Abstract
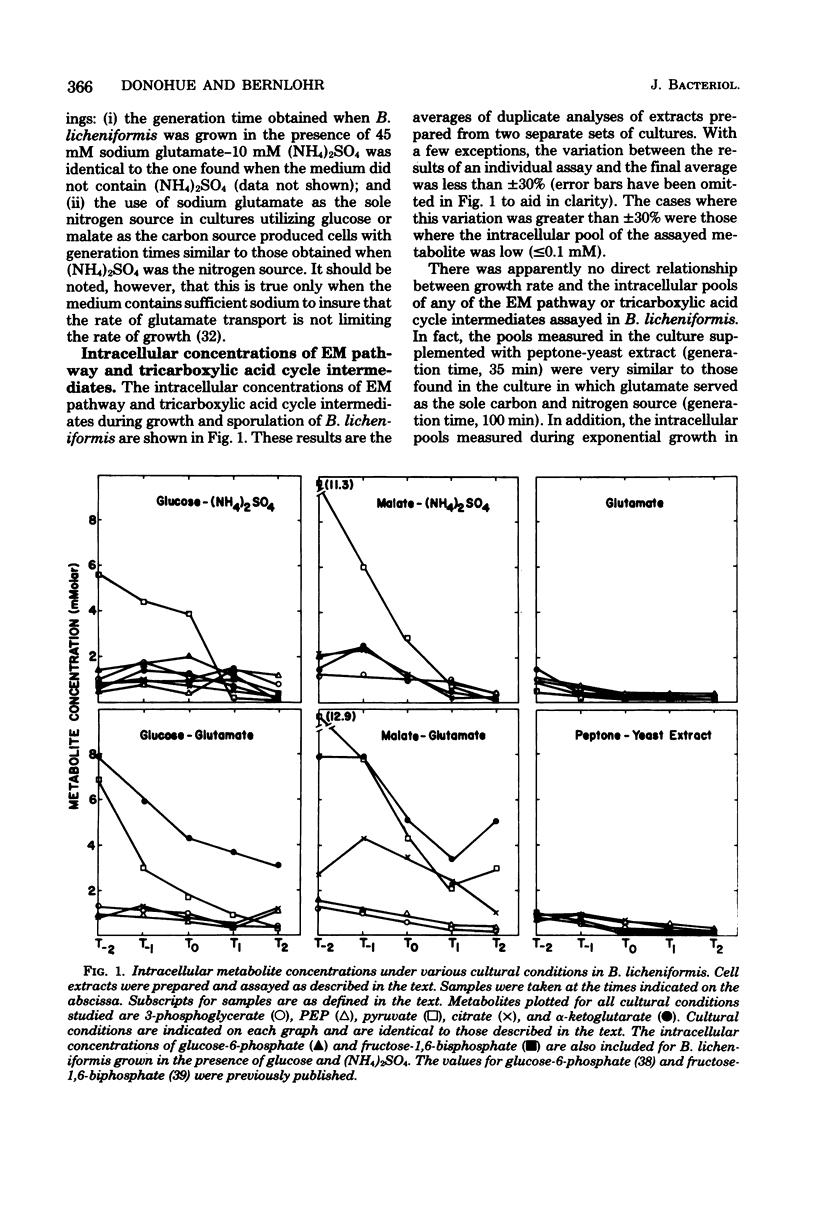
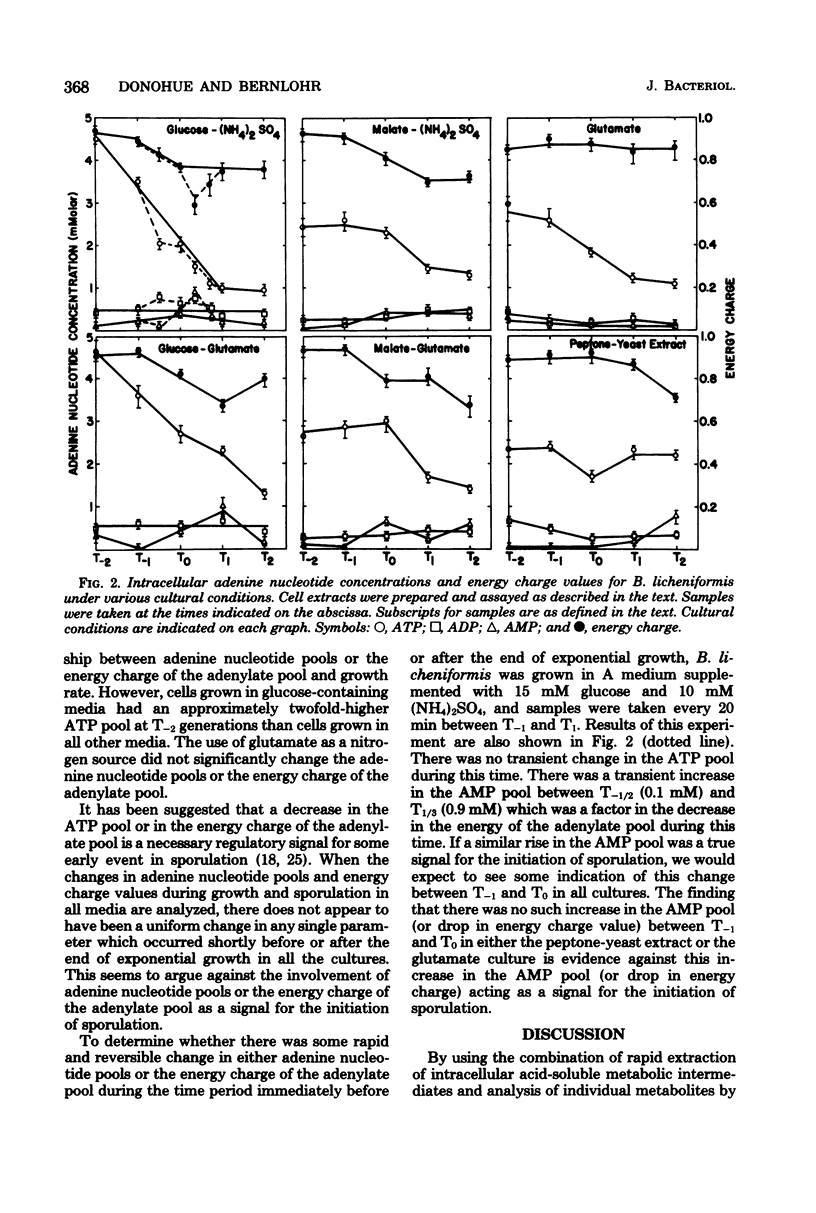
Intracellular concentrations of adenine nucleotides and intermediates of the Embden-Meyerhof pathway and the tricarboxylic acid cycle have been determined during growth and sporulation of Bacillus licheniformis in a variety of different media. The ATP pool was independent of growth rate and nitrogen source, but the use of glucose as a carbon source resulted in a twofold elevation in the ATP pool during exponential growth. The intracellular phosphoenolpyruvate pool was at least twofold higher during gluconeogenesis than during glycolysis. The finding that the use of glutamate as the sole nitrogen source resulted in at least a fivefold elevation of the alpha-ketoglutarate pool suggests a role for alpha-ketoglutarate in the repression of the enzymes of the tricarboxylic acid cycle responsible for alpha-ketoglutarate synthesis. Not one of the metabolites assayed appears to function as a signal of the nutrient deprivation which accompanies the initiation of sporulation.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernlohr R. W., Clark V. Characterization and regulation of protease synthesis and activity in Bacillus licheniformis. J Bacteriol. 1971 Jan;105(1):276–283. doi: 10.1128/jb.105.1.276-283.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernlohr R. W., Haddox M. K., Goldberg N. D. Cyclic guanosine 3':5'-monophosphate in Escherichia coli and Bacillus lichenformis. J Biol Chem. 1974 Jul 10;249(13):4329–4331. [PubMed] [Google Scholar]
- Buono F., Testa R., Lundgren D. G. Physiology of growth and sporulation in Bacillus cereus. I. Effect of glutamic and other amino acids. J Bacteriol. 1966 Jun;91(6):2291–2299. doi: 10.1128/jb.91.6.2291-2299.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascieri M., Amann R. P., Hammerstedt R. H. Adenine nucleotide changes at initiation of bull sperm motility. J Biol Chem. 1976 Feb 10;251(3):787–793. [PubMed] [Google Scholar]
- Clark V. L., Peterson D. E., Bernlohr R. W. Changes in free amino acid production and intracellular amino acid pools of Bacillus licheniformis as a function of culture age and growth media. J Bacteriol. 1972 Nov;112(2):715–725. doi: 10.1128/jb.112.2.715-725.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole H. A., Wimpenny J. W., Hughes D. E. The ATP pool in Escherichia coli. I. Measurement of the pool using modified luciferase assay. Biochim Biophys Acta. 1967;143(3):445–453. doi: 10.1016/0005-2728(67)90050-3. [DOI] [PubMed] [Google Scholar]
