In spite of the development and wide use of antibiotics, pneumonia is still the leading cause of infection-related mortality worldwide (101), and antibiotic resistance in the major pathogens of pneumonia has become more frequent during the past several decades. In order to defeat and prevent antibiotic resistance, antibiotics need to be used based on pharmacokinetics (PK) and pharmacodynamics (PD) (6, 10, 35).
With regard to the PK/PD of antibiotics, considerable effort has been devoted to directly measure the concentrations of antibiotics at infection sites, because the distributions of antibiotics may be different among a variety of tissues. However, even beyond the fact that these measurements are carried out in normal tissues, the techniques used for the measurement are variable in accuracy and reproducibility and the interpretation of their results is hindered by many confounding factors (74, 75).
For pulmonary infections, concentrations of antibiotics in epithelial lining fluid (ELF) for extracellular pathogens and in alveolar macrophage (AM) cells for intracellular pathogens are thought to reflect antibiotic activity in pneumonia. Antibiotics whose concentrations are high at these extravascular sites, such as macrolides and fluoroquinolones, tend to be promoted for treatment of pulmonary infection over antibiotics like beta-lactams and aminoglycosides, even though clinical trials do not show differences in clinical outcome or even bacteriological response.
In fact, it is less clear why the ratios of ELF to plasma concentrations are diverse between antibiotics and even between members of the same antibiotic class. The measured ELF-to-plasma concentration ratios may differ based on physicochemical characteristics intrinsic to the molecules. And also, as the ELF concentration of antibiotics is commonly measured by bronchoalveolar lavage (BAL), technical factors or errors in the method of measurement may create these differences. It is believed that these factors need to be clarified before the concept of ELF concentration should be connected to antibiotic outcomes such as bacterial eradication or clinical response. In the current review, data from published human studies were extracted and analyzed to interpret ELF concentrations of antibiotics measured by BAL, considering possible confounding factors.
DATA SOURCES AND ANALYSIS
For the evaluation, Medline (January 1982 to December 2006) was searched for studies measuring concentrations of antibiotics in ELF. The following were searched to identify relevant publications: human studies; studies of ELF sampled by BAL; and data on antibiotic concentrations measured simultaneously in serum, ELF, and AMs. Measurements at steady state were preferred over those at non-steady-state conditions. Under the criteria, the following antibiotics from a total of 45 publications, 44 original articles, and 1 review article were included in the evaluation: 3 beta-lactams (amoxicillin, cefdinir, and meropenem) (22, 32, 34), 2 macrolides (azithromycin and clarithromycin) (15, 19, 21, 44, 53, 77, 80, 86-88), 2 ketolides (cethromycin and telithromycin) (27, 57, 60, 72, 78), 13 fluoroquinolones (3-5, 8, 9, 15, 33, 45, 51, 52, 79, 87, 91, 93, 97, 99, 100), linezolid (26, 54), tigecycline (23), 2 rifamycins (rifampin and rifapentine) (18, 31, 103), 4 other antituberculosis antibiotics (ethambutol, ethionamide, isoniazid, and pyrazinamide) (20, 24, 29, 30), and an antifungal azole (itraconazole) (25).
POSSIBLE CONFOUNDING FACTORS IN INTERPRETING ELF CONCENTRATIONS OF ANTIBIOTICS MEASURED BY BAL
ELF is measured on the interior surface of the alveolar wall. The blood-alveolar barrier is composed of two membranes, the capillary wall and alveolar wall, which are separated by a fluid-filled interstitial space (Fig. 1), so the antibiotics measured in ELF represent portions which diffuse readily across the alveolar capillary wall, the interstitial fluid, and the alveolar epithelial cells. While the fenestrated pulmonary capillary bed is expected to permit passive diffusion of antibiotics with a molecular weight ≤1,000, the alveolar epithelial cells would not be expected to permit passive diffusion of antibiotics between cells, for the cells are linked by tight junctions (38). Thus, to reach ELF, the antibiotic must pass through the alveolar epithelial cells themselves.
FIG. 1.
Schematic diagram of the blood-alveolar antibiotic barrier (adapted from reference 38 with the permission of the publisher). The blood-alveolar barrier is composed of two membranes, the capillary wall and alveolar wall, which are separated by a fluid-filled interstitial space. Antibiotics need to diffuse across the alveolar capillary wall, the interstitial fluid, and the alveolar epithelial cells to reach ELF. Cells can carry antibiotics to the ELF also.
From the viewpoint of the anatomy of the blood-alveolar barrier, a number of factors are thought to influence the entry of antibiotics into the ELF. First, because only the free fraction of antibiotics is believed to equilibrate between serum and interstitial fluid, different degrees of protein binding will influence antibiotic concentrations in interstitial fluid and in ELF. Second, degrees of drug passage through the alveolar epithelial cells will depend on the lipophilicity and diffusibility of the antibiotics, similar to the drug entry into the central nervous system.
Measurement problems may also confound the interpretation of the ELF concentrations of antibiotics. In measurement experiments, ELF is a mixture of components, each of which can itself bring properties unique to some of the antibiotics under study. Besides the fluid component, cells, especially AM cells, are included in the composition of ELF. The cells may be lysed during the measurement of antibiotic concentration in BAL-derived fluids. Therefore, in the interpretation of the high ELF concentrations of some antibiotics, it may be argued that some studies have encountered contamination from released cellular components. When the concentration of an antibiotic in cells is higher than the concentration of the antibiotic in serum, lysis of some or all cells could artificially increase the measured ELF concentration of the antibiotic. The amount of error will presumably vary with the amount in the cells and the numbers of cells present.
The possibility of technical errors must also be considered. The volume of ELF sampled by BAL and the amount of antibiotic contained in the sample are corrected for drug-free saline added during the BAL procedure. This correction is usually performed by measurement of urea content. Urea is used as an endogenous marker of ELF because urea, small and relatively nonpolar, can travel across membranes freely to reach the outer surfaces of alveoli. The concentration of urea in ELF is considered to be same as the serum urea concentration, implying complete distribution. Therefore, the volume of ELF (VELF) is adjusted for excess exogenous water using the following equation: VELF = VBAL × UreaBAL/Ureaserum, where UreaBAL and Ureaserum are the concentrations of urea in BAL fluid and serum, respectively.
The “dwelling time” of fluid during the BAL can be a source of error in the urea method. From some studies, it has been shown that additional urea diffuses from the interstitium and other tissue when the dwelling times of BAL are prolonged. In situations where the dwelling time is over 1 min, ELF volume is expected to be overestimated by 100 to 300% (7, 70, 83). In addition, the urea concentration in BAL fluid can be increased by urea from blood contaminated during the procedure of BAL (19, 21, 44). Finally, although antibiotics are assumed to diffuse as fast as urea by use of this correction, certainly this seems unlikely with at least some antibiotics and antibiotic classes, such as vancomycin and protein-bound cephalosporins.
None of these potential confounding physiological principles, protein binding and limitation of passage through alveolar epithelial cells, potential lysis of cells, and technical error such as prolonged dwelling time, have been considered in the interpretation of ELF concentrations of antibiotics. For this review, we developed a simulation to estimate ELF concentrations of antibiotics in consideration of the impact of protein binding, different lipid solubilities and molecular weights, and lysis of cells in ELF.
CONCENTRATION OF PROTEIN AND VOLUME OF CELLS IN ELF
The concentration of protein in ELF needs to be known for assessment of the unbound free-drug concentrations in ELF and has been reported to be much lower than serum level. Total protein concentrations in ELF measured in children with congestive heart disease were reported as 3.9 mg/ml and 8.0 mg/ml depending on the children's infection status; these values were only 6 and 12% of plasma concentration of protein (55 to 85 mg/ml) (47). The low concentration of protein in ELF in this study might be the result of dilution by increased volume of alveolar fluid or diminished protein production within the alveolar space due to congestive heart disease. However, protein levels in ELF measured in healthy infants with normal lung were similar also: 4.6 mg/ml when sampled by tracheal aspiration and 3.3 mg/ml when sampled by nonbronchoscopic BAL (NB-BAL) (39). Although lung diseases increased the protein level in ELF, the degree of increase was less than two times of the level of that for infants without lung diseases. Concentrations of albumin in ELF were even much lower (0.68 by tracheal aspiration and 0.89 mg/ml by nonbronchoscopic BAL, respectively, in the infants with normal lungs) than serum levels (35 to 55 mg/ml). Animal studies showed similar results (50, 81). At these low levels of protein and albumin in ELF, protein binding of antibiotics is expected to be negligible, especially for antibiotics with low levels of protein binding ratio (36). Therefore, in this review, protein binding of antibiotics in ELF was not considered and the measured total antibiotic concentrations in ELF were regarded as equivalent to the free (unbound) fractions of the antibiotics.
To estimate the influence of released intracellular antibiotic content on concentrations of the antibiotics in ELF, the volume of cells in ELF needs to be measured. However, we were unable to find any publication which directly measured the volume of cells in ELF. Therefore, for the current review, the volume of cells in ELF was calculated by multiplication of usual cell counts in ELF with the known volume of AMs, neutrophils, and lymphocytes (37, 65, 83, 92, 96). In the calculation of cell contribution to the ELF amount, cell volume was estimated to constitute 3.8 to 10.0% of ELF volume (Table 1), and this range was applied to each antibiotic in relation to its intracellular content.
TABLE 1.
Volume of cells in ELF
| Cell type (%) | No. of cells/100 μl of ELF | Mean cell vol (10−6 μl) | Vol (μl)/100 μl of ELF |
|---|---|---|---|
| Macrophages (83) | (1.49-1.99) × 106 | 2.5-5.0 | 3.73-9.95 |
| Lymphocytes (17) | (0.31-0.41) × 106 | 0.2 | 0.06-0.08 |
| Neutrophils (1) | 0.02 × 106 | 0.3 | 0.01 |
| Total (100) | (1.8-2.4) × 106 | 3.80-10.04 |
FACTORS CONSIDERED IN THE SIMULATION OF ESTIMATED ELF CONCENTRATIONS OF ANTIBIOTICS
The simulation of estimated ELF concentrations of antibiotics was performed on the premise that unbound (free) concentrations of antibiotics in serum, calculated from in vitro protein binding and total serum level of the antibiotics, equilibrate with the free concentrations in interstitial fluid. In fact, since the effect of protein binding is buffered by relatively voluminous extravascular fluid, the percentage of protein binding in vitro does not contribute to the same extent in vivo (98). Furthermore, interstitial fluid is not protein free, which influences the unbound free levels of antibiotics in both serum and interstitial fluid. While in vivo measurement of free antibiotic concentrations from human serum drawn after administration of the drugs would reflect the actual unbound antibiotic concentrations, most of the protein binding fractions of antibiotics have been measured in vitro using equilibrium dialysis. However, to simplify the simulation, the extent of protein binding in serum was assumed to be same as the in vitro protein binding fraction at steady state, and protein binding of antibiotics in interstitial fluid was not considered in this review.
