Abstract
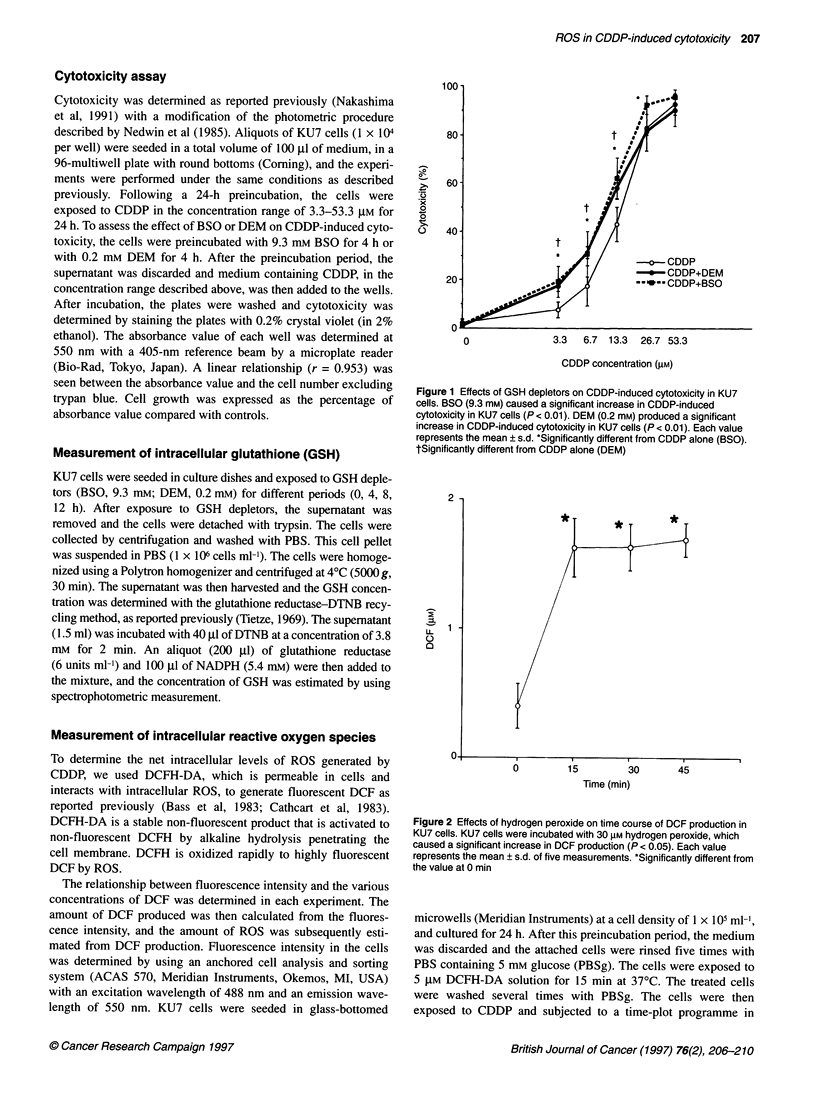
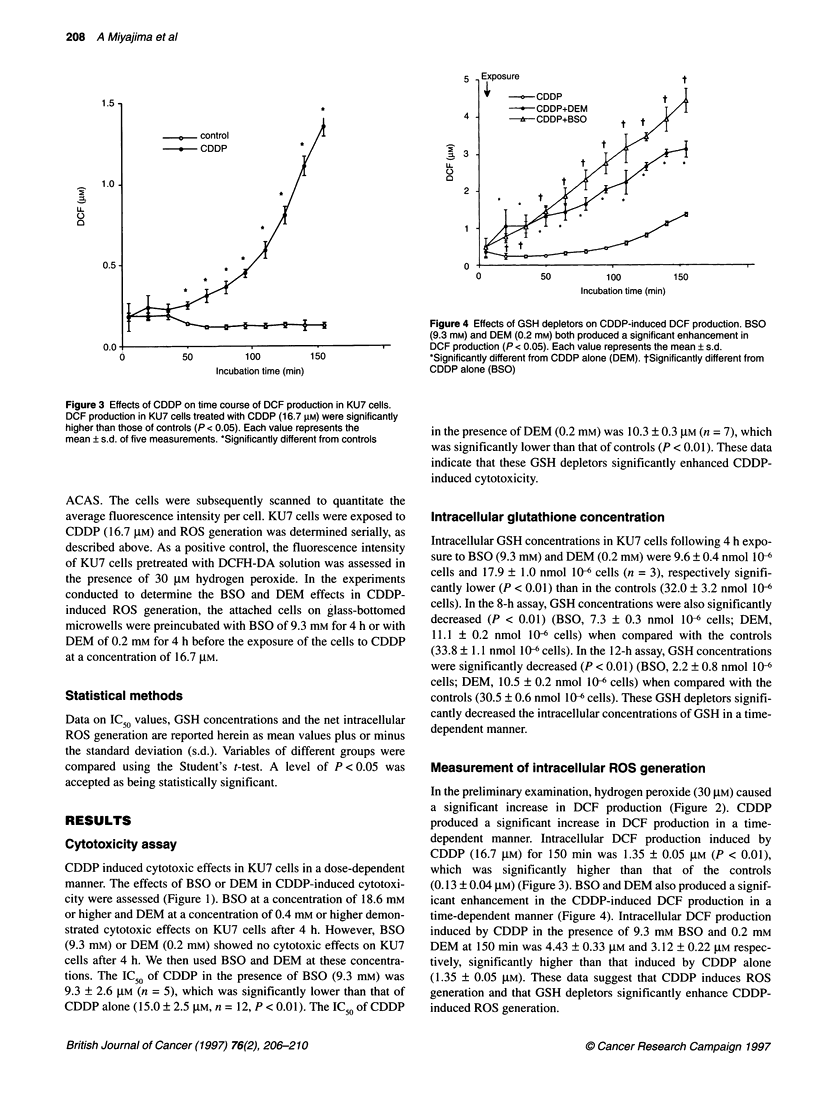
This study was undertaken to investigate the intracellular induction of reactive oxygen species (ROS) by cis-dichlorodiammineplatinum (CDDP) and the augmentation of their cytotoxicity in bladder cancer cells (KU7) by enhancement of ROS generation by the glutathione (GSH) depletors buthionine sulphoximine (BSO) and diethylmaleate (DEM). CDDP-induced cytotoxicity in KU7 cells and its modulation by GSH depletors were determined using spectrophotometric measurement with crystal violet staining. The effects of GSH depletors on intracellular GSH levels were confirmed using the GSH reductase-DTNB recycling method. Intracellular ROS generation induced by CDDP with or without GSH depletors was estimated from the amount of intracellular dichlorofluorescein (DCF), an oxidized product of dichlorofluorescein (DCFH), which was measured with an anchored cell analysis and sorting system. The cytotoxic effects of CDDP (IC50 15.0 +/- 2.5 microM) were significantly enhanced by BSO (IC50 9.3 +/- 2.6 microM, P < 0.01) and DEM (IC50 10.3 +/- 0.3 microM, P <0.01). BSO and DEM produced a significant depletion in intracellular GSH levels (9.6 +/- 0.4 nmol 10(-6) cells, 17.9 +/- 1.0 nmol 10(-6) cells) compared with the controls (30.5 +/- 0.6 nmol 10(-6) cells). Intracellular DCF production in KU7 cells treated with CDDP (1.35 +/- 0.33 microM) was significantly enhanced by the addition of BSO (4.43 +/- 0.33 microM) or DEM (3.12 +/- 0.22 microM) at 150 min. These results suggest that ROS may play a substantial role in CDDP-induced cytotoxicity and that GSH depletors augment its cytotoxicity through an enhancement of ROS generation in bladder cancer cells.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. E., Naganuma A., Meister A. Protection against cisplatin toxicity by administration of glutathione ester. FASEB J. 1990 Nov;4(14):3251–3255. doi: 10.1096/fasebj.4.14.2227215. [DOI] [PubMed] [Google Scholar]
- Bass D. A., Parce J. W., Dechatelet L. R., Szejda P., Seeds M. C., Thomas M. Flow cytometric studies of oxidative product formation by neutrophils: a graded response to membrane stimulation. J Immunol. 1983 Apr;130(4):1910–1917. [PubMed] [Google Scholar]
