Abstract
The concentration-dependent effects of echinocandins on the metabolic activity of Aspergillus spp. were comparatively studied by using nongerminated and germinated conidia. The susceptibilities of 11 Aspergillus fumigatus, 8 A. terreus and 8 A. flavus isolates to caspofungin, micafungin, and anidulafungin were studied by a CLSI (formerly NCCLS) M38-A broth microdilution-based method. After 48 h of incubation the minimum effective concentration (MEC) was defined microscopically. Metabolic activity was assessed by the 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide assay and modeled by using the sigmoid (Emax) or “bell-shaped” model. The median MEC values of caspofungin (0.5 to 1 μg/ml), micafungin (0.06 to 0.12 μg/ml), and anidulafungin (0.03 μg/ml) against nongerminated conidia increased by 0 to 1, 1 to 2, and 2 to 3 twofold dilutions, respectively (depending on the species), over those against germinated conidia. A similar shift to the right was demonstrated for the corresponding curves of metabolic activity. There was a significant correlation between the degrees of maximal metabolic inhibition caused by different echinocandins at both the species level (greater inhibition for A. flavus) and the strain level (r = 0.84 to 0.93; P < 0.0001). Paradoxical increases in metabolism in the presence of higher concentrations of caspofungin, micafungin, and anidulafungin were detected in 6, 2, and 5 of the A. fumigatus isolates, respectively; 5, 1, and 2 of the A. terreus isolates, respectively; and 1, 0, and 0 of the A. flavus isolates, respectively. Based on the model, 50% of the maximal paradoxical increase was detected with 4.2, 11.1, and 10.8 μg/ml of caspofungin, micafungin, and anidulafungin, respectively. All echinocandins therefore exerted comparable levels of maximal metabolic inhibition against Aspergillus spp. at concentrations that were differentially increased for germinated versus nongerminated conidia. The paradoxical increase in metabolism occurred more frequently and at lower concentrations with caspofungin than with micafungin and anidulafungin.
The echinocandins are a group of recently introduced cyclic lipopeptide agents that inhibit the (1,3)-β-d-glucan synthase activity of Candida spp. and Aspergillus spp. (10). In vitro and at clinically relevant concentrations, these compounds do not usually cause the complete inhibition of Aspergillus growth but induce morphological hyphal changes. The minimum effective concentration (MEC), defined as the lowest drug concentration at which short, stubby, and highly branched hyphae are observed, has been introduced for the determination of echinocandin activity against Aspergillus spp. in vitro (3, 13, 16, 24). The MEC, however, represents a subjective, qualitative assessment of hyphal morphology and does not provide a quantification of the antifungal activity at different concentrations.
We recently developed a new, objective, and quantitative method for assessment of the in vitro activity of caspofungin against Aspergillus fumigatus, A. terreus, and A. flavus by measuring the drug-induced changes in the metabolic activities of these species by using an optimized 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) assay (1). This method generated descriptive concentration-effect curves of the activity of caspofungin against Aspergillus spp. and demonstrated inter- and intraspecies differences in the degrees of metabolic inhibition associated with the formation of aberrant hyphae. This inhibition was greater for A. flavus than for the other two species. For almost half of the A. fumigatus and A. terreus isolates, a paradoxical increase in metabolism was detected at higher caspofungin concentrations. These concentration-dependent metabolic changes in the absence and the presence of the paradoxical response were described by using the sigmoid (Emax) and “bell-shaped” models, respectively, and the 50% effective concentrations (EC50s) generated by these models were a good approximation of the microscopically defined MEC (1).
While the mechanisms and potential in vivo and clinical significance of the concentration-dependent effects against Aspergillus spp. revealed above remain to be investigated, it would be important to discern whether these effects are caspofungin specific or are shared with the other echinocandins. Of particular interest would be an investigation of whether those species and isolates with lower degrees of metabolic inhibition by caspofungin are less inhibited by the other echinocandins as well (i.e., if there is metabolic cross-resistance among these agents), whether the EC50 values generated by the model also approximate the microscopic MECs of micafungin and anidulafungin, and whether the paradoxical increase in metabolism is observed for these echinocandins as well. Notably, recent studies on the paradoxical effect of caspofungin against Candida spp. suggested that this was less frequently observed with other echinocandins (7, 29).
We therefore comparatively studied the in vitro activities of caspofungin, micafungin, and anidulafungin against a large collection of A. fumigatus, A. terreus, and A. flavus isolates using the quantitative methodology described above. These studies were performed with both nongerminated and germinated conidia in order to investigate the possibility of the partial attenuation of activity for some or all of these compounds under circumstances in which they are added after the germination of Aspergillus conidia, which is the most likely scenario in vivo.
MATERIALS AND METHODS
Isolates.
A total of 27 clinical isolates of Aspergillus spp. were used, including 11 isolates of A. fumigatus, 8 of A. terreus, and 8 of A. flavus. The conidia were harvested after the isolates were subcultured on potato dextrose agar at 35°C for 5 to 7 days and were suspended in normal saline containing 0.025% Tween 20. The conidial suspensions were counted with a hemacytometer (27), and the inoculum sizes were verified by quantitative colony counts on Sabouraud dextrose agar plates. Reference strains Candida krusei ATCC 6258 and Candida parapsilosis ATCC 22019 were used as quality controls.
Medium.
RPMI 1640 medium with l-glutamine but without bicarbonate, buffered to pH 7.0 with 0.165 M 3-N-morpholinopropanesulfonic acid (Cambrex Bio Science, Walkersville, MD), was used as the assay medium.
XTT and menadione.
The tetrazolium salt XTT (Sigma-Aldrich, St. Louis, MO) was dissolved in normal saline at 0.5 mg/ml. Menadione (Sigma-Aldrich) was dissolved in absolute ethanol at 10 mg/ml (58 × 10−3 M) and was subsequently added to the XTT solution at a concentration of 31.25 μM.
Susceptibility testing.
