Abstract
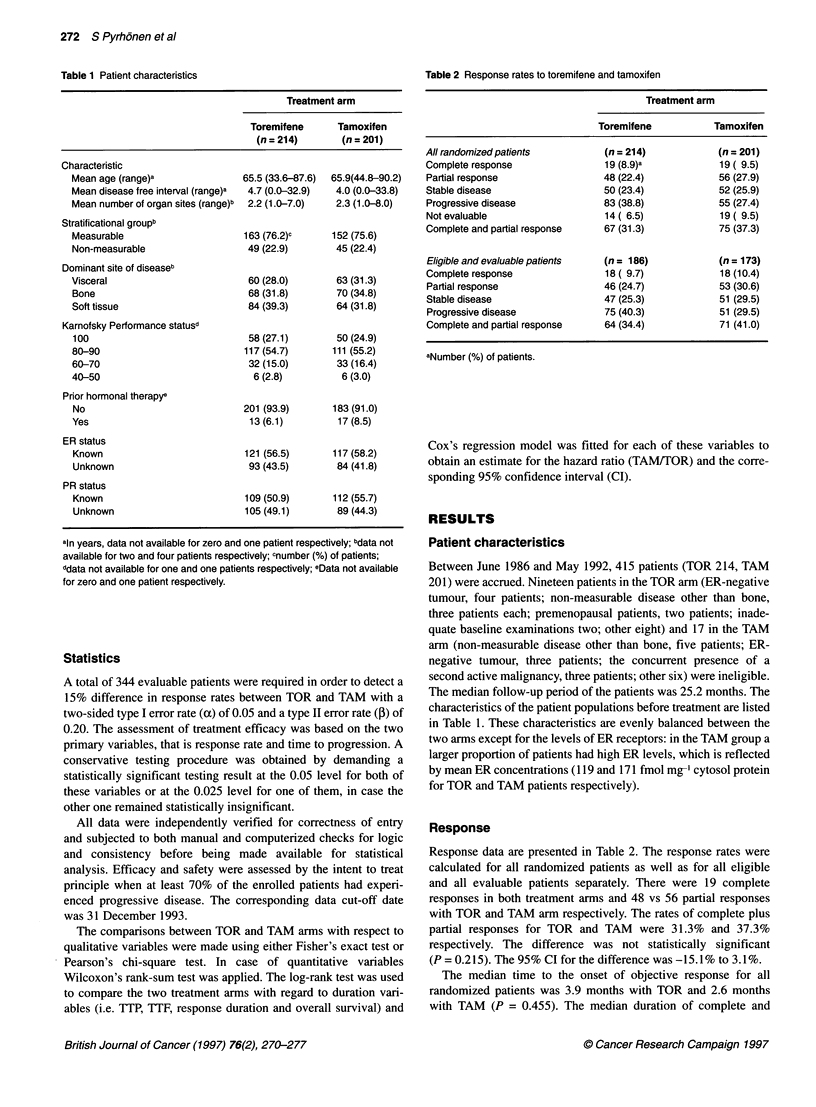
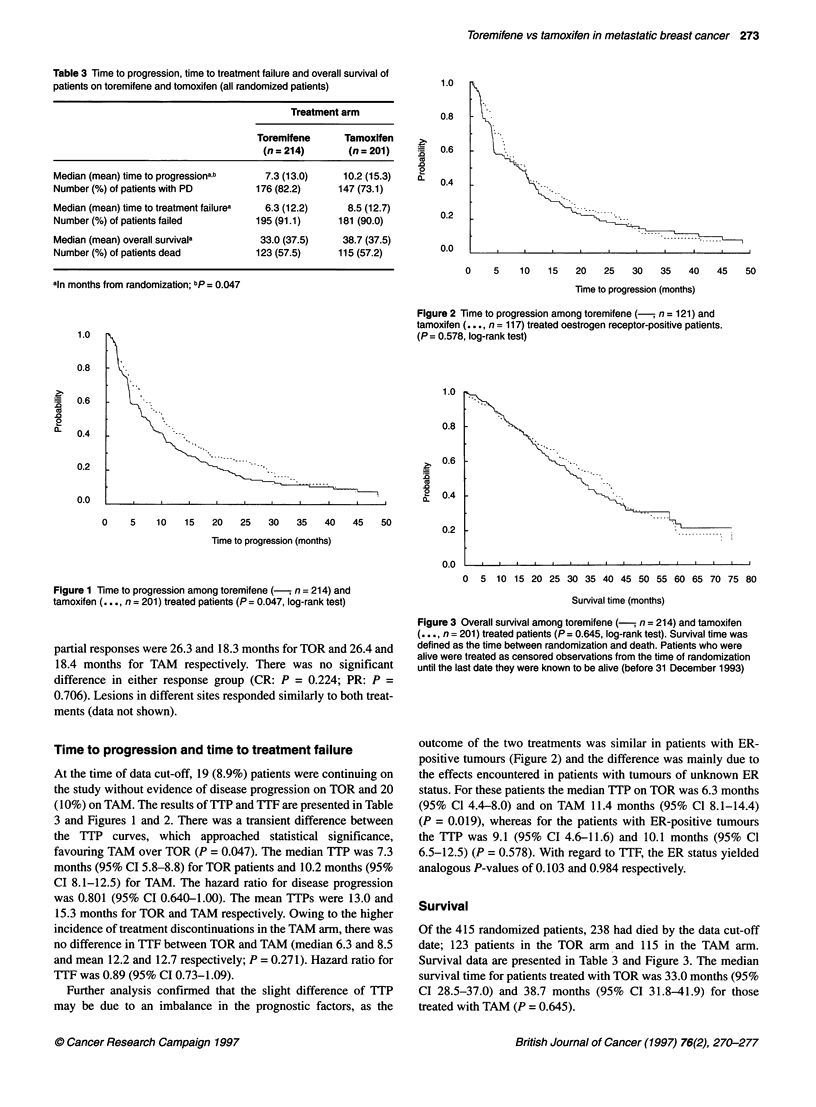
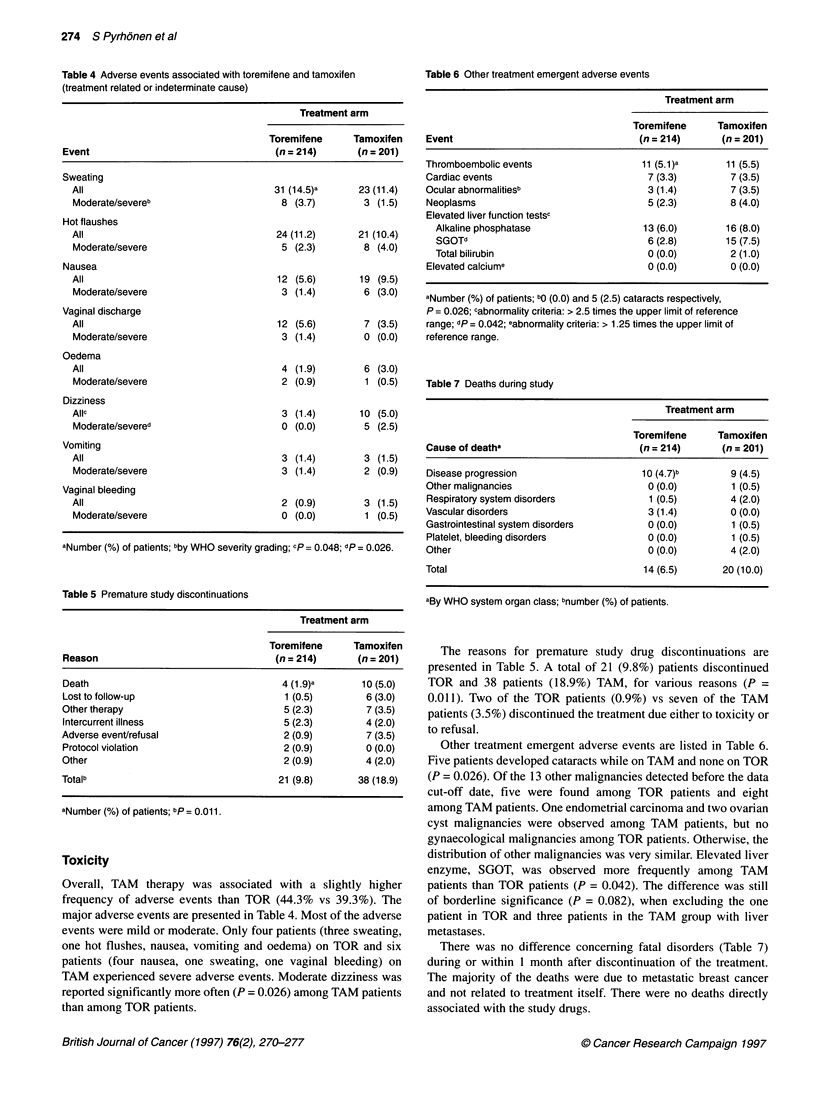
The study was planned to compare, in a prospective double-blind randomized trial, the efficacy and safety of toremifene (TOR) and tamoxifen (TAM) in post-menopausal patients with advanced breast cancer who have not had prior systemic therapy for advanced disease. Four hundred and fifteen post-menopausal patients with oestrogen receptor (ER)-positive or ER-unknown advanced breast cancer were randomly assigned to receive daily either 60 mg TOR or 40 mg TAM. The patients were stratified to measurable and non-measurable but evaluable groups. They were assessed for response to therapy, time to progression (TTP), time to treatment failure (TTF), response duration, overall survival and drug toxicity. Two hundred and fourteen patients were randomized into TOR and 201 into TAM treatment. The response rate (complete + partial) was 31.3% for TOR and 37.3% for TAM (P = 0.215). The 95% confidence interval (CI) for the 6% difference was -15.1% to 3.1%. The median TTP was 7.3 months for TOR and 10.2 months for TAM (P = 0.047). The 95% CI for the hazard ratio of 0.80 was 0.64-1.00. A percentage of the TOR patients (9.8%) and the TAM patients (18.9%) discontinued the treatment prematurely (P = 0.011) for various reasons. Consequently, the median TTF of 6.3 vs 8.5 months did not differ significantly (P = 0.271). The hazard ratio was 0.89 and the subsequent 95% CI 0.73-1.09. The median overall survival was 33.0 months for TOR and 38.7 months for TAM (P = 0.645). The hazard ratio was 0.94 with 95% CI of 0.73-1.22. The transient difference in TTP may be related to an imbalance in ER content of the tumours. When only patients with ER-positive tumours were considered (n = 238), no difference between two treatments was seen (P = 0.578). TAM was associated with an overall slightly higher frequency of adverse drug reactions than TOR (44.3 vs 39.3%) and a higher discontinuation rate due to these events (3.5% vs 0.9%). Treatment-emerged moderate dizziness (P = 0.026) and cataracts (P = 0.026) were more frequent among TAM than among TOR patients. In conclusion, TOR (60 mg day(-1)) and TAM (40 mg day(-1)) are equally effective and safe in the treatment of advanced post-menopausal ER-positive or ER-unknown breast cancer.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonomi P., Gale M., Von Roenn J., Anderson K., Johnson P., Wolter J., Economou S. Quantitative estrogen and progesterone receptor levels related to progression-free interval in advanced breast cancer patients treated with megestrol acetate or tamoxifen. Semin Oncol. 1988 Apr;15(2 Suppl 1):26–33. [PubMed] [Google Scholar]
- Fisher B., Costantino J. P., Redmond C. K., Fisher E. R., Wickerham D. L., Cronin W. M. Endometrial cancer in tamoxifen-treated breast cancer patients: findings from the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14. J Natl Cancer Inst. 1994 Apr 6;86(7):527–537. doi: 10.1093/jnci/86.7.527. [DOI] [PubMed] [Google Scholar]
- Fornander T., Hellström A. C., Moberger B. Descriptive clinicopathologic study of 17 patients with endometrial cancer during or after adjuvant tamoxifen in early breast cancer. J Natl Cancer Inst. 1993 Nov 17;85(22):1850–1855. doi: 10.1093/jnci/85.22.1850. [DOI] [PubMed] [Google Scholar]
- Hamm J. T., Tormey D. C., Kohler P. C., Haller D., Green M., Shemano I. Phase I study of toremifene in patients with advanced cancer. J Clin Oncol. 1991 Nov;9(11):2036–2041. doi: 10.1200/JCO.1991.9.11.2036. [DOI] [PubMed] [Google Scholar]
- Han X. L., Liehr J. G. Induction of covalent DNA adducts in rodents by tamoxifen. Cancer Res. 1992 Mar 1;52(5):1360–1363. [PubMed] [Google Scholar]
- Hard G. C., Iatropoulos M. J., Jordan K., Radi L., Kaltenberg O. P., Imondi A. R., Williams G. M. Major difference in the hepatocarcinogenicity and DNA adduct forming ability between toremifene and tamoxifen in female Crl:CD(BR) rats. Cancer Res. 1993 Oct 1;53(19):4534–4541. [PubMed] [Google Scholar]