- Bulkley G. B. The role of oxygen free radicals in human disease processes. Surgery. 1983 Sep;94(3):407–411. [PubMed] [Google Scholar]
- Busciglio J., Yankner B. A. Apoptosis and increased generation of reactive oxygen species in Down's syndrome neurons in vitro. Nature. 1995 Dec 21;378(6559):776–779. doi: 10.1038/378776a0. [DOI] [PubMed] [Google Scholar]
- Cathcart R., Schwiers E., Ames B. N. Detection of picomole levels of hydroperoxides using a fluorescent dichlorofluorescein assay. Anal Biochem. 1983 Oct 1;134(1):111–116. doi: 10.1016/0003-2697(83)90270-1. [DOI] [PubMed] [Google Scholar]
- Coyle J. T., Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993 Oct 29;262(5134):689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- Evans D. L., Dive C. Effects of cisplatin on the induction of apoptosis in proliferating hepatoma cells and nonproliferating immature thymocytes. Cancer Res. 1993 May 1;53(9):2133–2139. [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Gordon J. A., Gattone V. H., 2nd Mitochondrial alterations in cisplatin-induced acute renal failure. Am J Physiol. 1986 Jun;250(6 Pt 2):F991–F998. doi: 10.1152/ajprenal.1986.250.6.F991. [DOI] [PubMed] [Google Scholar]
- Ishikawa T., Ali-Osman F. Glutathione-associated cis-diamminedichloroplatinum(II) metabolism and ATP-dependent efflux from leukemia cells. Molecular characterization of glutathione-platinum complex and its biological significance. J Biol Chem. 1993 Sep 25;268(27):20116–20125. [PubMed] [Google Scholar]
- Matsuki N., Torii Y., Saito H. Effects of iron and deferoxamine on cisplatin-induced emesis: further evidence for the role of free radicals. Eur J Pharmacol. 1993 Dec 1;248(4):329–331. doi: 10.1016/0926-6917(93)90008-e. [DOI] [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- Meijer C., Mulder N. H., Hospers G. A., Uges D. R., de Vries E. G. The role of glutathione in resistance to cisplatin in a human small cell lung cancer cell line. Br J Cancer. 1990 Jul;62(1):72–77. doi: 10.1038/bjc.1990.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer C., Mulder N. H., Timmer-Bosscha H., Zijlstra J. G., de Vries E. G. Role of free radicals in an adriamycin-resistant human small cell lung cancer cell line. Cancer Res. 1987 Sep 1;47(17):4613–4617. [PubMed] [Google Scholar]