The susceptibilities of all Aspergillus isolates to caspofungin, micafungin, and anidulafungin were studied by a broth microdilution method based on the recommendations in CLSI (formerly NCCLS) document M38-A (23), with minor changes. Caspofungin (Merck & Co. Inc., Whitehouse Station, NJ), micafungin (Astellas Pharma US Inc., Deerfield, IL), and anidulafungin (Vicuron Pharmaceuticals Inc., distributed by McKesson Bioservices, Rockville, MD) were dissolved in normal saline. Twofold serial dilutions of these agents in 100 μl of the assay medium were initially prepared in flat-bottom 96-well microtitration plates (Costar 3596; Corning Inc., Corning, NY) in order to obtain final concentrations of caspofungin ranging from 0.015 to 16 μg/ml at a total volume of 200 μl after addition of the fungal inoculum. For micafungin and anidulafungin, the concentrations studied were 0.008 to 2 and 8 and 16 μg/ml, respectively. For those isolates demonstrating a paradoxical increase in metabolic activity at higher drug concentrations (see Results), three additional concentrations (32, 64, and 128 μg/ml) of all the echinocandins were studied.
For the studies with the nongerminated conidia, 100 μl of medium containing 5 × 104 conidia/ml were added to the drug-containing wells described above, yielding a final concentration of 2.5 × 104 conidia/ml, and the plates were incubated at 37°C for 48 h. The MEC was then defined microscopically by two of the investigators (C.A. and J.M.) as the lowest drug concentrations that produced short, stubby, and highly branched hyphae (3, 13). In case discordant MEC values were found, the highest concentration was reported.
For the studies with the germinated conidia, plates with 100 μl of medium/well containing 5 × 104 conidia/ml were incubated at 37°C for 10 h for the A. fumigatus and A. flavus isolates and for 15 h for the A. terreus isolates, based on the findings of preliminary germination studies demonstrating >90% germination for all isolates after these incubation periods. Subsequently, 100 μl of the twofold drug dilutions prepared as described above was added to each well in order to yield the desired final concentrations, as described above for the nongerminated conidia. After further incubation for 48 h with the drug at 37°C, the MEC was defined microscopically, as described above.
For each of the three echinocandins, a row of wells with drug dilutions was filled with medium up to a total volume of 200 μl, without the fungal inoculum, and served to provide the background absorbance values in subsequent spectrophotometric measurements of the absorbance of XTT.
In order to eliminate the possible effects of the inoculum or the drug concentration or the possible effects of variations in the comparisons of the three echinocandins or the nongerminated and the germinated conidia, for each Aspergillus isolate the same inoculum preparation was used for comparative studies of all three echinocandins against the nongerminated and the germinated conidia. In addition, for each echinocandin, the same drug dilution preparations were used for studies of nongerminated versus germinated conidia. The experiments were repeated in triplicate.
Metabolic assay.
The concentration-dependent effects of caspofungin, micafungin, and anidulafungin on the metabolic activities of the Aspergillus spp. were comparatively studied by using the optimized XTT assay developed previously (1). Briefly, immediately after determination of the MECs for all three echinocandins against nongerminated or germinated conidia, 50 μl of the XTT-menadione solution described above was added to each well, resulting in final concentrations of 100 μg/ml XTT and 6.25 μM menadione. The plates were incubated at 37°C for 2 h and were subsequently shaken for 1 to 2 min (Plate Shake 1296-004; Wallac OY, Turku, Finland) for further dissolution of the formazan derivatives. The color absorbance (A) was then measured at dual wavelengths (450 nm, with a reference wavelength of 630 nm) with a microtitration plate spectrophotometric reader (Elx808; Bio-Tek Instruments, Winooski, VT). The percent metabolic activity (Y) for each well with drug in relation to that of the drug-free control well was calculated, after subtraction of the background absorbance, as [(Adrug well − Abackground drug well)/(Acontrol − Abackground control)] × 100.
In cases in which a paradoxical increase in metabolic activity was observed at higher drug concentrations for some isolates, it was considered significant if it exceeded the minimum metabolic activity detected at lower concentrations (usually at the MEC or 1 to 2 dilutions higher) by at least 40% and was consistent in all three experiments (1, 20).
Modeling of in vitro echinocandin activities against Aspergillus spp.
Nonlinear regression analysis of the data was applied by including all three replicate Y values as individual points in the same fitting process. This resulted in a single estimated parameter value for each isolate. No weighting was applied. For those isolates for which a paradoxical response was not detected, the Emax model (sigmoid curve with variable slope) was used. The Emax model is described by the equation
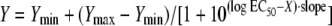 |
(1) |
where X is the log10 drug concentration. The four parameters generated by the model are the values of the maximum Y (Ymax) and the minimum Y (Ymin); EC50, which is the drug concentration that shows metabolic activity that is halfway between Ymax and Ymin [i.e., Ymin + 0.5·(Ymax − Ymin)]; and the slope, which describes the steepness of the curve (22). For every isolate, the EC5 value was also calculated. EC5 is the drug concentration showing metabolic activity equal to
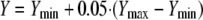 |
(2) |
From equations 1 and 2 and by solving for X, EC5 was calculated as described previously (2): 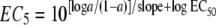 , where a is equal to 0.05.
, where a is equal to 0.05.
The bell-shaped model was used for those isolates demonstrating a paradoxical increase in metabolic activity at higher drug concentrations, after appropriate initial values were chosen. This model is described by the equation 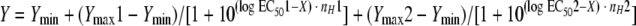 . The biphasic curve begins at Ymax1, turns over at Ymin, and then approaches Ymax2. EC501 and EC502 are the corresponding EC50 values, respectively, and nH1 and nH2 are the corresponding slope factors, respectively, for each phase of the curve. As shown in the equation presented above, the bell-shaped model combines two sigmoid concentration-effect relationships and, despite its name, does not describe a normal distribution. Instead, the bell-shaped equation is appropriate for the description of asymmetric biphasic concentration-effect curves (1, 21). For every isolate for which the bell-shaped model was used, the EC51 value (i.e., the EC5 value corresponding to the first [descending] phase of the curve) was also calculated, based on the following equation:
. The biphasic curve begins at Ymax1, turns over at Ymin, and then approaches Ymax2. EC501 and EC502 are the corresponding EC50 values, respectively, and nH1 and nH2 are the corresponding slope factors, respectively, for each phase of the curve. As shown in the equation presented above, the bell-shaped model combines two sigmoid concentration-effect relationships and, despite its name, does not describe a normal distribution. Instead, the bell-shaped equation is appropriate for the description of asymmetric biphasic concentration-effect curves (1, 21). For every isolate for which the bell-shaped model was used, the EC51 value (i.e., the EC5 value corresponding to the first [descending] phase of the curve) was also calculated, based on the following equation: 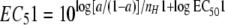 , where a is equal to 0.05. The goodness of fit for both models was assessed by the determination of R2 values, the runs test, and visual inspection. Model building was performed by using Prism software (version 4.0b; GraphPad, San Diego, CA).