It is known that the protein binding of antibiotics does not change much at the albumin levels normally achieved in the body during therapy (36). Therefore, in this review, fixed protein binding ratios of each antibiotic were applied across all the concentrations. One exception was azithromycin, whose protein binding was reported to vary between 7.1% and 50% depending on the drug concentration (41). Serum protein binding values for each antibiotic used for the current review are listed in Table 2, which were retrieved from the database of Clarke's Analysis of Drugs and Poisons (70a) and others (1, 12, 42, 55, 64, 69).
TABLE 2.
Physical and chemical properties of the antibiotics investigated
| Antibiotic | Protein binding (%) | PC (log) | Mol wt | Ka | Referencesb |
|---|---|---|---|---|---|
| Amoxicillin | 20 | 0.614 | 365.41 | 0.82 | 70a, S |
| Cefdinir | 60-73 | −0.725 | 395.42 | 0.39 | 69, S |
| Meropenem | 2 | −0.6 | 383.46 | 0.56 | 70a, M |
| Azithromycin | 7.1-50 | 3.329 | 748.98 | 1.36 | 70a, S |
| Clarithromycin | 80 | 3.159 | 747.95 | 1.32 | 70a, S |
| Cethromycin | 90 | 5.24 | 765.93 | 1.76 | 65, S |
| Telithromycin | 70 | 5.093 | 812 | 1.72 | 2, S |
| Ciprofloxacin | 30 | 1.308 | 331.34 | 0.97 | 70a, S |
| Clinafloxacin | 2-7 | 1.63 | 365.79 | 1.03 | 12, S |
| Garenoxacin | 75 | 1.615 | 426.41 | 1.02 | 42, S |
| Gatifloxacin | 20 | 1.586 | 375.39 | 1.02 | 69, S |
| Levofloxacin | 30-40 | 1.485 | 361.37 | 1.00 | 70a, S |
| Lomefloxacin | 10 | 2.334 | 351.35 | 1.18 | 12, S |
| Moxifloxacin | 39-52 | 1.974 | 401.43 | 1.10 | 69, S |
| Pefloxacin | 25 | 2.164 | 333.36 | 1.15 | 70a, S |
| Rufloxacin | 60 | 1.894 | 363.41 | 1.09 | 12, S |
| Sparfloxacin | 40 | 2.866 | 392.4 | 1.29 | 12, S |
| Trovafloxacin | 87 | 1.566 | 416.35 | 1.01 | 12, S |
| Grepafloxacin | 50 | 2.261 | 359.39 | 1.17 | 12, S |
| Pyrazinamide | 50 | −0.368 | 123.11 | 0.66 | 70a, S |
| Ethionamide | 30 | 1.220 | 166.24 | 0.98 | 70a, S |
| Linezolid | 31 | −0.923 | 337.35 | 0.50 | 69, S |
| Itraconazole | 99.8 | 3.291 | 705.63 | 1.35 | 70a, S |
| Tigecycline | 79-89 | 2.09 | 585.65 | 1.11 | 23, M |
| Rifampin | 80 | 0.486 | 822.94 | 0.76 | 70a, S |
| Ethambutol | 10-40 | −0.053 | 204.31 | 0.71 | 70a, S |
| Isoniazid | 0 | −0.887 | 137.14 | 0.55 | 70a, S |
| Rifapentine | 97.7 | 1.981 | 877.03 | 1.07 | 55, S |
Constant K = 0.96 + 0.091·ln (PC·MW−1/2).
S, SciFinder Scholar database (American Chemical Society; 2004); M, MDL quantitative structure-activity relationship (Elsevier Science, Inc.; 2004).
To describe the distribution of drugs into extravascular compartments, the steady-state area under the concentration-time curve (AUC) ratio at the extravascular site compared to the simultaneous serum level should be preferred, for there is a time lag between the serum concentration curve and extravascular concentration curve for drugs (Fig. 2) (85). In the exemplary case of Fig. 2, the simultaneous extravascular concentration/serum concentration ratio is <1 at the time point of the peak in serum, while it is >1 after the time point of the peak in the extravascular space. In contrast, the ratio of AUC is well established to approximate the overall intercompartmental drug equilibration. Therefore, for the present review, AUCs of antibiotics in ELF and serum were calculated by the trapezoidal rule when multiple measurements were available. When AUCs could not be obtained, simultaneous concentrations in ELF and serum were compared.
FIG. 2.
Different concentration-time profiles for cefdinir in plasma (•) and in blister fluid (♦) after single doses of 300 mg (a) and 600 mg (b) (adapted from reference 85 with permission). The simultaneous blister fluid/plasma concentration ratios were <1 at time points of peak in plasma, while they were >1 after the time points of peak in blister fluid. AUC ratios of blister fluid/plasma were 86.3% and 92.4%, respectively. The ratio of AUC is a better parameter to represent tissue penetration of antibiotics.
The influence of lipophilicity and diffusibility of antibiotics on penetration of the drugs through cellular barriers has been evaluated in a study for drug entry into the cerebrospinal fluid (CSF) through the blood-CSF barrier (73). As the octanol/water partition coefficient (PC), a measure of lipophilicity, and the square root of the molecular weight (MW1/2), a measure of diffusibility, correlated with the ratio of CSF concentration/free serum concentration (Ccsf/Cfs) (or ratio of AUC in CSF/free AUC in serum, AUCcsf/AUCfs), the relationship was expressed by the equation: Ccsf/Cfs (or AUCcsf/AUCfs) = 0.96 + 0.091·ln (PC·MW−1/2). For the current review, this equation was adopted to evaluate if the ratio of ELF concentration/free serum concentration of antibiotics could be explained on the basis of the penetration capacities of the antibiotics. Logarithmic values of the PCs (log PC) and MWs of the antibiotics were found in the SciFinder Scholar database (American Chemical Society; 2004) and others (Table 2).
STEPS SIMULATING ESTIMATED ELF CONCENTRATIONS OF ANTIBIOTICS
First, ELF concentrations were simulated with consideration of protein binding in serum and capacity for penetration through the alveolar epithelium. Because unbound free antibiotics in serum are expected to freely equilibrate with the interstitial levels of antibiotics, the concentration or AUC in ELF (Celf or AUCelf)-to-serum level ratios can be expressed as follows: Celf/Cfs (or AUCelf/AUCfs) = Celf/Cinterstitial fluid (or AUCelf/AUCinterstitial fluid) = 0.96 + 0.091·ln (PC·MW−1/2).
With a measured PC and an MW of each antibiotic, the formula 0.96 + 0.09·ln (PC·MW−1/2) shall be expressed as a constant (K), and the above equation can be simplified to Celf/Cfs (or AUCelf/AUCfs) = K. Constant K reflects the capacities of antibiotics to penetrate into the ELF. From the above equation, we can conclude that, as Celf/(Cfs·K) (or AUCelf/[AUCfs·K]) approached 1.0, the ratio of ELF concentration/free serum concentration of an antibiotic can be explained on the basis of the penetration capacity of the antibiotic related to its lipophilicity and diffusibility. Values of the constant K calculated from the PC and MW of each antibiotic are listed in Table 2.
Second, the expected ELF concentrations were challenged by additionally considering lysis of some fraction of the cellular content of the ELF. Lysis of cells in ELF is expected during the processing of BAL specimens. The resulting measured ELF concentrations reflect contamination with intracellular antibiotics, but the original ELF concentrations can be calculated using the following equation: mCelf × (Velf + Vcell) = oCelf × Velf + Ccell × Vcell, where mCelf is the measured ELF concentration, Velf is the volume of ELF, Vcell is the volume of lysed cells, oCelf is the original ELF concentration, and Ccell is the intracellular concentration. This equation can be solved for oCelf as follows: oCelf = mCelf × (1 + Vcell/Velf) − Ccell × Vcell/Velf.
Expected original AUCs also could be obtained from the original ELF concentrations calculated using the above equation. Then, the ratios of expected original Celf/Cfs (or AUCelf/AUCfs) divided by the constant K were plotted against the extent of cell lysis. As the equation approached 1.0, with a larger extent of cell lysis, we concluded that the measured ELF concentration might be explained by contamination of antibiotics from lysed cells and that the actual ELF concentration could be the same as the free serum concentration. The range of volume proportion of lysed cells in ELF (Vcell/Velf) used for the adjustment ranged from 0 to 0.1.
BETA-LACTAMS
Measured ELF concentrations of the beta-lactams amoxicillin, cefdinir, and meropenem were well below serum concentrations, and their respective concentrations in AM cells were negligible (22, 32, 34) (Table 3). When unbound free serum concentrations were used instead of total concentrations, the Celf/Cfs ratios (or AUCelf/AUCfs) increased somewhat, but they were <0.5.
TABLE 3.
