Abstract
Nontypeable Haemophilus influenzae (NTHi) commonly causes otitis media, chronic bronchitis in emphysema, and early airway infections in cystic fibrosis. Long-term, low-dose azithromycin has been shown to improve clinical outcomes in chronic lung diseases, although the mechanism of action remains unclear. The inhibition of bacterial biofilms by azithromycin has been postulated to be one mechanism mediating these effects. We hypothesized that subinhibitory concentrations of azithromycin would affect NTHi biofilm formation. Laboratory strains of NTHi expressing green fluorescent protein and azithromycin-resistant clinical isolates were grown in flow-cell and static-culture biofilm models. Using a range of concentrations of azithromycin and gentamicin, we measured the degree to which these antibiotics inhibited biofilm formation and persistence. Large biofilms formed over 2 to 4 days in a flow cell, displaying complex structures, including towers and channels. Subinhibitory concentrations of azithromycin significantly decreased biomass and maximal thickness in both forming and established NTHi biofilms. In contrast, subinhibitory concentrations of gentamicin had no effect on biofilm formation. Furthermore, established NTHi biofilms became resistant to gentamicin at concentrations far above the MIC. Biofilm formation of highly resistant clinical NTHi isolates (azithromycin MIC of >64 μg/ml) was similarly decreased at subinhibitory azithromycin concentrations. Clinically obtainable azithromycin concentrations inhibited biofilms in all but the most highly resistant isolates. These data show that subinhibitory concentrations of azithromycin have antibiofilm properties, provide mechanistic insights, and supply an additional rationale for the use of azithromycin in chronic biofilm infections involving H. influenzae.
Nontypeable Haemophilus influenzae (NTHi) remains one of the most common human bacterial respiratory pathogens. It causes substantial morbidity and mortality and exerts an enormous economic burden. In adults, chronic lower airway bacterial infections are the fourth leading cause of mortality (21), with an estimated cost of over 23 billion dollars per year (43). NTHi is the bacterium most commonly isolated from patients with chronic obstructive pulmonary disease (COPD) (40) and is most commonly the first lower airway bacterial pathogen in cystic fibrosis (CF) (36). Additionally, since the introduction of heptavalent pneumococcal vaccination, H. influenzae has become the most common pathogen in otitis media (2, 4). In children, otitis media is second only to acute upper respiratory illnesses for causing sick visits to physicians, resulting in 15 million office visits per year (39), with an economic burden in excess of 5 billion dollars per year (10).
NTHi biofilm formation may enable it to survive and cause chronic upper and lower respiratory tract infections despite aggressive treatment with antibiotics. For example, H. influenzae persists in COPD (23, 26), CF (35), and middle ear infections (31, 32) despite intensive antibiotic therapies. Utilizing a novel in vitro coculture model, we recently described NTHi cells forming adherent, antibiotic-resistant biofilms on airway epithelia (42). Additionally, lung lavage samples from very young patients with CF displayed structures consistent with those of NTHi biofilms (42). Communal bacteria in a biofilm can survive antibiotic concentrations up to 1,000-fold higher than the same bacteria in an individual, free-living, planktonic state (17). Therefore, clinically attainable antibiotic concentrations may not adequately clear infections, allowing the bacterial population to recover, persist, and spread.
Long-term, low-dose azithromycin improves clinical outcomes in CF (7, 37, 38, 45) and diffuse panbronchiolitis (20), although its mechanism of action remains poorly understood. Two prominent hypotheses are that azithromycin has beneficial immunomodulatory properties (16, 19, 41) or that it inhibits bacterial biofilm formation (9, 12, 18). This study tested the hypothesis that subinhibitory concentrations of azithromycin affect NTHi biofilm formation.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
NTHi strain 2019 is a clinical isolate obtained from a patient with COPD (3). Strain 2019wecA is deficient in undecaprenyl-phosphate-N-acetylglucosaminyltransferase (13). Plasmid pRSM2211, expressing GFPmut3, was kindly provided by Lauren Bakaletz (22) and was electroporated into NTHi strain 2019 and NTHi strain 2019wecA by methods described previously (24). Clinical azithromycin-resistant strains (S34, S43, S44, S52, S53, S57, and S61) had identified ribosomal mutations conferring resistance to azithromycin at >64 μg/ml (33). All strains were reconstituted from frozen glycerol stock cultures and propagated on brain heart infusion (BHI) agar or broth (Difco, Detroit, MI) supplemented with 10 μg of hemin (Sigma Chemical Co., St. Louis, MO) per ml and 10 μg of NAD (Sigma) per ml at 37°C. For pRSM2211 selection, 20 μg/ml kanamycin (Sigma) was added to BHI broth or agar.
Antimicrobial assays.
Azithromycin and gentamicin MICs for NTHi strain 2019 containing pRSM2211 were determined using CLSI (formerly NCCLS) methods by broth microdilution and Etest (AB Biodisk, Piscataway, NJ) by the Clinical Microbiology Laboratory at the University of Iowa.
Growth curves.
We performed growth curves on NTHi strain 2019 in 100% medium using an initial 1 ml of a culture grown overnight that was diluted in 24 ml of BHI broth supplemented with NAD and hemin with an initial OD at 600 nm of <0.050. Medium containing antibiotic had azithromycin (Pfizer, New York, NY), gentamicin (Celgro, Warren, NJ), or erythromycin (Hospira, Lake Forest, IL) added at doses ranging from 0.03 μg/ml to 2 μg/ml. We then incubated the bacteria in a shaker at 37°C and plotted growth curves from measurements of absorbance at 600 nm performed hourly using a Biophotometer (Eppendorf, Westbury, NY). Growth curves were additionally performed in flow-cell medium (1% supplemented BHI broth in 150 mM NaCl) with and without the addition of the same antibiotic concentrations describe above. Growth curves measured in static-culture biofilm assays are described below.