, where a is equal to 0.05. The goodness of fit for both models was assessed by the determination of R2 values, the runs test, and visual inspection. Model building was performed by using Prism software (version 4.0b; GraphPad, San Diego, CA).
Statistical analysis.
Comparisons of the MECs and model-derived quantitative parameters (EC5, Ymin, slope) were made between (i) the three echinocandins for each species, (ii) the three Aspergillus species for each drug, and (iii) germinated and nongerminated conidia for each species and drug. Differences were assessed after log10 transformation of the values and by passing Bartlett's test for equal variances by one-way analysis of variance, followed by Bonferroni's posttest. In case normality was not restored after log10 transformation of the values or the variances differed significantly according to Bartlett's test, the differences described above were assessed by using the nonparametric Kruskal-Wallis test, followed by Dunn's test for multiple comparisons. The correlation among the Ymin values obtained with the three echinocandins for each Aspergillus isolate was assessed with the Pearson correlation coefficient if the values obtained had a normal distribution, based on the D'Agostino and Pearson omnibus normality test, or with the Spearman nonparametric correlation coefficient if the values did not pass the normality test. Statistical analysis also was performed by using GraphPad Prism software (version 4.0b).
RESULTS
Susceptibility testing.
Of the three echinocandins studied, anidulafungin exhibited the lowest MEC values and caspofungin exhibited the highest MEC values against nongerminated conidia of Aspergillus spp. (P was <0.01 for all comparisons) (Table 1). Against germinated Aspergillus conidia, however, micafungin and anidulafungin had comparable MEC values, and these were lower than those of caspofungin. Differences between the echinocandins in the changes in the MEC values in the presence of germinated and nongerminated conidia were observed; while for caspofungin the MEC remained unchanged or increased by a maximum of 1 twofold dilution, for anidulafungin the MEC increased by a median of 2 to 3 dilutions, depending on the Aspergillus spp. For micafungin the MEC increased by a median of 1 dilution for A. fumigatus and A. terreus and 2 dilutions for A. flavus (Table 1). On microscopic examination, the transition from normal to short, stubby, and highly branched hyphae in the presence of increasing drug concentrations was found to occur more gradually for micafungin (often over a range of 3 to 4 drug dilutions) than for the other two echinocandins with both nongerminated and germinated conidia.
TABLE 1.
MEC values for germinated and nongerminated Aspergillus conidia
| Species (no. of strains) | Conidial state | Median (range) MEC (μg/ml)
|
||
|---|---|---|---|---|
| Caspofungina | Micafungina | Anidulafungina | ||
| A. fumigatus (11) | Nongerminated | 1 (0.5-1) | 0.06 (0.06-0.12) | 0.03 (0.015-0.03) |
| Germinated | 1 (1-1) | 0.25 (0.12-0.25) | 0.12 (0.12-0.25) | |
| Log2 of ratiob | 0 (0-1) | 1 (1-2)c | 2 (2-3)c | |
| A. terreus (8) | Nongerminated | 0.5 (0.5-1) | 0.12 (0.06-0.12) | 0.03 (0.015-0.03) |
| Germinated | 1 (1-2) | 0.25 (0.12-0.25) | 0.25 (0.25-0.5) | |
| Log2 of ratio | 1 (0-1)c | 1 (1-1)c | 3 (3-4)c | |
| A. flavus (8) | Nongerminated | 1 (0.5-1) | 0.12 (0.03-0.12) | 0.03 (0.03-0.06) |
| Germinated | 1 (1-1) | 0.5 (0.25-1) | 0.25 (0.12-0.25) | |
| Log2 of ratio | 0 (0-1) | 2 (2-3)c | 2.5 (2-3)c | |
For comparisons of the MECs between echinocandins, P was <0.01 for all comparisons, with the exception of the MEC values of micafungin versus those of anidulafungin for germinated conidia.
The ratio is expressed as the number of twofold drug dilutions (log2 of MEC for germinated conidia/MEC for nongerminated ratio).
For comparisons of the MECs between nongerminated and germinated conidia, P was <0.01 for the comparison of the corresponding MEC values.
Concentration-dependent effects of echinocandins on Aspergillus spp.
A substantial decrease in metabolic activity associated with the formation of short, aberrant hyphae was observed for all Aspergillus isolates in the presence of increasing concentrations of any of the three echinocandins. These metabolic properties are exemplified in Fig. 1. Of the total 81 drug-isolate pairs (3 echinocandins·27 isolates) studied with both nongerminated and germinated conidia, these concentration-dependent effects were described with the Emax model in 59 drug-isolate pairs for nongerminated conidia (median R2, 0.96; R2 range, 0.81 to 0.99) and germinated conidia (median R2, 0.94; R2 range, 0.75 to 0.98). The bell-shaped model described the metabolic changes in the remaining 22 drug-isolate pairs for nongerminated conidia (median R2, 0.96; R2 range, 0.83 to 0.99) and germinated conidia (median R2, 0.93; R2 range, 0.76 to 0.99). No significant deviation from the models was detected by use of the runs test. In most cases, the 95% confidence intervals provided by the models for the EC50 and the EC5 values were <1 log2, while for the Ymin values, Ymax values, and slopes, the ratio of the standard error to the best-fit value was <35%. In cases in which wider 95% confidence intervals or greater standard errors were obtained, however, the values generated by the models were not excluded from the analysis, as they still represented the best estimate for the parameters of interest.
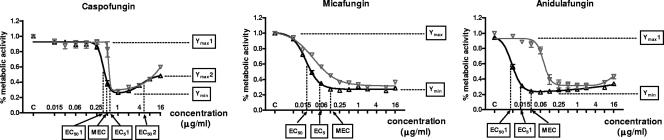
FIG. 1.
Representative concentration-effect curves of the metabolic activity of an A. fumigatus isolate (nongerminated [▵] and germinated [▿] conidia) in the presence of increasing concentrations of caspofungin, micafungin, and anidulafungin. For caspofungin and anidulafungin, the curves were generated with the bell-shaped model, and for micafungin, the curves were generated with the Emax model. The parameters calculated by the models, as well as the microscopically defined MECs, are presented.
Slopes and EC5 values.