Ratios of antibiotic concentrations (or AUCs) in ELF or cells compared to serum (or unbound free serum) levelsa
| Antibiotic | Celf/Cs | Ccell/Cs | Celf/Cfs | Ccell/Cfs | AUCelf/AUCs | AUCcell/AUCs | AUCelf/AUCfs | AUCcell/AUCfs | Reference(s) |
|---|---|---|---|---|---|---|---|---|---|
| Beta-lactams | |||||||||
| Amoxicillin | 0.13 | 0.00 | 0.16 | 0.00 | 34 | ||||
| Cefdinir | 0.12 | 0.00 | 0.35 | 0.00 | 32 | ||||
| Cefdinir | 0.15 | 0.00 | 0.44 | 0.00 | 32 | ||||
| Meropenem | 0.28 | 0.25 | 0.28 | 0.26 | 22 | ||||
| Meropenem | 0.43 | 0.15 | 0.44 | 0.15 | 22 | ||||
| Macrolides | |||||||||
| Azithromycin | 6.30-31.33 | 718.00-6,841.33 | 8.18-62.67 | 932.47-13,682.67 | 13.31 | 2,284.69 | 20.83 | 3,574.83 | 15 |
| Azithromycin | 12.63-24.40 | 533.75-1,010.00 | 19.73-48.80 | 833.98-2,020.00 | 21.29 | 748.46 | 34.95 | 1,228.54 | 88 |
| Azithromycin | 2.53-57.78 | 415.73-9,806.45 | 3.28-115.56 | 539.91-19,612.90 | 24.10 | 3,234.81 | 34.01 | 4,564.69 | 77 |
| Azithromycin | 4.60-20.43 | 1,756.49-5,242.86 | 5.60-26.53 | 2,142.06-6,808.91 | 5.59 | 1,828.54 | 8.87 | 3,320.07 | 87 |
| Clarithromycin | 5.17 | 94.12 | 25.83 | 470.58 | 53,86 | ||||
| Clarithromycin | 12.38-20.00 | 141.94-432.17 | 61.89-100.00 | 709.68-2,160.87 | 15.42 | 191.18 | 77.11 | 955.88 | 86,88 |
| Clarithromycin | 13.32-60.75 | 98.73-721.40 | 66.59-303.75 | 493.65-3,607.00 | 30.27 | 248.47 | 151.36 | 1,242.34 | 21,86 |
| Clarithromycin | 10.34-25.29 | 444.94-2,131.58 | 51.70-126.43 | 2224.68-10657.89 | 15.33 | 626.31 | 76.65 | 3,131.54 | 80,86 |
| Clarithromycin | 39.60 | 181.00 | 198.00 | 905.00 | 19,86 | ||||
| Clarithromycin | 4.13-38.97 | 169.33-657.69 | 20.65-194.87 | 846.67-3,288.46 | 8.34 | 217.55 | 41.71 | 1,087.73 | 44 |
| Telithromycin | 4.83-14.91 | 42.98-418.18 | 16.08-49.70 | 143.27-1,393.94 | 6.44 | 124.92 | 21.48 | 416.42 | 72 |
| Telithromycin | 5.38-6.65 | 36.39-154.60 | 17.94-22.16 | 121.29-515.33 | 57 | ||||
| Telithromycin | 8.01-14.22 | 37.27-2,019.63 | 26.68-47.39 | 124.23-6,732.08 | 9.23 | 338.47 | 30.76 | 1128.24 | 60 |
| Telithromycin | 2.27-6.27 | 48.94-240.27 | 7.57-20.91 | 163.15-800.91 | 3.15 | 84.18 | 10.49 | 280.62 | 78 |
| Cethromycin | 7.50-20.00 | 67.50-317.50 | 75.00-200.00 | 675.00-3,175.00 | 12.64 | 178.27 | 126.39 | 1782.71 | 27 |
| Cethromycin | 7.11-10.00 | 90.40-670.00 | 71.05-100.00 | 904.00-6,700.00 | 7.87 | 207.43 | 78.74 | 2,074.34 | 27 |
| Fluoroquinolones | |||||||||
| Ciprofloxacin | 0.00-0.89 | 12.36-28.75 | 0.00-1.27 | 17.66-41.07 | 0.82 | 15.62 | 1.174 | 22.31 | 45 |
| Ciprofloxacin | 0.90 | 2.32 | 1.29 | 3.31 | 91 | ||||
| Ciprofloxacin | 1.85 | 7.7 | 2.64 | 11.00 | 97 | ||||
| Ciprofloxacin | 2.13 | 11.84 | 3.04 | 16.91 | 9 | ||||
| Clinafloxacin | 1.77 | 10.20 | 1.86 | 10.73 | 51 | ||||
| Garenoxacin | 0.92-1.65 | 10.58-18.27 | 3.67-6.59 | 42.33-73.09 | 1.34 | 13.89 | 5.38 | 55.57 | 3 |
| Gatifloxacin | 1.51-1.75 | 17.51-36.67 | 1.89-2.19 | 21.89-45.84 | 1.77 | 24.94 | 2.21 | 31.17 | 52 |
| Grepafloxacin | 10.49-14.88 | 99.96-268.65 | 15.35-29.76 | 199.92-537.30 | 12.03 | 175.75 | 24.05 | 351.50 | 33 |
| Levofloxacin | 1.49-3.00 | 5.38-6.14 | 2.29-4.61 | 8.27-9.45 | 2.08 | 5.90 | 3.20 | 9.07 | 15 |
| Levofloxacin | 1.17-2.10 | 11.95-23.00 | 1.80-3.24 | 18.39-35.38 | 1.94 | 15.71 | 2.99 | 24.16 | 45 |
| Levofloxacin | 0.86-2.26 | 8.77-8.94 | 1.32-3.48 | 13.50-13.75 | 1.90 | 8.83 | 2.92 | 13.59 | 45 |
| Levofloxacin | 0.80-3.00 | 4.00-9.60 | 1.23-4.62 | 6.15-14.77 | 2.15 | 7.23 | 3.30 | 11.13 | 5 |
| Levofloxacin | 1.53-2.58 | 11.23-17.70 | 2.36-3.97 | 17.27-27.23 | 1.75 | 12.82 | 2.69 | 19.72 | 87 |
| Levofloxacin | 1.72-2.06 | 12.47-22.22 | 2.64-3.17 | 19.19-34.18 | 1.59 | 14.44 | 2.45 | 22.22 | 87 |
| Levofloxacin | 2.69 | 4.90 | 4.14 | 7.54 | 28 | ||||
| Lomefloxacin | 1.86 | 20.47 | 2.07 | 22.74 | 9 | ||||
| Moxifloxacin | 5.18-7.00 | 17.61-70.39 | 9.58-12.96 | 32.61-130.36 | 5.18 | 13.59 | 9.59 | 25.16 | 93 |
| Moxifloxacin | 3.61-7.32 | 14.76-55.77 | 6.54-13.56 | 27.33-103.28 | 4.92 | 27.33 | 9.11 | 50.61 | 15 |
| Pefloxacin | 13.10-13.44 | 13.78-18.69 | 17.46-17.91 | 18.37-24.92 | 79 | ||||
| Rufloxacin | 5.08-11.67 | 9.97-27.17 | 12.71-29.17 | 24.93-67.92 | 7.66 | 21.55 | 19.14 | 53.88 | 99 |
| Sparfloxacin | 8.17-17.00 | 42.63-126.33 | 13.61-28.33 | 71.04-210.56 | 12.60 | 61.24 | 21.00 | 102.07 | 100 |
| Sparfloxacin | 12.50 | 44.75 | 20.83 | 74.58 | 100 | ||||
| Sparfloxacin | 19.27-65.71 | 29.18-71.63 | 32.12-109.52 | 48.64-119.39 | 31.93 | 40.71 | 53.22 | 67.85 | 91 |
| Temafloxacin | 3.13 | 5.7 | 4.22 | 7.70 | 97 | ||||
| Temafloxacin | 3.06 | 8.81 | 4.14 | 11.91 | 8 | ||||
| Trovafloxacin | 2.16-5.53 | 13.32-22.55 | 16.62-42.54 | 102.46-173.46 | 3.65 | 17.55 | 28.09 | 135.01 | 4 |
| Trovafloxacin | 5.85 | 24.10 | 45.00 | 185.38 | 4 | ||||
| Others | |||||||||
| Pyrazinamide | 13.60-24.76 | 0.49-1.09 | 27.20-49.51 | 0.98-2.17 | 20 | ||||
| Ethionamide | 6.57-10.33 | 0.33-0.71 | 9.39-14.76 | 0.48-1.02 | 30 | ||||
| Linezolid | 2.38-4.22 | 0.11-0.17 | 3.45-6.12 | 0.16-0.24 | 3.29 | 0.15 | 4.77 | 0.22 | 26 |
| Linezolid | 8.35 | 0.71 | 12.10 | 1.03 | 54 | ||||
| Itraconazole | 0.14-0.56 | 2.33-5.44 | 14.29-55.56 | 233.33-544.44 | 0.22 | 2.94 | 21.51 | 293.60 | 25 |
| Tigecyline | 1.32 | 77.46 | 5.27 | 309.83 | 23 | ||||
| Rifampin | 0.17-0.31 | 0.71-1.50 | 0.83-1.56 | 3.54-7.50 | 18 | ||||
| Rifampin | 0.34 | 16.26 | 1.70 | 81.30 | 103 | ||||
| Ethambutol | 0.92-1.13 | 19.17-48.24 | 1.22-1.51 | 25.56-64.31 | 26 | ||||
| Isoniazid fast acetylator | 1.74-5.88 | 1.48-13.42 | 1.74-5.88 | 1.48-13.42 | 29 | ||||
| Isoniazid slow acetylator | 1.37-5.69 | 0.74-4.40 | 1.37-5.69 | 0.74-4.40 | 29 | ||||
| Rifapentine | 0.14-0.24 | 0.18-0.38 | 6.14-10.60 | 7.67-16.35 | 0.21 | 0.26 | 9.28 | 11.12 | 31 |
Cs, serum concentration; Cfs, free serum concentration; AUCs, area under the concentration-time curve in serum.
However, as the ratios were divided by the low constant Ks of the beta-lactams, derived primarily from their poor lipophilicity, the ratio Celf/Cfs·K (or AUCelf/AUCfs·K) for cefdinir and a data set for meropenem approached 0.8 to 1.0, whereas the Celf/(Cfs·K) ratio from another data set for meropenem was still 0.5 and that for amoxicillin was very low, at 0.2. While the constant K depends on PC, there was a variety of reported PCs for amoxicillin. Whereas the SciFinder Scholar database reported the log PC of amoxicillin as 0.61, another source reported it as −1.87 rather than 0.61 (58). If a log PC of −1.87 was used, the Celf/(Cfs·K) for amoxicillin was increased to 0.55.
The low ELF concentrations of the beta-lactams might be related to the sampling time. In the studies measuring ELF concentrations of amoxicillin and cefdinir, BAL was performed around the time points when serum concentrations were expected to achieve their highest values (1 to 2 h and 4 h, respectively). As shown in the Fig. 2, peak times of interstitial concentration of the antibiotics lag behind the time points of peak serum concentrations. Therefore, when ELF was obtained for measurement of the beta-lactams, interstitial concentrations should be lower than serum concentrations, which would be reflected as lower ELF/serum concentration ratios. Comparison of AUCs with multiple sampling rather than single-time-point measurements would be needed.
On the other hand, as stated earlier, the volume of ELF measured by BAL could be overestimated by severalfold due to technical errors such as prolonged dwelling time of the lavage fluid. When the ELF volume is overestimated, the concentrations of solutes in the fluid would be calculated to be lower than the true values. Considering these possible technical errors might account for observations with amoxicillin and a data set for meropenem, where low ELF concentrations could not be adequately explained on the basis of low overall binding to serum proteins and low capacity for penetration through alveolar epithelial cells.
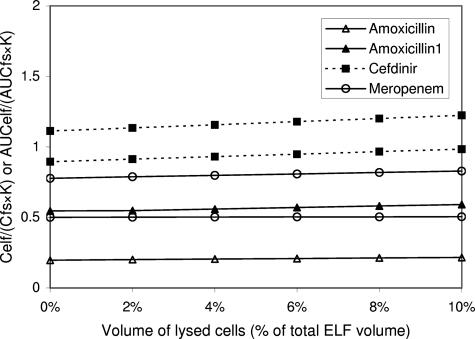
Although the suspected lysis of cells in ELF increased the Celf/(Cfs·K) of the beta-lactams, the extent of the increase was negligible (Fig. 3). Such behavior is consistent with drugs that do not penetrate cells, and thus lysis of cells would not add measurable amounts of drug to the supernatant.
FIG. 3.
Impact of number of cells lysed during the processing of BAL specimens on the measurement of ELF concentrations of amoxicillin, cefdinir, and meropenem. AUC (or concentration) ratios between ELF concentrations and unbound free serum concentrations divided by constant K, AUCelf/(AUCfs·K) (or Celf/[Cfs·K]) are plotted against the volume percentage of lysed cells in ELF. Constant K reflects the capacity of antibiotics to penetrate through alveolar epithelial cells (see text). While multiple lines of the same antibiotic are drawn based on different data sets, the plot of amoxicillin is represented with two different PCs: log PC for amoxicillin, 0.61; log PC for amoxicillin1, −1.87. The AUCelf/(AUCfs·K) (or Celf/[Cfs·K]) ratio for cefdinir and a data set for meropenem are around 1 already without consideration of cell lysis because of its low constant K, derived from poor lipophilicity (low log PC). The low ELF concentrations of those beta-lactams seem to be related to their low capacity for penetration through alveolar epithelial cells. Increasing the extent of the cell lysis changes the ratios just a little. The contribution of the low intracellular concentrations of the antibiotics to the low Celf/Cfs ratios is considered to be small. The possibility of technical errors needs to be excluded for amoxicillin and another data set of meropenem.
