Abstract
Steady-state concentrations of telavancin, a novel, bactericidal lipoglycopeptide, were determined in the plasma, pulmonary epithelial lining fluid (ELF), and alveolar macrophages (AMs) of 20 healthy subjects. Telavancin at 10 mg of drug/kg of body weight/day was administered as a 1-h intravenous infusion on three successive days, with bronchoalveolar lavage performed on five subjects, each at 4, 8, 12, and 24 h after the last dose. Plasma samples were collected before the first and third infusions and at 1, 2, 3, 4, 8, 12, and 24 h after the third infusion. The plasma telavancin concentration-time profile was as reported previously. Telavancin (mean ± standard deviation) penetrated well into ELF (3.73 ± 1.28 μg/ml at 8 h and 0.89 ± 1.03 μg/ml at 24 h) and extensively into AMs (19.0 ± 16.8 μg/ml at 8 h, 45.0 ± 22.4 μg/ml at 12 h, and 42.0 ± 31.4 μg/ml at 24 h). Mean concentrations in AMs and plasma at 12 h were 45.0 μg/ml and 22.9 μg/ml (mean AM/plasma ratio, 1.93), respectively, and at 24 h were 42.0 μg/ml and 7.28 μg/ml (mean AM/plasma ratio, 6.67), respectively. Over the entire dosing interval, telavancin was present in ELF and AMs at concentrations up to 8-fold and 85-fold, respectively, above its MIC90 for methicillin-resistant Staphylococcus aureus (0.5 μg/ml). Pulmonary surfactant did not affect telavancin's in vitro antibacterial activity. Telavancin was well tolerated. These results support the proposal for further clinical evaluation of telavancin for treating gram-positive respiratory infections.
Telavancin is a novel, rapidly bactericidal lipoglycopeptide that operates through multiple mechanisms involving the inhibition of peptidoglycan biosynthesis and perturbation of bacterial cell membrane function (15). This compound is currently undergoing late-stage clinical development for the treatment of serious infections due to gram-positive pathogens, with a focus on methicillin-resistant Staphylococcus aureus (MRSA). Recently published phase 2, randomized, double-blind trial data have shown that treatment with telavancin is well tolerated and is at least as effective as vancomycin for complicated skin and skin structure infections (32, 33).
The antibacterial and pharmacokinetic profile of telavancin suggests that this agent may also have clinical utility in the treatment of lower respiratory tract infections. In recently reported studies, telavancin exhibited potent in vitro activity against methicillin-susceptible and -resistant strains of S. aureus (MIC90, 0.5 μg/ml) (poster P2-033 presented by D. C. Draghi, B. M. Benton, M. E. Jones, K. M. Krause, C. Thornsberry, and D. F. Sahm at the 10th Western Pacific Congress on Chemotherapy and Infectious Diseases, Fukuoka, Japan, 3 to 6 December 2006) and Streptococcus pneumoniae (MIC90, 0.03 μg/ml) (poster E-0719 presented by C. Thornsberry, D. C. Draghi, B. M. Benton, M. E. Jones, K. M. Krause, and D. F. Sahm at the 46th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA, 27 to 30 September 2006), which account for approximately one-third of the pathogens isolated from patients with hospital-acquired pneumonia (20). Furthermore, in a murine model of pneumonia induced by MRSA, telavancin at exposures approximating those for human clinical use was associated with greater reductions in bacterial titers and mortality than either vancomycin or linezolid at equivalent exposures (26). In a subsequent phase 1 ex vivo study involving 54 healthy volunteers, peak and trough serum concentrations of telavancin were found to be highly bactericidal against a strain of MRSA and penicillin-resistant S. pneumoniae, particularly at doses of 7.5 to 15 mg of drug/kg of body weight/day over successive days (30).
The purpose of the present study was to determine if bioactive steady-state concentrations of telavancin could be attained at respiratory sites believed to be important for the treatment of pneumonia (3). In addition, we measured the effect of pulmonary surfactant, a key component of epithelial lining fluid (ELF), on the in vitro activities of telavancin and comparator antibiotics against clinically relevant respiratory pathogens.
MATERIALS AND METHODS
Methodology.
This open-label, single-arm, multiple-dose study was approved by the Institutional Review Board of Chesapeake Research Review, Inc., a commercial organization with membership in the Association for the Accreditation of Human Research Protection Programs, Inc. (AAHRPP). The study was conducted at Pulmonary Associates, Phoenix, AZ, and was undertaken in full compliance with the Good Clinical Practice guidelines of the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use and the principles of the Declaration of Helsinki.
Subjects.