Flow-cell biofilm assay.
We utilized a flow-cell system where NTHi bacteria expressing green fluorescent protein (GFP) attached to the underside of a glass coverslip develop into a biofilm in slow-flowing medium (5). Flow-cell medium consisted of 1% BHI broth supplemented with NAD and hemin (both at 1% final concentrations) in 150 mM NaCl with a 0.08-ml/min flow rate. From preliminary studies, we found that these conditions reproducibly produced NTHi biofilms. Medium with antibiotic contained azithromycin (Pfizer), erythromycin (Hospira), or gentamicin (Celgro) at the concentrations indicated. The entire apparatus, including medium, pump, and flow cells, was incubated at 37°C. All confocal microscopy experiments used NTHi strain 2019 or 2019wecA containing pRSM2211 expressing GFPmut3 imaged on a Bio-Rad 1024 confocal microscope. Kanamycin was used for plasmid selection pressure prior to flow-cell inoculation. In separate experiments, we found that pRSM2211 was highly stable in NTHi strain 2019 and showed no decrease in fluorescence until passaged 10 to 15 times without antibiotic selection. Staining of dead cells with propidium iodide (44) and biofilm quantification using COMSTAT software (15) were performed as previously described.
Static-culture biofilm assay.
We assayed the biofilm formation of NTHi isolates in 96-well culture microplates as previously described (13, 29, 42), except that we utilized flat-bottom polystyrene 96-well tissue culture plates to allow bacterial OD measurements during the biofilm formation process. Mid-log-phase bacteria were incubated in supplemented BHI broth with the addition of azithromycin (Pfizer) ranging from 0.06 to 64 μg/ml. Over the initial 7 h, bacterial growth curves were plotted from hourly measurements of absorbance at 490 nm using a VERSAmax microplate reader (Molecular Devices, Sunnyvale, CA) (n = 4). Biofilm formation was measured at 24 h. We measured both growth over the initial 7 h and biofilm formation after crystal violet staining at 24 h from the same wells. This was possible because biomass forms on the side walls of the polystyrene well at the air-liquid interface for H. influenzae (data not shown) in a fashion similar to that seen with other bacteria (29), and planktonic growth is measured by the OD from the center of the well. This ensured that both growth and biofilm data came from the same conditions.
Statistical analyses.
All statistical analyses were performed with paired two-tailed Student's t tests using Microsoft Excel. P values of <0.05 were considered to be statistically significant. Because of regional variations in the flow cell, five stacked images were obtained for each condition. Averaged biomass and maximal thickness values from different flow-cell experiments were used for statistical analyses. To avoid selection bias, the following imaging protocol was used: imaging was initiated at the middle of the flow-cell channel starting at ∼3 mm from the inflow port; the remaining stacked images were obtained from four successive, adjacent, and distal optical fields.
RESULTS
Establishing a flow-cell model for NTHi.
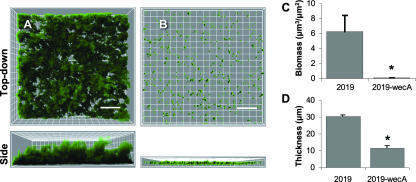
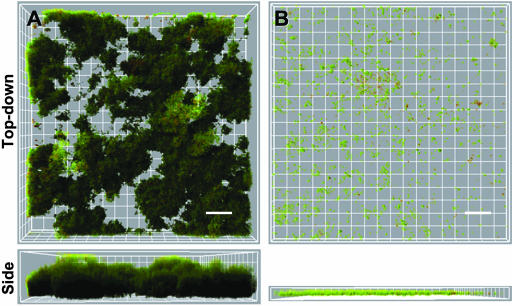
We optimized flow-cell culturing conditions to ensure biofilm formation that was neither too permissive nor too restrictive. Conditions that are too permissive may allow strains that form biofilms poorly to appear to be more robust. For example, too slow a flow rate may not apply enough shear stress to detach weakly formed biofilms. Conditions that are too restrictive may not allow even good biofilm-forming strains to form robust biofilms. Wild-type NTHi strain 2019 formed large biofilm structures with typical tower and channel structures over 2 days in the flow cell (Fig. 1A). Strain 2019wecA lacks undecaprenyl-phosphate-N-acetylglucosaminyltransferase and cannot make the secreted exopolysaccharide comprising NTHi's biofilm matrix. We used 2019wecA as a negative control to validate flow-cell conditions because it produces little or no biofilm in other models (13, 42). This strain formed biofilms poorly in the flow cell (Fig. 1B) and produced significantly diminished biomass and maximal thickness as determined by COMSTAT image analysis software (Fig. 1C and D). Maximal thickness represents the tallest bacterial structures, whereas biomass represents the average bacterial volume of the towers and channels. These data validated the conditions used in this model.
FIG. 1.
NTHi 2019 forms robust biofilms, and 2019wecA poorly forms biofilms. NTHi strain 2019wecA was used as a negative control because it is deficient in the ability to form the exopolysaccharide composing NTHi's biofilm and makes biofilms poorly (13, 42) (see the text). GFP-expressing NTHi 2019 (A) and 2019-wecA (B) were grown in a flow cell for 2 days. Bacterial biofilms were measured by confocal laser microscopy. Biofilm mass (C) (P < 0.05) and maximal thickness (D) (P < 0.01) were significantly decreased in the 2019wecA mutant. Representative top-down views (top) and side views (bottom) are shown. Each grid represents 10 μm, and scale bars represent 30 μm (n = 3).