In conclusion, the low measured ELF concentrations of beta-lactams in comparison to their corresponding serum levels could be the result of low capacity of their unbound free fractions for penetration through the alveolar epithelial cell barriers and technical errors which further lower measured concentrations of these antibiotics in the ELF. While the low intracellular concentrations of the antibiotics might contribute to the low ELF/free serum concentration, the influence of cells and cell lysis should be trivial.
MACROLIDES AND KETOLIDES
Measured ELF concentrations of macrolides (azithromycin and clarithromycin) and ketolides (telithromycin and cethromycin) and their derived AUCs were consistently higher than serum levels by as much as 10-fold (15, 19, 21, 27, 44, 55, 57, 60, 72, 77, 78, 80, 86-88) (Table 3). In addition, when protein binding of the antibiotics in serum was considered, the ratios became even higher. Although all those antibiotics had very high lipophilicity, their constant Ks reached just around 1.0. Theoretically, the maximum of constant K determined by simple diffusion should be 1. Therefore, the high measured AUCelf/AUCfs (or Celf/Cfs) of macrolides and ketolides could not approach 1.0 when they were divided by the constant K. This result indicates that the high ratios of ELF concentration to serum concentration for macrolides and ketolides could not be explained solely on the basis of good penetration across the alveolar epithelium.
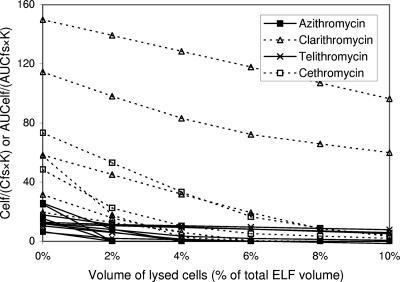
On the other hand, the intracellular concentrations of azithromycin, clarithromycin, telithromycin, and cethromycin were very high, approaching several thousandfold in excess of simultaneous serum concentrations in some cases. Because of the high ratios of intracellular level/free serum level, the high measured ELF concentrations of these antibiotics could be explained only if some or all of the high-drug-content cells in ELF were lysed during BAL. The subsequent measurements of drug content were thus performed with both the supernatant and the cell mass. Therefore, the expected original AUCelf/AUCfs (or Celf/Cfs) values for those antibiotics, calculated with consideration of contamination from cells, declined quickly as the extent of cell lysis increased (Fig. 4 and 5).
FIG. 4.
Impact of number of cells lysed during the processing of BAL specimens on the measurement of ELF concentrations of azithromycin, clarithromycin, telithromycin, and cethromycin. AUCelf/(AUCfs·K) (or Celf/[Cfs·K]) ratios are plotted against the volume percentage of lysed cells in ELF. Constant K reflects the capacity of antibiotics for penetration through alveolar epithelial cells (see text). Multiple lines of the same antibiotic are drawn based on different data sets. Because of the very high intracellular concentrations, the rapid drop of AUCelf/(AUCfs·K) (or Celf/Cfs·K) ratios from very high levels to around 1 with increasing lysis of cells is a general pattern in macrolides and ketolides. It means that lysis of a proportion of cells in the media may explain the high concentrations of macrolides and ketolides in ELF. While the ratios fail to reach ≤1 in some data sets for the antibiotics, the variety seems to be derived from very high ELF and intracellular concentrations of the antibiotics compared to their free serum levels, for trivial errors in measurement of the antibiotic concentrations in those sites will skew the concentration ratios by a great extent.
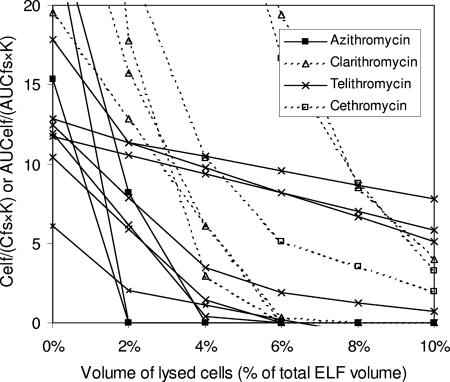
FIG. 5.
Impact of number of cells lysed during the processing of BAL specimens on the measurement of ELF concentrations of azithromycin, clarithromycin, telithromycin, and cethromycin. AUCelf/(AUCfs·K) (or Celf/[Cfs·K]) ratios are plotted against the volume percentage of lysed cells in ELF. Constant K reflects the capacity of antibiotics for penetration through alveolar epithelial cells (see text). Multiple lines of the same antibiotic are drawn based on different data sets. In general, the AUCelf/(AUCfs·K) (or Celf/[Cfs·K]) ratios for these antibiotics drop rapidly with lysis of cells.
Although there were several exceptions, expected original AUCelf/(AUCfs·K) (or Celf/[Cfs·K]) for the macrolides and ketolides reached ≤1 at ≤10% cell lysis in most settings. While clarithromycin and telithromycin failed to achieve the low ratio of AUCelf/(AUCfs·K) (or Celf/[Cfs·K]) in some settings, they showed rapid drops of the ratios to ≤1 also in other settings. Because the measured ELF and AM concentrations of clarithromycin and telithromycin were very high compared to their free serum levels, a trivial change of antibiotic concentrations in any of those sites would skew their concentration ratios by great extent. The AUCelf/(AUCfs·K) for cethromycin also did not reach 1 at ≤10% cell lysis; however, the range of the ratio was just 2 to 3 when the supposed cell lysis was 10%. Despite these some exceptions, the rapid drop of AUCelf/(AUCfs·K) (or Celf/[Cfs·K]) with lysis of cells was a general pattern in macrolides and ketolides.
In summary, the high concentrations of macrolides and ketolides in ELF might be explained by the possible contamination of intracellular antibiotics occurring during the process of BAL. This would be the case even when the actual concentrations of the antibiotics in ELF were low and in fact might be quite similar to free serum concentrations.
FLUOROQUINOLONES
Observations on the measured ratio of ELF concentrations to serum concentrations were more complex for fluoroquinolones. In some cases, ciprofloxacin, levofloxacin, and garenoxacin showed lower ELF concentrations than total serum levels at certain settings of sampling (3, 45). However, all fluoroquinolones achieved higher ELF levels than their free serum concentrations (3-5, 8, 9, 15, 33, 45, 51, 52, 79, 87, 91, 93, 97, 99, 100) (Table 3). The constant Ks of all the fluoroquinolones were calculated around 1.0 due to their high lipophilicities. While most of the fluoroquinolones achieved higher concentrations in AM cells than their free serum levels, the ratios of AM concentrations/free serum concentrations were modest compared to those for macrolides and ketolides. Therefore, the decreases in the AUCelf/(AUCfs·K) (or Celf/[Cfs·K]) expected when intracellular antibiotics contaminated the ELF were less than those for macrolides and ketolides.
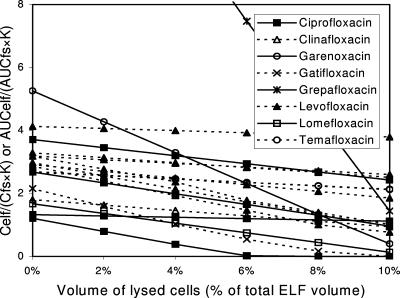
In general, fluoroquinolones were thought to be divided into two groups by their achieved AUCelf/AUCfs (or Celf/Cfs) ratios. The first group included ciprofloxacin, clinafloxacin, garenoxacin, gatifloxacin, levofloxacin, and lomefloxacin, whose AUCelf/AUCfs (or Celf/Cfs) ratios were less than 10 (mostly <5). Their AUCelf/(AUCfs·K) (or Celf/[Cfs·K]) ratios reached around 1.0 with higher degree of lysis of ELF cells in most settings (Fig. 6).
FIG. 6.
Impact of number of cells lysed during the processing of BAL specimens on the measurement of ELF concentrations of some fluoroquinolones. AUCelf/(AUCfs·K) (or Celf/[Cfs·K]) ratios are plotted against the volume percentage of lysed cells in ELF. Constant K reflects the capacity of antibiotics for penetration through alveolar epithelial cells (see text). Multiple lines of the same antibiotic are drawn based on different data sets. AUCelf/(AUCfs·K) (or Celf/[Cfs·K]) ratios for these fluoroquinolones reach around 1 with a higher degree of lysis of ELF cells in most settings although the extent of decrease is less than those for macrolides and ketolides due to their modest intracellular concentrations. Grepafloxacin shows a pattern resembling those for macrolides and ketolides because of its very high intracellular concentration. Lysis of a proportion of cells in ELF may explain the high measured ELF concentrations of these fluoroquinolones.
The second group, which included moxifloxacin, pefloxacin, rufloxacin, sparfloxacin, and trovafloxacin, achieved AUCelf/AUCfs (or Celf/Cfs) ratios higher than 10. Their AUCelf/(AUCfs·K) (or Celf/[Cfs·K]) ratios did not reach 1 (>5) even when lysis of the maximum volume of cells in ELF (10% of ELF volume) was considered (Fig. 7).
FIG. 7.
Impact of number of cells lysed during the processing of BAL specimens on the measurement of ELF concentrations of some fluoroquinolones. AUCelf/(AUCfs·K) (or Celf/[Cfs·K]) ratios are plotted against the volume percentage of lysed cells in ELF. Constant K reflects the capacity of antibiotics for penetration through alveolar epithelial cells (see text). Multiple lines of the same antibiotic are drawn based on different data sets. AUCelf/(AUCfs·K) (or Celf/[Cfs·K]) ratios for these fluoroquinolones fail to reach 1 even when lysis of the maximum volume of cells in ELF (10% of ELF volume) is considered. Lysis of a proportion of cells in ELF may not explain the high measured ELF concentrations of these fluoroquinolones. The possibility of technical errors needs to be excluded.
Among fluoroquinolones, grepafloxacin was exceptional in having the highest overall intracellular concentration, which was as high as several hundred times the serum concentration. Because of that, the change of the AUCelf/(AUCfs·K) (or Celf/[Cfs·K]) due to lysis of ELF cells in grepafloxacin resembled those for macrolides and ketolides (Table 3; Fig. 6).
Overall, the fluoroquinolones have been best studied of all the antibiotic classes, but the results vary greatly, even for the same drug. This points to the general difficulty in reproducing BAL fluid/ELF ratios given technical differences in the method.
OTHER ANTIBIOTICS
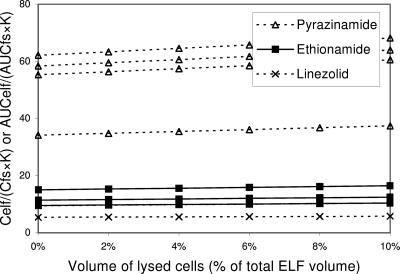
The antibiotics pyrazinamide, ethionamide, and linezolid showed high measured ELF concentrations in spite of their relatively low concentrations in AM cells (Table 3) (20, 26, 30, 54). While the ELF/free serum concentration ratios for linezolid and ethionamide were modest (linezolid, 1.6 to 6.1; ethionamide, 9.4 to 14.8), the ratio for pyrazinamide was relatively high (27.2 to 49.5). Different penetration capacities would not account for this pattern of ELF data: the K constants were 0.50 for linezolid, 0.98 for ethionamide, and 0.66 for pyrazinamide. The high ELF concentrations could not be explained using a correction for contamination by lysed cells too, for their intracellular concentrations were lower than or similar to their free serum levels (Fig. 8).
