Liposomal amphotericin B compared with amphotericin B deoxycholate in the treatment of documented and suspected neutropenia-associated invasive fungal infections. Br. J. Haematol. 103:205-212. [DOI] [PubMed] [Google Scholar]
- 27.Madias, N. E., and H. J. Adrogue. 2003. Cross-talk between two organs: how the kidney responds to disruption of acid-base balance by the lung. Nephron Physiol. 93:61-66. [DOI] [PubMed] [Google Scholar]
- 28.McCurdy, D. K., M. Frederic, and J. R. Elkinton. 1968. Renal tubular acidosis due to amphotericin B. N. Engl. J. Med. 278:124-130. [DOI] [PubMed] [Google Scholar]
- 29.Mills, W., R. Chopra, D. C. Linch, and A. H. Goldstone. 1994. Liposomal amphotericin B in the treatment of fungal infections in neutropenic patients: a single-centre experience of 133 episodes in 116 patients. Br. J. Haematol. 86:754-760. [DOI] [PubMed] [Google Scholar]
- 30.Morgan, J., K. A. Wannemuehler, K. A. Marr, S. Hadley, D. P. Kontoyiannis, T. J. Walsh, S. K. Fridkin, P. G. Pappas, and D. W. Warnock. 2005. Incidence of invasive aspergillosis following hematopoietic stem cell and solid organ transplantation: interim results of a prospective multicenter surveillance program. Med. Mycol. 43(Suppl. 1):S49-S58. [DOI] [PubMed] [Google Scholar]
- 31.Olson, J. A., J. P. Adler-Moore, J. Schwartz, G. M. Jensen, and R. T. Proffitt. 2006. Comparative efficacies, toxicities, and tissue concentrations of amphotericin B lipid formulations in a murine pulmonary aspergillosis model. Antimicrob. Agents Chemother. 50:2122-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ortoneda, M., J. Capilla, F. J. Pastor, I. Pujol, and J. Guarro. 2002. Efficacy of liposomal amphotericin B in treatment of systemic murine fusariosis. Antimicrob. Agents Chemother. 46:2273-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patterson, T. F., P. Miniter, J. Dijkstra, F. C. Szoka, Jr., J. L. Ryan, and V. T. Andriole. 1989. Treatment of experimental invasive aspergillosis with novel amphotericin B/cholesterol-sulfate complexes. J. Infect. Dis. 159:717-724. [DOI] [PubMed] [Google Scholar]
- 34.Perfect, J. R., and K. A. Wright. 1994. Amphotericin B lipid complex in the treatment of experimental cryptococcal meningitis and disseminated candidosis. J. Antimicrob. Chemother. 33:73-81. [DOI] [PubMed] [Google Scholar]
- 35.Proffitt, R. T., A. Satorius, S.-M. Chiang, L. Sullivan, and J. P. Adler-Moore. 1991. Pharmacology and toxicology of a liposomal formulation of amphotericin B (AmBisome) in rodents. J. Antimicrob. Chemother. 28(Suppl. B):49-61. [DOI] [PubMed] [Google Scholar]
- 36.Ringdén, O., E. Andstrom, M. Remberger, B.-M. Svahn, and J. Tollemar. 1994. Safety of liposomal amphotericin B (AmBisome) in 187 transplant recipients treated with cyclosporin. Bone Marrow Transplant. 14(Suppl. 5):10-14. [PubMed] [Google Scholar]
- 37.Seabury, J. H., and H. E. Dascomb. 1960. Experience with amphotericin B. Ann. N. Y. Acad. Sci. 89:202-220. [DOI] [PubMed] [Google Scholar]
- 38.Sokol-Anderson, M. L., J. Brajtburg, and G. Medoff. 1986. Amphotericin B-induced oxidative damage and killing of Candida albicans. J. Infect. Dis. 154:76-83. [DOI] [PubMed] [Google Scholar]
- 39.Wheat, L. J., G. Cloud, P. C. Johnson, P. Connolly, M. Goldman, A. Le Monte, D. E. Fuller, T. E. Davis, and R. Hafner. 2001. Clearance of fungal burden during treatment of disseminated histoplasmosis with liposomal amphotericin B versus itraconazole. Antimicrob. Agents Chemother. 45:2354-2357. [DOI] [PMC free article] [PubMed] [Google Scholar]