Determination of subinhibitory concentrations of azithromycin and gentamicin.
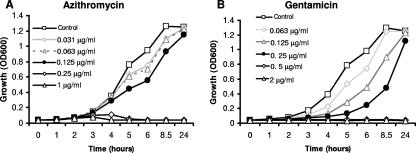
Based on liquid culture growth curves, we selected working azithromycin (0.125 μg/ml) and gentamicin (0.25 μg/ml) concentrations for flow-cell experiments (Fig. 2A and B). The rationale for the selected concentrations of both azithromycin and gentamicin was that both were the highest concentrations that allowed near-maximal bacterial density by the 24-h time point (Fig. 2A and B). Maximal doubling times were 44, 51, and 63 min for the no-antibiotic control, azithromycin at 0.125 μg/ml, and gentamicin at 0.25 μg/ml, respectively. Thus, the selected concentrations of both antibiotics showed some growth inhibition, with gentamicin being more severely delayed. We additionally performed growth curves in flow-cell medium (1% medium in isotonic NaCl). However, static cultures of NTHi grew poorly in this medium even without antibiotics, attaining a maximum OD at 600 nm of ∼0.150. Despite the poor growth, bacteria under conditions of 0.125 μg/ml azithromycin showed some growth at one-third the increase in the OD seen without antibiotic. However, bacteria under conditions of 0.25 μg/ml gentamicin showed no growth (data not shown). MICs for NTHi strain 2019 were 0.5 μg/ml for azithromycin and 2 μg/ml for gentamicin as determined by broth microdilution and Edisk, respectively. Thus, the selected antibiotic concentrations were 0.25 times the MIC for azithromycin and 0.125 times the MIC for gentamicin. It should be noted, however, that these growth curve and MIC data are not in a continuous-flow system and therefore are best approximations of relevant antibiotic concentrations in the flow-cell model.
FIG. 2.
Azithromycin and gentamicin growth curves. NTHi strain 2019 expressing GFP was grown with various concentrations of azithromycin (A) and gentamicin (B) as indicated. The azithromycin (0.125 μg/ml) and gentamicin (0.25 μg/ml) concentrations (black circles) were chosen for further studies, because these were the highest concentrations of antibiotic in which the growth reached near that of the nonantibiotic control over time (24-h time point). Both of these concentrations caused delayed growth, with gentamicin being more profoundly altered.
Azithromycin inhibits biofilm formation and also targets established biofilms.
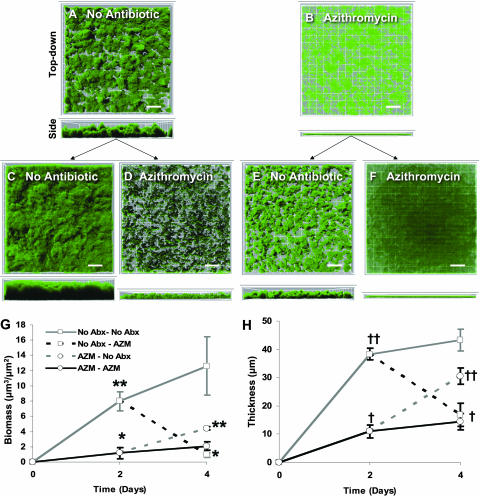
Using these sub-MIC concentrations of azithromycin and gentamicin, we next tested if they inhibited biofilm formation or affected established biofilms in a flow cell. In the absence of azithromycin, NTHi formed biofilms by 2 days of growth (Fig. 3A). The addition of subinhibitory concentrations of azithromycin substantially decreased biofilm formation (Fig. 3B). Compared to the antibiotic-free control, azithromycin significantly decreased biomass (Fig. 3G) and maximal thickness (Fig. 3H). These data show that azithromycin inhibits NTHi biofilm formation. We hypothesized that subinhibitory concentrations of azithromycin would only decrease biofilm formation and not affect established NTHi biofilms, as previously shown with Pseudomonas aeruginosa (11). To test whether azithromycin affected established biofilms, we continued these 2-day flow-cell experiments for an additional 2 days in either the presence or absence of azithromycin (Fig. 3C to F). Contrary to our hypothesis, azithromycin also significantly reduced the biomasses and maximum thicknesses of established NTHi biofilms (Fig. 3D, G, and H). Control biofilms in the absence of antibiotic from days 0 to 4 continued to increase in size over days 2 to 4 (Fig. 3C). After inhibiting NTHi biofilm formation with azithromycin over days 0 to 2 (Fig. 3B), the removal of azithromycin on days 2 to 4 allowed subsequent biofilm formation (Fig. 3E), with significant increases in both biomass and maximal thickness (Fig. 3G and H). Continuous azithromycin exposure from days 0 to 4 resulted in minimal biofilm formation over days 2 to 4 (Fig. 3B and F). These data show that subinhibitory concentrations of azithromycin not only decrease NTHi biofilm formation but also diminish established biofilms.
FIG. 3.