FIG. 8.
Impact of number of cells lysed during the processing of BAL specimens on the measurement of ELF concentrations of pyrazinamide, ethionamide, and linezolid. AUCelf/(AUCfs·K) (or Celf/[Cfs·K]) ratios are plotted against the volume percentage of lysed cells in ELF. Constant K reflects the capacity of antibiotics for penetration through alveolar epithelial cells (see text). Multiple lines of the same antibiotic are drawn based on different data sets. AUCelf/(AUCfs·K) (or Celf/]Cfs·K]) ratios for these antibiotics above 1 increase with increasing extent of cell lysis in ELF because intracellular concentrations are lower than free serum levels. The high ELF concentrations of these antibiotics cannot be explained by the contamination of lysed cells. The possibility of technical errors needs to be excluded.
Interestingly, a recent study using mini-BAL rather than the traditional BAL revealed concentrations of linezolid in ELF that were comparable to simultaneous serum levels (14). The mini-BAL instilled 40 ml of saline instead of 200 ml. It is not known what difference the lower volume of lavage fluid would make to the interpretation of the amount of solutes in ELF. However, this study suggests that technical errors in the process of BAL could be involved in the high measured ELF concentrations of linezolid, which may also apply to the other two antibiotics: ethionamide and pyrazinamide.
The measured ELF concentrations of itraconazole were lower than total serum levels. However, due to itraconazole's high rate of protein binding (99.8%), the ELF/free serum concentration ratios were increased by as much as 10-fold (Table 3) (25). Expected ELF concentrations of itraconazole considering cell lysis showed a pattern similar to those for macrolides and ketolides: high ratio of ELF concentration/free serum concentration without cell lysis, very high intracellular concentrations compared to free serum levels, and expected ELF concentrations matching free serum concentrations with cell lysis (Fig. 9). Tigecycline was also similar to itraconazole in that its high ratio of ELF concentration/free serum concentration was explained by the very high intracellular/free serum concentration ratio (23).
FIG. 9.
Impact of number of cells lysed during the processing of BAL specimens on the measurement of ELF concentrations of itraconazole, tigecycline, rifampin, and ethambutol. AUCelf/(AUCfs·K) (or Celf/[Cfs·K]) ratios are plotted against the volume percentage of lysed cells in ELF. Constant K reflects the capacity of antibiotics for penetration through alveolar epithelial cells (see text). Multiple lines of the same antibiotic are drawn based on different data sets. Itraconazole and tigecycline show pattern similar to those for macrolides and ketolides: high AUCelf/(AUCfs·K) without cell lysis, very high intracellular concentrations compared to free serum levels, and expected ELF concentrations matching free serum concentrations with cell lysis. Rifampin and ethambutol resemble fluoroquinolones whose Celf/ (Cfs ·K) ratios are modest and whose expected ELF concentrations match free serum concentrations with cell lysis.
Because of the modest Celf/Cfs and Ccell/Cfs ratios, the pattern of decrease of Celf/(Cfs·K) ratios for rifampin due to lysis of cells resembled that for the first group of fluoroquinolones, such as ciprofloxacin and levofloxacin (Fig. 9) (18, 103). Ethambutol also showed a pattern similar to that for rifampin and the first group of fluoroquinolones, with modest Celf/Cfs ratios and expected ELF concentrations matching free serum concentrations with cell lysis (Table 3; Fig. 9) (24).
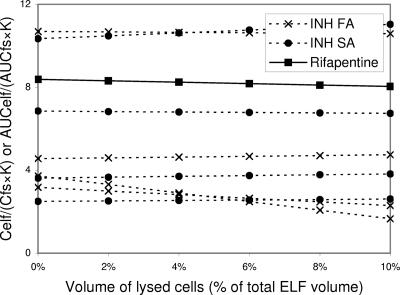
The antimycobacterial agents isoniazid and rifapentine showed a mixed pattern (Table 3) (29, 31). Although AUCelf/(AUCfs·K) (or Celf/[Cfs·K]) ratios for the two antibiotics did not reach 1.0 even with lysis of the maximum volume of cells in ELF, their ratios of concentration in ELF to free serum levels were modest (Fig. 10).
FIG. 10.
Impact of number of cells lysed during the processing of BAL specimens on the measurement of ELF concentrations of isoniazid (in fast acetlylators and slow acetlylators [INH FA and SA, respectively]) and rifapentine. AUCelf/(AUCfs·K) (or Celf/[Cfs·K]) ratios are plotted against the volume percentage of lysed cells in ELF. Constant K reflects the capacity of antibiotics for penetration through alveolar epithelial cells (see text). Multiple lines of the same antibiotic are drawn based on different data sets. Ratios of concentration in ELF compared to free serum levels of the antimycobacterial agents are just modest and AUCelf/(AUCfs·K) (or Celf/[Cfs·K]) ratios increase or decrease depending on intracellular/free serum concentration ratios, which fail to reach 1 with lysis of the maximum volume of cells in ELF. The moderately high ELF/free concentrations of these antibiotics compared to free serum levels cannot be explained by the contamination of lysed cells. The possibility of technical errors needs to be excluded.
On the other hand, pyrazinamide, ethionamide, isoniazid, rifampin, and ethambutol were sampled once at 4 h after administration, which was after the peak time points of serum concentrations (1 to 2.5 h). Although interstitial concentrations of the antibiotics around ELF could be higher than serum levels at the time points, it is not certain how much the late sampling time affected the ELF concentrations. While very high ELF concentrations of pyrazinamide and ethionamide may not be explained by the possible modest high interstitial/serum concentration ratio achieved after peak time of serum concentration, the ELF concentrations of isoniazid, rifampin, and ethambutol, a little higher than serum levels, might be understood on the basis of the late sampling time. Comparisons of AUCs rather than single-time-point concentrations between ELF and serum are needed to clarify the issue with those antibiotics.
DISCUSSION
Effects of penetration capacity on ELF concentrations of antibiotics.
In this review, an equation derived from a study evaluating the capacity of drugs for penetration into the CSF was adopted to estimate the capacity of the antibiotics to penetrate into the ELF. The blood-CSF barrier was considered to be similar to the blood-ELF barrier in the aspects that the capillary wall is fenestrated in both structures and epithelial cell linings of the barriers (alveolar cell lining and choroidal epithelial lining) are sealed with tight junctions (46, 89). Most studies investigating drug penetration to the central nervous system have been performed with brain tissue (2, 13, 16, 17, 40, 48, 49, 56, 59, 68, 71, 76, 84, 95). However, the blood-brain barrier (BBB) is different from blood-CSF and blood-ELF barriers in that the brain capillary wall is not fenestrated and endothelial cells are also sealed with tight junctions. In addition, accessory structures such as pericytes, astrocytes, and the basement membrane contribute to the BBB (16, 56, 63, 84). The equation adopted for this review also has advantages in that only data obtained from humans were included and AUCs of the free fraction of drugs were used to derive the equation (73).
At the present time, it is not certain if penetration of drugs into ELF follows the same pattern of penetration through the blood-CSF barrier. In fact, many other mechanisms of drug transport are present in drug delivery across the blood-CSF barrier. Besides passive diffusion, carrier-mediated transport, active efflux transport, and receptor-mediated transport are all potentially involved in the process (82, 94). It is not known whether these mechanisms are present also in the ELF epithelium. Even for passive diffusion through the BBB, many other equations have been evaluated (13, 17, 59, 68, 76, 95). Although the equation used in this review might not predict actual passive penetration of drugs through the alveolar epithelium exactly, it is still believed to reflect the general concept that passive diffusion through cells depends on lipophilicity and MW.
In this review, the discrepancy between serum and ELF concentrations could be explained with passive diffusion of just a few antibiotics: beta-lactams in negative way. Most antibiotics included in this review showed high ELF/free serum concentration ratios, which were not explainable by their limited penetration ability. In this regard, the possible error from estimating the ability of an antibiotic to penetrate into ELF with the adopted equation is not believed to be responsible for large errors in the current evaluation.
Although a study evaluating the ELF/plasma concentration ratio of vancomycin by the BAL technique was found, it was not included in this evaluation because simultaneous measurement of AM concentration was not performed (66). While the PC of vancomycin is reported to be −0.31 by material safety data sheets from the company, calculation of PC with an atom/fragment contribution method yields −0.84 (interactive KowWin; Syracuse Research Corporation). When vancomycin protein binding is assumed to be 55% and with an MW of 1,449.3 (SciFinder Scholar database) and two PC values applied, the lower ELF concentrations of vancomycin (ELF/serum concentration ratio = 0.18) from this study could also be explained by protein binding and low penetration capacity. The Celf/Cfs·K ratios using PC values of −0.31 and −0.84 were 0.74 and 0.92, respectively.
Effects of cells on ELF concentration of antibiotics.
This study shows that the ELF concentrations of some antibiotics, which were measured as higher than their serum levels by the BAL technique, might be explained by possible contamination from high achieved intracellular concentrations and subsequent lysis of these cells during the measurement of ELF content. The hypothesis can be applied to azithromycin, clarithromycin, ketolides (telithromycin and cethromycin), fluoroquinolones, itraconazole, tigecycline, rifampin, and ethambutol. This effect is similar to the problem of measuring tissue content using homogenization (75).
Whereas the antibiotics that concentrate inside cells show tissue-to-serum ratios above 1:1, antibiotics excluded from cells show ratios approximately 0.2:1 in homogenization experiments (74, 75). Data in tissue homogenization experiments, like ELF studies, are also variable between studies, even with the same antibiotic. Likely reasons for this variability include less-than-complete equilibration/diffusion because of non-steady-state conditions, variable field contaminations by blood and/or white blood cells, infected tissue versus noninfected tissue, and binding to extraneous proteins beyond blood and tissue fragments (e.g., albumin).
It is not known exactly how many cells are present or what fraction are lysed during the BAL procedure. We assumed lysis could be complete, but in some procedures we may not expect that all the cells in ELF were lysed when the fluid is sampled and measured. In the case of incomplete lysis, lower ELF concentrations than expected could result and the data could lead to discordant conclusions for relative ELF penetration within a drug class. Likewise, the same problems could occur if studies extracted variable amounts of cells. Finally, the situation of still larger ELF contents of antibiotics in settings of pulmonary infection versus the absence of infection could mainly depend on the numbers of cells in BAL-derived fluids (11, 67).
Effects of technical errors on ELF concentration of antibiotics.
For the group of antibiotics whose measured ELF concentrations were higher or lower than the expected ELF concentrations, including both penetration capacity and lysis of cells, there may be a yet-undiscovered permeability barrier or even an active transport process that could change the patterns of uptake or excretion around and through lung alveolar epithelial cells. However, in order to settle on that conclusion, the possibility of other technical errors first must be excluded.