- Davison J. A., Fynn G. H. The assay of ATP by the luciferin-luciferase method. Interference by a bacterial phosphatase enzyme stable to perchlorate treatment. Anal Biochem. 1974 Apr;58(2):632–637. doi: 10.1016/0003-2697(74)90233-4. [DOI] [PubMed] [Google Scholar]
- Decker K., Pfitzer S. Determination of steady-state concentrations of adenine nucleotides in growing C. kluyveri cells by biosynthetic labeling. Anal Biochem. 1972 Dec;50(2):529–539. doi: 10.1016/0003-2697(72)90063-2. [DOI] [PubMed] [Google Scholar]
- Decker S. J., Lang D. R. Bacillus megaterium mutant deficient in membrane-bound adenosine triphosphatase activity. J Bacteriol. 1977 Jul;131(1):98–104. doi: 10.1128/jb.131.1.98-104.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diesterhaft M. D., Freese E. Role of pyruvate carboxylase, phosphoenolpyruvate carboxykinase, and malic enzyme during growth and sporulation of Bacillus subtilis. J Biol Chem. 1973 Sep 10;248(17):6062–6070. [PubMed] [Google Scholar]
- Gancedo J. M., Gancedo C. Concentrations of intermediary metabolites in yeast. Biochimie. 1973;55(2):205–211. doi: 10.1016/s0300-9084(73)80393-1. [DOI] [PubMed] [Google Scholar]
- Hammerstedt R. H. An automated method for ATP analysis utilizing the luciferin-luciferase reaction. Anal Biochem. 1973 Apr;52(2):449–455. doi: 10.1016/0003-2697(73)90047-x. [DOI] [PubMed] [Google Scholar]
- Hanson R. S., Cox D. P. Effect of different nutritional conditions on the synthesis of tricarboxylic acid cycle enzymes. J Bacteriol. 1967 Jun;93(6):1777–1787. doi: 10.1128/jb.93.6.1777-1787.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess B., Haeckel R., Brand K. FDP-activation of yeast pyruvate kinase. Biochem Biophys Res Commun. 1966 Sep 22;24(6):824–831. doi: 10.1016/0006-291x(66)90322-6. [DOI] [PubMed] [Google Scholar]
- Holms W. H., Hamilton I. D., Robertson A. G. The rate of turnover of the adenosine triphosphate pool of Escherichia coli growing aerobically in simple defined media. Arch Mikrobiol. 1972;83(2):95–109. doi: 10.1007/BF00425016. [DOI] [PubMed] [Google Scholar]
- Hunsley J. R., Suelter C. H. Yeast pyruvate kinase. II. Kinetic properties. J Biol Chem. 1969 Sep 25;244(18):4819–4822. [PubMed] [Google Scholar]