Azithromycin at sub-MIC concentrations inhibits biofilm formation and decreases established NTHi biofilms. Representative top-down view (upper) and side view (lower) confocal images of NTHi biofilms in flow cells from a single experiment are shown. NTHi forms robust biofilms in the absence of antibiotics over 2 days (A) and 4 days (C). After establishing biofilms from days 0 to 2, azithromycin (0.125 μg/ml) added to the medium for days 2 to 4 decreased both biofilm volume and thickness (D). From the time of inoculation, azithromycin (0.125 μg/ml) inhibited NTHi biofilm formation over 2 days (B) and 4 days (F). When azithromycin was removed starting at day 2, biofilms began to form on days 2 to 4 (E). Total biomass and maximum thickness of NTHi were quantified utilizing MatLab and COMSTAT on days 2 and 4. Gray lines indicate no antibiotic, and black lines indicate azithromycin (0.125 μg/ml). No Abx-No Abx, no antibiotic from days 0 to 4; No Abx-AZM, no antibiotic from days 0 to 2 and azithromycin from days 2 to 4; AZM-No Abx, azithromycin from days 0 to 2 and no antibiotic from days 2 to 4; AZM-AZM, azithromycin from days 0 to 4. *, P < 0.05 versus no antibiotics at day 2; **, P < 0.05 versus azithromycin at day 2; †, P < 0.01 versus no antibiotics at day 2; ††, P < 0.01 versus azithromycin at day 2 (n = 5). Squares indicate 12 μm, and scales bars represent 24 μm.
Other antibiotics do not affect NTHi biofilms at subinhibitory concentrations.
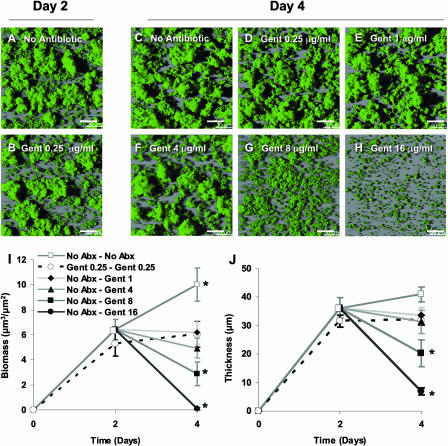
To test the specificity of azithromycin's biofilm inhibition, we investigated if other antibiotics with similar mechanisms of action exhibited similar properties. We chose gentamicin to test the specificity of the antibiofilm effects of azithromycin because it also inhibits protein synthesis by ribosomal binding and is commonly used in clinical practice. Despite a more profound delay on the growth curve (Fig. 2A and B), subinhibitory concentrations of gentamicin (0.25 μg/ml) showed no inhibition of biofilm formation over 2 days (Fig. 4B). In contrast to azithromycin, once NTHi biofilms formed under antibiotic-free conditions over 2 days, they became much more resistant to subsequent exposure to gentamicin. Sustained concentrations four times the MIC (8 μg/ml) were required to significantly decrease NTHi biofilms (Fig. 4G). Furthermore, concentrations 8 times the MIC (16 μg/ml) were required to decrease biomass and thickness, similar to those under conditions of 0.125 μg/ml azithromycin, at 0.25 times the MIC (Fig. 3B versus 4H). Therefore, in contrast to azithromycin, NTHi showed the more typical pattern of increased resistance to gentamicin once the bacteria formed a biofilm. We additionally tested another macrolide antibiotic, erythromycin, to see if it possessed antibiofilm properties similar to those displayed by azithromycin. Erythromycin concentrations (0.25 μg/ml) resulting in growth curves similar to those of azithromycin or gentamicin showed only a modest effect and did not significantly inhibit NTHi biofilms (data not shown). Thus, two related antibiotics did not exhibit azithromycin's antibiofilm properties at subinhibitory concentrations.
FIG. 4.
Established NTHi biofilms display gentamicin resistance. Representative top-down confocal images of NTHi biofilms in flow cells from a single experiment are shown. The gentamicin (Gent) (0.25 μg/ml) condition did not significantly reduce biomass or maximal thickness on days 2 (A versus B) or 4 (C versus D). After establishing NTHi biofilms under antibiotic-free conditions from days 0 to 2, biofilms were exposed 1 to 16 μg/ml of gentamicin as indicated (E to H). Biomass was decreased in a dose-dependent manner; however, biofilm structures persisted in all but the 16-μg/ml condition. Total biomass and maximum thickness of NTHi biofilms grown under the indicated conditions were quantified utilizing MatLab and COMSTAT on days 2 and 4. No Abx-No Abx, no antibiotic from days 0 to 4; Gent 0.25-Gent 0.25, 0.25 μg/ml gentamicin from days 0 to 4; No Abx-Gent, no antibiotic from days 0 to 2 and 1, 4, 8, or 16 μg/ml gentamicin from days 2 to 4 (n = 3). Scale bars indicate 30 μm. *, P < 0.05 versus the no-antibiotic condition at day 2.
Sub-MIC concentrations of azithromycin inhibit biofilm formation by resistant clinical NTHi strains.