Of the three echinocandins studied, the steepest concentration-effect curves were obtained for caspofungin, with median slopes of −4.94 for nongerminated conidia and −6.67 for germinated conidia; and the shallowest curves were obtained for micafungin, with median slopes of −2.42 and −2.15 for nongerminated and germinated conidia, respectively (P < 0.001). Anidulafungin curves had intermediate slopes, with median values of −3.91 for nongerminated conidia and −3.37 for germinated conidia. These differences in the steepnesses of the curves among the three echinocandins correlated inversely with the distance between the MEC values defined microscopically and the corresponding EC50 or EC501 values generated with the Emax or bell-shaped model (Fig. 1).
The median differences between the MEC and the EC50 or EC501 values for nongerminated and germinated conidia were 1.02 and 0.64 twofold dilutions, respectively, for caspofungin; 2.43 and 3.40 dilutions, respectively, for micafungin; and 2.03 and 2.15 dilutions, respectively, for anidulafungin. Consequently, while the EC50 or EC501 values provided a good approximation of the MEC for caspofungin, they tended to be 2 or more dilutions less than the MECs of micafungin and anidulafungin. In contrast, the EC5 or EC51, which corresponded to a percentage of metabolic activity that was much closer to the Ymin than the EC50 or EC501, approximated the MECs for all three echinocandins, as the median differences between the MEC and the EC5 or EC51 values for nongerminated and germinated conidia were 0.006 and −0.11 dilution, respectively, for caspofungin; 0.48 and 1.55 dilutions, respectively, for micafungin; and 0.68 and 0.90, respectively, for anidulafungin. Consistent with the proximity of the model-derived EC5 (or EC51) and microscopically defined MEC values was the fact that the differences in EC5 (or EC51) values between echinocandins for germinated and nongerminated conidia paralleled the differences in the MEC values (Table 2).
TABLE 2.
EC5 (or EC51) values generated with the Emax (or bell-shaped) model for the metabolic activities of germinated and nongerminated Aspergillus conidia in the presence of echinocandins
| Species (no. of strains) | Conidial state | Median (range) EC5 (or EC51) (μg/ml)
|
||
|---|---|---|---|---|
| Caspofungina | Micafungina | Anidulafungina | ||
| A. fumigatus (11) | Nongerminated | 0.67 (0.51-1.43) | 0.04 (0.01-0.15) | 0.03 (0.004-0.04) |
| Germinated | 1.2 (1.05-1.99) | 0.05 (0.04-0.12) | 0.11 (0.03-0.54) | |
| Log2 of ratiob | 0.96 (−0.05-1.96)c | 0.67 (−1.49-1.93) | 2.4 (0.7-4.2)c | |
| A. terreus (8) | Nongerminated | 0.65 (0.29-1.23) | 0.07 (0.02-0.25) | 0.01 (0.008-0.03) |
| Germinated | 1.06 (0.62-2.53) | 0.14 (0.03-0.4) | 0.10 (0.06-0.14) | |
| Log2 of ratio | 1.12 (−0.23-1.39)c | 2.04 (1.6-2.26)c | 2.97 (1-3.82)c | |
| A. flavus (8) | Nongerminated | 0.64 (0.42-0.96) | 0.05 (0.02-0.11) | 0.01 (0.007-0.03) |
| Germinated | 0.87 (0.62-1.11) | 0.14 (0.06-0.61) | 0.09 (0.06-0.38) | |
| Log2 of ratio | 0.43 (−0.18-0.87) | 1.57 (0.64-2.44)c | 2.96 (1.02-4.81)c | |
For comparisons of the EC5 values between the echinocandins, P was <0.01 for all comparisons, with the exception of the EC5 values of micafungin versus anidulafungin for germinated conidia.
The ratios are expressed as the numbers of twofold drug dilutions (log2) for germinated conidia to those for nongerminated conidia.
For comparisons of the EC5 values between nongerminated and germinated conidia, P was <0.05 for comparisons of the corresponding EC5 values.
Maximal metabolic inhibition (Ymin) of Aspergillus spp.
The degree of maximal metabolic inhibition of Aspergillus spp. by the three echinocandins, manifested by the Ymin values generated with the Emax or bell-shaped model, presented in Table 3. Notable interspecies differences were observed, with A. flavus (nongerminated or germinated conidia) showing greater metabolic inhibition in the presence of all three echinocandins than that of A. fumigatus or A. terreus. For the last two species, significant intraspecies variation was observed, as manifested by the relatively wide range of Ymin values. For each drug and species, the Ymin values obtained for germinated and nongerminated conidia did not differ significantly (median difference range, 1 to 10%), suggesting that the degree of metabolic inhibition induced by the echinocandins was not significantly altered in the presence of germinated conidia in comparison to that in the presence of nongerminated conidia.
TABLE 3.
Ymin values and percent difference in Ymin values generated by use of the Emax (or bell-shaped) model for the metabolic activity of germinated and nongerminated Aspergillus conidia in the presence of echinocandins
| Species (no. of strains) | Conidial state | Median (range) Ymin (%)
|
||
|---|---|---|---|---|
| Caspofungina | Micafungina | Anidulafunginb | ||
| A. fumigatus (11) | Nongerminated | 26 (21-50) | 25 (19-50) | 26 (21-51) |
| Germinated | 26 (13-49) | 29 (24-55) | 36 (21-65) | |
| Differencec | 3 (−9-11) | 3 (−14-19) | 6 (−17-18) | |
| A. terreus (8) | Nongerminated | 34 (14-62) | 29 (19-64) | 30 (17-62) |
| Germinated | 35 (15-66) | 37 (16-81) | 44 (24-76) | |
| Difference | 3 (−4-12) | 7 (−3-18) | 10 (0-21) | |
| A. flavus (8) | Nongerminated | 17 (14-23) | 17 (12-22) | 16 (13-22) |
| Germinated | 21 (16-28) | 20 (18-32) | 16 (14-22) | |
| Difference | 3 (−1-10) | 5 (−3-13) | 1 (−7-8) | |
P was <0.05 for nongerminated A. flavus conidia versus nongerminated A. fumigatus or A. terreus conidia and for germinated A. flavus conidia versus germinated A. terreus conidia.
P was <0.01 for nongerminated A. flavus conidia versus nongerminated A. fumigatus or A. terreus conidia and for germinated A. flavus conidia versus germinated A. fumigatus or A. terreus conidia.
The differences between germinated and nongerminated conidia are expressed here as the percent difference in metabolic activity.