Overestimation of the volume of ELF due to prolonged dwelling time of BAL fluid may explain the unexpectedly lower ELF concentrations of some antibiotics like amoxicillin. Other technical uncertainties regarding the BAL, such as the proper volume of instilled fluid, confuse the interpretation of ELF concentrations of some antibiotics.
Direct measurement of diffusion at bronchial sites.
To overcome possible technical errors caused by cells and cell lysis in sampling ELF by BAL, antibiotic concentrations in ELF have been sampled directly by approaching the alveolar wall as only a diffusion barrier. This would be similar in principle to the use of microdialysis for measurement of tissue concentration. In this regard, a new technique (bronchoscopic microsampling [BMS] method) is now being applied to measure drug concentrations in ELF (102).
Concentrations of levofloxacin in ELF measured by BMS showed marked differences from levofloxacin ELF concentrations measured by BAL (102). Although ELF concentrations of levofloxacin measured by the BAL technique were higher than serum levels by up to threefold, the BMS method revealed that ELF concentrations of levofloxacin were lower than serum concentrations before 2 h of oral administration and were same as the serum level thereafter. BMS studies measuring telithromycin and gatifloxacin also showed that concentrations of the antibiotics were significantly lower in ELF obtained by BMS than in ELF obtained by BAL (61, 62). This may be explained by slow diffusion of those antibiotics in comparison to urea, as a yet poorly unrecognized technical problem with the fluid washout method currently used to measure ELF concentrations of antibiotics by BAL.
Actual lung site of infection.
One might hypothesize that both free-ELF measurement and the BMS imply a 1:1 diffusion at steady state, regardless of the antibiotic used to measure these fluids. Even if there are real differences in Celf/Cfs ratio between antibiotics, it is an open question which medium correctly represents the lung site of infection.
Because lung infection can disrupt the alveolar wall and invade the interstitial space, superficial areas like ELF may not represent the actual site of lung infections. In these cases as well, it may still be best to approximate serum levels as a target in relation to MIC, arguing that diffusion into infection sites is at least as good as it is into other freely perfused capillary beds. In addition, the inflammation and alveolar cell damage created by bacterial invasion and infection result in increased vascular permeability. Cellular mass at a site of active infection also increases because of the margination of white blood cells to the site, and, with reference to antibiotics that enter cells, these cells may carry increased amounts of antibiotic with them (43, 90).
Thus, for many good reasons, the ELF levels of antibiotics measured in healthy persons may not be an accurate measure at the actual antibiotic concentrations at the site of infection, and in fact the cellular lysis portions of this analysis may become more important for the extrapolation of volunteer data to infected patients. On the other hand, it appears that total serum concentrations of macrolides and ciprofloxacin as AUIC >100 and >125, respectively, predict outcomes in human infections, including pneumonia (40a, 90a)
CONCLUSIONS
Low ELF ratios of beta-lactams could be explained by the poor diffusion and free fraction alone. Vancomycin might be another example of a drug with low ELF concentration due to limited penetration and protein binding. The high ELF ratios for most fluoroquinolones, macrolides, ketolides, and some other antibiotics were well described by inclusion of known intracellular concentrations and the anticipated range of cell lysis. Fundamentally, ELF may not represent the lung site where antibiotics act against infection. In view of the technical and interpretive problems with conventional ELF and especially BAL, the lung microdialysis experiments or the BMS method may offer an overall better correlation with microbiological outcomes. Development of more-relevant methods to measure tissue level of antibiotics appears essential if we are to truly assess real PK/PD differences between antibiotics in serum and antibiotics at the infection site. Further evaluation of the issue is needed, and, while these are reaching consensus, we continue to express PK/PD parameters using serum concentration of total drug because these values do correlate with microbiological outcomes in patients.
Footnotes
Published ahead of print on 10 September 2007.
REFERENCES
- 1.Ackermann, G., and A. C. Rodloff. 2003. Drugs of the 21st century: telithromycin (HMR 3647)—the first ketolide. J. Antimicrob. Chemother. 51:497-511. [DOI] [PubMed] [Google Scholar]
- 2.Alavijeh, M. S., M. Chishty, M. Z. Qaiser, and A. M. Palmer. 2005. Drug metabolism and pharmacokinetics, the blood-brain barrier, and central nervous system drug discovery. NeuroRx 2:554-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrews, J., D. Honeybourne, G. Jevons, M. Boyce, R. Wise, A. Bello, and D. Gajjar. 2003. Concentrations of garenoxacin in plasma, bronchial mucosa, alveolar macrophages and epithelial lining fluid following a single oral 600 mg dose in healthy adult subjects. J. Antimicrob. Chemother. 51:727-730. [DOI] [PubMed] [Google Scholar]
- 4.Andrews, J. M., D. Honeybourne, N. P. Brenwald, D. Bannerjee, M. Iredale, B. Cunningham, and R. Wise. 1997. Concentrations of trovafloxacin in bronchial mucosa, epithelial lining fluid, alveolar macrophages and serum after administration of single or multiple oral doses to patients undergoing fibre-optic bronchoscopy. J. Antimicrob. Chemother. 39:797-802. [DOI] [PubMed] [Google Scholar]
- 5.Andrews, J. M., D. Honeybourne, G. Jevons, N. P. Brenwald, B. Cunningham, and R. Wise. 1997. Concentrations of levofloxacin (HR 355) in the respiratory tract following a single oral dose in patients undergoing fibre-optic bronchoscopy. J. Antimicrob. Chemother. 40:573-577. [DOI] [PubMed] [Google Scholar]
- 6.Appelbaum, P. C. 2000. Microbiological and pharmacodynamic considerations in the treatment of infection due to antimicrobial-resistant Streptococcus pneumoniae. Clin. Infect. Dis. 31(Suppl. 2):S29-S34. [DOI] [PubMed] [Google Scholar]
- 7.Baldwin, D. R., D. Honeybourne, and R. Wise. 1992. Pulmonary disposition of antimicrobial agents: methodological considerations. Antimicrob. Agents Chemother. 36:1171-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baldwin, D. R., R. Wise, J. M. Andrews, J. P. Ashby, and D. Honeybourne. 1992. The distribution of temafloxacin in bronchial epithelial lining fluid, alveolar macrophages and bronchial mucosa. Eur. Respir. J. 5:471-476. [PubMed] [Google Scholar]
- 9.Baldwin, D. R., R. Wise, J. M. Andrews, M. Gill, and D. Honeybourne. 1993. Comparative bronchoalveolar concentrations of ciprofloxacin and lomefloxacin following oral administration. Respir. Med. 87:595-601. [DOI] [PubMed] [Google Scholar]
- 10.Ball, P., F. Baquero, O. Cars, T. File, J. Garau, K. Klugman, D. E. Low, E. Rubinstein, and R. Wise. 2002. Antibiotic therapy of community respiratory tract infections: strategies for optimal outcomes and minimized resistance emergence. J. Antimicrob. Chemother. 49:31-40. [DOI] [PubMed] [Google Scholar]
- 11.Baselski, V. S., and R. G. Wunderink. 1994. Bronchoscopic diagnosis of pneumonia. Clin. Microbiol. Rev. 7:533-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergogne-Berezin, E. 2002. Clinical role of protein binding of quinolones. Clin. Pharmacokinet. 41:741-750. [DOI] [PubMed] [Google Scholar]
- 13.Bodor, N., and P. Buchwald. 1999. Recent advances in the brain targeting of neuropharmaceuticals by chemical delivery systems. Adv. Drug Deliv. Rev. 36:229-254. [DOI] [PubMed] [Google Scholar]
- 14.Boselli, E., D. Breilh, T. Rimmele, S. Djabarouti, J. Toutain, D. Chassard, M. C. Saux, and B. Allaouchiche. 2005. Pharmacokinetics and intrapulmonary concentrations of linezolid administered to critically ill patients with ventilator-associated pneumonia. Crit. Care Med. 33:1529-1533. [DOI] [PubMed] [Google Scholar]
- 15.Capitano, B., H. M. Mattoes, E. Shore, A. O'Brien, S. Braman, C. Sutherland, and D. P. Nicolau. 2004. Steady-state intrapulmonary concentrations of moxifloxacin, levofloxacin, and azithromycin in older adults. Chest 125:965-973. [DOI] [PubMed] [Google Scholar]
- 16.Chen, Y., G. Dalwadi, and H. A. Benson. 2004. Drug delivery across the blood-brain barrier. Curr. Drug Deliv. 1:361-376. [DOI] [PubMed] [Google Scholar]
- 17.Clark, D. E. 2003. In silico prediction of blood-brain barrier permeation. Drug Discov. Today 8:927-933. [DOI] [PubMed] [Google Scholar]
- 18.Conte, J. E., J. A. Golden, J. E. Kipps, E. T. Lin, and E. Zurlinden. 2004. Effect of sex and AIDS status on the plasma and intrapulmonary pharmacokinetics of rifampicin. Clin. Pharmacokinet. 43:395-404. [DOI] [PubMed] [Google Scholar]
- 19.Conte, J. E., Jr., J. Golden, S. Duncan, E. McKenna, E. Lin, and E. Zurlinden. 1996. Single-dose intrapulmonary pharmacokinetics of azithromycin, clarithromycin, ciprofloxacin, and cefuroxime in volunteer subjects. Antimicrob. Agents Chemother. 40:1617-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conte, J. E., Jr., J. A. Golden, S. Duncan, E. McKenna, and E. Zurlinden. 1999. Intrapulmonary concentrations of pyrazinamide. Antimicrob. Agents Chemother. 43:1329-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conte, J. E., Jr., J. A. Golden, S. Duncan, E. McKenna, and E. Zurlinden. 1995. Intrapulmonary pharmacokinetics of clarithromycin and of erythromycin. Antimicrob. Agents Chemother. 39:334-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conte, J. E., Jr., J. A. Golden, M. J. Kelley, and E. Zurlinden. 2005. Intrapulmonary pharmacokinetics and pharmacodynamics of meropenem. Int. J. Antimicrob. Agents 26:449-456. [DOI] [PubMed] [Google Scholar]
- 23.Conte, J. E., Jr., J. A. Golden, M. J. Kelley, and E. Zurlinden. 2005. Steady-state serum and intrapulmonary pharmacokinetics and pharmacodynamics of tigecycline. Int. J. Antimicrob. Agents 25:523-529. [DOI] [PubMed] [Google Scholar]
- 24.Conte, J. E., Jr., J. A. Golden, J. Kipps, E. T. Lin, and E. Zurlinden. 2001. Effects of AIDS and gender on steady-state plasma and intrapulmonary ethambutol concentrations. Antimicrob. Agents Chemother. 45:2891-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conte, J. E., Jr., J. A. Golden, J. Kipps, M. McIver, and E. Zurlinden. 2004. Intrapulmonary pharmacokinetics and pharmacodynamics of itraconazole and 14-hydroxyitraconazole at steady state. Antimicrob. Agents Chemother. 48:3823-3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conte, J. E., Jr., J. A. Golden, J. Kipps, and E. Zurlinden. 2002. Intrapulmonary pharmacokinetics of linezolid. Antimicrob. Agents Chemother. 46:1475-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conte, J. E., Jr., J. A. Golden, J. Kipps, and E. Zurlinden. 2004. Steady-state plasma and intrapulmonary pharmacokinetics and pharmacodynamics of cethromycin. Antimicrob. Agents Chemother. 48:3508-3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conte, J. E., Jr., J. A. Golden, M. McIver, and E. Zurlinden. 2006. Intrapulmonary pharmacokinetics and pharmacodynamics of high-dose levofloxacin in healthy volunteer subjects. Int. J. Antimicrob. Agents 28:114-121. [DOI] [PubMed] [Google Scholar]
- 29.Conte, J. E., Jr., J. A. Golden, M. McQuitty, J. Kipps, S. Duncan, E. McKenna, and E. Zurlinden. 2002. Effects of gender, AIDS, and acetylator status on intrapulmonary concentrations of isoniazid. Antimicrob. Agents Chemother. 46:2358-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conte, J. E., Jr., J. A. Golden, M. McQuitty, J. Kipps, E. T. Lin, and E. Zurlinden. 2000. Effects of AIDS and gender on steady-state plasma and intrapulmonary ethionamide concentrations. Antimicrob. Agents Chemother. 44:1337-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Conte, J. E., Jr., J. A. Golden, M. McQuitty, J. Kipps, E. T. Lin, and E. Zurlinden. 2000. Single-dose intrapulmonary pharmacokinetics of rifapentine in normal subjects. Antimicrob. Agents Chemother. 44:985-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cook, P. J., J. M. Andrews, R. Wise, and D. Honeybourne. 1996. Distribution of cefdinir, a third generation cephalosporin antibiotic, in serum and pulmonary compartments. J. Antimicrob. Chemother. 37:331-339. [DOI] [PubMed] [Google Scholar]
- 33.Cook, P. J., J. M. Andrews, R. Wise, D. Honeybourne, and H. Moudgil. 1995. Concentrations of OPC-17116, a new fluoroquinolone antibacterial, in serum and lung compartments. J. Antimicrob. Chemother. 35:317-326. [DOI] [PubMed] [Google Scholar]
- 34.Cook, P. J., J. M. Andrews, J. Woodcock, R. Wise, and D. Honeybourne. 1994. Concentration of amoxycillin and clavulanate in lung compartments in adults without pulmonary infection. Thorax 49:1134-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Craig, W. A. 2001. The hidden impact of antibacterial resistance in respiratory tract infection. Re-evaluating current antibiotic therapy. Respir. Med. 95(Suppl. A):S12-S19. [DOI] [PubMed] [Google Scholar]
- 36.Craig, W. A., and B. Suh. 1996. Protein binding and the antimicrobial effects: methods for the determination of protein binding, p. 367-402. In V. Lorian (ed.), Antibiotics in laboratory medicine, 4th ed. Williams and Wilkins, Baltimore, MD.