Eligible male and female subjects gave written informed consent and were required to be 18 to 50 years of age and within 15% of the appropriate weight range based on sex, height, and frame (based on the Metropolitan Life Insurance Company height and weight tables [21]). The subjects were in good health, based on the results of routine clinical laboratory tests, physical examinations, and electrocardiograms that were performed at screening. The subjects could not have a history of drug hypersensitivity or allergic reactions related to glycopeptide antibiotics, lidocaine, or benzodiazepines and must have abstained from the use of tobacco and other nicotine-containing products for the previous 6 months and until the final study evaluation was completed.
Exclusion criteria included a clinically significant systemic disease or the existence of any surgical or medical condition that, in the judgment of the investigator, might interfere with the distribution, metabolism, or excretion of telavancin. Subjects with any unstable cardiac condition or clinically significant abnormal plasma levels of potassium and/or magnesium were also ineligible for study participation. We also excluded subjects who (i) had received an investigational medication/device within 30 days of study admission, (ii) required long-term medication (including the use of over-the-counter drugs, such as nonsteroidal anti-inflammatory drugs), (iii) had abused narcotics or alcohol over the past 2 years (based on medical history and urine toxicology), (iv) had experienced clinically significant changes in their baseline status within 7 days of the first dose of telavancin, (v) consumed more than six cups of coffee (or a caffeine equivalent) per day, (vi) had recently donated or lost more than 400 ml of blood, (vii) tested positive for HIV or hepatitis B or C antibodies, or (viii) were unable to undergo bronchoscopy. In addition, we excluded female subjects of childbearing potential if they were pregnant (a negative pregnancy test was documented before treatment), nursing, or unable to use a highly effective method of birth control during the study period and for at least 1 month after the last dose of study medication.
Treatment regimen and sample collection.
Twenty subjects were to receive three consecutive daily intravenous infusions of telavancin at 10 mg/kg over a 60-minute period. Plasma samples were collected before the first and third infusions and at 1, 2, 4, 6, 8, 12, and 24 h after completion of the third infusion. Cohorts of five subjects were assigned nonrandomly to one of four groups for bronchoalveolar lavage (BAL) sampling according to the time of the bronchoscopy: 4, 8, 12, and 24 h following the initiation of the last infusion. The 4- and 8-h sampling times were chosen to reflect the estimated period of maximum intrapulmonary concentrations of telavancin. The 12-h sampling time represented the midpoint of the dosing interval, and the 24-h sampling time was chosen to correspond with the telavancin trough concentration at steady state. No tobacco, alcohol, grapefruit juice, or caffeine-containing products, such as tea, chocolate, and coffee, were permitted during the study.
Blood samples.
Blood samples (5 ml each) were drawn into heparinized glass tubes and chilled on ice until centrifugation. The plasma was then divided equally into two aliquots and kept frozen at −70°C until analysis. One sample was retained at the study site, and the second sample was sent to a central laboratory for analysis (Covance Bioanalytical Services, LLC, Madison, WI). A portion of the plasma sample obtained at the time of bronchoscopy (0.5 ml) was sent to the Hartford Hospital Laboratory (Hartford, CT) for determination of the urea concentration (not a Good Laboratory Practice assay).
Bronchoscopy and BAL.
Standardized bronchoscopy and BAL were performed in the clinical trial center at 4, 8, 12, and 24 h after the administration of the last dose in accordance with previously published methods (2-6, 9-11, 27, 28). Vital signs were recorded before and at the completion of the procedure.
All subjects received 3 ml of 4% lidocaine as a gargle, which was expectorated, followed by 3 to 5 ml of 2% topical lidocaine spray on the vocal cords and 5 to 10 ml of 0.5% lidocaine in the lower airway. In addition, 2% lidocaine jelly was applied to the nasal passageway to facilitate insertion of the fiber-optic bronchoscope (Olympus P-10; Olympus America, Inc., Melville, NY). The bronchoscope was passed via the nares and wedged into a segment of the right middle lobe. A 50-ml aliquot of sterile normal saline (0.9%, wt/vol) was instilled through the bronchoscope, suctioned, and then discarded to prevent contamination of the BAL specimens from larger airway secretions and from premedications (1, 5, 9, 14, 22, 23, 28). This procedure was repeated with three additional 50-ml instillations, which were retained for analysis after collection.
The BAL aspirates were pooled, the volume was recorded, and 1 ml of 25% human serum albumin was added for every 25 ml of BAL fluid to normalize the concentration of solutes. Approximately 4 ml of pooled BAL fluid was divided into two samples, which were processed in duplicate at the local clinical laboratory for the total cell count and a differential analysis. The remaining pooled BAL fluid was immediately placed on ice before being centrifuged at 400 × g for 5 min. Following centrifugation, the BAL supernatant was collected without disturbing the pellet, containing alveolar macrophages (AMs), which was retained in the tube. Both the BAL and AM pellet samples were flash frozen and stored at −70°C until they were assayed at Covance. The AM pellets were reconstituted in 980 μl of 2% formic acid in 90:10 methanol-acetonitrile, subjected to two freeze (acetone/ice bath)-thaw cycles, sonicated for 10 min, and centrifuged. A small aliquot of BAL supernatant was processed at the Hartford Hospital Laboratory for determination of the urea concentration.