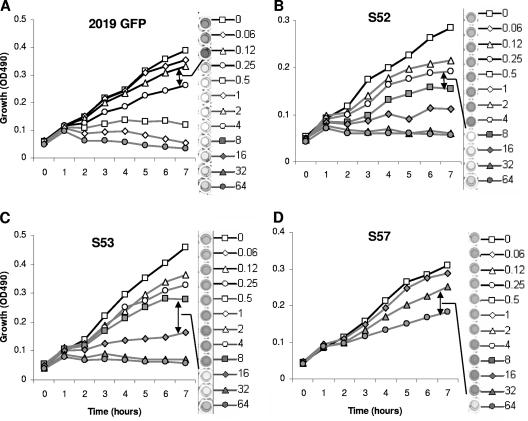
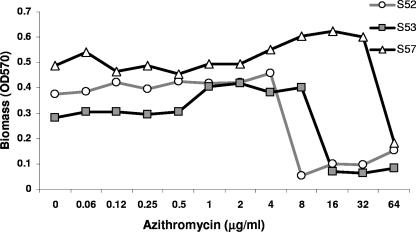
We next investigated if sub-MIC concentrations of azithromycin inhibited biofilm formation by highly resistant clinical strains of NTHi. These studies also gave insights into azithromycin's antibiofilm mechanism of action. All clinical isolates used in these studies had identified ribosomal mutations decreasing antibiotic binding and conferring resistance to azithromycin at >64 μg/ml (33). Using PCR for capsular typing, we verified all clinical strains as being NTHi (data not shown) (8). If biofilm inhibition occurred only at concentrations that impacted growth, this would suggest that the inhibition of biofilms occurred primarily through growth inhibition. In contrast, if azithromycin concentrations that did not affect growth inhibited biofilms, this would support growth-independent mechanisms. Isolates S34, S43, S44, and S61 formed biofilms poorly in this model but were resistant to azithromycin at >64 μg/ml (data not shown). Isolates S52, S53, and S57 formed biofilms. Azithromycin inhibited biofilm formation at concentrations that clearly allowed growth, at 0.25, 8, 16, and 64 μg/ml for strains 2019-GFP, S52, S53, and S57, respectively (Fig. 5). This result shows a second model where azithromycin inhibits biofilm formation at concentrations that allow the growth of NTHi. These data show that the finding that azithromycin affects biofilm formation at subinhibitory concentrations is a common phenomenon, even in highly resistant clinical isolates. Notably, inhibition did not appear to exhibit a dose response; rather, it had an “on-off” appearance once concentrations exceeded an inhibitory threshold for the isolate (Fig. 6). It also suggests that slower rates of growth may be associated with azithromycin's inhibition of biofilms. However, it does not exclude the possibility of a growth-independent mechanism(s).
FIG. 5.
Azithromycin inhibits biofilm formation in azithromycin-resistant clinical isolates. Isolates were incubated overnight in 96-well plates in the presence of 0.06 to 64 μg/ml azithromycin. Growth curves were measured over the initial 7 h of incubation. Biomass was assayed using crystal violet as described in Materials and Methods. NTHi 2019 expressing GFP (A) shows a similar pattern of biofilm inhibition at subinhibitory azithromycin concentrations compared to highly azithromycin-resistant clinical NTHi isolates (B to D). Arrows indicate the threshold at which biofilm formation was diminished. Representative growth curve and biofilm formation data from a single experiment are shown (n = 3).
FIG. 6.
Biofilm formation in resistant clinical isolates is inhibited by azithromycin above a threshold concentration. We graphed the biomass formed in the static-culture biofilms exposed to the various concentrations of azithromycin shown in Fig. 5. We measured growth over the initial 7 h and biofilm formation at 24 h from the same wells. All three clinical isolates showed a marked decrease in biofilm formation above a threshold concentration of azithromycin. Representative data are shown (n = 3).
Concentrations of azithromycin that inhibit biofilms do not kill NTHi.
Additionally, we verified that the concentrations of azithromycin that affect both biofilm formation and established biofilms were submicrobicidal and did not kill NTHi in the flow cell. Because the data from the growth curves in both 100% and 1% flow-cell media came from static-culture ODs, these results may not adequately reflect the conditions present in a continuous-flow system like the flow cell. Therefore, we performed propidium iodine staining of dead NTHi cells in the flow cell to verify bacterial viability. Live NTHi cells express GFP, and only dead bacteria take up propidium iodide. Therefore, live bacteria will fluoresce green and dead cells will fluoresce red in this model. The vast majority of bacteria in both the nonantibiotic control and azithromycin-treated groups remained alive in the flow cell (Fig. 7). The viability of the bacteria was further evidenced by the growth of the bacteria from days 2 to 4 after the removal of azithromycin in the crossover studies (Fig. 3E). Additionally, the growth curves from azithromycin-resistant clinical isolates clearly showed biofilm inhibition at concentrations that permit bacterial growth (Fig. 5A to D).
FIG. 7.
NTHi remains viable after exposure to azithromycin. NTHi strain 2019 expressing GFP was grown in the absence (A) or presence (B) of azithromycin (0.125 μg/ml) for 2 days. Biofilms were then stained with propidium iodide for 15 min. Permeabilized (dead) bacteria stain red, whereas live bacteria fluoresce green. Representative top-down views (top) and side views (bottom) are shown (n = 3). Each grid represents 10 μm, and scales bars represent 20 μm.
DISCUSSION
This report demonstrates that azithromycin both decreases biofilm formation and diminishes established NTHi biofilms at subinhibitory concentrations. Gentamicin and erythromycin, which have similar mechanisms of antimicrobial action, did not exhibit similar effects. The concentrations of azithromycin that inhibited biofilm formation allowed bacterial growth, and the bacteria remained viable after azithromycin exposure. Subinhibitory concentrations of azithromycin that affected NTHi biofilms delayed growth, suggesting that ribosomally mediated growth inhibition may be one mechanism of its antibiofilm properties. However, the biofilm and growth curve data suggest that azithromycin's inhibition of biofilms may not simply be a growth inhibition phenomenon. These studies also validated the flow-cell model for use with H. influenzae biofilms, and this model may be useful for future NTHi biofilm studies.
Although we hypothesized that subinhibitory concentrations of azithromycin would decrease biofilm formation only, they also decreased the volume and height of established biofilms. This differs from data for P. aeruginosa, where azithromycin inhibited biofilm formation only and had no affect once biofilms became established (11). The ability to decrease established biofilms makes these results more clinically relevant, since many patients would likely have established biofilms by the time treatment with azithromycin would be initiated.