- 37.Crapo, J. D., B. E. Barry, P. Gehr, M. Bachofen, and E. R. Weibel. 1982. Cell number and cell characteristics of the normal human lung. Am. Rev. Respir. Dis. 126:332-337. [DOI] [PubMed] [Google Scholar]
- 38.Cunha, B. A. 1991. Antibiotic pharmacokinetic considerations in pulmonary infections. Semin. Respir. Infect. 6:168-182. [PubMed] [Google Scholar]
- 39.Dargaville, P. A., M. South, P. Vervaart, and P. N. McDougall. 1999. Validity of markers of dilution in small volume lung lavage. Am. J. Respir. Crit. Care Med. 160:778-784. [DOI] [PubMed] [Google Scholar]
- 40.Egleton, R. D., and T. P. Davis. 1997. Bioavailability and transport of peptides and peptide drugs into the brain. Peptides 18:1431-1439. [DOI] [PubMed] [Google Scholar]
- 40a.Forrest, A., D. E. Nix, C. H. Ballow, T. F. Goss, M. C. Birmingham, and J. J. Schentag. 1993. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob. Agents Chemother. 37:1073-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Foulds, G., R. M. Shepard, and R. B. Johnson. 1990. The pharmacokinetics of azithromycin in human serum and tissues. J. Antimicrob. Chemother. 25(Suppl. A):73-82. [DOI] [PubMed] [Google Scholar]
- 42.Gajjar, D. A., A. Bello, Z. Ge, L. Christopher, and D. M. Grasela. 2003. Multiple-dose safety and pharmacokinetics of oral garenoxacin in healthy subjects. Antimicrob. Agents Chemother. 47:2256-2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gladue, R. P., G. M. Bright, R. E. Isaacson, and M. F. Newborg. 1989. In vitro and in vivo uptake of azithromycin (CP-62,993) by phagocytic cells: possible mechanism of delivery and release at sites of infection. Antimicrob. Agents Chemother. 33:277-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gotfried, M. H., L. H. Danziger, and K. A. Rodvold. 2003. Steady-state plasma and bronchopulmonary characteristics of clarithromycin extended-release tablets in normal healthy adult subjects. J. Antimicrob. Chemother. 52:450-456. [DOI] [PubMed] [Google Scholar]
- 45.Gotfried, M. H., L. H. Danziger, and K. A. Rodvold. 2001. Steady-state plasma and intrapulmonary concentrations of levofloxacin and ciprofloxacin in healthy adult subjects. Chest 119:1114-1122. [DOI] [PubMed] [Google Scholar]
- 46.Graff, C. L., and G. M. Pollack. 2004. Drug transport at the blood-brain barrier and the choroid plexus. Curr. Drug Metab. 5:95-108. [DOI] [PubMed] [Google Scholar]
- 47.Grigg, J., S. Kleinert, R. L. Woods, C. J. Thomas, P. Vervaart, J. L. Wilkinson, and C. F. Robertson. 1996. Alveolar epithelial lining fluid cellularity, protein and endothelin-1 in children with congenital heart disease. Eur. Respir. J. 9:1381-1388. [DOI] [PubMed] [Google Scholar]
- 48.Habgood, M. D., D. J. Begley, and N. J. Abbott. 2000. Determinants of passive drug entry into the central nervous system. Cell Mol. Neurobiol. 20:231-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hammarlund-Udenaes, M., L. K. Paalzow, and E. C. de Lange. 1997. Drug equilibration across the blood-brain barrier-pharmacokinetic considerations based on the microdialysis method. Pharm. Res. 14:128-134. [DOI] [PubMed] [Google Scholar]
- 50.Hennig-Pauka, I., M. Ganter, G. F. Gerlach, and H. J. Rothkotter. 2001. Enzyme activities, protein content and cellular variables in the pulmonary epithelial lining fluid in selected healthy pigs. J. Vet. Med. A Physiol. Pathol. Clin. Med. 48:631-639. [DOI] [PubMed] [Google Scholar]
- 51.Honeybourne, D., J. M. Andrews, B. Cunningham, G. Jevons, and R. Wise. 1999. The concentrations of clinafloxacin in alveolar macrophages, epithelial lining fluid, bronchial mucosa and serum after administration of single 200 mg oral doses to patients undergoing fibre-optic bronchoscopy. J. Antimicrob. Chemother. 43:153-155. [DOI] [PubMed] [Google Scholar]
- 52.Honeybourne, D., D. Banerjee, J. Andrews, and R. Wise. 2001. Concentrations of gatifloxacin in plasma and pulmonary compartments following a single 400 mg oral dose in patients undergoing fibre-optic bronchoscopy. J. Antimicrob. Chemother. 48:63-66. [DOI] [PubMed] [Google Scholar]
- 53.Honeybourne, D., F. Kees, J. M. Andrews, D. Baldwin, and R. Wise. 1994. The levels of clarithromycin and its 14-hydroxy metabolite in the lung. Eur. Respir. J. 7:1275-1280. [DOI] [PubMed] [Google Scholar]
- 54.Honeybourne, D., C. Tobin, G. Jevons, J. Andrews, and R. Wise. 2003. Intrapulmonary penetration of linezolid. J. Antimicrob. Chemother. 51:1431-1434. [DOI] [PubMed] [Google Scholar]
- 55.Jarvis, B., and H. M. Lamb. 1998. Rifapentine. Drugs 56:607-616. [DOI] [PubMed] [Google Scholar]
- 56.Jong, A., and S. H. Huang. 2005. Blood-brain barrier drug discovery for central nervous system infections. Curr. Drug Targets Infect. Disord. 5:65-72. [DOI] [PubMed] [Google Scholar]
- 57.Kadota, J., Y. Ishimatsu, T. Iwashita, Y. Matsubara, K. Tomono, M. Tateno, R. Ishihara, C. Muller-Serieys, and S. Kohno. 2002. Intrapulmonary pharmacokinetics of telithromycin, a new ketolide, in healthy Japanese volunteers. Antimicrob. Agents Chemother. 46:917-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kasim, N. A., M. Whitehouse, C. Ramachandran, M. Bermejo, H. Lennernas, A. S. Hussain, H. E. Junginger, S. A. Stavchansky, K. K. Midha, V. P. Shah, and G. L. Amidon. 2004. Molecular properties of WHO essential drugs and provisional biopharmaceutical classification. Mol. Pharm. 1:85-96. [DOI] [PubMed] [Google Scholar]
- 59.Kelder, J., P. D. Grootenhuis, D. M. Bayada, L. P. Delbressine, and J. P. Ploemen. 1999. Polar molecular surface as a dominating determinant for oral absorption and brain penetration of drugs. Pharm. Res. 16:1514-1519. [DOI] [PubMed] [Google Scholar]
- 60.Khair, O. A., J. M. Andrews, D. Honeybourne, G. Jevons, F. Vacheron, and R. Wise. 2001. Lung concentrations of telithromycin after oral dosing. J. Antimicrob. Chemother. 47:837-840. [DOI] [PubMed] [Google Scholar]
- 61.Kikuchi, J., K. Yamazaki, E. Kikuchi, A. Ishizaka, and M. Nishimura. 2007. Pharmacokinetics of gatifloxacin after a single oral dose in healthy young adult subjects and adult patients with chronic bronchitis, with a comparison of drug concentrations obtained by bronchoscopic microsampling and bronchoalveolar lavage. Clin. Ther. 29:123-130. [DOI] [PubMed] [Google Scholar]
- 62.Kikuchi, J., K. Yamazaki, E. Kikuchi, A. Ishizaka, and M. Nishimura. 2007. Pharmacokinetics of telithromycin using bronchoscopic microsampling after single and multiple oral doses. Pulm. Pharmacol. Ther. 20:549-555. [DOI] [PubMed] [Google Scholar]
- 63.Kim, J. H., J. A. Park, S. W. Lee, W. J. Kim, Y. S. Yu, and K. W. Kim. 2006. Blood-neural barrier: intercellular communication at glio-vascular interface. J. Biochem. Mol. Biol. 39:339-345. [DOI] [PubMed] [Google Scholar]
- 64.Kim, M. K., W. Zhou, P. R. Tessier, D. Xuan, M. Ye, C. H. Nightingale, and D. P. Nicolau. 2002. Bactericidal effect and pharmacodynamics of cethromycin (ABT-773) in a murine pneumococcal pneumonia model. Antimicrob. Agents Chemother. 46:3185-3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Krombach, F., S. Munzing, A. Allmeling, J. T. Gerlach, J. Behr, and M. Dorger. 1997. Cell size of alveolar macrophages: an interspecies comparison. Environ. Health Perspect. 105S:1261-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lamer, C., V. de Beco, P. Soler, S. Calvat, J. Y. Fagon, M. C. Dombret, R. Farinotti, J. Chastre, and C. Gibert. 1993. Analysis of vancomycin entry into pulmonary lining fluid by bronchoalveolar lavage in critically ill patients. Antimicrob. Agents Chemother. 37:281-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Linder, J., and S. Rennard. 1988. Bronchoalveolar lavage, p. 67-96. ASCP Press, Chicago, IL.