Telavancin assays.
Concentrations of telavancin in plasma, ELF, and AMs were determined using validated liquid chromatography (LC) with a tandem mass spectrometric detection system (MS/MS; Theravance, Inc., internal report). The plasma, BAL, and AM specimens containing telavancin and spiked with an analytical internal standard (AMI-13616; Covance material identification no. 11400 and 11425) were diluted with acidified water and filtered using a centrifugal filter device. The compounds were analyzed using the LC-MS/MS system, in which separation occurred on a 50- by 4.6-mm Merck Chromolith SpeedROD RP-18e high-performance LC column, with positive electrospray ionization MS/MS detection on a Sciex API 3000 or 4000 mass spectrometer.
The AM pellet and BAL supernatant matrices were spiked with 25 μg/ml lidocaine, aliquots of which were processed and screened for significant interference in the chromatographic regions of interest. Additional lidocaine-fortified aliquots were spiked at medium quality control concentrations (1.6 μg/ml) of telavancin (with an internal standard added) and processed. In all cases, the telavancin and AMI-13616 regions were free from significant interference in the lidocaine-fortified blank samples (<20.0% of the lower limit of quantification or <5.0% of the internal-standard response), and the deviation of the mean from the theoretical value was within ±15.0% for the lidocaine-fortified samples spiked with 1.6 μg/ml of telavancin. It was, therefore, concluded that the method demonstrated acceptable specificity under conditions where samples were fortified with lidocaine.
The lower limits of quantification of the assays were 0.25 μg/ml for plasma, 0.02 μg/ml for the BAL supernatant, and 0.05 μg/ml for the AM pellet. This method was linear over the range of 0.25 to 200 μg/ml for the plasma samples, 0.02 to 5.00 μg/ml for the BAL supernatant, and 0.05 to 5.00 μg/ml for the AM pellet. The overall precision and accuracy of the quality controls and standards were >15% for all specimen concentrations (16-18). An intrapulmonary telavancin concentration of ≥0.5 μg/ml over the 24-h dosage interval was considered clinically meaningful, as this concentration is the telavancin MIC90 value for MRSA (poster P2-033 presented by D. C. Draghi, B. M. Benton, M. E. Jones, K. M. Krause, C. Thornsberry, and D. F. Sahm at the 10th Western Pacific Congress on Chemotherapy and Infectious Diseases, Fukuoka, Japan, 3 to 6 December 2006). The overall intra-assay and interassay precision levels for the calibration standards and quality control samples were within 8.5% for the plasma samples, 7.9% for the AM pellet, and 7.1% for the BAL supernatant, based upon percentages relative to the standard deviation.
Urea assay.
The urea concentration in plasma and BAL fluid was assayed by a colorimetric method (Center for Anti-Infective Research & Development, Hartford Hospital, Hartford, CT).
Calculations of ELF volume and telavancin concentrations in ELF and AMs.
The amount of ELF recovered was calculated according to the urea dilution method described by Rennard et al. (25). Urea, a small hydrophobic molecule, is used as an endogenous marker of ELF recovered by BAL, since its concentration in ELF is assumed to be the same as that in plasma (3).
The concentration of telavancin in ELF was estimated from the concentration of the drug in BAL fluid, the volume of BAL fluid collected, and the ratio of urea concentration in BAL fluid to that in plasma, as determined by published methods (12, 25). Thus, the concentration of telavancin in ELF was determined as [TLV]BAL·VBAL/VELF, where [TLV]BAL is the concentration of telavancin in BAL fluid, VBAL is the volume of the aspirated BAL fluid (total), and VELF is VBAL·[urea]BAL/[urea]plasma, where [urea]BAL is the concentration of urea in the BAL fluid (supernatant) and [urea]plasma is the concentration of urea in the plasma specimens.
The concentration of telavancin in the AM pellets was determined as [TLV]pellet/VAM, where [TLV]pellet is the concentration of telavancin in the pellet obtained from the saline instillations and VAM is the volume of alveolar cells in the 1-ml cell suspension. A differential cell count was performed in order to determine the number of macrophages and monocytes present. A mean macrophage cell volume of 2.42 μl/106 cells was used in the calculations for the volume of the alveolar cells in the pellet suspension (5).
Pharmacokinetic analysis.