Although having a mechanism of action similar to that of azithromycin, gentamicin did not posses antibiofilm properties at subinhibitory concentrations. With the biofilm models used in these studies, we demonstrated that gentamicin and erythromycin do not show antibiofilm properties at subinhibitory concentrations (Fig. 4). We hypothesize that azithromycin's inhibition of NTHi biofilms at subinhibitory concentrations is a specific property not shared by other antibiotics with similar structures or mechanisms of action. Studies of other bacteria have shown that azithromycin possesses unique antibiofilm properties. Of 21 commonly used antibiotics, azithromycin and, to a lesser degree, clarithromycin were the only antibiotics shown to affect P. aeruginosa biofilms at subinhibitory concentrations (25).
Diminishing established NTHi biofilms required a much higher dose of gentamicin. The concentrations of gentamicin that diminished established NTHi biofilms approached the high end or exceeded clinically attainable concentrations. The standard dosing of gentamicin of three times a day has target peak serum levels of 5 to 10 μg/ml, and once-a-day dosing rarely exceeds 15 μg/ml (30). Furthermore, gentamicin levels in airway secretions are roughly threefold lower than serum levels, so bacteria in the airway would be unlikely to encounter gentamicin concentrations much above 4 μg/ml (30). Established NTHi biofilm inhibition required sustained gentamicin concentrations four times the MIC (8 μg/ml) to significantly decrease biomass (Fig. 4G) and eight times the gentamicin MIC (16 μg/ml) to decrease biomass and thickness similar to azithromycin at 0.25 times the MIC (0.125 μg/ml) (Fig. 3B versus 4H). As the methods used for these studies involved continuous exposure, we predict that shorter exposure times of gentamicin in clinical settings would have even less inhibitory effects. Finally, these studies used a relatively gentamicin-sensitive NTHi strain. Biofilms of gentamicin-resistant isolates would likely display resistance to even higher concentrations of gentamicin. On the other hand, azithromycin decreased forming and established biofilms in clinically relevant concentrations. The 0.125-μg/ml concentration of azithromycin used in these studies is approximately that found in serum (28). More importantly, azithromycin concentrates in human tissues, attaining 3 μg/ml in airway surface liquid (28) and 9 μg/ml in CF sputum (1). This study showed that even the most azithromycin resistant of over 6,000 clinical NTHi isolates were susceptible to biofilm inhibition at sub-MIC azithromycin concentrations (Fig. 5). This suggests that clinically attainable azithromycin concentrations would inhibit NTHi biofilms in all but the most resistant clinical isolates.
These data suggest that although growth inhibition is important, there may be additional factors that mediate biofilm inhibition. At the concentrations used in this study, gentamicin had more profound effects on delaying growth than azithromycin based on the growth curves using 100% (Fig. 2) and flow-cell (data not shown) media. If azithromycin's antibiofilm effects were purely a growth inhibition phenomenon, we would expect the gentamicin condition to have exhibited more biofilm inhibition. Additionally, if growth were the sole mediator of biofilm inhibition, we would also expect the inhibition of biofilm formation to be proportional to the growth of the bacteria. Rather, we saw an “on-off” appearance with increasing concentrations of azithromycin (Fig. 6). Further study is needed to clarify the mechanism by which azithromycin inhibits NTHi biofilms. Because NTHi biofilms may be involved in the pathogenesis of COPD, otitis media, and early CF infections (6, 14, 27, 34, 42), these results could have important clinical implications and provide an additional rationale for the long-term use of azithromycin in NTHi-related diseases. However, chronic antibiotic use would have to be weighed against the possibility of selecting for resistant organisms and adverse side effects.
In conclusion, we show that azithromycin decreased both biofilm formation and established H. influenzae biofilms at subinhibitory concentrations. This antibiofilm effect was not seen with gentamicin or erythromycin and may be a property specific to azithromycin. Biofilm inhibition occurred only at concentrations that delayed growth; however, the pattern of inhibition suggests that there may be factors in addition to growth inhibition that mediate azithromycin's antibiofilm properties. These data provide a rationale for the use of azithromycin in diseases involving chronic NTHi biofilms, such as COPD, otitis media, and early infections in CF.
Acknowledgments
We thank Paul B. McCray, Jr., and Michael A. Apicella for their guidance, mentoring, and critical commentary. We also thank Lauren Bakaletz for generously providing plasmid pRSM2211. We additionally acknowledge Niu Zhang for her technical expertise.
These studies were primarily supported by grants from the National Institutes of Health (HL67992 to T.D.S.), with additional support from the American Lung Association (RG-11408-N to T.D.S.), the Cystic Fibrosis Foundation (RDP R458 to T.D.S.), and the Internal Funding Initiative at the University of Iowa.
Footnotes
Published ahead of print on 22 October 2007.