- Hayes D. F., Van Zyl J. A., Hacking A., Goedhals L., Bezwoda W. R., Mailliard J. A., Jones S. E., Vogel C. L., Berris R. F., Shemano I. Randomized comparison of tamoxifen and two separate doses of toremifene in postmenopausal patients with metastatic breast cancer. J Clin Oncol. 1995 Oct;13(10):2556–2566. doi: 10.1200/JCO.1995.13.10.2556. [DOI] [PubMed] [Google Scholar]
- Heier J. S., Dragoo R. A., Enzenauer R. W., Waterhouse W. J. Screening for ocular toxicity in asymptomatic patients treated with tamoxifen. Am J Ophthalmol. 1994 Jun 15;117(6):772–775. doi: 10.1016/s0002-9394(14)70321-6. [DOI] [PubMed] [Google Scholar]
- Hietanen T., Baltina D., Johansson R., Numminen S., Hakala T., Helle L., Valavaara R. High dose toremifene (240 mg daily) is effective as first line hormonal treatment in advanced breast cancer. An ongoing phase II multicenter Finnish-Latvian cooperative study. Breast Cancer Res Treat. 1990 Aug;16 (Suppl):S37–S40. doi: 10.1007/BF01807143. [DOI] [PubMed] [Google Scholar]
- Hirsimäki P., Hirsimäki Y., Nieminen L., Payne B. J. Tamoxifen induces hepatocellular carcinoma in rat liver: a 1-year study with two antiestrogens. Arch Toxicol. 1993;67(1):49–54. doi: 10.1007/BF02072035. [DOI] [PubMed] [Google Scholar]
- Jaiyesimi I. A., Buzdar A. U., Decker D. A., Hortobagyi G. N. Use of tamoxifen for breast cancer: twenty-eight years later. J Clin Oncol. 1995 Feb;13(2):513–529. doi: 10.1200/JCO.1995.13.2.513. [DOI] [PubMed] [Google Scholar]
- Jönsson P. E., Malmberg M., Bergljung L., Ingvar C., Ericsson M., Ryden S., Nilsson I., Terje I. J. Phase II study of high dose toremifene in advanced breast cancer progressing during tamoxifene treatment. Anticancer Res. 1991 Mar-Apr;11(2):873–875. [PubMed] [Google Scholar]
- Kallio S., Kangas L., Blanco G., Johansson R., Karjalainen A., Perilä M., Pippo I., Sundquist H., Södervall M., Toivola R. A new triphenylethylene compound, Fc-1157a. I. Hormonal effects. Cancer Chemother Pharmacol. 1986;17(2):103–108. doi: 10.1007/BF00306736. [DOI] [PubMed] [Google Scholar]