Individual plasma drug concentration-time data were used to calculate telavancin pharmacokinetic parameters using noncompartmental analysis with WinNonlin pharmacokinetic software (version 4.0.1; Pharsight Corporation, Mountain View, CA). The maximum concentration in plasma (Cmax), the time to Cmax, and the minimum concentration in plasma (Cmin) were the observed values. The area under the concentration-time curve (AUC) was calculated from 0 to 24 h postdose (AUC0-24) using the linear trapezoidal method. The terminal-phase elimination rate constant (kel) was calculated as the negative gradient of the log-linear terminal portion of the plasma drug concentration-time curve using linear regression. Clearance at steady state was expressed as the ratio of the dose to the AUC0-24. The half-life (t1/2) of the terminal elimination phase was estimated by use of the following equation: 0.693/kel. Telavancin concentrations in each of the three anatomical matrices under study at synchronous time points from 0 to 24 h were determined to gauge the relative disposition of the antibacterial agent.
Safety and tolerability.
Safety was evaluated by conducting physical examinations, electrocardiographs, and clinical laboratory tests (hematology, serum chemistry, and urinalysis) at screening and at specified times throughout the study. The subjects were continuously observed and questioned for the possible occurrence of adverse events. The study investigator assessed adverse events for severity and relationship to the study drug. A subject experiencing an adverse event was followed by the investigator until it was determined that the adverse event had resolved or a stable clinical endpoint was reached.
Microbiology. (i) Organisms.
Reference strains of MRSA (ATCC 33591) and S. pneumoniae (ATCC 49619), both obtained from the American Type Culture Collection (ATCC; Manassas, VA), were selected for in vitro study.
(ii) Antimicrobial agents.
Telavancin powder was manufactured by Theravance, Inc., South San Francisco, CA. Daptomycin (Cubicin) and a commercially available pulmonary surfactant therapy containing 25 mg/ml phospholipids (Survanta) were purchased from Broadway Pharmacy, Burlingame, CA. Teicoplanin (Targocid) was a gift of Paul Tulkens from the Université Catholique de Louvain, Brussels, Belgium. Vancomycin and ceftriaxone were purchased from Sigma Chemical Company, St. Louis, MO.
(iii) In vitro susceptibility testing.
Susceptibility tests were performed by reference broth microdilution methodology as defined by the Clinical and Laboratory Standards Institute (CLSI) (8). The test medium was supplemented with 50 mg/liter Ca2+ for daptomycin susceptibility testing. Combinations of the study drug (telavancin, daptomycin, ceftriaxone, or vancomycin) and the pulmonary surfactant were tested using the standard checkerboard methodology described previously for the in vitro evaluation of antimicrobial combinations (13) and the CLSI broth microdilution methodology (8). The highest concentration of surfactant tested (1 mg/ml) corresponded to a 25-fold dilution of Survanta.
RESULTS
Subject demographics and baseline characteristics.
Twenty Caucasian subjects (7 men, 13 women) with a mean age of 30 years (range, 21 to 42 years) and a mean weight of 73.1 kg (range, 46.7 to 103.4 kg) were enrolled in and completed the study. All subjects were included in the telavancin pharmacokinetic and safety analyses, including two subjects who narrowly failed to meet the inclusion/exclusion criteria by being slightly overweight (based on the Metropolitan Life Insurance height and weight tables [21]). One of these subjects also had a lactate dehydrogenase value above the upper limit of normal at screening. A third subject also took a dose of ibuprofen between screening and day 1. None of these minor protocol deviations were expected to have a clinically significant effect on the study findings. The last infusion for one subject was interrupted because of intravenous infiltration, but data for the subject were included in the analysis because approximately 94% of the target dose had been administered.
Pharmacokinetics.
The mean pharmacokinetic parameters of telavancin in plasma for all 20 subjects are summarized in Table 1. Plasma telavancin concentrations peaked immediately after the 1-h infusion before declining in an exponential manner over the next 23 h.
TABLE 1.
Noncompartmental pharmacokinetics of telavancin, following administration of a 60-minute intravenous infusion of 10 mg/kg once daily for 3 days, in 20 healthy adult subjectsa
| Parameter | Mean (SD) |
|---|---|
| Cmax (μg/ml) | 116 (30) |
| Tmax (h) | 1.00 (0) |
| AUC0-24 (μg·h/ml) | 785 (111) |
| t1/2 (h) | 7.41 (1.08) |
| CLss (ml/h/kg) | 13.0 (1.9) |
| MRT (h) | 9.42 (1.54) |
| Vdss (ml/kg) | 122 (22) |
| Cmin (μg/ml) | 8.11 (2.28) |
CLss, clearance at steady state; MRT, mean residence time; Tmax, time to Cmax; Vdss, volume of distribution at steady state.