REFERENCES
- 1.Baumann, U., M. King, E. M. App, S. Tai, A. Konig, J. J. Fischer, T. Zimmermann, W. Sextro, and H. von der Hardt. 2004. Long term azithromycin therapy in cystic fibrosis patients: a study on drug levels and sputum properties. Can. Respir. J. 11:151-155. [DOI] [PubMed] [Google Scholar]
- 2.Block, S. L., J. Hedrick, C. J. Harrison, R. Tyler, A. Smith, R. Findlay, and E. Keegan. 2004. Community-wide vaccination with the heptavalent pneumococcal conjugate significantly alters the microbiology of acute otitis media. Pediatr. Infect. Dis. J. 23:829-833. [DOI] [PubMed] [Google Scholar]
- 3.Campagnari, A. A., M. R. Gupta, K. C. Dudas, T. F. Murphy, and M. A. Apicella. 1987. Antigenic diversity of lipooligosaccharides of nontypable Haemophilus influenzae. Infect. Immun. 55:882-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casey, J. R., and M. E. Pichichero. 2004. Changes in frequency and pathogens causing acute otitis media in 1995-2003. Pediatr. Infect. Dis. J. 23:824-828. [DOI] [PubMed] [Google Scholar]
- 5.Christensen, B. B., C. Sternberg, J. B. Andersen, R. J. Palmer, Jr., A. T. Nielsen, M. Givskov, and S. Molin. 1999. Molecular tools for study of biofilm physiology. Methods Enzymol. 310:20-42. [DOI] [PubMed] [Google Scholar]
- 6.Ehrlich, G. D., R. Veeh, X. Wang, J. W. Costerton, J. D. Hayes, F. Z. Hu, B. J. Daigle, M. D. Ehrlich, and J. C. Post. 2002. Mucosal biofilm formation on middle-ear mucosa in the chinchilla model of otitis media. JAMA 287:1710-1715. [DOI] [PubMed] [Google Scholar]
- 7.Equi, A., I. M. Balfour-Lynn, A. Bush, and M. Rosenthal. 2002. Long term azithromycin in children with cystic fibrosis: a randomised, placebo-controlled crossover trial. Lancet 360:978-984. [DOI] [PubMed] [Google Scholar]
- 8.Falla, T. J., D. W. Crook, L. N. Brophy, D. Maskell, J. S. Kroll, and E. R. Moxon. 1994. PCR for capsular typing of Haemophilus influenzae. J. Clin. Microbiol. 32:2382-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Favre-Bonte, S., T. Kohler, and C. Van Delden. 2003. Biofilm formation by Pseudomonas aeruginosa: role of the C4-HSL cell-to-cell signal and inhibition by azithromycin. J. Antimicrob. Chemother. 52:598-604. [DOI] [PubMed] [Google Scholar]
- 10.Gates, G. A. 1996. Cost-effectiveness considerations in otitis media treatment. Otolaryngol. Head Neck Surg. 114:525-530. [DOI] [PubMed] [Google Scholar]
- 11.Gillis, R. J., and B. H. Iglewski. 2004. Azithromycin retards Pseudomonas aeruginosa biofilm formation. J. Clin. Microbiol. 42:5842-5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gillis, R. J., K. G. White, K. H. Choi, V. E. Wagner, H. P. Schweizer, and B. H. Iglewski. 2005. Molecular basis of azithromycin-resistant Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 49:3858-3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greiner, L. L., H. Watanabe, N. J. Phillips, J. Shao, A. Morgan, A. Zaleski, B. W. Gibson, and M. A. Apicella. 2004. Nontypeable Haemophilus influenzae strain 2019 produces a biofilm containing N-acetylneuraminic acid that may mimic sialylated O-linked glycans. Infect. Immun. 72:4249-4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall-Stoodley, L., F. Z. Hu, A. Gieseke, L. Nistico, D. Nguyen, J. Hayes, M. Forbes, D. P. Greenberg, B. Dice, A. Burrows, P. A. Wackym, P. Stoodley, J. C. Post, G. D. Ehrlich, and J. E. Kerschner. 2006. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA 296:202-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heydorn, A., A. T. Nielsen, M. Hentzer, C. Sternberg, M. Givskov, B. K. Ersboll, and S. Molin. 2000. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 146:2395-2407. [DOI] [PubMed] [Google Scholar]
- 16.Hodge, S., G. Hodge, S. Brozyna, H. Jersmann, M. Holmes, and P. N. Reynolds. 2006. Azithromycin increases phagocytosis of apoptotic bronchial epithelial cells by alveolar macrophages. Eur. Respir. J. 28:486-495. [DOI] [PubMed] [Google Scholar]
- 17.Hoiby, N. 2002. Understanding bacterial biofilms in patients with cystic fibrosis: current and innovative approaches to potential therapies. J. Cyst. Fibros. 1:249-254. [DOI] [PubMed] [Google Scholar]
- 18.Ichimiya, T., K. Takeoka, K. Hiramatsu, K. Hirai, T. Yamasaki, and M. Nasu. 1996. The influence of azithromycin on the biofilm formation of Pseudomonas aeruginosa in vitro. Chemotherapy 42:186-191. [DOI] [PubMed] [Google Scholar]
- 19.Kadota, J., O. Sakito, S. Kohno, H. Sawa, H. Mukae, H. Oda, K. Kawakami, K. Fukushima, K. Hiratani, and K. Hara. 1993. A mechanism of erythromycin treatment in patients with diffuse panbronchiolitis. Am. Rev. Respir. Dis. 147:153-159. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi, H., H. Takeda, S. Sakayori, Y. Kawakami, Y. Otsuka, M. Tamura, K. Konishi, S. Tanimoto, M. Fukakusa, K. Shimada, et al. 1995. Study on azithromycin in treatment of diffuse panbronchiolitis. Kansenshogaku Zasshi 69:711-722. (In Japanese.) [DOI] [PubMed] [Google Scholar]
- 21.Kochanek, K. D., S. L. Murphy, R. N. Anderson, and C. Scott. 2004. Deaths: final data for 2002. Natl. Vital Stat. Rep. 53:1-115. [PubMed] [Google Scholar]