- 68.Liu, X., M. Tu, R. S. Kelly, C. Chen, and B. J. Smith. 2004. Development of a computational approach to predict blood-brain barrier permeability. Drug Metab. Dispos. 32:132-139. [DOI] [PubMed] [Google Scholar]
- 69.Mandell, G. L., J. E. Bennett, and R. Dolin. 2005. Principles and practice of infectious diseases, 6th ed. Elsevier Churchill Livingstone, Philadelphia, PA.
- 70.Marcy, T. W., W. W. Merrill, J. A. Rankin, and H. Y. Reynolds. 1987. Limitations of using urea to quantify epithelial lining fluid recovered by bronchoalveolar lavage. Am. Rev. Respir. Dis. 135:1276-1280. [DOI] [PubMed] [Google Scholar]
- 70a.Moffat, A. C., M. D. Osselton, and B. Widdap (ed.). 2004. Clarke's analysis of drugs and poisons, 3rd ed. Pharmaceutical Press, London, United Kingdom.
- 71.Motl, S., Y. Zhuang, C. M. Waters, and C. F. Stewart. 2006. Pharmacokinetic considerations in the treatment of CNS tumours. Clin. Pharmacokinet. 45:871-903. [DOI] [PubMed] [Google Scholar]
- 72.Muller-Serieys, C., P. Soler, C. Cantalloube, F. Lemaitre, H. P. Gia, F. Brunner, and A. Andremont. 2001. Bronchopulmonary disposition of the ketolide telithromycin (HMR 3647). Antimicrob. Agents Chemother. 45:3104-3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nau, R., F. Sorgel, and H. W. Prange. 1994. Lipophilicity at pH 7.4 and molecular size govern the entry of the free serum fraction of drugs into the cerebrospinal fluid in humans with uninflamed meninges. J. Neurol. Sci. 122:61-65. [DOI] [PubMed] [Google Scholar]
- 74.Nix, D. E., S. D. Goodwin, C. A. Peloquin, D. L. Rotella, and J. J. Schentag. 1991. Antibiotic tissue penetration and its relevance: impact of tissue penetration on infection response. Antimicrob. Agents Chemother. 35:1953-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nix, D. E., S. D. Goodwin, C. A. Peloquin, D. L. Rotella, and J. J. Schentag. 1991. Antibiotic tissue penetration and its relevance: models of tissue penetration and their meaning. Antimicrob. Agents Chemother. 35:1947-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Norinder, U., and M. Haeberlein. 2002. Computational approaches to the prediction of the blood-brain distribution. Adv. Drug Deliv. Rev. 54:291-313. [DOI] [PubMed] [Google Scholar]
- 77.Olsen, K. M., G. San Pedro, L. P. Gann, P. O. Gubbins, D. M. Halinski, and G. D. Campbell, Jr. 1996. Intrapulmonary pharmacokinetics of azithromycin in healthy volunteers given five oral doses. Antimicrob. Agents Chemother. 40:2582-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ong, C. T., P. K. Dandekar, C. Sutherland, C. H. Nightingale, and D. P. Nicolau. 2005. Intrapulmonary concentrations of telithromycin: clinical implications for respiratory tract infections due to Streptococcus pneumoniae. Chemotherapy 51:339-346. [DOI] [PubMed] [Google Scholar]
- 79.Panteix, G., R. Harf, J. F. Desnottes, H. Gosselet, M. Leclercq, N. Diallo, N. Couprie, A. Desbos, M. Perrin Fayolle, and M. Ballereau. 1994. Accumulation of pefloxacin in the lower respiratory tract demonstrated by bronchoalveolar lavage. J. Antimicrob. Chemother. 33:979-985. [DOI] [PubMed] [Google Scholar]
- 80.Patel, K. B., D. Xuan, P. R. Tessier, J. H. Russomanno, R. Quintiliani, and C. H. Nightingale. 1996. Comparison of bronchopulmonary pharmacokinetics of clarithromycin and azithromycin. Antimicrob. Agents Chemother. 40:2375-2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pusch, R., M. Kleen, O. Habler, F. Krombach, C. Vogelmeier, M. Welte, and B. Zwissler. 1997. Biochemical and cellular composition of alveolar epithelial lining fluid in anesthetized healthy lambs. Eur. J. Med. Res. 2:499-505. [PubMed] [Google Scholar]
- 82.Redzic, Z. B., and M. B. Segal. 2004. The structure of the choroid plexus and the physiology of the choroid plexus epithelium. Adv. Drug Deliv. Rev. 56:1695-1716. [DOI] [PubMed] [Google Scholar]
- 83.Rennard, S. I., G. Basset, D. Lecossier, K. M. O'Donnell, P. Pinkston, P. G. Martin, and R. G. Crystal. 1986. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J. Appl. Physiol. 60:532-538. [DOI] [PubMed] [Google Scholar]
- 84.Ricci, M., P. Blasi, S. Giovagnoli, and C. Rossi. 2006. Delivering drugs to the central nervous system: a medicinal chemistry or a pharmaceutical technology issue? Curr. Med. Chem. 13:1757-1775. [DOI] [PubMed] [Google Scholar]
- 85.Richer, M., S. Allard, L. Manseau, F. Vallee, R. Pak, and M. LeBel. 1995. Suction-induced blister fluid penetration of cefdinir in healthy volunteers following ascending oral doses. Antimicrob. Agents Chemother. 39:1082-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rodvold, K. A. 1999. Clinical pharmacokinetics of clarithromycin. Clin. Pharmacokinet. 37:385-398. [DOI] [PubMed] [Google Scholar]
- 87.Rodvold, K. A., L. H. Danziger, and M. H. Gotfried. 2003. Steady-state plasma and bronchopulmonary concentrations of intravenous levofloxacin and azithromycin in healthy adults. Antimicrob. Agents Chemother. 47:2450-2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rodvold, K. A., M. H. Gotfried, L. H. Danziger, and R. J. Servi. 1997. Intrapulmonary steady-state concentrations of clarithromycin and azithromycin in healthy adult volunteers. Antimicrob. Agents Chemother. 41:1399-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Saunders, N. R., M. D. Habgood, and K. M. Dziegielewska. 1999. Barrier mechanisms in the brain. I. Adult brain. Clin. Exp. Pharmacol. Physiol. 26:11-19. [DOI] [PubMed] [Google Scholar]
- 90.Schentag, J. J., and C. H. Ballow. 1991. Tissue-directed pharmacokinetics. Am. J. Med. 91:5S-11S. [DOI] [PubMed] [Google Scholar]
- 90a.Schentag, J. J., K. P. Klugman, V. L. Yu, M. H. Adelman, G. J. Wilton, C. C. Chiou, M. Patel, B. Lavin, and J. A. Paladino. 2007. Streptococcus pneumoniae bacteremia: pharmacodynamic correlations with outcome and macrolide resistance—a controlled study. Int. J. Antimicrob. 30:264-269. [DOI] [PubMed] [Google Scholar]
- 91.Schuler, P., K. Zemper, K. Borner, P. Koeppe, T. Schaberg, and H. Lode. 1997. Penetration of sparfloxacin and ciprofloxacin into alveolar macrophages, epithelial lining fluid, and polymorphonuclear leucocytes. Eur. Respir. J. 10:1130-1136. [DOI] [PubMed] [Google Scholar]
- 92.Segel, G. B., G. R. Cokelet, and M. A. Lichtman. 1981. The measurement of lymphocyte volume: importance of reference particle deformability and counting solution tonicity. Blood 57:894-899. [PubMed] [Google Scholar]
- 93.Soman, A., D. Honeybourne, J. Andrews, G. Jevons, and R. Wise. 1999. Concentrations of moxifloxacin in serum and pulmonary compartments following a single 400 mg oral dose in patients undergoing fibre-optic bronchoscopy. J. Antimicrob. Chemother. 44:835-838. [DOI] [PubMed] [Google Scholar]
- 94.Strazielle, N., S. T. Khuth, and J. F. Ghersi-Egea. 2004. Detoxification systems, passive and specific transport for drugs at the blood-CSF barrier in normal and pathological situations. Adv. Drug Deliv. Rev. 56:1717-1740. [DOI] [PubMed] [Google Scholar]
- 95.Subramanian, G., and D. B. Kitchen. 2003. Computational models to predict blood-brain barrier permeation and CNS activity. J Comput. Aided Mol. Des. 17:643-664. [DOI] [PubMed] [Google Scholar]
- 96.Ting-Beall, H. P., D. Needham, and R. M. Hochmuth. 1993. Volume and osmotic properties of human neutrophils. Blood 81:2774-2780. [PubMed] [Google Scholar]
- 97.Wise, R. 1991. Comparative penetration of selected fluoroquinolones into respiratory tract fluids and tissues. Am. J. Med. 91:67S-70S. [DOI] [PubMed] [Google Scholar]
- 98.Wise, R. 1983. Protein binding of beta-lactams: the effects on activity and pharmacology particularly tissue penetration. II. Studies in man. J. Antimicrob. Chemother. 12:105-118. [DOI] [PubMed] [Google Scholar]
- 99.Wise, R., J. Andrews, B. P. Imbimbo, I. Greaves, and D. Honeybourne. 1993. The penetration of rufloxacin into sites of potential infection in the respiratory tract. J. Antimicrob. Chemother. 32:861-866. [DOI] [PubMed] [Google Scholar]
- 100.Wise, R., and D. Honeybourne. 1996. A review of the penetration of sparfloxacin into the lower respiratory tract and sinuses. J. Antimicrob. Chemother. 37(Suppl. A):57-63. [DOI] [PubMed] [Google Scholar]
- 101.World Health Organization. 2004. World health report 2004. Changing history. World Health Organization, Geneva, Switzerland.
- 102.Yamazaki, K., S. Ogura, A. Ishizaka, T. Oh-hara, and M. Nishimura. 2003. Bronchoscopic microsampling method for measuring drug concentration in epithelial lining fluid. Am. J. Respir. Crit. Care Med. 168:1304-1307. [DOI] [PubMed] [Google Scholar]
- 103.Ziglam, H. M., D. R. Baldwin, I. Daniels, J. M. Andrew, and R. G. Finch. 2002. Rifampicin concentrations in bronchial mucosa, epithelial lining fluid, alveolar macrophages and serum following a single 600 mg oral dose in patients undergoing fibre-optic bronchoscopy. J. Antimicrob. Chemother. 50:1011-1015. [DOI] [PubMed] [Google Scholar]