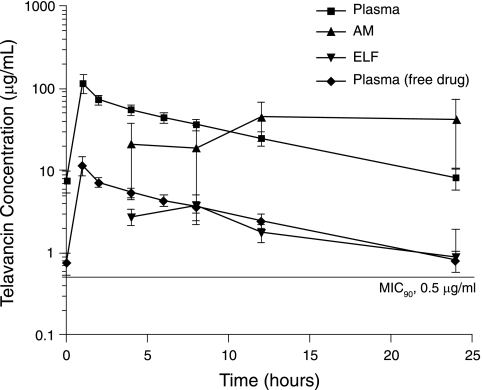
Good penetration of telavancin into the ELF and extensive penetration into the AMs of healthy subjects were observed throughout the dosing interval (Fig. 1). Telavancin mean concentrations in ELF peaked 8 h after the start of the infusion (at ∼3.7 μg/ml) before gradually declining over the second half of the 24-h dosing interval to a nadir of 0.9 μg/ml. In contrast, the maximum observed mean telavancin concentrations in AMs (45 μg/ml) occurred later in the dosing interval (at the 12-h time point) and remained high (42 μg/ml) at the 24-h time point. The mean ratio of the telavancin concentration in AMs to that in ELF was 8.3 (range, 0.42 to 16.3) at 4 h and 4.8 (range, 1.3 to 8.8) at 8 h after the start of the infusion. This relative difference in telavancin distributions increased substantially as drug concentrations in AMs increased from 8 to 24 h, while those in ELF declined. Similarly, the ratio of the telavancin concentration in AMs to that in plasma varied as the concentration of the drug in plasma changed over the dosing interval. Mean telavancin concentrations in AMs and plasma were 45.0 μg/ml and 22.9 μg/ml, respectively (mean AM/plasma ratio, 1.93), by the midpoint of the dosing interval (12 h), versus 42.0 μg/ml and 7.28 μg/ml, respectively (mean AM/plasma ratio, 6.67), at 24 h postdose.
FIG. 1.
Penetration of telavancin (10 mg/kg/day) into AMs and ELF in 20 healthy subjects undergoing fiber-optic bronchoscopy. Telavancin concentrations in AMs and ELF are expressed as mean values (± standard deviations) at the four sampling times (4, 8, 12, and 24 h postinfusion). Telavancin concentrations in plasma are expressed as mean values (± standard deviations) at the eight sampling times (0, 1, 2, 4, 6, 8, 12, and 24 h after the start of the infusion). The free drug concentration was calculated from the total concentration by assuming 90% plasma binding. The MIC90 for recent S. aureus isolates, including MRSA, is shown.
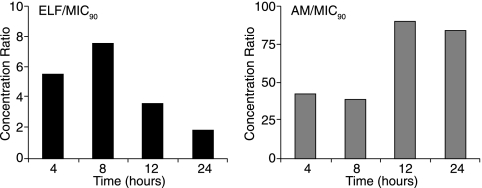
Telavancin was present in ELF and AMs at concentrations greater than the MIC90 for MRSA (0.5 μg/ml) over the entire dosing interval (Fig. 2).
FIG. 2.
Mean ratios of telavancin concentrations in ELF and AMs to the telavancin MIC90 value for MRSA (ATCC 33591) at the four sampling times (4, 8, 12, and 24 h after the start of the last of three daily infusions of 10 mg/kg telavancin).
Microbiology.
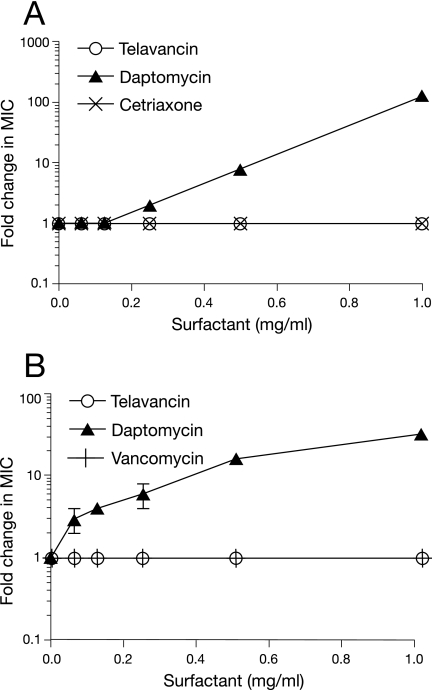
Pulmonary surfactant had no detectable effect on the in vitro activity of telavancin against MRSA or S. pneumoniae (Fig. 3). In contrast, the antibacterial activity of daptomycin (serving as a positive control in these studies) was significantly diminished by pulmonary surfactant in a concentration-dependent manner (Fig. 3). At the highest concentration of pulmonary surfactant tested (1 mg/ml), daptomycin was 32-fold less active against the MRSA isolate and 128-fold less active against the S. pneumoniae isolate. The activities of the other comparator agents, vancomycin and ceftriaxone, were unaffected by pulmonary surfactant (Fig. 3).
FIG. 3.
Effect of pulmonary surfactant on the in vitro activities of telavancin and comparator agents against S. pneumoniae (A) and MRSA (B). Antibacterial activity was determined by broth microdilution in cation-adjusted Mueller-Hinton broth (supplemented with 50 mg/liter Ca2+ for daptomycin susceptibility testing). Data are expressed as the median and range ratios of activity in the presence or absence of surfactant determined from two separate experiments.