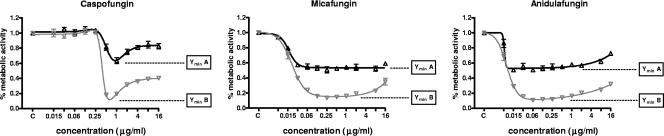
For the same species (nongerminated and germinated conidia), the medians and ranges of the Ymin values obtained in the presence of each of the three echinocandins were comparable (Table 3). Moreover, a significant correlation of the Ymin values obtained for each Aspergillus isolate was demonstrated among the echinocandins. For example, the relationship between YminA and YminB for two isolates (A and B, respectively) of A. terreus was preserved across all three echinocandins (Fig. 2).
FIG. 2.
Comparative concentration-effect curves for two A. terreus isolates (nongerminated conidia of isolates A [▵] and B [▿]) in the presence of echinocandins. There is a marked correlation in the degree of metabolic inhibition caused by these agents; the Ymin values obtained for isolate A are consistently and significantly higher than those obtained for isolate B for all three echinocandins.
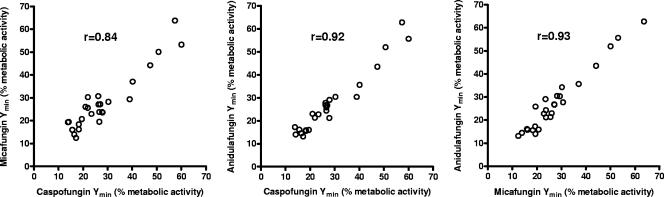
When all isolates (nongerminated conidia) were grouped together, the correlation coefficients were 0.84 for the Ymin values of micafungin compared with those of caspofungin, 0.92 for anidulafungin compared with those of caspofungin, and 0.93 for anidulafungin compared with those of micafungin (P < 0.0001) (Fig. 3). This correlation could be demonstrated even when the species with the greater variation in Ymin values (A. fumigatus, A. terreus) were analyzed separately. For A. fumigatus, the coefficients ranged from 0.84 to 0.91 (P < 0.001) for correlations between all three echinocandins; for A. terreus, the coefficients ranged from 0.91 to 0.98 (P < 0.004). The correlation in the degrees of metabolic inhibition among the three echinocandins also persisted for germinated conidia, with coefficients ranging from 0.81 to 0.88 (P < 0.0001) for the Ymin values of all Aspergillus isolates, 0.72 to 0.94 (P < 0.03) for A. fumigatus, and 0.82 to 0.94 (P < 0.02) for A. terreus isolates.
FIG. 3.
Correlation of Ymin values obtained for all Aspergillus isolates (nongerminated conidia) in the presence of caspofungin, micafungin, and anidulafungin. r, correlation coefficient (P < 0.0001).
Paradoxical increase in metabolic activity.
A paradoxical increase in the metabolic activity of nongerminated and germinated Aspergillus conidia in the presence of higher drug concentrations was detected for caspofungin in six and seven of the A. fumigatus isolates, respectively; five and five of the A. terreus isolates, respectively; and one and zero of the A. flavus isolates, respectively. Some, but not all, of these isolates exhibited this phenomenon for the other two echinocandins as well. In particular, for micafungin with nongerminated and germinated conidia, it was detected in two and one of the A. fumigatus isolates, respectively; one and one of the A. terreus isolates, respectively; and zero and zero of the A. flavus isolates, respectively. For anidulafungin with nongerminated and germinated conidia, it was detected in five and six of the A. fumigatus isolates, respectively; two and two of the A. terreus isolates, respectively; and zero and zero of the A. flavus isolates, respectively. In most cases, the paradoxical response obtained with germinated conidia was observed for those isolates for which it was observed with nongerminated conidia, as suggested by the small differences in the corresponding numbers. Examination of the wells under an inverted microscope suggested that the paradoxical increase in metabolic activity was associated with the progressive elongation of the aberrant hyphae at higher drug concentrations compared to the increase observed at the MEC (Fig. 4).
FIG. 4.
Photomicrographs of wells after 48 h of incubation of an A. fumigatus isolate (nongerminated conidia) demonstrating a paradoxical increase in metabolic activity at higher concentrations of anidulafungin. Anidulafungin was used at concentrations of 0.03 μg/ml (MEC) (A), 2 μg/ml (B), and 16 μg/ml (C). The short, stubby hyphae observed at the MEC (A) tend to become progressively more elongated at the higher concentrations (B and C), without, however, restoration of the normal hyphal mat that would be observed in the absence of drug. Magnifications, ×10.
By subtracting the Ymin value from the Ymax2 value generated with the bell-shaped model for those isolates demonstrating the paradoxical response, the increases in the percentages of metabolic activity at higher concentrations were comparable for the three echinocandins, with median increases of 23% (range, 6% to 38%) for caspofungin, 16% (range, 14% to 35%) for micafungin, and 29% (range, 18% to 35%) for anidulafungin (for the nongerminated conidia). However, the second (ascending) parts of the bell-shaped curves, associated with the paradoxical increase in fungal metabolism, were shallower for micafungin and anidulafungin (median nH2 values, −1.92 and −1.47, respectively) than those of caspofungin (median nH2 value, −3.13) (see also Fig. 1 and 2). In agreement with this finding, the EC502 values of micafungin (median, 11.1 μg/ml; range, 1.78 to 16.7 μg/ml) were comparable to those of anidulafungin (median, 10.8 μg/ml; range, 1.3 to 19.1 μg/ml) but higher than those of caspofungin (median, 4.2 μg/ml; range, 0.5 to 17.8 μg/ml). These results suggest that the paradoxical effect occurred at higher concentrations for micafungin and anidulafungin than for caspofungin. Furthermore, considering the lower MEC and EC51 or EC501 values obtained for micafungin and anidulafungin than those obtained for caspofungin (Tables 1 and 2), it became apparent that the difference between the concentrations at which the echinocandins exerted the paradoxical effect (EC502) and those concentrations at which they induced the formation of aberrant hyphae (MEC, EC51, EC501) was considerably greater for micafungin and anidulafungin than for caspofungin. Indeed, the median differences between the EC502 and the EC501 values for micafungin and anidulafungin were 9.4 and 9.8 twofold drug dilutions, respectively, while for caspofungin the median difference was only 3.5 drug dilutions.