- Meister A. Mitochondrial changes associated with glutathione deficiency. Biochim Biophys Acta. 1995 May 24;1271(1):35–42. doi: 10.1016/0925-4439(95)00007-q. [DOI] [PubMed] [Google Scholar]
- Meister A. Selective modification of glutathione metabolism. Science. 1983 Apr 29;220(4596):472–477. doi: 10.1126/science.6836290. [DOI] [PubMed] [Google Scholar]
- Micetich K., Zwelling L. A., Kohn K. W. Quenching of DNA:platinum(II) monoadducts as a possible mechanism of resistance to cis-diamminedichloroplatinum(II) in L1210 cells. Cancer Res. 1983 Aug;43(8):3609–3613. [PubMed] [Google Scholar]
- Mickisch G. H., Roehrich K., Koessig J., Forster S., Tschada R. K., Alken P. M. Mechanisms and modulation of multidrug resistance in primary human renal cell carcinoma. J Urol. 1990 Sep;144(3):755–759. doi: 10.1016/s0022-5347(17)39586-1. [DOI] [PubMed] [Google Scholar]
- Mimnaugh E. G., Dusre L., Atwell J., Myers C. E. Differential oxygen radical susceptibility of adriamycin-sensitive and -resistant MCF-7 human breast tumor cells. Cancer Res. 1989 Jan 1;49(1):8–15. [PubMed] [Google Scholar]
- Mãrtensson J., Meister A., Mrtensson J. Glutathione deficiency decreases tissue ascorbate levels in newborn rats: ascorbate spares glutathione and protects. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4656–4660. doi: 10.1073/pnas.88.11.4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårtensson J., Jain A., Stole E., Frayer W., Auld P. A., Meister A. Inhibition of glutathione synthesis in the newborn rat: a model for endogenously produced oxidative stress. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9360–9364. doi: 10.1073/pnas.88.20.9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima J., Brookins J., Beckman B., Fisher J. W. Increased erythropoietin secretion in human hepatoma cells by N6-cyclohexyladenosine. Am J Physiol. 1991 Sep;261(3 Pt 1):C455–C460. doi: 10.1152/ajpcell.1991.261.3.C455. [DOI] [PubMed] [Google Scholar]
- Nedwin G. E., Svedersky L. P., Bringman T. S., Palladino M. A., Jr, Goeddel D. V. Effect of interleukin 2, interferon-gamma, and mitogens on the production of tumor necrosis factors alpha and beta. J Immunol. 1985 Oct;135(4):2492–2497. [PubMed] [Google Scholar]