- Kangas L. Agonistic and antagonistic effects of antiestrogens in different target organs. Acta Oncol. 1992;31(2):143–146. doi: 10.3109/02841869209088894. [DOI] [PubMed] [Google Scholar]
- Kangas L., Nieminen A. L., Blanco G., Grönroos M., Kallio S., Karjalainen A., Perilä M., Södervall M., Toivola R. A new triphenylethylene compound, Fc-1157a. II. Antitumor effects. Cancer Chemother Pharmacol. 1986;17(2):109–113. doi: 10.1007/BF00306737. [DOI] [PubMed] [Google Scholar]
- Karlsson S., Hirsimäki Y., Mäntylä E., Nieminen L., Kangas L., Hirsimäki P., Perry C. J., Mulhern M., Millar P., Handa J. A two-year dietary carcinogenicity study of the antiestrogen toremifene in Sprague-Dawley rats. Drug Chem Toxicol. 1996 Nov;19(4):245–266. doi: 10.3109/01480549608998236. [DOI] [PubMed] [Google Scholar]
- Kivinen S., Mäenpä J. Effect of toremifene on clinical chemistry, hematology and hormone levels at different doses in healthy postmenopausal volunteers: phase I study. J Steroid Biochem. 1990 Jun 22;36(3):217–220. doi: 10.1016/0022-4731(90)90008-g. [DOI] [PubMed] [Google Scholar]
- Lahti E., Blanco G., Kauppila A., Apaja-Sarkkinen M., Taskinen P. J., Laatikainen T. Endometrial changes in postmenopausal breast cancer patients receiving tamoxifen. Obstet Gynecol. 1993 May;81(5 ):660–664. [PubMed] [Google Scholar]
- Metzler M., Degen G. H. Sex hormones and neoplasia: liver tumors in rodents. Arch Toxicol Suppl. 1987;10:251–263. doi: 10.1007/978-3-642-71617-1_23. [DOI] [PubMed] [Google Scholar]
- Miller A. B., Hoogstraten B., Staquet M., Winkler A. Reporting results of cancer treatment. Cancer. 1981 Jan 1;47(1):207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Montandon F., Williams G. M. Comparison of DNA reactivity of the polyphenylethylene hormonal agents diethylstilbestrol, tamoxifen and toremifene in rat and hamster liver. Arch Toxicol. 1994;68(4):272–275. doi: 10.1007/s002040050068. [DOI] [PubMed] [Google Scholar]