- 22.Mason, K. M., R. S. Munson, Jr., and L. O. Bakaletz. 2003. Nontypeable Haemophilus influenzae gene expression induced in vivo in a chinchilla model of otitis media. Infect. Immun. 71:3454-3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miravitlles, M., C. Espinosa, E. Fernandez-Laso, J. A. Martos, J. A. Maldonado, M. Gallego, et al. 1999. Relationship between bacterial flora in sputum and functional impairment in patients with acute exacerbations of COPD. Chest 116:40-46. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell, M. A., K. Skowronek, L. Kauc, and S. H. Goodgal. 1991. Electroporation of Haemophilus influenzae is effective for transformation of plasmid but not chromosomal DNA. Nucleic Acids Res. 19:3625-3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moskowitz, S. M., J. M. Foster, J. Emerson, and J. L. Burns. 2004. Clinically feasible biofilm susceptibility assay for isolates of Pseudomonas aeruginosa from patients with cystic fibrosis. J. Clin. Microbiol. 42:1915-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy, T. F., A. L. Brauer, A. T. Schiffmacher, and S. Sethi. 2004. Persistent colonization by Haemophilus influenzae in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 170:266-272. [DOI] [PubMed] [Google Scholar]
- 27.Murphy, T. F., and C. Kirkham. 2002. Biofilm formation by nontypeable Haemophilus influenzae: strain variability, outer membrane antigen expression and role of pili. BMC Microbiol. 2:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olsen, K. M., G. San Pedro, L. P. Gann, P. O. Gubbins, D. M. Halinski, and G. D. Campbell, Jr. 1996. Intrapulmonary pharmacokinetics of azithromycin in healthy volunteers given five oral doses. Antimicrob. Agents Chemother. 40:2582-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Toole, G. A., and R. Kolter. 1998. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol. Microbiol. 28:449-461. [DOI] [PubMed] [Google Scholar]
- 30.Panidis, D., S. L. Markantonis, E. Boutzouka, S. Karatzas, and G. Baltopoulos. 2005. Penetration of gentamicin into the alveolar lining fluid of critically ill patients with ventilator-associated pneumonia. Chest 128:545-552. [DOI] [PubMed] [Google Scholar]
- 31.Park, C. W., J. H. Han, J. H. Jeong, S. H. Cho, M. J. Kang, K. Tae, and S. H. Lee. 2004. Detection rates of bacteria in chronic otitis media with effusion in children. J. Korean Med. Sci. 19:735-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pereira, M. B., M. R. Pereira, V. Cantarelli, and S. S. Costa. 2004. Prevalence of bacteria in children with otitis media with effusion. J. Pediatr. (Rio J.) 80:41-48. (In Portugese.) [PubMed] [Google Scholar]
- 33.Peric, M., B. Bozdogan, M. R. Jacobs, and P. C. Appelbaum. 2003. Effects of an efflux mechanism and ribosomal mutations on macrolide susceptibility of Haemophilus influenzae clinical isolates. Antimicrob. Agents Chemother. 47:1017-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Post, J. C. 2001. Direct evidence of bacterial biofilms in otitis media. Laryngoscope 111:2083-2094. [DOI] [PubMed] [Google Scholar]
- 35.Roman, F., R. Canton, M. Perez-Vazquez, F. Baquero, and J. Campos. 2004. Dynamics of long-term colonization of respiratory tract by Haemophilus influenzae in cystic fibrosis patients shows a marked increase in hypermutable strains. J. Clin. Microbiol. 42:1450-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenfeld, M., R. L. Gibson, S. McNamara, J. Emerson, J. L. Burns, R. Castile, P. Hiatt, K. McCoy, C. B. Wilson, A. Inglis, A. Smith, T. R. Martin, and B. W. Ramsey. 2001. Early pulmonary infection, inflammation, and clinical outcomes in infants with cystic fibrosis. Pediatr. Pulmonol. 32:356-366. [DOI] [PubMed] [Google Scholar]
- 37.Saiman, L., B. C. Marshall, N. Mayer-Hamblett, J. L. Burns, A. L. Quittner, D. A. Cibene, S. Coquillette, A. Y. Fieberg, F. J. Accurso, and P. W. Campbell III. 2003. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA 290:1749-1756. [DOI] [PubMed] [Google Scholar]
- 38.Saiman, L., N. Mayer-Hamblett, P. Campbell, and B. C. Marshall. 2005. Heterogeneity of treatment response to azithromycin in patients with cystic fibrosis. Am. J. Respir. Crit. Care Med. 172:1008-1012. [DOI] [PubMed] [Google Scholar]
- 39.Schappert, S. M., and C. W. Burt. 2006. Ambulatory care visits to physician offices, hospital outpatient departments, and emergency departments: United States, 2001-02. Vital Health Stat. 13:1-66. [PubMed] [Google Scholar]
- 40.Sethi, S., N. Evans, B. J. Grant, and T. F. Murphy. 2002. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N. Engl. J. Med. 347:465-471. [DOI] [PubMed] [Google Scholar]
- 41.Shinkai, M., G. H. Foster, and B. K. Rubin. 2006. Macrolide antibiotics modulate ERK phosphorylation and IL-8 and GM-CSF production by human bronchial epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 290:L75-85. [DOI] [PubMed] [Google Scholar]
- 42.Starner, T. D., N. Zhang, G. Kim, M. A. Apicella, and P. B. McCray, Jr. 2006. Haemophilus influenzae forms biofilms on airway epithelia: implications in cystic fibrosis. Am. J. Respir. Crit. Care Med. 174:213-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sullivan, S. D., S. D. Ramsey, and T. A. Lee. 2000. The economic burden of COPD. Chest 117:5S-9S. [DOI] [PubMed] [Google Scholar]
- 44.Teitzel, G. M., and M. R. Parsek. 2003. Heavy metal resistance of biofilm and planktonic Pseudomonas aeruginosa. Appl. Environ. Microbiol. 69:2313-2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolter, J., S. Seeney, S. Bell, S. Bowler, P. Masel, and J. McCormack. 2002. Effect of long term treatment with azithromycin on disease parameters in cystic fibrosis: a randomised trial. Thorax 57:212-216. [DOI] [PMC free article] [PubMed] [Google Scholar]