Safety and tolerability.
The most common adverse events reported for subjects receiving telavancin were taste disturbance (n = 15), foamy urine (n = 13), postprocedural pain (n = 5), and headache (n = 2). Other than one subject who developed hypokalemia at the end of the study, which resolved during follow-up, no significant abnormalities in the clinical laboratory test results were noted.
DISCUSSION
The observed and calculated pharmacokinetic parameters of telavancin at 10 mg/kg in plasma were in close agreement with previously published data for this dosage (30). The t1/2 of 7 to 8 h, together with a Cmin value of 8.11 μg/ml, supports once-daily dosing for this compound. Telavancin penetrated well into ELF and extensively into AMs in healthy subjects who had no evidence of pulmonary inflammation or infection. In ELF, telavancin was present at concentrations exceeding the MIC90 for MRSA by approximately two- to eightfold over the entire dosing interval. The relative concentration of telavancin in AMs to the MIC90 for MRSA was even greater (ratio, 38 to 84). It cannot be determined from these data whether or not steady-state telavancin concentrations had been achieved in ELF or AMs. Thus, a longer period before the collection of BAL specimens may have resulted in higher concentrations in ELF and AMs than were observed in this study.
Over the 24-h dosing interval, Cmin values for telavancin were 8.11 μg/ml in plasma, 0.9 μg/ml in ELF, and 42.0 μg/ml in AMs. The intrapulmonary pharmacokinetic disposition of twice-daily intravenous infusions of 1 g vancomycin has also been examined in healthy volunteers in a study of virtually identical design (29). In that study, vancomycin concentrations at 12 h were 5.1 μg/ml in plasma, 2.4 μg/ml in ELF, and 45.2 μg/ml in AMs. However, we acknowledge the difficulty in making comparisons given interstudy differences. In our current study of an in vitro susceptibility assay designed to mimic the physiology of the lung, telavancin MICs were 2- to 16-fold less than those of vancomycin against a range of commonly encountered respiratory pathogens. The ELF AUC0-24 was approximately 45 μg/h/ml, so for an MIC90 of 0.5 μg/ml, the AUC/MIC90 ratio was 90 μg/h/ml. According to Reyes et al. (26), telavancin was effective in a murine pneumonia model at a dose of 40 mg/kg every 12 h (intravenously or subcutaneously administered). The corresponding AUC0-24 for free drug in mice was 52.3 μg/h/ml and is similar to the ELF AUC0-24 in humans. Therefore, it seems apparent that the concentrations of telavancin found in plasma, ELF, and AMs over the 24-h dosing interval are microbiologically relevant. Thus, these findings support the proposal for further studies of telavancin for the treatment of pneumonia.
The penetration of lung tissues by antimicrobial agents does not always predict the eradication of susceptible pathogens, as the agents' pharmacodynamics can be adversely affected by the unique physiology of the respiratory system (24, 31). The pulmonary surfactant, even at the highest test concentration (1 mg/ml), did not affect the activity of telavancin in our in vitro assay. The activities of vancomycin and ceftriaxone were also unaffected by the surfactant, whereas the antibacterial activity of daptomycin was antagonized in a surfactant concentration-dependent manner. The latter finding is consistent with a previous in vitro simulation of daptomycin activity against a susceptible strain of S. pneumoniae in lung tissue, which showed that daptomycin was inactivated by pulmonary surfactant (31).
Telavancin was well tolerated at a dosage of 10 mg/kg/day infused over 1 h. The adverse events reported are consistent with those observed in previous clinical studies with the drug (7, 19, 30, 32-34).
In conclusion, this is the first study to determine the intrapulmonary distribution of telavancin in healthy subjects. The data presented herein demonstrated that telavancin penetrated well into ELF and extensively into AMs at concentrations that exceed the MIC90 for MRSA. Since the in vitro antibacterial activity of telavancin was unaffected by the presence of the pulmonary surfactant, these results support the clinical evaluation of this new agent for the treatment of pneumonia due to gram-positive pathogens.
Acknowledgments
This study was supported by a research grant from Astellas Pharma, Inc., and Theravance, Inc. Jeng-Pyng Shaw, Bret M. Benton, Kevin M. Krause, Michael R. Goldberg, Michael M. Kitt, and Steven L. Barriere are employees of Theravance, Inc.
We thank Elaine F. Griffin for assistance with the preparation of the manuscript.
Footnotes
Published ahead of print on 8 October 2007.