Finally, for all isolates that were further studied by using echinocandin concentrations that exceeded those achieved at currently approved dosages (32, 64, and 128 μg/ml), a marked decrease in fungal metabolism was observed at 64 or 128 μg/ml of caspofungin, resulting in <5% metabolic activity and the absence of visual growth (MIC-0) at 128 μg/ml. In contrast, no inhibition of growth or a significant reduction of metabolism was detected at 64 or 128 μg/ml for the other two echinocandins, for which the percent metabolic activity remained at or close to the Ymax2 values.
DISCUSSION
The present study comparatively investigated the in vitro activities of echinocandins against nongerminated and germinated Aspergillus conidia by using, besides the MEC, a previously optimized metabolic assay (1) that provides quantification of the concentration-dependent drug effects and, therefore, more information over a range of concentrations. Previous comparative studies of the in vitro activities of echinocandins against Aspergillus spp. have used the MEC endpoint (12, 26, 28); in addition, the activities of these agents against germinated conidia have not heretofore been systematically assessed.
The transition from normal to short, stubby, and highly branched hyphae was observed to occur more gradually with micafungin than with the other two echinocandins, a fact that could potentially increase the subjectivity in MEC determination. These microscopic findings correlated with the differences in the steepnesses of the concentration-effect curves of metabolic activity among the echinocandins, with caspofungin having the steepest curves and micafungin having the shallowest curves. Consequently, the EC5 or EC51 value, which corresponded to a percent metabolic activity that was much closer to the Ymin than the EC50 or EC501, was the model-derived parameter that approximated the MEC for all three echinocandins. A plausible explanation for the shallower metabolic curves for micafungin and anidulafungin could be that these echinocandins may exhibit a more gradual concentration-dependent access or binding to their fungal cell target than caspofungin. This postulation could be related to the fact that the micafungin and anidulafungin molecules have similar structures in their aromatic side chains, while caspofungin has an aliphatic side chain (18, 31, 33).
The lower MEC and EC5 or EC51 values demonstrated in the present study for micafungin and anidulafungin than those for caspofungin are consistent with the findings of other in vitro studies (12, 25, 26, 28). Previous studies of host defense mechanisms against A. fumigatus have also shown that all three echinocandins have activity against germinated conidia, but there was no direct comparison with their activities against nongerminated conidia (5, 8, 9). The mechanism for the significant increase in the MEC and the EC5 or EC51 values in the presence of germinated conidia for anidulafungin and, to a less extent, for micafungin demonstrated here is not fully understood. A simple hypothesis could be that the germinated conidia have a much greater fungal biomass than nongerminated conidia and that the significantly increased amount of (1,3)-β-d-glucan synthase in germinated conidia cannot be inhibited by the very low anidulafungin or micafungin concentrations that are effective against nongerminated conidia. Notably, the greater increases in the MEC and EC5 or EC51 values in the presence of germinated conidia were observed for anidulafungin, which had the lowest MEC and EC5 or EC51 values against nongerminated conidia.
The increases in the MEC and the EC5 or EC51 values of anidulafungin and micafungin against germinated conidia may have practical implications. First, as calculation of the optimal echinocandin dosages for in vivo or clinical studies may take into consideration the MEC value, this parameter may be more relevant for germinated conidia than for nongerminated conidia. Wiederhold et al. demonstrated that the pharmacokinetic/pharmacodynamic parameter that is the most closely associated with the in vivo efficacy of caspofungin was the peak plasma concentration/MEC ratio (32). If such a parameter, for example, is to be calculated for anidulafungin or micafungin, then the MEC value, based on our findings, would preferably be that for germinated conidia. Second, the differences in the MEC and the EC5 or EC51 values for germinated conidia versus those for nongerminated conidia may suggest that anidulafungin has a relatively greater efficacy against Aspergillus infections in the prophylactic setting than in the therapeutic setting.
Significant inter- and intraspecies variations in the maximal percentages of metabolic inhibition of Aspergillus spp. were demonstrated for all echinocandins, with greater inhibition achieved for A. flavus. These results are in agreement with those published previously for caspofungin (1) but appear to be somewhat different from those of two other previous studies, which demonstrated a diminished inhibitory effect of micafungin against A. flavus compared to that against A. fumigatus or A. terreus by the XTT assay (15, 17). However, in both of those studies, the final concentration of menadione added to the wells was 25 μM, i.e., a concentration fourfold higher than the one (6.25 μM) used in the present study. We have previously shown that the 25 μM menadione concentration may mask the degree of metabolic inhibition caused by the echinocandins against Aspergillus spp. and that the 6.25 μM concentration is more appropriate for this purpose (1). Furthermore, the fluorescence staining for the determination of viability that was additionally performed in one of those studies (17) showed that micafungin caused hyphal damage for A. flavus at least comparable to that for A. fumigatus and A. terreus, in agreement with the findings of the present study.
The inter- and intraspecies differences in the levels of metabolic inhibition of Aspergillus spp. by echinocandins demonstrated here may have in vivo or clinical significance that warrants further investigation. Mice infected with A. flavus appeared to have better survival or microbiological clearance than those infected with A. fumigatus or A. terreus following treatment with caspofungin (4, 6). Maertens et al., in their study of caspofungin as salvage treatment for invasive aspergillosis, reported that microbiological eradication was achieved in 54% of 13 patients with A. flavus infection but only 28% of 47 patients with A. fumigatus infection (19). In a recent study of micafungin use, alone or in combination with other antifungal agents, for the treatment of invasive aspergillosis, a favorable response was observed in 29.4% of 102 patients with A. fumigatus infection, 48.4% of 31 patients with A. flavus infection, and 0% of 10 patients with A. terreus infection (11). Although these data may be confounded by a number of factors, the trends observed in those studies are in agreement with our finding of the greater metabolic inhibition of A. flavus than of A. fumigatus or A. terreus in the presence of echinocandins, despite the similar MEC values among these species.
The degrees of maximal metabolic inhibition caused by the echinocandins were comparable at the species and the strain levels, with a significant correlation of the Ymin values obtained for each isolate of A. fumigatus and A. terreus (which showed the greatest interspecies variations in metabolic inhibition) in the presence of caspofungin, micafungin, or anidulafungin. This finding may have practical implications if the degree of metabolic inhibition indeed proves to correlate with the in vivo or clinical outcome of echinocandin treatment.