- Pavlidis N. A., Petris C., Briassoulis E., Klouvas G., Psilas C., Rempapis J., Petroutsos G. Clear evidence that long-term, low-dose tamoxifen treatment can induce ocular toxicity. A prospective study of 63 patients. Cancer. 1992 Jun 15;69(12):2961–2964. doi: 10.1002/1097-0142(19920615)69:12<2961::aid-cncr2820691215>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Pyrhönen S., Valavaara R., Heikkinen M., Rissanen P., Blanco G., Nordman E., Holsti L. R., Hajba A. Treatment of advanced breast cancer with 20 mg toremifene, a phase II study. Preliminary communication. J Steroid Biochem. 1990 Jun 22;36(3):227–228. doi: 10.1016/0022-4731(90)90011-g. [DOI] [PubMed] [Google Scholar]
- Pyrhönen S., Valavaara R., Vuorinen J., Hajba A. High dose toremifene in advanced breast cancer resistant to or relapsed during tamoxifen treatment. Breast Cancer Res Treat. 1994;29(3):223–228. doi: 10.1007/BF00666475. [DOI] [PubMed] [Google Scholar]
- Rutqvist L. E., Johansson H., Signomklao T., Johansson U., Fornander T., Wilking N. Adjuvant tamoxifen therapy for early stage breast cancer and second primary malignancies. Stockholm Breast Cancer Study Group. J Natl Cancer Inst. 1995 May 3;87(9):645–651. doi: 10.1093/jnci/87.9.645. [DOI] [PubMed] [Google Scholar]
- Santen R. J., Manni A., Harvey H., Redmond C. Endocrine treatment of breast cancer in women. Endocr Rev. 1990 May;11(2):221–265. doi: 10.1210/edrv-11-2-221. [DOI] [PubMed] [Google Scholar]
- Spinelli C., Berti P., Ricci E., Miccoli P. Multicentric breast tumour: an anatomical-clinical study of 100 cases. Eur J Surg Oncol. 1992 Feb;18(1):23–26. [PubMed] [Google Scholar]
- Styles J. A., Davies A., Lim C. K., De Matteis F., Stanley L. A., White I. N., Yuan Z. X., Smith L. L. Genotoxicity of tamoxifen, tamoxifen epoxide and toremifene in human lymphoblastoid cells containing human cytochrome P450s. Carcinogenesis. 1994 Jan;15(1):5–9. doi: 10.1093/carcin/15.1.5. [DOI] [PubMed] [Google Scholar]
- Tominaga T., Abe O., Izuo M., Nomura Y. [Phase II study of NK 622 (toremifene citrate) in advanced breast cancer, a multicentral cooperative dose finding study]. Gan To Kagaku Ryoho. 1993 Jan;20(1):79–90. [PubMed] [Google Scholar]