REFERENCES
- 1.Andrews, J. M., D. Honeybourne, G. Jevons, N. P. Brenwald, B. Cunningham, and R. Wise. 1997. Concentrations of levofloxacin (HR 355) in the respiratory tract following a single oral dose in patients undergoing fibre-optic bronchoscopy. J. Antimicrob. Chemother. 40:573-577. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin, D. R., D. Honeybourne, and R. Wise. 1992. Pulmonary disposition of antimicrobial agents: in vivo observations and clinical relevance. Antimicrob. Agents Chemother. 36:1176-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldwin, D. R., D. Honeybourne, and R. Wise. 1992. Pulmonary disposition of antimicrobial agents: methodological considerations. Antimicrob. Agents Chemother. 36:1171-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baldwin, D. R., S. R. Maxwell, D. Honeybourne, J. M. Andrews, J. P. Ashby, and R. Wise. 1991. The penetration of cefpirome into the potential sites of pulmonary infection. J. Antimicrob. Chemother. 28:79-86. [DOI] [PubMed] [Google Scholar]
- 5.Baldwin, D. R., R. Wise, J. M. Andrews, J. P. Ashby, and D. Honeybourne. 1990. Azithromycin concentrations at the sites of pulmonary infection. Eur. Respir. J. 3:886-890. [PubMed] [Google Scholar]
- 6.Baldwin, D. R., R. Wise, J. M. Andrews, and D. Honeybourne. 1991. Microlavage: a technique for determining the volume of epithelial lining fluid. Thorax 46:658-662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barriere, S., F. Genter, E. Spencer, M. Kitt, D. Hoelscher, and J. Morganroth. 2004. Effects of a new antibacterial, telavancin, on cardiac repolarization (QTc interval duration) in healthy subjects. J. Clin. Pharmacol. 44:689-695. [DOI] [PubMed] [Google Scholar]
- 8.Clinical and Laboratory Standards Institute. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard, 6th ed. CLSI document M7-A6. Clinical and Laboratory Standards Institute, Wayne, PA.
- 9.Conte, J. E., Jr., J. Golden, S. Duncan, E. McKenna, E. Lin, and E. Zurlinden. 1996. Single-dose intrapulmonary pharmacokinetics of azithromycin, clarithromycin, ciprofloxacin, and cefuroxime in volunteer subjects. Antimicrob. Agents Chemother. 40:1617-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conte, J. E., Jr., J. A. Golden, S. Duncan, E. McKenna, and E. Zurlinden. 1999. Intrapulmonary concentrations of pyrazinamide. Antimicrob. Agents Chemother. 43:1329-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conte, J. E., Jr., J. A. Golden, S. Duncan, E. McKenna, and E. Zurlinden. 1995. Intrapulmonary pharmacokinetics of clarithromycin and of erythromycin. Antimicrob. Agents Chemother. 39:334-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conte, J. E., Jr., J. A. Golden, J. Kipps, and E. Zurlinden. 2002. Intrapulmonary pharmacokinetics of linezolid. Antimicrob. Agents Chemother. 46:1475-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eliopoulos, G. M., and R. C. Moellering, Jr. 1996. Antimicrobial combinations, p. 330-396. In V. Lorian (ed.), Antibiotics in laboratory medicine, 4th ed. Williams & Wilkins, Baltimore, MD.
- 14.Gotfried, M. H., L. H. Danziger, and K. A. Rodvold. 2001. Steady-state plasma and intrapulmonary concentrations of levofloxacin and ciprofloxacin in healthy adult subjects. Chest 119:1114-1122. [DOI] [PubMed] [Google Scholar]
- 15.Higgins, D. L., R. Chang, D. V. Debabov, J. Leung, T. Wu, K. M. Krause, E. Sandvik, J. M. Hubbard, K. Kaniga, D. E. Schmidt, Jr., Q. Gao, R. T. Cass, D. E. Karr, B. M. Benton, and P. P. Humphrey. 2005. Telavancin, a multifunctional lipoglycopeptide, disrupts both cell wall synthesis and cell membrane integrity in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 49:1127-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LaFayette, A. J. 9 December 2004. Abbreviated validation to lower the range of a method for the determination of TD-6424 in human bronchial alveolar lavage fluid (BAL supernatant) by liquid chromatography and mass spectroscopy (LC/MS/MS) detection. Covance document 7057-234. Covance, Madison, WI.
- 17.LaFayette, A. J. 15 March 2006. Validation of a method for the determination of TD-6424 in human bronchial alveolar lavage fluid and alveolar macrophages by liquid chromatography and mass spectroscopy (LC/MS/MS) detection. Covance document 7057-212. Covance, Madison, WI.
- 18.LaFayette, A. J. 15 July 2004. Validation of a method for the determination of TD-6424, AMI-11352, and AMI-999 in human plasma by liquid chromatography and mass spectroscopy (LC/MS) detection. Covance document 7057-192. Covance, Madison, WI.