- Hutchison K. W., Hanson R. S. Adenine nucleotide changes associated with the initiation of sporulation in Bacillus subtilis. J Bacteriol. 1974 Jul;119(1):70–75. doi: 10.1128/jb.119.1.70-75.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klofat W., Picciolo G., Chappelle E. W., Freese E. Production of adenosine triphosphate in normal cells and sporulation mutants of Bacillus subtilis. J Biol Chem. 1969 Jun 25;244(12):3270–3276. [PubMed] [Google Scholar]
- Knowles C. J., Smith L. Measurements of ATP levels of intact Azotobacter vinelandii under different conditions. Biochim Biophys Acta. 1970 Mar 3;197(2):152–160. doi: 10.1016/0005-2728(70)90026-5. [DOI] [PubMed] [Google Scholar]
- Lam K. B., Marmur J. Isolation and characterization of Saccharomyces cerevisiae glycolytic pathway mutants. J Bacteriol. 1977 May;130(2):746–749. doi: 10.1128/jb.130.2.746-749.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry O. H., Carter J., Ward J. B., Glaser L. The effect of carbon and nitrogen sources on the level of metabolic intermediates in Escherichia coli. J Biol Chem. 1971 Nov;246(21):6511–6521. [PubMed] [Google Scholar]
- Lundin A., Thore A. Comparison of methods for extraction of bacterial adenine nucleotides determined by firefly assay. Appl Microbiol. 1975 Nov;30(5):713–721. doi: 10.1128/am.30.5.713-721.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod R. A., Thurman P., Rogers H. J. Comparative transport activity of intact cells, membrane vesicles, and mesosomes of Bacillus licheniformis. J Bacteriol. 1973 Jan;113(1):329–340. doi: 10.1128/jb.113.1.329-340.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeba P., Sanwal B. D. The regulation of pyruvate kinase of Escherichia coli by fructose diphosphate and adenylic acid. J Biol Chem. 1968 Jan 25;243(2):448–450. [PubMed] [Google Scholar]
- Malcovati M., Kornberg H. L. Two types of pyruvate kinase in Escherichia coli K12. Biochim Biophys Acta. 1969 Apr 22;178(2):420–423. doi: 10.1016/0005-2744(69)90417-3. [DOI] [PubMed] [Google Scholar]
- Marschke C. K., Bernlohr R. W. Purification and characterization of phosphofructokinase of Bacillus licheniformis. Arch Biochem Biophys. 1973 May;156(1):1–16. doi: 10.1016/0003-9861(73)90335-4. [DOI] [PubMed] [Google Scholar]
- Ohné M., Rutberg B. Repression of sporulation in Bacillus subtilis by L-malate. J Bacteriol. 1976 Feb;125(2):453–460. doi: 10.1128/jb.125.2.453-460.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opheim D. J., Bernlohr R. W. Purification and regulation of fructose-1,6-bisphosphatase from Bacillus licheniformis. J Biol Chem. 1975 Apr 25;250(8):3024–3033. [PubMed] [Google Scholar]