The paradoxical increase in metabolism, which occurred at higher concentrations for some of the A. fumigatus and A. terreus isolates but uncommonly for A. flavus, was associated with the progressive elongation of aberrant hyphae (Fig. 4) and was detected in decreasing order of frequency with caspofungin, anidulafungin, and micafungin. It seems that a subpopulation of isolates from each species is prone to this phenomenon, which is then variably expressed with the different echinocandins. Possible mechanisms of this paradoxical effect and its uncommon occurrence in A. flavus in the presence of caspofungin have already been described (1, 14, 30). The differential frequencies among the echinocandins parallel those detected in other studies on the effects of these agents against Candida spp. (7, 29). Certain comparative features of the activities of the echinocandin described for the inhibitory metabolic effects associated with the transition from normal to aberrant hyphae were also observed for the paradoxical effect. First, the maximal levels of inhibition of metabolism were comparable among the three agents, as were the percentages of increase in metabolic activity at higher concentrations. Second, the slopes of the descending parts of the concentration-effect curves (describing the inhibition of metabolic activity) were shallower for micafungin and anidulafungin than for caspofungin, as were the slopes for the ascending parts of the curves (describing the paradoxical increase of metabolism at higher concentrations).
The present study therefore revealed certain comparative features of echinocandin in an analysis of the concentration-dependent activities of the echinocandins against Aspergillus spp. Common features among the echinocandins were the comparable degrees of maximal metabolic inhibition at the species level (greater inhibition for A. flavus) and the strain level, as well as the comparable increases in metabolic activity at higher concentrations for isolates demonstrating the paradoxical effect. However, the concentrations of the echinocandins at which metabolic inhibition occurred for Aspergillus spp., as well as the corresponding MEC values, increased differentially for germinated and nongerminated conidia, with a greater increase obtained with anidulafungin and a minimal increase obtained with caspofungin. Shallower metabolic curves were observed for micafungin and anidulafungin, for which the paradoxical increase in metabolism occurred at lower frequencies and at higher concentrations than those of caspofungin.
Acknowledgments
This work was supported by the Intramural Research Program of the National Cancer Institute.
Footnotes
Published ahead of print on 15 October 2007.
REFERENCES
- 1.Antachopoulos, C., J. Meletiadis, T. Sein, E. Roilides, and T. J. Walsh. 2007. Concentration-dependent effects of caspofungin on the metabolic activity of Aspergillus species. Antimicrob. Agents Chemother. 51:881-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antachopoulos, C., J. Meletiadis, T. Sein, E. Roilides, and T. J. Walsh. 2007. Use of high inoculum for early metabolic signalling and rapid susceptibility testing of Aspergillus species. J. Antimicrob. Chemother. 59:230-237. [DOI] [PubMed] [Google Scholar]
- 3.Arikan, S., M. Lozano-Chiu, V. Paetznick, and J. H. Rex. 2001. In vitro susceptibility testing methods for caspofungin against Aspergillus and Fusarium isolates. Antimicrob. Agents Chemother. 45:327-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowman, J. C., G. K. Abruzzo, A. M. Flattery, C. J. Gill, E. J. Hickey, M. J. Hsu, J. Nielsen Kahn, P. A. Liberator, A. S. Misura, B. A. Pelak, T. C. Wang, and C. M. Douglas. 2006. Efficacy of caspofungin against Aspergillus flavus, Aspergillus terreus, and Aspergillus nidulans. Antimicrob. Agents Chemother. 50:4202-4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brummer, E., S. D. Chauhan, and D. A. Stevens. 1999. Collaboration of human phagocytes with LY 303366 for antifungal activity against Aspergillus fumigatus. J. Antimicrob. Chemother. 43:491-496. [DOI] [PubMed] [Google Scholar]
- 6.Cacciapuoti, A., J. Halpern, C. Mendrick, C. Norris, R. Patel, and D. Loebenberg. 2006. Interaction between posaconazole and caspofungin in concomitant treatment of mice with systemic Aspergillus infection. Antimicrob. Agents Chemother. 50:2587-2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chamilos, G., R. E. Lewis, N. Albert, and D. P. Kontoyiannis. 2007. The paradoxical effect of echinocandins across Candida species in vitro: evidence for echinocandin-specific and Candida species-related differences. Antimicrob. Agents Chemother. 51:2257-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiller, T., K. Farrokhshad, E. Brummer, and D. A. Stevens. 2001. The interaction of human monocytes, monocyte-derived macrophages, and polymorphonuclear neutrophils with caspofungin (MK-0991), an echinocandin, for antifungal activity against Aspergillus fumigatus. Diagn. Microbiol. Infect. Dis. 39:99-103. [DOI] [PubMed] [Google Scholar]
- 9.Choi, J. H., E. Brummer, and D. A. Stevens. 2004. Combined action of micafungin, a new echinocandin, and human phagocytes for antifungal activity against Aspergillus fumigatus. Microbes Infect. 6:383-389. [DOI] [PubMed] [Google Scholar]
- 10.Denning, D. W. 2003. Echinocandin antifungal drugs. Lancet 362:1142-1151. [DOI] [PubMed] [Google Scholar]
- 11.Denning, D. W., K. A. Marr, W. M. Lau, D. P. Facklam, V. Ratanatharathorn, C. Becker, A. J. Ullmann, N. L. Seibel, P. M. Flynn, J. A. van Burik, D. N. Buell, and T. F. Patterson. 2006. Micafungin (FK463), alone or in combination with other systemic antifungal agents, for the treatment of acute invasive aspergillosis. J. Infect. 53:337-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Espinel-Ingroff, A. 1998. Comparison of in vitro activities of the new triazole SCH56592 and the echinocandins MK-0991 (L-743,872) and LY303366 against opportunistic filamentous and dimorphic fungi and yeasts. J. Clin. Microbiol. 36:2950-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Espinel-Ingroff, A. 2003. Evaluation of broth microdilution testing parameters and agar diffusion Etest procedure for testing susceptibilities of Aspergillus spp. to caspofungin acetate (MK-0991). J. Clin. Microbiol. 41:403-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gardiner, R. E., P. Souteropoulos, S. Park, and D. S. Perlin. 2005. Characterization of Aspergillus fumigatus mutants with reduced susceptibility to caspofungin. Med. Mycol. 43(Suppl. 1):S299-S305. [DOI] [PubMed] [Google Scholar]