- 19.Laohavaleeson, S., J. L. Kuti, and D. P. Nicolau. 2007. Telavancin: a novel lipoglycopeptide for serious Gram-positive infections. Expert Opin. Investig. Drugs 16:347-357. [DOI] [PubMed] [Google Scholar]
- 20.Mathai, D., M. T. Lewis, K. C. Kugler, M. A. Pfaller, R. N. Jones, and SENTRY Participants Group (North America). 2001. Antibacterial activity of 41 antimicrobials tested against over 2773 bacterial isolates from hospitalized patients with pneumonia: I—results from the SENTRY Antimicrobial Surveillance Program (North America, 1998). Diagn. Microbiol. Infect. Dis. 39:105-116. [DOI] [PubMed] [Google Scholar]
- 21.Metropolitan Life Insurance Company. 1983. Metropolitan height and weight tables. Stat. Bull. Metrop. Life Insur. Co. 64:2-9. [PubMed] [Google Scholar]
- 22.Olsen, K. M., G. S. San Pedro, L. P. Gann, P. O. Gubbins, D. M. Halinski, and G. D. Campbell, Jr. 1996. Intrapulmonary pharmacokinetics of azithromycin in healthy volunteers given five oral doses. Antimicrob. Agents Chemother. 40:2582-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel, K. B., D. Xuan, P. R. Tessier, J. H. Russomanno, R. Quintiliani, and C. H. Nightingale. 1996. Comparison of bronchopulmonary pharmacokinetics of clarithromycin and azithromycin. Antimicrob. Agents Chemother. 40:2375-2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pennington, J. E. 1981. Penetration of antibiotics into respiratory secretions. Rev. Infect. Dis. 3:67-73. [DOI] [PubMed] [Google Scholar]
- 25.Rennard, S. I., G. Basset, D. Lecossier, K. M. O'Donnell, P. Pinkston, P. G. Martin, and R. G. Crystal. 1986. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J. Appl. Physiol. 60:532-538. [DOI] [PubMed] [Google Scholar]
- 26.Reyes, N., R. Skinner, K. Kaniga, K. M. Krause, J. Shelton, G. P. Obedencio, A. Gough, M. Conner, and S. S. Hegde. 2005. Efficacy of telavancin (TD-6424), a rapidly bactericidal lipoglycopeptide with multiple mechanisms of action, in a murine model of pneumonia induced by methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 49:4344-4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodvold, K. A., L. H. Danziger, and M. H. Gotfried. 2003. Steady-state plasma and bronchopulmonary concentrations of intravenous levofloxacin and azithromycin in healthy adults. Antimicrob. Agents Chemother. 47:2450-2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodvold, K. A., M. H. Gotfried, L. H. Danziger, and R. J. Servi. 1997. Intrapulmonary steady-state concentrations of clarithromycin and azithromycin in healthy adult volunteers. Antimicrob. Agents Chemother. 41:1399-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodvold, K. A., M. H. Gotfried, J. S. Loutit, and S. B. Porter. 2004. Plasma and intrapulmonary concentrations of oritavancin and vancomycin in normal healthy adults, abstr. O254. Clin. Microbiol. Infect. 10(Suppl. 3):44. [Google Scholar]
- 30.Shaw, J. P., J. Seroogy, K. Kaniga, D. L. Higgins, M. Kitt, and S. Barriere. 2005. Pharmacokinetics, serum inhibitory and bactericidal activity, and safety of telavancin in healthy subjects. Antimicrob. Agents Chemother. 49:195-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silverman, J. A., L. I. Mortin, A. D. Vanpraagh, T. Li, and J. Alder. 2005. Inhibition of daptomycin by pulmonary surfactant: in vitro modeling and clinical impact. J. Infect. Dis. 191:2149-2152. [DOI] [PubMed] [Google Scholar]
- 32.Stryjewski, M. E., V. H. Chu, W. D. O'Riordan, B. L. Warren, L. M. Dunbar, D. M. Young, M. Vallee, V. G. Fowler, Jr., J. Morganroth, S. L. Barriere, M. M. Kitt, and G. R. Corey for the FAST 2 Investigator Group. 2006. Telavancin versus standard therapy for treatment of complicated skin and skin structure infections caused by gram-positive bacteria: FAST 2 study. Antimicrob. Agents Chemother. 50:862-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stryjewski, M. E., W. D. O'Riordan, W. K. Lau, F. D. Pien, L. M. Dunbar, M. Vallee, V. G. Fowler, Jr., V. H. Chu, E. Spencer, S. L. Barriere, M. M. Kitt, C. H. Cabell, and G. R. Corey. 2005. Telavancin versus standard therapy for treatment of complicated skin and soft-tissue infections due to gram-positive bacteria. Clin. Infect. Dis. 40:1601-1607. [DOI] [PubMed] [Google Scholar]
- 34.Sun, H. K., K. Duchin, C. H. Nightingale, J.-P. Shaw, J. Seroogy, and D. P. Nicolau. 2006. Tissue penetration of telavancin after intravenous administration in healthy subjects. Antimicrob. Agents Chemother. 50:788-790. [DOI] [PMC free article] [PubMed] [Google Scholar]