- Valavaara R., Pyrhönen S., Heikkinen M., Rissanen P., Blanco G., Thölix E., Nordman E., Taskinen P., Holsti L., Hajba A. Toremifene, a new antiestrogenic compound, for treatment of advanced breast cancer. Phase II study. Eur J Cancer Clin Oncol. 1988 Apr;24(4):785–790. doi: 10.1016/0277-5379(88)90316-1. [DOI] [PubMed] [Google Scholar]
- Valavaara R., Tuominen J., Johansson R. Predictive value of tumor estrogen and progesterone receptor levels in postmenopausal women with advanced breast cancer treated with toremifene. Cancer. 1990 Dec 1;66(11):2264–2269. doi: 10.1002/1097-0142(19901201)66:11<2264::aid-cncr2820661103>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Vancutsem P. M., Lazarus P., Williams G. M. Frequent and specific mutations of the rat p53 gene in hepatocarcinomas induced by tamoxifen. Cancer Res. 1994 Jul 15;54(14):3864–3867. [PubMed] [Google Scholar]
- Vogel C. L., Shemano I., Schoenfelder J., Gams R. A., Green M. R. Multicenter phase II efficacy trial of toremifene in tamoxifen-refractory patients with advanced breast cancer. J Clin Oncol. 1993 Feb;11(2):345–350. doi: 10.1200/JCO.1993.11.2.345. [DOI] [PubMed] [Google Scholar]
- Vuorio T., Wärri A., Sandberg M., Alitalo K., Vuorio E. Expression of the c-Ha-ras and neu oncogenes in DMBA-induced, anti-estrogen-treated rat mammary tumors. Int J Cancer. 1988 Nov 15;42(5):774–779. doi: 10.1002/ijc.2910420524. [DOI] [PubMed] [Google Scholar]
- White I. N., de Matteis F., Davies A., Smith L. L., Crofton-Sleigh C., Venitt S., Hewer A., Phillips D. H. Genotoxic potential of tamoxifen and analogues in female Fischer F344/n rats, DBA/2 and C57BL/6 mice and in human MCL-5 cells. Carcinogenesis. 1992 Dec;13(12):2197–2203. doi: 10.1093/carcin/13.12.2197. [DOI] [PubMed] [Google Scholar]
- Williams G. M., Iatropoulos M., Cheung R., Radi L., Wang C. X. Diethylstilbestrol liver carcinogenicity and modification of DNA in rats. Cancer Lett. 1993 Feb;68(2-3):193–198. doi: 10.1016/0304-3835(93)90146-z. [DOI] [PubMed] [Google Scholar]