- 15.Heyn, K., A. Tredup, S. Salvenmoser, and F. M. Muller. 2005. Effect of voriconazole combined with micafungin against Candida, Aspergillus, and Scedosporium spp. and Fusarium solani. Antimicrob. Agents Chemother. 49:5157-5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurtz, M. B., I. B. Heath, J. Marrinan, S. Dreikorn, J. Onishi, and C. Douglas. 1994. Morphological effects of lipopeptides against Aspergillus fumigatus correlate with activities against (1,3)-β-d-glucan synthase. Antimicrob. Agents Chemother. 38:1480-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis, R. E., and D. P. Kontoyiannis. 2005. Micafungin in combination with voriconazole in Aspergillus species: a pharmacodynamic approach for detection of combined antifungal activity in vitro. J. Antimicrob. Chemother. 56:887-892. [DOI] [PubMed] [Google Scholar]
- 18.Liu, J., and M. K. Balasubramanian. 2001. 1,3-beta-Glucan synthase: a useful target for antifungal drugs. Curr. Drug Targets Infect. Disord. 1:159-169. [DOI] [PubMed] [Google Scholar]
- 19.Maertens, J., I. Raad, G. Petrikkos, M. Boogaerts, D. Selleslag, F. B. Petersen, C. A. Sable, N. A. Kartsonis, A. Ngai, A. Taylor, T. F. Patterson, D. W. Denning, and T. J. Walsh. 2004. Efficacy and safety of caspofungin for treatment of invasive aspergillosis in patients refractory to or intolerant of conventional antifungal therapy. Clin. Infect. Dis. 39:1563-1571. [DOI] [PubMed] [Google Scholar]
- 20.Meletiadis, J., J. W. Mouton, J. F. Meis, B. A. Bouman, P. J. Donnelly, and P. E. Verweij. 2001. Comparison of spectrophotometric and visual readings of NCCLS method and evaluation of a colorimetric method based on reduction of a soluble tetrazolium salt, 2,3-bis [2-methoxy-4-nitro-5-[(sulfenylamino) carbonyl]-2H-tetrazolium-hydroxide], for antifungal susceptibility testing of Aspergillus species. J. Clin. Microbiol. 39:4256-4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Motulsky, H. J., and A. Christopoulos. 2003. Complex dose-response curves, p. 290-295. In H. J. Motulsky and A. Christopoulos (ed.), Fitting models to biological data using linear and nonlinear regression. GraphPad Software Inc., San Diego, CA.
- 22.Motulsky, H. J., and A. Christopoulos. 2003. Introduction to dose-response curves, p. 256-265. In H. J. Motulsky and A. Christopoulos (ed.), Fitting models to biological data using linear and nonlinear regression. GraphPad Software Inc., San Diego, CA.
- 23.NCCLS. 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard. NCCLS document M38-A. NCCLS, Wayne, PA.
- 24.Odds, F. C., M. Motyl, R. Andrade, J. Bille, E. Canton, M. Cuenca-Estrella, A. Davidson, C. Durussel, D. Ellis, E. Foraker, A. W. Fothergill, M. A. Ghannoum, R. A. Giacobbe, M. Gobernado, R. Handke, M. Laverdiere, W. Lee-Yang, W. G. Merz, L. Ostrosky-Zeichner, J. Peman, S. Perea, J. R. Perfect, M. A. Pfaller, L. Proia, J. H. Rex, M. G. Rinaldi, J. L. Rodriguez-Tudela, W. A. Schell, C. Shields, D. A. Sutton, P. E. Verweij, and D. W. Warnock. 2004. Interlaboratory comparison of results of susceptibility testing with caspofungin against Candida and Aspergillus species. J. Clin. Microbiol. 42:3475-3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paderu, P., G. Garcia-Effron, S. Balashov, G. Delmas, S. Park, and D. S. Perlin. 2007. Serum differentially alters the antifungal properties of echinocandin drugs. Antimicrob. Agents Chemother. 51:2253-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfaller, M. A., F. Marco, S. A. Messer, and R. N. Jones. 1998. In vitro activity of two echinocandin derivatives, LY303366 and MK-0991 (L-743,792), against clinical isolates of Aspergillus, Fusarium, Rhizopus, and other filamentous fungi. Diagn. Microbiol. Infect. Dis. 30:251-255. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez-Tudela, J. L., E. Chryssanthou, E. Petrikkou, J. Mosquera, D. W. Denning, and M. Cuenca-Estrella. 2003. Interlaboratory evaluation of hematocytometer method of inoculum preparation for testing antifungal susceptibilities of filamentous fungi. J. Clin. Microbiol. 41:5236-5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Serrano Mdel, C., A. Valverde-Conde, M. M. Chavez, S. Bernal, R. M. Claro, J. Peman, M. Ramirez, and E. Martin-Mazuelos. 2003. In vitro activity of voriconazole, itraconazole, caspofungin, anidulafungin (VER002, LY303366) and amphotericin B against aspergillus spp. Diagn. Microbiol. Infect. Dis. 45:131-135. [DOI] [PubMed] [Google Scholar]
- 29.Stevens, D. A., M. Espiritu, and R. Parmar. 2004. Paradoxical effect of caspofungin: reduced activity against Candida albicans at high drug concentrations. Antimicrob. Agents Chemother. 48:3407-3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stevens, D. A., M. Ichinomiya, Y. Koshi, and H. Horiuchi. 2006. Escape of Candida from caspofungin inhibition at concentrations above the MIC (paradoxical effect) accomplished by increased cell wall chitin; evidence for β-1,6-glucan synthesis inhibition by caspofungin. Antimicrob. Agents Chemother. 50:3160-3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagner, C., W. Graninger, E. Presterl, and C. Joukhadar. 2006. The echinocandins: comparison of their pharmacokinetics, pharmacodynamics and clinical applications. Pharmacology 78:161-177. [DOI] [PubMed] [Google Scholar]
- 32.Wiederhold, N. P., D. P. Kontoyiannis, J. Chi, R. A. Prince, V. H. Tam, and R. E. Lewis. 2004. Pharmacodynamics of caspofungin in a murine model of invasive pulmonary aspergillosis: evidence of concentration-dependent activity. J. Infect. Dis. 190:1464-1471. [DOI] [PubMed] [Google Scholar]
- 33.Wiederhold, N. P., and R. E. Lewis. 2003. The echinocandin antifungals: an overview of the pharmacology, spectrum and clinical efficacy. Expert Opin. Investig. Drugs 12:1313-1333. [DOI] [PubMed] [Google Scholar]