- Sausville E. A., Stein R. W., Peisach J., Horwitz S. B. Properties and products of the degradation of DNA by bleomycin and iron(II). Biochemistry. 1978 Jul 11;17(14):2746–2754. doi: 10.1021/bi00607a008. [DOI] [PubMed] [Google Scholar]
- Schulze-Osthoff K., Bakker A. C., Vanhaesebroeck B., Beyaert R., Jacob W. A., Fiers W. Cytotoxic activity of tumor necrosis factor is mediated by early damage of mitochondrial functions. Evidence for the involvement of mitochondrial radical generation. J Biol Chem. 1992 Mar 15;267(8):5317–5323. [PubMed] [Google Scholar]
- Shibayama T., Tachibana M., Deguchi N., Jitsukawa S., Tazaki H. SCID mice: a suitable model for experimental studies of urologic malignancies. J Urol. 1991 Oct;146(4):1136–1137. doi: 10.1016/s0022-5347(17)38025-4. [DOI] [PubMed] [Google Scholar]
- Sugihara K., Nakano S., Gemba M. Effect of cisplatin on in vitro production of lipid peroxides in rat kidney cortex. Jpn J Pharmacol. 1987 May;44(1):71–76. doi: 10.1254/jjp.44.71. [DOI] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Torii Y., Mutoh M., Saito H., Matsuki N. Involvement of free radicals in cisplatin-induced emesis in Suncus murinus. Eur J Pharmacol. 1993 Aug 2;248(2):131–135. doi: 10.1016/0926-6917(93)90034-n. [DOI] [PubMed] [Google Scholar]
- Uslu R., Bonavida B. Involvement of the mitochondrion respiratory chain in the synergy achieved by treatment of human ovarian carcinoma cell lines with both tumor necrosis factor-alpha and cis-diamminedichloroplatinum. Cancer. 1996 Feb 15;77(4):725–732. [PubMed] [Google Scholar]
- Yamauchi N., Kuriyama H., Watanabe N., Neda H., Maeda M., Niitsu Y. Intracellular hydroxyl radical production induced by recombinant human tumor necrosis factor and its implication in the killing of tumor cells in vitro. Cancer Res. 1989 Apr 1;49(7):1671–1675. [PubMed] [Google Scholar]
- Zwelling L. A., Anderson T., Kohn K. W. DNA-protein and DNA interstrand cross-linking by cis- and trans-platinum(II) diamminedichloride in L1210 mouse leukemia cells and relation to cytotoxicity. Cancer Res. 1979 Feb;39(2 Pt 1):365–369. [PubMed] [Google Scholar]