- Opheim D., Bernlohr R. W. Purification and regulation of glucose-6-phosphate dehydrogenase from Bacillus licheniformis. J Bacteriol. 1973 Dec;116(3):1150–1159. doi: 10.1128/jb.116.3.1150-1159.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertierra A. G., Cooper R. A. Pyruvate formation during the catabolism of simple hexose sugars by Escherichia coli: studies with pyruvate kinase-negative mutants. J Bacteriol. 1977 Mar;129(3):1208–1214. doi: 10.1128/jb.129.3.1208-1214.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phibbs P. V., Jr, Bernlohr R. W. Purification, properties, and regulation of glutamic dehydrogenase of Bacillus licheniformis. J Bacteriol. 1971 May;106(2):375–385. doi: 10.1128/jb.106.2.375-385.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P. Inability of detect cyclic AMP in vegetative or sporulating cells or dormant spores of Bacillus megaterium. Biochem Biophys Res Commun. 1973 May 15;52(2):365–372. doi: 10.1016/0006-291x(73)90720-1. [DOI] [PubMed] [Google Scholar]
- Siegel W. H., Donohue T., Bernlohr R. W. Determination of pools of tricarboxylic acid cycle and related acids in bacteria. Appl Environ Microbiol. 1977 Nov;34(5):512–517. doi: 10.1128/aem.34.5.512-517.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R. P., Setlow B., Setlow P. Levels of small molecules and enzymes in the mother cell compartment and the forespore of sporulating Bacillus megaterium. J Bacteriol. 1977 Jun;130(3):1130–1138. doi: 10.1128/jb.130.3.1130-1138.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague G. F., Jr Isolation and characterization of a Saccharomyces cerevisiae mutant deficient in pyruvate kinase activity. J Bacteriol. 1977 Apr;130(1):232–241. doi: 10.1128/jb.130.1.232-241.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuominen F. W., Bernlohr R. W. Pyruvate kinase of the spore-forming bacterium, Bacillus licheniformis. I. Purification, stability, regulation of synthesis, and evidence for multiple molecular states. J Biol Chem. 1971 Mar 25;246(6):1733–1745. [PubMed] [Google Scholar]
- Tuominen F. W., Bernlohr R. W. Pyruvate kinase of the spore-forming bacterium, Bacillus licheniformis. II. Kinetic properties. J Biol Chem. 1971 Mar 25;246(6):1746–1755. [PubMed] [Google Scholar]
- Wiener S., Wiener R., Urivetzky M., Meilman E. Coprecipitation of ATP with potassium perchlorate: the effect of the firefly enzyme assay of ATP in tissue and blood. Anal Biochem. 1974 Jun;59(2):489–500. doi: 10.1016/0003-2697(74)90302-9. [DOI] [PubMed] [Google Scholar]



